Abstract
Gastrointestinal symptoms in Crohn’s disease (CD) are common and affect the quality of life of patients; consequently, a growing number of studies have been published on diet interventions in this group. The role of the gut microbiota in the pathogenesis and the progression of inflammatory bowel diseases (IBD), including CD, has been widely discussed. Mainly, a decreased abundance of Firmicutes, species of the Bifidobacterium genus, and the Faecalibacterium prausnitzii species as well as a reduced general diversity have been described. In this review article, we summarize available data on the influence of reduction diets on the microbiome of patients with CD. One of the most frequently used elimination diets in CD patients is the low-FODMAP (Fermentable Oligosaccharides, Disaccharides, Monosaccharides, and Polyols) diet. Although many papers show it may reduce abdominal pain, diarrhea, or bloating, it also reduces the intake of prebiotic substances, which can negatively affect the gut microbiota composition, decreasing the abundance of Bifidobacterium species and Faecalibacterium prausnitzii. Other elimination diets used by IBD patients, such as lactose-free or gluten-free diets, have also been shown to disturb the microbial diversity. On the other hand, CDED (Crohn’s disease exclusion diet) with partial enteral nutrition not only induces the remission of CD but also has a positive influence on the microbiota. The impact of diet interventions on the microbiota and, potentially, on the future course of the disease should be considered when nutritional guidelines for IBD patients are designed. Dietetic recommendations should be based not only on the regulation of the symptoms but also on the long-term development of the disease.
Keywords: Crohn’s disease, elimination diets, microbiome
1. Introduction
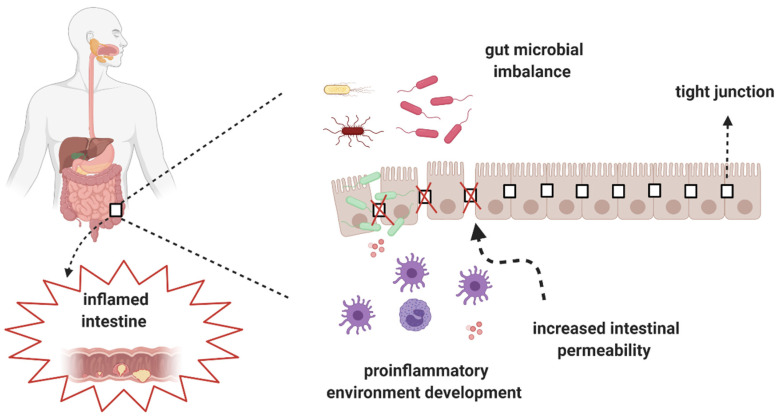
Crohn’s disease (CD) is an inflammatory bowel disease (IBDs) that may affect every segment of the gastrointestinal tract [1,2] and cause complications, such as lesions and fistulas. The most common symptoms of CD are abdominal pain, diarrhea, rectal bleeding, unintentional weight loss, fever, and chronic fatigue [3,4]. The etiopathogenesis of CD is multifactorial and includes, among others, genetic background, environmental factors, and gut microbiota imbalance (dysbiosis). The association between gut dysbiosis and the development of CD has been recognized during the last several years. In patients with CD, gut dysbiosis and increased gut permeability are observed (Figure 1) [5].
Figure 1.
Gut microbial imbalance and development of CD.
Currently, there are therapeutic methods that modulate and restore the gut microbiota balance, such as those based on the administration of prebiotics, probiotics, and symbiotics [6]. Nevertheless, the composition and activity of the gut microbiota may also be altered via nutrition (Table 1). Restrictive diets may reduce the symptoms of CD, influencing the composition of the microbiome. These changes may have the potential to affect the course of the disease. Therefore, in this review, we concentrate on the role of selected diets (low-FODMAP, gluten-free, lactose-free, ketogenic, specific carbohydrate diet, Paleo diet, and Crohn’s disease exclusion diet) in the treatment of CD, presenting their impact on the composition as well as the activity of the gut microbiota.
Table 1.
Summary of studies in terms of the effect of nutrition on the microbiome.
| Trial Name | Type of Nutrition Intervention; Population | Alternations in Microbiota |
|---|---|---|
| Staudacher HM, 2012 | Low-FODMAP; IBS | ↓ Bifidobacterium |
| Cox S.R, 2020 | Low-FODMAP; Crohn disease and Colitis ulcerosa | ↓ Bifidobacterium, in particular: Bifidobacterium adolescentis, Bifidobacterium longum, and Faecalibacterium prausnitzii |
| Schreiner P, 2019 | Gluten-free diet, Crohn disease and Colitis ulcerosa | ↓ Total amount and biodiversity |
| De Palma G, 2009 | Gluten-free diet, healthy people | ↓ Bifidobacterium, Lactobacillus, and Bifidobacterium longum ↑ Enterobacteriaceae and Escherichia coli |
| Marc J.B., 2016 | Gluten-free diet, healthy people | ↓ Veillonellaceae, Ruminococcus bromii, Roseburia faecis ↑ Clostridiacea, Coriobacteiaceae |
| Levine A, 2019 | CDED, children with Crohn disease | ↓ fecal Proteobacteria |
| Olson C.A., 2018 | Ketogenic diet; children with drug-resistant epilepsy | ↑ Parabacteroides |
| Lawrece D., 2014 | Ketogenic diet with an increased animal protein content; healthy people | ↑ Bacteroides, Alistipes, Bilophila |
| Devkota S., 2013 | Ketogenic diet with an increased saturated fatty acids content; healthy people | ↑ Bilophila wadsworthia |
| A Dubrovsky, 2018 | SCD, IBD patients and healthy control | ↑ Fusobacterium ulcerans |
| Schnorr SL, 2014 | Paleo diet, Hadza hunter–gatherers vs. Italian control | ↑ Prevotella (Bacteroidetes), Treponema (Spirochaetes), and unclassified Bacteroidetes |
| Halmos E., 2015 | Low-FODMAP; IBS | ↓ Total amount ↓ Cluster Clorstridium XIVa, Akkermansia municiphila, Ruminococcus |
FODMAP-Fermentable Oligosaccharides, Disaccharides, Monosaccharides, and Polyols; IBD—Inflammatory bowel disease; IBS—Irritable bowel syndrome; CDED—Crohn’s Disease Exclusion Diet; SCD—Specific Carbohydrate Diet; ↓—Decreased abundance, ↑—Increased abundance.
2. Alterations of the Gut Microbiota in CD
The human microbiome varies depending on the body site and consists of bacteria, archaea, fungi, protozoa, and viruses. Three main bacterial phyla and some genera dominate in the gastrointestinal tract, i.e., Bacteroidetes 9–42%, Firmicutes 30–52%, and Acinobacteria 1–13%, and Lactobacilli, Streptococci, and Escherichia coli (E.coli), respectively [5]. Intestinal microorganisms are associated with the development and activity of IBDs, including CD. Several theories support the role of the microbiome in the pathogenesis of IBD. IBD could be due firstly, to the presence of long-lasting infections caused by pathogenic species such as Mycobacterium avium subspecies paratuberculosis or adhesion-invasive Escherichia coli (AIEC); secondly, to immoderate bacterial translocation and a shift from a balanced to a dysbiotic microbiota composition [6]. On the other hand, a protective effect is observed by specific bacterial metabolites such as Short-chain fatty acids (SCFAs), which reduce local inflammation [7]. A study by Imhann showed alterations of the gut microbiome (mainly, lower abundance of the Roseburia genus) in healthy individuals with a high genetic risk of IBD [8]. In a recent meta-analysis, Paramsothy showed that fecal microbiota transplantation (FMT) has a positive effect on remission induction in ulcerative colitis (UC) patients. It was shown that 50.5% of CD patients achieved remission; however, the studies were heterogeneous, and future well-designed randomized trials are needed to confirm the validity of this method for CD treatment [1]. The intestinal microbiota in CD is characterized by a decrease in the diversity and total number of microorganisms of up to 50% compared to healthy individuals [2,3]. Moreover, a reduced abundance of Firmicutes is noticed, which is associated with a parallel increase in Proteobacteria [4]. Of the Firmicutes phylum, the less common species in IBD are Clostridium leptum and Feacalibacterium prausnitzii [2,9]. Commensal bacteria belonging to Clostridium cluster IVa are also characterized by reduced abundance [10]. A significant relative decrease of Clostridium leptum and Bifidobacteria from the Bifidobacteriaceae family and the Lachnospiraceae family has been observed in patients with CD compared to healthy subjects [11]. On the other hand, studies showed an increased number of Actinobacteria, Proteobacteria [2,12,13,14], and Enterobacteriaceae both in remission and in active CD [4]. Potentially unfavorable and proinflammatory intestinal microorganisms such as Raminococcus gnavus [10], Clostridium difficile, Escherichia coli, Campylobacter concisus [15,16], and Shigella flexneri [17] are present in increased numbers in comparison to healthy controls, shortening the remission of the disease [18]. A high abundance of the specific Escherichia coli (AIEC) strain LF82 has been demonstrated to be associated with ileal CD. AIEC strains were isolated from almost one-third of ileal specimens in CD as compared with 6% in ileal controls and less than 5% in colonic samples from both IBD patients and controls [19]. Colonization by this bacterium caused intestinal inflammation, increased the expression of proinflammatory cytokines, and stimulated the function of T-helper 17 lymphocytes (Th17) cells in vivo [20,21]. The prevalence of this invasive strain was associated with inflammation of the ileum but not of the colon [19]. Th17 produce the proinflammatory cytokine interleukin -23 (IL-23), whose increased production is correlated with the occurrence and development of IBDs [22].
