Abstract
Lysophosphatidic acid (LPA) is a bioactive lipid mediator primarily derived from membrane phospholipids. LPA initiates cellular effects upon binding to a family of G protein-coupled receptors, termed LPA receptors (LPAR1 to LPAR6). LPA signaling drives cell migration and proliferation, cytokine production, thrombosis, fibrosis, angiogenesis, and lymphangiogenesis. Since the expression and function of LPA receptors are critical for cellular effects, selective antagonists may represent a potential treatment for a broad range of illnesses, such as cardiovascular diseases, idiopathic pulmonary fibrosis, voiding dysfunctions, and various types of cancers. More new LPA receptor antagonists have shown their therapeutic potentials, although most are still in the preclinical trial stage. This review provided integrative information and summarized preclinical findings and recent clinical trials of different LPA receptor antagonists in cancer progression and resistance. Targeting LPA receptors can have potential applications in clinical patients with various diseases, including cancer.
Keywords: antagonist, cancer, clinical trial, lysophosphatidic acid, lysophosphatidic receptor, therapy
1. Introduction
Lysophosphatidic acid (LPA) and LPA receptors (LPARs), including LPAR1 to LPAR6, are integral parts of signaling pathways involved in cellular proliferation/migration/survival, vascular homeostasis, stromal remodeling, lymphocytes trafficking, and immune regulation [1,2,3]. In addition, autotaxin (ATX) is a secreted glycoprotein and functions as a pivotal enzyme to produce extracellular LPA [4,5]. Figure 1 illustrates the extracellular and intracellular biosynthesis of LPA. Consequently, aberrant ATX-LPA-LPAR axis may be involved in the development and progression of many pathologic conditions such as cancer and metastasis [6,7], radio- and chemo-resistances [8,9,10,11,12], fibrotic diseases [13], neuropathic pain [14], arthritis [15], metabolic syndromes [16], and atherosclerosis [17]. Understanding ATX/LPA expression and LPAR-mediated signals elucidated our understanding of the disease mechanisms and highlighted the therapeutic potential of the druggable ATX-LPAR axis. To date, enormous in vivo and in vitro investigations have demonstrated pharmacological antagonization of LPAR to be of paramount significance in reversing pathologic responses. This article sought to update current progress regarding LPAR antagonists in clinical and preclinical settings, emphasizing compounds being evaluated in completed and ongoing clinical trials.
Figure 1.
The biosynthesis of lysophosphatidic acid (LPA). Extracellular LPA is mainly synthesized by autotaxin (ATX) via conversion of lysophospholipids, which are hydrolyzed from phospholipids through lecithin-cholesterol acyltransferase (LCAT)/phospholipase A1 (PLA1) or PLA1/PLA2 mechanism. Phosphatidic acid (PA) on the plasma membrane is another resource of extracellular LPA. PA can be generated from phospholipids and diacylglycerol (DAG) via phospholipase (PLD) and diacylglycerol kinase (DGK), respectively. PA is converted to LPA through PLA1 and PLA2. On the other hand, monoacylglycerol (MAG) and glycerol 3-phosphate (G3P) on the mitochondria generated intracellular LPA via acylglycerol kinase (AGK) and glycerophosphate acyltransferase (GPAT), respectively.
2. LPA Receptor-Mediated Signaling in Cancer Biology
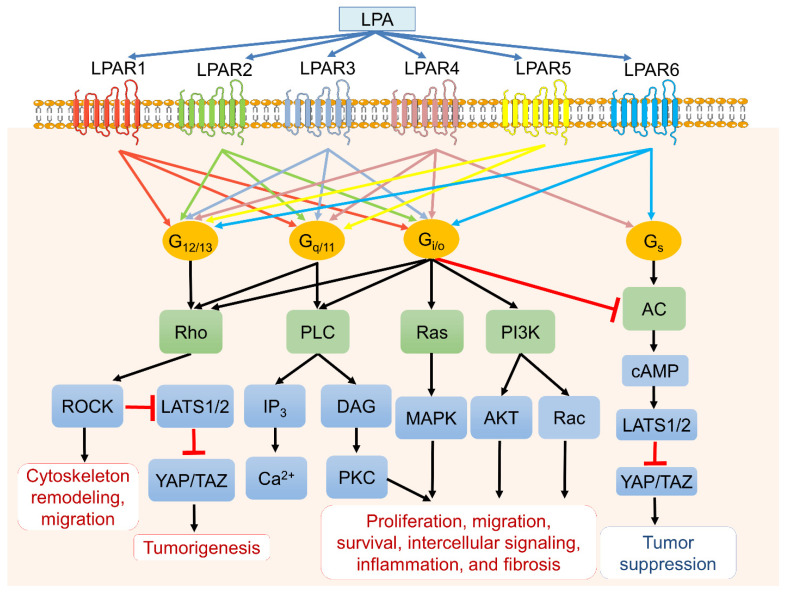
ATX-LPA-LPAR signaling is a complex network and intertwines with multiple cellular signaling to contribute a plethora of activities such as proliferation, survival, migration, metastasis, angiogenesis, and inflammation in cancers [6]. Individual LPARs favor different Gα proteins for their downstream signals and cellular functions. In brief, the endothelial cell differentiation gene (EGD) family LPARs (LPAR1 to LPAR3) bind to Gi/o and trigger the Ras/Raf/MAPK signaling pathway, phospholipase C (PLC), and the PI3K-Akt pathway [1,3,18,19]. Gq/11 protein couples LPAR1–5 to mediate PLC and calcium mobilization [20], whereas G12/13 interacts with all LPARs, leading to cell migration and invasion through Rho and Rho-associated protein kinase (ROCK) activation [21]. Signaling through Gs would activate the cAMP-dependent protein kinase A (PKA) signaling pathway and the large tumor suppressor 1 and 2 (LATS1/2). It would subsequently inhibit downstream transcriptional co-activators Yes-associated protein (YAP) and PDZ-binding motif (TAZ), which usually drive cancer cell survival, proliferation, invasive migration, and metastasis [22,23]. Interestingly, the ROCK activation would suppress LATS1/2 and subsequently activate YAP and TAZ, resulting in tumorigenesis (Figure 2).
Figure 2.
LPA, LPA receptors (LPARs), and downstream signaling pathways. LPA binds six primary LPA transmembrane receptors (LPAR1 to LPAR6) with varying affinities that couple to four different heterotrimeric G proteins (G12/13, Gq/11, Gi/o, and Gs) and trigger various downstream signaling cascades. LPA subsequently mediates cellular events such as cell proliferation, survival, apoptosis, migration, cytoskeleton reorganization, fibrosis, and inflammation.
The LPA-LPAR signaling pathway is one of the most investigated mechanisms because overexpression of one or more of these receptors was found in several types of cancers. Therefore, the concept to modulate cancer by agonizing or antagonizing LPARs is naturally generated. The following sessions would discuss all LPARs in detail.
2.1. LPAR 1
Studies show that LPAR1 enhances metastasis and tumor motility [18]. Aberrant LPAR1 expressions were observed in many cancer cell lines and primary tumors, including ovarian cancer [24], breast cancer [25], liver cancer [26], gastric cancer [27], pancreatic cancer [28,29], lung cancer [30,31], glioblastoma (GBM) [32,33,34], and osteosarcoma [35]. Ovarian cancer is the most investigated cancer in studying the malignancy of LPA signaling. High LPAR1 expressions in ovarian serous cystadenocarcinoma correlate with high proliferation, invasion, migration, and poorer prognosis than those with low expressions [36]. LPAR1 also promotes the development of intratumoral heterogeneity by regulating PI3K/AKT signaling [36]. Retaining the stemness phenotype of ovarian cancer, an autocrine loop via the ATX-LPA-LPAR1-AKT1 signaling axis is critical [37]. In breast cancer, overexpression of LPAR1 in MCF-10A mammary epithelial cells causes cells to acquire an invasive phenotype [38], which correlates with the heparin-binding EGF-like growth factor [39], and mediate basal breast metastasis through LPAR1-PI3K-ZEB1-miR-21 pathways [25]. For hepatocellular carcinoma, LPA-LPAR1 enhances cancer invasion via inducing MMP-9 expression through coordinate activation of PI3K and p38 MAPK signaling cascade [26]. Similarly, increased cancer cell invasiveness mediated by LPAR1 was found in pancreatic cancer [28,29]. For lung A549 cancer cells, the LPAR1/Gi/MAP kinase/NF-κB pathway is involved in LPA-induced oncogenesis, and using the LPAR1/3 antagonist Ki16425 to block LPAR1-mediated signaling would significantly reduce tumor volume [31]. In GBM, LPAR1 expression is also significantly higher than other gliomas [32]. Of interest, the LPA pathway of microglia-and-GBM interaction is a target to improve survival because microglia-derived LPA and ATX upon hypoxia stress may promote GBM proliferation and migration [32]. A recent report indicates LPAR1/PKCα/progesterone receptor pathway is involved in GBM migration [40]. In prostate PC-3 cancer cells, hyperglycemia triggers enhanced vascular endothelial growth factor-C (VEGF-C) expression via the LPAR1/3-Akt-ROS-LEDGF signaling [40]. The LPA-mediated VEGF-C expression can be modified by calreticulin, a multifunctional chaperon protein. In addition, pharmacological LPAR1 receptor antagonism may significantly reduce tumoral lymphatic vessel density and nodal metastasis in tumor-bearing nude mice, suggesting the key role of LPAR1 in prostate cancer lymphatic metastasis [41].
