Abstract
The lifelong infection with the human T lymphotropic virus type 1 (HTLV-1) has been associated with a variety of clinical manifestations; one of the less-explored is HTLV-1-associated pulmonary disease. Imaging of lung damage caused by the HTLV-1 hyperinflammatory cascade can be similar to sequelae from TB infection. Our study aims to describe the pulmonary lesions of HTLV-1-positive patients without past or current active TB and evaluate pulmonary function. We found that nine out of fourteen patients with no known TB disease history presented bronchiectasis, mainly found bilaterally while five presented pulmonary fibrosis. A normal pattern was found in most patients with a pulmonary functional test. Furthermore, there was no association between the PVL and the chest-CT scan findings, nor with spirometry results. However, the sample size was insufficient to conclude it.
Keywords: HTLV-1, pulmonary disease, pulmonary function, epidemiology
1. Introduction
The lifelong infection with the human T lymphotropic virus type 1 (HTLV-1) has been associated with a variety of clinical manifestations including adult T cell leukemia/lymphoma (ATLL), HTLV-1 associated myelopathy/tropical spastic paraparesis (HAM/TSP), uveitis, Sjögren’s syndrome, and others [1]. A less-explored manifestation, bronchiectasis, has been consistently reported among HTLV-1-positive patients from Australia, Japan, United Kingdom, and Brazil [2,3,4]. Other chronic lung diseases such as bronchitis and pulmonary fibrosis have also been described in HTLV-1 carriers [4,5,6,7]. These lesions can occur in symptomatic patients and less frequently in asymptomatic patients; however, this has hardly been recognized [8,9]. While these lesions arise due to an exaggerated immune response to the virus, the acquired immunodeficiency conveys a higher risk of opportunistic infections such as cryptococcosis and tuberculosis (TB), developing severe forms of these diseases [4,10].
In particular, the coinfection of TB and HTLV-1 should be taken into account given its high prevalence in areas where both diseases are endemic, the increased likelihood of hospitalization, and the high mortality rates among co-infected individuals [11,12,13,14,15]. Persistent pulmonary lesions occur in up to half of active TB survivors despite adequate treatment [16]. TB lesions may encompass cavitation, fibrosis, or bronchiectasis [17]. Imaging of pulmonary sequelae from TB disease can be similar to those caused by the hyper-inflammatory cascade of HTLV-1, usually described as peripheral lung parenchymal lesions mainly centrilobular nodules, ground-glass opacities, and bronchiectasis [3]. This hypothesizes the need to consider HTLV-1 in the differential diagnosis.
Peru is a TB-endemic country with an estimated incidence of 119 cases per 100,000 inhabitants [18]. Additionally, the prevalence of HTLV-1 has been estimated between 1–2% of the Peruvian population [19]. The co-infection of HTLV-1 with TB has been estimated between 2.8% to 5.6% among drug-susceptible and multidrug-resistant TB, respectively [13,20]. Few studies have characterized the lung lesions of HTLV-1 infected patients amongst those without known TB history. The study aimed to describe the pulmonary lesions and evaluate pulmonary function of HTLV-1-positive patients without past or current active TB disease.
2. Results
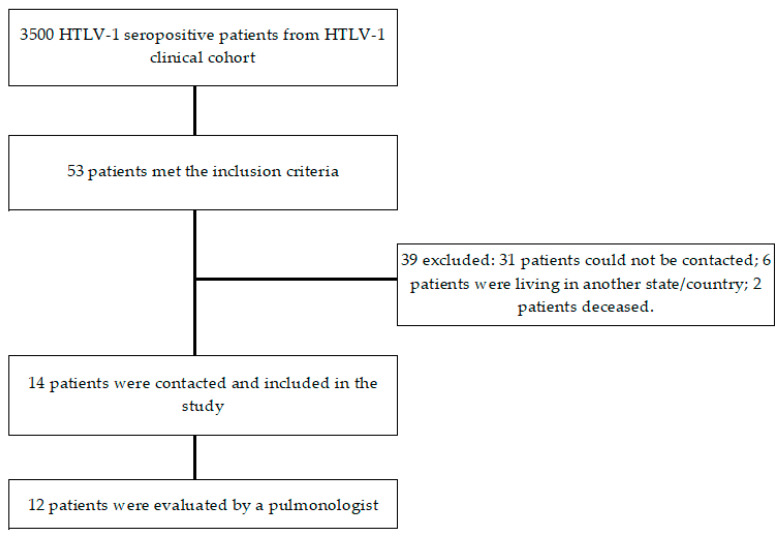
From 3500 patients registered in the HTLV-1 clinical cohort database, 53 met the inclusion criteria; nonetheless, only 14 patients (26%) were included in the study (Appendix A). Among these, eight (57%) were female and the median age was 60.6 years old (IQR: 52–69). Although six patients were originally from the Peruvian highlands, 13 were currently living in Lima, a coastal region. Four patients were obese (BMI ≥ 30 kg/m2) and one was overweight (BMI ≥ 25–29.9 kg/m2); median BMI was 28 kg/m2. Other comorbidities are listed in Table 1. Five presented at least one HTLV-associated disease, these are described in Table 2.
