Abstract
Extracellular vesicles (EVs) are membrane-bound lipid particles that are secreted by all cell types and function as cell-to-cell communicators through their cargos of protein, nucleic acid, lipids, and metabolites, which are derived from their parent cells. There is limited information on the isolation and the emerging therapeutic role of periodontal and dental pulp cell-derived small EVs (sEVs, <200 nm, or exosome). In this review, we discuss the biogenesis of three EV subtypes (sEVs, microvesicles and apoptotic bodies) and the emerging role of sEVs from periodontal ligament (stem) cells, gingival fibroblasts (or gingival mesenchymal stem cells) and dental pulp cells, and their therapeutic potential in vitro and in vivo. A review of the relevant methodology found that precipitation-based kits and ultracentrifugation are the two most common methods to isolate periodontal (dental pulp) cell sEVs. Periodontal (and pulp) cell sEVs range in size, from 40 nm to 2 μm, due to a lack of standardized isolation protocols. Nevertheless, our review found that these EVs possess anti-inflammatory, osteo/odontogenic, angiogenic and immunomodulatory functions in vitro and in vivo, via reported EV cargos of EV–miRNAs, EV–circRNAs, EV–mRNAs and EV–lncRNAs. This review highlights the considerable therapeutic potential of periodontal and dental pulp cell-derived sEVs in various regenerative applications.
Keywords: extracellular vesicles, exosomes, nanomedicine, regeneration, cell-free therapy
1. Introduction
Extracellular vesicles (EVs) are membrane-bound bilayered lipid particles that are secreted from both prokaryotic and eukaryotic cells, carrying a cargo of biological molecules (i.e., protein, nucleic acid, lipids and metabolites) from their parent cells [1]. Initially, EVs were considered ‘cellular dust’, generated by cellular metabolism, until their biological role in the mineralization of bone was recognized [2,3]. A principal role of EVs is as an intercellular communicator of biological information into a recipient cell. This interaction can trigger signaling cascades and modulate cell behavior [4]. The biological function of EVs is defined by the parent cells from which they originate. EVs are involved in almost all cellular interactions, especially tumor metastasis, tissue homeostasis, and inflammatory regulation [4,5]. Due to their constituent biological molecules, EVs hold great promise as a therapeutic delivery system in regenerative medicine.
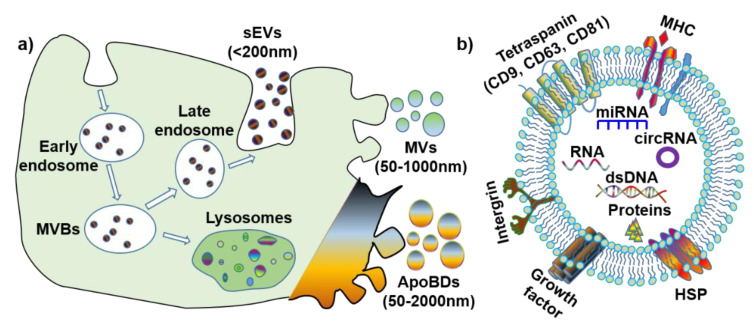
The definition, terminology and subtypes of EVs are still being debated. The International Society of Extracellular Vesicles (ISEV) recommends a division of EV subtypes based on their size: medium/large EV (>150 nm) and small EV (<150 nm) [6]. However, considering the discrepancies in the published literature, for simplification purposes, this review will define EV subtypes based on both their size and biogenesis (Figure 1a): small extracellular vesicles (also known as exosomes) (sEVs, <200 nm), microvesicles (MVs, 50–1000 nm) and apoptotic bodies (ApoBDs, 50–2000 nm). Furthermore, it is noteworthy that all EVs have various membrane proteins (e.g., tetraspanin, MHC, and HSP) and components (e.g., dsDNA, RNA, microRNA, circular RNA [7], and proteins) (Figure 1b).
Figure 1.
The biogenesis and contents of extracellular vesicles (EVs). (a) Biogenesis and size of three EV subtypes. (b) Common surface markers and cargos of EVs. sEVs: small extracellular vesicles; MVs: microvesicles; ApoBDs: apoptotic bodies; MVBs: multi-vesicular bodies; MHC: major histocompatibility complex; HSP: heat-shock protein; dsDNA: double-stranded DNA; miRNA: microRNA; circRNA: circular RNA.
1.1. Small EV (Exosomes)
Small EVs (sEV), or exosomes, originate from endosomes, and are biological nanoparticles that are smaller than 200 nm [5]. Further, sEVs are produced through an endocytic pathway, and their particle size is partially overlaid with that of microvesicles and apoptotic bodies. The biogenesis process for sEV is unique, whereby the endosomal network is the source of sEV that produce, classify, distribute and define the proper destination of the secreted sEV [2,8]. Endosome production can be categorized into the following three subtypes, according to each stage of development: early endosomes, late endosomes, and recycling endosomes. Early endosomes are formed by inward budding of the cell membrane, before a second inward budding of the endosomal membrane that results in the formation of late endosomes—intraluminal vesicles (ILVs). Late endosomes containing IVLs are named multi-vesicular bodies (MVBs), and the MVBs either fuse with lysosomes to degrade or follow the endocytic pathway for sEV generation. Once fusion with the plasma membrane is completed, the small membrane-enclosed vesicles are released into the extracellular matrix.
The biogenesis of sEV is affected by the following two main pathways that can induce multi-vesicular bodies (MVBs) generation: the endosomal sorting complex, required for the transport (ESCRT)-dependent pathway and ESCRT-independent pathway [9]. For the ESCRT-dependent pathway, ESCRT I and ESCRT II mediate the invagination of the late endosomal membrane, and ESCRT III will be recruited to the invaginated membrane sites. The cargo proteins are then deubiquitinated, and this stimulates the departure of the vesicle and the formation of MVBs. In the ESCRT-independent pathway, the neutral sphigomylinase2 (nSMase2) takes sphingolipids as substrates and converts sphingolipids to ceramide at the endosomal membrane. Following this, the microdomain is prepared for merging into a larger structure, which accelerates the endosomal budding and biogenesis of MVBs [10]. Moreover, sEVs that are produced by these different pathways possess different biomarkers, except CD63, which is the most common biomarker for all sEVs [11,12]. With respect to the ESCRT-dependent pathway, if endocytosis is mediated by Ras-related protein 27A/B (RAB27A/B), TSG101 is a biomarker of sEV. If the endocytosis is mediated by phospholipase D2 (PLD2) and RAB7, through the ESCRT-independent pathway, the biomarkers of sEV are alix, syntenin, and syndecan. As for the RAB11/35-mediated ESCRT-independent pathway, CD81, Wnt and proteolipid protein (PLP) are the preferred biomarkers.
The function of sEV in intercellular communication is determined by the interconnection between sEV surface proteins and receptors on the recipient cells that subsequently activates a variety of signaling pathways [5]. Further, sEVs arising from different cell types have different cargos that dictate and direct different biological effects. The sEVs are highly abundant in biofluids [13,14,15], and they have been demonstrated to be associated with immune response, viral pathogenicity, osteogenesis, odontogenesis, neuroprotection, angiogenesis, and anti-tumor functions [16]. For example, oral cancer cell-derived sEVs create a mechanism that can promote tumor progression by modifying vesicular contents and establishing a distant premetastatic niche with molecules that favor cancer cell proliferation, migration, invasion, metastasis, angiogenesis, and even drug resistance [17]. Evidence that sEVs play an important role in cell differentiation suggests that sEVs may have a potential role in tissue regeneration.
1.2. Microvesicles
Microvesicles (MVs) are membrane vesicles of different sizes, surrounded by a lipid layer of membrane, and they range in size from 50 nm to 1 μm. Microvesicles are generated by the outward budding of the plasma membrane, and are abundant in tissues/cells and biofluids [18]. The contents of MVs are similar to that of sEV. The MV components of note include CD40, selectins, integrins, cytoskeletal proteins, and cholesterol [19].
The biogenesis of MV involves the contraction of cytoskeletal proteins and phospholipid redistribution, contributing to a dynamic interplay in the plasma membrane and the resultant formation of microvesicles. Within the plasma membrane, the aminophospholipid translocase regulates phospholipid distribution, transferring phospholipids from one leaflet to another. Once phosphatidylserine (PS) is translocated to the leaflet of the outer membrane, the outward blebbing of the membrane and microvesicle formation is initiated. The interaction between actin and myosin causes the cytoskeletal structure contraction, which mediates membrane budding [20].
MVs have been reported to maintain tissue homeostasis during tissue regeneration, angiogenesis, anti-tumor effects, and in pathologies such as tumorigenesis, chronic inflammation, and atherosclerosis [19]. MVs that are produced by blood cells (e.g., neutrophils, macrophages, and platelets) are involved in the pro-coagulatory response [21]. MVs can be both pro-inflammatory and anti-inflammatory; this is determined by the induction or stimulation that is received by their parent cells. MVs that are produced by tumor cells enhance invasiveness and accelerate cancer progression, as well as strengthen the drug resistance of tumor cells [22]. This indicates that MVs are potential therapeutic agents for tissue regeneration; however, the function of MVs in periodontal tissue healing and regeneration requires further investigation.
1.3. Apoptotic Bodies
Apoptotic bodies (ApoBDs) are produced by cells undergoing apoptosis, and vary in size from 50 nm to 2 µm [23,24]. ApoBDs result from the formation of subcellular fragments when an apoptotic cell disassembles. They are comprised of molecular components from living cells and provide a rich molecular pool for recipient cells. However, ApoBDs are engulfed by macrophages and digested by phagolysosomes shortly after they are released [25]. ApoBDs and apoptosis are not related to an inflammatory reaction, the constituents in dying cells and ApoBDs are not released automatically to the environment, and anti-inflammatory cytokines are not generated during engulfing. ApoBDs have phosphatidylserine (PS) on their surfaces, to attract engulfing cells, and are considered to be specific biomarkers for ApoBDs [26]. Autoimmune diseases may be associated with defects in the clearance of ApoBDs. ApoBDs may stimulate the formation of thrombus and improve anti-cancer immunity.
Increasing evidence suggests that ApoBDs have important immune regulatory roles, in autoimmunity, cancer, and infection [24], as well as promoting osteogenesis [27]. For example, ApoBDs that are derived from mature osteoclasts can induce osteoblast differentiation by activating the protein kinase B/phosphoinositide 3-kinases (PI3K/AKT) pathway [27]. However, knowledge of their function and role is still limited and more studies are required in this field.
