Abstract
Patients suffering from critical illness have host inflammatory responses against injuries, such as infection and trauma, that can lead to tissue damage, organ failure, and death. Modulation of host immune response as well as infection and damage control are detrimental factors in the management of systemic inflammation. The gut is the motor of multiple organ failure following injury, and it is recognized that gut dysfunction is one of the causative factors of disease progression. The gut microbiota has a role in maintaining host immunity, and disruption of the gut microbiota might induce an immunosuppressive condition in critically ill patients. Treatment with probiotics and synbiotics has been reported to attenuate systemic inflammation by maintaining gut microbiota and to reduce postoperative infectious complications and ventilator-associated pneumonia. The administration of prophylactic probiotics/synbiotics could be an important treatment option for preventing infectious complications and modulating immunity. Further basic and clinical research is needed to promote intestinal therapies for critically ill patients.
Keywords: microbiota, gut, ICU, immune, ventilator, inflammation, probiotics, prebiotics, synbiotics, critically
1. Introduction
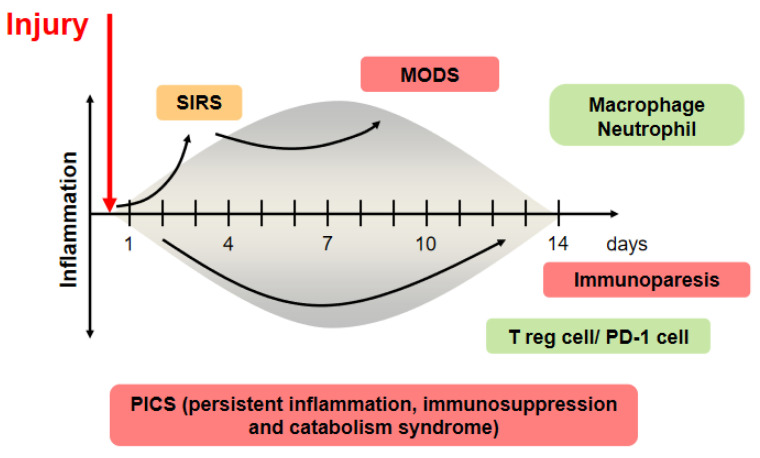
Patients suffering from critical illness can have a life-threatening host response to injuries, such as infection, trauma, burn, and cardiac arrest, that can lead to tissue damage, organ failure, and death. Sepsis management is a major challenge for healthcare systems throughout the world. About 50 million patients with sepsis were reported, with about 20% of all global deaths [1]. Trauma is the leading cause of death of children, adolescents, and younger adults [2]. These responses to injury affect cells and mediators of the innate and adaptive immune systems against pathogen-associated pattern molecules or damage-associated pattern molecules and have been referred to as systemic inflammatory response syndrome (SIRS) and compensatory anti-inflammatory response syndrome [3]. In contrast, prolonged SIRS suppresses Th1-type immune reactivity, and it also increases Th2-type cytokine production and activity of regulatory T cells. These conditions can easily progress to persistent inflammation, immunosuppression, and catabolism syndrome (PICS), which results in multiple immunologic and physiologic defects that are difficult to survive [4]. Modulation of host response against injury has been an important topic in this field (Figure 1).
Figure 1.
The concept of injury-induced imbalances of the immune system.
Gut dysfunction, including the intestinal epithelium, intestinal immune system, and intestinal microbiota, is one of the causative factors of disease progression [5]. A loss of beneficial microbes, expansion of pathobionts, and loss of diversity are defined as “dysbiosis”, which can change host immunity [6]. Especially, the gut microbiota and microbial metabolites, such as short-chain fatty acids (SCFAs) and trimethylamine N-oxide, are associated with human diseases, such as allergic and immune disorders, cancer, cardiovascular disease, and neurological disorders [7]. This review summarizes how gut microbiota leads to systemic inflammation and intestinal therapy in the critically ill setting.
Once an insult occurs, it causes significant phenotypic changes in the immune system. The injured host develops inflammation following severe injury, which is called systemic inflammatory response syndrome (SIRS). If the SIRS state is prolonged, it progresses to the multiple organ dysfunction syndrome (MODS) stage. Inflammation and immunosuppression coincidentally begin at the onset of sepsis, and persistent inflammation, immunosuppression, and catabolism syndrome (PICS) develops in this concept [8]. This pro-inflammatory state has been shown to be mainly driven by macrophages, with regulatory T cells being the mediators of this compensatory response following injury.
2. Gut Origin of Sepsis and Systemic Inflammation
Injury to the gut has been known to affect systemic organs and the gut itself. The gut is the motor of multiple organ failure [9], and gut dysfunction is known as a causative factor in the progression of diseases such as sepsis, infection, shock, trauma, burn, and bleeding. In 1994, Moore et al. reported that in a rat intestinal reperfusion model, with 45-min occlusion of the supramesenteric artery following intraperitoneal injection of lipopolysaccharide, white blood cells accumulated and permeability in the lung increased, as did lung weight [10]. This result suggested that injury to the gut caused lung inflammation and systemic inflammation. One of the mechanisms is a bacterial translocation. In a mouse burn model, Escherichia coli was identified in the spleen and liver, five minutes after injury [11]. The mechanism has not been elucidated completely, but gut-derived factors were carried in the mesenteric lymph rather than the portal circulation by intestinal lymphatic division in a rat shock model [12]. In the intestinal epithelium, burn increases gut epithelial cell death by apoptosis and permeability [13]. The inhibition of apoptosis could attenuate survival in P. aeruginosa infection mouse models [14]. In addition, intestinal tight junction proteins, such as claudin-5 and occludin, decreased and could change gut permeability following injury in a cecal ligation puncture model [15]. These reports indicated that direct or indirect injury to the gut could cause intestinal damages and bacterial translocation, which lead to an inflammatory response in multiple organs.
In clinical research, bacterial translocation was present in the mesenteric lymph nodes of about 15% of patients with laparotomy [16] and of about 35% of patients with hepatectomy for biliary cancer [17]. Obligate anaerobes, such as Clostridium coccoides and Bacteroides fragilis groups, dominated in the lymph. These results suggest that the gut barrier following injury could allow bacteria and gut-derived mediators into the mesenteric lymph and into the bloodstream and activate a systemic inflammatory response.
3. Gut Microbiota in Critically Ill Patients
Under critically ill conditions, it is difficult to keep healthy gut microbiota because of disease and the various kinds of treatments, such as histamine H2 receptor blocker for bleeding prevention, catecholamines for blood pressure control, broad-spectrum antibiotics for infection control, and mechanical ventilation for respiratory failure. As shown in Table 1, the patients had about 10,000 times lower total anaerobes, including Bifidobacterium and Lactobacillus, and 100 times higher Staphylococcus as compared with those in healthy persons [18]. Total organic acids, acetic acid, and butyric acid derived from gut microbiota were significantly decreased compared with those in healthy persons (Table 2). Especially, butyric acid was almost depleted in the gut in critically ill conditions. Fecal pH was markedly increased in the patients compared with those in healthy persons. These results highlighted the deterioration of the gut microbiota and environment in the process of critical illness. The numbers of total obligate anaerobes and total facultative anaerobes were associated with bacteremia and mortality in patients with critically illness [19].