2.2. LPAR2
LPAR2 activation has been shown to associate with cell survival because of its anti-apoptosis function. For ovarian cancer, tumors with overexpression of LPAR2 were associated with poorer survivals compared with controls [42]. Furthermore, LPAR2 signaling promotes invasion and metastasis through the production of VEGF [43], EGFR [44], interleukin-8 [45], and urokinase plasminogen activation [46], implying the multiple hyper-vascularization processes. LPAR2-Gi-Src-EGFR-ERK signaling cascade may mediate cell movement and LPA-stimulated COX-2 expression [47]. Together with LPAR1, LPAR2 regulates phosphorylation of ezrin/radixin/moesin (ERM) proteins, known as membrane-cytoskeleton linkers, and leads to promotion of ovarian OVCAR-3 cancer cell migration through cytoskeletal reorganization and formation of membrane protrusions [48]. The metastatic activity of gastric SGC-7901 cells was enhanced as well through LPA-LPAR2-Notch pathway activation [27]. LPAR2 is the major LPAR in colon cancer, and most of the cellular signals by LPAR2 were primarily mediated through interaction with scaffold proteins Na+/H+ exchanger regulatory factor 2 (NHERF2) [49]. In another two reports, LPA-LPAR2 may facilitate colon cancer proliferation via transcription factor Kruppel-like factor 5 (KLF5) and hypoxia-inducible factor 1α (HIF-1α) activations. The LPAR2 associated HIF-1α expression also promoted breast cancer proliferation/migration and conferred poor prognosis in the Chinese population [50]. Regarding the link between chronic inflammation and cancer, Lin et al. found genetic LPAR2 depletion may attenuate colon cancer development in a colitis mice model triggered by azoxymethane and dextran sulfate sodium [51]. Noteworthy, LPAR2 activation may exert anti-migration effects by blocking EGF-induced migration and invasion of pancreatic Panc-1 cancer cells through the G12/13/Rho signaling pathway [52]. Gi2 protein is also involved in enhanced ovarian cancer invasion and migration via the HIF1α-LPA-LPAR2 axis [24]. The distinct structure of LPAR2 from other LPARs is its carboxyl-terminal tail contains a zinc finger-binding motif to interact with TRIP6 and pro-apoptotic Siva-1. TRIP6 has a PSD95/Dlg/ZO-1 (PDZ)-binding motif to interact with scaffold proteins, particularly NHERF2 [53]. Siva-1 is an early response gene activated by DNA damage that promotes apoptosis through binding up the antiapoptotic Bxl-XL protein. Moreover, Siva-1 acts with p53 and the ubiquitin ligase Mdm2 in the nucleus complexes, and the polyubiquitinated complex would be degraded once the LPA-LPAR2 axis is activated. The functional significance of the LPAR2-activated assembly leads to up-regulation of ERK1/2, PI3K-Akt, and NFκB prosurvival pathways and the subsequent inhibition of apoptosis [54]. LPAR2 can protect cancer cells against apoptotic stress after irradiation and chemotherapy by augmenting DNA damage repair response and inhibiting the mitochondrial apoptosis cascade [55].
2.3. LPAR3
LPAR3 is the predominant receptor subtype in colon, liver, and lung cancers. LPAR3-expressing cells significantly promote motility and invasiveness through Ras-, Rac-, Rho-, and PI3K-signaling pathways [20]. In hepatocellular carcinoma, Zuckerman et al. reported distinct LPAR3 expressions within the tumor and normal tissues, and LPAR3 may enhance liver cancer migration via the LPAR3-Gi-ERK/MAPK pathway [56]. Okabe et al. found LPAR3 contributes to hepatocellular carcinoma proliferation and invasion via the β-catenin pathway in rat hepatic RH7777 cancer cells. They also demonstrated that tumor cells with high LPAR3 expression were resistant to cisplatin and doxorubicin through multidrug-resistance-related up-regulation of genes [20]. In melanoma, LPAR3 is essential to promote viability and proliferation, and the Src homology 3 domain is required for LPAR3 to mediate viability in melanoma SK-MEL-2 cells [57,58]. In ovarian cancer, LPAR3 promotes cell expansion and invasion in SKOV-3 cells, and tumors with overexpression of LPAR3 were associated with poor survival [42]. Besides Gq and Gi proteins, LPAR3 can also activate G12/13, increase dephosphorylation and nuclear translocation of YAP, and induce migration of ovarian cancer cells [59]. In addition, the LPA/LPAR3 signaling may initiate mutation-independent epithelial-to-mesenchymal transition (EMT) through β1-integrin-dependent activation of Wnt/β-catenin signaling [60]. Pharmacological suppression of LPAR3 would suppress motility and invasion in various cancers, including hamster pancreatic cancer cells [61], human triple-negative breast cancers [62], fibrosarcoma HT1080 cells, and osteosarcoma HOS cells [63]. Direct targeting of LPAR3 by miR-15b has been shown to repress cell proliferation and drive the senescence and apoptosis of ovarian cancer cells through the PI3K/Akt pathway [64], suggesting the potential mRNA treatment against LPAR3.
2.4. LPAR4
In contrast to LPAR1–3, LPAR4 and LPAR5 negatively affected cancer cell proliferation and motility [65]. LPAR4 attenuates tumor motility and colony formation in colon cancer cell lines. Knockdown of LPAR4 in the long-term 5FU treated DLD1 cells increased cell motility [66,67]. Similarly, LPAR4 depletion increases tumor motility in pancreatic cancer cells [65] and increases tumor proliferation in head and neck carcinoma [68]. Another recent study by Eino et al. found that LPAR4 is critical for developing a fine capillary network in brain tumors [69]. LPAR4 promotes endothelial cell-cell adhesion and VCAM-1 expression via RhoA/ROCK signaling, which may enhance anti-PD1 therapy efficacy and lymphocyte infiltration [69]. However, a contradictory pro-tumorigenesis was found in fibrosarcoma. In HT1080 cells, LPAR4 promotes cell invasion and invadopodium formation via cAMP/EPAC/Rac1 signaling [70]. Of interest, LPAR4/6 are necessary for embryogenic angiogenesis to activate YAP and transcriptional coactivator TAZ via the G12/G13 signaling pathway [71]. In the malignancies, YAP promotes cancer proliferation and migration in bladder cancers through YAP-Mask2 [72] and lung cancers through LKB1-YAP-human telomerase RAN (hTERC), respectively [73]. These suggested the involvement of LPAR4 in YAP-mediated cancer progression.
2.5. LPAR5
LPAR5 was considered a negative regulator in cancer cell motility and survival [69]. The inhibitory effect of LPAR5 on cell motility has been shown in pancreatic cancer [69] and sarcoma [74]. Nevertheless, contradictory effects of LPAR5 were found in different cancers. Okabe et al. reported upregulation of the LPAR5 gene with aberrant unmethylated status enhanced cell proliferation and motility in rat liver-derived hepatoma RH7777 and lung-derived adenocarcinoma RLCNR cells [75]. Blocking LPAR5 in thyroid cancer with a selective LPA5 antagonist TCLPA5 attenuated cancer proliferation and migration via PI3K/Akt signaling in vivo and in vitro [76]. Moreover, depletion of LPAR5 in murine B16-F10 melanoma resulted in fewer lung metastasis [77]. Interestingly, LPAR5 appears to mediate chemorepulsion in response to LPA. The underlying mechanism was proposed to be mediated via a non-canonical elevation of cAMP along with reduced PIP3 signaling in melanoma B16 cells [78]. LPAR5 expression is markedly increased in long-term cisplatin-treated melanoma cells [8]. Therefore, LPAR5 knockdown significantly conferred chemo-resistance and enhanced cancer cell survival [8]. In addition to the cancer cell growth and metastasis, LPAR5 was shown to suppress the function of CD8-positive cytotoxic T cells by inhibiting intracellular Ca2+ mobilization and ERK activation, suggesting LPAR5 might act as a mediator of immune suppression [79].
2.6. LPAR6
Reports regarding LPAR6 in cancer are relatively limited compared with other LPARs [2]. Several articles investigated the role of LPAR6 in liver, pancreatic, and colon cancers. LPAR6 expression in hepatocellular carcinoma correlated with poorer survival [80] and increased microvascular invasion [81]. Moreover, LPAR6 promotes hepatocellular carcinoma proliferation via the NCOA3-LPAR6-HGF signaling cascade, and the tumor-suppressive effect by depletion of LPAR6 is similar to that of anti-HGF treatment [82]. In pancreatic cancer, LPAR6 knockdown also inhibited cancer invasion and colony formation [67]. However, LPAR6 can, by contrast, be a negative regulator in different cancers. LPAR6 knockdown caused the formation of larger colonies [83] and enhanced motility in colon DLD1 and HCT116 cancer cells [67]. The role of LPAR6 in various cancer types should be further characterized in the future.
3. LPARs and Cancer Resistance to Chemotherapy and Radiation
Radiation therapy and chemotherapy are both primary cancer treatments. However, cancers often developed resistance and long-term side effects after radiotherapy and chemotherapy. Therefore, it is crucial to understand the molecular and physiological changes in patients with cancer receiving these therapies, which will help develop a better treatment for the patients. Remarkedly, the ENPP2 gene, which encoded LPA generating enzyme ATX, was found to serve as one of 90 drug-resistance genes [84]. Up-regulation of the ATX-LPA axis was found in refractory diseases treated by radiation and chemotherapy [85]. These results suggested ATX-LPA axis might be involved in chemo- and radio-resistances in cancers.
Chemotherapies such as docetaxel and doxorubicin would induce cancer cell apoptosis through ceramide formation [86,87]. Ceramide, belonging to sphingolipids and converted to sphingosine by ceramidases, promotes apoptosis by releasing cytochrome C from mitochondria and activates caspases. The pro-apoptotic effects of ceramide are counteracted by LPA-LPAR1-induced nuclear factor erythroid-2-related factor-2 (Nrf2) stabilization, which upregulates multidrug-resistant transporters and antioxidant proteins [88]. Another pathway of LPA-mediated chemo-resistance is to enhance sphingosine 1-phosphate (S1P) by activating phospholipase D (PLD) [89] and increasing sphingosine kinase 1 (Sphk1) [90]. Histone deacetylase (HDAC) activation is also involved in chemo-resistance. LPA activated HDAC and subsequently prevented cancer apoptosis which was induced by HDAC inhibitors [91]. Therefore, targeting ATX was considered the center to overcome drug resistance. For example, ONO-8430506, an ATX inhibitor, had a synergic effect with doxorubicin to reduce tumor growth and lung metastasis in an orthotopic mice breast cancer model [92]. In addition, novel benzene-sulfonamide analogues acting as ATX antagonists can reduce paclitaxel resistance in 4T1 murine breast cancer cells and B16 murine melanoma cells [93].
