Abstract
In a project designed to investigate the specific and infraspecific taxa of Matthiola endemic to Sicily (Italy) as new potential sources of bioactive compounds in this work, the infraspecific taxa of Matthiola fruticulosa were studied, namely, subsp. fruticulosa and subsp. coronopifolia. HPLC–PDA/ESI–MS and SPME–GC/MS analyses of hydroalcoholic extracts obtained from the aerial parts of the two subspecies led to the detection of 51 phenolics and 61 volatile components, highlighting a quite different qualitative–quantitative profile. The antioxidant properties of the extracts were explored through in vitro methods: 1,1-diphenyl-2-picrylhydrazyl (DPPH), reducing power and Fe2+ chelating activity assays. The results of the antioxidant tests showed that the extracts possess a different antioxidant ability: particularly, the extract of M. fruticulosa subsp. fruticulosa exhibited higher radical scavenging activity than that of subsp. coronopifolia (IC50 = 1.25 ± 0.02 mg/mL and 2.86 ± 0.05 mg/mL), which in turn displayed better chelating properties (IC50 = 1.49 ± 0.01 mg/mL and 0.63 ± 0.01 mg/mL). Lastly, Artemia salina lethality bioassay was performed for toxicity assessment. The results of the bioassay showed lack of toxicity against brine shrimp larvae for both extracts. The data presented indicate the infraspecific taxa of M. fruticulosa as new and safe sources of antioxidant compounds.
Keywords: native plants, Sicily, natural resource, Matthiola fruticulosa, chemical composition, biological activity
1. Introduction
Brassicaceae plants have been an interesting research topic for years due to their chemical composition characterized by the presence of a variety of bioactive metabolites with valuable potential applications in improving nutrition and human health [1].
In recent years, our research team has focused on the study of taxa that grow spontaneously in Sicily (Italy) included in the Brassicaceae family, with the aim of discovering new sources of bioactive compounds that could be used in the pharmaceutical, nutraceutical, and cosmetic fields. In a previous article, we reported the characterization of the phenolic and volatile components and the in vitro antioxidant properties of the aerial part hydroalcoholic extract of M. incana subsp. incana (L.) R. Br. [2]. Based on the promising results obtained, it seemed interesting to extend our research to the other specific and infraspecific taxa comprised in the genus Matthiola R. Br. endemic to Sicily. In particular, the infraspecific taxa of Matthiola fruticulosa (L.) Maire were selected for the current study.
Matthiola fruticulosa (L.) Maire (synonyms: Cheiranthus fruticulosus L., Matthiola tristis (L.) R. Br.) is a perennial species that reaches up to 60 cm in height, sparsely pubescent to densely white tomentose, woody at the base. The leaves are linear or oblong, entire to sinuate-pinnatifid, and the flowers are gathered in terminal racemes with yellowish to purplish-violet petals. The fruit is a siliqua, erect or patent, more or less cylindrical [3,4,5].
Matthiola fruticulosa is native to Southern Europe (Southeastern Europe: Albania, Bulgaria, Croatia, Greece, Italy, Macedonia, Montenegro, Serbia; Southwestern Europe: France, Portugal, Spain), Northern Africa (Algeria, Libya, Morocco, Tunisia), and Western Asia (Cyprus, Turkey) [3,5,6].
In the “Flora Europaea” and “Flora Hellenica”, as well as in the consulted taxonomic database “The Plant List”, three different subspecies are indicated under M. fruticulosa: the nominal subspecies (i.e., subsp. fruticulosa), subsp. valesiaca (Gay ex Gaudin) P. W. Ball, and subsp. perennis (P. Conti) P. W. Ball [5,7,8]. On the other hand, in the latest edition of the “Flora d’Italia” only two infraspecific subdivisions are recognized for this taxon: the nominal subspecies, which prefers calcareous grounds, and the subsp. coronopifolia (Sm.) Giardina and Raimondo (synonyms: Cheiranthus coronopifolius Sm., M. tristis var. montana Lojac., M. tristis var. bicornis Lojac., M. fruticulosa var. tricornis Lojac.), which prefers clayey and marly substrates [3,9]. Both subspecies occur in Sicily, as reported in the “Checklist of the vascular flora of Sicily” by Raimondo et al. [4].
The use of M. fruticulosa as a folk remedy has been documented: actually, its utilization in traditional medicine in Libya for the treatment of kidney stones and piles has been reported [10]. Despite this, as far as we know, in the literature there are no bibliographic data about the phytochemical composition and the evaluation of the biological properties of this taxon, except for the not very recent characterization of a few glucosinolates in the seeds and aerial parts of M. fruticulosa collected in Spain [11].
These premises prompted us to design a study aimed at defining the phytochemical profile and the antioxidant properties of the hydroalcoholic extracts obtained from the aerial parts of both M. fruticulosa infraspecific taxa growing wild in Sicily, namely, subsp. fruticulosa and subsp. coronopifolia. A comprehensive insight into the qualitative–quantitative profile of the phenolic and the volatile constituents contained in the extracts was achieved by HPLC–PDA/ESI–MS and SPME–GC/MS analyses. Three different in vitro methods, which are based on different mechanisms, were used to assess the antioxidant activity. Lastly, the brine shrimp (Artemia salina Leach) lethality bioassay was utilized to evaluate the toxicity of the extracts.
Both infraspecific taxa of M. fruticulosa were found to be safe sources of bioactive compounds with antioxidant properties significantly different from a phytochemical point of view.
2. Results and Discussion
2.1. Phytochemical Investigations
2.1.1. Determination of Polyphenolic Compounds by HPLC–PDA/ESI–MS
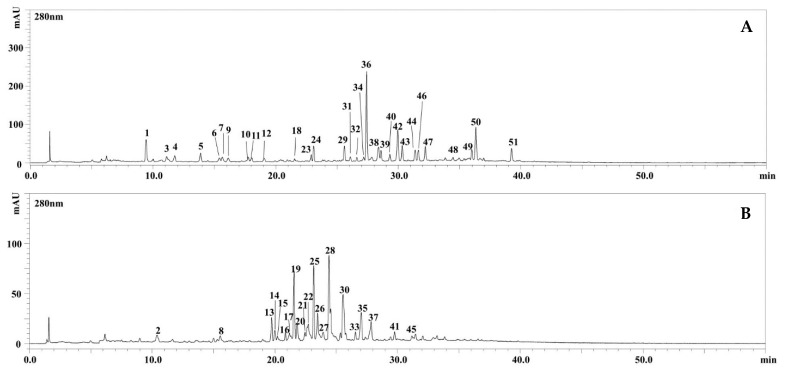
The polyphenolic characterization of the hydroalcoholic extracts of the aerial parts of the two subspecies of M. fruticulosa is reported here for the first time. In Figure 1, the HPLC–PDA chromatograms (λ = 280 nm) of the polyphenolic compounds occurring in the extracts of M. fruticulosa subsp. fruticulosa (A) and subsp. coronopifolia (B) are shown. A total of 51 compounds were detected, 31 in M. fruticulosa subsp. fruticulosa and 22 in subsp. coronopifolia (Table 1). Among them, 20 phenolic compounds were tentatively identified in M. fruticulosa subsp. fruticulosa according to retention times, PDA, MS, and literature data, 11 belonging to a class of flavonoids and 9 to phenolic acids; on the other hand, 11 compounds were characterized in M. fruticulosa subsp. coronopifolia, 6 flavonoids, and 5 phenolic acids. The identified flavonoid compounds belong to a class of flavonols, namely, quercetin, kaempferol, and isorhamnetin, and of flavones, namely, luteolin and its derivative isoorientin, while the phenolic acids are benzoic acids, hydroxybenzoic acid and syringic acid, and cinnamic acids, sinapic acid and ferulic acid. Except for peak n. 6 in M. fruticulosa subsp. fruticulosa, the rest of the polyphenolic compounds occurred in a glycosylated form.
Figure 1.
HPLC–PDA chromatograms of the polyphenolic compounds, extracted at 280 nm wavelength, of the hydroalcoholic extracts obtained from the aerial parts of M. fruticulosa subsp. fruticulosa (A) and subsp. coronopifolia (B). For peak identification, see Table 1.
Table 1.
