Abstract
Wine can be defined as a complex microbial ecosystem, where different microorganisms interact in the function of different biotic and abiotic factors. During natural fermentation, the effect of unpredictable interactions between microorganisms and environmental factors leads to the establishment of a complex and stable microbiota that will define the kinetics of the process and the final product. Controlled multistarter fermentation represents a microbial approach to achieve the dual purpose of having a less risky process and a distinctive final product. Indeed, the interactions evolved between microbial consortium members strongly modulate the final sensorial properties of the wine. Therefore, in well-managed mixed fermentations, the knowledge of molecular mechanisms on the basis of yeast interactions, in a well-defined ecological niche, becomes fundamental to control the winemaking process, representing a tool to achieve such objectives. In the present work, the recent development on the molecular and metabolic interactions between non-Saccharomyces and Saccharomyces yeasts in wine fermentation was reviewed. A particular focus will be reserved on molecular studies regarding the role of nutrients, the production of the main byproducts and volatile compounds, ethanol reduction, and antagonistic actions for biological control in mixed fermentations.
Keywords: multistarter fermentation, yeast interactions, molecular mechanisms, non-Saccharomyces yeasts
1. Introduction
Nowadays, the use of commercial Saccharomyces cerevisiae strains in wine production is a common practice. In the past, wine was produced through spontaneous fermentation conducted by microbiota naturally colonizing grapes and winery. In this way, many yeast strains and species contributed to wine fermentation determining unpredictable interactions, sometimes resulting in failure. For these reasons, the practice of controlled fermentation widespread since the 1970s, through the inoculum of pure S. cerevisiae starter cultures, quickly established a predictable process with a dominant yeast population carrying out well-managed, certain, and reliable results [1]. If the advantages correlated to pure wine fermentation such as the process management and quality of the final wine, the massive and widespread use of commercial S. cerevisiae strains reduce the microbial biodiversity of the process with a consequent reduction in wine complexity [2]. Indeed, the sensory profile of wines produced by monoculture-inoculated fermentations differs substantially from wines spontaneously fermented. This is demonstrated by the comparative study of the biochemical characteristics of wines inoculated with those from spontaneous fermentations, which are distinctly different [3]. Certainly, spontaneous fermentations imply a higher risk of sluggish and/or stuck fermentation, spoilage contamination, and a relatively undesired final aroma when compared to pure processes characterized by many defined and desirable characteristics but less complex flavor profiles [4].
For this reason, in the last few years, wine researchers have explored the controlled use of non-Saccharomyces starter cultures in addition to commercial S. cerevisiae strains [5,6]. It is certainly known that non-Saccharomyces yeasts are generally unable to complete alcoholic fermentation. For this, they are used in pairs with conventional starters. The goal to take advantage of multistarter fermentation, avoiding the risks of stuck fermentations, is reached by setting the conditions for which all participating yeasts cohabit in a stable way. This can be achieved by inoculating the two starter strains in coculture (non-Saccharomyces and S. cerevisiae strains) or first the non-Saccharomyces yeast followed by S. cerevisiae to finish the fermentation. This last modality is known as sequential inoculation [7,8]. During recent years, the controlled use of non-Saccharomyces yeasts in winemaking has grown enormously, quickly becoming a biotechnological tool to better understand the impact of the multistarter process on the chemical and sensorial properties of wine [9,10]. In this regard, analyzing comprehensive studies on controlled mixed fermentations in wine, two aspects emerged: (i) the wide intraspecific variability of non-Saccharomyces yeasts for the oenological characters; (ii) their different and specific behavior in coculture due to molecular interactions with S. cerevisiae. Experimental research based on these aspects strongly highlighted a significant role in the focus and application of non-Saccharomyces yeasts, determining the effect on the analytical and sensory profile and the aromatic complexity of wine. Indeed, it is well established that there are several specific purposes for the use of mixed cultures in fermented beverage production such as the improvement of specific wine traits, the enhancement of the complexity or peculiar structural/aromatic features of the final product, ethanol reduction, or the control of spontaneous or undesired microorganisms. In this context, the planned choice of the strain and the study of yeast interactions play an important role in the achievement of the desired features influencing the growth and/or metabolic pathway of mixed cultures. Moreover, a driving force of the market supported by a great demand of consumers is continuously increasing the interest in yeast–yeast interaction in mixed fermentation to provide biotechnological solutions to improve sensory characteristics [7].
Further efforts are needed to understand the modalities of such interactions and how each species contributes to fermentation. However, the available knowledge already defines the potentiality of well-managed mixed fermentations for the control of undesirable or spoilage microorganisms, in view of a sustainable perspective of organic wine production. Indeed, with countless possible yeast combinations, the potential of mixed fermentation in natural product discovery seems quite promising.
Here we reviewed the recent development in the studies on the molecular and metabolic interactions between non-Saccharomyces and Saccharomyces yeasts in wine fermentation. After a brief overview of the methods used for the molecular study, we examined the recent developments in the metabolic regulation in wine yeast interactions focusing on nutrients uptake, byproducts, and volatile compounds production, as well as ethanol reduction and antagonistic actions for biological control.
2. Methodological Approaches for the Study of Metabolic Interactions
Metabolomics is a branch of biochemistry involving comprehensive study on metabolites, bioactive compounds, or products of microbial metabolism [11]. In wine microbiology, there are few metabolomic studies, mainly focused on the evaluation of primary and secondary metabolites, their concentrations, and their relationships on the quality of wine. The most recent analytical techniques developed for metabolomics studies on wine allow the screening of hundreds of yeast metabolites using high-throughput methods [12].
The possibility to use a metabolomics approach in the study of the metabolic interactions between yeasts during mixed fermentations greatly increases the probability of understanding the molecular mechanisms underlying the interactions. For example, data obtained from Fourier transform ion cyclotron resonance mass spectrometry (FT-ICR-MS) and/or liquid chromatography coupled with tandem mass spectrometry (LC-MS/MS) analyses represent a useful metabolic footprint that discriminate wines based on the inoculated yeasts (in single or mixed culture) but also reveals differences related to the final aroma, providing a metabolomic picture of the wine [13].
Moreover, ultra-high-resolution mass spectrometry (uHRMS) is another tool to analyze the yeast metabolome after alcoholic fermentation. Roullier-Gall et al. [13] reported the change in wine chemical composition from pure and mixed-culture fermentation with Lachancea thermotolerans, Starmerella bacillaris, Metschnikowia pulcherrima, and S. cerevisiae. The authors, using this methodology, clearly differentiated wines according to pure or mixed fermentation. Again, uHRMS confirmed that cell–cell contact influences the metabolism of L. thermotolerans and S. cerevisiae [14]. For these reasons, metabolomics seems to be a suitable tool to better understand the microbial interaction during multistarter fermentation.
Interactions between different mixed cultures of yeasts studied by gas-chromatograph analysis demonstrated that wines produced from pure cultures had a different composition of volatile compounds compared to wines produced by cocultivation [15]. Also, NMR-based metabolomics was recently used to identify metabolites that discriminate between single and mixed cultures of two yeast during fermentation [16].
Other “omics” technologies were used to discover new relevant aspects of microbial interactions [13]. Transcriptomic analysis combined with physiological data can provide an integrated view into the response of a yeast to the environment during mixed-culture fermentation [17]. For instance, the available nutrients such as nitrogen and vitamins represent a factor that may determine population dynamics, fermentative activity, and byproducts formation.
3. Nutrient Uptake and Metabolic Response in Yeast-Yeast Interactions
Several biotic and abiotic mechanisms influence the yeast-yeast interactions during wine fermentation, determining, in a natural process, the microbial dynamics and the final dominance of one or few yeast strains. Although a wide variability, both in terms of quality and quantity, of non-Saccharomyces yeasts derived from grapes is present at the beginning of the process, S. cerevisiae is always the dominant species of fermentation. It has been well established that the capacity of S. cerevisiae strains to quickly consume the nitrogen and carbon sources available in grape juice, together with the high ethanol tolerance, gives them a key adaptive advantage over other yeasts [18]. Moreover, the abiotic stressful conditions during alcoholic wine fermentation are also important factors that positively impact S. cerevisiae dominance at the expense of non-Saccharomyces species. The availability and the nature of yeast assimilable nitrogen (YAN) compounds is a key factor for process management because, unlike other factors, it can be easily controlled by adding different organic and/or inorganic sources. Indeed, nitrogen is an essential nutrient during wine fermentation that increases biomass production and stimulates the rate of sugar utilization, while its deficiency can cause stuck or sluggish fermentations.
The relationship established between yeasts and nutrients is a very complex issue, even more so in a multispecies system such as wine fermentation [19]. Currently, little data on nitrogen needs, sources, and preferences are available among mixed fermentations [20], probably due to a wide variety of matrix parameters, yeast couples, and culture conditions.
Gobert et al. [21] studied the specific use uptake of amino acids and ammonium by three non-Saccharomyces yeast strains belonging to S. bacillaris, M. pulcherrima, and Pichia membranifaciens species in mixed fermentation with S. cerevisiae. The analysis of the YAN in mixed cultures showed that non-Saccharomyces yeasts have specific amino acid consumption profiles: histidine, methionine, threonine, and tyrosine were not consumed by S. bacillaris; aspartic acid was assimilated very slowly by M. pulcherrima; glutamine was not assimilated by P. membranifaciens. Differently, cysteine seems to be a preferred nitrogen source for all non-Saccharomyces yeasts. In sequential fermentation, these specific profiles of amino acid consumption by non-Saccharomyces yeasts may account for some of the interactions such as reduced performances of S. cerevisiae and volatile profile changes.
A possible explanation of specific non-Saccharomyces nitrogen uptake modality in a multispecies context could be their ability to change the transcriptional behavior in response to nutrient availability when compared with a single-species context. Using the transcriptomic approach, Barbosa and coauthors [17] showed that S. cerevisiae reduced its global transcription activity in coinoculation with Hanseniaspora guilliermondii. In particular, genes related to the biosynthesis of vitamins were upregulated, while genes involved in the uptake and biosynthesis of amino acids were downregulated. In another mixed fermentation model composed by S. cerevisiae/Hanseniaspora uvarum and S. cerevisiae/Candida sake, the results of the transcriptomic analysis showed a partial relief of nitrogen catabolite repression in S. cerevisiae as metabolic stimulation [22]. In a more recent work [23], a transcriptomic analysis of RNA-seq analysis demonstrated that S. cerevisiae in mixed fermentation with Torulaspora delbrueckii reduced the ammonium effects during fermentation. This behavior was already observed by Barbosa and coworkers in the H. guillermondii/S. cerevisiae system [17].
With the same intent, Lleixà et al. [24], analyzing the nitrogen consumption, together with the catabolism repression, demonstrated the positive involvement of Hanseniaspora vineae to produce wine with improved fermentation capacity and increased aromatic properties. This study also contributed to clarifying the controversial role of this apiculate yeast. Although it has been demonstrated that H. vineae increases the fruity aromas, producing high amounts of acetate esters (primarily 2-phenylethyl acetate), this yeast represents a concern in the fermentation of mixed cultures due to its consumption of nutrients, particularly nitrogen. However, these mechanisms of regulation and consumption are unclear. In this study, the use of synthetic must with standardized nitrogen content demonstrated a similar behavior of nitrogen regulation in H. vineae and S. cerevisiae, indicating the presence of specific nitrogen catabolism repression mechanism in the non-Saccharomyces yeast. In this case, the model of mixed fermentation contributed to the better understanding of nitrogen metabolism in an unexplored non-Saccharomyces species.
Recently, Su et al. [25] expanded the knowledge about the preferences and consumption rates of individual nitrogen sources by some non-Saccharomyces yeasts (T. delbrueckii, M. pulcherrima, and Metschnikowia fructicola) in a wine environment. They evidenced that during alcoholic fermentation, the non-Saccharomyces strains consumed different nitrogen sources in a similar order as S. cerevisiae, but not as quickly. Furthermore, when all the nitrogen sources were supplied in the same amount, their assimilation order was similarly affected for both S. cerevisiae and non-Saccharomyces strains. Another recent work confirmed that the management of the yeast assimilable nitrogen may be a powerful tool to modulate wine aroma profiles in mixed wine fermentation [26]. However, also from this last study emerged that further investigations should be carried out to clarify the metabolic fluxes of amino acid metabolism and volatile compounds production.