2. The Source and Characteristics of Periodontal (Dental Pulp) Cells
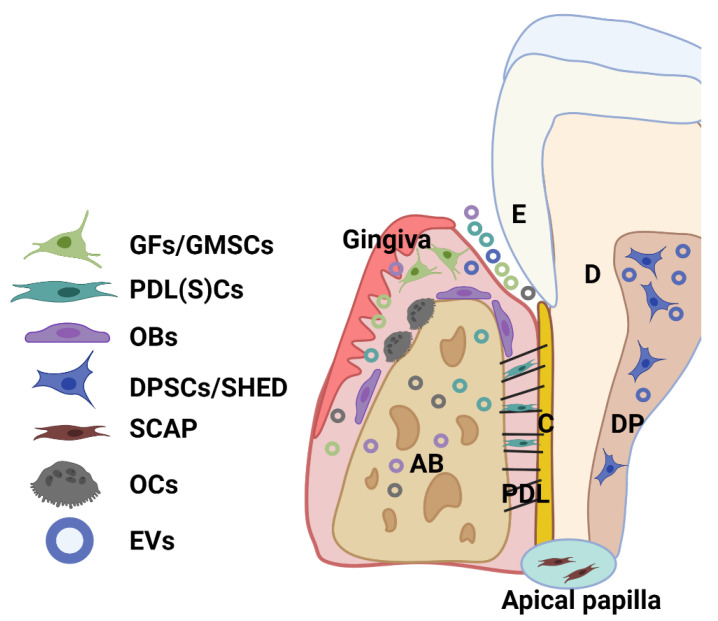
Dental tissue-derived (or stem) cells have remarkable characteristics for therapeutic application, being easily accessible and a rich source of stem cells with a well-known regenerative capacity. A great variety of multipotent adult or postnatal stem cells can be retrieved from dental tissues, especially from periodontal tissue and dental pulp from extracted permanent teeth (dental pulp stem cells—DPSCs) and exfoliated deciduous teeth (SHED). A healthy periodontium consists of soft (periodontal ligament-PDL and gingiva) and hard (alveolar bone and cementum) tissue, and cells residing within the healthy periodontal tissues include periodontal ligament (stem) cells (PDLSCs), PDL and gingival fibroblasts (PDLF, GFs), or gingival stem cells (GMSCs), osteoblasts (OBs), osteoclasts (OCs), and various immune cells (Figure 2) [28,29]. Moreover, stem cells can be obtained from dental apical papilla tissues (SCAP) and dental follicles (DFSCs, or DFCs) of the developing tooth [28,29]. Importantly, EV that is derived from these cells can be detected within periodontal tissues and biofluid (i.e., gingival crevicular fluid) (Figure 2).
Figure 2.
Schematic showing the main cell population and cell products (EVs) within a healthy periodontium. Various cells reside in the periodontium, such as periodontal ligament (stem) cells (PDLSCs), fibroblasts (GFs) and stem cells (GMSCs) from the gingiva, osteoblasts (OBs), osteoclasts (OCs), and various immune cells. AB: alveolar bone; C: cementum; D: dentin; DP: dental pulp; PDL: periodontal ligament; SHED: dental pulp cells from human exfoliated deciduous teeth (SHED); SCAP: cells from periodontal apical papilla tissues (SCAP).
Dental mesenchymal stem cells originate from the neural crest ectomesenchyme and reside in stromal niches (perivasculature and peripheral nerve-associated glia cells). The current consensus holds that both perivascular cells [30] and glia cells [31] are responsible for dental MSCs origin, as revealed in mouse experiments [31]. Much like bone-marrow-derived MSCs that originate from mesoderm [32], dental stem cells express MSCs markers and exhibit multipotent linage regeneration (i.e., osteogenic, chondrogenic, neurogenic) and immunomodulatory capabilities. These properties make these cells suitable candidates for therapeutic application (reviewed by Chalisserry et al. in [33]) in neurological disorders, angiogenesis, dentin-pulp regeneration and periodontal regeneration. PDLSCs, GFs, DPSCs, SHED, and DFSCs have been demonstrated to promote multiple-tissue regeneration, both in vitro and in vivo [34,35,36,37,38,39,40]. However, cell therapy has several challenges, including high cost, insufficient cell number, and associated regulatory barriers. On the other hand, a cell-free approach, centered around cell products (i.e., EVs derived from these cells), has been proposed, and there is an emerging focus on cell-derived EVs as potential therapeutic agents to promote periodontal regeneration. The utilization of sEVs for dental tissue regeneration is emerging as a viable cell-free treatment option, with ‘proof of concept’ studies reported using bone marrow or adipose MSC-derived sEVs (reviewed in [41,42,43]); yet, periodontal or dental pulp cell sources are likely to uniquely reflect the functional complexity of the periodontium and oral cavity.
The following sections will summarize the current methods for cell-derived sEV isolation and characterization, with particular emphasis on sEVs from periodontal and dental pulp cells.
3. Cell-Derived sEV Isolation Methods
3.1. General Concepts
Although sEVs have been studied for decades, there is still no standardized protocol for their isolation. Despite the presence of recommended guidelines for EV isolation and characterization, such as the Minimal Information for Studies of Extracellular Vesicles 2014 (MISEV2014) and MISEV 2018, these guidelines are not always followed.
Prior to the isolation of sEV, sequential centrifugation is commonly used to remove cell debris and large EVs, as follows:
Step 1: the cell conditional media (CM) is harvested and centrifuged at 300–400× g to remove cells, and the supernatant (SN) is collected;
Step 2: the SN collected in step 1 is centrifuged at 2000–3000× g to remove cells debris and apoptotic bodies. The SN is collected from this step;
Step 3: SN from step 2 is centrifuged at 10,000–20,000× g to remove the aggregates of biopolymers, microvesicles, and the other structures with a buoyant density higher than sEVs. The SN is collected from this step;
Step 4: then, the following isolation methods are used to enrich the sEVs: ultracentrifugation, sucrose gradient centrifugation, size exclusion chromatography, precipitation-based isolation, immunoaffinity chromatography, and ultrafiltration.
Given the growing interest in EVs, technical standardization is critical, as many different methodologies have been utilized for isolation and analysis. The influence of these various techniques on the downstream composition and functionality of EV cargos remains unclear; accordingly, the ISEV position papers [6,44] have raised the need to define ‘good practices’ and ultimately archive standardization. However, many researchers are not following these four steps, due to a lack of standardized protocols. Here, our review briefly introduces each isolation method, and discusses its merits and disadvantages (listed in Table 1).
Table 1.
Representative advantages and disadvantages of various EV isolation methods.
| Method | Time | Advantages | Disadvantage |
|---|---|---|---|
| Ultracentrifuge (100,000×–200,000× g for 1–2 h |
1.5 h to 10 h |
|
|
| Floatation-related methods (sucrose gradient centrifugation) | 250 min to 1 day |
|
• Fails to separate large vesicles with similar sedimentation rates |
| Size exclusion chromatography (SEC) | ~30 min (including column washing) |
|
• sEV and microvesicles cannot be separated |
| Precipitation based isolation (sodium acetate, PEG, protamine) | Overnight incubation |
|
|
| Immunoaffinity chromatography | ~240 min | • Very pure EV subpopulation (i.e., CD9+ EV) |
|
| Membrane filtration/Ultrafiltration | ~130 min |
|
• High contamination of non-EV protein |
3.2. Ultracentrifuge
Ultracentrifugation is the gold-standard method for isolating sEV, as the equipment is relatively easy to access and the methodology is technically straightforward. The method involves an ultracentrifugation step at 100,000×–200,000× g to pellet sEV [45]. However, ultracentrifugation has disadvantages, in that it leads to a low recovery rate of sEV, it is time consuming (1.5–10 h), contains non-vesicular macromolecule contamination, and results in EV aggregation.
3.3. Floatation-Related Methods (Sucrose Gradient Centrifugation)
Floatation-related methods distribute molecules based on the buoyant density, and the protein aggregates and sEV can be sufficiently separated. Differential gradient centrifugation (usually takes 250 min—1 day) takes advantage of buoyant density to fractionate EVs using sucrose or idoxanol gradients [45]. The sEVs can be separated by the discontinuous gradient sucrose solution, with each layer containing the desired size of EV. Other chemical reagents (i.e., iodixanol) can also be utilized instead of sucrose, for continuous EV harvest with no layers. Non-vesicular protein contaminants are distributed at a reduced level within this method, resulting in less protein contamination. However, sucrose gradient centrifugation cannot separate large particles that have a similar sedimentation rate.
3.4. Size Exclusion Chromatography (SEC)
SEC can be used to isolate small sEV, based on the size of the molecules, where large particles pass through the gel earlier than the small-sized molecules. The small-sized particles are trapped in the tiny pores on the surface of the gel, while the larger molecules can bypass the gel or receive less interference from the gel [46]. This technique has been well established with commercialized SEC columns, including qEV (iZON Science), Exo-spin™ SEC columns (Cell Guidance Systems Ltd.) and Pure-EVs SEC columns (HandaBioMed, Lonza). SEC has been proposed as an effective alternative method for pure sEV isolation, with a key advantage being its time efficiency (~30 min, including 10 mL of column washing with PBS). However, the similarly sized sEV and microvesicles cannot be separated by SEC.
3.5. Precipitation-Based Isolation (Sodium Acetate, PEG, Protamine)
Precipitation-based isolation has the following two mechanisms: polymeric precipitation and neutralizing charges [47]. In polymeric precipitation, a soluble polymer, usually polyethylene glycol (PEG), is mixed with EV samples and the mixture is incubated overnight, and EVs are sedimented by low-speed centrifugation at 1500 g. PEG precipitation enables a simple process for a large number of samples. Commercial kits, such as ExoQuick (System Biosciences), total exosome isolation reagent (Invitrogen), EXO-Prep (HansaBioMed), exosome purification kit (Norgen Biotek), and miRCURY exosome isolation kit (Exiqon), are based on this principle. For the other precipitation method, all EVs possess negative charges, so positively charged molecules (i.e., sodium acetate and protamine) are chosen for the precipitation. This method is popular due to its straightforward protocol; however, these precipitation methods lead to low sEV purity due to co-precipitation of the components from CM or biofluids, such as protein, DNA and RNA, and hence further purification is required.
3.6. Immunoaffinity Chromatography
The monoclonal antibodies (mAbs) against specific sEV surface proteins (i.e., CD 9) are fixed on the column, to capture a specific sEV population [48]. Once the CM passes through the column, the EVs, which express certain exosomal markers on their membrane, will be captured by the mAbs. This method leads to a very pure EV population, but low yield and scalability. This is attributed to the fact that this step needs to be repeated several times to ensure the mAbs can capture sufficient EVs (~240 min).
3.7. Ultrafiltration
Semi-permeable membranes (ranging from 3 kDa to 100 kDa) are adapted for sEV fractionation within filtration-based isolation; the membrane function is determined by its pore size. However, sEVs cannot be fractionated according to their biogenesis or biomarkers, but it is normally used to concentrate sEVs. It is still an efficient way to eliminate the minimal sample volume (~130 min) with a simple procedure, and has been proven to yield higher recovery of sEVs than ultracentrifugation [49]. However, ultrafiltration might lead to low EV protein, but a rather higher concentration of non-EV proteins (i.e., albumin).
