Abstract
The intake of food may be an initiator of adverse reactions. Food intolerance is an abnormal non-immunological response of the organism to the ingestion of food or its components in a dosage normally tolerated. Despite the fact that food intolerance is spread throughout the world, its diagnosing is still difficult. Histamine intolerance (HIT) is the term for that type of food intolerance which includes a set of undesirable reactions as a result of accumulated or ingested histamine. Manifestations may be caused by various pathophysiological mechanisms or a combination of them. The problem with a “diagnosis” of HIT is precisely the inconstancy and variety of the manifestations in the same individual following similar stimuli. The diagnosing of HIT therefore requires a complex time-demanding multidisciplinary approach, including the systematic elimination of disorders with a similar manifestation of symptoms. Among therapeutic approaches, the gold standard is a low-histamine diet. A good response to such a diet is considered to be confirmation of HIT. Alongside the dietary measures, DAO supplementation supporting the degradation of ingested histamine may be considered as subsidiary treatment for individuals with intestinal DAO deficiency. If antihistamines are indicated, the treatment should be conscious and time-limited, while 2nd or 3rd generation of H1 antihistamines should take precedence.
Keywords: histamine intolerance, histamine, diamine oxidase, DAO, low-histamine diet, probiotics, food intolerance
1. Introduction
The intake of food may be an initiator of adverse reactions. We are referring to any kind of abnormal reaction related to the intake of foods. Adverse food reactions are nowadays rather accepted in practice but are however less frequently objectively examined [1]. In addition to specific and well-differentiated disorders, allergic reactions and food aversions, we also class food intolerance among them. Distinguishing food intolerance from an allergic reaction to food is possible on the basis of key pathophysiological differences through the use of relevant diagnostic approaches. Food allergy is an inadequate response of the immune system to an antigen (in the majority of cases of a protein nature) ingested in food, that is accompanied by IgE or non-IgE (cellular) immunological mechanisms. Its cumulative prevalence is 3–6%, appearing much more frequently in children [2]. Testing for foods IgG or IgA antibodies is not of fundamental clinical importance [3,4]. Levels of these antibodies may rather reflect an intestinal permeability disorder regardless of its origin, which is often post-infectious [5]. One specific form of an adverse food reaction with an immunopathological feature is celiac disease, in which genetic disposition and epigenetic influences lead to adverse immunity reactions to gluten.
Food intolerance is an abnormal non-immunological response of the organism to the ingestion of food or its components in a dosage normally tolerated [6]. It is at the same time a simplified term for non-allergic food hypersensitivity according to the World Health Organization (WHO) [7]. Food hypersensitivity belongs among the most frequently occurring undesirable reactions to food. It affects from 15–20% of the population and can be the result of the pharmacological effects of food ingredients, non-celiac gluten sensitivity or malfunction of enzyme(s) or transport [8].
Despite the fact that food intolerance is spread throughout the world, its diagnosing is difficult and demanding. For example, in patients with suspected histamine intolerance (HIT) it is necessary to carefully consider other possible reasons for the manifestations of symptoms (Table 1) [1]. Food intolerance (and especially HIT) requires a comprehensive understanding of the symptoms, especially their diversity, severity and time of onset [6].
Table 1.
Symptoms and differential diagnostics in patients with suspected adverse reactions to ingested histamine. Adapted according to Reese et al. 2017 [1].
| Symptoms | Differential Diagnosis |
|---|---|
| Flushing | Neuroendocrine tumors |
| Itching | Urticaria, pruritus sine materia, prurigo |
| Nausea/vomiting/abdominal pain | Peptic ulcer disease, hiatal hernia, gastroesophageal reflux disease |
| Diarrhea and abdominal pain | Chronic inflammatory bowel disorders, disorders of carbohydrate metabolism |
| (lactose intolerance, fructose malabsorption), celiac disease | |
| Rhinitis | Allergic and non-allergic rhinitis of other origin |
| Dyspnea, dysphonia | Allergic and non-allergic asthma |
| Hypotension, vertigo, tachycardia | Anaphylaxis |
| Important differential diagnostic information is achieved by the analysis of symptoms with respect to their onset time. Adverse food reactions only considered if the symptoms manifested in less than 4 h from food intake. | |
In this review, we provide a critical overview on possible benefits of published diagnostic approaches. We present the current knowledge of the therapeutic options and suggest the management of HIT accordingly. Another strength of this review lies in its comprehensiveness of tables summarizing data of clinical importance.
1.1. Histamine Intolerance (HIT)
HIT is the term for that type of food intolerance which includes a set of undesirable reactions as a result of accumulated or ingested histamine. In the German guideline from 2017, German and Swiss specialists prefer the term “adverse reactions to ingested histamine” [1]. In older publications, this type of intolerance is designated by the expressions pseudoallergy, enteral histaminosis or histamine sensitivity. HIT is defined as a condition caused by an imbalance between the histamine released from food and the ability of the organism to degrade such an amount. It is accompanied by decreased activity of the DAO enzyme, leading to an increased concentration of histamine in plasma and the emergence of adverse reactions. In some publications, the state of decreased activity of DAO is referred to as a DAO deficiency. DAO deficiency predisposes a certain subgroup of the population to HIT. It can be of genetic, pathologic or pharmacological origin [9].
It is necessary to distinguish HIT from histamine intoxication designated as scrombroid syndrome, scombroidosis or histamine poisoning. The term originates from the name of the mackerel fish family (Scombridae), after the consumption of which the intoxication was most often observed. The Scombridae family includes tuna, herring and mackerel. Histamine poisoning is considered worldwide as one of the most frequent intoxications caused by the consuming of fish (Dalgaard, 2008 in [10]). According to Colombo et al., from 103 analysed samples which caused histamine poisoning, 101 showed fish or seafood sources, and only two contained cheese [10]. Manifestations of histamine intoxication may include rash, abdominal pain, vomiting, diarrhoea and shortness of breath, and the intoxication may also have a fatal outcome [11].
The term HIT is used in a similar manner as the concept of lactose intolerance (which occurs due to a lack of the lactase enzyme), since it is presumed that the HIT symptoms are related to a lack or diminished activity of the enzyme DAO. Ingested exogenous histamine is distributed into the blood stream and may trigger symptoms in the susceptible population. It should be stated that with HIT, the amount of histamine taken in is much lower than with histamine poisoning. HIT manifestations also have a milder course in comparison with intoxication [10].
With intolerances, gender-specific variations generally apply; women are affected with intolerances more frequently than men, although this distinction is not satisfactorily explained [12]. Increased sensitivity to the intake of histamine was observed in women in the premenstrual phase [13]. Serum diamine oxidase (DAO) levels in premenopausal women appear to be associated with the menstrual cycle, with higher DAO activity measured during the luteal phase compared to the follicular phase [14]. Painful menstruation may be associated with increased sensitivity to histamine. Administration of H1 antihistamines on the first day of menstruation has had a preventive effect on dysmenorrhea. High levels of histamine metabolites in urine during the ovulatory phase could be related to the effect of oestrogens (especially oestradiol) [15].
Manifestations of HIT
One of the reasons why the adverse reactions caused by the intake of histamine cannot be clearly defined and outlined, as against other sicknesses, is their heterogeneity. Due to the fact that histamine enters into the circulation and that histamine receptors occur ubiquitously in the human body, a typical clinical picture cannot be strictly defined. The adverse manifestations related to the intake of histamine are usually complex and may affect different organ systems. Paradoxically, if the set of manifestations appears in various ways, unexpectedly and randomly, and at the same time following the ingestion of food, the symptoms may have their origin in histamine intake.
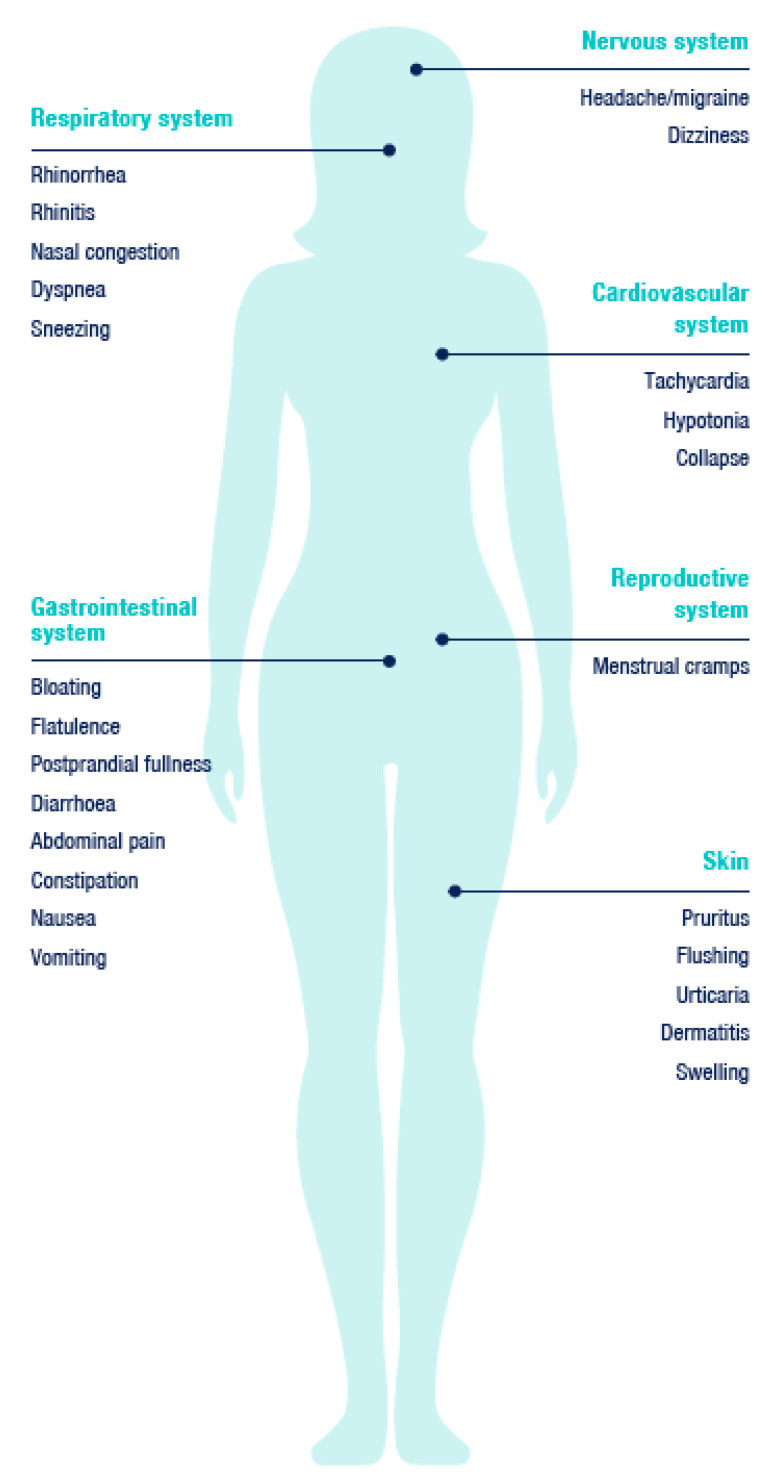
As typical signs, we can observe skin manifestations—for example erythema in the facial area (flushing), pruritus, urticarial rash on the body. Gastrointestinal symptoms include diarrhoea (±vomiting) but also constipation and abdominal pain. Manifestations in the cardiovascular system, such as low blood pressure (counter-regulatory hypertension may subsequently occur) and tachycardia are less frequent [1], as are manifestations in the nervous and respiratory systems (Figure 1) [9].
