Abstract
Background
Acute cellular rejection (ACR) frequently occurs after liver transplantation (LT) and can result in permanent damage of the liver allograft. Specific and sensitive biomarkers for predicting and monitoring ACR are vital for guiding post-transplantation care. In the present study, we aimed to investigate the function of high-mobility group box 1 (HMGB1) in predicting ACR and prognosis after LT.
Material/Methods
A total of 113 LT recipients were enrolled in the study, including 62 patients in an ACR group and 51 patients in a non-rejection group. Using tissues from the 113 patients, HMGB1 expression was examined by immunohistochemistry, and the total score for HMGB1 expression was calculated by multiplying the percentage of immunoreactive cells score and the staining intensity score. We then analyzed the association between HMGB1 expression and clinical features. Finally, the function of HMGB1 in predicting the prognosis of LT was determined using Kaplan-Meier (K-M) survival and Cox multivariate analyses.
Results
Immunohistochemical staining results demonstrated that the expression of HMGB1 was significantly increased in the ACR group, compared with that in the non-rejection group (P<0.05). Clinical characteristic analysis revealed that high HMGB1 levels were related to ACR (P<0.05). Moreover, K-M survival analysis showed that patients with high HMGB1 expression displayed poorer prognosis (P<0.05). Cox multivariate analysis demonstrated that HMGB1 was an independent prognostic predictor for post-LT survival (odds ratio, 3.283; P=0.008).
Conclusions
LT recipients’ HMGB1 levels may be a useful and noninvasive biomarker for the prediction of ACR and prognosis after LT.
Keywords: Graft Rejection; High Mobility Group Proteins; Immunity, Innate; Liver Transplantation
Background
Acute cellular rejection (ACR) is a common cause of liver insufficiency after liver transplantation (LT), with an incidence of 30% to 70%, and can lead to transplant failure [1,2]. Although LT is valid for treating end-stage liver disease, ACR remains a significant post-transplant complication [3]. ACR causes infection and cancer recurrence in recipients due to the increased use of immunosuppressants [4]. To avoid the increased use of immunosuppressive agents, accurate biomarkers are necessary to predict and identify the risk of ACR early [5,6]. Liver histopathology is the criterion standard for the diagnosis of graft dysfunction after LT via liver biopsy. Expert LT pathologists are required to obtain accurate pathological results [7]. Laboratory tests are also used to examine allograft rejection, but the specificity and sensitivity of these laboratory indexes are not sufficient [8]. Therefore, it is essential to explore specific and noninvasive prognostic markers that can monitor the risk of ACR and be useful in treating transplant rejection.
High-mobility group box 1 (HMGB1) exerts a proinflammatory cytokine-like function by binding DNA and regulating transcription. HMGB1 is generated during systemic disorders and serves as an alarm signal to initiate an inflammatory reaction [9–11]. Accumulating evidence shows that HMGB1 is a vital early participant in liver, kidney, and heart injury and inflammation after ischemia-reperfusion (I/R) [12–14]. In a liver I/R mouse model, increased HMGB1 expression was found in the early stage of reperfusion, and the application of an extracellular HMGB1-neutralizing antibody exerted an obvious protective effect on liver injury after I/R [15]. The absence of hepatocyte-specific HMGB1 aggravates liver I/R injury and inflammation, which is at least partly due to enhanced DNA injury and nuclear instability, thereby increasing histone release [16].
Moreover, anti-histone H1/HMGB1 antibodies have been shown to play an immunosuppressive role [17,18], and a previous study demonstrated that blocking extracellular HMGB1 can significantly prolong the survival rate of transplanted hearts in mice [19]. These results suggest that HMGB1 may play a pro-injury and inflammatory role in transplantation. However, the function of HMGB1 in ACR after LT has not been fully elucidated.