3.8. Current Isolation Challenge
As mentioned previously, the current challenges of sEV isolation include time-consuming procedures, impurities, insufficient EV yield, and low scalability [50]. Although many researchers have investigated combinations of these isolation methods, an urgent demand has arisen to investigate high-yielding and time-effective isolation protocols. Currently, there is no optimal sEV isolation method; however, a combination of ultracentrifugation, SEC, and ultrafiltration has been used for the pure sEV population, which is a critical factor for downstream therapeutic applications.
4. sEV Isolation and Characterization Methods for Periodontal (and Dental Pulp) Cells
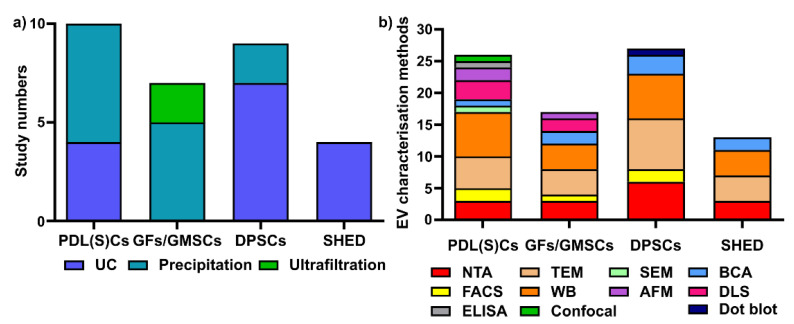
To date, there are no standardized protocols for sEV isolation and characterization. From the 33 studies that are reported in this review, we have summarized periodontal (dental pulp) cell-derived sEV isolation and characterisation methods [51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83]. Various isolation methods have been used for periodontal (dental pulp) cells sEVs, including ultracentrifugation (UC), precipitation-based methods, and ultrafiltration (Figure 3a). Regarding EV characterization, the latest MISEV 2018 guidelines [6] suggest that all EV researchers should characterize sEV from at least three different aspects, such as EV particle numbers, EV morphology, and EV-enriched protein markers. However, most of the current studies did not follow the MISEV guidelines, and this requires additional attention for all EV research.
Figure 3.
Various sEV isolation methods (a) and characterization methods (b) are used for periodontal (dental pulp) cells. UC: ultracentrifugation; NTA: nanoparticle tracking analysis; TEM: transmission electron microscopy; WB: Western blot; SEM: scanning electron microscopy; AFM: atomic force microscopy; BCA: bicinchoninic acid assay; DLS: dynamic light scattering; ELISA: enzyme-linked immunosorbent assay; confocal: confocal microscopy.
Different sEV isolation methods have been utilized for various cells (Figure 3a), with precipitation and ultracentrifugation methods being the two most commonly used techniques. In PDL(S)C-derived sEV isolation (10 studies), the precipitation-based method (i.e., a commercial ExoQuick kit) is the most commonly used (n = 6, 60%), followed by ultracentrifugation (n = 4, 40%). Among six studies in GFs/GMSC-derived sEVs, the precipitation-based method (n = 4, 66.7%) and ultrafiltration (n = 2, 33.3%) were used for GFs/GMMSCs–sEV isolation. Regarding DPSC-derived sEV, most researchers selected ultracentrifugation (n = 7, 77.8%), with one study using the precipitation-based method (n = 2, 22.2%). For SHED–sEVs, all of the studies (n = 4, 100%) used the ultracentrifugation method to isolate sEVs from SHED.
Concerning EV characterisation [6], NTA and DLS are common methods to quantify EV particle number, size, and distribution; TEM, SEM, and AFM can be used for EV morphology and size; BCA is for EV protein quantification; and WB is to determine EV-enriched protein markers. We have summarized the various EV characterisation methods for periodontal cell-derived sEVs (Figure 3b). For PDL(S)Cs—sEV (10 studies included in this review), WB is the most commonly used characterization method (n = 7 studies), followed by TEM (n = 5), NTA (n = 3), AFM (n = 2), flow cytometry (n = 2), SEM (n = 1), BCA assay (n = 1), ELISA (n = 1), and confocal microscopy (n = 1). In GFs/GMMSCs–sEVs, WB (n = 4) was utilized to detect CD9, CD63, and TSG101, as well as TEM (n = 4), NTA (n = 3), DLS (n = 2), BCA assay (n = 2), AFM (n = 1), and FACS (n = 1). The characterisation of DPSC–EVs are mostly performed using WB (n = 7), TEM (n = 8), NTA (n = 6), BCA assay (n = 3), FACS (n = 2), and dot blot (n = 1). For the characterization of SHED–sEVs (4 studies), TEM and WB (n = 4) are most commonly applied; NTA (n = 3) and BCA (n = 2) were also used for OBs–sEVs.
In summary, ultracentrifugation and precipitation-based methods are the two most common methods used for periodontal (dental pulp) cells sEV isolation. WB, TEM and NTA are the most common methods for periodontal (dental pulp) cell-derived sEVs characterisation.
5. The Function of sEVs Derived from Periodontal (Dental Pulp) Cells
Current studies mainly focus on small EV biogenesis and function in the periodontal regeneration field; thus, this review summarizes 33 studies [51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83] on periodontal cell-, gingival cell- and dental pulp (DPSCs and SHED) cell-derived sEV isolation, characterization, and their therapeutic role in tissue regeneration. Most of this research has focused on the function of sEVs in cell differentiation, and 11 studies investigated the cargos of sEVs (i.e., miRNA [52,53,61,63,64,72,75,81], circRNA [51,71], lncRNA [51], and EV-mRNA [67,72]) during this process.
5.1. Periodontal Ligament Fibroblasts or Stem Cells (hPDL(S)Cs)–sEV
A total of 10 studies investigated sEVs derived from human PDL (stem) cells or fibroblasts [51,52,53,54,55,56,57,58,59,60], and are summarized in Table 2. Eight studies isolated sEV from hPDLSCs [51,52,53,55,56,58,59,60], with one study each using sEVs from a human PDL fibroblast (hPDLFs) cell line [54] and hPDLCs [57]. Three of these studies investigated hPDLCs sEV function in vivo, using animal models [56,59,60].
Table 2.
The isolation, characterization, and function of PDL(S)C-derived EVs.
| Reference | Cell Source | EV Isolation | EV Characterization | Key Findings |
|---|---|---|---|---|
| Xie et al., 2021 [51] |
|
|
|
|
| Zhang et al., 2020 [52] |
|
|
|
|
| Chiricosta et al., 2020 [53] |
|
|
|
|
| Zhao et al., 2019 [54] |
|
|
|
|
| Čebatariūn-ienė et al., 2019 [55] |
|
|
|
|
| Pizzicannel-la et al., 2019 [56] |
|
|
|
|
| Wang et al., 2019 [57] |
|
|
|
|
| Kang et al., 2018 [58] |
|
|
|
|
| Diomede et al., 2018 [59] |
|
|
|
|
| Rajan et al., 2016 [60] |
|
|
|
|
Abbreviations: TGFβ, transforming growth factor β; MAPK, mitogen-activated protein kinase; mTOR, mechanistic target of rapamycin; FoxO, forkhead box protein O; VEGFA, vascular endothelial growth factor A; Ras, Ras GTPase; LPS, lipopolysaccharide; MFG-E8, milk fat globule-EGF factor 8 protein; TLR4, Toll-like receptor 4; PKB/Akt, protein kinase B; GSK-3β, glycogen synthase kinase 3; ALP, alkaline phosphatase; OPN, osteopontin; BSP, bone sialoprotein; CP23, cementum protein 23; BMP2, bone morphogenetic protein 2; RUNX2, runt-related transcription factor 2; COL1A1, alpha-1 type I collagen; VEGFR2, vascular endothelial growth factor receptor 2; IL-1β, interleukin-1 beta; IL-6, interleukin-6; TNF-α, tumor necrosis factor-α; MMP8, matrix metalloproteinase-8; TUFT1, Tuftelin 1; TFIP11, Tuftelin-interacting protein 11; IL-17, interleukin-17; IFN-γ, interferon-γ; IL-10, interleukin-10; STAT1, signal transducer and activator of transcription 1; Bax, Bcl-2-associated X protein.
According to the latest MISEV 2018 guidelines [6], it is critical to consider several factors influencing the collection of EV, including characteristics of primary cell source (donor health status, age, gender), primary cell passage number, confluence at harvest, culture volume, media change frequency, CM harvesting conditions, as well as all culture media composition and preparation details. Thus, our review includes detailed donor information for primary cells (if mentioned), the cell culture condition prior to CM collection, and detailed sEV isolation protocols. This will allow future EV researchers to select appropriate protocols for CM harvesting and sEV isolation.
Donor age was disclosed in only two studies (18–30 [51] and 18–21 years old [55]), while there is no clear information in the other studies [52,53,54,56,57,58,59,60]. The cells at passages 2–3 were used in five studies [51,52,53,56,59], passage 3–7 in one study [54], with no passage information provided in the remaining studies [55,57,58,60]. Since fetal calf serum (FBS or FCS) contains a large amount of EV, it is crucial to state how cells are cultured before CM harvesting. Currently, either EV-depleted FBS or FBS starvation is used before CM harvest for PDLCs–sEV isolation; from the 10 studies that were reviewed, 5 did not state how the cells were cultured before CM collection [53,56,58,59,60], 4 studies used EV-depleted FBS [52,54,55,57], and one study used FBS starvation [51]. While it is of considerable importance to clearly articulate the cell source, passage number, and CM harvest condition, this is something that is currently under-reported in many studies.
The following three aspects of hDPL(S)Cs–sEV analysis were evaluated in the 10 studies that have been included in this review: (1) EV size, (2) EV content (protein, RNA, etc.), and (3) EV function in cell differentiation in vitro and in vivo. Regarding the size of hPDL(S)Cs–sEV, three studies did not characterize the sEV size [52,53,60]. There is a large deviation for the reported EV size: <200 nm in five studies [51,54,55,57,58], two populations (90 ± 20 nm and 1200 ± 400 nm) in one study [59], and 100–710 nm in one study [56]. It is noted that two studies engineered the hPDLCs–EV using polyethyleneimine (PEI, yielding PEI–EV) [56,59]. The following two factors may contribute to this deviation: the EV isolation method (UC or precipitation methods), and the EV size characterization methods (TEM, or DLS, or NTA). We will define EV size <200 nm as sEV, and unclear EV size as EV.
