Abstract
The present paper reports the determination of the activation energies and the optimum temperatures of starch hydrolysis by porcine pancreas α-amylase. The parameters were estimated based on the literature data on the activity curves versus temperature for starch hydrolysis by α-amylase from porcine pancreas. It was assumed that both the hydrolysis reaction process and the deactivation process of α-amylase were first-order reactions by the enzyme concentration. A mathematical model describing the effect of temperature on porcine pancreas α-amylase activity was used. The determine deactivation energies Ea were from 19.82 ± 7.22 kJ/mol to 128.80 ± 9.27 kJ/mol, the obtained optimum temperatures Topt were in the range from 311.06 ± 1.10 K to 326.52 ± 1.75 K. In turn, the values of deactivation energies Ed has been noted in the range from 123.57 ± 14.17 kJ/mol to 209.37 ± 5.17 kJ/mol. The present study is related to the starch hydrolysis by α-amylase. In the industry, the obtained results the values Ea, Ed, Topt can be used to design and optimize starch hydrolysis by α-amylase porcine pancreas. The obtained results might also find application in research on the pharmaceutical preparations used to treat pancreatic insufficiency or prognosis of pancreatic cancer.
Keywords: porcine pancreas α-amylase, activation energy, deactivation energy, optimum temperature
1. Introduction
The starch molecules are glucose polymers linked together by α-1,4 and α-1,6 glucosidic bonds. Starch is insoluble in water at room temperature [1,2]. These products are the source of complex carbohydrates. They are synthesized naturally in a variety of plants. Plants with a high starch content include corn, potato, rice, sorghum, wheat, cassava and rhizome and bulbil of Chinese jam. Starchy substances are a major part of the human diet for most people in the world, as well as many other animals [1,3].
The enzymes α-amylases (E.C. 3.2.1.1) catalyze the hydrolysis of α-1,4 glycosidic bonds present in starch, glycogen and other related carbohydrates to low molecular weight products, such as glucose, maltose and maltotriose [4,5,6,7,8]. These enzymes are present in plants, animals and microorganisms [9] and have extensive applications in medicine [10,11,12,13,14], textiles [11], detergent [11], fermentation [11] and the food industry [4,11].
Amylases have potential application in various branches of industrial processes [7] and have been used in baking [9,11], brewing [9,11] and saccharification of starch.
During the baking process, gelatinization of the starch granules occurs, which together with the hydrolysis of the starch by α-amylase to cause its liquefaction [9]. In beer, brewing is the process-mashing (malting) in which enzymatic degradation of starch into fermentable sugars (maltose) occurs by inter alia α-amylase [9].
The saccharification of starch is an enzymatic hydrolysis of starch byα-amylase which takes place in three stages. The first is gelling, which is aimed at dissolving the starch granules. In turn, the second step consists of partial hydrolysis of the suspension, thus, it may lead to reduce its viscosity. The final stage of depolymerization is mainly the formation of mono-, di- and tri-saccharides. This process is called saccharification, due to the formation of saccharides [3].
The α-amylase which is extremely similar to human pancreatic α-amylase and is often used in industry is the three-dimensional structure of porcine pancreatic (Sus scrofa). Molecular cloning and primary structure analysis of porcine pancreatic α-amylase showed the highest homology to the human pancreatic α-amylase sequence (87.1%) among all the amylases known [15].
In addition to industrial use, α-amylase from porcine pancreas is used for example in health food research [16,17,18], to assay resistant starch (RS), not broken down by human enzymes in the small intestine [19] and is also used in medical diagnostics [12,13,14]. One of the problems is effective diagnostic methods allowing for the prognosis of pancreatic cancer of cancer, which is an exceptionally aggressive tumour with high mortality. Stotz et al. [12] hypothesized that the level of α-amylase and lipase quantities in the peripheral blood and the calculation of the lipase and α-amylase ratio at the time of localized pancreatic cancer might represent a novel marker for individualized patient risk assessment pancreatic cancer.
