Abstract
To date, the only treatment for celiac disease (CD) consists of a strict lifelong gluten-free diet (GFD), which has numerous limitations in patients with CD. For this reason, dietary transgressions are frequent, implying intestinal damage and possible long-term complications. There is an unquestionable need for non-dietary alternatives to avoid damage by involuntary contamination or voluntary dietary transgressions. In recent years, different therapies and treatments for CD have been developed and studied based on the degradation of gluten in the intestinal lumen, regulation of the immune response, modulation of intestinal permeability, and induction of immunological tolerance. In this review, therapeutic lines for CD are evaluated with special emphasis on phase III and II clinical trials, some of which have promising results.
Keywords: celiac disease, gluten-free diet, gluten, gliadin, gluten immunogenic peptides, non-dietary therapies
1. Introduction
Celiac disease (CD) is a chronic immune-mediated enteropathy triggered by exposure to dietary gluten in genetically predisposed individuals [1,2]. The pooled global prevalence of CD has been reported to be approximately 1%, however, the prevalence values for CD varies in South America, Africa, North America, Asia, Europe, and Oceania; the prevalence is higher in female vs. male individuals and is 4–8 times higher among non-Hispanic white people compared with other races. Moreover, there has been an increase in the diagnosis rate in the last 10 years [3,4,5,6,7]. CD is characterized by intestinal and/or extraintestinal manifestations, elevation of specific antibodies such as anti-gliadin and anti-tissue transglutaminase (anti-tTG), and the presence of HLA-DQ2/DQ8 haplotypes [8,9,10,11].
Gluten is a complex mixture of seed storage proteins known as prolamins, found in cereals grains such as wheat, barley, rye, oats, and their derivatives. The viscoelastic network generated by gluten enables an excellent aerated structure, contributing to the baking quality of these cereals [3,4,5,6,7,8,9,10,11,12]. Gluten proteins are characterized by high proline and glutamine content. Therefore, these proteins are partially degraded to peptides by digestive proteases of the gastrointestinal track that persist in the intestine and potentiate their deamidation through tTG [13].
The prevailing hypothesis of immunopathogenesis is the two-signal model, which establishes that gluten has a dual effect on the duodenum of celiac patients mediated by innate and adaptive immune systems [14,15]. Certain peptides, such as the 19-mer gliadin peptide, trigger an innate immune response mainly characterized by the production of interleukin-15 (IL-15) by epithelial cells and the disruption of the epithelial barrier caused by increased permeability and induction of enterocyte apoptosis [16,17]. Consequently, other peptides such as the 33-mer gliadin can now reach the lamina propria to be deamidated by tTG, providing a negative charge to gliadin peptides that activate the immune-adaptive system. The affinity of the HLA-DQ2/8 peptide is enhanced and expressed on the surface of dendritic cells (DCs) [18,19,20]. DCs present a gluten antigen to T-cells and drive the progression of the proinflammatory response, thereby contributing to the symptomatology of the disease [21,22].
2. Gluten-Free Diet: Challenge and Gluten Exposure
Currently, the only available treatment for CD is a strict, lifelong gluten-free diet (GFD). Dietary gluten restriction is a safe and effective therapy; however, unintentional gluten exposure on a GFD is common and intermittent. Recent findings suggest that most CD patients can only attain a gluten-reduced diet instead of the recommended strict GFD. Gluten exposure may be more common than realized and is distinct from lapses in an otherwise intentionally strict GFD [23,24].
Among the main causes of gluten exposure in a GFD is the ubiquitous nature of gluten, food cross-contamination, and the limitations and socio-emotional toll [25]. In addition, many of the manufactured gluten-free products tend to be less healthy than their gluten analogues since high amounts of lipids, sugars, and other additives are incorporated in their production to simulate the viscoelastic properties of gluten proteins [26]. Although it is well known that legislation on the labeling of gluten-free products is based on the limitation of 20 parts per million (ppm) of gluten [27], there is no clear consensus on the safe amount of daily gluten intake due to the threshold for triggering symptoms has interindividual variability. Total daily gluten consumption that seems to be safe for most CD patients is <50 mg gluten; nevertheless, little amounts as 10 mg of daily gluten for some CD patients could promote development of intestinal mucosal abnormalities [28].
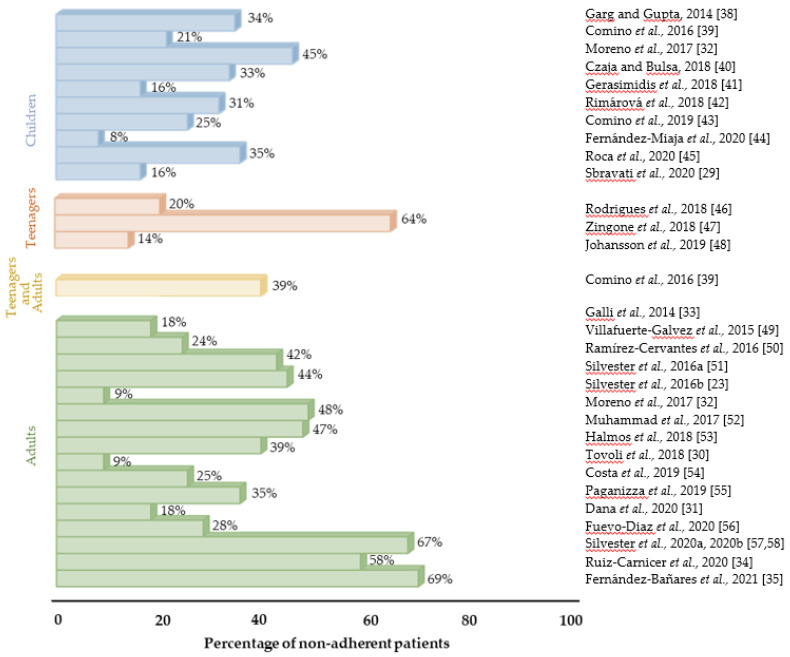
Several studies based on nutritional questionnaires, serological tests, and evaluating gluten immunogenic peptides in feces and urine, have reported variable gluten exposure rates in patients with CD, reaching up to 69% in adults, 64% in adolescents, and 45% in children (Figure 1) despite their best efforts to avoid it. Studies reporting gluten exposure rates may compromise high rates of ongoing symptoms [29,30,31] and enteropathy [32,33,34,35] in patients with CD, leading to comorbidities such as anemia, severe malabsorption, and various forms of malignancies [36]. Hence, it is important to drive efforts to develop non-dietary adjunctive or alternative therapies for CD treatment [37]. Recently, researchers have attempted to meet the requests of celiac patients seeking therapies aside from GFD. In this review, we summarize the spectrum of potential therapeutic agents to improve CD management and their research status, highlighting several drug candidates in phase II/III clinical trials.
Figure 1.
Studies reporting gluten exposure rates in CD patients on a supposed GFD. CD, celiac disease; GFD, gluten-free diet [23,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58].
3. Potential Alternative or Adjuvant Non-Dietary Treatments for CD
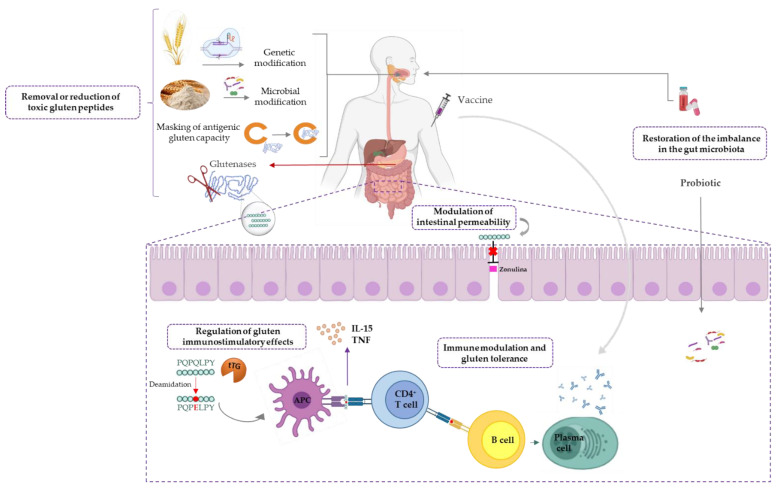
The emerging therapeutic options for CD can be broadly classified into one of the following strategies—(1) removal of toxic gluten peptides before reaching the intestine, (2) regulation of the immunostimulatory effects of toxic gluten peptides, (3) modulation of intestinal permeability, (4) immune modulation and induction of gluten tolerance, and (5) restoration of the imbalance in the gut microbiota (Figure 2).
Figure 2.
Emerging therapeutic approaches for non-dietary CD treatment. APC, antigen-presenting cell; CD, celiac disease; IL-15, interleukin 15; TNF, tumor necrosis factor; tTG, tissue transglutaminase.
Many of the sequential steps in CD pathogenesis are well-elucidated; hence, multiple well-defined targets for research and drug development are available (Table 1). Likewise, therapies focused on the regulation of the immunostimulatory effects have been described for other related pathologies, and due to their efficacy, their indications have been extended to CD.
Table 1.
Summary of strategies for CD grouped according to their goals.
| Strategy | Goal | Therapy | References | |
|---|---|---|---|---|
| Removal of toxic gluten peptides before reaching the intestine | Genetic modification of gluten-containing cereals | Genetically modified wheat flours | [59,60,61] | |
| Microbial gluten modification | Pretreatment with probiotic bacteria of the genus Lactobacillus (VSL#3) | [62] | ||
| Pretreatment with microbial transglutaminase (m-TG) and N-methyl-lysine | [63] | |||
| Masking of antigenic gluten capacity | Polymeric resins HEMA-co-SS | [64,65] | ||
| AGY-010 | [66] | |||
| Luminal gluten detoxification | Prolyl endopeptidases (PEPs) | Flavobacterium meningosepticum (FM-PEP) | [67,68] | |
| Myxococcus xanthus (MX-PEP) | [69] | |||
| Sphingomonas capsulata (SC-PEP) | [70,71] | |||
| Aspergillus niger (AN-PEP) | [72] | |||
| Gluten hydrolytic enzyme cocktail | SC-PEP and EPB-2 (ALV003) | [73] | ||
| FM-PEP and EPB-2 | [74] | |||
| Subtilisin derived from Rothia mucilaginosa (Sub-A) | [75] | |||
| Cysteine endopeptidase derived from Hordeum vulgare (EP-B2) | [21] | |||
| Elastase derived from Homo sapiens (CEL-3B) | [22] | |||
| Regulation of the immunostimulatory effects of toxic gluten peptides | Immune response regulation | Inhibition of transglutaminase (ZED 1227) | [76] | |
| Blocker of HLA DQ binding to T-cells | [77] | |||
| NK lymphocyte activation blocker: NKG2D receptor antagonists | [78] | |||
| Lymphocyte recruitment blocker | Anti-α4 integrin (natalizumab) | [79] | ||
| Anti-integrin α4β7 (vedolizumab) | ||||
| Binding inhibitors CD40-CD40L | ||||
| Binding inhibitors CXCL10- CXCR3 | ||||
| Binding inhibitors CCL25-CCR9 | ||||
| Anti-cytokines | Anti-IL-15, PRV-015, CALY-002 (AMG714) | [76,80] | ||
| Anti-TNF-α (infliximab and adalimumab) | ||||
| Anti-TNF- γ (fontolizumab) | ||||
| Inhibition of the proinflammatory cascade | Anti-inflammatories (generic corticosteroids, budesonide, mesalazine) | [81] | ||
| Modulation of intestinal permeability | Barrier enhancing therapies | Larazotide acetate (AT-1001 and INN-202) | [82,83] | |
| Immune modulation and induction of tolerance to gluten | Immunomodulation and gluten tolerance | Vaccine Nexvax2 | [84,85] | |
| TAK-101 (CNP-101 and TIMP-GLIA) | [86] | |||
| KAN-101 | [87] | |||
| Hookworm infection (Necator americanus) | [88] | |||
| Mucosal tolerance due to genetic modification | [89] | |||
| Restoration of the imbalance in the gut microbiota | Probiotic supplementation | Microbial therapies | [90,91] | |
TNF, tumor necrosis factor; IgA, immunoglobulin A; Il-15, interleukin 15; NK, natural killer; PEP, prolyl endopeptidase; P-HEMA-co-SS, poly-hydroxyethylmethacrylateco-styrene sulfonate.
