Abstract
There is a need to establish the role of antiandrogens as an alternative or concomitant therapy for hidradenitis suppurativa (HS). Thus, the objectives of this study are (1) to assess the effectiveness of oral contraceptive pills (OCPs) at week 12 in HS women, and (2) to describe the clinical profile of patients receiving oral contraceptive pills (OCPs). A prospective observational study was designed. This study included 100 participants, 50 women with HS who started OCPs for the first time at our HS Clinic and 50 participants without OCP treatment. The main outcome of interest was the percentage of reduction in total abscess and inflammatory nodule (AN) count at week 12. Thirty-three women received combined OCPs and 17 non-combined OCP. HS patients with OCPs treatment were younger (31.7 vs. 40.9 years, p < 0.001), thinner (28.62 vs. 33.35 kg/m2), and have a higher number of areas affected (2.32 vs. 1.38, p = 0.02) than those without OCPs. After 12-weeks of treatment, it was observed that the percentage of AN reduction was higher in HS women receiving OCP than in patients without OCP (53.9% vs. 38.42%, p = 0.049). It was observed that OCP prescription (β = 3.79, p = 0.034) and concomitant therapy (β = 3.91, p = 0.037) were independently associated with a higher % AN when controlling for disease duration, concomitant therapy, and treatment with/without OCP (R2 = 0.67). The factors potentially associated with the percentage AN reduction at week 12 in HS women treated with OCPs were disease duration (β = −1.327, p = 0.052), concomitant therapy (β = 11.04, p = 0.079), and HS worsening with the menstrual cycle (β = 10.55, p = 0.087). In conclusion, OCPs might be effective for improving AN count in women with HS. Women whose HS worsens in relation to the menstrual cycle and have a shorter disease may benefit more from the therapeutic effect of OCPs.
Keywords: contraceptives, hidradenitis suppurativa, premenstrual syndrome
1. Introduction
Hidradenitis suppurativa (HS) is a chronic, recurrent, debilitating inflammatory skin disease of the hair follicle that usually presents after puberty with painful, deep-seated inflamed lesions in the apocrine gland-bearing areas of the body, most commonly the axillae, inguinal, and anogenital regions [1,2]. It has an estimated prevalence rate of around 1% [3], and it disproportionally affects women of childbearing age [4].
The etiopathogeneses of HS seems to be multifactorial but this is not completely understood [5]. HS was originally only regarded as a cutaneous disorder but, today, a growing body of evidence links HS with several dermatological [6] and non-dermatological disorders [7,8,9]. Thus, its inclusion in autoinflammatory systemic diseases should be mandatory for both clinicians and scientists [10,11]. It has been observed that sex hormones may play a role in its pathogenesis [12], as disease severity may vary in intensity according to the menstrual cycle and pregnancy [13]. In fact, HS outbreaks are usually perimenstrual, and they may decrease during pregnancy and menopause [14]. Levels of estradiol and progesterone decrease during the premenstrual period, which may indicate that HS is influenced by fluctuations in the hormones involved in the menstrual cycle [15]. This correlation could be explained because estrogen may inhibit proinflammatory Th1 and Th17 cytokines and thus favor an immunosuppressive environment [13].
Moreover, parallels have been drawn between HS and acne vulgaris [16]. Androgens increase the keratinization of the hair follicle, leading to follicular obstruction, favoring the appearance of outbreaks and exacerbations of HS [17]. Weight gain is also known to contribute to HS pathogenesis [18], and it has been observed that high levels of testosterone and low levels of sex hormone-binding globulin (SHGB) are associated with high BMI [19].
While decreasing levels of progesterone and estrogen seem to coincide with disease flares in premenopausal women, this association is speculative and requires experimental confirmation [12]. To date, recommendations on hormonal therapies are based on limited evidence [20] and there is a need to establish the role of antiandrogens as an alternative or concomitant therapy for HS [21]. Patients reporting HS flares around menses or with features of polycystic ovarian syndrome may be more likely to benefit [22,23]. Therefore, it is important to evaluate whether oral contraceptive pills (OCPs) could improve HS and to assess the kind of women who may benefit from these treatments. Thus, the objectives of this study are (1) to describe the clinical profile of patients receiving oral contraceptive therapy for HS, (2) to assess treatment safety, tolerability and effectiveness at week 12.
2. Materials and Methods
Design.
A prospective observational study was conducted between October 2019 and March 2020 in the Hospital Universitario Virgen de las Nieves, Granada, Spain.
Participants.
This study included all patients diagnosed with HS who started oral contraceptive pills (OCPs) for first time at the HS Clinic of Hospital Universitario Virgen de las Nieves, Granada, Spain, which began its activity in February 2017. The recruitment and the assessment of participants were carried out by the chief dermatologist of the HS Clinic. Subjects were identified by replacing their original subject ID with a new random subject ID.
Inclusion criteria: Women of childbearing age (15–49 years) with menstrual cycles who were prescribed OCPs for the treatment of HS. Concomitant treatment with clindamycin gel 1% twice daily or oral doxycycline 100 mg twice daily depending on the severity of HS was prescribed according to current guidelines [24]. For each participant, a control was chosen. A control was the first HS patient that attend our HS clinic after a case inclusion and that was only receiving clindamycin or doxycycline, without OCPs treatment.
Exclusion criteria: Women with HS who did not sign the written consent form or young women under 18 whose legal representative did not sign the informed consent. Climacteric women. Women who were already taking OCPs before visiting our HS Unit.