Increased concentration of the Bacteroides B. fragilis and B. vulgatus [23] was another observation in CD patients. These species demonstrate resistance to antibiotics and may turn out to be potential pathogens [24]. Bacteroides fragilis is a common commensal microorganism, although in inflammatory bowel diseases, its enterotoxic strain (ETBF) is dominant. It produces a zinc-dependent proinflammatory toxin and causes colitis with severe inflammation and overproduction of IL-17 [25,26]. A western diet with high amounts of saturated fatty acids (SFAs) contributes to the development of IBDs. Animal models have proven that the consumption of saturated fats from milk changes the composition of bile acids. This enables sulphate-reducing bacteria to grow, which in turn can produce large quantities of potentially mucosa-toxic hydrogen sulfide and induce helper T-cells (Th1) [27].
The characteristics of the microbiota in IBDs show that potentially proinflammatory microorganisms dominate over anti-inflammatory ones. For instance, Faecalbacterium prausnitzii can synthesize the microbiota anti-inflammatory molecule (MAM), which inhibits the nuclear factor kappa-light-chain-enhancer of activated B cells (NFkB) pathway [28]. The reduced presence of these microorganisms in CD causes lower synthesis of MAM, which may be associated with decreased anti-inflammatory potential. Some families of the Firmicutes phylum, including Lachnospiraceae and Ruminoccaceae, represented by Faecalibacterium prausniztii, are the main producers of butyrate. The decreased abundance of these species, by as much as 5 to 10 times compared to healthy controls, makes the mucosa less rich in this compound [29,30]. Short-chain fatty acids (SCFAs) are the main energy source for colonocytes, keep the intestinal mucosa healthy, and have therapeutic potential for IBDs [31]. Therefore, reduction of SCFAs in the gut may lead to the development of inflammation and increase intestinal permeability [32], which is a characteristic feature of IBD [33]. Antibiotics used in IBDs may also increase intestinal dysbiosis, thus reducing the production of SCFAs, including butyrate, which may further increase intestinal barrier integrity disorders [34]. Intestinal microorganisms can also participate in immunological reactions through their specific antigens, triggering TLRs (Toll-like receptors) and stimulating the local expansion of T lymphocytes. Specific peptides synthesized by some microorganisms contribute to the release of cytokines and the production of antibodies [35,36,37,38]. Commensal and probiotic strains can also prevent excessive inflammation by controlling the level of immunological activation in response to pathogens or other harmful antigens. These regulatory effects can be mediated by inducing the production of regulatory and anti-inflammatory cytokines (i.e., interleukin-10 (IL-10) and transforming growth factor β (TGF-β)) [39]. For instance, Bacteroides fragilis in combination with a polysaccharide A (PSA) molecule induces the synthesis of anti-inflammatory IL-10 using TLRs. The presence of both this bacterium and the cytokine in IBDs is reduced, which may be related to the occurrence of inflammation [40,41]. Dysbiosis occurring in CD disturbs the immune function of the microbiota, which facilitates the development of chronic inflammation.
It has been shown that the composition of the microbiome can change even within 24 h after the introduction of a nutritional intervention or a change in the diet itself [42]. For this reason, patients with diagnosed IBDs should plan their nutrition in such a way as to support the proper development of the intestinal microbiota.
3. Low-FODMAP Diet
The low-FODMAP diet is a very restrictive diet requiring the almost complete exclusion of substances defined as FODMAPs (Fermentable Oligosaccharides, Disaccharides, Monosaccharides, and Polyols). These include monosaccharides, disaccharides oligosaccharides, and intestinal fermenting polyols, such as fructose, lactose, or xylitol [43]. The low-FODMAP diet has been proven to minimize gastrointestinal disorders, such as abdominal pain and bloating in IBS or IBDs [44,45,46]. A low intake of saccharides fermenting in the intestines reduces the frequency of diarrhea, but it was shown that this diet does not provide similar effects in minimizing constipation [47]. Moreover, a diet with low FODMAPs can improve the quality of life of IBD patients [48].
The compounds excluded during this diet belong to the group of prebiotics, which can selectively stimulate the growth of microorganisms such as Bifidobacterium or Faecalibacterium prausnitzii. Moreover, they are substrates for the production of short-chain fatty acids [49,50]. It was noted that the relative and the absolute number of butyrate-producing bacteria decreases after a low-FODMAP dietary intervention [51]. Fructooligosaccharides may increase the abundance of Bifidobacterium and positively modify the immune function of dendritic intestinal mucosa cells [52]. These compounds may reduce the production of proinflammatory cytokines, e.g., IL-6, and increase that of the anti-inflammatory cytokine IL-10 [53].
A low-FODMAP diet has a significant effect on intestinal microbial composition. The total number of microorganisms may decrease up to six times after a 4-week intervention [54]. Compared to a typical Australian diet, the low FODMAP diet resulted in a decrease in the total number of intestinal bacteria, especially of Clostridium cluster IV, including Faecalibacterium prausnitzii, Bifidobacterium, and Lactobacillus [55]. A characteristic change induced by the low-FODMAP diet is the reduced number of Bifidobacterium [54]. In a recent study by Cox, a decreased abundance of Bifidobacterium adolescentis and Bifidobacterium longum was observed [56]. Faecalibacterium prausnitzii, Ruminococcus spp., and Bifidobacterium longum, which take part in starch degradation, demonstrated to influence diet interventions in IBD [57]. Despite the limited amount of microorganisms regulating the immune response, no significant effect on inflammation markers (calprotectin or C-Reactive Protein (CRP)) was observed [56]. It has been noted that a short-term (4-week) low-FODMAP diet may even increase the levels of inflammatory markers [48].
4. Gluten-Free Diet
Gluten is a mixture of many proteins, mainly gliadins and glutenins. It is a form of “storage” in wheat grains. Similar compounds are found in rye (secalin), oats (avena), barley (hordein) and are collectively referred to as gluten. This compound is found in basic grains, which make up a significant part of the diet. It is contained in bread, pasta, and all other grain products, so a gluten-free diet excludes many basic energy sources and needs to be supervised by a dietitian [58,59].
A gluten-free diet is the only appropriate dietary management of celiac disease [60]. The prevalence of celiac disease or tissue transglutaminase Antibodies (anti-tTG) antibodies themselves, typical of this disease, is higher among people with diagnosed IBD than in healthy people [61]. These patients are also more likely to develop non-celiac gluten sensitivity, which causes gastrointestinal symptoms, such as abdominal pain and diarrhea after ingestion of foods containing this compound [62]. Consequently, a gluten-free diet is one of the most commonly considered elimination diets among CD patients [63].
Despite some benefits such as reducing gastrointestinal symptoms, a gluten-free diet has an impact on the composition of the intestinal microbiota [64]. Similar to the low-FODMAP diet, this dietary intervention causes a decrease in the abundance of Bifidobacterium microorganisms [65]. This refers mainly to Bifidobacterium longum species, but also to Lactobacillus. On the other hand, the number of Enterobacteriaceae and Escherichia coli increases [66]. A decreasing abundance of commensal bacteria combined with an increase in the number of potentially pathogenic microorganisms may have a proinflammatory and potentially negative effect on the intestinal mucosa [66]. A gluten-free diet also contributes to the decrease in Faecalibacterium prausnitzii abundance. As a result of unfavorable changes in the microbiota, the stimulation of host immunity is less affected, which is manifested particularly by a reduced production of IL-10 [67]. In patients with diagnosed celiac disease, a probiotic therapy minimalized gastrointestinal complaints due to changes in the intestinal microflora. In an Italian study, a 6-week Pentabiocel probiotic intake resulted in an increased amount of Bifidobacterium, Staphylococcus, and lactic acid bacteria, which reduced IBS-type symptoms in patients on a gluten-free diet. The study product consisted of a multistrain mixture containing five strains of lactic acid bacteria and bifidobacteria: Lactobacillus casei LMG 101/37 P-17504 (5 × 109 CFU/sachet), Lactobacillus plantarum CECT 4528 (5 × 109 CFU/sachet), Bifidobacterium animalis subsp. lactis Bi1 LMG P-17502 (10 × 109 CFU/sachet), Bifidobacterium breve Bbr8 LMG P-17501 (10 × 109 CFU/sachet), B. breve Bl10 LMG P-17500 (10 × 109 CFU/sachet). The probiotic was given as a sachet once per day [68]. The composition and functioning of intestinal microorganisms are very sensitive to the type of food consumed. It has been shown that even a short-term introduction of a gluten-free diet may adversely affect the diversity of the microbiota by, among other mechanisms, reducing butyrate synthesis. This compound is the main source of energy for the microbiota and enables its growth [69].
5. Lactose-Free Diet
Lactose is a disaccharide consisting of glucose and galactose linked by a B-1→ 4 bond. The hydrolysis of this bond requires β-galactosidase, which breaks down lactose into monosaccharides, allowing these compounds to be absorbed by the intestine [70]. Low activity of this enzyme may lead to digestive symptoms similar to those of IBS, such as flatulence or diarrhea, after consumption of food containing lactose [71]. The activity of β-galactosidase is higher in children and young people and decreases with age [70]. Moreover, lactase activity is proportional to the amount of lactose in the diet [72]. It has been shown that the intestine of lactose-intolerant individuals after intake of this disaccharide produces significantly more SCFAs than the intestine of healthy patients. Increased bacterial fermentation can lead to the gastrointestinal complaints observed in lactose-intolerant people [73].