Table 1.
Fecal flora in patients with severe SIRS.
| SIRS Patients | Normal | |
|---|---|---|
| Total obligate anaerobes | 8.3 ± 2.3 * | 10.5 ± 0.5 |
| Bacteroidaceae | 7.3 ± 3.0 * | 10.1 ± 0.4 |
| Bifidobacterium | 4.8 ± 3.3 * | 9.6 ± 0.7 |
| Clostridium | 2.1 ± 1.0 | 2.1 ± 0.7 |
| Veillonella | 3.1 ± 1.8 * | 7.0 ± 1.2 |
| Total facultative anaerobes | 7.8 ± 1.4 | 7.5 ± 0.4 |
| Lactobacillus | 2.7 ± 1.5 * | 5.0 ± 1.0 |
| Enterobacteriaceae | 4.1 ± 2.7 * | 7.4 ± 0.8 |
| Enterococcus | 6.4 ± 2.5 | 7.0 ± 0.9 |
| Staphylococcus | 5.3 ± 1.7 * | 2.7 ± 0.8 |
| Pseudomonas | 2.8 ± 1.4 * | ND |
| Candida | 2.5 ± 1.0 | 2.0 ± 0.5 |
(* p < 0.05 NS group vs. S group; data are mean ± SE) NS: Non–Synbiotics, S: Synbiotics.
Table 2.
Fecal organic acid concentrations and pH in patients with severe SIRS.
| SIRS Patients | Normal | |
|---|---|---|
| Total organic acid | 30.3 ± 20.3 * | 88.4 ± 21.2 |
| Succinic acid | 2.0 ± 2.5 | 0.9 ± 1.2 |
| Lactic acid | 3.8 ± 5.5 | 0.5 ± 0.3 |
| Formic acid | 1.7 ± 2.9 | 0.4 ± 0.3 |
| Acetic acid | 18.7 ± 15.9 * | 50.8 ± 13.1 |
| Propionic acid | 2.5 ± 4.6 * | 18.7 ± 6.8 |
| Isobutyric acid | 0.1 ± 0.5 | 1.1 ± 0.3 |
| Butyric acid | 0.9 ± 2.3 * | 16.6 ± 6.7 |
| Isovaleric acid | 0.5 ± 1.9 | 1.4 ± 0.7 |
| Valeric acid | 0.1 ± 0.7 | 0.6 ± 0.4 |
| pH | 7.4 ± 0.6 * | 6.6 ± 0.3 |
(* p < 0.05 NS group vs. S group; data are mean ± SE).
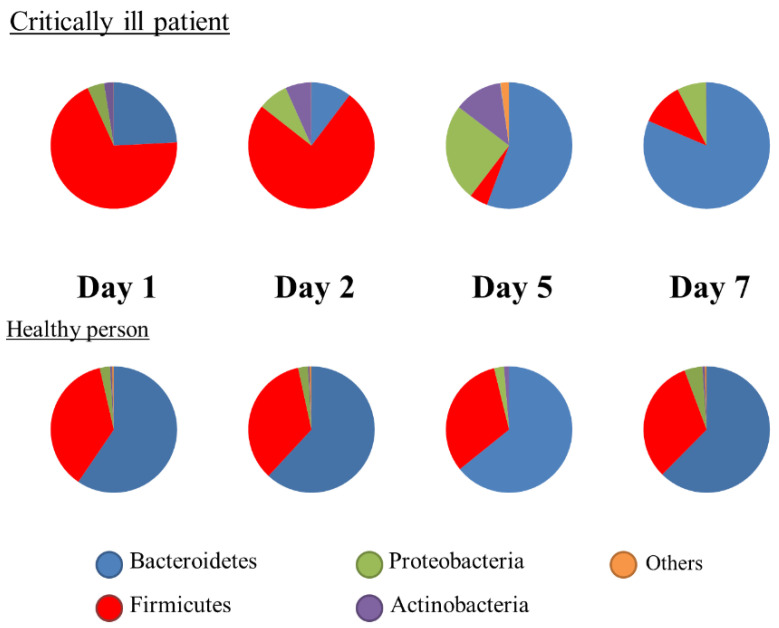
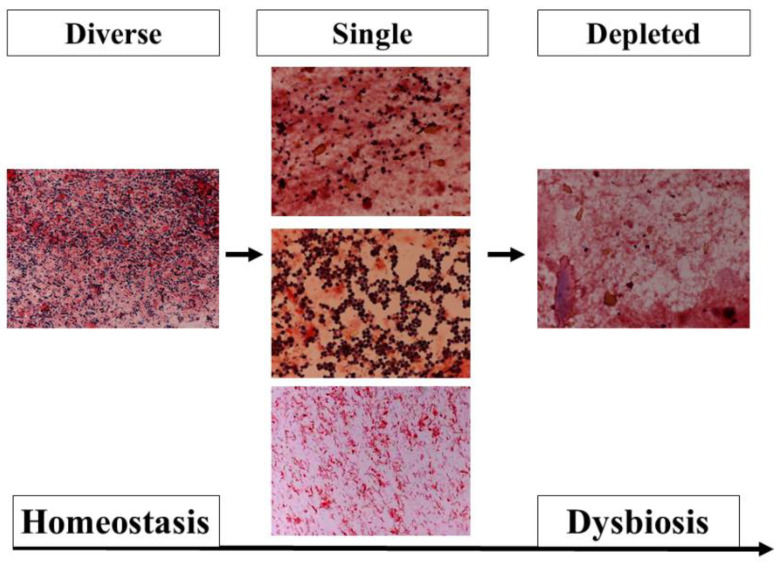
Metagenomic analysis using the 16S rRNA gene has been developed and shown to be different with ethnicity, diet, nutrition, lifestyle, and disease [20]. This method is not a quantitative analysis; rather, the results were expressed as proportions. At the phylum level, the Firmicutes or Bacteroidetes were predominant in healthy adults. In intensive care unit (ICU) patients, both phyla were altered significantly (Figure 2), and the ratio of Bacteroidetes to Firmicutes (B/F ratio) was associated with mortality [21]. The gut microbiota begins to change within the first 6 h [22]. Ojima et al. reported that gut microbiota under broad-spectrum antibiotics dramatically changed and stabilized within the first week [23]. To assess biomarkers at the bedside, metagenomic analysis has not been adequately used until now. However, fecal gram staining can be used as a quick marker for dysbiosis [24] (Figure 3).