Regarding the EV content, it seems that hPDLCs–EV contain miRNAs [52,53] and circular RNAs [51] that may alter the recipient cells functions. RNA sequencing of hPDLSCs–EV (where EV size was unclear) revealed that hPDLSCs–EV contains 955 non-coding transcripts, with five representative miRNAs, including MIR24-2, MIR142, MIR296, MIR335, and MIR490 [53]. The hDPLSCs–EV–miR-17-5p can regulate the angiogenesis of human umbilical vein endothelial cells (HUVECs) during inflammatory stimulation by TNF-α [52]. Furthermore, circular RNA and long non-coding RNAs (lncRNAs) were also found in the sEV from hPDLSCs, after five and seven days of osteogenic differentiation, with 69–557 circRNAs and 2907–11,581 lncRNAs detected by RNA sequencing. Compared with the sEV from hPDLSCs before osteoinduction, 3 sEV–circRNAs and 2 sEV–lncRNAs were upregulated, while 39 sEV–circRNAs and 5 sEV–lncRNAs were downregulated after 5 and 7 days of osteoinduction. RT-qPCR validation showed that three sEV–circRNAs (hsa_circ_0087960, hsa_circ_0000437, and hsa_circ_0000448) were upregulated after osteogenic differentiation, while one was downregulated (hsa_circ_0000448). However, three selected lncRNAs (small nucleolar RNA host gene5—SNHG5, LOC100130992, and ATP6VB1-AS1) showed no difference between the groups [51].
There is increasing evidence demonstrating that hPDL(S)Cs–EV can modulate in vitro angiogenesis (in HUVECs [52]), osteogenesis (in MG-63 OBs [54] and hPDLSCs [55,56,59]), anti-inflammation (in LPS-treated hPDLSCs [55,60] and J774.1 macrophages [57]), and immunoregulation (induced M1 polarization in THP-1 cells [58]) via modulating the TGF-beta pathway, MAPK pathway, mTOR pathway and FoxO signaling pathways [51], and PI3K/Akt signaling [55] and NF-kB signaling pathways [57]. The in vivo function of hPDL(S)Cs–EV was explored, either in rat calvaria defect [56,59] or intravenous administration in mouse multiple sclerosis disease [60] models. Pizzicannel-la et al. [56] created a calvarial defect, with a diameter of 4 mm and a height of 0.25 mm in male Wistar rats (300–350 g; n = 4 for each group). The hPDLSCs–EV and hPDLSCs–PEI–EV were loaded on collagen membranes and transplanted into the rat calvaria defect for 6 weeks, leading to enhanced bone and vascularization compared to the no-EV groups, with the PEI–EV group inducing better osteogenesis and vascularization compared to the EV group. Diomede et al. [59] revealed similar results, showing that hPDLSCs–PEI–EV leads to increased blood vessel formation after 6 weeks of the transplantation of hPDLSCs–EV- and hPDLSCs–PEI–EV-loaded collagen membranes into a rat calvarial defect. Rajan et al. [60] established a mouse model of MS disease, and intravenous administration of hPDLSCs–EVs decreased apoptosis and inflammation in the diseased mice.
In summary, the size of hPDL(S)Cs–EV ranges from 20 nm to 1600 nm when using different EV isolation methods, with under-reporting of sufficient detail about the cell source and cell culture conditions before CM collection. The hPDL(S)Cs–EV contains miRNAs, circRNAs, and lncRNAs, and they modulate the angiogenesis, osteogenesis, and inflammation of recipient cells, through TGF-β, MAPK, mTOR and FoxO pathways [51], and PI3K/Akt [55] and NF-kB signaling pathway [57]. However, none of the three in vivo studies [56,59,60] used either a periodontal defect or a periodontitis animal model.
5.2. Human Gingival Fibroblasts (hGFs)–sEV
Table 3 summarizes seven studies [61,62,63,64,65,66,67]) of EV from fibroblasts (hGFs [62,63] or MSCs (hGMSCs [61,64,65,66,67]) from human gingiva tissues. There are two studies that investigated the in vivo role of hGMSCs–EV using animal models [66,67]. The cells from either 20-to-40-year-old donors [66] or unclear age human donors [62,63,64,65,67] were used at passage 2 [64], passage 4–6 [62], <6 passage [66], or unclear [63,65,67]. EV-depleted FBS [61,63,66], FBS starvation [65], and unclear cell culture conditions [62,64,67] were applied in the studies before CM collection for EV isolation. The size of hGFs/hGMSCs–EV varied from different studies, as follows: <200 nm [61,62,63,66], 50–500 nm [65], unclear size [64], and a combination of two populations (93 ± 24 nm and 1200 ± 400 nm) [67]. Engineered hGMSCs–PEI–EV had the following two populations: 250 ± 50 nm and 3600 ± 500 nm [67].
Table 3.
The isolation, characterization, and function of EV from hGFs or hGSMCs.
| Reference | Cell Source | EV Isolation | EV Characterization | Key Findings |
|---|---|---|---|---|
| Nakao et al., 2021 [61] |
|
|
|
|
| Yin et al., 2020 [62] |
|
|
|
|
| Zhuang et al., 2020 [63] |
|
|
|
|
| Silvestro et al., 2020 [64] |
|
|
|
|
| Coccè et al., 2019 [65] |
|
|
|
|
| Mao et al., 2019 [66] |
|
|
|
|
| Diomede et al., 2018 [67] |
|
|
|
|
Abbreviation: MTS, 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium; Ki-67, antigen KI-67/marker of proliferation Ki-67; PCNA, proliferating cell nuclear antigen; CXCL12, C-X-C motif chemokine 12/ stromal cell-derived factor 1; FGF, fibroblast growth factor; GDNF, glial cell-derived neurotrophic factor; PTX, paclitaxel; c-JUN, jun proto-oncogene/AP-1 transcription factor subunit; Notch1, Notch homolog 1(translocation-associated).
RNA sequencing data from Silvestro et al. showed that hGMSCs–EV comprises 15,380 genes (for interleukins, TGF-β, BMPs, GDFs, Wnt, VEGF, FGF, and neurotrophins), and 1155 non-coding RNA (lncRNAs and miRNAs—miR1302, miR451, miR24, miR219 and miR194) [64]. The miRNA microarray data from Nako et al. [61] showed that 655 universal differentially expressed miRNAs were found in Exo-TNF compared to Exo-Ctrl, particularly miR-1260b (ranked in the top three of the most highly upregulated miRNAs, by using TNF- α preconditioning). RNA sequencing from Diomede et al. [67] demonstrated that 31 ossification genes were enhanced in hGMSCs–PEI–EV compared to hGMSCs-EV through the TGF-β signaling pathway.
The in vitro functional assays showed that hGFs/hGMSCs–sEV facilitates cell proliferation (in hGFs [62] and Schwann cells line [66]), anti-osteoclastogenic [61] and osteogenic differentiation (in hBMSCs [63] and hGMSCs [67]), as well as an anti-carcinogenesis effect (in human pancreatic carcinoma and squamous carcinoma cells [65]). This may be mediated by an miR-1260b/Wnt 5A/RANKL pathway [61], miR-23a/CXCL12 axis [63], interleukins, TGF-β, BMPs, GDFs, Wnt, VEGF, FGF, and neurotrophins [64], and TGF-β signaling [67].
In their in vivo investigations, Nakao et al. [61] created a ligature-induced periodontitis mice model, and locally injected hGMSCs–sEV or TNF-α-preconditioned GMSC-derived exosomes (hGMSCs–sEV–TNF) into the palatal gingiva of the ligated second maxillary molar. One week post-injection, both the interventions significantly reduced periodontal bone loss compared to the PBS control group, while hGMSCs–sEV–TNF further reduced the distance from the cementoenamel junction to the alveolar bone crest (CEJ–ABC) and the number of tartrate-resistant acid phosphatase (TRAP)-positive osteoclasts, indicating an anti-osteoclastic property for hGMSCs–sEV [61]. Moreover, Mao et al. [66] transplanted hGMSCs–sEV-loaded gelfoam sheets into the crush-injury sites of sciatic nerves in C57BL/6J mice, and the EV group had comparable beneficial effects on the functional recovery of the injured sciatic nerves of mice compared to the hGMSCs group. Further, hGMSCs–sEV enhanced the expression of neuronal and Schwann cell markers (β-tubulin III and S100 calcium-binding protein B—S100B) at one-month post-injury, compared with hGMSCs controls, suggesting that hGMSCs–sEV can promote neuron regeneration in vivo. Diomede et al. [67] loaded hGMSCs–EV or hGMSCs–PEI–EV into 3D-printed PLA scaffolds with/without hGMSCs, and transplanted them into rat calvaria defects for 6 weeks. Both the hGMSCs–EV and hGMSCs–PEI–EV groups enhanced bone and blood vessel formation, yet hGMSCs–PEI–EV performed better than the EV group.
In summary, the EV diameter from hGFs/hGSMCs is different among studies, ranging from 50 nm to 1600 nm. EV–mRNAs and EV–miRNAs [61,64,67] may contribute to their in vitro and in vivo function in cell proliferation [62,66], and reduce bone resorption [61], osteogenic differentiation [63,67] and nerve regeneration [66]. More in vivo studies are required in order to explore the function of EV from gingival tissue-derived cells.
5.3. Human Dental Pulp Cells (hDPSCs)–sEV
Table 4 summarizes nine studies investigating EV from human primary DPSCs [68,69,70,71,72,73,74,75,76], with three of these including in vivo models [69,72,76]. Cells were isolated from donors who were 24–41 years old [69], 16–25 years old [70], 20 years old [71], 19–28 years old [73], 22–36 years old [75], or an unclear donor age [68,72,74,76]. The cells at passage 2 [71], passage 3–6 [70], passage <4 [76], passage 3–7 [75], passage 3–5 [73], or unclear passage number [68,69,72,74] were used in these studies. Prior to CM collection, the cells were cultured in EV-depleted FBS [68,70,71,72,73] or FBS starvation [69,74,75,76]. The mode size of hPDSCs–sEV was smaller than <200 nm in most studies [68,69,70,71,72,75], with one study reporting 50–400 nm [72], 80–400 nm [73], and 30–250 nm [74], and unclear EV size [76].
Table 4.
The isolation, characterization, and function of hDPSCs–EV.
| Reference | Cell Source | EV Isolation | EV Characterization | Key Findings |
|---|---|---|---|---|
| Faruqu et al., 2020 [68] |
|
|
|
|
| Zhou et al., 2020 [69] |
|
|
|
|
| Ivica et al., 2020 [70] |
|
|
|
|
| Xie et al., 2020 [71] |
|
|
|
|
| Shen et al., 2020 [72] |
|
|
|
|
| Li et al., 2019 [73] |
|
|
|
|
| Ji et al., 2019 [74] |
|
|
|
|
| Hu et al., 2019 [75] |
|
|
|
|
| Huang et al., 2016 [76] |
|
|
|
|
Abbreviations: circPAR1, circular Prader–Willi/Angelman region-1; DMP-1, dentin matrix acidic phosphoprotein 1; DSPP, dentin phosphophoryn; TGFR1, transforming growth factor beta receptor I; p-Smad2/3, mothers against decapentaplegic homolog 2/3 or SMAD family member 2/3; Smad4, SMAD family member 4/mothers against decapentaplegic homolog 4; UTR, untranslated region; LTBP1, latent TGF-beta binding protein.