3.1. Removal or Reduction of Toxic Gluten Peptides
Therapies aimed at eliminating or reducing gluten peptides can act in food before marketing, during digestion in the human tract, or masking the antigenic capacity before reaching the intestinal mucosa.
3.1.1. Genetic Modification of Gluten-Containing Cereals
The development of cereals with reduced or absent immunogenic gluten proteins is important for the management of CD. The wheat variants currently used have been reported to be more immunogenic than the ancestral or wild variants such as those belonging to the genera Tritordeum or Triticum [92,93]. Genetic advances in plants have successfully allowed the production of wheat lines with very low or completely lacking gluten content through the hybridization of wheat species [94]. A recent study described the traditional breeding and characterization of a novel ultralow gluten barley variety in which the gluten content was reduced to below 5 ppm by combining three recessive alleles, which act independently to lower the hordein content in the parental varieties [59].
RNA interference to silence the expression of gluten proteins that contain immunogenic epitopes for CD has been employed as a genetic engineering strategy [95]. This approach has allowed the development of wheat lines that contain very few immunogenic epitopes of CD, and, therefore, could be consumed by patients with non-celiac gluten sensitivity, since it produces no adverse clinical symptoms [96,97]. Currently, several studies are in progress to understand the effects of these new lines in patients with CD.
The use of CRISPR/Cas9 (Clustered Regulatory Interspaced Palindromic Repeats associated protein 9) technology can precisely and efficiently reduce the amount of α-gliadin in the seed kernel, providing bread and durum lines with reduced immunoreactivity for the celiac community [60,94]. However, it is likely that the deleted gliadin genes need to be replaced by non-immunogenic gliadin variants to obtain adequate elasticity. Additionally, governmental regulations for genetic modification of food products require expensive and time-consuming food safety assessments to be met before product marketing [94].
3.1.2. Microbial Gluten Modification
The addition of diverse microorganisms in sourdough for fermentation has been studied because it contains proteases capable of hydrolyzing gluten peptides rich in glutamine and proline residues. Diverse studies using species of the genus Lactobacillus have reported that this baking method could obtain safe breads for celiac patients [62,98]. The well-known probiotic preparation VSL#3 comprises eight strains belonging to the genera Bifidobacterium, Lactobacillus, and Streptococcus. This cocktail was assayed during the food processing step and produced tolerable predigested gliadins without α-gliadin peptides p62-75 and 33-mer, but with the palatability of gluten-free products [99]. This study demonstrated the improvement in the symptoms of adult CD patients with irritable bowel syndrome (IBS) [100]. Furthermore, the probiotic preparation was capable of stabilizing intraepithelial junctions, promoting the barrier effect that prevents the entry of toxic peptides into the lamina propria [91,101]. However, individual probiotic strains are inadequate to break down gliadin compared to the group efficacy [101,102].
Another investigated approach in the preclinical phase consists of the pretreatment of flours or sourdoughs with microbial transglutaminase (m-TG) and N-methyl-lysine [103,104]. The use of N-methyl lysine and m-TG derived from Streptomyces mobaraensis provoked gluten modification and loss of affinity for the HLA-DQ2 molecule, which leads to less activation of intestinal T lymphocytes [105]. Although the effect of standard bakery concentrations of microbial transglutaminase (m-TG) in wheat bread preparation on the immunoreactivity of sera of CD patients was investigated, its use in food preparation remains a subject of debate [63].
3.1.3. Masking of Antigenic Gluten Capacity
The gluten-binding polymer BL-7010 or copolymer poly-hydroxyethylmethacrylate-co-styrene sulfonate (P-HEMA-co-SS) complex is a non-absorbable synthetic origin blocking agent that binds intraluminal gluten [64]. Therefore, digestive enzymes cannot access the cleavage sites, preventing the degradation of immunogenic peptides that are not absorbed by the intestine and do not induce an immune response. The effect of BL-7010 has been investigated in intestinal biopsy samples from patients with CD [64,65,106]. Attenuation of the immune response and the high safety profile in animal models were observed; however, this phase II therapy was discontinued in 2017.
Recent studies have developed neutralizing anti-gliadin antibodies extracted from egg yolk (AGY-010). IgY antibodies have shown effectiveness in neutralizing and absorbing gliadin, as well as resistance to stomach conditions [66]. This therapy is currently in phase II studies and a study is ongoing to evaluate its efficacy and safety in CD patients [107]. As the use of egg yolk antibodies might be inefficient for large-scale clinical production, parallel recombinant antibody fragments in single-chain format have been produced for the same purpose [108].
3.1.4. Luminal Gluten Detoxification
Oral enzyme therapy is focused on the inactivation of gluten peptides in the human gastrointestinal tract before reaching the intestine. Gluten-degrading enzymes seem to hold the most promise as attractive therapies for helping patients with CD to avoid accidental gluten ingestion and to promote better overall health. A prerequisite is that such enzymes should be active under gastro-duodenal conditions, quickly neutralize the T-cell-activating gluten peptides and be safe for human consumption [67,68,70,109].
Glutenases have been identified in bacteria, fungi, plants, and even insects (Table 2). Although the enzymes studied are endopeptidases, interesting exopeptidases have also been described [110]. Endopeptidases are further subdivided depending on their catalytic mechanism; among them, prolyl endopeptidases (PEPs) are especially effective in hydrolyzing peptide bonds on the carboxyl side of internal proline residues in gluten-derived oligopeptides [69]. The potential synergism between gluten-degrading enzymes that differ in their cleavage specificities and optimum pH values raises the possibility of a mixture that would more effectively eliminate the antigenicity of ingested gluten fractions [111].
Table 2.
Summary of glutenases used in enzyme therapy and classified according to origin of isolation, producer organism, and catalytic mechanism. ND, not determined.
| Source of Enzymes | Peptidase Type | Organism | Isolated Enzyme | References |
|---|---|---|---|---|
| Bacterial peptidases | Prolyl endopeptidase | S. capsulata | SC-PEP | [68] |
| M. xanthus | MX-PEP | [65] | ||
| F.meningosepticum | FM-PEP | [66] | ||
| Chryseobacterium taeanense | PEP 2RA3 | [109] | ||
| Subtilisin | Rothia aeria | ND | [112] | |
| R. mucilaginosa | Sub-A | [112] | ||
| Bacillus licheniformis | ND | [113] | ||
| Pseudolysin | Pseudomonas aeruginosa | lasB | [114] | |
| Thermolysin | Bacillus thermoproteolyticus | ND | [113] | |
| Serine peptidase | Bacillus tequilensis | ND | [115] | |
| ND | Bacillus spp GS 188 | ND | [116] | |
| Serine carboxyl peptidase | Actinoallomurus A8 | E40 | [117] | |
| Fungal peptidases | Prolyl endopeptidase | A. niger | AN-PEP | [72] |
| Aspergillopepsin | A. niger | ASP | [118] | |
| Exopeptidase | Aspergillus oryzae | AO-DPP-IV | [119] | |
| Plant peptidases | Cysteine endopeptidase | H. vulgare | EP-B2 | [120] |
| Carica papaya | Caricain | [121] | ||
| Triticum aestivum | Triticain-α | [122] | ||
| H. vulgare | HvPap-6 CysProt | [123] | ||
| Insect peptidases | Prolyl peptidase | Rhizopertha dominica | ND | [123] |
| Prolidase | Tenebrio molitor | ND | [124] | |
| Human peptidases | Elastase | Homo sapiens | CEL3B | [22] |
| Homo sapiens | CEL2A | [22] | ||
| Carboxypeptidase | Homo sapiens | CBPA1 | [22] |
Among the bacterial enzymes capable of degrading gluten, PEPs are produced by F. meningosepticum [68,69], S. capsulata [70,71] and M. xanthus [69]. These three enzymes showed high specificity against reference chromogenic substrates and the potential to successfully degrade the immunogenic sequences of gluten. The cysteine endoprotease EP-B2 and PEP from F. meningosepticum complement each other in terms of their gluten hydrolytic properties; however, significant efforts have been made to increase their thermostability to be suitable for industrial applications [111].
Fungal PEP from A. niger, known as AN-PEP, exhibits post-proline cleavage activity and is highly efficient in degrading gluten [72]. A clinical study with Tolerase G, an AN-PEP-based supplement, reduced the amount of gluten exposed in the duodenum efficiently, despite not completely degrading the gluten [72]. The enzyme preparation consisting of AN-PEP from A. niger and DPP-IV from A. oryzae (STAN 1) administered orally in celiac patients appeared to be modest because of the non-specificity of AN-PEP and the very limited proteolytic effect of DPP-IV. Therefore, these studies were stalled in phase II in 2017. In the genus Aspergillus, another enzyme was detected with gluten-degrading activity, termed aspergillopepsin (ASP) from A. niger, although ASP needs to be used as a complementary enzyme because of its incomplete degradation [118]. In this sense, a dietary supplement has been widely used in the food and feed industry containing ASP from A. niger and DPP-IV from A. oryzae, which successfully degraded small amounts of gluten in vitro [119].
As previously argued, the combination of enzymes appears to be a future direction in enzyme therapy. The enzymatic cocktail, latiglutenase or IMGX-003 (formerly ALV003), consists of a 1:1 combination of cysteine endoprotease from barley EP-B2 (IMGX-001), and PEP from S. capsulate SC-PEP (IMGX-002). A phase II gluten challenge to investigate its effect on both mucosal and symptomatic protection in CD patients is in progress. Initial findings with latiglutenase have been shown to mitigate gluten-induced intestinal mucosal injury as well as to reduce the severity and frequency of symptoms in patients with CD [73,125]. Evidence of symptom relief was particularly pronounced in patients with positive serology despite following a GFD [61,126,127].