The study of α-amylase activity, important in the diagnosis of pancreatic cancer in humans, is also used in the industrial hydrolysis of starch by α-amylase. The processes involving α-amylase cannot be designed without knowing the kinetic parameters of the process. Therefore, studies on the effect of temperature on α-amylase activity are required. Hydrolysis with porcine pancreas α-amylase is usually carried out at temperatures higher than 310 K [7,16,20,21,22,23,24,25], thus, a significant inactivation of the enzyme may occur. Therefore, it is necessary to determine the activation energy Ea, the deactivation energy Ed and the optimum temperature Topt for porcine pancreas α-amylase. The determination of parameters Ea, Ed, Topt based on experimental data on the effect of temperature on the activity of α-amylase from porcine pancreas has not been presented in previous studies.
The purpose of the present work was to estimate parameters of the activation energies Ea, the deactivation energies Ed and the optimum temperatures of starch hydrolysis by pancreas α-amylase, whose obtained values can be used in works focused on prognosis for a pancreatic tumour or in industrial purposes can be used to designed and optimized starch hydrolysis by α-amylase porcine pancreas.
2. Results and Discussion
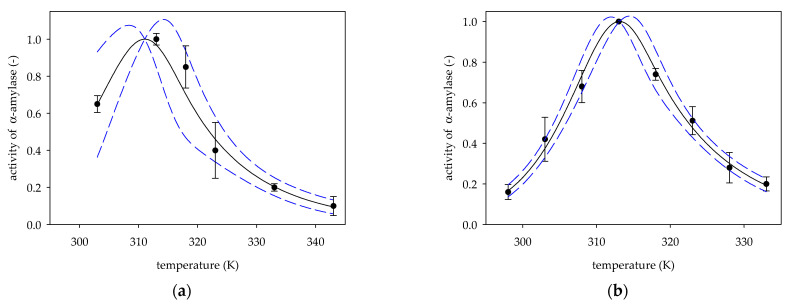
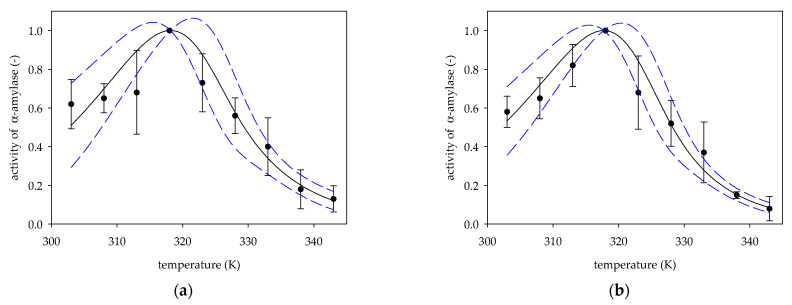
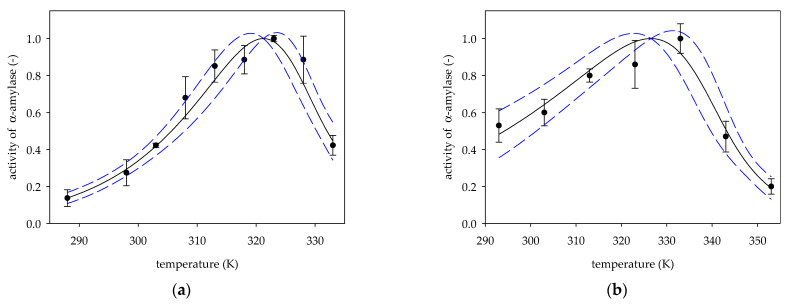
Based on experimental data on the change in the activity of α-amylase from the porcine pancreas [7,16,20,21,22] vs. temperature, values of deactivation energies Ed, β parameters and temperatures optimal Topt were determined from Equation (6). Figure 1, Figure 2 and Figure 3 show experimental data on α-amylase activity by hydrolysis of starch as a substrate, along with activity curves plotted based on Equation (6) for the values of the specified parameters Ed, Topt, β listed in Table 1.
Figure 1.