Variables of interest.
Main variables of interest:
-
(1)
Type of OCP prescribed. Combined OCP: one that includes a combination of estrogen and progestin. Non-combined OCP: one that only includes progestin. The type of OCP prescribed was selected according to strict clinical criteria, avoiding combined OCP use in patients with thrombotic risk [25,26]. All women in the combined OCP group were receiving ethinyl estradiol 0.02 + drospirenone 3 mg/24 h with 7 days rest per month, and all women in the non-combined OCP only received the progestogen desogestrel 75 mcgr/24 h.
-
(2)Treatment effectiveness at week 12 assessed by:
- The main outcome of interest was the percentage of total abscess and inflammatory nodule (AN) reduction [27].
- The reduction in 55% of International Hidradenitis Suppurativa Severity Score System (IHS4)-55. The IHS4 score was calculated by the number of nodules (multiplied by 1) plus the number of abscesses (multiplied by 2) plus the number of draining tunnels (multiplied by 4). Recently, in the 2021 Europea Hidradenitis Suppurativa Forum the 55% reduction of IHS4 has been proposed as a binary outcome with good correlation with HiSCR, which do not require a minimum baseline AN [28].
- Subjective severity improvement. This was evaluated by a numerical rating system (NRS), for pain, odor, suppuration, itching and general condition. It consists of a line numbered from 0 to 10, where 0 is absence of pain or discomfort, and 10 is the maximum degree of pain or discomfort [29].
Other variables of interest:
Clinical, sociodemographic and biometric variables were recorded by means of clinical interview and physical examination. Sociodemographic characteristics included sex, age, civil status, level of education, family history of HS, body mass index (BMI), smoking habit, alcohol consumption and comorbidities. Clinical features included age at HS onset, disease duration, Hurley stage, number of affected areas, nodules, abscesses and draining tunnels count and concomitant treatments.
Ethics.
All patients agreed with the treatment regimen and signed a written consent form to use their personal data for the present study. This study was approved by the Ethics Committee of the Hospital Universitario Virgen de las Nieves and is in accordance with the Helsinki Declaration.
Statistical analysis.
Descriptive statistics were used to evaluate the characteristics of the sample. The Shapiro-Wilk test was used to check the normality of the variables. Continuous data were expressed as mean ± standard deviation (SD) or as the median (25th–75th percentile). The absolute and relative frequency distributions were estimated for qualitative variables. The student’s t-test or the Wilcoxon test were used to compare nominal and continuous data, and the χ2 test or Fisher’s exact test were applied to nominal data where necessary. Multivariate logistic regression analyses were performed to independently assess the potential effect of OCPs on disease severity. Epidemiological and statistical criteria were used to model variable selection. Significance was set for all tests at two tails, p < 0.05. Statistical analyses were performed using JMP version 14.1.0 (SAS institute, Cary, NC, USA).
3. Results
3.1. Baseline Characteristics
One hundred participants were included in the study: 50 HS women receiving OCPs and 50 without OCPs. Their sociodemographic and clinical features are summarized in Table 1. HS patients with OCPs treatment were younger (31.7 vs. 40.9 years, p < 0.001) thinner (28.62 vs. 33.35 kg/m2) and have a higher number of areas affected (2.32 vs. 1.38, p = 0.02) than those without OCPs.
Table 1.
Baseline features of the sample.
| Patients with OCPs Treatment (n = 50) |
Patients without OCPs Treatment (n = 50) |
p | ||
|---|---|---|---|---|
| Baseline demographic features | ||||
| Age (years) | 31.7 (10.41) | 40.9 (14.06) | p < 0.001 | |
| Family history (yes) | 40% (20) | 34% (17) | 0.679 | |
| Disease duration (years) | 13.04 (8.80) | 12.78 (10.04) | 0.891 | |
| BMI (kg/m2) | 28.62 (6.41) | 33.35 (7.43) | 0.001 | |
| Smoking habit (yes) | 42% (21) | 42% (21) | 1.00 | |
| Baseline clinical features of the sample | ||||
| Hurley stage | I | 42% (21) | 28% (14) | 0.275 |
| II | 46% (23) | 52% (26) | ||
| III | 12% (6) | 20% (10) | ||
| Number of affected areas | 2.32 (1.11) | 1.78 (1.17) | 0.020 | |
| IHS4 at baseline | 6.58 (4.66) | 8.24 (6.81) | 0.158 | |
| AN at baseline | 3.24 (2.33) | 3.5 (3.02) | 0.631 | |
| VAS for pain | 4.62 (3.40) | 4.88 (3.46) | 0.706 | |
| VAS for malodor | 2.86 (3.39) | 3.74 (3.66) | 0.215 | |
| VAS for itching | 4.32 (3.76) | 4.44 (3.15) | 0.863 | |
| VAS for suppuration | 3.42 (3.73) | 4.46 (3.38) | 0.147 | |
| VAS global | 4.92 (2.66) | 5.42 (2.96) | 0.376 | |
| Concomitant therapy | Oral doxycycline | 60% (30) | 64% (32) | 0.680 |
| Clindamycin gel | 40% (20) | 36% (18) | ||
AN, total abscess and inflammatory nodule count; BMI, Body Mass Index; IHS4, Hidradenitis Suppurativa Severity Score System; OCP, oral contraceptive pills; VAS, Visual Analog Scale. Data are expressed as relative (absolute) frequencies and means (standard deviation (SD)).
