Abstract
Miso is a traditional Japanese seasoning paste produced by fermenting soybeans using the power of koji mold. A recent Japanese cohort study has shown that increased consumption of fermented soybean products is associated with a reduced risk of death in both men and women. In this review, we briefly explain what miso means in the Japanese culture and food industry, varieties of miso available today, and steps involved in miso making. Then, we review early and latest scientific researches in koji mold species, their safety, and beneficial enzymes they produce during fermentation and maturation processes, which play a major part in determining the quality and sensory profile of miso.
Keywords: Aspergillus oryzae, koji, miso, soybean, enzymes, peptidase, fermentation
1. Introduction
The earliest form of miso called kokusho (soybeans and grains fermented with salt) is said to have originated from ancient China or perhaps in Japan thousands of years ago [1]. The use of miso spread among Japanese people during the Edo Period (1603–1868), and miso constitutes one of the hallmarks of the country’s salted and fermented soybean seasoning along with soy sauce. Miso was integrated into local food cultures across Japan and evolved into various types, reflecting regional differences in climate and ingredient availability [2,3]. This is the reason miso comes in so many varieties with different colors and flavors. Today, rice miso is the most manufactured variety, while barley, soybean, and mixed miso are also available.
This review describes the manufacturing process of miso, including the critical step of koji making, microbes used for fermentation, and enzymes found in koji. Some of the latest topics on regular consumption of miso and its health benefits are also discussed.
2. Miso Varieties and Its Culinary Scene
Miso is regulated by the Food Labeling Standards as specified by the Food Labeling Act [4]. Miso is defined as a semisolid paste primarily made from soybeans, which are combined, fermented, and matured with soybeans and/or grains cultured with koji mold and salt. According to the ingredients used, miso is classified into four types: rice, barley, soybean, and mixed miso. Rice miso is made from rice, soybeans, and salt. Rice is first fermented with koji mold to produce koji, which is then used for the fermentation and maturation of soybeans. Barley miso is produced in much the same way, except that barley or naked barley is used instead of rice. Soybean miso is made from soybeans and salt using soybean koji for fermentation and maturation. Mixed miso can be any combination of rice, barley, and/or soybean miso or any miso produced using a mixture of rice, barley, and/or soybean koji. Miso can also be classified by taste and color. The proportion of koji (the rice-to-soybean ratio for rice miso and the barley-to-soybean ratio for barley miso) determines the sweetness. Higher proportions of rice or barley koji create a sweeter taste, while lower proportions of koji produce saltiness. There are red, yellow, and white miso according to the color of the finished product.
The most typical use of miso in Japanese cuisine is miso soup. Seasonal ingredients (vegetables, seaweed, and seafood) are cooked in dashi soup stock made from dried bonito, dried kelp, or other flavoring ingredients, and a spoonful of miso paste is dissolved in the soup.
In 2013, washoku was added to UNESCO’s Intangible Cultural Heritage List as traditional dietary cultures of the Japanese. Extending in a north-to-southwardly direction, Japan has a rich and diverse natural environment with four distinct seasons. Washoku is a cooking and serving practice that was born and nurtured in this unique environment and essentially represents Japanese people’s spirit of respect for nature [5]. The taste of miso is formed by complex interactions of sweetness, saltiness, umami, acidity, bitterness, and astringency. It can mask meaty and fishy odors, while adding umami and depth to a variety of dishes. Miso is one of the most fundamental fermented seasoning at the heart of washoku and essential to the everyday diet of Japanese people. It is also a part of the “One Soup Three Dishes” principle of washoku serving. Japanese people feel a sense of nostalgia and hometown familiarity along with comfort and warmth when they drink miso soup.
3. Process of Miso Making
Rice miso is the most common variety produced throughout Japan and accounts for as high as about 80% of the total production [6]. On the other hand, barley miso is manufactured mainly in the southern parts of Japan—Kyushu, Shikoku, and Chugoku regions, and soybean miso is mainly preferred in Chubu, the middle part of Japan.
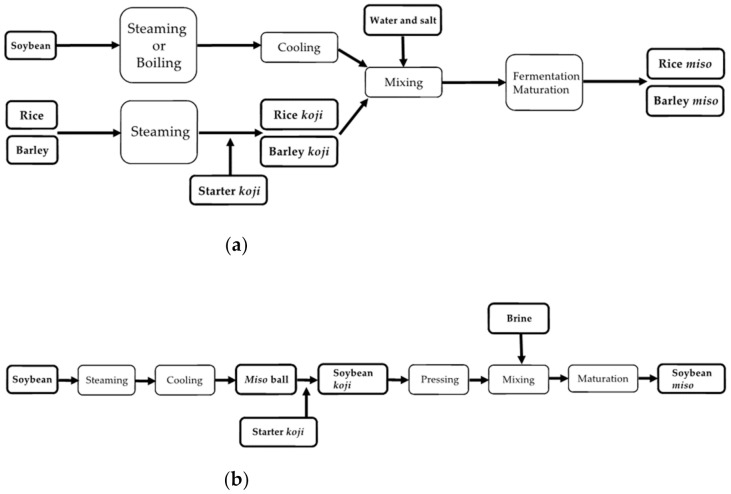
Since old times, koji making has been considered the most critical step, followed by soybean processing and mixing, among many steps of miso making [7]. Figure 1 summarizes these steps in miso making.
Figure 1.
Overview of the miso making process for (a) rice and barley miso and (b) soybean miso.
3.1. Rice Miso
The following explains the process of rice miso making [8,9]. Soybeans are washed and soaked in water typically for 15 to 17 h. In the next step, they are either steamed or boiled usually under pressure. After steaming or boiling, soybeans are then cooled down.
Rice koji making starts from the selection of rice grains and soaking followed by steaming for complete conversion of raw starch (β-starch) to gelatinized starch (α-starch). Near-saturated steam is used for 40 to 60 min. Steamed rice is cooled and then inoculated with koji mold. Although this differs depending on the culture room conditions, the first teire or mixing (discussed later) is usually done around 18 h after inoculation and the second teire usually around 26 h. It takes a total of 42 to 48 h to produce rice koji. After inoculation, the temperature is maintained at 28–32 °C during loading into the culture room (or hikikomi; see below) and at 40 °C or below during koji fermentation.
Mixing miso ingredients is called shikomi. Rice koji, steamed or boiled soybeans, water, salt, yeast, and lactic acid bacteria are mixed thoroughly and transferred into a fermentation/maturation tank. Steamed or boiled soybeans are crushed before mixing, typically by processing through a chopper with a 5–6 mm mesh. They are then immediately mixed with rice koji, salt water, and other ingredients. Generally, salt-tolerant yeast Zygosaccharomyces rouxii and salt-tolerant lactic acid bacterium Tetragenococcus halophilus are used for shikomi.
Mixed ingredients are fermented and matured in a tank. Temperature control and the timing of inversion called tenchi-gaeshi are essential steps during fermentation and maturation. Tenchi-gaeshi is a process in which miso is transferred upside down into another tank. The purpose is to
-
(1)
Ensure uniform fermentation and maturation across all parts of the tank;
-
(2)
Provide aeration to facilitate the yeast’s aerobic fermentation and growth (yeast does not grow under anaerobic conditions, although it generates alcohol that affects the flavor);
-
(3)
Release fermentation heat.
Fermentation and maturation are greatly affected by the enzymatic activities of koji and temperature of miso. For miso production based on both enzymatic and microbial activities, the temperature is maintained between 25 and 30 °C. The length of the maturation period is determined by what type of miso we want to produce and varies from 1 month to years.
This is how miso is made through fermentation. There is another type of miso, which is produced based on enzymatic degradation. There are two varieties of “enzymatically degraded” miso: white sweet types such as Kyoto white miso and red sweet types such as Edo sweet miso. These miso use higher percentages of koji, and they are sweeter and lower in salt (5 to 7%). The maturation takes place solely through koji enzyme activities and completes within a matter of several days at around 50 °C of controlled temperature (high-temperature digestion).
3.2. Barley Miso
The following explains the process of barley miso making [8]. Barley miso is produced much like rice miso with the following specific modifications.
Because barley absorbs water very quickly, the optimal soaking time is defined for each water temperature. During soaking, barley grains swell and form hard and tight clumps that must be loosened. They also form clumps during steaming and have to be broken up before loading into the koji facility to prevent uneven growth of the koji mold. The barley koji making process is similar to that of rice koji except that the higher protein content of barley makes it more susceptible to bacterial contamination, higher exothermic temperature, and clumping of koji.