Table 1.
Sociodemographic characteristics of HTLV-1 patients with lung diseases without prior known TB disease.
| Characteristics (n = 14) | Frequency |
|---|---|
| Female sex, (%) | 8 (57) |
| Age, years, median (IQR) | 60.6 (52–69) |
| BMI, kg/m2 | |
| Normal (18.5–24.9) | 6/11 |
| Overweight (25–29.9) | 1/11 |
| Obese (>30) | 4/11 |
| Place of origin | |
| Highlands | 5/14 |
| Coast region | 9/14 |
| Place of residence | |
| Lima | 12/14 |
| Outside Lima | 2/14 |
| Pneumoconiosis-risk occupation | 3/12 |
| Comorbidities | 4/12 |
| Cardiovascular: high blood pressure, heart failure | 4/12 |
| Endocrine: diabetes mellitus type II, hypothyroidism | 3/12 |
| Cancer | 1/12 |
| Lung damage-associated drugs: losartan and sertraline | 3/12 |
| Previous HTLV-1 associated disease | 5/12 |
| Infective dermatitis | 1/12 |
| Tropical spastic paraparesis | 4/12 |
| Strongyloidiasis | 1/12 |
| Uveitis | 1/12 |
| Sjögren’s syndrome | 1/12 |
HTLV-1: human T lymphotropic virus type 1; TB: tuberculosis infection; BMI: body mass index; MCTD: mixed connective tissue disorder.
Table 2.
HTLV-1-associated disease among the patients included in the study.
| Patient ID | HTLV-1 Associated Disorder |
|---|---|
| P2 | HAM/TSP |
| P4 | Strongyloidiasis |
| P6 | Strongyloidiasis, HAM/TSP, uveitis |
| P11 | Infective dermatitis, HAM/TSP, Sjögren’s syndrome |
| P15 | HAM/TSP |
P: patient; HTLV-1: human T lymphotropic virus type 1; HAM/TSP: HTLV-1 associated myelopathy/tropical spastic paraparesia.
Regarding risk factors associated with pulmonary disease, six patients reported daily exposure to wood-burning stoves for an average of 24 years (0.2–70 years), five were active smokers, and another four reported exposures to secondhand smoke. Three patients had occupational exposure to construction, carpentry, and cooking fumes. Likewise, two patients reported long-term use of losartan and one reported the use of sertraline. Additionally, five patients reported at least one episode of wheezing cough during their adulthood (≥18 years old) and only one had episodes of wheezing in childhood, although none have been previously diagnosed with asthma. Eight patients reported gastroesophageal reflux symptoms; three had allergic rhinitis and five patients reported chronic dry cough. Six participants reported between 2–5 respiratory infections per year, with none requiring hospitalization. Previous influenza and pneumococcal vaccination was reported in seven and three patients, respectively.
Only 12 patients were evaluated by a pulmonologist. Five patients had active cough; five with productive cough, two with purulent sputum based on SCC, and two reported hemoptysis. Four patients presented with active dyspnea, of whom two obtained a score of 2 on the mMRC, one had a score of 3 and only one had a score of 4. All patients were tachypneic (≥20 breaths/minute) at physical examination, with a median of 23 (IQR: 20–24); the median oxygen saturation was 94.7% (IQR: 92.6–96.8%).
Imaging studies by CT scans of the lungs were done in all patients, of which twelve showed abnormal findings. Figure 1 and Figure 2 show some of the most frequent lesions found in the participants’ CT scans. Most of them were localized in the lower lobe of either lung. Bronchiectasis were present in nine patients, and five were bilateral. Ground-glass opacities (GGO) were found in seven patients, whereas bilateral pleural thickness in five patients. All lung lesions are shown in Table 3 and a detailed descriptions of radiological lung findings on chest CT scans are shown in Appendix B. Pulmonary function by spirometry was successfully assessed in 12 patients as two were unable to tolerate the test. Only two had abnormal pattern findings: one had a restrictive pattern, and the other an obstructive pattern. PVL was measured in all patients; the median PVL value was 1925 copies/mL (IQR = 981–2868 copies/mL).
