Abstract
Activation of Transient Receptor Potential (TRP) channels can disrupt endothelial barrier function, as their mediated Ca2+ influx activates the CaM (calmodulin)/MLCK (myosin light chain kinase)-signaling pathway, and thereby rearranges the cytoskeleton, increases endothelial permeability and thus can facilitate activation of inflammatory cells and formation of pulmonary edema. Interestingly, TRP channel subunits can build heterotetramers, whereas heteromeric TRPC1/4, TRPC3/6 and TRPV1/4 are expressed in the lung endothelium and could be targeted as a protective strategy to reduce endothelial permeability in pulmonary inflammation. An update on TRP heteromers and their role in lung inflammation will be provided with this review.
Keywords: heteromeric TRP assemblies, pulmonary inflammation, endothelial permeability, TRPC3/6, TRPV1/4, TRPC1/4
1. Introduction
Pulmonary microvascular endothelial cells are a key constituent of the blood air barrier that has to be extremely thin (<1 µm) to allow for rapid and efficient alveolo-capillary gas exchange. Integrity of this barrier is mandatory to prevent airborne pathogens from entering the blood circulation and causing systemic infections and to avoid fluids such as plasma or blood entering the alveolar space. Yet, over the past years a considerable body of work has demonstrated that activation of Transient Receptor Potential (TRP) channels can disrupt endothelial barrier function via a Ca2+-dependent increase of endothelial permeability, resulting in the formation of lung edema and an inflammatory response characterized by immune cell infiltration [1,2,3,4]. TRP channels form a family of transmembrane cation-permeable channels that can be categorized into six subfamilies: TRPA (ankyrin), TRPC (canonical), TRPM (melastatin), TRPML (mucolipin), TRPP (polycystin) and TRPV (vanilloid) [5]. Members of all six TRP channel subfamilies are expressed in the lung endothelium [6,7,8,9] where they can mediate Ca2+ influx and signaling as a key second messenger regulating cellular adaptation in various physiological and pathophysiological scenarios including endothelial permeability, vasodilation, angiogenesis, thrombosis, and inflammation [3,10,11,12]. For example, TRPV1 has been demonstrated to initiate inflammatory responses in pulmonary endothelial cells [13] while TRPV4-mediated Ca2+ influx can cause gap formation between endothelial cells, promoting infiltration of immune cells such as monocytes and polymorphonuclear leukocytes (PMNs) into the alveolar space, as well as pulmonary edema formation and lung injury [14]. The subcellular mechanisms that determine why Ca2+ entry via different Ca2+ channels result in differential cellular responses are so far poorly understood, but likely relate to highly localized signaling effects in cellular sub-compartments [15].
To build a functional ion channel, TRP subunits have to assemble as a tetrameric structure [16,17]. Different TRP subunit assemblies differ in their specificities, ion conductances and activation mechanisms. For instance, a TRP channel consisting of four TRPV4 subunits reacts to arachidonic acid [18] and can be activated by a phosphorylation in Ser824 [19]. Interestingly, studies have shown that TRP channel subunits not only form homomeric, but also heteromeric structures [20,21], and that such heteromeric assembly determines channel ion conductance, e.g., for Ca2+. The stoichiometry of TRP heteromers influences the Ca2+ influx and is therefore important to investigate. For example, it has been found that heteromeric TRPV5/6 would have a reduced opening probability for Ca2+, if more TRPV6 subunits built the TRPV5/6 channel [17]. As Ca2+ is a key intracellular second messenger initiating stress responses, cytoskeletal reorganization, and junctional disassembly [20], TRP heteromers and their distinct Ca2+ gating can be expected to play a critical role in the regulation of endothelial permeability. Thus, it was found that heteromeric TRPC1/4 and TRPC3/6 could increase endothelial permeability [1,22].
In this review, we present an update on the molecular mechanisms of TRP channel heteromerization for well-characterized (TRPC1/4, TRPC3/6) as well as newly emerging (TRPV1/4) TRP heteromeric assemblies in the pulmonary endothelium. All three heteromeric assemblies are expressed in the lung and of relevance in lung inflammation. Pulmonary endothelial cells can initiate and amplify inflammatory processes in various diseases or syndromes such as ARDS (acute respiratory distress syndrome) or COVID-19 (coronavirus disease 2019) [14,23,24]. In this context, Ca2+ as a second messenger plays a key role in activating inflammatory signaling pathways and is regulated, amongst others, by heteromeric TRP assemblies. Here, we will provide a comprehensive overview on the role of these assemblies in acute pulmonary vascular inflammation and discuss relevant methodological approaches and limitations for their analysis.
2. Methodological Approaches and Limitations in the Analysis of Heteromeric TRP Assemblies
Homo- and heteromeric TRP assemblies are analyzed with respect to their functional properties, their structure and their mechanisms of regulation. In general, TRP channel complexes are difficult to analyze as low expression levels of endogenous heteromeric assemblies or moderate TRP channel subunit interactions require very sensitive methods and cannot be detected by less sensitive but commonly used strategies such as co-immunoprecipitation (Co-IP) [25]. As a consequence, most studies addressing the heteromeric structure of TRP channels rely on overexpression systems in HEK293 cells as a versatile tool to construct, modify and test TRP heteromeric assemblies which may, however, result in artificial heteromeric channel formation due to high protein expression.
The initial proof of an interaction between TRP channel subunits can be demonstrated by using the Proximity Ligation Assay [26,27] but is commonly performed by Co-IP [28]. Pitfalls of Co-IP are false positive or negative results due to low protein purity and due to subunit disassembly as a result of plasma membrane treatment with harsh lysis or permeabilization buffers. Hence, Co-IP should be combined with a second, more sensitive method like the yeast two-hybrid (Y2H) technique or Förster resonance energy transfer (FRET). Y2H systems face some difficulties when used for transmembrane proteins but have been successfully modified to demonstrate a heteromeric Drosophila retinal-specific TRP and TRPL (like) assembly [29] into a membrane-based yeast two-hybrid system for protein interactions in the plasma membrane [30]. The most sensitive approach, however, to investigate TRP heteromers directly in living cells remains FRET, which has been used extensively for the study of heteromeric assemblies within the TRPV and TRPC subfamilies [28].
To examine which structural domains of TRP channel subunits contribute to the stabilization of TRP heteromers, loss of function mutations are commonly utilized, e.g., for the analysis of the heteromeric assemblies of TRPV5/6 [17] or TRPC3/6 [28]. For functional analyses of TRP channel subunit heteromerization, whole-cell patch-clamp recordings are able to identify modulations in cation conductances relative to the corresponding homomeric assemblies (e.g., for TRPV5/6 [17]).
In in silico analyses, interaction sites for protein interaction can be predicted by molecular docking [31]. Such analyses, however, require detailed structural input of putative heteromeric TRP subunits and their interaction sites. Thus far, such data are largely lacking due to the difficulties inherent in the assessment of the stoichiometric structure of a single channel by X-ray crystallography due to TRP channels’ flexibility and multi-domain topology [32]. This knowledge gap can be filled in part by analysis of single channel structure by cryo-electron microscopy [33]. Both X-ray crystallography and cryo-electron microscopy are two indispensable tools, generating valid input data for a broad range of in silico studies, uncovering stoichiometric changes and potential of modulating their properties by heteromerization.
Atomic force microscopy (AFM) provides a very sensitive method for determination of TRP heteromer’s stoichiometry, after TRP subunits were marked with a monoclonal antibody each, as shown for TRPP2/V4 with a 2:2 stoichiometry in HEK293 by Stewart and colleagues in 2010 [34]. In 2017, Single Channel Single Molecule Determination (SC-SMD) has been established successfully to investigate, for instance, the stoichiometry of heteromeric TRP channels by tagging TRP channel subunits in a heteromeric assembly with different fluorescent molecules which then can be detected by Total Internal Reflection Fluorescence (TIRF) microscopy. Likewise, combination of cross-linking TRP channel subunits with each other in a heteromeric assembly followed by mass spectrometry analysis [35] may present a versatile alternative for future analyses of physical interactions and stoichiometry in heteromeric TRP assemblies.
At present, FRET analyses and whole-cell patch-clamp recordings present the most commonly used and sensitive methods to probe for structural and functional interactions of heteromeric TRP assemblies. AFM, SC-SMD and cross-linking in combination with mass spectrometry present novel and promising strategies for future analyses of heteromeric TRP channel subunit assemblies with regard to their individual stoichiometry (an overview of methodological approaches is provided in Table 1).
Table 1.
Overview of methods for heteromeric TRP channels analyses.
| Method | Application | Limitation | Example |
|---|---|---|---|
| AFM (atomic force microscopy) [36] | for stoichiometric determination in cells expressing tagged channels; allows for detection at the single channel level as it yields three dimensional structures in an Ångström (Å) range | not applicable for electrophysiological analysis | TRPP2/V4 [34] |
| Co-IP (co-immuno- precipitation) [37] |
proof of interactions between TRP channel subunits in cells expressing tagged channels; allows for differentiation of heteromeric or homomeric assemblies |
not applicable for structural and electrophysiological analysis | TRPV1/4 [38] |
| cross-linking in combination with mass spectrometry [39] | proof of interactions between TRP channel subunits and for structural and stoichiometric determination in native cells; provides a 3D-structure of interacting proteins, the distance between TRP channel subunits in the cross-linking reaction depends on the length of the crosslinker | not applicable for electrophysiological analysis | no applications made for heteromeric TRP channels yet |
| cryo-electron microscopy [40] | for structural analysis in cells overexpressing channels; allows for detection at the single channel level as it shows three dimensional structures, but sensitivity depends on the investigated protein’s electron irradiation and scattering | not applicable for electrophysiological analysis | no applications made for heteromeric TRP channels yet |
| FRET (Förster resonance energy transfer) [41] | proof of interactions between fluorescently tagged TRP channel subunits by live cell imaging; quantifies interaction over short distances (10–100 Å), yet fluorophores can alter binding properties | not applicable for structural, stoichiometric and electrophysiological analysis | TRPV1/4 [38] |
| molecular docking [31] | for prediction of structural domains that stabilize the TRP heteromer; method is based on acquired input data |
not applicable for electrophysiological analysis | no applications made for heteromeric TRP channels yet |
| PLA (proximity ligation assay) [42] | proof of interactions between TRP channel subunits in native cells; quantifies interactions in a 30-nm range, allows quantification of heteromeric channel assemblies, but not at the single channel level; strongly depends on antibody specificity |
not applicable for structural, stoichiometric and electrophysiological analysis | TRPV1/4 [27] |
| whole-cell patch-clamp recordings [43] | for electrophysiological analysis in native cells or cells expressing tagged channels; measures single ion channel current in a picoampere range | not applicable for structural and stoichiometric determinations | TRPC1/4 [44] |
| SC-SMD (single channel single molecule determinations) [45] | proof of interactions between TRP channel subunits and for structural and stoichiometric analysis in cells expressing tagged channels; detection at the single channel level as it yields three dimensional structures of proteins in a nanometer range | not applicable for electrophysiological analysis | no applications made for heteromeric TRP channels yet |
| x-ray crystallography [46] | for structural and stoichiometric analysis in cells overexpressing channels; detection at the single channel level as it yields three dimensional structures of molecules in an (Å) range, but is difficult to perform for transmembrane proteins | not applicable for electrophysiological analysis | no applications made for heteromeric TRP channels |
| Y2H (yeast two-hybrid technique) [47] | proof of interactions between TRP channel subunits in living native cells; detection of protein interactions by yeast reproduction, but not on a single channel level, difficult application for transmembrane proteins, and high rate of false positive results | not applicable for structural, stoichiometric and electrophysiological analysis | Drosophila retinal-specific TRP/L [29] |
3. Heteromeric TRP Channel Assemblies and Their Molecular Mechanisms
3.1. Introduction to TRP Channel Assemblies
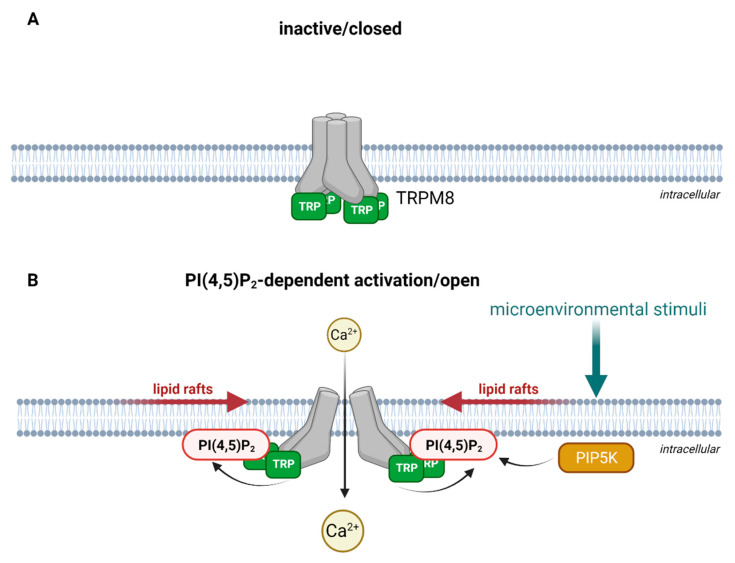
TRP channel subunits are membrane proteins, consisting of six transmembrane spans and a pore loop between the fifth and the sixth span that is cation-permeable [48]. The intracellular N- and C-termini are fitted with specific sequence motifs that are essential for the multimeric assembly of TRP channels. The N-terminus contains several ankyrin repeat domains (ARDs) [49], consisting of short sequences of 33 amino acid residues that form an anti-parallel helix-turn-helix structure followed by a β-hairpin loop. Several of these helix-turn-helix structures are packed into a bundle. These bundles form in turn a hand-shaped structure [50,51]. The C-terminus of TRPC, TRPV and TRPM subtypes contain a so-called TRP box, which maintains the channel in a closed confirmation in a phosphatidylinositol 4,5-bisphosphate (PI(4,5)P2)-dependent manner [52,53] as shown in Figure 1 upon the example of TRPM8.