There are mainly two types of barley miso: (1) light-colored miso with a higher proportion of koji, shorter maturation period, characteristic koji aroma, and salt content of 9 to 11%, and (2) red miso with a longer maturation period and higher salt content of 10 to 12%.
3.3. Soybean Miso
The following explains the process of soybean miso making [8]. Soybeans and salt are the only ingredients in soybean miso. Soybeans are washed and then soaked. The soaking process is the critical point that directly determines the product quality. The restricted water absorption technique is used to strictly control the amount of water and soaking time to produce soybeans with an ideal moisture content after steaming for the inoculation of koji mold. In general, the optimal weight of soaked and drained soybeans is 1.5 to 1.6 times the original weight. After soaking, soybeans are usually steamed under pressure.
The first step of koji making is to make miso balls. Steamed soybeans are cooled and then molded into balls using a specialized machine. These balls are inoculated with a koji starter and transferred to a koji facility. The size of a miso ball ranges from 15 to 40 mm in diameter and influences the quality of finished miso. Small miso balls tend to allow higher enzymatic activities, while larger miso balls have higher lactic acid contents, which give a fresher taste. Aspergillus oryzae or Aspergillus sojae is typically used as a starter.
The key point in soybean koji making is to maintain low temperatures to promote the growth of koji mold and lactic acid bacteria while suppressing Bacillus spp. The temperature is maintained at 27–28 °C in the initial phase. After germination of koji spores, the koji temperature is increased gradually up to 35–37 °C and generally maintained at 33–35 °C in the later phase.
For shikomi, soybean koji is pressed with a roller mill and mixed with saturated salt water and additional salt.
Soybean miso is matured for at least 6 to 12 months without heat treatment and around 4 to 6 months with heat treatment.
4. Filamentous Fungi, Koji Mold, in Japanese Fermented Soybean Paste
4.1. Brewer’s Koji Mold
4.1.1. Koji Mold for Sake, Miso and Soy Sauce
Koji mold is a filamentous fungus used in the making of a variety of fermented foods such as sake (rice wine), soy sauce, miso, and mirin. It belongs to Aspergillus oryzae, also known as, yellow koji mold, which can be further classified into many varieties or strains depending on the application. Brewer’s starter koji is commercially available as koji spores and marketed by starter koji manufacturers. To manufacture a starter koji, a stock koji strain is inoculated to steamed brown rice or wheat bran and cultured for a period longer than typical koji mold culture to ensure good sporulation. Spores are then dried and harvested. Some koji starters contain cereals used as the growth medium. In others, spores are separated by sieving and sold as a uniform powder after the addition of a bulking agent such as starch and calcium carbonate.
Koji mold for sake making is characterized by a higher capability to produce amylase, an enzyme which efficiently breaks down rice starch into glucose. For clear sake, it is important to prevent coloration of sake by choosing a strain that does not produce too many spores or pigmentation.
Soy sauce is a liquid seasoning obtained by the hydrolysis of soy proteins into free amino acids. Because the primary role of koji mold for soy sauce making is to break down soy proteins, the koji mold should have higher protease activities than amylase activities. A. sojae is also used for some soy sauce products. This species is known for its high capacity to produce proteases, dark green-colored spores, and short conidiophores. Shorter hyphae are advantageous in that they allow better air permeability due to less intertwining of hyphae and that the teire process during koji making is easier as koji clumps would break up easily.
A. oryzae is the primary koji mold used in miso koji, while A. sojae is used in some instances. Strains with moderate protease and amylase activities are selected and maintained. During the fermentation and maturation of miso, soy proteins are hydrolyzed to release amino acids that add umami, while rice and barley starches are broken down into glucose and other sugars. Therefore, koji mold for miso making should be able to break down both proteins and starches. The balance between proteinase and amylase activities is the key factor when selecting excellent koji mold for miso.
4.1.2. Starter Koji for Miso Making
A variety of koji starters are available for miso making to meet the requirements of wide-ranging miso types that vary in ingredients, ingredient proportions, salt concentrations, and color. In one study, 94 koji mold strains were isolated from koji starters used for miso making, and 92 of them were A. oryzae, whereas only two were A. sojae, which were isolated from koji starters for soybean miso [10].
The properties and specifications of koji starters include enzyme production ability, growth rate, hyphae length, growing form on steamed rice, color, and aroma. These properties can be divided into those important for handling during koji making and those associated with koji properties. The properties required for a strain used in soy sauce making are clear: high capability to produce proteases and faster spore formation. For miso, the definition is not so clear, but reported properties include
-
(1)
A broader spectrum of protease activities;
-
(2)
About 8% higher amylase activities and 20% higher protease activities compared to brewer’s strains;
-
(3)
Correlations among amylase activities and among protease activities but not between amylases and proteases [11].
These properties are likely the result of artificial selection of strains to meet the diverse needs of miso. The strains provided from the starter koji manufacturers have undergone selection by miso makers. The diverse miso qualities, which reflect social trends of the time and consumer preferences, seem to have played key roles in the properties of koji mold for miso making.
Note that the properties of a starter koji strain are not directly linked to the quality of miso. The quality of koji can vary depending on the handling during the koji making process, and the quality of final miso products is determined by many factors including soybean processing, shikomi proportions, control during maturation, and lactic acid bacteria and yeast activities during maturation.
4.1.3. Safety of Koji Mold
The safety of A. oryzae in food has been empirically demonstrated by the fact that A. oryzae has been used for the fermentation of foods for over a thousand years in Japan. Based on the molecular phylogenetic analysis, A. oryzae belongs to the Aspergillus section Flavi. Because the Aspergillus section Flavi includes an aflatoxigenic species Aspergillus flavus, concerns were raised that A. oryzae could also produce aflatoxins. In the 1970s, government research organizations, universities, and manufacturers initiated a collaborative research and found that none of the starter koji strains were aflatoxigenic [12].
Later, this result was further confirmed by molecular studies. The aflatoxin biosynthetic gene system of A. flavus is made up of a cluster of more than 25 genes. By PCR analysis, 15 of 39 strains of A. oryzae were shown to have deletions in five genes within the cluster that is homologous to the aflatoxin biosynthetic gene cluster of A. flavus. In the remainder of the strains examined, the genes within the homolog cluster were dysfunctional [13]. In 2005, the whole genome sequencing was completed for A. oryzae strain RIB40, and this strain contained the aflatoxin biosynthesis gene homolog cluster. Using the sequences of seven homolog genes (aflT, nor-1, aflR, norA, avnA, verb, and vbs) found in this strain, 196 strains of koji mold were analyzed by PCR. It was found that they could be classified into three groups: 105 strains having seven of those genes (Group 1), 81 strains having three genes (Group 2), and eight strains having one gene (Group 3), and that half of the strains had deletions in cluster genes [14]. In addition, the expression of aflR, which encodes a transcription factor for the entire cluster, was absent in the expressed sequence tag data of A. oryzae RIB40, strongly indicating that the aflatoxin biosynthesis gene cluster is unlikely expressed [15]. RT-PCR analysis has also shown that the expression of aflR is very low in the 10 strains in Group 1. Furthermore, the expression of avnA, verB, omtA, and vbs cluster genes was not detected [14].
Thus, koji mold does not produce toxins during culture, and genomic studies have shown deletions in the aflatoxin biosynthesis gene cluster as well as mutations in the transcription factor gene aflR and other cluster genes. These findings support that koji mold is not toxigenic from both toxicological and genetic perspectives.
4.2. Koji Making and Enzyme Production
The characteristics of starter koji strains are not the only determinant of the quality of final miso products. Process conditions during koji making also have a significant influence on enzyme production and coloration of koji.
4.2.1. Amount of Starter Koji
Unlike industrial microbial culture, koji making does not need precultures and starts as soon as starter koji is inoculated to steamed rice or barley. Therefore, it is best to inoculate as many spores as possible. For koji making for miso production, 5 × 105 spores per 1 g of rice is considered optimal. In a laboratory setting, changes in the spore count affect the length of the growth induction phase but not the final enzymatic activities or fungal count in koji obtained after culture [16]. In the industrial setting, however, the inoculation amount should be optimized based on production conditions, because the rate of respiratory heat generation affects the temperature control management and enzyme production efficiency.
