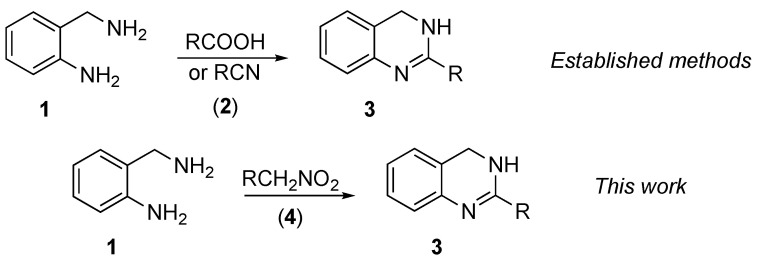
Abstract
Nitroalkanes activated with polyphosphoric acid serve as efficient electrophiles in reactions with various nucleophilic amines. Strategically placed second functionality allows for the design of annulation reactions enabling preparation of various heterocycles. This strategy was employed to develop an innovative synthetic approach towards 3,4-dihydroquinazolines from readily available 2-(aminomethyl)anilines.
Keywords: nitroalkanes, heterocyclic compounds, quinozalines, annulation
1. Introduction
3,4-Dihydroquinazolines belong to privileged scaffolds widely used in drug design, with their importance in medicinal chemistry being difficult to overstate. This structural fragment is found in thousands of natural products and synthetic medicinal agents, including many alkaloids with valuable biological activities. Among them are cytotoxic alkaloid chaetominine isolated from endophytic fungi of the Chaetomium genus [1] and 3-hydroxyfumiquinazoline A isolated from the fungus Aspergillus fumigatus, which has demonstrated promising antifungal and insecticidal activities [2] (Figure 1). Additionally, a family of trigonoliimine alkaloids were isolated from the leaves of tropical plants from the genus Trigonostemon [3]. Due to their highly unusual polycyclic scaffolds and newly discovered anti-cancer and antiviral activities, these trigoliimines are in focus of multiple synthetic exercises [4,5,6,7,8]. Still, elaboration of novel synthetic methods allowing for the efficient assembly of the 3,4-dihydroquinazoline core is exceedingly desired. Herein, we wish to disclose an account of our recent progress towards preparation of these important heterocycles via annulation involving electrophilically activated nitroalkanes.
Figure 1.
Biologically active natural 3,4-dihydroquinazolines.
2. Results and Discussion
Annulation of 2-(aminomethyl)anilines to afford a 3,4-dihydroquinozaline core is one of the most common reactions, returning almost 600,000 matches in a SciFinder database search. Since 2-(aminomethyl)aniline (1) can be viewed as a bis-nucleophilic synthon, all these annulations are designed as double-fold attacks at reagents that can serve as a 1,1-bis-electrophilic synthetic equivalent (Scheme 1). Typically, carboxylic acids [9,10,11,12,13], nitriles [14], or other related carbonyl derivatives [15,16,17] (2) are employed (Scheme 1). Oxidative protocols utilizing aldehydes as alkylating reagents were also reported [18,19,20]. Lately, our research group was instrumental in designing novel multistep cascade transformations employing nitroalkanes as electrophilic or bis-electrophilic synthons.
Scheme 1.
Annulations of 2-(aminomethyl)anilines to afford 3,4-dihydroquinazolines.
Although nitroalkanes (4) normally behave as pronucleophilic species; in a polyphosphoric acid medium they are stabilized in a form of phosphorylated nitronates (5) with profound electrophilic properties. These phosphorylated nitronates tend to react with various carbon-based nucleophiles, for instance electron-rich arenes, allowing for Friedel–Crafts-type C–H functionalization reactions, often with subsequent accompanying rearrangements [21,22,23,24]. Furthermore, it was found that nitrogen-based nucleophiles (6), such as aliphatic amines [25,26,27], anilines [28,29,30,31], or hydrazines [32,33], can be utilized instead of water in a process mechanistically related to the classical Nef reaction [34,35,36]. Employing strategically placed second nucleophilic functionality (carbon or heteroatom-based), the resulting amidinium species (7) can be transformed into a variety of heterocyclic compounds, such as benzoxazoles (8) [23,28] or benzimidazoles (9) [28], perimidines (10) [29], 6,7-dihydro-1H-cyclopenta[gh]perimidines (11) [31], 1,3,4-oxa-di-azoles (12) [32,33], imidazolines (13) [25], 3,4-dihydro-iso-quinolines (14) [27], imidazo [1,5-a]pyridines (15) [26], and [1,2,4]triazolo[4,3-a]pyridines (16) [37] (Scheme 2).
Scheme 2.
Synthesis of nitrogen-based heterocycles employing electrophilically activated nitroalkanes.
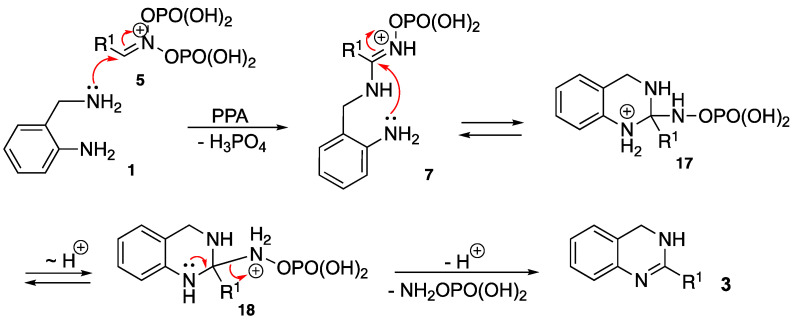
It occurred to us that a similar strategy can be applied for the straightforward preparation of 3,4-dihydroquinazolines as well. In reaction with bis-nucleophilic 2-(aminomethyl)anilines (1), the phosphorylated nitronate species 5 would, after elimination of orthophosphoric acid entity, afford an amidinium intermediate 7 (Scheme 3). The latter would then experience intramolecular 6-exo-trig nucleophilic attack of the aniline group to the iminium moiety to furnish heterocyclic ring 17. Subsequent proton transfer to the exocyclic nitrogen atom would provide intermediate 18, which after elimination of hydroxylamine phosphate would afford the desired product 3 (Scheme 3).
Scheme 3.
Mechanistic rationale of the featured annulation.
With this idea in mind, we attempted to carry out reactions between 2-(1-amino ethyl)aniline (1a) and 1-nitropropane (4c) in a molar ratio of 1:2. In the initial experiment, a mixture of these reagents was heated in 86% orthophosphoric acid at 110 °C (Table 1, entry 1) or 120 °C (entry 2). However, the reaction did not proceed under these conditions. Next, a mixture of 86% orthophosphoric acid and 87% polyphosphoric acids (approximately corresponding to anhydrous H3PO4) or molten 87% polyphosphoric acid (PPA) were employed as the media for the reactions carried out at 120 °C. Again, the reactions did not proceed, and the starting amine 1a remained intact in both cases (entries 3, 4). As it was shown in our previous report [25] that addition of phosphorous acid to the reaction medium could enable stubborn steric-restricted annulations, we also decided to attempt to stimulate the reactivity using this trick. To this end, the reaction of 1a and 4c was carried out in a mixture of H3PO3 and 87% PPA (1:1 m/m) at 130 °C. Formation of product 3ac was detected, albeit in a low yield (entry 5). To improve the reaction performance, we attempted to increase the temperature. At 150 °C, the reaction outcome still remained quite marginal (entry 6). However, at 160 °C, a synthetically meaningful result was obtained (entry 7). We also tested the reaction in pure phosphorous acid without addition of PPA. This reaction was accompanied with partial thermal decomposition of the medium, which complicated the isolation and rendered notably lower yield (entry 8).
Table 1.
Optimization of reaction conditions for annulation.

| Medium | t, °C | Time, h | Yield, % 1 | |
|---|---|---|---|---|
| 1 | H3PO4 86%, 2 g | 110 | 4 | 0 |
| 2 | H3PO4 86%, 2 g | 120 | 4 | 0 |
| 3 | H3PO4 86%, 1 g PPA 87%, 1 g | 120 | 4 | 0 |
| 4 | PPA 87%, 2 g | 120 | 4 | 0 |
| 5 | PPA 87%, 1 g; H3PO3, 1 g | 130 | 4 | 9 |
| 6 | PPA 87%, 1 g; H3PO3, 1 g | 150 | 2 | 40 |
| 7 | PPA 87%, 1 g; H3PO3, 1 g | 160 | 2 | 63 2 |
| 8 | H3PO3, 2 g | 160 | 2 | 51 2 |
1 NMR yields are provided unless specified otherwise. 2 Isolated yield of purified product is reported.
With optimized conditions in hand, we then proceeded to investigate the scope of the featured transformation. The results presented in Scheme 4 demonstrate that 3,4-dihydroquinazolines substituted with alkyl groups at C-2, C-4, or C-6 can be routinely obtained via this method in good to excellent yields. It should be pointed out that alkyl substituents at these positions as well as the length of the alkyl chain in the nitroalkane reagent do not seem to affect the efficiency of the featured transformation. Aromatic halide substituent in substrate 1d was also well tolerated, allowing for efficient preparation of brominated product 3cd (Scheme 4).