Figure 1.
Schematic illustration of PI(4,5)P2-dependent activation as exemplified for TRPM8. (A) In the absence of PI(4,5)P2, the TRP-box holds the channel in a closed conformation. For simplification, other structural domains of TRPM8 are not illustrated. (B) Microenvironmental stimuli activate PI(4,5)P2 production via phosphorylation of phosphatidylinositol 4-phosphate by PIP5K (phosphatidylinositol-4-phosphate 5-kinase) in lipid rafts. The interaction of phosphatidylinositol 4,5-bisphosphate (PI(4,5)P2) the with the TRP-box activates TRPM8 and opens the channel for Ca2+ entry. Created with BioRender.com.
Additionally, coiled-coil domains (CCDs) consisting of seven α-helices that interact with each other have been reported in TRP channels [54,55] with the potential to build coiled-coil structures [56]. CCDs have been also described to be differentially localized to C- and N-termini depending on the individual TRP subfamily and to promote multimeric assembly, channel maturation and trafficking [55]. For instance, the TRPM subfamily and TRPA1 contain CCDs on their C-terminus [57,58] whereas TRPC1 and TRPC4 have their CCDs on the N-terminus [59].
3.2. Heteromeric TRP Channel Assemblies
Heteromeric assemblies of TRP channel subunits are known to be formed within their subfamily and moreover with TRP channel subunits from other subfamilies, as it has been demonstrated for heteromeric assemblies of TRPP2/C1 in pig kidney epithelial cells [21], or TRPC1/V6 and TRPML/V5 in HEK293 cells [60,61]. Heteromerization of three different TRP channel subunits has also been described, e.g., for TRPC3/C6/C7 [62], TRPC1/C4/C5 [63], or TRPV4/C1/P2 [64] although heteromerization of two TRP channel subunits seems to be more commonly found [20]. It seems fair to speculate that with an increasing number of different TRP channel subunits being co-expressed in the same tissue or cell type, the probability for heteromerization increases which in turn may serve as a mechanism to fine-tune cellular responses to tissue-specific environmental stimuli [65]. For example, TRPV4, TRPC1 and TRPC6 have been shown to heteromerize in dorsal root ganglion neurons where they mediate mechanical hyperalgesia and primary afferent nociceptor sensitization [66] whereas TRPV4, TRPC1 and TRPC6 alone have no influence on the baseline mechanical nociceptive threshold [66,67,68]. Other examples for processes specifically regulated by heteromeric TRP channels include TRPC1/4 which mediates a large depolarizing plateau potential in lateral septal neurons that is the cause for epileptiform burst firing [69], TRPV1/4 induced angiogenesis in retinal microvascular endothelial cells (RMECs) in contrast to TRPV4 alone which inhibits angiogenesis in endothelial cells from prostate cancer [70], or TRPC3/6 which plays an important role in T-lymphocyte apoptosis and is increased in septic rat peripheral blood T-lymphocytes [71], indicating that heteromerization may also occur as an adaptive response to an inflammatory milieu.
3.3. Molecular Mechanisms for Stabilization of Heteromeric TRP Channel Assemblies
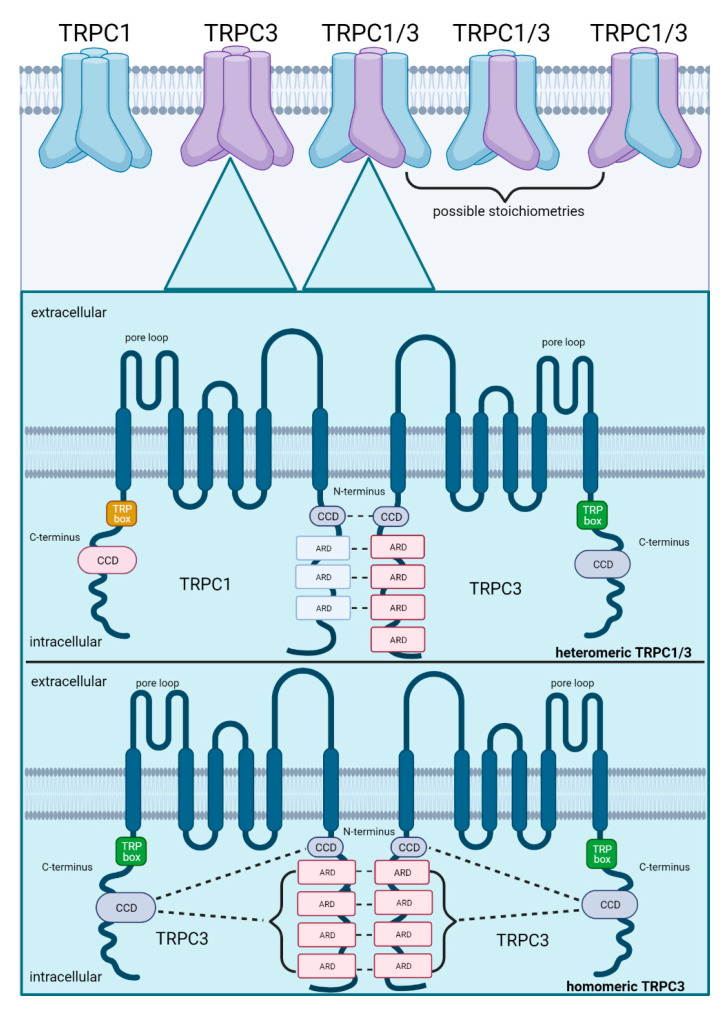
Thus far, the most important identified structural domains for stabilization of heteromeric TRP assemblies are ARDs and CCDs located on the N- and C-termini of the TRP subunits. To exemplify the molecular mechanisms that stabilize heteromeric assemblies, the well-described heteromeric TRPC1/3 is illustrated in Figure 2. Heteromeric TRPC1/3 is stabilized by non-covalent interactions between N-terminal ARDs and CCDs of the individual TRPC channel subunits whereas the homomeric TRPC3 assembly is stabilized by interactions between CCDs on the C-terminus with ARDS and CCDs on the N-terminus, indicating different molecular mechanisms for stabilization. Co-expression and heteromerization of TRPC1 and TRPC3 has been confirmed in skeletal myocytes [72]. In contrast to homomeric TRPC1 which promotes muscle regeneration after muscle atrophy [73], heteromeric TRPC1/3 plays an important role in the differentiation of myoblasts during myogenesis [72], consolidating the notion that heteromeric TRP assemblies are linked to other signal cascades than their homomers. The exact stoichiometry of TRPC1/3 heteromers (1:3, 2:2 or 3:1 for TRPC1:TRPC3, as shown in Figure 2) remains, however, unclear and may potentially vary with respective consequences for channel properties.
Figure 2.
Schematic illustration of subunit assembly for heteromeric TRPC1/3 as compared to homomeric TRPC3. Co-expression of both subunits increases the probability of heteromerization, which is facilitated by their 28.7% amino acid congruency. Possible stoichiometries in a heteromeric assembly comprise 1:3, 2:2 or 3:1 (TRPC1:TRPC3). TRPC1/3 is stabilized by non-covalent interactions between ARDs (ankyrin repeat domains) and CCDs (coiled-coil domains) on their N-termini. In contrast, homomeric assembly of TRPC3 is stabilized by interactions between ARDs on their N-termini and by interactions between the CCDs on their C-termini with the ARDs and CCDs on their N-termini. Created with BioRender.com.
3.4. Properties of Heteromeric TRP Channel Assemblies
In line with different functional effects from homomeric channels, heteromeric assemblies of TRP channel subunits have a unique cation conductance that is unrelated to the additive conductance of the individual channel subunits. This was shown, for example, for the heteromeric assembly of TRPV1 and TRPV3 in HEK293 cells, which has an intermediate conductance compared to the homomeric assemblies of TRPV1 and TRPV3 [74]. Similarly, an intermediate conductance as compared to the homomeric assemblies was reported for TRPC1/P2 [21]. As such, TRP channels may adapt their individual conductivity (and putatively also their opening probability and their intracellular localization) by forming heteromers, thereby modulating cation influx and downstream cellular responses [21,29,62,75].