In the present study, we focused on evaluating the association between HMGB1 and the incidence of post-LT ACR in a group of 113 patients with LT. For this purpose, the expression of HMGB1 was examined in the recipient liver samples by immunohistochemistry, and the incidence of ACR in the first 2 years after LT was recorded and analyzed.
Material and Methods
Patients and Samples
The study enrolled 113 patients who received LT at the First Affiliated Hospital of Zhejiang University (Hangzhou, China) between 2006 and 2014, including 62 patients with ACR (rejection group) and 51 patients without ACR (non-rejection group). All recipients were diagnosed with end-stage liver disease caused by benign hepatic disease. Liver tissue specimens were removed and paraffin-embedded for further study. The immunosuppressive scheme received by all patients was tacrolimus-based, including corticosteroids and mycophenolate mofetil. All ACR diagnoses were based on clinically increased liver transaminase levels and pathological evaluations by needle core liver biopsy, which were independently confirmed by 2 expert pathologists. The study was approved by the Ethics Review Committee of the First Affiliated Hospital of Zhejiang University, which abided by the Helsinki Declaration on ethical principles. Written informed consent was obtained from all patients.
Tissue Microarray and Immunohistochemical Staining
The level of HMGB1 in the liver tissues of the 113 patients was detected by tissue microarray (TMA) using formalin-fixed paraffin sections. We placed the tissue samples on the TMA blindly to avoid bias in the immunohistochemical (IHC) staining results. To avoid tissue loss and tissue heterogeneity, each sample was placed in triplicate, and 2 experts conducted semi-quantitative scoring of the staining intensity and percentage of immunoreactive cells, without any knowledge of patient information. Staining intensity was divided into 3 levels: “0” for weak staining, “1” for moderate staining, and “2” for strong staining. For the percentage of immunoreactive cells, we defined “0” as <20% positivity, “1” as 21% to 40%, “2” as 41% to 60%, “3” as 61% to 80%, and “4” as 81% to 100%. The percentage of immunoreactive cells multiplied by the staining intensity score was used to calculate the total expression score of HMGB1. The total score ranged between 0 and 8. We defined a high HMGB1 expression score as ≥4.
Statistical Analysis
Statistical analysis was performed using SPSS version 18.0 (Chicago, IL, USA). The number, mean±standard deviation, or median±interquartile range was used to present the data. Categorical variables were analyzed using Fisher’s exact test. The relationship between HMGB1 expression levels and clinical outcomes was assessed using the chi-squared test by analyzing the IHC staining results. The Kaplan-Meier (K-M) method was used to analyze overall survival, and survival differences were determined using the log-rank test. The Cox regression model was used for multivariate analysis. A P value <0.05 was considered statistically significant, with a confidence interval of 95%.
Results
HMGB1 Expression in the Livers of Patients with ACR
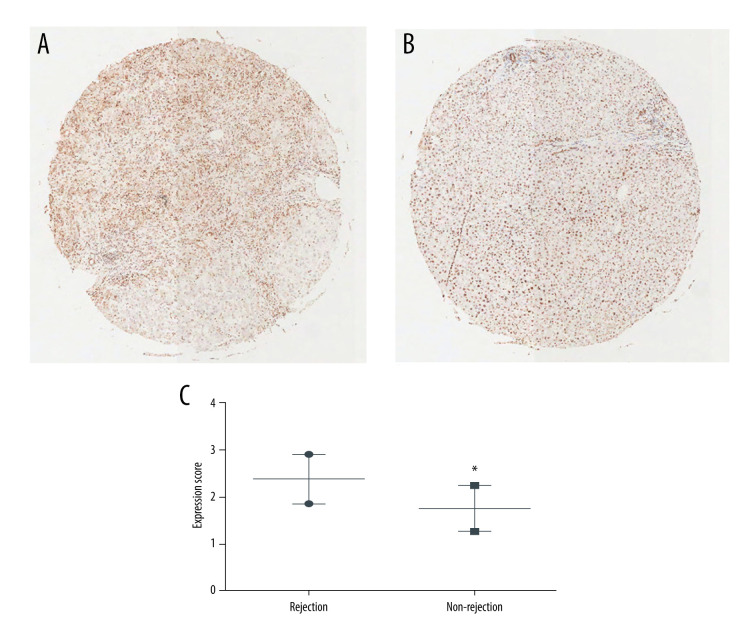
To validate our hypothesis that the LT recipient HMGB1 expression levels were related to post-transplant ACR, we first examined the expression of HMGB1 in the 113 patient tissues, including those of the 62 patients with ACR and 51 patients without ACR. The demographic and clinical features of the patients are presented in Table 1. No differences were found in age, sex, donor age, cause of disease, model for end-stage liver disease (MELD) scores, Child-Pugh scores, serum creatinine concentration, and ABO compatibility between the 2 groups. HMGB1 expression in the 2 groups was examined by IHC staining in a tissue microarray. The results indicated that HMGB1 expression levels were significantly higher in the rejection group (P<0.05; Figure 1).