4.2.2. Blending Different Koji Starters
Commercial starter koji can be a single strain or a mixture of multiple strains. Multiple-strain koji starters are formulated by each manufacturer to achieve the best balance for the growth of each strain based on their experimental data. Some miso manufacturers endeavor to add more strains in the hope of incorporating different benefits. Often, however, blending strains with different growth rates would not produce the desired effects, and it could even reduce enzyme production efficiency. Experimental validation is recommended for any blend of koji starters before starting commercial application.
4.2.3. Effects of Additives
Powdered starter koji is used with additives to ensure uniform distribution, facilitate enzyme production, and enhance ingredients. Calcium carbonate is used for uniform distribution and improved protease production. The addition of sodium phosphate and sodium glutamate, sodium succinate and other compounds has been shown to increase protease production [17].
4.2.4. Conditions for Koji Making
The factors that can be artificially controlled during koji making are ambient temperature, humidity, ventilation rate, and culture length. The koji growth and enzyme production are also affected by these factors, especially by temperature, moisture, and culture length. The optimum temperature and humidity are similar for all koji strains and typically 35–38 °C and 95%, respectively. Nonetheless, each strain has its own unique growth rate and enzyme productivity, and the culture environment should be optimized to achieve the desired enzyme profiles.
Temperature Transition
In koji making for rice miso, the baseline temperature is 35 °C, and temperature is adjusted accordingly in the last half of the culture when enzymes are produced until the desired enzymatic activities are achieved. For example, the temperature is maintained at 2–5 °C above the baseline for sweet miso to increase amylase production. For barley miso, it is reduced by 2–5 °C. Temperature adjustment is necessary because the optimum temperature for synthesis is different for each enzyme. Different enzymatic activities are desired for different types of miso, and the amount of enzymes to be produced can be adjusted by the temperature during koji making. Note that the production of protease enzymes cannot be enhanced at a temperature above 40 °C.
Ambient Humidity
In koji making, the relative humidity around steamed rice is in equilibrium with water activity (Aw) of the moisture contained in steamed rice. Initially, it is 98% and then changes with the Aw of koji.
The Aw of koji decreases during koji making due to (1) water generated through the metabolism of koji mold and evaporation of water by metabolic heat, and (2) low molecular weight sugars generated by the breakdown of starch by amylases. At the end of the koji making, the lowest limit of Aw (0.90) is reached for koji mold growth. Optimal enzyme production is achieved at moisture levels that are slightly lower than those for optimal growth. Koji making starts at optimal Aw for growth, undergoes optimal Aw for enzyme production and ends at low Aw that limits the growth [18].
Duration of Koji Making
For each Koji enzyme, the time course of synthesis follows a different pattern. By changing the time to finish the koji culture, we can adjust the enzymatic contents and activities in the final koji. In commercial koji plants, however, it is often impossible to change the time as desired due to shikomi and other process schedules and employees’ working hours. The duration of koji making can be shortened by selecting strains with higher enzyme production efficiency. The enzymatic activities in starter koji for miso making can vary 2- to 4-fold depending on the strains. High productivity strains can reduce the time required for koji making. In addition, increasing the amount of starter koji can also shorten the length of the growth induction phase. A combination of these can be used to reduce the entire duration of koji making.
4.3. Enzymes in Koji
In brewery and fermented food production, the most important role of koji is to provide enzymes. In miso making, proteins and polysaccharides in soybeans, rice, and barley are hydrolyzed into amino acids and monosaccharides, respectively, which determine the taste, aroma, digestibility, physiological benefits, and other functional qualities of miso. Proteases, amylases, and lipases contained in koji are responsible for the breakdown of these components in miso ingredients. In addition to these enzymes that are already known, extracts of koji likely contain almost all types of enzymes. This is why koji is called a “gold mine of enzymes”.
The sequence analysis of A. oryzae genome was completed in 2005. It was cleared that A. oryzae genome contained 13,572 genes and 25% of these genes were identified as unknown genes. Discovery of new and novel enzymes from the A. oryzae genome is expected [19].
5. Koji Making (Seikiku) [20]
5.1. Rice Koji
5.1.1. Roles of Koji Making
Rice miso accounts for the majority of miso produced in Japan. In this section, we will mainly discuss rice koji, which is used in rice miso.
Koji making has four roles in miso making:
Growth and elaboration of fungal hyphae around and into the ingredients by solid-state culture;
production of amylases, (neutral) proteases and other enzymes important for miso fermentation and maturation (hypha extension into the ingredients (hazekomi) enhances this process);
growth of salt-tolerant yeast and lactic acid bacteria that are essential for maturation and production of precursors of aromatic components in miso (the growth of koji mold facilitates this process); and
elimination of ingredient odors.
In addition, the extension of koji hyphae creates numerous spaces inside the ingredients and facilitates the hydrolytic actions of enzymes. In addition to amylases and neutral proteases secreted from koji mold, other types of enzymes that are retained within the koji bodies also facilitate the maturation process. Similarly, intracellular nucleic acid degradation products also contribute to the taste of miso.
5.1.2. Growth Conditions of Koji Mold in Koji Making Process
Three to 5 h after attachment to the solid medium (i.e., steamed rice), starter koji spores germinate. The hyphae grow and start to secrete high molecular weight hydrolytic enzymes such as amylase and proteases from their ends. After germination, a lower koji temperature is maintained to suppress contamination with Bacillus subtilis and other unwanted bacteria. As hyphae grow and extend, respiratory heat generation, oxygen consumption, and carbon dioxide production increase markedly. To encourage healthy growth, it is necessary to increase the ventilation rate to provide adequate oxygen, lower the koji temperature, and remove carbon dioxide. As the culture continues, koji mold extends its hyphae into rice grains. This is called hazekomi. In thoroughly cooked rice, koji hyphae can grow deeper into rice grains by hydrolyzing starches. In undercooked rice, in which gelatinization is incomplete, the tip of the hypha cannot dig forward and extends only on the surface of the grain. This results in the insufficient production of enzymes and failure to achieve the enzyme activities needed for miso maturation.
5.1.3. Optimal Koji Making Conditions for Enzyme Production
The most critical enzymes in miso making are amylases, which hydrolyze starches in miso ingredients, and proteases, which break down proteins also in miso ingredients. For sweeter miso, koji is used in higher proportions. The ability to increase the production of glucose and oligosaccharides from starches is required to enhance sweetness. For this reason, koji is cultured at a relatively higher temperature of 35–38 °C to ensure higher amylase production. For salty miso, it is important to have higher protease activities to facilitate hydrolysis of proteins into amino acids and peptides, which constitute umami in this type of miso. For this purpose, the temperature is maintained at 30 °C or below during koji making.
5.1.4. Koji Making Methods
Koji making is largely divided into manual and mechanical methods. The tray koji method is a traditional manual method passed down from generation to generation. In this method, koji is cultured in a culture room using wooden trays called futa and wooden beds called toko for insulation. Because the processing volume is limited, it is not suitable for large-scale miso making. However, this method is still used by manufacturers who advertise hand-crafted miso making and for experimental shikomi. This method consists of loading (hikikomi), kneading (kirikaeshi), piling (morikomi), mixing (teire), rearranging (tsumikae), and finishing (de-koji).
Loading (hikikomi): Steamed rice and other ingredients, if used, are cooled down, inoculated with starter koji (tanetsuke) and laid out on a toko bed in a culture room (hikikomi). The bed is then covered with cloth to maintain temperature and moisture. The temperature of steamed rice is controlled between 27 and 30 °C.
Kneading (kirikaeshi): Spores grow on steamed rice and start to generate heat around 10 h after inoculation, leading to rapid heat generation around the 16th hour. To prevent excessive temperature rise, koji is mixed and kneaded by hand. This process is called kirikaeshi. It also prevents rice grains from sticking to each other as the mold hyphae continue to grow.
Piling (morikomi): After kneading, the batch is transferred to futa trays. A thin wooden plate that is slightly raised at its center is laid in each futa tray to help heat dissipation.
Mixing (teire): After being transferred to futa trays, koji mold continues to grow. Koji is mixed manually before the temperature becomes too high. This teire process is usually done twice and is aimed at controlling the temperature and providing oxygen while reducing carbon dioxide concentration.
Rearranging (tsumikae): The temperature is not even across the culture room. Futa trays are rearranged to even out the temperature history for each tray.
Finishing (de-koji): About 40 h after loading, koji is taken out of the culture room. The optimal timing of unloading is different for each strain of koji mold.
Mechanical/automatic koji making.