Interestingly, heteromeric TRP channels do not only show a unique cation conductance but also a distinct activation of downstream signaling pathways which is dependent on their cell type expression. Heteromeric TRPC1/3 as well as the heteromeric TRPC3/6/7 channel assembly have been reported to act as store-operated channels [73,76,77]. That notwithstanding, these assemblies activate different signaling pathways, as TRPC1/3-mediated Ca2+ influx plays a critical role in the differentiation of neuronal cells [73], whereas TRPC3/6/7 mediates an inflammatory response in astrocytes [62]. Heteromeric assemblies of TRP channel subunits have also been demonstrated in endothelial cells, e.g., TRPV4/C1/P2 in rat mesenteric artery endothelial cells (RMAECs) [64] and TRPV4/C1 in human umbilical vein endothelial cells (HUVECs) [7]. Heteromeric TRPV4/C1/P2 was shown to cause a flow-mediated Ca2+ influx, which is considered to play a major role in vasodilation, as it leads to the production of endothelial vasodilators like NO. A vasodilatory function in vascular endothelial cells has similarly been suggested for TRPV4/C1 which can be activated by stromal interaction molecule (STIM1) and Ca2+ release-activated calcium channel protein (Orai1), resulting in store-operated Ca2+ influx [7]. In addition to vasoregulation, maintenance and regulation of barrier properties is a basic function of pulmonary microvascular endothelial cells that is similarly regulated by TRP channel mediated Ca2+ influx. The resulting increase in intracellular Ca2+ concentration activates the calmodulin (CaM)/myosin light chain kinase (MLCK)-signaling pathway which in turn triggers cell contraction and additional Ca2+-dependent signaling cascades such as disassembly of intercellular junctions, ultimately resulting in increased endothelial permeability. Amongst others, TRPC1/4, TRPC3/6 and TRPV1/4 have been shown to be expressed and regulate barrier function in pulmonary microvascular endothelial cells. TRPC1/4 and TRPC3/6 present some of the best characterized heteromeric TRP channels, whereas TRPV1/4 is a recently discovered TRP heteromer. All three TRP heteromers will be described below in greater detail as they regulate barrier function and play an important role in lung inflammation.
4. Heteromeric TRPC1/4 Assembly
4.1. Heteromeric TRPC1/4 Structure
In pulmonary microvascular endothelial cells, TRPC1/4—just like TRPV4/C1 —interacts with Orai1, which in turn increases a store-operated Ca2+ influx through the heteromeric TRPC1/4 assembly [78]. The resulting Ca2+ influx via TRPC1/4 can increase pulmonary endothelial permeability and thus promote permeability-type lung edema in acute lung injury [1]. TRPC1 and TRPC4 share 41.3% of their primary structure and are therefore likely to co-assemble [20]. Typically, the quaternary structure of the canonical TRP subfamily is maintained by non-covalent interactions between the subunits’ C-termini and N-termini [79]. Specifically, for stabilization of the heteromeric TRPC1/4 assembly the CCD on the N-terminus and the structural domain from 725 to 745 on the C-terminus of TRPC1 interact with the CCD on the N-terminus and the structural domain from 673 to 725 on the C-terminus of TRPC4 [59].
4.2. TRPC1/4 Regulation
Regarding its activation mechanism, heteromeric TRPC1/4 assembly was not only found to be activated by Orai1 but also by a Gαq-protein-coupled receptor in HEK293 whereby the Gαq subunit upon dissociation from the G-protein-coupled-receptor (GPCR) activates TRPC1/4 directly [80]. Additionally, Phospholipase Cβ (PLCβ) is activated by Gαq-coupled GPCRs, inducing hydrolysis of phosphatidylinositol 4,5-bisphosphate (PI(4,5)P2), decreasing PI(4,5)P2 concentrations. The resulting decrease in PI(4,5)P2 levels reduces the activity of the TRPC1/4 channel, indicating that the activation of TRPC1/4 by the Gαq-PLCβ pathway is self-limited [80]. Interestingly, TRPC1/4 was found to differ in its PI(4,5)P2 sensitivity [81] and to have a reduced Ca2+ conductance compared to homomeric TRPC4 [44], underlining that heteromerization changes the channel’s regulation and cation conductance. Although the Ca2+ conductance for TRPC1/4 is surprisingly lower compared to homomeric TRPC4, its mediated store-operated Ca2+ influx may be increased by prolonged channel opening that could be further extended by PI(4,5)P2 release which increases TRPC1/4-mediated Ca2+ influx [80].
4.3. TRPC1/4 Function
The resulting Ca2+ influx was shown to facilitate gap formation and thus increased endothelial permeability during acute pulmonary inflammation [1,82]. In contrast to that, TRPC1/4 is suggested to be protective in hypoxia-induced pulmonary arterial hypertension [83], where activation of nuclear factor-kappa B (NF-κB) and increased Galectin-3 expression shifts cellular response towards apoptosis by inactivation of TRPC1/4 and resulting suppression of autophagy [83]. TRPC1/4-mediated Ca2+ influx can on the one hand increase microvascular endothelial permeability and thus increase endothelial inflammation [1]. On the other hand, however, TRPC1/4-mediated Ca2+ influx has a protective effect in hypoxia-induced pulmonary arterial hypertension by maintaining autophagic responses [83], suggesting that the effect of TRPC1/4-mediated Ca2+ influx depends on the specific physiological or pathophysiological context, respectively.
5. Heteromeric TRPC3/6 Assembly
5.1. Heteromeric TRPC3/6 Structure
In human microvascular endothelial cells (HMVECs), TRPC3/6 can be activated by vascular endothelial growth factor (VEGF) and results in a Ca2+ influx [22]. The amino acid sequences of TRPC3 and TRPC6 show a 71.9% congruency and as such, of all TRP channel subunits they are the most probable pair to form a heteromer [20]. As mentioned before, the N-terminus of TRPC3 consists of four ARDs and a CCD whereas a CCD was also found on its C-terminus [54]. Heteromeric TRPC3/6 has been identified in FRET analyses, demonstrating that the 131 amino acids on the N-terminus, including the four ARDs, are crucial for stabilization of heteromeric TRPC3/6 assembly [28].
5.2. TRPC3/6 Regulation
TRPC3/6 is activated by intracellular diacylglycerol (DAG) [84] as a result of PI(4,5)P2 hydrolysis by PLC isoforms like PLCβ or PLCγ in response to, e.g., inflammatory stimuli [85]. In parallel with DAG, PI(4,5)P2 hydrolysis forms inositol 1,4,5-trisphosphate (IP3), inducing a store-operated Ca2+ entry (SOCE) through TRPC3/6 [76,86]. Whether TRPC3/6 acts as a store depletion- or GPCR-activated channel depends on its cell type-specific expression. In rat prostate smooth muscle cells [87], cardiac myocytes [88] and Jurkat T-cells [84], heteromeric TRPC3/6 has been shown to get activated by GPCR, whereas it shows a store-operated activity in mesangial cells [89]. Additionally, the TRPC3/6-mediated increase in intracellular Ca2+ concentrations can activate hypertrophic and anti-apoptotic signaling pathways. For example, brain-derived neurotrophic factor-mediated TRPC3/6 activation inhibited apoptosis in neonatal rat ventricular myocytes [90], whereas angiotensin II induced DAG release causes an activation of TRPC3/6 in cardiomyocytes, initiating a hypertrophic response [88].
5.3. TRPC3/6 Function
In pulmonary microvascular endothelial cells, TRPC3/6-mediated responses play a crucial role in acute pulmonary inflammation when VEGF is released and activates the heteromeric channel [91]. On the one hand TRPC3/6 activation promotes loss of endothelial barrier function in acute pulmonary inflammation [91]. TRPC3/6-mediated signaling might also be important to maintain endothelial barrier integrity on the other hand, as pro-angiogenic endothelial responses—mediated via VEGF and TRPC3/6—may counteract endothelial senescence and decrease permeability [92].
6. Heteromeric TRPV1/4 Assembly
6.1. TRPV1/4 Structure
Heteromeric TRPV1/4 assembly has been demonstrated by FRET analyses [74]. Yet, little is known so far about this heteromer. Typical for TRP heteromers, its cation conductance is dependent on its stoichiometry, with conductance closer resembling the homomeric TRPV1 assembly—characterized by very long openings and a low conductance in whole-patch clamp-recordings in HEK293 cells—with integration of more TRPV1 channel subunits into the heteromeric assembly [74]. Homomeric TRPV1 assemblies are known for their capsaicin-induced activation, which is dependent on an alanin residue in position 578 between the 5th and 6th span of the cation-permeable pore loop [93]. Interestingly, deletion of the amino acids 233 to 295 in the N-terminus reduces capsaicin-induced channel activity while FRET analyses demonstrated a weaker signal for TRPV1 tetramer formation, suggesting this amino acid, which includes the first two ARDs, as a possible stabilizing region for heteromerization with TRPV4. Additionally, the transmembrane domains have been proposed to stabilize the heteromeric assembly of TRPV1 and TRPV4, albeit this hypothesis remains to be proven [20].
6.2. TRPV1/4 Regulation
Both TRPV1 and TRPV4 channels can be activated by lipid-derived molecules like arachidonic acid [84,85] and by a variety of toxins (e.g., scorpion venom, botulinum neurotoxin and lipopolysaccharides) [94,95] and play important roles in mechano-, thermo-, osmo- and nociception depending on their cell type expression [67,96,97,98,99,100].
6.3. TRPV1/4 Function
In RMECs, TRPV1/4 was shown to regulate angiogenesis [27]. Heteromeric TRPV1/4 may also alter membrane potential similar to homomeric TRPV4-mediated Ca2+ influx which increases K+ efflux via activation of Ca2+-dependent K+ channels, such as intermediate and small Ca2+-activated K+ channels (IK and SK), causing hyperpolarization which in turn amplifies Ca2+ influx [101,102]. As such, TRPV1/4-mediated Ca2+ influx and endothelial hyperpolarization may stabilize each other in a positive feedback loop, thereby driving TRPV1/4 mediated endothelial responses such as angiogenesis but considering that angiogenesis does not take place in matured lungs.
7. Other Heteromeric TRP Assemblies
Heteromeric assembly of TRP channels thus emerges as a way for cells to combine and finetune individual properties of each channel to generate various channel complexes from the same genetic repertoire. As such, heteromeric assemblies may play an important role in regulating the Ca2+ responses to specific stimuli, in a cell- or tissue-specific manner, and/or as a function of the individual biochemical or biomechanical cellular environment. Over the past decades different endothelial and non-endothelial variations of heteromeric TRP channel complexes have been identified, e.g., TRP channel subunits TRPC1 and TRPC4 were found to heteromerize also with TRPC5 [28]. Heteromeric TRPC1/4/5 is considered as a GPCR-activated channel and has been reported to have a lower Ca2+ conductance but longer openings compared to homomeric TRPC4 and TRPC5 assemblies [103]. TRPC1/4/5 increases action potential-triggered excitatory postsynaptic currents in the hippocampus and has thus been proposed to play an important role in spatial working memory and flexible relearning [104]. Interestingly, heteromeric assembly has also been reported for TRPC1, TRPC4 and TRPC6 and to increase SOCE upon its upregulation in pulmonary arteries. Notably, this effect was not solely attributable to pulmonary arterial smooth muscle cells but also involved other pulmonary cells like endothelial cells, ultimately promoting pulmonary vascular remodeling in Sprague Dawley rats in response to smoking exposure [105].
