Abstract
Cardiovascular diseases (CVDs) can originate from early life. Accumulating evidence suggests that gut microbiota in early life is linked to CVDs in later life. Gut microbiota-targeted therapy has gained significant importance in recent decades for its health-promoting role in the prevention (rather than just treatment) of CVDs. Thus far, available gut microbiota-based treatment modalities used as reprogramming interventions include probiotics, prebiotics, and postbiotics. The purpose of this review is, first, to highlight current studies that link dysbiotic gut microbiota to the developmental origins of CVD. This is followed by a summary of the connections between the gut microbiota and CVD behind cardiovascular programming, such as short chain fatty acids (SCFAs) and their receptors, trimethylamine-N-oxide (TMAO), uremic toxins, and aryl hydrocarbon receptor (AhR), and the renin-angiotensin system (RAS). This review also presents an overview of how gut microbiota-targeted reprogramming interventions can prevent the developmental origins of CVD from animal studies. Overall, this review reveals that recent advances in gut microbiota-targeted therapy might provide the answers to reduce the global burden of CVDs. Still, additional studies will be needed to put research findings into practice.
Keywords: aryl hydrocarbon receptor, cardiovascular disease, hypertension, gut microbiota, postbiotics, short chain fatty acid, prebiotics, probiotics, trimethylamine-N-oxide, developmental origins of health and disease (DOHaD)
1. Introduction
Cardiovascular diseases (CVDs), a cluster of disorders of the heart and blood vessels, are the leading cause of death worldwide [1]. Despite many advances in drug treatment, the prevalence of CVDs has continued to rise. Of note, most CVDs affect older adults. However, CVDs can originate from early life, from not only infancy and childhood, but tracing back to the prenatal stage as well (and gradually progressing across one’s life span) [2]. The cardiovascular system is one of the first body systems to appear within the fetus, which is vulnerable to the adverse intrauterine exposure to environmental insults [2]. The developmental origins of health and disease (DOHaD) theory posits that exposure to various insults during critical periods in fetal development leads to structural changes and functional adaption, resulting in increased risk of adult diseases, including CVDs [3]. Important support for the DOHaD concept came from epidemiological reports following birth cohorts in severe famines, which demonstrated that malnutrition during gestation induced a cluster of CVD risk factors, such as hypertension, dyslipidemia, obesity, kidney disease, type 2 diabetes, and cardiovascular morbidity in adult offspring [4,5]. Additionally, an observational study evaluating 2302 mother–child dyads reported that a better gestational CV health score in the mothers was associated with better CV health in the offspring from ages 10 to 14 years [6].
CVD risk can be shaped by a number of modifiable environmental risk factors often linked to diet. Diet influences the host’s health status by modulating the composition of the gut microbiota. Emerging evidence supports that gut microbiota may indirectly or directly influence cardiovascular risk [7,8,9]. Gut microbiota derived metabolites can act as a mediator of microbial influence through circulation on various target organs, including the cardiovascular systems [7,8]. Thus far, scientists have proposed several mechanisms by which dysbiotic gut microbiota contributes to CVD, such as alterations of microbiota-derived metabolite short-chain fatty acids (SCFA), increases of trimethylamine-N-oxide (TMAO), inhibition of nitric oxide (NO), and aberrant activation of the renin-angiotensin system (RAS) [7,8,9,10,11]. A meta-analysis study summarized 19 studies with 19,256 participants and found that individuals with high concentrations of TMAO and its precursors were associated with increased risks of major adverse cardiovascular events and all-cause mortality [12]. Maternal nutritional insults have been shown to alter gut microbiota balance, resulting in an increased risk of adult diseases [13]. However, relatively little is known about whether (and how) various early life insults could affect gut microbiota, resulting in CVDs in adult offspring.
Nevertheless, developmental programming, besides determining the risk for CVDs in adulthood, also offers an innovative approach to prevent CVDs by so-called reprogramming [14]. By switching therapy from adulthood to early life before disease occurs, we have the potential to postpone or reduce undesirable programming processes that would lead to CVDs. Over the year, the gut microbiota has gained more attention because, unlike non-modifiable CV risk factors, it can be modified through agents that modulate the intestinal bacterial flora, including prebiotics, probiotics, synbiotics, etc. [7,15,16,17]. Accordingly, one may assume that early gut microbiota-targeted therapy may serve as a reprogramming strategy to prevent the developmental origins of CVD.
The purpose of our scoping review is to provide insight on gut microbiota implicated in the developmental programming of CVD. Hence, we examine mechanisms linking gut microbiota to cardiovascular programming that, in light of the available evidence, can be considered therapeutic targets. In particular, we focus on addressing probiotics, prebiotics, and postbiotics as a reprogramming strategy for prevention of developmental programming of CVD.
We searched the PubMed/MEDLINE databases for studies published in English between January 1980 and May 2021, using the following search terms: “cardiovascular disease”, “developmental programming”, “DOHaD”, “reprogramming”, “gut microbiota”, “probiotics”, “prebiotics”, “synbiotics”, “postbiotics”, “mother”, “pregnancy”, “gestation”, “offspring”, “progeny”, “atherosclerosis”, “heart”, “vascular”, “endothelial dysfunction”, “stroke”, “thrombosis”, “aryl hydrocarbon receptor” and “hypertension”. Additional studies were then selected and assessed based on fitting references in eligible papers. We found that there are more than 3000 publications related to gut microbiota and CVD-related disorders. However, only approximately 10% belong to the research of DOHaD. Among them, hypertension accounts for nearly 80% of searchable publications.
2. Developmental Programming of CVD: Human Evidence
Epidemiological studies have consistently indicated that people exposed to famine during early life have disclosed strong relationships with CVD risk factors, such as obesity, dyslipidemia, hypertension, and type 2 diabetes [4,18,19]. Additionally, extensive evidence indicates that other early life adverse influences contribute to CVD in later life, including maternal illness, pregnancy complications, medication use in pregnancy, and in utero exposure to environmental pollutants [20,21,22]. In one example, the offspring of a diabetic pregnancy conveyed high risks for type 2 diabetes and obesity in adulthood—both risk factors are associated with CVD [23]. Prior research also suggested an association between medication use in pregnant women, such as glucocorticoid [24] and non-steroidal anti-inflammatory drugs [25], and adverse cardiovascular renal outcomes in their progeny. Several other risk factors in early life affecting cardiovascular outcomes in offspring have been reported, such as vitamin D deficiency [26], gestational hypertension [27], short-term breastfeeding [28], and excessively rapid weight gain postnatally [29]. Moreover, early life toxic environmental exposure, such as endocrine-disrupting chemicals, is linked to cardiometabolic traits in childhood [30]. The association between low birth weight (LBW), a clinical marker of inappropriate fetal growth, and later cardiovascular risk is another line of evidence for the developmental origins of CVD [31,32,33]. Previous studies on twins have reported that the lighter twins developed endothelial dysfunction and arterial narrowing and were more likely to die from ischemic heart disease [32,33].
However, epidemiological studies often do not provide information in which a direct cause–effect relationship can be established. Instead, the results from animal models can help one understand which developmental stage is critical for cardiovascular programming, to discover molecular mechanisms underlying the developmental origins of CVD, and to develop novel reprogramming strategies.
3. Implications of Gut Microbiota in the Developmental Origins of CVD
3.1. Animal Models Related to Gut Microbiota
Animal models have provided compelling evidence that adverse early life conditions induce cardiovascular programming, coinciding with altered gut microbiota. Table 1 summarizes animal studies documenting the association between gut microbiota, early life insults, and subsequent CVDs in adult offspring [34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51]. CVDs often occur after a prolonged asymptomatic phase in childhood. For the sake of brevity, the present review is solely restricted to data obtained from adult offspring.
Table 1.
Eligible animal models reporting cardiovascular outcomes related to alterations of gut microbiota.
