Abstract
Consolidation and reconsolidation are independent memory processes. Consolidation stabilizes new memories, whereas reconsolidation restabilizes memories destabilized when reactivated during recall. However, the biological role of the destabilization/reconsolidation cycle is still unknown. It has been hypothesized that reconsolidation links new information with reactivated memories, but some reports suggest that new and old memories are associated through consolidation mechanisms instead. Object-recognition memory (ORM) serves to judge the familiarity of items and is essential for remembering previous events. We took advantage of the fact that ORM consolidation, destabilization, and reconsolidation can be pharmacologically dissociated to demonstrate that, depending on the activation state of hippocampal dopamine D1/D5 receptors, the memory of a novel object presented during recall of the memory of a familiar one can be formed via reconsolidation or consolidation, but only reconsolidation can link them. We also found that recognition memories formed through reconsolidation can be destabilized even if indirectly reactivated. Our results indicate that dopamine couples novelty detection with memory destabilization to determine whether a new recognition trace is associated with an active network and suggest that declarative reminders should be used with caution during reconsolidation-based psychotherapeutic interventions.
Keywords: CaMKII, PKMζ, memory schemata, indirect recall, memory updating
New memories are initially unstable and require a protein-synthesis–dependent stabilization process, known as consolidation, to persist. Consolidated memories are long lasting; however, they may be destabilized when reactivated at recall. Reconsolidation is the protein-synthesis–dependent process that restabilizes memories destabilized by recall, but its biological role is not fully understood (1).
Object-recognition memory (ORM) is a form of declarative memory that confers the ability to identify familiar elements, a vital competence used on a daily basis, frequently together with the explicit recall of the episodes during which those elements were previously encountered (2). In rats, ORM consolidation necessitates a functional hippocampus. The hippocampus is also necessary for ORM reconsolidation, but only when recall occurs in the presence of a novel object (3). Indeed, presentation of a novel object during ORM recall activates the reconsolidation marker Zif268 (4) and is accompanied by a brief depotentiation period followed by a lasting increase in hippocampal synaptic efficacy (5). Moreover, the amnesia caused by ORM reconsolidation inhibitors is prevented by impairing novelty detection through hippocampal dopamine D1/D5 receptor blockade (6), suggesting that reconsolidation links the memory of the novel object to the reactivated recognition trace. However, some memory types are linked via consolidation mechanisms instead, without affecting memory stability (7). Thus, we wondered if the memory of a novel object presented during recall of the memory of a familiar one can be stored through consolidation rather than reconsolidation and pondered whether any of these processes actually update the reactivated recognition trace. These are important questions insofar as ORM is essential for remembering previous events, and it has been suggested that reconsolidation modulation improves the effectiveness of psychotherapies aiming to erase or modify the memory of disturbing experiences (1).
Here we report that hippocampal dopamine D1/D5 receptors control whether new ORMs are linked to old ones through reconsolidation or whether they are consolidated as independent traces, and that the individual components of the recognition memory systems formed by reconsolidation can be destabilized even when indirectly reactivated.
Results and Discussion
First, we analyzed whether the memory of a novel object presented alongside a familiar one can be formed via consolidation when the induction of reconsolidation is prevented by blocking memory destabilization. To do that, we utilized the novel object-recognition task, an incidental episodic-like learning paradigm based on rodents’ innate preference for novelty that involves exposure to two different but behaviorally equivalent novel objects in a familiar environment (3), and capitalized on the fact that ORM consolidation, destabilization, and reconsolidation can be pharmacologically dissociated. Indeed, ORM consolidation, but not reconsolidation, requires hippocampal CaMKII (8), whereas ORM reconsolidation, but not consolidation, necessitates hippocampal PKMζ activity (9). On the other hand, hippocampal dopamine D1/D5 receptors are unnecessary for ORM consolidation, recall, or reconsolidation but control ORM destabilization and, hence, their inhibition impedes the amnesic effect of reconsolidation blockers (6).