The activity of α-amylase porcine pancreas by measurements: (a) Akhond et al. [7]; (b) Aksoy et al. [20]; (●) experimental data, (solid line) Equation (6); (dotted line) 95% confidence band.
Figure 2.
The activity of α-amylase porcine pancreas by measurements Gopal et al. [15]: (a) isoform I; (b) isoform II; (●) experimental data, (solid line) Equation (6); (dotted line) 95% confidence band.
Figure 3.
The activity of α-amylase porcine pancreas by measurements: (a) Louati et al. [21]; (b) Guoet al. [22]; (●) experimental data, (solid line) Equation (6); (dotted line) 95% confidence band.
Table 1.
The values of kinetic parameters estimated for α-amylase porcine pancreas.
| Figure | t (min) | β | Ref. | ||||
|---|---|---|---|---|---|---|---|
| 1a | 3 | 311.06 ± 1.10 | 164.9 ± 19.14 | 1.46 ± 0.29 | 92.08 ± 23.07 | 1.79 | [7] |
| 1b | 5 | 313.12 ± 0.55 | 209.37 ± 5.17 | 1.68 ± 0.12 | 128.80 ± 9.27 | 1.63 | [20] |
| 2a | 60 | 318.17 ± 1.36 | 152.83 ± 11.06 | 0.83 ± 0.24 | 54.75 ± 17.02 | 2.79 | [15] |
| 2b | 60 | 317.74 ± 1.04 | 164.06 ± 9.23 | 0.71 ± 0.16 | 51.41 ± 12.71 | 3.19 | [15] |
| 3a | 15 | 321.24 ± 1.04 | 162.70 ± 19.21 | 0.76 ± 0.17 | 54.07 ± 15.88 | 3.01 | [21] |
| 3b | 30 | 326.52 ± 1.75 | 123.57 ± 14.17 | 0.34 ± 0.10 | 19.82 ± 7.22 | 6.23 | [22] |
The obtained parameters Topt, β, Ed for α-amylase from porcine pancreas are presented in Table 1, according to the increasing value of the temperatures optimal Topt. Then, based on the value of the deactivation energy Ed and the parameter β, the activation energy value Ea was calculated based on Equation (8). The obtained Ea values are presented in Table 1.
In addition, Figure 1, Figure 2 and Figure 3 present standard deviation errors for experimental points, while the 95% confidence limits were marked for the obtained curves.
Table 2 presents statistical data obtained during the determination of the parameters of porcine pancreatic α-amylase. High values of regression coefficient R2 (above 0.93) and standard errors of estimation RSS below 0.19 were obtained; while statistical variability of Ed and Topt parameters in most of the analyzed cases p < 0.0001. F-Fisher test values were from 44.93 to 170.77 with a low probability value [p 0.0031] which confirmed, that when determining the parameters, it was appropriated to apply Equation (6).
Table 2.
The statistical data obtained by determining the kinetic parameters of α-amylase porcine pancreas.
| Figure | R 2 | RSS | p | F | P | Ref. | ||
|---|---|---|---|---|---|---|---|---|
| β | ||||||||
| 1a | 0.9789 | 0.1370 | 0.0033 | <0.0001 | 0.0151 | 69.56 | 0.0031 | [7] |
| 1b | 0.9856 | 0.0080 | <0.0001 | <0.0001 | <0.0001 | 170.77 | <0.0001 | [20] |
| 2a | 0.9374 | 0.1912 | <0.0001 | <0.0001 | 0.0132 | 44.93 | 0.0002 | [15] |
| 2b | 0.9679 | 0.1592 | <0.0001 | <0.0001 | 0.0041 | 90.43 | <0.0001 | [15] |
| 3a | 0.9817 | 0.1034 | 0.0001 | <0.0001 | 0.0044 | 160.97 | <0.0001 | [21] |
| 3b | 0.9751 | 0.1117 | 0.0010 | <0.0001 | 0.0185 | 78.41 | 0.0006 | [22] |
This work aimed to identify the activation energy Ea, the deactivation energy Ed and the optimum temperature Topt of starch hydrolysis by porcine pancreas α-amylase. Knowing the obtained values can be used in works focused on prognosis for a pancreatic tumour.