| Animal Models | Cardiovascular Outcomes | Programming Mechanisms Related Gut Microbiota | Species/ Gender |
Age at Measure | Ref. |
|---|---|---|---|---|---|
| Maternal high-fructose diet | Hypertension | Decreased SCFA receptor GPR41 and GPR43 expression | SD rat/M | 12 weeks | [34] |
| Maternal high-fructose diet | Hypertension | Decreased plasma TMA level; reduced phylum Verrucomicrobia and genus Akkermansia abundance | SD rat/M | 12 weeks | [35] |
| Maternal plus post-weaning high-fructose diet | Hypertension | Decreased abundance of genera Bacteroides, Dysgonomonas, and Turicibacter | SD rat/M | 12 weeks | [36] |
| Maternal high-fructose diet and TCDD exposure | Hypertension | Increased abundance of genus Gordonibacter | SD rat/M | 12 weeks | [37] |
| Maternal adenine-induced chronic kidney disease | Hypertension | A decreased α-diversity and an increased F/B ratio; A decreased abundance of the genus Bifidobacterium | SD rat/M | 12 weeks | [38] |
| Maternal minocycline administration | Hypertension | An increase F/B ratio, and decreased genera Lactobacillus, Ruminococcus, and Odoribacter abundance | SD rat/M | 12 weeks | [39] |
| Maternal ADMA and TMAO exposure | Hypertension | Decreased abundance of family Erysipelotrichaceae | [40] | ||
| Maternal hypertension | Hypertension | An increased abundance of the genera Bifidobacterium, Lactobacillus, Turicibacter, and Akkermansia | SHR/M | 12 weeks | [41] |
| Maternal hypertension | Hypertension | An increased F/B ratio | SHR/M | 12 weeks | [42] |
| Maternal high-fat diet | Obesity and insulin resistance | Decreased gut microbiota richness | C57BL/6J mouse/M and F | 12 weeks | [43] |
| Maternal high-fat diet | Obesity and nonalcoholic fatty liver disease | Decreased α-diversity | C57BL/6J mouse/M and F | 17 weeks | [44] |
| Maternal high-fat and high-cholesterol diet | Hypertension, endothelial dysfunction, increased lipid profile and insulin resistance | Decreased α-diversity | Wistar rat/M | 90 days | [45] |
| Maternal plus post-weaning high-fat diet | Hypertension | An increased F/B ratio; a reduction of genera Lactobacillus and Akkermansia | SD rat/M | 16 weeks | [46,47] |
| Maternal L-NAME administration plus post-weaning high-fat diet | Hypertension | An increased F/B ratio | SD rat/M | 16 weeks | [48] |
| Maternal Western-style diet | Obesity and nonalcoholic fatty liver disease | An increase in abundance of genus Ruminococcus | C57BL/6J mouse/M | 20 weeks | [49] |
| Maternal dyslipidemia | Hypertension and increased lipid profile | A decrease of genera Lactobacillus | Wistar rat/M and F | 24 weeks | [50] |
| Prenatal androgen exposure | Hypertension, decreased heart rate, obesity, and increased thickness of left ventricle | An increased abundance of bacteria associated with production of SCFAs. | Wistar rat/F | 4 months | [51] |
Studies tabulated according to animal models, and age at measure; SD rats—Sprague-Dawley rats; SHR—spontaneously hypertensive rat; M—male; F—female; TCDD—2,3,7,8-tetrachlorodibenzo-p-dioxin; ADMA—asymmetric dimethylarginine; TMAO—trimethylamine N-oxide; L-NAME—NG-nitro-L-arginine-methyl ester; F/B ratio—Firmicutes to Bacteroidetes (F/B) ratio.
A variety of environmental insults in early life were reported to induce cardiovascular programming related to alterations of gut microbiota, including maternal high-fructose diet [34,35,36], maternal high-fructose diet plus 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) exposure [37], maternal chronic kidney disease [38], maternal minocycline administration [39], maternal asymmetric dimethylarginine (ADMA), trimethylamine N-oxide (TMAO) exposure [40], maternal hypertension [41,42], maternal high-fat and/or post-weaning high-fat diet [43,44,45,46,47], maternal NG-nitro-L-arginine-methyl ester (L-NAME) administration plus post-weaning high-fat diet [48], maternal Western-style diet [49], maternal dyslipidemia [50], and prenatal androgen exposure [51].
The most common cardiovascular outcome being studied in altered gut microbiota-related animal models is hypertension [34,35,36,37,38,39,40,41,42,45,46,47,48,50,51], followed by endothelial dysfunction [44] and ventricular hypertrophy [51]. Abnormalities in gut microbiota were also related CV risks, such as obesity [43,44,49,51], insulin resistance [43,45], increased lipid profile [45,50], and nonalcoholic fatty liver disease [44,48]. Abnormalities in gut microbial richness and diversity have been linked to a higher risk of developing other CVDs, such as coronary artery disease, cardiomyopathy, and heart failure [52]. However, little information exists regarding the role of gut microbiota in the developmental origins of those CVDs.
As Table 1 illustrates, the rat is the most common species used. The possible reason might be that rat models provide a low-cost option with a short life cycle that is easy to handle [53,54]. Although other species, such as rabbits, sheep, and pigs, are used in DOHaD research [53,54], limited information is provided on using large animals to study gut microbiota and the developmental origins of CVD.
In adulthood, every one month of the rat is equivalent to three human years [55]. Table 1 lists the timing of cardiovascular outcomes determined from 12 weeks to 4 months of age in rats, which corresponds to humans from childhood to early adulthood. Accordingly, knowledge gaps about long-term adverse effects of early life insults on CVD and gut microbiota in older adult offspring remain large.
3.2. Potential Mechanisms Underlying the Developmental Origins of CVD
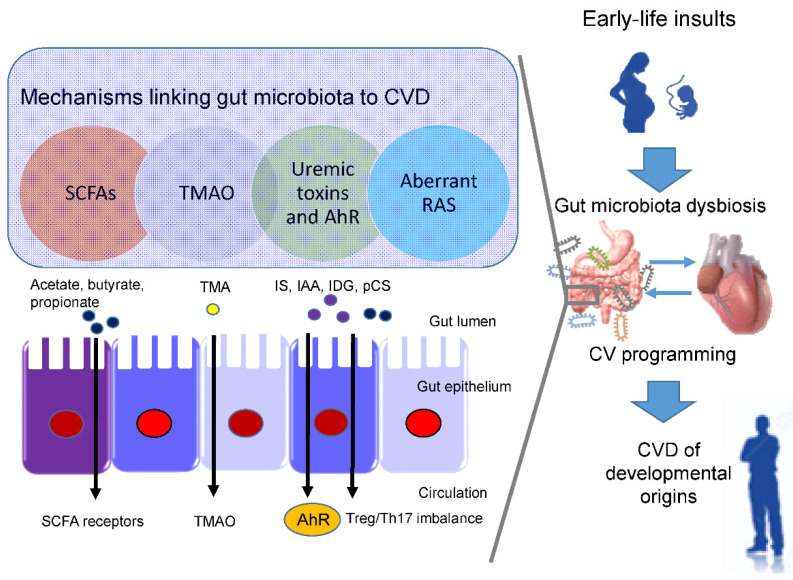
In view of the fact that a variety of insults during fetal development generates similar cardiovascular outcomes (e.g., hypertension) in adulthood, this raises the possibility that a common pathway is involved in the pathogenesis of the developmental origins of CVD. Presently, several mechanisms have been linked to the developmental origins of CVD, such as oxidative stress, nitric oxide (NO) deficiency, dysregulated nutrient-sensing signals, gut microbiome dysbiosis, epigenetic regulation, sex differences, etc. [2,14,20,21,22,54]. Some of them are interrelated to gut microbiota dysbiosis. Recent evidence has been accumulated by deciphering the role of gut microbiota in the developmental origins of CVD, including alterations of SCFAs and their receptors, increases of TMAO, uremic toxins, and aryl hydrocarbon receptor (AhR), and aberrant renin-angiotensin system (RAS) [10,11] (Figure 1). Each of them will be discussed in turn.
Figure 1.
Overview of the common mechanisms linking gut microbiota dysbiosis to cardiovascular programming. CVD—cardiovascular disease. SCFA—short chain fatty acid. TMAO—trimethylamine N-oxide. AhR—aryl hydrocarbon receptor. RAS—renin-angiotensin system. Treg—T regulatory cells. TH17—T helper 17 cells. IS—indoxyl sulfate. IAA—indoleacetic acid. IDG—3-indoxyl β-d-glucuronide. pCS-p—Cresyl sulfate. TMA—trimethylamine.
3.3. SCFAs and Their Receptors
Short chain fatty acids (SCFAs) are formed during bacterial fermentation of carbohydrates in the gut. SCFAs have one to six carbon atoms (C1-C6), mainly consisting of acetate (C2), propionate (C3), and butyrate (C4) [56]. SCFAs are generally known to induce vasodilatation, in favor of antihypertension [56].
In spontaneously hypertensive rats (SHRs), hypertension is related to decreased abundance of acetate- and butyrate-producing bacteria [57]. SCFAs exert their regulation on BP mainly by activating their SCFA receptors, including G protein-coupled receptor 41 (GPR41), 43 (GPR43), 109A (GRP109A), and olfactory receptor 78 (Olfr78) [56]. The Olfr78 is expressed in the renal juxtaglomerular apparatus, where it mediates renin secretion in response to SCFAs to elevate BP [58]. In addition to hypertension, the metabolism of SCFAs is also a factor contributing to obesity [59].
In a maternal minocycline exposure model, minocycline-induced hypertension is associated with decreased plasma levels of acetate and butyrate [39]. Another report demonstrated that maternal garlic oil therapy protects against increases in blood pressure induced by high-fat diets in adult male rat offspring, which coincided with increases of acetate, butyrate, and propionate levels, as well as their producing microorganisms [60]. Additionally, SCFA supplementation in pregnancy and lactation have shown positive benefits on hypertension of developmental origins [35,61]. These findings support the notion that SCFAs and their receptors might be a key mechanism underlying developmental programming of hypertension.
3.4. TMAO
Emerging evidence indicates that a high TMAO level correlates with CVD mortality [62]. Besides CVD, TMAO also contributes to other cardiovascular risks, such as chronic kidney disease (CKD), type II diabetes, insulin resistance, and NAFAD [63]. TMAO can activate p38 mitogen-activated protein kinase (MAPK) signaling, nuclear factor-κB (NF-κB) signaling, inflammatory gene expression, and leukocyte-endothelial cell adhesion in atherosclerosis development [64]. TMAO is a small colorless amine oxide produced by gut microbiota metabolism. Its production is a two-step process. The first step involves gut microbial trimethylamine (TMA) formation from dietary precursors (e.g., choline and carnitine); in the second step, TMA is oxidized to TMAO by flavin-containing monooxygenases (FMOs) in the liver [65]. TMAO is then either excreted by the kidney or transported to the tissues.
Maternal TMAO administration can induce hypertension in adult male offspring [40]. Conversely, 3,3-dimethyl-1-butanol (DMB, a TMA inhibitor) treatment during pregnancy and lactation protected adult offspring against hypertension programmed by a maternal high-fructose diet, which was associated with the reduction of TMA and TMAO levels [35]. Moreover, perinatal resveratrol therapy prevented maternal CKD-induced hypertension in adult male rat offspring and was associated with a decreased TMAO-to-TMA ratio [38]. These observations suggest a pathogenic association between the TMA-TMAO pathway and the developmental origins of CVD.