3.2. Effectiveness and Safety of Oral Contreceptives Pills and Factors Potentially Related to the Reduction in the Number of Abscesses and Nodules at 12 Weeks of Treatment
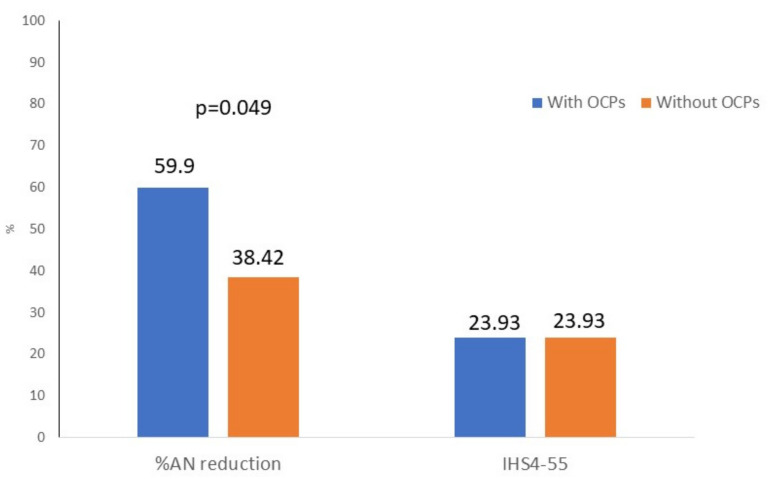
After the follow-up, it was observed that the percentage of AN reduction was higher in HS women receiving OCP than in patients without OCP (53.9% vs. 38.42%, p = 0.049). No differences were observed in IHS4-55, Figure 1. Factors associated with a higher % AN reduction are shown in Table 2.
Figure 1.
Differences in effectiveness evaluated by percentage of AN count reduction and IHS4-55.
Table 2.
Factors associated with % AN reduction at week 12.
| % AN Reduction | p | ||
|---|---|---|---|
| OCP treatment | Yes | 53.90 (5.49) | 0.049 |
| No | 38.42 (5.49) | ||
| Age b | −0.48 (0.30) | 0.111 | |
| Family history a | Yes | 42.79 (6.50) | 0.515 |
| No | 48.14 (4.98) | ||
| Disease duration b | −0.64 (0.41) | 0.129 | |
| BMI b | −0.42 (0.54) | 0.439 | |
| Smoking habit a | Yes | 46.15 (6.11) | 0.999 |
| No | 46.17 (6.11) | ||
| Concomitant therapy a | Oral doxycycline | 51.83 (4.94) | 0.066 |
| Clindamycin gel | 36.91 (6.32) | ||
| Hurley stage a | I | 54.76 (6.34) | 0.2647 |
| II | 40.60 (5.61) | ||
| III | 44.38 (9.82) | ||
| Number of affected areas b | 3.47 (3.39) | 0.310 | |
AN, total abscess and inflammatory nodule count; AN12, AN count reduction at week 12; BMI, Body Mass Index; OCP, oral contraceptive pills. a The Student’s t test for independent samples or the ANOVA test was used to evaluate the association between AN count reduction week 12 (AN12) and categoric variables, data are expressed as a mean (standard deviation). b Simple linear regression was performed to evaluate the association between AN12 and continuous variables, the data are expressed as a beta coefficient (standard deviation).
A multivariate regression model was constructed, adjusted for disease duration, concomitant therapy and treatment with/without OCP. It was observed that OCP prescription (β = 3.79, p = 0.034) and concomitant therapy (β = 3.91, p = 0.037) were independently associated with a higher % AN reduction at week 12 when controlling for the other variables included in the model (R2 = 0.67).
The factors potentially associated with the percentage AN reduction at week 12 in HS women treated with OCPs are reflected in Table 3. A multivariate regression model was constructed, adjusted by disease duration, concomitant therapy, number of affected areas and HS worsening with the menstrual cycle. It was observed that disease duration (β = −1.327, p = 0.052), concomitant therapy (β = 11.04, p = 0.079) and HS worsening with the menstrual cycle (β = 10.55, p = 0.087) were almost independently associated with a higher % AN reduction at week 12 when controlling for the other variables included in the model (R2 = 0.20).
Table 3.
Factors potentially associated with % AN reduction in women treated with OCPs.
| AN12 | p | ||
|---|---|---|---|
| Age b | −0.30 (0.57) | 0.593 | |
| Family history a | Yes | 53.92 (9.25) | 0.998 |
| No | 53.89 (7.55) | ||
| Disease duration b | −1.21 (0.65) | 0.067 | |
| Irregular menses a | Yes | 56.53 (6.76) | 0.445 |
| No | 46.41 (11.40) | ||
| Worsening in relation to the menstrual cycle a | Yes | 69.22 (6.72) | 0.083 |
| No | 47.33 (0.083) | ||
| BMI b | −0.72 (0.92) | 0.433 | |
| Smoking habit a | Yes | 49.76 (8.99) | 0.548 |
| No | 56.90 (7.65) | ||
| OCP type a | Combined OCP | 59.60 (7.06) | 0.173 |
| Non-combined OCP | 42.84 (9.83) | ||
| Concomitant therapy a | Oral doxycycline | 57.78 (7.50) | 0.417 |
| Clindamycin gel | 48.08 (9.18) | ||
| Hurley stage a | I | 59.52 (9.03) | 0.620 |
| II | 51.96 (8.63) | ||
| III | 41.67 (16.89) | ||
| Number of affected areas b | −5.46 (5.24) | 0.303 | |
AN, total abscess and inflammatory nodule count; AN12, AN count reduction at week 12; BMI, Body Mass Index; OCP, oral contraceptive pills. a The Student’s t test for independent samples or the ANOVA test was used to evaluate the association between AN count reduction week 12 (AN12) and categoric variables, data are expressed as a mean (standard deviation). b Simple linear regression was performed to evaluate the association between AN12 and continuous variables, the data are expressed as a beta coefficient (standard deviation).