An engineered synthetic gluten-degrading enzyme, KumaMax, with technological improvements, is being studied. KumaMax showed similar in vitro results to IMGX-003, although it is still under development [128]. The gluten-degrading enzyme subtilisin-A (Sub-A) from B. licheniformis was modified by PEGylation and subjected to microencapsulation. The effectiveness was confirmed in vitro and in vivo and showed a significant increase in protection against acid exposure [113].
Investigating the effect of glutenases on the symptoms and biomarkers in CD patients with randomized, placebo-controlled studies is mandatory; however, this is not as straightforward as it might seem.
3.2. Immune Response Regulation
As inflammatory mediators are common in CD and other gastrointestinal pathologies, certain therapies aimed at avoiding chronic gastrointestinal inflammation could be applied in CD.
tTG plays a critical role in the pathogenesis of CD through the deamidation and transamidation of gluten peptides, which leads to an immune response with inflammation of the intestinal mucosa [129,130]. Hence, the inhibition of tTG results in the abolishment of gluten peptide presentation by HLA-DQ2/DQ8, preventing the immune response. Three varieties of tTG-2 inhibitors have been well described, namely, irreversible inhibitors, reversible inhibitors, and competitive amine inhibitors. ZED-1227 is a highly specific orally active irreversible inhibitor with promising preliminary preclinical results. A phase II clinical study with ZED-1227 is ongoing in EU countries in healthy volunteers [76]. Nevertheless, tTG plays a critical role in gut wound healing, and its safety and efficacy require further study [131]. Among competitive inhibitors, cystamine is currently the only competitive commercially available tTG-2 inhibitor despite that it has not been explored for its potential role in CD. Recently, Palansky et al. [132] discovered that disulfiram, an FDA-approved drug for alcohol abuse, is also a tTG inhibitor. This is the first clinically approved compound to show human tTG inhibitory activity, raising further interesting possibilities for the future in terms of tTG inhibition as a therapeutic strategy in CD [133].
Another attractive therapeutic target to prevent the activation of the immune response is the HLA-DQ2 blocker. Gluten-like molecules in which proline residues have been replaced by azidoprolines do not elicit an immune response in T-cells isolated from individuals with CD [8]. Cyclic and dimeric peptides have also been developed that bind DQ2, partially blocking T-cell proliferation and antigen presentation. However, these molecules do not fully block the activation of T-cells; therefore, other nontoxic antagonists with high affinity are currently being studied [129].
Some studies have highlighted the role of IL-15 and the receptor activator NKG2D and other immune soluble factors as targets of CD treatment. IL-15 plays a critical role in the activation of intraepithelial lymphocytes and participates in both innate and adaptive responses. NKG2D is the receptor of T-cells and natural killer cells [134]. The first monoclonal antibody (moAb) studied against the IL-15 receptor was Hu-Mik-Beta-1, and positive results were obtained in refractory CD. However, this therapy was stuck in phase I. Second, PRV-015 (also known as AMG 714) is a fully human moAb that has emerged as a leading investigational candidate for nonresponsive CD (NRCD), in which patients maintain disease activity despite an ongoing GFD. Phase II studies have shown a reduction in inflammation and symptoms in a clinical trial with patients with refractory CD type 2 [80]. Lastly, CALY-002 is a moAb whose safety, tolerability, pharmacokinetics, and pharmacodynamics are being evaluated in phase II studies in both CD and eosinophilic esophagitis [135].
Tumor necrosis factor (TNF)-γ secreted by T-cells in response to gluten is another therapeutic target under study. Fontolizumab was initially developed for inflammatory bowel disease (IBD) treatment and has been proposed for CD, although clinical trials for this indication have not yet been registered. Infliximab and adalimumab moAbs targeting TNF-α have been used in clinical practice for IBD and could be useful in treating CD [76,136].
Among T-cell-targeted therapies aimed at blocking lymphocyte recruitment, natalizumab is an anti-α4 used in Crohn’s disease and could be useful in CD, although its side effects are very high [79,137]. Vedolizumab is scheduled to start phase II studies that block α4β7 integrin [138]. In addition, chemokine receptor inhibitors such as CXCR3 and its specific ligands CXCL10 and CXCL11 have also been studied [79]. These molecules are among the main determinants in the recruitment of immune cells to the intestinal lamina propria and are involved in the uptake of lymphocytes in the presence of gliadin peptides. CCL25 and its receptor CCR9 appear to be a therapeutic alternative in the future, although to date it has only been studied in animal models with Crohn’s disease [139,140].
Anti-inflammatory drugs such as corticosteroids and budesonide are generally used to treat the symptoms of refractory CD. Likewise, mesalazine has been proposed, although it must be remembered that most of these formulations are prepared to be released in the colon and the inflammation in CD affects the small intestine [66]. Recent studies have shown that mesalazine has a beneficial effect on the molecules and biological mediators of inflammation that occur in the mucosa of celiac patients [81].
3.3. Barrier Enhancing Therapies
Increased intestinal permeability has been implicated in CD due to both transcellular and paracellular epithelial permeability, with apical junctional protein complexes called tight junctions being key components in the latter process [141].
Larazotide acetate, formerly known as AT-1001 or INN-202, is a locally acting octapeptide with a sequence analogous to a portion of Vibrio cholerae zonula occludens toxin [141]. In cultured intestinal epithelial monolayers, larazotide acetate enhanced actin rearrangement and prevented the disassembly of tight junctions [142,143]. In addition, larazotide acetate prevents the passage of gluten peptides to the lamina propria by closing the intercellular junctions of the enterocytes, which could help prevent the development of the immune cascade in celiac patients. Therefore, larazotide acetate is the most advanced experimental drug, showing a reduction in symptoms as well as a reduction in anti-tTG antibody levels. Three phase II studies of larazotide acetate have been completed and published in CD patients undergoing a gluten challenge, but only an excellent safety profile and efficacy with low dose have been reported in patients with NRCD. Therefore, larazotide acetate has moved forward to a phase III registration study for this indication [82,83,144].
3.4. Immunomodulation and Gluten Tolerance
Vaccine therapy is the preferred option among alternative treatments to a GFD in patients with CD. It is based on immunization with gluten epitopes, which induces the expansion of regulatory T-cells, restoring oral tolerance to gluten [145]. The Nexvax2 vaccine (ImmusanT, Cambridge, MA, USA) comprises the use of three gluten epitopes chosen based on a study by Tye-Din et al. [145]. This study examined epitopes within wheat, barley, and rye with the ability to induce and stimulate T-cells isolated from the serum of patients with CD on a gluten-containing diet. Nexvax2 is one of several CD drugs that has reached phase II clinical trials [141]. However, although Nexvax2 showed a good safety profile, its efficacy has yet to be demonstrated. Nexvax2 is specific only for individuals with the HLA-DQ2 genotype. Therefore, another vaccine should be investigated in patients with HLA-DQ8 genotyping [84,85].
Biodegradable nanoparticles encapsulated with gliadin proteins TAK-101 (formerly known as CNP-101 and TIMP-GLIA) seem to be a first-in-class agent that induces antigen-specific immune tolerance to CD [141]. TAK-101 binds inflammatory cells to initiate tolerogenic immune reprogramming. According to the clinicaltrials.gov, the phase II developmental trial of TAK-101 for treating patients with CD was estimated to be completed in July 2019, but it is still in the active phase, not the recruiting phase [146].
A new therapy in phase I focuses on restoring normal immune tolerance by targeting specific receptors in the liver, named KAN-101 [141]. The tolerogenic nanoparticles for intravenous injection trigger a cascade of events that drive the re-education of T-cells so that they do not respond to gluten antigens [87].
The administration of N. americanus infective larvae in patients with CD interferes with the host immune response due to its survival in the intestine. Studies of duodenal biopsies from CD individuals infected with N. americanus and exposed to gluten have shown a reduction in the production of IL-2, IFN-γ, and IL-17. In addition, the absence of histological lesions and even a decrease in anti-tTG antibody levels have been demonstrated [88]. N. americanus is currently in phase II clinical trials, although problems with CD patient acceptance for routine clinical use are anticipated [66,147].
Finally, other studies based on the tolerance of the mucosa to genetic modification are in the initial phase of investigations. These studies specifically focused on organoids derived from the human intestine, providing a model to study the response to gluten and the effects of molecules derived from the microbiota in patients with CD [89].
3.5. Restoration of the Imbalance in the Gut Microbiota
The gut microbiota is involved in the initiation and perpetuation of intestinal inflammation in several chronic diseases. Indeed, several studies have identified certain microorganisms in CD patients and healthy subjects. Therefore, alteration of the microbiota could play a significant role in the pathogenesis of CD. Recent studies have focused on the role of the gut microbiota in CD and the complex relationship between its composition, genetic background, GFD, and persistence of clinical symptoms [90,148]. The specific mechanisms by which microorganisms can participate in the development of responses to gluten are broad and include the metabolism of trigger antigen responses, enhancement of the intestinal barrier, and modulation of adaptive and innate immune responses [149].
Recent data have shown that genetics (HLA-DQ-2 or DQ-8) may predispose individuals with CD to dysbiosis [90,148]. Palma et al. [150] studied the effects of following a GFD on the composition of gut microbiota in healthy subjects. A significant decrease of Bifidobacterium, Clostridium lituseburense, and Faecalibacterium prausnitzii and an increase in Enterobacteriaceae and Escherichia coli counts were found. Therefore, the supplementation with a probiotic to restore the imbalance in the gut microbiota might be a reasonable therapeutic option by downregulating the proinflammatory immune response in CD patients [90]. The design of specific probiotics comprises advanced genomic and metabolomics techniques using the interactions between the human body-microbiota and intra-microbiota, eventually leading to tailored specific probiotic therapies for microbiome regulation and health sustainability.
Probiotics play an important role in preventing the overgrowth of potentially pathogenic bacteria and maintaining the integrity of the gut mucosal barrier. The beneficial effects of probiotics have been previously studied in adult patients with IBS. Oral administration of a probiotic mixture of Lactobacillus plantarum 14D-CECT 4528, Lactobacillus casei, Bifidobacterium breve Bbr8 LMG P-17501, B. breve Bl10 LMG P-17500, and Bifidobacterium animalis under randomized, double-blind, and placebo-controlled conditions showed the improvement in symptoms of adult CD patients with IBS [100]. In the future, microorganisms or even genetically engineered microorganisms could be used to act as living enzyme machinery as well as vectors for the delivery of endopeptidases capable of digesting gluten in the stomach, thereby allowing celiac patients to have a controlled dietary gluten intake [91,151].
In conclusion, probiotics are not expected to provide a rapid cure for complex diseases such as CD, but rather to alleviate the severity of symptoms [99]. More studies are needed to address how the gut microbiome can modulate or alter the course of the disease. To date, there are no guidelines available that recommend probiotic use in patients with CD. However, the data suggest a strong adjunctive role in the management of symptoms and bacterial overgrowth.