3.5. Uremic Toxins and Aryl Hydrocarbon Receptor
Uremic toxins play an important role in cardiovascular morbidity and mortality in patients with CKD [66]. One example is TMAO, a cardiovascular risk factor and uremic toxins. Additionally, several tryptophan metabolites are known as gut microbiota-derived uremic toxins, such as indoxyl sulfate (IS), indoleacetic acid (IAA), and indoxyl--D glucuronide (IDG) [67]. These tryptophan-derived uremic toxins have proinflammatory, procoagulant, prooxidant, and pro-apoptotic effects, all of which are implicated in the pathogenesis of CVD [68]. Among them, IS and IAA were related to the risk for cardiovascular morbidity and mortality in uremic patients [69]. In CKD, IS can activate monocytes, intensify inflammatory process, augment oxidative stress and impair hemostatic system, all of which are major contributors to the development of CVD [70,71].
Moreover, IAA and several microbial tryptophan catabolites are ligands for aryl AhR [72]. AhR signaling modulates pro-inflammatory T helper 17 (TH17) axis and triggers inflammation, by which activation of AhR by its ligands is closely associated with the development of CVD [73,74]. An imbalance of T regulatory cells (Treg) and TH17 cells is involved in the development of hypertension [75]. In patients with CKD, Treg/Th17 imbalance has been associated with the pathogenesis of cardiovascular complications [76].
In a maternal CKD-induced hypertension model, maternal tryptophan therapy preventing offspring hypertension was associated with mediation of the AhR signaling pathway [77]. On the other hand, antagonizing AhR signaling by resveratrol has been reported to protect adult offspring against hypertension in several hypertension models of developmental origins [36,78,79]. Prior research demonstrated that IS and AhR both can promote thrombosis [80,81]. As thrombus formation, secondary to atherosclerotic plaque disruption, plays a major role in the development of several CVDs [82], attention will need to be paid to better understand the interplay between AhR and uremic toxins underlying developmental programming of thrombotic-related CVDs.
Another uremic toxin is p-Cresyl sulfate (pCS), derived from aromatic amino acids metabolized by gut bacteria [83]. Increased levels of pCS have been related to worsening CV outcomes in patients with CKD [83]. Previous work demonstrated that p-Cresyl sulfate could increase expression of proinflammatory cytokines and adhesion molecules, therefore mechanistically promoting atherogenesis [84].
3.6. RAS
The RAS is a complex network that is implicated in CVD [85]. Within the RAS, regulation is achieved through a cascade of proteases that generate several bioactive peptides [86]. The classical RAS is composed of angiotensin-converting enzyme (ACE), angiotensin (ANG) II, and angiotensin II type 1 receptor (AT1R). Activation of the classical RAS triggers vasoconstriction and inflammation under pathophysiological conditions, thus promoting hypertension and cardiovascular damage [85]. Emerging evidence suggests that aberrant RAS plays a key role in cardiovascular programming and RAS-based interventions can be used as a reprogramming strategy to prevent DOHaD-related disorders [86]. Treating young offspring with renin inhibitor aliskiren [87], ACE inhibitor captopril [88], or angiotensin receptor blocker (ARB) losartan [89] between 2 and 4 weeks of age was shown to protect against hypertension programmed by various maternal insults in adulthood.
Angiotensin-converting enzyme 2 (ACE2), a homologue of ACE, converts ANG II to ANG-(1–7) that negatively regulates the RAS [90]. Via regulation of intestinal amino acid transport, previous research showed that ACE2 plays a crucial non-catalytic role in gut biology and modulation of gut microbiota [91]. One previous study reported that administration with the ACE2 activator or with ANG-(1–7) during pregnancy could alleviate cardiovascular dysfunction in adult SHR offspring [92]. Another report showed that the antihypertensive effect of probiotics might be due to their ability to produce ACE inhibitory peptides [93].
Since gut microbiome dysbiosis has been connected to CVD by modulating the gut RAS [94], these findings support the notion that the interplay between gut microbiota and the RAS implicates the pathogenesis of cardiovascular programming, although this remains speculative.
4. Preventing the Developmental Origins of CVD by Gut Microbiota-Targeted Therapy
4.1. Gut Microbiota-Targeted Therapy
As mentioned, gut microbiota is closely related to the developmental origins of CVD. Researchers have gradually turned their attention to focus on gut microflora and related metabolites as a potential target for therapeutics [95,96]. Probiotics are live microorganisms, which, when administered in adequate amounts, confer a health benefit to the host [97]. Prebiotics can promote the growth of probiotics and inhibit the growth of pathogen [97]. Synbiotics refer to dietary supplements, combining probiotics and prebiotics. A substantial body of research supports that prebiotics and/or probiotics supplementation have possible benefits for the prevention and management of CVD [8,15,95,96].
Additionally, fecal microbial transplantation (FMT) is being extensively studied in microbiome-associated pathologies, such as CVD [98]. However, its potential application in CVD remains limited, mainly as it brings both beneficial and harmful bacteria to the patients, which may lead to adverse complications.
Another gut microbiota-targeted modality is the use of microbial derived metabolites, namely postbiotics. Postbiotics include any substance produced or leased through the metabolism of the gut microbes, which have a positive effect on the host [99]. As postbiotics do not contain live microorganisms, they appear to lack serious side effects while maintaining similar effectiveness to probiotics [99].
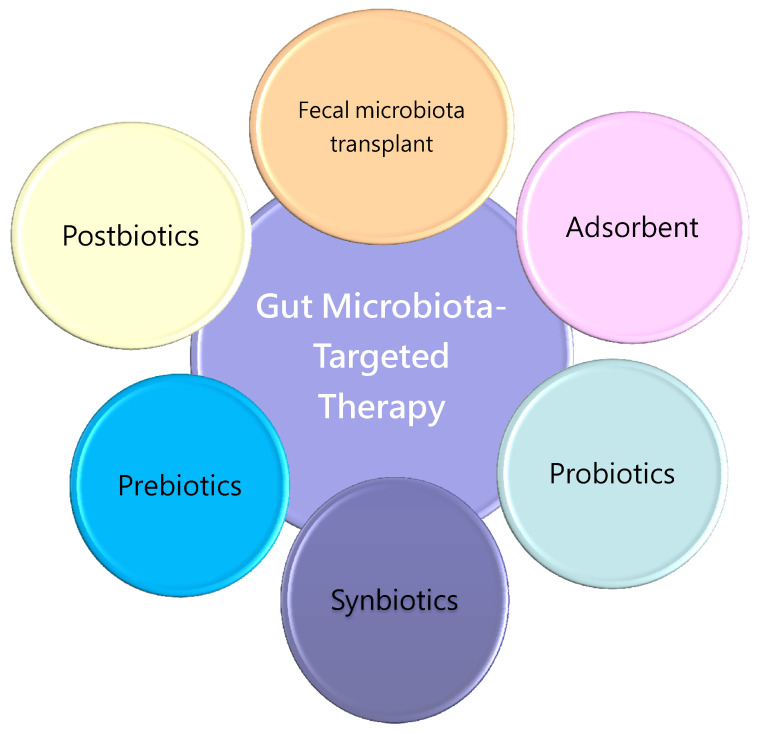
Moreover, microbiota-derived uremic toxins can be reduced by AST-120, an oral charcoal adsorbent [100]. In patients with CKD, AST-120 treatment has shown cardiovascular benefits [101,102]. However, the influence of adsorbents on gut microbiota compositions, and their related metabolites in other CVD populations instead of CKD, remains largely unknown. We propose the notion of the schema summary to address the potential gut microbiota-targeted therapies as a reprogramming strategy involved in the the developmental origins of CVD, which is illustrated in Figure 2.
Figure 2.
Schema outlining the potential gut microbiota-targeted therapies as a reprogramming strategy to prevent cardiovascular disease of developmental origins.
4.2. Uses of Probiotics and Prebiotics in Pregnant Women
In clinical practice, the most commonly used gut microbiota-targeted modalities are probiotics and prebiotics. When discussing the therapeutic benefits of probiotics and prebiotics in clinical utility, particular attention should be paid to their safety. Human studies regarding probiotic supplementation during pregnancy are limited [103]. Probiotic supplementation for pregnant women is generally safe and may have a protective role in preeclampsia [104], gestational diabetes [105], vaginal infections [106], and spontaneous preterm delivery [107]. Likewise, little is known about the use of prebiotics in pregnant women. One report demonstrated that supplementation with indigestible oligosaccharide prebiotics increased the number of maternal fecal Bifidobacterium spp., while this bifidogenic effect may not be transferred to the neonatal gut [108]. Although results from human studies on probiotics supplementation in the treatment of maternal conditions during pregnancy are beneficial, essentially no information exists regarding the effectiveness in protecting offspring against adult diseases.
4.3. Uses of Probiotics and Prebiotics in Newborn and Infants
In humans, microbiota colonization evolves continuously after birth, and by the age of three, the microbiota resembles adult-like composition [109]. Accordingly, manipulation of infant gut microbiome, through prebiotic and probiotic supplementation, may provide opportunities to promote health in later life [110]. Breast milk is the primary source of oral feeding. With prebiotic and probiotic properties, breast milk is crucial for the establishment of health-promoting microorganisms and plays specific effects on infantile immune function [111].