Figure 1.
Typical manifestations related to the intake of exogenous histamine. Adapted according to [9].
The problem with a “diagnosis” of HIT is precisely the inconstancy and variety of the manifestations in the same individual following similar stimuli. In cross-over placebo-controlled trials in which symptoms were assessed, the subjects reacted randomly to the histamine provocation test. Although the total score of symptoms when histamine was administered was significantly higher as compared to the placebo, with many individuals no relationship between the ingestion of the histamine and the individual symptoms could be established [16].
New findings have been recorded just recently. A 45-year-old woman who experienced Nissen’s fundoplication for long-lasting laryngopharyngeal reflux developed episodes of throat clearing and coughing. Laryngopharyngeal reflux indicates the return flow of gastric contents to the laryngopharynx and upper aerodigestive space. It is a clinical unit different from the gastroesophageal reflux disease. In this case, consultations with nutrition specialists led to considerations of HIT. A low-histamine diet led to a significant improvement in the patient’s symptoms. For patients who do not respond according to expectations to typical laryngopharyngeal reflux treatment, a potential link to HIT should be taken into consideration [17], with a 3-month diet treatment prior to a possible operation.
In another study, 30 laryngopharyngeal reflux patients with chronic coughs underwent a histamine provocation test. Using a visual analogue scale, videolaryngostroboscopy findings and voice and throat symptoms were assessed directly before and after the exposure test. Moreover, the correlation between the relative changes in spirometry values in relation to changes in vocal fold oedema was also evaluated, along with redness and changes in the voice and throat symptoms reported by the patients during the histamine provocation test. The relative changes in inspiratory and expiratory air flow and voice and throat symptoms during the histamine challenge test correlated. Histamine induced oedema of the vocal folds, visible by videolaryngostroboscopic imaging, did not significantly influence spirometric air flow values [18].
1.2. Histamine
Histamine is a neuro-immuno-endocrine system mediator. In the human organism it influences the whole spectrum of physiologic functions of various tissues and cells, including immunity. From a chemical perspective, it is a ubiquitously occurring biogenic amine. In the organism, its synthesis is ensured by decarboxylation of the amino acid L-histidine by the L-histidine decarboxylase enzyme. In the human organism, histamine is primarily stored in the mast cells and basophils, but its presence has also been found in the enterochromaffin cells [19] and in the histaminergic neurons [20]. Histamine acts in the organism as an agonist of histamine H1, H2, H3 and H4 receptors. H1 and H2 receptors appear ubiquitously, with H2 mostly present in the digestive tract (stomach, duodenum, small intestine). The H3 receptors are abundant in the nervous system. The H4 receptors are present in certain tissues (skin, tonsils), but in a small amount [21]. Among other processes, histamine mediates inflammatory responses, vasodilation, gastric acid production in enterochromaffin cells, congestion and bronchospasm, and secretion in the respiratory system. Its pleiotropic effect was found in the nervous system, where it acts as a neuromediator and a neurohormone, influencing e.g., thermoregulation, alertness, appetite and cognitive and behavioural functions [22]. The microbiome can also be a source of histamine in the macroorganism [9]. Its production has been described in some species (see the Microbiome and HIT section). Food is the main exogenous source of histamine [23].
Metabolism of Histamine
The quantity of endogenous histamine is controlled on a genetic level. In genes encoding the enzymes responsible for the synthesis and degradation of histamine, similarly as in histamine receptor-encoding genes, genetic polymorphisms have been identified [21]. Genetic polymorphisms for histamine receptors and for DAO are most likely associated with several specific symptoms and their combinations [24]. In certain polymorphisms of the gene encoding DAO (and similarly, the H3 receptor), diminished activity of this enzyme has been reported, which increases the risk of migraines. Reduced DAO activity however has also been recorded in healthy individuals. In addition to genetic predisposition, several factors (e.g., variability of histamine content in food etc.) appear to be responsible for the manifestation of symptoms; hence the functional and clinical significance of genetic polymorphisms remains elusive [21,24].
The half-life of histamine in plasma is relatively short, a few minutes [25]. Histamine is metabolized in several pathways in the organism. As clinically most significant is considered enzymatic degradation mediated by the DAO enzyme, with a second pathway represented by the histamine-N-methyl transferase enzyme (HNMT) [1].
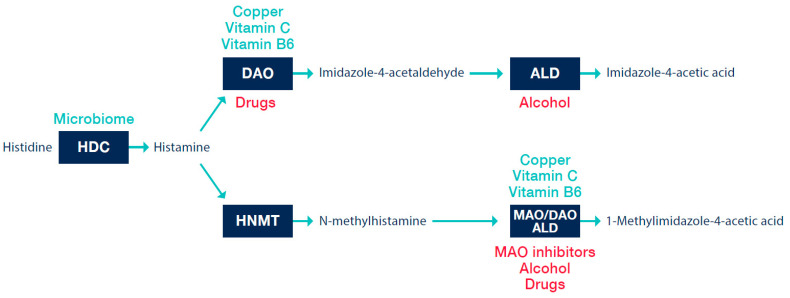
The DAO enzyme is also identified in the literature according to the gene that encodes it, AOC1 Amine Oxidase Copper Containing 1 [26], formerly known as histaminase. The DAO molecule contains copper and is the essential enzyme responsible for the degradation of histamine from the extracellular space [27]. The product of oxidative deamination of histamine is imidazole-4-acetaldehyde (Figure 2).
Figure 2.
Histamine metabolism in vivo. HDC—histidine decarboxylase; DAO—diamine oxidase; ALD—aldehyde dehydrogenase; HNMT—histamine-N-methyl transferase; MAO—monoamine oxidase. The green are factors potentiating an endogenous capacity of enzymatic reaction. The red are factors directly/indirectly inhibiting an enzymatic reaction. Adapted according to [9,13,21,27].
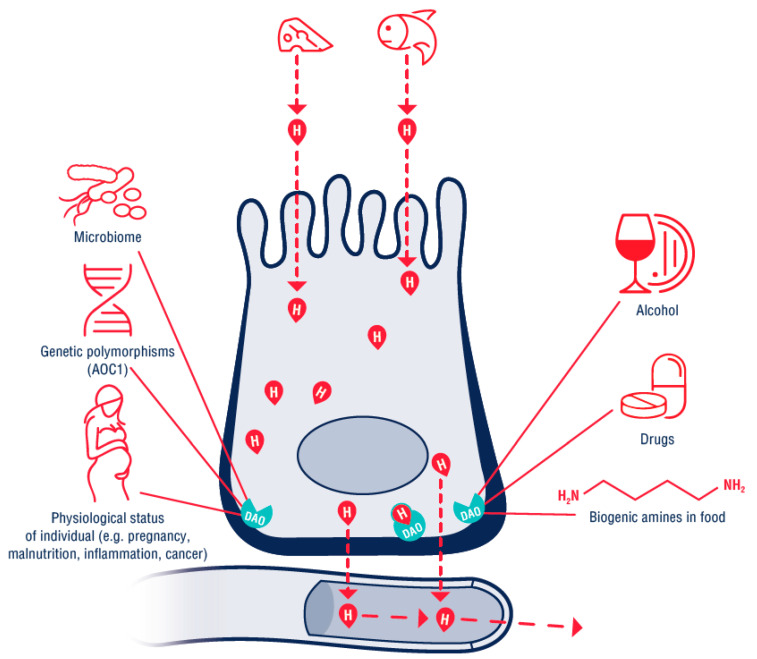
DAO is found in the epithelial cells of the (small) intestine, the placenta, the kidneys, the thymus and seminal plasma [28]. The physiological function of the DAO enzyme includes regulation of the inflammation processes, proliferation, allergic response and ischemia [29]. During digestion, the DAO enzyme is continuously synthesized in the mucosa of the small intestine. It is stored in vesicular structures on the basolateral membrane of the enterocytes and acts as a metabolic barrier against exogenous diamines, including histamine [30]. The accumulation of ingested histamine and its subsequent penetration into the circulation as a result of reduced or slowed catabolism by the DAO enzyme at the level of the small intestinal epithelium is considered as a possible reason for the HIT syndrome [6]. The activity and plasmatic level of the DAO may be dependent on the genetic variability of the relevant genes (AOC1 on the 7th chromosome) [21], or on the physiological state of the organism [13]. During pregnancy, greatly increased concentrations (up to 150-fold) of serum and plasma DAO were measured [27]—the placenta is a producer of this enzyme. This is regarded as the reason why during pregnancy, in women suffering from HIT manifestations, a lessening or complete regression of HIT symptoms is observed. DAO activity or histamine release may be influenced by a number of commonly used medicaments, such as N-acetylcysteine, ambroxol, verapamil, propafenone, amiloride, cefuroxime, clavulanic acid or non-steroidal anti-inflammatory drugs, metamizole, as well as radiological contrast agents (Figure 3) [13,31]. We summarize an extended list of substances possibly interfering with the activity of DAO in Table 2.