4. Clinical Trials
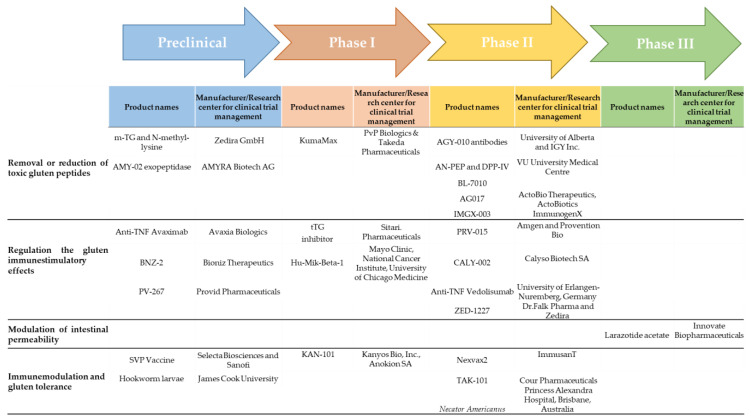
Clinical endpoints are variables to quantify the potential effect of the treatment or intervention under study and reflect or characterize how a subject “feels, functions, or survives” [152]. Many major disease areas have established clinical trial endpoints because a fair number of registration trials have already been conducted and drugs approved for marketing. As in CD, there are no approved products and little experience, and agreed endpoints are lacking. Certain treatments in CD could control symptoms and prevent worsening of damage, while others are, at least initially, focused primarily on healing and maintenance of healing, with little effect on symptoms. Therefore, different endpoints or endpoint instruments are needed [153]. To date, only larazotide acetate is currently in phase III studies; most of them at phase II and a few phase I trials have explored its efficacy. Some therapies are being evaluated in preclinical trials and are postulated to be promising treatments for CD pathogenesis (Figure 3). We are facing many promising and emerging options for the treatment of CD.
Figure 3.
Clinical and preclinical development pipeline for CD. CD, celiac disease; PEP, prolyl endopeptidases; TNF, tumor necrosis factor; tTG, tissue transglutaminase.
5. Conclusions
Although a GFD has been shown to be safe and effective in most celiac patients, the limitations caused by dietary gluten restriction and high gluten exposure rates raise the need to develop new therapies for CD. Different non-dietary therapeutic strategies are currently in the development phase and in clinical research, which could be a useful option in the medium- or long-term in patients with CD. To date, larazotide acetate is the most advanced experimental drug that has shown a reduction in symptoms as well as anti-tTG antibody titers. Promising PRV-015 immunotherapy requires more assays to establish rational targets for disease prevention. The use of glutenases as food preprocessors has proven to be very effective; however, the use of oral glutenases is perhaps the most accepted strategy for patients with CD and one of the most numerous options in terms of ongoing studies. All efforts are now being made to assess the effectiveness of these enzymes as a supplement to a GFD, highlighting the phase II results of IMGX-003 being very promising. Vaccine therapy has limitations, such as that it can engage only known or previously investigated immunogenic epitopes and effectiveness with the specific HLA-DQ2 genotype. However, if successful, it has the potential to have prolonged benefits on patients.
In addition, other many interesting drugs are in early research stages, such as tTG inhibitors, HLA blockers, and probiotics, although probiotics will probably need to be combined with long-term dietary changes. While several trials are ongoing or underway for CD, there is no consensus on outcome measures in CD patient trials.
Preventing the onset of CD entirely would be the most beneficial and desirable approach; however, recent approaches argue whether ingesting certain amounts of gluten plays a complementary or “adjuvant” role to a GFD and not as a substitute to a GFD in patients with CD. Nevertheless, some of these therapies could also be effective in other gluten-related pathologies in which a minimal amount of gluten is tolerable.
Great efforts are ongoing to determine the effectiveness and the dose limit of gluten ingested in these therapies. It is also obvious that the possibility of using synergistic strategies could increase the maximum safe doses allowed for CD; therefore, this issue will be the next challenge.
Author Contributions
Conceptualization, V.S. and M.d.L.M.; data curation, V.S. and M.d.L.M.; formal analysis, V.S. and M.d.L.M.; investigation, V.S., Á.R.-C., C.S. and M.d.L.M.; methodology, V.S. and M.d.L.M.; resources, V.S. and M.d.L.M.; writing—original draft, V.S., Á.R.-C., C.S. and M.d.L.M.; writing—review and editing, V.S., Á.R.-C., C.S. and M.d.L.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grant from Federación de Asociaciones de Celíacos de España (FACE) (SUBN/2019/005).
Conflicts of Interest
The authors have no potential conflict of interest to declare. None of the authors have any conflicts of interest that could affect the performance of the work or the interpretation of the data.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lebwohl B., Cao Y., Zong G., Hu F.B., Green P.H.R., Neugut A.I., Rimm E.B., Sampson L., Dougherty L.W., Giovannucci E., et al. Long-term gluten consumption in adults without celiac disease and risk of coronary heart disease: Prospective cohort study. BMJ. 2017;357:j1892. doi: 10.1136/bmj.j1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al-Toma A., Volta U., Auricchio R., Castillejo G., Sanders D.S., Cellier C., Mulder C.J., Lundin K.E.A. European Society for the Study of Coeliac Disease (ESsCD) guideline for coeliac disease and other gluten-related disorders. United Eur. Gastroenterol. J. 2019;7:583–613. doi: 10.1177/2050640619844125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lebwohl B., Sanders D.S., Green P.H.R. Coeliac disease. Lancet. 2018;391:70–81. doi: 10.1016/S0140-6736(17)31796-8. [DOI] [PubMed] [Google Scholar]
- 4.Ludvigsson J.F., Murray J.A. Epidemiology of celiac disease. Gastroenterol. Clin. N. Am. 2019;48:1–18. doi: 10.1016/j.gtc.2018.09.004. [DOI] [PubMed] [Google Scholar]
- 5.Singh P., Arora A., Strand T.A., Leffler D.A., Catassi C., Green P.H., Kelly C.P., Ahuja V., Makharia G.K. Global prevalence of celiac disease: Systematic review and meta-analysis. Clin. Gastroenterol. Hepatol. 2018;16:823–836.e2. doi: 10.1016/j.cgh.2017.06.037. [DOI] [PubMed] [Google Scholar]
- 6.Glissen J.R.B., Singh P. Coeliac disease. Paediatr. Int. Child. Health. 2019;39:23–31. doi: 10.1080/20469047.2018.1504431. [DOI] [PubMed] [Google Scholar]
- 7.Mardini H.E., Westgate P., Grigorian A.Y. Racial differences in the prevalence of celiac disease in the US population: National Health and Nutrition Examination Survey (NHANES) 2009–2012. Dig. Dis. Sci. 2015;60:1738–1742. doi: 10.1007/s10620-014-3514-7. [DOI] [PubMed] [Google Scholar]
- 8.Husby S., Koletzko S., Korponay-Szabo I.R., Mearin M.L., Phillips A., Shamir R., Troncone R., Giersiepen K., Branski D., Catassi C., et al. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition guidelines for the diagnosis of coeliac disease. J. Pediatr. Gastroenterol. Nutr. 2012;54:136–160. doi: 10.1097/MPG.0b013e31821a23d0. [DOI] [PubMed] [Google Scholar]
- 9.Rubio-Tapia A., Hill I.D., Kelly C.P., Calderwood A.H., Murray J.A. American College of Gastroenterology. ACG clinical guidelines: Diagnosis and management of celiac disease. Am. J. Gastroenterol. 2013;108:656–676. doi: 10.1038/ajg.2013.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ludvigsson J.F., Bai J.C., Biagi F., Card T.R., Ciacci C., Ciclitira P.J., Green P.H., Hadjivassiliou M., Holdoway A., van Heel D.A., et al. Diagnosis and management of adult coeliac disease: Guidelines from the British Society of Gastroenterology. Gut. 2014;63:1210–1228. doi: 10.1136/gutjnl-2013-306578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fasano A., Sapone A., Zevallos V., Schuppan D. Nonceliac gluten sensitivity. Gastroenterology. 2015;148:1195–1204. doi: 10.1053/j.gastro.2014.12.049. [DOI] [PubMed] [Google Scholar]
- 12.Mena M.C., Sousa C. Analytical tools for gluten detection. Policies and regulation. In: Arranz E., Fernández-Bañares F., editors. Advances in the Understanding of Gluten Related Pathology and the Evolution of Gluten-Free Foods. OmniaScience; Barcelona, Spain: 2015. pp. 527–564. [Google Scholar]
- 13.Caio G., Volta U., Sapone A., Leffler D.A., De Giorgio R., Catassi C., Fasano A. Celiac disease: A comprehensive current review. BMC Med. 2019;17:142. doi: 10.1186/s12916-019-1380-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sharma N., Bhatia S., Chunduri V., Kaur S., Sharma S., Kapoor P., Kumari A., Garg M. Pathogenesis of celiac disease and other gluten related disorders in wheat and strategies for mitigating them. Front. Nutr. 2020;7:6. doi: 10.3389/fnut.2020.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lindfors K., Ciacci C., Kurppa K., Lundin K.E.A., Makharia G.K., Mearin M.L., Murray J.A., Verdu E.F., Kaukinen K. Coeliac disease. Nat. Rev. Dis. Primers. 2019;5:3. doi: 10.1038/s41572-018-0054-z. [DOI] [PubMed] [Google Scholar]
- 16.Maiuri L., Ciacci C., Auricchio S., Brown V., Quaratino S., Londei M. Interleukin 15 mediates epithelialmchanges in celiac disease. Gastroenterology. 2000;119:996–1006. doi: 10.1053/gast.2000.18149. [DOI] [PubMed] [Google Scholar]
- 17.Maiuri L., Ciacci C., Ricciardelli I., Vacca L., Raia V., Auricchio S., Picard J., Osman M., Quaratino S., Londei M. Association between innate response to gliadin and activation of pathogenic T cells in coeliac disease. Lancet. 2003;362:30–37. doi: 10.1016/S0140-6736(03)13803-2. [DOI] [PubMed] [Google Scholar]
- 18.Qiao S.W., Bergseng E., Molberg O., Xia J., Fleckenstein B., Khosla C., Sollid L.M. Antigen presentation to celiac lesion-derived t cells of a 33-mer gliadin peptide naturally formed by gastrointestinal digestion. J. Immunol. 2004;173:1757–1762. doi: 10.4049/jimmunol.173.3.1757. [DOI] [PubMed] [Google Scholar]
- 19.Ráki M., Tollefsen S., Molberg Ø., Lundin K.E.A., Sollid L.M., Jahnsen F.L. A Unique dendritic cell subset accumulates in the celiac lesion and efficiently activates gluten-reactive T cells. Gastroenterology. 2006;131:428–438. doi: 10.1053/j.gastro.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 20.Tollefsen S., Arentz-Hansen H., Fleckenstein B., Molberg Ø., Ráki M., Kwok W.W., Jung G., Lundin K.E.A., Sollid L.M. HLA-DQ2 and -DQ8 signatures of gluten T cell epitopes in celiac disease. J. Clin. Investig. 2006;116:2226–2236. doi: 10.1172/JCI27620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martinez M., Gómez-Cabellos S., Giménez M.J., Barro F., Diaz I., Diaz-Mendozam M. Plant Proteases: From key enzymes in germination to allies for fighting human gluten-related disorders. Front. Plant Sci. 2019;10:721. doi: 10.3389/fpls.2019.00721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gutiérrez S., Pérez-Andrés J., Martínez-Blanco H., Ferrero M.A., Vaquero L., Vivas S., Casqueiro J., Rodríguez-Aparicio L.B. The human digestive tract has proteases capable of gluten hydrolysis. Mol. Metab. 2017;6:693–702. doi: 10.1016/j.molmet.2017.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Silvester J.A., Graff L.A., Rigaux L., Walker J.R., Duerksen D.R. Symptomatic suspected gluten exposure is common among patients with coeliac disease on a gluten-free diet. Aliment. Pharmacol. Ther. 2016;44:612–619. doi: 10.1111/apt.13725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stefanolo J.P., Tálamo M., Dodds S., Temprano M.P., Costa A.F., Moreno M.L., Pinto-Sánchez M.I., Smecuol E., Vázquez H., Gonzalez A., et al. Real-world gluten exposure in patients with celiac disease on gluten-free diets, determined from gliadin immunogenic peptides in urine and fecal samples. Clin. Gastroenterol. Hepatol. 2021;19:484–491.e1. doi: 10.1016/j.cgh.2020.03.038. [DOI] [PubMed] [Google Scholar]
- 25.Wolf R.L., Lebwohl B., Lee A.R., Zybert P., Reilly N.R., Cadenhead J., Amengual C., Green P.H.R. Hypervigilance to a gluten-free diet and decreased quality of life in teenagers and adults with celiac disease. Dig. Dis. Sci. 2018;63:1438–1448. doi: 10.1007/s10620-018-4936-4. [DOI] [PubMed] [Google Scholar]
- 26.Khoury D.E., Balfour-Ducharme S., Joye I.J. A review on the gluten-free diet: Technological and nutritional challenges. Nutrients. 2018;10:1410. doi: 10.3390/nu10101410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Codex Standard 118-1979. [(accessed on 7 January 2021)]; Available online: http://www.fao.org/fao-who-codexalimentarius/sh-proxy/en/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCXS%2B118-1979%252FCXS_118e_2015.pdf.