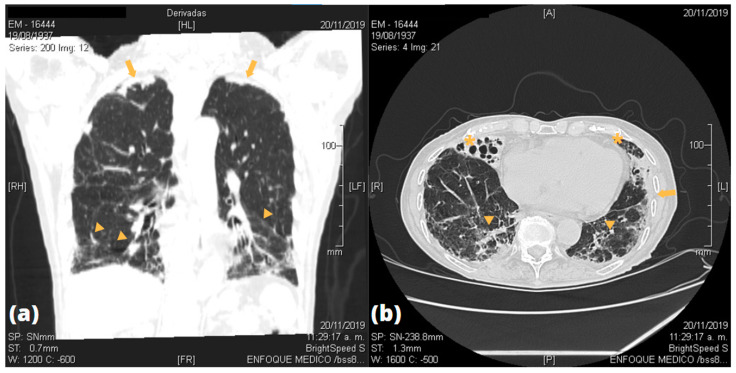
Figure 1.
Images of an 85 years-old female patient with multiple HTLV-1 associated disorders (infective dermatitis, TSP/HAM, Sjögren’s syndrome). (a) Coronal CT scan shows peripheral images of ground-glass pattern in the lower lobes (arrowheads) and apical pleural thickening (arrows). (b) Transverse CT scan shows bilateral saccular bronchiectasis in the lower lobes (asterisks), thickening of bronchovascular bundles, and presence of ground-glass pattern (arrowheads) with pleural thickening (arrow).
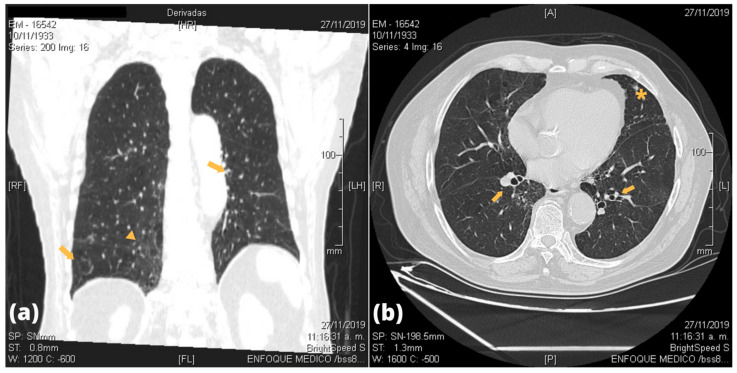
Figure 2.
Images of an 86 years-old male patient with TSP/HAM and neurogenic bladder. (a) Coronal CT scan shows diffuse ground-glass pattern (arrowheads) in the right paramediastinal region, elevation of the left hemidiaphragm and presence of bronchiectasis (arrows). (b) Transverse CT scan shows diffuse ground-glass pattern, bronchiectasis (arrows), and presence of a nodular image (asterisk).
Table 3.
Radiological findings on chest-CT scan of HTLV-1 infected patients.
| Radiological Lung Lesion | Frequency |
|---|---|
| Lobe compromise | |
| Lower lobe: RL, LF | 10/14, 9/14 |
| Medial lobe: RL | 7/14 |
| Upper lobe: RL, LF | 7/14, 5/14 |
| Bronchial lesions | |
| Bronchiectasis | 9/14 |
| Bilateral | 5/9 |
| Atelectasis | 2/14 |
| Alveolar damage | 0/14 |
| Parenchymal damage | |
| GGO/infiltrates | 7/14 |
| Fibro-retractable tracts | 10/14 |
| Bilateral pleural thickness | 5/14 |
CT: computed tomography; HTLV-1: human T lymphotropic virus type 1; RL: right lobe; LF: left lobe; GGO: ground-glass opacities.
Moreover, we did not find significant difference in PVL among those with a history of HTLV-associated disease (p = 0.22) nor in those with abnormal findings on chest-CT scan (p = 0.28). Likewise, there was no difference between PVL median among those with bronchiectasis (p = 1.0) nor pulmonary fibrosis (p = 0.08). Median PVL was 1925 copies/mL (IQR: 981–2868 copies/mL) and 1205 copies/mL among those with abnormal pattern on spirometry (p = 0.59).
3. Discussion
Our study aims to describe the pulmonary damage of HTLV-1 infected patients either on chest-CT scan and in the pulmonary functional tests as well as clinical characteristics of pulmonary disease. We found that most patients with no known TB disease history presented bronchiectasis (mainly found bilaterally) and pulmonary fibrosis. Nonetheless, a normal pattern was found in most patients with a pulmonary functional test. Furthermore, there was no association between the PVL and the chest CT scan findings, nor with spirometry results.
Despite it being a neglected tropical disease, the multi-systemic impact of HTLV-1 infection on the health of patients has been thoroughly studied [21]. Lung injuries due to viral damage are a less-explored spectrum in the context of HTLV-1 infection, albeit the tropism has an estimated prevalence of 30.1% among HTLV-1 carriers [9,22,23]. However, the differential diagnosis, which includes TB disease, may cause similar findings on imaging studies and therefore should be evaluated, especially in hyperendemic countries such as Peru. By excluding patients with known prior active TB disease and who had a documented lung disease, we attempted to isolate the extension of pulmonary damage caused by the virus.