Figure 3.
The function of diamine oxidase (DAO) in the enterocyte. The red drop with H shows the histamine released from food. The histamine passes through the enterocyte into the circulation. The DAO enzyme on the basolateral membrane creates a barrier, and the histamine obtained from the food is metabolized (the red drop with H with a green contour). DAO activity is directly/indirectly dependent on internal and external factors such as the polymorphisms of the AOC1 gene, the physiological status of the organism, alcohol, other biogenic amines and medication intake. AOC1—amine oxidase copper containing 1 gene.
Table 2.
Drugs and substances with possible effects on the metabolism and the distribution of histamine in the organism, which encompass decreasing DAO activity. Adapted according to [13,31,32,33,34].
| DAO blocking foods | |
| Alcohol | wine and spirits especially |
| OTC drugs interfering with DAO activity (decreasing DAO activity) | |
| Expectorants, mucolytics | ambroxol, N-acetylcysteine |
| Nonsteroidal anti-inflammatory drugs | acetylsalicylic acid, ibuprofen |
| Rx drugs interfering with histamine metabolism or distribution | |
| Prokinetics | metoclopramide |
| Antiinfectives | clavulanic acid, isoniazid, cefuroxime, cefotiame, pentamidine, chloroquine, doxycycline, neomycin B, acriflavine, D-cycloserine |
| Bronchodilators | aminophylline, theophylline |
| Diuretics | amiloride, furosemide |
| Antidepressants | amitriptyline, monoaminooxidase 1 inhibitors |
| Anxiolytics | diazepam, barbiturates |
| Antipsychotics | haloperidol |
| Cytostatics | cyclophosphamide |
| Antihypertensives | verapamil, dihydrazine, alprenolol |
| Cardiotonics | dobutamine, dopamine |
| Opioids | pethidine, morphine, codeine |
| Analgesics | metamizole |
| Local anaesthetics | lidocaine, prilocaine, marcaine, procaine |
| General anaesthetics | thiopental |
| Muscle relaxants | pancuronium, alcuronium, D-tubocurarine |
| Antiarrhytmics | propafenone, verapamil, quinidine |
| Antihistamines decreasing DAO activity | |
| H1/H2 receptor blockers | cimetidine, promethazine |
| Other substances interfering with histamine metabolism | |
| Radiocontrast agents | iodine containing |
OTC-over the counter.
HNMT is a cytosolic enzyme whose role is to regulate intracellular histamine levels [6]. The inactivation of intracellular histamine is mediated by the methylation of the imidazole nucleus; this metabolite is subsequently oxidized [13]. Although it is also found in the gastrointestinal tract, it is unlikely to play a major role in the degradation of exogenous histamine or histamine produced by the gut microbiome [9].
1.3. Biogenic Amines in Food
Biogenic amines may be present in greater or lesser amounts in any food. Processing and storage are generally inevitable in cases where the ingredients spoil quickly and/or are rich in proteins. Storage raises the risk of accumulation of biogenic amines. It seems that their accumulation is totally dependent on the microorganisms that create histamine during food storage (especially in case of foods with a high L-histidine content) [35]. Overall, the fresher the food, the lower the probability of biogenic amine formation.
Amines are classified as monoamines, diamines and polyamines, depending on how many amine groups they contain. Among the most important biogenic amines found in food are monoamine tyramine, diamines histamine, putrescine and cadaverine, as well as the polyamines spermine and spermidine [36].
In the context of HIT syndrome, of clinical significance is tyramine, which may be present in excessive amounts in certain types of ripening cheeses. In sensitive people, this is related to increased blood pressure and the consequent occurrence of migraine pains [37].
Biogenic amines may contribute to histamine toxicity by saturating enzymes responsible for the degradation of histamine in the mucosa (DAO, HNMT). The diamines putrescine and cadaverine are considered to be the amines with the greatest influence on the metabolism of histamine. This is due to the fact that the DAO enzyme breaks down them preferentially [13,23,36]. Foods with a high biogenic amine content are generally considered as risky and should be omitted from low-histamine diets [9,23].
Values of Biogenic Amines and Histamine in Foods
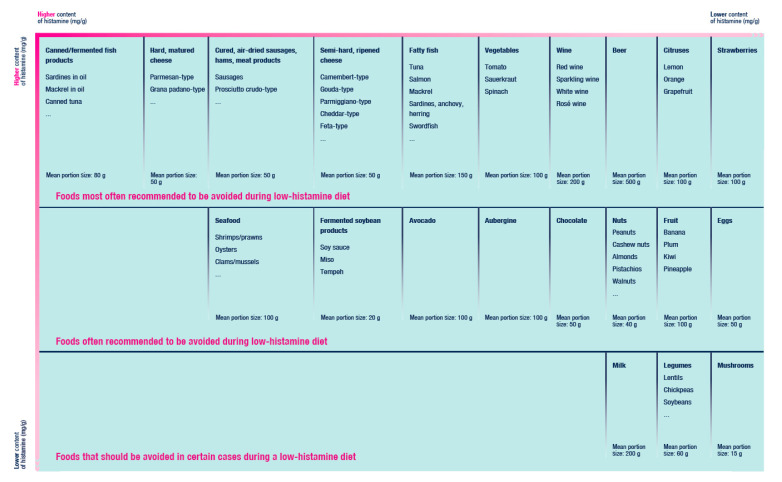
A diet that ensures the complete elimination of histamine is unattainable [38]. The content of biogenic amines and histamine in foods differs in dependence on their source, freshness, types, pH, salt content, content of proteins (and L-histidine), processing and storage [13,23,39]. The wide range of content of histamine and/or other biogenic amines for individual foods makes these parameters inconclusive and so we do not regard the listing of specific value intervals per 100 g of food as authoritative. In Figure 4, we present a list of foods that are most often recommended to be excluded from diet in case of suspected HIT.
Figure 4.
Foods that are most often recommended to be excluded from low-histamine diets. Adapted according to [9].
In Table 3, we list foods that in usual quantities are considered safe from triggering HIT symptoms.
Table 3.
Foods that in usual quantities are considered safe from triggering HIT symptoms. Adapted according to [40].
| Water, coffee, tea, homemade juices from allowed fruits and vegetables |
| Bread, pastry, potatoes, rice, pasta, cereals, millet, buckwheat, corn |
| Yoghurt, fresh soft cheese |
| Lettuce, cauliflower, broccoli, chicory, carrot, garlic, onion, cucumber, pumpkin, zucchini, pepper, radish, artichoke, rhubarb, asparagus |
| Apple, pear, cherry, amarelle, peach, apricot, watermelon, blueberries |
| Spices, herbs |
| Vegetable oil, vinegar |
| FRESH/IMMEDIATELY FROZEN meat: poultry, veal, beef, lamb, pork |
| FRESH/IMMEDIATELY FROZEN fish: cod/pollock, trout, zander, halibut |
| Ham (fresh, cooked and high-quality), eggs (cooked) |
| Jam made from allowed fruits, honey, butter, margarine |
In general, biogenic amines are thermostable. If they are already present in the food, heat treatment does not significantly degrade them [36]. However, boiling in water can reduce the biogenic amines content in certain types of vegetables, most likely by transferring them from the food to the water. Boiling spinach reduced the histamine level by 83% when compared to raw spinach, while analysis confirmed the transfer of the histamine from the spinach to the water (Latorre-Moratalla et al., 2015, in [23]). Heat treatment needs not always lead to a reduction of the biogenic amines contained in the food however. Heat treatment in the form of boiling and grilling showed an increase of the biogenic amines content in mg/100 g in aubergine, green and yellow beans [41,42]. In a work by Chung, the histamine content in mg/100 g in grilled seafood and meat increased, whereas boiling these foods reduced the histamine content in the meat. Boiling the vegetable had no influence on the content of histamine or reduced it only minimally [43].
1.4. Factors Contributing to Increased Sensitivity to Histamine
Among the factors increasing the sensitivity of individuals to the ingestion of histamine are classed other biogenic amines, alcohol (blocks the enzyme DAO, can release endogenous histamine), specific medications (with an inhibitory effect on DAO) and malnutrition, leading to an insufficiency of enzyme cofactors (vitamin C, copper, vitamin B6) [13].
1.5. Microbiome and HIT
In 2018, Schink compared microbial patterns from 33 healthy individuals with 33 persons with suspected HIT, 8 of whom had decreased DAO enzyme activity in serum. In comparison with those patients suspected of HIT presence, the healthy people showed a greater abundance of the Bifidobacteriaceae family, with a median of 0.3% [44]. To this family belongs the Bifidobacterium genus, which confers health benefits to the host [45]. In persons having decreased DAO activity in serum, a greater abundance of the Proteobacteria genus was observed. The higher ratio in favour of Proteobacteria genus, which competes with strict anaerobes (including bacteria of the genus Bifidobacterium), may predict dysbiosis and/or impaired intestinal epithelial function [44]. If the bifidobacteria were used as a starting culture in the production of fermented sausages, the end products contained lower amounts of biogenic amines [46].
Some bacterial strains also have an enzyme that ensures endogenous histamine synthesis in the human body. It should be emphasized that the presence of bacterial L-histidine decarboxylase is strain-, not species-, specific [47]. Accordingly, it is not possible to extrapolate this property from one strain to another, although within the same species. Hence, it is imperative to always assess independently the amount of produced histamine for the individual strains of bacterial species.
Certain strains which are considered potentially probiotic, for example Lactobacillus saerimneri 30a, produce a significant amount of histamine and other biogenic amines [48,49,50]. In the wide commercially used bacteria Limosilactobacillus reuteri DSM 17938, the presence of genes responsible for the synthesis of this enzyme was not proven [51]. From the 15 strains of the bacteria Lactobacillus acidophilus, Lacticaseibacillus casei, Lactobacillus delbrueckii ssp. bulgaricus, Lactobacillus lactis ssp. lactis, Lactococcus lactis ssp. lactis and Lactiplantibacillus plantarum, only two strains, L. casei TISTR 389 and L. bulgaricus TISTR 895, appeared to be potentially histamine-producing [47].
