Abstract
The YABBY family of plant-specific transcription factors play important regulatory roles during the development of leaves and floral organs, but their functions in Brassica species are incompletely understood. Here, we identified 79 YABBY genes from Arabidopsis thaliana and five Brassica species (B. rapa, B. nigra, B. oleracea, B. juncea, and B. napus). A phylogenetic analysis of YABBY proteins separated them into five clusters (YAB1–YAB5) with representatives from all five Brassica species, suggesting a high degree of conservation and similar functions within each subfamily. We determined the gene structure, chromosomal location, and expression patterns of the 21 BnaYAB genes identified, revealing extensive duplication events and gene loss following polyploidization. Changes in exon–intron structure during evolution may have driven differentiation in expression patterns and functions, combined with purifying selection, as evidenced by Ka/Ks values below 1. Based on transcriptome sequencing data, we selected nine genes with high expression at the flowering stage. qRT-PCR analysis further indicated that most BnaYAB family members are tissue-specific and exhibit different expression patterns in various tissues and organs of B. napus. This preliminary study of the characteristics of the YABBY gene family in the Brassica napus genome provides theoretical support and reference for the later functional identification of the family genes.
Keywords: Brassica napus, YABBY gene family, whole genome, phylogenetic analysis, expression pattern
1. Introduction
The YABBY family is a small gene family unique to seed plants [1]. In angiosperms, YABBY members regulate the growth of leaves [2,3,4,5] and floral organs [6,7]. YABBY proteins belong to the zinc finger protein superfamily and are characterized by a C2C2 zinc finger domain at their N terminus and a C-terminal helix–loop–helix motif (called the YABBY domain). The C2C2 and YABBY domains are highly conserved across family members, while the rest of the YABBY proteins have low sequence similarity [5,8,9,10,11]. Phylogenetic analysis divided the angiosperm YABBY family into five subfamilies: CRABS CLAW (CRC), FILAMENTOUS FLOWER (FIL)/YABBY3 (YAB3), INNER NO OUTER (INO), YABBY2 (YAB2), and YABBY5 (YAB5) [2,12]. FIL and YAB3 are highly similar and are expressed in the abaxial side of the initial primordium, which determines the fate of abaxial cells. FIL and YAB3 may be derived from a gene duplication event and are therefore included in the same subfamily [6,13,14]. All five subfamilies are represented in early-diverging Amborellales, Nymphaeales, and Austrobaileyales (ANA) angiosperms, indicating that the last common ancestor of current flowering plants harbored at least five YABBY genes [7,15,16].
YABBY members exist in many species, but the family is small. The Arabidopsis (Arabidopsis thaliana) YABBY gene family consists of six members [3,5,17,18]. FIL, YAB2, YAB3, and YAB5 regulate gene expression in vegetative tissues [19,20], including the polar development of lateral organs, the formation of edges, the maturity of leaves, and the development of shoot apical meristems and phyllodes. FIL is expressed in both leaf primordia and floral organs [3,21,22]. As a transcriptional regulator, FIL not only is involved in the formation of floral organs and leaf development [17,23], but also affects anthocyanin accumulation [24]. INO and CRC genes are more tissue-specific: INO plays an important part in the development of the outer integument [18], while CRC was the first gene identified as having a role in the formation of nectaries in flowers at the base of stamen filaments in Arabidopsis [5,13]. The number of YABBY genes has been determined in multiple plant species: 9 YABBY genes in tomato (Solanum lycopersicum) that map to 7 of the 12 tomato chromosomes [25], 8 in rice (Oryza sativa) that form four subgroups [4], 12 in Chinese cabbage (Brassica rapa) mapping to 6 of its 20 chromosomes [26], and 13 in maize (Zea mays) [27]. The genomes of tree cotton (Gossypium arboreum) and Gossypium raymondii each encode 12 YABBY genes, while that of upland cotton (Gossypium hirsutum) harbors 23 YABBY genes [28]. Fifty-five YABBY genes were identified in seven Magnolia species, comprising 5 INO, 6 CRC, 8 YAB2, 22 YAB5, and 14 FIL members [29]. Eight orchid species have 54 YABBY genes separated into 15 CRC/DL, 8 INO, 17 YAB2, and 14 FIL members [30]. As the terrestrial plants bryophytes and stone pine (Pinus pinea) have no YABBY members, it is thought that this gene family is specific to seed plants [16].
The Brassica genus includes the diploid species B. rapa, Brassica oleracea (CC, 2n = 18), and black mustard (Brassica nigra) and the amphidiploid species rapeseed (Brassica napus), brown mustard (Brassica juncea), and Ethiopian mustard (Brassica carinata). B. rapa, B. oleracea, and B. nigra are diploid species that can generate tetraploid species through mutual hybridization and natural chromosomal doubling [31]. These species provide an excellent evolutionary model for the expansion of the Brassica genus [32]. Rapeseed (B. napus) is a very important oil crop worldwide and has growth habits similar to those of the model plant Arabidopsis. Rapeseed oil has high nutritional value, and its stems and leaves can be used as animal feed and for some industrial uses [33]. Although the YABBY gene family has been described in Arabidopsis and other plants [3,5,17,18], it remains to be characterized in B. napus.
In this study, using whole-genome analysis, we explored the evolutionary relationship of YABBY genes by systematically identifying YABBY genes in five Brassica species. We then conducted a phylogenetic and gene structure analysis of the resulting 79 genes from these five species and Arabidopsis. Focusing on YABBY genes in B. napus, we listed all cis-acting elements in their promoters and identified differentially expressed genes in floral organs, seeds, and siliques via transcriptome deep sequencing (RNA-seq) data from the rapeseed cultivar ZS11, which we validated by RT-qPCR. Our results provide theoretical support and reference basis for the functional identification of the YABBY genes in B. napus.
2. Materials and Methods
2.1. Identification of YABBY Family Genes in Brassica
Arabidopsis YABBY protein sequences were downloaded from the TAIR10 database (ftp://ftp.Arabidopsis.org (accessed on 25 January 2021)) and used as queries for the Basic Local Alignment Sequence Tool for Protein (BLASTP) [34] to search the predicted proteomes from five Brassica species on the Brassica Database (BRAD; http://brassicadb.cn/ (accessed on 25 January 2021)) website with E-value < 1 × 10−20. The physicochemical properties of predicted YABBY proteins, such as isoelectric points (pI) and predicted molecular weights (MW), were determined with the ProtParam tool on the ExPASy server [35] (https://web.expasy.org/protparam/ (accessed on 3 March 2021)).
2.2. Phylogenetic Analysis
A multiple sequence alignment and phylogenetic analysis were used to explore the evolutionary relationship among the YABBY proteins from the six species under study. The phylogenetic tree was generated in MEGA 7.0 (Tokyo Metropolitan University, Tokyo, Japan) [36] using the neighbor-joining (NJ) method with default parameters for multiple sequence alignment, with a bootstrap number set to 1000 to estimate branch lengths. The tree was visualized with Evolview (https://evolgenius.info//evolview-v2/ (accessed on 2 February 2021)) software.
2.3. Gene Structure, Prediction of Conserved Sequences, and Protein Domain Analysis
For each YABBY gene obtained from the five Brassica species, the corresponding annotation was extracted from the GFF file of the appropriate genome and provided as input to the gene structure display server (GSDS v2.0; http://gsds.gao-lab.org/ (accessed on 29 January 2021)) to analyze gene structure. The online tool Multiple Expectation Maximization for Motif Elucidation suite (MEME v4.12.0, http://meme-suite.org/tools/meme (accessed on 28 January 2021)) [37] was used to predict conservative structural elements; the number of motifs was set to 10, and the width was set to 6–300 each motif with an E-value < 1 × 10−10 was retained. Conserved protein domains were identified through the National Center for Biotechnological Information Conserved Domain Database (NCBI-CDD; https://www.ncbi.nlm.nih.gov/Structure/bwrpsb/bwrpsb.cgi (accessed on 29 January 2021)) [38]. The results of gene structure and protein domain were displayed using TBtools [39].
2.4. Chromosomal Location and Collinearity Analysis
Using the information contained in the GFF annotation file of the B. napus genome, all YABBY genes were mapped to their respective chromosomes, and their locations were visualized with TBtools. The MCScanX algorithm [40] was used to predict collinearity between genomes, and Circos was used for visualization [41].