Lung infection due to non-tuberculous mycobacteria (NTM) often occurs in the context of pre-existing lung or chronic diseases [24]; however, the prevalence in developing countries appears to be low compared to developed ones [25]. While the prevalence in Peru is unknown, with only eight cases reported [26,27,28], the national burden of TB disease would make lung lesions by NTM a trivial proportion.
The impact of HTLV-1 infection on pulmonary functional tests has been described as normal pattern on asymptomatic carriers, whilst abnormal findings, either restrictive or obstructive, among those with HAM/TSP, although studies are scarce [29].
The chronic hyperinflammatory cascade found in the bronchoalveolar lavage plays a critical role in the development of pulmonary changes in HTLV-1 patients, activating the recruitment and adhesion of cytokines as well as fibrosis of the lung tissue, leading to structural damage of the lungs [4]. Higher PVL is associated with lung injuries, particularly with bronchiectasis [5,7]; however, we did not find such association in our study. Bronchitis/bronchoalveolitis pattern was found in 30% of HTLV-1 patients with abnormal radiological findings in a Japanese study, whilst bronchiectasis were reported in 19.2% of Australian Aboriginal population with an increased mortality in younger ages associated with bronchiectasis complications [5,30]
While viral lung damage seems certain, there are many environmental factors that might contribute to the development of pulmonary diseases, and that are likely to be expressed in older patients. Exposure to environmental factors is a less controlled factor in studies although the impact on lung health has been well recognized and the household air pollution has been estimated as the leading environmental cause of death worldwide [31,32,33] Biomass fuel and domestic smoking such as wood-burning stoves, a common practice in the Peruvian Andean region, are a known risk factor for several pulmonary diseases including non-TB bronchiectasis, chronic bronchitis, cor pulmonale, and convey 2.5-times greater risk to develop chronic obstructive pulmonary disease [34,35,36]. Four out of the nine patients who presented bronchiectasis on chest-CT scan reported daily use of wood-burning stoves as the main source of cooking. On the other hand, work-related exposure such as pneumoconiosis and drug-related damage may lead to interstitial lung diseases [37]. We found that among those with GGO suggesting fibrotic changes on chest-CT scan, one was an active smoker, four has been daily exposed to secondhand smoke but none had been exposed to mines, paint, lead, or industrial smoke. Furthermore, none of the three patients who reported long-term use of drugs related to lung damage (losartan and sertraline) presented GGO findings on imaging.
The clinical implications of lung damage in HTLV-1 patients need further attention. Chronic cough and dyspnea were the most common symptoms found in our population and the median oxygen saturation was below the normal range. These implications should be assessed by physicians in a rehabilitation program to minimize the burden on quality of life and daily activities. Several authors have recommended lung damage screening through chest-CT scan and a detailed physical examination in HTLV-1 patients [3,7]
Our study is limited due to the small number of participants, it was only possible to evaluate less than a third of the cases identified in the cohort, and therefore any associations should be further evaluated in a larger population with adequate funding to achieve a better follow-up. Additionally, as it has been found lung damage in patients with sub-clinical tuberculosis [38], having no prior history of active TB pulmonary disease as exclusion criteria to rule out the infection and TB-related lung lesions is insufficient. All patients should undergo greater sensitive tests, such as interferon-gamma release assay or protein purified derivative skin test, to confirm the absence of TB exposure. It is necessary to evaluate the risk factors for the development of pulmonary diseases in greater detail, with our study we cannot assert that HTLV-1 infection was the only cause of the pulmonary lesions. Finally, imaging, pulmonary function tests and PVL measurements were assessed in different points from infection or symptom onset, varying greatly between patients. Far more prospective studies on the natural history of HTLV-1 are needed to understand our remaining questions.
4. Methods
4.1. Study Design and Population
We conducted a cross-sectional study between September to December 2019 among HTLV-1-infected patients. Participants were searched from the records of the HTLV-1 clinical cohort of the Instituto de Medicina Tropical Alexander von Humboldt, the largest cohort in Peru with approximately 3500 patients and relatives. The cohort database was searched for patients with (1) diagnosis of HTLV-1 infection using two enzyme-linked immunosorbent assays and/or one confirmatory test (Western blot), (2) a documented lung disease: including bronchitis, pulmonary fibrosis, bronchiectasis or asthma, and (3) without clinical history of tuberculosis infection or current active tuberculosis lung disease. History of tuberculosis disease was evaluated through a detailed review of the medical records, previous imaging results and during an interview.