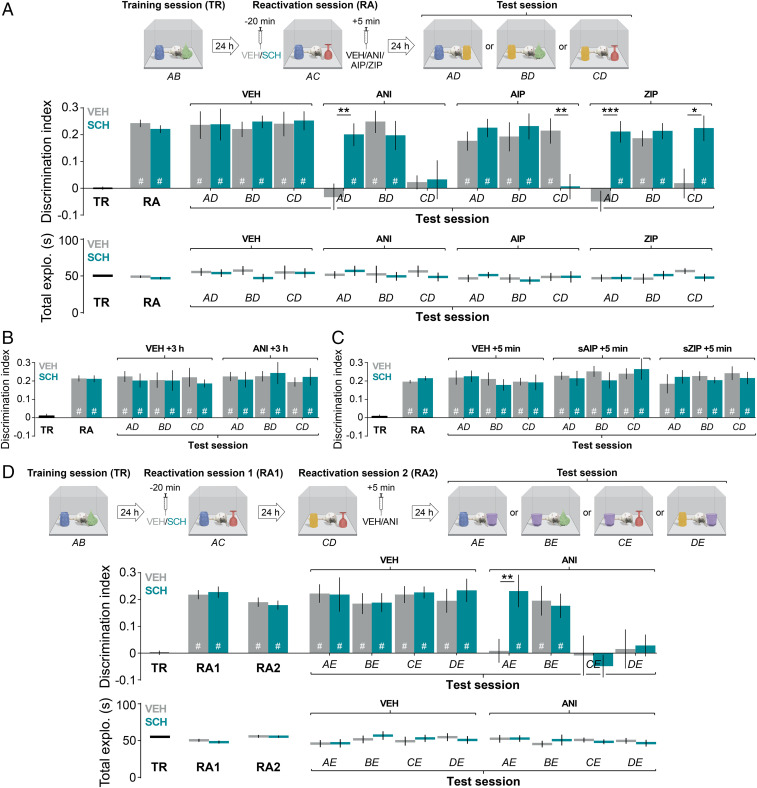
Rats were trained in the novel object-recognition paradigm using objects A and B and 24 h posttraining received intra-CA1 infusions of vehicle (0.9% saline) or the dopamine D1/D5 receptor antagonist SCH23390 (1.5 µg/side). Twenty minutes later, animals were subjected to an ORM reactivation session in the presence of familiar object A and novel object C, and 5 min thereafter, were given vehicle, the protein synthesis inhibitor anisomycin (160 µg/side), the CaMKII inhibitor AIP (5 nmol/side), or the PKMζ inhibitor ZIP (1 nmol/side) in dorsal-CA1. Retention was tested 24 h postreactivation in the presence of novel object D. As seen in Fig. 1A, animals given vehicle after the reactivation session discriminated objects A, B, and C from object D regardless of whether they had received vehicle or SCH23390 before that session, showing that they remembered objects A and B and acquired the memory for object C during the reactivation session, thus confirming that prereactivation inhibition of hippocampal D1/D5 receptors has no effect on ORM retention (6). In contrast, animals that received vehicle before and anisomycin after the reactivation session discriminated object B, but not objects A and C, from object D, whereas animals given prereactivation SCH23390 and postreactivation anisomycin discriminated objects A and B, but not object C, from object D. Three implications can be derived from these results. Firstly, they ratify that recall in the presence of a novel object destabilizes and renders sensitive to protein synthesis inhibition the memory of the familiar object that was present during the reactivation session but not that of the familiar object that was absent in that session, corroborating that these two memories are independent (3). Secondly, they verify that ORM destabilization requires hippocampal dopamine D1/D5 receptor activation (6). Thirdly, they show that D1/D5 receptor inhibition does not protect the memory of the novel object from the amnesia induced by anisomycin, suggesting that this memory can be formed via a protein-synthesis–dependent process that does not require destabilization of the reactivated recognition trace. Animals that received vehicle before the reactivation session and AIP after that session discriminated objects A, B, and C from object D, confirming that hippocampal CaMKII is unnecessary for ORM reconsolidation but essential for its consolidation (8). Animals given SCH23390 before and AIP after the reactivation session also remembered objects A and B, but were unable to discriminate object C from object D, suggesting that the protein-synthesis–dependent process that mediates object C memory formation when object A memory destabilization is blocked requires hippocampal CaMKII activity. Rats that received vehicle before and ZIP after ORM reactivation only remembered object B, whereas those that received prereactivation SCH23390 and postreactivation ZIP also remembered objects A and C, concurring with findings showing that hippocampal PKMζ is involved in ORM reconsolidation but not in ORM consolidation (9), and demonstrating that the protein-synthesis– and CaMKII-dependent process that mediates object C memory formation when object A memory destabilization is inhibited does not require PKMζ. Test session total exploration time did not differ among groups. Neither anisomycin given 3 h after the reactivation session nor scrambled-AIP (5 nmol/side) or scrambled-ZIP (1 nmol/side) given 5 min after that session affected ORM retention regardless of whether animals had received vehicle or SCH23390 before reactivation (Fig. 1 B and C). Together, these results indicate that the memory of an object perceived for the first time during recall of a related representation can be formed through consolidation or reconsolidation mechanisms, depending on the activation state of hippocampal dopamine D1/D5 receptors. If D1/D5 receptor tone is high, the reactivated ORM is destabilized and the new one is formed through PKMζ-dependent reconsolidation. Conversely, if D1/D5 receptor tone is low, the reactivated ORM remains stable and the new one is formed through CaMKII-dependent consolidation.