Figure 2.
Changes in the composition of gut microbiota. Representative cases showing the proportions of microbiota.
Figure 3.
Pattern classification of fecal Gram staining. (Left) In the diverse pattern, many kinds of bacteria cover the field. (Middle) In the single pattern, a few kinds of bacteria dominantly cover the field. These images show that Gram-positive cocci (Upper), Gram-negative rods (Middle), and fungus (Bottom) dominantly cover the field. (Right) In the depleted pattern, most bacteria are depleted in the field. The dysbiosis progresses from a diverse pattern to a single pattern, and from a single pattern to a depleted pattern.
4. Immune Reactions through Gut Microbiota
The human gut microbiota has no less than 100 times more bacterial than human genes; therefore, metabolism is a combination of microbial- and human-derived actions [25]. In this mutualistic relationship, there is immunological tolerance of many bacteria. Obligate anaerobic bacteria, a main gut microbiota, are the main inhibitors of bacterial overgrowth, which is defined as ‘‘colonization resistance” [26]. In addition, the gut microbiota has a role in producing immune signals that affect the host’s metabolism, immunity, and response to infection by the immune system [27]. The germ-free mouse has a smaller mucus barrier, Peyer’s patches, and lamina propria and also decreased T cells and B cells compared with the normal mouse [28]. The signals from microbial components and metabolites, such as SCFAs, coupled with pathogen recognition receptors, such as Toll-like receptors and nucleotide oligomerization domain (NOD)-like receptors (NLRs) in the intestine, orchestrate host-microbiota interaction and multifactorial diseases. In the absence of the microbiota, reduced myeloid-cell development in the bone marrow causes delayed clearance of systemic bacterial infection. For adaptive immunity, Ivanov et al. revealed that segmented filamentous bacteria induce Th17 cells [29]. Atarashi et al. revealed that 17 species of bacteria, including Clostridiales, induced regulatory T cells [30]. ICU patients were significantly lower in the proportions of the class Clostridia than the healthy controls [31]. IFN-γ-producing CD8 + cells were induced by 11 species of bacteria included in the phyla Bacteroidetes, Firmicutes, and Fusobacterium [31]. Thus, the gut microbiota can help to shape the balance of immune cells and modulate immune status [32]. In autopsy cases of sepsis, the numbers of CD4 + T cells, CD8 + T cells, and HLA-DR+ cells were decreased, whereas those of PD-1, regulatory T cells, and myeloid-derived suppressor cells were increased [33]. As the extreme balance of Bacteroidetes and Firmicutes ratio was associated with mortality, the disrupted balance of commensal gut microbiota following injury might be associated with imbalance of immunity. There is a missing link between gut microbiota and systemic immunity, but residual pathogenic bacteria might induce an inflammatory reaction, or a depleted microbiota might decrease an innate immune response, resulting in prolonged immunosuppression (Figure 1).
5. Effects of Probiotics and Synbiotics during Critical Illness
5.1. Influence of Probiotics on Gut and Immunity
Probiotics are defined as live microorganisms, which confer a health benefit on the host [34]. Probiotics, most commonly Lactobacillus and Bifidobacterium, have been shown to have preventive effects in many kinds of diseases, including antibiotic-induced diarrhea, acute diarrhea, and necrotizing enterocolitis [35]. Prebiotics are currently defined as non-digestible food ingredients that beneficially affect the host by selectively stimulating the growth and/or activity of one or a limited number of bacteria in the colon [36]. Synbiotics are combinations of probiotics and prebiotics. For critically ill patients, synbiotics have prophylactic effects for complications of abdominal surgery, trauma, and ventilator-associated pneumonia (VAP) [37].
The mechanisms of probiotics have been explained with improving barrier function and immunity through the actions of cell components and metabolites of probiotics [38,39]. There are direct effects, including microorganism-associated molecular patterns (MAMPs) and pattern recognition receptors (PRRs), in the gut mucosa [40]. MAMPs involve flagellin, lipopolysaccharide, lipoteichoic acid, peptidoglycan, etc. For example, flagellins of the probiotic E. coli Nissle 1917 were shown to induce beta-defensin via Toll-like receptor 5 [41]. Peptidoglycan from the gut translocates to the circulation and increases the killing capacity of neutrophils via NOD1 [42]. For probiotic metabolites, probiotic bifidobacteria were shown to produce a high concentration of acetic acid and to lower the pH of the gut in a enterohemorrhagic E. coli 0157 mouse model [43]. Bifidobacterium breve have a positive correlation with acetic acid levels and intestinal epithelium expression of tight junction-related genes [44]. L. casei increased pulmonary natural killer cell activity, and interleukin-12 production was increased in a mouse model of influenza virus infection [45]. In a mouse model of diarrhea associated with clindamycin antibiotics administration, C. butyricum decreased the level of inflammatory cytokines and intestinal barrier-related proteins, such as IL-6, IFN-γ, mucin-2 in the colon [46]. Lactobacillus increased regulatory T cells with better survival in a mouse model of pseudomonas pneumonia [47]. These data suggest that probiotics might modulate the host response and prevent systemic inflammation.
5.2. Effectiveness of Probiotics and Synbiotics for Diarrhea
Diarrhea has been reported to prolong ICU stay and increase mortality in the ICU [48]. Hickson et al. reported that in 135 hospital patients with antibiotics and L. casei, L. bulgaricus, and Streptococcus thermophiles, 12% in the probiotic group exhibited diarrhea compared with 34% in the control group [49]. A meta-analysis revealed that Saccharomyces boulardii, L. rhamnosus GG, or B. longum were used to show the prevention of diarrhea in the hospital setting [50].
In ICU settings, Bleichner et al. reported that in 128 ICU patients, the number of days with diarrhea was reduced in the S. boulardii-treated group [51]. Shimizu et al. reported that B. breve, L. casei, and galactooligosaccharides had less complications of diarrhea and ventilator-associated diarrhea in 178 ventilated critically ill patients [52]. Prophylactic probiotics and synbiotics might keep gut microbiota and reduce the incidence of diarrhea. For refractory diarrhea, fecal microbiota transplantation (FMT) is a strong reconstruction of the gut microbiota [53], which has been recommended for patients with multiple recurrences of C. difficile infection [54]. A case of antibiotics-associated diarrhea was also reported among patients with diarrhea of more than 5 L/day, in which the gut microbiota was recovered and symptoms were resolved by FMT [55]. In severe gut microbiota disruption, more effective probiotics/synbiotics could be required in the ICU.