Mechanical or automatic koji making has become popular in recent years to replace labor-intensive manual methods. Various systems are commercially available from different fermentation and brewing machinery manufacturers. These systems are designed to deliver temperature- and humidity-controlled fresh air around and into koji to prevent excessive heat accumulation inside the koji and to exchange oxygen and carbon dioxide. Depending on the ventilation method used, koji making machines are divided into a surface ventilation system and interior ventilation system. The interior ventilation system is further divided into fixed bed, shelf bed, rotating drum, and rotating disc systems depending on how koji ingredients are loaded. The apparatus is ventilated before the koji temperature reaches 40 °C due to respiratory heat. Humidification of the air may be stopped just before unloading the koji.
5.1.5. Quality of Koji
The quality criteria desired for koji at the time of finishing are as follows:
Contains enzymatic activities required for the type of miso to be manufactured;
Sufficient depth of hazekomi with minimal coloration and brilliant color;
Aromatic without foul odor from bacterial contamination;
Fluffy and soft texture;
Minimal sporulation and coloration with high amylase activities for white or yellow miso through shorter culture time; and
High protease activities through slightly longer culture for red miso.
5.2. Barley Koji
Barley koji tends to generate more respiratory heat than rice koji, because barley is richer in protein, inorganic salts, and vitamins. The surface moisture of steamed barley evaporates easily, making barley grains too dry for the optimal growth of koji mold. Therefore, in the early phase of koji making it is essential to maintain the humidity close to the saturation point and deliver highly humid air at a temperature as close as possible to the koji temperature. At the same time, caution needs to be taken because sticky barley with too much moisture facilitates the growth of unwanted bacteria. For light-colored barley miso containing a high proportion of koji, the koji temperature is controlled to reach 36–38 °C at 10 to 18 h after loading to obtain higher amylase activities. It is then decreased to 30–32 °C. For red barley miso, on the other hand, high temperature must be avoided to enhance both protease and amylase activities. It is usually controlled at a slightly low temperature around 29–31 °C. Occasionally, finished koji is mixed with salt to prevent continued heat generation during storage. Finished koji is mixed with salt (about 1/3 of salt used in the initial ingredients) to suppress respiratory heat generation. Salted koji must be used within 2 days, because enzymatic activities will decrease.
5.3. Soybean Koji
To make rice and barley miso, rice and barley are steamed, inoculated with starter koji, and mixed with salt and soybeans. To make soybean miso, soybeans are steamed, cooled down to 40 °C, and made into miso balls using a specialized machine called tamanigiri-ki and then used for koji making. Different sizes and shapes of miso balls are used at each manufacturer. The reason for this extra step is to facilitate the growth of facultative anaerobic lactic acid bacteria inside the balls to lower pH and prevent the growth of B. subtilis, which has a negative effect on the growth of koji mold.
Although some manufacturers prefer 45 mm or larger miso balls, most common miso balls are 19–24 mm in diameter. Larger miso balls have more space to grow facultative anaerobic lactic acid bacteria, resulting in significantly higher amounts of lactic acid, which tend to delay the maturation of miso. To prevent miso balls sticking to each other, miso balls are usually coated with roasted and powdered barley called kosen or hattaiko for better aeration. During koji making, a lower temperature is maintained in the early phase to suppress the growth of B. subtilis and then increased to dissipate the moisture from miso balls to induce the growth of koji mold into the balls.
6. Koji Enzymes Involved in Miso Making
The degradation of starches and proteins in soybeans, rice, barley, and naked barley is the key process in miso making. This is where koji enzymes work. A. oryzae is the main koji mold used in miso making [21]. A. oryzae produces amylases (e.g., Taka-amylase and glucoamylase) and proteinases in large quantities [22]. To make miso, koji, salt water, and steam-boiled grains are mixed. The high salt content and anaerobic conditions prohibit the growth of koji mold, and only the enzymes produced during koji making can act on the ingredients.
Starches in the miso ingredients are broken down into glucose by Taka-amylase and glucoamylase. These glucose molecules allow the growth of salt-tolerant yeasts Z. rouxii and Candida versatilis, and alcohols produced by these yeasts enhance the flavor of miso [23]. On the other hand, proteins are made up of 20 types of amino acids, which have side chains with different properties and size. No single protease can hydrolyze all of the peptide bonds formed between these different types of amino acids. Thus, more diverse types of proteases are involved in miso making compared to amylases, and genomic analysis has identified about 130 different proteases in A. oryzae [24]. Because protein breakdown is especially important in miso making, this section focuses primarily on proteinases in koji mold.
Because the pH remains around 5 to 6 during miso maturation, proteases that work in a weakly acidic environment are the key. Acid proteases likely play a major part, while neutral and alkaline proteases may also be involved [25]. For exopeptidases, acid carboxypeptidase and a trace amount of leucine aminopeptidase seem to have an important role.
6.1. Acidic Endopeptidases
Genomic analysis of koji mold identified two acidic proteases, aspartic endopeptidase and glutamic peptidase [24]. As a mammalian protease belonging to the pepsin family, aspartic endopeptidase has two aspartate residues in its active site and is optimally active at around pH 3. Koji mold has 11 aspartic endopeptidase genes, and sequence homology analysis of encoded amino acids suggests that five are extracellular, three are vacuolar, and three are glycosylphosphatidylinositol-anchored. In the making of clear rice wine and shochu (Japanese distilled spirits), PepO (PepA), the major aspartic endopeptidase in koji mold, has been shown to break down rice proteins to release amino acids and peptides [26,27,28]. PepO appears to preferentially hydrolyze peptide bonds where hydrophobic and aromatic amino acid repeats are present. It is thought that as the acidity and alcohol concentration increase, substrate proteins are denatured, and hydrophobic side chains become exposed and accessible to the enzyme [29].
In a recent animal study, A. oryzae-derived PepO has shown a promising role as a prebiotic, and dietary supplementation of a very small amount of PepO significantly increased beneficial Bifidobacterium [30]. It has been reported that miso retains acid protease activities for a long period [31,32] indicating that the consumption of miso may have a similar prebiotic effect.
Aorsin is a member of the sedolisin family and has an optimum pH of around 4 [33]. Koji mold carries two aorsin genes, and enzymological properties have been reported for aorsin A. Aorsin A primarily cleaves the peptide bond C-terminal to the arginine residue. The second aorsin (aorsin B) is specific to aspartic and phenylalanine residues [29].
6.2. Neutral Endopeptidases
A. oryzae contains genes that encode neutral endopeptidases, fungalysin, and deuterolysin. Fungalysin is a thermolysin-type metalloendopeptidase with Zn2+ in the active center shared by filamentous fungi [34]. Koji mold has two enzymes, neutral protease I (NpI), and neutral protease III (NpIII). Whereas NpIII is yet to be characterized, NpI is most active at around pH 7 and specifically cleaves a peptide bond at the C terminus of hydrophobic and bulky amino acid repeats [29].
Deuterolysin is also a metalloendopeptidase with Zn2+ in its active center, but Zn2+ is positioned at a different amino acid [35]. Its molecular weight is about 19 kDa, much smaller than fungalysin (~45 kDa). The optimum pH is 7 to 8. Two deuterolysins, DeuA and DeuB, are found in koji mold [36]. DeuA, also called NpII, is highly thermostable and still active after 10 min of heat treatment at 100 °C [37]. In contrast, DeuB is not heat-tolerant and almost completely inactivated after 10 min at 80 °C. Because DeuA is mainly produced in solid-state culture, DeuA is considered the major deuterolysin in miso making. These enzymes efficiently break down basic proteins such as salmine, clupeine, and histone, but their efficiency is very low for casein and hemoglobin. This is why these enzymes are often overlooked in casein-based enzyme activity studies.
6.3. Alkaline Endopeptidases
Oryzin is an extracellular alkaline protease produced by koji mold [38,39]. It is optimally active at a pH of 10 to 10.5 and hydrolyzes a peptide bond at the C terminus of hydrophobic and aromatic amino acids with bulky side chains, such as leucine, tyrosine and phenylalanine.
6.4. Exopeptidases
During miso maturation, proteins in miso ingredients are broken down by the endopeptidases discussed above, and the resulting peptides are further broken down into amino acids and oligopeptides by exopeptidases.