Scheme 4.
Examples of 3,4-dihydroquinazolines obtained in annulation of 2-(aminomethyl)anilines and nitroalkanes. Isolated yield of purified products is listed.
An attempt to use ethyl 2-nitroacetate as an electrophilic component in the described transformation proved unfruitful. The reaction with 1a produced complex mixtures of unidentified products, and none of them was isolated in a meaningful yield. More interesting results were obtained in reactions employing α-nitroacetophenone (4g). The products of these reactions, 3ag and 3cg, were identified as 3,4-dihydroquinozalines bearing phenyl substituents at C-2 (Scheme 4 and Scheme 5). It is believed that the mechanism of this transformation involves initial formation of imine 19 via condensation of ketone 4g with one of the available amino groups. Subsequent 6-endo-trig nucleophilic cyclization took place, providing cyclic aminal 20, which underwent further elimination of nitromethane moiety to afford the observed products (Scheme 5).
Scheme 5.
Mechanistic rationale of the annulation involving α-nitroacetophenone.
3. Materials and Methods
General. NMR spectra, 1H and 13C were measured in solutions of CDCl3 or DMSO-d6 on a Bruker AVANCE-III HD instrument (at 400.40 or 100.61 MHz, respectively). Residual solvent signals were used as internal standards, in DMSO-d6 (2.50 ppm for 1H, and 40.45 ppm for 13C nuclei) or in CDCl3 (7.26 ppm for 1H, and 77.16 ppm for 13C nuclei). Signals in 13C NMR spectra marked with an asterisk (*) were assigned based on 2D 1H-13C HMBC and 1H-13C HSQC experiments. HRMS spectra were measured on a Bruker maXis impact (electrospray ionization, in MeCN solutions, employing HCO2Na-HCO2H for calibration). IR spectra were measured on an FT-IR spectrometer Shimadzu IRAffinity-1S equipped with an ATR sampling module. The reaction progress, the purity of isolated compounds, and the Rf values were monitored with TLC on Silufol UV-254 plates. Column chromatography was performed on silica gel (32–63 μm, 60 Å pore size). Melting points were measured with a Stuart SMP30 apparatus. Polyphosphoric acid (87%) was prepared by dissolving a precisely measured amount of P2O5 in 85% orthophosphoric acid. All the reagents and solvents were purchased from commercial venders and used as received.
2-(1-Aminoethyl)aniline (1a):
This compound was obtained via transformation of acetophenone into the corresponding oxime and its subsequent reduction with either sodium borohydride in the presence of TiCl4 in dimethoxyethane (DME) (method A), or with zinc dust in hydrochloric acid (method B).
Accordingly, 1-(2-nitrophenyl)ethan-1-one oxime was obtained via published procedure [38] by addition of hydroxylamine hydrochloride (4.45 g, 64.0 mmol) and sodium acetate (6.56 g, 80 mmol) to a solution of 2-nitroacetophenone (6.61 g, 40.0 mmol) in an ethanol and water mixture (4:1, 100 mL). The reaction mixture was boiled at reflux for 2 h (monitored with TLC), and cooled to RT. Then a third of the solvent volume was removed in vacuum, and the residual solution was poured into 100 mL of crushed ice and water mixture. Formed white flaky precipitate was filtered and dried in air. Yield: 6.45 g (90%). Colorless crystalline mass, mp 92–95 °C (Literature data: mp 91 °C [39], mp 115.5–116.5 °C (aq. methanol) [40].
The oxime was subsequently reduced via one of the following procedures:
Method A:
A solution of 1-(2-nitrophenyl)ethan-1-one oxime (1.80 g, 10.0 mmol) in anhydrous DME was added slowly under argon atmosphere to a stirred and chilled in ice-bath mixture of sodium borohydride (1.59 g, 42 mmol) and titanium (IV) chloride (3.99 g, 21 mmol) in anhydrous DME (40 mL), and the mixture was stirred at room temperature for 20 h [41]. Then, the reaction mixture was carefully poured into ice-cold water (100 mL), neutralized with aqueous ammonia (25%), and concentrated in vacuum. The residual solid was extracted in a Soxhlet apparatus for 8 h into isopropyl alcohol (230 mL). The extract was concentrated in vacuum to obtain crude 2-(1-aminoethyl)aniline chlorohydride as off-white powder, mp 172–179 °C (Literature data: mp 187 °C (decomp) [42]. Yield: 1.58 g (9.1 mmol, 91%). For isolation of 2-(1-aminoethyl)aniline as free base, the salt was dissolved in water, washed with ethyl acetate, basified with aqueous NaOH (20%), and extracted with ethyl acetate. Combine organic extracts were washed with brine, dried with sodium sulphate, and concentrated in vacuum.
Method B:
To a solution of 1-(2-nitrophenyl)ethan-1-one oxime (2.07 g, 11.5 mmol) in a mixture of ethanol (45 mL), water (12 mL) and concentrated HCl (24 mL) stirred at 0 °C zinc duct was added in small portions over 30 min (9.03 g, 138.1 mmol) [43]. The reaction mixture was stirred at 50 °C for 1.5 h (the reaction progress was monitored with TLC), then cooled down to room temperature, and filtered. The filtrate was concentrated in vacuum, and the residue was basified with aqueous ammonia (25%) to pH 9 and extracted with ethyl acetate (3 × 50 mL). Combined organic extracts were dried with sodium sulphate and concentrated in vacuum. The residual amber-colored oil was dissolved in diluted aqueous HCl (70 mL, pH of resulting solution c.a. 4–5), and washed with ethyl acetate (3 × 25 mL). The aqueous phase was basified with ammonia (25%) to pH 9 and extracted with ethyl acetate (3 × 25 mL). Combined organic phases were washed with brine, dried with sodium sulphate, and concentrated. 2-(1-Aminoethyl)aniline was obtained as pale-yellow oil, slowly solidifying upon standing. Grinding of this crystalline mass afforded pale-yellow powder, mp 85–89 °C. Yield: 1.19 g (8.4 mmol, 73%). 1H NMR (400 MHz, DMSO-d6) δ 7.04 (dd, J = 7.5, 1.6 Hz, 1H), 6.88 (td, J = 7.6, 1.6 Hz, 1H), 6.56 (dd, J = 7.9, 1.3 Hz, 1H), 6.48 (td, J = 7.4, 1.3 Hz, 1H), 4.03 (q, J = 6.6 Hz, 1H), 1.26 (d, J = 6.6 Hz, 3H).
2-(Aminomethyl)-4-methylaniline (1b):
This compound was obtained starting from p-toluidine via iodination and subsequent transformation of the resulting 2-iodo-4-methylaniline into 2-amino-5-methylbenzonitrile, which was reduced with lithium aluminum hydride in tetrahydrofuran (THF).
Accordingly, 2-iodo-4-methylaniline was obtained according to the known procedure [44,45]. To this end, a mixture of p-toluidine (2.65 g, 24.7 mmol), iodine (6.15 g, 24.2 mmol), and sodium bicarbonate (2.70 g, 32.1 mmol), methylene chloride (50 mL) and water (25 mL) was stirred at room temperature for 14 h. The organic phase was separated, and the aqueous layer was extracted with methylene chloride (3 × 10 mL). Combined organic extracts were washed with brine (20 mL) and dried with anhydrous sodium sulphate. Solvent was evaporated in vacuum, and the residue was purified by preparative column chromatography eluting with a mixture of ethyl acetate and light petroleum ether (1:20). 2-Iodo-4-methylaniline was obtained as yellow oil, Rf 0.47 (EtOAc/hexane, 1:15). Yield: 5.03 g (21.1 mmol, 87%). This material was used for the following step without additional purification.
2-Amino-5-methylbenzonitrile was synthesized according to the known procedure [46]. To a stirred solution of 2-iodo-4-methylaniline (4.80 g, 20.5 mmol) in DMF (25 mL) was added copper (I) cyanide (2.75 g, 30.8 mmol). The reaction mass was stirred and heated at 130 °C for 3 h, then cooled to room temperature. Aqueous sodium cyanide (100 mL of 10% solution) was added (caution, toxic) and the mixture was stirred for an additional 1 h. The aqueous phase was extracted with ethyl acetate (3 × 40 mL). Combined organic extracts were washed with brine, dried with sodium sulphate, and concentrated in vacuum. The residual crude oil was purified by preparative column chromatography on silica gel eluting with a mixture of ethyl acetate and light petroleum ether (1:5). 2-Amino-5-methylbenzonitrile was obtained as light-caramel crystals, mp 59–61 °C (Literature data: mp 60.5–61 °C [47]), Rf 0.66 (EtOAc/hexane, 1:2). Yield: 2.11 g (16.0 mmol, 78%).