In addition to nitrogen metabolism, non-Saccharomyces/S. cerevisiae mixed fermentation may affect carbon-related byproducts, such as acetic acid (Figure 1). In this regard, several studies on different non-Saccharomyces species in sequential fermentation with S. cerevisiae highlighted their effect on the capacity to produce wines with low volatile acidity [27,28,29].
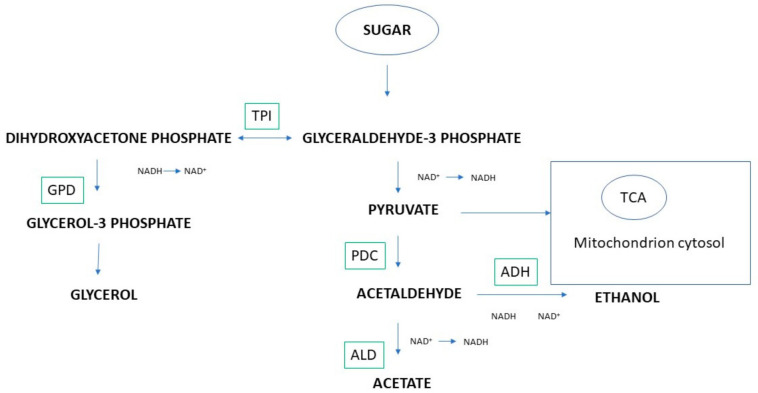
Figure 1.
Overview of sugar catabolism in yeast cells during alcoholic fermentation. GPD: glycerol-3-phosphate dehydrogenase; PDC: pyruvate decarboxylase; ADH: alcohol dehydrogenase; ALD: aldehyde dehydrogenase; TPI: triosephosphate isomerase. Pyruvate originating is partially shuttled to the mitochondrion and incorporated into the tricarboxylic acid (TCA) cycle.
The competition for nutrients and metabolic resources during mixed fermentation also concerns the uptake and the metabolic flux of carbon sources. The evaluation of specific phenotypic traits of S. bacillaris in relation to central carbon metabolite and nitrogen sources showed that the non-Saccharomyces yeast exhibited low activity through the acetaldehyde pathway, which triggers an important redistribution of fluxes through the central carbon metabolic network [30]. In particular, the formation of metabolites derived from the two glycolytic intermediates glyceraldehyde-3-phosphate and pyruvate is substantially increased during fermentation by S. bacillaris. Effectively, the low production of ethanol and acetic acid by some non-Saccharomyces in general and by S. bacillaris in particular is strictly linked to the low activity of the acetaldehyde pathway. It is already known that this behavior has large-scale effects on the metabolic fluxes, requiring increased production of glycerol to overcome the lower production of ethanol and to maintain the redox balance of cells [31]. Furthermore, Englezos and coworkers [32] again reinforced the evidence of a metabolic reorientation of fluxes around the pyruvic acid and glyceraldehyde-3-phosphate nodes as a consequence of reduced carbon channeling toward the acetaldehyde pathway always in the S. bacillaris model. As a direct consequence, increased production of pyruvate and amino acids and larger amounts of alcohols derived from alanine, leucine, valine, and isobutanol, as well as metabolites from glyceraldehyde-3-phosphate, are shown.
The gene expression involved in pyruvate dehydrogenase bypass and glycerol pyruvic fermentation was already investigated by Sadoudi et al. [33] to monitor the acetic acid and glycerol production in S. cerevisiae and the sequential formation of M. pulcherrima in Sauvignon Blanc. In this case, S. cerevisiae exhibited a high expression of pyruvate decarboxylase PDC1 and PDC5, acetaldehyde dehydrogenase ALD6, alcohol dehydrogenase ADH1, and glycerol-3-phosphate dehydrogenase PDC1 genes during the first 3 days of fermentation in sequential fermentation. These results highlighted that the metabolic pathway of these two fermentation products can be affected by the presence of M. pulcherrima, determining an increase in glycerol content and a decrease in acetic acid.
In a comparative transcriptomic study carried out on an S. cerevisiae wine strain and Saccharomyces kudriavzevii, del Real and coworkers [34] demonstrated that in mixed fermentation, S. cerevisiae accelerated the nutrient uptake and utilization to outcompete the coinoculated yeast through a cell-to-cell contact mechanism. It was seen that S. kudriavzevii exhibited a specific response to competition that involved carbon and nitrogen nutrient uptake.
Another recent study investigated sources consumed and metabolites produced from central carbon metabolism in mixed fermentation under enological conditions with M. pulcherrima, M. fructicola, Hanseniaspora opuntiae, H. uvarum, and S. cerevisiae [35]. The competition for the resources seems to be the cause of strong mortality of all non-Saccharomyces species, particularly in mixed fermentation. This study also confirmed the importance of the evaluation of cell population dynamics and their metabolite kinetics such as glycerol and lipid uptake on the limitation of some non-Saccharomyces growth.
The respiratory metabolism, dependent on the overexpression of genes related to sugar consumption and cell proliferation under anaerobic and aerobic conditions in different non-Saccharomyces species, was investigated by Tronchoni et al. [36]. The transcriptional response to cocultivation of S. cerevisiae and T. delbrueckii was analyzed, demonstrating a metabolic interaction in HSP12 and PAU gene expression (encodes one of the two major small heat-shock proteins and fermentative growth, respectively). They found that HSP12 gene expression was stimulated in both yeasts, while PAU genes were stimulated only in S. cerevisiae.
In a more recent work on transcriptomic response of coculture, M. pulcherrima determined in S. cerevisiae an overexpression of the glucofermentative pathway much stronger than with the other species. In addition, in response to M. pulcherrima, great repression of the respiration pathway of S. cerevisiae was found [37]. The hypothesis formulated by the authors is a direct interaction stress response between S. cerevisiae and the non-Saccharomyces yeast. Under excess sugar conditions, respiration is inhibited while the transcription of the hexose transporters is induced, improving the fermentation rate.
The transcriptomic analysis of S. cerevisiae/L. thermotolerans mixed fermentations revealed a clear response of both yeast species to the presence of the other. In this case, it seems that genes involved in the response were related to the competition of micronutrients uptake (such as copper and iron) and those required for cell wall structure and integrity [38].
From grape to wine, the role of nutrients in winemaking is complex. The review of recent studies showed that YAN preferentially consumed by non-Saccharomyces yeast strains during the first stage of fermentation varies in the function of multiple factors, such as intraspecific diversity of non-Saccharomyces and competition with S. cerevisiae. However, any study carried out on the effect of nitrogen uptake during mixed fermentation depth explains why some YAN sources are preferentially consumed by non-Saccharomyces yeasts with respect to others. For this, the study of the gene expression involved in nitrogen and carbon regulation of S. cerevisiae together with transcriptional approaches, although still little explored, would be useful tools for increasing knowledge (Table 1).
Table 1.
The main metabolic response in yeast–yeast interactions.
| Species | Metabolic Pathway/Gene Regulation | Fermentation Condition |
References |
|---|---|---|---|
| S. bacillaris | Histidine, methionine, threonine, and tyrosine were not consumed; cysteine preferred the nitrogen source | Mixed fermentation | [21,30,31,32] |
| Low activity of the acetaldehyde pathway | |||
| M. pulcherrima | Aspartic acid assimilated slowly, cysteine preferred nitrogen source | Mixed fermentation | [21] |
| P. membranifaciens | Glutamine was not assimilated; cysteine preferred the nitrogen source | Mixed fermentation | [21] |
| S. cerevisiae | Genes related to the biosynthesis of vitamins were upregulated while genes involved in the uptake and biosynthesis of amino acids were downregulated | Mixed fermentation with H. guilliermondii | [17] |
| Stimulation of nitrogen metabolism | Mixed fermentation with C. sake and H. uvarum | [22] | |
| Reduced the ammonium effects at fermentation kinetics; stimulated PAU and HSP12 genes | Mixed fermentation with T. delbrueckii | [23,36] | |
| High expression of pyruvate decarboxylase (PDC1) and (PDC5), acetaldehyde dehydrogenase (ALD6), alcohol dehydrogenase (ADH1) and glycerol-3-phosphate dehydrogenase (PDC1); overexpression of the glucofermentative pathway and great repression of the respiration pathway | Mixed fermentation with M. pulcherrima | [33,37] | |
| Accelerated the nutrient uptake | Mixed fermentation with S. kudriavzevii | [34] | |
| H. vineae | Nitrogen catabolism repression | Mixed fermentation synthetic must | [24] |
| T. delbrueckii | Stimulation HSP12 gene (encodes one of the two major small heat-shock proteins) | Mixed fermentation | [36] |
4. Metabolic Regulation in Ethanol Reduction Using Coculture
Over recent years, there has been increasing interest in the lowering of the alcohol content in wines for human health and quality improvement. Global warming and overripened grapes due to modified consumer requests caused an alcohol concentration increase [39,40].
Among the metabolic strategies that have been applied to reduce ethanol yields, gene modification strategies on S. cerevisiae strains to overproduce glycerol have proven to be the most effective [41], although redox imbalances can arise, leading to an increase in undesired metabolites, including acetic acid, acetaldehyde, and acetoin, compromising the final wine quality [42]. Therefore, multiple functional genetic approaches have been explored to limit ethanol production in S. cerevisiae, including both the ALD6 encoding aldehyde dehydrogenase gene deletion and BDH1 encoding 2,3-butanediol dehydrogenase gene overexpression to limit acetic acid and acetoin production, respectively [43,44,45]. However, in this case, the difficult gene regulation such as the principal limitation of classical genetic engineering led to uncontrolled processes.
Recently, systemic ‘omics approaches proved to be useful in identifying specific genetic targets of modification by providing an integrated view of cell physiology, firstly describing wine yeast metabolism in detail, to understand the complex regulatory networks that occur in this organism during wine fermentation [46].
Indeed, integrated ‘omics approaches based on genomics, proteomics, and metabolomics on single S. cerevisiae fermentation require a broad and in-depth study of the cellular mechanisms involved in the control of the overall fermentation process and also in the reduction of ethanol as a final result. Then, following a biological strategy, the metabolic study of the coinoculation of non-Saccharomyces yeasts could be an alternative way to reach ethanol reduction by exploiting the reduced alcoholic fermentation efficiency of the non-Saccharomyces coinoculated strain. The metabolic flux distribution during fermentation differs from S. cerevisiae and non-Saccharomyces yeasts. In some non-Saccharomyces yeast species/strains, the diversion of alcoholic fermentation with an abundant formation of secondary compounds may in part explain the low ethanol yield. In addition, the different regulations of respirofermentative metabolism (Crabtree effect) may contribute to achieving ethanol reduction.
For these reasons, the knowledge of genes involved in the metabolic activity of non-Saccharomyces in mixed fermentation is a requirement to manage their use to obtain wine with low ethanol content. In this regard, Milanovic et al. [28] investigated the metabolic interaction of Starmerella bombicola (formerly Candida stellata) in an immobilized form in mixed fermentation with S. cerevisiae. After considerable evidence that the use of S. bombicola increased the glycerol content, improved the analytical profile of wine, and reduced ethanol content, it was evaluated as the metabolic mechanism through the expression with real-time RT-PCR. S. bombicola influenced the gene’s expression in S. cerevisiae: alcohol dehydrogenase (ADH1) gene expression was higher in the mixed fermentation than the pure culture differently by pyruvate decarboxylase (PDC1). This transcriptomic approach allowed us to understand that PDC1 and ADH1 genes are highly induced at the initial phase of fermentation, while at the end of the process, the expression level of PDC1 was much higher in the pure culture.
Applied studies on the use of M. pulcherrima in mixed fermentations showed a relevant ethanol reduction in different fermentation conditions [47,48]. A recent study on the metabolic flux of M. pulcherrima strains in sequential fermentation with S. cerevisiae showed an ethanol reduction and a higher concentration of TCA cycle byproducts (i.e., fumarate and succinate) and glycerol and lower concentrations of acetic acid [49].
