Abstract
A set of twenty-four 3-hydroxynaphthalene-2-carboxanilides, disubstituted on the anilide ring by combinations of methoxy/methyl/fluoro/chloro/bromo and ditrifluoromethyl groups at different positions, was prepared. The compounds were tested for their ability to inhibit photosynthetic electron transport (PET) in spinach (Spinacia oleracea L.) chloroplasts. N-(3,5-Difluorophenyl)-, N-(3,5-dimethylphenyl)-, N-(2,5-difluorophenyl)- and N-(2,5-dimethylphenyl)-3-hydroxynaphthalene-2-carboxamides showed the highest PET-inhibiting activity (IC50 ~ 10 µM) within the series. These compounds were able to inhibit PET in photosystem II. It has been found that PET-inhibiting activity strongly depends on the position of the individual substituents on the anilide ring and on the lipophilicity of the compounds. The electron-withdrawing properties of the substituents contribute towards the PET activity of these compounds.
Keywords: hydroxynaphthalene-carboxamides, PET inhibition, spinach chloroplasts, structure-activity relationships
1. Introduction
Due to population growth, there is a constant pressure on farmers to multiply yields to ensure sufficient food. On the other hand, this challenge is difficult to meet due to deteriorating conditions for agriculture, such as the loss of quality agricultural land, desiccation or, conversely, heavy rains and floods, climate change and the rise of many plant and crop destroyers. One way to combat pathogens of plants is to use pesticides to help farmers increase productivity per hectare by protecting plants from pests, diseases and weeds. For example, food crops must compete with approximately 30,000 weed species. Herbicides are still used widely around the world because manual weeding has never been an effective method of weed control, especially when large-scale farming is used. Herbicides are often used instead of tillage because the use of herbicides reduces erosion, fuel consumption, greenhouse gas emissions and nutrient leakage, and saves water compared to plowing. Of course, the question remains as to what extent the negative chemical effects of herbicides harm non-target organisms and degrade soil and water resources [1,2,3].
Herbicides can be classified according to the type/chemical structure of the active ingredient, mechanism of action, method and time of application, mobility, type of formulation or residual effect [4,5,6]. There are currently about 20 different mechanisms of action of herbicides [4,5,6,7,8,9,10,11], but over 50% of commercially available herbicides act by reversibly binding to photosystem II (PS II), resulting in disruption of photosynthetic electron transport (PET) [4,6,7,8]. PS II uses light energy to oxidize water and reduce plastoquinone, which consists of parts QA and QB. The plastoquinone QA acting as a single electron acceptor is permanently bound to PS II; the plastoquinone QB acting as a two-electron acceptor is loosely bound; after reduction, it separates from the reaction center and diffuses into the hydrophobic membrane nucleus, the QB binding site being occupied by the oxidized molecule plastoquinone [12]. Herbicides belonging to inhibitors of PS II inhibit photosynthetic electron transfer (PET) by binding to the QB binding niche on the D1 protein of the PS II complex in chloroplast thylakoid membranes, leading to inhibition of PET from QA to QB, blocking CO2 fixation and inhibition of ATP production [6,9,10,11].
Studies of large libraries of structurally diverse PS II inhibitors have confirmed the hydrophobic nature of the binding domain, with lipophilicity being the dominant determinant of Hill inhibitory activity [12,13,14,15,16,17,18]. Significant amounts of herbicides acting as PET inhibitors in PS II contain an amide (–CONH–) and/or carbamate (–HNCOO–) bond in their structure that is capable of forming hydrogen bonds between the amide/carbamate group and target proteins in the photosynthetic centers of thylakoid membranes, leading to conformational changes and PET inhibition [19,20,21,22,23,24,25]. Both the N- and O-terminal ends of the CONH linker are substituted and the substituents further modify the bond properties and strength of the basic scaffold [26]. Amides are thought to be inhibitors of PS II, causing the displacement of QB from its binding pocket in the D1 protein [27], and halogenated substituents have been found to contribute to increased PET inhibitory activity [18,23,24,28,29,30].
Our team has long been investigating the effects of a wide range of variously substituted napthalenecarboxanilides [23,24,27,29,30,31] and quinolinecarboxanilides [32] on PS II. A series of ring-monosubstituted anilides of 3-hydroxynaphthalene-2-carboxylic acid was published by Kos et al. [29] and some interesting biological activity was found, including herbicidal activity. Since monosubstituted derivatives of 3-hydroxy-N-arylnaphthalene-2-carboxanilides showed PET inhibition in spinach chloroplasts (Spinacia oleracea L.), select new, variously disubstituted, derivatives were evaluated for their PET-inhibiting activity.
2. Results and Discussion
2.1. Chemistry
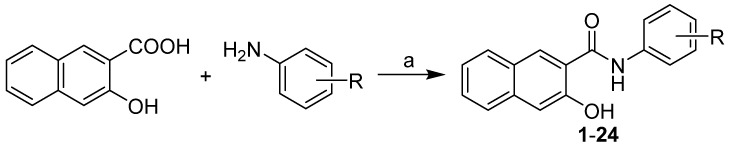
All compounds were prepared by the reaction of 3-hydroxynaphthalene-2-carboxylic acid with appropriate disubstituted anilines with the addition of phosphorus trichloride in dry chlorobenzene under microwave conditions (Scheme 1) [29,31], which resulted in a series of target N-(disubstituted-phenyl)-3-hydroxynaphthalene-2-carboxamides 1–24, see Table 1.
Scheme 1.