Finally, 2-(aminomethyl)-4-methylaniline (1b) was obtained according to the known method [48], replacing borane with lithium aluminum hydride (mole ratio LiAlH4/nitrile 4:1). To this end, to a stirred under argon atmosphere solution of LiAlH4 (2.15 g, 56.7 mmol) in anhydrous THF (20 mL), a solution of 2-amino-5-methylbenzonitrile 1.83 g (13.8 mmol) in dry THF (10 mL) was added dropwise over 30 min at room temperature. The mixture was stirred for 5 h, and the reaction progress was monitored with TLC. After complete consumption of nitrile, water (5 mL) was carefully added dropwise, followed by 20% aqueous sodium hydroxide (2 mL). Formed precipitate was filtered, and the filter cake was washed with several portions of hot chloroform (combined volume 100 mL). Combined organic phases were washed with brine, dried with sodium sulphate and concentrated to obtain the titled compound as light-yellow crystalline powder, mp 72–75 °C. Yield: 1.75 g (10.4 mmol, 93%). 1H NMR (400 MHz, Chloroform-d) δ 6.96–6.81 (m, 2H), 6.60 (d, J = 7.9 Hz, 1H), 3.86 (s, 2H), 2.23 (s, 3H).
2-(Aminomethyl)aniline (1c):
This compound was obtained analogously to diamine 1a from 2-nitrobenzaldehyde via its conversion to the corresponding oxime and subsequent reduction either with sodium borohydride in DME in the presence of TiCl4 (method A), or with zinc dust in hydrochloric acid (method B).
2-Nitrobenzaldehyde oxime was obtained in a way similar to preparation of 2-nitroacetophenone oxime described above. To this end, a mixture of 2-nitrobenzaldehyde (6.04 g, 40.0 mmol), hydroxylamine hydrochloride (4.45 g, 64.0 mmol), and sodium acetate (6.56 g, 80 mmol) was boiled in a mixture of ethanol and water (4:1, 100 mL) for 1 h. Crude product was re-crystallized from benzene to afford colorless crystals, mp 99–101 °C. (Literature data, mp 102–103 °C [49]). Yield: 4.98 g (30 mmol, 75%). This material was subjected to the subsequent reduction using one of the following procedures.
Method A:
The reaction was performed in a way similar to the one described above for the preparation of 2-(1-aminoethyl)aniline (1a), adding dropwise a solution of 2-nitrobenzaldehyde oxime (1.66 g, 10.0 mmol) in dry DME (10 mL) to a stirred ice-cold mixture of sodium borohydride 1.59 g (42 mmol), titanium (IV) chloride (3.99 g, 21 mmol), and anhydrous DME (40 mL). The mixture was stirred at room temperature for 20 h [43]. After aqueous workup and neutralization with aqueous ammonia (25%), crude material was extracted in Soxhlet apparatus with isopropyl alcohol (210 mL). The extract was concentrated to 2/3 of the initial volume, and the formed precipitate was filtered off to afford 2-(aminomethyl)aniline hydrochloride as off-white flakes, mp 197–199 °C (Literature data: mp 197–199 °C (methanol/diethyl ether) [50]). Yield: 0.75 g (4.8 mmol, 48%).
Transformation of this hydrochloride into free amine was performed by dissolving the salt in water, washing the solution with ethyl acetate, basification of the aqueous phase with aqueous ammonia to pH 9, and extraction with ethyl acetate in the same manner as described above for material 1a. Combined organic extracts were dried and concentrated in vacuum to afford free titled amine.
Method B:
Reduction with zinc dust was performed according to the typical protocol described above for preparation of 1a [43]. Zinc dust 9.03 g (138.1 mmol) was added in small portions to a mixture of 2-nitrobenzaldehyde oxime 1.91 g (11.5 mmol), ethanol (45 mL), water (12 mL), and concentrated HCl (24 mL) vigorously stirred at 0 °C. The reaction mixture was stirred for 1.5 h at 50 °C, and the reaction progress was monitored with TLC. The aqueous work up, extraction, and isolation of the product was performed in the same manner as described above in protocol for preparation of compound 1a. The titled material was afforded as light-yellow crystals, mp 59–60 °C (Literature data: mp 58–59 °C (MeOH, CHCl3) [51]). Yield: 0.97 g (7.9 mmol, 69%). 1H NMR (400 MHz, Chloroform-d) δ 7.14–7.01 (m, 2H), 6.76–6.65 (m, 2H), 3.90 (s, 2H).
2-(1-Aminoethyl)-4-bromoaniline (1d):
This compound was obtained according to the procedure analogous to those described above for diamines 1a and 1c via transformation of 1-(2-amino-5-bromophenyl)ethan-1-one (1.07 g, 5.0 mmol) into the corresponding oxime and its subsequent reduction with zinc dust in hydrochloric acid (method B) according to published literature procedure [41,43]. Overall yield: 0.24 g (1.1 mmol, 22%). Light yellow viscous oil. 1H NMR (400 MHz, Chloroform-d) δ 7.17 (d, J = 2.4 Hz, 1H), 7.13 (dd, J = 8.4, 2.4 Hz, 1H), 6.52 (d, J = 8.3 Hz, 1H), 4.13 (q, J = 6.6 Hz, 1H), 1.45 (d, J = 6.6 Hz, 3H). 13C NMR (101 MHz, Chloroform-d) δ 145.2, 131.3, 130.5, 129.9, 118.0, 109.5, 50.1, 22.7. IR, vmax/cm−1: 3446, 3364, 2969, 1607, 1414, 1281, 1099, 814. HRMS (ES TOF) calculated for (M + H)+ C8H12BrN2 215.0177, found 215.0178 (0.5 ppm).
Preparation of 3,4-dihydroquinazolines (3aa–3cf) via annulation of 2-aminobenzylamines and 1-nitroalkanes (General procedure):
A 10 mL round-bottomed flask equipped with reflux condenser and magnetic stirring bar was charged with 2-aminobenzylamine (1a–c, 1 mmol), 1-nitroalkane (4a–f, 2.0 mmol), 87% PPA (1 g) and phosphorous acid (1 g). The mixture was vigorously stirred and heated to 160 °C for 2 h monitoring the reaction progress with TLC. Note: in reactions involving nitromethane (4a: R3 = H) or nitroethane (4b: R3 = Me) an additional portion of nitroalkane (1.0 mmol) should be added after 1.5 h to compensate for evaporation. After consumption of the starting amine, the reaction mass was cooled down to 80–85 °C, transferred to crushed ice (10–15 g), neutralized with aqueous ammonia (25%) to pH 8, and extracted with ethyl acetate (3 × 25 mL). The combined organic extracts were washed with brine, dried with sodium sulphate, and concentrated in vacuum to obtain the crude material, which can be further purified by preparative column chromatography on silica gel.
4-Methyl-3,4-dihydroquinazoline (3aa):
Off-white powder, mp 62–65 °C (Literature data: mp 81–83 °C [52]), Rf 0.33 (EtOAc/EtOH/TEA, 32:8:1). Yield: 92 mg (0.63 mmol, 63%). 1H NMR (400 MHz, DMSO-d6) δ 7.06 (q, J = 6.8, 6.1 Hz, 2H), 6.98–6.88 (m, 2H), 6.76 (d, J = 7.8 Hz, 1H), 4.60 (q, J = 6.5 Hz, 1H), 1.28 (d, J = 6.5 Hz, 3H). 13C NMR (101 MHz, DMSO-d6) δ 146.4, 140.3*, 127.3, 127.0*, 125.6, 123.4, 120.3*, 47.6, 25.7. IR, vmax/cm−1: 3195, 2974, 1614, 1590, 1479, 1452, 1371, 1250, 759. HRMS (ES TOF) calculated for (M + H)+ C9H11N2 147.0918, found 147.0917 (−1.2 ppm).