Within the TRPV family, TRPV1/2, TRPV1/4 and TRPV5/6 are known heteromers of which the heteromerization of TRPV5 and TRPV6 is the best characterized but is most prominent in epithelia [17,28,74]. Both TRPV channels, TRPV5 and TRPV6, share 73.9% of their amino acid sequence [20]. In 2003, Hoendrop and co-workers first described a heteromeric assembly of both channels that was stabilized by N-glycosylation in the extracellular loop of a transmembrane domain [17]. One year later, FRET analyses by Hellwig and co-workers confirmed the heteromeric formation of TRPV5/6 in HEK293 cells [38]. In TRPV5/6 assemblies, five CaM binding sites have been identified [106]. TRPV5/6 regulates intracellular Ca2+ influx and becomes inactivated when Ca2+/CaM-complexes bind to their CaM binding sites [106], thus establishing a negative regulatory feedback loop. TRPV5 and TRPV6 have been proposed to play an important role in non-small lung cancer, based on the fact that their expression in lung tumor tissue of patient with non-small lung cancer is reduced. Patients with reduced expression of TRPV5 and TRPV6 had also a reduced median survival after surgical resection [107,108]. This suggests that dysregulated Ca2+ homeostasis due to loss of TRPV5 and TRPV6 may contribute relevantly to the malignancy of tumor cells. Different stoichiometric configurations in heteromerization of TRPV5/6 (e.g., 1:3, 2:2 or 3:1 for TRPV5:TRPV6) again give rise to different Ca2+ conductance of the heteromeric assembly thus fine-tuning cellular Ca2+ signaling [17]. Specifically, as the number of TRPV6 subunits in a heteromeric assembly of TRPV5/6 increases, the Ca2+-dependent inactivation accelerates [17]. We conclude that TRP heteromers play a crucial role in endothelial and non-endothelial cells by regulating cellular Ca2+ influx by modulating channel conductances and opening phases, thus regulating vascular inflammation and physiological functions.
8. Role of Heteromeric TRP Assemblies in Lung Inflammation
8.1. Introduction to Lung Inflammation
Barrier function and vasoregulation are primary functions of pulmonary microvascular endothelial cells and are impaired in acute pulmonary endothelial inflammation [109,110,111]. Frequently, this “dysfunction” of the pulmonary endothelium establishes a vicious circle as increased endothelial permeability will promote lung inflammation and vice versa. In this scenario, Ca2+ influx is a key mechanism of pulmonary endothelial permeability and as such, heteromeric TRP assemblies play an important role in endothelial inflammation.
8.2. Activation of Heteromeric TRPC1/4 and TRPC3/6 in Lung Inflammation
To initiate an inflammatory response in specific, pathogens can be recognized by different endothelial receptors: Toll-like receptors (TLR), cytosolic NOD-like receptors (NLR), RIG-I-like receptors (RLR) and DNA sensors, which are commonly summarized as pathogen recognition receptors (PRR) [109]. Once activated, pathogen recognition receptors activate the NF-κB signaling pathway which in turn leads to the production of cytokines in pulmonary endothelial cells [111]. By releasing pro-inflammatory cytokines, monocytes and PMNs are recruited and start or aggravate the inflammatory response [109,111]. Among the PRRs, TLR4 is most important for recognizing lipopolysaccharides (LPS) from Gram-negative bacteria [112]. The activation of TLR4 increases the production of DAG, which in turn activates TRPC1/4 and TRPC3/6 [1,81,84] (Table 2), thus potentially further increasing Ca2+ influx via these assemblies in addition to their store-operated Ca2+ influx (Figure 3).
Table 2.
An overview of activated receptors in lung inflammation and their link to TRP channels.
| Receptor | Function in Lung Inflammation | Involved TRP Channels |
|---|---|---|
| TLR4 (Toll-like receptor) | activation of NF-κB signaling pathway and thus initiation of cytokine production; DAG production [111] | DAG production activates TRPC1/4, TRPC3/6, and TRPC6 [1,81,84] |
| PAR1 (protease-activated receptor 1) | secretion of VEGF and formation of IP3 [91,114] | activation of TRPC1/4 and TRPC3/6 via IP3-dependent Ca2+ release from the endoplasmic reticulum [84,115] |
| VEGFR (vascular endothelial growth factor receptor) | DAG production [115] | DAG production activates TRPC1/4, TRPC3/6, and TRPC6 [84,104,123] |
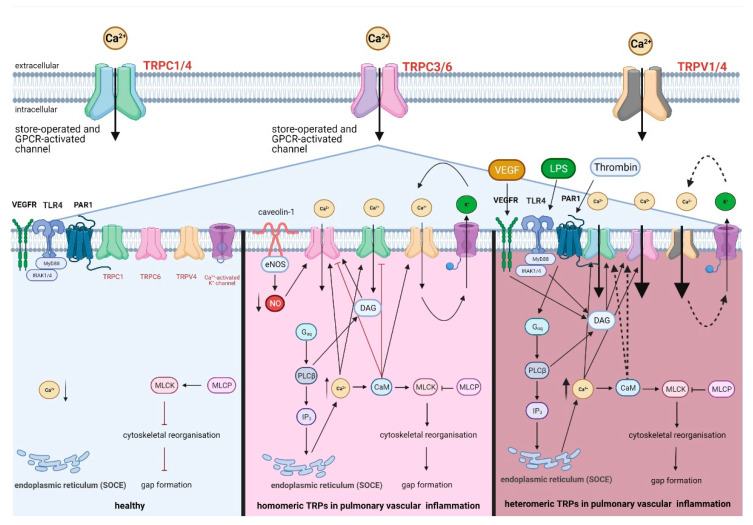
Figure 3.
Regulation of endothelial permeability in acute pulmonary inflammation by homomeric and heteromeric TRP assemblies. Under physiological conditions (left), pro-inflammatory and barrier-disruptive receptors including TLR4 (Toll-like receptors), PAR1 (protease-activated receptor 1) and VEGFR (vascular endothelial growth factor receptor) are not activated by their respective ligands, and intracellular Ca2+ concentration is maintained low by closed confirmations of TRP homomers. In vascular inflammation with TRP homomers (centre), TRPC6 is activated by DAG (diacylglycerol) and by inhibition of NO production through eNOS (endothelial NO-synthase) uncoupling and eNOS interaction with caveolin-1. In parallel, TRPC1 is activated by IP3 (inositol 1,4,5-trisphosphate)-dependent Ca2+ release. TRPC6-mediated Ca2+ influx can then be inhibited in a Ca2+/CaM (calmodulin)-dependent manner as part of a negative feedback regulation which could (potentially) also be the case for TRPC1. TRPV4-mediated Ca2+ influx increases K+ efflux via intermediate and small Ca2+-activated K+ channels (IK and SK), causing endothelial hyperpolarization which in turn increases Ca2+ influx. In vascular inflammation with TRP heteromers (right), activation of PAR1, TLR4 and VEGFR by their respective ligands thrombin, lipopoly-saccharide (LPS) or VEGF, respectively, causes GPCR-operated activation of TRPC1/4 and TRPC3/6, and the resulting Ca2+ influx is stabilized by SOCE. The Ca2+ influx may be further stabilized via K+ efflux maintaining TRPV1/4 activation (dashed lines) and via Ca2+/CaM-complexes binding to their CaM binding sites in TRPC1/4 and TRPC3/6 (dashed lines). Heteromeric and homomeric TRP channel-mediated Ca2+ influx activates the CaM/MLCK (myosin light chain kinase)-signaling pathway by binding to CaM which in turn activates MLCK. Activated MLCK inhibits MLCP, thereby activating MLC II (myosin light chain II), actin-myosin interaction and formation of F-actin stress fibers. The resulting tensile force on endothelial cell–cell-junctions (inside-out-signaling) causes inter-endothelial gap formation and endothelial barrier failure. Created with BioRender.com.
Additionally, thrombin activity is increased due to a dysregulation of coagulation in pulmonary inflammation [113]. Thrombin binds to and cleaves a specific GPCR, namely protease-activated receptor 1 (PAR1), leading to the secretion of VEGF and VEGFR-mediated signaling (Table 2), which in turn contributes to an increase of endothelial permeability [91,114] by DAG-dependent activation of heteromeric TRPC3/6 [84,115] as illustrated in Figure 3. PAR1 could also activate TRPC1/4 in pulmonary endothelial cells [116], as the Gαq subunit of PAR1 is released and initiates the formation of IP3 and thus, Ca2+ release from the endoplasmic reticulum (Figure 3). Heteromeric TRPC1/4 and TRPC3/6 could act in parallel and synergistically as GPCR- and Ca2+ store depletion-activated channels in acute pulmonary inflammation. The GPCR-induced Ca2+ influx via TRPC1/4 and TRPC3/6 heteromers may be further stabilized by their activation via Ca2+ store depletion from the endoplasmic reticulum [78,84,89], while TRPV1/4 mediated Ca2+ influx is amplified by Ca2+-dependent K+ efflux via IK and SK driving hyperpolarization. Both scenarios will ultimately result in prolonged and increased Ca2+ signaling in pulmonary microvascular endothelial cells, thereby promoting barrier loss and inflammatory signaling.
8.3. Activation of Homomeric TRPC1, TRPC6, TRPV1, and TRPV4 in Lung Inflammation
Platelet-activating factor is released during pulmonary inflammation and activates acid sphingomyelinase which produces ceramides, recruiting caveolin-1, endothelial NO-synthase (eNOS) and TRPC6 channels into caveolae [117]. Caveolar recruitment of caveolin-1 and eNOS inhibits NO production, increasing the activity of homomeric TRPC6 which in turn—via Ca2+ influx—activates the CaM/MLCK-signaling pathway [117]. When Ca2+/CaM-complexes bind to their CaM binding sites, TRPC6-mediated Ca2+ influx gets eventually inactivated, thereby limiting the endothelial response, which can be also applicable to TRPC1, under the assumption that TRPC1 itself induces a relevant Ca2+ influx and does not only modulate Ca2+ influxes through other Ca2+ permeable channels [118,119,120]. Other important homomeric TRP channels in lung inflammation are TRPV1 and TRPV4. TRPV1 contributes to ER-stress, is activated by high temperatures like fever in pulmonary microvascular endothelial cells [13] and in vascular inflammation in other vascular beds [121]. TRPV4 is a crucial regulator of lung vascular permeability [116]. Its Ca2+ influx is stabilized by hyperpolarization [101,102] and interestingly, by Ca2+/CaM-complexes binding to its CaM binding sites on its C-terminus [122]. This unveils a positive Ca2+/CaM-feedback loop for TRPV4 in contrast to TRPC1 and TRPC6. The Ca2+ influx stimulates the CaM/MLCK-signaling pathway, thus promoting endothelial barrier failure, e.g., in ventilator-induced lung injury in patients with the ARDS [14].
8.4. Comparison of Heteromeric TRP Assemblies to Homomeric TRP Assemblies in Lung Inflammation
TRPC1 and TRPC6 contribute to a self-limited Ca2+ influx whereas TRPV4 leads to an altered increased Ca2+ influx. An altered increased Ca2+ could be also assumed for TRP heteromers, as heteromers of TRPC1 and TRPC6, for example, are not reported to get inhibited by the negative Ca2+/CaM-feedback mechanism (Figure 3). One reason for that could be that CaM binding sites are covered in a heteromeric assembly and therefore not reachable for Ca2+/CaM-complexes. Another hypothesis could be that the binding of Ca2+/CaM-complexes to their binding sites has a positive effect on heteromers of TRPC1 and TRPC6, similarly to TRPV4. Additionally, a K+ efflux-mediated hyperpolarization caused by Ca2+ influx could increase the opening phase of TRPV1/4 just as it was found for TRPV4 (Figure 3). By loss of negative feedback loops and by activation through simultaneous upregulated activation mechanisms, TRP heteromers could have a longer opening phase. Therefore, TRP heteromers could induce an altered increased Ca2+ influx that promotes further endothelial loss of barrier integrity and thus would have a major pathophysiological relevance, e.g., in inflammatory processes by facilitating the invasion of immunological cells into the lung, such as monocytes, PMNs, T-helper 2 cells, mast cells and eosinophils.