Despite the use of probiotics being considered safe in healthy infants and children, the effect of probiotics on the developing immune system and safety in neonates is largely unknown. Likewise, adverse effects of prebiotics added to infant formula are rarely documented. However, uncertainties remain about the doses and species necessary to confer beneficial effects, duration of supplementation, and the long-term effects of prebiotic and probiotic supplementation in neonates and infants.
4.4. Reprogramming Strategy for the Developmental Origins of CVD
Due to ethical considerations in humans, experiments to develop reprogramming interventions, targeting gut microbiota to prevent the developmental programming of CVD, have provided compelling evidence in animal models.
As mentioned in Table 1, various early life insults can alter the offspring’s gut microbial composition, leading to adverse CV outcomes. Conversely, early intervention targeting on gut microbiota has shown benefits against cardiovascular programming. Although novel gut microbiota-targeted modalities are tested in established CVD, few of them have been examined in early life for the developmental origins of CVD.
Table 2 summarizes studies that document microbiota-based reprogramming interventions in animal models, of cardiovascular programming, restricting those applying to critical periods during early development [34,35,37,39,47,112,113]. Moreover, we merely considered studies reporting CV outcomes with long enough follow-up of the offspring.
Table 2.
Summary of gut microbiota-targeted modalities used as reprogramming interventions for cardiovascular disease of developmental origins.
| Gut Microbiota-Targeted Intervention | Animal Models | Species/Gender | Age at Evaluation | Reprogramming Effects | Ref. |
|---|---|---|---|---|---|
| Probiotics | |||||
| Lactobacillus casei 2 × 10⁸ CFU/day via oral gavage during pregnancy and lactation | Maternal high-fructose diet | SD rat/M | 12 weeks | Prevented hypertension | [34] |
| Lactobacillus casei 2 × 10⁸ CFU/day via oral gavage during pregnancy and lactation | Perinatal high-fat diet | SD rat/M | 16 weeks | Prevented hypertension | [47] |
| Lactiplantibacillus plantarum WJL 1 × 10⁸ CFU/day via oral gavage during pregnancy and lactation | Maternal high-fat and high-cholesterol diet | Wistar rat/M | 90 days | Prevented cardiovascular dysfunction | [45] |
| Prebiotics | |||||
| 5% w/w long chain inulin during pregnancy and lactation | Maternal high-fructose diet | SD rat/M | 12 weeks | Prevented hypertension | [34] |
| 5% w/w long chain inulin during pregnancy and lactation | Perinatal high-fat diet | SD rat/M | 16 weeks | Prevented hypertension | [47] |
| 10% w/w oligofructose during pregnancy and lactation | Maternal high-fat/-sucrose diet | SD rat/M | 21 weeks | Attenuated hepatic steatosis and insulin resistance | [112] |
| Postbiotics | |||||
| Magnesium acetate 200 mmol/L in drinking water during pregnancy and lactation | Maternal high-fructose diet | SD rat/M | 12 weeks | Prevented hypertension | [35] |
| 1% conjugated linoleic acid during pregnancy and lactation | Maternal high-fat diet | SD rat/M | 18 weeks | Prevented hypertension and endothelial dysfunction | [113] |
| Others | |||||
| 1% DMB in drinking water during pregnancy and lactation | Maternal high-fructose diet | SD rat/M | 12 weeks | Prevented hypertension | [35] |
| 1% DMB in drinking water during pregnancy and lactation | Maternal high-fructose diet and TCDD exposure | SD rat/M | 12 weeks | Prevented hypertension | [37] |
Studies tabulated according to types of intervention, animal models, and age at evaluation. DMB-3,3—maternal dimethyl-1-butanol. TCDD—2,3,7,8-tetrachlorodibenzo-p-dioxin. SD—Sprague–Dawley rat.
As shown in Table 2, gut microbiota-based interventions have been dominated by experiments using rats. Available gut microbiota-targeted treatment modalities used as reprogramming interventions include probiotics, prebiotics, and postbiotics.
Although several probiotic microorganisms are shown to benefit cardiovascular health [15], there is still limited information regarding their role on the developmental origins of CVD. Supplementation with Lactobacillus casei throughout gestation and lactation periods protected adult male rat offspring against hypertension induced by the maternal high-fructose diet [34] or high-fat diet [47]. Another study demonstrated that maternal Lactiplantibacillus plantarum WJL treatment prevented cardiovascular dysfunction programmed by the maternal high-fat and high-cholesterol diet [45].
So far, only oligofructose and inulin have been investigated in regards to their reprogramming effects in the developmental origins of CVD [34,47,112], despite several types of prebiotics providing various health benefits [114]. Maternal oligofructose therapy attenuated hepatic steatosis and insulin resistance induced in adult offspring born to dams received high-fat/-sucrose diets [112]. Using a high-fat model [47], our prior research reported that inulin treatment protected against hypertension in adult rat offspring, coinciding with alterations of gut microbiota, and particularly increased the proportion of Lactobacillus, a well-known probiotic strain. Similarly, inulin supplementation during gestation and lactation protected adult offspring against hypertension programmed by the maternal high-fructose diet, which was related to an increased plasma propionate level [35].
Since SCFAs are products of fermentation of polysaccharides by gut microbiota, they have been used as postbiotics for CVD [115]. Although acetate, butyrate, and propionate have been shown to reduce BP in animal models of hypertension [116,117], only maternal acetate supplementation was reported to protect offspring hypertension programmed by a high-fructose diet [35]. Another example of postbiotics use in the developmental origins of CVD is conjugated linoleic acid [113]. In humans, conjugated linoleic acid production is performed by gut microbiota, mainly by the Bifidobacterium species [118].
In addition to metabolites, postbiotics include many different constituents, such as microbial cell fractions, extracellular polysaccharides, extracellular vesicles, functional proteins, cell lysates, cell wall-derived muropeptides, etc. [115]. However, essentially no information exists regarding the use of other types of postbiotics in the developmental origins of CVD.
There are other gut microbiota-related interventions applied to preventing the developmental origins of CVD. DMB, a structural analogue of choline, is able to inhibit microbe-dependent TMA and host TMAO formation. At least two studies have reported that maternal DMB therapy protected hypertension in adult offspring born to mothers exposed to high-fructose diets [35] or high-fructose diets plus TCDD exposure [37].
Of note, there are prebiotic-like components commonly found in consumable functional foods, such as flavonoids, polyphenols, and vitamins. Take resveratrol, a polyphenolic compound found in grapes and wine, as an example. A growing body of studies firmly supports that the beneficial effects of resveratrol on CVD are related to its prebiotic effect on gut microbiota [119,120]. As we reviewed elsewhere, early resveratrol therapy could be a reprogramming agent to protect against the developmental origins of CVD [121]. One study demonstrated that maternal resveratrol therapy protected against maternal CKD-induced hypertension in adult progeny, which was associated with restoration of microbial richness and diversity, and increased abundance of Lactobacillus and Bifidobacterium [38]. In another study, using a maternal L-NAME plus high-fat diet model, maternal resveratrol therapy protected against hypertension of developmental origins, coinciding with reduction of the Firmicutes to Bacteroidetes ratio, a microbial marker for hypertension [10,11]. Likewise, prebiotic-rich food, such as garlic, was used as used as a strategy to reprogram and prevent high-fat diet-induced hypertension in adult progeny [60]. Therefore, there is an urgent need to gain a greater understanding of other prebiotic-like components implicated on the developmental origins of CVD.
5. Conclusions and Perspectives
Previous studies have indicated that early gut microbiome and microbial metabolites might influence cardiovascular programming and exert adverse cardiovascular effects in later life. This review sought to highlight the value of gut microbiota-targeted interventions if applied early, to treat (and help prevent) CVD.
At face value, it would be logical to consider early life probiotics or prebiotics supplementation in potential reprogramming interventions in regards to the developmental origins of CVD. However, there are many aspects still unsolved. At a deeper level, little reliable information currently exists regarding the reprogramming effects of gut microbiota-targeted interventions in human trials. Future work in large prospective trials is needed to better identify (and appreciate) probiotic species and improve formulation of prebiotics for the developmental origins of CVD.
Animal studies suggest certain prebiotics may contribute to the prevention of the developmental origins of CVD, although the exact mechanisms have not been fully elucidated. What is missing from the literature is a greater understanding of whether the uses of numbers of prebiotic-like components or prebiotic-rich food in pregnancy and lactation can also alter gut microbiota and derived metabolites to prevent offspring against the developmental origins of CVD.
Due to the complex nature of postbiotics, the long-term effects of early life postbiotics interventions in various models of cardiovascular programming, either individually or in combination, are incomplete, or are awaiting further clarification. In exception for definitions provided by the International Scientific Association for Probiotics and Prebiotics (ISAPP), and the Food and Agriculture Organization of the United Nations–World Health Organization (FAO–WHO) for prebiotics and probiotics [122,123], so far there is a lack of a clear definition for postbiotics. A clear definition is important from a regulatory perspective, as postbiotics can be made with various probiotic species via a wide range of inactivation methods.
In conclusion, gut microbiota is a meaningfully pathogenetic link in the developmental origins of CVD. After all of this remarkable growth in gut microbiota-based interventions and greater understanding of cardiovascular programming, we expect that translating this emerging body of evidence into clinical practice is a future strategy that could reduce the global burden of CVD.
Author Contributions
Conceptualization, C.-N.H. and Y.-L.T.; data curation, C.-N.H., C.-Y.H., W.-H.H. and Y.-L.T.; funding acquisition, Y.-L.T.; project administration, C.-N.H. and Y.-L.T.; writing—original draft, C.-N.H., C.-Y.H., W.-H.H. and Y.-L.T.; writing—review and editing, C.-N.H., C.-Y.H., W.-H.H. and Y.-L.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Chang Gung Memorial Hospital, Kaohsiung, Taiwan, grants CMRPG8J0253, CORPG8J0121, CORPG8L0121, CORPG8L0261, and CORPG8L0301.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data will be available upon request.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Health Organization The Top 10 Causes of Death. [(accessed on 9 December 2020)];2020 Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death.