The hPDSCs–EV modulates angiogenesis in endothelial cells [69], migration/proliferation (in hBMMSCs [70], Schwann cells (SCs) [73], and CD4+ T cells [74]), osteogenic differentiation in hDPSCs [71], anti-inflammation (in DPSCs [72] and CD4+ T cells [74]), and odontogenic differentiation of Schwann cells (SCs) [73], hDPSCs [75,76], and hMSCs [76]. This may be regulated through hDPSCs–sEV–circPAR1 binding with hsa-miR-31 [71], hDPSCs–sEV–miR-1246 [72] and hDPSCs–sEV–miR-27a-5p [75]. RNA sequencing data from Hu et al. [75] demonstrated that 7 increased sEV–miRNAs and 21 decreased sEV–miRNAs were found in odontogenic differentiated hDPSCs–sEV, and these miRNAs are associated with the TGFβ1/smads signaling pathway. The authors concluded that sEV–miR-27a-5p can modulate odontogenic differentiation via the TGFβ1/smads signaling pathway, by downregulating latent-transforming growth factor beta-binding protein 1 (LTBP1).
With respect to in vivo studies, Zhou et al. [69] created a full-thickness excisional skin wound-healing model in male C57BL/6 mice (8 weeks old), and then subcutaneously injected hDPSCs–sEV (200 μg in 100 μL) from healthy or periodontitis patients derived hDPSCs–sEV (200 μg in 100 μL PBS) for 4, 9, and 14 days. Both the sEV groups promoted the wound healing process and vascularization compared to the PBS control group, while hDPSCs–sEV from the periodontitis patients increased the wound closure rate and the number of newly formed microvessels, with more CD31- and VEGF-positive cells compared to the sEV from a healthy patient. Shen et al. [72] established a ligation-induced periodontitis model in 6–8-week-old male C57BL/6J mice, and a chitosan hydrogel (CS) loaded with 50 μg of hDPSCs–sEV (hPSDCs–sEV–CS group) was locally injected after ligature removal, with a local injection of PBS or hPSDCs–sEV used as the controls. The results showed that the hDPSCs–sEV–CS group led to increased bone formation, a thick layer of epithelial layers, less inflammatory cells, and a lower amount of TRAP-positive osteoclasts, at 10 days post-treatment. Furthermore, hPSDCs–sEV–CS treatment significantly reduced pro-inflammatory cytokines (IL-23, IL-1α, TNF-α, IL-12, IL-1β, IL-27, and IL-17), and NF-κB p65 and p38 MAPK signaling, in periodontal tissues compared with other groups. RNA sequencing analysis of the periodontium showed that 7351 differentially expressed genes (DEGs) were found between the hDPSCs–sEV–CS and CS groups. GO term enrichment analysis of the top 200 DEGs demonstrated that they are associated with chemotaxis pathways and the immune response, which were downregulated in the hDPSCs–sEV–CS group. Most importantly, hDPSCs–sEV–CS induced macrophages converting from a proinflammatory phenotype to an anti-inflammatory phenotype in the periodontium of periodontitis mice, with more CD206+ anti-inflammatory macrophages and significantly decreased CD86+ in pro-inflammatory macrophages [72]. This indicates that hDPSCs–sEV can promote bone formation, epithelium re-growth, and reduce inflammation in a periodontitis mice model. Furthermore, Huang et al. [76] loaded hDPSCs–EV into clinical-grade type I collagen membranes, and then placed them on a human tooth root slice (3–4 mm in thickness), before subcutaneously transplanting into athymic nude mice for 2 weeks. They resulted in enhanced dental-pulp-like tissues, with increased odontogenic proteins (dentin matrix acidic phosphoprotein 1—DMP1, and dentin phosphophoryn—DSPP) and endothelial cell marker protein (von Willebrand factor—vWF).
To summarize, hDPSCs–EV, ranging from 30 nm to 400 nm among nine studies, and containing circRNA [71], miRNAs [72,75], and mRNAs [72], may modulate angiogenesis [69], migration/proliferation [70,73,74], osteogenic differentiation [71], anti-inflammation [72,74] and odontogenic differentiation [73,75,76] in recipient cells. Among the three in vivo studies, the skin wound-healing model [69], periodontitis disease model [72], and subcutaneous transplantation [76] were employed, and the results showed that hPDSCs–EV can promote angiogenesis, osteogenesis, dentin-pulp regeneration, and reduce inflammation and osteoclastic activity. Further in vivo studies are required to validate the function of hDPSCs–EV.
5.4. SHED/SCAP/DFCs–sEVs
Table 5 summarizes seven investigations (five in vivo studies) examining sEVs from dental cells, including SCAP [77,78], SHED [79,80,81,82], and DFCs [83]. Cells were isolated from 5–8-year-old donors [81], 12–15 years old [77,78], 13–19 years old [83], or unknown age [79,80,82], at passage 3–4 [79], 4–7 [80], 4 [81], 3–6 [82], 5 [83], or unknown [77,78]. EV-depleted FBS [77,79,81,82] and FBS-starvation [78,80,83] were applied for CM collection.
Table 5.
The isolation, characterization, and function of sEVs from SHED, SCAP and DFCs.
| Reference | Cell Source | EV Isolation | EV Characterization | Key Findings |
|---|---|---|---|---|
| Liu et al., 2021 [77] |
|
|
|
|
| Zhuang et al., 2021 [78] |
|
|
|
|
| Wang et al., 2020 [79] |
|
|
|
|
| Wei et al., 2020 [80] |
|
|
|
|
| Luo et al., 2019 [81] |
|
|
|
|
| Wu et al., 2019 [82] |
|
|
|
|
| Shi et al., 2020 [83] |
|
|
|
|
Abbreviations: Cdc42, cell division control protein 42 homolog; RANKL/OPG, receptor activator of nuclear factor kappa-B ligand/osteoprotegerin.
The size of SHED/SCAP/DFCs–sEVs was smaller than 200 nm in all the studies; these sEVs promote angiogenesis in HUVECs [77,82], anti-inflammation in mBMSCs [80] and chondrocytes [81], osteogenesis in PDLCs [79], mBMSCs [80] and rBMSCs [82], and dentinogenesis in BMMSCs [78,83] in vitro, by the Cdc42 pathway [77], Wnt/β-catenin and BMP/Smad signaling pathways [79], miR-100–5p/mTOR pathway [81], and AMPK pathway [82].
The function of SCAP–sEVs was investigated in vivo on gingival soft tissue [77] and dentin-pulp regeneration [78]. Liu et al. [77] created full-thickness circular gingival wounds in C57BL/6J mice, using a biopsy punch (soft tissue defects with a diameter of 2.0 mm). Following this, 40 μg of SCAP–sEVs, SCAP–siCdc42–sEVs, or PBS, was injected submucosally into the palates of the wounds sites. Seven days post-injection, SCAP–sEVs promoted palatal gingival tissue regeneration by enhancing vascularization in the early phase [77]. Zhuang et al. [78] loaded 50 μg/mL SCAP–sEVs and 4 × 105 BMMSCs with gelatin sponge onto a dentin slice, before subcutaneously transplanting them into immunodeficient mice. Significant dentin-pulp regeneration was observed 12 weeks post-transplantation in the SCAP–sEVs group compared to the PBS control group.
The action of SHED–sEVs on periodontitis disease and periodontal defect in vivo has been investigated in a mouse [80] and rat model [82], respectively. Wei et al. [80] locally injected 20 µg of SHED–sEVs into buccal and lingual sides of the first molar once per week, over 2 weeks, in ligature-induced periodontitis mice. After 2 weeks, SHED–sEVs reduced bone loss, with a decreased CEJ–ABC distance compared to the controls. Moreover, Wu et al. [82] generated a periodontal defect (4 × 2 × 1.5 mm3) in their rat model, at the buccal alveolar bone of the first-to-third mandibular molars. SHED–sEVs were loaded into a β-TCP scaffold before placing them into the periodontal defect for four weeks, resulting in enhanced neovascularization and new bone formation compared to the β-TCP/PBS scaffold.
In their study, Shi et al. [83] injected gelatin hydrogels (100 µL), loaded with LPS–DFCs–sEVs (sEVs derived from LPS-treated DFCs) or DFCs–sEVs, into the periodontal pocket of the right maxillary second molar in a ligature-induced periodontitis rat model. The intervention was once a week for up to 8 weeks, and resulted in significantly reduced alveolar bone loss and TRAP-positive osteoclasts, as well as enhanced well-oriented PDL fibers in the LPS–DFCs–sEVs group.
In summary, SHED/SCAP/DFCs–sEVs are smaller than 200 nm, and those containing miR-100–5p [81] may modulate angiogenesis [77,82], inflammation [80,81], osteogenesis [79,80,82], and dentinogenesis [78,83] in vitro. More importantly, five in vivo studies showed that SHED/SCAP/DFCs–sEVs can promote angiogenesis [77,82], dentin-pulp complex [78], alveolar bone [82], and well-organized PDL fiber formation [82]. It is noted that two studies utilized a ligature-induced periodontitis disease model [80,83] and one study used a periodontal defect model [82]. More studies are needed to further validate the in vivo functional role of SHED/SCAP/DFCs–sEVs.
6. Summary and Discussion
Periodontal cells (PDLCs/SCAP and GFs/GMSCs) and dental pulp (DPSCs/SHED)-derived EVs can play an important role in augmenting the function of recipient cells, such as proliferation and osteo/odontogenic differentiation, as well as anti-inflammation and anti-cancer properties [51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83]. In particular, one study of GMSCs–sEVs [61] and DPSCs–sEVs [72], two studies of SHED–sEVs [80,82], and one study of DFCs–sEVs [83] can promote alveolar bone, vasculature and well-organized PDL fibers regeneration, and reduced inflammation in a periodontitis animal model or a periodontal defect model. As such, these EVs may serve as potential ‘cell-free’ therapeutics to facilitate periodontal regeneration; however, more in vivo studies are required to confirm this concept.