3.3. Type of Oral Contraceptive Pills
Thirty-three women received combined OCPs and 17 non-combined OCP. Demographic and clinical features depending on OCP type are described in Table 4. Women prescribed non-combined OCPs were older than those prescribed combined OCPs (42.58 vs. 26.09, <0.001) and had a longer disease duration (19.09 vs. 9.87 years, p = 0.001). Women taking non-combined OCPs were also more frequently active smokers (76.5% vs. 24.26%, p < 0.001). Moreover, women taking non-combined OCPs were in Hurley stage III and had severe disease assessed by IHS4 and global VAS.
Table 4.
Baseline features of the sample depending on the type of oral contraceptive pills prescribed.
| Combined OCP (n = 33) | Non-Combined OCP (n = 17) | |||
|---|---|---|---|---|
| Baseline demographic features | ||||
| Age (years) | 26.09 (7.26) | 42.59 (5.95) | <0.001 * | |
| Educational level | Mandatory incomplete | 12.1% (4/33) | 17.6% (3/17) | 0.755 |
| Mandatory | 57.6% (19/33) | 47.1% (8/17) | ||
| Superior | 30.3% (10.33) | 35.3% (6/17) | ||
| Family history (yes) | 36.4% (12/33) | 47.1% (8/17) | 0.465 | |
| Disease duration (years) | 9.48 (5.23) | 19.94 (10.27) | 0.001 * | |
| Irregular menses (yes) | 27.3% (9/33) | 23.5% (4/17) | 0.775 | |
| Worsening in relation to the menstrual cycle (yes) | 27.3% (9/33) | 29.4% (5/17) | 0.873 | |
| BMI (kg/cm2) | 27.95 (5.79) | 29.92 (7.50) | 0.308 | |
| Smoking habit (yes) | 24.26% (8/33) | 76.5% (13/17) | <0.001 * | |
| Baseline clinical features of the sample | ||||
| Hurley stage | I | 57.6% (19/33) | 11.8% (2/17) | 0.001 * |
| II | 39.4% (13/33) | 58.8% (10/17) | ||
| III | 3% (1/33) | 29.4% (5/17) | ||
| Number of affected areas | 2.18 (1.13) | 2.59 (1.06) | 0.323 | |
| IHS4 at baseline | 5.33 (4.34) | 9.00 (4.42) | 0.007 * | |
| AN at baseline | 3.06 (2.90) | 2.71 (1.40) | 0.56 | |
| Number of inflammatory nodules | 2.00 (2.19) | 1.12 (1.22) | 0.074 | |
| Number of abscesses | 1.06 (1.39) | 1.59 (1.23) | 0.193 | |
| Number of draining tunnels | 0.30 (0.59) | 1.18 (1.13) | 0.007 * | |
| VAS for pain | 3.48 (3.12) | 6.82 (2.83) | 0.001 * | |
| VAS for malodor | 4.76 (3.63) | 1.88 (2.83) | 0.003 * | |
| VAS for itching | 3.39 (3.57) | 6.12 (3.53) | 0.014 * | |
| VAS for suppuration | 5.71 (3.70) | 2.24 (3.19) | 0.001 * | |
| VAS global | 4.12 (2.63) | 6.47 (1.97) | 0.001 * | |
| Concomitant therapy | Oral doxycycline | 57.6% (19/33) | 64.7% (11/17) | 0.626 |
| Clindamycin gel | 42.4% (14/33) | 35.3% (6/17) | ||
AN, total abscess and inflammatory nodule count; BMI, Body Mass Index; IHS4, Hidradenitis Suppurativa Severity Score System; OCP, oral contraceptive pills; VAS, Visual Analog Scale. Data are expressed as relative (absolute) frequencies and means (standard deviation (SD). The Student’s t test for independent samples was used to compare continuous variables and the chi-square test or Fisher’s exact test, as appropriate, were applied to compare categoric data. Two-tailed * p < 0.05 was considered statistically significant in all tests.
A multivariate logistic regression model was constructed, adjusted for age, Hurley stage, smoking habit and HS worsening with the menstrual cycle to assess independent factors associated with the type of OCP prescribed. It was observed that for every year that the patient’s age increases, the probability of being prescribed a non-combined OCP instead of a combined OCP was 1.37 times higher. Moreover, for every Hurley stage increase, the probability of being prescribed a non-combined OCP instead of a combined OCP was 11.73 times higher, Table 5.
Table 5.
Multivariate analysis of the factors related to OCPs type prescribed.
| Variable | aOR | p |
|---|---|---|
| Age (years) | 1.37 | 0.007 * |
| Hurley stage | 11.73 | 0.040 * |
| Smoking habit | 3.41 | 0.328 |
| Worsening in relation to the menstrual cycle | 0.63 | 0.743 |
| R2 Cox y Snell = 0.59 | ||
A logistic regression model was constructed to determine the variables influencing of the type of OCP prescribed (dependent variable) adjusted for age (continuous), Hurley stage (I, II, III), smoking habit (no, yes) and HS worsening in relation to the menstrual cycle (no, yes). Adjusted odds ratios (aOR) are presented. Two-tailed * p < 0.05 was considered statistically significant in all tests.
