Abstract
This cross-sectional study aimed to investigate the association between hyperuricemia and the frequency of coffee, tea, and soft drink consumption, based on data from the Korean Genome and Epidemiology Study (KoGES) (2004–2016). We used the KoGES health examinee data, obtained from urban residents aged ≥ 40 years. Information on the participants’ medical history, nutrition (total calorie, protein, fat, and carbohydrate intake), frequency of alcohol consumption, smoking status, household income, and frequency of coffee/green tea/soft drink intake was collected. A logistic regression model was used to analyze the data. Subgroup analyses were performed according to the participant’s age and sex. Among 173,209 participants, there were 11,750 and 156,002 individuals with hyperuricemia and non-hyperuricemia controls, respectively. In an adjusted model, frequent coffee and green tea consumption did not increase the risk of hyperuricemia, compared to the “no intake” reference group. However, an adjusted odds ratio of hyperuricemia was 1.23 (95% confidence interval, 1.11–1.35, p < 0.001) for participants who reported consuming soft drinks ≥ 3 times per day, compared to the respective “no drink” reference group. Even after adjusting for nutritional and sociodemographic factors, frequent soft drink intake was associated with an increased risk of hyperuricemia. Meanwhile, neither coffee nor green tea intake was associated with an increased risk of hyperuricemia.
Keywords: hyperuricemia, soft drink intake, nutrition, cross-sectional study
1. Introduction
Hyperuricemia is the main risk factor for gout and plays a major role in its pathogenesis [1]. Gout is a common form of inflammatory arthritis, characterized by sudden and severe pain, and swelling of the affected joint [2], impairing patients’ quality of life [3]. In addition to being a risk factor for inflammatory arthritis, elevated levels of serum uric acid are associated with an increased risk of cardiovascular disorders [4]. According to the National Health and Nutrition Examination Survey, 21% of Northern America’s adult population aged > 20 years are affected by hyperuricemia [5]. In Korea, the age-standardized prevalence of hyperuricemia has been estimated at 11.4% [6]. Hyperuricemia is prevalent worldwide, and the number of people affected by it has increased over the past two decades [7].
Various factors affect serum uric acid concentration, including dietary habits, and the diagnosis of obesity and metabolic syndrome [8]. Studies have shown that a high intake of purine-rich food (for example bacon, meat, and pork) and beer can increase the risk of hyperuricemia [9,10]. Meanwhile, the intake of dairy products (for example milk and yogurt) has been shown to reduce serum uric acid level [10,11]. Low-calorie intake and a diet that involves restricted carbohydrate consumption can also induce hyperuricemia [12,13]. This evidence suggests that achieving a nutritional balance (including in total calorie, protein, fat, and carbohydrate intake) is paramount before commencing further investigations into the causes of hyperuricemia.
Tea and coffee are among the most commonly consumed beverage types worldwide [14,15], while the consumption of soft drinks continues to increase [16]. Recent studies have reported an association between serum uric acid level and the intake of coffee, tea, and soft drinks [17,18,19]. It has been hypothesized that the fructose present in soft drinks may increase the risk of hyperuricemia [18]; however, the exact mechanism through which these beverages may induce hyperuricemia remains unclear.
Thus, in the present study, we aimed to investigate the association between hyperuricemia and the frequency of coffee, tea, and soft drink consumption. We adjusted the nutritional and sociodemographic factors in a cross-sectional design, using population-based data. The secondary aim of this study was to estimate the risk of hyperuricemia, stratified by age and sex.
2. Materials and Methods
The present study protocol was approved by the ethics committee of Hallym University (No. 2019-02-020); the requirement for informed consent was waived. This cross-sectional study was based on the Korean Genome and Epidemiology Study (KoGES) dataset (2004–2016), details of which have been previously reported [20]. From the KoGES Consortium, we extracted the health examinee (HEXA) data, obtained from urban residents aged ≥ 40 years. Baseline data and follow-up data were acquired during 2004–2013 and 2012–2016, respectively.
2.1. Participant Selection
Of 173,209 participants, we excluded participants with missing data on height or weight (n = 698); smoking history (n = 494); alcohol drinking habits (n = 1463); nutritional status (n = 1977); history of hypertension, diabetes mellitus, or hyperlipidemia (n = 125); serum uric acid levels (n = 46); coffee, green tea, or soft drink intake (n = 654). The final sample included 11,750 and 156,002 participants with hyperuricemia and non-hyperuricemia controls, respectively (Figure 1). The frequency of coffee, green tea, and soft drink consumption was investigated.
Figure 1.
The selection of the study’s participants. A total of 11,750 participants with hyperuricemia and 156,002 participants with non-hyperuricemia controls were analyzed.