Table 1.
Demographic and clinical characteristics of liver transplant patients.
| Characteristics, n (%) | Rejection (n=62) | Non-rejection (n=51) | P value |
|---|---|---|---|
| Age | 42.7±9.88 | 42.5±9.78 | NS |
|
| |||
| Sex | |||
| Male | 50 | 45 | NS |
| Female | 12 | 6 | |
|
| |||
| Donor age | 32.1±11.58 | 30.3±10.21 | NS |
|
| |||
| Primary diagnosis | |||
| HBV cirrhosis | 53 | 39 | NS |
| Other | 9 | 12 | |
|
| |||
| MELD scores | 23.6±10.29 | 23.9±10.22 | NS |
|
| |||
| Child-Pugh scores | 10.0±2.21 | 10.0±2.15 | NS |
|
| |||
| Serum creatinine (μmol/L) | 99.5±112.17 | 101.7±114.23 | NS |
|
| |||
| ABO compatible (%) | |||
| Compatible | 51 | 41 | NS |
| Incompatible | 11 | 10 | |
NS – no significance (P>0.05); HBV – hepatitis B virus; MELD – model for end-stage liver disease.
Figure 1.
High-mobility group box 1 (HMGB1) expression in resected recipient livers. (A) Typical immunohistochemical (IHC) staining results were obtained from the rejection group. (B) Typical IHC staining results were obtained from the non-rejection group. (C) HMGB1 expression levels in the 2 groups. The data are expressed as means±standard deviations. * P<0.05 vs the rejection group.
Correlation of HMGB1 Expression with Recipient Survival and ACR
Based on the expression of HMGB1 in recipient liver tissues, we divided the patients into a high-expression group (n=38; IHC staining score ≥4) and a low-expression group (n=75; IHC staining score <4). No differences in age, sex, MELD scores, and Child-Pugh scores were observed (Table 2). However, HGMB1 expression was clearly related to ACR (P=0.039). Moreover, K-M survival analysis showed that the survival of patients with low HMGB1 levels was improved, compared with that of patients with high HMGB1 levels (P<0.05; Figure 2).
Table 2.
Characteristics of recipients between the HMGB1 high-expression group and low-expression group.
| HMGB1 expression level | High expression (n=38) | Low expression (n=75) | P value |
|---|---|---|---|
| Age | 42.6±9.89 | 43.2±9.93 | NS |
|
| |||
| Sex (Male/Female) | 34/4 | 61/14 | NS |
|
| |||
| Child-Pugh scores | 10.0±2.17 | 9.7±2.10 | NS |
|
| |||
| MELD scores | 23.6±10.27 | 21.7±9.50 | NS |
|
| |||
| Rejection (n) | 26 | 36 | 0.039 |
| Non-rejection (n) | 12 | 39 | |
NS – no significance; HMGB1 – high-mobility group box 1; MELD – model for end-stage liver disease.