9. Summary
Here, we discuss that TRP heteromers—similar to their homomeric TRP counterparts like TRPC1, TRPV4 or TRPC6 [4,14] —may play a crucial role in regulating endothelial barrier function. Heteromerization multiplies the variability of TRP channel compositions within their own subfamily and hence, of cellular responses to biochemical or biomechanical stimuli. Heteromeric TRP channels could also disinhibit reactions through loss of negative feedback loops. Therefore, TRP heteromers could be contributed a major, so far misunderstood, role in pulmonary vascular physiology and pathophysiology.
In pulmonary inflammation, the three here described TRP heteromers TRPC1/4, TRPC3/6 and TRPV1/4 have—to various extents—been shown to play a functional role in the regulation of barrier integrity. The knowledge of structural and functional advantages of TRP heteromerization remains to date largely elusive. Structural heteromeric compositions bind and stabilize via non-covalent interactions between ARDs or CCDs on their N- and C-termini [54,59] (Figure 2) and anchor in the plasma membrane by direct binding of PI(4,5)P2 to the C-terminus [124]. Functionally, TRPC1/4 and TRPC3/6 can be activated either by GPCR or Ca2+ store depletion from the endoplasmic reticulum [78,80,84]. As PLC-mediated PI(4,5)P2 hydrolysis from the phospholipid bilayer has a double-edged role on TRP heteromeric function in that it either diminishes channel activity by loss of PI(4,5)P2 relevant for anchoring of the heteromeric assembly to the plasma membrane and/or increases heteromeric channel activity via an increase in DAG [80,84]. In vascular inflammation, TRPC1/4 and TRPC3/6 may be simultaneously activated by GPCR and Ca2+ store depletion, with Ca2+ influx being initially triggered by GPCR signaling and subsequently stabilized by a SOCE mediated response.
The Ca2+ conductance in endothelial TRP heteromers has been found to be surprisingly intermediate compared to their corresponding homomeric assemblies [74]. As such, the question arises whether and how an intermediate Ca2+ conductance may increase endothelial permeability. A conceivable mechanism could be synergistic effects of an intermediate conductance with a prolonged opening by simultaneous activation in a GPCR- and Ca2+ store released-manner [7,73,78,125]. This might be very likely, as heteromeric TRP assemblies are activated during inflammation not only in pulmonary endothelial cells, but also in other cells [126].
For most heteromeric TRP assemblies the actual stoichiometry remains so far unclear. To obtain better in-depth insight into the structural makeup of TRP channel heteromeric assemblies and to foster our understanding in their physiological function, AFM or SC-SMD may be used for future identification of TRP heteromer’s stoichiometry in channel expressing cells. Both methods present relatively new approaches and allow for the analysis of heteromeric TRP channels at the single channel level, as they yield the three-dimensional structure of ion channels at the nanometer (for SC-SMD) or Ångström range (for AFM). With their high sensitivity, both methods represent promising alternatives to investigate the structure and the stoichiometry of heteromeric TRP channels.
Heteromeric TRP channel assemblies may amplify Ca2+ influx by prolonged openings, thus promoting a CaM/MLCK-signaling-dependent increase in endothelial permeability. Consistently, activation of heteromeric TRP channels can disrupt endothelial barrier function more profoundly as compared to homomeric TRP channels, and as such, more differentiated T-helper 2 cells, mast cells, neutrophils and eosinophils can invade lung tissues. In line with an important role of TRP channels in immune cell dynamics, TRPC6-positive cells correlated positively with the number of eosinophils in histological specimens from nasal tissue in patients with eosinophilic chronic rhinosinusitis with nasal polyps [127]. TRPC6 plays also an important role in allergic inflammation of the lung, as TRPC6 deficiency inhibited the immunological functions of T-helper 2, mast cells and eosinophils [128]. Analogously, heteromeric TRP channel assemblies containing TRPC6 may further amplify the invasion of eosinophils and therefore increase allergic inflammatory processes in the lung.
Heteromeric TRP assemblies may present possible therapeutic targets to reduce pulmonary vascular inflammation in diseases such as pneumonia, ARDS, COVID-19 or eosinophilic lung diseases.
Abbreviations
| AFM | atomic force microscopy |
| ARDS | Acute Respiratory Distress Syndrome |
| ARDs | ankyrin repeat domains |
| CaM | calmodulin |
| CCDs | coiled-coil domains |
| Co-IP | co-immunoprecipitation |
| DAG | diacylglycerol |
| eNOS | endothelial NO-synthase |
| FRET | Förster resonance energy transfer |
| GPCR | G-protein-coupled receptor |
| HEK293 | human embryonic kidney cells 293 |
| HMVECs | human microvascular endothelial cells |
| HUVECs | human umbilical vein endothelial cells |
| IK | intermediate conductance Ca2+-activated K+ channel |
| IP3 | inositol 1,4,5-trisphosphate |
| LPS | lipopolysaccharide |
| RMAECs | rat mesenteric artery endothelial cells |
| MLCII | myosin light chain II |
| MLCK | myosin light chain kinase |
| MLCP | myosin light chain phosphatase |
| MRLC | myosin II regulatory light chains |
| NF-κB | nuclear factor-kappa B |
| Orai1 | Ca2+ release-activated calcium channel protein |
| PAR1 | protease-activated receptor 1 |
| PI(4,5)P2 | phosphatidylinositol 4,5-bisphosphate |
| PLA | proximity ligation assay |
| PLCβ/γ | phospholipase C β/γ |
| PMNs | polymorphonuclear leukocytes |
| PRR | pathogen recognition receptors |
| RMECs | retinal microvascular endothelial cells |
| ROS | reactive oxygen species |
| SC-SMD | single channel single molecule detection |
| SK | small Ca2+-dependent K+ channel |
| SOCE | store-operated Ca2+ entry |
| STIM1 | stromal interaction molecule |
| TIRF | total internal reflection fluorescence microscopy |
| TLR | Toll-like receptors |
| TRP | transient receptor potential |
| VEGF | vascular endothelial growth factor |
| Y2H | yeast two-hybrid |
Author Contributions
M.Z., W.M.K. and L.M. contributed to the conception and design of the work, drafted the review and revised it critically for intellectual content. All authors have approved the final version of the article and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed. All authors have read and agreed to the published version of the manuscript.
Funding
This review received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cioffi D.L., Lowe K., Alvarez D.F., Barry C., Stevens T. TRPing on the lung endothelium: Calcium channels that regulate barrier function. Antioxid. Redox Signal. 2009;11:765–776. doi: 10.1089/ars.2008.2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yin J., Kuebler W.M. Mechanotransduction by TRP channels: General concepts and specific role in the vasculature. Cell Biochem. Biophys. 2010;56:1–18. doi: 10.1007/s12013-009-9067-2. [DOI] [PubMed] [Google Scholar]
- 3.Simmons S., Erfinanda L., Bartz C., Kuebler W.M. Novel mechanisms regulating endothelial barrier function in the pulmonary microcirculation. J. Physiol. 2019;597:997–1021. doi: 10.1113/JP276245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Villalta P.C., Townsley M.I. Transient receptor potential channels and regulation of lung endothelial permeability. Pulm Circ. 2013;3:802–815. doi: 10.1086/674765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Montell C., Birnbaumer L., Flockerzi V., Bindels R.J., Bruford E.A., Caterina M.J., Clapham D.E., Harteneck C., Heller S., Julius D., et al. A unified nomenclature for the superfamily of TRP cation channels. Mol. Cell. 2002;9:229–231. doi: 10.1016/S1097-2765(02)00448-3. [DOI] [PubMed] [Google Scholar]
- 6.Paria B.C., Vogel S.M., Ahmmed G.U., Alamgir S., Shroff J., Malik A.B., Tiruppathi C. Tumor necrosis factor-alpha-induced TRPC1 expression amplifies store-operated Ca2+ influx and endothelial permeability. Am. J. Physiol Lung Cell Mol. Physiol. 2004;287:L1303–L1313. doi: 10.1152/ajplung.00240.2004. [DOI] [PubMed] [Google Scholar]
- 7.Ma X., Cheng K.T., Wong C.O., O’Neil R.G., Birnbaumer L., Ambudkar I.S., Yao X. Heteromeric TRPV4-C1 channels contribute to store-operated Ca(2+) entry in vascular endothelial cells. Cell Calcium. 2011;50:502–509. doi: 10.1016/j.ceca.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yin J., Michalick L., Tang C., Tabuchi A., Goldenberg N., Dan Q., Awwad K., Wang L., Erfinanda L., Nouailles G., et al. Role of Transient Receptor Potential Vanilloid 4 in Neutrophil Activation and Acute Lung Injury. Am. J. Respir. Cell Mol. Biol. 2016;54:370–383. doi: 10.1165/rcmb.2014-0225OC. [DOI] [PubMed] [Google Scholar]
- 9.Hecquet C.M., Zhang M., Mittal M., Vogel S.M., Di A., Gao X., Bonini M.G., Malik A.B. Cooperative interaction of trp melastatin channel transient receptor potential (TRPM2) with its splice variant TRPM2 short variant is essential for endothelial cell apoptosis. Circ. Res. 2014;114:469–479. doi: 10.1161/CIRCRESAHA.114.302414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Earley S., Brayden J.E. Transient receptor potential channels in the vasculature. Physiol. Rev. 2015;95:645–690. doi: 10.1152/physrev.00026.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moccia F., Guerra G. Ca(2+) Signalling in Endothelial Progenitor Cells: Friend or Foe? J. Cell Physiol. 2016;231:314–327. doi: 10.1002/jcp.25126. [DOI] [PubMed] [Google Scholar]
- 12.Smani T., Gomez L.J., Regodon S., Woodard G.E., Siegfried G., Khatib A.M., Rosado J.A. TRP Channels in Angiogenesis and Other Endothelial Functions. Front. Physiol. 2018;9:1731. doi: 10.3389/fphys.2018.01731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thomas K.C., Roberts J.K., Deering-Rice C.E., Romero E.G., Dull R.O., Lee J., Yost G.S., Reilly C.A. Contributions of TRPV1, endovanilloids, and endoplasmic reticulum stress in lung cell death in vitro and lung injury. Am. J. Physiol. Lung Cell Mol. Physiol. 2012;302:L111–L119. doi: 10.1152/ajplung.00231.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Michalick L., Erfinanda L., Weichelt U., van der Giet M., Liedtke W., Kuebler W.M. Transient Receptor Potential Vanilloid 4 and Serum Glucocorticoid-regulated Kinase 1 Are Critical Mediators of Lung Injury in Overventilated Mice In Vivo. Anesthesiology. 2017;126:300–311. doi: 10.1097/ALN.0000000000001443. [DOI] [PubMed] [Google Scholar]