2.1. The Activation Energy Ea
The obtained values of the activation energy Ea of starch hydrolysis by α-amylase from porcine pancreas were in the range from 19.82 ± 7.22 kJ/mol to 128.80 ± 9.27 kJ/mol.
In turn, in [23,24] determined values of Ea activation energy of the starch hydrolysis were equal to 48.91 kJ/mol and 50.16 kJ/mol, respectively.
The analysis of the data presented in Table 2 allows concluding that there is a correlation between the values of the activation energy Ea, deactivation energy Ed and parameter β. Indeed, it has been reported that for most of the analyzed cases, with the simultaneous increase in the values of parameter β and Ed increase, the values of Ea increase. The longer the starch hydrolysis by α-amylase is carried out, the lower the value of the β parameter. Determining the influence of the time of measurement α-amylase activity on the value β parameter of will be the aim of further research.
According to the calculations for the measurement performed by Aksoy et al. [20], the energy value Ea was six as high compared to the Ea values obtained by Guo et al. [22]. The observed difference may be due to the different time at which the α-amylase activity is determined. Indeed, the measurements time of hydrolysis starch time was equal to 5 min and 30 min in the studies of Aksoy et al. [20] and Guo et al. [22], respectively.
Additionally, α-amylase from porcine pancreas used in to study by Guo et al. [22] was from Shanghai Kaiyang Biological Technology Co (Shanghai, China). The highest Ea value was obtained for α-amylase from Merck AG (Darmstadt, Germany) for measurements carried out by Aksoy et al. [20].
2.2. The Activation Energy of the Deactivation Process Ed
In the present study, the obtained values of the deactivation energy process were, in the range from 123.57 ± 14.17 kJ/mol to 209.37 ± 5.17 kJ/mol (Table 2).
The lowest value Ed was obtained in the hydrolysis starch by α-amylase from porcine pancreas from Biological Technology Co., Ltd. (Shanghai, China) for measurements Guo et al. [22]. Simultaneously, the Ea value for this α-amylase was also the lowest. The lowest Ed value was obtained for α-amylase from Merck AG (Germany) for measurements Aksoy et al. [20]. In addition, the Ea value for this α-amylase was the highest.
The differences in the obtained activation energy value of the deactivation process Ed can be caused by the use of α-amylase from a different company.
2.3. Optimum Temperature Topt
The determined values of the optimum temperature Topt of starch hydrolysis by α-amylase from porcine pancreas were different by about fifteen degrees and are in the range from 311.06 ± 1.10 K to 326.52 ± 1.75 K (Table 2). The highest Topt value was obtained in the hydrolysis starch by α-amylase from porcine pancreas from Biological Technology Co., Ltd. (Shanghai, China) for measurements Guo et al. [22]. The lowest value Topt was obtained in the hydrolysis starch by α-amylase from porcine pancreas from Sigma-Aldrich for measurements Akhond et al. [7]. Additionally, it should be noted that the measurement was performed in the shortest time, i.e., 3 min.
In works [23,24], a Topt of starch hydrolysis by porcine pancreatic α-amylase were presented and equals 313 K and 327 K, respectively.
3. Materials and Methods
3.1. The Effect of Temperature on α-Amylase Activity
The value of activation energy, Ea can be determined from the curve of the dependence of the logarithm of the reaction rate (ln v) on the reciprocal of temperature (1/T), the so-called Arrhenius dependence [23,26]. However, the determined values of Ea and Ed by application of the traditional method is burdened with an error. Many researchers have studied the kinetic parameters of α-amylase of other origins [24,26]; however, the parameters Topt, Ea and Ed were not obtained simultaneously for α-amylase porcine pancreas.
When studying α-amylase activity during the hydrolysis of starch, it is assumed that the change in substrate concentration S during reaction time t describes by the first-order equations due to the concentration of the enzyme
| (1) |
where k is the kinetic constant of the enzymatic reaction (1/min) and E is the concentration of the active enzyme (M).