4. Discussion
To the best of our knowledge, this study describes for the first time a higher AN reduction in HS women treated with OCPs and describe the clinical profile of these patients. We found two patient profiles: those receiving combined OCPs and those receiving progestin only. We observed an improvement in inflammatory activity after using OCPs and identified clinical factors potentially associated with a greater treatment effect.
The profile of HS patients being treated with OCPs is that of a young overweight woman, with a family history of HS and mild-moderate disease. The mean age of the population studied is between 20 and 30 years, similar to other HS populations [30], as OCPs should be avoided in older or post-menopausal women [31]. Smoking habit, a risk factor for developing HS, but also a relative contraindication for OCP use, was relatively frequent in our population (44%), in agreement with previous reports [32]. Nevertheless, the thrombotic risk is determined by estrogen, so older women who smoke were mainly prescribed non-combined OCPs. Regarding educational level, the rate of highly educated people in our study was low. This may be related to the disease chronicity in which relapses are frequent, which may have a great impact on the patient’s quality of life and influence their social, occupational, and psychological lives [33]. Over half of our population was overweight. Obesity is also a risk factor for developing HS [34] because adipocytes stimulate the overproduction of proinflammatory cytokines and HS relapses increase because of the mechanical irritation, occlusion and maceration. Two-fifths of our population had a family history of HS. Reports have previously shown a probable genetic component in HS, as having a family history of the disease doubles the risk of suffering from HS [33]. Moreover, we found that more than 90% of the patients receiving OCPs were in stage I and II of the Hurley classification. This is explained because only concomitant treatment with topical clindamycin or oral doxycycline was allowed, therapies used in mild-moderate disease [20].
Our study shows a decrease in AN count in patients undergoing treatment with OCPs and oral antibiotics for 12 weeks without serious adverse effects compared to those women not receiving OCPs. We only observed a reduction in AN but not in IHS4-55. IHS4-55 is a scoring tool that considers both AN and draining tunnel count [28]. The effect of OCPs might be only on inflammatory lesions (nodules and abscesses) and do not work on draining tunnels, explaining the differences in AN but not in IHS4-55. Drospirenone has anti-mineralocorticoid and anti-androgenic power that may reduce the symptoms of HS [21]. Although some studies show that OCPs in combination with drospirenone is more effective than when prescribed alone [35], similar reduction in AN count between the two OCPs were found in our study. This may be because contraceptives combined with drospirenone show efficacy over a longer time period, from 6 months to 1 year [35]. Moreover, one of the benefits of OCPs, whether anti or proandrogenic, is to avoid hormonal imbalances and the worsening associated with menstruation, which may explain similar results using both OCPs.
Factors independently associated with an increase in OCP effectiveness were a shorter HS duration, worsening in relation to the menstrual cycle and being receiving a concomitant therapy. A long disease duration is related to the presence of scars and draining tunnels [36], when OCPs are less effective. It was shown that the worsening of HS with the menstrual cycle was almost independently related to the percentage of AN reduction in patients with OCP treatment. OCPs inhibit the hypothalamic-pituitary axis, repressing basal plasma levels of follicle-stimulating hormone (FSH) and luteinizing hormone (LH) and eliminating oscillation cyclical surges in FSH, LH and estrogen levels [37]. Intraindividual variations in the menstrual cycle [38] may explain why some women with HS get worse in relation to their menstrual cycle and improve more with OCP use, as those women that worsen in relation to the menstrual cycle could have higher level levels of progesterone and estrogen before menstruation [12]. It is possible that the assessment of a hormonal profile could help to select the women who would best respond to OCPs. Moreover, the genetic heterogeneity in HS [39] and the differential response to therapies influenced by patients’ comorbidities [40] may justify variations in womens’ OCP response.
Clinicians should also consider that women with painful menstrual periods and menstrual migraines may take non-steroidal anti-inflammatory drugs (NSAIDs), a possible trigger factor in HS. So, treating this syndrome with OCPs may remove these symptoms and decrease the menstrual use of NSAIDs and HS exacerbation [41,42].
Regarding OCP type, combined OCPs were more frequently prescribed than progesterone-only. We found that the use of non-combined OCPs was independently associated with higher age and more severe disease. Combined OCPs should be avoided in women with risk factors of thrombosis: >35 years or smokers [31]. So, our data may be explained because older people [43] and higher Hurley stages [44] are associated with greater cardiovascular risk.
The results of this study should be considered in light of some limitations: (1) the small sample size, (2) the short follow-up, (3) concomitant treatment allowed (both for HS as clindamycin and doxycycline and NSAIDs), (4) a higher percentage of Hurley III patients in the control group, although no statistically significant differences were found between groups and the AN count at baseline was similar between patients with OCPS and without OCPs.
5. Conclusions
In conclusion, to the best of our knowledge, this is the first report to describe the profile of patients receiving OCPs in a real-life setting and finds that women whose HS worsens in relation to the menstrual cycle and have a shorter HS duration may benefit more from the therapeutic effect of OCPs. The increase in the number of effective therapies and the needs of HS patients makes necessary a multidisciplinary approach, including dermatologist, gynecologists, endocrinologists, and surgeons, to improve the management of HS patients.