Besides ATX antagonists, targeting individual LPARs also has potency against resistance [94]. Recent studies emphasized the effects of LPAR2 and LPAR5 on drug resistance. Interestingly, LPAR2 promoted the acquisition of chemo-resistance, whereas LPAR5 suppressed chemo-protection [9,10]. Administration of the LPAR2 agonist, GRI-977143, in human lung A549 adenocarcinoma cells with long-term cisplatin treatment increased cell survival rate [10]. Similar results for LPAR2 mediated cisplatin-resistance was found in melanoma A375 cells [11] and fibrosarcoma HT1080 cells [12]. The Gi and LPAR2 interacting proteins, TRIP6 and NHERF2, were believed to involve LPAR2-mediated chemo-resistance [12,54]. Furthermore, LPAR2-mediated small GTPase Arf6 activation contributes to Sunitinib and Temsirolimus resistance in renal 786-O renal cell carcinoma cells [95]. Silencing of ArfGAPs, AMAP1, and EPB41L5 enhanced the drug sensitivity, suggesting Arf6-mediated LPAR2 recycling might be the key for chemotherapy resistance. Interestingly, the decline of G protein signaling regulators (RGS) might also participate in LPA-mediated chemo-resistance via reducing de-activation of G protein-coupled receptors (GPCR). For example, RGS10 and RGS17 knockdown conferred cisplatin-resistance in ovarian cancer cells [96], whereas increasing RGS17 in nasopharyngeal cancer improves sensitivity to 5-fluorouracil [97]. The detailed mechanism of LPAR5 is not clarified, so further investigation is required. Notably, LPAR2 is imperative to protect normal organs, especially intestinal epithelium and myeloid progenitors, against ceramide-induced cell death. Deng et al. found the LPA administration protected IEC-6 cells from camptothecin-induced apoptosis through inhibiting caspase-3 activation mediated by the attenuation of caspase-9 activation [55]. The role of LPAR3 in chemo-resistance remains controversial. A selective LPAR3 agonist, OMPT, would reduce the cell viability of cisplatin-treated lung A549 adenocarcinoma cells [10]. Another article indicated that LPAR3 conferred chemo-resistance by upregulating multidrug resistance-related genes because the cell survival in LPAR3-expressing rat hepatoma cells treated with cisplatin or doxorubicin was higher than controls [20]. Conclusively, the above information provides a horizon to ameliorate drug resistance by targeting LPAR2, LPAR3, and LPAR5 in different cancers.
For radiotherapy, it is therapeutically important to lower the radio-resistance of cancer cells but to protect normal tissue injury from radiation. In response to radiation injury, adipose tissue increased ATX production and LPARs expressions to enhance LPA signaling [98]. Since ATX responses were found to precede the radiation-induced inflammatory cascade in several in vivo studies [99], the ATX-LPA-inflammatory cycle may play a vital role in desensitizing cancer cells to radiotherapy. Different LPARs mediate radio-resistance in different cancer cells, and LPAR1 is a pronounced receptor to mediate radio-resistance because of its ability to stabilize Nrf2 via PI3K signaling [86,88,98]. Nrf2 mediates multiple antioxidant enzymes, such as glutathione S-transferase A2 (GSTA2) and NADPH quinone oxidoreductase 1 (NQO1), to protect cancer cells from oxidative stress [100]. Nrf2 over-expression enables cancer cells against radiation injury by cross-talk with multiple LPA-induced DNA repair proteins expressions (e.g., ATM, ATR, PARP-1) to activate NF-κB signaling [101,102]. It implies that Nrf2 destabilization may be a potential strategy to overcome radio-resistance [103].
Interestingly, not only ATX, but LPAR2 expression are also upregulated in response to radiation. Radiation-induced DNA double-strand breaks via ATM-mediated NF-κB activation, resulting in increment of serum ATX/LPA levels and subsequently LPAR2 activation to accelerate γH2AX histones resolution [101]. In breast cancer adipose tissue, Meng et al. found radiation-induced LPAR1, LPAR2, ATX, cyclooxygenase-2, and numerous inflammatory mediators increased in response to γ-radiation ranging from 0.25 to 5 Gy, resulting in blunting radio-sensitivity of breast cancer cells [98]. LPAR2 is imperative to protect normal organs, especially intestinal epithelium and myeloid progenitors, against ceramide-induced cell death. In a radiation-exposed murine model, intraperitoneal administration of synthetic LPA, octadecenyl thiophosphate (OTP), may reduce radiation-associated mortality under lethal dose (6–12 Gy) [104]. By contrast, LPAR2 knockout mice (LPAR2−/−) can no longer be rescued from radiation-induced apoptosis upon OTP administration. In another whole-body radiation mice model, administration of the LPAR2 agonist, GRI977143, significantly increased mean survival by saving apoptotically condemned cells from radiation-induced cell death [105]. In contrast, DBIBB, a more specific LPAR2 agonist without binding affinity to other LPARs, was found to alleviate the acute hematopoietic and gastrointestinal radiation responses for wild-type C57BL/6 mice when drug treatment was delayed to 72 h post-irradiation. In human hematopoietic progenitors, DBIBB significantly enhanced cell survival and the differentiation of the myeloid cell lineage after irradiation [106]. Moreover, DBIBB treatment increased the post-radiation survival of the rat intestinal crypt epithelium-like cell line IEC-6 cells by augmenting DNA repair via LPAR2 signaling [106].
4. LPA and Chemotherapy-Induced Neuropathic Pain (NP)
Cancer patients treated with chemotherapy, such as paclitaxel, usually suffer with severe peripheral neuropathic pain [14]. Mechanisms that underlie NP are complex for multiple pathways activated through inflammatory molecules, growth factors, lipid metabolites, and cellular responses in peripheral and central nervous systems. Aberrant LPA production and signaling were pivotal for NP initiation in which the LPA-LPAR1 axis mediated peripheral mechanisms [107], including dorsal root demyelination, PKCγ, calcium channel subunit α2δ1 up-regulation [107], neuronal growth cones retraction, and morphological changes of Schwann cells [108]. LPAR3, together with LPAR1, was also responsible for NP by amplifying central LPA production via glial cells [109]. Interestingly, Uchida et al. found that LPA production significantly increased within 24 h after the first paclitaxel treatment [14]. The paclitaxel-induced mechanical allodynia disappeared in LPAR1- and LPAR3- knockout mice [14], as well as mice pretreated with LPAR1/3 antagonist Ki16425 [110]. Moreover, significant attenuation of nerve demyelination was also observed in LPAR1-/LPAR3- knockout mice [110]. In addition, the LPAR5 signaling was shown to enhance microglial migration/cytotoxicity and induced a distinct pro-inflammatory signature via the protein kinase D (PKD) pathway [111]. In summary, antagonizing LPAR1/3/5 may be a promising strategy to manage chemotherapy-induced NP.
5. Application of LPAR Agonist/Antagonist in Cancer
Many LPAR antagonists were developed to attenuate proliferation, progression, invasion, and metastasis in some preclinical studies. BrP-LPA, a pan-LPAR antagonist, was used to treat breast MDA-MB-231 cancer cells, and Zhang et al. found more decreased intra-tumoral blood vessel density treated by BrP-LPA (10 mg/kg) compared with those by paclitaxel (10 mg/kg) [112]. Through LPAR2, BrP-LPA may also sensitize vascular endothelial cells in mice GL-261 glioma cells to improve malignant glioma response to radiation therapy [113]. Furthermore, Ki16425 is one of the most investigated agents targeting both LPAR1 and LPAR3, and its anti-tumor effects were validated in several cancer cells [31,114,115]. As discussed above, Ki16425 may benefit the reduction of chemotherapy-induced NP [110]. A more potent dual LPAR1/3 antagonist, Debio-0719, was found to reduce pulmonary and bone metastases of murine 4T1 breast cancer cells without affecting primary tumor size [116]. Similar results were observed in the MDA-MB-231T experimental metastasis mouse model, suggesting Debio-0719, a potent therapeutic agent to prevent breast cancer metastasis and induce dormancy at secondary tumor sites [117]. Another LPAR1/3 antagonist, Ki16198, effectively suppressed pancreatic cancer invasion and metastasis partially through inhibiting MMP production [118].
Other LPAR antagonists were less discussed, while H2L5186303 for LPAR2 attenuated fibrosarcoma HT1080 cells invasiveness [119]. The 4-methylene-2-octyl-5-oxotetra-hydrofuran-3-carboxylic acid (C75) and xanthenylacetic acid (XAA) for LPAR6 ameliorated hepatocellular carcinoma growth through modulating mitochondria homeostasis and arresting cancer cells at the G1-phase cell cycle [120].
As mentioned above, targeting LPAR5 was proposed to be a good option against cancer development in certain cancers [8,9,65]. LPAR5 antagonist TCLPA5 attenuated cancer proliferation and migration in thyroid cancer [76]. Moreover, depletion of LPAR5 in murine B16-F10 melanoma resulted in fewer lung metastasis [77], suggesting pharmaceutic inhibition of LPAR5 may also manage melanoma-mediated metastasis. On the other hand, since LPAR5 contributes to NP by affecting microglia biology and induces a distinct pro-inflammatory phenotype [111], various antagonists such as compound 7e [121] and AS2717638 [122] were considered to control chemotherapy-induced NP [76,111].
6. Clinical Trials of LPAR Antagonists
Since the extensive involvement of LPARs in cancers, questions have emerged regarding their clinical significance. However, as of now, there are no therapeutic clinical trials of LPAR antagonists in cancer therapy. A diagnostic trial (NCT00986206) enrolled 525 patients with ovarian cancer, or at risk for ovarian cancer, and aimed to develop a serum- or plasma-based assay to quantitate LPA levels in the early detection of ovarian cancer [123], but there was no conclusive result.
Apart from cancer, LPAR1 antagonists, BMS-986020, and BMS-986278 focused on idiopathic pulmonary fibrosis (IPF) and aimed to prove the application of LPAR. In the case of BMS-986020, clinical trials including NCT01766817 (Phase 2) [124,125], NCT02068053 (Phase 1) [126], and NCT02101125 (Phase 1) [127] were completed. The NCT01766817 study revealed that BMS-986020 treatment significantly slowed the pulmonary function decline compared with placebo [124]. Regarding BMS-986278, the Phase 1 study, NCT03429933, was completed [128], and a Phase 2 trial, NCT04308681, is recruiting participants [129]. Interestingly, there is a diagnostic trial recruiting IPF patients to validate the safety, tolerability, kinetics, and repeatability of an LPAR1 PET ligand 18F-BMS-986327 [130]. Moreover, a Phase 2 study (NCT01651143) focused on SAR-100842, a potent, selective oral antagonist of the LPAR1, for diffuse cutaneous systemic sclerosis. The results suggested that SAR-100842 is safe, moderately effective, and well-tolerated in patients, whereas a further larger controlled trial is required to confirm the clinical efficacy [131]. Table 1 summarizes the clinical trials targeting LPARs.