2-Methyl-4-methyl-3,4-dihydroquinazoline (3ab):
Pale-yellow crystals, mp 107–110 °C (Literature data: mp 145–146 °C [52]), Rf 0.62 (EtOAc/EtOH/TEA, 32:8:1). Yield: 133 mg (0.83 mmol, 83%). 1H NMR (400 MHz, DMSO-d6) δ 7.05 (td, J = 7.5, 1.8 Hz, 1H), 6.95 (dd, J = 7.6, 1.7 Hz, 1H), 6.89 (td, J = 7.3, 1.3 Hz, 1H), 6.75 (dd, J = 7.8, 1.3 Hz, 1H), 4.59 (q, J = 6.4 Hz, 1H), 1.87 (s, 3H), 1.27 (d, J = 6.4 Hz, 3H). 1H NMR (400 MHz, Chloroform-d) δ 7.15 (td, J = 7.5, 1.6 Hz, 1H), 7.06–6.90 (m, 3H), 5.22 (s, 1H), 4.75 (q, J = 6.5 Hz, 1H), 2.06 (s, 3H), 1.44 (d, J = 6.5 Hz, 3H). 13C NMR (101 MHz, Chloroform-d) δ 154.6, 140.7, 128.1, 125.3, 125.2, 124.4, 121.8, 49.2, 25.9, 22.7. 13C NMR (101 MHz, DMSO-d6) δ 127.3, 125.1, 122.8, 48.4, 25.6, 21.7. IR, vmax/cm−1: 3253, 2973, 1617, 1576, 1484, 1436, 1395, 1274, 752. HRMS (ES TOF) calculated for (M + H)+ C10H13N2 161.1070, found 161.1073 (2.0 ppm).
2-Ethyl-4-methyl-3,4-dihydroquinazoline (3ac):
Viscous glassy oil of amber color, Rf 0.42 (EtOAc/EtOH/TEA, 32:8:1). Yield: 110 mg (0.63 mmol, 63%). 1H NMR (400 MHz, DMSO-d6) δ 7.04 (td, J = 7.5, 1.7 Hz, 1H), 6.94 (dd, J = 7.5, 1.7 Hz, 1H), 6.88 (td, J = 7.3, 1.3 Hz, 1H), 6.77 (dd, J = 7.8, 1.3 Hz, 1H), 4.58 (q, J = 6.5 Hz, 1H), 2.13 (q, J = 7.6 Hz, 2H), 1.25 (d, J = 6.5 Hz, 3H), 1.10 (t, J = 7.6 Hz, 3H). 13C NMR (101 MHz, DMSO-d6) δ 158.9*, 140.9*, 127.2, 126.9*, 125.2, 124.4*, 122.8, 48.1*, 28.3, 25.5, 11.4. IR, vmax/cm−1: 3234, 2979, 1658, 1576, 1487, 1451, 1398, 1299, 754. HRMS (ES TOF) calculated for (M + H)+ C11H15N2 175.1235, found 175.1230 (−2.8 ppm).
4-Methyl-2-propyl-3,4-dihydroquinazoline (3ad):
Viscous glassy oil of amber color, Rf 0.62 (EtOAc/EtOH/TEA, 32:8:1). Yield: 128 mg (0.68 mmol, 68%). 1H NMR (400 MHz, DMSO-d6) δ 7.04 (td, J = 7.5, 1.7 Hz, 1H), 6.94 (dd, J = 7.7, 1.7 Hz, 1H), 6.88 (td, J = 7.3, 1.3 Hz, 1H), 6.77 (dd, J = 7.8, 1.3 Hz, 1H), 4.58 (q, J = 6.4 Hz, 1H), 2.08 (td, J = 7.3, 1.7 Hz, 2H), 1.60 (q, J = 7.4 Hz, 2H), 1.24 (d, J = 6.4 Hz, 3H), 0.90 (t, J = 7.4 Hz, 3H). 13C NMR (101 MHz, Chloroform-d) δ 159.2, 137.7, 128.4, 125.5, 125.2, 124.6, 120.5, 49.1, 36.8, 25.9, 21.1, 13.7. IR, vmax/cm−1: 3224, 2964, 1658, 1614, 1578, 1481, 1452, 1368, 1327, 1272, 751. HRMS (ES TOF) calculated for (M + H)+ C12H17N2 189.1381, found 189.1386 (2.8 ppm).
2-Butyl-4-methyl-3,4-dihydroquinazoline (3ae):
Viscous glassy oil of amber color, Rf 0.73 (EtOAc/EtOH/TEA, 32:8:1). Yield: 123 mg (0.61 mmol, 61%). 1H NMR (400 MHz, DMSO-d6) δ 7.04 (td, J = 7.5, 1.7 Hz, 1H), 6.94 (dd, J = 7.6, 1.7 Hz, 1H), 6.88 (td, J = 7.3, 1.3 Hz, 1H), 6.77 (dd, J = 7.8, 1.3 Hz, 1H), 4.58 (q, J = 6.4 Hz, 1H), 2.15–2.08 (m, 2H), 1.61–1.52 (m, 2H), 1.35–1.28 (m, 2H), 1.24 (d, J = 6.4 Hz, 3H), 0.89 (t, J = 7.4 Hz, 3H). 13C NMR (101 MHz, Chloroform-d) δ 159.2, 137.7, 128.4, 125.5, 125.2, 124.6, 120.5, 49.1, 36.8, 25.9, 21.1, 13.7. IR, vmax/cm−1: 3297, 2959, 1655, 1616, 1578, 1481, 1455, 1375, 1272, 749. HRMS (ES TOF) calculated for (M + H)+ C13H19N2 203.1543, found 203.1543 (0.0 ppm).
4-Methyl-2-pentyl-3,4-dihydroquinazoline (3af):
Viscous glassy oil of amber color, Rf 0.78 (EtOAc/EtOH/TEA, 32:8:1). Yield: 110 mg (0.51 mmol, 51%). 1H NMR (400 MHz, DMSO-d6) δ 7.04 (td, J = 7.5, 1.7 Hz, 1H), 6.94 (dd, J = 7.6, 1.7 Hz, 1H), 6.88 (td, J = 7.3, 1.3 Hz, 1H), 6.76 (d, J = 7.8 Hz, 1H), 4.57 (q, J = 6.4 Hz, 1H), 2.16–2.03 (m, 2H), 1.58 (p, J = 7.4 Hz, 2H), 1.30 (dp, J = 9.1, 5.2 Hz, 4H), 1.24 (d, J = 6.4 Hz, 3H), 0.87 (t, J = 6.9 Hz, 3H). 13C NMR (101 MHz, DMSO-d6) δ 157.6*, 141.5*, 127.4*, 127.3, 125.2, 123.0*, 122.8, 48.3*, 35.1, 30.9, 26.4, 25.6, 22.0, 14.0. IR, vmax/cm−1: 3205, 2964, 1653, 1617, 1581, 1482, 1455, 1373, 1272, 752. HRMS (ES TOF) calculated for (M + H)+ C14H21N2 217.1693, found 217.1699 (2.7 ppm).
4-Methyl-2-phenyl-3,4-dihydroquinazoline (3ag):
A light-yellow, glassy oil (Literature data: mp 70 °C [52]), Rf 0.38 (EtOAc/EtOH, 4:1). Yield: 102 mg (0.46 mmol, 46%). 1H NMR (400 MHz, Chloroform-d) δ 7.81–7.72 (m, 2H), 7.45–7.33 (m, 3H), 7.18–7.08 (m, 2H), 7.03–6.98 (m, 1H), 6.95 (dd, J = 7.6, 1.4 Hz, 1H), 4.84 (q, J = 6.5 Hz, 1H), 1.46 (d, J = 6.5 Hz, 3H). 13C NMR (101 MHz, Chloroform-d) δ 154.1, 141.7, 135.8, 130.7, 128.8 (2C), 128.2, 126.7 (2C), 126.1, 125.2, 124.9, 49.5, 29.9, 25.8. IR, vmax/cm−1: 3311, 2935, 1547, 1477, 1440, 1330, 1272, 1115, 1067, 1031. HRMS (ES TOF) calculated for (M + H)+ C15H15N2 223.1228, found 223.1230 (0.9 ppm).
6-Methyl-3,4-dihydroquinazoline (3ba):
A light-yellow, viscous, glassy oil crystallizing over time, Rf 0.23 (EtOAc/EtOH/TEA, 40:20:1). Yield: 102 mg (0.70 mmol, 70%). 1H NMR (400 MHz, Chloroform-d) δ 7.14 (s, 1H), 6.97–6.90 (m, 1H), 6.78 (d, J = 7.9 Hz, 1H), 6.68 (d, J = 1.9 Hz, 1H), 4.60 (s, 2H), 2.26 (s, 3H). 13C NMR (101 MHz, Chloroform-d) δ 147.1, 137.0*, 134.1, 129.0, 127.0, 120.8, 116.6, 44.2, 21.2. IR, vmax/cm−1: 3186, 2983, 1747, 1660, 1624, 1597, 1373, 1318, 1265, 1243, 828. HRMS (ES TOF) calculated for (M + H)+ C9H11N2 147.0914, found 147.0917 (2.1 ppm).