4.2. Procedures
After providing written informed consent, all participants underwent a non-contrast chest computed tomography (CT) scan and a spirometry test. Blood samples were drawn to measure HTLV-1 proviral load by RT-PCR. Participants were then evaluated by a pulmonologist, who performed a focused physical exam and recorded the patient’s medical history, HTLV-1-asssociated comorbidities, risk factors for asthma, bronchiectasis, pulmonary fibrosis, infections and vaccination history. The Sputum Color Chart (SCC) was applied to characterize the sputum if they presented it at the time of the interview. This chart uses eight photographs of sputum of patients with bronchiectasis, and correlate bacterial colonization with three typical gradations of color (purulence): clear (mucoid), pale yellow/pale green (mucopurulent), and dark yellow/dark green (purulent) [39]. The modified Medical Research Council (mMRC) breathlessness scale was used to characterize shortness of breath by severity, from grade 0 to 4 as follows: (0) get breathless with strenuous exercise; (1) get short of breath when hurrying on level ground or walking up a slight hill; (2) need to walk slower due to breathlessness; (3) stop for breath after walking about 100 m; and (4) feel too breathless to leave the house or get dressed [40].
Imaging findings on chest CTs were classified as following: (a) bronchial lesions, (b) alveolar damage, (c) parenchymal damage and d) pleural damage. Chest-CT scans were reported by a radiologist and were later reviewed by a pulmonologist. Spirometry results were interpreted by the pulmonologist and categorized as normal, obstructive, or restrictive patterns. Spirometry measures included forced vital capacity, forced expiratory volume in one second, the FEV1/FVC ratio, forced expiratory flow at 50% and the peak expiratory flow.
4.3. Statistical Analyses
Participant data was anonymized and entered into a secure database. Analyses were performed using Stata SE 16.1 (StataCorp, US). A descriptive analysis was performed using percentages (%) to describe frequencies for categorical variables; median with interquartile range was used to describe numerical data. In the bivariate analysis, the chi-squared test was used for categorical variables; the Mann–Whitney U test and Kruskal–Wallis rank test were used for two or more continuous variables, respectively.
4.4. Ethical Aspects
The study was approved by the Institutional Ethics Committee of Universidad Peruana Cayetano Heredia (SIDISI: 104427). All participants provided written informed consent before imaging/laboratory procedures and specialist evaluation.
Acknowledgments
The authors would like to acknowledge the tremendous effort of the IMTAvH HTLV-1 Clinical Cohort to provide care to the patients and facilitate their follow-up. The authors would like to dedicate this manuscript to Yessica Ramos, who played a vital role in this and other research studies of the IMTAvH HTLV-1 Clinical Cohort. The authors would also like to thank the extraordinary support of Alberto Hurtado School of Medicine, Universidad Peruana Cayetano Heredia for the publication of this manuscript.
Appendix A
Figure A1.
Flow chart of participants in the study.
Appendix B
Table A1.
Detailed radiological findings on chest-CT scan of HTLV-1 infected patients.
| Patients | Bronchial Lesion | Alveolar Lesion | Parenchymal Lesion | Pleural Lesion |
|---|---|---|---|---|
| P1 | None | None | Scarce right inferior fibrous sequelae tracts | None |
| P2 | Bilateral tubular bronchiectasis | None | Bilateral GGO; scarce right inferior fibrous retractile tracts | None |
| P3 | Bilateral varicose bronchiectasis | None | None | None |
| P4 | None | None | Bilateral apical fibrous retractile tracts | Bilateral apical pleural thickening |
| P5 | Right medial bronchiectasis | None | Bilateral inferior GGO; bilateral apical fibrous retractile tracts; right medial air cyst | Bilateral apical pleural thickening |
| P6 | Right medial and left inferior bronchiectasis | None | Bilateral inferior fibrous retractile tracts; right apical interstitial infiltrate | None |
| P7 | Left atelectasis | None | Bilateral inferior interstitial infiltrate | None |
| P8 | Bilateral cylindrical hilio-perihiliar bronchiectasis; laminar atelectasis | None | Bilateral apical fibrous retractile tracts | Right apical pleural thickening |
| P9 | Bilateral tubular bronchiectasis | None | Bilateral GGO with fibrous tracts | None |
| P11 | Right medial varicose bronchiectasis | None | Bilateral inferior GGO and interlobular septal interstitial thickening; bilateral apical thick fibrous sequelae tracts | Bilateral apical pleural thickening |
| P12 | Right tubular bronchiectasis | None | Bilateral inferior opacities and fibro retractable tracts | Bilateral pleural thickening |
| P13 | Bilateral tubular and varicose bronchiectasis | None | Right inferior GGO; bilateral inferior intra- and interlobular thickening | None |
| P14 | None | None | None | None |
| P15 | None | None | Subpleural infiltrate | None |
CT: Computed Tomography; HTLV-1: Human T lymphotropic virus type 1; P: patient; GGO: ground-glass opacities.