- 15.Townsley M.I. Permeability and calcium signaling in lung endothelium: Unpack the box. Pulm Circ. 2018;8:2045893217738218. doi: 10.1177/2045893217738218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kedei N., Szabo T., Lile J.D., Treanor J.J., Olah Z., Iadarola M.J., Blumberg P.M. Analysis of the native quaternary structure of vanilloid receptor 1. J. Biol. Chem. 2001;276:28613–28619. doi: 10.1074/jbc.M103272200. [DOI] [PubMed] [Google Scholar]
- 17.Hoenderop J.G., Voets T., Hoefs S., Weidema F., Prenen J., Nilius B., Bindels R.J. Homo- and heterotetrameric architecture of the epithelial Ca2+ channels TRPV5 and TRPV6. EMBO J. 2003;22:776–785. doi: 10.1093/emboj/cdg080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gonzalez-Ramirez R., Chen Y., Liedtke W.B., Morales-Lazaro S.L. TRP Channels and Pain. In: Emir T.L.R., editor. Neurobiology of TRP Channels. CRC Press; Boca Raton, FL, USA: 2017. pp. 125–147. [PubMed] [Google Scholar]
- 19.Cao S., Anishkin A., Zinkevich N.S., Nishijima Y., Korishettar A., Wang Z., Fang J., Wilcox D.A., Zhang D.X. Transient receptor potential vanilloid 4 (TRPV4) activation by arachidonic acid requires protein kinase A-mediated phosphorylation. J. Biol. Chem. 2018;293:5307–5322. doi: 10.1074/jbc.M117.811075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schaefer M. Homo- and heteromeric assembly of TRP channel subunits. Pflug. Arch. 2005;451:35–42. doi: 10.1007/s00424-005-1467-6. [DOI] [PubMed] [Google Scholar]
- 21.Bai C.X., Giamarchi A., Rodat-Despoix L., Padilla F., Downs T., Tsiokas L., Delmas P. Formation of a new receptor-operated channel by heteromeric assembly of TRPP2 and TRPC1 subunits. EMBO Rep. 2008;9:472–479. doi: 10.1038/embor.2008.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheng H.W., James A.F., Foster R.R., Hancox J.C., Bates D.O. VEGF activates receptor-operated cation channels in human microvascular endothelial cells. Arter. Thromb. Vasc. Biol. 2006;26:1768–1776. doi: 10.1161/01.ATV.0000231518.86795.0f. [DOI] [PubMed] [Google Scholar]
- 23.Ackermann M., Verleden S.E., Kuehnel M., Haverich A., Welte T., Laenger F., Vanstapel A., Werlein C., Stark H., Tzankov A., et al. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in Covid-19. N. Engl. J. Med. 2020;383:120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gross C.M., Kellner M., Wang T., Lu Q., Sun X., Zemskov E.A., Noonepalle S., Kangath A., Kumar S., Gonzalez-Garay M., et al. LPS-induced Acute Lung Injury Involves NF-kappaB-mediated Downregulation of SOX18. Am. J. Respir. Cell Mol. Biol. 2018;58:614–624. doi: 10.1165/rcmb.2016-0390OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singh A.K., McGoldrick L.L., Sobolevsky A.I. Expression, Purification, and Crystallization of the Transient Receptor Potential Channel TRPV6. Methods Mol. Biol. 2019;1987:23–37. doi: 10.1007/978-1-4939-9446-5_2. [DOI] [PubMed] [Google Scholar]
- 26.Fredriksson S., Gullberg M., Jarvius J., Olsson C., Pietras K., Gustafsdottir S.M., Ostman A., Landegren U. Protein detection using proximity-dependent DNA ligation assays. Nat. Biotechnol. 2002;20:473–477. doi: 10.1038/nbt0502-473. [DOI] [PubMed] [Google Scholar]
- 27.O’Leary C., McGahon M.K., Ashraf S., McNaughten J., Friedel T., Cincola P., Barabas P., Fernandez J.A., Stitt A.W., McGeown J.G., et al. Involvement of TRPV1 and TRPV4 Channels in Retinal Angiogenesis. Invest. Ophthalmol Vis. Sci. 2019;60:3297–3309. doi: 10.1167/iovs.18-26344. [DOI] [PubMed] [Google Scholar]
- 28.Hofmann T., Schaefer M., Schultz G., Gudermann T. Subunit composition of mammalian transient receptor potential channels in living cells. Proc. Natl. Acad. Sci. USA. 2002;99:7461–7466. doi: 10.1073/pnas.102596199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu X.Z., Li H.S., Guggino W.B., Montell C. Coassembly of TRP and TRPL produces a distinct store-operated conductance. Cell. 1997;89:1155–1164. doi: 10.1016/S0092-8674(00)80302-5. [DOI] [PubMed] [Google Scholar]
- 30.Lentze N., Auerbach D. Membrane-based yeast two-hybrid system to detect protein interactions. Curr. Protoc. Protein Sci. 2008;52 doi: 10.1002/0471140864.ps1917s52. [DOI] [PubMed] [Google Scholar]
- 31.Das S., Chakrabarti S. Classification and prediction of protein-protein interaction interface using machine learning algorithm. Sci. Rep. 2021;11:1761. doi: 10.1038/s41598-020-80900-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Singh A.K., McGoldrick L.L., Saotome K., Sobolevsky A.I. X-ray crystallography of TRP channels. Channels (Austin) 2018;12:137–152. doi: 10.1080/19336950.2018.1457898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Samanta A., Hughes T.E.T., Moiseenkova-Bell V.Y. Cryo Electron Microscopy of TRP Channels. Methods Mol. Biol. 2019;1987:39–50. doi: 10.1007/978-1-4939-9446-5_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stewart A.P., Smith G.D., Sandford R.N., Edwardson J.M. Atomic force microscopy reveals the alternating subunit arrangement of the TRPP2-TRPV4 heterotetramer. Biophys. J. 2010;99:790–797. doi: 10.1016/j.bpj.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sinz A., Arlt C., Chorev D., Sharon M. Chemical cross-linking and native mass spectrometry: A fruitful combination for structural biology. Protein Sci. 2015;24:1193–1209. doi: 10.1002/pro.2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Silva L.P. Imaging proteins with atomic force microscopy: An overview. Curr. Protein Pept. Sci. 2005;6:387–395. doi: 10.2174/1389203054546389. [DOI] [PubMed] [Google Scholar]
- 37.Tang Z., Takahashi Y. Analysis of Protein-Protein Interaction by Co-IP in Human Cells. Methods Mol. Biol. 2018;1794:289–296. doi: 10.1007/978-1-4939-7871-7_20. [DOI] [PubMed] [Google Scholar]
- 38.Hellwig N., Albrecht N., Harteneck C., Schultz G., Schaefer M. Homo- and heteromeric assembly of TRPV channel subunits. J. Cell Sci. 2005;118:917–928. doi: 10.1242/jcs.01675. [DOI] [PubMed] [Google Scholar]
- 39.Iacobucci C., Gotze M., Sinz A. Cross-linking/mass spectrometry to get a closer view on protein interaction networks. Curr. Opin. Biotechnol. 2020;63:48–53. doi: 10.1016/j.copbio.2019.12.009. [DOI] [PubMed] [Google Scholar]
- 40.Danev R., Yanagisawa H., Kikkawa M. Cryo-Electron Microscopy Methodology: Current Aspects and Future Directions. Trends Biochem. Sci. 2019;44:837–848. doi: 10.1016/j.tibs.2019.04.008. [DOI] [PubMed] [Google Scholar]
- 41.Kaminski C.F., Rees E.J., Schierle G.S. A quantitative protocol for intensity-based live cell FRET imaging. Methods Mol. Biol. 2014;1076:445–454. doi: 10.1007/978-1-62703-649-8_19. [DOI] [PubMed] [Google Scholar]
- 42.Alam M.S. Proximity Ligation Assay (PLA) Curr. Protoc. Immunol. 2018;123:e58. doi: 10.1002/cpim.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jackson M.B. Whole-cell voltage clamp recording. Curr. Protoc. Neurosci. 2001;6:6. doi: 10.1002/0471142301.ns0606s00. [DOI] [PubMed] [Google Scholar]
- 44.Storch U., Forst A.L., Philipp M., Gudermann T., Mederos y Schnitzler M. Transient receptor potential channel 1 (TRPC1) reduces calcium permeability in heteromeric channel complexes. J. Biol. Chem. 2012;287:3530–3540. doi: 10.1074/jbc.M111.283218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ceballos L.G., Asanov A., Vaca L. Single-Channel Single-Molecule Detection (SC-SMD) System. Methods Mol. Biol. 2018;1843:189–201. doi: 10.1007/978-1-4939-8704-7_16. [DOI] [PubMed] [Google Scholar]
- 46.Smyth M.S., Martin J.H. x ray crystallography. Mol. Pathol. 2000;53:8–14. doi: 10.1136/mp.53.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Paiano A., Margiotta A., De Luca M., Bucci C. Yeast Two-Hybrid Assay to Identify Interacting Proteins. Curr. Protoc. Protein Sci. 2019;95:e70. doi: 10.1002/cpps.70. [DOI] [PubMed] [Google Scholar]
- 48.Venkatachalam K., Montell C. TRP channels. Annu. Rev. Biochem. 2007;76:387–417. doi: 10.1146/annurev.biochem.75.103004.142819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Phelps C.B., Procko E., Lishko P.V., Wang R.R., Gaudet R. Insights into the roles of conserved and divergent residues in the ankyrin repeats of TRPV ion channels. Channels (Austin) 2007;1:148–151. doi: 10.4161/chan.4716. [DOI] [PubMed] [Google Scholar]
- 50.Li J., Mahajan A., Tsai M.D. Ankyrin repeat: A unique motif mediating protein-protein interactions. Biochemistry. 2006;45:15168–15178. doi: 10.1021/bi062188q. [DOI] [PubMed] [Google Scholar]
- 51.Mosavi L.K., Minor D.L., Jr., Peng Z.Y. Consensus-derived structural determinants of the ankyrin repeat motif. Proc. Natl. Acad. Sci. USA. 2002;99:16029–16034. doi: 10.1073/pnas.252537899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li M., Yu Y., Yang J. Structural biology of TRP channels. Adv. Exp. Med. Biol. 2011;704:1–23. doi: 10.1007/978-94-007-0265-3_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rohacs T., Lopes C.M., Michailidis I., Logothetis D.E. PI(4,5)P2 regulates the activation and desensitization of TRPM8 channels through the TRP domain. Nat. Neurosci. 2005;8:626–634. doi: 10.1038/nn1451. [DOI] [PubMed] [Google Scholar]