Theoretical mathematical approaches such as the central composite design (CCD) and response surface methodology (RSM) were used as predictive models to investigate the potential application of non-Saccharomyces yeasts in ethanol reduction. For example, the cohabitation mechanisms between S. bacillaris (synonym Candida zemplinina) in combination with S. cerevisiae were established, both in coculture and sequential cultures, involved in ethanol reduction [50,51].
With a similar approach, Maturano and coworkers [52] recently evaluated the possibility of using mixed fermentations between a commercial starter strain of S. cerevisiae and H. uvarum and Candida membranaefaciens, respectively. In this case, the microbiological strategy based on the calibrated use of mixed fermentations clearly showed a significantly reduced ethanol yield when compared with S. cerevisiae pure culture.
Although many scientific works supported the use of mixed fermentations for ethanol reduction in wine, integrated studies on metabolic interactions between S. cerevisiae and non-Saccharomyces widely demonstrated that some associations could lead to the production of unwanted compounds such as acetic acid and ethyl acetate [53]. Non-Saccharomyces yeasts are characterized by respirofermentative regulatory mechanisms different from S. cerevisiae, and this characteristic could be used to reduce ethanol content in wine [47,54,55]. Experimental works exploring this concept are scarce due to the lack of information regarding the exact metabolic characteristics of different yeasts. The use of non-Saccharomyces yeasts to reduce ethanol content in wine via respiration was evaluated by Quiros et al. [54]. A screening of several non-Saccharomyces yeasts under aerated conditions showed that the same strains were suitable for lowering ethanol levels by respiration, in particular belonging to M. pulcherrima. The study of the oxidative–fermentative metabolism of different non-Saccharomyces yeasts strains allowed selecting H. uvarum, Hanseniaspora osmophila, S. bacillaris, and C. membranifaciens as candidates to design cocultures [56].
In this scenario (Table 2), the rich research activity around non-Saccharomyces wine yeasts and their metabolic traits opened new opportunities to exploit yeast metabolism with the aim of reducing the ethanol content of wines. Further investigations are needed to support the study of this aspect.
Table 2.
Metabolic regulation in ethanol reduction in different wine yeasts.
| Species | Metabolic Pathway/Gene Regulation | Fermentation Condition |
References |
|---|---|---|---|
| S. cerevisiae | Gene modification: aldehyde dehydrogenase (ALD6) gene deletion; 2,3-butanediol dehydrogenase (BDH1) gene overexpression | Pure culture | [43,44,45] |
| S. bombicola | Alcohol dehydrogenase (ADH1) gene expression increased; pyruvate decarboxylase (PDC1) decrease | Immobilized form in mixed fermentation | [28] |
| M. pulcherrima | higher concentrations of TCA cycle byproducts (i.e., fumarate and succinate) | Sequential culture | [49] |
| H. uvarum and C. membranaefaciens | calibrate the use of mixed fermentation clearly showed a significantly reduced ethanol yield | Mixed fermentation | [52] |
| M. pulcherrima | Metabolism respirofermentative | Pure culture | [54] |
| H. uvarum, H. osmophila, S. bacillaris and C. membranafaciens | Oxidative–fermentative metabolism | Coculture | [56] |
5. Metabolic Regulation of Volatile Compounds in Mixed Fermentation
The term “volatilome” describes the volatile organic compounds (VOCs) produced by microorganisms during alcoholic fermentation [57]. VOCs are the main responsible for the aromatic composition of wine and are closely related to yeast species, strains, and fermentation conditions [58]. During recent years, several studies were focused on the impact of non-Saccharomyces yeasts in mixed fermentation with S. cerevisiae on the aroma composition of wine, but little research has focused on the molecular mechanisms that attend in these interactions. [59,60,61,62].
During alcoholic fermentation, S. cerevisiae strains metabolize sugar into ethanol, fatty acids, higher alcohols, and esters, responsible for the final flavor of wine (Figure 2). The metabolic pathway involved in the production of these aroma compounds is related to the different fermentation conditions such as availability of precursors, different types of stress, the cellular redox potential and the energy status of the cell, and different types of stress [63,64,65].
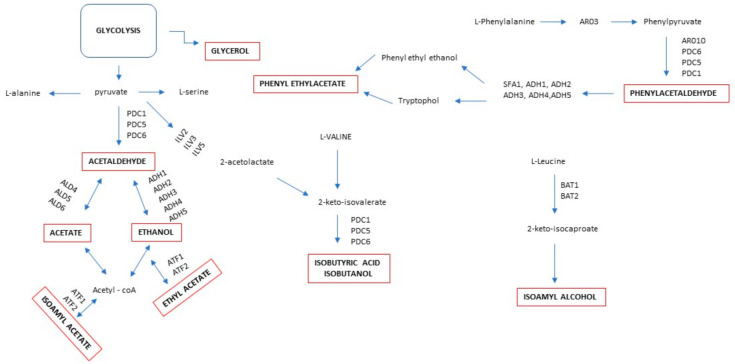
Figure 2.
The main metabolic pathway involved in the production of aroma compounds. PDC1, PDC5, and PDC6 genes coding pyruvate decarboxylase; ALD4, ALD5, and ALD6 genes coding aldehyde dehydrogenase; ADH1, ADH2, ADH3, ADH4, and ADH5 genes coding alcohol dehydrogenase; ATF1 and ATF2 genes coding alcohol acetyl transferase; ILV2, ILV3, and ILV5 genes coding acetolactate synthase; AR03 and AR010 genes coding phenyl pyruvate and phenyl acetaldehyde; SFA1 synonyms of ADH5; BAT1 and BAT2 gene coding the mitochondrial-targeting signal.
The chemical and biological interactions among different yeasts during mixed fermentations underline the difficulty to understand the contribution of genes in the production of aroma compounds, making it difficult to understand every single mechanism that can influence this aspect. Several studies reported the differences in the metabolism of S. cerevisiae in single culture and in coculture with non-Saccharomyces yeasts, but a few studies have investigated the gene regulation of yeast interactions. Indeed, it is necessary to extend the genetic knowledge of non-Saccharomyces species with a sparsely annotated genome than a well-annotated species S. cerevisiae.
T. delbrueckii is one of the most investigated species that contributes positively to the flavor of alcoholic beverages, suggesting its profitable involvement in mixed fermentation with S. cerevisiae [66,67,68,69,70,71]. A recent investigation started to elucidate the metabolic differences regarding the production of aroma compounds [72]. In pure culture on synthetic grape juice medium, T. delbrueckii produced higher levels of ethyl propanoate, contrastingly to, S. cerevisiae, which exhibited a wider range of acetate and ethyl esters. This trend could be explained by transcriptome analysis, which showed the lack of the ATF1-2 gene in T. delbrueckii responsible for the production of acetate esters. Regarding genes related to ethyl esters, transcriptome analyses revealed different expressions between two strains, with overexpression of ETH1 in T. delbreuckii and a lower expression of EEB1 (biosynthesis of ethyl esters). Moreover, the low production of higher alcohols in T. delbreuckii is related to the catabolism of branched-chain amino acids (BCAAs; leucine, valine, and isoleucine) through the Ehrlich pathway and regulated by BAT1, BAT2, and BAP2 genes that are not transcribed in T. delbreuckii. Agarbati and colleagues [73] assessed the possible employment of T. delbrueckii to produce Verdicchio wine with reduced sulfites through sequential fermentation with S. cerevisiae to determine an increase in aroma compounds such as phenyl ethyl acetate and ethyl hexanoate. The modality of inoculation also affects the volatile thiol production in T. delbrueckii during alcoholic fermentation. In sequential fermentation with S. cerevisiae, higher levels of 3-sulfanylhexan-1-ol (3SH) and its acetate ester were observed in comparison with pure culture, highlighting a synergistic interaction between the two species. To understand this result, the metabolism of the precursors responsible for these aromatic compounds, glutathionylated conjugate precursor (Glut-3SH) and cysteinylated conjugate precursor (Cys-3SH), were analyzed [74]. The results showed that S. cerevisiae metabolized the two precursor forms, while T. delbrueckii was able to metabolize the glutathionylated precursor. Consequently, the presence of T. delbrueckii during mixed fermentation led to an increase in Glut-3SH degradation and Cys-3SH production. This overproduction was dependent on the T. delbrueckii biomass. In sequential culture, thus favoring T. delbrueckii development, the higher availability of Cys-3SH throughout AF resulted in more abundant 3SH and 3SHA production by S. cerevisiae. H. vinae also is a non-Saccharomyces species used in mixed fermentation to improve the aromatic profile of wines by the production of 2-phenylethyl acetate, acetate esters, medium-chain fatty acid ethyl esters, benzenoids, and terpenes [75]. The study of the genome sequencing of H. vinae in sequential fermentation in synthetic medium revealed that the increase in 2-phenylethyl acetate and phenylpropanoids was linked to gene duplications of aromatic amino acid aminotransferases encoded by ARO8 and ARO9 genes and phenylpyruvate decarboxylases encoded by ARO10 [76]. The absence of the branched-chain amino acid transaminases (BAT2) and acyl-coenzyme A (acyl-CoA)/ethanol O-acyltransferases (EEB1) genes in H. vineae reduced the production of branched-chain higher alcohols, fatty acids, and ethyl esters, respectively.
Another important group of volatile compounds that affects wine flavor comprises sulfur compounds. It has long been shown that H2S can be produced by yeasts using elemental sulfur with reducing compounds [77]. H2S is converted to cysteine, methionine, and glutathione via a series of enzyme-catalyzed reactions. During the growth phase of yeast, sulfur amino acids are used in protein synthesis, while later in the fermentation process, they can be excreted from the cell and appear in the finished wine. When cellular nitrogen levels are limited, intracellular metabolic pathways can lead to the production of unpleasant H2S. Therefore, in the issue of mixed fermentation, the understanding of the metabolic mechanisms that lead to the formation of these compounds is essential to avoid high levels of H2S by non-Saccharomyces strong H2S producers. However, very few reports are available in the literature concerning the production of sulfur compounds by non-Saccharomyces yeasts. Moreira et al. [78,79] studied the effect of pure and mixed cultures of H. guilliermondii, H. uvarum, and S. cerevisiae in sulfur production in wine. The levels of heavy sulfur compounds in mixed cultures with S. cerevisiae of both apiculate yeasts were low and like those obtained in a pure culture of S. cerevisiae, highlighting the positive role of mixed fermentation.
Transcriptional analysis on non-Saccharomyces and S. cerevisiae could be a suitable strategy to understand and set up strategies to modulate the transcriptional response of specific genes that are linked to the production of aroma compounds of both S. cerevisiae and non-Saccharomyces yeasts (Table 3). Indeed, a recent review, focusing on the strategies for enhancing aroma production by yeast during wine fermentation, highlighted the relevant role of selected non-Saccharomyces yeasts in mixed fermentation to expand the aroma profiles of the wines [80]. About this, it is necessary to improve genetic information about non-Saccharomyces yeasts to delineate the genes and metabolic pathways involved in the production of aromatic compounds in mixed fermentation. In this regard, a recent work developed genetic tools for H. uvarum to reduce ethyl acetate through the disruption of the HuATF1 genes that encodes a putative alcohol acetyltransferase [81].
Table 3.