Figure 2.
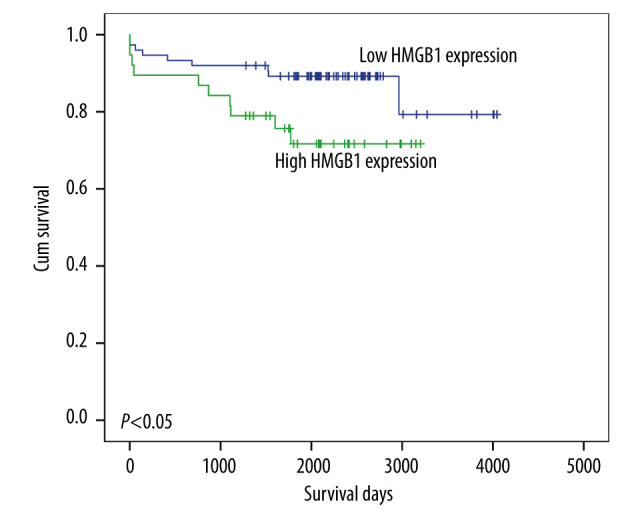
Survival of recipients with high or low high-mobility group box 1 (HMGB1) levels. The Kaplan-Meier method was used to analyze overall survival, and survival differences were examined by the log-rank test.
Validation of HMGB1 Levels as a Potential Prognostic Factor in Patients with LT
To confirm that HMGB1 expression level was a prognostic marker for LT, we used a Cox regression model to examine whether patient HMGB1 expression was an independent prognostic factor. Our results suggested that female sex was a protective prognostic factor (hazard ratio [HR], 0.111; P<0.05) (Table 3). Notably, HMGB1 expression level was clearly related to poor overall survival (odds ratio [OR], 3.283; P<0.05), indicating that a high HMGB1 level may be an independent and adverse prognostic marker in LT recipients.
Table 3.
Multivariate analyses of overall survival in all recipient populations.
| Variables | B | S.E. | Wald | df | Sig. | Exp(B) |
|---|---|---|---|---|---|---|
| Sex (Female vs Male) | −2.020 | 0.597 | 13.59 | 1 | 0.000 | 0.111 |
| Age, years (>50 vs ≤50) | 0.420 | 0.630 | 0.444 | 1 | 0.505 | 1.521 |
| Donor age (>40 vs ≤40) | 0.362 | 0.559 | 0.401 | 1 | 0.656 | 1.378 |
| MELD scores (≤22 vs >22) | 0.026 | 0.727 | 0.001 | 1 | 0.972 | 1.026 |
| Child-Pugh status (Child C vs Child A and B) | −1.476 | 0.849 | 3.019 | 1 | 0.082 | 0.229 |
| HMGB1 expression (high vs low) | 1.189 | 0.679 | 3.067 | 1 | 0.008 | 3.283 |
HMGB1 – high-mobility group box 1; MELD – model for end-stage liver disease.
Discussion
ACR is one of the principal reasons for graft dysfunction after LT [20]. Liver biopsy is still the most accurate method for ACR diagnosis, but noninvasive and sensitive biomarkers remain necessary owing to the cost, inconvenience, and invasiveness of liver biopsy [21]. In the present study, we demonstrated that HMGB1 overexpression was present in LT recipients with ACR. We also found the main cause of death in the high-expression HMGB1 group was loss of transplanted liver function, followed by death due to abdominal bleeding, infection, biliary tract complications, and other causes due to liver failure during follow-up. However, the main cause of death in the low-expression HMGB1 group was primary disease, followed by progressive loss of liver function caused by chronic rejection, cardiovascular disease, and other causes. Our results suggested that HMGB1 level was an independent predictor of patient prognosis and HMGB1 can be used as a new biomarker for liver ACR prediction and monitoring.