2,6-Dimethyl-3,4-dihydroquinazoline (3bb):
A light-yellow crystalline powder, Rf 0.16 (EtOAc/EtOH/TEA, 40:20:1). Yield: 99 mg (0.62 mmol, 62%). 1H NMR (400 MHz, Chloroform-d) δ 6.96–6.91 (m, 1H), 6.80 (d, J = 8.0 Hz, 1H), 6.69 (t, J = 1.4 Hz, 1H), 4.58 (s, 2H), 2.25 (s, 3H), 2.01 (s, 3H). 13C NMR (101 MHz, Chloroform-d) δ 154.7*, 138.1*, 133.8, 128.7, 126.3, 119.6, 44.6, 22.7, 21.0. IR, vmax/cm−1: 3215 2906, 1747, 1655, 1619, 1586, 1499, 1381, 1323, 1243, 826. HRMS (ES TOF) calculated for (M + H)+ C10H13N2 161.1071, found 161.1073 (1.6 ppm).
2-Ethyl-6-methyl-3,4-dihydroquinazoline (3bc):
A light-yellow, viscous, glassy oil crystallizing over time, Rf 0.31 (EtOAc/EtOH/TEA, 40:20:1). Yield: 134 mg (0.77 mmol, 77%). 1H NMR (400 MHz, DMSO-d6) δ 6.87–6.80 (m, 1H), 6.71–6.65 (m, 1H), 6.63 (d, J = 7.9 Hz, 1H), 4.40 (s, 2H), 2.17 (s, 3H), 2.10 (q, J = 7.6 Hz, 2H), 1.07 (t, J = 7.6 Hz, 3H). 13C NMR (101 MHz, DMSO-d6) δ 160.1, 153.3, 139.0*, 131.5, 127.79, 126.0, 119.7, 43.9*, 28.1, 20.5, 11.2. IR, vmax/cm−1: 3258, 2993, 1653, 1622, 1496, 1373, 1270, 1238, 959, 824. HRMS (ES TOF) calculated for (M + H)+ C11H15N2 175.1234, found 175.1230 (−2.4 ppm).
6-Methyl-2-propyl-3,4-dihydroquinazoline (3bd):
A light-yellow, viscous, glassy oil crystallizing over time, Rf 0.47 (EtOAc/EtOH/TEA, 40:20:1). Yield: 162 mg (0.86 mmol, 86%). 1H NMR (400 MHz, Chloroform-d) δ 6.96–6.90 (m, 1H), 6.80 (d, J = 8.0 Hz, 1H), 6.72–6.65 (m, 1H), 4.57 (s, 2H), 2.25 (s, 3H), 2.22–2.18 (m, 2H), 1.70–1.64 (m, 2H), 0.97 (t, J = 7.4 Hz, 3H). 13C NMR (101 MHz, Chloroform-d) δ 157.9, 136.2, 134.1, 133.3, 128.3, 125.9, 119.5, 44.3, 38.1, 20.7, 20.5, 13.6. IR, vmax/cm−1: 3253, 2964, 2930, 1747, 1655, 1622, 1586, 1508, 1489, 1378, 1320, 1241, 829. HRMS (ES TOF) calculated for (M + H)+ C12H17N2 189.1390, found 189.1386 (−2.0 ppm).
2-Butyl-6-methyl-3,4-dihydroquinazoline (3be):
A light-yellow, viscous, glassy oil crystallizing over time, Rf 0.55 (EtOAc/EtOH/TEA, 40:20:1). Yield: 162 mg (0.80 mmol, 80%). 1H NMR (400 MHz, Chloroform-d) δ 6.95–6.90 (m, 1H), 6.80 (d, J = 7.9 Hz, 1H), 6.69 (d, J = 1.9 Hz, 1H), 4.58 (s, 2H), 2.25 (s, 3H), 2.24–2.20 (m, 2H), 1.65–1.59 (m, 2H), 1.42–1.36 (m, 2H), 0.92 (t, J = 7.3 Hz, 3H). 13C NMR (101 MHz, Chloroform-d) δ 159.9, 138.4*, 133.3, 129.9, 128.6, 126.3, 119.8, 44.7, 36.4, 29.6, 22.1, 21.0, 14.0. IR, vmax/cm−1: 3263, 3195, 2964, 2935, 1742, 1653, 1622, 1586, 1510, 1489, 1378, 1322, 1270, 1241, 822. HRMS (ES TOF) calculated for (M + H)+ C13H19N2 203.1539, found 203.1543 (1.9 ppm).
6-Methyl-2-pentyl-3,4-dihydroquinazoline (3bf):
A light-yellow, viscous, glassy oil, Rf 0.66 (EtOAc/EtOH/TEA, 40:20:1). Yield: 169 mg (0.78 mmol, 78%). 1H NMR (400 MHz, Chloroform-d) δ 6.93 (dd, J = 7.8, 2.2 Hz, 1H), 6.80 (d, J = 8.1 Hz, 1H), 6.69 (d, J = 1.9 Hz, 1H), 4.58 (s, 2H), 2.26–2.25 (m, 3H), 2.24–2.20 (m, 2H), 1.67–1.61 (m, 2H), 1.33 (dq, J = 6.6, 3.0 Hz, 4H), 0.91–0.86 (m, 3H). 13C NMR (101 MHz, Chloroform-d) δ 158.4*, 135.2*, 133.7, 129.9, 128.6, 126.3, 119.8, 44.5, 36.6, 31.7, 27.2, 22.6, 21.0, 14.1. IR, vmax/cm−1: 3292, 3195, 2964, 2939, 1747, 1655, 1588, 1511, 1492, 1381, 1323, 1251, 821. HRMS (ES TOF) calculated for (M + H)+ C14H21N2 217.1702, found 217.1699 (−1.1 ppm).
3,4-Dihydroquinazoline (3ca):
Off-white solid, mp 123–125 °C (Literature data: mp 123–126.5 °C [53]), Rf 0.37 (EtOAc/EtOH/TEA, 12:6:1). Yield: 107 mg (0.81 mmol, 81%). 1H NMR (400 MHz, Chloroform-d) δ 7.15 (s, 1H), 7.06 (td, J = 7.6, 1.5 Hz, 1H), 6.92 (td, J = 7.5, 1.2 Hz, 1H), 6.84–6.74 (m, 2H), 5.88 (s, 1H), 4.58 (s, 2H). 13C NMR (101 MHz, Chloroform-d) δ 147.2, 139.0, 128.0, 126.0, 124.6, 120.1, 119.7, 44.0. IR, vmax/cm−1: 3344, 3234, 3041, 2872, 1662, 1493, 1375, 1146, 1077, 913. HRMS (ES TOF) calculated for (M + H)+ C8H9N2 133.0756, found 133.0760 (2.8 ppm).
2-Methyl-3,4-dihydroquinazoline (3cb):
Yellow gummy oil, Rf. 0.40 (EtOAc/EtOH/TEA 12:6:1). Yield: 105 mg (0.72 mmol, 72%). 1H NMR (400 MHz, Chloroform-d) δ 7.05 (t, J = 1.0 Hz, 1H), 6.90 (td, J = 7.4, 1.3 Hz, 1H), 6.81 (ddd, J = 7.0, 5.6, 1.3 Hz, 2H), 5.81 (s, 1H), 4.55 (s, 2H), 1.97 (s, 3H). 13C NMR (101 MHz, Chloroform-d) δ 155.4, 140.3, 128.0, 125.6, 124.1, 120.0, 118.8, 44.5, 22.1. IR, vmax/cm−1: 3209, 3017, 2877, 2829, 1660, 1587, 1481, 1334, 1272. HRMS (ES TOF) calculated for (M + H)+ C9H11N2 147.0917, found 147.0917 (−0.5 ppm).
2-Ethyl-3,4-dihydroquinazoline (3cc):
Colorless solid, mp 101–103 °C (Literature data mp 103–104 °C (benzene, petroleum ether) [54]), Rf 0.43 (EtOAc/EtOH/TEA, 10:4:1). Yield: 112 mg (0.70 mmol, 70%). 1H NMR (400 MHz, Chloroform-d) δ 7.17–7.09 (m, 1H), 6.98 (td, J = 7.4, 1.2 Hz, 1H), 6.90 (dd, J = 7.8, 1.4 Hz, 2H), 5.57 (s, 1H), 4.64 (s, 2H), 2.30 (q, J = 7.6 Hz, 2H), 1.24 (t, J = 7.6 Hz, 3H). 13C NMR (101 MHz, Chloroform-d) δ 161.3, 138.3, 127.9, 125.6, 123.8, 119.7, 119.6, 43.4, 29.2, 11.4. IR, vmax/cm−1: 2922, 2848, 1975, 1944, 1836, 1766, 1674, 1604, 1457, 954. HRMS (ES TOF) calculated for (M + H)+ C10H13N2 161.1068, found 161.1073 (3.0 ppm).