As stated in the latest MISEV 2018 guidelines [6], it is critical to clearly describe the primary cell source (i.e., donor age, health status, gender), primary cell passage number, cell culture conditions (using either EV-depleted FBS or FBS starvation before CM collection), and detailed EV isolation and characterization protocols. Among 33 studies in our review, only two studies used human or mouse cell lines [54,57], and 31 studies isolated EV from primary cells, with only 12 out of 31 studies stating a clear age range for the human or mouse donors [51,55,66,69,70,71,73,75,77,78,81,83], and 13 out of 31 studies were unclear about cell passage numbers [55,57,58,60,63,65,67,68,69,72,74,77,78]. Since FBS is largely EV contaminated, EV-depleted FBS or FBS starvation should be used for cell culture before CM collection. EV-depleted FBS was used in 12 studies [61,63,66,68,70,71,72,73,77,79,81,82], FBS starvation in 8 studies [65,69,74,75,76,78,80,83], and unclear cell culture conditions in 11 studies. Although all the studies used the two most common sEV (or exosome) isolation methods (precipitation and ultracentrifugation), the EV size in these studies (excluding studies with no EV characterization) is not consistent, with 22 studies generating <200 nm sEVs [51,54,55,57,58,61,62,63,66,68,69,70,71,72,75,77,78,79,80,81,82,83]. This may be attributed to the different CM collection, EV isolation and characterization methods among the studies. Thus, appropriate methods should be chosen to prepare CM, and isolate and characterize cell-derived EV according to the MISEV guidelines. Our review has defined <200nm EV as sEV (small EV) and unclear size or >200 nm as simply EV.
Among 33 studies, 12 studies performed in vivo research to investigate the EV function of hPDLCs–sEV [56,59,60], hGMSCs–EV [66,67], hDPSCs–EV [69,72,76], SCAP–sEVs [77,78], SHED–sEVs [80,82] and DFCs–sEVs [83]. Furthermore, three studies engineered the EV using polyethyleneimine (PEI), yielding PEI–EV [56,59,67], and all three studies reported that the PEI–EV group enhanced in vivo osteo/odontogenic and angiogenic properties compared to the EV group. Animal studies employed either defect or disease models, such as calvaria defects [56,59,67], nerve injury model [66,67], skin wound-healing model [69], subcutaneous transplantation [76], and multiple sclerosis [60], ligation-induced periodontitis [69,72,80,83] and a periodontal defect [82].
EVs were administrated either by loading into biomaterials, such as collagen membrane [56,59,76], gelfoam sheets [66], gelatin sponge [78] and 3D-printed PLA scaffold [67], or via intravenous administration [60], subcutaneous injection [69], local injection [61,72,80,83], or submucosal injection [77]. More pre-clinical models (i.e., periodontal defects or periodontitis disease models) and EV delivery systems need to be investigated to explore the potential of periodontal cell-derived EV in the regeneration of anatomically complex tissues, such as the periodontium.
All of the above factors are critical for a successful therapeutic outcome; thus, it is of great importance to follow the relevant guidelines and consider the above-discussed variables with more comparisons between different parameters.
7. Conclusions and Future Perspectives
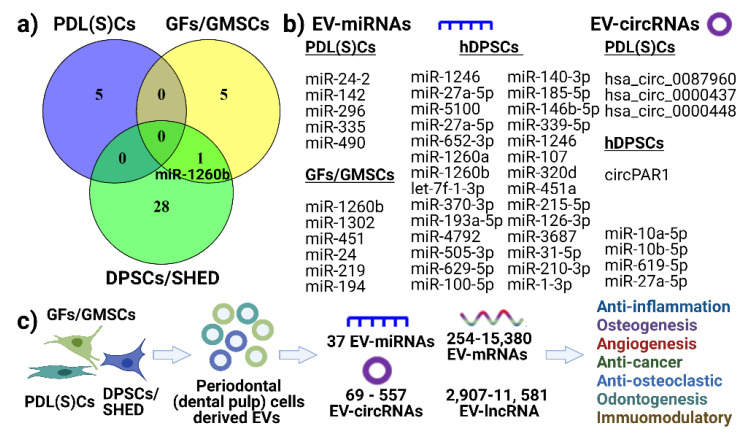
This review demonstrates that sEV can be isolated from periodontal and pulp cells, with 11 studies investigated the EV cargos, including sEV–miRNA [52,53,61,63,64,72,75,81], EV–circRNA [51,71], EV–lncRNA [51] and EV–mRNA [67,72]. We summarize the common EV–miRNA and EV–circRNA within periodontal (or dental pulp) cells (Figure 4a,b). From the included studies, except for one common EV–miRNA (miR-1260b) between DPSCs/SHED and GFs/GMSCs, there appears to be no common EV–miRNA detected between these cell types (shown in Venn diagram, Figure 4a). We also listed reported EV–miRNAs and EV–circRNAs from PDL(S)Cs–EV and hDPSCs-–EV (Figure 4b). However, this needs further confirmation with more studies. Furthermore, 38 EV–miRNAs, 69–557 EV–circRNAs, 254–15,380 EV–mRNAs and 2907–11,581 EV–lncRNAs were reported for EV from periodontal (dental pulp) cells by RNA sequencing analysis. We have outlined that these EVs possess anti-inflammation, osteo/odontogenesis, anti-osteoclastogenesis, angiogenesis and immunomodulatory functions in vitro and in vivo. Thus, we propose that periodontal cell-derived EVs can modulate the cell function via EV cargos (Figure 4c). However, more studies for periodontal cell-derived EVs are required to further confirm this concept.
Figure 4.
Summary of EV–miRNAs, circular RNAs (a,b) and proposed (c) function of periodontal cell-derived EV on recipient cells function. (a) Venn diagram showing no common EV–miRNAs found from PDL(S)Cs, GFs/GMSCs and DPSCs. (b) Listed EV–miRNAs and EV–circRNAs. (c) Proposed mechanism of how periodontal cell-derived EVs modulate inflammation, angiogenesis, osteo/odontogenesis via EV cargos, such as miRNA, mRNAs, lncRNAs, and circRNAs.
Given that cell source, CM collection, and EV isolation and characterization are critical in obtaining pure EV populations, future studies should take these factors into account and follow the latest MISEV guidelines. Researchers should consider adding EV purity (EV particles per μg protein), DNase/RNase/proteinase treatment and EV engineering before in vivo therapeutic research. Although current research has not yet standardized these factors, data from all 33 studies in this review suggest that periodontal (dental pulp) cell-derived EVs can function as potential therapeutics to promote periodontal regeneration and impart anti-inflammatory properties. However, investigating the effect of periodontal cell-derived EV on in vivo periodontal regeneration models is required to understand their potential therapeutic role in periodontal regeneration.
Funding
This work was supported by the Australian Dental Research Foundation (ADRF) grant number 534-2019. Karan Gulati is supported by the National Health and Medical Research Council (NHMRC) Early Career Fellowship (APP1140699).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Raposo G., Stahl P.D. Extracellular vesicles: A new communication paradigm? Nat. Rev. Mol. Cell Biol. 2019;20:509–510. doi: 10.1038/s41580-019-0158-7. [DOI] [PubMed] [Google Scholar]
- 2.Johnstone R.M., Adam M., Hammond J., Orr L., Turbide C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes) J. Biol. Chem. 1987;262:9412–9420. doi: 10.1016/S0021-9258(18)48095-7. [DOI] [PubMed] [Google Scholar]
- 3.Hirsch J.G., Fedorko M.E., Cohn Z.A. Vesicle fusion and formation at the surface of pinocytic vacuoles in macrophages. J. Cell Biol. 1968;38:629. doi: 10.1083/jcb.38.3.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van Niel G., D’Angelo G., Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018;19:213–228. doi: 10.1038/nrm.2017.125. [DOI] [PubMed] [Google Scholar]
- 5.Kalluri R., LeBleu V.S. The biology, function, and biomedical applications of exosomes. Science. 2020;367 doi: 10.1126/science.aau6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Théry C., Witwer K.W., Aikawa E., Alcaraz M.J., Anderson J.D., Andriantsitohaina R., Antoniou A., Arab T., Archer F., Atkin-Smith G.K., et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles. 2018;7:1535750. doi: 10.1080/20013078.2018.1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiao K., Walsh L.J., Ivanovski S., Han P. The emerging regulatory role of circular RNAs in periodontal tissues and cells. Int J. Mol. Sci. 2021;22:4636. doi: 10.3390/ijms22094636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pan B.-T., Teng K., Wu C., Adam M., Johnstone R.M. Electron microscopic evidence for externalization of the transferrin receptor in vesicular form in sheep reticulocytes. J. Cell Biol. 1985;101:942–948. doi: 10.1083/jcb.101.3.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boulbitch A. Deflection of a cell membrane under application of a local force. Phys. Rev. E. 1998;57:2123. doi: 10.1103/PhysRevE.57.2123. [DOI] [Google Scholar]
- 10.Bratton D.L., Fadok V.A., Richter D.A., Kailey J.M., Guthrie L.A., Henson P.M. Appearance of phosphatidylserine on apoptotic cells requires calcium-mediated nonspecific flip-flop and is enhanced by loss of the aminophospholipid translocase. J. Biol. Chem. 1997;272:26159–26165. doi: 10.1074/jbc.272.42.26159. [DOI] [PubMed] [Google Scholar]
- 11.Pols M.S., Klumperman J. Trafficking and function of the tetraspanin CD63. Exp. Cell Res. 2009;315:1584–1592. doi: 10.1016/j.yexcr.2008.09.020. [DOI] [PubMed] [Google Scholar]
- 12.Beinert T., Münzing S., Possinger K., Krombach F. Increased expression of the tetraspanins CD53 and CD63 on apoptotic human neutrophils. J. Leukoc. Biol. 2000;67:369–373. doi: 10.1002/jlb.67.3.369. [DOI] [PubMed] [Google Scholar]
- 13.Han P., Bartold P.M., Salomon C., Ivanovski S. Salivary small extracellular vesicles associated miRNAs in periodontal status—A pilot study. Int. J. Mol. Sci. 2020;21:2809. doi: 10.3390/ijms21082809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Han P., Bartold P.M., Salomon C., Ivanovski S. Salivary outer membrane vesicles and DNA methylation of small extracellular vesicles as biomarkers for periodontal status: A pilot study. Int. J. Mol. Sci. 2021;22:2423. doi: 10.3390/ijms22052423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Han P., Lai A., Salomon C., Ivanovski S. Detection of salivary small extracellular vesicles associated inflammatory cytokines gene methylation in gingivitis. Int. J. Mol. Sci. 2020;21:5273. doi: 10.3390/ijms21155273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shi Q., Huo N., Wang X., Yang S., Wang J., Zhang T. Exosomes from oral tissue stem cells: Biological effects and applications. Cell Biosci. 2020;10:108. doi: 10.1186/s13578-020-00471-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhan C., Yang X., Yin X., Hou J. Exosomes and other extracellular vesicles in oral and salivary gland cancers. Oral Dis. 2020;26:865–875. doi: 10.1111/odi.13172. [DOI] [PubMed] [Google Scholar]