Certain strains of the following bacteria, yeasts and moulds genera and species have the capacity for histamine formation. In Table 4, we summarize microbial genera and species in which the presence of gene for the L-histidine decarboxylase was demonstrated.
Table 4.
Microbial genera or species which may have the capacity for histamine formation. Adapted according to [39,50,52,53].
| Bacteria | ||
|---|---|---|
| Acinetobacter spp. | Chryseobacterium spp. | Pediococcus pentosaceus |
| Alcalingenes faecalis | Klebsiella oxytoca | Proteus spp. |
| Klebsiella pneumoniae | ||
| Arizona spp. | Providencia spp. | |
| Kluyvera spp. | Providencia heimbachae | |
| Cedecea spp. | ||
| Lacticaseibacillus casei | Pseudomonas putida | |
| Citrobacter freundii | Lacticaseibacillus paracasei | Pseudomonas lundensis |
| Citrobacter braakii | Lacticaseibacillus rhamnosus | Pseudomonas stutzeri |
| Lactiplantibacillus plantarum | ||
| Lactobacillus curvatus | ||
| Edwardsiella spp. | Lactobacillus delbrueckii | Psychrobacter spp. |
| Lactobacillus helveticus | ||
| Enterococcus casseliflavus | Lactococcus lactis ssp. lactis | Raoultella planticola |
| Enterococcus faecalis | Latilactobacillus spp. | Raoultella ornithinolytica |
| Enterococcus faecium | Lentilactobacillus buchneri | |
| Lentilactobacillus hilgardii | Salmonella enterica ssp. arizonae | |
| Enterobacter spp. | Lentilactobacillus parabuchneri | |
| Leuconostoc spp. | ||
| Escherichia coli | Levilactobacillus brevis | Serratia spp. |
| Escherichia fergusonii | Limosilactobacillus reuteri | |
| Limosilactobacillus vaginalis | Sphingobacterium spp. | |
| Hafnia alvei | ||
| Hafnia paralvei | Microbacterium foliorum | Streptococcus thermophilus |
| Halomonas spp. | Morganella morganii | Tetragenococcus halophilus |
| Yeasts and moulds | ||
| Debaryomyces hansenii | Geotrichum candidum | |
The presence of such bacteria in the gastrointestinal tract could for certain individuals increase sensitivity to ingested histamine. In a study from 2013, the effect of the histamine-producing strain of the Lacticaseibacillus rhamnosus species was studied in mice. Frei et al. came to the conclusion that alteration of the innate immune response (e.g., dendritic cells) may be mediated through the H2 receptor not only by endogenous histamine, but also by histamine produced by the microbiome [53]. The L. rhamnosus LGG and L. rhamnosus Lc705 strains suppressed the expression of the H4 receptor of mast cells and decreased mast cell activation and IgE response [54].
Since microorganisms play a crucial role in histamine formation; they have been studied for their ability to degrade biogenic amines in foods, particularly histamine and tyramine [55,56,57]. The Lactiplantibacillus plantarum D-103 strain was able to degrade histamine up to 100% in histamine MRS broth [57]. Although the microbial catabolic activities responsible for histamine degradation have yet to be completely elucidated, microbial copper-containing amine oxidases, such as histamine oxidase, are most likely involved [55,57]. The capacity of microorganisms to degrade biogenic amines is strain-specific [55]. In the future, eligible strains could be exploited to control the accumulation of biogenic amines in certain foods (e.g., cheese, wine, miso) [55,56,57]. This approach would require an excellent knowledge of the microbial metabolism, since some by-products of the enzymatic reactions (e.g., hydrogen peroxide) are not desirable [55]. Moreover, some strains could have properties for concomitant degradation and production of biogenic amines [56].
2. Diagnostic Approaches
HIT is currently not a nosological unit. Direct HIT-specific diagnostic criteria or markers are lacking [9]. Generally, for intolerances and malabsorption disorders, several diagnostic strategies using various tests have been proposed. In distinction to some other disorders such as for example celiac disease, there is no valid diagnostic laboratory assay for HIT that has been was consensually accepted [1,6,9,24].
It is proposed that the manifestation of HIT symptoms has its origin in a reduced level/activity of DAO. Based on this, the measurement of concentration or activity of this enzyme should be useful for the diagnosing of HIT. However, the problem resides in the fact that a reference value for DAO levels in serum has not yet been established [24,27]. Moreover, a measured DAO value and/or activity in a serum may be incoherent with the current level/functional activity of DAO in the intestinal mucosa.
2.1. DAO Enzyme Activity in Serum
The most studied but still controversial laboratory diagnostic approach is the analysis of DAO enzyme activity in serum. The tests measure the amount of histamine that degrades over a specified time in a blood sample, using enzyme-linked immunosorbent assay (ELISA) or radioimmunoassay (RIA). The threshold for serum DAO enzyme activity by which the presence of HIT should be considered has been proposed at 10 U/mL [58,59]. A number of authors have pointed out the potential contribution of this diagnostic approach in the search for the causes of the symptoms in patients with suspected HIT [9,59,60,61]. The reason for the controversy was that DAO was not detected in the blood by monoclonal antibodies, at least not in relevant amounts [62]. Accordingly, the DAO enzyme activity in serum could not be considered as conclusive [1]. In 2017, however, Boehm et al. demonstrated that ELISA is able to reliably and accurately quantify human DAO in different biological fluids and concluded that the potential of DAO as a biomarker in various diseases can be evaluated [27]. Still, in some patients, the relation between DAO activity in blood serum and the clinical history of symptomatic patients suffering from symptoms typical for HIT could not be confirmed. This can be explained by the fact that the activity of the DAO enzyme may naturally vary (over the course of the day and/or over the month) in an individual [63]. Measuring the DAO enzyme activity in serum may be considered as a part of the complementary examinations in certain cases if the determination of DAO activity in the intestinal mucosa is not feasible.
2.2. DAO Activity in the Intestinal Mucosa
Determination of DAO activity in the intestinal mucosa could represent a reliable diagnostic method. The determination is conditioned by a biopsy of colon tissue during colonoscopy. There are only a few studies addressing this diagnostic approach. However, they showed reduced DAO catabolic activity in patients with urticaria [64] and a food allergy [65] with associated higher histamine levels; the importance of this approach however needs to be confirmed by more robust data [1,9].
2.3. Faecal Histamine Levels
As was stated, the microbiota of the gastrointestinal tract can be a significant source of histamine [50]. The determination of faecal histamine levels is therefore not considered a reliable method [1].
2.4. Skin Prick Test
In HIT diagnostics the skin prick test variant, whose results are read after a longer time (50 min) than in the standard prick test (20 min), has been applied. The resolution of the redness from the puncture occurs later in symptomatic patients in comparison with the controls, which may signal a reduced ability of the body to degrade intracutaneously administered histamine. The limitation of this test resides in the fact that it need not necessarily reflect the degradation of histamine in the small intestine. Moreover, it is difficult, on the basis of this test, to differentiate HIT from other, for example allergic, disorders [66].
2.5. Histamine Levels in Plasma
Fourteen patients with suspected HIT were given orally 75 mg of histamine or a placebo, and plasmatic levels of histamine were measured at 10 min. intervals over 1 h. These results were compared with 4 healthy individuals. In this small sample, no significant differences were found in plasma histamine levels between the experimental and placebo groups. After the administration of the placebo, 33% of the patients had an increased level of histamine in the plasma, while about 50% exhibited symptoms [67].
2.6. The Histamine Challenge Test
The histamine challenge (provocation) test provides diagnostic output as well as a determination of the individual threshold dose of ingested histamine. The threshold dose of ingested histamine is the amount of histamine capable of triggering symptoms in an individual. On the other hand, it is difficult (or even impossible) to estimate the histamine content for each food because it varies depending on multiple factors [13,23,39]. The main disadvantage of this test is that it demands practically uninterrupted supervision by specialist personnel over a relatively long time-interval. In a number of the publications, the provocative dose was set at 75 mg of histamine [1], which should refer to a dose harmless to a healthy individual and a commonly occurring dietary dose [68]. However, a consensus on the critical value for histamine intoxication is lacking [10]. In a small placebo-controlled study, a dosage of 75 mg histamine immediately caused symptoms (tachycardia, sneezing, itchy nose, rhinorrhoea) in 5 of the 10 healthy patients tested [68]. An Austrian study from 2011 also questioned the decisiveness of the test. The patients were given 75 mg histamine, and those who reacted to it (n = 39), were divided into groups and given tea with/without a histamine and a capsule with a DAO enzyme, or a placebo. According to the study’s author, symptoms with patients suspected of HIT in individual organs manifested in a variety of situations. In some cases, the patients reacted unexpectedly, even randomly. For the diagnosing of HIT therefore, the reproducibility of the individual symptoms may not in itself be informative; a scoring system evaluating all symptoms could be more appropriate [16]. In addition, the histamine challenge (provocation) test itself carries the risk of serious side effects [9].
2.7. Single Nucleotide Polymorphisms (SNPs) of AOC1 Gene Evaluation
Currently, there are known 4 different SNPs of the AOC1 gene associated with a predisposition to HIT [21]. Genetic testing of AOC1 gene SNPs is a non-invasive procedure, where SNPs evaluated from blood or oral mucosa samples could be provided in ambulant care or at home. The results could be read in days. This simple test may play a part in the complementary testing of suspected individuals in order to support the results of other diagnostic outcomes.
2.8. Determination of Histamine and Its Metabolite 1-Methylhistamine from Urine
In 2017, Comas-Basté proposed a new diagnostic approach. The principle of this non-invasive test is the determination of histamine and its metabolite 1-methylhistamine from urine by ultra-high performance liquid chromatography and fluorimetry [69]. This approach however is waiting for validation [1,9].