2.5. Analysis of Selection Pressure and Cis-Regulatory Elements
TBtools was used to calculate the nonsynonymous substitution rate (Ka), synonymous substitution rate (Ks), and Ka/Ks ratio for each pair of duplicated genes in B. napus with default parameter settings. Promoter sequences (2000 bp of sequence upstream of the transcription start site) for BnaYABBY genes were downloaded from the NCBI database (https://blast.ncbi.nlm.nih.gov/ (accessed on 30 January 2021)) and scanned for cis-regulatory elements on the New PLACE database (https://www.dna.affrc.go.jp/PLACE/?action=newpLACE (accessed on 30 January 2021)) [42]. TBtools was used to draw a heatmap to visualize the number of cis-elements.
2.6. Tissue Expression Analysis
The transcriptome data (PRJNA358784) of 49 tissues and organs of ‘ZS11’ in different developmental stages were selected to analyze the expression of YABBY. The log2 FPKM was calculated for each gene and sample and visualized with TBtools and HeatMap.
2.7. Plant Materials
Seeds for the B. napus cultivar ZS11 were obtained from the Rapeseed Engineering Research Center of Southwest University in Chongqing, China (CERCR). The plants were grown in the field in Chongqing. Samples were collected at different growth stages (initial flowering stage, full flowering stage, and green pod stage) and from various tissues (leaves, petals, stamens, nectaries, seeds, siliques) and immediately frozen in liquid nitrogen and stored at −80 °C until use.
2.8. Total RNA Extraction and RT-qPCR Analysis
Total RNA was extracted from collected samples with the DNAaway RNA Mini-prep Kit (Sangon Biotech, Shanghai, China) and used as a template for first-strand cDNA synthesis with TransScript One-Step gDNA Removal and cDNA Synthesis SuperMix (TransGen Biotech, Beijing, China). qPCR was performed using the ChamQ Universal SYBR qPCR Master Mix (Vazyme Biotech, Nanjing, China) on a Bio-Rad CFX96 Real-Time System (Bio-Rad Laboratories, Hercules, CA, USA) as previously described [43]. BnACTIN7 (EV116054) was employed as a reference gene and determined by the 2−∆∆Ct method [44,45]. The experiment was repeated three times, and the values represent the mean ± standard error (SE). The qRT-PCR primers were obtained from the qPCR Primer Database (Table S2) [46]. GraphPad Prism 5.0 software was used to visualize the results [47,48].
3. Results
3.1. Identification and Evolutionary Relationships of YABBY Genes
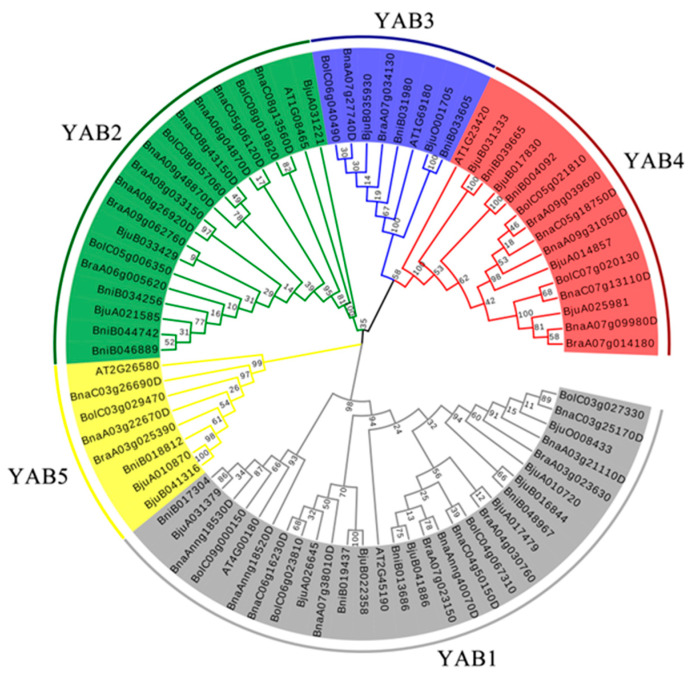
Using the six Arabidopsis YABBY proteins [2,5,49] as query, we performed a BLASTP search for related proteins across the Brassica genus, leading to the identification of 10 putative YABBY members from B. rapa, 11 from B. oleracea, 12 from B. nigra, 19 from B. juncea, and 21 from B. napus (Table 1 and Table S3). Following the classification of the YABBY family in Arabidopsis, phylogenetic analysis clustered the 79 YABBY members into five subfamilies, YAB1, YAB2, YAB3, YAB4, and YAB5 (Figure 1 and Table S3). Arabidopsis YAB1 (FIL) and YAB3 are closely related and defined one subgroup comprising the largest proportion of family members, with 29 (or 36.7%) YABBY proteins. The YAB2 subgroup contained 19 members, the YAB3 subgroup contained 15 members, and the YAB3 (which includes Arabidopsis CRC) and YAB5 subgroups were the smallest, with 8 members apiece.
Table 1.
Numbers of YABBY genes in Arabidopsis thaliana and five Brassica species.
| Type | A. thaliana | B. rapa | B. oleracea | B. nigra | B. juncea | B. napus |
|---|---|---|---|---|---|---|
| YAB1 | 2 | 3 | 4 | 4 | 8 | 8 |
| YAB2 | 1 | 3 | 3 | 3 | 3 | 6 |
| YAB3 | 1 | 1 | 1 | 2 | 2 | 1 |
| YAB4 | 1 | 2 | 2 | 2 | 4 | 4 |
| YAB5 | 1 | 1 | 1 | 1 | 2 | 2 |
Figure 1.
Phylogenetic tree of YABBY proteins from Arabidopsis and five Brassica species. YABBYs cluster into five subfamilies (YAB1–YAB5), indicated by different colors. Bju, Brassica juncea; Bna, Brassica napus; Bni, Brassica niger; Bol, Brassica oleracea; Bra, Brassica rapa.
The encoded proteins were 158 to 247 amino acids in length, with a predicted molecular weight (MW) ranging from 17.9 and 27.8 kDa and a pI between 5.5 and 9.95. The coding sequences of members of the YAB1 and YAB2 subgroups were on average longer than for the other subgroups: the coding sequences of BnaYAB1 were between 1806 and 5159 bp, while those of BnaYAB2 ranged from 1375 to 5795 bp. By contrast, the coding sequence of BnaYAB3 was 1594 bp, and those of the four BnaYAB4 members were between 1663 and 1978 bp; the coding sequences of the two BnaYAB5 members were 2933 and 3086 bp (Table S4).
3.2. Chromosomal Locations of BnaYAB Genes and Duplication Analysis
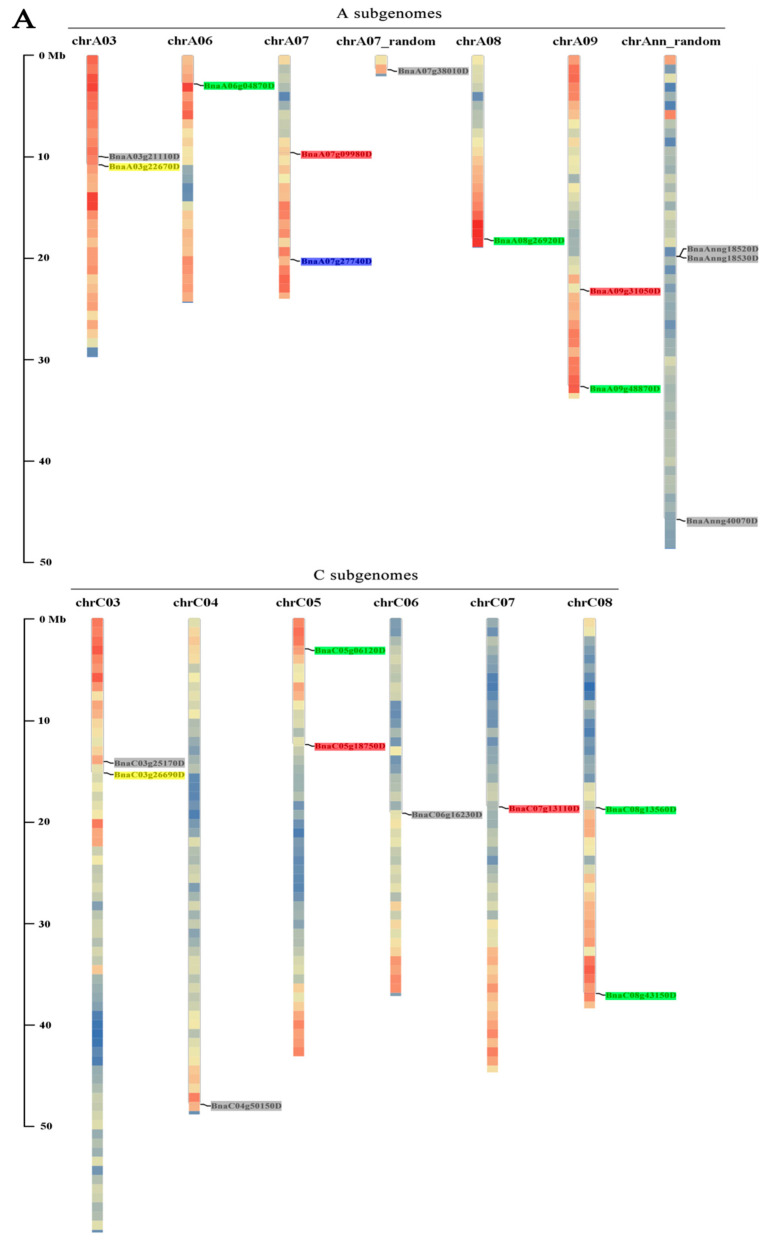
We next extracted the chromosomal coordinates of each BnaYAB gene from the GFF files downloaded from the BRAD. Accordingly, we mapped 18 BnaYAB genes to the 12 current B. napus chromosomes, with the remaining 3 BnaYAB genes mapping to the pseudochromosome Ann. Chromosomes A03, A07, A09, C03, C05, and C08 each harbored two YABBY genes, while chromosomes A06, A07-random, A08, C04, C06, and C07 each carried a single YABBY gene (Figure 2A).