Table 1.
A summary of clinical trials targeting LPA receptors (LPARs).
| No. | ClinicalTrials.gov Identifier | Mechanism | Project Title | Study Design | Outcome |
|---|---|---|---|---|---|
| 1 | NCT01766817 | LPAR1 antagonist (BMS-986020) | Safety and efficacy of a lysophosphatidic acid receptor antagonist in idiopathic pulmonary fibrosis | Phase 2; parallel-arm, multicenter, randomized, double-blind, placebo-controlled trial; 143 patients with idiopathic pulmonary fibrosis were randomized and treated. | BMS-986020 600 mg bid treatment for 26 weeks significantly slowed the lung function decline compared with placebo [124,125]. |
| 2 | NCT02068053 | LPAR1 antagonist (BMS-986020) | Absorption, distribution, metabolism, and excretion (ADME) study of BMS-986020 | Phase 1; a single group assignment to investigate the pharmacokinetic, biotransformation, routes of elimination, and mass balance of BMS-986020 in humans; 6 healthy participants. | It was completed in April 2014. No results were posted [126]. |
| 3 | NCT02101125 | LPAR1 antagonist (BMS-986020) | Drug interaction study with Rosuvastatin | Phase 1; an open-label, single-sequence study to evaluate the effect of concomitant administration of BMS-986020 on the single-dose pharmacokinetics of Rosuvastatin in healthy subjects; 26 healthy participants. | It was completed in May 2014. No results were posted [127]. |
| 4 | NCT03429933 | LPAR1 antagonist (BMS-986278) | A study of experimental medication BMS-986278 given to healthy participants | Phase 1; a double-blind, placebo-controlled, randomized, single and multiple ascending dose study of oral BMS-986278 administration in healthy participants; 112 healthy participants. | It was completed in March 2019. No results were posted [128]. |
| 5 | NCT04308681 | LPAR1 antagonist (BMS-986278) | A study measuring the effectiveness, safety, and tolerability of BMS-986278 in participants with lung fibrosis | Phase 2; a multicenter, randomized, double-blind, placebo-controlled study; 360 patients with lung fibrosis. | It started in July 2020 and is currently still recruiting [129]. |
| 6 | NCT04069143 | LPAR1 tracer (BMT-136088) | Safety, tolerability, kinetics, and repeatability of the novel LPA1 PET ligand 18F-BMS-986327 | Phase 1; an open-label study; 20 participants (healthy or with idiopathic pulmonary fibrosis). | It started in October 2019 and is currently still recruiting [130]. |
| 7 | NCT01651143 | LPAR1 antagonist (SAR100842) | Proof of biological activity of SAR100842 in systemic sclerosis | Phase 2; a double-blind, randomized, placebo-controlled study; 32 patients with diffuse cutaneous systemic sclerosis. | SAR100842 was well tolerated in patients. The modified Rodnan skin thickness score improved during the study, although the difference was not significant [131]. |
7. Limitation of LPAR Antagonist in Cancer
Despite the apparent relevance of LPA signaling in cancer initiation, progression, metastasis, and developments of resistance against chemo- and radio-induced cancer cell death, no inhibitors targeting LPARs have progressed to cancer-related clinical trials thus far. One possible reason is that ATX, lipid phosphate phosphatase, and non-GPCR LPARs signaling have been implicated in tumor growth and metastasis, suggesting the limitation by attenuating LPA-GPCR signaling only. Another reason is that the LPA receptor subtypes might exert distinct effects depending on the type and cellular origin of the individual carcinoma and thereby complicate the use of LPAR antagonists. The third reason is that LPAR antagonists might have cross-activities on multiple receptors and other key targets, making individuals more complicated by jeopardizing physiological activities that required specific signaling. Nevertheless, clinical trials of LPAR antagonists on cancer research could be anticipated to be conducted in the future, especially concerning its role as adjuvants to traditional chemo- and/or radio-therapy to reduce resistance, prolong cancer remission, and lower treatment-associated adverse events. Additionally, LPA signaling antagonism could have high clinical significance to minimize side effects, such as drug-induced pain, acute radiation syndrome, or radiation-induced fibrosis. To conclude, further perspectives should shed light on precise targeting of particular LPARs in each cancer to make LPAR antagonists clinically beneficial, thus improving the prognosis of cancer patients.
8. Conclusions
In conclusion, it deserves our attention that multiple therapeutic agents undergo clinical trials or preclinical evaluation for various diseases via inhibition of LPA signaling. Their safety is generally acceptable, and the LPAR antagonists are potentially effective and novel for improving pain and current cancer therapies. In general, being inflammatory mediators, LPA signaling inhibitors could be potential therapeutic modalities for chemoprevention, enhancing the efficacy of chemotherapy and radiotherapy and improving prognosis.
Author Contributions
Conceptualization: C.-C.C.; methodology: C.-C.C.; formal analysis: Y.-C.L. and C.-C.C.; investigation: all authors; resources: Y.-H.L. and C.-C.C.; data curation: Y.-C.L. and C.-C.C.; writing—original draft preparation: Y.-H.L. and C.-C.C.; writing—review and editing: Y.-C.L. and C.-C.C.; visualization: C.-C.C.; supervision: Y.-C.L. and C.-C.C.; project administration: C.-C.C.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Choi J.W., Herr D.R., Noguchi K., Yung Y.C., Lee C.W., Mutoh T., Lin M.E., Teo S.T., Park K.E., Mosley A.N., et al. LPA receptors: Subtypes and biological actions. Annu. Rev. Pharmacol. Toxicol. 2010;50:157–186. doi: 10.1146/annurev.pharmtox.010909.105753. [DOI] [PubMed] [Google Scholar]
- 2.Taniguchi R., Inoue A., Sayama M., Uwamizu A., Yamashita K., Hirata K., Yoshida M., Tanaka Y., Kato H.E., Nakada-Nakura Y., et al. Structural insights into ligand recognition by the lysophosphatidic acid receptor LPA6. Nature. 2017;548:356–360. doi: 10.1038/nature23448. [DOI] [PubMed] [Google Scholar]
- 3.Geraldo L., Spohr T., Amaral R., Fonseca A., Garcia C., Mendes F.A., Freitas C., Fabio dosSantos M., Lima F. Role of lysophosphatidic acid and its receptors in health and disease: Novel therapeutic strategies. Signal Transduct. Target Ther. 2021;6:45. doi: 10.1038/s41392-020-00367-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang X., Li M., Yin N., Zhang J. The expression regulation and biological function of autotaxin. Cells. 2021;10:939. doi: 10.3390/cells10040939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gellett A.M., Kharel Y., Sunkara M., Morris A.J., Lynch K.R. Biosynthesis of alkyl lysophosphatidic acid by diacylglycerol kinases. Biochem. Biophys. Res. Commun. 2012;422:758–763. doi: 10.1016/j.bbrc.2012.05.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gotoh M., Fujiwara Y., Yue J., Liu J., Lee S., Fells J., Uchiyama A., Murakami-Murofushi K., Kennel S., Wall J., et al. Controlling cancer through the autotaxin-lysophosphatidic acid receptor axis. Biochem. Soc. Trans. 2012;40:31–36. doi: 10.1042/BST20110608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benesch M., MacIntyre I., McMullen T., Brindley D.N. Coming of age for autotaxin and lysophosphatidate signaling: Clinical applications for preventing, detecting and targeting tumor-promoting inflammation. Cancers. 2018;10:73. doi: 10.3390/cancers10030073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Minami K., Ueda N., Maeda H., Ishimoto K., Otagaki S., Tsujiuchi T. Modulation of chemoresistance by lysophosphatidic acid (LPA) signaling through LPA5 in melanoma cells treated with anticancer drugs. Biochem. Biophys. Res. Commun. 2019;517:359–363. doi: 10.1016/j.bbrc.2019.07.092. [DOI] [PubMed] [Google Scholar]
- 9.Ishimoto K., Minami A., Minami K., Ueda N., Tsujiuchi T. Different effects of lysophosphatidic acid receptor-2 (LPA2) and LPA5 on the regulation of chemoresistance in colon cancer cells. J. Recept. Signal Transduct. Res. 2021;41:93–98. doi: 10.1080/10799893.2020.1794002. [DOI] [PubMed] [Google Scholar]
- 10.Ueda N., Minami K., Ishimoto K., Tsujiuchi T. Effects of lysophosphatidic acid (LPA) receptor-2 (LPA2) and LPA3 on the regulation of chemoresistance to anticancer drug in lung cancer cells. Cell Signal. 2020;69:109551. doi: 10.1016/j.cellsig.2020.109551. [DOI] [PubMed] [Google Scholar]
- 11.Minami K., Ueda N., Ishimoto K., Tsujiuchi T. Lysophosphatidic acid receptor-2 (LPA2)-mediated signaling enhances chemoresistance in melanoma cells treated with anticancer drugs. Mol. Cell. Biochem. 2020;469:89–95. doi: 10.1007/s11010-020-03730-w. [DOI] [PubMed] [Google Scholar]
- 12.Minami K., Ueda N., Ishimoto K., Kurisu R., Takamoto M., Ikeda H., Tsujiuchi T. Cooperation of G12/13 and Gi proteins via lysophosphatidic acid receptor-2 (LPA2) signaling enhances cancer cell survival to cisplatin. Biochem. Biophys. Res. Commun. 2020;532:427–432. doi: 10.1016/j.bbrc.2020.08.087. [DOI] [PubMed] [Google Scholar]