Synthesis of 3-hydroxy-N-arylnaphthalene-2-carboxanilides 1–24. Reagents and conditions: (a) PCl3, chlorobenzene, MW, 45 min [29,31].
Table 1.
Structure of ring-disubstituted 3-hydroxynaphthalene-2-carboxanilides 1–24, calculated values of Clog P for compounds, electronic σ parameters of anilide (Ar) and IC50 [µM] values related to photosynthetic electron transport (PET) inhibition in spinach chloroplasts of tested compounds in comparison with the 3-(3,4-dichlorophenyl)-1,1-dimethylurea (DCMU) standard.
| ||||
|---|---|---|---|---|
| Comp. | R | Clog P 2 | σ(Ar) 3 | PET Inhibition IC50 [µM] |
| 1 1 | 2,5-OCH3 | 3.9563 | 0.08 | 183 |
| 2 1 | 3,5-OCH3 | 4.5463 | 0.93 | 24.5 |
| 3 1 | 2,5-CH3 | 4.7942 | 0.59 | 11.6 |
| 4 1 | 2,6-CH3 | 4.1442 | 0.58 | 28.5 |
| 5 1 | 3,5-CH3 | 5.4442 | 0.59 | 9.9 |
| 6 1 | 2,5-F | 4.4799 | 1.24 | 11.2 |
| 7 1 | 2,6-F | 3.8799 | 1.44 | 78.7 |
| 8 1 | 3,5-F | 5.0799 | 1.12 | 9.8 |
| 9 1 | 2,5-Cl | 5.3699 | 1.22 | 321 |
| 10 1 | 2,6-Cl | 4.5199 | 1.33 | 156 |
| 11 1 | 3,4-Cl | 6.0999 | 1.19 | 47.5 |
| 12 1 | 3,5-Cl | 6.2199 | 1.11 | 39.2 |
| 13 | 2,4-Br | 5.6399 | 1.11 | 296 |
| 14 | 2,5-Br | 5.6399 | 1.23 | 161 |
| 15 1 | 3,5-CF3 | 6.8207 | 1.05 | 15.9 |
| 16 | 2-OCH3-5-F | 4.2725 | 0.14 | 79.1 |
| 17 | 2-F-6-OCH3 | 3.6725 | 0.16 | 507 |
| 18 | 3-F-5-OCH3 | 4.8625 | 0.99 | 31.6 |
| 19 | 2-Cl-5-OCH3 | 4.5825 | 1.13 | 171 |
| 20 | 2-F-4-Cl | 5.0499 | 1.17 | 1405 |
| 21 1 | 3-F-4-Br | 5.7999 | 1.16 | 527 |
| 22 1 | 3-F-5-CF3 | 6.0131 | 1.04 | 31.0 |
| 23 1 | 2-Cl-5-CF3 | 5.7331 | 1.19 | 13.2 |
| 24 1 | 2-Br-4-CF3 | 5.8531 | 1.32 | 621 |
| DCMU | – | – | – | 2.1 |
1 Compounds described in [31]; 2 ChemBioDraw Ultra 13.0 (CambridgeSoft, PerkinElmer Inc., MA, USA); 3 calculated using ACD/Percepta ver. 2012 (Advanced Chemistry Development, Toronto, ON, Canada).
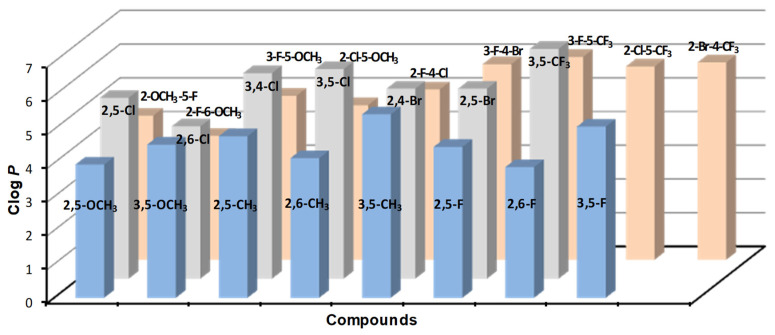
Lipophilicity is an extremely important parameter in the design of any biologically active compound, as it primarily ensures sufficient penetration across biological membranes to reach the target site of action [33]. In general, it can be stated that a higher value of lipophilicity is required for agrochemicals acting in plant leaves due to the permeability of a stronger and more lipophilic cuticle [34]. Lipophilicity, expressed as Clog P values (predicted by ChemBioDraw Ultra 13.0), of the investigated compounds is listed in Table 1. Clog P values ranged from 3.6 (compound 17, R = 2-F-6-OCH3) to 6.8 (compound 15, R = 3,5-CF3). When comparing the general effect of substituents at the same positions on lipophilicity, the order of the groups with respect to the increasing contribution of lipophilicity is as follows: OCH3 < F < CH3 < Cl < Br < CF3; thus, in general, any substitution by a fluorine or methoxy moiety significantly decreases lipophilicity. In addition to the type of substituent, their mutual position on the aniline ring also has a significant effect on the lipophilicity value. For example, in series with dichlorinated derivatives, lipophilicity increases as follows: 2.6 < 2.5 < 3.4 < 3.5. In series with different moieties of disubstituted compounds, the Clog P values are significantly decreased when a fluorine or methoxy moiety is introduced, especially in positions C(2)′ and C(6)′, as mentioned above. The predicted Clog P values are presented in the illustrated order in Figure 1, where they are simultaneously divided into three groups according to the nature of the substitution. The first group consists of methoxy-, methyl- and fluoro-disubstituted compounds 1–8; the second group consists of dichloro, dibromo and 3,5-CF3 derivatives 9–15; and derivatives 16–24, disubstituted by two different substituents, are in the third group. This division proves important for the description of PET inhibition, see below.