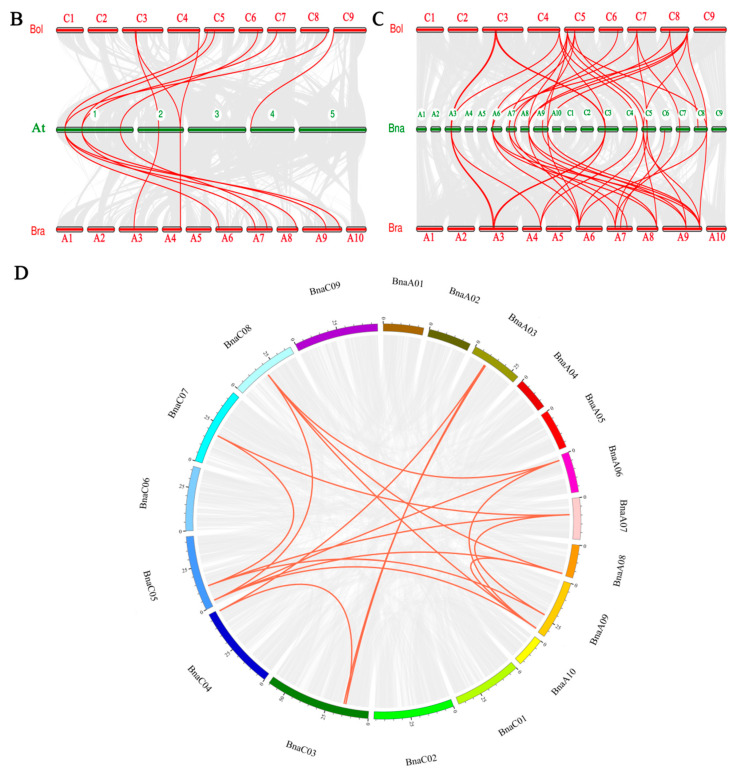
Figure 2.
Chromosomal locations of BnaYAB genes in the B. napus genome and syntenic analysis of YABBY genes in Arabidopsis, B. napus, B. rapa, and B. oleracea. (A) Chromosomal location of the 21 BnaYAB genes in the B. napus genome. Genes from the same subgroup are highlighted in the same color, as defined in the phylogenetic tree (Figure 1). Each chromosome is drawn as a heatmap of gene density. (B) Collinearity analysis of YABBY family genes between Arabidopsis, B. rapa, and B. oleracea, indicated as connecting lines. (C) Collinearity analysis of YABBY family genes between B. napus, B. rapa, and B. oleracea, indicated as connecting lines. (D) Chromosomal locations of the 21 BnaYAB genes in the B. napus genome, indicated as connecting lines. A and C denote the two main subgenomes in B. napus. Mb, megabase; Ann, pseudomolecule chromosomes. The 5 Arabidopsis chromosomes (1–5), 19 B. napus chromosomes (A01–A10 and C01–C09), 10 B. rapa chromosomes (A01–A10), and 9 B. oleracea chromosomes (C01–C09) are shown. Gene pairs are represented by red lines.
Partial and tandem gene duplications are important in the generation of new gene functions and the expansion of gene families [50]. Therefore, we performed a collinearity analysis on the YABBY family between Arabidopsis and three of the five Brassica species. The syntenic relationships between Arabidopsis, B. oleracea, and B. rapa; between B. napus, B. oleracea, and B. rapa; and between the 21 BnaYAB genes are shown in Figure 2B–D, respectively. We identified pairs of homologs between the Brassica species: 9 between Arabidopsis and B. oleracea, 8 between Arabidopsis and B. rapa, 26 between B. napus and B. oleracea, and 28 between B. napus and B. rapa. We also grouped YABBY genes in B. napus into 18 gene pairs. The copy numbers of YABBY genes varied from one to three in Arabidopsis and B. rapa (Table 1, Figure 2B) and from one to eight in B. oleracea and B. napus (Table 1, Figure 2C), indicating that several gene copies may have been lost or duplicated during evolution in various cases. For example, putative homologs to Arabidopsis YAB3 were present only in B. oleracea and appeared to have been lost in B. rapa, whereas Arabidopsis FIL had two putative homologs in B. oleracea and one in B. rapa (Figure 2B).
We estimated the Ka, Ks, and Ka/Ks ratio of homologous genes in B. napus (Table S5): their Ka/Ks ratios were all less than 1. These results suggested that the BnaYAB gene family underwent purifying selection after duplication.
3.3. Structures of BnaYAB Genes, Conserved Motifs, and BnaYAB Protein Domain Analyses
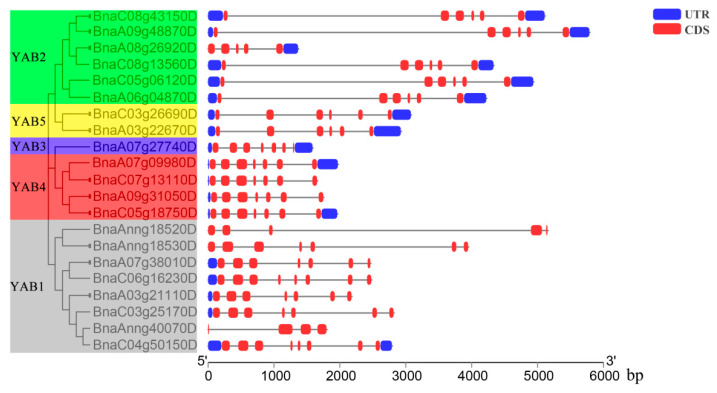
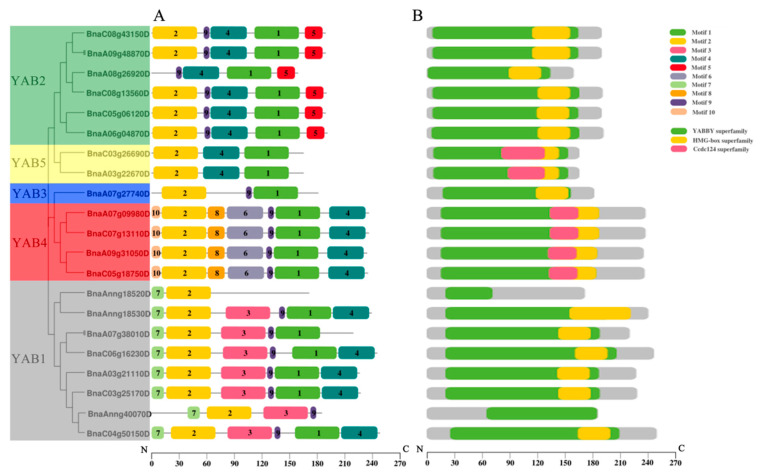
Gene structure reflects the evolution of a gene family. We therefore compared the coding sequences (CDS) and genomic sequences of all BnaYAB loci to analyze their exon/intron structures and displayed the results alongside the phylogenetic tree (Figure 3). The number of exons varied from four to eight. For example, members of the YAB1 subfamily harbored four to eight exons, as compared to five or six for YAB2, seven for YAB3 and YAB4, and six for YAB5 subfamily members (Figure 3 and Table S2). BnaYAB genes belonging to the same subgroup thus shared highly similar exon/intron characteristics, while those from different subgroups had more varied structures.