The most important exopeptidases in miso making are said to be serine-type carboxypeptidases. These enzymes cleave the C-terminal amino acid one by one, and the released free amino acids are thought to play an important role in umami and flavor formation in miso. Ten extracellular secreted enzymes belonging to the MEROPS (the peptidase database; https://www.ebi.ac.uk/merops/, accessed on 7 May 2021) S10 family have been identified in koji mold [40]. Of these, carboxypeptidase I from A. oryzae strain TK3 [41], OcpO specifically found in a liquid culture of A. oryzae strain IAM2640 [42], carboxypeptidases O1 and O2 found from solid-state cultures [43], and their orthologs in the strain RIB40, CpI, OcpO, OcpA, and OcpB [44,45], have been enzymologically characterized. The optimum pH is below 4 for all of these enzymes. Their specificity is somewhat different, and the optimum substrates are Z-Tyr-Leu and Z-Phe-Leu for OcpO, Z-Phe-Leu for OcpA and Z-Phe-Leu and Z-Leu-Tyr for OcpB.
Aminopeptidases also likely have a role in releasing amino acids from peptides. Aminopeptidases cleave the N-terminal amino acid one by one at neutral pH. It is estimated that 23 different aminopeptidases are present in koji mold, and four of them are secreted into the extracellular space. All of these enzymes belong to the metallopeptidase family. The two leucine aminopeptidases, LapA and LapB, share 56% identity in their amino acid sequences [46,47]. LapA has an optimum pH of around 8.5 and cleaves hydrophobic and basic amino acids such as leucine, phenylalanine, methionine, lysine, and arginine but not acidic amino acids such as aspartic acid. LapB has a wider substrate specificity with strong affinity for leucine, lysine, alanine, and glutamic acid and also cleaves valine, proline, and isoleucine. Its optimum pH is alkaline, between 9.5 and 10. The other two enzymes have been partially isolated and likely have a similar substrate specificity to LapA according to Kusumoto et al. [29].
In addition to aminopeptidases, koji mold also contains dipeptidyl peptidases and tripeptidyl peptidases that catalyze the cleavage of the terminal peptide bond. Although both peptidases have serine in the active site, dipeptidyl peptidases are serine endopeptidases, while tripeptidyl peptidases belong to the sedolisin family.
Dipeptidyl peptidases release a dipeptide from the N terminus one by one, and three extracellular enzymes, DppB, DppE, and DppF, are found from A. oryzae [48]. The optimum pH is around 7 for all of these enzymes. DppB acts on substrates in which the second N-terminal amino acid is proline, and it is a homolog of the mammalian dipeptidyl peptidase IV first discovered by Tachi et al. [49]. Compared to DppIV previously found in A. fumigatus, DppB shows a stricter substrate specificity and unlikely acts on substrates if the second N-terminal amino acid is not proline. It works more preferentially when the N-terminal amino acid is arginine, alanine, and glycine in this order, indicating that the size and polarity of the side chain may influence the specificity. DppE and DppF, which correspond to DppV of A. fumigatus, work more actively when the second N-terminal amino acid is alanine or phenylalanine. These two enzymes may work synergistically, as their specificities are different.
6.5. Pro-Xaa Peptidases
Recently, four peptidases (AoS28A, AoS28B, AoS28C, and AoS28D) belonging to the MEROPS S28 family have been newly identified from A. oryzae [50]. This family contains human lysosomal Pro-Xaa endopeptidase. AoS28A and AoS28B hydrolyze a peptide bond at the C terminus that contains proline and have an optimum pH of 4 and 4.5, respectively. Their specificities are different and determined by amino acids flanking the proline residue, indicating that they work synergistically. On the other hand, AoS28D is a Pro-Xaa carboxypeptidase that cleaves the C-terminal amino acid when the second C-terminal amino acid is proline [51]. The serine-type carboxypeptidases discussed above do not have these catalytic activities, but AoS28D can release Xaa from -Pro-Xaa at pH 3.8 and 7.0 and also Xaa-amide from -Pro-Xaa-amide. It can release C-terminal amino acids including phenylalanine, isoleucine, threonine, glutamine, glutamic acid, arginine, and lysine but cannot release proline from -Pro-Pro. Thus, AoS28 enzymes are capable of hydrolysis of the proline-containing C-terminal peptides, which are usually difficult to hydrolyze. It is likely that AoS28A and AoS28B evolved as endopeptidases, while AoS28D evolved as an exopeptidase.
6.6. Glutaminase
Glutamic acid is the umami component in soy sauce and miso. Glutamic acid is one of the amino acids released by protein hydrolysis [52]. It has been shown that Koji mold not only releases glutamic acid from proteins, but also converts glutamine to glutamic acid enhancing umami during soy sauce making. Glutaminase is the enzyme responsible for the conversion of glutamine to glutamic acid. It catalyzes the hydrolysis of glutamine into glutamic acid and ammonia. Twelve glutaminase genes are present in A. oryzae [53]. Because these include orthologs of GahA and GahB found in A. sojae, which convert glutamine to glutamic acid at the C terminus, A. oryzae likely increases glutamic acid through a similar mechanism [54].
7. Z. rouxii and T. halophilus in Miso Making
The main microbes working during the fermentation and maturation process of miso are yeast and lactic acid bacteria, which are usually added at the time of shikomi. Z. rouxii plays a main part, while C. versatilis and C. etchellsii are also working in late maturation. Z. rouxii is a heterothallic, spore-forming yeast. C. versatilis and C. etchellsii are non-spore-forming yeasts that grow in the late maturation phase and produce aromatic components. In general, miso yeast is the term referring to Z. rouxii.
Under aerobic conditions, Z. rouxii breaks down glucose into carbon dioxide gas and water and grows rapidly using glucose as energy. Under anaerobic conditions, it shifts to the fermentation of glucose and produces alcohol and carbon dioxide gas. Glycerol, succinic acid and acetaldehyde are also produced as by-products. Glycerol and succinic acid add depth to miso taste, while acetaldehyde masks the ingredient odor. Alcohol reacts with organic acids to form various esters. Z. rouxii also produces higher alcohols such as isoamyl alcohol by decarboxylation of amino acids.
Z. rouxii is tolerant to up to 4M of salt, and as the salt concentration increases, the optimum growth pH narrows down to 5.0.
The main lactic acid bacterium used in miso making is T. halophilus, which is a Gram-positive, non-spore-forming, homofermentative tetrad cocci. It was once called Pediococcus halophilus as proposed by R. H. Mees in 1934 until its scientific name was changed to Tetragenococcus halophilus in the 1990s [55]. It is a halophilic bacterium that grows most efficiently at 5–10% salt and can survive with a higher salt concentration up to 24%. The optimum growth temperature is 25–30 °C, and the optimum growth pH is between 5.5 and 9.0. It is sensitive to acidic conditions. T. halophilus is characterized by wide-ranging properties. For example, the amount of lactic acid production is influenced by sugar and oxygen levels [56], and different strains show different sugar fermentation efficiency and amino acid hydrolysis [57,58].
8. Changes in Miso Components during Fermentation and Maturation
8.1. Macronutrients
Macronutrient compositions of finished commercial miso products are shown in Table 1. The water content may vary from 40 to 50%, but it is generally between 44 and 46%. The salt content varies widely from 5 to 13%, but typically it is in the 11 to 13% range. The water and salt are the important factors that change the components in miso and play various roles such as inhibition of unwanted microbial growth, promotion of growth and metabolism of fermentation microbes and control of enzymatic actions.
Table 1.
Concentration of nutrients in miso (per 100 g) [59].
| Nutrients | Rice Miso, Sweet |
Rice Miso, Light Yellow | Rice Miso, Red |
Barley Miso | Soybean Miso |
|---|---|---|---|---|---|
| Water (g) | 42.6 | 45.4 | 45.7 | 44.0 | 44.9 |
| Protein (g) | 9.7 | 12.5 | 13.1 | 9.7 | 17.2 |
| Lipid (g) | 3.0 | 6.0 | 5.5 | 4.3 | 10.5 |
| Carbohydrate (g) | 37.9 | 21.9 | 21.1 | 30.0 | 14.5 |
| Dietary fiber, total (g) | 5.6 | 4.9 | 4.1 | 6.3 | 6.5 |
| Salt equivalents (g) | 6.1 | 12.4 | 13.0 | 10.7 | 10.9 |
The pH of miso is around 6 at the start of fermentation/maturation but decreases to around 5 during maturation. This is the reason pH is used as a process parameter that indicates the degree of maturation. The Aw also decreases during maturation, and it is usually 0.80 ± 0.05 in matured miso.
The protein content of rice miso is generally between 12 and 13%. Soon after the ingredients are mixed, proteins are degraded rapidly, releasing a large quantity of free amino acids during early maturation. Matured miso is rich in free glutamic acid, arginine, lysine, and leucine [60].
Carbohydrates in miso are mostly starches from rice and also include polysaccharides such as arabinogalactan from soybeans.