The change in α-amylase dimensionless activity a is also described by the first-order kinetics [26,27] with the following equation
| (2) |
where kd is the kinetic constant of the enzymatic reaction (1/min).
The solution of Equation (2) for the initial condition a (t = 0) = 1 is
| (3) |
Kinetic constants k and deactivation constant kd depend on temperature T and are described by the Arrhenius equations as:
| (4) |
| (5) |
where , are pre-exponential factors of the hydrolysis reaction rate or deactivation process of α-amylase (1/min), Ea is the activation energy for the enzymatic reaction (kJ/mol) while Ed is the activation energy of the deactivation process (kJ/mol), R is the gas constant 8.315 (J/(mol·K)) and T is the absolute temperature (K).
Equations (1)–(5) are the basis for the derived dependence of the change in the dimensionless activity of the enzyme on the temperature measurement T as follows:
| (6) |
where Topt is the temperature at which α-amylase shows maximum activity (K) and dimensionless parameter β determines the relationship
| (7) |
where ta is the reaction time of starch hydrolysis by α-amylase from porcine pancreas (min).
The full analysis of the solution of Equation (6) was presented in an earlier publication of Wojcik and Miłek [28].
It means that knowing the value of the activation energy of the deactivation reaction Ed and the parameter β, the activation energy Ea is determined by the following equation
| (8) |
Equations (6)–(8) were used to determine the kinetic parameters of inulin hydrolysis by exo-inulinases Aspergillus niger [29], olive oil hydrolysis by porcine pancreas lipase [30,31], p-nitrophenyl palmitate hydrolysis by lipases from Rhizopus oryzae 3562 and Enterobacter aerogenes [32], hydrolysis of starch by α-amylase Bacillus licheniformis [33], inulin hydrolysis by endo-inulinase A. niger [34] and inulin hydrolysis by inulinase K. marxianus [28].
Based on Equation (6), the parameters , β and Topt were estimated by non-linear regression according to the methods of least squares [25,27,35,36,37] determining the residual sum of squared (RSS) from the equation:
| (9) |
where is α-amylase dimensionless activity determined experimentally and is α-amylase dimensionless activity calculated from Equation (6).
3.2. Assay of α-Amylase Activity
Literature data [7,16,20,21,22] for porcine pancreas α-amylase from various companies have been analyzed.
Amylase activity was determined according to Miller [38]. The reaction mixture consisted of starch, buffer and enzyme solution and incubated for different time (min) at 90 °C. The reaction was stopped by the addition of 3,5 dinitrosalicylate (DNS) reagent. The quantity of reducing sugar was measured spectrophotometrically. The unit of amylase was defined as the amount of enzyme which produced 1 μmol of reducing sugar as glucose in 1 min underspecified condition.
Table 3 presents the conditions for measuring α-amylase activity during the hydrolysis of starch with the various buffer pH, the various duration of measurement and the used the initial concentration of starch [7,16,20,21,22].
Table 3.
Conditions for measuring α-amylase porcine pancreas activity.
| BufferpH | t (min) | λ (nm) | Concentration of Starch | Source Inulinase | Ref. |
|---|---|---|---|---|---|
| 6.9 sodium phosphate | 3 | 540 | 0.5% | Sigma-Aldrich (St. Louis, MO, USA) | [7] |
| 6.9 phosphate | 5 | 600 | 2% | Merck AG (Germany) | [20] |
| 6.9 sodium phosphate | 60 | 540 | 1% | Sigma Chemical Company | [16] |
| 7.0 MOPS | 15 | 575 | 0.5% | Sigma | [21] |
| 7.0 MES | 30 | 520 | - | Shanghai Kaiyang BiologicalTechnology Co., Ltd. (Shanghai, China) | [22] |
4. Conclusions
The following method of determining parameters was used: the optimum temperatures Topt, activation energies Ea and deactivation energies Ed of olive oil hydrolysis by α-amylase from porcine pancreas reaction based on four curves of changes activity of α-amylase from porcine pancreas depending on the temperature of hydrolysis. For the optimum temperatures Topt, the difference between the obtained values is fifteen degrees. The differences in the calculated values of the deactivation energy Ed are equal to about 110 kJ/mol, for the activation energy of the reaction Ea equal to about 85 kJ/mol. The reason for the differences in the obtained values Ea, Ed, Topt is, above all, different origins of α-amylase from porcine pancreas. The lowest values Ea and Ed, together with the highest Topt was obtained for the enzyme derived from Shanghai Kaiyang Biological Technology Co., Ltd. The highest values Ea and Ed, together with lower Topt obtained for an enzyme derived from Merck AG (Germany). Additionally, it is essential to mention that the noted differences in values of parameters can be caused by the various duration of the α-amylase activity assay, different pH values of the hydrolyzed starch used to test the α-amylase activity as well as different concentrations of the starch.