Figure 3.
Exon–intron structures of the 21 BnaYAB genes, ordered based on their phylogenetic positions.
Reflecting the phylogenetic analysis, prediction of functional motifs showed that members of each subgroup share the same conserved motifs, implying that they may have similar functions. YAB1 subfamily members were characterized by motifs 2 and 7, which were the only motifs identified in BnaAnng18520D. YAB2 subfamily members typically harbored motifs 1, 2, 4, 5, and 9, with motif 2 at the N terminus and motif 5 at the C terminus, although BnaA08g26920D appeared to lack motif 2. The one YAB3 subfamily member contained only motifs 1, 2, and 9, while YAB4 subfamily members all had the same set of motifs (motifs 1, 2, 4, 6, 8, 9, and 10). YAB5 subfamily members had motifs 1, 2, and 4 (Figure 4A). We also scanned YABBY proteins in the NCBI conserved domain database [38]. In addition to the YABBY superfamily domain (which was a prerequisite during our identification of YABBY members), most proteins contained the HMG-box superfamily domain, with the exception of BnaAnng18520D and BnaAnng40070D. The members of the YAB4 and YAB5 subfamilies also had a coiled-coil domain-containing protein 124 (Ccdc124) superfamily domain (Figure 4B).
Figure 4.
Analysis of protein motifs and conserved domains in B. napus YABBY proteins. (A) Protein motifs. Conserved motifs in the 21 BnaYAB proteins, arranged based on their phylogenetic positions. Weblogo plots of the ten conserved motifs are shown in Figure S1. (B) Conserved domains. Conserved domains are represented by different colored boxes.
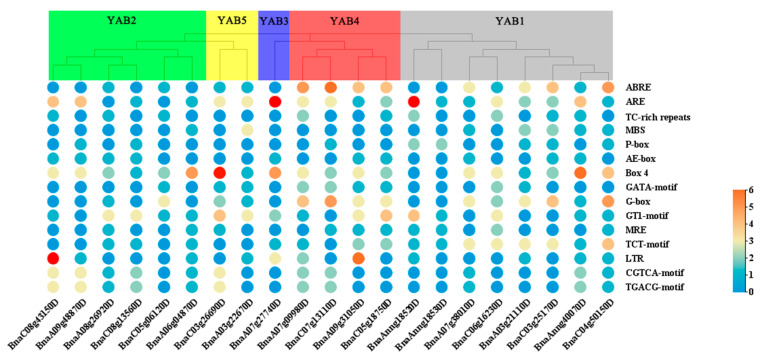
3.4. Cis-Regulatory Elements in BnaYAB Promoters
To explore the possible regulatory mechanisms of BnaYAB gene expression in abiotic or biotic stress responses, we identified 15 different cis-regulatory elements which can be classified into four types. First, we identified phytohormone response elements, such as those for abscisic acid (ABRE), gibberellin (P-box), and methyl jasmonate (CGTCA-motif and TGACG-motif). We also detected stress-responsive elements, including those related to drought (MBS), defense and adversity (TC-rich repeats), and low temperature (LTR). A third type of cis-elements consisted of several light-responsive elements: AE-box, Box 4, GATA-motif, G-box, GT1-motif, MRE, and TCT-motif. Finally, we noted the presence of anaerobic induction necessary (ARE) elements (Figure 5 and Table S1). BnaYAB genes are therefore likely to participate in a number of physiological and biochemical functions, such as adversity response, phytohormone pathways, and light signaling. The complement of cis-regulatory elements was specific to each BnaYAB gene, indicating that different members of the YABBY gene family may help regulate different aspects of plant development.
Figure 5.
Analysis of major cis-acting elements in BnaYAB promoters. The number of cis-regulatory elements is indicated as a heatmap (Table S1).
3.5. Transcriptional Patterns of BnaYAB Genes
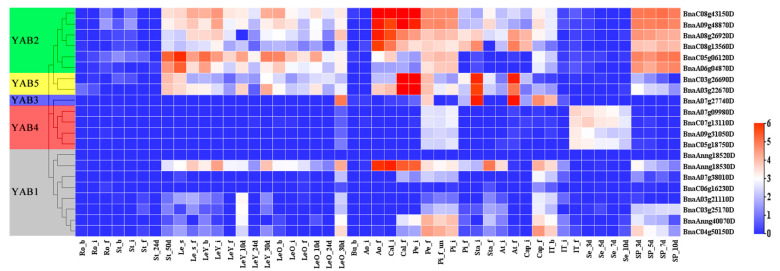
We determined the expression pattern of YABBY genes by analyzing a public transcriptome dataset of B. napus cultivar ZS11, including roots, stems, young leaves, old leaves, buds, flower stalks, sepals, petals, unpollinated pistils, pistils, stamens, anthers, filaments, seeds, and siliques (Table S6). The expression patterns of most BnaYAB genes are tissue-specific (Figure 6 and Table S7). For example, BnaYAB genes are seldom expressed in rhizomes, while most BnaYAB1 and BnaYAB4 subfamily members showed little or no expression in each tissue over the course of their development. BnaYAB2, BnaYAB3, and BnaYAB5 were expressed in various tissues at the flowering stage, indicating that they may regulate the development of flowers. With the exception of BnaAnng18530D, which is highly expressed in various tissues during flowering, the remaining BnaYAB1 members were barely expressed in most tissues (Figure 6 and Table S7). Most members of the BnaYAB2 subfamily shared the same expression pattern, with high expression in flower tissues and siliques. The two BnaYAB5 members, BnaA03g22670D and BnaC03g26690D, also exhibited the same expression pattern. The expression patterns of BnaYAB genes were thus largely consistent with their locations along the phylogenetic tree (Figure 1). These results indicate that most BnaYAB genes are tissue-specific and that related genes exhibit similar expression patterns (Figure 1 and Figure 6).
Figure 6.
Heatmap representation of the expression patterns of BnaYAB genes in the ZS11 cultivar. The abbreviations below the heatmap indicate the different tissues and organs/developmental stages (Table S6) relative expression is shown as a heatmap (Table S7).
3.6. Gene Expression Analysis in Brassica napus L.
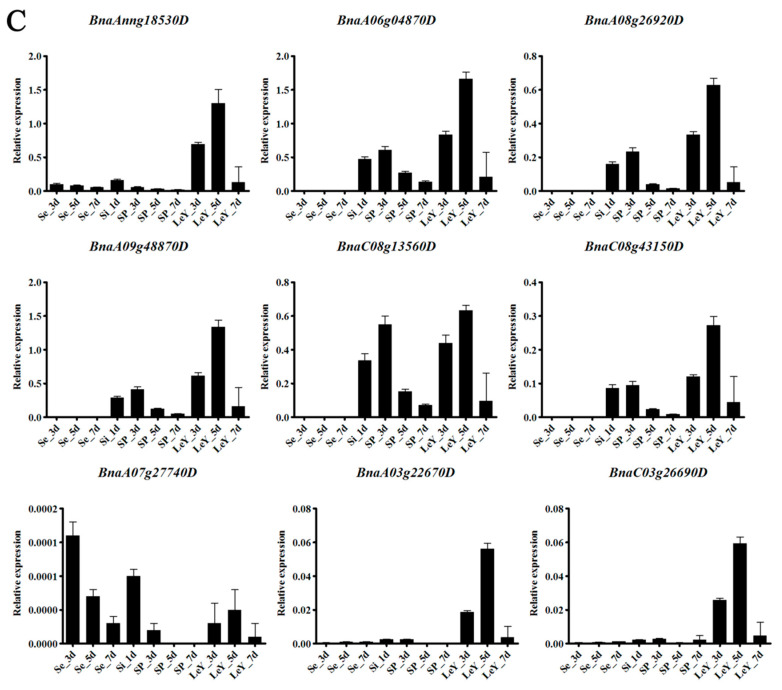
To clarify the tissue expression characteristics of YABBY genes, we selected nine genes with significant expression differences for RT-qPCR analysis (Table S2), and the results were consistent with RNA-seq (Figure 7).
Figure 7.
Relative expression of nine BnaYAB genes in different tissues and growth stages of B. napus. Relative expression levels in different tissues at the initial flowering stage (A), at the full flowering stage (B), and during the green pod stage (C). The abbreviations along the x-axes represent the different samples (Table S6). A red box indicates that the gene is only highly expressed in one tissue.