Figure 1.
Graphical comparison of lipophilicity of investigated compounds expressed as Clog P, predicted by ChemBioDraw Ultra 13.0.
Electronic contributions of substituents are another important parameter, especially for substituted aromatic rings (anilines, phenols). The electron σ parameters of the whole substituted anilide ring, predicted by the ADC/Percepta program, are listed in Table 1. As with the lipophilicity values, the σ values are in a wide range. Based on the results of the prediction program, the weakest electron-withdrawing properties have the substitution 2,5-OCH3-Ph of compound 1 (σ = 0.08), while the strongest electron-withdrawing properties have fluoro-substituted derivative 7 (2,6-F-Ph, σ = 1.44). These values affect the electron density at the amide linker and thus the overall binding to the putative site of action of these compounds, which is on the acceptor side of PS II, at the section between P680 (primary donor of PS II) and QB [23,24,25,29].
2.2. Inhibition of Photosynthetic Electron Transport (PET) in Spinach Chloroplasts
The PET-inhibition of the studied compounds was expressed by the negative logarithm of the IC50 value (concentration (in µM) of the compounds causing a 50% decrease in the oxygen evolution rate relative to the untreated control). The evaluated disubstituted 3-hydroxynaphthalene-2-carboxanilides showed a wide range of PET inhibition in spinach (Spinacia oleracea L.) chloroplasts with the IC50 values ranging from 9.8 to 1405 µM, see Table 1. N-(3,5-Difluorophenyl)-(8) and N-(3,5-dimethylphenyl)-(5), N-(2,5-difluorophenyl)-(6) and N-(2,5-dimethylphenyl)-(3) 3-hydroxynaphthalene-2-carboxamides demonstrated the highest PET-inhibiting activity (IC50 ~ 10 µM) within the whole investigated series. Acceptable activity was also found for N-(2-chloro-5-trifluoromethylphenyl)-(23) and N-(3,5-ditrifluoromethylphenyl)-3-hydroxynaphthalene-2-carboxamides (15) with IC50 13.2 and 15.9 µM, respectively. On the other hand, derivatives 21 (3-F-4-Br), 24 (2-Br-4-CF3) and 20 (R = 2-F-4-Cl) were completely inactive (IC50 = 527, 621 and 1405 µM, respectively).
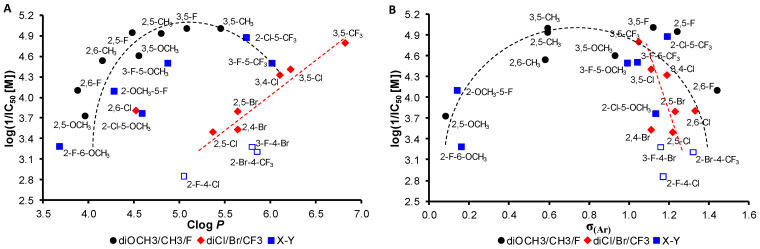
The results of this screening indicate that the position of the substituents is crucial for the activity, with the 3,5 positions being the most preferred (i.e., both meta positions are substituted). However, 2,5-disubstituted derivatives also showed PET-inhibiting activity when substituted with moieties with suitable properties, including electronic properties and lipophilicity. As mentioned above, lipophilicity tends to affect biological activity. The dependence of the PET-inhibiting activity, expressed as log(1/IC50 [M]), of the investigated compounds in spinach chloroplasts on lipophilicity (Clog P) is shown in Figure 2A. It can be stated that most of the evaluated compounds substituted by OCH3/CH3/F can be traced to a quasi-parabolic dependence with the optimum Clog P ca. 5. The active compounds have a range of lipophilicity values from 4.4 to 5.7. On the other hand, a linear dependence can be observed for the dichloro-, dibromo- and bis(trifluoromethyl)-substituted compounds, i.e., markedly lipophilic groups. The inhibition of PET increases with increasing lipophilicity.
Figure 2.
Dependence of PET-inhibiting activity log(1/IC50 [M]) of all discussed compounds 1–24 in spinach chloroplasts on lipophilicity expressed as Clog P (A) and electronic σ parameters of whole N-aryl part of individual anilides (B). Empty squares are not involved in SAR discussion due to their inactivity.
Figure 2B shows the dependence of the PET-inhibiting activity, expressed as log(1/IC50 [M]), on the electronic σ(Ar) properties of the whole anilide substituents. As can be seen, electronic properties play a secondary role compared to lipophilicity and substituent position; however, the quasi-parabolic (for OCH3/CH3/F substituted compounds) or linear (for disubstituted compounds by Cl/Br/CF3 moieties) trend is evident. It can be stated that electron-withdrawing properties in the range of σ(Ar) from approximately 0.6 to 1.2 are preferred.
Based on the structural similarity of the test compounds to previously performed experiments with salicylanilides or hydroxynaphthanilides, the same mechanism of action can be supposed, i.e., inhibition on the acceptor side of PS II, at the section between P680 (primary donor of PS II) and plastoquinone QB [20,21,22,23,24,27,29,30,31]. Furthermore, it should be noted that plastoquinone QB on the acceptor side of PS II has been found to be the site of inhibitory action of other amide-based derivatives [6,13,14,15,25,35], such as N-phenylpyrazine-2-carboxamides [19], N-substituted 2-aminobenzothiazoles [22] or 8-hydroxyquinoline-2-carboxanilides [32].