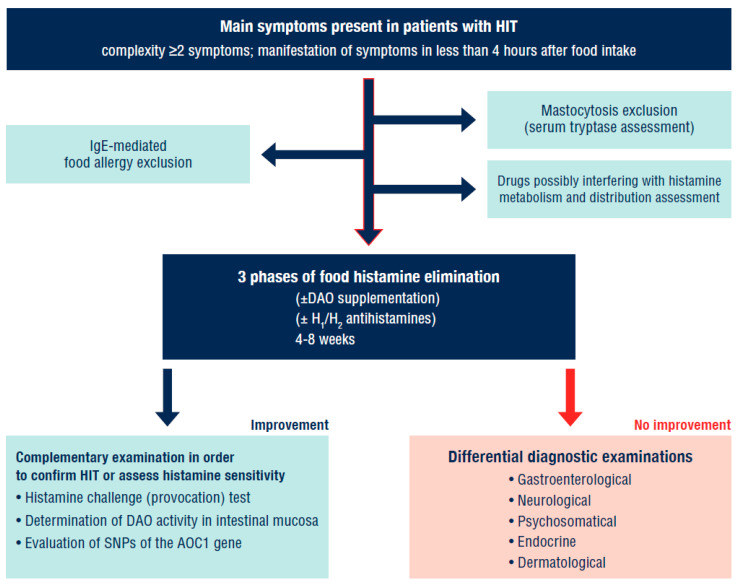
As there is currently no validated diagnostic method which would unequivocally identify the intake of exogenous histamine as a trigger of symptoms, we would recommend the approach described in Figure 5. In future it may be optimal to add the determination of histamine and its metabolites in urine among the diagnostic procedures carried out before the actual elimination of food histamine.
Figure 5.
Diagnostic approach in a patient with suspected HIT. IgE—immunoglobulin E; DAO—diamine oxidase; HIT—histamine intolerance; SNPs—single nucleotide polymorphisms; AOC1—amine oxidase copper containing 1. Adapted according to [1,6,9].
Concerns have been raised regarding fact that HIT suspected patients often receive questionnaires from non-scientific sources and after filling them out they come to the specialist already convinced of their diagnosis and accordingly refuse further examination. Questionnaires from non-scientific sources are not considered to be a reliable diagnostic approach [70].
2.9. Differential Diagnostic Exclusion of Other Diseases
Symptoms triggered by the ingestion of histamine are non-specific. Within diagnostic approaches, the exclusion of allergic disorders is recommended as the first step (IgE-mediated food allergy). The excessive formation of histamine may also be related to rare, relatively well-differentiated specific disorders. For the exclusion of mastocytosis in HIT suspected patients, their serum tryptase levels should be examined [6]. The repeated taking of samples is optimal—during the course of the symptoms and then after their diminution. Chronic disorders (Crohn’s disease, celiac disease) should be excluded, along with a potential infectious noxa (Helicobacter pylori, zoonoses). During these first examinations, it is recommended for the patient to compile a patient’s diary, where the food consumed and symptoms manifested will be recorded. A comprehensible smartphone application developed in cooperation with specialists could be the option.
After the differential diagnostic exclusion of other possible causes of the symptoms, there follows a low-histamine diet, which should be divided into 3 phases (Table 5). When the effect of the diet is elusive, histamine exposure could follow (e.g., the consumption of sauerkraut). The onset of symptoms shortly after exposure and their subsequent remission after dieting resumption may indicate HIT.
Table 5.
Phases of dietary measures in patients with suspected HIT. Adapted according to [1].
| Phase | Objective | Recommendation | Duration |
|---|---|---|---|
| Phase 1: Elimination phase | Reduction in symptoms to a maximum possible level | • Change in diet composition - introduction of mixed diet measures with accent on fresh vegetables and reduction of biogenic amine intake, in particular histamine • Nutrient optimization |
10–14 days |
| Phase 2: Test phase | Reintroducing foods excluded in Phase 1, after taking into account individual risk factors (stress, menstruation, medication use etc.) | • Targeted gradual reintroduction of suspected foods taking into consideration patient’s individual dietary preferences • Assessment of individual sensitivity to ingested histamine |
Up to 6 weeks |
| Phase 3: Long-term diet | Maintenance of high-quality of life Continual balanced diet | • Individual nutritional recommendations based on individual sensitivity to ingested histamine taking exogenous risk factors into consideration | _ |
If the response to a low-histamine diet is insufficient and no remission of symptoms has been observed, it is recommended to perform further professional examinations with specialists (gastroenterologist, neurologist, endocrinologist, dermatologist).
The HIT syndrome does not necessarily indicate one (transient) feature of the organism. Patients with inflammatory bowel diseases showed decreased serum DAO activity [13,71]. Similarly, in patients with ulcerative colitis, decreased DAO activity in the colonic mucosa has been shown [72]. The AOC1 gene polymorphism was related to the severity of the course of the ulcerative colitis [73]. The hypothesis that decreased DAO activity resulting from a gene polymorphism could act as a risk factor for the development of inflammatory bowel disease has not been proven [74]. It should be stated that with non-celiac gluten sensitivity have been reported intestinal and extra-intestinal symptoms which are very similar to those observed in HIT. Gluten-containing cereals are found in many foods, including bread, pasta, pizza, bulgur and couscous and in beverages such as beer. The majority of these foods and drinks however also contain histamine and/or are usually consumed with other products with a histamine content. Many bakery products containing gluten, and also beer, contain yeasts, thus live microorganisms which may contribute to the production of histamine are used in the process of their production. Bulgur, pasta and pizza are usually consumed with tomatoes and other vegetables which, due to their histamine content or ability to release endogenous histamine, could trigger symptoms in sensitive people [75].
In 18 of 20 patients with refractory celiac disease, Schnedl et al. diagnosed other intolerances/malabsorption and/or H. pylori infection. HIT syndrome was diagnosed in 11 patients. HIT would seem to play a significant role among patients with celiac disease who do not respond to therapeutic measures [76].
3. Therapeutic Approaches with HIT
Among therapeutic approaches, the gold standard is a low-histamine diet. A good response to such a diet is considered to be confirmation of HIT. Alongside the dietary measures, DAO supplementation supporting the degradation of ingested histamine is recommended as subsidiary treatment for individuals with intestinal DAO deficiency [9]. In acute and clinically more severe cases, or in cases where the ingested histamine cannot be completely removed by following the low-histamine diet [38], H1 (or possibly H2) antihistamines can be used (preferably short-term use).
3.1. Low-Histamine Diet
The principle of the low-histamine diet consists in a choice of foods where an excessive amount of histamine or biogenic amines respectively is not to be expected. In the first phase of the low-histamine diet, foods that typically contain a high amount of histamine (and other biogenic amines) should be completely excluded. There is a great variability between studies regarding type of foods that is recommended to avoid during elimination diet. Some of routinely excluded foods contain only low levels of biogenic amines and become designated as histamine liberators [77]. In Figure 4, we present examples of foods that are often recommended to exclude in low-histamine diet.
Foods that under normal circumstances do not contain high levels of biogenic amines should be consumed as fresh as possible (Table 3).
The low-histamine diet should be temporary and must not stress the patient. The patient should be aware that after certain period some eliminated foods may again be reintroduced to the menu.
Reese et al. proposed 3 phases during which the foods responsible for the symptoms are progressively eliminated and reintroduced respectively (Table 5). Adherence to a low-histamine diet has clearly led to improved gastrointestinal, cutaneous and neurological manifestations [23,78], and in some cases simultaneous raising of DAO serum levels was observed [79].
3.2. Exogenous Supply of DAO
Similarly as in lactose intolerance, lactase supplementation is used, the exogenous supply of the DAO enzyme has been proposed for HIT [9,16,32]. The European Food Safety Authority (EFSA) has authorized a porcine kidney extract containing 0.3 mg of DAO enzyme as a novel food. From 2002, it could be distributed on the market as a nutrition supplement and from 2013 as a food for special medical purposes. According to EFSA, the maximum daily dose of exogenously ingested enzyme is 3 × 0.3 mg, which corresponds to 0.9 mg of DAO. The dosage form must be gastro-resistant in order to deliver the enzyme undamaged to the place of presumed effect (small intestine) [80]. It has been demonstrated that DAO exogenous supplementation has improved the symptoms in clinical practice (Table 6).
Table 6.
Summary of clinical studies testing the effects of exogenous supplementation of diamine oxidase obtained from porcine kidneys. DAO—diamine oxidase; DBPC—double-blind placebo controlled; N/A—not available; 5-HT—5 hydroxytryptamine; UAS7—Urticaria Activity Score 7; NS—non-significant; C1—before treatment (consultation no. 1); C2—after treatment (consultation no. 2) [16,58,59,81,82].
| Study | Trial Design | Intervention | Control | Sample size | Duration of Intervention | Reported Outcomes |
|---|---|---|---|---|---|---|
| Komericki, 2011 | DBPC cross-over study | 0.5 mg DAO | Placebo | 39 | N/A | A significant improvement in symptoms after DAO administration as compared with placebo. |
| Manzotti, 2016 | Restrospective observational study | 2 × 0.3 mg DAO | N/A | 14 | 14 | 13 patients (93%) reported improvement in ≥ 1 of symptoms. |
| Yacoub, 2018 | DBPC cross-over study | 2 × 0.3 mg DAO | Placebo | 20 | 30 | A significant improvement in Urticaria Activity Score 7 (UAS7) in patients with urticaria unsatisfactorily controlled by antihistamines (p < 0.05). Mild significant reduction in antihistamines consumption (p < 0.05). |
| Izquierdo-Casas, 2019 | Randomized DBPC study | 3 × 0.6 mg DAO | Placebo | 82 | C1 without an intervention (30 days) + C2 with intervention (30 days) | A significant reduction in number (p < 0.001) and duration (p < 0.05) of migraine episodes in intervention group compared with baseline. A decrease in the percentage of patients using selective 5-HT receptor agonists (triptans). |
| Schnedl, 2019 | Open label interventional study | 3 × 0.3 mg DAO | N/A | 28 | 28 + 28 (follow-up) | Significant reduction in frequency and intensity of symptoms. 61% of patients showed mild increase in serum DAO levels (NS). During follow-up, without DAO supplementation, the symptoms sum score increased again and serum DAO levels slightly decreased. |
Despite the promising results, studies dealing with DAO exogenous supplementation are scarce. In addition, the studies conducted investigated only a small sample of patients. It is therefore necessary to confirm the clinical significance of exogenous supplementation of the DAO enzyme by more robust, well-designed clinical trials.