Acknowledgments
We would like to thank all the individuals who generously shared their time to participate in this research.
Author Contributions
Conceptualization, A.M.-L. and A.L.-G.; methodology, A.M.-L., T.M.-V. and A.V.-A.; software, C.C.-B.; validation, A.M.-L., A.L.-G., S.A.-S. and T.M.-V.; formal analysis, A.M.-L. and A.L.-G.; investigation, A.M.-L., A.V.-A. and A.L.-G.; resources, A.V.-A. and C.C.-B.; data curation, A.V.-A.; writing—original draft preparation, A.V.-A. and T.M.-V.; writing—review and editing, A.M.-L.; visualization, A.M.-L. and S.A.-S.; S.A.-S.; project administration, A.M.-L.; funding acquisition, A.M.-L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of Hospital Universitario Virgen de las Nieves, Granada, Spain (protocol code V01 and date of approval 19 May 2019).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Nguyen T.V., Damiani G., Orenstein L.A., Hamzavi I., Jemec G. Hidradenitis suppurativa: An update on epidemiology, phenotypes, diagnosis, pathogenesis, comorbidities and quality of life. J. Eur. Acad. Dermatol. Venereol. 2021;35:50–61. doi: 10.1111/jdv.16677. [DOI] [PubMed] [Google Scholar]
- 2.Orenstein L.A.V., Nguyen T.V., Damiani G., Sayed C., Jemec G.B.E., Hamzavi I. Medical and Surgical Management of Hidradeni-tis Suppurativa: A Review of International Treatment Guidelines and Implementation in General Dermatology Practice. Dermatology. 2020;236:393–412. doi: 10.1159/000507323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alikhan A., Lynch P.J., Eisen D. Hidradenitis suppurativa: A comprehensive review. J. Am. Acad. Dermatol. 2009;60:539–561. doi: 10.1016/j.jaad.2008.11.911. [DOI] [PubMed] [Google Scholar]
- 4.Revuz J.E., Canoui-Poitrine F., Wolkenstein P., Viallette C., Gabison G., Pouget F., Poli F., Faye O., Roujeau J.C., Bonnelye G., et al. Prevalence and factors associated with hidradenitis suppurativa: Results from two case-control studies. J. Am. Acad. Dermatol. 2008;59:596–601. doi: 10.1016/j.jaad.2008.06.020. [DOI] [PubMed] [Google Scholar]
- 5.Karagiannidis I., Nikolakis G., Sabat R., Zouboulis C.C. Hidradenitis suppurativa/Acne inversa: An endocrine skin disorder? Rev. Endocr. Metab. Disord. 2016;17:335–341. doi: 10.1007/s11154-016-9366-z. [DOI] [PubMed] [Google Scholar]
- 6.Kridin K., Patel P.M., Jones V.A., Damiani G., Amber K.T., Cohen A.D. Hidradenitis suppurativa is associated with acne keloi-dalis nuchae: A population-based study. Arch. Dermatol. Res. 2021;313:333–337. doi: 10.1007/s00403-020-02105-x. [DOI] [PubMed] [Google Scholar]
- 7.Conic R.R.Z., Fabbrocini G., Marasca C., Bragazzi N.L., Watad A., Adawi M., Damiani G. Burden of Ocular Comorbidities in Patients With Hidradenitis Suppurativa. JAMA Dermatol. 2021;157:226. doi: 10.1001/jamadermatol.2020.5087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Damiani G., Di Meo N., Marzano A.V. A unique pneumopathy in a patient with skin nodules and abscesses. Intern. Emerg. Med. 2017;12:637–640. doi: 10.1007/s11739-017-1658-0. [DOI] [PubMed] [Google Scholar]
- 9.Damiani G., Leone S., Fajgenbaum K., Bragazzi N., Pacifico A., Conic R.R., Pigatto P.D., Maiorana C., Poli P., Berti E., et al. Nonalcoholic fatty liver disease prevalence in an Italian cohort of patients with hidradenitis suppurativa: A multi-center retrospective analysis. World J. Hepatol. 2019;11:391–401. doi: 10.4254/wjh.v11.i4.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Damiani G., Della Valle V., Iannone M., Dini V., Marzano A.V. Autoinflammatory Disease Damage Index (ADDI): A possible newborn also in hidradenitis suppurativa daily practice. Ann. Rheum. Dis. 2016;76:e25. doi: 10.1136/annrheumdis-2016-210901. [DOI] [PubMed] [Google Scholar]
- 11.Marzano A.V., Damiani G., Genovese G., Gattorno M. A dermatologic perspective on autoinflammatory diseases. Clin. Exp. Rheumatol. 2018;36(Suppl. 110):32–38. [PubMed] [Google Scholar]
- 12.Riis P.T., Ring H.C., Themstrup L., Jemec G.B. The Role of Androgens and Estrogens in Hidradenitis Suppurativa-A Systemat-ic Review. Acta Dermatovenerol. Croat. 2016;24:239–249. [PubMed] [Google Scholar]
- 13.Vossen A.R., van Straalen K.R., Prens E.P., van der Zee H.H. Menses and pregnancy affect symptoms in hidradenitis suppurativa: A cross-sectional study. J. Am. Acad. Dermatol. 2017;76:155–156. doi: 10.1016/j.jaad.2016.07.024. [DOI] [PubMed] [Google Scholar]
- 14.Von der Werth J.M., Williams H.C. The natural history of hidradenitis suppurativa. J. Eur. Acad. Dermatol. Venereol. 2000;14:389–392. doi: 10.1046/j.1468-3083.2000.00087.x. [DOI] [PubMed] [Google Scholar]