The incidence of lactose intolerance is not higher in inflammatory bowel diseases than in the general population; however, it can be observed more often in the presence of active CD. Moreover, the consumption of dairy products can reduce the risk of IBD [74,75]. Individuals suffering from gastrointestinal disorders after ingestion of lactose often exclude dairy products from their diet without attempting to introduce lactose-free dairy products. The health benefits of fermented milk include prevention of gastrointestinal infections, reduction of serum cholesterol levels, and antimutagenic activity. The consumption of fermented products by lactose-intolerant individuals and patients suffering from atherosclerosis is recommended [76,77]. However, milk-derived saturated fat can alter the composition of bile acids and allow the growth of sulfate-reducing bacteria such as Bilophila wadsworthia, producing toxic hydrogen sulfide [78] and worsening colitis in murine IL-10 knockout models [27].
Lactose has a prebiotic effect, inducing microbiota growth and development and promoting its diversity. It increases the abundance of probiotic Lactobacillus and Bifidobacterium species as well as of Firmicutes [79,80]. On the other hand, dairy sugar reduces the number of potentially pathogenic microorganisms, such as Clostridium perfringens, Escherichia coli, and Proteobacteria [79,81]. Intestinal bacteria also affect digestibility and lactose absorption. The importance of the microbiota in the proper distribution of lactose was confirmed by a study by Almeida. Lactose-intolerant patients were supplemented with a probiotic yogurt containing L. casei Shirota and B. breve. A 4-week intervention resulted in a decreased amount of exhaled hydrogen and gastrointestinal complaints after lactose intake, and this effect lasted for 3 months on average [82]. Some probiotic strains such as Lactobacillus and Bifidobacterium may also increase β-galactosidase activity [82,83,84]. Lactose supplementation is also effective in enhancing the production of SCFAs, including butyric acid [79].
Individuals with diagnosed lactose intolerance are advised not to eliminate all dairy products but to choose lactose-free products. A diet containing lactose-free milk allowed to maintain an optimal balance of the microflora in an experimental model. The mice received lactose-free or whole milk, glycomacropeptide- or soy protein (control)-supplemented diets for one month. A lactose-free milk diet was as efficient as the control diet in retaining fecal microbiota diversity in mice. Both milk diets had a significant effect on the relative abundance of health-relevant taxa (e.g., Ruminococcaceae, Lachnospiraceae). The glycomacropeptide prebiotic activity previously observed in vitro was not replicated in vivo. However, these data indicate the novel prebiotic potential of bovine milk for human nutrition [85]. Moreover, patients excluding all dairy products, including lactose-free ones, may develop nutritional deficiencies of many valuable nutrients, including calcium [86]. Lactose, synergistically with bovine milk oligosaccharides, may also influence the growth of Bifidobacterium longum subsp. longum and Parabacteroides distasonis, while inhibiting the growth of Clostridium perfingers and Escherichia coli [81]. Moreover, it has been shown that oligosaccharides from cow’s milk, can reduce intestinal permeability [87]. Due to limited scientific data, the European Society for Clinical Nutrition and Metabolism (ESPEN) guidelines do not recommend the routine use of lactose-free diets if no intolerance is diagnosed [88].
6. Ketogenic Diet
The ketogenic diet is characterized by a very low carbohydrate content, i.e., below 50 g per day, which is equal to 5–10% of the energy value of daily food [89]. This diet is suitable for patients with drug-resistant epilepsy [90].
The mechanisms underlying the protective effect of the ketogenic diet in epilepsy are not quite known. This diet causes enrichment and colonization of the gut by Akkermansia and Parabacteroides, which may have anticonvulsant effects [91]. This change in the intestinal microbiota is beneficial, but in IBDs, it results in an increase in the abundance of bacteria with potentially pro-inflammatory activity, e.g., Parabacteroides, inducing the progression of the disease [92]. A very-low-calorie ketogenic diet (VLCKD) causes a reduction in the total amount of intestinal microorganisms in the mouse model of autism spectrum disorders [93].
The elimination of carbohydrates leads to a decrease in prebiotic substances in the diet, which are the energy source for the growth and development of the microbiota. For this reason, the effect on intestinal bacterial diversity is similar to that of a low-FODMAP diet and is characterized by reduced numbers of Bifidobacterium and of the main butyrate producer, i.e., Faecalibacterium prausnitzii [50,94]. This will result in a reduced amount of SCFAs [95]. Butyrate contributes to the maintenance of the intestinal barrier, which is an important part of the pathomechanism of IBDs [96].
A ketogenic diet is associated with an increased intake of protein, which also influences the microbiota. This effect depends on the origin of this macronutrient. It was shown that consumption of animal protein, especially from red meat, leads to an increase in anaerobic bacteria with potential pro-inflammatory effects, such as Bacteroides, Alistipes, and Bilophila [97]. Furthermore, it may raise the risk of IBD development by increasing the production of hydrogen sulfide by sulphate-reducing bacteria, including Desulfovibrio spp. [98]. Additionally, the fermentation of animal proteins reduces the production of SCFAs, especially butyrate, and the abundance of Bifidobacterium in fecal microbiota [99]. On the other hand, a higher intake of plant proteins or whey proteins has the opposite effect. It was demonstrated that the consumption of whey protein and pea extracts increases the amount of Bifidobacterium and Lactobacillus, while whey further reduces the potentially pathogenic Bacteroides fragilis and Clostridium perfingers [100,101]
Another nutrient that occurs in excess in a ketogenic diet is fat, which can affect the microbiota of the digestive tract. Among all fat fractions, the development of inflammatory bowel diseases is mostly affected by trans and saturated fats [102]. It has been shown that a high-fat diet, particularly rich in SFA (saturated fatty acids), leads to an increased number of sulphate-reducing bacteria, which may raise the risk of developing intestinal inflammation [27]. Even MUFA (monounsaturated fatty acids) can cause changes in the composition of the microbiota if they occur in excess. Meanwhile, a high SFA content in the diet may reduce the overall diversity of the microbiota, including Bifidobacterium [103]. On the other hand, excessive amounts of PUFA (polyunsaturated fatty acids) do not affect the richness of the microbiota [103].
7. Specific Carbohydrate Diet (SCD)
The SCD recommends excluding complex carbohydrates in favor of more simple carbohydrates, easier to digest and absorb [104]. Originally, this diet was used for children with celiac disease (in the 1920s) [105]. In the 1980s, it gained the status of a dietary novelty for the treatment of people with inflammatory bowel diseases [106].
The regimen has been mostly promoted on the Internet by non-licensed individuals, with little supporting evidence. Indeed, the data do not strongly support the role of carbohydrates in initiating/exacerbating intestinal inflammation [107]. SCD, being a very restrictive diet, limiting the short-term consumption of most disaccharides and starches, seems to be contributing to the reduction of intestinal symptoms in IBD patients [108]. A study of the gut microbiota conducted in 2014 in eight participants aged 16–50 years with a diagnosis of CD, showed that in patients receiving the SCD diet (unlike in those receiving a diet low in plant fiber), the diversity of the microbiome increased, resulting in 134 bacteria belonging to 32 different classes. The bacterial families overrepresented in the gut ecosystem included more than 20 species of non-pathogenic bacteria of the Clostridia family [109]. A case study published in 2018 [110], in which SCD was used for 2 weeks in a 20-year-old patient with ulcerative colitis (UC), demonstrated that such dietary intervention significantly changed the microbiome. A decrease in the abundance of the most dominant species of fecal bacteria—Fusobacterium ulcerans—was observed, with a simultaneous increase in the diversity and evenness of other intestinal bacteria species. Hoffman demonstrated that carbohydrate intake might increase the counts of Candida and Methanobrevibacter archaea which utilize starch, producing simple carbohydrates. Those carbohydrates are substrates for Prevotella and Ruminococcus, producing resources consumed by Methanobrevibacter for methane (CH4) and carbon dioxide (CO2) production [111].
8. Paleo Diet
The Paleolithic diet was firstly described by the gastroenterologist Walter L. Voegtlin and initially named the Stone Age Diet [112]. It is based on the premise that we have not evolved sufficiently to metabolize food that is available today to humans. The creators of this diet assumed that food products that appeared as a result of the agricultural revolution (grains, milk) are harmful to human physiology because the human digestive tract is not sufficiently developed to digest such food particles. As a consequence, the Paleolithic diet is devoid of refined sugars, grains, processed vegetables and fruits, and domestic animal meats [113]. It is assumed that exposure to foods that were not present at the time of human evolution may cause modern diseases such as IBD. It seems that the microbiota of the Paleo diet can be compared to the stool microbiota of Hadza hunter–gatherers from Tanzania. Research on this unique ethnic group whose diets are based on foods available seasonally (in the rainy season, the predominant food is plant food, baobab, honey, rich in simple sugars, starch, proteins, but low in tuber fat) [114]. It was elegantly documented that in Hadza’s gut, the Firmicutes phylum dominates (72%), followed by Bacteroidetes (17%), Proteobacteria (6%), and Spirochaetes (3%). At the species level, Prevotella (Bacteroidetes), Treponema (Spirochaetes), and unclassified Bacteroidetes are noted, while Bifidobacteria is absent. Of note, some Firmicutes species, in particular Prevotella and Treponema, harbors several fiber-degrading enzymes [114].
To date, there are no data on the role of the Paleolithic diet in the treatment of IBD, except for rare and isolated, rather positive case reports [115]. However, the elevated intake of dietary fiber is positively correlated with SCFAs synthesis to further modulate GALT immune response via attenuating proinflammatory cytokines production [116] As demonstrated by Hallert et al., the increased consumption of oat bran (6 g/day) alleviated significantly the symptoms of UC [117]. Kanauchi et al. treated [118] 18 UC patients with 20–30 g/day of germinated barley foodstuff and found that Bifidobacterium and Eubaterium limosum abundance increased, which further led to improvement of bowel-related symptoms. Of note, the Paleo diet was found to increase vitamin D deficiency, thereby elevating the risk hospitalization [119].