The obtained results the values Ea, Ed, Topt can be used to design and optimize starch hydrolysis α-amylase by porcine pancreas in the food, pharmaceutical and industrial industries, among others.
Acknowledgments
The author would like to thank Marek Wójcik.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data are contained within the article.
Conflicts of Interest
The author declares no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Khlestkin V., Eltsov I. Different reactivity of raw starch from diverse potato genotypes. Molecules. 2021;26:226. doi: 10.3390/molecules26010226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albani J.R. Principles and Applications of Fluorescence Spectroscopy. Blackwell Publishing; Oxford, UK: 2007. Starch hydrolysis by amylase; pp. 59–78. [Google Scholar]
- 3.Zhang B., Guo K., Lin L., Wei C. Comparison of structural and functional properties of starches from the rhizome and bulbil of Chinese Yam (Dioscorea opposita Thunb.) Molecules. 2018;23:427. doi: 10.3390/molecules23020427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khan M.J., Husain Q., Azam A. Immobilization of porcine pancreatic α-amylase on magnetic Fe2O3 nanoparticles: Applications to the hydrolysis of starch. Biotechnol. Bioprocess Eng. 2012;17:377–384. doi: 10.1007/s12257-011-0105-8. [DOI] [Google Scholar]
- 5.Li Z., Guo K., Lin L., He W., Zhang L., Wei C. Comparison of physicochemical properties of starches from flesh and peel of green banana fruit. Molecules. 2018;23:2312. doi: 10.3390/molecules23092312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xiao H., Lin Q., Liu G.-Q. Effect of cross-linking and enzymatic hydrolysis composite modification on the properties of rice starches. Molecules. 2012;17:8136–8146. doi: 10.3390/molecules17078136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Akhond M., Pashangeh K., Karbalaei-Heidari H.R., Absalan G. Efficient immobilization of porcine pancreatic α-amylase on amino-functionalized magnetite nanoparticles: Characterization and stability evaluation of the immobilized enzyme. Appl. Biochem. Biotechnol. 2016;180:954–968. doi: 10.1007/s12010-016-2145-1. [DOI] [PubMed] [Google Scholar]
- 8.Couto S.R., Sanromán M.Á. Application of solid-state fermentation to food industry—A review. J. Food Eng. 2006;76:291–302. doi: 10.1016/j.jfoodeng.2005.05.022. [DOI] [Google Scholar]
- 9.Balakrishnan D., Kumar S.S., Sugathan S. Green Bioprocesses. Enzymes in Industrial Food Processing. Springer; Singapore: 2019. Amylases for food applications—Updated information; pp. 199–228. [Google Scholar]
- 10.Azzopardi E., Lloyd C., Teixeira S.R., Conlan R.S., Whitaker I. Clinical applications of amylase: Novel perspectives. Surgery. 2016;160:26–37. doi: 10.1016/j.surg.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 11.Vaidya S., Srivastava P.K., Rathore P., Pandey A.K. Amylases: A prospective enzyme in the field of biotechnology. J. Appl. Biosci. 2015;41:1–18. [Google Scholar]