Lipids in miso are mainly derived from soybeans and are typically around 6% in rice miso. During maturation, lipase from koji mold hydrolyzes some lipids into fatty acids and glycerol. A part of the fatty acids reacts with ethanol, which is generated by yeast fermentation, to form ethyl esters, the important constituents of miso aroma.
8.2. Organic Acids
Lactic acid, acetic acid, citric acid, and succinic acid make up a large part of the organic acids in miso. Lactic acid and acetic acid are produced by lactic acid bacteria, while a small quantity of succinic acid is generated by lactic acid bacteria and yeast. Citric acid is derived from soybeans and remains in miso. Lactic acid bacteria utilize citric acid to generate more lactic acid and acetic acid. This is how lactic acid, acetic acid and succinic acid continue to increase during the maturation of rice miso.
8.3. Color and Aromatic Compounds
The Maillard reaction (amino-carbonyl reaction) plays a large part in component changes during miso maturation. First described by L. C. Maillard in 1912, the Maillard reaction is a non-enzymatic, browning reaction between amino compounds and carbonyl compounds resulting in the formation of high-molecular weight polymers, melanoidins. The Maillard reaction not only adds color, but also generates a range of aromatic compounds.
The characteristic rich and mellow aroma of miso is known for its susceptibility to change during fermentation/maturation and heat treatment. Over 200 aromatic compounds have been identified from rice miso [61]. 4-Hydroxy-2 (or 5)-ethyl-5 (or 2)-methyl-3 (2H)-furanone (HEMF) that produces a sweet caramel-like aroma with a very narrow threshold value has been extensively studied. HEMF is associated with the sensory analysis results of rice miso, and it is identified as one of the critical compounds responsible for the appealing aroma of miso [62]. In rice miso, HEMF is not detected immediately after the mixing of miso ingredients but starts to increase with maturation and then decreases during the late maturation phase [63]. 4-Ethylguaiacol is an important aroma component that allows us to distinguish miso types containing different koji, and HEMF and methionol are important aromatic components produced by yeast. The combination and concentrations of these three components seem to determine the aromatic property of miso [64]. More recently, Kumazawa et al. [65] identified 16 new aromatic components in rice miso and found that trace amounts of low-threshold components other than HEMF also contribute to miso aroma. Further understanding of aroma components in miso awaits future research.
9. Nutritional Function of Miso
The health benefits of miso have been passed down orally through hundreds of years by way of proverbs and folklore. Due to its high salt content, however, specific health benefits of miso have been discussed with caution in modern medicine and public health. In fact, restricted miso consumption is sometimes recommended in Japan to reduce salt intake. While miso has recently become a popular research topic as a functional food [66], studies are mostly conducted in cell cultures and laboratory animals and rarely involve human participants. Two published studies of rice miso, however, found that long-term standard consumption of miso soup did not affect blood pressure in human subjects [67,68]. While growing evidence suggests no association between miso soup consumption and high blood pressure [69], multipronged studies are necessary to understand the exact mechanisms.
Cohort studies are another approach to examine the benefit of miso as a part of regular Japanese diet. In a recent cohort study [70], Katagiri et al. followed 92,915 men and women aged between 45 and 74 years, who responded to dietary habit surveys conducted between 1995 and 1998, to prospectively evaluated the relationship between the risk for mortality and consumption of soybean products and fermented soybean products up to 2012. The study showed that while no association was found between the risk of death and consumption of all types of soybean products combined, the risk of death decreased with increasing consumption of fermented soybean products in both men and women.
Dietary sodium intake is much higher in the Japanese population than in Western countries, and the majority of it comes from seasoning. However, a study by Okada et al. [71] has shown that the portion size of soy sauce or miso is not associated with hypertension. These results were based on 25,738 Japanese men and women aged 20 years or older who participated in the national health and nutrition surveys between 2012 and 2016. The study explains that people who consumed soy sauce or miso in larger portion sizes more likely consumed larger quantities of vegetables, fruits, and fish, resulting in a higher intake of potassium, which has been shown to decrease blood pressure.
One of the nutritional advantages of miso is that soy proteins and starches are predigested and easily absorbed as amino acids and saccharides. In particular, because miso is eaten as a soup or as aemono (chopped fish, shellfish, or vegetables, dressed with sauce such as miso or vinegar), it is an excellent companion to a balanced diet consisting of vegetables, seafood and meat. The health benefits of this type of diet are beyond calculation.
10. Conclusions
Globally, soybeans are the dominant oilseed crop, while in Japan they are considered as an important food source. Especially, miso is an essential part of the Japanese diet as it contains a wide variety of nutritious fermentation products derived from soybeans and grains as a result of the activities of koji enzymes and beneficial microbes. It is truly a gift from the Japanese tradition.
We highly anticipate that the increasing popularity of miso in the world will play a role in facilitating further globalization of Japanese culinary culture.
Author Contributions
Conceptualization, K.I. and M.K.; investigation, resources, data curation and writing the original manuscript, K.-I.K., Y.Y., R.T., M.K., T.K., K.I. and Y.K.; supervision, Y.K.; project administration, K.I. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Yamamoto Y., Tanaka H. An Introductory Guide to Miso and Shoyu. 5th ed. The Japan Food Journal Co., Ltd.; Tokyo, Japan: 2013. pp. 3–6. (In Japanese) [Google Scholar]
- 2.Kusumoto K., Rai A.K. Miso, the traditional fermented soybean paste of Japan. In: Ray R.C., Montet D., editors. Fermented Foods, Part II: Technological Interventions. CRC Press; Boca Raton, FL, USA: 2017. pp. 122–134. [Google Scholar]
- 3.Shurtleff W., Aoyagi A. The Book of Miso. 2nd ed. Soyinfo Center; Lafayette, CA, USA: 2018. pp. 30–44. [Google Scholar]
- 4.Food Labeling Standards of Japan [in Japanese] [(accessed on 7 May 2021)]; Available online: https://www.caa.go.jp/policies/policy/food_labeling/food_labeling_act/pdf/food_labeling_cms101_201009_4.pdf.
- 5.Announcement by the Japanese Ministry of Agriculture, Forestry and Fisheries. Washoku, the Traditional Dietary Culture of the Japanese, Has Been Registered on UNESCO’s Intangible Cultural Heritage List [in Japanese] [(accessed on 24 June 2021)]; Available online: https://www.maff.go.jp/j/keikaku/syokubunka/ich/
- 6.Miso/Soy Sauce. JETRO. [(accessed on 7 July 2021)];2020 Available online: https://www.jetro.go.jp/en/trends/foods/ingredients/misoshoyu.html.