9. Crohn’s Disease Exclusion Diet (CDED)
The exclusive enteral nutrition (EEN) has been described as an efficient method of inducing remission in children in comparison with steroid therapy [120]. The CD exclusion diet is a combination of enteral nutrition and whole-food restrictive diet. Oral nutrition is based on the elimination of all substances that could be allergenic or increase gastrointestinal disorders, such as milk lactose. For this reason, the diet is low in fat and animal protein and, at the same time, rich in compound carbohydrates and dietary fiber. It does not include gluten, dairy, and certain food additives such as emulsifiers, maltodextrins, carrageenan, and sulfites. In the second period, a fixed portion of whole-grain bread is allowed, as are small amounts of nuts, fruits, legumes, and vegetables. Patients with strictures continue the quantitative restriction of fruits and vegetables on an individual basis. Positive results are especially recorded in children and young adults [121,122,123,124,125,126].
In a 12-week intervention in 74 patients (mean age 14.2 ± 2.7 years), it was shown that a CDED diet in combination with enteral nutrition was better tolerated than enteral nutrition alone and was more effective in inducing remission [124]. For 6 weeks, the children received 50% of their caloric requirements from an oral diet and 50% from enteral nutrition (Modulen formula), whereas for 7–12 weeks, the industrial diet accounted for only 25% of their requirements. CRP (C-reactive protein) decreased in both groups (i.e., CDED and exclusive enteral nutrition (EEN)): in EEN, from 24 mg/dL to 4.1 mg/dL and in CDED, from 23.6 mg/dL to 5 mg/dL. Both EEN and partial enteral nutrition (PEN) affect the composition of the intestinal microbiota. For the first 6 weeks, both diets showed a similar effect on microbial activity: a reduced bacterial abundance of Actinobacteria and Proteobacteria and increased commensal Clostridia. However, between 6 and 12 weeks in EEN, these changes were reversed, while in CDED + PEN, the microbial composition remained similar [124]. These differences in the microbiota during the treatment may indicate a better effect of CDED + PEN compared to PEN. Another study that confirmed the effectiveness of the CDED diet is the Sigall-Boneh clinical trial [121]. Children and young adults (47 patients, mean age, 16.1 ± 5.6 years; 34 children) with active disease defined by a pediatric Crohn’s disease activity index >7.5 or a Harvey–Bradshaw index ≥4 received a 6-week structured CDED. Response and remission were achieved in 37 (78.7%) and 33 (70.2%) patients, respectively. The mean pediatric Crohn’s disease activity index decreased from 27.7 ± 9.4 to 5.4 ± 8 (p < 0.001), the Harvey–Bradshaw index from 6.4 ± 2.7 to 1.8 ± 2.9 (p < 0.001). Remission was observed in 70% of the children and 69% of the adults. Normalization of previously elevated CRP occurred in 21 of 30 (70%) patients in remission [121].
10. Conclusions
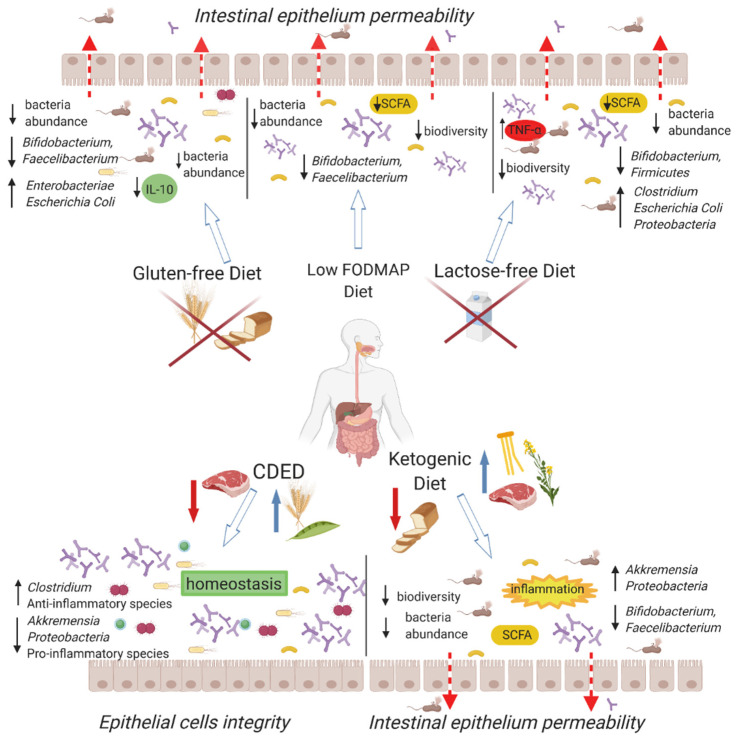
In patients suffering from CD, gut dysbiosis with low diversity of intestinal microbes is observed. Several therapeutic methods are used to alter the composition as well as the activity of the gut microbiota (Figure 2). The low-FODMAP diet may reduce the diversity of the intestinal microbiome, already impoverished by the very presence of CD. Bacteria, such as Bifidobacterium or Faecalbacterium prausnitzii are important elements, maintaining intestinal barrier integrity. A decreased abundance of those species is observed in patients with the disease. Dietary patterns eliminating prebiotic substances may exacerbate these deficiencies. Gluten-free, lactose-free, and SDC diets have similar effects on the microbiome as those of the low-FODMAP diet. The ketogenic diet, rarely used in CD, is based on animal protein, containing large amounts of saturated fats derived from meat. Excessive amounts of saturated fats lead to an increase in the number of bacteria with pro-inflammatory activity and a decrease in that of commensal ones, which may promote the development of IBDs. On the other hand, CDED provides beneficial changes in the fecal microbiome and the course of the disease, reducing the amount of bacteria with proinflammatory activity and increasing that of anti-inflammatory ones. Elimination diets appear to be effective in minimizing gastrointestinal symptoms associated with Crohn’s disease. Unfortunately, most of them can have negative effects on the microbiome and cause nutritional deficiencies. Professional dietitians with clinical experience need to be engaged for the treatment of CD patients. Nutrition recommendations should consider the effect on the quality of life and potential long-term consequences on the course of the disease.
Figure 2.
Relationship between diet and microbiome, its function, and intestinal barrier condition. Each of the indicated types of nutrition interacts with the microbiota in a characteristic way. IL-10—interleukin 10, CDED—Crohn disease exclusion diet, TNF-α–tumor necrosis factor. SCFA: Short-chain fatty acids; FODMAP: Fermentable Oligosaccharides, Disaccharides, Monosaccharides, and Polyols.
Author Contributions
Conceptualization, E.S. (Eliza Starz) and M.F. Investigation, all. Writing—Original Draft Preparation, E.S. (Eliza Starz) and M.F. Writing—Review, E.S. (Eliza Starz), M.F., K.W., K.K.-S., L.S., K.S.-Ż., K.P. and E.S. (Ewa Stachowska). Editing, Final approval, E.S. (Eliza Starz), M.F., K.W., K.K.-S., L.S., K.S.-Ż., K.P. and E.S. (Ewa Stachowska). Supervision, M.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Paramsothy S., Paramsothy R., Rubin D.T., Kamm M.A., Kaakoush N.O., Mitchell H.M., Castaño-Rodríguez N. Faecal microbiota transplantation for inflammatory bowel disease: A systematic review and meta-analysis. J. Crohn’s Colitis. 2017;11:1180–1199. doi: 10.1093/ecco-jcc/jjx063. [DOI] [PubMed] [Google Scholar]
- 2.Frank D.N., St. Amand A.L., Feldman R.A., Boedeker E.C., Harpaz N., Pace N.R. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc. Natl. Acad. Sci. USA. 2007;104:13780–13785. doi: 10.1073/pnas.0706625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ott S.J., Musfeldt M., Wenderoth D.F., Hampe J., Brant O., Fölsch U.R., Timmis K.N., Schreiber S. Reduction in diversity of the colonic mucosa associated bacterial microflora in patients with active inflammatory bowel disease. Gut. 2004;53:685–693. doi: 10.1136/gut.2003.025403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seksik P., Rigottier-Gois L., Gramet G., Sutren M., Pochart P., Marteau P., Jian R., Doré J. Alterations of the dominant faecal bacterial groups in patients with Crohn’s disease of the colon. Gut. 2003;52:237–242. doi: 10.1136/gut.52.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Riaz Rajoka M.S., Shi J., Mehwish H.M., Zhu J., Li Q., Shao D., Huang Q., Yang H. Interaction between diet composition and gut microbiota and its impact on gastrointestinal tract health. Food Sci. Hum. Wellness. 2017;6:121–130. doi: 10.1016/j.fshw.2017.07.003. [DOI] [Google Scholar]
- 6.Liu S., Zhao W., Lan P., Mou X. The microbiome in inflammatory bowel diseases: From pathogenesis to therapy. Protein Cell. 2021;12:331–345. doi: 10.1007/s13238-020-00745-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Postler T.S., Ghosh S. Understanding the holobiont: How microbial metabolites affect human health and shape the immune system. Cell Metab. 2017;26:110–130. doi: 10.1016/j.cmet.2017.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Imhann F., Vich Vila A., Bonder M.J., Fu J., Gevers D., Visschedijk M.C., Spekhorst L.M., Alberts R., Franke L., van Dullemen H.M., et al. Interplay of host genetics and gut microbiota underlying the onset and clinical presentation of inflammatory bowel disease. Gut. 2018;67:108–119. doi: 10.1136/gutjnl-2016-312135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peterson D.A., Frank D.N., Pace N.R., Gordon J.I. Metagenomic approaches for defining the pathogenesis of inflammatory bowel diseases. Cell Host Microbe. 2008;3:417–427. doi: 10.1016/j.chom.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Joossens M., Huys G., Cnockaert M., De Preter V., Verbeke K., Rutgeerts P., Vandamme P., Vermeire S. Dysbiosis of the faecal microbiota in patients with Crohn’s disease and their unaffected relatives. Gut. 2011;60:631–637. doi: 10.1136/gut.2010.223263. [DOI] [PubMed] [Google Scholar]