- 13.Radhakrishnan R., Ha J.H., Jayaraman M., Liu J., Moxley K.M., Isidoro C., Sood A.K., Song Y.S., Dhanasekaran D.N. Ovarian cancer cell-derived lysophosphatidic acid induces glycolytic shift and cancer-associated fibroblast-phenotype in normal and peritumoral fibroblasts. Cancer Lett. 2019;442:464–474. doi: 10.1016/j.canlet.2018.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Uchida H., Nagai J., Ueda H. Lysophosphatidic acid and its receptors LPA1 and LPA3 mediate paclitaxel-induced neuropathic pain in mice. Mol. Pain. 2014;10:71. doi: 10.1186/1744-8069-10-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Orosa B., García S., Conde C. The autotaxin-lysophosphatidic acid pathway in pathogenesis of rheumatoid arthritis. Eur. J. Pharmacol. 2015;765:228–233. doi: 10.1016/j.ejphar.2015.08.028. [DOI] [PubMed] [Google Scholar]
- 16.Kraemer M.P., Mao G., Hammill C., Yan B., Li Y., Onono F., Smyth S.S., Morris A.J. Effects of diet and hyperlipidemia on levels and distribution of circulating lysophosphatidic acid. J. Lipid Res. 2019;60:1818–1828. doi: 10.1194/jlr.M093096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou Y., Little P.J., Ta H.T., Xu S., Kamato D. Lysophosphatidic acid and its receptors: Pharmacology and therapeutic potential in atherosclerosis and vascular disease. Pharmacol. Ther. 2019;204:107404. doi: 10.1016/j.pharmthera.2019.107404. [DOI] [PubMed] [Google Scholar]
- 18.Yung Y.C., Stoddard N.C., Chun J. LPA receptor signaling: Pharmacology, physiology, and pathophysiology. J. Lipid Res. 2014;55:1192–1214. doi: 10.1194/jlr.R046458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu Y. Targeting Lysophosphatidic Acid in Cancer: The issues in moving from bench to bedside. Cancers. 2019;11:1523. doi: 10.3390/cancers11101523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Okabe K., Hayashi M., Kato K., Okumura M., Fukui R., Honoki K., Fukushima N., Tsujiuchi T. Lysophosphatidic acid receptor-3 increases tumorigenicity and aggressiveness of rat hepatoma RH7777 cells. Mol. Carcinog. 2013;52:247–254. doi: 10.1002/mc.21851. [DOI] [PubMed] [Google Scholar]
- 21.Lee M., Choi S., Halldén G., Yo S.J., Schichnes D., Aponte G.W. P2Y5 is a G(alpha)i, G(alpha)12/13 G protein-coupled receptor activated by lysophosphatidic acid that reduces intestinal cell adhesion. Am. J. Physiol. Gastrointest. Liver Physiol. 2009;297:G641–G654. doi: 10.1152/ajpgi.00191.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rao R., Salloum R., Xin M., Lu Q.R. The G protein Gαs acts as a tumor suppressor in sonic hedgehog signaling-driven tumorigenesis. Cell Cycle. 2016;15:1325–1330. doi: 10.1080/15384101.2016.1164371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luo J., Yu F.-X. GPCR-Hippo Signaling in Cancer. Cells. 2019;8:426. doi: 10.3390/cells8050426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ha J.H., Ward J.D., Radhakrishnan R., Jayaraman M., Song Y.S., Dhanasekaran D.N. Lysophosphatidic acid stimulates epithelial to mesenchymal transition marker Slug/Snail2 in ovarian cancer cells via Gαi2, Src, and HIF1α signaling nexus. Oncotarget. 2016;7:37664–37679. doi: 10.18632/oncotarget.9224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sahay D., Leblanc R., Grunewald T.G., Ambatipudi S., Ribeiro J., Clézardin P., Peyruchaud O. The LPA1/ZEB1/miR-21-activation pathway regulates metastasis in basal breast cancer. Oncotarget. 2015;6:20604–20620. doi: 10.18632/oncotarget.3774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park S.Y., Jeong K.J., Panupinthu N., Yu S., Lee J., Han J.W., Kim J.M., Lee J.S., Kang J., Park C.G., et al. Lysophosphatidic acid augments human hepatocellular carcinoma cell invasion through LPA1 receptor and MMP-9 expression. Oncogene. 2011;30:1351–1359. doi: 10.1038/onc.2010.517. [DOI] [PubMed] [Google Scholar]
- 27.Ren Z., Zhang C., Ma L., Zhang X., Shi S., Tang D., Xu J., Hu Y., Wang B., Zhang F., et al. Lysophosphatidic acid induces the migration and invasion of SGC-7901 gastric cancer cells through the LPA2 and Notch signaling pathways. Int. J. Mol. Med. 2019;44:67–78. doi: 10.3892/ijmm.2019.4186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fukushima K., Otagaki S., Takahashi K., Minami K., Ishimoto K., Fukushima N., Honoki K., Tsujiuchi T. Promotion of cell-invasive activity through the induction of LPA receptor-1 in pancreatic cancer cells. J. Recept. Signal Transduct. Res. 2018;38:367–371. doi: 10.1080/10799893.2018.1531889. [DOI] [PubMed] [Google Scholar]
- 29.Fukushima K., Takahashi K., Yamasaki E., Onishi Y., Fukushima N., Honoki K., Tsujiuchi T. Lysophosphatidic acid signaling via LPA1 and LPA3 regulates cellular functions during tumor progression in pancreatic cancer cells. Exp. Cell Res. 2017;352:139–145. doi: 10.1016/j.yexcr.2017.02.007. [DOI] [PubMed] [Google Scholar]
- 30.Obo Y., Yamada T., Furukawa M., Hotta M., Honoki K., Fukushima N., Tsujiuchi T. Frequent mutations of lysophosphatidic acid receptor-1 gene in rat liver tumors. Mutat. Res. 2009;660:47–50. doi: 10.1016/j.mrfmmm.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 31.Zhao P.F., Wu S., Li Y., Bao G., Pei J.Y., Wang Y.W., Ma Q., Sun H.J., Damirin A. LPA receptor1 antagonists as anticancer agents suppress human lung tumours. Eur. J. Pharmacol. 2020;868:172886. doi: 10.1016/j.ejphar.2019.172886. [DOI] [PubMed] [Google Scholar]
- 32.Amaral R.F., Geraldo L.H.M., Einicker-Lamas M.E., Spohr T.C.L.S., Mendes F., Lima F.R.S. Microglial lysophosphatidic acid promotes glioblastoma proliferation and migration via LPA1 receptor. J. Neurochem. 2021;156:499–512. doi: 10.1111/jnc.15097. [DOI] [PubMed] [Google Scholar]
- 33.Loskutov Y.V., Griffin C.L., Marinak K.M., Bobko A., Margaryan N.V., Geldenhuys W.J., Sarkaria J.N., Pugacheva E.N. LPA signaling is regulated through the primary cilium: A novel target in glioblastoma. Oncogene. 2018;37:1457–1471. doi: 10.1038/s41388-017-0049-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Valdés-Rives S.A., Arcos-Montoya D., de la Fuente-Granada M., Zamora-Sánchez C.J., Arias-Romero L.E., Villamar-Cruz O., Camacho-Arroyo I., Pérez-Tapia S.M., González-Arenas A. LPA1 receptor promotes progesterone receptor phosphorylation through PKCα in human glioblastoma cells. Cells. 2021;10:807. doi: 10.3390/cells10040807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Okabe K., Hayashi M., Fujii M., Honoki K., Mori T., Fukushima N., Tsujiuchi T. Mutations of lysophosphatidic acid receptor genes in human osteosarcoma cells. Pathobiology. 2010;77:278–282. doi: 10.1159/000319875. [DOI] [PubMed] [Google Scholar]
- 36.Cui R., Cao G., Bai H., Zhang Z. LPAR1 regulates the development of intratumoral heterogeneity in ovarian serous cystadenocarcinoma by activating the PI3K/AKT signaling pathway. Cancer Cell Int. 2019;19:201. doi: 10.1186/s12935-019-0920-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seo E.J., Kwon Y.W., Jang I.H., Kim D.K., Lee S.I., Choi E.J., Kim K.H., Suh D.S., Lee J.H., Choi K.U., et al. Autotaxin regulates maintenance of ovarian cancer stem cells through lysophosphatidic acid-mediated autocrine mechanism. Stem Cells. 2016;34:551–564. doi: 10.1002/stem.2279. [DOI] [PubMed] [Google Scholar]
- 38.Li T.T., Alemayehu M., Aziziyeh A.I., Pape C., Pampillo M., Postovit L.M., Mills G.B., Babwah A.V., Bhattacharya M. Beta-arrestin/Ral signaling regulates lysophosphatidic acid-mediated migration and invasion of human breast tumor cells. Mol. Cancer Res. 2009;7:1064–1077. doi: 10.1158/1541-7786.MCR-08-0578. [DOI] [PubMed] [Google Scholar]
- 39.David M., Sahay D., Mege F., Descotes F., Leblanc R., Ribeiro J., Clézardin P., Peyruchaud O. Identification of heparin-binding EGF-like growth factor (HB-EGF) as a biomarker for lysophosphatidic acid receptor type 1 (LPA1) activation in human breast and prostate cancers. PLoS ONE. 2014;9:e97771. doi: 10.1371/journal.pone.0097771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang Y.L., Lin Y.C., Lin C.C., Chen W.M., Chen B.P.C., Lee H. High glucose induces VEGF-C expression via the LPA1/3-Akt-ROS-LEDGF signaling axis in human prostate cancer pc-3 cells. Cell. Physiol. Biochem. 2018;50:597–611. doi: 10.1159/000494177. [DOI] [PubMed] [Google Scholar]
- 41.Lin Y.C., Chen C.C., Chen W.M., Lu K.Y., Shen T.L., Jou Y.C., Shen C.H., Ohbayashi N., Kanaho Y., Huang Y.L., et al. LPA1/3 signaling mediates tumor lymphangiogenesis through promoting CRT expression in prostate cancer. Biochim. Biophys. Acta Mol. Cell Biol. Lipids. 2018;1863:1305–1315. doi: 10.1016/j.bbalip.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 42.Yu S., Murph M.M., Lu Y., Liu S., Hall H.S., Liu J., Stephens C., Fang X., Mills G.B. Lysophosphatidic acid receptors determine tumorigenicity and aggressiveness of ovarian cancer cells. J. Natl. Cancer Inst. 2008;100:1630–1642. doi: 10.1093/jnci/djn378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fujita T., Miyamoto S., Onoyama I., Sonoda K., Mekada E., Nakano H. Expression of lysophosphatidic acid receptors and vascular endothelial growth factor mediating lysophosphatidic acid in the development of human ovarian cancer. Cancer Lett. 2003;192:161–169. doi: 10.1016/S0304-3835(02)00713-9. [DOI] [PubMed] [Google Scholar]