HPLC–PDA/ESI–MS (negative and positive ionization modes) polyphenolic fingerprint of M. fruticulosa subsp. fruticulosa (A) and subsp. coronopifolia (B) extracts. Results are expressed as mg/g extract ± S.D. (n = 3).
| No | tR (min) |
UVmax (nm) |
[M − H]− | [M + H]+ |
Tentative
Identification |
mg/g Extract | |
|---|---|---|---|---|---|---|---|
| A | B | ||||||
| 1 | 9.3 | 260 | 299 | - | Hydroxybenzoic acid-hexoside | 6.21 ± 1.73 | n.d. |
| 2 | 10.4 | 276 | 359 | - | Syringic acid-hexoside | n.d. | 1.64 ± 0.03 |
| 3 | 11.0 | 264, 297 | 418 | - | 4-(methylthio)but-3-enyl glucosinolate | n.q. | n.d. |
| 4 | 11.7 | 293 | 418 | - | 4-(methylthio)but-3-enyl glucosinolate isomer | n.q. | n.d. |
| 5 | 13.8 | 257, 295 | 387 | - | Unknown | n.q. | n.d. |
| 6 | 15.3 | 277 | 325 | - | 5-hydroxyferuloylmalate | 1.71 ± 0.30 | n.d. |
| 7 | 15.5 | 315 | 289 | - | Unknown | n.q. | n.d. |
| 8 | 15.6 | 308 | 413 | - | Unknown | n.d. | n.q. |
| 9 | 16.0 | 232 | 329 | - | Unknown | n.q. | n.d. |
| 10 | 17.6 | 330 | 517 | - | Feruloyl-dihexoside | 3.01 ± 0.67 | n.d. |
| 11 | 17.9 | 329 | 355 | - | Ferulic acid-dihexoside | 3.23 ± 0.78 | n.d. |
| 12 | 19.0 | 330 | 385 | - | Sinapoylhexoside | 3.91 ± 0.72 | n.d. |
| 13 | 19.7 | 269sh, 329 | 437 | - | Unknown | n.d. | n.q. |
| 14 | 20.0 | 267sh, 314 | 725,433,285 | 595 | Kaempferol derivative | n.d. | 1.82 ± 0.01 |
| 15 | 20.2 | 267sh, 328 | 411 | - | Unknown | n.d. | n.q. |
| 16 | 20.9 | 268sh, 329 | 423,379 | - | Unknown | n.d. | n.q. |
| 17 | 21.2 | 337 | 371 | - | Hydroxyferuloyl-hexoside | n.d. | 1.62 ± 0.07 |
| 18 | 21.4 | 349 | 755,609,285 | 449 | Kaempferol derivative | 1.93 ± 0.36 | n.d. |
| 19 | 21.6 | 269sh, 328 | 595,440,285 | 339 | Kaempferol-dihexoside | n.d. | 10.99 ± 0.26 |
| 20 | 21.8 | 269sh, 318 | 739,579,285 | - | Kaempferol-trihexoside | n.d. | 3.23 ± 0.15 |
| 21 | 22.5 | 329 | 739,579 | 595 | Sinapoylhydroxyferuloyl-dihexoside | n.d. | 1.53 ± 0.18 |
| 22 | 22.6 | 349 | 741,301 | - | Quercetin derivative | n.d. | 3.03 ± 0.37 |
| 23 | 22.8 | 266, 337 | 185 | - | Unknown | n.q. | n.d. |
| 24 | 23.0 | 329 | 739,579 | 595 | Sinapoylhydroxyferuloyl-dihexoside isomer | 4.93 ± 1.25 | n.d. |
| 25 | 23.2 | 268sh, 336 | 387,501 | 438 | Unknown | n.d. | n.q. |
| 26 | 23.5 | 269sh, 314 | 387 | - | Unknown | n.d. | n.q. |
| 27 | 23.9 | 311 | 251 | - | Unknown | n.d. | n.q. |
| 28 | 24.4 | 266, 349 | 725 | 433 | Dihydroxyferuloyl-hexoside | n.d. | 10.83 ± 0.04 |
| 29 | 25.5 | 255, 350 | 725 | 433 | Dihydroxyferuloyl-hexoside isomer | 5.63 ± 1.30 | n.d. |
| 30 | 25.6 | 330 | 755,515,435,285 | - | Kaempferol derivative | n.d. | 11.93 ± 0.82 |
| 31 | 26.0 | 353 | 579,303,285 | 449 | Kaempferol-trihexoside | 2.74 ± 1.79 | n.d. |
| 32 | 26.5 | 266, 346 | 458 | - | Unknown | n.q. | n.d. |
| 33 | 26.6 | 338 | 593,447 | - | Isoorientin-hexoside | n.d. | 2.76 ± 0.68 |
| 34 | 27.0 | 352 | 771,301 | 303 | Quercetin-p-coumaroylhexoside | 3.65 ± 0.47 | n.d. |
| 35 | 27.0 | 339 | 695 | 433 | Unknown | n.d. | n.q. |
| 36 | 27.3 | 266, 346 | 709,563,431 | 287 | Feruloylhydroxyferuloyl-dihexoside | 26.74 ± 2.48 | n.d. |
| 37 | 27.9 | 318 | 725,563,593 | - | Dihydroxyferuloyl-dihexoside isomer | n.d. | 2.77 ± 0.52 |
| 38 | 28.2 | 265, 347 | 755,609,285 | - | Kaempferol derivative | 10.12 ± 3.28 | n.d. |
| 39 | 28.5 | 329 | 739,579 | - | Sinapoylhydroxyferuloyl-dihexoside isomer | 4.74 ± 0.88 | n.d. |
| 40 | 29.2 | 254, 351 | 593,285 | 463,287 | Luteolin-dihexoside | 3.75 ± 2.36 | n.d. |
| 41 | 29.8 | 329 | 613,518 | - | Unknown | n.d. | n.q. |
| 42 | 29.8 | 265,348 | 785,755,593,285 | 287 | Kaempferol-feruloyldihexoside | 19.85 ± 3.16 | n.d. |
| 43 | 30.2 | 265,348 | 785,755,593,285 | - | Kaempferol-feruloyldihexoside isomer | 10.25 ± 2.86 | n.d. |
| 44 | 31.3 | 265, 345 | 593,285 | 287 | Kaempferol-dihexoside | 6.16 ± 0.85 | n.d. |
| 45 | 31.5 | 329 | 759,449 | - | Unknown | n.d. | n.q. |
| 46 | 31.5 | 254, 352 | 623,447,315 | 479,317 | Isorhamnetin-dihexoside | 12.96 ± 3.94 | n.d. |
| 47 | 32.1 | 254, 352 | 623,447,315 | 479,317 | Isorhamnetin-dihexoside isomer | 17.63 ± 1.85 | n.d. |
| 48 | 34.4 | 330 | 447,409,285 | 181 | Kaempferol-hexoside | 3.14 ± 0.26 | n.d. |
| 49 | 35.9 | 330 | 535 | 495,287 | Unknown | n.q. | n.d. |
| 50 | 36.2 | 330 | 539 | 317,287 | Unknown | n.q. | n.d. |
| 51 | 39.2 | 310 | 674 | 339 | Unknown | n.q. | n.d. |
n.d.: not detected; n.q.: not quantified.
In addition to polyphenols, 4-(methylthio)but-3-enyl glucosinolate (glucoraphenin) and its isomer (peaks n. 3 and n. 4) were detected in traces in the extract of M. fruticulosa subsp. fruticulosa only. The presence of this glucosinolate compound in a 70% methanol extract of M. fruticulosa aerial parts collected in Spain was previously reported by Gmelin and Kjær [12].
Interestingly, from the comparison of the phenolics detected in the two subspecies, a quite different profile was highlighted since no compounds in common were found.
Regarding quantitative determination, the total amount of polyphenols identified in the extract of M. fruticulosa subsp. fruticulosa was found to be approximately three fold higher than that of subsp. coronopifolia (151.6 mg/g extract and 51.8 mg/g extract, respectively), with the flavonoids being 91.7 mg/g extract and 33.5 mg/g extract and the phenolic acids 59.9 mg/g extract and 18.3 mg/g extract. In addition, peak n. 36, feruloylhydroxyferuloyl-dihexoside, was the most abundant polyphenolic compound in M. fruticulosa subsp. fruticulosa (26.74 mg/g ± 2.48), whereas in M. fruticulosa subsp. coronopifolia, peak n. 30, kaempferol derivative, turned out to be the main component (11.93 mg/g ± 0.82).