- 2.Blackmore H.L., Ozanne S.E. Programming of cardiovascular disease across the life-course. J. Mol. Cell. Cardiol. 2015;83:122–130. doi: 10.1016/j.yjmcc.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 3.Hanson M., Gluckman P. Developmental origins of noncommunicable disease: Population and public health implications. Am. J. Clin. Nutr. 2011;94:1754S–1758S. doi: 10.3945/ajcn.110.001206. [DOI] [PubMed] [Google Scholar]
- 4.Roseboom T., de Rooij S., Painter R. The Dutch famine and its long-term consequences for adult health. Early Hum. Dev. 2006;82:485–491. doi: 10.1016/j.earlhumdev.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 5.Luyckx V.A., Bertram J.F., Brenner B.M., Fall C., Hoy W.E., Ozanne S.E., Vikse B.E. Effect of fetal and child health on kidney development and long-term risk of hypertension and kidney disease. Lancet. 2013;382:273–283. doi: 10.1016/S0140-6736(13)60311-6. [DOI] [PubMed] [Google Scholar]
- 6.Perak A.M., Lancki N., Kuang A., Labarthe D.R., Allen N.B., Shah S.H., Lowe L.P., Grobman W.A., Lawrence J.M., Lloyd-Jones D.M., et al. HAPO Follow-up study cooperative research group. Associations of maternal cardiovascular health in pregnancy with offspring cardiovascular health in early adolescence. JAMA. 2021;325:658–668. doi: 10.1001/jama.2021.0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scarmozzino F., Poli A., Visioli F. Microbiota and cardiovascular disease risk: A scoping review. Pharmacol. Res. 2020;159:104952. doi: 10.1016/j.phrs.2020.104952. [DOI] [PubMed] [Google Scholar]
- 8.Tang W.W., Kitai T., Hazen S.L. Gut microbiota in cardiovascular health and disease. Circ. Res. 2017;120:1183–1196. doi: 10.1161/CIRCRESAHA.117.309715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tang W.H.W., Bäckhed F., Landmesser U., Hazen S.L. Intestinal microbiota in cardiovascular health and disease: JACC State-of-the-Art review. J. Am. Coll. Cardiol. 2019;73:2089–2105. doi: 10.1016/j.jacc.2019.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khodor S.A., Reichert B., Shatat I.F. The microbiome and blood pressure: Can microbes regulate our blood pressure? Front. Pediatr. 2017;5:138. doi: 10.3389/fped.2017.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang T., Richards E.M., Pepine C.J., Raizada M.K. The gut microbiota and the brain-gut-kidney axis in hypertension and chronic kidney disease. Nat. Rev. Nephrol. 2018;14:442–456. doi: 10.1038/s41581-018-0018-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heianza Y., Ma W., Manson J.E., Rexrode K.M., Qi L. Gut microbiota metabolites and risk of major adverse cardiovascular disease events and death: A systematic review and meta-analysis of prospective studies. J. Am. Heart Assoc. 2017;6:e004947. doi: 10.1161/JAHA.116.004947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chu D.M., Meyer K.M., Prince A.L., Aagaard K.M. Impact of maternal nutrition in pregnancy and lactation on offspring gut microbial composition and function. Gut Microbes. 2016;7:459–470. doi: 10.1080/19490976.2016.1241357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tain Y.L., Joles J.A. Reprogramming: A preventive strategy in hypertension focusing on the kidney. Int. J. Mol. Sci. 2015;17:23. doi: 10.3390/ijms17010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ettinger G., MacDonald K., Reid G., Burton J.P. The influence of the human microbiome and probiotics on cardiovascular health. Gut Microbes. 2014;5:719–728. doi: 10.4161/19490976.2014.983775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harkins C.P., Kong H.H., Segre J.A. Manipulating the Human Microbiome to Manage Disease. JAMA. 2020;323:303–304. doi: 10.1001/jama.2019.19602. [DOI] [PubMed] [Google Scholar]
- 17.Oniszczuk A., Oniszczuk T., Gancarz M., Szymańska J. Role of gut microbiota, probiotics and prebiotics in the cardiovascular diseases. Molecules. 2021;26:1172. doi: 10.3390/molecules26041172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hult M., Tornhammar P., Ueda P., Chima C., Bonamy A.-K.E., Ozumba B., Norman M. Hypertension, diabetes and overweight: Looming legacies of the biafran famine. PLoS ONE. 2010;5:e13582. doi: 10.1371/journal.pone.0013582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li C., Lumey L.H. Exposure to the Chinese famine of 1959-61 in early life and long-term health conditions: A systematic review and meta-analysis. Int. J. Epidemiol. 2017;46:1157–1170. doi: 10.1093/ije/dyx013. [DOI] [PubMed] [Google Scholar]
- 20.Thornburg K.L. The programming of cardiovascular disease. J. Dev. Orig. Health Dis. 2015;6:366–376. doi: 10.1017/S2040174415001300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Santos M.S., Joles J.A. Early determinants of cardiovascular disease. Best Pract. Res. Clin. Endocrinol. Metab. 2012;26:581–597. doi: 10.1016/j.beem.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 22.Tain Y.L., Hsu C.N. Interplay between oxidative stress and nutrient sensing signaling in the developmental origins of cardiovascular disease. Int. J. Mol. Sci. 2017;18:841. doi: 10.3390/ijms18040841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dabelea D. The predisposition to obesity and diabetes in offspring of diabetic mothers. Diabetes Care. 2007;30(Suppl. 2):S169–S174. doi: 10.2337/dc07-s211. [DOI] [PubMed] [Google Scholar]
- 24.Dalziel S.R., Walker N.K., Parag V., Mantell C., Rea H.H., Rodgers A., Harding J.E. Cardiovascular risk factors after antenatal exposure to betamethasone: 30-year follow-up of a randomised controlled trial. Lancet. 2005;365:1856–1862. doi: 10.1016/S0140-6736(05)66617-2. [DOI] [PubMed] [Google Scholar]
- 25.Antonucci R., Zaffanello M., Puxeddu E., Porcella A., Cuzzolin L., Pilloni M.D., Fanos V. Use of non-steroidal anti-inflammatory drugs in pregnancy: Impact on the fetus and newborn. Curr. Drug Metab. 2012;13:474–490. doi: 10.2174/138920012800166607. [DOI] [PubMed] [Google Scholar]
- 26.Hrudey E.J., Reynolds R.M., Oostvogels A.J.J.M., Brouwerv I.A., Vrijkotte T. The association between maternal 25-hydroxyvitamin D concentration during gestation and early childhood cardio-metabolic outcomes: Is there interaction with pre-pregnancy bmi? PLoS ONE. 2015;10:e0133313. doi: 10.1371/journal.pone.0133313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fraser A., Nelson S.M., Macdonald-Wallis C., Sattar N., Lawlor D. Hypertensive disorders of pregnancy and cardiometabolic health in adolescent offspring. Hypertension. 2013;62:614–620. doi: 10.1161/HYPERTENSIONAHA.113.01513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hosaka M., Asayama K., Staessen J.A., Ohkubo T., Hayashi K., Tatsuta N., Kurokawa N., Satoh M., Hashimoto T., Hirose T., et al. Breastfeeding leads to lower blood pressure in 7-year-old Japanese children: Tohoku study of child development. Hypertens. Res. 2012;36:117–122. doi: 10.1038/hr.2012.128. [DOI] [PubMed] [Google Scholar]
- 29.Keijzer-Veen M.G., Finken M.J.J., Nauta J., Dekker F.W., Hille E.T., Frölich M., Wit J.M., Van Der Heijden A. Is blood pressure increased 19 years after intrauterine growth restriction and preterm birth? A prospective follow-up study in the Netherlands. Pediatrics. 2005;116:725–731. doi: 10.1542/peds.2005-0309. [DOI] [PubMed] [Google Scholar]
- 30.Tang-Peronard J.L., Andersen H.R., Jensen T.K., Heitmann B.L. Endocrine-disrupting chemicals and obesity development in humans: A review. Obes. Rev. 2011;12:622–636. doi: 10.1111/j.1467-789X.2011.00871.x. [DOI] [PubMed] [Google Scholar]
- 31.Barker D.J., Winter P.D., Osmond C., Margetts B., Simmonds S.J. Weight in infancy and death from ischaemic heart disease. Lancet. 1989;2:577–580. doi: 10.1016/S0140-6736(89)90710-1. [DOI] [PubMed] [Google Scholar]
- 32.Halvorsen C.P., Andolf E., Hu J., Pilo C., Winbladh B., Norman M. Discordant twin growth in utero and differences in blood pressure and endothelial function at 8 years of age. J. Intern. Med. 2006;259:155–163. doi: 10.1111/j.1365-2796.2005.01593.x. [DOI] [PubMed] [Google Scholar]
- 33.Vågerö D., Leon D.A. Ischaemic heart disease and low birth weight: A test of the fetal-origins hypothesis from the Swedish Twin Registry. Lancet. 1994;343:260–263. doi: 10.1016/S0140-6736(94)91112-6. [DOI] [PubMed] [Google Scholar]
- 34.Hsu C.-N., Lin Y.-J., Hou C.-Y., Tain Y.-L. Maternal administration of probiotic or prebiotic prevents male adult rat offspring against developmental programming of hypertension induced by high fructose consumption in pregnancy and lactation. Nutrients. 2018;10:1229. doi: 10.3390/nu10091229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hsu C.N., Chang-Chien G.P., Lin S., Hou C.Y., Tain Y.L. Targeting on gut microbial metabolite trimethylamine-N-Oxide and short-chain fatty acid to prevent maternal high-fructose-diet-induced developmental programming of hypertension in adult male offspring. Mol. Nutr. Food Res. 2019;63:e1900073. doi: 10.1002/mnfr.201900073. [DOI] [PubMed] [Google Scholar]