During the early flowering stage (Figure 7A) BnaAnng18530D, BnaA06g04870D, BnaA08g26920D, BnaA09g48870D, and BnaC08g13560D were highly expressed in petals, with little or no expression in other tissues. In addition, BnaC08g43150D was expressed only in petals, BnaA03C0367026690 was expressed in petals and nectaries, and BnaA07g27740D was expressed at relatively high levels only in nectaries. Most genes displayed the same expression pattern during the full blooming stage (Figure 7B) as during the initial flowering stage, albeit at slightly lower levels. BnaYAB1 and BnaYAB2 subfamily members also showed expression in sepals, with BnaC08g13560D having the highest expression in this tissue, exceeding that seen in petals. During the days after flowering (DAF) stage (Figure 7C), all BnaYAB genes were expressed at low levels, if at all, in seed coats, seeds, and siliques 1 day after flowering. BnaYAB1 and BnaYAB2 members were expressed in leaves during the green pod stage, but at low levels. These results demonstrated that most BnaYAB genes are tissue-specific and that BnaYAB transcription is differentially regulated in different organs. In addition, we observed that BnaYAB transcription decreases as B. napus flowers develop.
4. Discussion
Brassica napus, one of the most important oil crops in the world, resulted from the hybridization of B. rapa and B. oleracea followed by natural chromosome doubling [51,52,53]. The availability of the genome sequences for Brassica crops such as B. rapa, B. oleracea, and B. napus [54,55,56] has paved the way for the systematic analysis of several gene families encoding transcription factors such as APETALA2 (AP2)/ERF, basic helix–loop–helix (bHLH), GRAS, CONSTANS-like (COL), and WRKY [57,58,59,60,61,62]. Here, we embarked on a comparative genomics study of the YABBY gene family in B. napus, other Brassica species, and the related model plant Arabidopsis. YABBY proteins are a family of transcription factors unique to seed plants that regulate the development of lateral organs and play an important role in the differentiation of the plant dorsal axis, floral organ development, and phytohormone responses [15,25,63]. Their characterization would therefore contribute to our understanding of organ formation, development, and differentiation, in particular that of floral organs, which could offer a means of increasing yield in B. napus.
Each Arabidopsis YABBY gene should have three homologs in the genomes of B. rapa and B. oleracea and six copies in the B. napus genome, based on their evolutionary histories [55,56,64]. Starting with 6 YABBY genes in Arabidopsis, we identified 21 genes in B. napus, 10 in B. rapa, 11 in B. oleracea, 12 in B. nigra, and 19 in B. juncea, numbers that do not agree with expectations. However, each Arabidopsis YABBY gene had one to six putative homologs in B. napus and one to three putative homologs in B. rapa and B. oleracea (Table 1, Figure 2B–D), possibly reflecting gene loss or duplications that might be expected following hybridization and whole-genome duplication [64,65,66]. It is speculated that the YABBY genes have undergone strong selection during evolution, and the retained genes should have important functions in B. napus. Most YABBY genes have more than one orthologous gene in B. napus, which indicates that the YABBY gene family has expanded. However, only a few AtYABBY genes have fewer than six orthologous genes in B. napus, which is less than expected and suggests that the YABBY gene family has shrunk during the diversification of B. napus. Notably, the number of YABBY genes in B. napus is exactly the sum of the numbers of YABBY genes in B. rapa and B. oleracea, indicating that there was no gene loss during this evolutionary process and pointing to a loss of YABBY genes in the Arabidopsis lineage. The evolution of gene families, such as the YABBY family here, is thus complex and varies with the family under consideration [67,68], warranting separate study in each case. A phylogenetic analysis of YABBY proteins from Arabidopsis and five Brassica species separated all members into five subfamilies (Figure 1). Notably, the gene redundancy patterns of their constituent members are not the same.
We analyzed the results of gene structure and conserved protein motifs and found that genes clustered in the same subfamily have similar characteristics. For example, members of the BnaYAB4 subfamily all contained seven exons (Figure 3). Most BnaYAB members also displayed motifs 1 and 2, whereas motif 5 was specific to the BnaYAB5 subfamily and motifs 6, 8, and 10 were specific to the BnaYAB4 subfamily; most BnaYAB1 subfamily members carried motifs 3 and 7 (Figure 4A). Of the 21 B. napus YABBY proteins, 19 harbored an HMG-box, a domain that is also present in HIGH MOBILITY GROUP A (HMGA, At1g14900), which modulates flowering in Arabidopsis [69]. In addition, BnaYAB4 and BnaYAB5 members also had a Ccdc124 domain (Figure 4B). Ccdc124 is conserved across eukaryotes, contains a coiled-coil domain (CCD) found in most centrosome proteins, and participates in cell division [70], hinting that an analysis of the transcriptional regulation of these two subfamilies might illuminate their role in plant cell division. The expression levels of members of the BnaYAB4 and BnaYAB5 subfamilies were much lower than those of the members of the other three subfamilies (Figure 6). The above conclusions indicate that gene redundancy and gene elimination occurred during the evolution of the B. napus YABBY gene family, contributing to its diversification [71].
Cis-regulatory elements are the targets of transcription factors and thus partially determine gene expression patterns [72]. The promoters of this gene family mainly contain phytohormone response elements, such as those for abscisic acid (ABRE); stress response elements, such as drought (MBS); and light response elements, such as AE-box and anaerobic inducible elements (ARE) (Figure 5). The numerous light response elements suggest that the expression of YABBY genes in B. napus might be tightly controlled by photosynthesis. All promoters of genes in the BnaYAB4 subfamily contained ABREs, which mediate transcriptional regulation in response to abscisic acid [73]. This plant hormone is typically involved in abiotic stress responses and participates in the metabolic regulation of drought stress [74], stomatal closure, and the regulation of gene expression [75]. The B. napus genome thus harbors more YABBY genes than that of Arabidopsis, with highly complex gene structures, which may explain the high variability and tolerance of these plants under different conditions. In addition, the cis-regulatory elements identified in their promoters shape their participation in plant growth and development, offering new directions for further research on BnaYAB genes.
Arabidopsis FIL, YAB2, and YAB5 are expressed in leaves, cotyledons, and floral organs and can control the growth of side branches [3,76,77]. We were interested in BnaYAB genes that regulate the development of floral organs and cotyledons; therefore, we analyzed public transcriptome data for B. napus (Table S7, Figure 6) and discovered that many family members are highly expressed during the flowering stage and in floral tissues, resulting in a list of nine differentially expressed genes from subfamilies BnaYAB1-BnaYAB3 and BnaYAB5. Arabidopsis YABBY4 (also named INNER NO OUTER (INO)) and SUPERMAN (SUP) regulate the asymmetric growth of the outer skin of bitegmic ovules [78,79], with INO expressed only in the outermost cell layer of the outer envelope and promoting the growth of the outer envelope. INO expression is tissue-specific [18,80], a feature that is supported by its low expression levels in tissues and cotyledons at the flowering stage. We validated the differential expression of the selected genes by RT-qPCR. RNA-seq analysis showed that BnaYAB genes have similar expression patterns in tissues of different periods. For example, BnaAnng18530D, BnaA06g04870D, BnaA08g26920D, BnaA09g48870D, BnaC08g13560D, and BnaC08g43150D were highly expressed in petals at the initial flowering stage, while BnaA07g27740D from the CRC subfamily was expressed only in nectaries. BnaA03g22670D and BnaC03g26690D were also highly expressed in nectaries, suggesting that they participate in nectary formation [15,55]. The expression levels of the above-mentioned genes decreased at the full blooming stage and were low in all tissues during the green pod stage (Figure 7), indicating that they play different roles at different stages, thus validating the goals of this study, although the specific underlying regulatory mechanisms should be studied in more detail.
5. Conclusions
In this study, we conducted a systematic exploration of the YABBY gene family and identified 79 YABBY genes from Arabidopsis and five Brassica species (B. rapa, B. nigra, B. oleracea, B. napus, and B. juncea) that belonged to the previously described subgroups YAB1, YAB2, YAB3, YAB4, and YAB5. We analyzed the chromosomal location, gene structure, and expression patterns of the B. napus YABBY genes and the conserved domains and evolutionary relationship of the YABBY proteins. During polyploidization, the YABBY gene family underwent tandem duplications and gene loss, with evidence of purifying selection. Exon–intron structural changes may have led to changes in coding regions and affected gene expression patterns and protein functions. Finally, we combined RNA-seq and RT-qPCR analysis to explore the specific expression of BnaYAB genes. The results of this study offer guiding principles for the evolution of the YABBY gene family, providing a basis for polyploid analysis and laying theoretical support for further research on the function of BnaYAB genes.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/genes12070981/s1. Figure S1: Weblogo plots of the 10 conserved motifs; Table S1: Major cis-regulatory elements of YABBY genes in Brassica napus L.; Table S2: Primers used to amplify the YABBY genes and reference genes using qRT-PCR; Table S3: List of YABBY genes identified in the A. thaliana and Brassica genomes; Table S4: List of YABBY genes identified in Brassica napus; Table S5: One-to-one orthologous relationships in Brassica napus; Table S6: B. napus ZS11 tissues and organs used in this study; Table S7: The FPKM values of YABBY family genes in B. napus by RNA-Seq analysis.