Author Contributions
Conceptualization, R.C., O.G. and E.G.; formal analysis R.C, M.G.-Z. and A.S.; data curation, R.C. and T.W.-T.; writing—original draft preparation, R.C., M.G.-Z., T.W.-T. and A.S.; writing—review and editing, R.C., M.G.-Z., T.W.-T., A.S., F.M., O.G. and E.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of Universidad Peruana Cayetano Heredia (SIDISI 104427, 15 March 2019).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study before imaging procedures, spirometry and specialist evaluation.
Data Availability Statement
The data presented in this study are openly available in FigShare at 10.6084/m9.figshare.14602896.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Eusebio-Ponce E., Anguita E., Paulino-Ramirez R., Candel F.J. HTLV-1 infection: An emerging risk. Pathogenesis, epidemiology, diagnosis and associated diseases. Rev. Esp. Quim. 2019;32:485–496. [PMC free article] [PubMed] [Google Scholar]
- 2.Einsiedel L., Fernandes L., Spelman T., Steinfort D., Gotuzzo E. Bronchiectasis Is Associated with Human T-Lymphotropic Virus 1 Infection in an Indigenous Australian Population. Clin. Infect. Dis. 2012;54:43–50. doi: 10.1093/cid/cir766. [DOI] [PubMed] [Google Scholar]
- 3.Honarbakhsh S., Taylor G.P. High prevalence of bronchiectasis is linked to HTLV-1-associated inflammatory disease. BMC Infect. Dis. 2015;15:258. doi: 10.1186/s12879-015-1002-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dias A.R.N., Falcão L.F.M., Falcão A.S.C., Normando V.M.F., Quaresma J.A.S. Human T Lymphotropic Virus and Pulmonary Diseases. Front. Microbiol. 2018;9:1879. doi: 10.3389/fmicb.2018.01879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Einsiedel L., Pham H., Wilson K., Walley R., Turpin J., Bangham C., Gessain A., Woodman R. Human T-Lymphotropic Virus type 1c subtype proviral loads, chronic lung disease and survival in a prospective cohort of Indigenous Australians. PLoS Negl. Trop. Dis. 2018;12:e0006281. doi: 10.1371/journal.pntd.0006281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Couderc L.J., Rain B., Desgranges C. Pulmonary fibrosis in association with human T cell lymphotropic virus type 1 (HTLV-1) infection. Respir. Med. 2000;94:1010. doi: 10.1053/rmed.2000.0836. [DOI] [PubMed] [Google Scholar]
- 7.Normando V.M.F., Dias Á.R.N., da Silva A.L.S.E., da Silva Pinto D., de Souza Santos M.C., Rodrigues C.L., de Oliviera E.M., de Souza Filho L.E.C., de Brito Vieira W., Andriolo R.B., et al. HTLV-I induces lesions in the pulmonary system: A systematic review. Life Sci. 2020;256:117979. doi: 10.1016/j.lfs.2020.117979. [DOI] [PubMed] [Google Scholar]
- 8.Nakagawa M., Izumo S., Ijichi S., Kubota H., Arimura K., Kawabata M., Osame M. HTLV-I-associated myelopathy: Analysis of 213 patients based on clinical features and laboratory findings. J. NeuroVirol. 1995;1:50–61. doi: 10.3109/13550289509111010. [DOI] [PubMed] [Google Scholar]
- 9.Okada F., Ando Y., Yoshitake S., Yotsumoto S., Matsumoto S., Wakisaka M., Maeda T., Mori H. Pulmonary CT Findings in 320 Carriers of Human T-Lymphotropic Virus Type 1. Radiology. 2006;240:559–564. doi: 10.1148/radiol.2402050886. [DOI] [PubMed] [Google Scholar]
- 10.Tanaka T., Sekioka T., Usui M., Imashuku S. Opportunistic Infections in Patients with HTLV-1 Infection. Case Rep. Hematol. 2015;2015:943867. doi: 10.1155/2015/943867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bastos M.d.L., Santos S.B., Souza A., Finkmoore B., Bispo O., Barreto T., Cardoso I., Bispo I., Bastos F., Pereira D., et al. Influence of HTLV-1 on the clinical, microbiologic and immunologic presentation of tuberculosis. BMC Infect. Dis. 2012;12:199. doi: 10.1186/1471-2334-12-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pedral-Sampaio D.B., Neto M.E., Pedrosa C., Brites C., Duarte M., Harrington W., Jr. Co-Infection of Tuberculosis and HIV/HTLV Retroviruses: Frequency and Prognosis Among Patients Admitted in a Brazilian Hospital. Braz. J. Infect. Dis. 1997;1:31–35. [PubMed] [Google Scholar]