Comparing the polyphenolic profiles of the two subspecies here analyzed with that of the aerial part extract of M. incana subsp. incana previously investigated, substantial quali-quantitative differences were highlighted [2]. Indeed, the total amount of polyphenols detected in the M. incana subsp. incana extract (161.31 mg/g extract) was quite similar to that of M. fruticulosa subsp. fruticulosa and about three times higher than that of subsp. coronopifolia, with the flavonoids being the most representative group (155.85 mg/g extract), while the two phenolic acids identified (i.e., vanillic and sinapic acids) occurred in small amounts. On the other hand, phenolic acids were contained in greater numbers in the extracts from the two subspecies of M. fruticulosa, accounting their amounts to be about one-third of the total phenols quantified for both of them.
2.1.2. Identification of Volatile Compounds by SPME–GC/MS
Table 2 reports the volatile profile of the hydroalcoholic extracts obtained from the aerial parts of M. fruticulosa subsp. fruticulosa and subsp. coronopifolia. As can be observed in the table, the 61 volatiles identified can be classified into several main groups: aldehydes (mainly with alkanals, 2-alkenals, and 2,4-alcadienals, although also aromatic), acids, alcohols (mainly unsaturated and branched), ketones, esters (mainly methyl esters), sulfides, nitriles, and terpenoids.
Table 2.
Composition as volatile constituents and classes of substances of the hydroalcoholic extracts obtained from the aerial parts of M. fruticulosa subsp. fruticulosa and subsp. coronopifolia.
| Compound | LRI * on DB-5ms |
LRI * on VF-AXms |
M. fruticulosa Subsp. fruticulosa |
M. fruticulosa Subsp. coronopifolia |
||
|---|---|---|---|---|---|---|
| Amount † X ± Dev St |
Percentage X ± Dev St |
Amount † X ± Dev St |
Percentage X ± Dev St |
|||
| Aldehydes | ||||||
| 2-Methylbutanal | 675 | 913 | -§ | - | 1262 ± 143 | 3.73 ± 0.39 |
| Heptanal | 903 | 1198 | - | - | 51 ± 7 | 0.15 ± 0.02 |
| (E)-2-Heptenal | 957 | 1338 | - | - | 396 ± 43 | 1.17 ± 0.19 |
| Benzaldehyde | 962 | 1529 | 261 ± 47 | 0.22 ± 0.02 | 298 ± 38 | 0.88 ± 0.11 |
| Octanal | 1004 | 1298 | 508 ± 51 | 0.43 ± 0.05 | 101 ± 19 | 0.30 ± 0.05 |
| (E,E)-2,4-Heptadienal | 1014 | 1497 | - | - | 247 ± 34 | 0.73 ± 0.14 |
| Phenylacetaldehyde | 1043 | 1640 | - | - | 213 ± 37 | 0.63 ± 0.11 |
| Nonanal | 1105 | 1398 | 5554 ± 939 | 4.72 ± 0.74 | - | - |
| (E)-2-Nonenal | 1161 | 1543 | 72 ± 12 | 0.06 ± 0.01 | - | - |
| Decanal | 1206 | 1502 | 115 ± 19 | 0.10 ± 0.02 | 116 ± 14 | 0.34 ± 0.06 |
| (E)-2-Decenal | 1263 | 1647 | - | - | 101 ± 12 | 0.30 ± 0.05 |
| (Z)-9-Octadecenal | 2006 | 2693 | 242 ± 42 | 0.21 ± 0.03 | - | - |
| All | 6752 ± 385 | 5.73 ± 0.30 | 2787 ± 55 | 8.24 ± 0.16 | ||
| Acids | ||||||
| Benzoic acid | 1165 | 2433 | 522 ± 60 | 0.47 ± 0.08 | - | - |
| Octanoic acid | 1174 | 2072 | 262 ± 29 | 0.22 ± 0.04 | 636 ± 96 | 1.88 ± 0.31 |
| Nonanoic acid | 1269 | 2178 | 180 ± 26 | 0.15 ± 0.02 | 231 ± 30 | 0.68 ± 0.09 |
| Decanoic acid | 1366 | 2284 | - | - | 205 ± 36 | 0.61 ± 0.11 |
| All | 995 ± 41 | 0.84 ± 0.05 | 1072 ± 62 | 3.17 ± 0.20 | ||
| Alcohols | ||||||
| (E)-2-Hepten-1-ol | 973 | 1515 | - | - | 409 ± 78 | 1.21 ± 0.19 |
| 1-Octen-3-ol | 980 | 1452 | - | - | 2719 ± 342 | 8.04 ± 1.09 |
| 2-Ethyl-1-hexanol | 1029 | 1489 | 164 ± 20 | 0.14 ± 0.02 | - | - |
| (E)-2-Octen-1-ol | 1068 | 1617 | - | - | 244 ± 35 | 0.72 ± 0.14 |
| 1-Octanol | 1072 | 1561 | - | - | 393 ± 71 | 1.16 ± 0.21 |
| 2-Methyl-1-octanol | 1100 | - | - | - | 148 ± 27 | 0.44 ± 0.08 |
| All | 164 ± 20 | 0.14 ± 0.02 | 3914 ± 161 | 11.58 ± 0.51 | ||
| Ketones | ||||||
| 4-Methyl-2-pentanone | 736 | 1010 | - | - | 487 ± 90 | 1.44 ± 0.23 |
| 2-Hexanone | 790 | 1083 | - | - | 265 ± 36 | 0.78 ± 0.15 |
| 4-Methyl-3-penten-2-one | 801 | 1132 | 2267 ± 228 | 1.92 ± 0.39 | 4097 ± 746 | 12.12 ± 2.43 |
| Acetylacetone | 815 | 1196 | - | - | 278 ± 50 | 0.82 ± 0.14 |
| 2-Heptanone | 891 | 1185 | - | - | 201 ± 29 | 0.60 ± 0.09 |
| 3-Methyl-2-heptanone | 936 | 1210 | 446 ± 77 | 0.38 ± 0.07 | 499 ± 77 | 1.48 ± 0.19 |
| Acetophenone | 1066 | 1656 | 249 ± 39 | 0.21 ± 0.04 | - | - |
| Hexahydrofarnesyl acetone | 1844 | 2121 | 318 ± 58 | 0.27 ± 0.04 | 4835 ± 717 | 14.3 ± 2.01 |
| All | 3279 ± 125 | 2.78 ± 0.20 | 10663 ± 394 | 31.54 ± 1.20 | ||
| Esters | ||||||
| Methyl heptanoate | 1023 | 1293 | 693 ± 136 | 0.59 ± 0.09 | - | - |
| Methyl benzoate | 1094 | 1628 | 6239 ± 1218 | 5.3 ± 1.02 | - | - |
| Methyl octanoate | 1123 | 1394 | 363 ± 65 | 0.31 ± 0.05 | 94 ± 10 | 0.28 ± 0.05 |
| Ethyl octanoate | 1196 | 1439 | 126 ± 19 | 0.11 ± 0.01 | 135 ± 14 | 0.40 ± 0.05 |
| Methyl decanoate | 1323 | 1594 | 216 ± 34 | 0.18 ± 0.03 | 64 ± 8 | 0.19 ± 0.03 |
| Ethyl 9-decenoate | 1386 | 1678 | 180 ± 24 | 0.15 ± 0.03 | 395 ± 66 | 1.17 ± 0.19 |
| Ethyl decanoate | 1394 | 1639 | 1078 ± 128 | 0.92 ± 0.14 | 1193 ± 216 | 3.53 ± 0.56 |
| Fumaric acid, pent-4-en-2-yl propyl ester | 1492 | - | 371 ± 55 | 0.32 ± 0.05 | 146 ± 18 | 0.43 ± 0.08 |
| Ethyl dodecanoate | 1590 | 1840 | - | - | 60 ± 8 | 0.18 ± 0.04 |
| Methyl tetradecanoate | 1726 | 1997 | - | - | 40 ± 6 | 0.12 ± 0.01 |
| Methyl hexadecanoate | 1926 | 2199 | 264 ± 39 | 0.22 ± 0.02 | 192 ± 35 | 0.57 ± 0.09 |
| Isopropyl hexadecanoate | 2023 | 2232 | - | - | 53 ± 8 | 0.16 ± 0.03 |
| All | 9530 ± 412 | 8.09 ± 0.35 | 2373 ± 73 | 7.02 ± 0.19 | ||
| Sulfur compounds | ||||||
| Dimethyl disulfide | 743 | 1082 | 855 ± 111 | 0.73 ± 0.12 | 855 ± 137 | 2.53 ± 0.49 |
| Dimethyl trisulfide | 968 | 1391 | 80953 ± 14196 | 68.73 ± 13.01 | 539 ± 97 | 1.6 ± 0.29 |
| Dimethyl tetrasulfide | 1215 | 1750 | 9554 ± 1063 | 8.11 ± 1.29 | - | - |
| All | 91362 ± 8219 | 77.57 ± 7.55 | 1395 ± 119 | 4.13 ± 0.40 | ||