Author Contributions
X.X. conceived and designed the experiments; J.X. and D.W. conducted the experiments; Y.P., Q.W., and W.W. collected and analyzed the data; Y.X., T.L., and K.Z. carried out the experiments and utilized software; J.X. and X.X. wrote the manuscript; X.X. and J.L. reviewed the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Department of Agriculture Projects of Modern Agricultural Technology System (CARS-12), the Chongqing Basic Scientific and Advanced Technology Research (cstc2015jcyjBX0001), and the 111 Project (B12006).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data used in this study are presented in the supplementary information.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Floyd S.K., Bowman J.L. The Ancestral Developmental Tool Kit of Land Plants. Int. J. Plant Sci. 2007;168:1–35. doi: 10.1086/509079. [DOI] [Google Scholar]
- 2.Bowman J.L. The YABBY gene family and abaxial cell fate. Curr. Opin. Plant Biol. 2000;3:17–22. doi: 10.1016/S1369-5266(99)00035-7. [DOI] [PubMed] [Google Scholar]
- 3.Baum S.F., Eshed Y., Otsuga D., Drews G.N., Siegfried K.R., Bowman J.L. Members of the YABBY gene family specify abaxial cell fate in Arabidopsis. Development. 1999;126:4117–4128. doi: 10.1242/dev.126.18.4117. [DOI] [PubMed] [Google Scholar]
- 4.Toriba T., Harada K., Takamura A., Nakamura H., Ichikawa H., Suzaki T., Hirano H.Y. Molecular characterization the YABBY gene family in Oryza sativa and expression analysis of OsYABBY1. Mol. Genet Genom. 2007;277:457–468. doi: 10.1007/s00438-006-0202-0. [DOI] [PubMed] [Google Scholar]
- 5.Bowman J.L., Smyth D.R. CRABS CLAW, a gene that regulates carpel and nectary development in Arabidopsis, encodes a novel protein with zinc finger and helix-loop-helix domains. Development. 1999;126:2387–2396. doi: 10.1242/dev.126.11.2387. [DOI] [PubMed] [Google Scholar]
- 6.Ha C.M., Jun J.H., Fletcher J.C. Control of Arabidopsis leaf morphogenesis through regulation of the YABBY and KNOX families of transcription factors. Genetics. 2010;186:197–206. doi: 10.1534/genetics.110.118703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamada T., Yokota S., Hirayama Y., Imaichi R., Kato M., Gasser C.S. Ancestral expression patterns and evolutionary diversification of YABBY genes in angiosperms. Plant J. 2011;67:26–36. doi: 10.1111/j.1365-313X.2011.04570.x. [DOI] [PubMed] [Google Scholar]
- 8.Sieber P., Petrascheck M., Barberis A., Schneitz K. Organ polarity in Arabidopsis NOZZLE physically interacts with members of the YABBY family. Plant Physiol. 2004;135:2172–2185. doi: 10.1104/pp.104.040154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Golz J.F., Roccaro M., Kuzoff R., Hudson A. GRAMINIFOLIA promotes growth and polarity of Antirrhinum leaves. Development. 2004;131:3661–3670. doi: 10.1242/dev.01221. [DOI] [PubMed] [Google Scholar]
- 10.Bowman J.L., Eshed Y. Formation and maintenance of the shoot apical meristem. Trends Plant Sci. 2000;5:110–115. doi: 10.1016/S1360-1385(00)01569-7. [DOI] [PubMed] [Google Scholar]
- 11.Kanaya E., Nakajima N., Okada K. Non-sequence-specific DNA Binding by the FILAMENTOUS FLOWER Protein from Arabidopsis thalianaIs Reduced by EDTA. J. Biol. Chem. 2002;277:11957–11964. doi: 10.1074/jbc.M108889200. [DOI] [PubMed] [Google Scholar]
- 12.Yamada T., Ito M., Kato M. YABBY2-Homologue Expression in Lateral Organs of Amborella trichopoda (Amborellaceae) Int. J. Plant Sci. 2004;165:917–924. doi: 10.1086/423793. [DOI] [Google Scholar]
- 13.Lee J.Y., Baum S.F., Oh S.H., Jiang C.Z., Chen J.C., Bowman J.L. Recruitment of CRABS CLAW to promote nectary development within the eudicot clade. Development. 2005;132:5021–5032. doi: 10.1242/dev.02067. [DOI] [PubMed] [Google Scholar]
- 14.Eshed Y., Izhaki A., Baum S.F., Floyd S.K., Bowman J.L. Asymmetric leaf development and blade expansion in Arabidopsis are mediated by KANADI and YABBY activities. Development. 2004;131:2997–3006. doi: 10.1242/dev.01186. [DOI] [PubMed] [Google Scholar]
- 15.Bartholmes C., Hidalgo O., Gleissberg S. Evolution of the YABBY gene family with emphasis on the basal eudicot Eschscholzia californica (Papaveraceae) Plant Biol. 2011;14:11–23. doi: 10.1111/j.1438-8677.2011.00486.x. [DOI] [PubMed] [Google Scholar]
- 16.Finet C., Floyd S.K., Conway S.J., Zhong B., Scutt C.P., Bowman J.L., Zhong B. Evolution of the YABBY gene family in seed plants. Evol. Dev. 2016;18:116–126. doi: 10.1111/ede.12173. [DOI] [PubMed] [Google Scholar]
- 17.Sawa S., Watanabe K., Goto K., Liu Y.G., Shibata D., Kanaya E., Morita E.H., Okada K. FILAMENTOUS FLOWER, a meristem and organ identity gene of Arabidopsis, encodes a protein with a zinc finger and HMG-related domains. Genes Dev. 1999;13:1079–1088. doi: 10.1101/gad.13.9.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Villanueva J.M., Broadhvest J., Hauser B.A., Meister R.J., Schneitz K., Gasser C.S. INNER NO OUTER regulates abaxial- adaxial patterning in Arabidopsis ovules. Genes Dev. 1999;13:3160–3169. doi: 10.1101/gad.13.23.3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eckardt N.A. YABBY genes and the development and origin of seed plant leaves. Plant Cell. 2010;22:2103. doi: 10.1105/tpc.110.220710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sarojam R., Sappl P.G., Goldshmidt A., Efroni I., Floyd S.K., Eshed Y., Bowman J.L. Differentiating Arabidopsis shoots from leaves by combined YABBY activities. Plant Cell. 2010;22:2113–2130. doi: 10.1105/tpc.110.075853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pfannebecker K.C., Lange M., Rupp O., Becker A., Purugganan M. Seed plant-specific gene lineages involved in carpel development. Mol. Biol. Evol. 2017;34:925–942. doi: 10.1093/molbev/msw297. [DOI] [PubMed] [Google Scholar]
- 22.Filyushin M.A., Slugin M.A., Dzhos E.A., Kochieva E.Z., Shchennikova A.V. Coexpression of YABBY1 and YABBY3 genes in lateral organs of tomato species (Solanum, Section Lycopersicon) Dokl. Biochem. Biophys. 2018;478:50–54. doi: 10.1134/S160767291801012X. [DOI] [PubMed] [Google Scholar]
- 23.Stahle M.I., Kuehlich J., Staron L., von Arnim A.G., Golz J.F. YABBYs and the transcriptional corepressors LEUNIG and LEUNIG_HOMOLOG maintain leaf polarity and meristem activity in Arabidopsis. Plant Cell. 2009;21:3105–3118. doi: 10.1105/tpc.109.070458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boter M., Golz J.F., Gimenez-Ibanez S., Fernandez-Barbero G., Franco-Zorrilla J.M., Solano R. Filamentous flower is a direct target of JAZ3 and modulates responses to jasmonate. Plant Cell. 2015;27:3160–3174. doi: 10.1105/tpc.15.00220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Knaap E.V.D., Gonzalez G., Xiao H., Huang Z.J., Houten J.V. Genome-wide identification, phylogeny and expression analysis of SUN, OFP and YABBY gene family in tomato. Mol. Genet. Genom. 2013;288:111–129. doi: 10.1007/s00438-013-0733-0. [DOI] [PubMed] [Google Scholar]
- 26.Hou H., Wu P., Gao L., Zhang C.W., Hou X.L. Characterization and expression profile analysis of YABBY family genes in Pak-choi (Brassica rapa ssp. chinensis) under abiotic stresses and hormone treatments. Plant Growth Regul. 2019;87:421–432. doi: 10.1007/s10725-019-00475-5. [DOI] [Google Scholar]
- 27.Cao Y., Lang Z.H., Wang L. Characteristics and Expression Analysis of Transcription Factor YABBY Family in Maize. J. Agric. Sci. Tech. Iran. 2015;17:32–41. [Google Scholar]