Metabolic regulation of volatile compounds in different wine yeasts.
| Yeast Species |
Volatile Compounds | Gene/Metabolic Regulation | Fermentation Condition | References |
|---|---|---|---|---|
| T. delbrueckii | Increase ethyl propanoate | Lack of the ATF1-2 gene | pure culture on synthetic grape juice medium | [72] |
| Ethyl esters | Overexpression EHT1 gene and low expression of EEB1 gene | |||
| Low production higher alcohols | Catabolism of branched-chain amino acids (BCAAs; leucine, valine, and isoleucine) regulated by BAT1, BAT2, and BAP2 genes that are not transcribed in T. delbreuckii | |||
| Higher levels of 3-sulfanylhexan-1-ol and acetate ester | Glut-3SH (glutathionylated conjugate precursor) and Cys-3SH (cysteinylated conjugate precursor) | Sequential fermentation | [74] | |
| H. vinae | 2-phenylethyl acetate, acetate esters, medium-chain fatty acid ethyl esters, benzenoids, and terpenes | ARO8 and ARO9 genes coding aromatic amino acid aminotransferases; ARO10 gene coding phenylpyruvate decarboxylases. | Mixed fermentation | [75] |
| Reduced production of branched-chain higher alcohols, fatty acids, and ethyl esters | Absence of the branched-chain amino acid transaminases (BAT2) and acyl-coenzyme A (acyl-CoA)/ethanol O-acyltransferases (EEB1) genes | Sequential fermentation in synthetic grape juice | [76] | |
| H. uvarum | Reduce ethyl acetate | Disruption of the HuATF1 genes | Mixed fermentation | [81] |
6. Yeast-Yeast Interaction and Antimicrobial Activity
Among the wide applications of non-Saccharomyces/S. cerevisiae mixed fermentations in winemaking, the control of undesired microorganisms is one of the actual and promising features. Indeed, there is a growing global interest in biocontrol procedures in foods and in beverages, based on the environmentally sustainable trend. The regulation of the growth of undesired microorganisms could be exploited through antagonistic action, cell-to-cell contact, or through the production of antimicrobial compounds such as mycocins, small peptides, or extracellular vesicles (Table 4). Mycocins are clearly involved in the yeast–yeast interactions in mixed fermentations. Several works have focused on the study of non-Saccharomyces strains able to counteract the development of Brettanomyces spp., a relevant dangerous yeast in the cellar [82,83,84].
Table 4.
Main antimicrobial activity in yeast–yeast interactions.
| Antimicrobial Features |
Yeast Specie | Antimicrobial Activity | Wine Management | Gene Regulation | References |
|---|---|---|---|---|---|
| Mycocins | Tetrapisispora phaffii | Kpkt_β-glucanase activity | Prefermentative stage of mixed wine fermentation | TpBGL2 chromosomal gene | [91,123] |
| Pichia anomala | Pikt_ubiquitin-like protein | Biocontrol agent against wine-spoilage yeasts | - | [92,93] | |
| Kluyveromyces wickerhamii | Kwkt_ β-1,6-glucosidase activity | Biocontrol agent against wine-spoilage yeasts | - | [92,95] | |
| Pichia membranifaciens | - | Mixed wine fermentation | CTT1, HSP12, GPD1, GPP2, TRK2, PDR12, ENA1, SCH9, HAL9, YAP1, XBP1, and STL1 genes involved in osmotic shock | [96,97,98,100] | |
| Pichia membranifaciens | - | Mixed wine fermentation | Stimulates markers of cellular apoptosis | [98,99,100] | |
| Candida pyralidae | CpKT1_β-glucanase activity | Mixed wine fermentation | - | [102] | |
| Wickerhamomyces anomalus | WA18_9UDP-glycosyltransferase | Mixed wine fermentation | - | [104] | |
| Torulaspora delbrueckii | TdKT_glucanase and chitinase activities | Biocontrol agent against wine-spoilage yeasts | - | [105] | |
| Torulaspora delbrueckii | - | Biocontrol agent against wine-spoilage yeasts | Encoded by TdV-Mbarr-1 dsRNA | [106,107] | |
| Saccharomyces eubayanus | SeKT_β-glucanase and chitinase activities | Biocontrol agent against wine-spoilage yeasts | - | [108,109] | |
| Extracellular antimicrobial peptides | Saccharomyces cerevisiae | AMPs_fragments of glyceraldehyde 3-phosphate dehydrogenase | Mixed wine fermentation | - | [112] |
| Candida intermedia | AMPs_stimulate reactive oxygen species (ROS) | Biocontrol agent against wine-spoilage yeasts | - | [114,115] | |
| Extracellular vesicles and compounds | Saccharomyces cerevisiae | Evs_glycolytic enzymes | Biocontrol agent | - | [122] |
| Torulaspora delbrueckii | Evs_glycolytic enzymes | Biocontrol agent | - | [37] | |
| Lachancea thermotolerans | Evs_glycolytic enzymes | Biocontrol agent | - | [122] | |
| Metschnikowia pulcherrima | Pulcherriminic acid | Biocontrol agent against wine-spoilage yeasts | - | [123,124] | |
| Cell-to-cell contact | Lachancea thermotolerans/Saccharomyces cerevisiae | Cell-to-cell contact | Mixed wine fermentation | - | [126] |
| Starmerella bacillaris/Saccharomyces cerevisiae | Cell-to-cell contact | Mixed wine fermentation | - | [127] |
(-): unavailable information.
The mycocin Kpkt produced by Tetrapisispora phaffii was first described as antispoilage yeast [85]. Kpkt acts through a specific β-glucanase activity causing irreversible modifications on the cell wall structure, and it is codified by the TpBGL2 chromosomal gene [86,87,88]. The recombinant toxin (rKpkt) was recently obtained by transferring the Kpkt-coding gene in Komagataella phaffii (formerly Pichia pastoris) [89]. The recombinant Kpkt, when expressed in K. phaffii, displayed a wider spectrum of action than in its native yeast [90], reinforcing the idea of the possible application of mycocins and/or killer yeasts in the food and beverages industries. T. phaffii was used in mixed fermentations at the prefermentative stage to control wild yeasts such as Hanseniaspora, Zygosaccharomyces, and Saccharomycodes as substitutes of sulfur dioxide [91].
Most studies on mycocins are focused on counteracting the development of the Dekkera/Brettanomyces wine-spoilage yeasts. In this context, Pikt and Kwkt mycocins, produced by Pichia anomala and Kluyveromyces wickerhamii, respectively [92], could be used to counteract Dekkera/Brettanomyces. In particular, Pikt is a ubiquitin-like protein of about 8 kDa able to interact with β-1,6-glucan of the cell wall of sensitive yeasts [93]. Kwkt, a protein of about 72 kDa of molecular mass, without any glycosyl residue [94] and β-1,6-glucosidase activity, seems to be involved in blocking the cell cycle function of sensitive yeasts [95]. Through fluorescence techniques of cell death due to alterations in yeast cell permeability and cell metabolism (cytofluorimetric evaluation), it was demonstrated that both Pikt and Kwkt caused the irreversible death of this yeast.
Differently, sulfur dioxide induced a viable but noncultivable (VBNC) state of Brettanomyces with the consequent recovery of yeasts when fresh medium was replaced [95].
P. membranifaciens yeast was also described as a killer yeast able to produce two mycocins: PMKT and PMKT2. The first mycocin binds linear (1→6)-β-d-glucans in the cell wall and Cwp2p plasma membrane receptor of sensitive yeasts, leading to alterations in ionic exchange via the plasma membrane [96]. PMKT induces the activation of genes such as CTT1, HSP12, GPD1, GPP2, TRK2, PDR12, ENA1, SCH9, HAL9, YAP1, XBP1, and STL1, involved in resisting osmotic shock [97,98]. PMKT2, a protein with an apparent molecular mass of 30 kDa, binds mannoproteins and induces cell cycle blockage in an early S-phase of sensitive yeasts, and stimulates markers of cellular apoptosis such as the cytochrome c release, DNA strand breaks, metacaspase activation, and production of reactive oxygen species at a low dose [98,99].
The killer activity of P. membranifaciens was exploited in mixed fermentation with S. cerevisiae and Brettanomyces bruxellensis (inoculum ratio of 1:1) in mixed wine fermentation. P. membranifaciens inhibited B. bruxellensis growth [100] without deleterious effects on the fermentation performance of S. cerevisiae.
Other mycocins active against B. bruxellensis produced by Candida pyralidae and denominated CpKT1 and CpKT2 (both about 50 kDa) were partially characterized: both were active and stable at pH 3.5–4.5 and in a temperature range between 15 and 25 °C. Furthermore, their killer activity was not reduced/suppressed by the ethanol and sugars presence, highlighting their compatibility with winemaking conditions [101]. Subsequently, CpKT1 was described as a protein with β-glucanase activity able to lead to cell membrane and cell wall damage. Both CpKT1 and C. pyralidae yeast were used in mixed fermentations in red grape juice containing B. bruxellensis with a consequent decrease in spoilage yeast concentration [102]. A strain of Wickerhamomyces anomalus was proposed as a valid biocontrol agent against Brettanomyces/Dekkera spp. [103]. The killer activity of W. anomalus is expressed through the release of KTCf20, a mycocin that binds β-1,3- and β-1,6-glucans of sensitive yeasts’ cell walls. This mycocin was able to counteract the growth of Brettanomyces/Dekkera, P. guilliermondii, and P. membranifaciens. Moreover, they showed that W. anomalus did not negatively affect the presence of S. cerevisiae strains in mixed fermentations. Another mycocin produced by another strain of W. anomalus and active against B. bruxellensis was also reported by Comitini et al. [104]. The mycocin denominated WA18 is a protein with 99% UDP-glycosyltransferase protein identity and branched beta-glucans, representing the first mycocin receptors on the surface of the sensitive yeasts. In accordance with de Ullivarri and coworkers [103], they confirmed the compatibility of W. anomalus strain in mixed fermentation with S. cerevisiae yeast.
It was also described that T. delbrueckii is able to release the TdKT mycocin (>30 kDa) with glucanase and chitinase activities. This mycocin is stable in wine environmental conditions, and pustulan and chitin seem to be the first toxin targets in the cell wall, causing cell wall damage, necrosis/apoptotic cell death of sensitive yeasts such as B. bruxellensis, and other potential wine-spoilage yeasts [105]. Moreover, Ramírez et al. [106] isolated and selected wine T. delbrueckii strains producing killer toxin Kbarr-1. This toxin is encoded by a dsRNA, TdV-Mbarr-1, structurally like M dsRNAs of S. cerevisiae, which both seem to be evolutionarily related [107].
Until now, non-Saccharomyces yeasts were able to exploit their killer activity through the production of mycocins active against other non-Saccharomyces yeasts. However, it was also described as the application of Saccharomyces spp. killer strains/mycocins versus grape/wine-spoilage yeasts. Recently, a strain of Saccharomyces eubayanus was studied able to secrete a mycocin (SeKT) with a molecular mass of about 70 kDa that reduces the levels of volatile phenols produced by wine-spoilage yeasts such as B. bruxellensis, Meyerozyma guilliermondii, P. membranifaciens, and Pichia manshurica. This mycocin acts through β-glucanase and chitinase activities, leading to cell wall disruption [108,109].
In the yeast–yeast interactions in wine fermentation, Saccharomyces yeasts play an important role in antimicrobial activity through the production of mycocins and secretion of extracellular antimicrobial peptides (AMPs), recently named “saccharomycin”, derived from the glycolytic enzyme glyceraldehyde 3-phosphate dehydrogenase (GAPDH), with a wide range of action. AMPs are low-molecular-weight proteins active against bacteria, viruses, and fungi. Only a limited number of articles have reported AMPs secreted by yeast with activity against non-Saccharomyces wine-related strains. The most studied are AMPs from S. cerevisiae with activity against a variety of wine-related yeasts [110,111]. These peptides are C-terminal fragments of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) with a molecular mass close to 1.6 kDa, with interesting antifungal properties against H. guilliermondii, Kluyveromyces marxianus, L. thermotolerans, and T. delbrueckii [112]. The membrane functionality seems to have a key role in the antimicrobial activity of AMPs by the production of amphiphilic structures that interact with the exposed receptors (carbohydrate molecules). This was recently demonstrated by Caldeira et al. [113] by structural characterization of saccharomycin carried out by nuclear magnetic resonance (NMR). Moreover, Pena and Gang [114] and Pena et al. [115] found AMPs, of about 5 kDa produced by Candida intermedia, able to stimulate the production of reactive oxygen species (ROS) in sensitive yeasts. These AMPs showed an effective antimicrobial activity in wine against two strains of P. guilliermondii and B. bruxellensis, considered an excellent application of a new strategy for the biocontrol of spoilage yeasts.