- 11.Manichanh C., Rigottier-Gois L., Bonnaud E., Gloux K., Pelletier E., Frangeul L., Nalin R., Jarrin C., Chardon P., Marteau P., et al. Reduced diversity of faecal microbiota in Crohn’s disease revealed by a metagenomic approach. Gut. 2006;55:205–211. doi: 10.1136/gut.2005.073817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matsuoka K., Kanai T. The gut microbiota and inflammatory bowel disease. Semin. Immunopathol. 2015;37:47–55. doi: 10.1007/s00281-014-0454-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prosberg M., Bendtsen F., Vind I., Petersen A.M., Gluud L.L. The association between the gut microbiota and the inflammatory bowel disease activity: A systematic review and meta-analysis. Scand. J. Gastroenterol. 2016;51:1407–1415. doi: 10.1080/00365521.2016.1216587. [DOI] [PubMed] [Google Scholar]
- 14.Kostic A.D., Xavier R.J., Gevers D. The microbiome in inflammatory bowel disease: Current status and the future ahead. Gastroenterology. 2014;146:1489–1499. doi: 10.1053/j.gastro.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mahendran V., Riordan S.M., Grimm M.C., Tran T.A.T., Major J., Kaakoush N.O., Mitchell H., Zhang L. Prevalence of Campylobacter species in adult Crohn’s disease and the preferential colonization sites of Campylobacter species in the human intestine. PLoS ONE. 2011;6:e25417. doi: 10.1371/journal.pone.0025417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Man S.M., Zhang L., Day A.S., Leach S.T., Lemberg D.A., Mitchell H. Campylobacter concisus and other Campylobacter species in children with newly diagnosed Crohn’s disease. Inflamm. Bowel Dis. 2010;16:1008–1016. doi: 10.1002/ibd.21157. [DOI] [PubMed] [Google Scholar]
- 17.Lee K.N., Lee O.Y. Intestinal microbiota in pathophysiology and management of irritable bowel syndrome. World J. Gastroenterol. 2014;20:8886–8897. doi: 10.3748/wjg.v20.i27.8886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ananthakrishnan A.N., Binion D.G. Impact of Clostridium difficile on inflammatory bowel disease. Expert Rev. Gastroenterol. Hepatol. 2010;4:589–600. doi: 10.1586/egh.10.55. [DOI] [PubMed] [Google Scholar]
- 19.Darfeuille-Michaud A., Boudeau J., Bulois P., Neut C., Glasser A.L., Barnich N., Bringer M.A., Swidsinski A., Beaugerie L., Colombel J.F. High prevalence of adherent-invasive Escherichia coli associated with ileal mucosa in Crohn’s disease. Gastroenterology. 2004;127:412–421. doi: 10.1053/j.gastro.2004.04.061. [DOI] [PubMed] [Google Scholar]
- 20.Carvalho F.A., Barnich N., Sivignon A., Darcha C., Chan C.H.F., Stanners C.P., Darfeuille-Michaud A. Crohn’s disease adherent-invasive Escherichia coli colonize and induce strong gut inflammation in transgenic mice expressing human CEACAM. J. Exp. Med. 2009;206:2179–2189. doi: 10.1084/jem.20090741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Viladomiu M., Kivolowitz C., Abdulhamid A., Dogan B., Victorio D., Castellanos J.G., Woo V., Teng F., Tran N.L., Sczesnak A., et al. IgA-coated E. Coli enriched in Crohn’s disease spondyloarthritis promote TH17-dependent inflammation. Sci. Transl. Med. 2017;9 doi: 10.1126/scitranslmed.aaf9655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ma C., Panaccione R., Khanna R., Feagan B.G., Jairath V. IL12/23 or selective IL23 inhibition for the management of moderate-to-severe Crohn’s disease? Best Pract. Res. Clin. Gastroenterol. 2019;38–39 doi: 10.1016/j.bpg.2019.02.006. [DOI] [PubMed] [Google Scholar]
- 23.Ruseler-van Embden J.G., Both-Patoir H.C. Anaerobic gram-negative faecal flora in patients with Crohn’s disease and healthy subjects. Antonie Van Leeuwenhoek. 1983;49:125–132. doi: 10.1007/BF00393670. [DOI] [PubMed] [Google Scholar]
- 24.Wexler H.M. Bacteroides: The good, the bad, and the nitty-gritty. Clin. Microbiol. Rev. 2007;20:593–621. doi: 10.1128/CMR.00008-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rabizadeh S., Rhee K.J., Wu S., Huso D., Gan C.M., Golub J.E., Wu X., Zhang M., Sears C.L. Enterotoxigenic Bacteroides fragilis: A potential instigator of colitis. Inflamm. Bowel Dis. 2007;13:1475–1483. doi: 10.1002/ibd.20265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prindiville T.P., Sheikh R.A., Cohen S.H., Tang Y.J., Cantrell M.C., Silva J. Bacteroides fragilis enterotoxin gene sequences in patients with inflammatory bowel disease. Emerg. Infect. Dis. 2000;6:171–174. doi: 10.3201/eid0602.000210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Devkota S., Wang Y., Musch M.W., Leone V., Fehlner-Peach H., Nadimpalli A., Antonopoulos D.A., Jabri B., Chang E.B. Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in Il10-/- mice. Nature. 2012;487:104–108. doi: 10.1038/nature11225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Quévrain E., Maubert M.A., Michon C., Chain F., Marquant R., Tailhades J., Miquel S., Carlier L., Bermúdez-Humarán L.G., Pigneur B., et al. Identification of an anti-inflammatory protein from Faecalibacterium prausnitzii, a commensal bacterium deficient in Crohn’s disease. Gut. 2016;65:415–425. doi: 10.1136/gutjnl-2014-307649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marchesi J.R., Holmes E., Khan F., Kochhar S., Scanlan P., Shanahan F., Wilson I.D., Wang Y. Rapid and noninvasive metabonomic characterization of inflammatory bowel disease. J. Proteome Res. 2007;6:546–551. doi: 10.1021/pr060470d. [DOI] [PubMed] [Google Scholar]
- 30.Miquel S., Martín R., Rossi O., Bermúdez-Humarán L.G., Chatel J.M., Sokol H., Thomas M., Wells J.M., Langella P. Faecalibacterium prausnitzii and human intestinal health. Curr. Opin. Microbiol. 2013;16:255–261. doi: 10.1016/j.mib.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 31.Hamer H.M., Jonkers D., Venema K., Vanhoutvin S., Troost F.J., Brummer R.J. Review article: The role of butyrate on colonic function. Aliment. Pharmacol. Ther. 2008;27:104–119. doi: 10.1111/j.1365-2036.2007.03562.x. [DOI] [PubMed] [Google Scholar]
- 32.Nozu T., Miyagishi S., Nozu R., Takakusaki K., Okumura T. Butyrate inhibits visceral allodynia and colonic hyperpermeability in rat models of irritable bowel syndrome. Sci. Rep. 2019;9 doi: 10.1038/s41598-019-56132-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Michielan A., D’Incà R. Intestinal Permeability in Inflammatory Bowel Disease: Pathogenesis, Clinical Evaluation, and Therapy of Leaky Gut. Mediators Inflamm. 2015;2015 doi: 10.1155/2015/628157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Holota Y., Dovbynchuk T., Kaji I., Vareniuk I., Dzyubenko N., Chervinska T., Zakordonets L., Stetska V., Ostapchenko L., Serhiychuk T., et al. The long-term consequences of antibiotic therapy: Role of colonic short-chain fatty acids (SCFA) system and intestinal barrier integrity. PLoS ONE. 2019;14 doi: 10.1371/journal.pone.0220642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Balfour Sartor R. Bacteria in Crohn’s disease: Mechanisms of inflammation and therapeutic implications. J. Clin. Gastroenterol. 2007;41:37–43. doi: 10.1097/MCG.0b013e31802db364. [DOI] [PubMed] [Google Scholar]
- 36.Fong F.L.Y., Kirjavainen P., Wong V.H.Y., El-Nezami H. Immunomodulatory effects of Lactobacillus rhamnosus GG on dendritic cells, macrophages and monocytes from healthy donors. J. Funct. Foods. 2015;13:71–79. doi: 10.1016/j.jff.2014.12.040. [DOI] [Google Scholar]
- 37.Chang Y.L., Rossetti M., Vlamakis H., Casero D., Sunga G., Harre N., Miller S., Humphries R., Stappenbeck T., Simpson K.W., et al. A screen of Crohn’s disease-associated microbial metabolites identifies ascorbate as a novel metabolic inhibitor of activated human T cells. Mucosal Immunol. 2019;12:457–467. doi: 10.1038/s41385-018-0022-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fernández-Tomé S., Marin A.C., Ortega Moreno L., Baldan-Martin M., Mora-Gutiérrez I., Lanas-Gimeno A., Moreno-Monteagudo J.A., Santander C., Sánchez B., Chaparro M., et al. Immunomodulatory Effect of Gut Microbiota-Derived Bioactive Peptides on Human Immune System from Healthy Controls and Patients with Inflammatory Bowel Disease. Nutrients. 2019;11:2605. doi: 10.3390/nu11112605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sanz Y. Microbiome and gluten. Ann. Nutr. Metab. 2015;67:28–41. doi: 10.1159/000440991. [DOI] [PubMed] [Google Scholar]