- 18.Cocucci E., Racchetti G., Meldolesi J. Shedding microvesicles: Artefacts no more. Trends Cell Biol. 2009;19:43–51. doi: 10.1016/j.tcb.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 19.Ratajczak M.Z., Ratajczak J. Extracellular microvesicles/exosomes: Discovery, disbelief, acceptance, and the future? Leukemia. 2020;34:3126–3135. doi: 10.1038/s41375-020-01041-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muralidharan-Chari V., Clancy J.W., Sedgwick A., D’Souza-Schorey C. Microvesicles: Mediators of extracellular communication during cancer progression. J. Cell Sci. 2010;123:1603–1611. doi: 10.1242/jcs.064386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tricarico C., Clancy J., D’Souza-Schorey C. Biology and biogenesis of shed microvesicles. Small GTPases. 2017;8:220–232. doi: 10.1080/21541248.2016.1215283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Panfoli I., Santucci L., Bruschi M., Petretto A., Calzia D., Ramenghi L.A., Ghiggeri G., Candiano G. Microvesicles as promising biological tools for diagnosis and therapy. Exp. Rev. Proteom. 2018;15:801–808. doi: 10.1080/14789450.2018.1528149. [DOI] [PubMed] [Google Scholar]
- 23.King K., Cidlowski J. Cell cycle regulation and apoptosis. Annu. Rev. Physiol. 1998;60:601–617. doi: 10.1146/annurev.physiol.60.1.601. [DOI] [PubMed] [Google Scholar]
- 24.Caruso S., Poon I.K.H. Apoptotic cell-derived extracellular vesicles: More than just debris. Front. Immunol. 2018;9 doi: 10.3389/fimmu.2018.01486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kakarla R., Hur J., Kim Y.J., Kim J., Chwae Y.-J. Apoptotic cell-derived exosomes: Messages from dying cells. Exp. Mol. Med. 2020;52 doi: 10.1038/s12276-019-0362-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Opferman J.T., Korsmeyer S.J. Apoptosis in the development and maintenance of the immune system. Nat. Immunol. 2003;4:410–415. doi: 10.1038/ni0503-410. [DOI] [PubMed] [Google Scholar]
- 27.Ma Q., Liang M., Wu Y., Ding N., Duan L., Yu T., Bai Y., Kang F., Dong S., Xu J. Mature osteoclast-derived apoptotic bodies promote osteogenic differentiation via RANKL-mediated reverse signaling. J. Biol. Chem. 2019;294:11240–11247. doi: 10.1074/jbc.RA119.007625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ivanovski S., Gronthos S., Shi S., Bartold P.M. Stem cells in the periodontal ligament. Oral Dis. 2006;12:358–363. doi: 10.1111/j.1601-0825.2006.01253.x. [DOI] [PubMed] [Google Scholar]
- 29.Guo T., Gulati K., Arora H., Han P., Fournier B., Ivanovski S. Orchestrating soft tissue integration at the transmucosal region of titanium implants. Acta Biomater. 2021;124:33–49. doi: 10.1016/j.actbio.2021.01.001. [DOI] [PubMed] [Google Scholar]
- 30.Shi X., Mao J., Liu Y. Pulp stem cells derived from human permanent and deciduous teeth: Biological characteristics and therapeutic applications. Stem Cells Transl. Med. 2020;9:445–464. doi: 10.1002/sctm.19-0398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaukua N., Shahidi M.K., Konstantinidou C., Dyachuk V., Kaucka M., Furlan A., An Z., Wang L., Hultman I., Ährlund-Richter L., et al. Glial origin of mesenchymal stem cells in a tooth model system. Nature. 2014;513:551–554. doi: 10.1038/nature13536. [DOI] [PubMed] [Google Scholar]
- 32.Komada Y., Yamane T., Kadota D., Isono K., Takakura N., Hayashi S., Yamazaki H. Origins and properties of dental, thymic, and bone marrow mesenchymal cells and their stem cells. PLoS ONE. 2012;7:e46436. doi: 10.1371/journal.pone.0046436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chalisserry E.P., Nam S.Y., Park S.H., Anil S. Therapeutic potential of dental stem cells. J. Tissue Eng. 2017;8 doi: 10.1177/2041731417702531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dan H.X., Vaquette C., Fisher A.G., Hamlet S.M., Xiao Y., Hutmacher D.W., Ivanovski S. The influence of cellular source on periodontal regeneration using calcium phosphate coated polycaprolactone scaffold supported cell sheets. Biomaterials. 2014;35:113–122. doi: 10.1016/j.biomaterials.2013.09.074. [DOI] [PubMed] [Google Scholar]
- 35.Vaquette C., Fan W., Xiao Y., Hamlet S., Hutmacher D.W., Ivanovski S. A biphasic scaffold design combined with cell sheet technology for simultaneous regeneration of alveolar bone/periodontal ligament complex. Biomaterials. 2012;33:5560–5573. doi: 10.1016/j.biomaterials.2012.04.038. [DOI] [PubMed] [Google Scholar]
- 36.Staples R.J., Ivanovski S., Vaquette C. Fibre guiding scaffolds for periodontal tissue engineering. J. Periodontal Res. 2020;55:331–341. doi: 10.1111/jre.12729. [DOI] [PubMed] [Google Scholar]
- 37.Vaquette C., Saifzadeh S., Farag A., Hutmacher D.W., Ivanovski S. Periodontal tissue engineering with a multiphasic construct and cell sheets. J. Dental Res. 2019;98:673–681. doi: 10.1177/0022034519837967. [DOI] [PubMed] [Google Scholar]
- 38.Vaquette C., Mitchell J., Fernandez-Medina T., Kumar S., Ivanovski S. Resorbable additively manufactured scaffold imparts dimensional stability to extraskeletally regenerated bone. Biomaterials. 2021;269:120671. doi: 10.1016/j.biomaterials.2021.120671. [DOI] [PubMed] [Google Scholar]
- 39.Han P., Ivanovski S., Crawford R., Xiao Y. Activation of the canonical Wnt signaling pathway induces cementum regeneration. J. Bone Miner. Res. 2015;30:1160–1174. doi: 10.1002/jbmr.2445. [DOI] [PubMed] [Google Scholar]
- 40.Han P., Lloyd T., Chen Z., Xiao Y. Proinflammatory cytokines regulate cementogenic differentiation of periodontal ligament cells by Wnt/Ca(2+) signaling pathway. J. Interferon Cytokine Res. 2016;36:328–337. doi: 10.1089/jir.2015.0111. [DOI] [PubMed] [Google Scholar]
- 41.Gholami L., Nooshabadi V.T., Shahabi S., Jazayeri M., Tarzemany R., Afsartala Z., Khorsandi K. Extracellular vesicles in bone and periodontal regeneration: Current and potential therapeutic applications. Cell Biosci. 2021;11:16. doi: 10.1186/s13578-020-00527-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gegout P.Y., Stutz C., Olson J., Batool F., Petit C., Tenenbaum H., Benkirane-Jessel N., Huck O. Interests of exosomes in bone and periodontal regeneration: A systematic review. Adv. Exp. Med. Biol. 2020 doi: 10.1007/5584_2020_593. [DOI] [PubMed] [Google Scholar]
- 43.Novello S., Pellen-Mussi P., Jeanne S. Mesenchymal stem cell-derived small extracellular vesicles as cell-free therapy: Perspectives in periodontal regeneration. J. Periodontal Res. 2021;56:433–442. doi: 10.1111/jre.12866. [DOI] [PubMed] [Google Scholar]
- 44.Witwer K.W., Buzás E.I., Bemis L.T., Bora A., Lässer C., Lötvall J., Nolte-‘t Hoen E.N., Piper M.G., Sivaraman S., Skog J., et al. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J. Extracell. Vesicles. 2013;2:20360. doi: 10.3402/jev.v2i0.20360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Greening D.W., Xu R., Ji H., Tauro B.J., Simpson R.J. Proteomic Profiling. Springer; Cham, Switzerland: 2015. A protocol for exosome isolation and characterization: Evaluation of ultracentrifugation, density-gradient separation, and immunoaffinity capture methods; pp. 179–209. [DOI] [PubMed] [Google Scholar]
- 46.Böing A.N., Van Der Pol E., Grootemaat A.E., Coumans F.A., Sturk A., Nieuwland R. Single-step isolation of extracellular vesicles by size-exclusion chromatography. J. Extracell. Vesicles. 2014;3:23430. doi: 10.3402/jev.v3.23430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Karttunen J., Heiskanen M., Navarro-Ferrandis V., Das Gupta S., Lipponen A., Puhakka N., Rilla K., Koistinen A., Pitkänen A. Precipitation-based extracellular vesicle isolation from rat plasma co-precipitate vesicle-free microRNAs. J. Extracell. Vesicles. 2019;8:1555410. doi: 10.1080/20013078.2018.1555410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oliveira-Rodríguez M., López-Cobo S., Reyburn H.T., Costa-García A., López-Martín S., Yáñez-Mó M., Cernuda-Morollón E., Paschen A., Valés-Gómez M., Blanco-López M.C. Development of a rapid lateral flow immunoassay test for detection of exosomes previously enriched from cell culture medium and body fluids. J. Extracell. Vesicles. 2016;5:31803. doi: 10.3402/jev.v5.31803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Oeyen E., Van Mol K., Baggerman G., Willems H., Boonen K., Rolfo C., Pauwels P., Jacobs A., Schildermans K., Cho W.C. Ultrafiltration and size exclusion chromatography combined with asymmetrical-flow field-flow fractionation for the isolation and characterisation of extracellular vesicles from urine. J. Extracell. Vesicles. 2018;7:1490143. doi: 10.1080/20013078.2018.1490143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Konoshenko M.Y., Lekchnov E.A., Vlassov A.V., Laktionov P.P. Isolation of extracellular vesicles: General methodologies and latest trends. Biomed. Res. Int. 2018;2018:8545347. doi: 10.1155/2018/8545347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xie L., Chen J., Ren X., Zhang M., Thuaksuban N., Nuntanaranont T., Guan Z. Alteration of circRNA and lncRNA expression profile in exosomes derived from periodontal ligament stem cells undergoing osteogenic differentiation. Arch. Oral Biol. 2021;121:104984. doi: 10.1016/j.archoralbio.2020.104984. [DOI] [PubMed] [Google Scholar]