- 13.Verdonck K., González E., Henostroza G., Nabeta P., Llanos F., Cornejo H., Vanham G., Seas C., Gotuzzo E. HTLV-1 infection is frequent among out-patients with pulmonary tuberculosis in northern Lima, Peru. Int. J. Tuberc. Lung Dis. 2007;11:1066–1072. [PubMed] [Google Scholar]
- 14.Verdonck K., González E., Schrooten W., Vanham G., Gotuzzo E., Verdonck K., González E., Schrooten W., Vanham G., Gotuzzo E. HTLV-1 infection is associated with a history of active tuberculosis among family members of HTLV-1-infected patients in Peru. Epidemiol. Infect. 2008;136:1076–1083. doi: 10.1017/S0950268807009521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grassi M.F.R., Dos Santos N.P., Lírio M., Kritski A.L., Almeida M.d.C.C., Santana L.P., Lazaro N., Dias J., Neto E.M., Galvao-Castro B. Tuberculosis incidence in a cohort of individuals infected with human T-lymphotropic virus type 1 (HTLV-1) in Salvador, Brazil. BMC Infect. Dis. 2016;16:491. doi: 10.1186/s12879-016-1428-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pasipanodya J.G., Miller T.L., Vecino M., Munguia G., Garmon R., Bae S., Drewyer G., Weis S.E. Pulmonary Impairment After Tuberculosis. Chest. 2007;131:1817–1824. doi: 10.1378/chest.06-2949. [DOI] [PubMed] [Google Scholar]
- 17.Ravimohan S., Kornfeld H., Weissman D., Bisson G.P. Tuberculosis and lung damage: From epidemiology to pathophysiology. Eur. Respir. Rev. 2018;27:170077. doi: 10.1183/16000617.0077-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.World Health Organization Global Tuberculosis Report 2020. [(accessed on 1 January 2021)];2020 Available online: https://www.who.int/teams/global-tuberculosis-programme/data.
- 19.Ita F., Mayer E.F., Verdonck K., Gonzalez E., Clark D., Gotuzzo E. Human T-lymphotropic virus type 1 infection is frequent in rural communities of the southern Andes of Peru. Int. J. Infect. Dis. 2014;19:46–52. doi: 10.1016/j.ijid.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 20.Cachay R., Schwalb A., Mora R., Caceres T., Gotuzzo E. Infección por virus linfotrópico de células T humanas tipo 1 en pacientes con diagnóstico de tuberculosis multidrogorresistente. Rev. Peru. Med. Exp. Salud Pública. 2019;36:150–151. doi: 10.17843/rpmesp.2019.361.4232. [DOI] [PubMed] [Google Scholar]
- 21.Zihlmann K.F., De Alvarenga A.T., Casseb J. Living Invisible: HTLV-1-Infected Persons and the Lack of Care in Public Health. PLoS Negl. Trop. Dis. 2012;6:e1705. doi: 10.1371/journal.pntd.0001705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamazato Y., Miyazato A., Kawakami K., Yara S., Kaneshima H., Saito A. High Expression of p40 tax and Pro-inflammatory Cytokines and Chemokines in the Lungs of Human T-Lymphotropic Virus Type 1-Related Bronchopulmonary Disorders. Chest. 2003;124:2283–2292. doi: 10.1378/chest.124.6.2283. [DOI] [PubMed] [Google Scholar]
- 23.Sugimoto M., Mita S., Tokunaga M., Yamaguchi K., Cho I., Matsumoto M., Mochizuki M., Araki S., Takatsuki K., Ando M. Pulmonary involvement in human T-cell lymphotropic virus type-I uveitis: T-lymphocytosis and high proviral DNA load in bronchoalveolar lavage fluid. Eur. Respir. J. 1993;6:938–943. [PubMed] [Google Scholar]
- 24.Griffith D.E., Aksamit T., Brown-Elliott B.A., Catanzaro A., Daley C., Gordin F., Holland S.M., Horsburgh R., Huitt G., Iademarco M.F., et al. An Official ATS/IDSA Statement: Diagnosis, Treatment, and Prevention of Nontuberculous Mycobacterial Diseases. Am. J. Respir. Crit. Care Med. 2007;175:367–416. doi: 10.1164/rccm.200604-571ST. [DOI] [PubMed] [Google Scholar]
- 25.von Reyn C.F., Arbeit R.D., Tosteson A.N., Ristola M.A., Barber T.W., Waddell R., Sox C.H., Brindle R.J., Gilks C.F., Ranki A., et al. The international epidemiology of disseminated Mycobacterium avium complex infection in AIDS. International MAC Study Group. AIDS. 1996;10:1025–1032. doi: 10.1097/00002030-199610090-00014. [DOI] [PubMed] [Google Scholar]