- 28.Cohen I.S., Day A.S., Shaoul R. Gluten in celiac disease-more or less? Rambam Maimonides Med. J. 2019;10:e0007. doi: 10.5041/RMMJ.10360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sbravati F., Pagano S., Retetangos C., Spisni E., Bolasco G., Labriola F., Filardi M.C., Grondona A.G., Alvisi P. Adherence to gluten-free diet in a celiac pediatric population referred to the general pediatrician after remission. J. Pediatr. Gastroenterol. Nutr. 2020;71:78–82. doi: 10.1097/MPG.0000000000002676. [DOI] [PubMed] [Google Scholar]
- 30.Tovoli F., Negrini G., Sansone V., Faggiano C., Catenaro T., Bolondi L., Granito A. Celiac disease diagnosed through screening programs in at-risk adults is not associated with worse adherence to the gluten-free diet and might protect from osteopenia/osteoporosis. Nutrients. 2018;10:1940. doi: 10.3390/nu10121940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dana Z.Y., Lena B., Vered R., Haim S., Efrat B. Factors associated with non adherence to a gluten free diet in adult with celiac disease: A survey assessed by BIAGI score. Clin. Res. Hepatol. Gastroenterol. 2020;44:762–767. doi: 10.1016/j.clinre.2019.12.014. [DOI] [PubMed] [Google Scholar]
- 32.Moreno M.L., Cebolla Á., Muñoz-Suano A., Carrillo-Carrion C., Comino I., Pizarro Á., León F., Rodríguez-Herrera A., Sousa C. Detection of gluten immunogenic peptides in the urine of patients with coeliac disease reveals transgressions in the gluten-free diet and incomplete mucosal healing. Gut. 2017;66:250–257. doi: 10.1136/gutjnl-2015-310148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Galli G., Esposito G., Lahner E., Pilozzi E., Corleto V.D., Di-Giulio E., Spiriti M.A., Annibale B. Histological recovery and gluten-free diet adherence: A prospective 1-year follow-up study of adult patients with coeliac disease. Aliment. Pharmacol. Ther. 2014;40:639–647. doi: 10.1111/apt.12893. [DOI] [PubMed] [Google Scholar]
- 34.Ruiz-Carnicer A., Garzón-Benavides M., Fombuena B., Segura V., García-Fernández F., Sobrino-Rodríguez S., Gómez-Izquierdo L., Montes-Cano M.A., Rodríguez-Herrera A., Millán R., et al. Negative predictive value of the repeated absence of gluten immunogenic peptides in the urine of treated celiac patients in predicting mucosal healing: New proposals for follow-up in celiac disease. Am. J. Clin. Nutr. 2020;112:1240–1251. doi: 10.1093/ajcn/nqaa188. [DOI] [PubMed] [Google Scholar]
- 35.Fernández-Bañares F., Beltrán B., Salas A., Comino I., Ballester-Clau R., Ferrer C., Molina-Infante J., Rosinach M., Modolell I., Rodríguez-Moranta F., et al. Persistent villous atrophy in de novo adult patients with celiac disease and strict control of gluten-free diet adherence: A multicenter prospective study (CADER Study) Am. J. Gastoenterol. 2021;116:1036–1043. doi: 10.14309/ajg.0000000000001139. [DOI] [PubMed] [Google Scholar]
- 36.Abu-Janb N., Jaana M. Facilitators and barriers to adherence to gluten-free diet among adults with celiac disease: A systematic review. J. Hum. Nutr. Diet. 2020;33:786–810. doi: 10.1111/jhn.12754. [DOI] [PubMed] [Google Scholar]
- 37.Caio G., Ciccocioppo R., Zoli G., Giorgio R.D., Volta U. Therapeutic options for coeliac disease: What else beyond gluten-free diet? Digest. Liver Dis. 2020;52:130–137. doi: 10.1016/j.dld.2019.11.010. [DOI] [PubMed] [Google Scholar]
- 38.Garg A., Gupta R. Predictors of compliance to gluten-free diet in children with celiac disease. Int. Sch. Res. Not. 2014;2014:248402. doi: 10.1155/2014/248402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Comino I., Fernández-Bañares F., Esteve M., Ortigosa L., Castillejo G., Fombuena B., Ribes-Koninckx C., Sierra C., Rodríguez-Herrera A., Salazar J.C., et al. Fecal gluten peptides reveal limitations of serological tests and food questionnaires for monitoring gluten-free diet in celiac disease patients. Am. J. Gastroenterol. 2016;111:1456–1465. doi: 10.1038/ajg.2016.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Czaja-Bulsa G., Bulsa M. Adherence to gluten-free diet in children with celiac disease. Nutrients. 2018;10:1424. doi: 10.3390/nu10101424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gerasimidis K., Zafeiropoulou K., Mackinder M., Ijaz U.Z., Duncan H., Buchanan E., Cardigan T., Edwards C.A., McGrogan P., Russell R.K. Comparison of clinical methods with the faecal gluten immunogenic peptide to assess gluten intake in coeliac disease. J. Pediatr. Gastroenterol. Nutr. 2018;67:356–360. doi: 10.1097/MPG.0000000000002062. [DOI] [PubMed] [Google Scholar]
- 42.Rimárová K., Dorko E., Diabelková J., Sulinová Z., Makovický P., Baková J., Uhrin T., Jenča A., Jenčová J., Petrášová A., et al. Compliance with gluten-free diet in a selected group of celiac children in the Slovak Republic. Cent. Eur. J. Public Health. 2018;26:S19–S24. doi: 10.21101/cejph.a5369. [DOI] [PubMed] [Google Scholar]
- 43.Comino I., Segura V., Ortigosa L., Espín B., Castillejo G., Garrote J.A., Sierra C., Millán A., Ribes-Koninckx C., Román E., et al. Prospective longitudinal study: Use of faecal gluten immunogenic peptides to monitor children diagnosed with coeliac disease during transition to a gluten-free diet. Aliment. Pharmacol. Ther. 2019;49:1484–1492. doi: 10.1111/apt.15277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fernández-Miaja M., Díaz-Martín J.J., Jiménez-Treviño S., Suárez-González M., Bousoño-García C. Estudio de la adherencia a la dieta sin gluten en pacientes celiacos [Study of adherence to the gluten-free diet in coeliac patients] An. Pediatr. 2020;94:377–384. doi: 10.1016/j.anpedi.2020.06.017. [DOI] [PubMed] [Google Scholar]
- 45.Roca M., Donat E., Masip E., Crespo-Escobar P., Cañada-Martínez A.J., Polo B., Ribes-Koninckx C. Analysis of gluten immunogenic peptides in feces to assess adherence to the gluten-free diet in pediatric celiac patients. Eur. J. Nutr. 2020;60:2131–2140. doi: 10.1007/s00394-020-02404-z. [DOI] [PubMed] [Google Scholar]
- 46.Rodrigues M., Yonamine G.H., Fernandes-Satiro C.A. Rate and determinants of nonadherence to a gluten-free diet and nutritional status assessment in children and adolescents with celiac disease in a tertiary Brazilian referral center: A cross-sectional and retrospective study. BMC Gastroenterol. 2018;18:15. doi: 10.1186/s12876-018-0740-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zingone F., Massa S., Malamisura B., Pisano P., Ciacci C. Coeliac disease: Factors affecting the transition and a practical tool for the transition to adult healthcare. United Eur. Gastroenterol. J. 2018;6:1356–1362. doi: 10.1177/2050640618787651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Johansson K., Norström F., Nordyke K., Myleus A. Celiac dietary adherence test simplifies determining adherence to a gluten-free diet in swedish adolescents. J. Pediatr. Gastroenterol. Nutr. 2019;69:575–580. doi: 10.1097/MPG.0000000000002451. [DOI] [PubMed] [Google Scholar]
- 49.Villafuerte-Galvez J., Vanga R.R., Dennis M., Hansen J., Leffler D.A., Kelly C.P., Mukherjee R. Factors governing long-term adherence to a gluten-free diet in adult patients with coeliac disease. Aliment. Pharmacol. Ther. 2015;42:753–760. doi: 10.1111/apt.13319. [DOI] [PubMed] [Google Scholar]
- 50.Ramírez-Cervantes K.L., Romero-López A.V., Núñez-Álvarez C.A., Uscanga-Domínguez L.F. Adherence to a gluten-free diet in mexican subjects with gluten-related ¡disorders: A high prevalence of inadvertent gluten intake. Rev. Investig. Clin. 2016;68:229–234. [PubMed] [Google Scholar]
- 51.Silvester J.A., Weiten D., Graff L.A., Walker J.R., Duerksen D.R. Is it gluten-free? Relationship between self-reported gluten-free diet adherence and knowledge of gluten content of foods. Nutrition. 2016;32:777–783. doi: 10.1016/j.nut.2016.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Muhammad H., Reeves S., Ishaq S., Mayberry J., Jeanes Y.M. Adherence to a gluten free diet is associated with receiving gluten free foods on prescription and understanding food labelling. Nutrients. 2017;9:705. doi: 10.3390/nu9070705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Halmos E.P., Deng M., Knowles S.R., Sainsbury K., Mullan B., Tye-Din J.A. Food knowledge and psychological state predict adherence to a gluten-free diet in a survey of 5310 Australians and New Zealanders with coeliac disease. Aliment. Pharmacol. Ther. 2018;48:78–86. doi: 10.1111/apt.14791. [DOI] [PubMed] [Google Scholar]
- 54.Costa A.F., Sugai E., Temprano M.P., Niveloni S.I., Vázquez H., Moreno M.L., Domínguez-Flores M.R., Muñoz-Suano A., Smecuol E., Stefanolo J.P., et al. Gluten immunogenic peptide excretion detects dietary transgressions in treated celiac disease patients. World J. Gastroenterol. 2019;25:1409–1420. doi: 10.3748/wjg.v25.i11.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Paganizza S., Zanotti R., D’Odorico A., Scapolo P., Canova C. Is adherence to a gluten-free diet by adult patients with celiac disease influenced by their knowledge of the gluten content of foods? Gastroenterol. Nurs. 2019;42:55–64. doi: 10.1097/SGA.0000000000000368. [DOI] [PubMed] [Google Scholar]
- 56.Fueyo-Díaz R., Magallón-Botaya R., Gascón-Santos S., Asensio-Martínez Á., Navarro G.P., Sebastián-Domingo J.J. The effect of self-efficacy expectations in the adherence to a gluten free diet in celiac disease. Psychol. Health. 2020;35:734–749. doi: 10.1080/08870446.2019.1675658. [DOI] [PubMed] [Google Scholar]