- 7.Kitagawa M. Ever-changing Miso: Past History and Future Prospects of Miso. J. Brew. Soc. Jpn. 2021;116:211–219. (In Japanese) [Google Scholar]
- 8.Zenkoku Miso Gijutsukai . New Handbook of Miso Technology. Zenkoku Miso Gijutsukai; Tokyo, Japan: 2006. pp. 21–85. (In Japanese) [Google Scholar]
- 9.Ohyama T., Takahashi Y., Joh T., Whitaker A.C., Nishiwaki T., Morobashi K., Watanabe S., Shimojo S. Traditional and Modern Japanese Soy Foods. Nova Science Publishers, Inc.; New York, NY, USA: 2013. pp. 91–104. [Google Scholar]
- 10.Narahara H. Koji mold and koji (part 2): Handling of koji mold in koji making [in Japanese] J. Brew. Soc. Jpn. 1994;89:954–964. doi: 10.6013/jbrewsocjapan1988.89.954. [DOI] [Google Scholar]
- 11.Narahara H. Starter koji and koji in miso (4) Miso Sci. Tech. 1999;47:13–21. (In Japanese) [Google Scholar]
- 12.Tanaka K., Kushiro M., Manabe M. A review of studies and measures to improve the mycotoxicological safety of traditional Japanese mold-fermented foods. Mycotoxin Res. 2006;22:153–158. doi: 10.1007/BF02959268. [DOI] [PubMed] [Google Scholar]
- 13.Kusumoto K., Nogata Y., Ohta A. Directed deletions in the aflatoxin biosynthesis gene homolog cluster of Aspergillus oryzae. Curr. Genet. 2000;37:104–111. doi: 10.1007/s002940050016. [DOI] [PubMed] [Google Scholar]
- 14.Tominaga M., Lee Y.-H., Hayashi R., Suzuki Y., Yamada O., Sakamoto K., Gotoh K., Akita O. Molecular analysis of an inactive aflatoxin biosynthesis gene cluster in Aspergillus oryzae RIB strains. Appl. Environ. Microbiol. 2006;72:484–490. doi: 10.1128/AEM.72.1.484-490.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Akao T., Sano M., Yamada O., Akeno T., Fujii K., Goto K., Ohashi-Kunihiro. S., Takase K., Yasukawa-Watanabe M., Yamaguchi K., et al. Analysis of expressed sequence tags from the fungus Aspergillus oryzae cultured under different conditions. DNA Res. 2007;14:47–57. doi: 10.1093/dnares/dsm008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Narahara H., Iwata M. Studies on the koji making process for rice koji: (1) factors affecting the growth of koji mold on steamed rice. Miso Sci. Tech. 1983;31:127–133. (In Japanese) [Google Scholar]
- 17.Shiota H., Sakurai Y. Protease production in rice koji (part 2) J. Brew. Soc. Jpn. 1963;58:392. doi: 10.6013/jbrewsocjapan1915.58.392. (In Japanese) [DOI] [Google Scholar]
- 18.Narahara H. Water activity and growth in koji. J. Brew. Soc. Jpn. 1988;83:729–733. doi: 10.6013/jbrewsocjapan1988.83.729. (In Japanese) [DOI] [Google Scholar]
- 19.Abe K., Gomi K., Hasegawa F., Machida M. Impact of Aspergillus oryzae genomics on industrial production of metabolites. Mycopathologia. 2006;162:143–153. doi: 10.1007/s11046-006-0049-2. [DOI] [PubMed] [Google Scholar]
- 20.Yoshii H. Koji making. In: Nojiro K., Kozaki M., Yoshii H., Koizumi T., editors. Kaitei Jozogaku. Kodansha Scientific; Tokyo, Japan: 1997. pp. 165–170. (In Japanese) [Google Scholar]
- 21.Murakami H. Aspergillus oryzae group (V) J. Brew. Soc. Jpn. 1971;66:1042–1045. doi: 10.6013/jbrewsocjapan1915.66.1042. (In Japanese) [DOI] [Google Scholar]
- 22.Machida M. Progress of Aspergillus oryzae genomics. Adv. Appl. Microbiol. 2002;51:81–106. doi: 10.1016/s0065-2164(02)51002-9. [DOI] [PubMed] [Google Scholar]
- 23.Sugawara E., Hashimoto S., Sakurai Y., Kobayashi A. Formation by yeast of the HEMF (4-hydroxy-2(or 5)-ethyl-5(or 2)-methyl-3(2H)-furanone) aroma component in miso with aging. Biosci. Biotechnol. Biochem. 1994;58:1134–1135. doi: 10.1271/bbb.58.1134. [DOI] [Google Scholar]
- 24.Machida M., Asai K., Sano M., Tanaka T., Kumagai T., Terai G., Kusumoto K., Arima T., Akita O., Kashiwagi Y. Genome sequencing and analysis of Aspergillus oryzae. Nature. 2005;438:1157–1161. doi: 10.1038/nature04300. [DOI] [PubMed] [Google Scholar]
- 25.Matsumoto I., Imai S. Enzymatic activities of rice koji and the quality of miso. J. Brew. Soc. Jpn. 1977;72:565–569. (In Japanese) [Google Scholar]
- 26.Gomi K., Arikawa K., Kamiya N., Kitamoto K., Kumagai C. Cloning and nucleotide sequence of the acid protease-encoding gene (pepA) from Aspergillus oryzae. Biosci. Biotechnol. Biochem. 1993;57:1095–1100. doi: 10.1271/bbb.57.1095. [DOI] [PubMed] [Google Scholar]
- 27.Takeuchi M., Ogura K., Hamamoto T., Kobayashi Y. Molecular cloning and sequence analysis of a gene encoding an aspartic proteinase from Aspergillus oryzae. Adv. Exp. Med. Biol. 1995;362:577–580. doi: 10.1007/978-1-4615-1871-6_77. [DOI] [PubMed] [Google Scholar]
- 28.Nunokawa Y. Fermentation of Seishu Moromi from an enzymological point of view. J. Brew. Soc. Jpn. 1981;76:6–11. doi: 10.6013/jbrewsocjapan1915.76.6. (In Japanese) [DOI] [Google Scholar]
- 29.Yamagata Y. Proteolytic enzymes of A. oryzae. Kagaku Seibutsu. 2016;54:109–116. doi: 10.1271/kagakutoseibutsu.54.109. (In Japanese) [DOI] [Google Scholar]
- 30.Yang Y., Sitanggang N.V., Kato N., Inoue J., Murakami T., Watanabe T., Iguchi T., Okazaki Y. Beneficial effects of protease preparations derived from Aspergillus on the colonic luminal environment in rats consuming a high-fat diet. Biomed. Rep. 2015;3:715–720. doi: 10.3892/br.2015.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ushiyama E., Ukaji Y. Amylases and proteases in soy sauce taste (6th report); characterization of alkaline, neutral, acid protease fractions. Chomi Kagaku. 1959;7:1–7. (In Japanese) [Google Scholar]
- 32.Harayama F., Yasuhira H. Comparison of hydrolytic action on soybean protein by the genus Aspergillus and Rhizopus. J. Brew. Soc. Jpn. 1988;83:828–833. doi: 10.6013/jbrewsocjapan1988.83.828. [DOI] [Google Scholar]
- 33.Lee B.R., Furukawa M., Yamashita K., Kanasugi Y., Kawabata C., Hirano K., Ando K., Ichishima E. Aorsin, a novel serine proteinase with trypsin-like specificity at acidic pH. Biochem. J. 2003;371:541–548. doi: 10.1042/bj20021691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nakadai T., Nasuno S., Iguchi N. Purification and properties of neutral proteinase I from Aspergillus oryzae. Agric. Biol. Chem. 1973;37:2695–2701. doi: 10.1271/bbb1961.37.2695. [DOI] [Google Scholar]
- 35.Fushimi N., Ee C.E., Nakajima T., Ichishima E. Aspzincin, a family of metalloendopeptidases with a new zinc-binding motif. Identification of new zinc-binding sites (His(128), His(132), and Asp(164)) and three catalytically crucial residues (Glu(129), Asp(143), and Tyr(106)) of deuterolysin from Aspergillus oryzae by site-directed mutagenesis. J. Biol. Chem. 1999;274:24195–24201. doi: 10.1074/jbc.274.34.24195. [DOI] [PubMed] [Google Scholar]
- 36.Maeda H., Katase T., Sakai D., Takeuchi M., Kusumoto K., Amano H., Ishida H., Abe K., Yamagata Y. A novel non-thermostable deuterolysin from Aspergillus oryzae. Biosci. Biotechnol. Biochem. 2006;80:1813–1819. doi: 10.1080/09168451.2016.1166933. [DOI] [PubMed] [Google Scholar]
- 37.Nakadai T., Nasuno S., Iguchi N. Purification and properties of neutral proteinase II from Aspergillus oryzae. Agric. Biol. Chem. 1973;37:2703–2708. doi: 10.1271/bbb1961.37.2703. [DOI] [Google Scholar]
- 38.Morihara K., Tsuzuki H. Comparison of the specificities of various serine proteinases from microorganisms. Arch. Biochem. Biophys. 1969;129:620–634. doi: 10.1016/0003-9861(69)90223-9. [DOI] [PubMed] [Google Scholar]
- 39.Nakadai T., Nasuno S., Iguchi N. Purification and properties of alkaline proteinase from Aspergillus oryzae. Agric. Biol. Chem. 1973;37:2685–2694. doi: 10.1080/00021369.1973.10861071. [DOI] [Google Scholar]
- 40.MEROPS the Peptidase Database. [(accessed on 7 May 2021)]; Available online: https://www.ebi.ac.uk/merops/