3. Materials and Methods
3.1. General Information
All reagents were purchased from Merck (Sigma-Aldrich, St. Louis, MO, USA) and Alfa (Alfa-Aesar, Ward Hill, MA, USA). Reactions were performed using a CEM Discover SP microwave reactor (CEM, Matthews, NC, USA). The melting points were determined on a Kofler hot-plate apparatus HMK (Franz Kustner Nacht KG, Dresden, Germany) and are uncorrected. Infrared (IR) spectra were recorded on a Smart MIRacle™ ATR ZnSe for Nicolet™ Impact 410 Fourier-transform IR spectrometer (Thermo Scientific, West Palm Beach, FL, USA). The spectra were obtained by the accumulation of 256 scans with 2 cm−1 resolution in the region of 4000–650 cm−1. All 1H- and 13C-NMR spectra were recorded in dimethyl sulfoxide-d6 (DMSO-d6) at 600 MHz for 1H and 150 MHz for 13C, on an Agilent VNMRS 600 MHz system (Agilent Technologies, Santa Clara, CA, USA). The 1H and 13C chemical shifts (δ) are reported in ppm. High-resolution mass spectra were measured using a high-performance liquid chromatograph Dionex UltiMate® 3000 (Thermo Scientific, West Palm Beach, FL, USA) coupled with an LTQ Orbitrap XLTM Hybrid Ion Trap-Orbitrap Fourier Transform Mass Spectrometer (Thermo Scientific) equipped with a HESI II (heated electrospray ionization) source in positive and negative mode.
3.2. Synthesis
General Procedure for the Synthesis of N-(Disubstituted phenyl)-3-hydroxynaphthalene-2-carboxamides 1–24
3-Hydroxynaphthalene-2-carboxylic acid (0.5 g, 2.65 mM) was suspended in dry chlorobenzene (20 mL) at ambient temperature and phosphorus trichloride (0.12 mL, 1.35 mM), and the corresponding substituted aniline (2.65 mM) was added dropwise. The reaction mixture was transferred to the microwave reactor, where the synthesis was performed (1st phase: 10 min, 100 °C; 2nd phase: 15 min, 120 °C; 3rd phase: 20 min, 130 °C; max 500 W). The mixture was then cooled to 60 °C, and the solvent was removed under reduced pressure. The residue was washed sequentially with hydrochloric acid and water, and the crude product was recrystallized from EtOH. All the compounds are presented in Table 1.
The synthesis and analytical data for anilides 1–12, 15 and 21–24 were described previously [31].
N-(2,4-Dibromophenyl)-3-hydroxynaphthalene-2-carboxamide (13). Yield 56%; mp 241–243 °C; IR (cm−1): 3221; 1641; 1625; 1603; 1575; 1524; 1462; 1448; 1398; 1363; 1345; 1321; 1290; 1240; 1206; 1175; 1146; 1081; 1035; 951; 913; 878; 867; 846; 825; 791; 767; 737; 688; 1H NMR (DMSO-d6), δ: 11.97 (s, 1H), 11.07 (s, 1H), 8.70 (s, 1H), 8.42 (d, J = 8.8 Hz, 1H), 7.99 (d, J = 8.2 Hz, 1H), 7.97 (d, J = 2.2 Hz, 1H), 7.78 (d, J = 8.3 Hz, 1H), 7.66 (dd, J = 2.2 Hz, J = 8.8 Hz, 1H), 7.53 (ddd, J = 1.2 Hz, J = 6.8 Hz, J = 8.3 Hz, 1H), 7.38 (s, 1H), 7.37 (ddd, J = 1.1 Hz, J = 6.8 Hz, J = 8.2 Hz, 1H); 13C NMR (DMSO-d6), δ: 163.6, 152.6, 136.2, 136.1, 134.4, 132.8, 131.3, 129.1, 128.6, 127.2, 125.7, 124.4, 124.0, 120.4, 116.3, 114.9, 110.8; HR-MS C17H12O2NBr2 [M + H]+ calculated 419.9229 m/z, found 419.9237 m/z.
N-(2,5-Dibromophenyl)-3-hydroxynaphthalene-2-carboxamide (14). Yield 49%; mp 233–235 °C; IR (cm−1): 3190; 1636; 1622; 1597; 1568; 1506; 1447; 1393; 1360; 1344; 1250; 1192; 1174; 1147; 1080; 1069; 1029; 962; 915; 902; 868; 848; 796; 770; 750; 736; 1H NMR (DMSO-d6), δ: 12.02 (s, 1H), 11.14 (s, 1H), 8.74 (d, J = 2.3 Hz, 1H), 8.71 (s, 1H), 8.00 (d, J = 8.2 Hz, 1H), 7.79 (d, J = 8.3 Hz, 1H), 7.69 (d, J = 8.5 Hz, 1H), 7.54 (ddd, J = 1.2 Hz, J = 6.8 Hz, J = 8.3 Hz, 1H), 7.39 (s, 1H), 7.38 (ddd, J = 1.1 Hz, J = 6.8 Hz, J = 8.2 Hz, 1H), 7.32 (dd, J = 2.4 Hz, J = 8.5 Hz, 1H); 13C NMR (DMSO-d6), δ: 163.6, 152.5, 138.1, 136.2, 134.2, 133.0, 129.1, 128.7, 128.1, 127.2, 125.7, 125.0, 124.0, 120.9, 120.3, 112.7, 110.8; MS C17H12O2NBr2 [M + H]+ calculated 419.9229 m/z, found 419.9239 m/z.