- 12.Stotz M., Barth D.A., Riedl J.M., Asamer E., Klocker E.V., Kornprat P., Hutterer G.C., Prinz F., Lackner K., Stöger H., et al. The lipase/amylase ratio (LAR) in peripheral blood might represent a novel prognostic marker in patients with surgically resectable pancreatic cancer. Cancers. 2020;12:1798. doi: 10.3390/cancers12071798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Quek A., Kassim N.K., Ismail A., Latif M.A.M., Shaari K., Tan D.C., Lim P.C. Identification of dipeptidyl peptidase-4 and α-amylase inhibitors from Melicope glabra (Blume) T. G. Hartley (Rutaceae) using liquid chromatography tandem mass spectrometry, in vitro and in silico methods. Molecules. 2021;26:1. doi: 10.3390/molecules26010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nazir N., Zahoor M., Ullah R., Ezzeldin E., Mostafa G.A.E. Curative effect of catechin isolated from Elaeagnus umbellata Thunb. berries for diabetes and related complications in streptozotocin-induced diabetic rats model. Molecules. 2021;26:137. doi: 10.3390/molecules26010137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gopal B.A., Muralikrishna G. Porcine pancreatic α-amylase and its isoforms: Purification and kinetic studies. Int. J. Food Prop. 2009;12:571–586. doi: 10.1080/10942910801947755. [DOI] [Google Scholar]
- 16.Oszmiański J., Lachowicz S., Nowicka P., Rubiński P., Cebulak T. Evaluation of innovative dried purée from Jerusalem artichoke—In vitro studies of its physicochemical and health-promoting properties. Molecules. 2021;26:2644. doi: 10.3390/molecules26092644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Riyaphan J., Jhong C.-H., Lin S.-R., Chang C.-H., Tsai M.-J., Lee D.-N., Sung P.-J., Leong M.K., Weng C.-F. Hypoglycemic efficacy of docking selected natural compounds against α-glucosidase and α-amylase. Molecules. 2018;23:2260. doi: 10.3390/molecules23092260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang M., Shi J., Wang L., Hu Y., Ye X., Liu D., Chen J. Inhibitory kinetics and mechanism of flavonoids from lotus (Nelumbo nucifera Gaertn.) leaf against pancreatic α-amylase. Int. J. Biol. Macromol. 2018;120:2589–2596. doi: 10.1016/j.ijbiomac.2018.09.035. [DOI] [PubMed] [Google Scholar]
- 19.Megazyme, Resistant Starch Assay Procedure. K-RSTAR 05/19. (100 Assays per Kit). AOAC Method 2002.02. AACC Method 32-40.01. Codex Type II Method. [(accessed on 27 May 2021)]; Available online: https://www.megazyme.com/documents/Booklet/K-RSTAR_DATA.pdf.
- 20.Aksoy S., Tumturk H., Hasirci N. Stability of α-amylase immobilized on poly(methylmethacrylate-acrylic acid) microspheres. J. Biotechnol. 1998;60:37–46. doi: 10.1016/S0168-1656(97)00179-X. [DOI] [PubMed] [Google Scholar]
- 21.Louati H., Zouari N., Fendri A., Gargouri Y. Digestive amylase of a primitive animal, the scorpion: Purification and biochemical characterization. J. Chromatogr. B. 2010;878:853–860. doi: 10.1016/j.jchromb.2010.01.047. [DOI] [PubMed] [Google Scholar]
- 22.Guo H., Tang Y., Yu Y., Xue L., Qian J.-Q. Covalent immobilization of α-amylase on magnetic particles as catalyst for hydrolysis of high-amylose starch. Int. J. Biol. Macromol. 2016;87:537–544. doi: 10.1016/j.ijbiomac.2016.02.080. [DOI] [PubMed] [Google Scholar]
- 23.Cipolla A., Delbrassine F., Da Lage J.-L., Feller G. Temperature adaptations in psychrophilic, mesophilic and thermophilic chloride-dependent alpha-amylases. Biochimie. 2012;94:1943–1950. doi: 10.1016/j.biochi.2012.05.013. [DOI] [PubMed] [Google Scholar]
- 24.D’Amico S., Marx J.-C., Gerday C., Feller G. Activity-Stability Relationships in Extremophilic Enzymes. J. Biol. Chem. 2003;278:7891–7896. doi: 10.1074/jbc.M212508200. [DOI] [PubMed] [Google Scholar]