HMGB1 is a DNA-binding protein expressed in almost all eukaryotic cells, which can stabilize the formation of nucleosomes and promote transcription as a nuclear factor [22]. Recent studies have shown that HMGB1 plays vital roles in response to tissue injury, suggesting that HMGB1 is a new molecular prototype of a damage-related molecular model [23,24]. During infection and inflammatory stimuli, HMGB1 is released by activated immunocytes, such as macrophages [25], dendritic cells [26], and natural killer cells [27]. The secreted HMGB1 then leads to an inflammatory reaction via cellular signal transduction mediated by its receptors, such as Toll-like receptor (TLR)2, TLR4 [28,29], and advanced glycation end products [29,30]. Moreover, HMGB1 levels are clearly augmented during severe sepsis in humans and animals, and death from sepsis can be prevented by specific HMGB1-neutralizing antibodies [31]. Ilmakunnas et al demonstrated that HMGB1 was exclusively released from the graft and that its levels correlated with postoperative alanine aminotransferase levels in 20 LT recipients [32]. These studies demonstrated that HMGB1 is an essential inducer of organ injury; however, its precise function in ACR is poorly understood. Here, we investigated the relationship between HMGB1 levels and ACR in LT recipients. We found that HMGB1 levels were higher in LT recipients with ACR and high HMGB1 levels were related to an increased incidence of ACR. Moreover, univariate and multivariate analyses indicated that high HMGB1 levels were correlated with poor prognosis. Thus, our study suggested that HMGB1 in the recipient liver is a predictive biomarker of ACR and prognosis after LT.
However, there are some limitations to this study. First, the data were retrospectively acquired. Second, the immune interactions of the recipient and the donor liver lead to ACR; however, we examined only recipient HMGB1 levels, ignored the level of HMGB1 in the patients’ serum, and the effect of donor HMGB1 levels remains unclear. Third, we failed to include some important information indicators in our study that can impact the prognosis of liver transplantation, such as donor-specific antibodies, trough level of immunosuppressive drugs, postoperative infection, and bacteremia. Finally, there was an insufficient number of enrolled samples, which may have caused some inconsistent results. For example, ABO incompatibility was not verified as an independent risk factor for rejection or prognosis.
Conclusions
The results of this study provide a strong theoretical basis for verifying whether HMGB1 expression level in LT recipients can be a useful noninvasive biomarker for predicting rejection and prognosis after LT in prospective trials.
Footnotes
Conflicts of Interest
None.
Declaration of Figures Authenticity
All figures submitted have been created by the authors who confirm that the images are original with no duplication and have not been previously published in whole or in part.
Source of support: This study was funded by the Zhejiang Natural Science Foundation (LQ20H030005), Zhejiang Health Technology Project (2020KY126), Zhejiang Health Technology Project (2019RC153), and China Postdoctoral Science Foundation (2019T120525)
References
- 1.Mor E, Solomon H, Gibbs JF, et al. Acute cellular rejection following liver transplantation: clinical pathologic features and effect on outcome. Semin Liver Dis. 1992;12:28–40. doi: 10.1055/s-2007-1007374. [DOI] [PubMed] [Google Scholar]
- 2.Fisher LR, Henley KS, Lucey MR. Acute cellular rejection after liver transplantation: Variability, morbidity, and mortality. Liver Transpl Surg. 1995;1:10–15. doi: 10.1002/lt.500010104. [DOI] [PubMed] [Google Scholar]