N-(5-Fluoro-2-methoxyphenyl)-3-hydroxynaphthalene-2-carboxamide (16). Yield 80%; mp 198–203 °C; IR (cm−1): 3194, 1640, 1625, 1615, 1601, 1538, 1488, 1432, 1393, 1356, 1346, 1249, 1214, 1176, 1148, 1065, 1038, 975, 866, 838, 786, 731, 711; 1H-NMR (DMSO-d6), δ: 11.86 (s, 1H), 11.25 (s, 1H), 8.70 (s, 1H), 8.40 (dd, J = 11.0 Hz, J=3.3 Hz, 1H), 7.93 (d, J = 8.1 Hz, 1H), 7.78 (d, J = 8.4 Hz, 1H), 7.53 (t, J = 7.5 Hz, 1H), 7.37 (s, 1H), 7.36 (t, J = 7.5 Hz, 1H), 7.12 (dd, J = 9.2 Hz, J = 5.1 Hz, 1H), 6.92 (td, J = 8.6 Hz, J = 3.3 Hz, 1H), 3.92 (s, 3H); 13C-NMR (DMSO-d6), δ: 163.0, 156.0 (d, J = 232.2 Hz), 152.4, 144.8 (d, J = 1.8 Hz), 136.0, 132.9, 129.1, 129.0 (d, J = 12.9 Hz), 128.5, 127.2, 125.7, 123.9, 121.0, 111.7 (d, J = 9.1 Hz), 110.8, 109.0 (d, J = 22.8 Hz), 106.9 (d, J = 29.6 Hz), 57.0; HR-MS: C18H13FNO3 [M − H]− calculated 310.0885 m/z, found 310.0881 m/z.
N-(2-Fluoro-6-methoxyphenyl)-3-hydroxynaphthalene-2-carboxamide (17). Yield 66%; mp 138–144 °C; IR (cm−1): 3259, 2836, 1651, 1622, 1596, 1532, 1515, 1506, 1466, 1438, 1279, 1249, 1216, 1167, 1146, 1087, 900, 873, 834, 789, 767, 747, 728; 1H-NMR (DMSO-d6), δ: 11.76 (s, 1H), 10.22 (s, 1H), 8.69 (s, 1H), 7.93 (d, J = 8.8 Hz, 1H), 7.78 (d, J = 8.4 Hz, 1H), 7.54 (t, J = 7.3 Hz, 1H), 7.30–7.40 (m, 3H), 6.99 (d, J = 8.4 Hz, 1H), 6.94 (t, J = 9.0 Hz, 1H), 3.84 (s, 3H); 13C-NMR (DMSO-d6), δ: 166.3, 158.1 (d, J = 246.4 Hz), 155.8 (d, J = 5.3 Hz), 154.6, 136.2, 131.0, 128.9, 128.5, 128.2 (d, J = 10.7 Hz), 126.8, 125.8, 123.9, 118.7, 113.7 (d, J = 15.3 Hz), 110.9, 107.9 (d, J = 26.4 Hz), 107.7, 56.3; HR-MS: C18H13FNO3 [M − H]− calculated 310.0885 m/z, found 310.0880 m/z.
N-(3-Fluoro-5-methoxyphenyl)-3-hydroxynaphthalene-2-carboxamide (18). Yield 59%; mp 227–230 °C; IR (cm−1): 3147, 1644, 1622, 1595, 1557, 1520, 1456, 1448, 1359, 1261, 1224, 1212, 1191, 1141, 1129, 1063, 999, 987, 872, 858, 816, 767, 745, 690; 1H-NMR (DMSO-d6), δ: 11.12 (s, 1H), 10.64 (s, 1H), 8.41 (s, 1H), 7.93 (d, J = 8.4 Hz, 1H), 7.76 (d, J = 8.1 Hz, 1H), 7.51 (t, J = 7.0 Hz, 1H), 7.32-7.40 (m, 3H), 7.21 (s, 1H), 6.62 (d, J = 11.0 Hz, 1H), 3.78 (s, 3H); 13C-NMR (DMSO-d6), δ: 165.7, 162.9 (d, J = 238.5 Hz), 160.7 (d, J = 12.9 Hz), 153.3, 140.6 (d, J = 13.7 Hz), 135.7, 130.5, 128.7, 128.1, 126.9, 125.8, 123.8, 122.5, 110.5, 102.0 (d, J = 2.0 Hz), 99.4 (d, J = 27.3 Hz), 97.0 (d, J = 25.0 Hz), 55.6; HR-MS: C18H13FNO3 [M − H]− calculated 310.0885 m/z, found 310.0881 m/z.
N-(2-Chloro-5-methoxyphenyl)-3-hydroxynaphthalene-2-carboxamide (19). Yield 58%; mp 187–188 °C; IR (cm−1): 3177, 2954, 2834, 1638, 1624, 1598, 1539, 1462, 1447, 1427, 1358, 1305, 1274, 1262, 1220, 1167, 1147, 1135, 1063, 1028, 960, 916, 866, 845, 787, 771, 745, 719; 1H-NMR (DMSO-d6), δ: 11.97 (s, 1H), 11.17 (s, 1H), 8.73 (s, 1H), 8.25 (d, J = 2.9 Hz, 1H), 7.99 (d, J = 8.2 Hz, 1H), 7.78 (d, J = 8.3 Hz, 1H), 7.53 (ddd, J = 8.3 Hz, J = 6.8 Hz, J = 1.2 Hz, 1H), 7.46 (d, J = 8.8 Hz, 1H), 7.38 (ddd, J = 8.2 Hz, J = 6.8 Hz, J = 1.2 Hz, 1H), 7.38 (s, 1H), 6.78 (dd, J = 8.8 Hz, J = 3.0 Hz, 1H), 3.80 (s, 3H); 13C-NMR (DMSO-d6), δ: 163.4, 158.5, 152.5, 136.1, 132.9, 129.6, 129.1, 128.6, 127.2, 125.7, 124.0, 120.6, 114.2, 110.8, 110.4, 108.0, 55.5; HR-MS: C18H15ClNO3 [M + H]+ calculated 328.0735 m/z, found 328.0737 m/z.