2-Propyl-3,4-dihydroquinazoline (3cd):
Yellow gummy oil, Rf 0.43 (EtOAc/EtOH, 5:1). Yield: 113 mg (0.65 mmol, 65%). 1H NMR (400 MHz, Chloroform-d)) δ 7.15 (td, J = 7.6, 1.5 Hz, 1H), 6.99 (td, J = 7.4, 1.3 Hz, 1H), 6.90 (d, J = 7.3 Hz, 2H), 4.65 (s, 2H), 4.11 (s, 1H), 2.31–2.21 (m, 2H), 1.77–1.65 (m, 2H), 1.01 (t, J = 7.3 Hz, 3H). 13C NMR (101 MHz, Chloroform-d) δ 158.87, 140.89, 128.40, 126.05, 124.40, 120.47, 120.16, 45.11, 38.65, 21.14, 14.24. IR, vmax/cm−1: 2607, 2327, 1648, 1585, 1498, 1397, 1327, 1245, 1057, 886. HRMS (ES TOF) calculated for (M + H)+ C11H15N2 175.1225, found 175.1230 (2.9 ppm).
2-Butyl-3,4-dihydroquinazoline (3ce):
Yellow gummy oil, Rf 0.50 (EtOAc/EtOH, 5:2). Yield: 105 mg (0.56 mmol, 56%). 1H NMR (400 MHz, DMSO) δ 7.04 (ddd, J = 7.8, 5.6, 3.3 Hz, 1H), 6.91–6.83 (m, 2H), 6.75 (d, J = 7.8 Hz, 1H), 4.44 (s, 2H), 2.17–2.04 (m, 2H), 1.55 (tt, J = 8.9, 6.8 Hz, 2H), 1.40–1.24 (m, 2H), 0.88 (t, J = 7.4 Hz, 3H). 13C NMR (101 MHz, DMSO-d6) δ 159.4, 140.5, 128.0, 126.1, 123.6, 119.8, 118.7, 44.3, 34.6, 29.1, 22.2, 14.2. IR, vmax/cm−1: 3195, 3002, 2853, 2356, 1773, 1580, 1436, 1149, 1055. HRMS (ES TOF) calculated for (M + H)+ C12H17N2 189.1386, found 189.1386 (0.1 ppm).
2-Pentyl-3,4-dihydroquinazoline (3cf):
Colorless gummy oil, Rf 0.67 (EtOAc/EtOH, 5:2). Yield: 101 mg (0.50 mmol, 50%). 1H NMR (400 MHz, DMSO) δ 7.04 (ddd, J = 8.2, 5.6, 3.2 Hz, 1H), 6.87 (d, J = 3.3 Hz, 2H), 6.74 (d, J = 7.8 Hz, 1H), 4.43 (s, 2H), 2.15–2.04 (m, 2H), 1.57 (p, J = 7.4 Hz, 2H), 1.36–1.17 (m, 4H), 0.87 (t, J = 6.8 Hz, 3H). 13C NMR (101 MHz, DMSO-d6) δ 158.8, 141.4, 127.8, 125.9, 123.2, 120.1, 119.0, 44.6, 35.3, 31.4, 26.6, 22.3, 14.3. IR, vmax/cm−1: 3195, 3026, 2858, 1640, 1433, 1151, 1045, 995, 896. HRMS (ES TOF) calculated for (M + H)+ C13H19N2 203.1540, found 203.1543 (1.5 ppm).
2-Phenyl-3,4-dihydroquinazoline (3cg):
Yield: 114 mg (0.55 mmol, 55%) off-white solid, mp: 133–135 °C (Literature data [48]), Rf 0.45 (dichloromethane/MeOH, 95:5). 1H NMR (400 MHz, Chloroform-d) δ 7.97 (d, J = 7.6 Hz, 2H), 7.53 (d, J = 7.9 Hz, 1H), 7.34 (t, J = 7.3 Hz, 1H), 7.30–7.26 (m, 2H), 7.20 (t, J = 7.6 Hz, 1H), 7.13 (d, J = 7.4 Hz, 1H), 6.94 (d, J = 7.5 Hz, 1H), 4.70 (s, 2H).
6-Bromo-2-ethyl-4-methyl-3,4-dihydroquinazoline (3dc):
Light yellow glassy oil, Rf 0.20 (EtOAc/EtOH, 4:1). Yield: 186 mg (0.74 mmol, 74%). 1H NMR (400 MHz, Chloroform-d) δ 7.23 (dd, J = 8.4, 2.3 Hz, 1H), 7.04 (d, J = 2.3 Hz, 1H), 6.89–6.85 (m, 1H), 5.07 (br. s, 1H), 4.68 (q, J = 6.5 Hz, 1H), 2.27 (q, J = 7.6 Hz, 2H), 1.39 (d, J = 6.5 Hz, 3H), 1.21 (t, J = 7.7 Hz, 3H). 13C NMR (101 MHz, Chloroform-d) δ 159.4, 140.5, 131.0, 128.1, 127.6, 124.1, 116.4, 48.7, 29.7, 25.8, 11.6. IR, vmax/cm−1: 3287, 2973, 1607, 1580, 1540, 1472, 1440, 1404, 1294, 1262, 1064,879. HRMS (ES TOF) calculated for (M + H)+ C11H14BrN2 253.0331, found 253.0335 (1.7 ppm).
4. Conclusions
In conclusion, a new protocol was developed, allowing for the assembly of 3,4-dihydroquinazolines from readily available 2-(aminomethyl)anilines and electrophilically activated nitroalkanes. Typically, reactions proceeding via nucleophilic attacks of nitronates with nucleophilic aniline moieties can be performed routinely. However, the featured transformation, involving the reaction of aliphatic amine groups, required careful optimization of the reaction conditions. It was found that switching to a mixture of 87% PPA and H3PO3 as a reaction medium allowed the transformation to be carried out efficiently, obtaining adequate to high yields of 3,4-dihydroquinazolines from a range of linear nitroalkanes (from nitromethane to 1-nitrohexane). Reaction with α-nitroacetophenone allowed for efficient preparation of phenyl-substituted derivatives. Evidently, the described method largely depends on availability and stability of the corresponding starting materials. Since nitroalkanes are activated at quite high temperatures and are used in the reaction as surrogates for much more available and reactive carboxylic acid derivatives, it is hard to expect that the method as is would be strongly competitive. This reaction might become invaluable, however, as a part of multi-step cascade transformations involving PPA as an “intelligent” reaction medium, which are being developed lately in our laboratories.
Author Contributions
A.V.A.—conceptualization, supervision, funding acquisition; I.Y.G.—methodology, investigation; N.A.A.—investigation, formal analysis; V.V.M.—investigation; D.A.A.—investigation; M.A.N.—formal analysis, writing (review and editing); M.R.—conceptualization, supervision, writing (original draft, review, and editing). All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Russian Foundation for Basic Research (grants #20-33-90027 and 19-03-00308a) and the Ministry of Education and Science (grant #0795-2020-0031).
Institutional Review Board Statement
Not applicable. This study did not involve human or animal subject.
Informed Consent Statement
Not applicable. This study did not involve human or animal subject.