- 15.Clark A.K., Quinonez R.L., Saric S., Sivamani R.K. Hormonal therapies for hidradenitis suppurativa: Review. Dermatol. Online J. 2017;23:23. doi: 10.5070/D32310036990. [DOI] [PubMed] [Google Scholar]
- 16.Pink A., Anzengruber F., Navarini A. Acne and hidradenitis suppurativa. Br. J. Dermatol. 2018;178:619–631. doi: 10.1111/bjd.16231. [DOI] [PubMed] [Google Scholar]
- 17.Khandalavala B.N., Do M.V. Finasteride in Hidradenitis Suppurativa: A “Male” Therapy for a Predominantly “Female” Disease. J. Clin. Aesthet. Dermatol. 2016;9:44–50. [PMC free article] [PubMed] [Google Scholar]
- 18.Collier E., Shi V.Y., Parvataneni R.K., Lowes M.A., Hsiao J.L. Special considerations for women with hidradenitis suppurativa. Int. J. Women’s Dermatol. 2020;6:85–88. doi: 10.1016/j.ijwd.2020.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tin T.S., Reeves G.K., Key T.J. Body size and composition, physical activity and sedentary time in relation to endogenous hormones in premenopausal and postmenopausal women: Findings from the UK Biobank. Int. J. Cancer. 2020;147:2101–2115. doi: 10.1002/ijc.33010. [DOI] [PubMed] [Google Scholar]
- 20.Alikhan A., Sayed C., Alavi A., Alhusayen R., Brassard A., Burkhart C., Crowell K., Eisen D.B., Gottlieb A.B., Hamzavi I., et al. North American clinical management guidelines for hidradenitis suppurativa: A publication from the United States and Canadian Hidradenitis Suppurativa Foundations: Part I: Diagnosis, evaluation, and the use of complementary and procedural management. J. Am. Acad. Dermatol. 2019;81:76–90. doi: 10.1016/j.jaad.2019.02.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nikolakis G., Kyrgidis A., Zouboulis C.C. Is There a Role for Antiandrogen Therapy for Hidradenitis Suppurativa? A System-atic Review of Published Data. Am. J. Clin. Dermatol. 2019;20:503–513. doi: 10.1007/s40257-019-00442-w. [DOI] [PubMed] [Google Scholar]
- 22.Mortimer P., Dawber R., Gales M.A., Moore R. A double-blind controlled cross-over trial of cyproterone acetate in females with hidradenitis suppurativa. Br. J. Dermatol. 1986;115:263–268. doi: 10.1111/j.1365-2133.1986.tb05740.x. [DOI] [PubMed] [Google Scholar]
- 23.Kraft J.N., Searles G.E. Hidradenitis Suppurativa in 64 Female Patients: Retrospective Study Comparing Oral Antibiotics and Antiandrogen Therapy. J. Cutan. Med. Surg. 2007;11:125–131. doi: 10.2310/7750.2007.00019. [DOI] [PubMed] [Google Scholar]
- 24.Gulliver W., Zouboulis C.C., Prens E., Jemec G.B., Tzellos T. Evidence-based approach to the treatment of hidradenitis suppura-tiva/acne inversa, based on the European guidelines for hidradenitis suppurativa. Rev. Endocr. Metab. Disord. 2016;17:343–351. doi: 10.1007/s11154-016-9328-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Serfaty D. Update on the contraceptive contraindications. J. Gynecol. Obstet. Hum. Reprod. 2019;48:297–307. doi: 10.1016/j.jogoh.2019.02.006. [DOI] [PubMed] [Google Scholar]
- 26.Rivlin K., Isley M.M. Patient-centered Contraceptive Counseling and Prescribing. Clin. Obstet. Gynecol. 2018;61:27–39. doi: 10.1097/GRF.0000000000000337. [DOI] [PubMed] [Google Scholar]
- 27.Kimball A., Sobell J., Zouboulis C.P.D., Gu Y., A Williams D., Sundaram M., Teixeira H., Jemec G. HiSCR (Hidradenitis Suppurativa Clinical Response): A novel clinical endpoint to evaluate therapeutic outcomes in patients with hidradenitis suppurativa from the placebo-controlled portion of a phase 2 adalimumab study. J. Eur. Acad. Dermatol. Venereol. 2015;30:989–994. doi: 10.1111/jdv.13216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zouboulis C.C., Tzellos T., Kyrgidis A., Jemec G.B.E., Bechara F.G., Giamarellos-Bourboulis E.J., Ingram J.R., Kanni T., Karagiannidis I., Martorell A., et al. Development and validation of the International Hidradenitis Suppurativa Severity Score System (IHS 4), a novel dynamic scoring system to assess HS severity. Br. J. Dermatol. 2017;177:1401–1409. doi: 10.1111/bjd.15748. [DOI] [PubMed] [Google Scholar]
- 29.Szczęch J., Kaaz K., Lelonek E., Szepietowski J. Clinical Characteristics of Pruritus and Pain in Patients with Hidradenitis Suppurativa. Acta Derm. Venereol. 2018;98:191–194. doi: 10.2340/00015555-2815. [DOI] [PubMed] [Google Scholar]
- 30.Tavora I.G., Bissoli G.C., Miot H.A., Schmitt J.V. Clinical manifestations and quality of life in hidradenitis suppurativa patients: Survey of participants from an internet support group. An. Bras. Dermatol. 2019;94:298–303. doi: 10.1590/abd1806-4841.20197687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.US Food and Drug Administration.ALESSE® 28 Tablets (Levonorgestrel and Ethinyl Estradiol Tablets) [(accessed on 28 August 2020)]; Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/020683s012lbl.pdf.