Other Potential Sources of DAO
DAO need not be necessarily of animal origin. In 2020, Comas-Basté carried out screening of the ability of the family of the Leguminosae (legumes) plant to break down histamine. The goal was to identify plants with DAO enzyme activity (in vitro) and then to consider their potential usage as an active component of enzyme supplements. Lyophilization of the sprouts kept their enzyme activity unchanged for at least 12 months. The results show that in the future certain edible legumes could be suitable for the manufacture of supplements with DAO content for the management of HIT [83].
3.3. Antihistamines
The treatment of patients with antihistamines is empirical. There exist no randomized clinical trials that prove the contribution of this therapy in HIT. Therapeutic dosages and the choice of the generation and type of antihistamine (H1/H2) are in the competence of the clinician after the manifestation of the symptoms (gastrointestinal, neurological, dermatological) are taken into account; in consideration of effectiveness and safety however, 2nd or 3rd generation of H1 antihistamines should take precedence. H2 blockers could be used in patients with dominant gastrointestinal symptoms (hyperacidity and reflux as manifestations of HIT are also questions to discuss).
Treatment with antihistamines should be conscious and time-limited and should help create a picture of whether H1/H2 receptor blockade attenuates manifestations [1]. However, it may also be used as a therapeutic-diagnostic test.
3.4. Complementary Strategies in HIT Management
Some authors regard supplementation of cofactors of the DAO enzyme as optional adjunctive therapy. Vitamin C, copper or vitamin B6 supplementation may be considered [32,33,40,84].
Supplementation with probiotic microorganisms could lead to such modulations of microbiome that would reduce the production of the microbial enzyme L-histidine decarboxylase. The precondition therefore is the administration of strains that do not produce L-histidine decarboxylase. In an ideal case, these would be strains capable at the same time of degrading histamine (or other biogenic amines). Clinical trials assessing the possible impact of probiotic administration in HIT are not found in literature up to the present.
Arising from experimental trials, it would seem at present that members of Bifidobacterium genus could be considered as candidates for appropriate supplementation, but studies confirming this assumption are warranted [44,46].
4. Conclusions
HIT represents a set of diverse symptoms that appear following the ingestion of food with a content of such amounts of histamine that usually do not provoke symptoms in a healthy person. Manifestations may be caused by various pathophysiological mechanisms, or a combination of them. It would seem that symptoms typical for HIT in an individual may potentiate the presence of other disorders such as, e.g., celiac disease or H. pylori infection. The diagnosing of HIT therefore requires a complex time-demanding multidisciplinary approach, including the systematic elimination of disorders with a similar manifestation of symptoms. A low-histamine diet is currently a suitable (not however sole) diagnostic and at the same time therapeutic measure. Recently, evidence has been growing regarding the therapeutic contribution of food for special medical purposes containing a DAO enzyme of animal origin. H1/H2 antihistamines may also be considered in management of HIT, with the daily dosage (usually higher than the standard) determined by a clinician on the basis of the patient’s clinical condition. Pharmacological treatment should have a limited time duration. The aim of the therapeutic approach should be to develop such dietary habits which will not in the future lead to the triggering of symptoms.
Acknowledgments
The authors would like to thank Kevin M. Slavin for his prompt and precise revision of the English text. The authors would like to thank also Miroslava Nikodemová and Mária Škultéty for help with visualization.
Author Contributions
Each author of the manuscript has contributed to conception of this review. M.H. (Martin Hrubisko) and R.D. wrote the draft of the manuscript. M.H. (Martin Huorka) made suggestions and corrections. M.W. made suggestions and critical supervision. All authors declare an approval of the final manuscript. Authors M.H. (Martin Hrubisko) and R.D. contributed equally to this article 35%. Author M.H. (Martin Huorka) contributed 10%. Author M.W. contributed 20%. All authors have read and agreed to the published version of the manuscript.
Funding
Publication of this manuscript was funded by the authors.
Conflicts of Interest
The authors declare no conflict of interest at the time of submission of the manuscript.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Reese I., Ballmer-Weber B., Beyer K., Fuchs T., Kleine-Tebbe J., Klimek L., Lepp U., Niggemann B., Saloga J., Schäfer C., et al. German guideline for the management of adverse reactions to ingested histamine. Allergo J. Int. 2017;26:72–79. doi: 10.1007/s40629-017-0011-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sicherer S.H. Epidemiology of food allergy. J. Allergy Clin. Immunol. 2011;127:594–602. doi: 10.1016/j.jaci.2010.11.044. [DOI] [PubMed] [Google Scholar]
- 3.Stapel S.O., Asero R., Ballmer-Weber B.K., Knol E.F., Strobel S., Vieths S., Kleine-Tebbe J. Testing for IgG4 against foods is not recommended as a diagnostic tool: EAACI task force report. Allergy. 2008;63:793–796. doi: 10.1111/j.1398-9995.2008.01705.x. [DOI] [PubMed] [Google Scholar]
- 4.Burks A.W., Tang M., Sicherer S., Muraro A., Eigenmann P.A., Ebisawa M., Fiocchi A., Chiang W., Beyer K., Wood R., et al. ICON: Food allergy. J. Allergy Clin. Immunol. 2012;129:906–920. doi: 10.1016/j.jaci.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 5.Matricon J., Meleine M., Gelot A., Piche T., Dapoigny M., Müller E., Ardid D. Associations between immune activation, intestinal permeability and the irritable bowel syndrome. Aliment. Pharmacol. Ther. 2012;36:1009–1031. doi: 10.1111/apt.12080. [DOI] [PubMed] [Google Scholar]
- 6.Tuck C.J., Biesiekierski J.R., Schmid-Grendelmeier P., Pohl D. Food intolerances. Nutrients. 2019;11:1684. doi: 10.3390/nu11071684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johansson S.G.O., Bieber T., Dahl R., Friedmann P.S., Lanier B.Q., Lockey R.F., Motala C. Revised nomenclature for allergy for global use: Report of the nomenclature review committee of the World Allergy Organization, October 2003. J. Allergy Clin. Immunol. 2004;113:832–836. doi: 10.1016/j.jaci.2003.12.591. [DOI] [PubMed] [Google Scholar]
- 8.Lomer M.C.E. The aetiology, diagnosis, mechanisms and clinical evidence for food intolerance. Aliment. Pharmacol. Ther. 2015;41:262–275. doi: 10.1111/apt.13041. [DOI] [PubMed] [Google Scholar]
- 9.Comas-Basté O., Sánchez-Pérez S., Veciana-Nogués M.T., Latorre-Moratalla M., del Carmen Vidal-Carou M. Histamine intolerance: The current state of the art. Biomolecules. 2020;10:1181. doi: 10.3390/biom10081181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Colombo F.M., Cattaneo P., Confalonieri E., Bernardi C. Histamine food poisonings: A systematic review and meta-analysis. Crit. Rev. Food Sci. Nutr. 2018;58:1131–1151. doi: 10.1080/10408398.2016.1242476. [DOI] [PubMed] [Google Scholar]
- 11.Yu Y., Wang P., Bian L., Hong S. Rare death via histamine poisoning following crab consumption: A case report. J. Forensic Sci. 2017;63:980–982. doi: 10.1111/1556-4029.13611. [DOI] [PubMed] [Google Scholar]
- 12.Afify S.M., Pali-Schöll I. Adverse reactions to food: The female dominance—A secondary publication and update. World Allergy Organ. J. 2017;10:43. doi: 10.1186/s40413-017-0174-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maintz L., Novak N. Histamine and histamine intolerance. Am. J. Clin. Nutr. 2007;85:1185–1196. doi: 10.1093/ajcn/85.5.1185. [DOI] [PubMed] [Google Scholar]
- 14.Hamada Y., Shinohara Y., Yano M., Yamamoto M., Yoshio M., Satake K., Toda A., Hirai M., Usami M. Effect of the menstrual cycle on serum diamine oxidase levels in healthy women. Clin. Biochem. 2013;46:99–102. doi: 10.1016/j.clinbiochem.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 15.Jarisch R. Histamine Intolerance. Springer; Berlin, Germany: 2014. Histamine intolerance in women; pp. 109–115. [Google Scholar]
- 16.Komericki P., Klein G., Reider N., Hawranek T., Strimitzer T., Lang R., Kranzelbinder B., Aberer W. Histamine intolerance: Lack of reproducibility of single symptoms by oral provocation with histamine: A randomised, double-blind, placebo-controlled cross-over study. Wien. Klin. Wochenschr. 2010;123:15–20. doi: 10.1007/s00508-010-1506-y. [DOI] [PubMed] [Google Scholar]
- 17.Alnouri G., Cha N., Sataloff R.T. Histamine sensitivity: An uncommon recognized cause of living laryngopharyngeal reflux symptoms and signs—A case report. Ear Nose Throat J. 2020 doi: 10.1177/0145561320951071. [DOI] [PubMed] [Google Scholar]
- 18.Ansaranta M., Kauppi P., Malmberg L.P., Vilkman E., Geneid A. Inspiratory and expiratory flow changes, voice symptoms and laryngeal findings during Histamine Challenge tests. Folia Phoniatr. Logop. 2019;72:29–35. doi: 10.1159/000495783. [DOI] [PubMed] [Google Scholar]