- 25.Guimarães J.R., Giordano R.D.L.C., Fernandez-Lafuente R., Tardioli P.W. Evaluation of strategies to produce highly porous cross-linked aggregates of porcine pancreas lipase with magnetic properties. Molecules. 2018;23:2993. doi: 10.3390/molecules23112993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haq I.-U., Javed M.M., Hameed U., Adnan F. Kinetics and thermodynamic studies of alfa amylase from Bacillus licheniformis mutant. Pak. J. Bot. 2010;42:3507–3516. [Google Scholar]
- 27.Ademakinwa A., Agunbiade M., Ayinla Z., Agboola F. Optimization of aqueous two-phase partitioning of Aureobasidium pullulans α-amylase via response surface methodology and investigation of its thermodynamic and kinetic properties. Int. J. Biol. Macromol. 2019;140:833–841. doi: 10.1016/j.ijbiomac.2019.08.159. [DOI] [PubMed] [Google Scholar]
- 28.Wójcik M., Miłek J. A new method to determine optimum temperature and activation energies for enzymatic reactions. Bioprocess Biosyst. Eng. 2016;39:1319–1323. doi: 10.1007/s00449-016-1596-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miłek J. Application of the new method to determine of the kinetic parameters of inulin hydrolysis by exo-inulinase Aspergillus niger. J. Therm. Anal. Calorim. 2021 doi: 10.1007/s10973-020-10495-3. [DOI] [Google Scholar]
- 30.Miłek J. Calculation of temperature optimum as well as activation and deactivation energy for the olive oil hydrolysis with porcine pancreas lipase. Przem. Chem. 2020;99:585–587. doi: 10.15199/62.2020.4.14. [DOI] [Google Scholar]
- 31.Miłek J. The effect of pH on determination of activation energies and the optimum temperatures of hydrolysis of olive oil by lipase from porcine pancreas. Acta Bioeng. Biomech. 2021 doi: 10.37190/ABB-01827-2021-02. unpublished work. [DOI] [PubMed] [Google Scholar]
- 32.Miłek J. Determination of the activation energies and optimum temperature for the hydrolysis of p-nitrophenyl palmitate catalyzed by lipases. Przem. Chem. 2021;100:103–104. doi: 10.15199/62.2021.1.14. [DOI] [Google Scholar]
- 33.Miłek J. Determination the optimum temperature and activation energy for the hydrolysis of starch catalyzed by α-amylase Bacillus licheniformis. Przem. Chem. 2020;99:880–881. doi: 10.15199/62.2020.6.9. [DOI] [Google Scholar]
- 34.Miłek J. Determination the optimum temperatures and activation energies of inulin hydrolysis by endo-inulinase Aspergillus niger. Chem. Proc. Eng. 2020;41:229–236. doi: 10.24425/CPE.2020.132545. [DOI] [Google Scholar]
- 35.Miłek J. Estimation of the kinetic parameters for h2o2 enzymatic decomposition and for catalase deactivation. Braz. J. Chem. Eng. 2018;35:995–1004. doi: 10.1590/0104-6632.20180353s20160617. [DOI] [Google Scholar]
- 36.Miłek J. Thermodynamics and kinetics of thermal deactivation of catalase Aspergillus niger. Pol. J. Chem. Technol. 2020;22:67–72. doi: 10.2478/pjct-2020-0018. [DOI] [Google Scholar]
- 37.Apar D.K., Özbek B. α-Amylase inactivation by temperature during starch hydrolysis. Process. Biochem. 2004;39:1137–1144. doi: 10.1016/S0032-9592(03)00224-3. [DOI] [Google Scholar]
- 38.Miller G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959;31:426–428. doi: 10.1021/ac60147a030. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are contained within the article.