Among yeast–yeast interactions, the culturability loss of non-Saccharomyces yeasts has received growing interest due to new findings on the role of excreted compounds in the interaction between Saccharomyces and non-Saccharomyces yeasts [116,117,118]. Branco et al. [111] showed that VBNC status was related to interaction through secreted compounds. The loss of culturability was investigated in more non-Saccharomyces wine yeasts as starters in mixed fermentation with S. cerevisiae [119] with the aim of understanding their final impact on wine quality. Another work [120] confirmed that some metabolites produced by S. cerevisiae played the main role in the decreased cultivability of the other Saccharomyces and non-Saccharomyces yeasts, indicating that the interactions are species and strain specific. For example, it was found that the main cause for the lack of cultivability of H. uvarum did not seem to be due to cell-to-cell contact but rather compounds related to fermentations such as ethanol and/or certain metabolites secreted by S. cerevisiae. Another possible mechanism of interaction in mixed fermentations is the possible involvement of extracellular vehicles (EVs). In this regard, recent work on exo-proteome in pure and mixed fermentations non-Saccharomyces and S. cerevisiae investigated proteomic analysis of EV-enriched fractions from six different species. Results showed a wide diversity of proteins secreted, indicating the presence of interactions and the possible involvement of EVs [121,122]. The EV-enriched fractions from different species such as S. cerevisiae, T. delbrueckii, and L. thermotolerans showed enrichment in glycolytic enzymes and cell-wall-related proteins and particularly the enzyme exo-1,3-β-glucanase. However, this protein was not involved in the here-observed negative impact of T. delbrueckii extracellular fractions on the growth of other yeast species. These findings suggest that EVs may play a role in fungal interactions during wine fermentation and other aspects of wine yeast biology.
Another antimicrobial interaction in wine fermentation is the release of extracellular compounds such as peptides, acids, and other small molecules. M. pulcherrima exerts its antagonistic action through pulcherriminic acid (precursor of pulcherrimin pigment) production, depleting the iron present in the medium and consequently not making it available for other yeasts. M. pulcherrima, when inoculated in coculture with H. guilliermondii, P. membranifaciens, and B. bruxellensis, exerted an antimicrobial action, while in coculture with S. cerevisiae, it did not show antimicrobial activity [123]. More recently, Kántor et al. [124] demonstrated the in vitro antimicrobial action of M. pulcherrima against Candida spp. and P. manshurica grape/wine-related yeasts.
The death of some wine-related yeast species during mixed-culture fermentations has always been attributed to their inability to survive in the presence of selective growth factors. However, it was shown that mechanisms based on cell-to-cell contact were able to influence yeast population dynamics during wine fermentation [125]. Double-compartment fermentation system used for L. thermotolerans/S. cerevisiae mix fermentations showed a reduction in the death rate of L. thermotolerans than in noncompartmentalized mixed-culture fermentations, despite the two fermentation systems showing comparable amounts of the antimicrobial peptidic fraction [126]. Similarly, S. bacillaris died earlier when tested in mixed fermentation with S. cerevisiae using the flask compared to when both yeast species were kept physically separate [127]. These results highlight that the death of these non-Saccharomyces yeasts seems to be not caused by a nutrient limitation or growth-inhibitory compound accumulation but rather by cell-to-cell contact mechanisms. Moreover, the cell-to-cell contact mechanism seems also to influence the metabolic behavior of the yeast strains with the consequent production (or not) of specific chemical compounds, affecting the aroma characteristics of wine [128].
7. Conclusions
Controlled mixed fermentations undoubtedly have a positive impact on the analytical and sensory characteristics of wines and may have a positive role in the biocontrol procedures to reduce the use of chemical compounds. On the other hand, the behavior of the coculture cannot be accurately predicted, and fermentations carried out in a reproducible manner remains a big challenge.
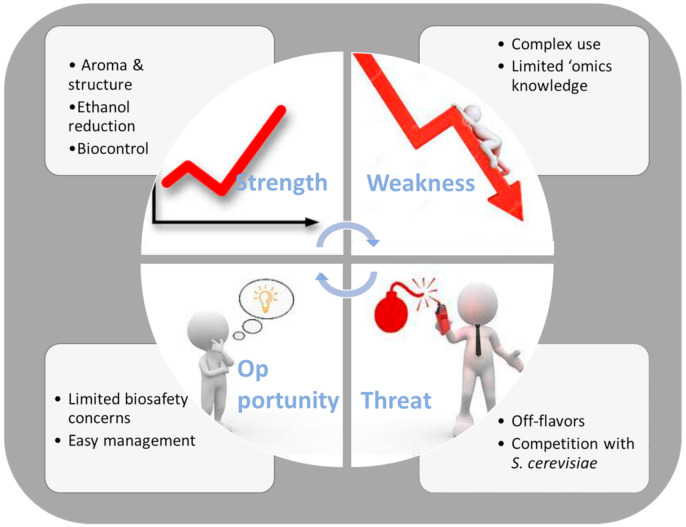
For this, further knowledge is needed on yeast–yeast interactions and in relation to the environmental factors, as the molecular mechanisms of these interactions are largely unknown. While S. cerevisiae is genetically well characterized, genome information on non-Saccharomyces is still in an early stage, and further investigations should be carried out to obtain a more clearer picture in identifying certain key genes and pathways. Figure 3 summarizes the overviewed functions of the use of non-Saccharomyces wine yeasts by means of SWOT analysis. where each item assesses the strengths, weaknesses, opportunities, and threats of this issue.
Figure 3.
SWOT diagram, summarizing the strengths (S), weaknesses (W), opportunities (O), and threats (T) of the current management of non-Saccharomyces yeasts.
Abbreviations
| 3SH | 3-Sulfanylhexan-1-ol |
| ADH | Alcohol dehydrogenase |
| ALD | Aldehyde dehydrogenase |
| AMPs | Antimicrobial peptides |
| Cys-3SH | Cysteinylated conjugate precursor |
| EVs | Extracellular vehicles |
| GAPDH | Glyceraldehyde 3-phosphate dehydrogenase |
| Glut-3SH | Glutathionylated conjugate precursor |
| GPD | Glycerol-3-phosphate dehydrogenase |
| PDC | Pyruvate decarboxylase |
| TCA | Tricarboxylic acid cycle |
| TPI | Triosephosphate isomerase |
| VBNC | Viable but noncultivable |
| VOCs | Volatile organic compounds |
| YAN | Yeast assimilable nitrogen |
Author Contributions
Conceptualization: F.C. and M.C.; data collection: A.A. and L.C.; writing—original draft preparation: A.A.; funding acquisition: M.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received external funding: A.A. was supported by a grant from Polytechnic University of Marche, D.R. n. 1312, 23 October 2019.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ciani M., Comitini F. Red Wine Technology. Volume 4. Academic Press; Cambridge, MA, USA: 2019. Use of non-Saccharomyces yeasts in red winemaking; pp. 51–68. [Google Scholar]
- 2.Conacher C.G., Luyt N.A., Naidoo-Blassoples R.K., Rossouw D., Setati M.E., Bauer F.F. The ecology of wine fermentation: A model for the study of complex microbial ecosystems. Appl. Microbiol. Biotechnol. 2021;105:3027–3043. doi: 10.1007/s00253-021-11270-6. [DOI] [PubMed] [Google Scholar]
- 3.Varela C., Siebert T., Cozzolino D., Rose L., McLean H., Henschke P.A. Discovering a chemical basis for differentiating wines made by fermentation with ‘wild’indigenous and inoculated yeasts: Role of yeast volatile compounds. Aust. J. Grape Wine Res. 2009;15:238–248. doi: 10.1111/j.1755-0238.2009.00054.x. [DOI] [Google Scholar]
- 4.Jolly N.P., Varela C., Pretorius I.S. Not your ordinary yeast: Non-Saccharomyces yeasts in wine production uncovered. FEMS Yeast Res. 2014;14:215–237. doi: 10.1111/1567-1364.12111. [DOI] [PubMed] [Google Scholar]
- 5.Del Fresno J.M., Morata A., Loira I., Bañuelos M.A., Escott C., Benito S., González Chamorro C., Suárez-Lepe J.A. Use of non-Saccharomyces in single-culture, mixed and sequential fermentation to improve red wine quality. Eur. Food Res. Technol. 2017;243:2175–2185. doi: 10.1007/s00217-017-2920-4. [DOI] [Google Scholar]
- 6.Muñoz-Redondo J.M., Puertas B., Cantos-Villar E., Jiménez-Hierro M.J., Carbú M., Garrido C., Ruiz-Moreno M.J., Moreno-Rojas J.M. Impact of Sequential Inoculation with the Non-Saccharomyces T. delbrueckii and M. pulcherrima Combined with Saccharomyces cerevisiae Strains on Chemicals and Sensory Profile of Roseé Wines. J. Agric. Food Chem. 2021;69:1598–1609. doi: 10.1021/acs.jafc.0c06970. [DOI] [PubMed] [Google Scholar]
- 7.Ciani M., Comitini F., Mannazzu I., Domizio P. Controlled mixed culture fermentation: A new perspective on the use of non-Saccharomyces yeasts in winemaking. FEMS Yeast Res. 2010;10:123–133. doi: 10.1111/j.1567-1364.2009.00579.x. [DOI] [PubMed] [Google Scholar]
- 8.Cañas P.M., Romero E.G., Pérez-Martín F., Seseña S., Palop M.L. Sequential inoculation versus co-inoculation in Cabernet Franc wine fermentation. Food Sci. Technol. Int. 2015;21:203–212. doi: 10.1177/1082013214524585. [DOI] [PubMed] [Google Scholar]
- 9.García M., Esteve-Zarzoso B., Crespo J., Cabellos J.M., Arroyo T. Yeast monitoring of wine mixed or sequential fermentations made by native strains from DO “Vinos de Madrid” using real-time quantitative PCR. Front. Microbiol. 2017;8:2520. doi: 10.3389/fmicb.2017.02520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu K., Jin G.J., Xu Y.H., Tao Y.S. Wine aroma response to different participation of selected Hanseniaspora uvarum in mixed fermentation with Saccharomyces cerevisiae. Food Res. Int. 2018;108:119–127. doi: 10.1016/j.foodres.2018.03.037. [DOI] [PubMed] [Google Scholar]
- 11.Daviss B. Growing pains for metabolomics: The newest’omic science is producing results and more data than researchers know what to do with. Scientist. 2005;19:25–29. [Google Scholar]
- 12.Schmitt-Kopplin P., Harir M., Tziotis D., Gabelica Z., Hertkorn N. Ultrahigh resolution Fourier transform ion cyclotron resonance mass spectrometry for the analysis of natural organic matter from various environmental systems. In: Lebedev A., editor. Comprehensive Environmental Mass Spectrometry. ILM Publications; St. Albans, UK: 2012. pp. 443–459. [Google Scholar]
- 13.Roullier-Gall C., David V., Hemmler D., Schmitt-Kopplin P., Alexandre H. Exploring yeast interactions through metabolic profiling. Sci. Rep. 2020;10:6073. doi: 10.1038/s41598-020-63182-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petitgonnet C., Klein G.L., Roullier-Gall C., Schmitt-Kopplin P., Quintanilla-Casas B., Vichi S., Diane Julien-David D., Alexandre H. Influence of cell-cell contact between L. thermotolerans and S. cerevisiae on yeast interactions and the exo-metabolome. Food Microbiol. 2019;83:122–133. doi: 10.1016/j.fm.2019.05.005. [DOI] [PubMed] [Google Scholar]
- 15.Howell K.S., Cozzolino D., Bartowsky E.J., Fleet G.H., Henschke P.A. Metabolic profiling as a tool for revealing Saccharomyces interactions during wine fermentation. FEMS Yeast Res. 2006;6:91–101. doi: 10.1111/j.1567-1364.2005.00010.x. [DOI] [PubMed] [Google Scholar]