| Nitriles | ||||||
| 4-Methylpentanenitrile | 840 | 1253 | - | - | 778 ± 110 | 2.3 ± 0.47 |
| Hexanenitrile | 877 | 1308 | - | - | 333 ± 61 | 0.98 ± 0.16 |
| Heptanenitrile | 978 | 1408 | 855 ± 103 | 0.73 ± 0.09 | - | - |
| 4-(Methylthio)-butanenitrile | 1082 | 1806 | - | - | 1629 ± 257 | 4.79 ± 0.85 |
| Benzyl nitrile | 1137 | 1893 | 409 ± 66 | 0.35 ± 0.07 | - | - |
| All | 1264 ± 87 | 1.08 ± 0.08 | 2730 ± 165 | 8.08 ± 0.57 | ||
| Terpenoids | ||||||
| Isophorone | 1126 | 1621 | - | - | 35 ± 4 | 0.10 ± 0.01 |
| Cryptone | 1175 | 1675 | - | - | 289 ± 37 | 0.85 ± 0.13 |
| Safranal | 1200 | 1649 | 891 ± 127 | 0.76 ± 0.11 | 208 ± 39 | 0.61 ± 0.13 |
| β-Cyclocitral | 1220 | 1626 | 246 ± 49 | 0.21 ± 0.03 | 179 ± 33 | 0.53 ± 0.09 |
| Pulegone | 1233 | 1637 | - | - | 166 ± 22 | 0.49 ± 0.07 |
| Thymoquinone | 1250 | - | - | - | 171 ± 19 | 0.50 ± 0.07 |
| 2-Isopropyl-5-methyl-3-cyclohexen-1-one | 1255 | - | - | - | 6605 ± 1113 | 19.54 ± 3.76 |
| Carvacrol | 1291 | 2225 | - | - | 89 ± 14 | 0.26 ± 0.04 |
| Geranylacetone | 1447 | 1857 | - | - | 86 ± 13 | 0.26 ± 0.05 |
| All | 1136 ± 96 | 0.97 ± 0.08 | 7827 ± 372 | 23.15 ± 1.26 | ||
| Hydrocarbons | ||||||
| 9-Octadecene | 1744 | 1795 | 554 ± 80 | 0.47 ± 0.07 | - | - |
| 9-Eicosene | 1948 | 1914 | 280 ± 38 | 0.24 ± 0.04 | 50 ± 7 | 0.15 ± 0.03 |
| All | 834 ± 63 | 0.71 ± 0.06 | 50 ± 7 | 0.15 ± 0.03 | ||
| Others | ||||||
| All | 2463 ± 368 | 2.09 ± 0.42 | 995 ± 179 | 2.94 ± 0.58 | ||
* Linear retention indexes calculated according to the Van Den Dool and Kratz equation; † peak area arbitrary scale; § not detected.
As regards M. fruticulosa subsp. fruticulosa, the extract of the aerial parts was constituted mainly of sulfides (primarily dimethyl trisulfide and dimethyl tetrasulfide), which accounted for more than 77% of all volatiles. Among the other chemical classes, esters (8.09%), aldehydes (5.73%), and ketones (2.78%) were the most represented, with methyl benzoate, nonanal, and 4-methyl-3-penten-2-one as the main compounds in the three classes, respectively. These five volatiles, namely, dimethyl trisulfide, dimethyl tetrasulfide, methyl benzoate, nonanal, and 4-methyl-3-penten-2-one, accounted for more than 88% of the volatile fraction of the M. fruticulosa subsp. fruticulosa extract.
The extract of the M. fruticulosa subsp. coronopifolia aerial parts showed a volatile profile in which ketones (31.54%) and terpenes (23.15%) prevailed, although the other classes were also well represented, ranging between 3.17% (acids) and 11.58% (alcohols). The main volatile compounds were 2-isopropyl-5-methyl-3-cyclohexen-1-one among terpenoids; hexahydrofarnesyl acetone, 4-methyl-3-penten-2-one, and 4-methyl-2-pentanone among ketones; 1-octen-3-ol among alcohols; 4-(methylthio)-butanenitrile and 4-methylpentanenitrile among nitriles; 2-methylbutanal among aldehydes; ethyl decanoate among esters; and dimethyl disulfide and octanoic acid among sulfides and acids, respectively.
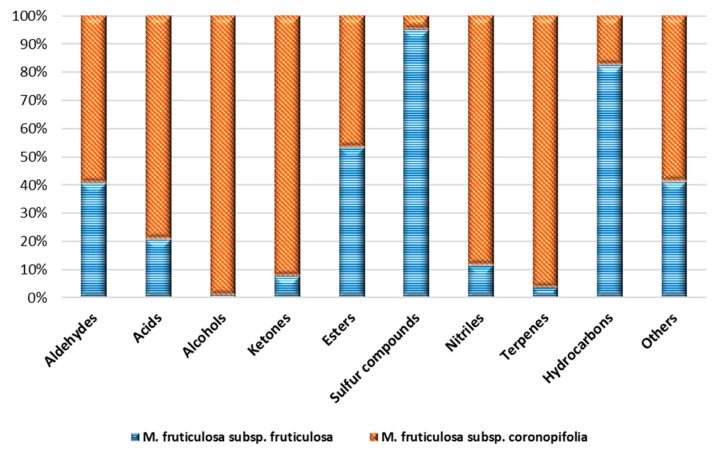
Figure 2 shows the normalized percentage composition, as classes of substances, of the two subspecies of M. fruticulosa, highlighting considerable differences between them.
Figure 2.
Normalized percentage composition, as classes of substances, of the volatile profile of M. fruticulosa subsp. fruticulosa and subsp. coronopifolia extracts.
In plants, several biosynthetic pathways occur, leading to a wide range of volatile organic compounds as secondary metabolites; thus, differences in the volatile profiles of different plants may reflect differences in their metabolic pathways. These differences have been observed in plants of the same species and even in different cultivars of the same subspecies [13]. The volatile compounds identified in the samples of M. fruticulosa subsp. fruticulosa and subsp. coronopifolia can be classified into terpenoids, carotenoid derivatives, fatty acid derivatives, amino acid derivatives, and benzenoid compounds.
The terpenoids safranal, β-cyclocitral, isophorone, and geranylacetone derive from the enzymatic degradation of carotenoids; the first two compounds were present in both subspecies, whereas the others only in subsp. coronopifolia, in any case at a low percentage. The other terpenoid compounds were detected only in the extract of M. fruticulosa subsp. coronopifolia, and all of them were oxygenated monoterpenes; these compounds have not been reported previously among volatiles of Matthiola species. Terpenoids in plants originate from two distinct metabolic pathways: the cytosolic mevalonic acid pathway (MVA) and the plastidial methylerythritol phosphate pathway (MEP). Isopentenyl pyrophosphate (IPP) and dimethylallyl pyrophosphate (DMAPP) are the end products in both pathways and are the precursors of a very large number of compounds by numerous enzyme-catalyzed reactions, including cyclization, hydroxylation, dehydrogenation, oxidation and/or reduction, and isomerization.
Aldehydes, alcohols, and esters, containing 6 to 10 carbon atoms, originate from unsaturated fatty acids through the lipoxygenase pathway. In particular, linoleic and linolenic acid are the precursors of the so-called green leaf volatiles, namely, C6 aldehydes, alcohols, and their ester. Linoleic acid is responsible also for the formation of 1-octen-3-one and 1-octen-3-ol, whereas nonanal, 1-nonanol, heptanal, and 1-heptanol are formed from oleic and palmitoleic acids. Among fatty acid derivative compounds, nonanal was the main volatile in subsp. fruticulosa, whereas 1-octen-3-ol was the most abundant in subsp. coronopifolia, highlighting possible differences between the two subspecies in both their fatty acid composition and the set of lipoxygenases.