- 36.Tain Y.L., Lee W.C., Wu K.L.H., Leu S., Chan J.Y.H. Resveratrol prevents the development of hypertension programmed by maternal plus post-weaning high-fructose consumption through modulation of oxidative stress, nutrient-sensing signals, and gut microbiota. Mol. Nutr. Food Res. 2018;62:e1800066. doi: 10.1002/mnfr.201800066. [DOI] [PubMed] [Google Scholar]
- 37.Hsu C.N., Chan J.Y.H., Yu H.R., Lee W.C., Wu K.L.H., Chang-Chien G.P., Lin S., Hou C.Y., Tain Y.L. Targeting on gut microbiota-derived metabolite trimethylamine to protect adult male rat offspring against hypertension programmed by combined maternal high-fructose intake and dioxin exposure. Int. J. Mol. Sci. 2020;21:5488. doi: 10.3390/ijms21155488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hsu C.N., Hou C.Y., Chang-Chien G.P., Lin S., Yang H.W., Tain Y.L. Perinatal resveratrol therapy prevents hypertension programmed by maternal chronic kidney disease in adult male offspring: Implications of the gut microbiome and their metabolites. Biomedicines. 2020;8:567. doi: 10.3390/biomedicines8120567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hsu C.N., Chan J.Y.H., Wu K.L.H., Yu H.R., Lee W.C., Hou C.Y., Tain Y.L. Altered gut microbiota and its metabolites in hypertension of developmental origins: Exploring differences between fructose and antibiotics exposure. Int. J. Mol. Sci. 2021;22:2674. doi: 10.3390/ijms22052674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hsu C.N., Hou C.Y., Chang-Chien G.P., Lin S., Chan J.Y.H., Lee C.T., Tain Y.L. Maternal resveratrol therapy protected adult rat offspring against hypertension programmed by combined exposures to asymmetric dimethylarginine and trimethylamine-N-oxide. J. Nutr. Biochem. 2021;93:108630. doi: 10.1016/j.jnutbio.2021.108630. [DOI] [PubMed] [Google Scholar]
- 41.Hsu C.N., Hou C.Y., Chang-Chien G.P., Lin S., Tain Y.L. Maternal N-acetylcysteine therapy prevents hypertension in spontaneously hypertensive rat offspring: Implications of hydrogen sulfide-generating pathway and gut microbiota. Antioxidants. 2020;9:856. doi: 10.3390/antiox9090856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li H.B., Yang T., Richards E.M., Pepine C.J., Raizada M.K. Maternal Treatment with captopril persistently alters gut-brain communication and attenuates hypertension of male offspring. Hypertension. 2020;75:1315–1324. doi: 10.1161/HYPERTENSIONAHA.120.14736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang M., Zhang Y., Miller D., Rehman N.O., Cheng X., Yeo J.Y., Joe B., Hill J.W. Microbial reconstitution reverses early female puberty induced by maternal high-fat diet during lactation. Endocrinology. 2020;161:bqz041. doi: 10.1210/endocr/bqz041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wankhade U.D., Zhong Y., Kang P., Alfaro M., Chintapalli S.V., Thakali K.M., Shankar K. Enhanced offspring predisposition to steatohepatitis with maternal high-fat diet is associated with epigenetic and microbiome alterations. PLoS ONE. 2017;12:e0175675. doi: 10.1371/journal.pone.0175675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guimarães K.S.L., Braga V.A., Noronha S.I.S.R., Costa W.K.A.D., Makki K., Cruz J.C., Brandão L.R., Chianca Junior D.A., Meugnier E., Leulier F., et al. Lactiplantibacillus plantarum WJL administration during pregnancy and lactation improves lipid profile, insulin sensitivity and gut microbiota diversity in dyslipidemic dams and protects male offspring against cardiovascular dysfunction in later life. Food Funct. 2020;11:8939–8950. doi: 10.1039/D0FO01718C. [DOI] [PubMed] [Google Scholar]
- 46.Hsu C.N., Hou C.Y., Lee C.T., Chan J.Y.H., Tain Y.L. The Interplay between maternal and post-weaning high-fat diet and gut microbiota in the developmental programming of hypertension. Nutrients. 2019;11:1982. doi: 10.3390/nu11091982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hsu C.N., Hou C., Chan J.Y.H., Lee C.T., Tain Y.L. Hypertension programmed by perinatal high-fat diet: Effect of maternal gut microbiota-targeted therapy. Nutrients. 2019;11:2908. doi: 10.3390/nu11122908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen H.E., Lin Y.J., Lin I.C., Yu H.R., Sheen J.M., Tsai C.C., Huang L.T., Tain Y.L. Resveratrol prevents combined prenatal NG-nitro-L-arginine-methyl ester (L-NAME) treatment plus postnatal high-fat diet induced programmed hypertension in adult rat offspring: Interplay between nutrient-sensing signals, oxidative stress and gut microbiota. J. Nutr. Biochem. 2019;70:28–37. doi: 10.1016/j.jnutbio.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 49.Friedman J.E., Dobrinskikh E., Alfonso-Garcia A., Fast A., Janssen R.C., Soderborg T.K., Anderson A.L., Reisz J.A., D’Alessandro A., Frank D.N., et al. Pyrroloquinoline quinone prevents developmental programming of microbial dysbiosis and macrophage polarization to attenuate liver fibrosis in offspring of obese mice. Hepatol. Commun. 2018;2:313–328. doi: 10.1002/hep4.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.de Oliveira Y., Cavalcante R.G.S., Cavalcanti Neto M.P., Magnani M., Braga V.A., de Souza E.L., de Brito Alves J.L. Oral administration of Lactobacillus fermentum post-weaning improves the lipid profile and autonomic dysfunction in rat offspring exposed to maternal dyslipidemia. Food Funct. 2020;11:5581–5594. doi: 10.1039/D0FO00514B. [DOI] [PubMed] [Google Scholar]
- 51.Sherman S.B., Sarsour N., Salehi M., Schroering A., Mell B., Joe B., Hill J.W. Prenatal androgen exposure causes hypertension and gut microbiota dysbiosis. Gut Microbes. 2018;9:400–421. doi: 10.1080/19490976.2018.1441664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Marzullo P., Di Renzo L., Pugliese G., De Siena M., Barrea L., Muscogiuri G., Colao A., Savastano S. Obesity Programs of nutrition, Education, Research and Assessment (OPERA) Group. From obesity through gut microbiota to cardiovascular diseases: A dangerous journey. Int. J. Obes. Suppl. 2020;10:35–49. doi: 10.1038/s41367-020-0017-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McMullen S., Mostyn A. Animal models for the study of the developmental origins of health and disease. Proc. Nutr. Soc. 2009;68:306–320. doi: 10.1017/S0029665109001396. [DOI] [PubMed] [Google Scholar]
- 54.Hsu C.N., Tain Y.L. Animal models for DOHaD research: Focus on hypertension of developmental origins. Biomedicines. 2021;9:623. doi: 10.3390/biomedicines9060623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sengupta P. The laboratory rat: Relating its age with human’s. Int. J. Prev. Med. 2013;4:624–630. [PMC free article] [PubMed] [Google Scholar]
- 56.Pluznick J.L. Microbial short-chain fatty acids and blood pressure regulation. Curr. Hypertens. Rep. 2017;19:25. doi: 10.1007/s11906-017-0722-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang T., Santisteban M.M., Rodriguez V., Li E., Ahmari N., Carvajal J.M., Zadeh M., Gong M., Qi Y., Zubcevic J., et al. Gut dysbiosis is linked to hypertension. Hypertension. 2015;65:1331–1340. doi: 10.1161/HYPERTENSIONAHA.115.05315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pluznick J.L., Protzko R.J., Gevorgyan H., Peterlin Z., Sipos A., Han J., Brunet I., Wan L.X., Rey F., Wang T., et al. Olfactory receptor responding to gut microbiota-derived signals plays a role in renin secretion and blood pressure regulation. Proc. Natl. Acad. Sci. USA. 2013;110:4410–4415. doi: 10.1073/pnas.1215927110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ratajczak W., Rył A., Mizerski A., Walczakiewicz K., Sipak O., Laszczyńska M. Immunomodulatory potential of gut microbiome-derived short-chain fatty acids (SCFAs) Acta Biochim. Pol. 2019;66:1–12. doi: 10.18388/abp.2018_2648. [DOI] [PubMed] [Google Scholar]
- 60.Hsu C.N., Hou C.Y., Chang-Chien G.P., Lin S., Tain Y.L. Maternal garlic oil supplementation prevents high-fat diet-induced hypertension in adult rat offspring: Implications of H2S-generating pathway in the gut and kidneys. Mol. Nutr. Food Res. 2021:e2001116. doi: 10.1002/mnfr.202001116. [DOI] [PubMed] [Google Scholar]
- 61.Marques F.Z., Nelson E., Chu P.-Y., Horlock D., Fiedler A., Ziemann M., Tan J.K., Kuruppu S., Rajapakse N.W., El-Osta A., et al. High-fiber diet and acetate supplementation change the gut microbiota and prevent the development of hypertension and heart failure in hypertensive mice. Circulation. 2017;135:964–977. doi: 10.1161/CIRCULATIONAHA.116.024545. [DOI] [PubMed] [Google Scholar]