- 16.Peng C., Viana T., Petersen M.A., Larsen F.H., Arneborg N. Metabolic footprint analysis of metabolites that discriminate single and mixed yeast cultures at two key time-points during mixed culture alcoholic fermentations. Metabolomics. 2018;14:93. doi: 10.1007/s11306-018-1391-3. [DOI] [PubMed] [Google Scholar]
- 17.Barbosa C., Mendes-Faia A., Lage P., Mira N.P., Mendes-Ferreira A. Genomic expression program of Saccharomyces cerevisiae along a mixed-culture wine fermentation with Hanseniaspora guilliermondii. Microb. Cell Factories. 2015;14:124. doi: 10.1186/s12934-015-0318-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brice C., Cubillos F.A., Dequin S., Camarasa C., Martínez C. Adaptability of the Saccharomyces cerevisiae yeasts to wine fermentation conditions relies on their strong ability to consume nitrogen. PLoS ONE. 2018;13:e0192383. doi: 10.1371/journal.pone.0192383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kemsawasd V., Viana T., Ardö Y., Arneborg N. Influence of nitrogen sources on growth and fermentation performance of different wine yeast species during alcoholic fermentation. Appl. Microbiol. Biotechnol. 2015;99:10191–10207. doi: 10.1007/s00253-015-6835-3. [DOI] [PubMed] [Google Scholar]
- 20.Andorrà I., Berradre M., Rozès N., Mas A., Guillamón J.M., Esteve-Zarzoso B. Effect of pure and mixed cultures of the main wine yeast species on grape must fermentations. Eur. Food Res. Technol. 2010;231:215–224. doi: 10.1007/s00217-010-1272-0. [DOI] [Google Scholar]
- 21.Gobert A., Tourdot-Maréchal R., Morge C., Sparrow C., Liu Y., Quintanilla-Casas B., Vichi S., Alexandre H. Non-Saccharomyces Yeasts Nitrogen Source Preferences: Impact on Sequential Fermentation and Wine Volatile Compounds Profile. Front. Microbiol. 2017;8:2175. doi: 10.3389/fmicb.2017.02175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Curiel J.A., Morales P., Gonzalez R., Tronchoni J. Different non-Saccharomyces yeast species stimulate nutrient consumption in S. cerevisiae mixed cultures. Front. Microbiol. 2017;8:2121. doi: 10.3389/fmicb.2017.02121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ruiz J., de Celis M., de Toro M., Mendes-Ferreira A., Rauhut D., Santos A., Belda I. Phenotypic and transcriptional analysis of Saccharomyces cerevisiae during wine fermentation in response to nitrogen nutrition and co-inoculation with Torulaspora delbrueckii. Food Res. Int. 2020;137:109663. doi: 10.1016/j.foodres.2020.109663. [DOI] [PubMed] [Google Scholar]
- 24.Lleixà Daga J. Ph.D. Thesis. Universitat Rovira i Virgili; Terragona, Spain: 2019. Influence of Non-Saccharomyces Yeast on Winemaking and Quality. [Google Scholar]
- 25.Su Y., Seguinot P., Sanchez I., Ortiz-Julien A., Heras J.M., Querol A., Camarasa C., Guillamón J.M. Nitrogen sources preferences of non-Saccharomyces yeasts to sustain growth and fermentation under winemaking conditions. Food Microbiol. 2020;85:103287. doi: 10.1016/j.fm.2019.103287. [DOI] [PubMed] [Google Scholar]
- 26.Rollero S., Bloem A., Brand J., Ortiz-Julien A., Camarasa C., Divol B. Nitrogen metabolism in three non-conventional wine yeast species: A tool to modulate wine aroma profiles. Food Microbiol. 2021;94:103650. doi: 10.1016/j.fm.2020.103650. [DOI] [PubMed] [Google Scholar]
- 27.Comitini F., Gobbi M., Domizio P., Romani C., Lencioni L., Mannazzu I., Ciani M. Selected non-Saccharomyces wine yeasts in controlled multistarter fermentations with Saccharomyces cerevisiae. Food Microbiol. 2011;28:873–882. doi: 10.1016/j.fm.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 28.Milanovic V., Ciani M., Oro L., Comitini F. Starmerella bombicola influences the metabolism of Saccharomyces cerevisiae at pyruvate decarboxylase and alcohol dehydrogenase level during mixed wine fermentation. Microb. Cell Factories. 2012;11:18. doi: 10.1186/1475-2859-11-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rantsiou K., Dolci P., Giacosa S., Torchio F., Tofalo R., Torriani S., Suzzi G., Rolle L., Cocolin L. Candida zemplinina can reduce acetic acid produced by Saccharomyces cerevisiae in sweet wine fermentations. Appl. Environ. Microbiol. 2012;78:1987. doi: 10.1128/AEM.06768-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Englezos V., Rantsiou K., Cravero F., Torchio F., Pollon M., Fracassetti D., Ortiz-Julien A., Gerbi V., Rolle L., Cocolin L. Volatile profile of white wines fermented with sequential inoculation of Starmerella bacillaris and Saccharomyces cerevisiae. Food Chem. 2018;257:350–360. doi: 10.1016/j.foodchem.2018.03.018. [DOI] [PubMed] [Google Scholar]
- 31.Ciani M., Ferraro L. Combined use of immobilized Candida stellata cells and Saccharomyces cerevisiae to improve the quality of wines. J. Appl. Microbiol. 1998;85:247–254. doi: 10.1046/j.1365-2672.1998.00485.x. [DOI] [PubMed] [Google Scholar]
- 32.Englezos V., Cocolin L., Rantsiou K., Ortiz-Julien A., Bloem A., Dequin S., Camarasa C. Specific phenotypic traits of Starmerella bacillaris related to nitrogen source consumption and central carbon metabolite production during wine fermentation. Appl. Environ. Microbiol. 2018;84:e00797-18. doi: 10.1128/AEM.00797-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sadoudi M., Rousseaux S., David V., Alexandre H., Tourdot-Maréchal R. Metschnikowia pulcherrima influences the expression of genes involved in PDH bypass and glyceropyruvic fermentation in Saccharomyces cerevisiae. Front. Microbiol. 2017;8:1137. doi: 10.3389/fmicb.2017.01137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alonso del Real J., Pérez-Torrado R., Querol A., Barrio E. Dominance of wine Saccharomyces cerevisiae strains over S. kudriavzevii in industrial fermentation competitions is related to an acceleration of nutrient uptake and utilization. Environ. Microbiol. 2019;21:1627–1644. doi: 10.1111/1462-2920.14536. [DOI] [PubMed] [Google Scholar]
- 35.Harlé O., Legrand J., Tesnière C., Pradal M., Mouret J.R., Nidelet T. Investigations of the mechanisms of interactions between four nonconventional species with Saccharomyces cerevisiae in oenological conditions. PLoS ONE. 2020;15:e0233285. doi: 10.1371/journal.pone.0233285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tronchoni J., Curiel J.A., Morales P., Torres-Pérez R., Gonzalez R. Early transcriptional response to biotic stress in mixed starter fermentations involving Saccharomyces cerevisiae and Torulaspora delbrueckii. Int. J. Food Microbiol. 2017;241:60–68. doi: 10.1016/j.ijfoodmicro.2016.10.017. [DOI] [PubMed] [Google Scholar]
- 37.Mencher A., Morales P., Curiel J.A., Gonzalez R., Tronchoni J. Metschnikowia pulcherrima represses aerobic respiration in Saccharomyces cerevisiae suggesting a direct response to co-cultivation. Food Microbiol. 2021;94:103670. doi: 10.1016/j.fm.2020.103670. [DOI] [PubMed] [Google Scholar]
- 38.Shekhawat K., Patterton H., Bauer F.F., Setati M.E. RNA-seq based transcriptional analysis of Saccharomyces cerevisiae and Lachancea thermotolerans in mixed-culture fermentations under anaerobic conditions. BMC Genom. 2019;20:145. doi: 10.1186/s12864-019-5511-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gobbi M., De Vero L., Solieri L., Comitini F., Oro L., Giudici L., Ciani M. Fermentative aptitude of non-Saccharomyces wine yeast for reduction in the ethanol content in wine. Eur. Food Res. Technol. 2014;239:41–48. doi: 10.1007/s00217-014-2187-y. [DOI] [Google Scholar]
- 40.Varela J., Varela C. Microbiological strategies to produce beer and wine with reduced ethanol concentration. Curr. Opin. Biotechnol. 2019;56:88–96. doi: 10.1016/j.copbio.2018.10.003. [DOI] [PubMed] [Google Scholar]
- 41.Varela C., Kutyna D.R., Solomon M.R., Black C.A., Borneman A., Henschke P.A., Pretorius I.S., Chambers P.J. Evaluation of gene modification strategies for the development of low-alcohol-wine yeasts. Appl. Environ. Microbiol. 2012;78:6068–6077. doi: 10.1128/AEM.01279-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goold H.D., Kroukamp H., Williams T.C., Paulsen I.T., Varela C., Pretorius I.S. Yeast’s balancing act between ethanol and glycerol production in low-alcohol wines. Microb. Biotechnol. 2017;10:264–278. doi: 10.1111/1751-7915.12488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eglinton J.M., Heinrich A.J., Pollnitz A.P., Langridge P., Henschke P.A., de Barros Lopes M. Decreasing acetic acid accumulation by a glycerol overproducing strain of Saccharomyces cerevisiae by deleting the ALD6 aldehyde dehydrogenase gene. Yeast. 2002;19:295–301. doi: 10.1002/yea.834. [DOI] [PubMed] [Google Scholar]
- 44.Cambon B., Monteil V., Remize F., Camarasa C., Dequin S. Effects of GPD1 overexpression in Saccharomyces cerevisiae commercial wine yeast strains lacking ALD6 genes. Appl. Environ. Microbiol. 2006;72:4688. doi: 10.1128/AEM.02975-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ehsani M., Fernández M.R., Biosca J.A., Julien A., Dequin S. Engineering of 2, 3-butanediol dehydrogenase to reduce acetoin formation by glycerol-overproducing, low-alcohol Saccharomyces cerevisiae. Appl. Environ. Microbiol. 2009;75:3196. doi: 10.1128/AEM.02157-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Varela C., Schmidt S.A., Borneman A.R., Pang C.N.I., Krömerx J.O., Khan A., Song X., Hodson M.P., Solomon M., Mayr C.M., et al. Systems-based approaches enable identification of gene targets which improve the flavour profile of low-ethanol wine yeast strains. Metab. Eng. 2018;49:178–191. doi: 10.1016/j.ymben.2018.08.006. [DOI] [PubMed] [Google Scholar]
- 47.Morales P., Rojas V., Quirós M., Gonzalez R. The impact of oxygen on the final alcohol content of wine fermented by a mixed starter culture. Appl. Microbiol. Biotechnol. 2015;99:3993–4003. doi: 10.1007/s00253-014-6321-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Canonico L., Comitini F., Ciani M. Metschnikowia pulcherrima selected strain for ethanol reduction in wine: Influence of cell immobilization and aeration condition. Foods. 2019;8:378. doi: 10.3390/foods8090378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hranilovic A., Gambetta J.M., Jeffery D.W., Grbin P.R., Jiranek V. Lower-alcohol wines produced by Metschnikowia pulcherrima and Saccharomyces cerevisiae co-fermentations: The effect of sequential inoculation timing. Int. J. Food Microbiol. 2020;329:108651. doi: 10.1016/j.ijfoodmicro.2020.108651. [DOI] [PubMed] [Google Scholar]
- 50.Englezos V., Rantsiou K., Torchio F., Rolle L., Gerbi V., Cocolin L. Exploitation of the non-Saccharomyces yeast Starmerella bacillaris (synonym Candida zemplinina) in wine fermentation: Physiological and molecular characterizations. Int. J. Food Microbiol. 2015;199:33–40. doi: 10.1016/j.ijfoodmicro.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 51.Englezos V., Rantsiou K., Cravero F., Torchio F., Ortiz-Julien A., Gerbi V., Rolle L., Cocolin L. Starmerella bacillaris and Saccharomyces cerevisiae mixed fermentations to reduce ethanol content in wine. Appl. Microbiol. Biotechnol. 2016;100:5515–5526. doi: 10.1007/s00253-016-7413-z. [DOI] [PubMed] [Google Scholar]