- 26.Cordero F.M., Miranda J.G., Mercado J.H., Leiva P.L., Ramos C.S. Compromiso intestinal por Mycobacterium avium en un paciente con VIH/ SIDA. Rev. Gastroenterol. Peru. 2014;34:59–61. [PubMed] [Google Scholar]
- 27.Carrasco J., Soto L., Samalvides F., Asencios L., Quispe N., Valencia E. Infección pulmonar por Mycobacterium avium en paciente VIH/SIDA: Primer reporte en Perú. Rev. Peru. Med. Exp. Salud Pública. 2014;31:156–159. doi: 10.17843/rpmesp.2014.311.23. [DOI] [PubMed] [Google Scholar]
- 28.Ticona-Huaroto C., Astocondor-Salazar L., Montenegro-Idrogo J., Valencia-Mesias G., Soria J. Infección por el complejo Mycobacterium avium—Intracellulare en pacientes con VIH/SIDA en un hospital peruano: Una serie de casos. Rev. Peru. Med. Exp. Salud Pública. 2017;34:323–327. doi: 10.17843/rpmesp.2017.342.2476. [DOI] [PubMed] [Google Scholar]
- 29.Falcão L.F.M., Falcão A.S.C., Sousa R.C.M., Vieira W.d.B., de Oliveira R.T.M., Normando V.M.F., da Silva Dias G.A., De Souza Santos M.C., Rocha R.S.B., Yoshikawa G.T., et al. CT Chest and pulmonary functional changes in patients with HTLV-associated myelopathy in the Eastern Brazilian Amazon. PLoS ONE. 2017;12:e0186055. doi: 10.1371/journal.pone.0186055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamashiro T., Kamiya H., Miyara T., Gibo S., Ogawa K., Akamine T., Moromizato H., Yara S., Murayama S. CT Scans of the Chest in Carriers of Human T-cell Lymphotropic Virus Type 1: Presence of Interstitial Pneumonia. Acad. Radiol. 2012;19:952–957. doi: 10.1016/j.acra.2012.03.020. [DOI] [PubMed] [Google Scholar]
- 31.Jacobson S., Massoud R. Human T-Cell Lymphotropic Virus Type 1 Infection. In: Jackson A.C., editor. Viral Infections of the Human Nervous System. Springer; Basel, Switzerland: 2013. pp. 183–207. [Google Scholar]
- 32.Diette G.B., Accinelli R.A., Balmes J.R., Buist A.S., Checkley W., Garbe P., Hansel N.N., Kapil V., Gordon S., Lagat D.K., et al. Obstructive lung disease and exposure to burning biomass fuel in the indoor environment. Glob. Heart. 2012;7:265–270. doi: 10.1016/j.gheart.2012.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.World Health Organization . Global Health Risks: Mortality and Burden of Disease Attributable to Selected Major Risks. World Health Organization; Geneva, Switzerland: 2009. [Google Scholar]
- 34.Dennis R.J., Maldonado D., Norman S., Baena E., Martinez G. Woodsmoke Exposure and Risk for Obstructive Airways Disease Among Women. Chest. 1996;109:115–119. doi: 10.1378/chest.109.1.115. [DOI] [PubMed] [Google Scholar]
- 35.Sandoval J., Salas J., Martinez-Guerra M.L., Gómez A., Martinez C., Portales A., Palomar A., Villegas M., Barrios R. Pulmonary Arterial Hypertension and Cor Pulmonale Associated with Chronic Domestic Woodsmoke Inhalation. Chest. 1993;103:12–20. doi: 10.1378/chest.103.1.12. [DOI] [PubMed] [Google Scholar]
- 36.Hu G., Zhou Y., Tian J., Yao W., Li J., Li B., Ran P. Risk of COPD from exposure to biomass smoke: A metaanalysis. Chest. 2010;138:20–31. doi: 10.1378/chest.08-2114. [DOI] [PubMed] [Google Scholar]
- 37.Estarriol M.H., Goday M.R., Turmo L.B. Lesiones Pulmonares Inducidas por Fármacos. Elsevier; Amsterdam, The Netherlands: 2002. [(accessed on 1 January 2021)]. Available online: http://www.elsevier.es/es-revista-medicina-integral-63-articulo-lesiones-pulmonares-inducidas-por-farmacos-13034633. [Google Scholar]
- 38.Dutta N.K., Karakousis C. Latent Tuberculosis Infection: Myths, Models, and Molecular Mechanisms. Microbiol. Mol. Biol. Rev. 2014;78:343–371. doi: 10.1128/MMBR.00010-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Murray M.P., Pentland J.L., Turnbull K., MacQuarrie S., Hill A.T. Sputum colour: A useful clinical tool in non-cystic fibrosis bronchiectasis. Eur. Respir. J. 2009;34:361–364. doi: 10.1183/09031936.00163208. [DOI] [PubMed] [Google Scholar]
- 40.Williams N. The MRC breathlessness scale. Occup. Med. 2017;67:496–497. doi: 10.1093/occmed/kqx086. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are openly available in FigShare at 10.6084/m9.figshare.14602896.