- 44.Huang M.C., Lee H.Y., Yeh C.C., Kong Y., Zaloudek C.J., Goetzl E.J. Induction of protein growth factor systems in the ovaries of transgenic mice overexpressing human type 2 lysophosphatidic acid G protein-coupled receptor (LPA2) Oncogene. 2004;23:122–129. doi: 10.1038/sj.onc.1206986. [DOI] [PubMed] [Google Scholar]
- 45.Fang X., Yu S., Bast R.C., Liu S., Xu H.J., Hu S.X., LaPushin R., Claret F.X., Aggarwal B.B., Lu Y., et al. Mechanisms for lysophosphatidic acid-induced cytokine production in ovarian cancer cells. J. Biol. Chem. 2004;279:9653–9661. doi: 10.1074/jbc.M306662200. [DOI] [PubMed] [Google Scholar]
- 46.Pustilnik T.B., Estrella V., Wiener J.R., Mao M., Eder A., Watt M.A., Bast R.C., Jr., Mills G.B. Lysophosphatidic acid induces urokinase secretion by ovarian cancer cells. Clin. Cancer Res. 1999;5:3704–3710. [PubMed] [Google Scholar]
- 47.Jeong K.J., Park S.Y., Seo J.H., Lee K.B., Choi W.S., Han J.W., Kang J.K., Park C.G., Kim Y.K., Lee H.Y. Lysophosphatidic acid receptor 2 and Gi/Src pathway mediate cell motility through cyclooxygenase 2 expression in CAOV-3 ovarian cancer cells. Exp. Mol. Med. 2008;40:607–616. doi: 10.3858/emm.2008.40.6.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Park J., Jang J.H., Oh S., Kim M., Shin C., Jeong M., Heo K., Park J.B., Kim S.R., Oh Y.S. LPA-induced migration of ovarian cancer cells requires activation of ERM proteins via LPA1 and LPA2. Cell Signal. 2018;44:138–147. doi: 10.1016/j.cellsig.2018.01.007. [DOI] [PubMed] [Google Scholar]
- 49.Yun C.C., Sun H., Wang D., Rusovici R., Castleberry A., Hall R.A., Shim H. LPA2 receptor mediates mitogenic signals in human colon cancer cells. Am. J. Physiol. Cell Physiol. 2005;289:C2–C11. doi: 10.1152/ajpcell.00610.2004. [DOI] [PubMed] [Google Scholar]
- 50.Li M., Xiao D., Zhang J., Qu H., Yang Y., Yan Y., Liu X., Wang J., Liu L., Wang J., et al. Expression of LPA2 is associated with poor prognosis in human breast cancer and regulates HIF-1α expression and breast cancer cell growth. Oncol. Rep. 2016;36:3479–3487. doi: 10.3892/or.2016.5206. [DOI] [PubMed] [Google Scholar]
- 51.Lin S., Wang D., Iyer S., Ghaleb A.M., Shim H., Yang V.W., Chun J., Yun C.C. The absence of LPA2 attenuates tumor formation in an experimental model of colitis-associated cancer. Gastroenterology. 2009;136:1711–1720. doi: 10.1053/j.gastro.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Komachi M., Tomura H., Malchinkhuu E., Tobo M., Mogi C., Yamada T., Kimura T., Kuwabara A., Ohta H., Im D.S., et al. LPA1 receptors mediate stimulation, whereas LPA2 receptors mediate inhibition, of migration of pancreatic cancer cells in response to lysophosphatidic acid and malignant ascites. Carcinogenesis. 2009;30:457–465. doi: 10.1093/carcin/bgp011. [DOI] [PubMed] [Google Scholar]
- 53.Lin F.T., Lai Y.J., Makarova N., Tigyi G., Lin W.C. The lysophosphatidic acid 2 receptor mediates down-regulation of Siva-1 to promote cell survival. J. Biol. Chem. 2007;282:37759–37769. doi: 10.1074/jbc.M705025200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shuyu E., Lai Y.J., Tsukahara R., Chen C.S., Fujiwara Y., Yue J., Yu J.H., Guo H., Kihara A., Tigyi G., et al. Lysophosphatidic acid 2 receptor-mediated supramolecular complex formation regulates its antiapoptotic effect. J. Biol. Chem. 2009;284:14558–14571. doi: 10.1074/jbc.M900185200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Deng W., Wang D.A., Gosmanova E., Johnson L.R., Tigyi G. LPA protects intestinal epithelial cells from apoptosis by inhibiting the mitochondrial pathway. Am. J. Physiol. Gastrointest. Liver Physiol. 2003;284:G821–G829. doi: 10.1152/ajpgi.00406.2002. [DOI] [PubMed] [Google Scholar]
- 56.Zuckerman V., Sokolov E., Swet J.H., Ahrens W.A., Showlater V., Iannitti D.A., Mckillop I.H. Expression and function of lysophosphatidic acid receptors (LPARs) 1 and 3 in human hepatic cancer progenitor cells. Oncotarget. 2016;7:2951–2967. doi: 10.18632/oncotarget.6696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Altman M.K., Gopal V., Jia W., Yu S., Hall H., Mills G.B., McGinnis A.C., Bartlett M.G., Jiang G., Madan D., et al. Targeting melanoma growth and viability reveals dualistic functionality of the phosphonothionate analogue of carba cyclic phosphatidic acid. Mol. Cancer. 2010;9:140. doi: 10.1186/1476-4598-9-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jia W., Tran S.K., Ruddick C.A., Murph M.M. The Src homology 3 binding domain is required for lysophosphatidic acid 3 receptor-mediated cellular viability in melanoma cells. Cancer Lett. 2015;356:589–596. doi: 10.1016/j.canlet.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 59.Cai H., Xu Y. The role of LPA and YAP signaling in long-term migration of human ovarian cancer cells. Cell Commun. Signal. 2013;11:31. doi: 10.1186/1478-811X-11-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Burkhalter R.J., Westfall S.D., Liu Y., Stack M.S. Lysophosphatidic acid initiates epithelial to mesenchymal transition and induces β-Catenin-mediated transcription in epithelial ovarian carcinoma. J. Biol. Chem. 2015;290:22143–22154. doi: 10.1074/jbc.M115.641092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kato K., Yoshikawa K., Tanabe E., Kitayoshi M., Fukui R., Fukushima N., Tsujiuchi T. Opposite roles of LPA1 and LPA3 on cell motile and invasive activities of pancreatic cancer cells. Tumour. Biol. 2012;33:1739–1744. doi: 10.1007/s13277-012-0433-0. [DOI] [PubMed] [Google Scholar]
- 62.Sun K., Cai H., Duan X., Yang Y., Li M., Qu J., Zhang X., Wang J. Aberrant expression and potential therapeutic target of lysophosphatidic acid receptor 3 in triple-negative breast cancers. Clin. Exp. Med. 2015;15:371–380. doi: 10.1007/s10238-014-0306-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tanabe E., Kitayoshi M., Yoshikawa K., Shibata A., Honoki K., Fukushima N., Tsujiuchi T. Loss of lysophosphatidic acid receptor-3 suppresses cell migration activity of human sarcoma cells. J. Recept. Signal Transduct. Res. 2012;32:328–334. doi: 10.3109/10799893.2012.738689. [DOI] [PubMed] [Google Scholar]
- 64.Li G.C., Qin X.L., Song H.H., Li Y.N., Qiu Y.Y., Cui S.C., Wang Y.S., Wang H., Gong J.L. Upregulated microRNA-15b alleviates ovarian cancer through inhitbition of the PI3K/Akt pathway by targeting LPAR3. J. Cell. Physiol. 2019;234:22331–22342. doi: 10.1002/jcp.28799. [DOI] [PubMed] [Google Scholar]
- 65.Ishii S., Hirane M., Fukushima K., Tomimatsu A., Fukushima N., Tsujiuchi T. Diverse effects of LPA4, LPA5 and LPA6 on the activation of tumor progression in pancreatic cancer cells. Biochem. Biophys. Res. Commun. 2015;461:59–64. doi: 10.1016/j.bbrc.2015.03.169. [DOI] [PubMed] [Google Scholar]
- 66.Lee Z., Cheng C.T., Zhang H., Subler M.A., Wu J., Mukherjee A., Windle J.J., Chen C.K., Fang X. Role of LPA4/p2y9/GPR23 in negative regulation of cell motility. Mol. Biol. Cell. 2008;19:5435–5445. doi: 10.1091/mbc.e08-03-0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Takahashi K., Fukushima K., Onishi Y., Inui K., Node Y., Fukushima N., Honoki K., Tsujiuchi T. Lysophosphatidic acid (LPA) signaling via LPA4 and LPA6 negatively regulates cell motile activities of colon cancer cells. Biochem. Biophys. Res. Commun. 2017;483:652–657. doi: 10.1016/j.bbrc.2016.12.088. [DOI] [PubMed] [Google Scholar]
- 68.Matayoshi S., Chiba S., Lin Y., Arakaki K., Matsumoto H., Nakanishi T., Suzuki M., Kato S. Lysophosphatidic acid receptor 4 signaling potentially modulates malignant behavior in human head and neck squamous cell carcinoma cells. Int. J. Oncol. 2013;42:1560–1568. doi: 10.3892/ijo.2013.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Eino D., Tsukada Y., Naito H., Kanemura Y., Iba T., Wakabayashi T., Muramatsu F., Kidoya H., Arita H., Kagawa N., et al. LPA4-mediated vascular network formation increases the efficacy of anti-PD-1 therapy against brain tumors. Cancer Res. 2018;78:6607–6620. doi: 10.1158/0008-5472.CAN-18-0498. [DOI] [PubMed] [Google Scholar]
- 70.Harper K., Arsenault D., Boulay-Jean S., Lauzier A., Lucien F., Dubois C.M. Autotaxin promotes cancer invasion via the lysophosphatidic acid receptor 4: Participation of the cyclic AMP/EPAC/Rac1 signaling pathway in invadopodia formation. Cancer Res. 2010;70:4634–4643. doi: 10.1158/0008-5472.CAN-09-3813. [DOI] [PubMed] [Google Scholar]