- 52.Maturano Y.P., Mestre M.V., Kuchen B., Toro M.E., Mercado L.A., Vazquez F., Combina M. Optimization of fermentation-relevant factors: A strategy to reduce ethanol in red wine by sequential culture of native yeasts. Int. J. Food Microbiol. 2019;289:40–48. doi: 10.1016/j.ijfoodmicro.2018.08.016. [DOI] [PubMed] [Google Scholar]
- 53.Ciani M., Capece A., Comitini F., Canonico L., Siesto G., Romano P. Yeast interactions in inoculated wine fermentation. Front. Microbiol. 2016;7:555. doi: 10.3389/fmicb.2016.00555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Quirós M., Rojas V., Gonzalez R., Morales P. Selection of non-Saccharomyces yeast strains for reducing alcohol levels in wine by sugar respiration. Int. J. Food Microbiol. 2014;181:85–91. doi: 10.1016/j.ijfoodmicro.2014.04.024. [DOI] [PubMed] [Google Scholar]
- 55.Contreras A., Hidalgo C., Schmidt S., Henschke P.A., Curtin C., Varela C. The application of non-Saccharomyces yeast in fermentations with limited aeration as a strategy for the production of wine with reduced alcohol content. Int. J. Food Microbiol. 2015;205:7–15. doi: 10.1016/j.ijfoodmicro.2015.03.027. [DOI] [PubMed] [Google Scholar]
- 56.Mestre Furlani M.V., Maturano Y.P., Combina M., Mercado L.A., Toro M.E., Vazquez F. Selection of non-Saccharomyces yeasts to be used in grape musts with high alcoholic potential: A strategy to obtain wines with reduced ethanol content. FEMS Yeast Res. 2017;17 doi: 10.1093/femsyr/fox010. [DOI] [PubMed] [Google Scholar]
- 57.Ebert B.E., Halbfeld C.M., Blank L. Exploration and exploitation of the yeast volatilome. Curr. Metabolomics. 2017;5:102–118. doi: 10.2174/2213235X04666160818151119. [DOI] [Google Scholar]
- 58.Escribano R., González-Arenzana L., Portu J., Garijo P., López-Alfaro I., López R., Santamaría P., Gutiérrez A.R. Wine aromatic compound production and fermentative behaviour within different non-Saccharomyces species and clones. J. Appl. Microbiol. 2018;124:1521–1531. doi: 10.1111/jam.13735. [DOI] [PubMed] [Google Scholar]
- 59.Hazelwood L.A., Daran J.M., Van Maris A.J., Pronk J.T., Dickinson J.R. The Ehrlich pathway for fusel alcohol production: A century of research on Saccharomyces cerevisiae metabolism. Appl. Environ. Microbiol. 2008;74:2259–2266. doi: 10.1128/AEM.02625-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bisson L.F., Karpel J.E. Genetics of yeast impacting wine quality. Annu. Rev. Food Sci. Technol. 2010;1:139–162. doi: 10.1146/annurev.food.080708.100734. [DOI] [PubMed] [Google Scholar]
- 61.Procopio S., Brunner M., Becker T. Differential transcribed yeast genes involved in flavour formation and its associated amino acid metabolism during brewery fermentation. Eur. Food Res. Technol. 2014;239:421–439. doi: 10.1007/s00217-014-2236-6. [DOI] [Google Scholar]
- 62.Chidi B.S., Bauer F.F., Rossouw D. Organic acid metabolism and the impact of fermentation practices on wine acidity: A review. S. Afr. J. Enol. Vitic. 2018;39 doi: 10.21548/39-2-3172. [DOI] [Google Scholar]
- 63.Delneri D., Gardner D.C.J., Bruschi C.V., Oliver S.G. Disruption of seven hypothetical aryl alcohol dehydrogenase genes from Saccharomyces cerevisiae and construction of a multiple knock-out strain. Yeast. 1999;15:1681–1689. doi: 10.1002/(SICI)1097-0061(199911)15:15<1681::AID-YEA486>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 64.Dickinson J.R., Harrison S.J., Dickinson J.A., Hewlins M.J.E. An investigation of the metabolism of isoleucine to active amyl alcohol in Saccharomyces cerevisiae. J. Biol. Chem. 2000;275:10937–10942. doi: 10.1074/jbc.275.15.10937. [DOI] [PubMed] [Google Scholar]
- 65.Dickinson J.R., Salgado L.E., Hewlins M.J.E. The catabolism of amino acids to long chain and complex alcohols in Saccharomyces cerevisiae. J. Biol. Chem. 2003;278:8028–8034. doi: 10.1074/jbc.M211914200. [DOI] [PubMed] [Google Scholar]
- 66.Bely M., Stoeckle P., Masneuf-Pomarède I., Dubourdieu D. Impact of mixed Torulaspora delbrueckii–Saccharomyces cerevisiae culture on high-sugar fermentation. Int. J. Food Microbiol. 2008;122:312–320. doi: 10.1016/j.ijfoodmicro.2007.12.023. [DOI] [PubMed] [Google Scholar]
- 67.Sadoudi M., Tourdot-Maréchal R., Rousseaux S., Steyer D., Gallardo-Chacon J.J., Ballester J., Vichi S., Guérin-Schneider R., Caixach J., Alexandre H. Yeast-yeast interactions revealed by aromatic profile analysis of Sauvignon Blanc wine fermented by single or co-culture of non-Saccharomyces and Saccharomyces yeasts. Food Microbiol. 2012;32:243–253. doi: 10.1016/j.fm.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 68.van Breda V., Jolly N., van Wyk J. Characterisation of commercial and natural Torulaspora delbrueckii wine yeast strains. Int. J. Food Microbiol. 2013;163:80–88. doi: 10.1016/j.ijfoodmicro.2013.02.011. [DOI] [PubMed] [Google Scholar]
- 69.Taillandier P., Lai Q.P., Julien-Ortiz A., Brandam C. Interactions between Torulaspora delbrueckii and Saccharomyces cerevisiae in wine fermentation: Influence of inoculation and nitrogen content. World J. Microb. Biot. 2014;30:1959–1967. doi: 10.1007/s11274-014-1618-z. [DOI] [PubMed] [Google Scholar]
- 70.Velázquez R., Zamora E., Álvarez M.L., Hernández L.M., Ramírez M. Effects of new Torulaspora delbrueckii killer yeasts on the must fermentation kinetics and aroma compounds of white table wine. Front. Microbiol. 2015;6:1222. doi: 10.3389/fmicb.2015.01222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Canonico L., Comitini F., Ciani M. Influence of vintage and selected starter on Torulaspora delbrueckii/Saccharomyces cerevisiae sequential fermentation. Eur. Food Res. Technol. 2015;241:827–833. doi: 10.1007/s00217-015-2507-x. [DOI] [Google Scholar]
- 72.Tondini F., Lang T., Chen L., Herderich M., Jiranek V. Linking gene expression and oenological traits: Comparison between Torulaspora delbrueckii and Saccharomyces cerevisiae strains. Int. J. Food Microbiol. 2019;294:42–49. doi: 10.1016/j.ijfoodmicro.2019.01.014. [DOI] [PubMed] [Google Scholar]
- 73.Agarbati A., Canonico L., Comitini F., Ciani M. Improved Saccharomyces cerevisiae Strain in Pure and Sequential Fermentation with Torulaspora delbrueckii for the Production of Verdicchio Wine with Reduced Sulfites. Appl. Sci. 2020;10:6722. doi: 10.3390/app10196722. [DOI] [Google Scholar]
- 74.Renault P., Coulon J., Moine V., Thibon C., Bely M. Enhanced 3-sulfanylhexan-1-ol production in sequential mixed fermentation with Torulaspora delbrueckii/Saccharomyces cerevisiae reveals a situation of synergistic interaction between two industrial strains. Front. Microbiol. 2016;7:293. doi: 10.3389/fmicb.2016.00293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Benito Á., Calderón F., Benito S. The influence of non-Saccharomyces species on wine fermentation quality parameters. Fermentation. 2019;5:54. doi: 10.3390/fermentation5030054. [DOI] [Google Scholar]
- 76.Giorello F., Valera M.J., Martin V., Parada A., Salzman V., Camesasca L., Fariña L., Boido E., Medina K., Dellacassa E., et al. Genomic and transcriptomic basis of Hanseniaspora vineae’s impact on flavor diversity and wine quality. Appl. Environ. Microbiol. 2019;85:e01959-18. doi: 10.1128/AEM.01959-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rankine B.C. Nature, origin and prevention of hydrogen sulphide aroma in wines. J. Sci. Food Agric. 1963;14:79–91. doi: 10.1002/jsfa.2740140204. [DOI] [Google Scholar]
- 78.Moreira N., De Pinho P.G., Vasconcelos I. Method for analysis of heavy sulphur compounds using gas chromatography with flame photometric detection. Anal. Chim. Acta. 2004;513:183–189. doi: 10.1016/j.aca.2003.12.041. [DOI] [Google Scholar]
- 79.Moreira N., Pina C., Mendes F., Couto J.A., Hogg T., Vasconcelos I. Volatile compounds contribution of Hanseniaspora guilliermondii and Hanseniaspora uvarum during red wine vinifications. Food Control. 2011;22:662–667. doi: 10.1016/j.foodcont.2010.07.025. [DOI] [Google Scholar]
- 80.Van Wyk N., Grossmann M., Wendland J., Von Wallbrunn C., Pretorius I.S. The whiff of wine yeast innovation: Strategies for enhancing aroma production by yeast during wine fermentation. J. Agric. Food Chem. 2019;67:13496–13505. doi: 10.1021/acs.jafc.9b06191. [DOI] [PubMed] [Google Scholar]
- 81.Badura J., van Wyk N., Brezina S., Pretorius I.S., Rauhut D., Wendland J., von Wallbrunn C. Development of Genetic Modification Tools for Hanseniaspora uvarum. Int. J. Mol. Sci. 2021;22:1943. doi: 10.3390/ijms22041943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Boynton P.J. The ecology of killer yeasts: Interference competition in natural habitats. Yeast. 2019;36:473–485. doi: 10.1002/yea.3398. [DOI] [PubMed] [Google Scholar]
- 83.Mannazzu I., Domizio P., Carboni G., Zara S., Zara G., Comitini F., Budroni M., Ciani M. Yeast killer toxins: From ecological significance to application. Crit. Rev. Biotechnol. 2019;39:603–617. doi: 10.1080/07388551.2019.1601679. [DOI] [PubMed] [Google Scholar]
- 84.Pinto L., Baruzzi F., Cocolin L., Malfeito-Ferreira M. Emerging technologies to control Brettanomyces spp. in wine: Recent advances and future trends. Trends Food Sci. Technol. 2020;99:88–100. doi: 10.1016/j.tifs.2020.02.013. [DOI] [Google Scholar]
- 85.Ciani M., Fatichenti F. Killer Toxin of Kluyveromyces phaffii DBVPG 6076 as a Biopreservative Agent to Control apiculate wine yeasts. Appl. Environ. Microbiol. 2001;67:3058–3063. doi: 10.1128/AEM.67.7.3058-3063.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Comitini F., Di Pietro N., Zacchi L., Mannazzu I., Ciani M. Kluyveromyces phaffii killer toxin active against wine spoilage yeasts: Purification and characterization. Microbiology. 2004;150:2535–2541. doi: 10.1099/mic.0.27145-0. [DOI] [PubMed] [Google Scholar]
- 87.Comitini F., Mannazzu I., Ciani M. Tetrapisispora phaffii killer toxin is a highly specific β-glucanase that disrupts the integrity of the yeast cell wall. Microb. Cell Factories. 2009;8:55. doi: 10.1186/1475-2859-8-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Oro L., Zara S., Fancellu F., Mannazzu I., Budroni M., Ciani M., Comitini F. Tp BGL2 codes for a Tetrapisispora phaffii killer toxin active against wine spoilage yeasts. FEMS Yeast Res. 2014;14:464–471. doi: 10.1111/1567-1364.12126. [DOI] [PubMed] [Google Scholar]
- 89.Carboni G., Fancello F., Zara G., Zara S., Ruiu L., Marova I., Pinna G., Budroni M., Mannazzu I. Production of a lyophilized ready-to-use yeast killer toxin with possible applications in the wine and food industries. Int. J. Food Microbiol. 2020;335:108883. doi: 10.1016/j.ijfoodmicro.2020.108883. [DOI] [PubMed] [Google Scholar]