5.3. Prophylactic Effect of Probiotics and Synbiotics on Critical Illness
The effects of probiotics/synbiotics are based upon the alteration of gut microbiota and environment in critically ill patients. Those patients who received probiotics had significantly greater levels of Bifidobacterium, Lactobacillus, and microbial products, particularly SCFAs, than those who did not receive probiotics [56]. In meta-analyses, probiotics have been reported to reduce infectious complications of surgical procedures [57] and trauma [58]. Although the cause of upregulated immunity has not been elucidated, Sugawara et al. reported that patients taking probiotics before hepatectomy had more natural killer cell activity, increased lymphocyte counts, and lower IL-6 levels after hepatectomy than those not taking probiotics [59].
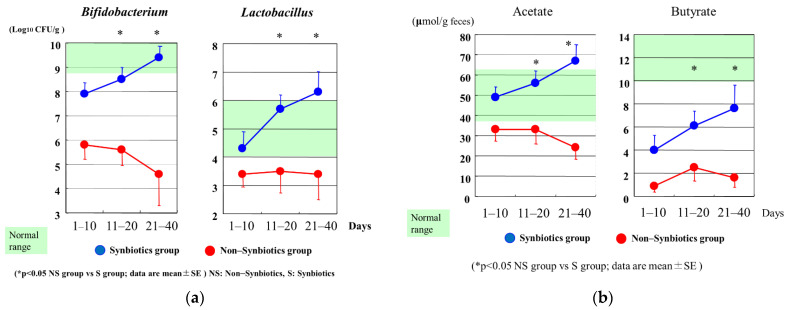
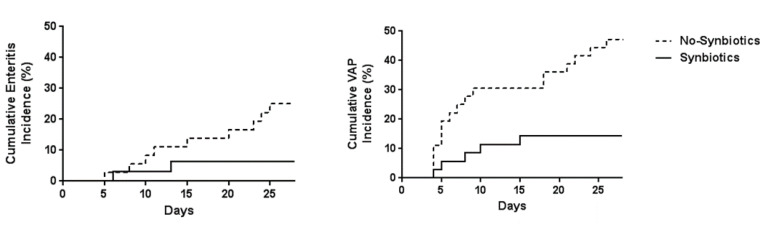
In the ICU setting, analysis of fecal flora confirmed that critically ill patients in the synbiotics group had significantly greater levels of beneficial Bifidobacterium, Lactobacillus, and total organic acids, particularly SCFAs, than those in the non-synbiotics group [60] (Figure 4). Synbiotics maintain the gut flora and environment and decrease the incidence of diarrhea and VAP in ventilated patients with sepsis [61] (Figure 5). In a meta-analysis, Batra et al. reported that in ventilated critically ill ICU patients, the administration of probiotics reduced their incidence of VAP, the duration of mechanical ventilation, length of ICU stay, and in-hospital mortality [62]. This research indicated that the administration of probiotics and synbiotics could maintain gut microbiota and upregulate immunity (Figure 6).
Figure 4.
(a) Serial changes in Bifidobacterium and Lactobacillus after admission. Bifidobacterium and Lactobacillus counts in the Synbiotics group were significantly increased compared with those in the Non–Synbiotics group. (b) Serial changes in acetate and butyrate after admission. Acetate and butyrate in the Synbiotics group were significantly increased compared with those in the Non–Synbiotics group.
Figure 5.
Effects of synbiotics on infectious complications. The cumulative incidence of enteritis and ventilator-associated pneumonia (VAP) were significantly lower in the Synbiotics group than in the Non-Synbiotics group by log-rank test (p < 0.05). (Cited from [62]).
Figure 6.
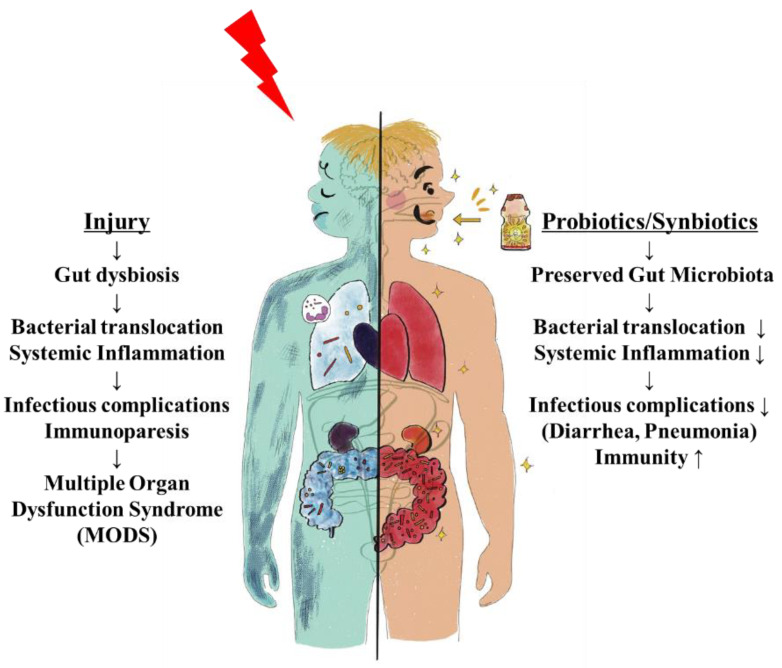
Gut origin hypothesis and Probiotics/Synbiotics. Deteriorated microbiota following injury cause systemic inflammation and infectious complications including pneumonia. If inflammation continues, immunoparesis and multiple organ failures develop. Probiotics and synbiotics help to maintain gut microbiota and prevent infectious complications and their subsequent sequelae.
The new coronavirus disease (COVID-19) causes gastrointestinal symptoms as well as respiratory symptoms. The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) uses angiotensin-converting enzyme 2 (ACE2). A deficiency in murine ACE2, which encodes a key regulatory enzyme of the renin–angiotensin system, results in highly increased susceptibility to diarrhea and intestinal inflammation [63]. In gut microbiota of COVID-19 patients, the decrease in normal gut microbiota bacteria, such as Eubacterium ventricosum, Faecalibacterium prausnitzii, Reseburia, and Lachnospiraceae, and the increase of opportunistic bacteria, such as C. hathewayi, Actinomyces viscosus, and B. nordii, was observed [64]. Probiotics/synbiotics are one of the promising therapies for COVID-19 to maintain gut microbiota and prevent the exacerbation of pneumonia [39]. In 200 adults with severe COVID-19 pneumonia, patients treated with several kinds of probiotics were associated with a reduced risk for death [65]. Intestinal therapy for COVID-19 might be important to prevent pneumonia and systemic inflammation.
5.4. Gut Dysmotility and Limitations for Intestinal Therapy
Gut motility in critically ill patients is often suppressed by various factors, for example, ischemia, analgesics, adrenergic drugs, fluid therapy, and diseases such as diabetes [66,67]. This intestinal motility failure leads to increased gut permeability for mediators and bacteria and the development of SIRS. Gastrointestinal complications in critically ill patients occurred about 60%, and enteral nutrition was stopped about 15% [68]. ICU patients with feeding intolerance had significantly deteriorated gut microbiota compared to those patients without feeding intolerance (p < 0.05) [69]. Patients with feeding intolerance had significantly higher rates of bacteremia and mortality. Interstitial cells of Cajal, which has a role of gut motility, form an extensive network associated with the myenteric plexus in the enteric nervous system [70]. These cells in critically ill patients were almost decreased in the colon compared with those in the controls [71].