- 57.Silvester J.A., Comino I., Kelly C.P., Sousa C., Duerksen D.R. The DOGGIE BAG study group. Most patients with celiac disease on gluten-free diets consume measurable amounts of gluten. Gastroenterology. 2020;158:1497–1499.e1. doi: 10.1053/j.gastro.2019.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Silvester J.A., Comino I., Rigaux L.N., Segura V., Green K.H., Cebolla Á., Weiten D., Dominguez R., Leffler A., Leon F., et al. Exposure sources, amounts and time course of gluten ingestion and excretion in patients with coeliac disease on a gluten-free diet. Aliment. Pharmacol. Ther. 2020;52:1469–1479. doi: 10.1111/apt.16075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tanner G.J., Blundell M.J., Colgrave M.L., Howitt C.A. Creation of the first ultra-low gluten barley (Hordeum vulgare L.) for coeliac and gluten-intolerant populations. Plant Biotechnol. J. 2016;14:1139–1150. doi: 10.1111/pbi.12482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sánchez-León S., Gil-Humanes J., Ozuna C.V., Giménez M.J., Sousa C., Voytas D.F., Barro F. Low-gluten, nontransgenic wheat engineered with CRISPR/Cas9. Plant Biotechnol. J. 2018;16:902–910. doi: 10.1111/pbi.12837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Syage J.A., Green P.H.R., Khosla C., Adelman D.C., Sealey-Voyksner J.A., Murray J.A. Latiglutenase treatment for celiac disease: Symptom and quality of life improvement for seropositive patients on a gluten-free diet. GastroHep. 2019;1:293–301. doi: 10.1002/ygh2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Håkansson Å., Andrén-Aronsson C., Brundin C., Oscarsson E., Molin G., Agardh D. Effects of Lactobacillus plantarum and Lactobacillus paracasei on the peripheral immune response in children with celiac disease autoimmunity: A randomized, double-blind, placebo-controlled clinical trial. Nutrients. 2019;11:1925. doi: 10.3390/nu11081925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ruh T., Ohsam J., Pasternack R., Yokoyama K., Kumazawa Y., Hils M. Microbial transglutaminase treatment in pasta-production does not affect the immunoreactivity of gliadin with celiac disease patients’ sera. J. Agric. Food Chem. 2014;62:7604–7611. doi: 10.1021/jf501275c. [DOI] [PubMed] [Google Scholar]
- 64.Liang L., Pinier M., Leroux J.C., Subirade M. Interaction of alpha-gliadin with poly (HEMA-co-SS): Structural characterization and biological implication. Biopolymers. 2009;91:169–178. doi: 10.1002/bip.21109. [DOI] [PubMed] [Google Scholar]
- 65.Pinier M., Fuhrmann G., Galipeau H.J., Rivard N., Murray J.A., David S.H., Drasarova H., Tuckova L., Leroux J.C., Verdu E. The copolymer P(HEMA-co-SS) binds gluten and reduces immune response in gluten-sensitized mice and human tissues. Gastroenterology. 2012;142:316–325.e12. doi: 10.1053/j.gastro.2011.10.038. [DOI] [PubMed] [Google Scholar]
- 66.Sánchez-Valverde F.V., Denis S.Z., Etayo V.E. Nuevas estrategias terapéuticas en la enfermedad celíaca. In: Allué P., editor. Enfermedad Celíaca: Presente y Future. Ergon; Madrid, Spain: 2013. pp. 127–134. [Google Scholar]
- 67.Chevallier S., Goeltz P., Thibault P., Banville D., Gagnon J. Characterization of a prolyl endopeptidase from flavobacterium meningosepticum. complete sequence and localization of the active-site serine. J. Biol. Chem. 1992;267:8192–8199. doi: 10.1016/S0021-9258(18)42426-X. [DOI] [PubMed] [Google Scholar]
- 68.Diefenthal T., Dargatz H., Witte V., Reipen G., Svendsen I. Cloning of proline-specific endopeptidase gene from Flavobacterium meningosepticum: Expression in Escherichia coli and purification of the heterologous protein. Appl. Microbiol. Biotechnol. 1993;40:90–97. doi: 10.1007/BF00170434. [DOI] [PubMed] [Google Scholar]
- 69.Shan L., Marti T., Sollid L.M., Gray G.M., Khosla C. Comparative biochemical analysis of three bacterial prolyl endopeptidases: Implications for coeliac sprue. Biochem. J. 2004;383 Pt 2:311–318. doi: 10.1042/BJ20040907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kabashima T., Fujii M., Meng Y., Ito K., Yoshimoto T. Prolyl endopeptidase from Sphingomonas capsulata: Isolation and characterization of the enzyme and nucleotide sequence of the gene. Arch. Biochem. Biophys. 1998;358:141–148. doi: 10.1006/abbi.1998.0836. [DOI] [PubMed] [Google Scholar]
- 71.Xiao B., Zhang C., Song X., Wu M., Mao J., Yu R., Zheng Y. Rationally engineered prolyl endopeptidases from Sphingomonas capsulata with improved hydrolytic activity towards pathogenic peptides of celiac diseases. Eur. J. Med. Chem. 2020;15:112499. doi: 10.1016/j.ejmech.2020.112499. [DOI] [PubMed] [Google Scholar]
- 72.Knorr V., Wieser H., Koehler P. Production of gluten-free beer by peptidase treatment. Eur. Food Res. Technol. 2016;242:1129–1140. doi: 10.1007/s00217-015-2617-5. [DOI] [Google Scholar]
- 73.Lähdeaho M.L., Kaukinen K., Laurila K., Vuotikka P., Koivurova O.P., Kärjä-Lahdensuu T., Marcantonio A., Adelman D.C., Mäki M. Glutenase ALV003 attenuates gluten-induced mucosal injury in patients with celiac disease. Gastroenterology. 2014;146:1649–1658. doi: 10.1053/j.gastro.2014.02.031. [DOI] [PubMed] [Google Scholar]
- 74.Osorio C.E., Wen N., Mejías J.H., Mitchell S., von Wettstein D., Rustgi S. Directed-Mutagenesis of Flavobacterium meningosepticum prolyl-oligopeptidase and a glutamine-specific endopeptidase from barley. Front. Nutr. 2020;7:11. doi: 10.3389/fnut.2020.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Darwish G., Helmerhorst E.J., Schuppan D., Oppenheim F.G., Wei G. Pharmaceutically modified subtilisins withstand acidic conditions and effectively degrade gluten in vivo. Sci. Rep. 2019;9:7505. doi: 10.1038/s41598-019-43837-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lähdeaho M.L., Scheinin M., Vuotikka P., Taavela J., Popp A., Laukkarinen J., Koffert J., Koivurova O.P., Pesu M., Kivelä L., et al. Safety and efficacy of AMG 714 in adults with coeliac disease exposed to gluten challenge: A phase 2a, randomised, double-blind, placebo-controlled study. Lancet Gastroenterol. Hepatol. 2019;4:948–959. doi: 10.1016/S2468-1253(19)30264-X. [DOI] [PubMed] [Google Scholar]
- 77.Kapoerchan V.V., Wiesner M., Hillaert U., Drijfhout J.W., Overhand M., Alard P., van der Marel G.A., Overkleeft H.S., Koning F. Design, synthesis and evaluation of high-affinity binders for the celiac disease associated HLA-DQ2 molecule. Mol. Immunol. 2010;47:1091–1097. doi: 10.1016/j.molimm.2009.10.036. [DOI] [PubMed] [Google Scholar]
- 78.Tang F., Sally B., Lesko K., Discepolo V., Abadie V., Ciszewski C., Semrad C., Guandalini S., Kupfer S.S., Jabri B. Cysteinyl leukotrienes mediate lymphokine killer activity induced by NKG2D and IL-15 in cytotoxic T cells during celiac disease. J. Exp. Med. 2015;212:1487–1495. doi: 10.1084/jem.20150303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Haghbin M., Rostami-Nejad M., Forouzesh F., Sadeghi A., Rostami K., Aghamohammadi E., Asadzadeh-Aghdaei H., Masotti A., Zali M.R. The role of CXCR3 and its ligands CXCL10 and CXCL11 in the pathogenesis of celiac disease. Medicine. 2019;98:e15949. doi: 10.1097/MD.0000000000015949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cellier C., Bouma G., van Gils T., Khater S., Malamut G., Crespo L., Collin P., Green P.H.R., Crowe S.E., Tsuji W., et al. Safety and efficacy of AMG 714 in patients with type 2 refractory coeliac disease: A phase 2a, randomised, double-blind, placebo-controlled, parallel-group study. Lancet Gastroenterol. Hepatol. 2019;4:960–970. doi: 10.1016/S2468-1253(19)30265-1. [DOI] [PubMed] [Google Scholar]
- 81.Benedetti E., Viscido A., Castelli V., Maggiani C., d’Angelo M., Di Giacomo E., Antonosante A., Picarelli A., Frieri G. Mesalazine treatment in organotypic culture of celiac patients: Comparative study with gluten free diet. J. Cell Physiol. 2018;233:4383–4390. doi: 10.1002/jcp.26217. [DOI] [PubMed] [Google Scholar]
- 82.Leffler D.A., Kelly C.P., Green P.H., Fedorak R.N., DiMarin A., Perrow W., Rasmussen H., Wang C., Bercik P., Bachir N.M., et al. Larazotide acetate for persistent symptoms of celiac disease despite a gluten-free diet: A randomized controlled trial. Gastroenterology. 2015;148:1311–1319.e6. doi: 10.1053/j.gastro.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Khaleghi S., Ju J.M., Lamba A., Murray J.A. The potential utility of tight junction regulation in celiac disease: Focus on larazotide acetate. Therap. Adv. Gastroenterol. 2016;9:37–49. doi: 10.1177/1756283X15616576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Daveson A.J.M., Ee H.C., Andrews J.M., King T., Goldstein K.E., Dzuris J.L., MacDougall J.A., Williams L.J., Treohan A., Cooreman M.P., et al. Epitope-specific immunotherapy targeting CD4-positive T cells in celiac disease: Safety, pharmacokinetics, and effects on intestinal histology and plasma cytokines with escalating dose regimens of NEXVAX2 in a randomized, double-blind, placebo-controlled phase 1 study. EBioMedicine. 2017;26:78–90. doi: 10.1016/j.ebiom.2017.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Goel G., King T., Daveson A.J., Andrews J.M., Krishnarajah J., Krause R., Brown G.J.E., Fogel R., Barish C.F., Epstein R., et al. Epitope-specific immunotherapy targeting CD4-positive T cells in coeliac disease: Two randomised, double-blind, placebo-controlled phase 1 studies. Lancet Gastroenterol. Hepatol. 2017;2:479–493. doi: 10.1016/S2468-1253(17)30110-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Database ClinicalTrials.gov. [(accessed on 5 January 2021)]; Available online: https://clinicaltrials.gov/ct2/show/NCT04530123.