- 71.Yasuda D., Kobayashi D., Akahoshi N., Ohto-Nakanishi T., Yoshioka K., Takuwa Y., Mizuno S., Takahashi S., Ishii S. Lysophosphatidic acid-induced YAP/TAZ activation promotes developmental angiogenesis by repressing Notch ligand Dll4. J. Clin. Investig. 2019;129:4332–4349. doi: 10.1172/JCI121955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dong L., Lin F., Wu W., Huang W., Cai Z. Transcriptional cofactor Mask2 is required for YAP-induced cell growth and migration in bladder cancer cell. J. Cancer. 2016;7:2132–2138. doi: 10.7150/jca.16438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.He L., Wu M.Z., Wang X.B., Qiu X.S., Wang E.H., Wu G.P. Tumor Suppressor LKB1 inhibits both the mRNA expression and the amplification of hTERC by the phosphorylation of YAP in lung cancer cells. J. Cancer. 2019;10:3632–3638. doi: 10.7150/jca.33237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Araki M., Kitayoshi M., Dong Y., Hirane M., Ozaki S., Mori S., Fukushima N., Honoki K., Tsujiuchi T. Inhibitory effects of lysophosphatidic acid receptor-5 on cellular functions of sarcoma cells. Growth Factors. 2014;32:117–122. doi: 10.3109/08977194.2014.911294. [DOI] [PubMed] [Google Scholar]
- 75.Okabe K., Hayashi M., Yamawaki Y., Teranishi M., Honoki K., Mori T., Fukushima N., Tsujiuchi T. Possible involvement of lysophosphatidic acid receptor-5 gene in the acquisition of growth advantage of rat tumor cells. Mol. Carcinog. 2011;50:635–642. doi: 10.1002/mc.20750. [DOI] [PubMed] [Google Scholar]
- 76.Zhao W.J., Zhu L.L., Yang W.Q., Xu S.J., Chen J., Ding X.F., Liang Y., Chen G. LPAR5 promotes thyroid carcinoma cell proliferation and migration by activating class IA PI3K catalytic subunit p110β. Cancer Sci. 2021;112:1624–1632. doi: 10.1111/cas.14837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lee S.C., Fujiwara Y., Liu J., Yue J., Shimizu Y., Norman D.D., Wang Y., Tsukahara R., Szabo E., Patil R., et al. Autotaxin and LPA1 and LPA5 receptors exert disparate functions in tumor cells versus the host tissue microenvironment in melanoma invasion and metastasis. Mol. Cancer Res. 2015;13:174–185. doi: 10.1158/1541-7786.MCR-14-0263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jongsma M., Matas-Rico E., Rzadkowski A., Jalink K., Moolenaar W.H. LPA is a chemorepellent for B16 melanoma cells: Action through the cAMP-elevating LPA5 receptor. PLoS ONE. 2011;6:e29260. doi: 10.1371/journal.pone.0029260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mathew D., Kremer K.N., Strauch P., Tigyi G., Pelanda R., Torres R.M. LPA5 is an inhibitory receptor that suppresses CD8 T-cell cytotoxic function via disruption of early TCR signaling. Front. Immunol. 2019;10:1159. doi: 10.3389/fimmu.2019.01159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mazzocca A., Dituri F., De Santis F., Filannino A., Lopane C., Betz R.C., Li Y.Y., Mukaida N., Winter P., Tortorella C., et al. Lysophosphatidic acid receptor LPAR6 supports the tumorigenicity of hepatocellular carcinoma. Cancer Res. 2015;75:532–543. doi: 10.1158/0008-5472.CAN-14-1607. [DOI] [PubMed] [Google Scholar]
- 81.Enooku K., Uranbileg B., Ikeda H., Kurano M., Sato M., Kudo H., Maki H., Koike K., Hasegawa K., Kokudo N., et al. Higher LPA2 and LPA6 mRNA Levels in hepatocellular carcinoma are associated with poorer differentiation, microvascular invasion and earlier recurrence with higher serum autotaxin levels. PLoS ONE. 2016;11:e0161825. doi: 10.1371/journal.pone.0161825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zheng X., Jia Y., Qiu L., Zeng X., Xu L., Wei M., Huang C., Liu C., Chen L., Han J. A potential target for liver cancer management, lysophosphatidic acid receptor 6 (LPAR6), is transcriptionally up-regulated by the NCOA3 coactivator. J. Biol. Chem. 2020;295:1474–1488. doi: 10.1074/jbc.RA119.009899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Takahashi K., Fukushima K., Otagaki S., Ishimoto K., Minami K., Fukushima N., Honoki K., Tsujiuchi T. Effects of LPA1 and LPA6 on the regulation of colony formation activity in colon cancer cells treated with anticancer drugs. J. Recept. Signal Transduct. Res. 2018;38:71–75. doi: 10.1080/10799893.2018.1426608. [DOI] [PubMed] [Google Scholar]
- 84.Vidot S., Witham J., Agarwal R., Greenhough S., Bamrah H.S., Tigyi G.J., Kaye S.B., Richardson A. Autotaxin delays apoptosis induced by carboplatin in ovarian cancer cells. Cell Signal. 2010;22:926–935. doi: 10.1016/j.cellsig.2010.01.017. [DOI] [PubMed] [Google Scholar]
- 85.Samadi N., Bekele R., Capatos D., Venkatraman G., Sariahmetoglu M., Brindley D.N. Regulation of lysophosphatidate signaling by autotaxin and lipid phosphate phosphatases with respect to tumor progression, angiogenesis, metastasis and chemoresistance. Biochimie. 2011;93:61–70. doi: 10.1016/j.biochi.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 86.Brindley D.N., Lin F.T., Tigyi G.J. Role of the autotaxin-lysophosphatidate axis in cancer resistance to chemotherapy and radiotherapy. Biochim. Biophys. Acta. 2013;1831:74–85. doi: 10.1016/j.bbalip.2012.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Huang W.C., Chen C.L., Lin Y.S., Lin C.F. Apoptotic sphingolipid ceramide in cancer therapy. J. Lipids. 2011;2011:565316. doi: 10.1155/2011/565316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Venkatraman G., Benesch M.G., Tang X., Dewald J., McMullen T.P., Brindley D.N. Lysophosphatidate signaling stabilizes Nrf2 and increases the expression of genes involved in drug resistance and oxidative stress responses: Implications for cancer treatment. FASEB J. 2015;29:772–785. doi: 10.1096/fj.14-262659. [DOI] [PubMed] [Google Scholar]
- 89.Gomez-Muñoz A., Martin A., O’Brien L., Brindley D.N. Cell-permeable ceramides inhibit the stimulation of DNA synthesis and phospholipase D activity by phosphatidate and lysophosphatidate in rat fibroblasts. J. Biol. Chem. 1994;269:8937–8943. doi: 10.1016/S0021-9258(17)37058-8. [DOI] [PubMed] [Google Scholar]
- 90.Shida D., Fang X., Kordula T., Takabe K., Lépine S., Alvarez S.E., Milstien S., Spiegel S. Cross-talk between LPA1 and epidermal growth factor receptors mediates up-regulation of sphingosine kinase 1 to promote gastric cancer cell motility and invasion. Cancer Res. 2008;68:6569–6577. doi: 10.1158/0008-5472.CAN-08-0411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ishdorj G., Graham B.A., Hu X., Chen J., Johnston J.B., Fang X., Gibson S.B. Lysophosphatidic acid protects cancer cells from histone deacetylase (HDAC) inhibitor-induced apoptosis through activation of HDAC. J. Biol. Chem. 2008;283:16818–16829. doi: 10.1074/jbc.M710177200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Benesch M.G., Tang X., Maeda T., Ohhata A., Zhao Y.Y., Kok B.P., Dewald J., Hitt M., Curtis J.M., McMullen T.P., et al. Inhibition of autotaxin delays breast tumor growth and lung metastasis in mice. FASEB J. 2014;28:2655–2666. doi: 10.1096/fj.13-248641. [DOI] [PubMed] [Google Scholar]
- 93.Banerjee S., Norman D.D., Lee S.C., Parrill A.L., Pham T.C., Baker D.L., Tigyi G.J., Miller D.D. Highly potent non-carboxylic acid autotaxin inhibitors reduce melanoma metastasis and chemotherapeutic resistance of breast cancer stem cells. J. Med. Chem. 2017;60:1309–1324. doi: 10.1021/acs.jmedchem.6b01270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rogers L.C., Davis R.R., Said N., Hollis T., Daniel L.W. Blocking LPA-dependent signaling increases ovarian cancer cell death in response to chemotherapy. Redox Biol. 2018;15:380–386. doi: 10.1016/j.redox.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hashimoto S., Mikami S., Sugino H., Yoshikawa A., Hashimoto A., Onodera Y., Furukawa S., Handa H., Oikawa T., Okada Y., et al. Lysophosphatidic acid activates Arf6 to promote the mesenchymal malignancy of renal cancer. Nat. Commun. 2016;7:10656. doi: 10.1038/ncomms10656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hooks S.B., Callihan P., Altman M.K., Hurst J.H., Ali M.W., Murph M.M. Regulators of G-Protein signaling RGS10 and RGS17 regulate chemoresistance in ovarian cancer cells. Mol. Cancer. 2010;9:289. doi: 10.1186/1476-4598-9-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yu Q., Zhang N., Jiang Y., Huang Y., Lian Y.Y., Liu T., Li N., Guan G. RGS17 inhibits tumorigenesis and improves 5-fluorouracil sensitivity in nasopharyngeal carcinoma. Onco Targets Ther. 2018;11:7591–7600. doi: 10.2147/OTT.S176002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Meng G., Tang X., Yang Z., Benesch M.G.K., Marshall A., Murray D., Hemmings D.G., Wuest F., McMullen T.P.W., Brindley D.N. Implications for breast cancer treatment from increased autotaxin production in adipose tissue after radiotherapy. FASEB J. 2017;31:4064–4077. doi: 10.1096/fj.201700159R. [DOI] [PubMed] [Google Scholar]