Nitriles are among the amino-acid-derivative compounds. In fact, amino acids are the precursors of glucosinolates, a group of sulfur-containing secondary metabolites characterizing Brassicaceae vegetables; from the glucosinolate hydrolysis, through the action of the myrosinase enzyme, isothiocyanates, nitriles, and thiocyanates may arise. In both subspecies of M. fruticulosa, only nitriles were detected, with a higher percentage in subsp. coronopifolia, where 4-(methylthio)-butanenitrile was the most abundant compound, followed by 4-methylpentanenitrile. 4-(Methylthio)-butanenitrile originates from glucoerucin, which has been identified as the main glucosinolate in M. fruticulosa seeds [11]. The absence of isothiocyanates suggests that in our samples the hydrolysis of glucosinolates is altered in favor of nitriles; many factors can modify the activity of myrosinase, favoring nitrile formation [14], such as the presence of ferrous ions, nitrile-specific proteins, acidic conditions, and, according to Wieczorek and Jelen [13], also frozen–thawed processes of the vegetable tissue.
The only sulfur compounds detected in the extracts of M. fruticulosa subsp. fruticulosa and subsp. coronopifolia were sulfides (i.e., dimethyl disulfide, dimethyl trisulfide, and dimethyl tetrasulfide). Sulfides are derivative compounds of the S-alk(en)yl-l-cysteine pathway and occur very frequently in the volatile fraction of Brassicaceae family plants, even being the most abundant volatiles in M. incana subsp. incana and Brassica incana grown wild in Sicily [2,15] and in different cultivars of Brassica oleracea [13].
Benzenoid compounds are derived from t-cinnamic acid with the propyl side chain shortened by two carbon atoms via either a non-β-oxidative or a β-oxidative pathway, and the formation of benzaldehyde and benzoyl-CoA as intermediates for benzoic acid production [16]. Benzaldehyde and benzoic acid were detected only in the M. fruticulosa subsp. fruticulosa extract; moreover, methyl benzoate was also present, being the most abundant compound among esters.
Finally, hexahydrofarnesyl acetone was among the most abundant volatiles of the M. fruticulosa subsp. coronopifolia extract; hexahydrofarnesyl acetone, or phytone, is a ketone very common in plants that arises from the oxidative degradation of (E)-phytol, a diterpene alcohol that occurs as a side chain of chlorophyll a [17].
2.2. Antioxidant Activity
In the last decades, oxidative stress has been recognized to play a critical role in the pathogenesis of several diseases. This has significantly increased research studies aimed at discovering new plant sources of antioxidants. The Brassicaceae family encloses many plant species that are potential sources of antioxidant compounds, including some belonging to the Matthiola genus, as also demonstrated in recent studies on M. incana infraspecific taxa carried out by our research team [2,18,19,20].
In this work, the antioxidant properties of the extracts of M. fruticulosa subsp. fruticulosa and subsp. coronopifolia were established using three in vitro tests to evaluate the different mechanisms through which the diverse antioxidant compounds contained in the phytocomplexes could exert their effect: DPPH assay, based on the hydrogen atom transfer (HAT) and electron transfer (ET) mechanisms; reducing power, an electron transfer (ET)-based assay; and ferrous ion (Fe2+) chelating activity assay.
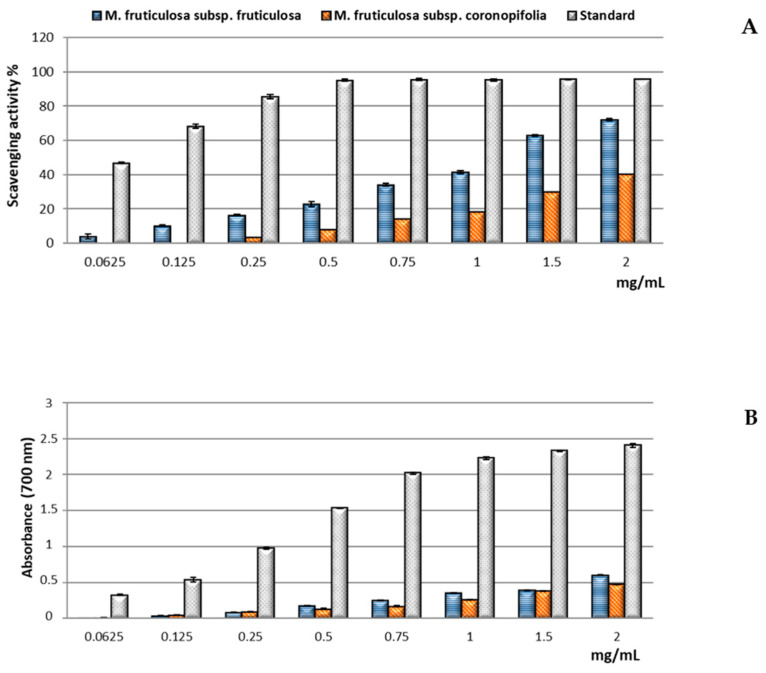
Figure 3A shows the results of the DPPH assay, utilized to determine the free scavenging ability of the extracts. Compared with the reference standard BHT, both extracts displayed lower activity in the range of concentrations tested, which increased with increasing dose. The extract of M. fruticulosa subsp. fruticulosa exhibited the best scavenging activity, reaching about 70% inhibition of the DPPH radical at the highest concentration tested. From the comparison of the calculated IC50 values, it was evidenced that the radical scavenging ability of the M. fruticulosa subsp. fruticulosa extract, showing an IC50 of 1.25 ± 0.02 mg/mL, was approximately 2.3-fold higher than that of the subsp. coronopifolia extract (IC50 = 2.86 ± 0.05 mg/mL). Comparing the free radical scavenging activity of the M. fruticulosa subsp. fruticulosa extract with that highlighted in other Matthiola species, the result showed an activity higher than that of the hydroalcoholic extracts (80% MeOH) of the aerial parts from the Matthiola incana infraspecific taxa endemic to Sicily, whose antioxidant properties had previously been investigated under the same experimental conditions, namely, M. incana subsp. incana, IC50 = 2.32 ± 0.24 mg/mL; subsp. rupestris, IC50 = 1.73 ± 0.02 mg/mL and 2.60 ± 0.01 mg/mL; and subsp. pulchella, IC50 = 3.69 ± 0.14 mg/mL [2,18]. On the other hand, Abdelshafeek et al. [20] reported a lower IC50 value (0.49 mg/mL) for a hydroalcoholic extract (80% MeOH) obtained by maceration from the aerial parts of M. longipetala subsp. longipetala collected in Libya. The results of the evaluation of the reducing power of the extracts of the two subspecies of M. fruticulosa, determined through the Fe3+–Fe2+ transformation method, showed that both extracts displayed mild activity compared with the standard BHT. This result agrees with those previously reported for the extracts of the aerial parts from the M. incana infraspecific taxa [2,18]. As shown in Figure 3B, the extracts exhibited similar reducing power, dose dependent, although the calculated ASE/mL values indicated greater reducing efficacy for the extract of subsp. coronopifolia (18.61 ± 0.06 mg/mL) than that of subsp. fruticulosa (38.17 ± 1.14 mg/mL). Both extracts are less active than that of M. incana subsp. incana (ASE/mL = 12.28 ± 0.42 mg/mL), which showed the best reducing power among the infraspecific taxa of M. incana [2,18].
Figure 3.
Free radical scavenging activity (DPPH assay) (A), reducing power (B), and ferrous ion chelating activity (C) of the hydroalcoholic extracts obtained from the aerial parts of M. fruticulosa subsp. fruticulosa and subsp. coronopifolia. Reference standard: BHT (A,B), EDTA (C). Values are expressed as the mean ± SD (n = 3).