- 3.Florman S, Schiano T, Kim L, et al. The incidence and significance of late acute cellular rejection (>1000 days) after liver transplantation. Clin Transplant. 2004;18:152–55. doi: 10.1046/j.1399-0012.2003.00139.x. [DOI] [PubMed] [Google Scholar]
- 4.Lai Q, Iesari S, Finkenstedt A, et al. Hepatocellular carcinoma recurrence after acute liver allograft rejection treatment: A multicenter European experience. Hepatobiliary Pancreat Dis Int. 2019;18:517–24. doi: 10.1016/j.hbpd.2019.05.006. [DOI] [PubMed] [Google Scholar]
- 5.Choudhary NS, Saigal S, Bansal RK, et al. Acute and chronic rejection after liver transplantation: What a clinician needs to know. J Clin Exp Hepatol. 2017;7:358–66. doi: 10.1016/j.jceh.2017.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fenkel JM, Halegoua-DeMarzio DL. Management of the liver transplant recipient: Approach to allograft dysfunction. Med Clin North Am. 2016;100:477–86. doi: 10.1016/j.mcna.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 7.Kumar S, Mohapatra N, Borle DP, Choudhury A, et al. Non invasive diagnosis of acute cellular rejection after liver transplantation – current opinion. Transpl Immunol. 2018;47:1–9. doi: 10.1016/j.trim.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 8.Okubo K, Wada H, Tanaka A, et al. Identification of novel and noninvasive biomarkers of acute cellular rejection after liver transplantation by protein microarray. Transplant Direct. 2016;2:e118. doi: 10.1097/TXD.0000000000000630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sims GP, Rowe DC, Rietdijk ST, et al. HMGB1 and RAGE in inflammation and cancer. Annu Rev Immunol. 2010;28:367–88. doi: 10.1146/annurev.immunol.021908.132603. [DOI] [PubMed] [Google Scholar]
- 10.Andersson U, Tracey KJ. HMGB1 is a therapeutic target for sterile inflammation and infection. Annu Rev Immunol. 2011;29:139–62. doi: 10.1146/annurev-immunol-030409-101323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andersson U, Yang H, Harris H. Extracellular HMGB1 as a therapeutic target in inflammatory diseases. Expert Opin Ther Targets. 2018;22:263–77. doi: 10.1080/14728222.2018.1439924. [DOI] [PubMed] [Google Scholar]
- 12.Zhao G, Fu C, Wang L, et al. Down-regulation of nuclear HMGB1 reduces ischemia-induced HMGB1 translocation and release and protects against liver ischemia-reperfusion injury. Sci Rep. 2017;7:46272. doi: 10.1038/srep46272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang J, Xia J, Zhang Y, et al. HMGB1-TLR4 signaling participates in renal ischemia reperfusion injury and could be attenuated by dexamethasone-mediated inhibition of the ERK/NF-kappaB pathway. Am J Transl Res. 2016;8:4054–67. [PMC free article] [PubMed] [Google Scholar]
- 14.Xue J, Ge H, Lin Z, et al. The role of dendritic cells regulated by HMGB1/TLR4 signalling pathway in myocardial ischaemia reperfusion injury. J Cell Mol Med. 2019;23:2849–62. doi: 10.1111/jcmm.14192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsung A, Sahai R, Tanaka H, et al. The nuclear factor HMGB1 mediates hepatic injury after murine liver ischemia-reperfusion. J Exp Med. 2005;201:1135–43. doi: 10.1084/jem.20042614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang H, Nace GW, McDonald KA, et al. Hepatocyte-specific high-mobility group box 1 deletion worsens the injury in liver ischemia/reperfusion: A role for intracellular high-mobility group box 1 in cellular protection. Hepatology. 2014;59:1984–97. doi: 10.1002/hep.26976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakano T, Kawamoto S, Lai CY, et al. Liver transplantation-induced antihistone H1 autoantibodies suppress mixed lymphocyte reaction. Transplantation. 2004;77:1595–603. doi: 10.1097/01.tp.0000123079.10650.71. [DOI] [PubMed] [Google Scholar]