- 52.Zhang Z., Shuai Y., Zhou F., Yin J., Hu J., Guo S., Wang Y., Liu W. PDLSCs regulate angiogenesis of periodontal ligaments via VEGF transferred by exosomes in periodontitis. Int. J. Med. Sci. 2020;17:558–567. doi: 10.7150/ijms.40918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chiricosta L., Silvestro S., Gugliandolo A., Marconi G.D., Pizzicannella J., Bramanti P., Trubiani O., Mazzon E. Extracellular vesicles of human periodontal ligament stem cells contain MicroRNAs associated to proto-oncogenes: Implications in cytokinesis. Front. Genet. 2020;11:582. doi: 10.3389/fgene.2020.00582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhao M., Dai W., Wang H., Xue C., Feng J., He Y., Wang P., Li S., Bai D., Shu R. Periodontal ligament fibroblasts regulate osteoblasts by exosome secretion induced by inflammatory stimuli. Arch. Oral Biol. 2019;105:27–34. doi: 10.1016/j.archoralbio.2019.06.002. [DOI] [PubMed] [Google Scholar]
- 55.Čebatariūnienė A., Kriaučiūnaitė K., Prunskaitė J., Tunaitis V., Pivoriūnas A. Extracellular vesicles suppress basal and Lipopolysaccharide-induced NFκB activity in human periodontal ligament stem cells. Stem Cells Dev. 2019;28:1037–1049. doi: 10.1089/scd.2019.0021. [DOI] [PubMed] [Google Scholar]
- 56.Pizzicannella J., Gugliandolo A., Orsini T., Fontana A., Ventrella A., Mazzon E., Bramanti P., Diomede F., Trubiani O. Engineered extracellular vesicles from human periodontal-ligament stem cells increase VEGF/VEGFR2 expression during bone regeneration. Front. Physiol. 2019;10 doi: 10.3389/fphys.2019.00512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang Z., Maruyama K., Sakisaka Y., Suzuki S., Tada H., Suto M., Saito M., Yamada S., Nemoto E. Cyclic stretch force induces periodontal ligament cells to secrete exosomes that suppress IL-1β production through the inhibition of the NF-κB signaling pathway in macrophages. Front. Immunol. 2019;10 doi: 10.3389/fimmu.2019.01310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kang H., Lee M.-J., Park S.J., Lee M.-S. Lipopolysaccharide-preconditioned periodontal ligament stem cells induce M1 polarization of macrophages through extracellular vesicles. Int. J. Mol. Sci. 2018;19:3843. doi: 10.3390/ijms19123843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Diomede F., D’aurora M., Gugliandolo A., Merciaro I., Ettorre V., Bramanti A., Piattelli A., Gatta V., Mazzon E., Fontana A. A novel role in skeletal segment regeneration of extracellular vesicles released from periodontal-ligament stem cells. Int. J. Nanomed. 2018;13:3805. doi: 10.2147/IJN.S162836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rajan T.S., Giacoppo S., Diomede F., Ballerini P., Paolantonio M., Marchisio M., Piattelli A., Bramanti P., Mazzon E., Trubiani O. The secretome of periodontal ligament stem cells from MS patients protects against EAE. Sci. Rep. 2016;6:38743. doi: 10.1038/srep38743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nakao Y., Fukuda T., Zhang Q., Sanui T., Shinjo T., Kou X., Chen C., Liu D., Watanabe Y., Hayashi C., et al. Exosomes from TNF-α-treated human gingiva-derived MSCs enhance M2 macrophage polarization and inhibit periodontal bone loss. Acta Biomater. 2021;122:306–324. doi: 10.1016/j.actbio.2020.12.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yin S., Jia F., Ran L., Xie L., Wu Z., Zhan Y., Zhang Y., Zhang M. Exosomes derived from idiopathic gingival fibroma fibroblasts regulate gingival fibroblast proliferation and apoptosis. Oral Dis. 2020 doi: 10.1111/odi.13707. [DOI] [PubMed] [Google Scholar]
- 63.Zhuang X.-M., Zhou B. Exosome secreted by human gingival fibroblasts in radiation therapy inhibits osteogenic differentiation of bone mesenchymal stem cells by transferring miR-23a. Biomed. Pharmacother. 2020;131:110672. doi: 10.1016/j.biopha.2020.110672. [DOI] [PubMed] [Google Scholar]
- 64.Silvestro S., Chiricosta L., Gugliandolo A., Pizzicannella J., Diomede F., Bramanti P., Trubiani O., Mazzon E. Extracellular vesicles derived from human gingival mesenchymal stem cells: A transcriptomic analysis. Genes. 2020;11:118. doi: 10.3390/genes11020118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Coccè V., Franzè S., Brini A.T., Giannì A.B., Pascucci L., Ciusani E., Alessandri G., Farronato G., Cavicchini L., Sordi V. In vitro anticancer activity of extracellular vesicles (EVs) secreted by gingival mesenchymal stromal cells primed with paclitaxel. Pharmaceutics. 2019;11:61. doi: 10.3390/pharmaceutics11020061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mao Q., Nguyen P.D., Shanti R.M., Shi S., Shakoori P., Zhang Q., Le A.D. Gingiva-derived mesenchymal stem cell-extracellular vesicles activate schwann cell repair phenotype and promote nerve regeneration. Tissue Eng. Part A. 2019;25:887–900. doi: 10.1089/ten.tea.2018.0176. [DOI] [PubMed] [Google Scholar]
- 67.Diomede F., Gugliandolo A., Cardelli P., Merciaro I., Ettorre V., Traini T., Bedini R., Scionti D., Bramanti A., Nanci A. Three-dimensional printed PLA scaffold and human gingival stem cell-derived extracellular vesicles: A new tool for bone defect repair. Stem Cell Res. Ther. 2018;9:104. doi: 10.1186/s13287-018-0850-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Faruqu F.N., Zhou S., Sami N., Gheidari F., Lu H., Al-Jamal K.T. Three-dimensional culture of dental pulp pluripotent-like stem cells (DPPSCs) enhances Nanog expression and provides a serum-free condition for exosome isolation. FASEB BioAdv. 2020;2:419–433. doi: 10.1096/fba.2020-00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhou H., Li X., Yin Y., He X.-T., An Y., Tian B.-M., Hong Y.-L., Wu L.-A., Chen F.-M. The proangiogenic effects of extracellular vesicles secreted by dental pulp stem cells derived from periodontally compromised teeth. Stem Cell Res. Ther. 2020;11 doi: 10.1186/s13287-020-01614-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ivica A., Ghayor C., Zehnder M., Valdec S., Weber F.E. Pulp-derived exosomes in a fibrin-based regenerative root filling material. J. Clin. Med. 2020;9:491. doi: 10.3390/jcm9020491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xie L., Guan Z., Zhang M., Lyu S., Thuaksuban N., Kamolmattayakul S., Nuntanaranont T. Exosomal circLPAR1 promoted osteogenic differentiation of homotypic dental pulp stem cells by competitively binding to hsa-miR-31. BioMed Res. Int. 2020;2020:6319395. doi: 10.1155/2020/6319395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shen Z., Kuang S., Zhang Y., Yang M., Qin W., Shi X., Lin Z. Chitosan hydrogel incorporated with dental pulp stem cell-derived exosomes alleviates periodontitis in mice via a macrophage-dependent mechanism. Bioact. Mater. 2020;5:1113–1126. doi: 10.1016/j.bioactmat.2020.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li J., Ju Y., Liu S., Fu Y., Zhao S. Exosomes derived from lipopolysaccharide-preconditioned human dental pulp stem cells regulate Schwann cell migration and differentiation. Connect. Tissue Res. 2019;62:277–286. doi: 10.1080/03008207.2019.1694010. [DOI] [PubMed] [Google Scholar]
- 74.Ji L., Bao L., Gu Z., Zhou Q., Liang Y., Zheng Y., Xu Y., Zhang X., Feng X. Comparison of immunomodulatory properties of exosomes derived from bone marrow mesenchymal stem cells and dental pulp stem cells. Immunol. Res. 2019;67:432–442. doi: 10.1007/s12026-019-09088-6. [DOI] [PubMed] [Google Scholar]
- 75.Hu X., Zhong Y., Kong Y., Chen Y., Feng J., Zheng J. Lineage-specific exosomes promote the odontogenic differentiation of human dental pulp stem cells (DPSCs) through TGFβ1/smads signaling pathway via transfer of microRNAs. Stem Cell Res. Ther. 2019;10:170. doi: 10.1186/s13287-019-1278-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Huang C.-C., Narayanan R., Alapati S., Ravindran S. Exosomes as biomimetic tools for stem cell differentiation: Applications in dental pulp tissue regeneration. Biomaterials. 2016;111:103–115. doi: 10.1016/j.biomaterials.2016.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liu Y., Zhuang X., Yu S., Yang N., Zeng J., Liu X., Chen X. Exosomes derived from stem cells from apical papilla promote craniofacial soft tissue regeneration by enhancing Cdc42-mediated vascularization. Stem Cell Res. Ther. 2021;12:76. doi: 10.1186/s13287-021-02151-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhuang X., Ji L., Jiang H., Liu Y., Liu X., Bi J., Zhao W., Ding Z., Chen X. Exosomes derived from stem cells from the apical papilla promote dentine-pulp complex regeneration by inducing specific dentinogenesis. Stem Cells Int. 2020;2020:5816723. doi: 10.1155/2020/5816723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang M., Li J., Ye Y., He S., Song J. SHED-derived conditioned exosomes enhance the osteogenic differentiation of PDLSCs via Wnt and BMP signaling in vitro. Differentiation. 2020;111 doi: 10.1016/j.diff.2019.10.003. [DOI] [PubMed] [Google Scholar]
- 80.Wei J., Song Y., Du Z., Yu F., Zhang Y., Jiang N., Ge X. Exosomes derived from human exfoliated deciduous teeth ameliorate adult bone loss in mice through promoting osteogenesis. J. Mol. Histol. 2020;51:455–466. doi: 10.1007/s10735-020-09896-3. [DOI] [PubMed] [Google Scholar]
- 81.Luo P., Jiang C., Ji P., Wang M., Xu J. Exosomes of stem cells from human exfoliated deciduous teeth as an anti-inflammatory agent in temporomandibular joint chondrocytes via miR-100-5p/mTOR. Stem Cell Res. Ther. 2019;10:216. doi: 10.1186/s13287-019-1341-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wu J., Chen L., Wang R., Song Z., Shen Z., Zhao Y., Huang S., Lin Z. Exosomes secreted by stem cells from human exfoliated deciduous teeth promote alveolar bone defect repair through the regulation of angiogenesis and osteogenesis. ACS Biomater. Sci. Eng. 2019;5:3561–3571. doi: 10.1021/acsbiomaterials.9b00607. [DOI] [PubMed] [Google Scholar]
- 83.Shi W., Guo S., Liu L., Liu Q., Huo F., Ding Y., Tian W. Small extracellular vesicles from lipopolysaccharide-preconditioned dental follicle cells promote periodontal regeneration in an inflammatory microenvironment. ACS Biomater. Sci. Eng. 2020;6:5797–5810. doi: 10.1021/acsbiomaterials.0c00882. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.