- 54.Tang Q., Guo W., Zheng L., Wu J.X., Liu M., Zhou X., Zhang X., Chen L. Structure of the receptor-activated human TRPC6 and TRPC3 ion channels. Cell Res. 2018;28:746–755. doi: 10.1038/s41422-018-0038-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tsuruda P.R., Julius D., Minor D.L., Jr. Coiled coils direct assembly of a cold-activated TRP channel. Neuron. 2006;51:201–212. doi: 10.1016/j.neuron.2006.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu J., Zheng Q., Deng Y., Cheng C.S., Kallenbach N.R., Lu M. A seven-helix coiled coil. Proc. Natl. Acad. Sci. USA. 2006;103:15457–15462. doi: 10.1073/pnas.0604871103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fujiwara Y., Minor D.L., Jr. X-ray crystal structure of a TRPM assembly domain reveals an antiparallel four-stranded coiled-coil. J. Mol. Biol. 2008;383:854–870. doi: 10.1016/j.jmb.2008.08.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Martinez G.Q., Gordon S.E. Multimerization of Homo sapiens TRPA1 ion channel cytoplasmic domains. PLoS ONE. 2019;14:e0207835. doi: 10.1371/journal.pone.0207835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Myeong J., Ko J., Hong C., Yang D., Lee K.P., Jeon J.H., So I. The interaction domains of transient receptor potential canonical (TRPC)1/4 and TRPC1/5 heteromultimeric channels. Biochem. Biophys. Res. Commun. 2016;474:476–481. doi: 10.1016/j.bbrc.2016.04.138. [DOI] [PubMed] [Google Scholar]
- 60.Schindl R., Fritsch R., Jardin I., Frischauf I., Kahr H., Muik M., Riedl M.C., Groschner K., Romanin C. Canonical transient receptor potential (TRPC) 1 acts as a negative regulator for vanilloid TRPV6-mediated Ca2+ influx. J. Biol. Chem. 2012;287:35612–35620. doi: 10.1074/jbc.M112.400952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Guo Z., Grimm C., Becker L., Ricci A.J., Heller S. A novel ion channel formed by interaction of TRPML3 with TRPV5. PLoS ONE. 2013;8:e58174. doi: 10.1371/journal.pone.0058174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen X., Lu M., He X., Ma L., Birnbaumer L., Liao Y. TRPC3/6/7 Knockdown Protects the Brain from Cerebral Ischemia Injury via Astrocyte Apoptosis Inhibition and Effects on NF-small ka, CyrillicB Translocation. Mol. Neurobiol. 2017;54:7555–7566. doi: 10.1007/s12035-016-0227-2. [DOI] [PubMed] [Google Scholar]
- 63.Chu W.G., Wang F.D., Sun Z.C., Ma S.B., Wang X., Han W.J., Wang F., Bai Z.T., Wu S.X., Freichel M., et al. TRPC1/4/5 channels contribute to morphine-induced analgesic tolerance and hyperalgesia by enhancing spinal synaptic potentiation and structural plasticity. Faseb J. 2020;34:8526–8543. doi: 10.1096/fj.202000154RR. [DOI] [PubMed] [Google Scholar]
- 64.Du J., Ma X., Shen B., Huang Y., Birnbaumer L., Yao X. TRPV4, TRPC1, and TRPP2 assemble to form a flow-sensitive heteromeric channel. Faseb J. 2014;28:4677–4685. doi: 10.1096/fj.14-251652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fonfria E., Murdock P.R., Cusdin F.S., Benham C.D., Kelsell R.E., McNulty S. Tissue distribution profiles of the human TRPM cation channel family. J. Recept. Signal. Transduct. Res. 2006;26:159–178. doi: 10.1080/10799890600637506. [DOI] [PubMed] [Google Scholar]
- 66.Alessandri-Haber N., Dina O.A., Chen X., Levine J.D. TRPC1 and TRPC6 channels cooperate with TRPV4 to mediate mechanical hyperalgesia and nociceptor sensitization. J. Neurosci. 2009;29:6217–6228. doi: 10.1523/JNEUROSCI.0893-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Alessandri-Haber N., Yeh J.J., Boyd A.E., Parada C.A., Chen X., Reichling D.B., Levine J.D. Hypotonicity induces TRPV4-mediated nociception in rat. Neuron. 2003;39:497–511. doi: 10.1016/S0896-6273(03)00462-8. [DOI] [PubMed] [Google Scholar]
- 68.Liedtke W., Friedman J.M. Abnormal osmotic regulation in trpv4-/- mice. Proc. Natl. Acad. Sci. USA. 2003;100:13698–13703. doi: 10.1073/pnas.1735416100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Phelan K.D., Mock M.M., Kretz O., Shwe U.T., Kozhemyakin M., Greenfield L.J., Dietrich A., Birnbaumer L., Freichel M., Flockerzi V., et al. Heteromeric canonical transient receptor potential 1 and 4 channels play a critical role in epileptiform burst firing and seizure-induced neurodegeneration. Mol. Pharm. 2012;81:384–392. doi: 10.1124/mol.111.075341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Thoppil R.J., Adapala R.K., Cappelli H.C., Kondeti V., Dudley A.C., Gary Meszaros J., Paruchuri S., Thodeti C.K. TRPV4 channel activation selectively inhibits tumor endothelial cell proliferation. Sci. Rep. 2015;5:14257. doi: 10.1038/srep14257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wu Q.Y., Sun M.R., Wu C.L., Li Y., Du J.J., Zeng J.Y., Bi H.L., Sun Y.H. Activation of calcium-sensing receptor increases TRPC3/6 expression in T lymphocyte in sepsis. Mol. Immunol. 2015;64:18–25. doi: 10.1016/j.molimm.2014.10.018. [DOI] [PubMed] [Google Scholar]
- 72.Liu X., Bandyopadhyay B.C., Singh B.B., Groschner K., Ambudkar I.S. Molecular analysis of a store-operated and 2-acetyl-sn-glycerol-sensitive non-selective cation channel. Heteromeric assembly of TRPC1-TRPC3. J. Biol. Chem. 2005;280:21600–21606. doi: 10.1074/jbc.C400492200. [DOI] [PubMed] [Google Scholar]
- 73.Wu X., Zagranichnaya T.K., Gurda G.T., Eves E.M., Villereal M.L. A TRPC1/TRPC3-mediated increase in store-operated calcium entry is required for differentiation of H19-7 hippocampal neuronal cells. J. Biol. Chem. 2004;279:43392–43402. doi: 10.1074/jbc.M408959200. [DOI] [PubMed] [Google Scholar]
- 74.Cheng W., Yang F., Takanishi C.L., Zheng J. Thermosensitive TRPV channel subunits coassemble into heteromeric channels with intermediate conductance and gating properties. J. Gen. Physiol. 2007;129:191–207. doi: 10.1085/jgp.200709731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cheung K.K., Yeung S.S., Au S.W., Lam L.S., Dai Z.Q., Li Y.H., Yeung E.W. Expression and association of TRPC1 with TRPC3 during skeletal myogenesis. Muscle Nerve. 2011;44:358–365. doi: 10.1002/mus.22060. [DOI] [PubMed] [Google Scholar]
- 76.Eder P., Groschner K. TRPC3/6/7: Topical aspects of biophysics and pathophysiology. Channels (Austin) 2008;2:94–99. doi: 10.4161/chan.2.2.6015. [DOI] [PubMed] [Google Scholar]
- 77.Tano J.Y., Smedlund K., Vazquez G. Endothelial TRPC3/6/7 proteins at the edge of cardiovascular disease. Cardiovasc. Hematol. Agents Med. Chem. 2010;8:76–86. doi: 10.2174/187152510790796138. [DOI] [PubMed] [Google Scholar]
- 78.Cioffi D.L., Wu S., Chen H., Alexeyev M., St Croix C.M., Pitt B.R., Uhlig S., Stevens T. Orai1 determines calcium selectivity of an endogenous TRPC heterotetramer channel. Circ. Res. 2012;110:1435–1444. doi: 10.1161/CIRCRESAHA.112.269506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang H., Cheng X., Tian J., Xiao Y., Tian T., Xu F., Hong X., Zhu M.X. TRPC channels: Structure, function, regulation and recent advances in small molecular probes. Pharm. Therap. 2020;209:107497. doi: 10.1016/j.pharmthera.2020.107497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Myeong J., Ko J., Kwak M., Kim J., Woo J., Ha K., Hong C., Yang D., Kim H.J., Jeon J.H., et al. Dual action of the Galphaq-PLCbeta-PI(4,5)P2 pathway on TRPC1/4 and TRPC1/5 heterotetramers. Sci. Rep. 2018;8:12117. doi: 10.1038/s41598-018-30625-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ko J., Myeong J., Shin Y.C., So I. Differential PI(4,5)P2 sensitivities of TRPC4, C5 homomeric and TRPC1/4, C1/5 heteromeric channels. Sci. Rep. 2019;9:1849. doi: 10.1038/s41598-018-38443-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kelly J.J., Moore T.M., Babal P., Diwan A.H., Stevens T., Thompson W.J. Pulmonary microvascular and macrovascular endothelial cells: Differential regulation of Ca2+ and permeability. Am. J. Physiol. 1998;274:L810–L819. doi: 10.1152/ajplung.1998.274.5.L810. [DOI] [PubMed] [Google Scholar]
- 83.Li Y., Chen X., Zeng X., Chen S., Yang X., Zhang L. Galectin-3 mediates pulmonary vascular endothelial cell dynamics via TRPC1/4 under acute hypoxia. J. Biochem. Mol. Toxicol. 2020;34:e22463. doi: 10.1002/jbt.22463. [DOI] [PubMed] [Google Scholar]
- 84.Carrillo C., Hichami A., Andreoletti P., Cherkaoui-Malki M., del Mar Cavia M., Abdoul-Azize S., Alonso-Torre S.R., Khan N.A. Diacylglycerol-containing oleic acid induces increases in [Ca(2+)](i) via TRPC3/6 channels in human T-cells. Biochim. Biophys. Acta. 2012;1821:618–626. doi: 10.1016/j.bbalip.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 85.Bijli K.M., Fazal F., Slavin S.A., Leonard A., Grose V., Alexander W.B., Smrcka A.V., Rahman A. Phospholipase C-epsilon signaling mediates endothelial cell inflammation and barrier disruption in acute lung injury. Am. J. Physiol. Lung Cell Mol. Physiol. 2016;311:L517–L524. doi: 10.1152/ajplung.00069.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Futosi K., Fodor S., Mocsai A. Reprint of Neutrophil cell surface receptors and their intracellular signal transduction pathways. Int. Immunopharmacol. 2013;17:1185–1197. doi: 10.1016/j.intimp.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 87.Thebault S., Zholos A., Enfissi A., Slomianny C., Dewailly E., Roudbaraki M., Parys J., Prevarskaya N. Receptor-operated Ca2+ entry mediated by TRPC3/TRPC6 proteins in rat prostate smooth muscle (PS1) cell line. J. Cell Physiol. 2005;204:320–328. doi: 10.1002/jcp.20301. [DOI] [PubMed] [Google Scholar]
- 88.Onohara N., Nishida M., Inoue R., Kobayashi H., Sumimoto H., Sato Y., Mori Y., Nagao T., Kurose H. TRPC3 and TRPC6 are essential for angiotensin II-induced cardiac hypertrophy. EMBO J. 2006;25:5305–5316. doi: 10.1038/sj.emboj.7601417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Meng K., Xu J., Zhang C., Zhang R., Yang H., Liao C., Jiao J. Calcium sensing receptor modulates extracellular calcium entry and proliferation via TRPC3/6 channels in cultured human mesangial cells. PLoS ONE. 2014;9:e98777. doi: 10.1371/journal.pone.0098777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hang P., Zhao J., Cai B., Tian S., Huang W., Guo J., Sun C., Li Y., Du Z. Brain-derived neurotrophic factor regulates TRPC3/6 channels and protects against myocardial infarction in rodents. Int. J. Biol. Sci. 2015;11:536–545. doi: 10.7150/ijbs.10754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hippenstiel S., Krull M., Ikemann A., Risau W., Clauss M., Suttorp N. VEGF induces hyperpermeability by a direct action on endothelial cells. Am. J. Physiol. 1998;274:L678–L684. doi: 10.1152/ajplung.1998.274.5.L678. [DOI] [PubMed] [Google Scholar]