2.2. Survey
Participant history of hypertension, diabetes mellitus, and hyperlipidemia was acquired during face-to-face interviews, conducted by expert interviewers. Although hyperuricemia does not have a consensus definition based on the level of serum uric acid, the present study defined hyperuricemia as serum uric acid levels of >7.0 mg/dL in men and of >6.0 mg/dL in women, based on the previous literature [5,21]. Body mass index (BMI) was defined as weight over height-squared (kg/m2), calculated based on health checkup data. Smoking history was classified as non-smoker (<100 cigarettes during lifetime), past smoker (ceased smoking > 1 year before study enrollment), and current smoker. Alcohol intake was categorized as nondrinker, past drinker, and current drinker. Nutritional intake (total calories [kcal/day], protein [g/day], fat [g/day], and carbohydrate [g/day]) was assessed based on a food-frequency questionnaire, which has been previously described and validated [22]. Household income was categorized as follows: undisclosed low income (up to $2000 per month), middle income ($2000–3999 per month), and high income (≥$4000 per month).
The frequency of coffee, green tea, and soft drink intake was classified as “never,” 1 time per month, 2–3 times per month, 1–2 times per week, 3–4 times per week, 5–6 times per week, 1–2 times per day, 3–4 time per day, and ≥5 times per day. Given the distribution of these categories in the study sample, we reclassified them into four groups as follows: never, 1 time a month through 6 times a week, 1–2 times a day, and ≥3 times a day.
2.3. Statistical Analyses
Chi-square tests were used to compare the rate of sex, income group, smoking, drinking and alcohol consumption, and the frequency and amount of coffee/green tea/soft drink consumption. To compare the age, BMI, and nutritional intake, an independent t-test was applied.
To investigate the impact of coffee, green tea, and soft drink intake on the risk of hyperuricemia, we computed three models: a crude, model 1 (adjusted for age, sex, BMI, income, smoking, past medical histories, alcohol consumption, and nutritional intake [total calories, protein, fat, carbohydrate intake]), and model 2 (fully-adjusted model, including variables from model l as well as independent variables, such as the frequency of coffee, green tea, and soft drink consumption). Effect estimates were expressed as odds ratios (OR).
In age-based subgroup analyses, the data were dichotomized at the median value (≤52 vs. ≥53 years).
Two-tailed analyses were performed, and p-values of <0.05 were considered indicative of statistical significance. Statistical analysis was performed using SPSS v. 24.0 (IBM Corp. IBM SPSS Statistics for Windows, Version 24.0, Armonk, NY, USA).
3. Results
Participant characteristics are presented in Table 1. The participants in the hyperuricemia group were older than the participants in the control group, and their BMI was higher than that of the control group. The proportion of men was significantly higher in the hyperuricemia group than in the control group. There were between-group differences in household income, smoking status, and alcohol consumption. The rate of hypertension, diabetes mellitus, and hyperlipidemia was significantly higher in the hyperuricemia group than in the control group. Nutritional status, including total calorie, protein, fat, and carbohydrate intake was significantly higher in the hyperuricemia group than in the control group. There were significant between-group differences in the frequency of coffee, tea, and soft drink intake.
Table 1.
General Characteristics of Participants.
| Characteristics | Total Participants | p-Value | ||
|---|---|---|---|---|
| Total | Hyperuricemia | Control | ||
| Total (n) | 167,752 (100.0) | 11,750 (100.0) | 156,002 (100.0) | |
| Age (mean, SD, y) | 53.1 (8.3) | 54.9 (8.7) | 53.0 (8.3) | <0.001 * |
| Sex (n, %) | <0.001 * | |||
| Men | 57,596 (34.3) | 7757 (66.0) | 49,839 (31.9) | |
| Women | 110,156 (65.7) | 3993 (34.0) | 106,163 (68.1) | |
| BMI (mean, SD, kg/m2) | 23.9 (2.9) | 25.5 (3.0) | 23.8 (2.9) | <0.001 * |
| Income (n, %) | <0.001 * | |||
| Missing, no response | 26,423 (15.8) | 2069 (17.6) | 24,354 (15.6) | |
| Lowest | 45,908 (27.4) | 3231 (27.5) | 42,677 (27.4) | |
| Middle | 60,465 (36.0) | 4014 (34.2) | 56,451 (36.2) | |
| Highest | 34,956 (20.8) | 2436 (20.7) | 32,520 (20.8) | |
| Smoking status (n, %) | <0.001 * | |||
| Nonsmoker | 122,197 (72.8) | 5755 (49.0) | 116,442 (74.6) | |