- 19.Waldum H.L., Sørdal Ø.F., Mjønes P.G. The enterochromaffin-like [ECL] cell—Central in gastric physiology and pathology. Int. J. Mol. Sci. 2019;20:2444. doi: 10.3390/ijms20102444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yoshikawa T., Nakamura T., Yanai K. Histaminergic neurons in the tuberomammillary nucleus as a control centre for wakefulness. Br. J. Pharmacol. 2021;178:750–769. doi: 10.1111/bph.15220. [DOI] [PubMed] [Google Scholar]
- 21.Kucher A.N. Association of polymorphic variants of key histamine metabolism genes and histamine receptor genes with multifactorial diseases. Russ. J. Genet. 2019;55:794–814. doi: 10.1134/S102279541907010X. [DOI] [Google Scholar]
- 22.Scammell T.E., Jackson A.C., Franks N.P., Wisden W., Dauvilliers Y. Histamine: Neural circuits and new medications. Sleep. 2019;42:183. doi: 10.1093/sleep/zsy183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sánchez-Pérez S., Comas-Basté O., Rabell-González J., Veciana-Nogués M.T., Latorre-Moratalla M.L., Vidal-Carou M.C. Biogenic amines in plant-origin foods: Are they frequently underestimated in low-histamine diets? Foods. 2018;7:205. doi: 10.3390/foods7120205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schnedl W.J., Enko D. Considering histamine in functional gastrointestinal disorders. Crit. Rev. Food Sci. Nutr. 2020:1–8. doi: 10.1080/10408398.2020.1791049. [DOI] [PubMed] [Google Scholar]
- 25.Laroche D., Vergnaud M.-C., Sillard B., Soufarapis H., Bricard H. Biochemical markers of anaphylactoid reactions to drugs comparison of plasma histamine and tryptase. J. Am. Soc. Anesthesiol. 1991;75:945–949. doi: 10.1097/00000542-199112000-00004. [DOI] [PubMed] [Google Scholar]
- 26.Vakal S., Jalkanen S., Dahlström K.M., Salminen T.A. Human copper-containing amine oxidases in drug design and development. Molecules. 2020;25:1293. doi: 10.3390/molecules25061293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boehm T., Pils S., Gludovacz E., Szoelloesi H., Petroczi K., Majdic O., Quaroni A., Borth N., Valent P., Jilma B. Quantification of human diamine oxidase. Clin. Biochem. 2017;50:444–451. doi: 10.1016/j.clinbiochem.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 28.Schwelberger H.G., Feurle J., Ahrens F. Characterization of diamine oxidase from human seminal plasma. J. Neural Transm. 2013;120:983–986. doi: 10.1007/s00702-013-0983-3. [DOI] [PubMed] [Google Scholar]
- 29.McGrath A.P., Hilmer K.M., Collyer C.A., Shepard E.M., Elmore B.O., Brown D.E., Dooley D.M., Guss M. Structure and inhibition of human diamine oxidase. Biochemistry. 2009;48:9810–9822. doi: 10.1021/bi9014192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ji Y., Sakata Y., Li X., Zhang C., Yang Q., Xu M., Wollin A., Langhans W., Tso P. Lymphatic diamine oxidase secretion stimulated by fat absorption is linked with histamine release. Am. J. Physiol. Gastrointest. Liver Physiol. 2013;304:G732–G740. doi: 10.1152/ajpgi.00399.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leitner R., Zoernpfenning E., Missbichler A. Evaluation of the inhibitory effect of various drugs/active ingredients on the activity of human diamine oxidase in vitro. Clin. Transl. Allergy. 2014;4:P23. doi: 10.1186/2045-7022-4-S3-P23. [DOI] [Google Scholar]
- 32.Kovacova-Hanuskova E., Buday T., Gavliakova S., Plevkova J. Histamine, histamine intoxication and intolerance. Allergol. Immunopathol. 2015;43:498–506. doi: 10.1016/j.aller.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 33.Rosell-Camps A., Zibetti S., Pérez-Esteban G., Vila-Vidal M., Ferrés-Ramis L., García-Teresa-García E. Histamine intolerance as a cause of chronic digestive complaints in pediatric patients. Rev. Esp. Enferm. Dig. 2013;105:201–207. doi: 10.4321/S1130-01082013000400004. [DOI] [PubMed] [Google Scholar]
- 34.Kacik J., Wróblewska B., Lewicki S., Zdanowski R., Kalicki B. Advances in Experimental Medicine and Biology. Volume 1039. Springer; Cham, Switzerland: 2017. Serum diamine oxidase in pseudoallergy in the pediatric population; pp. 35–44. [DOI] [PubMed] [Google Scholar]
- 35.Nei D. Evaluation of non-bacterial factors contributing to histamine accumulation in fish fillets. Food Control. 2014;35:142–145. doi: 10.1016/j.foodcont.2013.06.037. [DOI] [Google Scholar]
- 36.Verma N., Hooda V., Gahlaut A., Gothwal A., Hooda V. Enzymatic biosensors for the quantification of biogenic amines: A literature update. Crit. Rev. Biotechnol. 2019;40:1–14. doi: 10.1080/07388551.2019.1680600. [DOI] [PubMed] [Google Scholar]
- 37.D’Andrea G., D’Arrigo A., Carbonare M.D., Leon A. Pathogenesis of migraine: Role of neuromodulators. Headache. 2012;52:1155–1163. doi: 10.1111/j.1526-4610.2012.02168.x. [DOI] [PubMed] [Google Scholar]
- 38.Kohn J.B. Is there a diet for histamine intolerance? J. Acad. Nutr. Diet. 2014;114:1860. doi: 10.1016/j.jand.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 39.Moniente M., García-Gonzalo D., Ontañón I., Pagán R., Botello-Morte L. Histamine accumulation in dairy products: Microbial causes, techniques for the detection of histamine-producing microbiota, and potential solutions. Compr. Rev. Food Sci. Food Saf. 2021;20:1481–1523. doi: 10.1111/1541-4337.12704. [DOI] [PubMed] [Google Scholar]
- 40.Martin I.S.M., Brachero S., Vilar E.G. Histamine intolerance and dietary management: A complete review. Allergol. Immunopathol. 2016;44:475–483. doi: 10.1016/j.aller.2016.04.015. [DOI] [PubMed] [Google Scholar]
- 41.Scalzo R.L., Fibiani M., Francese G., D’Alessandro A., Rotino G.L., Conte P., Mennella G. Cooking influence on physico-chemical fruit characteristics of eggplant (Solanum melongena L.) Food Chem. 2016;194:835–842. doi: 10.1016/j.foodchem.2015.08.063. [DOI] [PubMed] [Google Scholar]
- 42.Preti R., Rapa M., Vinci G. Effect of steaming and boiling on the antioxidant properties and biogenic amines content in green bean (Phaseolus vulgaris) varieties of different colours. J. Food Qual. 2017;2017:5329070. doi: 10.1155/2017/5329070. [DOI] [Google Scholar]
- 43.Chung B.Y., Park S.Y., Byun Y.S., Son J.H., Choi Y.W., Cho Y.S., Kim H.O., Park C.W. Effect of different cooking methods on histamine levels in selected foods. Ann. Dermatol. 2017;29:706–714. doi: 10.5021/ad.2017.29.6.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schink M., Konturek P.C., Tietz E., Dieterich W., Pinzer T.C., Wirtz S., Neurath M.F., Zopf Y. Microbial patterns in patients with histamine intolerance. J. Physiol. Pharmacol. 2018;69:579–593. doi: 10.26402/jpp.2018.4.09. [DOI] [PubMed] [Google Scholar]
- 45.O’Callaghan A., van Sinderen D. Bifidobacteria and their role as members of the human gut microbiota. Front. Microbiol. 2016;7:925. doi: 10.3389/fmicb.2016.00925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mokhtar S., Mostafa G., Taha R., Eldeep G.S.S. Effect of different starter cultures on the biogenic amines production as a critical control point in fresh fermented sausages. Eur. Food Res. Technol. 2012;235:527–535. doi: 10.1007/s00217-012-1777-9. [DOI] [Google Scholar]
- 47.Priyadarshani D., Mesthri W., Rakshit S.K. Screening selected strains of probiotic lactic acid bacteria for their ability to produce biogenic amines (histamine and tyramine) Int. J. Food Sci. Technol. 2011;46:2062–2069. doi: 10.1111/j.1365-2621.2011.02717.x. [DOI] [Google Scholar]
- 48.Coton M., Romano A., Spano G., Ziegler K., Vetrana C., Desmarais C., Lonvaud-Funel A., Lucas P., Coton E. Occurrence of biogenic amine-forming lactic acid bacteria in wine and cider. Food Microbiol. 2010;27:1078–1085. doi: 10.1016/j.fm.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 49.Poveda J.M., Ruiz P., Sesena S., Palop M.L. Occurrence of biogenic amine-forming lactic acid bacteria during a craft brewing process. LWT Food Sci. Technol. 2017;85:129–136. doi: 10.1016/j.lwt.2017.07.003. [DOI] [Google Scholar]
- 50.Ferstl R., Frei R., Schiavi E., Konieczna P., Barcik W., Ziegler M., Lauener R.P., Chassard C., Lacroix C., Akdis C.A., et al. Histamine receptor 2 is a key influence in immune responses to intestinal histamine-secreting microbes. J. Allergy Clin. Immunol. 2014;134:744–746.e3. doi: 10.1016/j.jaci.2014.04.034. [DOI] [PubMed] [Google Scholar]
- 51.Spinler J.K., Sontakke A., Hollister E.B., Venable S.F., Oh P.L., Balderas M.A., Saulnier D.M., Mistretta T.-A., Devaraj S., Walter J., et al. From prediction to function using evolutionary genomics: Human-specific ecotypes of lactobacillus reuteri have diverse probiotic functions. Genome Biol. Evol. 2014;6:1772–1789. doi: 10.1093/gbe/evu137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wechsler D., Irmler S., Berthoud H., Portmann R., Badertscher R., Bisig W., Schafroth K., Fröhlich-Wyder M.-T. Influence of the inoculum level of Lactobacillus parabuchneri in vat milk and of the cheese-making conditions on histamine formation during ripening. Int. Dairy J. 2021;113:104883. doi: 10.1016/j.idairyj.2020.104883. [DOI] [Google Scholar]
- 53.Frei R., Ferstl R., Konieczna P., Ziegler M., Simon T., Rugeles T.M., Mailand S., Watanabe T., Lauener R., Akdis C.A., et al. Histamine receptor 2 modifies dendritic cell responses to microbial ligands. J. Allergy Clin. Immunol. 2013;132:194–204. doi: 10.1016/j.jaci.2013.01.013. [DOI] [PubMed] [Google Scholar]
