Abstract
Listeria spp. is a diverse genus of Gram-positive bacteria commonly present in the environment while L. monocytogenes and L. ivanovii are well known human and ruminant pathogens. The aim of the present study was to reveal the prevalence and genetic diversity of L. monocytogenes and other Listeria spp. and to identify the factors related to the abundance of pathogen at cattle farms. A total of 521 animal and environmental samples from 27 meat and dairy cattle farms were investigated and the genetic diversity of L. monocytogenes isolates was studied with WGS. The prevalence of Listeria was 58.9%, while of L. monocytogenes it was −11%. The highest prevalence of L. monocytogenes was found in the environment—soil samples near to manure storage (93%), mixed feed from the feeding trough and hay (29%), water samples from farms drinking trough (28%) and cattle feces (28%). Clonal complexes (CC) of CC37 (30%), CC11 (20%) and CC18 (17%) (all IIa serogroup) were predominant L. monocytogenes clones. CC18, CC37 and CC8 were isolated from case farms and CC37, CC11 and CC18 from farms without listeriosis history. Only one hypervirulent CC4 (1%) was isolated from the case farm. Sequence types (STs) were not associated with the isolation source, except for ST7, which was significantly associated with soil (p < 0.05). The contamination of soil, feeding tables and troughs with L. monocytogenes was associated with an increased prevalence of L. monocytogenes at farms. Our study indicates the importance of hygienic practice in the prevention of the dissemination of L. monocytogenes in the cattle farm environment.
Keywords: serogroups, clonal complexes, feed, soil, water, feces, epidemiology, WGS, Latvia
1. Introduction
Genus Listeria consists of 21 species of which Listeria monocytogenes and L. ivanovii were found to be pathogenic [1]. While L. ivanovii is associated with animal infection, L. monocytogenes is responsible for listeriosis in humans and animals [2]. In humans, listeriosis is characterized with gastroenteritis or severe manifestations including central nervous system disorders, miscarriage and even death may occur in immunocompromised individuals [3]. L. monocytogenes is a foodborne pathogen and products that do not undergo sufficient thermal treatment to eliminate the pathogen or that are consumed without any processing are considered to be high-risk foods. Outbreaks of listeriosis have been linked to the contamination of unpasteurized milk and milk products, soft cheeses, fish and seafood, ready-to-eat meat products with the growing importance of plant-based novel food vehicles [4,5,6]. Foods with L. monocytogenes may become contaminated before or during processing due to the occurrence and/or persistence of the pathogen in the animal farms and the food-processing environment [7].
Since the same L. monocytogenes genotypes were found in animals, farms and food producing environments, it has been assumed that the dairy cattle may serve as a source of milk contamination with public health implications [8]. L. monocytogenes primarily affects ruminants and may lead to significant economic losses. A typical manifestation of listeriosis in animals includes encephalitis and septicemia, which may cause fetal infection and abortion [9,10]. Listeriosis outbreaks in ruminants were attributed to the consumption of silage, which may become contaminated from external environments including plants and soil, water, manure and wildlife due to the widespread prevalence of Listeria spp. and L. monocytogenes in the environment [11,12]. However, the transmission of L. monocytogenes in farms and their importance for animal and public health is still not well understood [8].
Listeria spp. and L. monocytogenes were isolated from the farm and surrounding environment—water, soil and feed—indicating a variety of different sources of contamination [8,13]. Ruminants are frequently identified as asymptomatic carriers of L. monocytogenes that can directly excrete the pathogen [14,15,16]. This may contribute to the spread of infection between animals and the dissemination of the pathogen in the farm environment. Studies on the diversity of L. monocytogenes in animals and the environment may help to better understand the epidemiology of listeriosis in the ruminants.
The characterization of clinical and environmental isolates of L. monocytogenes is important since the circulation of the same type within farms or geographic regions was reported. In dairy cattle, L. monocytogenes genotypes associated with human outbreaks were found; therefore, the characterization of Listeria isolates has significant public health importance [17,18]. Novel typing methods, such as whole-genome sequencing (WGS), including core genome multilocus sequence typing (cgMLST), can successfully be applied to analyze the origins and contamination sources of L. monocytogenes. Until now, cgMLST showed s higher discriminatory accuracy compared to the pulsed-field gel electrophoresis (PFGE) method and multilocus sequence typing (MLST) that may provide new data on the epidemiology of L. monocytogenes from an animal and public health perspective [19].