| Past smoker | 24,651 (14.7) | 3499 (29.8) | 21,152 (13.6) | |
| Current smoker | 20,904 (12.5) | 2496 (21.2) | 18,408 (11.8) | |
| Alcohol consumption (n, %) | <0.001 * | |||
| Non drinker | 85,200 (50.8) | 3988 (33.9) | 81,212 (52.1) | |
| Past drinker | 6607 (3.9) | 732 (6.2) | 5875 (3.8) | |
| Current drinker | 75,945 (45.3) | 7030 (59.8) | 68,915 (44.2) | |
| Hypertension | 37,362 (22.3) | 4757 (40.5) | 32,605 (20.9) | <0.001 * |
| Diabetes mellitus | 13,243 (7.9) | 1237 (10.5) | 12,006 (7.7) | <0.001 * |
| Hyperlipidemia | 22,122 (13.2) | 2027 (17.3) | 20,095 (12.9) | <0.001 * |
| Nutritional intake | ||||
| Total calories (kcal/d) | 1758.6 (590.1) | 1786.4 (595.9) | 1756.5 (589.6) | <0.001 * |
| Protein (g/d) | 59.9 (27.1) | 61.1 (27.6) | 59.8 (27.1) | <0.001 * |
| Fat (g/d) | 28.2 (18.7) | 29.0 (19.0) | 28.1 (18.6) | <0.001 * |
| Carbohydrate (g/d) | 312.2 (96.5) | 315.5 (96.6) | 312.0 (96.5) | <0.001 * |
| Frequency of coffee | <0.001 * | |||
| No drink | 71,674 (16.7) | 1744 (14.8) | 26,327 (16.9) | |
| 1 time (m) though 6 times (w) | 27,483 (21.6) | 2438 (20.7) | 33,745 (21.6) | |
| 1–2 times (d) | 47,595 (42.2) | 4877 (41.5) | 65,876 (42.2) | |
| ≥3 times (d) | 21,000 (19.5) | 2691 (22.9) | 30,054 (19.3) | |
| Frequency of tea | <0.001 * | |||
| No drink | 71,674 (42.7) | 4680 (39.8) | 66,994 (42.9) | |
| 1 time (m) though 6 times (w) | 27,483 (16.4) | 1846 (15.7) | 25,637 (16.4) | |
| 1–2 times (d) | 47,595 (28.4) | 3391 (28.9) | 44,204 (28.3) | |
| ≥3 times (d) | 21,000 (12.5) | 1833 (15.6) | 19,167 (12.3) | |
| Frequency of soda drink | <0.001 * | |||
| No drink | 66,392 (39.6) | 4534 (38.6) | 61,858 (39.7) | |
| 1 time (m) though 6 times (w) | 72,468 (43.2) | 4921 (41.9) | 67,547 (43.3) | |
| 1–2 times (d) | 22,641 (13.5) | 1744 (14.8) | 20,897 (13.4) | |
| ≥3 times (d) | 6251 (3.7) | 551 (4.7) | 5700 (3.7) | |
d: day; w: week; m: month; * Independent t-test or Chi-square test. Significance at p < 0.05.
Table 2 presents the crude and adjusted ORs of hyperuricemia, based on the frequency of coffee, green tea, and soft drink intake. In model 1, the adjusted OR of hyperuricemia was 1.09 (95% confidence interval [CI], 1.02–1.16, p = 0.008) for participants who consumed soft drinks 1–2 times per day; it was 1.25 (95% CI, 1.13–1.37, p < 0.001) for participants who consumed soft drinks ≥ 3 times per day compared to the reference group of “never” consumption (Table 2). In model 2, the adjusted OR of hyperuricemia was 1.07 (95% CI, 1.01–1.14, p = 0.028) in participants who consumed soft drinks 1–2 times per day; it was 1.23 (95% CI, 1.11–1.35, p < 0.001) in participants who consumed soft drinks ≥ 3 times per day, compared to the reference group of “never”.
Table 2.
Crude and adjusted odd ratios (95% confidence interval) of coffee, green tea, and soft drink intake (frequency) for hyperuricemia.
| Characteristics | Odd Ratios for Hyperuricemia | |||||
|---|---|---|---|---|---|---|
| Crude | p-Value | Model 1 † | p-Value | Model 2 ‡ | p-Value | |
| Total participants (n = 167,752) | ||||||
| Coffee | ||||||
| No drink | 1.00 | 1.00 | 1.00 | |||
| 1 time (m) though 6 times (w) | 1.09 (1.02–1.16) | 0.007 * | 0.95 (0.89–1.02) | 0.154 | 0.99 (0.91–1.07) | 0.788 |
| 1–2 times (d) | 1.12 (1.06–1.18) | <0.001 * | 0.97 (0.91–1.03) | 0.279 | 0.98 (0.91–1.05) | 0.509 |
| ≥3 times (d) | 1.35 (1.27–1.44) | <0.001 * | 0.95 (0.88–1.01) | 0.105 | 0.95 (0.87–1.04) | 0.251 |
| Green tea | ||||||
| No drink | 1.00 | 1.00 | 1.00 | |||
| 1 time (m) though 6 times (w) | 1.03 (0.98–1.09) | 0.287 | 0.95 (0.89–1.00) | 0.063 | 0.95 (0.88–1.02) | 0.161 |
| 1–2 times (d) | 1.10 (1.05–1.15) | <0.001 * | 0.98 (0.93–1.03) | 0.407 | 0.99 (0.93–1.05) | 0.665 |
| ≥3 times (d) | 1.37 (1.29–1.45) | <0.001 * | 0.96 (0.90–1.02) | 0.204 | 0.99 (0.91–1.08) | 0.809 |
| Soft drink | ||||||
| No drink | 1.00 | 1.00 | 1.00 | |||
| 1 time (m) though 6 times (w) | 0.99 (0.95–1.04) | 0.776 | 1.02 (0.98–1.06) | 0.412 | 1.01 (0.97–1.06) | 0.615 |
| 1–2 times (d) | 1.14 (1.08–1.21) | <0.001 * | 1.09 (1.02–1.16) | 0.008 * | 1.07 (1.01–1.14) | 0.028 * |
| ≥3 times (d) | 1.32 (1.20–1.45) | <0.001 * | 1.25 (1.13–1.37) | <0.001 * | 1.23 (1.11–1.35) | <0.001 * |
* Logistic regression model, Significance at p < 0.05; † Model 1 was adjusted for age, sex, BMI, income, past medical histories, smoking, alcohol consumption, and nutritional intake; ‡ Model 2 was adjusted for model 1 plus frequency of coffee, green tea, and soft drinks.