Data Availability Statement
Supporting Information data include NMR spectral charts and are available.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Not applicable.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Jiao R.H., Xu S., Liu J.-Y., Ge H.M., Ding H., Xu C., Zhu A.H.L., Tan R.X. Chaetominine, a Cytotoxic Alkaloid Produced by Endophytic Chaetomium sp. IFB-E015. Org. Lett. 2006;8:5709–5712. doi: 10.1021/ol062257t. [DOI] [PubMed] [Google Scholar]
- 2.Li X.-J., Zhang Q., Zhang A.-L., Gao J.-M. Metabolites from Aspergillus fumigatus, an Endophytic Fungus Associated with Melia azedarach, and their Antifungal, Antifeedant, and Toxic Activities. J. Agric. Food Chem. 2012;60:3424–3431. doi: 10.1021/jf300146n. [DOI] [PubMed] [Google Scholar]
- 3.Tan C.-J., Di Y.-T., Wang Y.-H., Zhang Y., Si Y.-K., Zhang Q., Gao S., Hu X.-J., Fang X., Li S.-F., et al. Three New Indole Alkaloids from Trigonostemon lii. Org. Lett. 2010;12:2370–2373. doi: 10.1021/ol100715x. [DOI] [PubMed] [Google Scholar]
- 4.Buyck T., Wang Q., Zhu J. A Concise Total Synthesis of (±)-Trigonoliimine B. Org. Lett. 2012;14:1338–1341. doi: 10.1021/ol300249y. [DOI] [PubMed] [Google Scholar]
- 5.Feng P., Fan Y., Xue F., Liu W., Li S., Shi Y. An Approach to the Hexacyclic Skeleton of Trigonoliimines. Org. Lett. 2011;13:5827–5829. doi: 10.1021/ol202475r. [DOI] [PubMed] [Google Scholar]
- 6.Han S., Morrison K.C., Hergenrother P.J., Movassaghi M. Total Synthesis, Stereochemical Assignment, and Biological Activity of All Known (−)-Trigonoliimines. J. Org. Chem. 2013;79:473–486. doi: 10.1021/jo4020358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Han S., Movassaghi M. Concise Total Synthesis and Stereochemical Revision of all (−)-Trigonoliimines. J. Am. Chem. Soc. 2011;133:10768–10771. doi: 10.1021/ja204597k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao B., Hao X.-Y., Zhang J.-X., Liu S., Hao X.-J. Rapid Total Synthesis of (±)Trigonoliimine A via a Strecker/Houben–Hoesch Sequence. Org. Lett. 2013;15:528–530. doi: 10.1021/ol303344d. [DOI] [PubMed] [Google Scholar]
- 9.Beck H.P., Kohn T., Rubenstein S., Hedberg C., Schwandner R., Hasslinger K., Dai K., Li C., Liang L., Wesche H., et al. Discovery of potent LPA2 (EDG4) antagonists as potential anticancer agents. Bioorg. Med. Chem. Lett. 2008;18:1037–1041. doi: 10.1016/j.bmcl.2007.12.024. [DOI] [PubMed] [Google Scholar]
- 10.Bergman J., Brynolf A. Synthesis of Chrysogine, a Metabolite of Penicillium chrysogenum and some related 2-substituted 4-(3H)-Quinazolinones. Tetrahedron. 1990;46:1295–1310. doi: 10.1016/S0040-4020(01)86694-1. [DOI] [Google Scholar]
- 11.Matharu D.S., Flaherty D.P., Simpson D.S., Schroeder C.E., Chung D., Yan D., Noah J.W., Jonsson C.B., White E.L., Aubé J., et al. Optimization of Potent and Selective Quinazolinediones: Inhibitors of Respiratory Syncytial Virus That Block RNA-Dependent RNA-Polymerase Complex Activity. J. Med. Chem. 2014;57:10314–10328. doi: 10.1021/jm500902x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McAulay K., Hoyt E.A., Thomas M., Schimpl M., Bodnarchuk M.S., Lewis H.J., Barratt D., Bhavsar D., Robinson D.M., Deery M.J., et al. Alkynyl Benzoxazines and Dihydroquinazolines as Cysteine Targeting Covalent Warheads and their Application in Identification of Selective Irreversible Kinase Inhibitors. J. Am. Chem. Soc. 2020;142:10358–10372. doi: 10.1021/jacs.9b13391. [DOI] [PubMed] [Google Scholar]
- 13.Van Veghel D., Cleynhens J., Pearce L.V., DeAndrea-Lazarus I., Blumberg P.M., Van Laere K., Verbruggen A., Bormans G. New Transient Receptor Potential Vanilloid Subfamily Member 1 Positron Emission Tomography Radioligands: Synthesis, Radiolabeling, and Preclinical Evaluation. ACS Chem. Neurosci. 2013;4:624–634. doi: 10.1021/cn300233v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li C., An S., Zhu Y., Zhang J., Kang Y., Liu P., Wang Y., Li J. Copper-catalyzed intermolecular cyclization of nitriles and 2-aminobenzylamine for 3,4-dihydroquinazolines and quinazolines synthesis via cascade coupling and aerobic oxidation. RSC Adv. 2014;4:49888–49891. doi: 10.1039/C4RA09240F. [DOI] [Google Scholar]
- 15.Amini-Rentsch L., Vanoli E., Richard-Bildstein S., Marti R., Vilé G. A Novel and Efficient Continuous-Flow Route To Prepare Trifluoromethylated N-Fused Heterocycles for Drug Discovery and Pharmaceutical Manufacturing. Ind. Eng. Chem. Res. 2019;58:10164–10171. doi: 10.1021/acs.iecr.9b01906. [DOI] [Google Scholar]
- 16.Fan Z., Shi J., Luo N., Ding M., Bao X. Synthesis, Crystal Structure, and Agricultural Antimicrobial Evaluation of Novel Quinazoline Thioether Derivatives Incorporating the 1,2,4-Triazolo[4,3-a]pyridine Moiety. J. Agric. Food Chem. 2019;67:11598–11606. doi: 10.1021/acs.jafc.9b04733. [DOI] [PubMed] [Google Scholar]
- 17.Zhou H., Liu G.-S., Yao Z.-J. Short and Efficient Total Synthesis of Luotonin A and 22-Hydroxyacuminatine Using a Common Cascade Strategy. J. Org. Chem. 2007;72:6270–6272. doi: 10.1021/jo070837d. [DOI] [PubMed] [Google Scholar]
- 18.Reddy M.B., Prasanth K., Anandhan R. Visible-light induced copper(i)-catalyzed oxidative cyclization of o-aminobenzamides with methanol and ethanol via HAT. Org. Biomol. Chem. 2020;18:9601–9605. doi: 10.1039/D0OB02234A. [DOI] [PubMed] [Google Scholar]
- 19.Sen S., Hati S. Synthesis of Quinazolines and Dihydroquinazolines: O-Iodoxybenzoic Acid Mediated Tandem Reaction of o-Aminobenzylamine with Aldehydes. Synthesis. 2016;48:1389–1398. doi: 10.1055/s-0035-1560416. [DOI] [Google Scholar]
- 20.Wang G.-W., Miao C.-B., Kang H. Benign and Efficient Synthesis of 2-Substituted 4(3H)-Quinazolinones Mediated by Iron(III) Chloride Hexahydrate in Refluxing Water. Bull. Chem. Soc. Jpn. 2006;79:1426–1430. doi: 10.1246/bcsj.79.1426. [DOI] [Google Scholar]
- 21.Aksenov N.A., Aksenov A.V., Ovcharov S.N., Aksenov D.A., Rubin M. Electrophilically Activated Nitroalkanes in Reactions with Carbon Based Nucleophiles. Front. Chem. 2020;8:77. doi: 10.3389/fchem.2020.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aksenov A.V., Aksenov N.A., Orazova N.A., Aksenov D.A., Dmitriev M.V., Rubin M. Direct metal-free synthesis of diarylamines from 2-nitropropane via the twofold C–H functionalization of arenes. RSC Adv. 2015;5:84849–84855. doi: 10.1039/C5RA17668A. [DOI] [Google Scholar]
- 23.Aksenov N.A., Aksenov A.V., Nadein O.N., Aksenov D.A., Smirnov A.N., Rubin M. One-pot synthesis of benzoxazoles via the metal-free ortho-C–H functionalization of phenols with nitroalkanes. RSC Adv. 2015;5:71620–71626. doi: 10.1039/C5RA15128G. [DOI] [Google Scholar]
- 24.Aksenov A., Aksenov N., Nadein O., Aksenova I. Nitroethane in Polyphosphoric Acid: A New Reagent for Acetamidation and Amination of Aromatic Compounds. Synlett. 2010;2010:2628–2630. doi: 10.1055/s-0030-1258767. [DOI] [Google Scholar]
- 25.Aksenov A.V., Aksenov N.A., Arutiunov N.A., Malyuga V.V., Ovcharov S.N., Rubin M. Electrophilically activated nitroalkanes in reaction with aliphatic diamines en route to imidazolines. RSC Adv. 2019;9:39458–39465. doi: 10.1039/C9RA08630G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aksenov D.A., Arutiunov N.A., Maliuga V.V., Aksenov A.V., Rubin M. Synthesis of imidazo[1,5-a]pyridines via cyclocondensation of 2-(aminomethyl)pyridines with electrophilically activated nitroalkanes. Beilstein J. Org. Chem. 2020;16:2903–2910. doi: 10.3762/bjoc.16.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aksenov N.A., Malyuga V.V., Abakarov G.M., Voskressensky L.G., Aksenov A., Aksenov D.A. Synthesis of 3,4-dihydroisoquinolines using nitroalkanes in polyphosphoric acid. Russ. Chem. Bull. 2019;68:1047–1051. doi: 10.1007/s11172-019-2518-z. [DOI] [Google Scholar]