- 87.Database ClinicalTrials.gov. [(accessed on 6 January 2021)]; Available online: https://clinicaltrials.gov/ct2/show/NCT04248855.
- 88.Croese J., Giacomin P., Navarro S., Clouston A., McCann L., Dougall A., Ferreira I., Susianto A., O’Rourke P., Howlett M., et al. Experimental hookworm infection and gluten microchallenge promote tolerance in celiac disease. J. Allergy Clin. Immunol. 2015;135:508–516. doi: 10.1016/j.jaci.2014.07.022. [DOI] [PubMed] [Google Scholar]
- 89.Freire R., Ingano L., Serena G., Cetinbas M., Anselmo A., Sapone A., Sadreyev R.I., Fasano A., Senger S. Human gut derived-organoids provide model to study gluten response and effects of microbiota-derived molecules in celiac disease. Sci. Rep. 2019;9:7029. doi: 10.1038/s41598-019-43426-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cristofori F., Indrio F., Miniello V.L., De Angelis M., Francavilla R. Probiotics in celiac disease. Nutrients. 2018;10:1824. doi: 10.3390/nu10121824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ramedani N., Sharifan A., Gholam-Mostafaei F.S., Rostami-Nejad M., Yadegar A., Ehsani-Ardakani M.J. The potentials of probiotics on gluten hydrolysis; a review study. Gastroenterol. Hepatol. Bed Bench. 2020;13(Suppl. 1):S1–S7. [PMC free article] [PubMed] [Google Scholar]
- 92.Zanini B., Petroboni B., Not T., di Toro N., Villanacci V., Lanzarotto F., Pogna N., Ricci C., Lanzini A. Search for atoxic cereals: A single blind, cross-over study on the safety of a single dose of Triticum monococcum, in patients with celiac disease. BMC Gastroenterol. 2013;13:92. doi: 10.1186/1471-230X-13-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Vaquero L., Comino I., Vivas S., Rodríguez-Martín L., Giménez M.J., Pastor J., Sousa C., Barro F. Tritordeum: A novel cereal for food processing with good acceptability and significant reduction in gluten immunogenic peptides in comparison with wheat. J. Sci. Food Agric. 2018;98:2201–2209. doi: 10.1002/jsfa.8705. [DOI] [PubMed] [Google Scholar]
- 94.Jouanin A., Gilissen L.J.W.J., Schaart J.G., Leigh F.J., Cockram J., Wallington E.J., Boyd L.A., van den Broeck H.C., van der Meer I.M., America A.H.P., et al. CRISPR/Cas9 gene editing of gluten in wheat to reduce gluten content and exposure-reviewing methods to screen for coeliac safety. Front. Nutr. 2020;7:51. doi: 10.3389/fnut.2020.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Troncone R., Auricchio R., Granata V. Issues related to gluten-free diet in coeliac disease. Curr. Opin. Clin. Nutr. Metab. Care. 2008;11:329–333. doi: 10.1097/MCO.0b013e3282f795f8. [DOI] [PubMed] [Google Scholar]
- 96.Barro F., Lehisa J.C., Giménez M.J., García-Molina M.D., Ozun C.V., Comino I., Sousa C., Gil-Humanes J. Targeting of prolamins by RNAi in bread wheat: Effectiveness of seven silencing-fragment combinations for obtaining lines devoid of coeliac disease epitopes from highly immunogenic gliadins. Plant Biotechnol. J. 2016;14:986–996. doi: 10.1111/pbi.12455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Haro C., Villatoro M., Vaquero L., Pastor J., Giménez M.J., Ozuna C.V., Sánchez-León S., García-Molina M.D., Segura V., Comino I., et al. The dietary intervention of transgenic low-gliadin wheat bread in patients with non-celiac gluten sensitivity (NCGS) showed no differences with gluten free diet (GFD) but provides better gut microbiota profile. Nutrients. 2018;10:1964. doi: 10.3390/nu10121964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Picozzi C., Mariotti M., Cappa C., Tedesco B., Vigentini I., Foschino R., Lucisano M. Development of a Type I gluten-free sourdough. Lett. Appl. Microbiol. 2016;62:119–125. doi: 10.1111/lam.12525. [DOI] [PubMed] [Google Scholar]
- 99.Kõiv V., Tenson T. Gluten-degrading bacteria: Availability and applications. Appl. Microbiol. Biotechnol. 2021;105:3045–3059. doi: 10.1007/s00253-021-11263-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Francavilla R., Piccolo M., Francavilla A., Polimeno L., Semeraro F., Cristofori F., Castellaneta S., Barone M., Indrio F., Gobbetti M., et al. Clinical and microbiological effect of a multispecies probiotic supplementation in celiac patients with persistent ibs-type symptoms: A randomized, double-blind, placebo-controlled, multicenter trial. J. Clin. Gastroenterol. 2019;53:e117–e125. doi: 10.1097/MCG.0000000000001023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.De Angelis M., Rizzello C.G., Fasano A., Clemente M.G., De Simone C., Silano M., De Vincenzi M., Losito I., Gobbetti M. VSL#3 probiotic preparation has the capacity to hydrolyze gliadin polypeptides responsible for Celiac Sprue. Biochim. Biophys. Acta. 2006;1762:80–93. doi: 10.1016/j.bbadis.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 102.Harnett J., Myers S.P., Rolfe M. Probiotics and the microbiome in celiac disease: A Randomised Controlled Trial. Evid. Based Complement. Alternat. Med. 2016;2016:9048574. doi: 10.1155/2016/9048574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Vaquero L., Rodríguez-Martín L., León F., Jorquera F., Vivas S. Nuevas terapias en la enfermedad celiaca y sus complicaciones. Gastroenterol. Hepatol. 2018;41:191–204. doi: 10.1016/j.gastrohep.2017.12.002. [DOI] [PubMed] [Google Scholar]
- 104.Aaron L., Torsten M. Microbial transglutaminase: A new potential player in celiac disease. Clin. Immunol. 2019;199:37–43. doi: 10.1016/j.clim.2018.12.008. [DOI] [PubMed] [Google Scholar]
- 105.Zhou G., Sprengers D., Boor P.P.C., Doukas M., Schutz H., Mancham S., Pedroza-Gonzalez A., Polak W.G., de Jonge J., Gaspersz M., et al. Antibodies Against Immune Checkpoint Molecules Restore Functions of Tumor-Infiltrating T Cells in Hepatocellular Carcinomas. Gastroenterology. 2017;153:1107–1119.e10. doi: 10.1053/j.gastro.2017.06.017. [DOI] [PubMed] [Google Scholar]
- 106.McCarville J.L., Nisemblat Y., Galipeau H.J., Jury J., Tabakman R., Cohen A., Naftali E., Neiman B., Halbfinger E., Murray J.A., et al. BL-7010 demonstrates specific binding to gliadin and reduces gluten-associated pathology in a chronic mouse model of gliadin sensitivity. PLoS ONE. 2014;9:e109972. doi: 10.1371/journal.pone.0109972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Database ClinicalTrials.gov. [(accessed on 10 March 2021)]; Available online: https://clinicaltrials.gov/ct2/show/NCT03707730.