Pancreatitis is one of the critical illnesses in the abdomen, and gastrointestinal dysmotility can cause feeding intolerance and bacterial translocation [72]. Olah et al. [73] reported that the incidence of infectious complications with Lactobacillus plantarum decreased more than those without L. plantarum (4.5% vs. 30.4%). In contrast, Besselink et al. [74] reported that bowel ischemia and mortality rates in patients with six kinds of bacteria were significantly higher than those in patients without these bacteria (16 vs. 6%). However, in this study, the incidence of infectious complications showed no significant difference, and no bacteremia was caused from the administered bacteria. In addition, this study has been criticized from multiple perspectives [75,76]. Extensive burn can cause SIRS, sepsis, and multiple organ dysfunction syndrome. In a case of non-obstructive ileus in burn, the numbers of gut microbiota, mainly those of Bifidobacterium, decreased and Pseudomonas and Candida increased in major burn patients [77]. These results indicate that critically ill patients with gastrointestinal dysmotility have altered gut microbiota that could lead to an ‘‘undrained abscess’’. These studies further suggest that the effect and safety of probiotics differ with the bacteria administered and intestinal tolerance. Probiotics and synbiotics might not be indicated for severe intestinal dysmotility. Further studies are needed to determine an appropriate therapeutic indication for severe abdominal inflammation.
6. Summary
The gut microbiota has a role in maintaining host immunity. Deterioration of the gut microbiota in critical illnesses can lead to systemic inflammation response syndrome and multiple organ dysfunction syndrome.
Probiotics/synbiotics treatment can maintain the disrupted gut microbiota and reduce infectious complications in critically ill patients. Further basic and clinical research will be required to promote intestinal therapies for critically ill patients.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rudd K.E., Johnson S.C., Agesa K.M., Shackelford K.A., Tsoi D., Kievlan D.R., Colombara D.V., Ikuta K., Kissoon N., Finfer S., et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: Analysis for the Global Burden of Disease Study. Lancet. 2020;395:200–211. doi: 10.1016/S0140-6736(19)32989-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cunningham R.M., Walton M.A., Carter P.M. The Major Causes of Death in Children and Adolescents in the United States. N. Engl. J. Med. 2018;379:2468–2475. doi: 10.1056/NEJMsr1804754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stoecklein V.M., Osuka A., Lederer J.A. Trauma equals danger—Damage control by the immune system. J. Leukoc. Biol. 2012;92:539–551. doi: 10.1189/jlb.0212072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosenthal M.D., Moore F.A. Persistent Inflammation, Immunosuppression, and Catabolism: Evolution of Multiple Organ Dysfunction. Surg. Infect. (Larchmt) 2016;17:167–172. doi: 10.1089/sur.2015.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Otani S., Coopersmith C.M. Gut integrity in critical illness. J. Intensiv. Care. 2019;7:1–7. doi: 10.1186/s40560-019-0372-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Petersen C., Round J.L. Defining dysbiosis and its influence on host immunity and disease. Cell. Microbiol. 2014;16:1024–1033. doi: 10.1111/cmi.12308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rooks M.G., Garrett W.S. Gut microbiota, metabolites and host immunity. Nat. Rev. Immunol. 2016;16:341–352. doi: 10.1038/nri.2016.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gentile L.F., Cuenca A.G., Efron P.A., Ang D., Bihorac A., McKinley B.A., Moldawer L.L., Moore F.A. Persistent inflammation and immunosuppression: A common syndrome and new horizon for surgical intensive care. J. Trauma Acute Care Surg. 2012;72:1491–1501. doi: 10.1097/TA.0b013e318256e000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clark J.A., Coopersmith C.M. Intestinal crosstalk: A new paradigm for understanding the gut as the “motor” of critical illness. Shock. 2007;28:384–393. doi: 10.1097/shk.0b013e31805569df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moore E.E., Moore F.A., Franciose R.J., Kim F.J., Biffl W.L., Banerjee A. The Postischemic Gut Serves as a Priming Bed for Circulating Neutrophils that Provoke Multiple Organ Failure. J. Trauma Inj. Infect. Crit. Care. 1994;37:881–887. doi: 10.1097/00005373-199412000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Eaves-Pyles T., Alexander J.W. Rapid and Prolonged Impairment of Gut Barrier Function after Thermal Injury in Mice. Shock. 1998;9:95–100. doi: 10.1097/00024382-199802000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Magnotti L.J., Upperman J.S., Xu D.-Z., Lu Q., Deitch E.A. Gut-Derived Mesenteric Lymph but not Portal Blood Increases Endothelial Cell Permeability and Promotes Lung Injury After Hemorrhagic Shock. Ann. Surg. 1998;228:518–527. doi: 10.1097/00000658-199810000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.I Ramzy P., E Wolf S., Irtun O., Hart D.W., Thompson J.C., Herndon D.N. Gut epithelial apoptosis after severe burn: Effects of gut hypoperfusion11No competing interests declared. J. Am. Coll. Surg. 2000;190:281–287. doi: 10.1016/S1072-7515(99)00269-0. [DOI] [PubMed] [Google Scholar]
- 14.Coopersmith C.M., Stromberg P.E., Dunne W.M., Davis C.G., Amiot D.M., II, Buchman T.G., Karl I.E., Hotchkiss R.S. Inhibition of intestinal epithelial apoptosis and survival in a murine model of pneumonia-induced sepsis. JAMA. 2002;287:1716–1721. doi: 10.1001/jama.287.13.1716. [DOI] [PubMed] [Google Scholar]