- 41.Blinkovsky A.M., Byun T., Brown K.M., Golightly E.J. Purification, characterization, and heterologous expression in Fusarium venenatum of a novel serine carboxypeptidase from Aspergillus oryzae. Appl. Environ. Microbiol. 1999;65:3298–3303. doi: 10.1128/AEM.65.8.3298-3303.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takeuchi M., Ichishima E. A 155K acid carboxypeptidase O from Aspergillus oryzae. Agric. Biol. Chem. 1986;50:633–638. doi: 10.1271/bbb1961.50.633. [DOI] [Google Scholar]
- 43.Takeuchi M., Ushijima T., Ichishima E. A new acid carboxypeptidase, O-1, from Aspergillus oryzae. Curr. Microbiol. 1982;7:19–23. doi: 10.1007/BF01570974. [DOI] [Google Scholar]
- 44.Morita H., Okamoto A., Yamagata Y., Kusumoto K., Koide Y., Ishida H., Takeuchi M. Heterologous expression and characterization of CpI, OcpA, and novel serine-type carboxypeptidase OcpB from Aspergillus oryzae. Appl. Microbiol. Biotechnol. 2009;85:335–346. doi: 10.1007/s00253-009-2087-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morita H., Kuriyama K., Akiyama N., Okamoto A., Yamagata Y., Kusumoto K., Koide Y., Ishida H., Takeuchi M. Molecular cloning of ocpO encoding carboxypeptidase O of Aspergillus oryzae IAM2640. Biosci. Biotechnol. Biochem. 2010;74:1000–1006. doi: 10.1271/bbb.90863. [DOI] [PubMed] [Google Scholar]
- 46.Matsushita-Morita M., Tada S., Suzuki S., Hattori R., Marui J., Furukawa I., Yamagata Y., Amano H., Ishida H., Takeuchi M., et al. Overexpression and characterization of an extracellular leucine aminopeptidase from Aspergillus oryzae. Curr. Microbiol. 2011;62:557–564. doi: 10.1007/s00284-010-9744-9. [DOI] [PubMed] [Google Scholar]
- 47.Blinkovsky A.M., Byun T., Brown K.M., Golightly E.J., Klotz A.V. A non-specific aminopeptidase from Aspergillus. Biochim. Biophys. Acta. 2000;1480:171–181. doi: 10.1016/S0167-4838(00)00064-9. [DOI] [PubMed] [Google Scholar]
- 48.Maeda H., Sakai D., Kobayashi T., Morita H., Okamoto A., Takeuchi M., Kusumoto K., Amano H., Ishida H., Yamagata Y. Three extracellular dipeptidyl peptidases found in Aspergillus oryzae show varying substrate specificities. Appl. Microbiol. Biotechnol. 2016;100:4947–4958. doi: 10.1007/s00253-016-7339-5. [DOI] [PubMed] [Google Scholar]
- 49.Tachi H., Ito H., Ichishima E. An X-prolyl dipeptidyl-aminopeptidase from Aspergillus oryzae. Phytochemistry. 1992;31:3707–3709. doi: 10.1016/S0031-9422(00)97513-7. [DOI] [Google Scholar]
- 50.Eugster P.J., Salamin K., Grouzmann E., Monod M. Production and characterization of two major Aspergillus oryzae secreted prolyl endopeptidases able to efficiently digest proline-rich peptides of gliadin. Microbiology. 2015;161:2277–2288. doi: 10.1099/mic.0.000198. [DOI] [PubMed] [Google Scholar]
- 51.Salamin K., Eugster P.J., Jousson O., Waridel P., Grouzmann E., Monod M. AoS28D, a proline-Xaa carboxypeptidase secreted by Aspergillus oryzae. Appl. Microbiol. Biotechnol. 2017;101:4129–4137. doi: 10.1007/s00253-017-8186-8. [DOI] [PubMed] [Google Scholar]
- 52.Ito H. Taste of miso. J. Brew. Soc. Jpn. 1980;75:881–884. (In Japanese) [Google Scholar]
- 53.Ito K., Hanya Y., Koyama Y. Purification and characterization of a glutaminase enzyme accounting for the majority of glutaminase activity in Aspergillus sojae under solid-state culture. Appl. Microbiol. Biotechnol. 2013;97:8581–8590. doi: 10.1007/s00253-013-4693-4. [DOI] [PubMed] [Google Scholar]
- 54.Ito K., Matsushima K., Koyama Y. Gene cloning, purification, and characterization of a novel peptidoglutaminase-asparaginase from Aspergillus sojae. Appl. Environ. Microbiol. 2012;78:5182–5188. doi: 10.1128/AEM.00765-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Collins M.D., Williams A.M., Wallbanks S. The phylogeny of Aerococcus and Pediococcus as determined by 16S rRNA sequence analysis: Description of Tetragenococcus gen. nov. FEMS Microbiol. Lett. 1990;58:255–262. doi: 10.1111/j.1574-6968.1990.tb14006.x. [DOI] [PubMed] [Google Scholar]
- 56.Kanbe C., Uchida K. Oxygen consumption by Pediococcus halophilus. Agric. Biol. Chem. 1985;49:2931–2937. [Google Scholar]
- 57.Uchida K. Multiplicity in soy pediococci carbohydrate fermentation and its application for analysis of their flora. J. Gen. Appl. Microbiol. 1982;28:215–223. doi: 10.2323/jgam.28.215. [DOI] [Google Scholar]
- 58.Uchida K. Diversity and ecology of salt tolerant lactic acid bacteria: Tetragenococcus halophilus in soy sauce fermentation. Japan. J. Lact. Acid Bact. 2000;11:60–65. doi: 10.4109/jslab1997.11.60. [DOI] [Google Scholar]
- 59.Standard Tables of Food Composition in Japan—2015—(Seventh Revised Edition) [(accessed on 7 May 2021)]; Available online: https://www.mext.go.jp/en/policy/science_technology/policy/title01/detail01/sdetail01/sdetail01/1385122.htm.
- 60.Mochizuki T., Ouchi I., Matsumoto K. Amino acids in miso. J. Brew. Soc. Jpn. 1968;63:378–381. (In Japanese) [Google Scholar]
- 61.Honma N. Aroma and aroma components of miso (2) J. Brew. Soc. Jpn. 1987;82:548–553. (In Japanese) [Google Scholar]
- 62.Sugawara E., Saiga S., Kobayashi A. Relationship between aroma components and sensory evaluation of miso. Nippon Shokuhin Kogyo Gakkaishi. 1992;39:1098–1104. doi: 10.3136/nskkk1962.39.1098. (In Japanese) [DOI] [Google Scholar]
- 63.Sugawara E. Change in aroma components of miso with aging. Nippon Shokuhin Kogyo Gakkaishi. 1991;38:1093–1097. doi: 10.3136/nskkk1962.38.1093. (In Japanese) [DOI] [Google Scholar]
- 64.Sugawara E., Yonekura Y. Comparison of aroma components in five types of miso. Nippon Shokuhin Kogyo Gakkaishi. 1998;45:323–329. doi: 10.3136/nskkk.45.323. (In Japanese) [DOI] [Google Scholar]
- 65.Kumazawa K., Kaneko S., Nishimura O. Identification and characterization of volatile components causing the characteristic flavor in miso (Japanese fermented soybean paste) and heat-processed miso products. J. Agric. Food Chem. 2013;61:11968–11973. doi: 10.1021/jf404082a. [DOI] [PubMed] [Google Scholar]
- 66.Reports of Central Miso Research Institute [in Japanese] [(accessed on 7 July 2021)]; Available online: https://www.miso.jp/chumiken_hokoku.html.
- 67.Kitagawa M., Ito K., Yamada M., Koike S., Yamamoto T., Uehara Y. Long term intake of miso soup unaffected blood pressure in subjects with normal or stage I hypertension-double blind comparative interventional trial. Jpn Pharm. Ther. 2016;44:1601–1612. (In Japanese) [Google Scholar]
- 68.Kondo H., Sakuyama T.H., Yamakawa S., Kitagawa M., Yamada M., Itou S., Yamamoto T., Uehara Y. Long-term intake of miso soup decreases nighttime blood pressure in subjects with high-normal blood pressure or stage I hypertension. Hypertens Res. 2019;42:1757–1767. doi: 10.1038/s41440-019-0304-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ito K. Review of the health benefits of habitual consumption of miso soup: Focus on the effects on sympathetic nerve activity, blood pressure, and heart rate. Env. Health Prev Med. 2020;25:1–9. doi: 10.1186/s12199-020-00883-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Katagiri R., Sawada N., Goto A., Yamaji T., Iwasaki M., Noda M., Iso H., Tsugane S. Association of soy and fermented soy product intake with total and cause specific mortality: Prospective cohort study. BMJ. 2020;368:m34. doi: 10.1136/bmj.m34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Okada E., Saito A., Takimoto H. Association between the portion sizes of traditional Japanese seasonings—Soy sauce and miso—And blood pressure: Cross-sectional study using national health and nutrition survey, 2012–2016 data. Nutrients. 2018;10:1865. doi: 10.3390/nu10121865. [DOI] [PMC free article] [PubMed] [Google Scholar]