- 90.Carboni G., Marova I., Zara G., Zara S., Budroni M., Mannazzu I. Evaluation of Recombinant Kpkt Cytotoxicity on HaCaT Cells: Further Steps towards the Biotechnological Exploitation Yeast Killer Toxins. Foods. 2021;10:556. doi: 10.3390/foods10030556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Comitini F., Ciani M. The zymocidial activity of Tetrapisispora phaffii in the control of Hanseniaspora uvarum during the early stages of winemaking. Lett. Appl. Microbiol. 2010;50:50–56. doi: 10.1111/j.1472-765X.2009.02754.x. [DOI] [PubMed] [Google Scholar]
- 92.Comitini F., De Ingeniis J., Pepe L., Mannazzu I., Ciani M. Pichia anomala and Kluyveromyces wickerhamii killer toxins as new tools against Dekkera/Brettanomyces spoilage yeasts. FEMS Microbiol. Lett. 2004;238:235–240. doi: 10.1111/j.1574-6968.2004.tb09761.x. [DOI] [PubMed] [Google Scholar]
- 93.De Ingeniis J., Raffaelli N., Ciani M., Mannazzu I. Pichia anomala DBVPG 3003 secretes a ubiquitin-like protein that has antimicrobial activity. Appl. Environ. Microbiol. 2009;75:1129–1134. doi: 10.1128/AEM.01837-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Comitini F., Ciani M. Kluyveromyces wickerhamii killer toxin: Purification and activity towards Brettanomyces/Dekkera yeasts in grape must. FEMS Microbiol. Lett. 2011;316:77–82. doi: 10.1111/j.1574-6968.2010.02194.x. [DOI] [PubMed] [Google Scholar]
- 95.Oro L., Ciani M., Bizzaro D., Comitini F. Evaluation of damage induced by Kwkt and Pikt zymocins against Brettanomyces/Dekkera spoilage yeast, as compared to sulphur dioxide. J. Appl. Microbiol. 2016;121:207–214. doi: 10.1111/jam.13121. [DOI] [PubMed] [Google Scholar]
- 96.Santos A., San Mauro M., Abrusci C., Marquina D. Cwp2p, the plasma membrane receptor for Pichia membranifaciens killer toxin. Mol. Microbiol. 2007;64:831–843. doi: 10.1111/j.1365-2958.2007.05702.x. [DOI] [PubMed] [Google Scholar]
- 97.Santos A., Marquina D. Ion channel activity by Pichia membranifaciens killer toxin. Yeast. 2004;21:151–162. doi: 10.1002/yea.1069. [DOI] [PubMed] [Google Scholar]
- 98.Belda I., Ruiz J., Alonso A., Marquina D., Santos A. The biology of Pichia membranifaciens killer toxins. Toxins. 2017;9:112. doi: 10.3390/toxins9040112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Santos A., Alonso A., Belda I., Marquina D. Cell cycle arrest and apoptosis, two alternative mechanisms for PMKT2 killer activity. Fungal Genet. Biol. 2013;50:44–54. doi: 10.1016/j.fgb.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 100.Santos A., San Mauro M., Bravo E., Marquina D. PMKT2, a new killer toxin from Pichia membranifaciens, and its promising biotechnological properties for control of the spoilage yeast Brettanomyces bruxellensis. Microbiology. 2009;155:624–634. doi: 10.1099/mic.0.023663-0. [DOI] [PubMed] [Google Scholar]
- 101.Mehlomakulu N.N., Setati M.E., Divol B. Characterization of novel killer toxins secreted by wine-related non-Saccharomyces yeasts and their action on Brettanomyces spp. Int. J. Food. Microbiol. 2014;188:83–91. doi: 10.1016/j.ijfoodmicro.2014.07.015. [DOI] [PubMed] [Google Scholar]
- 102.Mehlomakulu N.N., Prior K.J., Setati M.E., Divol B. Candida pyralidae killer toxin disrupts the cell wall of Brettanomyces bruxellensis in red grape juice. J. Appl. Microbiol. 2017;122:747–758. doi: 10.1111/jam.13383. [DOI] [PubMed] [Google Scholar]
- 103.de Ullivarri M.F., Mendoza L.M., Raya R.R. Characterization of the killer toxin KTCf20 from Wickerhamomyces anomalus, a potential biocontrol agent against wine spoilage yeasts. Biol. Control. 2018;121:223–228. doi: 10.1016/j.biocontrol.2018.03.008. [DOI] [Google Scholar]
- 104.Comitini F., Agarbati A., Canonico L., Galli E., Ciani M. Purification and Characterization of WA18, a New Mycocin Produced by Wickerhamomyces anomalus Active in Wine against Brettanomyces bruxellensis Spoilage Yeasts. Microorganisms. 2021;9:56. doi: 10.3390/microorganisms9010056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Villalba M.L., Sáez J.S., Del Monaco S., Lopes C.A., Sangorrín M.P. TdKT, a new killer toxin produced by Torulaspora delbrueckii effective against wine spoilage yeasts. Int. J. Food Microbiol. 2016;217:94–100. doi: 10.1016/j.ijfoodmicro.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 106.Ramírez M., Velázquez R., Maqueda M., López-Piñeiro A., Ribas J.C. A new wine Torulaspora delbrueckii killer strain with broad antifungal activity and its toxin-encoding double-stranded RNA virus. Front. Microbiol. 2015;6:983. doi: 10.3389/fmicb.2015.00983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ramírez M., Velázquez R., Maqueda M., Martínez A. Genome organization of a new double-stranded RNA LA helper virus from wine Torulaspora delbrueckii killer yeast as compared with its Saccharomyces counterparts. Front. Microbiol. 2020;11:2977. doi: 10.3389/fmicb.2020.593846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mazzucco M.B., Ganga M.A., Sangorrín M.P. Production of a novel killer toxin from Saccharomyces eubayanus using agro-industrial waste and its application against wine spoilage yeasts. Antonie van Leeuwenhoek. 2019;112:965–973. doi: 10.1007/s10482-019-01231-5. [DOI] [PubMed] [Google Scholar]
- 109.Villalba M.L., Mazzucco M.B., Lopes C.A., Ganga M.A., Sangorrín M.P. Purification and characterization of Saccharomyces eubayanus killer toxin: Biocontrol effectiveness against wine spoilage yeasts. Int. J. Food Microbiol. 2020;331:108714. doi: 10.1016/j.ijfoodmicro.2020.108714. [DOI] [PubMed] [Google Scholar]
- 110.Albergaria H., Francisco D., Gori K., Arneborg N., Gírio F. Saccharomyces cerevisiae CCMI 885 secretes peptides that inhibit the growth of some non-Saccharomyces wine-related strains. Appl. Microbiol. Biotechnol. 2010;86:965–972. doi: 10.1007/s00253-009-2409-6. [DOI] [PubMed] [Google Scholar]
- 111.Branco P., Viana T., Albergaria H., Arneborg N. Antimicrobial peptides (AMPs) produced by Saccharomyces cerevisiae induce alterations in the intracellular pH, membrane permeability and culturability of Hanseniaspora guilliermondii cells. Int. J. Food Microbiol. 2015;205:112–118. doi: 10.1016/j.ijfoodmicro.2015.04.015. [DOI] [PubMed] [Google Scholar]
- 112.Branco P., Francisco D., Monteiro M., Almeida M.G., Caldeira J., Arneborg N., Prista C., Albergaria H. Antimicrobial properties and death-inducing mechanisms of saccharomycin, a biocide secreted by Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 2017;101:159–171. doi: 10.1007/s00253-016-7755-6. [DOI] [PubMed] [Google Scholar]
- 113.Caldeira J., Almeida G., Macedo A.L., Silva J.P., Albergaria H. Saccharomycin, a biocide from S. cerevisiae that kill-off other yeasts. Ann. Med. 2019;51(Suppl. 1):94–95. doi: 10.1080/07853890.2018.1562694. [DOI] [Google Scholar]
- 114.Peña R., Ganga M.A. Novel antimicrobial peptides produced by Candida intermedia LAMAP1790 active against the wine-spoilage yeast Brettanomyces bruxellensis. Antonie van Leeuwenhoek. 2019;112:297–304. doi: 10.1007/s10482-018-1159-9. [DOI] [PubMed] [Google Scholar]
- 115.Peña R., Vílches J., G-Poblete C., Ganga M.A. Effect of Candida intermedia LAMAP1790 Antimicrobial Peptides against Wine-Spoilage Yeasts Brettanomyces bruxellensis and Pichia guilliermondii. Fermentation. 2020;6:65. doi: 10.3390/fermentation6030065. [DOI] [Google Scholar]
- 116.Ciani M., Comitini F. Yeast interactions in multi-starter wine fermentation. Curr. Opin. Food Sci. 2015;1 doi: 10.1016/j.cofs.2014.07.001. [DOI] [Google Scholar]
- 117.Liu Y., Rousseaux S., Tourdot-Maréchal R., Sadoudi M., Gougeon R., Schmitt-Kopplin P., Alexandre H. Wine microbiome, a dynamic world of microbial interactions. Crit. Rev. Food Sci. Nutr. 2015;57:856–873. doi: 10.1080/10408398.2014.983591. [DOI] [PubMed] [Google Scholar]
- 118.Albergaria H., Arneborg N. Dominance of Saccharomyces cerevisiae in alcoholic fermentation processes: Role of physiological fitness and microbial interactions. Appl. Microbiol. Biotechnol. 2016;100:2035–2046. doi: 10.1007/s00253-015-7255-0. [DOI] [PubMed] [Google Scholar]
- 119.Masneuf-Pomarede I., Bely M., Marullo P., Albertin W. The genetics of non-conventional wine yeasts: Current knowledge and future challenges. Front. Microbiol. 2016;6:1563. doi: 10.3389/fmicb.2015.01563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wang C., Mas A., Esteve-Zarzoso B. The Interaction between Saccharomyces cerevisiae and Non-Saccharomyces Yeast during Alcoholic Fermentation Is Species and Strain Specific. Front. Microbiol. 2016;7:502. doi: 10.3389/fmicb.2016.00502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Stahl P.D., Raposo G. Exosomes and extracellular vesicles: The path forward. Essays Biochem. 2018;62:119–124. doi: 10.1042/EBC20170088. [DOI] [PubMed] [Google Scholar]
- 122.Mencher A., Morales P., Valero E., Tronchoni J., Patil K.R., Gonzalez R. Proteomic characterization of extracellular vesicles produced by several wine yeast species. Microb. Biotechnol. 2020;13:1581–1596. doi: 10.1111/1751-7915.13614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Oro L., Ciani M., Comitini F. Antimicrobial activity of Metschnikowia pulcherrima on wine yeasts. J. Appl. Microbiol. 2014;116:1209–1217. doi: 10.1111/jam.12446. [DOI] [PubMed] [Google Scholar]
- 124.Kántor A., Hutková J., Petrová J., Hleba L., Kačániová M. Antimicrobial activity of pulcherrimin pigment produced by Metschnikowia pulcherrima against various yeast species. J. Microbiol. Biotechnol. Food Sci. 2021;2021:282–285. doi: 10.15414/jmbfs.2015/16.5.3.282-285. [DOI] [Google Scholar]
- 125.Renault P.E., Albertin W., Bely M. An innovative tool reveals interaction mechanisms among yeast populations under oenological conditions. Appl. Microbiol. Biotechnol. 2013;97:4105–4119. doi: 10.1007/s00253-012-4660-5. [DOI] [PubMed] [Google Scholar]
- 126.Kemsawasd V., Branco P., Almeida M.G., Caldeira J., Albergaria H., Arneborg N. Cell-to-cell contact and antimicrobial peptides play a combined role in the death of Lachanchea thermotolerans during mixed-culture alcoholic fermentation with Saccharomyces cerevisiae. FEMS Microbiol. Lett. 2015;362:fnv103. doi: 10.1093/femsle/fnv103. [DOI] [PubMed] [Google Scholar]
- 127.Englezos V., Rantsiou K., Giacosa S., Segade S.R., Rolle L., Cocolin L. Cell-to-cell contact mechanism modulates Starmerella bacillaris death in mixed culture fermentations with Saccharomyces cerevisiae. Int. J. Food Microbiol. 2019;289:106–114. doi: 10.1016/j.ijfoodmicro.2018.09.009. [DOI] [PubMed] [Google Scholar]
- 128.Pietrafesa A., Capece A., Pietrafesa R., Bely M., Romano P. Saccharomyces cerevisiae and Hanseniaspora uvarum mixed starter cultures: Influence of microbial/physical interactions on wine characteristics. Yeast. 2020;37:609–621. doi: 10.1002/yea.3506. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.