- 92.Rossman M.J., Kaplon R.E., Hill S.D., McNamara M.N., Santos-Parker J.R., Pierce G.L., Seals D.R., Donato A.J. Endothelial cell senescence with aging in healthy humans: Prevention by habitual exercise and relation to vascular endothelial function. Am. J. Physiol. Heart Circ. Physiol. 2017;313:H890–H895. doi: 10.1152/ajpheart.00416.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chu Y., Cohen B.E., Chuang H.H. A single TRPV1 amino acid controls species sensitivity to capsaicin. Sci. Rep. 2020;10:8038. doi: 10.1038/s41598-020-64584-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Min J.W., Liu W.H., He X.H., Peng B.W. Different types of toxins targeting TRPV1 in pain. Toxicon. 2013;71:66–75. doi: 10.1016/j.toxicon.2013.05.016. [DOI] [PubMed] [Google Scholar]
- 95.Mazgaeen L., Gurung P. Recent Advances in Lipopolysaccharide Recognition Systems. Int. J. Mol. Sci. 2020;21:379. doi: 10.3390/ijms21020379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chen S.R., Pan H.L. Removing TRPV1-expressing primary afferent neurons potentiates the spinal analgesic effect of delta-opioid agonists on mechano-nociception. Neuropharmacology. 2008;55:215–222. doi: 10.1016/j.neuropharm.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nozadze I., Tsiklauri N., Gurtskaia G., Tsagareli M.G. Role of thermo TRPA1 and TRPV1 channels in heat, cold, and mechanical nociception of rats. Behav. Pharm. 2016;27:29–36. doi: 10.1097/FBP.0000000000000176. [DOI] [PubMed] [Google Scholar]
- 98.Zheng J., Liu F., Du S., Li M., Wu T., Tan X., Cheng W. Mechanism for Regulation of Melanoma Cell Death via Activation of Thermo-TRPV4 and TRPV2. J. Oncol. 2019;2019:7362875. doi: 10.1155/2019/7362875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Porcari C.Y., Debarba L.K., Amigone J.L., Caeiro X.E., Reis L.C., Cunha T.M., Mecawi A.S., Elias L.L., Antunes-Rodrigues J., Vivas L., et al. Brain osmo-sodium sensitive channels and the onset of sodium appetite. Horm. Behav. 2020;118:104658. doi: 10.1016/j.yhbeh.2019.104658. [DOI] [PubMed] [Google Scholar]
- 100.Li F., Yang W., Jiang H., Guo C., Huang A.J.W., Hu H., Liu Q. TRPV1 activity and substance P release are required for corneal cold nociception. Nat. Commun. 2019;10:5678. doi: 10.1038/s41467-019-13536-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bagher P., Beleznai T., Kansui Y., Mitchell R., Garland C.J., Dora K.A. Low intravascular pressure activates endothelial cell TRPV4 channels, local Ca2+ events, and IKCa channels, reducing arteriolar tone. Proc. Natl. Acad. Sci. USA. 2012;109:18174–18179. doi: 10.1073/pnas.1211946109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lin M.T., Jian M.Y., Taylor M.S., Cioffi D.L., Yap F.C., Liedtke W., Townsley M.I. Functional coupling of TRPV4, IK, and SK channels contributes to Ca(2+)-dependent endothelial injury in rodent lung. Pulm Circ. 2015;5:279–290. doi: 10.1086/680166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Minard A., Bauer C.C., Wright D.J., Rubaiy H.N., Muraki K., Beech D.J., Bon R.S. Remarkable Progress with Small-Molecule Modulation of TRPC1/4/5 Channels: Implications for Understanding the Channels in Health and Disease. Cells. 2018;7:52. doi: 10.3390/cells7060052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Broker-Lai J., Kollewe A., Schindeldecker B., Pohle J., Nguyen Chi V., Mathar I., Guzman R., Schwarz Y., Lai A., Weissgerber P., et al. Heteromeric channels formed by TRPC1, TRPC4 and TRPC5 define hippocampal synaptic transmission and working memory. EMBO J. 2017;36:2770–2789. doi: 10.15252/embj.201696369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhao L., Wang J., Wang L., Liang Y.T., Chen Y.Q., Lu W.J., Zhou W.L. Remodeling of rat pulmonary artery induced by chronic smoking exposure. J. Thorac Dis. 2014;6:818–828. doi: 10.3978/j.issn.2072-1439.2014.03.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kovalevskaya N.V., Bokhovchuk F.M., Vuister G.W. The TRPV5/6 calcium channels contain multiple calmodulin binding sites with differential binding properties. J. Struct. Funct. Genom. 2012;13:91–100. doi: 10.1007/s10969-012-9128-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Fan H., Shen Y.X., Yuan Y.F. Expression and prognostic roles of TRPV5 and TRPV6 in non-small cell lung cancer after curative resection. Asian Pac. J. Cancer Prev. 2014;15:2559–2563. doi: 10.7314/APJCP.2014.15.6.2559. [DOI] [PubMed] [Google Scholar]
- 108.Lehen’kyi V., Raphael M., Prevarskaya N. The role of the TRPV6 channel in cancer. J. Physiol. 2012;590:1369–1376. doi: 10.1113/jphysiol.2011.225862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Muller-Redetzky H.C., Suttorp N., Witzenrath M. Dynamics of pulmonary endothelial barrier function in acute inflammation: Mechanisms and therapeutic perspectives. Cell Tissue Res. 2014;355:657–673. doi: 10.1007/s00441-014-1821-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ince C., Mayeux P.R., Nguyen T., Gomez H., Kellum J.A., Ospina-Tascon G.A., Hernandez G., Murray P., De Backer D., Workgroup A.X. The Endothelium in Sepsis. Shock. 2016;45:259–270. doi: 10.1097/SHK.0000000000000473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Opitz B., van Laak V., Eitel J., Suttorp N. Innate immune recognition in infectious and noninfectious diseases of the lung. Am. J. Respir. Crit Care Med. 2010;181:1294–1309. doi: 10.1164/rccm.200909-1427SO. [DOI] [PubMed] [Google Scholar]
- 112.Imai Y., Kuba K., Neely G.G., Yaghubian-Malhami R., Perkmann T., van Loo G., Ermolaeva M., Veldhuizen R., Leung Y.H., Wang H., et al. Identification of oxidative stress and Toll-like receptor 4 signaling as a key pathway of acute lung injury. Cell. 2008;133:235–249. doi: 10.1016/j.cell.2008.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Glas G.J., Van Der Sluijs K.F., Schultz M.J., Hofstra J.J., Van Der Poll T., Levi M. Bronchoalveolar hemostasis in lung injury and acute respiratory distress syndrome. J. Thromb. Haemost. 2013;11:17–25. doi: 10.1111/jth.12047. [DOI] [PubMed] [Google Scholar]
- 114.Cirino G., Cicala C., Bucci M.R., Sorrentino L., Maraganore J.M., Stone S.R. Thrombin functions as an inflammatory mediator through activation of its receptor. J. Exp. Med. 1996;183:821–827. doi: 10.1084/jem.183.3.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Takahashi T., Yamaguchi S., Chida K., Shibuya M. A single autophosphorylation site on KDR/Flk-1 is essential for VEGF-A-dependent activation of PLC-gamma and DNA synthesis in vascular endothelial cells. EMBO J. 2001;20:2768–2778. doi: 10.1093/emboj/20.11.2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Tiruppathi C., Ahmmed G.U., Vogel S.M., Malik A.B. Ca2+ signaling, TRP channels, and endothelial permeability. Microcirculation. 2006;13:693–708. doi: 10.1080/10739680600930347. [DOI] [PubMed] [Google Scholar]
- 117.Uhlig S., Yang Y. Sphingolipids in acute lung injury. Handb. Exp. Pharm. 2013;216:227–246. doi: 10.1007/978-3-7091-1511-4_11. [DOI] [PubMed] [Google Scholar]
- 118.Polat O.K., Uno M., Maruyama T., Tran H.N., Imamura K., Wong C.F., Sakaguchi R., Ariyoshi M., Itsuki K., Ichikawa J., et al. Contribution of Coiled-Coil Assembly to Ca(2+)/Calmodulin-Dependent Inactivation of TRPC6 Channel and its Impacts on FSGS-Associated Phenotypes. J. Am. Soc. Nephrol. 2019;30:1587–1603. doi: 10.1681/ASN.2018070756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Singh B.B., Liu X., Tang J., Zhu M.X., Ambudkar I.S. Calmodulin regulates Ca(2+)-dependent feedback inhibition of store-operated Ca(2+) influx by interaction with a site in the C terminus of TrpC1. Mol. Cell. 2002;9:739–750. doi: 10.1016/S1097-2765(02)00506-3. [DOI] [PubMed] [Google Scholar]
- 120.Antoniotti S., Lovisolo D., Fiorio Pla A., Munaron L. Expression and functional role of bTRPC1 channels in native endothelial cells. Febs Lett. 2002;510:189–195. doi: 10.1016/S0014-5793(01)03256-2. [DOI] [PubMed] [Google Scholar]
- 121.Keeble J., Russell F., Curtis B., Starr A., Pinter E., Brain S.D. Involvement of transient receptor potential vanilloid 1 in the vascular and hyperalgesic components of joint inflammation. Arthritis Rheum. 2005;52:3248–3256. doi: 10.1002/art.21297. [DOI] [PubMed] [Google Scholar]
- 122.Strotmann R., Schultz G., Plant T.D. Ca2+-dependent potentiation of the nonselective cation channel TRPV4 is mediated by a C-terminal calmodulin binding site. J. Biol. Chem. 2003;278:26541–26549. doi: 10.1074/jbc.M302590200. [DOI] [PubMed] [Google Scholar]
- 123.Tauseef M., Knezevic N., Chava K.R., Smith M., Sukriti S., Gianaris N., Obukhov A.G., Vogel S.M., Schraufnagel D.E., Dietrich A., et al. TLR4 activation of TRPC6-dependent calcium signaling mediates endotoxin-induced lung vascular permeability and inflammation. J. Exp. Med. 2012;209:1953–1968. doi: 10.1084/jem.20111355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ufret-Vincenty C.A., Klein R.M., Hua L., Angueyra J., Gordon S.E. Localization of the PIP2 sensor of TRPV1 ion channels. J. Biol. Chem. 2011;286:9688–9698. doi: 10.1074/jbc.M110.192526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Cheng K.T., Liu X., Ong H.L., Swaim W., Ambudkar I.S. Local Ca(2)+ entry via Orai1 regulates plasma membrane recruitment of TRPC1 and controls cytosolic Ca(2)+ signals required for specific cell functions. PLoS Biol. 2011;9:e1001025. doi: 10.1371/journal.pbio.1001025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Xu M., Zhang Y., Wang M., Zhang H., Chen Y., Adcock I.M., Chung K.F., Mo J., Zhang Y., Li F. TRPV1 and TRPA1 in Lung Inflammation and Airway Hyperresponsiveness Induced by Fine Particulate Matter (PM2.5) Oxid. Med. Cell Longev. 2019;2019:7450151. doi: 10.1155/2019/7450151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tang R., Li Z.P., Li M.X., Li D.W., Ye H.B., Su K.M., Lin H., Zhang W.T. Pro-inflammatory role of transient receptor potential canonical channel 6 in the pathogenesis of chronic rhinosinusitis with nasal polyps. Int. Forum Allergy Rhinol. 2018;8:1334–1341. doi: 10.1002/alr.22208. [DOI] [PubMed] [Google Scholar]
- 128.Sel S., Rost B.R., Yildirim A.O., Sel B., Kalwa H., Fehrenbach H., Renz H., Gudermann T., Dietrich A. Loss of classical transient receptor potential 6 channel reduces allergic airway response. Clin. Exp. Allergy. 2008;38:1548–1558. doi: 10.1111/j.1365-2222.2008.03043.x. [DOI] [PubMed] [Google Scholar]