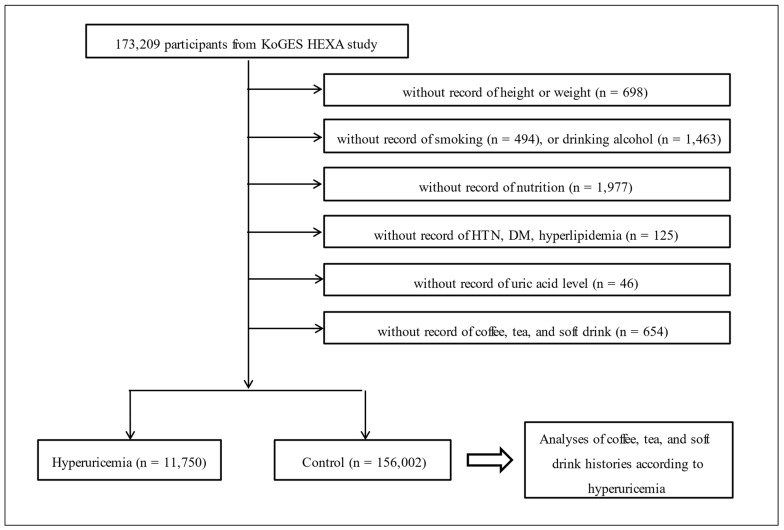
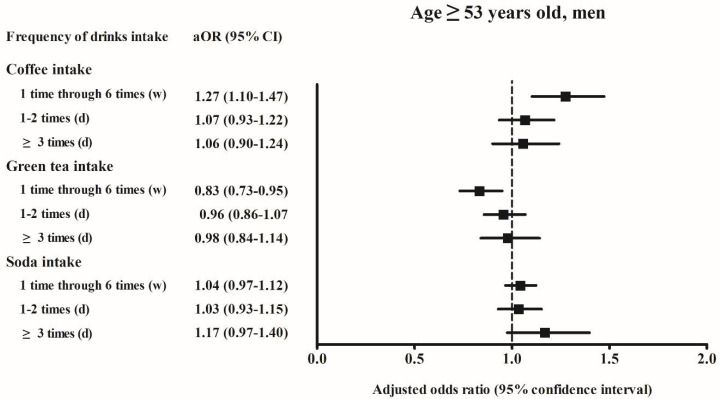
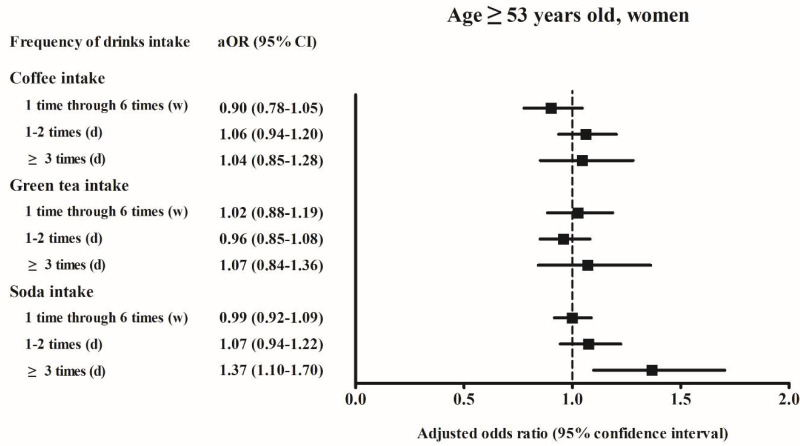
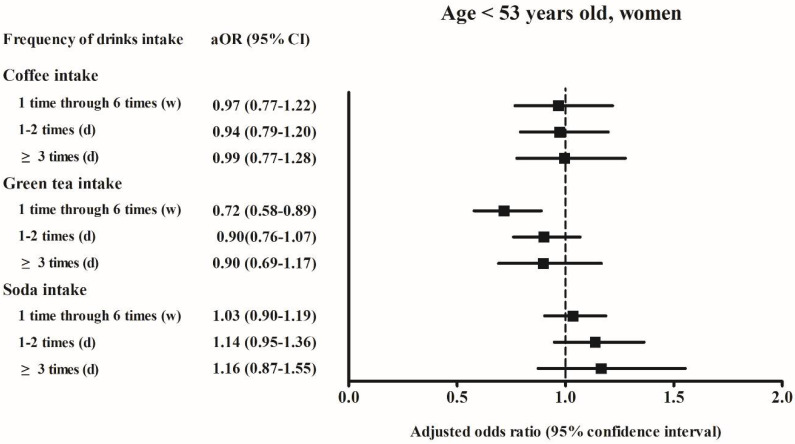
Among men aged < 53 years who consumed soft drinks ≥ 3 times per day, the adjusted OR of hyperuricemia was 1.26 (95% CI, 1.08–1.48, p = 0.004) compared to the reference group of “never”. Among women aged ≥ 53 years who consumed soft drinks ≥ 3 times per day, the adjusted OR of hyperuricemia was 1.37 (95% CI, 1.10–1.70, p = 0.005) compared to the reference group of “never” (Figure 2, Figure 3, Figure 4 and Figure 5).