- 28.Yang Z.E., Gong Q., Wang L.L., Jin Y.Y., Xi J.P., Li Z., Qin W.Q., Yang Z.R., Lu L.L., Chen Q.J., et al. Genome-Wide Study of YABBY Genes in Upland Cotton and Their Expression Patterns under Different Stresses. Front. Genet. 2018;9:33. doi: 10.3389/fgene.2018.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu X., Liao X.Y., Zheng Y., Zhu M.J., Lan S. Genome-wide identification of the yabby gene family in seven species of magnoliids and expression analysis in litsea. Plants. 2020;10:21. doi: 10.3390/plants10010021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen Y.Y., Hsiao Y.Y., Chang S.B., Zhang D., Tsai W.C. Genome-wide identification of yabby genes in orchidaceae and their expression patterns in phalaenopsis orchid. Genes. 2020;11:955. doi: 10.3390/genes11090955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nagaharu U. Genome analysis in Brassica with special reference to the experimental formation of B. napus and peculiar mode of fertilisation. J. Jpn. Bot. 1935;7:389–452. [Google Scholar]
- 32.Yu J.Y., Hu F., Dossa K., Wang Z.K., Ke T. Genome-wide analysis of UDP-glycosyltransferase super family in Brassica rapa and Brassica oleracea reveals its evolutionary history and functional characterization. BMC Genom. 2017;18:474. doi: 10.1186/s12864-017-3844-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jian H.J., Lu K., Bo Y., Wang T.Y., Li Z., Zhang A.X., Wang J., Liu L.Z., Qu C.M., Li J.N. Genome-Wide Analysis and Expression Profiling of the SUC and SWEET Gene Families of Sucrose Transporters in Oilseed Rape (Brassica napus L.) Front. Plant. Sci. 2016;7:1464. doi: 10.3389/fpls.2016.01464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Altschul S.F., Madden T.L., Schaffer A.A., Zhang J., Zhang Z., Miller W., Lipman D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Elisabeth G., Alexandre G., Christine H., Ivan I., Appel R.D., Amos B. ExPASy: The proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Res. 2003;13:3784–3788. doi: 10.1093/nar/gkg563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sudhir K., Glen S., Koichiro T. MEGA7: Molecular Evolutionary Genetics Analysis V ersion 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016;7:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bailey T.L., Boden M., Buske F.A., Frith M., Grant C.E., Clementi L., Ren J., Li W.W., Noble W.S. MEME SUITE: Tools for motif discovery and searching. Nucleic Acids Res. 2009;37:W202–W208. doi: 10.1093/nar/gkp335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marchler-Bauer A., Derbyshire M.K., Gonzales N.R., Lu S., Chitsaz F., Geer L.Y., Geer R.C., He J., Gwadz M., Hurwitz D.I., et al. CDD: NCBI’s conserved domain database. Nucleic Acids Res. 2014;43:D222–D226. doi: 10.1093/nar/gku1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen C., Chen H., Zhang Y., Thomas H.R., Frank M.H., He Y., Xia R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant. 2020;13:1194–1202. doi: 10.1016/j.molp.2020.06.009. [DOI] [PubMed] [Google Scholar]
- 40.Wang Y.P., Tang H.B., DeBarry J.D., Tan X., Li J.P., Wang X.Y., Lee T.h., Jin H.Z., Marler B., Guo H., et al. MCScanX: A toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012;40:e49. doi: 10.1093/nar/gkr1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Krzywinski M., Schein J., Birol I., Connors J., Gascoyne R., Horsman D., Jones S., Marra M. Circos: An information aesthetic for comparative genomics. Genome Res. 2009;19:1639–1645. doi: 10.1101/gr.092759.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Higo K., Ugawa Y., Iwamoto M., Korenaga T. Plant cis-acting regulatory DNA elements (PLACE) database: 1999. Nucleic Acids Res. 1999;27:297–300. doi: 10.1093/nar/27.1.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Qu C.M., Fu F.Y., Lu K., Zhang K., Wang R., Xu X.F., Wang M., Lu J.X., Wan H.F., Tang Z.L., et al. Differential accumulation of phenolic compounds and expression of related genes in black- and yellow-seeded Brassica napus. J. Exp. Bot. 2013;64:2885–2898. doi: 10.1093/jxb/ert148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Livak K., Schmittgen T. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−∆∆Ct Method. Methods. 2000;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 45.Wu G., Zhang L., Wu Y.H., Cao Y.L., Lu C.M. Comparison of Five Endogenous Reference Genes for Specific PCR Detection and Quantification of Brassica napus. J. Agric. Food Chem. 2010;58:2812–2817. doi: 10.1021/jf904255b. [DOI] [PubMed] [Google Scholar]
- 46.Lu K., Li T., He J., Chang W., Zhang R., Liu M., Yu M.N., Fan Y.H., Ma J.Q., Sun W., et al. qPrimerDB: A thermodynamics-based gene-specific qPCR primer database for 147 organisms. Nucleic Acids Res. 2018;46:D1229–D1236. doi: 10.1093/nar/gkx725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Steland A. Book Review: Fitting Models to Biological Data Using Linear and Nonlinear Regression. By Harvey Motulsky und Arthur Christopoulos. Biom. J. 2006;48:327. doi: 10.1002/bimj.200610209. [DOI] [Google Scholar]
- 48.Swift M.L. GraphPad Prism, Data Analysis, and Scientific Graphing. J. Chem. Inf. Modeling. 1997;37:411–412. doi: 10.1021/ci960402j. [DOI] [Google Scholar]
- 49.Sawa S., Ito T., Shimura Y., Okada K. FILAMENTOUS FLOWER controls the formation and development of Arabidopsis inflorescences and floral meristems. Plant. Cell. 1999;11:69–86. doi: 10.1105/tpc.11.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cannon S.B., Mitra A., Baumgarten A., Young N.D., May G. The roles of segmental and tandem gene duplication in the evolution of large gene families in Arabidopsis thaliana. BMC Plant Biol. 2004;4:10. doi: 10.1186/1471-2229-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhao J.J., Wang X.W., Deng B., Lou P., Wu J., Sun R.F., Xu Z.Y., Vromans J., Koornneef M., Bonnema G. Genetic relationships within Brassica rapa as inferred from AFLP fingerprints. Theor. App. Genet. 2005;110:1301–1314. doi: 10.1007/s00122-005-1967-y. [DOI] [PubMed] [Google Scholar]
- 52.Cheng F., Wu J., Wang X.W. Genome triplication drove the diversification of Brassica plants. Hortic. Res. 2014;1:14024. doi: 10.1038/hortres.2014.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cheng F., Sun R.F., Hou X.L., Zheng H.K., Zhang F.L., Zhang Y.Y., Liu B., Liang J.L., Zhuang M., Liu Y.X., et al. Subgenome parallel selection is associated with morphotype diversification and convergent crop domestication in Brassica rapa and Brassica oleracea. Nat. Genet. 2016;48:1218. doi: 10.1038/ng.3634. [DOI] [PubMed] [Google Scholar]
- 54.Cheng F., Liu S.Y., Wu J., Fang L., Sun S.L., Liu B., Li P.X., Hua W., Wang X.W. BRAD, the genetics and genomics database for Brassica plants. BMC Plant. Biol. 2011;11:136. doi: 10.1186/1471-2229-11-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu S.Y., Liu Y.M., Yang X.H., Tong C.B., Edwards D., Parkin I.A.P., Zhao M.X., Ma J.X., Yu J.Y., Huang S.M., et al. The Brassica oleracea genome reveals the asymmetrical evolution of polyploidy genomes. Nat Commun. 2014;5:3930. doi: 10.1038/ncomms4930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chalhoub B., Denoeud F., Liu S.Y., Parkin I.A.P., Tang H.B., Wang X.Y., Chiquet J., Belcram H., Tong C.B., Samans B., et al. Early allopolyploid evolution in the post-Neolithic Brassica napus oilseed genome. Science. 2014;345:950–953. doi: 10.1126/science.1253435. [DOI] [PubMed] [Google Scholar]