- 99.Meng G., Wuest M., Tang X., Dufour J., Zhao Y., Curtis J.M., McMullen T.P.W., Murray D., Wuest F., Brindley D.N. Repeated fractions of X-radiation to the breast fat pads of mice augment activation of the autotaxin-lysophosphatidate-inflammatory cycle. Cancers. 2019;11:1816. doi: 10.3390/cancers11111816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cloer E.W., Goldfarb D., Schrank T.P., Weissman B.E., Major M.B. NRF2 activation in cancer: From dna to protein. Cancer Res. 2019;79:889–898. doi: 10.1158/0008-5472.CAN-18-2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Balogh A., Shimizu Y., Lee S.C., Norman D.D., Gangwar R., Bavaria M., Moon C., Shukla P., Rao R., Ray R., et al. The autotaxin-LPA2 GPCR axis is modulated by γ-irradiation and facilitates DNA damage repair. Cell Signal. 2015;27:1751–1762. doi: 10.1016/j.cellsig.2015.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sekhar K.R., Freeman M.L. Nrf2 promotes survival following exposure to ionizing radiation. Free Radic. Biol. Med. 2015;88:268–274. doi: 10.1016/j.freeradbiomed.2015.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhou S., Ye W., Shao Q., Zhang M., Liang J. Nrf2 is a potential therapeutic target in radioresistance in human cancer. Crit. Rev. Oncol. Hematol. 2013;88:706–715. doi: 10.1016/j.critrevonc.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 104.Deng W., Shuyu E., Tsukahara R., Valentine W.J., Durgam G., Gududuru V., Balazs L., Manickam V., Arsura M., VanMiddlesworth L., et al. The lysophosphatidic acid type 2 receptor is required for protection against radiation-induced intestinal injury. Gastroenterology. 2007;132:1834–1851. doi: 10.1053/j.gastro.2007.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kiss G.N., Lee S.C., Fells J.I., Liu J., Valentine W.J., Fujiwara Y., Thompson K.E., Yates C.R., Sümegi B., Tigyi G. Mitigation of radiation injury by selective stimulation of the LPA(2) receptor. Biochim. Biophys. Acta. 2013;1831:117–125. doi: 10.1016/j.bbalip.2012.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Patil R., Szabó E., Fells J.I., Balogh A., Lim K.G., Fujiwara Y., Norman D.D., Lee S.C., Balazs L., Thomas F., et al. Combined mitigation of the gastrointestinal and hematopoietic acute radiation syndromes by an LPA2 receptor-specific nonlipid agonist. Chem. Biol. 2015;22:206–216. doi: 10.1016/j.chembiol.2014.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Inoue M., Rashid M.H., Fujita R., Contos J.J., Chun J., Ueda H. Initiation of neuropathic pain requires lysophosphatidic acid receptor signaling. Nat. Med. 2004;10:712–718. doi: 10.1038/nm1060. [DOI] [PubMed] [Google Scholar]
- 108.Plastira I., Bernhart E., Joshi L., Koyani C.N., Strohmaier H., Reicher H., Malle E., Sattler W. MAPK signaling determines lysophosphatidic acid (LPA)-induced inflammation in microglia. J. Neuroinflamm. 2020;17:127. doi: 10.1186/s12974-020-01809-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Velasco M., O’Sullivan C., Sheridan G.K. Lysophosphatidic acid receptors (LPARs): Potential targets for the treatment of neuropathic pain. Neuropharmacology. 2017;113:608–617. doi: 10.1016/j.neuropharm.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 110.Ueda H. Systems pathology of neuropathic pain and fibromyalgia. Biol. Pharm. Bull. 2019;42:1773–1782. doi: 10.1248/bpb.b19-00535. [DOI] [PubMed] [Google Scholar]
- 111.Plastira I., Bernhart E., Goeritzer M., DeVaney T., Reicher H., Hammer A., Lohberger B., Wintersperger A., Zucol B., Graier W.F., et al. Lysophosphatidic acid via LPA-receptor 5/protein kinase D-dependent pathways induces a motile and pro-inflammatory microglial phenotype. J. Neuroinflamm. 2017;14:253. doi: 10.1186/s12974-017-1024-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhang H., Xu X., Gajewiak J., Tsukahara R., Fujiwara Y., Liu J., Fells J.I., Perygin D., Parrill A.L., Tigyi G., et al. Dual activity lysophosphatidic acid receptor pan-antagonist/autotaxin inhibitor reduces breast cancer cell migration in vitro and causes tumor regression in vivo. Cancer Res. 2009;69:5441–5449. doi: 10.1158/0008-5472.CAN-09-0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Schleicher S.M., Thotala D.K., Linkous A.G., Hu R., Leahy K.M., Yazlovitskaya E.M., Hallahan D.E. Autotaxin and LPA receptors represent potential molecular targets for the radiosensitization of murine glioma through effects on tumor vasculature. PLoS ONE. 2011;6:e22182. doi: 10.1371/journal.pone.0022182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Su S.C., Hu X., Kenney P.A., Merrill M.M., Babaian K.N., Zhang X.Y., Maity T., Yang S.F., Lin X., Wood C.G. Autotaxin-lysophosphatidic acid signaling axis mediates tumorigenesis and development of acquired resistance to sunitinib in renal cell carcinoma. Clin. Cancer Res. 2013;19:6461–6472. doi: 10.1158/1078-0432.CCR-13-1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lou L., Chen Y.X., Jin L., Li X., Tao X., Zhu J., Chen X., Wu S., Ye W., He J., et al. Enhancement of invasion of hepatocellular carcinoma cells through lysophosphatidic acid receptor. J. Int. Med. Res. 2013;41:55–63. doi: 10.1177/0300060512474124. [DOI] [PubMed] [Google Scholar]
- 116.David M., Ribeiro J., Descotes F., Serre C.M., Barbier M., Murone M., Clézardin P., Peyruchaud O. Targeting lysophosphatidic acid receptor type 1 with Debio 0719 inhibits spontaneous metastasis dissemination of breast cancer cells independently of cell proliferation and angiogenesis. Int. J. Oncol. 2012;40:1133–1141. doi: 10.3892/ijo.2011.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Marshall J.C., Collins J.W., Nakayama J., Horak C.E., Liewehr D.J., Steinberg S.M., Albaugh M., Vidal-Vanaclocha F., Palmieri D., Barbier M., et al. Effect of inhibition of the lysophosphatidic acid receptor 1 on metastasis and metastatic dormancy in breast cancer. J. Natl. Cancer Inst. 2012;104:1306–1319. doi: 10.1093/jnci/djs319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Komachi M., Sato K., Tobo M., Mogi C., Yamada T., Ohta H., Tomura H., Kimura T., Im D.S., Yanagida K., et al. Orally active lysophosphatidic acid receptor antagonist attenuates pancreatic cancer invasion and metastasis in vivo. Cancer Sci. 2012;103:1099–1104. doi: 10.1111/j.1349-7006.2012.02246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Takahashi K., Minami K., Otagaki S., Ishimoto K., Fukushima K., Fukushima N., Honoki K., Tsujiuchi T. Lysophosphatidic acid receptor-2 (LPA2) and LPA5 regulate cellular functions during tumor progression in fibrosarcoma HT1080 cells. Biochem. Biophys. Res. Commun. 2018;503:2698–2703. doi: 10.1016/j.bbrc.2018.08.026. [DOI] [PubMed] [Google Scholar]
- 120.Gnocchi D., Kapoor S., Nitti P., Cavalluzzi M.M., Lentini G., Denora N., Sabbà C., Mazzocca A. Novel lysophosphatidic acid receptor 6 antagonists inhibit hepatocellular carcinoma growth through affecting mitochondrial function. J. Mol. Med. 2020;98:179–191. doi: 10.1007/s00109-019-01862-1. [DOI] [PubMed] [Google Scholar]
- 121.Kawamoto Y., Seo R., Murai N., Hiyama H., Oka H. Identification of potent lysophosphatidic acid receptor 5 (LPA5) antagonists as potential analgesic agents. Bioorg. Med. Chem. 2018;26:257–265. doi: 10.1016/j.bmc.2017.11.038. [DOI] [PubMed] [Google Scholar]
- 122.Murai N., Hiyama H., Kiso T., Sekizawa T., Watabiki T., Oka H., Aoki T. Analgesic effects of novel lysophosphatidic acid receptor 5 antagonist AS2717638 in rodents. Neuropharmacology. 2017;126:97–107. doi: 10.1016/j.neuropharm.2017.08.032. [DOI] [PubMed] [Google Scholar]
- 123.Lysophosphatidic Acid Assay in Patients with Ovarian Cancer or Who Are at Risk for Ovarian Cancer. [(accessed on 4 June 2021)]; Available online: https://clinicaltrials.gov/ct2/show/NCT00986206.
- 124.Palmer S.M., Snyder L., Todd J.L., Soule B., Christian R., Anstrom K., Luo Y., Gagnon R., Rosen G. Randomized, double-blind, placebo-controlled, phase 2 trial of BMS-986020, a lysophosphatidic acid receptor antagonist for the treatment of idiopathic pulmonary fibrosis. Chest. 2018;154:1061–1069. doi: 10.1016/j.chest.2018.08.1058. [DOI] [PubMed] [Google Scholar]
- 125.Safety and Efficacy of a Lysophosphatidic Acid Receptor Antagonist in Idiopathic Pulmonary Fibrosis. [(accessed on 4 June 2021)]; Available online: https://clinicaltrials.gov/ct2/show/NCT01766817.
- 126.Absorption, Distribution, Metabolism and Excretion (ADME) Study of BMS-986020. [(accessed on 4 June 2021)]; Available online: https://clinicaltrials.gov/ct2/show/NCT02068053.
- 127.Drug Interaction Study with Rosuvastatin. [(accessed on 4 June 2021)]; Available online: https://clinicaltrials.gov/ct2/show/NCT02101125.
- 128.A Study of Experimental Medication BMS-986278 Given to Healthy Participants. [(accessed on 4 June 2021)]; Available online: https://clinicaltrials.gov/ct2/show/NCT03429933.
- 129.A Study Measuring the Effectiveness, Safety, and Tolerability of BMS-986278 in Participants with Lung Fibrosis. [(accessed on 4 June 2021)]; Available online: https://clinicaltrials.gov/ct2/show/NCT04308681.
- 130.Safety, Tolerability, Kinetics, and Repeatability of the Novel LPA1 PET Ligand 18F-BMS-986327. [(accessed on 4 June 2021)]; Available online: https://clinicaltrials.gov/ct2/show/NCT04069143.
- 131.Allanore Y., Distler O., Jagerschmidt A., Illiano S., Ledein L., Boitier E., Agueusop I., Denton C.P., Khanna D. Lysophosphatidic acid receptor 1 antagonist SAR100842 for patients with diffuse cutaneous systemic sclerosis: A double-blind, randomized, eight-week placebo-controlled study followed by a sixteen-week open-label extension study. Arthritis Rheumatol. 2018;70:1634–1643. doi: 10.1002/art.40547. [DOI] [PubMed] [Google Scholar]