- 108.Stadlmann V., Harant H., Korschineck I., Hermann M., Forster F., Missbichler A. Novel avian single-chain fragment variable (scFv) targets dietary gluten and related natural grain prolamins, toxic entities of celiac disease. BMC Biotechnol. 2015;15:109. doi: 10.1186/s12896-015-0223-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Moreno M.L., Arévalo-Rodríguez M., Mellado E., Martínez-Reyes J.C., Sousa C. A new microbial gluten-degrading prolyl endopeptidase: Potential application in celiac disease to reduce gluten immunogenic peptides. PLoS ONE. 2019;14:e0218346. doi: 10.1371/journal.pone.0218346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Krishnareddy S., Stier K., Recanati M., Lebwohl B., Green P.H. Commercially available glutenases: A potential hazard in coeliac disease. Therap. Adv. Gastroenterol. 2017;10:473–481. doi: 10.1177/1756283X17690991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Moreno M.L., Sánchez-Muñoz D., Sanders D., Rodríguez-Herrera A., Sousa C. Verifying diagnosis of refractory celiac disease with urine gluten immunogenic peptides as biomarker. Front. Med. 2021;7:601854. doi: 10.3389/fmed.2020.601854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tian N., Wei G., Schuppan D., Helmerhorst E.J. Effect of Rothia mucilaginosa enzymes on gliadin (gluten) structure, deamidation, and immunogenic epitopes relevant to celiac disease. Am. J. Physiol. Gastrointest. Liver Physiol. 2014;307:G769–G776. doi: 10.1152/ajpgi.00144.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Socha P., Mickowska B., Urminská D., Kacmárová K. The use of different proteases to hydrolyze gliadins. J. Microbiol. Biotechnol. Food Sci. 2015;4:101–104. doi: 10.15414/jmbfs.2015.4.special2.101-104. [DOI] [Google Scholar]
- 114.Wei G., Tian N., Valery A.C., Zhong Y., Schuppan D., Helmerhorst E.J. Identification of pseudolysin (lasB) as an aciduric gluten-degrading enzymewith high therapeutic potential for celiac disease. Am. J. Gastroenterol. 2015;110:899–908. doi: 10.1038/ajg.2015.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wagh S.K., Gadge P.P., Padul M.V. Significant hydrolysis of wheat gliadin by bacillus tequilensis (10bT/HQ223107): A Pilot Study. Probiotics Antimicrob. Proteins. 2018;10:662–667. doi: 10.1007/s12602-017-9331-5. [DOI] [PubMed] [Google Scholar]
- 116.Rashmi B.S., Gayathri D., Vasudha M., Prashantkumar C.S., Swamy C.T., Sunil K.S., Somaraja P.K., Prakash P. Gluten hydrolyzing activity of Bacillus spp isolated from sourdough. Microb. Cell Factories. 2020;19:130. doi: 10.1186/s12934-020-01388-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cavaletti L., Taravella A., Carrano L., Carenzi G., Sigurta A., Solinas N., De Caro S., Di Stasio L., Piscascia S., Laezza M., et al. E40, a novel microbial protease efficiently detoxifying gluten proteins, for the dietary management of gluten intolerance. Sci. Rep. 2019;9:13147. doi: 10.1038/s41598-019-48299-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ehren J., Morón B., Martin E., Bethune M.T., Gray G.M., Khosla C. A foodgrade enzyme preparation with modest gluten detoxification properties. PLoS ONE. 2009;4:e6313. doi: 10.1371/journal.pone.0006313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Janssen G., Christis C., Kooy-Winkelaar Y., Edens L., Smith D., van Veelen P., Koning F. Ineffective degradation of immunogenic gluten epitopes by currently available enzyme supplements. PLoS ONE. 2015;10:e0128065. doi: 10.1371/journal.pone.0128065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bethune M.T., Ribka E., Khosla C., Sestak K. Transepithelial transport and enzymatic detoxification of gluten in gluten-sensitive rhesus macaques. PLoS ONE. 2008;3:e1857. doi: 10.1371/journal.pone.0001857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Cornell H.J., Doherty W., Stelmasiak T. Papaya latex enzymes capable of detoxification of gliadin. Amino Acids. 2009;38:155–165. doi: 10.1007/s00726-008-0223-6. [DOI] [PubMed] [Google Scholar]
- 122.Savvateeva L.V., Gorokhovets N.V., Makarov V.A., Serebryakova M.V., Solovyev A.G., Morozov S.Y., Reddy V.P., Zernii E.Y., Zamyatnin A.A., Jr., Aliev G. Glutenase and collagenase activities of wheat cysteine protease Triticain-α: Feasibility for enzymatic therapy assays. Int. J. Biochem. Cell Biol. 2015;62:115–124. doi: 10.1016/j.biocel.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 123.Mika N., Zorn H., Rühl M. Prolyl-specific peptidases for applications in food protein hydrolysis. Appl. Microbiol. Biotechnol. 2015;99:7837–7846. doi: 10.1007/s00253-015-6838-0. [DOI] [PubMed] [Google Scholar]
- 124.Tereshchenkova V.F., Goptar I.A., Zhuzhikov D.P., Belozersky M.A., Dunaevsky Y.E., Oppert B., Filippova I.Y., Elpidina E.N. Prolidase is a critical enzyme for complete gliadin digestion in Tenebrio molitor larvae. Arch. Insect Biochem. Physiol. 2017;95:e21395. doi: 10.1002/arch.21395. [DOI] [PubMed] [Google Scholar]
- 125.Murray J.A., Kelly C.P., Green P.H.R., Marcantonio A., Wu T.T., Mäki M., Adelman D.C., CeliAction Study Group of Investigators No difference between latiglutenase and placebo in reducing villous atrophy or improving symptoms in patients with symptomatic celiac disease. Gastroenterology. 2017;152:787–798.e2. doi: 10.1053/j.gastro.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 126.Syage J.A., Murray J.A., Green P.H.R., Khosla C. Latiglutenase improves symptoms in seropositive celiac disease patients while on a gluten-free diet. Dig. Dis. Sci. 2017;62:2428–2432. doi: 10.1007/s10620-017-4687-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Database ClinicalTrials.gov. [(accessed on 13 March 2021)]; Available online: https://clinicaltrials.gov/ct2/show/NCT03585478.
- 128.Mitea C., Havenaar R., Drijfhout J.W., Edens L., Dekking L., Koning F. Efficient degradation of gluten by a prolyl endoprotease in a gastrointestinal model: Implications for coeliac disease. Gut. 2008;57:25–32. doi: 10.1136/gut.2006.111609. [DOI] [PubMed] [Google Scholar]
- 129.Xia J., Bergseng E., Fleckenstein B., Siegel M., Kim C.Y., Khosla C., Sollid L.M. Cyclic and dimeric gluten peptide analogues inhibiting DQ2-mediated antigen presentation in celiac disease. Bioorg. Med. Chem. 2007;15:6565–6573. doi: 10.1016/j.bmc.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Esposito C., Caputo I., Troncone R. New therapeutic strategies for coeliac disease: Tissue transglutaminase as a target. Curr. Med. Chem. 2007;14:2572–2580. doi: 10.2174/092986707782023343. [DOI] [PubMed] [Google Scholar]
- 131.Alhassan E., Yadav A., Kelly C.P., Mukherjee R. Novel nondietary therapies for celiac disease. Cell Mol. Gastroenterol. Hepatol. 2019;8:335–345. doi: 10.1016/j.jcmgh.2019.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Palanski B.A., Khosla C. Cystamine and disulfiram inhibit human transglutaminase 2 via an oxidative mechanism. Biochemistry. 2018;57:3359–3363. doi: 10.1021/acs.biochem.8b00204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Database ClinicalTrials.gov. [(accessed on 1 April 2021)]; Available online: https://grantome.com/grant/NIH/R01-DK100619-01A1.
- 134.Sollid L.M., Khosla C. Novel therapies for coeliac disease. J. Intern. Med. 2011;269:604–613. doi: 10.1111/j.1365-2796.2011.02376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Database ClinicalTrials.gov. [(accessed on 1 April 2021)]; Available online: https://clinicaltrials.gov/ct2/show/NCT04593251.
- 136.Reinisch W. How to manage loss of response to anti-TNF in Crohn’s disease? Curr. Drug Targets. 2010;11:152–155. doi: 10.2174/138945010790309894. [DOI] [PubMed] [Google Scholar]
- 137.Phan-Ba R., Lambinet N., Louis E., Delvenne P., Tshibanda L., Boverie J., Moonen G., Belachew S. Natalizumab to kill two birds with one stone: A case of celiac disease and multiple sclerosis. Inflamm. Bowel Dis. 2011;17:E62–E63. doi: 10.1002/ibd.21716. [DOI] [PubMed] [Google Scholar]
- 138.Rath T., Billmeier U., Ferrazzi F., Vieth M., Ekici A., Neurath F.M., Atreya R. Effects of anti-integrin treatment with vedolizumab on immune pathways and cytokines in inflammatory bowel diseases. Front. Immunol. 2018;9:1700. doi: 10.3389/fimmu.2018.01700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Saruta M., Yu Q.T., Avanesyan A., Fleshner P.R., Targan S.R., Papadakis K.A. Phenotype and effector function of CC chemokine receptor 9-expressing lymphocytes in small intestinal Crohn’s disease. J. Immunol. 2007;178:3293–3300. doi: 10.4049/jimmunol.178.5.3293. [DOI] [PubMed] [Google Scholar]
- 140.Walters M.J., Wang Y., Lai N., Baumgart T., Zhao B.N., Dairaghi D.J., Bekker P., Ertl L.S., Penfold M.E., Jaen J.C., et al. Characterization of CCX282-B, an orally bioavailable antagonist of the CCR9 chemokine receptor, for treatment of inflammatory bowel disease. J. Pharmacol. Exp. Ther. 2010;335:61–69. doi: 10.1124/jpet.110.169714. [DOI] [PubMed] [Google Scholar]
- 141.Kivelä L., Caminero A., Leffler D.A., Pinto-Sanchez M.I., Tye-Din J.A., Lindfors K. Current and emerging therapies for coeliac disease. Nat. Rev. Gastroenterol. Hepatol. 2021;18:181–195. doi: 10.1038/s41575-020-00378-1. [DOI] [PubMed] [Google Scholar]
- 142.Gopalakrishnan S., Tripathi A., Tamiz A.P., Alkan S.S., Pandey N.B. Larazotide acetate promotes tight junction assembly in epithelial cells. Peptides. 2012;35:95–101. doi: 10.1016/j.peptides.2012.02.016. [DOI] [PubMed] [Google Scholar]
- 143.Gopalakrishnan S., Durai M., Kitchens K., Tamiz A.P., Somerville R., Ginski M., Paterson B.M., Murray J.A., Verdu E.F., Alkan S.S., et al. Larazotide acetate regulates epithelial tight junctions in vitro and in vivo. Peptides. 2012;35:86–94. doi: 10.1016/j.peptides.2012.02.015. [DOI] [PubMed] [Google Scholar]
- 144.Serena G., Kelly C.P., Fasano A. Nondietary therapies for celiac disease. Gastroenterol. Clin. N. Am. 2019;48:145–163. doi: 10.1016/j.gtc.2018.09.011. [DOI] [PubMed] [Google Scholar]
- 145.Tye-Din J.A., Stewart J.A., Dromey J.A., Beissbarth T., van Heel D.A., Tatham A., Henderson K., Mannering S.I., Gianfrani C., Jewell D.P., et al. Comprehensive, quantitative mapping of T cell epitopes in gluten in celiac disease. Sci. Transl. Med. 2010;2:41ra51. doi: 10.1126/scitranslmed.3001012. [DOI] [PubMed] [Google Scholar]
- 146.Database ClinicalTrials.gov. [(accessed on 10 April 2021)]; Available online: https://clinicaltrials.gov/ct2/show/NCT03738475.
- 147.Daveson A.J., Jones D.M., Gaze S., McSorley H., Clouston A., Pascoe A., Cooke S., Speare R., Macdonald G.A., Anderson R., et al. Effect of hookworm infection on wheat challenge in celiac disease-a randomised double-blinded placebo-controlled trial. PLoS ONE. 2011;6:e17366. doi: 10.1371/journal.pone.0017366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Chibbar R., Dieleman L.A. The gut microbiota in celiac disease and probiotics. Nutrients. 2019;11:2375. doi: 10.3390/nu11102375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Caminero A., Verdu E.F. Metabolism of wheat proteins by intestinal microbes: Implications for wheat related disorders. Gastroenterol. Hepatol. 2019;42:449–457. doi: 10.1016/j.gastrohep.2019.04.001. [DOI] [PubMed] [Google Scholar]
- 150.Palma M.L., Garcia-Bates T.M., Martins F.S., Douradinha B. Genetically engineered probiotic Saccharomyces cerevisiae strains mature human dendritic cells and stimulate Gag-specific memory CD8+ T cells ex vivo. Appl. Microbiol. Biotechnol. 2019;103:5183–5192. doi: 10.1007/s00253-019-09842-8. [DOI] [PubMed] [Google Scholar]
- 151.Francavilla R., Cristofori F., Vacca M., Barone M., De Angelis M. Advances in understanding the potential therapeutic applications of gut microbiota and probiotic mediated therapies in celiac disease. Expert. Rev. Gastroenterol. Hepatol. 2020;14:323–333. doi: 10.1080/17474124.2020.1745630. [DOI] [PubMed] [Google Scholar]
- 152.Strimbu K., Tavel J.A. What are biomarkers? Curr. Opin. HIV AIDS. 2010;5:463–466. doi: 10.1097/COH.0b013e32833ed177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Gottlieb K., Dawson J., Hussain F., Murray J.A. Development of drugs for celiac disease: Review of endpoints for Phase 2 and 3 trials. Gastroenterol. Rep. 2015;3:91–102. doi: 10.1093/gastro/gov006. [DOI] [PMC free article] [PubMed] [Google Scholar]