N-(4-Chloro-2-fluorophenyl)-3-hydroxynaphthalene-2-carboxamide (20). Yield 75%; mp 267–269 °C; IR (cm−1): 3194, 1647, 1627, 1601, 1552, 1489, 1449, 1414, 1393, 1357, 1338, 1259, 1207, 1174, 1147, 1118, 1064, 951, 918, 897, 870, 841, 820, 767, 740, 722, 667; 1H NMR (DMSO-d6) δ: 11.85 (s, 1H), 10.97 (s, 1H), 8.66 (s, 1H), 8.37 (t, J = 8.7 Hz, 1H), 7.97 (d, J = 8.2 Hz, 1H), 7.77 (d, J = 8.3 Hz, 1H), 7.56 (dd, J = 2.4 Hz, J = 10.8 Hz, 1H), 7.52 (ddd, J = 1.2 Hz, J = 6.8 Hz, J = 8.3 Hz, 1H), 7.37 (ddd, J = 1.1 Hz, J = 6.8 Hz, J = 8.2 Hz, 1H), 7.36 (s, 1H), 7.34 (ddd, J = 1.2 Hz, J = 2.4 Hz, J = 8.8 Hz, 1H). 13C NMR (DMSO-d6), δ: 163.8, 152.8 (d, J = 247.5 Hz), 152.8, 136.1, 132.4, 129.0, 128.5, 127.9 (d, J = 10.0 Hz), 127.1, 125.7, 125.7 (d, J = 10.7 Hz), 124.9 (d, J = 3.4 Hz), 124.0, 123.6, 120.4, 115.9 (d, J = 23.1 Hz), 110.9; HR-MS: C17H12ClFNO2 [M + H]+ calculated 316.0535 m/z, found 316.0535 m/z.
3.3. Study of Inhibition of Photosynthetic Electron Transport (PET) in Spinach Chloroplasts
Chloroplasts were prepared from spinach (Spinacia oleracea L.) according to Kralova et al. [36]. Screening was performed as described previously [e.g., 19–25,31]. A selective herbicide 3-(3,4-dichlorophenyl)-1,1-dimethylurea, DCMU (Diuron®, Merck, Darmstadt, Germany) was used as a standard. The results are summarized in Table 1.
4. Conclusions
A series of 3-hydroxynaphthalene-2-carboxanilides substituted with two similar or different atoms or groups on the anilide ring was prepared under microwave-assisted conditions and tested for their ability to inhibit photosynthetic electron transport (PET) in spinach (Spinacia oleracea L.) chloroplasts. N-(3,5-Difluorophenyl)-3-hydroxynaphthalene-2-carboxamide (8), N-(3,5-dimethylphenyl)-3-hydroxynaphthalene-2-carboxamide (5), N-(2,5-difluorophenyl)-3-hydroxynaphthalene-2-carboxamide (6) and N-(2,5-dimethylphenyl)-3-hydroxynaphthalene-2-carboxamide (3) exhibited the highest PET-inhibiting activity with their IC50 values ranging from 9.8 to 11.6 µM. The C(3,5)′ and C(2,5)′ disubstituted isomers were found to be the most active among the test compounds. Furthermore, for diOCH3/diCH3/diF substituted derivatives, a Clog P value of approximately 5 is important, while for diCl/diBr/diCF3 substituted derivatives, PET inhibition increases with increasing lipophilicity to a Clog P value of 6.8 of N-(3,5-ditrifluoromethylphenyl)-3-hydroxynaphthalene-2-carboxamides (15) with IC50 = 15.9 µM. The electronic properties of the substituents play a complementary role and the electron-withdrawing properties (σ(Ar) ca. 0.6 to 1.2) for PET activity seem to be more advantageous. Based on the structural similarity of the investigated compounds with previously published isomers, it can be concluded that these hydroxynaphthanilides inhibit PET in photosystem II.
Author Contributions
J.K., T.G. and I.J. synthesized and characterized the compounds. M.O. performed analytical measurement. J.K. performed biological screening. J.J. designed the compounds. J.K., T.G. and J.J. wrote the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Slovak Research and Development Agency (projects APVV-17-0373 and APVV-17-0318). This work is based on use of Large Research Infrastructure CzeCOS supported by the Ministry of Education, Youth and Sports of the Czech Republic within the CzeCOS program, grant number LM2018123; M.O. was supported by SustES—Adaptation strategies for sustainable ecosystem services and food security under adverse environmental conditions, project no. CZ.02.1.01/0.0/0.0/16_019/0000797.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.CropLife International; Brussels, Belgium: 2020. [(accessed on 19 May 2021)]. Importance & Benefits of Pesticides. Available online: https://pesticidefacts.org/topics/necessity-of-pesticides/ [Google Scholar]
- 2.Gianessi L.P. The increasing importance of herbicides in worldwide crop production. Pest. Manag. Sci. 2013;69:1099–1105. doi: 10.1002/ps.3598. [DOI] [PubMed] [Google Scholar]
- 3.Gianessi L., Williams A. The importance of herbicides for natural resource conservation in the USA. In: Songstad D., Hatfield J., Tomes D., editors. Convergence of Food Security, Energy Security and Sustainable Agriculture. Springer; Heidelberg, Germany: 2014. pp. 333–350. [Google Scholar]
- 4.Classification of Herbicides. Weed Management in Horticulture Crops. [(accessed on 19 May 2021)]; Available online: http://ecoursesonline.iasri.res.in/mod/page/view.php?id=12032.