- 62.Schiattarella G.G., Sannino A., Toscano E., Giugliano G., Gargiulo G., Franzone A., Trimarco B., Esposito G., Perrino C. Gut microbe-generated metabolite trimethylamine-N-oxide as cardiovascular risk biomarker: A systematic review and dose-response meta-analysis. Eur. Heart J. 2017;38:2948–2956. doi: 10.1093/eurheartj/ehx342. [DOI] [PubMed] [Google Scholar]
- 63.Wang Z., Zhao Y. Gut microbiota derived metabolites in cardiovascular health and disease. Protein Cell. 2018;9:416–431. doi: 10.1007/s13238-018-0549-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Seldin M.M., Meng Y., Qi H., Zhu W., Wang Z., Hazen S.L., Lusis A.J., Shih D.M. Trimethylamine N-Oxide promotes vascular inflammation through signaling of mitogen-activated protein kinase and nuclear factor-κB. J. Am. Heart Assoc. 2016;5:e002767. doi: 10.1161/JAHA.115.002767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Velasquez M.T., Ramezani A., Manal A., Raj D.S. Trimethylamine N-Oxide: The good, the bad and the unknown. Toxins. 2016;8:326. doi: 10.3390/toxins8110326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Velasquez M.T., Centron P., Barrows I., Dwivedi R., Raj D.S. Gut Microbiota and cardiovascular uremic toxicities. Toxins. 2018;10:287. doi: 10.3390/toxins10070287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hsu C.N., Tain Y.L. Developmental programming and reprogramming of hypertension and kidney disease: Impact of tryptophan metabolism. Int. J. Mol. Sci. 2020;21:8705. doi: 10.3390/ijms21228705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sallée M., Dou L., Cerini C., Poitevin S., Brunet P., Burtey S. The aryl hydrocarbon receptor-activating effect of uremic toxins from tryptophan metabolism: A new concept to understand cardiovascular complications of chronic kidney disease. Toxins. 2014;6:934–949. doi: 10.3390/toxins6030934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Leong S.C., Sirich T.L. Indoxyl sulfate-review of toxicity and therapeutic strategies. Toxins. 2016;8:358. doi: 10.3390/toxins8120358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kamiński T.W., Pawlak K., Karbowska M., Myśliwiec M., Pawlak D. Indoxyl sulfate-the uremic toxin linking hemostatic system disturbances with the prevalence of cardiovascular disease in patients with chronic kidney disease. BMC Nephrol. 2017;18:35. doi: 10.1186/s12882-017-0457-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hung S.C., Kuo K.L., Wu C.C., Tarng D.C. Indoxyl sulfate: A novel cardiovascular risk factor in chronic kidney disease. J. Am. Heart Assoc. 2017;6:e005022. doi: 10.1161/JAHA.116.005022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hubbard T.D., Murray I.A., Perdew G.H. Indole and tryptophan metabolism: Endogenous and dietary routes to ah receptor activation. Drug Metab. Dispos. 2015;43:1522–1535. doi: 10.1124/dmd.115.064246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang N. The role of endogenous aryl hydrocarbon receptor signaling in cardiovascular physiology. J. Cardiovasc. Dis. Res. 2011;2:91–95. doi: 10.4103/0975-3583.83033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wilck N., Matus M.G., Kearney S.M., Olesen S.W., Forslund K., Bartolomaeus H., Haase S., Mähler A., Balogh A., Markó L., et al. Salt-responsive gut commensal modulates TH17 axis and disease. Nature. 2017;551:585–589. doi: 10.1038/nature24628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Brito J.S., Borges N.A., Esgalhado M., Magliano D.C., Soulage C.O., Mafra D. Aryl hydrocarbon receptor activation in chronic kidney disease: Role of uremic toxins. Nephron. 2017;137:1–7. doi: 10.1159/000476074. [DOI] [PubMed] [Google Scholar]
- 76.Ren J., Crowley S.D. Role of T-cell activation in salt-sensitive hypertension. Am. J. Physiol. Heart Circ. Physiol. 2019;316:H1345–H1353. doi: 10.1152/ajpheart.00096.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hsu C.N., Lin I.C., Yu H.R., Huang L.T., Tiao M.M., Tain Y.L. Maternal tryptophan supplementation protects adult rat offspring against hypertension programmed by maternal chronic kidney disease: Implication of tryptophan-metabolizing microbiome and aryl hydrocarbon receptor. Int. J. Mol. Sci. 2020;21:4552. doi: 10.3390/ijms21124552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hsu C.N., Lin Y.J., Tain Y.L. Maternal exposure to bisphenol a combined with high-fat diet-induced programmed hypertension in adult male rat offspring: Effects of resveratrol. Int. J. Mol. Sci. 2019;20:4382. doi: 10.3390/ijms20184382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hsu C.N., Lin Y.J., Lu P.C., Tain Y.L. Maternal resveratrol therapy protects male rat offspring against programmed hypertension induced by tcdd and dexamethasone exposures: Is it relevant to aryl hydrocarbon receptor? Int. J. Mol. Sci. 2018;19:2459. doi: 10.3390/ijms19082459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kroll M.H. The AHR: Adaptive evolution or one-off? Blood. 2019;134:2337–2338. doi: 10.1182/blood.2019003943. [DOI] [PubMed] [Google Scholar]
- 81.Karbowska M., Kaminski T.W., Znorko B., Domaniewski T., Misztal T., Rusak T., Pryczynicz A., Guzinska-Ustymowicz K., Pawlak K., Pawlak D. Indoxyl sulfate promotes arterial thrombosis in rat model via increased levels of complex TF/VII, PAI-1, platelet activation as well as decreased contents of SIRT1 and SIRT3. Front. Physiol. 2018;9:1623. doi: 10.3389/fphys.2018.01623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nagareddy P., Smyth S.S. Inflammation and thrombosis in cardiovascular disease. Curr. Opin. Hematol. 2013;20:457–463. doi: 10.1097/MOH.0b013e328364219d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gryp T., Vanholder R., Vaneechoutte M., Glorieux G. p-Cresyl Sulfate. Toxins. 2017;9:52. doi: 10.3390/toxins9020052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jing Y.J., Ni J.W., Ding F.H., Fang Y.H., Wang X.Q., Wang H.B., Chen X.N., Chen N., Zhan W.W., Lu L., et al. p-Cresyl sulfate is associated with carotid arteriosclerosis in hemodialysis patients and promotes atherogenesis in apoE-/- mice. Kidney Int. 2016;89:439–449. doi: 10.1038/ki.2015.287. [DOI] [PubMed] [Google Scholar]
- 85.Paz Ocaranza M., Riquelme J.A., García L., Jalil J.E., Chiong M., Santos R.A.S., Lavandero S. Counter-regulatory renin-angiotensin system in cardiovascular disease. Nat. Rev. Cardiol. 2020;17:116–129. doi: 10.1038/s41569-019-0244-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hsu C.-N., Tain Y.-L. Targeting the renin–angiotensin–aldosterone system to prevent hypertension and kidney disease of developmental origins. Int. J. Mol. Sci. 2021;22:2298. doi: 10.3390/ijms22052298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hsu C.N., Wu K.L., Lee W.C., Leu S., Chan J.Y., Tain Y.L. Aliskiren administration during early postnatal life sex-specifically alleviates hypertension programmed by maternal high fructose consumption. Front. Physiol. 2016;7:299. doi: 10.3389/fphys.2016.00299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Manning J., Vehaskari V.M. Postnatal modulation of prenatally programmed hypertension by dietary Na and ACE inhibition. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005;288:R80–R84. doi: 10.1152/ajpregu.00309.2004. [DOI] [PubMed] [Google Scholar]
- 89.Sherman R.C., Langley-Evans S.C. Early administration of angiotensin-converting enzyme inhibitor captopril prevents the development of hypertension programmed by intrauterine exposure to a maternal low-protein diet in the rat. Clin. Sci. (Lond.) 1998;94:373–381. doi: 10.1042/cs0940373. [DOI] [PubMed] [Google Scholar]
- 90.Forrester S.J., Booz G.W., Sigmund C.D., Coffman T.M., Kawai T., Rizzo V., Scalia R., Eguchi S. Angiotensin II Signal Transduction: An Update on Mechanisms of Physiology and Pathophysiology. Physiol. Rev. 2018;98:1627–1738. doi: 10.1152/physrev.00038.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Oliveira Andrade J.M., de Farias Lelis D., Mafra V., Cota J. The angiotensin converting enzyme 2 (ACE2), gut microbiota, and cardiovascular health. Protein Pept. Lett. 2017;24:827–832. doi: 10.2174/0929866524666170728145333. [DOI] [PubMed] [Google Scholar]
- 92.Bessa A.S.M., Jesus É.F., Nunes A.D.C., Pontes C.N.R., Lacerda I.S., Costa J.M., Souza E.J., Lino-Júnior R.S., Biancardi M.F., Dos Santos F.C.A., et al. Stimulation of the ACE2/Ang-(1-7)/Mas axis in hypertensive pregnant rats attenuates cardiovascular dysfunction in adult male offspring. Hypertens. Res. 2019;42:1883–1893. doi: 10.1038/s41440-019-0321-8. [DOI] [PubMed] [Google Scholar]