Figure 2.
The adjusted odds ratio of the hyperuricemia according to the frequency of coffee/green tea/soda (soft drink) intake in the age ≥ 53 years old men. Fully-adjusted model (age, BMI, income, past medical histories, smoking, alcohol consumption, nutritional intake, frequency of coffee, green tea, and soft drink intake) was used.
Figure 3.
The adjusted odds ratio of the hyperuricemia according to the frequency of coffee/green tea/soda (soft drink) intake in the age ≥ 53 years old women. Fully-adjusted model (age, BMI, income, past medical histories, smoking, alcohol consumption, nutritional intake, frequency of coffee, green tea, and soft drink intake) was used.
Figure 4.
The adjusted odds ratio of the hyperuricemia according to the frequency of coffee/green tea/soda (soft drink) intake in the age < 53 years-old men. Fully-adjusted model (age, BMI, income, past medical histories, smoking, alcohol consumption, nutritional intake, frequency of coffee, green tea, and soft drink intake) was used.
Figure 5.
The adjusted odds ratio of the hyperuricemia according to the frequency of coffee/green tea/soda (soft drink) intake in the age < 53 years old women. Fully-adjusted model (age, BMI, income, past medical histories, smoking, alcohol consumption, nutritional intake, frequency of coffee, green tea, and soft drink intake) was used.
4. Discussion
In the present study, participants who reported frequent consumption of soft drinks were at increased risk of hyperuricemia compared to their counterparts who declared “never” in their consumption; this association remained after adjustments for sociodemographic and nutritional factors. Adjusting in relation to nutritional factors is paramount in this type of study, as nutrition can affect the level of serum uric acid. Previous studies on this topic have reported findings based on similar adjustments [18,23]. In their reports, the impact of total calorie, protein, alcohol, meat, seafood, and dairy product intake were considered [18,23]. However, our study involved adjustments for the intake of all three major nutrients (carbohydrate, fat, and protein), reported in grams per day, which is rare; nevertheless, this approach ensures the accuracy of analysis and ease of comparison between the present findings and those of previous studies.
Choi et al. reported in their prospective study that an intake of sugar-sweetened soft drinks was strongly related to the elevated risk of gout in men [24]. In their report, the relative risk of gout was 1.85 (95% CI, 1.08–3.16) in men who consumed sugar-sweetened soft drinks > 2 times per day, compared to men who consumed them < 1 time per month. Interestingly, intake of “diet” soft drinks (for example, low-calorie or caffeine-free Coca-Cola, or other types of low-calorie beverages) did not affect the risk of gout in that study. Another report from the Korean population demonstrated that increased soft drink intake was associated with the increased risk of hyperuricemia in men [25]. In their report, the adjusted OR of hyperuricemia was 1.35 (95% CI, 1.07–1.71) in the higher intake group (Q5), compared to the lower intake group (Q1–Q3). Serum uric acid level can be affected by the boosted uric acid production via fructose metabolism. Throughout the fructose metabolism in the cell after fructose uptake, adenosine triphosphate (ATP) depletion induces adenosine monophosphate (AMP) accumulation and AMP deaminase stimulation, leading to the production of uric acid in the cell [26].
In our study, men aged < 53 years and women aged ≥ 53 years who consumed soft drinks ≥ 3 times per day were at increased risk of hyperuricemia (Figure 2 and Figure 3). A previous study based on the national health and nutrition examination survey data reported that the increase in serum uric acid levels related to soft drink consumption was significantly larger among men than it was among women [18]. In an animal study based on rats, female sex hormones have shown to have a protective effect against hyperinsulinemia associated with fructose consumption [27]. In a separate study, hyperinsulinemia has been shown to decrease the rate of renal excretion of uric acid and correlate with elevated levels of serum uric acid [28]. In our study, women aged ≥ 53 years might have had lower estrogen levels than those observed among women aged < 53 years. The present study findings support the hypothesis that estrogen may have a protective effect against the development of hyperuricemia.