- 5.Forouzesh A., Zand E., Soufizadeh S., Foroushani S.S. Classification of herbicides according to chemical family for weed resistance management strategies—An update. Weed Res. 2015;55:334–358. doi: 10.1111/wre.12153. [DOI] [Google Scholar]
- 6.Herbicide Resistance Action Committee HRAC Mode of Action Classification 2020 Map. [(accessed on 19 May 2021)]; Available online: https://hracglobal.com/tools/hrac-mode-of-action-classification-2020-map.
- 7.Draber W., Tietjen K., Kluth J.F., Trebst A. Herbicides in photosynthesis research. Angew. Chem. 1991;3:1621–1633. doi: 10.1002/anie.199116211. [DOI] [Google Scholar]
- 8.Tischer W., Strotmann H. Relationship between inhibitor binding by chloroplasts and inhibition of photosynthetic electron-transport. Biochim. Biophys. Acta. 1977;460:113–125. doi: 10.1016/0005-2728(77)90157-8. [DOI] [PubMed] [Google Scholar]
- 9.Trebst A., Draber W. Structure activity correlations of recent herbicides in photosynthetic reactions. In: Greissbuehler H., editor. Advances in Pesticide Science. Pergamon Press; Oxford, UK: 1979. pp. 223–234. [Google Scholar]
- 10.Bowyer J.R., Camilleri P., Vermaas W.F.J. In: Herbicides, Topics in Photosynthesis. Baker N.R., Percival M.P., editors. Elsevier; Amsterdam, The Netherlands: 1991. pp. 27–85. [Google Scholar]
- 11.Izawa S. Acceptors and donors for chloroplast electron transport. In: Part C., Colowick P., Kaplan N.O., editors. Methods in Enzymology. Academic Press; New York, NY, USA: London, UK: 1980. pp. 413–434. [Google Scholar]
- 12.Whitmarsh J. Electron transport and energy transduction. In: Raghavendra A.S., editor. Photosynthesis: A Comprehensive Treatise. Cambridge University Press; Cambridge, UK: 1998. pp. 87–110. [Google Scholar]
- 13.Jablonkai I. Molecular mechanism of action of herbicides. In: Abd El-Ghany Hasaneen M.N., editor. Herbicides—Mechanisms and Mode of Action. IntechOpen; Rijeka, Croatia: 2011. [(accessed on 19 May 2021)]. Chapter 1. Available online: https://www.intechopen.com/books/herbicides-physiology-of-action-and-safety/modes-of-action-of-different-classes-of-herbicides. [Google Scholar]
- 14.Sherwani S.I., Arif I.A., Khan H.A. Modes of action of different classes of herbicides. In: Price A., Kelton J., Sarunaite L., editors. Herbicides—Physiology of Action, and Safety. IntechOpen; Rijeka, Croatia: 2015. [(accessed on 19 May 2021)]. Chapter 8. Available online: https://www.intechopen.com/books/herbicides-physiology-of-action-and-safety/modes-of-action-of-different-classes-of-herbicides. [Google Scholar]
- 15.Huppatz J.L., McFadden H.G. Understanding the topography of the photosystem II herbicide binding niche: Does QSAR help? Z. Naturforsch. 1993;48:140–145. doi: 10.1515/znc-1993-3-405. [DOI] [Google Scholar]
- 16.Lambreva M.D., Russo D., Polticelli F., Scognamiglio V., Antonacci A., Zobnina V., Campi G., Rea G. Structure/function/dynamics of photosystem II plastoquinone binding sites. Curr. Protein Pept. Sci. 2014;15:285–295. doi: 10.2174/1389203715666140327104802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trebst A. Inhibitors in the functional dissection of the photosynthetic electron transport system. Photosynth. Res. 2007;92:217–224. doi: 10.1007/s11120-007-9213-x. [DOI] [PubMed] [Google Scholar]
- 18.Teixeira R.R., de Andrade Barros M.V., Bressan G.C., Siqueira R.P., Dos Santos F.S., Bertazzini M., Kiralj R., Ferreira M.M.C., Forlani G. Synthesis, theoretical studies, and effect on the photosynthetic electron transport of trifluoromethyl arylamides. Pest. Manag. Sci. 2017;73:2360–2371. doi: 10.1002/ps.4623. [DOI] [PubMed] [Google Scholar]
- 19.Dolezal M., Zitko J., Osicka Z., Kunes J., Vejsova M., Buchta V., Dohnal J., Jampilek J., Kralova K. Synthesis, antimycobacterial, antifungal and photosynthesis-inhibiting activity of chlorinated N-phenylpyrazine-2-carboxamides. Molecules. 2010;15:8567–8581. doi: 10.3390/molecules15128567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Imramovsky A., Pesko M., Kralova K., Vejsova M., Stolarikova J., Vinsova J., Jampilek J. Investigating spectrum of biological activity of 4- and 5-chloro-2-hydroxy-N-[2-(arylamino)-1-alkyl-2-oxoethyl]benzamides. Molecules. 2011;16:2414–2430. doi: 10.3390/molecules16032414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Imramovsky A., Pesko M., Monreal-Ferriz J., Kralova K., Vinsova J., Jampilek J. Photosynthesis-inhibiting efficiency of 4-chloro-2-(chlorophenylcarbamoyl)phenyl alkyl-carbamates. Bioorg. Med. Chem. Lett. 2011;21:4564–4567. doi: 10.1016/j.bmcl.2011.05.118. [DOI] [PubMed] [Google Scholar]