- 40.Round J.L., Lee S.M., Li J., Tran G., Jabri B., Chatila T.A., Mazmanian S.K. The toll-like receptor 2 pathway establishes colonization by a commensal of the human microbiota. Science. 2011;332:974–977. doi: 10.1126/science.1206095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nunberg M.Y., Werner L., Kopylov U., Haberman Y., Lahad A., Weiss B., Shouval D.S. Impaired IL-10 receptor-mediated suppression in monocyte from patients with crohn disease. J. Pediatr. Gastroenterol. Nutr. 2018;66:779–784. doi: 10.1097/MPG.0000000000001795. [DOI] [PubMed] [Google Scholar]
- 42.Wu G.D., Chen J., Hoffmann C., Bittinger K., Chen Y.Y., Keilbaugh S.A., Bewtra M., Knights D., Walters W.A., Knight R., et al. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334:105–108. doi: 10.1126/science.1208344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Varney J., Barrett J., Scarlata K., Catsos P., Gibson P.R., Muir J.G. FODMAPs: Food composition, defining cutoff values and international application. J. Gastroenterol. Hepatol. 2017;32:53–61. doi: 10.1111/jgh.13698. [DOI] [PubMed] [Google Scholar]
- 44.Pedersen N., Ankersen D.V., Felding M., Wachmann H., Végh Z., Molzen L., Burisch J., Andersen J.R., Munkholm P. Low-FODMAP diet reduces irritable bowel symptoms in patients with inflammatory bowel disease. World J. Gastroenterol. 2017;23:3356–3366. doi: 10.3748/wjg.v23.i18.3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lis D.M., Stellingwerff T., Kitic C.M., Fell J.W., Ahuja K.D.K. Low FODMAP: A preliminary strategy to reduce gastrointestinal distress in athletes. Med. Sci. Sports Exerc. 2018;50:116–123. doi: 10.1249/MSS.0000000000001419. [DOI] [PubMed] [Google Scholar]
- 46.Altobelli E., Del Negro V., Angeletti P.M., Latella G. Low-FODMAP diet improves irritable bowel syndrome symptoms: A meta-analysis. Nutrients. 2017;9:940. doi: 10.3390/nu9090940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhan Y., Zhan Y., Dai S. Is a low FODMAP diet beneficial for patients with inflammatory bowel disease? A meta-analysis and systematic review. Clin. Nutr. 2018;37:123–129. doi: 10.1016/j.clnu.2017.05.019. [DOI] [PubMed] [Google Scholar]
- 48.Bodini G., Zanella C., Crespi M., Lo Pumo S., Demarzo M.G., Savarino E., Savarino V., Giannini E.G. A randomized, 6-wk trial of a low FODMAP diet in patients with inflammatory bowel disease. Nutrition. 2019;67–68 doi: 10.1016/j.nut.2019.06.023. [DOI] [PubMed] [Google Scholar]
- 49.Ramirez-Farias C., Slezak K., Fuller Z., Duncan A., Holtrop G., Louis P. Effect of inulin on the human gut microbiota: Stimulation of Bifidobacterium adolescentis and Faecalibacterium prausnitzii. Br. J. Nutr. 2008;101:541–550. doi: 10.1017/S0007114508019880. [DOI] [PubMed] [Google Scholar]
- 50.Wilson B., Whelan K. Prebiotic inulin-type fructans and galacto-oligosaccharides: Definition, specificity, function, and application in gastrointestinal disorders. J. Gastroenterol. Hepatol. 2017;32:64–68. doi: 10.1111/jgh.13700. [DOI] [PubMed] [Google Scholar]
- 51.Hill P., Muir J.G., Gibson P.R. Controversies and recent developments of the low-FODMAP diet. Gastroenterol. Hepatol. 2017;13:36–45. [PMC free article] [PubMed] [Google Scholar]
- 52.Lindsay J.O., Whelan K., Stagg A.J., Gobin P., Al-Hassi H.O., Rayment N., Kamm M.A., Knight S.C., Forbes A. Clinical, microbiological, and immunological effects of fructo-oligosaccharide in patients with Crohn’s disease. Gut. 2006;55:348–355. doi: 10.1136/gut.2005.074971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Benjamin J.L., Hedin C.R.H., Koutsoumpas A., Ng S.C., McCarthy N.E., Hart A.L., Kamm M.A., Sanderson J.D., Knight S.C., Forbes A., et al. Randomised, double-blind, placebo-controlled trial of fructo- oligosaccharides in active Crohn’s disease. Gut. 2011;60:923–929. doi: 10.1136/gut.2010.232025. [DOI] [PubMed] [Google Scholar]
- 54.Staudacher H.M., Lomer M.C.E., Anderson J.L., Barrett J.S., Muir J.G., Irving P.M., Whelan K. Fermentable carbohydrate restriction reduces luminal bifidobacteria and gastrointestinal symptoms in patients with irritable bowel syndrome. J. Nutr. 2012;142:1510–1518. doi: 10.3945/jn.112.159285. [DOI] [PubMed] [Google Scholar]
- 55.Halmos E.P., Christophersen C.T., Bird A.R., Shepherd S.J., Gibson P.R., Muir J.G. Diets that differ in their FODMAP content alter the colonic luminal microenvironment. Gut. 2015;64:93–100. doi: 10.1136/gutjnl-2014-307264. [DOI] [PubMed] [Google Scholar]
- 56.Cox S.R., Lindsay J.O., Fromentin S., Stagg A.J., McCarthy N.E., Galleron N., Ibraim S.B., Roume H., Levenez F., Pons N., et al. Effects of low-FODMAP diet on symptoms, fecal microbiome, and markers of inflammation in patients with quiescent inflammatory bowel disease in a randomized trial. Gastroenterology. 2020;158:176–188.e7. doi: 10.1053/j.gastro.2019.09.024. [DOI] [PubMed] [Google Scholar]
- 57.Mack A., Bobardt J.S., Haβ A., Nichols K.B., Schmid R.M., Stein-Thoeringer C.K. Changes in gut microbial metagenomic pathways associated with clinical outcomes after the elimination of malabsorbed sugars in an IBS cohort. Gut Microbes. 2019 doi: 10.1080/19490976.2019.1686322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Biesiekierski J.R. What is gluten? J. Gastroenterol. Hepatol. 2017;32:78–81. doi: 10.1111/jgh.13703. [DOI] [PubMed] [Google Scholar]
- 59.Vici G., Belli L., Biondi M., Polzonetti V. Gluten free diet and nutrient deficiencies: A review. Clin. Nutr. 2016;35:1236–1241. doi: 10.1016/j.clnu.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 60.Caruso R., Pallone F., Stasi E., Romeo S., Monteleone G. Appropriate nutrient supplementation in celiac disease. Ann. Med. 2013;45:522–531. doi: 10.3109/07853890.2013.849383. [DOI] [PubMed] [Google Scholar]
- 61.Manceñido Marcos N., Pajares Villarroya R., Salinas Moreno S., Arribas López M.R., Comas Redondo C. The association between de novo inflammatory bowel disease and celiac disease. Rev. Española Enfermedades Dig. 2019 doi: 10.17235/reed.2019.5535/2018. [DOI] [PubMed] [Google Scholar]
- 62.Limketkai B.N., Sepulveda R., Hing T., Shah N.D., Choe M., Limsui D., Shah S. Prevalence and factors associated with gluten sensitivity in inflammatory bowel disease. Scand. J. Gastroenterol. 2018;53:147–151. doi: 10.1080/00365521.2017.1409364. [DOI] [PubMed] [Google Scholar]
- 63.Casella G., Di Bella C., Salemme M., Villanacci V., Antonelli E., Baldini V., Bassotti G. Celiac disease, non-celiac gluten sensitivity and inflammatory bowel disease. Minerva Gastroenterol. Dietol. 2015;61:267–271. [PubMed] [Google Scholar]
- 64.Schreiner P., Yilmaz B., Rossel J.B., Franc Y., Misselwitz B., Scharl M., Zeitz J., Frei P., Greuter T., Vavricka S.R., et al. Vegetarian or gluten-free diets in patients with inflammatory bowel disease are associated with lower psychological well-being and a different gut microbiota, but no beneficial effects on the course of the disease. United Eur. Gastroenterol. J. 2019;7:767–781. doi: 10.1177/2050640619841249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Golfetto L., de Senna F.D., Hermes J., Beserra B.T.S., da Silva França F., Martinello F. Baixa contagem de bifidobactérias em pacientes adultos com doença celíaca, em dieta isenta de glúten. Arq. Gastroenterol. 2014;51:139–143. doi: 10.1590/S0004-28032014000200013. [DOI] [PubMed] [Google Scholar]
- 66.De Palma G., Nadal I., Collado M.C., Sanz Y. Effects of a gluten-free diet on gut microbiota and immune function in healthy adult human subjects. Br. J. Nutr. 2009;102:1154–1160. doi: 10.1017/S0007114509371767. [DOI] [PubMed] [Google Scholar]