Although a previous study reported that men aged > 50 years with dietary fructose intake are at increased risk of hyperuricemia [29], the reason for the significant association between soft drink consumption and hyperuricemia risk observed in men aged < 53 years in the present study remains unclear. Potentially confounding factors, such as lack of exercise routine in the middle-aged men [30] and emotional stress in the workplace [31], may affect the final results in a relatively young male group. Therefore, further studies are required to elucidate these associations.
A few studies have reported on the association between coffee consumption and serum uric acid levels. However, their findings were inconsistent. One recent meta-analysis demonstrated a lack of evidence for an association between coffee consumption and reduced serum uric acid levels [17]. Nevertheless, a previous study has reported on the protective effect of coffee consumption against hyperuricemia risk [32]. In the present study, frequent coffee intake was not significantly associated with hyperuricemia risk (Table 2). However, further studies are required to clarify this association.
In the present study, green tea consumption was not significantly associated with hyperuricemia risk. Previous studies in animals have shown that ingredients present in green tea may decrease serum uric acid levels [33,34]. Although a similar finding might have been expected in humans, previous studies have been inconclusive [35,36]. Consistent with our results, a recent meta-analysis has demonstrated lack of evidence that tea consumption is associated with serum uric acid level [19]. Although our results support this overall finding, further studies are needed before conclusions can be drawn.
The present study has several limitations. Firstly, we did not evaluate the exact amount of coffee, green tea, and soft drinks consumed, as the data were collected with a questionnaire that used frequency categories rather than volume estimates. Secondly, the influence of medication use on serum uric acid levels was not evaluated. Many types of medication, including anti-tubercular drugs, low-dose aspirin, chemotherapy drugs, and diuretics have been reported to increase the level of serum uric acid [37]. Aspirin is widely available in Korea without a prescription, therefore the amount of aspirin consumed cannot be estimated based on medical records.
Notwithstanding these weaknesses, this study has several strengths. Firstly, this study included a large sample size. The HEXA dataset included a large number of participants with hyperuricemia. To the best of our knowledge, this is the largest study of its kind done to-date. Secondly, this study accounted for candidate confounders, including nutritional intake and alcohol consumption. In addition, we applied model 2 (in Table 2) to our analysis in order to minimize the mutual influences of drinking habits. These aspects of the study design helped to ensure the validity of the presented findings.
5. Conclusions
This study has shown that frequent soft drink consumption is associated with an increased risk of hyperuricemia. Subgroup analysis revealed that this effect particularly affects men aged < 53 years and women aged ≥ 53 years. Further studies are required to fully elucidate the association between soft drink intake and the risk of hyperuricemia, including the underlying mechanism.
Acknowledgments
Data in this study were from the Korean Genome and Epidemiology Study (KoGES; 4851-302), National Research Institute of Health, Centers for Disease Control and Prevention, Ministry for Health and Welfare, Korea.
Author Contributions
Conceptualization, H.G.C. and J.S.L.; Methodology, J.H.W. and S.K.H.; Formal analysis, H.G.C., C.M. and D.M.Y.; Investigation, H.G.C.; writing-original draft preparation, T.J.K. and J.S.L.; writing-review and editing, T.J.K., J.S.L. and H.G.C.; supervision, H.G.C.; funding acquisition, H.G.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported in part by a research grant (NRF-2018-R1D1A1A0-2085328, NRF-2021-R1C1C100498611) from the National Research Foundation (NRF) of Korea, and Hallym University Research Fund (HURF 2019-31).
Institutional Review Board Statement
The present study protocol was approved by the ethics committee of Hallym University (No. 2019-02-020).
Informed Consent Statement
The requirement for informed consent was waived.
Data Availability Statement
Restrictions apply to the availability of these data. Data was obtained from Korean Genome and Epidemiologiy Study [KoGES] and are available at [www.kdca.go.kr] (accessed on 23 May 2021), with the permission of [KoGES].