- 32.Prens E.P., Lugo-Somolinos A.M., Paller A.S., Kerdel F., Duan Y., Teixeira H.D., Longcore M., Kimball A.B. Baseline Characteristics from UNITE: An Observational, International, Multicentre Registry to Evaluate Hidradenitis Suppurativa (Acne Inversa) in Clinical Practice. Am. J. Clin. Dermatol. 2020;21:579–590. doi: 10.1007/s40257-020-00504-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Napolitano M., Megna M., Timoshchuk E.A., Patruno C., Balato N., Fabbrocini G., Monfrecola G. Hidradenitis suppurativa: From patho-genesis to diagnosis and treatment. Clin. Cosmet. Investig. Dermatol. 2017;10:105–115. doi: 10.2147/CCID.S111019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Garcia-Martinez F.J., Pascual J.C., Lopez-Martin I., Pereyra-Rodriguez J.J. Martorell Calatayud, A.; Salgado-Boquete; Labandeira-García, J. [Update of hidradenitis suppurativa in Primary Care] Semergen. 2017;43:34–42. doi: 10.1016/j.semerg.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 35.Regidor P.A., Schindler A.E. Antiandrogenic and antimineralocorticoid health benefits of COC containing newer progestogens: Dienogest and drospirenone. Oncotarget. 2017;8:83334–83342. doi: 10.18632/oncotarget.19833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Montero-Vilchez T., Salvador-Rodriguez L., Sanchez-Diaz M., Cuenca-Barrales C., Martinez-Lopez A., Arias-Santiago S., Molina-Leyva A. Clinical selection criteria in new clinical trials of hidradenitis suppurativa: External validity and implications on the daily clinical practice. Dermatol. Ther. 2020;33:e14254. doi: 10.1111/dth.14254. [DOI] [PubMed] [Google Scholar]
- 37.Fraser I.S., Jansen R.P.S. Why do inadvertent pregnancies occur in oral contraceptive users?--Effectiveness of oral contraceptive regimens and interfering factors. Stud. Fam. Plan. 1984;15:99. doi: 10.2307/1966055. [DOI] [PubMed] [Google Scholar]
- 38.Nishihama Y., Yoshinaga J., Iida A., Konishi S., Imai H. Menstrual Cycle Length and Source of Its Variation in Female University Students Majoring in Nursing Sciences. Nippon. Eiseigaku Zasshi Jpn. J. Hyg. 2015;70:139–148. doi: 10.1265/jjh.70.139. [DOI] [PubMed] [Google Scholar]
- 39.Duchatelet S., Miskinyte S., Delage M., Ungeheuer M.-N., Lam T., Benhadou F., Del Marmol V., Vossen A.R.V., Prens E.P., Cogrel O., et al. Low Prevalence of GSC Gene Mutations in a Large Cohort of Predominantly Caucasian Patients with Hidradenitis Suppurativa. J. Investig. Dermatol. 2020;140:2085–2088.e14. doi: 10.1016/j.jid.2019.10.025. [DOI] [PubMed] [Google Scholar]
- 40.Zouboulis C.C., Hansen H., Caposiena C.R.D., Damiani G., Delorme I., Pascual J.C., Reguiai Z., Trigoni A., Vilarrasa E., Alfageme Roldán F. Adalimumab Dose Intensification in Recalcitrant Hidradenitis Suppurativa/Acne Inversa. Dermatology. 2020;236:25–30. doi: 10.1159/000503606. [DOI] [PubMed] [Google Scholar]
- 41.Collier E.K., Price K.N., Grogan T.R., Naik H.B., Shi V.Y., Hsiao J.L. Characterizing perimenstrual flares of hidradenitis suppurativa. Int. J. Women’s Dermatol. 2020;6:372–376. doi: 10.1016/j.ijwd.2020.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fernandez J.M., Hendricks A.J., Thompson A.M., Mata E.M., Collier E.K., Grogan T.R., Shi V.Y., Hsiao J.L. Menses, pregnancy, delivery, and menopause in hidradenitis suppurativa: A patient survey. Int. J. Women’s Dermatol. 2020;6:368–371. doi: 10.1016/j.ijwd.2020.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boreskie K.F., Rose A.V., Hay J.L., Kehler D.S., Costa E.C., Moffatt T.L., Arora R.C., Duhamel T.A. Frailty status and cardiovascular disease risk profile in middle-aged and older females. Exp. Gerontol. 2020;140:111061. doi: 10.1016/j.exger.2020.111061. [DOI] [PubMed] [Google Scholar]
- 44.Skroza N., Mambrin A., Proietti I., Balduzzi V., Bernardini N., Marchesiello A., Arora R.C., Duhamel T.A. Evaluation of Cardiovascular Risk in Hid-radenitis Suppurativa Patients Using Heart Rate Variability (HRV) Analysis. Cardiovasc. Ther. 2020;2020:1321782. doi: 10.1155/2020/1321782. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.