- 67.Vazquez-Roque M.I., Camilleri M., Smyrk T., Murray J.A., Marietta E., O’Neill J., Carlson P., Lamsam J., Janzow D., Eckert D., et al. A controlled trial of gluten-free diet in patients with irritable bowel syndrome-diarrhea: Effects on bowel frequency and intestinal function. Gastroenterology. 2013;144 doi: 10.1053/j.gastro.2013.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Francavilla R., Piccolo M., Francavilla A., Polimeno L., Semeraro F., Cristofori F., Castellaneta S., Barone M., Indrio F., Gobbetti M., et al. Clinical and Microbiological Effect of a Multispecies Probiotic Supplementation in Celiac Patients with Persistent IBS-type Symptoms: A Randomized, Double-Blind, Placebo-controlled, Multicenter Trial. J. Clin. Gastroenterol. 2019;53:E117–E125. doi: 10.1097/MCG.0000000000001023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bonder M.J., Tigchelaar E.F., Cai X., Trynka G., Cenit M.C., Hrdlickova B., Zhong H., Vatanen T., Gevers D., Wijmenga C., et al. The influence of a short-term gluten-free diet on the human gut microbiome. Genome Med. 2016;8:45. doi: 10.1186/s13073-016-0295-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Montgomery R.K., Krasinski S.D., Hirschhorn J.N., Grand R.J. Lactose and lactase—Who is lactose intolerant and why? J. Pediatric Gastroenterol. Nutr. 2007;45 doi: 10.1097/MPG.0b013e31812e68f6. [DOI] [PubMed] [Google Scholar]
- 71.Scrimshaw N.S., Murrary E.B. The acceptability of milk and milk products in populations with a high prevalence of lactose intolerance. Am. J. Clin. Nutr. 1988;48:1083–1159. doi: 10.1093/ajcn/48.4.1142. [DOI] [PubMed] [Google Scholar]
- 72.Hertzler S.R., Savaiano D.A. Colonic adaptation to daily lactose feeding in lactose maldigesters reduces lactose intolerance. Am. J. Clin. Nutr. 1996;64:232–236. doi: 10.1093/ajcn/64.2.232. [DOI] [PubMed] [Google Scholar]
- 73.He T., Priebe M.G., Harmsen H.J.M., Stellaard F., Sun X., Welling G.W., Vonk R.J. Colonic Fermentation May Play a Role in Lactose Intolerance in Humans. J. Nutr. 2006;136:58–63. doi: 10.1093/jn/136.1.58. [DOI] [PubMed] [Google Scholar]
- 74.Von Tirpitz C., Kohn C., Steinkamp M., Geerling I., Maier V., Möller P., Adler G., Reinshagen M. Lactose intolerance in active Crohn’s disease: Clinical value of duodenal lactase analysis. J. Clin. Gastroenterol. 2002;34:49–53. doi: 10.1097/00004836-200201000-00009. [DOI] [PubMed] [Google Scholar]
- 75.Szilagyi A., Galiatsatos P., Xue X. Systematic review and meta-analysis of lactose digestion, its impact on intolerance and nutritional effects of dairy food restriction in inflammatory bowel diseases. Nutr. J. 2016;15:67. doi: 10.1186/s12937-016-0183-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shiby V.K., Mishra H.N. Fermented Milks and Milk Products as Functional Foods-A Review. Crit. Rev. Food Sci. Nutr. 2013;53:482–496. doi: 10.1080/10408398.2010.547398. [DOI] [PubMed] [Google Scholar]
- 77.Rosa D.D., Dias M.M.S., Grześkowiak Ł.M., Reis S.A., Conceição L.L., Peluzio M.D.C.G. Milk kefir: Nutritional, microbiological and health benefits. Nutr. Res. Rev. 2017;30:82–96. doi: 10.1017/S0954422416000275. [DOI] [PubMed] [Google Scholar]
- 78.Devkota S., Chang E.B. Diet-induced expansion of pathobionts in experimental colitis: Implications for tailored therapies. Gut Microbes. 2013;4:172–174. doi: 10.4161/gmic.23589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Salli K., Anglenius H., Hirvonen J., Hibberd A.A., Ahonen I., Saarinen M.T., Tiihonen K., Maukonen J., Ouwehand A.C. The effect of 2′-fucosyllactose on simulated infant gut microbiome and metabolites; a pilot study in comparison to GOS and lactose. Sci. Rep. 2019;9 doi: 10.1038/s41598-019-49497-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pham H.T.T., Boger M.C.L., Dijkhuizen L., van Leeuwen S.S. Stimulatory effects of novel glucosylated lactose derivatives GL34 on growth of selected gut bacteria. Appl. Microbiol. Biotechnol. 2019;103:707–718. doi: 10.1007/s00253-018-9473-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jakobsen L.M.A., Sundekilde U.K., Andersen H.J., Nielsen D.S., Bertram H.C. Lactose and Bovine Milk Oligosaccharides Synergistically Stimulate B. longum subsp. longum Growth in a Simplified Model of the Infant Gut Microbiome. J. Proteome Res. 2019;18:3086–3098. doi: 10.1021/acs.jproteome.9b00211. [DOI] [PubMed] [Google Scholar]
- 82.Almeida C.C., Lorena S.L.S., Pavan C.R., Akasaka H.M.I., Mesquita M.A. Beneficial effects of long-term consumption of a probiotic combination of Lactobacillus casei Shirota and Bifidobacterium breve Yakult may persist after suspension of therapy in lactose-intolerant patients. Nutr. Clin. Pract. 2012;27:247–251. doi: 10.1177/0884533612440289. [DOI] [PubMed] [Google Scholar]
- 83.Saltzman J.R., Russell R.M., Golner B., Barakat S., Dallal G.E., Goldin B.R. A randomized trial of Lactobacillus acidophilus BG2FO4 to treat lactose intolerance. Am. J. Clin. Nutr. 1999;69:140–146. doi: 10.1093/ajcn/69.1.140. [DOI] [PubMed] [Google Scholar]
- 84.Li J., Zhang W., Wang C., Yu Q., Dai R., Pei X. Lactococcus lactis expressing food-grade β-galactosidase alleviates lactose intolerance symptoms in post-weaning Balb/c mice. Appl. Microbiol. Biotechnol. 2012;96:1499–1506. doi: 10.1007/s00253-012-3977-4. [DOI] [PubMed] [Google Scholar]
- 85.Ntemiri A., Ribière C., Stanton C., Ross R.P., O’Connor E.M., O’Toole P.W. Retention of microbiota diversity by lactose-free milk in a mouse model of elderly gut microbiota. J. Agric. Food Chem. 2019;67:2098–2112. doi: 10.1021/acs.jafc.8b06414. [DOI] [PubMed] [Google Scholar]
- 86.Nicklas T.A., Qu H., Hughes S.O., He M., Wagner S.E., Foushee H.R., Shewchuk R.M. Self-perceived lactose intolerance results in lower intakes of calcium and dairy foods and is associated with hypertension and diabetes in adults. Am. J. Clin. Nutr. 2011;94:191–198. doi: 10.3945/ajcn.110.009860. [DOI] [PubMed] [Google Scholar]
- 87.Hamilton X.M.K., Ronveaux C.C., Rust B.M., Newman J.W., Hawley M., Barile D., Mills D.A., Raybould H.E. Prebiotic milk oligosaccharides prevent development of obese phenotype, impairment of gut permeability, and microbial dysbiosis in high fat-fed mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2017;312:G474–G487. doi: 10.1152/ajpgi.00427.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bischoff S.C., Escher J., Hébuterne X., Kłęk S., Krznaric Z., Schneider S., Shamir R., Stardelova K., Wierdsma N., Wiskin A.E., et al. ESPEN practical guideline: Clinical Nutrition in inflammatory bowel disease. Clin. Nutr. 2020;39:632–653. doi: 10.1016/j.clnu.2019.11.002. [DOI] [PubMed] [Google Scholar]
- 89.Veech R.L. The therapeutic implications of ketone bodies: The effects of ketone bodies in pathological conditions: Ketosis, ketogenic diet, redox states, insulin resistance, and mitochondrial metabolism. Prostaglandins Leukot. Essent. Fat. Acids. 2004;70:309–319. doi: 10.1016/j.plefa.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 90.Sampaio L.P. de B. Ketogenic diet for epilepsy treatment. Arq. Neuropsiquiatr. 2016;74:842–848. doi: 10.1590/0004-282X20160116. [DOI] [PubMed] [Google Scholar]
- 91.Olson C.A., Vuong H.E., Yano J.M., Liang Q.Y., Nusbaum D.J., Hsiao E.Y. The gut microbiota mediates the anti-seizure effects of the ketogenic diet. Cell. 2018;173:1728–1741.e13. doi: 10.1016/j.cell.2018.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lopetuso L.R., Petito V., Graziani C., Schiavoni E., Paroni Sterbini F., Poscia A., Gaetani E., Franceschi F., Cammarota G., Sanguinetti M., et al. Gut microbiota in health, diverticular disease, irritable bowel syndrome, and inflammatory bowel diseases: Time for microbial marker of gastrointestinal disorders. Dig. Dis. 2018;36:56–65. doi: 10.1159/000477205. [DOI] [PubMed] [Google Scholar]
- 93.Newell C., Bomhof M.R., Reimer R.A., Hittel D.S., Rho J.M., Shearer J. Ketogenic diet modifies the gut microbiota in a murine model of autism spectrum disorder. Mol. Autism. 2016;7:37. doi: 10.1186/s13229-016-0099-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Staudacher H.M. Nutritional, microbiological and psychosocial implications of the low FODMAP diet. J. Gastroenterol. Hepatol. 2017;32:16–19. doi: 10.1111/jgh.13688. [DOI] [PubMed] [Google Scholar]