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Schumacher H.R., Jr. The pathogenesis of gout. Clevel. Clin. J. Med. 2008;75:S2–S4. doi: 10.3949/ccjm.75.Suppl_5.S2. [DOI] [PubMed] [Google Scholar]
- 2.Underwood M. Diagnosis and management of gout. BMJ. 2006;332:1315–1319. doi: 10.1136/bmj.332.7553.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roddy E., Zhang W., Doherty M. Is gout associated with reduced quality of life? A case-control study. Rheumatology. 2007;46:1441–1444. doi: 10.1093/rheumatology/kem150. [DOI] [PubMed] [Google Scholar]
- 4.Grassi D., Ferri L., Desideri G., Di Giosia P., Cheli P., Del Pinto R., Properzi G., Ferri C. Chronic Hyperuricemia, Uric Acid Deposit and Cardiovascular Risk. Curr. Pharm. Des. 2013;19:2432–2438. doi: 10.2174/1381612811319130011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhu Y., Pandya B.J., Choi H.K. Prevalence of gout and hyperuricemia in the US general population: The National Health and Nutrition Examination Survey 2007–2008. Arthritis Rheum. 2011;63:3136–3141. doi: 10.1002/art.30520. [DOI] [PubMed] [Google Scholar]
- 6.Kim Y., Kang J., Kim G.-T. Prevalence of hyperuricemia and its associated factors in the general Korean population: An analysis of a population-based nationally representative sample. Clin. Rheumatol. 2018;37:2529–2538. doi: 10.1007/s10067-018-4130-2. [DOI] [PubMed] [Google Scholar]
- 7.Aggarwal R., Dua D., Jain S.K., Sud R., Meena S.R. Hyperuricemia: A Lifestyle Change. Urol. Nephrol. Open Access J. 2018;6:119–121. doi: 10.15406/unoaj.2018.06.00217. [DOI] [Google Scholar]
- 8.Nakagawa T., Tuttle K.R., Short R.A., Johnson R.J. Hypothesis: Fructose-induced hyperuricemia as a causal mechanism for the epidemic of the metabolic syndrome. Nat. Clin. Pract. Nephrol. 2005;1:80–86. doi: 10.1038/ncpneph0019. [DOI] [PubMed] [Google Scholar]
- 9.Yu K.H., See L.C., Huang Y.C., Yang C.H., Sun J.H. Dietary factors associated with hyperuricemia in adults. Semin. Arthritis Rheum. 2008;37:243–250. doi: 10.1016/j.semarthrit.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 10.Choi H.K., Liu S., Curhan G. Intake of purine-rich foods, protein, and dairy products and relationship to serum levels of uric acid: The Third National Health and Nutrition Examination Survey. Arthritis Rheum. 2005;52:283–289. doi: 10.1002/art.20761. [DOI] [PubMed] [Google Scholar]
- 11.Zgaga L., Theodoratou E., Kyle J., Farrington S.M., Agakov F., Tenesa A., Walker M., McNeill G., Wright A.F., Rudan I., et al. The Association of Dietary Intake of Purine-Rich Vegetables, Sugar-Sweetened Beverages and Dairy with Plasma Urate, in a Cross-Sectional Study. PLoS ONE. 2012;7:e38123. doi: 10.1371/journal.pone.0038123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ogryzlo M.A. Hyperuricemia induced by high fat diets and starvation. Arthritis Rheum. 1965;8:799–822. doi: 10.1002/art.1780080443. [DOI] [PubMed] [Google Scholar]
- 13.Howard A.N. The historical development, efficacy and safety of very-low-calorie diets. Int. J. Obes. 1981;5:195–208. [PubMed] [Google Scholar]
- 14.Ozen A.E., Bibiloni M.D.M., Pons A., Tur J.A. Fluid intake from beverages across age groups: A systematic review. J. Hum. Nutr. Diet. 2014;28:417–442. doi: 10.1111/jhn.12250. [DOI] [PubMed] [Google Scholar]
- 15.Elmadfa I., Meyer A.L. Patterns of drinking and eating across the European Union: Implications for hydration status. Nutr. Rev. 2015;73:141–147. doi: 10.1093/nutrit/nuv034. [DOI] [PubMed] [Google Scholar]
- 16.Basu S., McKee M., Galea G., Stuckler D. Relationship of Soft Drink Consumption to Global Overweight, Obesity, and Diabetes: A Cross-National Analysis of 75 Countries. Am. J. Public Health. 2013;103:2071–2077. doi: 10.2105/AJPH.2012.300974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang Y., Yang T., Zeng C., Wei J., Li H., Xiong Y., Yang Y., Ding X., Lei G. Is coffee consumption associated with a lower risk of hyperuricemia or gout? A systematic review and meta-analysis. BMJ Open. 2016;6:e009809. doi: 10.1136/bmjopen-2015-009809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choi J.W.J., Ford E.S., Gao X., Choi H.K. Sugar-sweetened soft drinks, diet soft drinks, and serum uric acid level: The third national health and nutrition examination survey. Arthritis Rheum. 2007;59:109–116. doi: 10.1002/art.23245. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y., Cui Y., Li X.-A., Li L.-J., Xie X., Huang Y.-Z., Deng Y.-H., Zeng C., Lei G.-H. Is tea consumption associated with the serum uric acid level, hyperuricemia or the risk of gout? A systematic review and meta-analysis. BMC Musculoskelet. Disord. 2017;18:95. doi: 10.1186/s12891-017-1456-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim Y., Han B.G., the KoGES Group Cohort Profile: The Korean Genome and Epidemiology Study (KoGES) Consortium. Int. J. Epidemiol. 2017;46:1350. doi: 10.1093/ije/dyx105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin K.C., Lin H.Y., Chou P. Community based epidemiological study on hyperuricemia and gout in Kin-Hu, Kinmen. J. Rheumatol. 2000;27:1045–1050. [PubMed] [Google Scholar]