- 15.Yoseph B.P., Klingensmith N.J., Liang Z., Breed E., Burd E.M., Mittal R., Dominguez J.A., Petrie B., Ford M.L., Coopersmith C.M. Mechanisms of Intestinal Barrier Dysfunction in Sepsis. Shock. 2016;46:52–59. doi: 10.1097/SHK.0000000000000565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Boyle C.J., MacFie J., Mitchell C.J., Johnstone D., Sagar P.M., Sedman P.C. Microbiology of bacterial translocation in humans. Gut. 1998;42:29–35. doi: 10.1136/gut.42.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mizuno T., Yokoyama Y., Nishio H., Ebata T., Sugawara G., Asahara T., Nomoto K., Nagino M. Intraoperative Bacterial Translocation Detected by Bacterium-Specific Ribosomal RNA-Targeted Reverse-Transcriptase Polymerase Chain Reaction for the Mesenteric Lymph Node Strongly Predicts Postoperative Infectious Complications After Major Hepatectomy for Biliary Malignancies. Ann. Surg. 2010;252:1013–1019. doi: 10.1097/sla.0b013e3181f3f355. [DOI] [PubMed] [Google Scholar]
- 18.Shimizu K., Ogura H., Goto M., Asahara T., Nomoto K., Morotomi M., Yoshiya K., Matsushima A., Sumi Y., Kuwagata Y., et al. Altered Gut Flora and Environment in Patients with Severe SIRS. J. Trauma Inj. Infect. Crit. Care. 2006;60:126–133. doi: 10.1097/01.ta.0000197374.99755.fe. [DOI] [PubMed] [Google Scholar]
- 19.Shimizu K., Ogura H., Hamasaki T., Goto M., Tasaki O., Asahara T., Nomoto K., Morotomi M., Matsushima A., Kuwagata Y., et al. Altered Gut Flora Are Associated with Septic Complications and Death in Critically Ill Patients with Systemic Inflammatory Response Syndrome. Dig. Dis. Sci. 2010;56:1171–1177. doi: 10.1007/s10620-010-1418-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gupta V.K., Paul S., Dutta C. Geography, Ethnicity or Subsistence-Specific Variations in Human Microbiome Composition and Diversity. Front. Microbiol. 2017;8:1162. doi: 10.3389/fmicb.2017.01162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ojima M., Motooka D., Shimizu K., Gotoh K., Shintani A., Yoshiya K., Nakamura S., Ogura H., Iida T., Shimazu T. Metagenomic Analysis Reveals Dynamic Changes of Whole Gut Microbiota in the Acute Phase of Intensive Care Unit Patients. Dig. Dis. Sci. 2016;61:1628–1634. doi: 10.1007/s10620-015-4011-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.HayakawaTakashi M., Asahara T., Henzan N., Murakami H., Yamamoto H., Mukai N., Minami Y., Sugano M., Kubota N., Uegaki S., et al. Dramatic Changes of the Gut Flora Immediately After Severe and Sudden Insults. Dig. Dis. Sci. 2011;56:2361–2365. doi: 10.1007/s10620-011-1649-3. [DOI] [PubMed] [Google Scholar]
- 23.Ojima M., Shimizu K., Motooka D., Ishihara T., Nakamura S., Shintani A., Ogura H., Iida T., Yoshiya K., Shimazu T. Gut Dysbiosis Associated with Antibiotics and Disease Severity and Its Relation to Mortality in Critically Ill Patients. Dig. Dis. Sci. 2021:1–13. doi: 10.1007/s10620-021-07000-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shimizu K., Ogura H., Tomono K., Tasaki O., Asahara T., Nomoto K., Morotomi M., Matsushima A., Nakahori Y., Yamano S., et al. Patterns of Gram-Stained Fecal Flora as a Quick Diagnostic Marker in Patients with Severe SIRS. Dig. Dis. Sci. 2011;56:1782–1788. doi: 10.1007/s10620-010-1486-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gill S.R., Pop M., DeBoy R.T., Eckburg P.B., Turnbaugh P., Samuel B.S., Gordon J.I., Relman D., Fraser C., Nelson K.E. Metagenomic Analysis of the Human Distal Gut Microbiome. Science. 2006;312:1355–1359. doi: 10.1126/science.1124234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vollaard E.J., Clasener H.A. Colonization resistance. Antimicrob. Agents Chemother. 1994;38:409–414. doi: 10.1128/AAC.38.3.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thaiss C.A., Zmora N., Levy M., Elinav C.A.T.N.Z.M.L.E. The microbiome and innate immunity. Nat. Cell Biol. 2016;535:65–74. doi: 10.1038/nature18847. [DOI] [PubMed] [Google Scholar]
- 28.Round J.L., Mazmanian S.K. The gut microbiota shapes intestinal immune responses during health and disease. Nat. Rev. Immunol. 2009;9:313–323. doi: 10.1038/nri2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ivanov I.I., Atarashi K., Manel N., Brodie E.L., Shima T., Karaoz U., Wei D., Goldfarb K.C., Santee C.A., Lynch S.V., et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139:485–498. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Atarashi K., Tanoue T., Oshima K., Suda W., Nagano Y., Nishikawa H., Fukuda S., Saito T., Narushima S., Hase K., et al. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature. 2013;500:232–236. doi: 10.1038/nature12331. [DOI] [PubMed] [Google Scholar]
- 31.Tanoue T., Morita S., Plichta D.R., Skelly A.N., Suda W., Sugiura Y., Narushima S., Vlamakis H., Motoo I., Sugita K., et al. A defined commensal consortium elicits CD8 T cells and anti-cancer immunity. Nature. 2019;565:600–605. doi: 10.1038/s41586-019-0878-z. [DOI] [PubMed] [Google Scholar]
- 32.Honda K., Littman D.R. The microbiota in adaptive immune homeostasis and disease. Nat. Cell Biol. 2016;535:75–84. doi: 10.1038/nature18848. [DOI] [PubMed] [Google Scholar]
- 33.Hotchkiss R.S., Monneret G., Payen D. Sepsis-induced immunosuppression: From cellular dysfunctions to immunotherapy. Nat. Rev. Immunol. 2013;13:862–874. doi: 10.1038/nri3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.FAO. WHO . Report of a Joint FAO/WHO Expert Consultation on Evaluation of Health and Nutritional Properties of Probiotics in Food Including Powder Milk with Live Lactic acid Bacteria. WHO; Geneva, Switzerland: 2001. [Google Scholar]
- 35.Sanders M.E., Guarner F., Guerrant R., Holt P., Quigley E.M.M., Sartor R.B., Sherman P., A Mayer E. An update on the use and investigation of probiotics in health and disease. Gut. 2013;62:787–796. doi: 10.1136/gutjnl-2012-302504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gibson G.R., Roberfroid M.B. Dietary Modulation of the Human Colonic Microbiota: Introducing the Concept of Prebiotics. J. Nutr. 1995;125:1401–1412. doi: 10.1093/jn/125.6.1401. [DOI] [PubMed] [Google Scholar]
- 37.Shimizu K., Ogura H., Asahara T., Nomoto K., Morotomi M., Tasaki O., Matsushima A., Kuwagata Y., Shimazu T., Sugimoto H. Probiotic/Synbiotic Therapy for Treating Critically Ill Patients from a Gut Microbiota Perspective. Dig. Dis. Sci. 2012;58:23–32. doi: 10.1007/s10620-012-2334-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bron P.A., van Baarlen P., Kleerebezem M. Emerging molecular insights into the interaction between probiotics and the host intestinal mucosa. Nat. Rev. Genet. 2011;10:66–78. doi: 10.1038/nrmicro2690. [DOI] [PubMed] [Google Scholar]