- 95.Duncan S.H., Belenguer A., Holtrop G., Johnstone A.M., Flint H.J., Lobley G.E. Reduced dietary intake of carbohydrates by obese subjects results in decreased concentrations of butyrate and butyrate-producing bacteria in feces. Appl. Environ. Microbiol. 2007;73:1073–1078. doi: 10.1128/AEM.02340-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kelly C.J., Zheng L., Campbell E.L., Saeedi B., Scholz C.C., Bayless A.J., Wilson K.E., Glover L.E., Kominsky D.J., Magnuson A., et al. Crosstalk between microbiota-derived short-chain fatty acids and intestinal epithelial HIF augments tissue barrier function. Cell Host Microbe. 2015;17:662–671. doi: 10.1016/j.chom.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.David L.A., Maurice C.F., Carmody R.N., Gootenberg D.B., Button J.E., Wolfe B.E., Ling A.V., Devlin A.S., Varma Y., Fischbach M.A., et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505:559–563. doi: 10.1038/nature12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jantchou P., Morois S., Clavel-Chapelon F., Boutron-Ruault M.C., Carbonnel F. Animal protein intake and risk of inflammatory bowel disease: The E3N prospective study. Am. J. Gastroenterol. 2010;105:2195–2201. doi: 10.1038/ajg.2010.192. [DOI] [PubMed] [Google Scholar]
- 99.De Filippo C., Cavalieri D., Di Paola M., Ramazzotti M., Poullet J.B., Massart S., Collini S., Pieraccini G., Lionetti P. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc. Natl. Acad. Sci. USA. 2010;107:14691–14696. doi: 10.1073/pnas.1005963107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dominika Ś., Arjan N., Karyn R.P., Henryk K. The study on the impact of glycated pea proteins on human intestinal bacteria. Int. J. Food Microbiol. 2011;145:267–272. doi: 10.1016/j.ijfoodmicro.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 101.Meddah A.T.T., Yazourh A., Desmet I., Risbourg B., Verstraete W., Romond M.B. The regulatory effects of whey retentate from Bifidobacteria fermented milk on the microbiota of the simulator of the human intestinal microbial ecosystem (SHIME) J. Appl. Microbiol. 2001;91:1110–1117. doi: 10.1046/j.1365-2672.2001.01482.x. [DOI] [PubMed] [Google Scholar]
- 102.Manzel A., Muller D.N., Hafler D.A., Erdman S.E., Linker R.A., Kleinewietfeld M. Role of “western diet” in inflammatory autoimmune diseases. Curr. Allergy Asthma Rep. 2014;14 doi: 10.1007/s11882-013-0404-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wolters M., Ahrens J., Romaní-Pérez M., Watkins C., Sanz Y., Benítez-Páez A., Stanton C., Günther K. Dietary fat, the gut microbiota, and metabolic health—A systematic review conducted within the MyNewGut project. Clin. Nutr. 2019;38:2504–2520. doi: 10.1016/j.clnu.2018.12.024. [DOI] [PubMed] [Google Scholar]
- 104.Kakodkar S., Farooqui A.J., Mikolaitis S.L., Mutlu E.A. The specific carbohydrate diet for inflammatory bowel disease: A case series. J. Acad. Nutr. Diet. 2015;115:1226–1232. doi: 10.1016/j.jand.2015.04.016. [DOI] [PubMed] [Google Scholar]
- 105.Haas S.V., Haas M.P. The treatment of celiac disease with the specific carbohydrate diet; report on 191 additional cases. Am. J. Gastroenterol. 1955;23:344–360. [PubMed] [Google Scholar]
- 106.Limketkai B.N., Wolf A., Parian A.M. Nutritional interventions in the patient with inflammatory bowel disease. Gastroenterol. Clin. North Am. 2018;47:155–177. doi: 10.1016/j.gtc.2017.09.007. [DOI] [PubMed] [Google Scholar]
- 107.Hou J.K., Abraham B., El-Serag H. Dietary intake and risk of developing inflammatory bowel disease: A systematic review of the literature. Am. J. Gastroenterol. 2011;106:563–573. doi: 10.1038/ajg.2011.44. [DOI] [PubMed] [Google Scholar]
- 108.Omura K., Hirano K., Kanehira E., Kaito K., Tamura M., Nishida S., Kawakami K., Watanabe Y. Small amount of low-residue diet with parenteral nutrition can prevent decreases in intestinal mucosal integrity. Ann. Surg. 2000;231:112–118. doi: 10.1097/00000658-200001000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Walters S.S. Analysis of gut microbiome and diet modification in patients with crohn’s disease. SOJ Microbiol. Infect. Dis. 2014;2:1. doi: 10.15226/sojmid/2/3/00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dubrovsky A., Kitts C.L. Effect of the specific carbohydrate diet on the microbiome of a primary sclerosing cholangitis and ulcerative colitis patient. Cureus. 2018;10 doi: 10.7759/cureus.2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hoffmann C., Dollive S., Grunberg S., Chen J., Li H., Wu G.D., Lewis J.D., Bushman F.D. Archaea and fungi of the human gut microbiome: Correlations with Diet and Bacterial Residents. PLoS ONE. 2013;8 doi: 10.1371/journal.pone.0066019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Voegtlin W.L. The Stone Age Diet: Based on In-Depth Studies of Human Ecology and the Diet of Man. Vantage Press, Incorporated; New York, NY, USA: 1975. [Google Scholar]
- 113.Challa H.J., Bandlamudi M., Uppaluri K.R. Nutrition and Cardiometabolic Health. CRC Press; Boca Raton, FL, USA: 2019. Paleolithic diets; pp. 493–516. [Google Scholar]
- 114.Schnorr S.L., Candela M., Rampelli S., Centanni M., Consolandi C., Basaglia G., Turroni S., Biagi E., Peano C., Severgnini M., et al. Gut microbiome of the Hadza hunter-gatherers. Nat. Commun. 2014;5:3654. doi: 10.1038/ncomms4654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Eaton S.B., Konner M. Paleolithic nutrition. N. Engl. J. Med. 1985;312:283–289. doi: 10.1056/NEJM198501313120505. [DOI] [PubMed] [Google Scholar]
- 116.Galvez J., Rodríguez-Cabezas M.E., Zarzuelo A. Effects of dietary fiber on inflammatory bowel disease. Mol. Nutr. Food Res. 2005;49:601–608. doi: 10.1002/mnfr.200500013. [DOI] [PubMed] [Google Scholar]
- 117.Hallert C., Björck I., Nyman M., Pousette A., Grännö C., Svensson H. Increasing fecal butyrate in ulcerative colitis patients by diet: Controlled pilot study. Inflamm. Bowel Dis. 2003;9:116–121. doi: 10.1097/00054725-200303000-00005. [DOI] [PubMed] [Google Scholar]
- 118.Kanauchi O., Suga T., Tochihara M., Hibi T., Naganuma M., Homma T., Asakura H., Nakano H., Takahama K., Fujiyama Y., et al. Treatment of ulcerative colitis by feeding with germinated barley foodstuff: First report of a multicenter open control trial. J. Gastroenterol. 2002;37:67–72. doi: 10.1007/BF03326417. [DOI] [PubMed] [Google Scholar]
- 119.Ananthakrishnan A.N., Cagan A., Gainer V.S., Cai T., Cheng S.C., Savova G., Chen P., Szolovits P., Xia Z., De Jager P.L., et al. Normalization of plasma 25-hydroxy vitamin D is associated with reduced risk of surgery in Crohn’s disease. Inflamm. Bowel Dis. 2013;19:1921–1927. doi: 10.1097/MIB.0b013e3182902ad9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Dziechciarz P., Horvath A., Shamir R., Szajewska H. Meta-analysis: Enteral nutrition in active Crohn’s disease in children. Aliment. Pharmacol. Ther. 2007;26:795–806. doi: 10.1111/j.1365-2036.2007.03431.x. [DOI] [PubMed] [Google Scholar]
- 121.Sigall-Boneh R., Pfeffer-Gik T., Segal I., Zangen T., Boaz M., Levine A. Partial enteral nutrition with a Crohn’s disease exclusion diet is effective for induction of remission in children and young adults with Crohn’s disease. Inflamm. Bowel Dis. 2014;20:1353–1360. doi: 10.1097/MIB.0000000000000110. [DOI] [PubMed] [Google Scholar]
- 122.Levine A., Sigall Boneh R., Wine E. Evolving role of diet in the pathogenesis and treatment of inflammatory bowel diseases. Gut. 2018;67:1726–1738. doi: 10.1136/gutjnl-2017-315866. [DOI] [PubMed] [Google Scholar]
- 123.Boneh R.S., Shabat C.S., Yanai H., Chermesh I., Avraham S.B., Boaz M., Levine A. Dietary therapy with the Crohn’s disease exclusion diet is a successful strategy for induction of Remission in children and adults failing biological therapy. J. Crohn’s Colitis. 2017;11:1205–1212. doi: 10.1093/ecco-jcc/jjx071. [DOI] [PubMed] [Google Scholar]
- 124.Levine A., Wine E., Assa A., Sigall Boneh R., Shaoul R., Kori M., Cohen S., Peleg S., Shamaly H., On A., et al. Crohn’s disease exclusion diet plus partial enteral nutrition induces sustained remission in a randomized controlled trial. Gastroenterology. 2019;157:440–450.e8. doi: 10.1053/j.gastro.2019.04.021. [DOI] [PubMed] [Google Scholar]
- 125.Urlep D., Benedik E., Brecelj J., Orel R. Partial enteral nutrition induces clinical and endoscopic remission in active pediatric Crohn’s disease: Results of a prospective cohort study. Eur. J. Pediatr. 2019 doi: 10.1007/s00431-019-03520-7. [DOI] [PubMed] [Google Scholar]
- 126.Lane E.R., Lee D., Suskind D.L. Dietary Therapies in Pediatric Inflammatory Bowel Disease: An Evolving Inflammatory Bowel Disease Paradigm. Gastroenterol. Clin. N. Am. 2017;46:731–744. doi: 10.1016/j.gtc.2017.08.012. [DOI] [PubMed] [Google Scholar]