- 57.Song X.M., Huang Z.N., Duan W.K., Ren J., Hou X.L. Genome-wide analysis of the bHLH transcription factor family in Chinese cabbage (Brassica rapa ssp. pekinensis) Mol. Genet. Genom. 2014;289:77–91. doi: 10.1007/s00438-013-0791-3. [DOI] [PubMed] [Google Scholar]
- 58.Song X.M., Duan W.K., Huang Z.N., Liu G.F., Wu P., Liu T.K., Li Y., Hou X.L. Comprehensive analysis of the flowering genes in Chinese cabbage and examination of evolutionary pattern of CO-like genes in plant kingdom. Sci. Rep. 2015;5:14631. doi: 10.1038/srep14631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Song X.M., Li Y., Hou X.L. Genome-wide analysis of the AP2/ERF transcription factor superfamily in Chinese cabbage (Brassica rapa ssp. pekinensis) BMC Genom. 2013;14:573. doi: 10.1186/1471-2164-14-573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Song X.M., Wang J.P., Ma X., Li Y.X., Lei T.Y., Wang L., Ge W.N., Guo D., Wang Z.Y., Li C.J., et al. Origination, Expansion, Evolutionary Trajectory, and Expression Bias of AP2/ERF Superfamily in Brassica napus. Front. Plant Sci. 2016;7:1186. doi: 10.3389/fpls.2016.01186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Song X.M., Liu T.K., Duan W.K., Ma Q.H., Ren J., Wang Z., Li Y., Hou X.L. Genome-wide analysis of the GRAS gene family in Chinese cabbage (Brassica rapa ssp. pekinensis) Genomics. 2014;103:135–146. doi: 10.1016/j.ygeno.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 62.He Y.J., Mao S.S., Gao Y.L., Wu D.M., Cui Y.X., Li J.N., Qian W. Genome-wide identification and expression analysis of WRKY transcription factors under multiple stresses in Brassica napus. PLoS ONE. 2016;11:e0157558. doi: 10.1371/journal.pone.0157558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang X.L., Yang Z.P., Zhang J., Zhang L.G. Ectopic expression of BraYAB1-702, a member of YABBY gene family in Chinese cabbage, causes leaf curling, inhibition of development of shoot apical meristem and flowering stage delaying in Arabidopsis thaliana. Int. J. Mol. Sci. 2013;14:14872–14891. doi: 10.3390/ijms140714872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mun J.H., Kwon S.J., Yang T.J., Seol Y.J., Jin M. Genome-wide comparative analysis of the Brassica rapagene space reveals genome shrinkage and differential loss of duplicated genes after whole genome triplication. Genome Biol. 2009;10:R11. doi: 10.1186/gb-2009-10-10-r111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Moghe G.D., Hufnagel D.E., Tang H., Xiao Y., Dworkin I., Town C.D., Conner J.K., Shiu S.H. Consequences of Whole-Genome Triplication as Revealed by Comparative Genomic Analyses of the Wild Radish Raphanus raphanistrum and Three Other Brassicaceae Species. Plant. Cell. 2014;26:1925–1937. doi: 10.1105/tpc.114.124297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ma J.Q., Jian H.J., Yang B., Lu K., Zhang A.X., Liu P., Li J.N. Genome-wide analysis and expression profiling of the GRF gene family in oilseed rape (Brassica napus L.) Gene. 2017;620:36–45. doi: 10.1016/j.gene.2017.03.030. [DOI] [PubMed] [Google Scholar]
- 67.Wang X.W., Wang H.Z., Wang J., Sun R.F., Wu J., Liu S.Y., Bai Y.Q., Mun J.H., Bancroft I., Cheng F., et al. The genome of the mesopolyploid crop species Brassica rapa. Nat. Genet. 2011;43:1035–1039. doi: 10.1038/ng.919. [DOI] [PubMed] [Google Scholar]
- 68.Gao K., Hua Y.P., Song H.X., Guan C.Y., Zhang Z.H., Zhou T. Identification and Bioinformatics analysis of the PIN family gene in Brassica napus. Acta Agron. Sin. 2018;44:1334–1346. doi: 10.3724/SP.J.1006.2018.01334. [DOI] [Google Scholar]
- 69.Gupta R., Webster C.I., Walker A.R., Gray J.C. Chromosomal location and expression of the single-copy gene encoding high-mobility-group protein HMG-I/Y in Arabidopsis thaliana. Plant Mol. Biol. 1997;34:529–536. doi: 10.1023/A:1005828430861. [DOI] [PubMed] [Google Scholar]
- 70.Telkoparan P., Erkek S., Yaman E., Alotaibi H., Bayık D., Tazebay U.H. Coiled-Coil Domain Containing Protein 124 Is a Novel Centrosome and Midbody Protein That Interacts with the Ras-Guanine Nucleotide Exchange Factor 1B and Is Involved in Cytokinesis. PLoS ONE. 2013;8:e69289. doi: 10.1371/journal.pone.0069289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xu G.X., Guo C.C., Shan H.Y., Kong H.Z. Divergence of duplicate genes in exon-intron structure. Proc. Natl. Acad. Sci. USA. 2012;109:1187–1192. doi: 10.1073/pnas.1109047109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu Y., Wang L., Xing X., Sun L.P., Pan J.W., Kong X.P., Zhang M.Y., Li D.Q. ZmLEA3, a multifunctional group 3 LEA protein from maize (Zea mays L.), is involved in biotic and abiotic stresses. Plant Cell Physiol. 2013;54:944–959. doi: 10.1093/pcp/pct047. [DOI] [PubMed] [Google Scholar]
- 73.Osakabe Y., Yamaguchi-Shinozaki K., Shinozaki K., Tran L.S. ABA control of plant macroelement membrane transport systems in response to water deficit and high salinity. New Phytol. 2014;202:35–49. doi: 10.1111/nph.12613. [DOI] [PubMed] [Google Scholar]
- 74.Simpson S.D., Nakashima K., Narusaka Y., Seki M., Shinozaki K., Yamaguchi-Shinozaki K. Two different novel cis-acting elements of erd1, a clpA homologous Arabidopsis gene function in induction by dehydration stress and dark-induced senescence. Plant J. 2003;33:259–270. doi: 10.1046/j.1365-313X.2003.01624.x. [DOI] [PubMed] [Google Scholar]
- 75.Lee S.C., Luan S. Aba signal transduction at the crossroad of biotic and abiotic stress Responses. Plant Cell Environ. 2011;35:53–60. doi: 10.1111/j.1365-3040.2011.02426.x. [DOI] [PubMed] [Google Scholar]
- 76.De Almeida A.M.R., Yockteng R., Schnable J.C., Alvarez-Buylla E.R., Freeling M., Specht C.D. Co-option of the polarity gene network shapes filament morphology in angiosperms. Sci. Rep. 2014;4:06194. doi: 10.1038/srep06194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rudall P.J., Bateman R.M. Roles of synorganisation, zygomorphy and heterotopy in floral evolution: The gynostemium and labellum of orchids and other lilioid monocots. Biol. Rev. 2002;77:403–441. doi: 10.1017/S1464793102005936. [DOI] [PubMed] [Google Scholar]
- 78.Meister R.J., Oldenhof H., Bowman J.L., Gasser C.S. Multiple protein regions contribute to differential activities of YABBY proteins in reproductive development. Plant Physiol. 2005;137:651–662. doi: 10.1104/pp.104.055368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.McAbee J.M., Hill T.A., Skinner D.J., Izhaki A., Hauser B.A., Meister R.J., Venugopala Reddy G., Meyerowitz E.M., Bowman J.L., Gasser C.S. ABERRANT TESTA SHAPE encodes a KANADI family member, linking polarity determination to separation and growth of Arabidopsis ovule integuments. Plant J. 2006;46:522–531. doi: 10.1111/j.1365-313X.2006.02717.x. [DOI] [PubMed] [Google Scholar]
- 80.Filyushin M.A., Slugina M.A., Pyshnaya O.N., Kochieva E.Z., Shchennikova A.V. Structure analysis of INNER NO OUTER (INO) homologs in Capsicum species. Russ. J. Genet. 2018;54:753–757. doi: 10.1134/S1022795418050034. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data used in this study are presented in the supplementary information.