Fig. 1.
Dopamine D1/D5 receptors control whether ORMs are linked through reconsolidation. (A, Top) Experimental protocol. (Middle) One day after training (TR) with objects A and B, animals received vehicle (VEH) or SCH23390 (SCH) in dorsal-CA1 and 20 min later were subjected to an ORM reactivation session (RA) in the presence of familiar object A and novel object C. Five min later, animals received VEH, anisomycin (ANI), AIP, or ZIP in CA1. Retention was evaluated one day later by reexposing animals to objects A, B, or C alongside novel object D (test session; ANI: AD, t(18) = 3.546, P = 0.0023; AIP: CD, t(18) = 3.123, P = 0.0059; ZIP: AD, t(18) = 4.623, P = 0.0002, CD, t(18) = 2.823, P = 0.0113). (Bottom) Total exploration time at TR, RA, and test. (B) Rats were treated as in A, but received VEH or ANI 3 h post-RA. (C) Rats were treated as in A, but received VEH, scrambled-AIP (sAIP), or scrambled-ZIP (sZIP) 5 min post-RA. (D, Top) Experimental protocol. (Middle) Twenty-four hours post-TR, rats received VEH or SCH in dorsal-CA1 and 20 min later were subjected to a 5-min-long ORM reactivation session (RA1) during which they explored familiar object A and novel object C. One day later, animals were reexposed to object C alongside novel object D for 5 min (RA2) and 5 min later received VEH or ANI in dorsal-CA1. Retention was assessed 24 h later by reexposing animals to objects A, B, C, or D alongside novel object E (test session; ANI: AE, t(17) = 3.010, P = 0.0079). (D, Bottom) Total exploration time at TR, RAs, and test. Data are presented as mean ± SEM; n = 8 to 11 animals/group; #P < 0.05 in one-sample t test; *P < 0.05, **P < 0.01, ***P < 0.001 in unpaired t test.
Next, we analyzed whether consolidation and/or reconsolidation can link new and reactivated ORMs. We reasoned that if they could, then future recall of the new ORM should also reactivate the old one, making it susceptible to amnestic manipulations even if not directly recalled. To test this hypothesis, rats trained in the novel object-recognition task with objects A and B were subjected to an ORM reactivation session in the presence of familiar object A and novel object C 24 h posttraining. Twenty minutes before that, animals received intra-CA1 vehicle, to allow destabilization of the memory of object A and let object C memory formation occur through reconsolidation, or SCH23390, to block object A memory destabilization and enable object C memory consolidation. One day later, animals were subjected to a second reactivation session by reexposing them to object C alongside novel object D to induce hippocampus-dependent reconsolidation and 5 min thereafter were given vehicle or anisomycin in CA1. Retention was tested 24 h later in the presence of novel object E. As shown in Fig. 1D, animals that received vehicle after the second reactivation session discriminated object E from all other objects regardless of whether they had received vehicle or SCH23390 before the first reactivation session. In contrast, animals that received vehicle before the first reactivation session and anisomycin after the second one remembered only object B, indicating that inhibition of hippocampal protein synthesis after the second reactivation session did not hamper merely the memories of objects C and D, which were present in that session, but also the memory of object A, which was absent in that session and, therefore, could not have been directly recalled. SCH23390 administration before the first reactivation session prevented the amnesic effect of anisomycin on object A memory but not on object C and object D memories, demonstrating that reconsolidation, but not consolidation, links new and reactivated ORMs. Total exploration time during the test session did not differ among groups.