- 39.Mullish B.H., Marchesi J.R., McDonald J.A., Pass D.A., Masetti G., Michael D.R., Plummer S., Jack A.A., Davies T.S., Hughes T.R., et al. Probiotics reduce self-reported symptoms of upper respiratory tract infection in overweight and obese adults: Should we be considering probiotics during viral pandemics? Gut Microbes. 2021;13:1–9. doi: 10.1080/19490976.2021.1900997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lebeer S., Vanderleyden J., De Keersmaecker S.C. Host interactions of probiotic bacterial surface molecules: Comparison with commensals and pathogens. Nat. Rev. Genet. 2010;8:171–184. doi: 10.1038/nrmicro2297. [DOI] [PubMed] [Google Scholar]
- 41.Schlee M., Wehkamp J., Altenhoefer A., Oelschlaeger T.A., Stange E.F., Fellermann K. Induction of human beta-defensin 2 by the probiotic Escherichia coli Nissle 1917 is mediated through flagellin. Infect. Immun. 2007;75:2399–2407. doi: 10.1128/IAI.01563-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Clarke T.B., Davis K.M., Lysenko E.S., Zhou A.Y., Yu Y., Weiser J.N. Recognition of peptidoglycan from the microbiota by Nod1 enhances systemic innate immunity. Nat. Med. 2010;16:228–231. doi: 10.1038/nm.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Asahara T., Shimizu K., Nomoto K., Hamabata T., Ozawa A., Takeda Y. Probiotic Bifidobacteria Protect Mice from Lethal Infection with Shiga Toxin-Producing Escherichia coli O157:H7. Infect. Immun. 2004;72:2240–2247. doi: 10.1128/IAI.72.4.2240-2247.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Asahara T., Takahashi A., Yuki N., Kaji R., Takahashi T., Nomoto K. Protective Effect of a Synbiotic against Multidrug-Resistant Acinetobacter baumannii in a Murine Infection Model. Antimicrob. Agents Chemother. 2016;60:3041–3050. doi: 10.1128/AAC.02928-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yasui H., Kiyoshima J., Hori T. Reduction of Influenza Virus Titer and Protection against Influenza Virus Infection in Infant Mice Fed Lactobacillus casei Shirota. Clin. Vaccine Immunol. 2004;11:675–679. doi: 10.1128/CDLI.11.4.675-679.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hagihara M., Kuroki Y., Ariyoshi T., Higashi S., Fukuda K., Yamashita R., Matsumoto A., Mori T., Mimura K., Yamaguchi N., et al. Clostridium butyricum Modulates the Microbiome to Protect Intestinal Barrier Function in Mice with Antibiotic-Induced Dysbiosis. iScience. 2020;23:100772. doi: 10.1016/j.isci.2019.100772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Khailova L., Baird C.H., Rush A.A., McNamee E.N., Wischmeyer P.E. Lactobacillus rhamnosus GG improves outcome in experimental pseudomonas aeruginosa pneumonia: Potential role of regulatory T cells. Shock. 2013;40:496–503. doi: 10.1097/SHK.0000000000000066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tirlapur N., Puthucheary Z.A., Cooper J.A., Sanders J., Coen P.G., Moonesinghe S.R., Wilson A.P., Mythen M.G., Montgomery H.E. Diarrhoea in the critically ill is common, associated with poor outcome and rarely due to Clostridium difficile. Sci. Rep. 2016;6:24691. doi: 10.1038/srep24691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hickson M., D’Souza A.L., Muthu N., Rogers T., Want S., Rajkumar C., Bulpitt C.J. Use of probiotic Lactobacillus preparation to prevent diarrhoea associated with antibiotics: Randomised double blind placebo controlled trial. BMJ. 2007;335:80. doi: 10.1136/bmj.39231.599815.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.D’Souza A.L., Rajkumar C., Cooke J., Bulpitt C.J. Probiotics in prevention of antibiotic associated diarrhoea: Meta-analysis. BMJ. 2002;324:1361. doi: 10.1136/bmj.324.7350.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bleichner G., Blehaut H., Mentec H., Moyse D. Saccharomyces boulardii prevents diarrhea in critically ill tube-fed patients. Intensiv. Care Med. 1997;23:517–523. doi: 10.1007/s001340050367. [DOI] [PubMed] [Google Scholar]
- 52.Shimizu K., Ogura H., Kabata D., Shintani A., Tasaki O., Ojima M., Ikeda M., Shimazu T. Association of prophylactic synbiotics with reduction in diarrhea and pneumonia in mechanically ventilated critically ill patients: A propensity score analysis. J. Infect. Chemother. 2018;24:795–801. doi: 10.1016/j.jiac.2018.06.006. [DOI] [PubMed] [Google Scholar]
- 53.Alagna L., Haak B.W., Gori A. Fecal microbiota transplantation in the ICU: Perspectives on future implementations. Intensiv. Care Med. 2019;45:998–1001. doi: 10.1007/s00134-019-05645-7. [DOI] [PubMed] [Google Scholar]
- 54.McDonald L.C., Gerding D.N., Johnson S., Bakken J.S., Carroll K.C., E Coffin S., Dubberke E.R., Garey K.W., Gould C.V., Kelly C., et al. Clinical Practice Guidelines for Clostridium difficile Infection in Adults and Children: 2017 Update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA) Clin. Infect. Dis. 2018;66:987–994. doi: 10.1093/cid/ciy149. [DOI] [PubMed] [Google Scholar]
- 55.Wurm P., Spindelboeck W., Krause R., Plank J., Fuchs G., Bashir M., Petritsch W., Halwachs B., Langner C., Hogenauer C., et al. Antibiotic-Associated Apoptotic Enterocolitis in the Absence of a Defined Pathogen: The Role of Intestinal Microbiota Depletion. Crit. Care Med. 2017;45:e600–e606. doi: 10.1097/CCM.0000000000002310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shimizu K., Hiroshi O., Goto M., Tasaki O., Kuwagata Y., Tanaka H., Shimazu T., Sugimoto H., Asahara T., Nomoto K., et al. Synbiotics Reduce the Septic Complications in Patients with Severe Sirs. Crit. Care Med. 2005;33:A9. doi: 10.1097/00003246-200512002-00035. [DOI] [Google Scholar]
- 57.Wu X.D., Liu M.M., Liang X., Hu N., Huang W. Effects of perioperative supplementation with pro-/synbiotics on clinical outcomes in surgical patients: A meta-analysis with trial sequential analysis of randomized controlled trials. Clin. Nutr. 2018;37:505–515. doi: 10.1016/j.clnu.2016.10.015. [DOI] [PubMed] [Google Scholar]