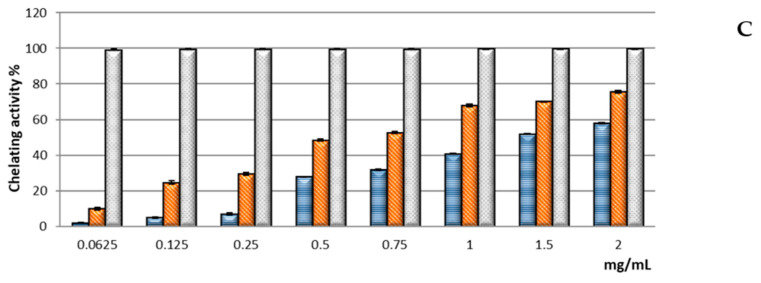
In the Fe2+ chelating activity assay, performed by evaluating the inhibiting effect on the Fe2+–ferrozine complex formation, both extracts of the two subspecies of M. fruticulosa showed good chelating properties, dose dependent, although lower than those of the reference standard EDTA (Figure 3C). Contrary to what has been observed in the other antioxidant assays, the extract of M. fruticulosa subsp. coronopifolia displayed the best chelating activity, reaching about 75% inhibition at the highest concentration tested. This was confirmed also by a comparison of the IC50 values calculated for the extracts, which indicated that the chelating efficacy of M. fruticulosa subsp. coronopifolia (0.63 ± 0.01 mg/mL) was approximately 2.5-fold higher than that of M. fruticulosa subsp. fruticulosa (1.49 ± 0.01 mg/mL). The chelating activity of the extract of M. fruticulosa subsp. coronopifolia was close to that previously found for that of M. incana subsp. incana (IC50 = 0.53 ± 0.02 mg/mL), the most effective among those from the infraspecific taxa of M. incana [2,18].
The results of the antioxidant tests clearly indicate that the extracts of the two subspecies of M. fruticulosa possess different antioxidant ability; in fact, the extract of M. fruticulosa subsp. fruticulosa exhibited higher radical scavenging activity than that of subsp. coronopifolia; conversely, the latter displayed better chelating properties than the former.
Among plant secondary metabolites, polyphenols represent the most important group of antioxidant compounds. Flavonoids and phenolic acids, the largest classes of plant phenolics, have been shown to be effective antioxidants in several in vitro and in vivo investigations [21,22,23]. The higher radical scavenging properties of the extract of M. fruticulosa subsp. fruticulosa seem to be related to the greater content of flavonoids and phenolic acids detected by HPLC–PDA/ESI–MS analysis, both present in quantities about three times higher than those of subsp. coronopifolia. Regarding the chelating properties, which are greater for M. fruticulosa subsp. coronopifolia, although the partial involvement of the phenolic constituents detected in the extracts cannot be ruled out, it is assumed that they may depend on other antioxidant phytochemicals.
2.3. Artemia salina Leach Lethality Bioassay
Artemiasalina Leach (brine shrimp) is a small crustacean widely utilized as a model organism in the toxicity assessment of plant extracts [24]. The brine shrimp lethality bioassay has been applied as an alternative method for the preliminary estimation of toxicity because it shows several advantages, such as rapidity, cost-effectiveness, ease of handling and maintenance under laboratory conditions, and adaptability to various testing conditions [25].
The results of the bioassay showed the absence of toxicity against brine shrimp larvae for both extracts of the M. fruticulosa subspecies. Indeed, the median lethal concentration values were found to be above 1000 μg/mL, thus indicating their potential safety based on Clarkson’s toxicity criterion [26]. The obtained results agree with those reported for the hydroalcoholic extracts of the aerial parts from the M. incana infraspecific taxa previously investigated, which were tested under the same experimental conditions [2,18].
3. Materials and Methods
3.1. Chemicals and Reagents
LC–MS-grade water (H2O), acetonitrile (ACN), gallic acid, catechin, chlorogenic acid, apigenin, luteolin, rutin, kaempferol, and quercetin were obtained from Merck Life Science (Merck KGaA, Darmstadt, Germany). LC–MS-grade formic acid was purchased from Riedel-de Haën (Seelze, Germany). Methanol (MeOH) was purchased from Carlo Erba (Milan, Italy). Unless indicated otherwise, all chemicals were purchased from Sigma-Aldrich (Milan, Italy).
3.2. Plant Material and Extraction Procedure
The aerial parts of the Matthiola fruticulosa subspecies were collected in Sicily (Italy): M. fruticulosa subsp. fruticulosa (L.) Maire was picked in May 2019 in the locality of Polizzi Generosa, Contrada Pietà (Palermo), on a dolomitic lithosol, about 1200 m (a.s.l., above sea level), and M. fruticulosa subsp. coronopifolia (Sm.) Giardina and Raimondo in the locality of Sutera (Caltanissetta), on chalky cliffs, about 400 m (a.s.l.), in June 2019. Voucher specimens were identified by Prof. F.M. Raimondo, PLANTA/Center for Research, Documentation and Training (Palermo), and Prof. V. Spadaro, University of Palermo, and have been deposited to the Herbarium Mediterraneum of the University of Palermo, Italy (PAL-Gr) (voucher numbers: Raimondo & Spadaro n. 03/19; Schimmenti n. 05/19).
After harvesting, the aerial parts of the two subspecies were immediately frozen; then after lyophilization, the plant material was subjected to a preventive maceration with 80% MeOH (1:10 w/v) for 150 min. The extraction was performed with 80% MeOH (1:10 w/v) in an ultrasonic bath at 50 °C for 15 min three times; then the filtrates were pooled and evaporated to dryness by a rotavapor. The yields of the extracts, referring to 100 g of lyophilized plant material, were 20.38% and 19.83% for M. fruticulosa subsp. fruticulosa and subsp. coronopifolia, respectively.
3.3. Phytochemical Investigations
3.3.1. Identification of Phenolic Compounds by HPLC–PDA/ESI–MS
The analyses were carried out using a Shimadzu HPLC system (Milan, Italy) equipped with a CBM-20A controller, LC-20AD pumps, a DGU-20A3 degasser, a SIL-20AC autosampler, an SPD-M20A photodiode array detector (PDA), and a triple quadrupole mass analyzer (LCMS-8050, Shimadzu, Kyoto, Japan), equipped with an ESI interface, in positive and negative ionization modes. Data acquisition was performed by Shimadzu LabSolutions software ver. 5.91.
Samples and sample preparation: An amount of 20 mg of M. fruticulosa subsp. fruticulosa and subsp. coronopifolia extracts was dissolved in 1 mL of MeOH.
Chromatographic conditions: Analyses were carried out on an Ascentis Express C18, 15 cm × 4.6 mm I.D. with a particle size of 2.7 µm (Merck Life Science, Merck KGaA, Darmstadt, Germany). The injection volume was 5 µL, the mobile phase consisted of water/formic acid (99.9:0.1, v/v) (solvent A) and ACN/formic acid (99.9:0.1, v/v) (solvent B), and the linear gradient profile was as follows: 0 min, 0% B; 5 min, 5% B; 15 min, 10% B; 30 min, 20% B; 60 min, 50% B; 70 min, 100% B; 71 min, 0% B. The flow rate was 1 mL/min, and it was split to 0.2 mL/min prior to MS detection.
PDA conditions: The wavelength range was 200–400 nm, and the chromatograms were extracted at a wavelength of 280 nm. Time constant was 0.08 s, and the sample frequency was 40 Hz.
MS conditions: The mass spectral range was 100–1000 m/z, the interval was 0.5 s, the nebulizing gas (N2) flow was 1.5 L/min, the interface temperature was 350 °C, the heat block was 300 °C, the DL temperature was 300 °C, the DL voltage was −34 V, the probe voltage was 4.5 kV, the Qarray voltage was 1.0 V, the RF voltage was 90 V, and the detection gain was 1.0 kV.
Quantitative determination was performed using the calibration curves of six standards, namely, gallic acid, hydroxybenzoic acid, chlorogenic acid, luteolin, kaempferol, and quercetin. Standard calibration curves were prepared in the concentration range of 0.1–50 mg/L with five different concentration levels [27].
3.3.2. Identification of Volatile Compounds by SPME–GC/MS
Extraction (HS–SPME): The hydroalcoholic extracts of the aerial parts of M. fruticulosa subsp. fruticulosa and subsp. coronopifolia were analyzed for their volatile composition by HS–SPME–GC/MS.
An amount of 30.0 mg of each dried extract was solubilized in 3.0 mL of saturated sodium chloride solution (final concentration of 10 mg/mL) into a 7 mL vial, then closed with a “mininert” valve (Supelco, Bellefonte, PA, USA). The samples were extracted using a DVB/CAR/PDMS fiber with a 50/30 μm film thickness (Supelco, Bellefonte, PA, USA) following the method reported by Miceli et al. [2].