- 54.Oksaharju A., Kankainen M., Kekkonen R.A., Lindstedt K.A., Kovanen P.T., Korpela R., Miettinen M. Probiotic lactobacillus rhamnosus downregulates FCER1 and HRH4 expression in human mast cells. World J. Gastroenterol. 2011;17:750. doi: 10.3748/wjg.v17.i6.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Alvarez M.A., Moreno-Arribas M.V. The problem of biogenic amines in fermented foods and the use of potential biogenic amine-degrading microorganisms as a solution. Trends Food Sci. Technol. 2014;39:146–155. doi: 10.1016/j.tifs.2014.07.007. [DOI] [Google Scholar]
- 56.Tittarelli F., Perpetuini G., Di Gianvito P., Tofalo R. Biogenic amines producing and degrading bacteria: A snapshot from raw ewes’ cheese. Lebensm. Wiss. Technol. 2019;101:1–9. doi: 10.1016/j.lwt.2018.11.030. [DOI] [Google Scholar]
- 57.Kung H.-F., Lee Y.-C., Huang Y.-L., Huang Y.R., Su Y.-C., Tsai Y.-H. Degradation of histamine by lactobacillus plantarum isolated from miso products. J. Food Prot. 2017;80:1682–1688. doi: 10.4315/0362-028X.JFP-17-135. [DOI] [PubMed] [Google Scholar]
- 58.Schnedl W.J., Schenk M., Lackner S., Enko D., Mangge H., Forster F. Diamine oxidase supplementation improves symptoms in patients with histamine intolerance. Food Sci. Biotechnol. 2019;28:1779–1784. doi: 10.1007/s10068-019-00627-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Manzotti G., Breda D., Di Gioacchino M., Burastero S. Serum diamine oxidase activity in patients with histamine intolerance. Int. J. Immunopathol. Pharmacol. 2016;29:105–111. doi: 10.1177/0394632015617170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mušič E., Korošec P., Šilar M., Adamič K., Košnik M., Rijavec M. Serum diamine oxidase activity as a diagnostic test for histamine intolerance. Wien. Klin. Wochenschr. 2013;125:239–243. doi: 10.1007/s00508-013-0354-y. [DOI] [PubMed] [Google Scholar]
- 61.Peralta T., Carla B., Beltran-Ortiz C., Magdalena D., Verónica R., Antonieta G.M., Pablo F. Histamine intolerance: Clinical characterization and determination of serum diamine oxidase (Dao) in a series of cases and controls. Res. Sq. 2020 doi: 10.21203/rs.3.rs-60226/v1. [DOI] [Google Scholar]
- 62.Schwelberger H.G., Feurle J., Houen G. New tools for studying old questions: Antibodies for human diamine oxidase. J. Neural Transm. 2012;120:1019–1026. doi: 10.1007/s00702-012-0936-2. [DOI] [PubMed] [Google Scholar]
- 63.Pinzer T.C., Tietz E., Waldmann E., Schink M., Neurath M.F., Zopf Y. Circadian profiling reveals higher histamine plasma levels and lower diamine oxidase serum activities in 24% of patients with suspected histamine intolerance compared to food allergy and controls. Allergy. 2017;73:949–957. doi: 10.1111/all.13361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lessof M.H., Gant V., Hinuma K., Murphy G.M., Dowling R.H. Recurrent urticaria and reduced diamine oxidase activity. Clin. Exp. Allergy. 1990;20:373–376. doi: 10.1111/j.1365-2222.1990.tb02796.x. [DOI] [PubMed] [Google Scholar]
- 65.Schwelberger H.G., Weidenhiller M., Hahn E.G., Raithel M., Kuefner M.A. Both catabolic pathways of histamine via histamine-N-methyltransferase and diamine oxidase are diminished in the colonic mucosa of patients with food allergy. Inflamm. Res. 2004;53:S31–S32. doi: 10.1007/s00011-003-0314-5. [DOI] [PubMed] [Google Scholar]
- 66.Wagner A., Buczyłko K., Zielińska-Bliźniewska H., Wagner W. Impaired resolution of wheals in the skin prick test and low diamine oxidase blood level in allergic patients. Adv. Dermatol. Allergol. Postȩp. Dermatol. Alergol. 2019;36:538–543. doi: 10.5114/ada.2019.89504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Giera B., Straube S., Konturek P., Hahn E.G., Raithel M. Plasma histamine levels and symptoms in double blind placebo controlled histamine provocation. Inflamm. Res. 2008;57:73–74. doi: 10.1007/s00011-007-0636-9. [DOI] [PubMed] [Google Scholar]
- 68.Wöhrl S., Hemmer W., Focke M., Rappersberger K., Jarisch R. Histamine intolerance-like symptoms in healthy volunteers after oral provocation with liquid histamine. Allergy Asthma Proc. 2004;25:305–311. [PubMed] [Google Scholar]
- 69.Comas-Basté O., Latorre-Moratalla M., Bernacchia R., Veciana-Nogués M., Vidal-Carou M. New approach for the diagnosis of histamine intolerance based on the determination of histamine and methylhistamine in urine. J. Pharm. Biomed. Anal. 2017;145:379–385. doi: 10.1016/j.jpba.2017.06.029. [DOI] [PubMed] [Google Scholar]
- 70.Mayo-Yáñez M., Díaz-Díaz A., Calvo-Henríquez C., Chiesa-Estomba C., Figueroa A., Martín-Martín C. Usefulness of the histamine intolerance assessment questionnaire for diagnosis. Rev. Française d’Allergol. 2021;61:87–91. doi: 10.1016/j.reval.2020.10.002. [DOI] [Google Scholar]
- 71.Honzawa Y., Nakase H., Matsuura M., Chiba T. Clinical significance of serum diamine oxidase activity in inflammatory bowel disease: Importance of evaluation of small intestinal permeability. Inflamm. Bowel Dis. 2011;17:E23–E25. doi: 10.1002/ibd.21588. [DOI] [PubMed] [Google Scholar]
- 72.Mennigen R., Kusche J., Streffer C., Krakamp B. Diamine oxidase activities in the large bowel mucosa of ulcerative colitis patients. Inflamm. Res. 1990;30:264–266. doi: 10.1007/BF01969056. [DOI] [PubMed] [Google Scholar]
- 73.García-Martín E., Mendoza J.L., Martínez C., Taxonera C., Urcelay E., Ladero J.M., de la Concha E.G., Díaz-Rubio M., Agúndez J.A.G. Severity of ulcerative colitis is associated with a polymorphism at diamine oxidase gene but not at histamine N-methyltransferase gene. World J. Gastroenterol. 2006;12:615. doi: 10.3748/wjg.v12.i4.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Palacios N.L., Agúndez J.A.G., Mendoza J.L., Garcia-Martin E., Martínez C., Ferrer M.E.F., Ladero J.M., Taxonera C., Díaz-Rubio M. Analysis of a non-synonymous single nucleotide polymorphism of the human diamine oxidase gene (ref. SNP ID: rs1049793) in patients with Crohn’s disease. Scand. J. Gastroenterol. 2009;44:1207–1212. doi: 10.1080/00365520903171250. [DOI] [PubMed] [Google Scholar]
- 75.Schnedl W.J., Lackner S., Enko D., Schenk M., Mangge H., Holasek S.J. Non-celiac gluten sensitivity: People without celiac disease avoiding gluten—Is it due to histamine intolerance? Inflamm. Res. 2017;67:279–284. doi: 10.1007/s00011-017-1117-4. [DOI] [PubMed] [Google Scholar]
- 76.Schnedl W.J., Mangge H., Schenk M., Enko D. Non-responsive celiac disease may coincide with additional food intolerance/malabsorption, including histamine intolerance. Med. Hypotheses. 2021;146:110404. doi: 10.1016/j.mehy.2020.110404. [DOI] [PubMed] [Google Scholar]
- 77.Sánchez-Pérez S., Comas-Basté O., Veciana-Nogués M., Latorre-Moratalla M., Vidal-Carou M. Low-histamine diets: Is the exclusion of foods justified by their histamine content? Nutrients. 2021;13:1395. doi: 10.3390/nu13051395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wagner N., Dirk D., Reese I., Peveling-Oberhag A., Rady-Pizarro U., Mitzel H., Staubach P. A popular myth—Low-histamine diet improves chronic spontaneous urticaria—Fact or fiction? J. Eur. Acad. Dermatol. Venereol. 2016;31:650–655. doi: 10.1111/jdv.13966. [DOI] [PubMed] [Google Scholar]
- 79.Lackner S., Malcher V., Enko D., Mangge H., Holasek S.J., Schnedl W.J. Histamine-reduced diet and increase of serum diamine oxidase correlating to diet compliance in histamine intolerance. Eur. J. Clin. Nutr. 2018;73:102–104. doi: 10.1038/s41430-018-0260-5. [DOI] [PubMed] [Google Scholar]
- 80.European Commission Commission Implementing Regulation (EU) 2020/973 of 6 July 2020: Authorising a Change of the Conditions of Use of the Novel Food ‘Protein Extract from Pig Kidneys’ and Amending Implementing Regulation (EU) 2017/2470 (Text with EEA Relevance) [(accessed on 23 March 2021)]; Available online: https://eur-lex.europa.eu/legal-content/SK/TXT/PDF/?uri=CELEX:32020R0973&from=EN. (In Slovak)
- 81.Yacoub M.-R., Ramirez G.A., Berti A., Mercurio G., Breda D., Saporiti N., Burastero S., Dagna L., Colombo G. Diamine oxidase supplementation in chronic spontaneous urticaria: A randomized, double-blind placebo-controlled study. Int. Arch. Allergy Immunol. 2018;176:268–271. doi: 10.1159/000488142. [DOI] [PubMed] [Google Scholar]
- 82.Izquierdo-Casas J., Comas-Basté O., Latorre-Moratalla M., Lorente-Gascón M., Duelo A., Soler-Singla L., Vidal-Carou M.C. Diamine oxidase (DAO) supplement reduces headache in episodic migraine patients with DAO deficiency: A randomized double-blind trial. Clin. Nutr. 2019;38:152–158. doi: 10.1016/j.clnu.2018.01.013. [DOI] [PubMed] [Google Scholar]
- 83.Comas-Basté O., Latorre-Moratalla M.L., Rabell-González J., Veciana-Nogués M.T., Vidal-Carou M.C. Lyophilised legume sprouts as a functional ingredient for diamine oxidase enzyme supplementation in histamine intolerance. Lebensm. Wiss. Technol. 2020;125:109201. doi: 10.1016/j.lwt.2020.109201. [DOI] [Google Scholar]
- 84.Maintz L., Benfadal S., Allam J.-P., Hagemann T., Fimmers R., Novak N. Evidence for a reduced histamine degradation capacity in a subgroup of patients with atopic eczema. J. Allergy Clin. Immunol. 2006;117:1106–1112. doi: 10.1016/j.jaci.2005.11.041. [DOI] [PubMed] [Google Scholar]