- 58.Gu W.J., Deng T., Gong Y.Z., Jing R., Liu J.C. The effects of probiotics in early enteral nutrition on the outcomes of trauma: A meta-analysis of randomized controlled trials. JPEN J. Parenter. Enteral Nutr. 2013;37:310–317. doi: 10.1177/0148607112463245. [DOI] [PubMed] [Google Scholar]
- 59.Sugawara G., Nagino M., Nishio H., Ebata T., Takagi K., Asahara T., Nomoto K., Nimura Y. Perioperative synbiotic treatment to prevent postoperative infectious complications in biliary cancer surgery: A randomized controlled trial. Ann. Surg. 2006;244:706–714. doi: 10.1097/01.sla.0000219039.20924.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shimizu K., Ogura H., Goto M., Asahara T., Nomoto K., Morotomi M., Matsushima A., Tasaki O., Fujita K., Hosotsubo H., et al. Synbiotics Decrease the Incidence of Septic Complications in Patients with Severe SIRS: A Preliminary Report. Dig. Dis. Sci. 2008;54:1071–1078. doi: 10.1007/s10620-008-0460-2. [DOI] [PubMed] [Google Scholar]
- 61.Shimizu K., Yamada T., Ogura H., Mohri T., Kiguchi T., Fujimi S., Asahara T., Ojima M., Ikeda M., Shimazu T. Synbiotics modulate gut microbiota and reduce enteritis and ventilator-associated pneumonia in patients with sepsis: A randomized controlled trial. Crit. Care. 2018;22:239. doi: 10.1186/s13054-018-2167-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Batra P., Soni K.D., Mathur P. Efficacy of probiotics in the prevention of VAP in critically ill ICU patients: An updated systematic review and meta-analysis of randomized control trials. J. Intensive Care. 2020;8:81. doi: 10.1186/s40560-020-00487-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hashimoto T., Perlot T., Rehman A., Trichereau J., Ishiguro H., Paolino M., Sigl V., Hanada T., Hanada R., Lipinski S., et al. ACE2 links amino acid malnutrition to microbial ecology and intestinal inflammation. Nat. Cell Biol. 2012;487:477–481. doi: 10.1038/nature11228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zuo T., Zhang F., Lui G.C., Yeoh Y.K., Li A.Y., Zhan H., Wan Y., Chung A.C., Cheung C.P., Chen N., et al. Alterations in Gut Microbiota of Patients With COVID-19 During Time of Hospitalization. Gastroenterology. 2020;159:944–955.e8. doi: 10.1053/j.gastro.2020.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ceccarelli G., Borrazzo C., Pinacchio C., Santinelli L., Innocenti G.P., Cavallari E.N., Celani L., Marazzato M., Alessandri F., Ruberto F., et al. Oral Bacteriotherapy in Patients With COVID-19: A Retrospective Cohort Study. Front. Nutr. 2021;7:613928. doi: 10.3389/fnut.2020.613928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fruhwald S., Holzer P., Metzler H. Intestinal motility disturbances in intensive care patients pathogenesis and clinical impact. Intensiv. Care Med. 2006;33:36–44. doi: 10.1007/s00134-006-0452-7. [DOI] [PubMed] [Google Scholar]
- 67.Caddell K.A., Martindale R., McClave S.A., Miller K. Can the Intestinal Dysmotility of Critical Illness be Differentiated from Postoperative Ileus? Curr. Gastroenterol. Rep. 2011;13:358–367. doi: 10.1007/s11894-011-0206-8. [DOI] [PubMed] [Google Scholar]
- 68.Montejo J.C. Enteral nutrition-related gastrointestinal complications in critically ill patients: A multicenter study. The Nutritional and Metabolic Working Group of the Spanish Society of Intensive Care Medicine and Coronary Units. Crit. Care Med. 1999;27:1447–1453. doi: 10.1097/00003246-199908000-00006. [DOI] [PubMed] [Google Scholar]
- 69.Shimizu K., Ogura H., Asahara T., Nomoto K., Morotomi M., Nakahori Y., Osuka A., Yamano S., Goto M., Matsushima A., et al. Gastrointestinal dysmotility is associated with altered gut flora and septic mortality in patients with severe systemic inflammatory response syndrome: A preliminary study. Neurogastroenterol. Motil. 2010;23:330-e157. doi: 10.1111/j.1365-2982.2010.01653.x. [DOI] [PubMed] [Google Scholar]
- 70.Shimojima N., Nakaki T., Morikawa Y., Hoshino K., Ozaki H., Hori M., Kitajima M. Interstitial Cells of Cajal in Dysmotility in Intestinal Ischemia and Reperfusion Injury in Rats. J. Surg. Res. 2006;135:255–261. doi: 10.1016/j.jss.2006.04.022. [DOI] [PubMed] [Google Scholar]
- 71.Shimizu K., Ogura H., Matsumoto N., Ikeda M., Yamamoto H., Mori M., Morii E., Shimazu T. Interstitial cells of Cajal are diminished in critically ill patients: Autopsy cases. Nutrition. 2020;70:110591. doi: 10.1016/j.nut.2019.110591. [DOI] [PubMed] [Google Scholar]
- 72.Wang X., Gong Z., Wu K., Wang B., Yuang Y. Gastrointestinal dysmotility in patients with acute pancreatitis. J. Gastroenterol. Hepatol. 2003;18:57–62. doi: 10.1046/j.1440-1746.2003.02898.x. [DOI] [PubMed] [Google Scholar]
- 73.Oláh A., Belágyi T., Issekutz Á., Gamal M.E., Bengmark S. Randomized clinical trial of specific lactobacillus and fibre supplement to early enteral nutrition in patients with acute pancreatitis. BJS. 2002;89:1103–1107. doi: 10.1046/j.1365-2168.2002.02189.x. [DOI] [PubMed] [Google Scholar]
- 74.Besselink M.G., van Santvoort H.C., Buskens E., Boermeester M.A., van Goor H., Timmerman H.M., Nieuwenhuijs V.B., Bollen T.L., van Ramshorst B., Witteman B.J., et al. Probiotic prophylaxis in predicted severe acute pancreatitis: A randomised, double-blind, placebo-controlled trial. Lancet. 2008;371:651–659. doi: 10.1016/S0140-6736(08)60207-X. [DOI] [PubMed] [Google Scholar]
- 75.Sheldon T. Dutch probiotics study is criticised for its “design, approval, and conduct”. BMJ. 2010;340:c77. doi: 10.1136/bmj.c77. [DOI] [PubMed] [Google Scholar]
- 76.Expression of concern–Probiotic prophylaxis in predicted severe acute pancreatitis: A randomised, double-blind, placebo-controlled trial. Lancet. 2010;375:875–876. doi: 10.1016/S0140-6736(10)60360-1. [DOI] [PubMed] [Google Scholar]
- 77.Shimizu K., Ogura H., Asahara T., Nomoto K., Matsushima A., Hayakawa K., Ikegawa H., Tasaki O., Kuwagata Y., Shimazu T. Gut microbiota and environment in patients with major burns–A preliminary report. Burns. 2015;41:e28–e33. doi: 10.1016/j.burns.2014.10.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.