Analysis (GC/MS): The volatiles were analyzed by a Shimadzu GC 2010 Plus gas chromatograph coupled to a TQMS 8040 triple quadrupole mass spectrometer (Shimadzu, Milan, Italy) on two different capillary columns: (1) VF-WAXms, 60 m, 0.25 mm i.d., 0.25 μm film thickness polar column (Agilent Technologies Italia S.p.A., Milan, Italy), and (2) DB-5ms, 30 m, 0.25 mm i.d., 0.25 μm film thickness polar column (Agilent Technologies Italia S.p.A., Milan, Italy).
The conditions were as follows: injection mode, splitless; oven temperature, (1) 45 °C held for 5 min, then increased to 80 °C at a rate of 10 °C/min and to 240 °C at 2 °C/min, held at 240 °C for 5 min, for VF-WAXms column, and (2) 45 °C increased to 160 °C at a rate of 3 °C/min and to 260 °C at 10 °C/min, held at 260 °C for 5 min, for DB-5ms column; carrier gas, helium at a constant flow of 1 mL/min; transfer line temperature, 250 °C; acquisition range, 40 to 360 m/z; scan speed, 1250. For the identification of the volatiles, mass spectral data, NIST 14 (NIST/EPA/NIH Mass Spectra Library, version 2.0, NIST Mass Spectrometry Data Center, National Institute of Standards and Technology, Gaithersburg, MD, USA) and FFNSC 3.0 databases, linear retention indexes (LRIs), literature data, and injection of the available standards were used [28].
3.4. Antioxidant Activity
3.4.1. DPPH Assay
The 1,1-diphenyl-2-picrylhydrazyl (DPPH) assay was used to determine the free radical scavenging activity of the hydroalcoholic extracts of M. fruticulosa subsp. fruticulosa and subsp. coronopifolia [29]. The extracts were tested in the range of 0.0625–2 mg/mL using butylated hydroxytoluene (BHT) as positive control. A 0.5 mL aliquot of each sample solution was added to 3 mL of DPPH methanol solution (0.1 mM). The mixture was left at room temperature in the dark for 20 min, and then absorbance was measured at 517 nm using a model UV-1601 spectrophotometer (Shimadzu). The results were obtained from the average of three independent experiments, and are reported as mean radical scavenging activity (%) ± standard deviation (SD) and mean 50% inhibitory concentration (IC50) ± SD.
3.4.2. Reducing Power Assay
The reducing power of the hydroalcoholic extracts of M. fruticulosa subsp. fruticulosa and subsp. coronopifolia was assessed by the Fe3+–Fe2+ transformation method [30]. The extracts were tested in the range of 0.0625–2 mg/mL using butylated hydroxytoluene (BHT) and ascorbic acid as positive controls. An amount of 1 mL of each sample solution was mixed with 2.5 mL of phosphate buffer (0.2 M, pH 6.6) and 2.5 mL of 1% potassium ferricyanide. After incubation at 50 °C for 20 min and rapid cooling, 2.5 mL of 10% trichloroacetic acid was added. Finally, 2.5 mL of the supernatant was mixed with 2.5 mL of distilled water and 0.5 mL of 0.1% ferric chloride; then the mixture was incubated for 10 min at room temperature in the dark, and the optical density change was measured at 700 nm. The results were obtained from the average of three independent experiments, and are expressed as mean absorbance values ± SD and ascorbic acid equivalent/mL (ASE/mL) ± SD.
3.4.3. Ferrous Ion (Fe2+) Chelating Activity Assay
The spectrophotometric measurement of the Fe2+–ferrozine complex was used to determine the Fe2+ chelating activity of the hydroalcoholic extracts of M. fruticulosa subsp. fruticulosa and subsp. coronopifolia according to the method previously reported by Decker and Welch [31]. The extracts were tested in the range of 0.0625–2 mg/mL using ethylenediaminetetraacetic acid (EDTA) as positive control. Briefly, 0.05 mL of 2 mM ferrous chloride was added to 1 mL of sample solution and 0.5 mL of methanol. The reaction was initiated by the addition of 0.2 mL of 5 mM ferrozine solution. After vigorous shaking, the mixture was incubated at room temperature in the dark for 10 min; then the optical density change was measured spectrophotometrically at 562 nm. The results were obtained from the average of three independent experiments and are reported as mean inhibition of the ferrozine–(Fe2+) complex formation (%) ± SD and IC50 ± SD.
3.5. Artemia salina Leach Lethality Bioassay
To establish the acute toxicity of the hydroalcoholic extracts of M. fruticulosa subsp. fruticulosa and subsp. coronopifolia, the brine shrimp (Artemia salina Leach) lethality bioassay was carried out according to the method of Meyer et al. [32]. Brine shrimp eggs were placed in a brine shrimp hatchery dish filled with sterile artificial seawater for 48 h. After hatching, active nauplii free from eggshells were collected from the brighter portion of the hatchery dish and used for the assay. Ten brine shrimp larvae were incubated for 24 h at 25–28 °C in 5 mL of artificial seawater mixed with different amounts of the extracts (10–1000 µg/mL). Then, the surviving larvae were counted using a magnifying glass, and median lethal concentration (LC50) values were determined by Litchfield and Wilcoxon’s method. The assay was carried out in triplicate. The toxicity level of the extracts was assessed according to the toxicity scale reported by Clarkson et al. [26]; extracts giving LC50 values greater than 1000 μg/mL were considered nontoxic.
4. Conclusions
In summary, in this work we explored for the first time the phytochemical profile and the antioxidant properties of the hydroalcoholic extracts of the aerial parts of M. fruticulosa subsp. fruticulosa and subsp. coronopifolia endemic to Sicily (Italy). From the comparison of the extracts of the two infraspecific taxa of M. fruticulosa, a quite different qualitative–quantitative profile of both phenolic and volatile compounds was highlighted, as well as a diverse antioxidant ability. It is interesting to note, in fact, that the extract of M. fruticulosa subsp. fruticulosa exhibited much higher radical scavenging activity, while that of subsp. coronopifolia was shown to be a better source of metal chelating antioxidants. Furthermore, the lack of toxicity against brine shrimp larvae indicates the potential safety of the extracts.
Overall, the data presented here improve the knowledge of the taxa included in the Matthiola genus, also indicating the infraspecific taxa of M. fruticulosa endemic to Sicily as new and safe sources of bioactive compounds, which increases the number of Brassicaceae plants hitherto known for their health-promoting properties. The present findings pave the way for new studies to further investigate the antioxidant properties of these taxa and to evaluate their potential protective effect against diseases related to oxidative stress.
Acknowledgments
Emilia Cavò thanks the Foundation “Prof. Antonio Imbesi” for the fellowship. The authors are thankful to Shimadzu and Merck Life Science Corporation for their continuous support.
Author Contributions
Conceptualization, M.F.T., V.S., F.M.R., and N.M.; investigation, M.F.T., E.C., V.M., F.C. (Francesco Cacciola), Y.O.E.M., C.C., F.C. (Fabrizio Cincotta), and N.M.; resources, V.S. and F.M.R.; data curation, M.F.T., F.C. (Francesco Cacciola), L.M., C.C., A.V., and N.M.; writing—original draft preparation, M.F.T., F.C. (Francesco Cacciola), C.C., and N.M.; writing—review and editing, M.F.T., V.S., F.M.R., L.M., A.V., and N.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are not available from the authors.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Favela-González K.M., Hérnandez-Almanza A., De la Fuente-Salcido N.M. The value of bioactive compounds of cruciferous vegetables (Brassica) as antimicrobials and antioxidants: A review. J. Food Biochem. 2020;44:e13414. doi: 10.1111/jfbc.13414. [DOI] [PubMed] [Google Scholar]
- 2.Miceli N., Cavò E., Ragusa S., Cacciola F., Dugo P., Mondello L., Marino A., Cincotta F., Condurso C., Taviano M.F. Phytochemical characterization and biological activities of a hydroalcoholic extract obtained from the aerial parts of Matthiola incana (L.) R. Br. subsp. incana (Brassicaceae) growing wild in Sicily (Italy) Chem. Biodivers. 2019;16:e1800677. doi: 10.1002/cbdv.201800677. [DOI] [PubMed] [Google Scholar]
- 3.Pignatti S. Flora d’Italia. Volume 2. Edagricole; Milano, Italy: 2017. Matthiola R. Br; pp. 912–916. [Google Scholar]
- 4.Raimondo F.M., Domina G., Spadaro V. Checklist of the vascular flora of Sicily. Quad. Bot. Amb. Appl. 2010;21:189–252. [Google Scholar]
- 5.Ball P.W. Matthiola R. Br. In: Tutin T.G., Heywood V.H., Burges N.A., Moore D.M., Valentine D.H., Walters S.M., Webb D.A., editors. Flora Europaea. Volume I. Cambridge University Press; Cambridge, UK: 1964. pp. 279–280. [Google Scholar]
- 6.Marhold K. Brassicaceae. [(accessed on 21 April 2021)];Euro+Med Plantbase-The Information Resource for Euro-Mediterranean Plant Diversity. Available online: http://www.emplantbase.org/home.html.