- 22.Ahn Y., Kwon E., Shim J.E., Park M.K., Joo Y., Kimm K., Park C., Kim D.H. Validation and reproducibility of food frequency questionnaire for Korean genome epidemiologic study. Eur. J. Clin. Nutr. 2007;61:1435–1441. doi: 10.1038/sj.ejcn.1602657. [DOI] [PubMed] [Google Scholar]
- 23.Bomback A.S., Derebail V., Shoham D.A., Anderson C.A., Steffen L.M., Rosamond W.D., Kshirsagar A.V. Sugar-sweetened soda consumption, hyperuricemia, and kidney disease. Kidney Int. 2010;77:609–616. doi: 10.1038/ki.2009.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Choi H.K., Curhan G. Soft drinks, fructose consumption, and the risk of gout in men: Prospective cohort study. BMJ. 2008;336:309–312. doi: 10.1136/bmj.39449.819271.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bae J., Chun B.-Y., Park P.S., Choi B.Y., Kim M.K., Shin M.-H., Lee Y.-H., Shin D.H., Kim S.-K. Higher consumption of sugar-sweetened soft drinks increases the risk of hyperuricemia in Korean population: The Korean Multi-Rural Communities Cohort Study. Semin. Arthritis Rheum. 2014;43:654–661. doi: 10.1016/j.semarthrit.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 26.Johnson R.J., Perez-Pozo S.E., Sautin Y., Manitius J., Sanchez-Lozada L.-G., Feig D.I., Shafiu M., Segal M., Glassock R.J., Shimada M., et al. Hypothesis: Could Excessive Fructose Intake and Uric Acid Cause Type 2 Diabetes? Endocr. Rev. 2009;30:96–116. doi: 10.1210/er.2008-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Galipeau D., Verma S., McNeill J.H. Female rats are protected against fructose-induced changes in metabolism and blood pressure. Am. J. Physiol. Circ. Physiol. 2002;283:H2478–H2484. doi: 10.1152/ajpheart.00243.2002. [DOI] [PubMed] [Google Scholar]
- 28.Choi H.K., Ford E.S., Li C., Curhan G. Prevalence of the metabolic syndrome in patients with gout: The Third National Health and Nutrition Examination Survey. Arthritis Rheum. 2007;57:109–115. doi: 10.1002/art.22466. [DOI] [PubMed] [Google Scholar]
- 29.Sun S.Z., Flickinger B.D., Williamson-Hughes P.S., Empie M.W. Lack of association between dietary fructose and hyperuricemia risk in adults. Nutr. Metab. 2010;7:16. doi: 10.1186/1743-7075-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ka T., Yamamoto T., Moriwaki Y., Kaya M., Tsujita J., Takahashi S., Tsutsumi Z., Fukuchi M., Hada T. Effect of exercise and beer on the plasma concentration and urinary excretion of purine bases. J. Rheumatol. 2003;30:1036–1042. [PubMed] [Google Scholar]
- 31.Uetani M., Suwazono Y., Kobayashi E., Inaba T., Oishi M., Nogawa K. A longitudinal study of the influence of shift work on serum uric acid levels in workers at a telecommunications company. Occup. Med. 2005;56:83–88. doi: 10.1093/occmed/kqi178. [DOI] [PubMed] [Google Scholar]
- 32.Pham N.M., Yoshida D., Morita M., Yin G., Toyomura K., Ohnaka K., Takayanagi R., Kono S. The Relation of Coffee Consumption to Serum Uric Acid in Japanese Men and Women Aged 49–76 Years. J. Nutr. Metab. 2010;2010:1–7. doi: 10.1155/2010/930757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jung M.H., Seong P.N., Kim M.H., Myong N.-H., Chang M.-J. Effect of green tea extract microencapsulation on hypertriglyceridemia and cardiovascular tissues in high fructose-fed rats. Nutr. Res. Pract. 2013;7:366–372. doi: 10.4162/nrp.2013.7.5.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen G., Tan M.-L., Li K.-K., Leung P.-C., Ko C.-H. Green tea polyphenols decreases uric acid level through xanthine oxidase and renal urate transporters in hyperuricemic mice. J. Ethnopharmacol. 2015;175:14–20. doi: 10.1016/j.jep.2015.08.043. [DOI] [PubMed] [Google Scholar]
- 35.Choi H.K., Curhan G. Coffee, tea, and caffeine consumption and serum uric acid level: The third national health and nutrition examination survey. Arthritis Rheum. 2007;57:816–821. doi: 10.1002/art.22762. [DOI] [PubMed] [Google Scholar]
- 36.Teng G.G., Tan C.S., Santosa A., Saag K.G., Yuan J.M., Koh W.P. Serum urate levels and consumption of common beverages and alcohol among Chinese in Singapore. Arthritis Care Res. 2013;65:1432–1440. doi: 10.1002/acr.21999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ben Salem C., Slim R., Fathallah N., Hmouda H. Drug-induced hyperuricaemia and gout. Rheumatology. 2017;56:679–688. doi: 10.1093/rheumatology/kew293. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Restrictions apply to the availability of these data. Data was obtained from Korean Genome and Epidemiologiy Study [KoGES] and are available at [www.kdca.go.kr] (accessed on 23 May 2021), with the permission of [KoGES].