In summary, our results show that reconsolidation links new information with preexisting declarative knowledge but, if reactivation-induced destabilization is prevented by blocking hippocampal dopamine D1/D5 receptors, the new information is stored using consolidation mechanisms and no linking occurs. This suggests that dopamine couples novelty detection during recall to declarative memory destabilization and determines whether a new trace will be connected to an active network, ultimately defining its future susceptibility to indirect reactivation. This observation has broad implications because learning seldom occurs in a cognitive vacuum, but new memories are usually acquired concomitantly with the recall of related knowledge. Our data agree with the view that reconsolidation updates memories to preserve their relevance (1) and are in line with the suggestion that this process is influenced by prior learning and requires hippocampal activity to integrate new elements into memory schemata (10–12). Reconsolidation-based psychotherapies aim to mitigate the nondeclarative aspects of traumatic experiences while leaving their declarative framework intact (13). However, in most cases, it is impossible or unethical to induce the recall of the distressing memory that lies at the root of the trauma, which to become susceptible to reconsolidation must be indirectly reactivated utilizing symbolic reminders. Thus, the clinical connotations of our results should not be underestimated, inasmuch as they suggest that using declarative cues during psychotherapy might cause unforeseeable memory changes.
Methods
Before training in the novel object-recognition task, rats were habituated to the empty training arena. Training occurred 24 h posthabituation. Exploration was defined as sniffing/touching objects with the muzzle/forepaws. Extended methods are provided in SI Appendix.
Supplementary Material
Acknowledgments
This work was supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES)/Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Brazil.
Footnotes
The authors declare no competing interest.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2025275118/-/DCSupplemental.
Data Availability
All study data are included in the article and/or supporting information.
References
- 1.Lee J. L. C., Nader K., Schiller D., An update on memory reconsolidation updating. Trends Cogn. Sci. 21, 531–545 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cole E., Simundic A., Mossa F. P., Mumby D. G., Assessing object-recognition memory in rats: Pitfalls of the existent tasks and the advantages of a new test. Learn. Behav. 47, 141–155 (2019). [DOI] [PubMed] [Google Scholar]
- 3.Rossato J. I., et al., On the role of hippocampal protein synthesis in the consolidation and reconsolidation of object recognition memory. Learn. Mem. 14, 36–46 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gonzalez M.C., Rossato J.I., Radiske A., Pádua Reis M., Cammarota M.. Recognition memory reconsolidation requires hippocampal Zif268. Sci. Rep. 12, 16620 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clarke J. R., Cammarota M., Gruart A., Izquierdo I., Delgado-García J. M., Plastic modifications induced by object recognition memory processing. Proc. Natl. Acad. Sci. U.S.A. 107, 2652–2657 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rossato J. I., et al., State-dependent effect of dopamine D1/D5 receptors inactivation on memory destabilization and reconsolidation. Behav. Brain Res. 285, 194–199 (2015). [DOI] [PubMed] [Google Scholar]
- 7.Tronel S., Milekic M. H., Alberini C. M., Linking new information to a reactivated memory requires consolidation and not reconsolidation mechanisms. PLoS Biol. 3, e293 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Furini C. R. G., et al., Molecular mechanisms in hippocampus involved on object recognition memory consolidation and reconsolidation. Neuroscience 435, 112–123 (2020). [DOI] [PubMed] [Google Scholar]
- 9.Rossato J. I., et al., PKMζ inhibition disrupts reconsolidation and erases object recognition memory. J. Neurosci. 39, 1828–1841 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Molitor R. J., Sherrill K. R., Morton N. W., Miller A. A., Preston A. R., Memory reactivation during learning simultaneously promotes dentate gyrus/CA2,3 pattern differentiation and CA1 memory integration. J. Neurosci. 41, 726–738 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Josselyn S. A., Frankland P. W., Memory allocation: Mechanisms and function. Annu. Rev. Neurosci. 41, 389–413 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Preston A. R., Eichenbaum H., Interplay of hippocampus and prefrontal cortex in memory. Curr. Biol. 23, R764–R773 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holmes E. A., Sandberg A., Iyadurai L., Erasing trauma memories. Br. J. Psychiatry 197, 414–415 (2010). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and/or supporting information.