- 7.The Plant List. Version 1.1. [(accessed on 21 April 2021)];2013 Available online: http://www.theplantlist.org.
- 8.Livaniou-Tiniakou A. Matthiola R. Br. In: Strid A., Tan K., editors. Flora Hellenica. Volume 2. Koeltz Scientific Books; Königstein, Germany: 2002. pp. 265–268. [Google Scholar]
- 9.Giardina G., Raimondo F.M., Spadaro V. A catalogue of plants growing in Sicily. Bocconea. 2007;20:5–582. [Google Scholar]
- 10.El-Mokasabi F.M., Al-Sanousi A.F., El-Mabrouk R.M. Taxonomy and ethnobotany of medicinal plants in eastern region of Libya. J. Environ. Sci. Toxicol. Food Technol. 2018;12:14–23. [Google Scholar]
- 11.Daxenbichler M.E., Spencer G.F., Carlson D.G., Rose G.B., Brinker A.M., Powell R.G. Glucosinolate composition of seeds from 297 species of wild plants. Phytochemistry. 1991;30:2623–2638. doi: 10.1016/0031-9422(91)85112-D. [DOI] [Google Scholar]
- 12.Gmelin R., Kjær A. Glucosinolates in Matthiola fruticulosa and related species: A reinvestigation. Phytochemistry. 1970;9:569–573. doi: 10.1016/S0031-9422(00)85692-7. [DOI] [Google Scholar]
- 13.Wieczorek M.N., Jeleń H.H. Volatile compounds of selected raw and cooked Brassica vegetables. Molecules. 2019;24:391. doi: 10.3390/molecules24030391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fenwick G.R., Heaney R.K., Mullin W.J., VanEtten C.H. Glucosinolates and their breakdown products in food and food plants. CRC Crit. Rev. Food Sci. Nutr. 1983;18:123–201. doi: 10.1080/10408398209527361. [DOI] [PubMed] [Google Scholar]
- 15.Speranza J., Taviano M.F., Ragusa S., Condurso C., Cincotta F., Verzera A., Day-Walsh P., Kroon P., Miceli N. Characterization of volatile components and in vitro inhibitory effect on gut microbial TMA production of the leaf hydroalcoholic extract of Brassica incana Ten. (Brassicaceae) growing wild in Sicily (Italy); Proceedings of the 115th Congresso della Società Botanica Italiana; Online. 9–11 September 2020; p. 194. [Google Scholar]
- 16.Moerkercke A.V., Schauvinhold I., Pichersky E., Haring M.A., Schuurink R.C. A plant thiolase involved in benzoic acid biosynthesis and volatile benzenoid production. Plant. J. 2009;60:292–302. doi: 10.1111/j.1365-313X.2009.03953.x. [DOI] [PubMed] [Google Scholar]
- 17.Schulz S., Yildizhan S., Van Loon J.J. The biosynthesis of hexahydrofarnesylacetone in the butterfly Pieris brassicae. J. Chem. Ecol. 2011;37:360–363. doi: 10.1007/s10886-011-9939-y. [DOI] [PubMed] [Google Scholar]
- 18.Miceli N., Cavò E., Spadaro V., Raimondo F.M., Ragusa S., Cacciola F., Oulad El Majdoub Y., Arena K., Mondello L., Condurso C., et al. Phytochemical profile and antioxidant activity of the aerial part extracts from Matthiola incana subsp. rupestris and subsp. pulchella (Brassicaceae) endemic to Sicily. Chem. Biodivers. 2021;18:e2100167. doi: 10.1002/cbdv.202100167. [DOI] [PubMed] [Google Scholar]
- 19.Taviano M.F., Miceli N., Acquaviva R., Malfa G.A., Ragusa S., Giordano D., Cásedas G., Les F., López V. Cytotoxic, antioxidant, and enzyme inhibitory properties of the traditional medicinal plant Matthiola incana (L.) R. Br. Biology. 2020;9:163. doi: 10.3390/biology9070163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abdelshafeek K.A., Abdelmohsen M.M., Hamed A., Shahat A.A. Investigation of some chemical constituents and antioxidant activity extracts of Matthiola longipetala subsp. longipetala. Chem. Nat. Compd. 2013;49:539–543. doi: 10.1007/s10600-013-0665-2. [DOI] [Google Scholar]
- 21.Singh D.P., Verma S., Prabh R. Investigations on antioxidant potential of phenolic acids and flavonoids: The common phytochemical ingredients in plants. J. Plant. Biochem. Physiol. 2018;6:1000219. doi: 10.4172/2329-9029.1000219. [DOI] [Google Scholar]
- 22.Kasote D.M., Katyare S.S., Hegde M.V., Bae H. Significance of antioxidant potential of plants and its relevance to therapeutic applications. Int. J. Biol. Sci. 2015;11:982–991. doi: 10.7150/ijbs.12096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fernandez-Panchon M.S., Villano D., Troncoso A.M., Garcia-Parrilla M.C. Antioxidant activity of phenolic compounds: From in vitro results to in vivo evidence. Crit. Rev. Food Sci. Nutr. 2008;48:649–671. doi: 10.1080/10408390701761845. [DOI] [PubMed] [Google Scholar]
- 24.Ntungwe N.E., Domínguez-Martín E.M., Roberto A., Tavares J., Isca V.M.S., Pereira P., Cebola M.-J., Rijo P. Artemia species: An important tool to screen general toxicity samples. Curr. Pharm. Des. 2020;26:2892–2908. doi: 10.2174/1381612826666200406083035. [DOI] [PubMed] [Google Scholar]
- 25.Libralato G., Prato E., Migliore L., Cicero A.M., Manfra L. A review of toxicity testing protocols and endpoints with Artemia spp. Ecol. Indic. 2016;69:35–49. doi: 10.1016/j.ecolind.2016.04.017. [DOI] [Google Scholar]
- 26.Clarkson C., Maharaj V.J., Crouch N.R., Grace O.M., Pillay P., Matsabisa M.G., Bhagwandin N., Smith P.J., Folb P.I. In vitro antiplasmodial activity of medicinal plants native to or naturalised in South Africa. J. Ethnopharmacol. 2004;92:177–191. doi: 10.1016/j.jep.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 27.Oulad El Majdoub Y., Alibrando F., Cacciola F., Arena K., Pagnotta E., Matteo R., Micalizzi G., Dugo L., Dugo P., Mondello L. Chemical characterization of three accessions of Brassica juncea L. extracts from different plant tissues. Molecules. 2020;25:5421. doi: 10.3390/molecules25225421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cincotta F., Verzera A., Tripodi G., Condurso C. Non-intentionally added substances in PET bottled mineral water during the shelf-life. Eur. Food Res. Technol. 2018;244:433–439. doi: 10.1007/s00217-017-2971-6. [DOI] [Google Scholar]
- 29.Ohnishi M., Morishita H., Iwahashi H., Shitzuo T., Yoshiaki S., Kimura M., Kido R. Inhibitory effects of chlorogenic acid on linoleic acid peroxidation and haemolysis. Phytochemistry. 1994;36:579–583. doi: 10.1016/S0031-9422(00)89778-2. [DOI] [Google Scholar]
- 30.Oyaizu M. Studies on products of browning reaction: Antioxidative activities of products of browning reaction prepared from glucosamine. Jpn J. Nutr. Diet. 1986;44:307–315. doi: 10.5264/eiyogakuzashi.44.307. [DOI] [Google Scholar]
- 31.Decker E.A., Welch B. Role of ferritin as a lipid oxidation catalyst in muscle food. J. Agric. Food Chem. 1990;38:674–677. doi: 10.1021/jf00093a019. [DOI] [Google Scholar]
- 32.Meyer B.N., Ferrigni N.R., Putnam J.E., Jacobson L.B., Nichols D.E., McLaughlin J.L. Brine shrimp: A convenient general bioassay for active plant constituents. Planta Med. 1982;45:31–34. doi: 10.1055/s-2007-971236. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.