- 93.Rubak Y.T., Nuraida L., Iswantini D., Prangdimurti E. Angiotensin-I-converting enzyme inhibitory peptides in milk fermented by indigenous lactic acid bacteria. Vet. World. 2020;13:345–353. doi: 10.14202/vetworld.2020.345-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Richards E.M., Pepine C.J., Raizada M.K., Kim S. The gut, its microbiome, and hypertension. Curr. Hypertens. Rep. 2017;19:36. doi: 10.1007/s11906-017-0734-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Thushara R.M., Gangadaran S., Solati Z., Moghadasian M.H. Cardiovascular benefits of probiotics: A review of experimental and clinical studies. Food Funct. 2016;7:632–642. doi: 10.1039/C5FO01190F. [DOI] [PubMed] [Google Scholar]
- 96.Barengolts E. Gut microbiota, prebiotics, probiotics, and synbiotics in management of obesity and prediabetes: Review of randomized controlled trials. Endocr. Pract. 2016;22:1224–1234. doi: 10.4158/EP151157.RA. [DOI] [PubMed] [Google Scholar]
- 97.Pandey K.R., Naik S.R., Vakil B.V. Probiotics, prebiotics and synbiotics-A review. J. Food Sci. Technol. 2015;52:7577–7587. doi: 10.1007/s13197-015-1921-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Leshem A., Horesh N., Elinav E. Fecal microbial transplantation and its potential application in cardiometabolic syndrome. Front. Immunol. 2019;10:1341. doi: 10.3389/fimmu.2019.01341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Żółkiewicz J., Marzec A., Ruszczyński M., Feleszko W. Postbiotics-A step beyond pre- and probiotics. Nutrients. 2020;12:2189. doi: 10.3390/nu12082189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sanaka T., Sugino N., Teraoka S., Ota K. Therapeutic effects of oral sorbent in undialyzed uremia. Am. J. Kidney Dis. 1988;12:97–103. doi: 10.1016/S0272-6386(88)80002-7. [DOI] [PubMed] [Google Scholar]
- 101.Lee C.T., Hsu C.Y., Tain Y.L., Ng H.Y., Cheng B.C., Yang C.C., Wu C.H., Chiou T.T., Lee Y.T., Liao S.C. Effects of AST-120 on blood concentrations of protein-bound uremic toxins and biomarkers of cardiovascular risk in chronic dialysis patients. Blood Purif. 2014;37:76–83. doi: 10.1159/000357641. [DOI] [PubMed] [Google Scholar]
- 102.Toyoda S., Hashimoto R., Tezuka T., Sakuma M., Abe S., Ishikawa T., Taguchi I., Inoue T. Antioxidative effect of an oral adsorbent, AST-120, and long-term outcomes in chronic kidney disease patients with cardiovascular disease. Hypertens. Res. 2020;43:1128–1131. doi: 10.1038/s41440-020-0465-6. [DOI] [PubMed] [Google Scholar]
- 103.Gomez Arango L.F., Barrett H.L., Callaway L.K., Nitert M.D. Probiotics and pregnancy. Curr. Diabet. Rep. 2015;15:567. doi: 10.1007/s11892-014-0567-0. [DOI] [PubMed] [Google Scholar]
- 104.Brantsaeter A.L., Myhre R., Haugen M., Myking S., Sengpiel V., Magnus P., Jacobsson B., Meltzer H.M. Intake of probiotic food and risk of preeclampsia in primiparous women: The norwegian mother and child cohort study. Am. J. Epidemiol. 2011;174:807–815. doi: 10.1093/aje/kwr168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Luoto R., Laitinen K., Nermes M., Isolauri E. Impact of maternal probiotic-supplemented dietary counselling on pregnancy outcome and prenatal and postnatal growth: A double-blind, placebo controlled study. Br. J. Nutr. 2010;103:1792–1799. doi: 10.1017/S0007114509993898. [DOI] [PubMed] [Google Scholar]
- 106.Vitali B., Cruciani F., Baldassarre M.E., Capursi T., Spisni E., Valerii M.C., Candela M., Turroni S., Brigidi P. Dietary supplementation with probiotics during late pregnancy: Outcome on vaginal microbiota and cytokine secretion. BMC Microbiol. 2012;12:236. doi: 10.1186/1471-2180-12-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Othman M., Neilson J.P., Alfirevic Z. Probiotics for preventing preterm labour. Cochrane Database Syst. Rev. 2007;1:CD005941. doi: 10.1002/14651858.CD005941.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jinno S., Toshimitsu T., Nakamura Y., Kubota T., Igoshi Y., Ozawa N., Suzuki S., Nakano T., Morita Y., Arima T., et al. Maternal prebiotic ingestion increased the number of fecal bifidobacteria in pregnant women but not in their neonates aged one month. Nutrients. 2017;9:196. doi: 10.3390/nu9030196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cheng J., Ringel-Kulka T., Heikamp-de Jong I., Ringel Y., Carroll I., de Vos W.M., Salojärvi J., Satokari R. Discordant temporal development of bacterial phyla and the emergence of core in the fecal microbiota of young children. ISME J. 2016;10:1002–1014. doi: 10.1038/ismej.2015.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bertelsen R.J., Jensen E.T., Ringel-Kulka T. Use of probiotics and prebiotics in infant feeding. Best Pract. Res. Clin. Gastroenterol. 2016;30:39–48. doi: 10.1016/j.bpg.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 111.Moossavi S., Miliku K., Sepehri S., Khafipour E., Azad M.B. The prebiotic and probiotic properties of human milk: Implications for infant immune development and pediatric asthma. Front. Pediatr. 2018;6:197. doi: 10.3389/fped.2018.00197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Paul H.A., Collins K.H., Nicolucci A.C., Urbanski S.J., Hart D.A., Vogel H.J., Reimer R.A. Maternal prebiotic supplementation reduces fatty liver development in offspring through altered microbial and metabolomic profiles in rats. FASEB J. 2019;33:5153–5167. doi: 10.1096/fj.201801551R. [DOI] [PubMed] [Google Scholar]
- 113.Gray C., Vickers M.H., Segovia S.A., Zhang X.D., Reynolds C.M. A maternal high fat diet programmes endothelial function and cardiovascular status in adult male offspring independent of body weight, which is reversed by maternal conjugated linoleic acid (CLA) supplementation. PLoS ONE. 2015;10:e0115994. doi: 10.1371/journal.pone.0115994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ashwini A., Ramya H.N., Ramkumar C., Reddy K.R., Kulkarni R.V., Abinaya V., Naveen S., Raghu A.V. Reactive mechanism and the applications of bioactive prebiotics for human health: Review. J. Microbiol. Methods. 2019;159:128–137. doi: 10.1016/j.mimet.2019.02.019. [DOI] [PubMed] [Google Scholar]
- 115.Wegh C.A.M., Geerlings S.Y., Knol J., Roeselers G., Belzer C. Postbiotics and their potential applications in early life nutrition and beyond. Int. J. Mol. Sci. 2019;20:4673. doi: 10.3390/ijms20194673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Robles-Vera I., Toral M., de la Visitación N., Sánchez M., Gómez-Guzmán M., Romero M., Yang T., Izquierdo-Garcia J.L., Jiménez R., Ruiz-Cabello J., et al. Probiotics prevent dysbiosis and the rise in blood pressure in genetic hypertension: Role of short-chain fatty acids. Mol. Nutr. Food Res. 2020;64:e1900616. doi: 10.1002/mnfr.201900616. [DOI] [PubMed] [Google Scholar]
- 117.Bartolomaeus H., Balogh A., Yakoub M., Homann S., Markó L., Höges S., Tsvetkov D., Krannich A., Wundersitz S., Avery E.G., et al. Short-chain fatty acid propionate protects from hypertensive cardiovascular damage. Circulation. 2019;139:1407–1421. doi: 10.1161/CIRCULATIONAHA.118.036652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gorissen L., De Vuyst L., Raes K., De Smet S., Leroy F. Conjugated linoleic and linolenic acid production kinetics by bifidobacteria differ among strains. Int. J. Food Microbiol. 2012;155:234–240. doi: 10.1016/j.ijfoodmicro.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 119.Bird J.K., Raederstorff D., Weber P., Steinert R.E. Cardiovascular and antiobesity effects of resveratrol mediated through the gut microbiota. Adv. Nutr. 2017;8:839–849. doi: 10.3945/an.117.016568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Song J.Y., Shen T.C., Hou Y.C., Chang J.F., Lu C.L., Liu W.C., Chen P.J., Chen B.H., Zheng C.M., Lu K.C. Influence of resveratrol on the cardiovascular health effects of chronic kidney disease. Int. J. Mol. Sci. 2020;21:6294. doi: 10.3390/ijms21176294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hsu C.N., Hou C.Y., Tain Y.L. Preventive aspects of early resveratrol supplementation in cardiovascular and kidney disease of developmental origins. Int. J. Mol. Sci. 2021;22:4210. doi: 10.3390/ijms22084210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gibson G.R., Hutkins R., Sanders M.E., Prescott S.L., Reimer R.A., Salminen S.J., Scott K., Stanton C., Swanson K.S., Cani P.D., et al. Expert consensus document: The international scientific association for probiotics and prebiotics (isapp) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Amp. Hepatol. 2017;14:491. doi: 10.1038/nrgastro.2017.75. [DOI] [PubMed] [Google Scholar]
- 123.Food and Agriculture Organization of the United Nations/World Health Organization (FAO/WHO) Joint Fao/Who Working Group on Drafting Guidelines for the Evaluation of Probiotics in Food. WHO; London, ON, Canada: 2002. Guidelines for the Evaluation of Probiotics in Food. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be available upon request.