- 18.Nakano T, Goto S, Lai CY, et al. Involvement of autoimmunity against nuclear histone H1 in liver transplantation tolerance. Transpl Immunol. 2008;19:87–92. doi: 10.1016/j.trim.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 19.Li JH, Zhao B, Zhu XH, et al. Blockade of extracellular HMGB1 suppresses xenoreactive B cell responses and delays acute vascular xenogeneic rejection. Am J Transplant. 2015;15:2062–74. doi: 10.1111/ajt.13275. [DOI] [PubMed] [Google Scholar]
- 20.Rook M, Rand E. Predictors of long-term outcome after liver transplant. Curr Opin Organ Transplant. 2011;16:499–504. doi: 10.1097/MOT.0b013e32834a945d. [DOI] [PubMed] [Google Scholar]
- 21.Rodriguez-Peralvarez M, Rico-Juri JM, Tsochatzis E, et al. Biopsy-proven acute cellular rejection as an efficacy endpoint of randomized trials in liver transplantation: A systematic review and critical appraisal. Transpl Int. 2016;29:961–73. doi: 10.1111/tri.12737. [DOI] [PubMed] [Google Scholar]
- 22.Muller S, Ronfani L, Bianchi ME. Regulated expression and subcellular localization of HMGB1, a chromatin protein with a cytokine function. J Intern Med. 2004;255:332–43. doi: 10.1111/j.1365-2796.2003.01296.x. [DOI] [PubMed] [Google Scholar]
- 23.Seong SY, Matzinger P. Hydrophobicity: An ancient damage-associated molecular pattern that initiates innate immune responses. Nat Rev Immunol. 2004;4:469–78. doi: 10.1038/nri1372. [DOI] [PubMed] [Google Scholar]
- 24.Lotze MT, Tracey KJ. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat Rev Immunol. 2005;5:331–42. doi: 10.1038/nri1594. [DOI] [PubMed] [Google Scholar]
- 25.Wang H, Bloom O, Zhang M, et al. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285:248–51. doi: 10.1126/science.285.5425.248. [DOI] [PubMed] [Google Scholar]
- 26.Dumitriu IE, Baruah P, Valentinis B, et al. Release of high mobility group box 1 by dendritic cells controls T cell activation via the receptor for advanced glycation end products. J Immunol. 2005;174:7506–15. doi: 10.4049/jimmunol.174.12.7506. [DOI] [PubMed] [Google Scholar]
- 27.Semino C, Angelini G, Poggi A, Rubartelli A. NK/iDC interaction results in IL-18 secretion by DCs at the synaptic cleft followed by NK cell activation and release of the DC maturation factor HMGB1. Blood. 2005;106:609–16. doi: 10.1182/blood-2004-10-3906. [DOI] [PubMed] [Google Scholar]
- 28.Park JS, Svetkauskaite D, He Q, et al. Involvement of toll-like receptors 2 and 4 in cellular activation by high mobility group box 1 protein. J Biol Chem. 2004;279:7370–77. doi: 10.1074/jbc.M306793200. [DOI] [PubMed] [Google Scholar]
- 29.Park JS, Gamboni-Robertson F, He Q, et al. High mobility group box 1 protein interacts with multiple Toll-like receptors. Am J Physiol Cell Physiol. 2006;290:C917–24. doi: 10.1152/ajpcell.00401.2005. [DOI] [PubMed] [Google Scholar]
- 30.Wang M, Wang L, Guo Y, et al. A high mobility group box 1 (HMGB1) gene from Chlamys farreri and the DNA-binding ability and pro-inflammatory activity of its recombinant protein. Fish Shellfish Immunol. 2014;36:393–400. doi: 10.1016/j.fsi.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 31.Abdulmahdi W, Patel D, Rabadi MM, et al. HMGB1 redox during sepsis. Redox Biol. 2017;13:600–7. doi: 10.1016/j.redox.2017.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ilmakunnas M, Tukiainen EM, Rouhiainen A, et al. High mobility group box 1 protein as a marker of hepatocellular injury in human liver transplantation. Liver Transpl. 2008;14:1517–25. doi: 10.1002/lt.21573. [DOI] [PubMed] [Google Scholar]