- 28.Aksenov A.V., Smirnov A.N., Aksenov N., Bijieva A.S., Aksenova I., Rubin M. Benzimidazoles and benzoxazoles via the nucleophilic addition of anilines to nitroalkanes. Org. Biomol. Chem. 2015;13:4289–4295. doi: 10.1039/C5OB00131E. [DOI] [PubMed] [Google Scholar]
- 29.Aksenov A.V., Aksenov N.A., Ovcharov D.S., Aksenov D.A., Griaznov G., Voskressensky L., Rubin M. Rational design of an efficient one-pot synthesis of 6H-pyrrolo[2,3,4-gh]perimidines in polyphosphoric acid. RSC Adv. 2016;6:82425–82431. doi: 10.1039/C6RA17269E. [DOI] [Google Scholar]
- 30.Aksenov A.V., Ovcharov D.S., Aksenov N.A., Aksenov D.A., Nadein O.N., Rubin M. Dual role of polyphosphoric acid-activated nitroalkanes in oxidative peri-annulations: Efficient synthesis of 1,3,6,8-tetraazapyrenes. RSC Adv. 2017;7:29927–29932. doi: 10.1039/C7RA04751G. [DOI] [Google Scholar]
- 31.Aksenov A.V., Aksenov N.A., Ovcharov D.S., Shcherbakov S.V., Smirnova A.N., Aksenova I.V., Goncharov V.I., Rubin M.A. Electrophilically activated nitroalkanes in the synthesis of 6,7-dihydro-1H-cyclopenta[g]perimidines. Russ. J. Org. Chem. 2017;53:1081–1084. doi: 10.1134/S107042801707020X. [DOI] [Google Scholar]
- 32.Aksenov N.A., Arutiunov N.A., Kirillov N.K., Aksenov D.A., Aksenov A.V., Rubin M. Preparation of 1,3,4-oxadiazoles and 1,3,4-thiadiazoles via chemoselective cyclocondensation of electrophilically activated nitroalkanes to (thio)semicarbazides or thiohydrazides. Chem. Heterocycl. Compd. 2020;56:1–6. doi: 10.1007/s10593-020-02775-5. [DOI] [Google Scholar]
- 33.Aksenov A.V., Khamraev V., Aksenov N.A., Kirilov N.K., Domenyuk D.A., Zelensky V.A., Rubin M. Electrophilic activation of nitroalkanes in efficient synthesis of 1,3,4-oxadiazoles. RSC Adv. 2019;9:6636–6642. doi: 10.1039/C9RA00976K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nef J.U. Ueber die Constitution der Salze der Nitroparaffine. Justus Liebigs Ann. Chem. 1894;280:263–291. doi: 10.1002/jlac.18942800209. [DOI] [Google Scholar]
- 35.Ballini R., Petrini M. Recent synthetic developments in the nitro to carbonyl conversion (Nef reaction) Tetrahedron. 2004;60:1017–1047. doi: 10.1016/j.tet.2003.11.016. [DOI] [Google Scholar]
- 36.Ballini R., Petrini M. The Nitro to Carbonyl Conversion (Nef Reaction): New Perspectives for a Classical Transformation. Adv. Synth. Catal. 2015;357:2371–2402. doi: 10.1002/adsc.201500008. [DOI] [Google Scholar]
- 37.Aksenov N.A., Aksenov A.V., Kirilov N.K., Arutiunov N.A., Aksenov D.A., Maslivetc V., Zhao Z., Du L., Rubin M., Kornienko A. Nitroalkanes as electrophiles: Synthesis of triazole-fused heterocycles with neuroblastoma differentiation activity. Org. Biomol. Chem. 2020;18:6651–6664. doi: 10.1039/D0OB01007C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang G., Zhao Y., Xuan L., Ding C. SO2F2-Activated Efficient Beckmann Rearrangement of Ketoximes for Accessing Amides and Lactams. Eur. J. Org. Chem. 2019;2019:4911–4915. doi: 10.1002/ejoc.201900844. [DOI] [Google Scholar]
- 39.Scheinbaum M.L. Addition of dinitrogen trioxide to nonconjugated dienes. J. Org. Chem. 1970;35:2785–2790. doi: 10.1021/jo00833a069. [DOI] [Google Scholar]
- 40.Pearson D.E., Cole W.E. The Beckmann Rearrangement. V. The Rearrangement Rates of Someortho-Substituted Acetophenone Oximes (1) J. Org. Chem. 1955;20:488–493. doi: 10.1021/jo01122a011. [DOI] [Google Scholar]
- 41.Kano S., Tanaka Y., Sugino E., Hibino S. Reduction of Some Functional Groups with Titanium(IV) Chloride/Sodium Borohydride. Synthesis. 1980;1980:695–697. doi: 10.1055/s-1980-29174. [DOI] [Google Scholar]
- 42.Bischler A. Zur Kenntniss der Phenmiazinderivate. Ber. Dtsch. Chem. Ges. 1893;26:1891–1903. doi: 10.1002/cber.189302602142. [DOI] [Google Scholar]
- 43.Aneja B., Irfan M., Kapil C., Jairajpuri M.A., Maguire R., Kavanagh K., Rizvi M., Manzoor N., Azam A., Abid M. Effect of novel triazole–amino acid hybrids on growth and virulence of Candida species: In vitro and in vivo studies. Org. Biomol. Chem. 2016;14:10599–10619. doi: 10.1039/C6OB01718E. [DOI] [PubMed] [Google Scholar]
- 44.Alcaide B., Almendros P., Busto E., Herrera F., Lázaro-Milla C., Luna A. Photopromoted Entry to Benzothiophenes, Benzoselenophenes, 3H-Indoles, Isocoumarins, Benzosultams, and (Thio)flavones by Gold-Catalyzed Arylative Heterocyclization of Alkynes. Adv. Synth. Catal. 2017;359:2640–2652. doi: 10.1002/adsc.201700427. [DOI] [Google Scholar]
- 45.Roman D.S., Takahashi Y., Charette A.B. Potassiumtert-Butoxide Promoted Intramolecular Arylation via a Radical Pathway. Org. Lett. 2011;13:3242–3245. doi: 10.1021/ol201160s. [DOI] [PubMed] [Google Scholar]
- 46.Kurosu M., Lin M.-H., Kishi Y. Fe/Cr- and Co/Cr-Mediated Catalytic Asymmetric 2-Haloallylations of Aldehydes. J. Am. Chem. Soc. 2004;126:12248–12249. doi: 10.1021/ja045557j. [DOI] [PubMed] [Google Scholar]
- 47.Cao S.-L., Han Y., Yuan C.-Z., Wang Y., Xiahou Z.-K., Liao J., Gao R.-T., Mao B.-B., Zhao B.-L., Li Z.-F., et al. Synthesis and antiproliferative activity of 4-substituted-piperazine-1-carbodithioate derivatives of 2,4-diaminoquinazoline. Eur. J. Med. Chem. 2013;64:401–409. doi: 10.1016/j.ejmech.2013.04.017. [DOI] [PubMed] [Google Scholar]
- 48.Chatterjee T., Kim D.I., Cho E.J. Base-Promoted Synthesis of 2-Aryl Quinazolines from 2-Aminobenzylamines in Water. J. Org. Chem. 2018;83:7423–7430. doi: 10.1021/acs.joc.8b00327. [DOI] [PubMed] [Google Scholar]
- 49.Wiley R.H., Wakefield B.J. Infrared Spectra of the Nitrile N-Oxides: Some New Furoxans. J. Org. Chem. 1960;25:546–551. doi: 10.1021/jo01074a014. [DOI] [Google Scholar]
- 50.Grosso J.A., Nichols D.E., Nichols M.B., Yim G.K.W. Synthesis and adrenergic blocking effects of 2-(alkylamino)-3,4-dihydroquinazolines. J. Med. Chem. 1980;23:1261–1264. doi: 10.1021/jm00185a026. [DOI] [PubMed] [Google Scholar]
- 51.Francesconi L.C., Yang Y.Y., Kung M.-P., Zhang X.X., Billings J.J., Guo Y.-Z., Kung H.F. Technetium-99 m N,N′-Bis(2-mercapto-2-methylpropyl)-2-aminobenzylamine: Technetium-99 m Complexes of a Novel Bis(aminoethanethiol) Ligand. J. Med. Chem. 1994;37:3282–3288. doi: 10.1021/jm00046a012. [DOI] [PubMed] [Google Scholar]
- 52.Kempter G., Ziegner H.J. Heterocycles from aminoketones. XVII. Quinazolines from 1,4-diamines. Z. Chem. 1971;11:12–13. doi: 10.1002/zfch.19710110108. [DOI] [Google Scholar]
- 53.Richards J.E., Hooper A.J.J., Bayfield O.W., Cockett M.C.R., Dear G.J., Holmes A.J., John R.O., Mewis R.E., Pridmore N., Roberts A.D., et al. Using hyperpolarised NMR and DFT to rationalise the unexpected hydrogenation of quinazoline to 3,4-dihydroquinazoline. Chem. Commun. 2018;54:10375–10378. doi: 10.1039/C8CC04826F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ried W., Stahlhofen P. Über heterocyclische Siebenringsysteme, IV. Mitteil.: Synthesen und Eigenschaften von 4.5-Benzo-[hept-1.2.6-oxdiazinen] Chem. Ber. 1954;87:1814–1824. doi: 10.1002/cber.19540871204. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Supporting Information data include NMR spectral charts and are available.