- 22.Fajkusova D., Pesko M., Keltosova S., Guo J., Oktabec Z., Vejsova M., Kollar P., Coffey A., Csollei J., Kralova K., et al. Anti-infective and herbicidal activity of N-substituted 2-aminobenzothiazoles. Bioorg. Med. Chem. 2012;20:7059–7068. doi: 10.1016/j.bmc.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 23.Gonec T., Kos J., Zadrazilova I., Pesko M., Keltosova S., Tengler J., Bobal P., Kollar P., Cizek A., Kralova K., et al. Antimycobacterial and herbicidal activity of ring-substituted 1-hydroxynaphthalene-2-carboxanilides. Bioorg. Med. Chem. 2013;21:6531–6541. doi: 10.1016/j.bmc.2013.08.030. [DOI] [PubMed] [Google Scholar]
- 24.Gonec T., Kos J., Pesko M., Dohanosova J., Oravec M., Liptaj T., Kralova K., Jampilek J. Halogenated 1-Hydroxynaphthalene-2-Carboxanilides Affecting Photosynthetic Electron Transport in Photosystem II. Molecules. 2017;22:1709. doi: 10.3390/molecules22101709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bak A., Pizova H., Kozik V., Vorcakova K., Kos J., Treml J., Odehnalova K., Oravec M., Imramovsky A., Bobal P., et al. SAR-mediated Similarity Assessment of the Property Profile for New, Silicon-Based AChE/BChE Inhibitors. Int. J. Mol. Sci. 2019;20:5385. doi: 10.3390/ijms20215385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pattabiraman V.R., Bode J.W. Rethinking amide bond synthesis. Nature. 2011;480:471–479. doi: 10.1038/nature10702. [DOI] [PubMed] [Google Scholar]
- 27.Gonec T., Zadrazilova I., Nevin E., Kauerova T., Pesko M., Kos J., Oravec M., Kollar P., Coffey A., O’Mahony J., et al. Synthesis and biological evaluation of N-alkoxyphenyl-3-hydroxynaphthalene-2-carboxanilides. Molecules. 2015;20:9767–9787. doi: 10.3390/molecules20069767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Teixeira R.R., Pereira J.L., Pereira W.L. Photosynthetic inhibitors. In: Najafpour M., editor. Applied Photosynthesis. InTech; Rijeka, Croatia: 2012. pp. 3–22. [Google Scholar]
- 29.Kos J., Zadrazilova I., Pesko M., Keltosova S., Tengler J., Gonec T., Bobal P., Kauerova T., Oravec M., Kollar P., et al. Antibacterial and herbicidal activity of ring-substituted 3-hydroxynaphthalene-2-carboxanilides. Molecules. 2013;18:7977–7997. doi: 10.3390/molecules18077977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gonec T., Kos J., Zadrazilova I., Pesko M., Govender R., Keltosova S., Chambel B., Pereira D., Kollar P., Imramovsky A., et al. Antibacterial and herbicidal activity of ring-substituted 2-hydroxynaphthalene-1-carboxanilides. Molecules. 2013;18:9397–9419. doi: 10.3390/molecules18089397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gonec T., Kralova K., Pesko M., Jampilek J. Antimycobacterial N-alkoxyphenylhydroxynaphthalene-carboxamides affecting photosystem II. Bioorg. Med. Chem. Lett. 2017;27:1881–1885. doi: 10.1016/j.bmcl.2017.03.050. [DOI] [PubMed] [Google Scholar]
- 32.Jampilek J., Kralova K., Pesko M., Kos J. Ring-substituted 8-hydroxyquinoline-2-carboxanilides as photosystem II inhibitors. Bioorg. Med. Chem. Lett. 2016;26:3862–3865. doi: 10.1016/j.bmcl.2016.07.021. [DOI] [PubMed] [Google Scholar]
- 33.Bak A., Kos J., Michnova H., Gonec T., Pospisilova S., Kozik V., Cizek A., Smolinski A., Jampilek J. Similarity-driven pharmacophore mapping for series of N-(disubstituted-phenyl)-3-hydroxynaphthalene-2-carboxamides. Int. J. Mol. Sci. 2020;21:6583. doi: 10.3390/ijms21186583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kerns E.H., Di L. Drug-Like Properties: Concepts. Structure Design and Methods: From ADME to Toxicity Optimization. Academic Press; San Diego, CA, USA: 2008. [Google Scholar]
- 35.Jampilek J. Potential of agricultural fungicides for antifungal drug discovery. Expert Opin. Drug Dis. 2016;11:1–9. doi: 10.1517/17460441.2016.1110142. [DOI] [PubMed] [Google Scholar]
- 36.Masarovicova E., Kralova K. Approaches to measuring plant photosynthesis activity. In: Pessarakli M., editor. Handbook of Photosynthesis. 2nd ed. Taylor & Francis Group; Boca Raton, FL, USA: 2005. pp. 617–656. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data is contained within the article.