WGS and cgMLST-based typing allows the genetic diversity of L. monocytogenes isolates to be characterized by sequence type (ST) and clonal complex (CC) that shows considerable differences in ecology, virulence and the clinical potential of the pathogenic isolates [19,20]. Hypervirulent clones have been reported to be more effective in the colonization of the intestinal tract accompanied with the wider dissemination of the pathogen in the organisms. CC1, CC2, CC4 and CC6 were reported to be associated with a clinical origin [19,20]. Hypervirulent CC1, CC4-CC127 were linked to rhombencephalitis and abortus in ruminants, while a high prevalence of CC2, CC4 and CC11 was found in subclinical cattle mastitis that potentially may serve as milk contaminants [21,22]. CC9 and CC121 are food-associated hypovirulent MLST clones which were implicated in listeriosis in immunocompromised individuals [19,23]. The reported prevalence of hypervirulent clones in animals, farm environments and foods highlights the importance of understanding the ecology and transmission of L. monocytogenes [16,21,24]. Studies on the prevalence and genetic diversity of L. monocytogenes in the cattle farm environment may provide a new insight on the epidemiology of L. monocytogenes. The aim of the present study was to study the prevalence of Listeria spp. and L. monocytogenes in the environment of cattle farms, characterize the genetic diversity of L. monocytogenes and to identify the risk factors related to clinical listeriosis in ruminant farms.
2. Results
2.1. Prevalence of Listeria spp. in the Farm Environment
Altogether six Listeria species were identified in environmental samples from farms with a prevalence of 58.9% (307/521). L. monocytogenes, L. innocua and L. seeligeri were isolated from all types of samples. L. fleishmanii was sporadically found in soil and feed, L. welshimeri in feed and water but L. ivanovii was found in water and animal feces (Table 1). The highest prevalence of L. monocytogenes was found in feces—25.2%, while the lowest was in feed—14.9%. The prevalence of L. monocytogenes at individual farms varied from 0 to 80%. The prevalence of L. monocytogenes of 33% in animals at case farms (11/33) was higher than the prevalence of 22% identified at control farms (17/78). The prevalence of L. innocua was significantly higher than the prevalence of other Listeria species (p < 0.001).
Table 1.
Prevalence of Listeria spp. in the cattle farm environment.
| Listeria Species | Sample | |||||||
|---|---|---|---|---|---|---|---|---|
| Soil (n = 133) | Feed (n = 141) | Water (n = 136) | Feces (n = 111) | |||||
| No. of Positive Samples (%) | 95% CI | No. of Positive Samples (%) | 95% CI | No. of Positive Samples (%) | 95% CI | No. of Positive Samples (%) | 95% CI | |
| L. monocytogenes | 25 (18.8) | 11.9–25.6 | 21 (14.9) | 9.5–21.9 | 26 (19.1) | 12.9–26.7 | 28 (25.2) | 17.5–34.4 |
| L. innocua a | 72 (54.1) | 45.3–62.8 | 51 (36.2) | 28.3–44.7 | 53 (38.9) | 30.7–47.7 | 37 (33.2) | 24.7–42.9 |
| L. seeligeri | 14 (10.5) | 5.8–17.0 | 11 (7.8) | 3.9–13.5 | 11 (8.1) | 4.1–14.0 | 17 (15.3) | 9.2–23.4 |
| L. fleishmanii | 1 (0.8) | 0.0–4.1 | 1 (0.8) | 0.0–3.9 | 0 (0) | 0 | 0 (0) | 0 |
| L. welshimeri | 0 (0) | 0.0–2.7 | 1 (0.8) | 0.0–3.9 | 2 (1.5) | 1.8–5.2 | 0 (0) | 0 |
| L. ivanovii | 0 (0) | 0.0–2.7 | 0 (0) | 0 | 2 (1.5) | 1.8–5.2 | 3 (2.7) | 0.6–7.7 |
| Total | 93 (69.9) | 61.3–77.6 | 71 (50.4) | 41.8–58.9 | 78 (57.4) | 48.6–65.8 | 65 (58.6) | 48.8–67.8 |
a Prevalence of L. innocua was significantly higher than the prevalence of other Listeria species (p < 0.001).
The highest prevalence of L. monocytogenes—29%—was identified in the mixed feed from the feeding trough and hay, while the lowest prevalence of 4% was found in silage. The highest prevalence of L. innocua of 65% was found in the mixed feed from the feeding trough, while the lowest prevalence of L. innocua of 14% was revealed in silage (Table 2). The highest prevalence of L. seeligeri of 19% was observed in hay, but the lowest prevalence of 4% was identified in grains and flours. L. monocytogenes counts were from 1.48 log cfu/g in the total mixed ratio (TMR) to 5.15 log cfu/g in the feed from the feeding troughs. L. innocua counts varied from 2.04 log cfu/g in grass to 5.77 log cfu/g in silage. L. seeligeri counts ranged from 1.83 log cfu/g in hay to 6.06 log cfu/g in silage. The significant differences between the mean counts of L. monocytogenes, L. innocua and L. seeligeri in different types of feeds were not identified (p > 0.05).
Table 2.
Prevalence of Listeria spp. in animal feed at cattle farms in Latvia.
| L. monocytogenes | L. innocua | L. seeligeri | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Feed | No. of Samples | No. of Listeria Positive Samples (%) | No. of Positive Samples (%) | Serogroups | ST a | CC b | Mean log cfu/g | No. Positive Samples (%) | Cfu/g | No. of Positive Samples (%) | Cfu/g |
| TMR c | 5 | 3 (60) | 1 (20) | ND | - | - | 1.48 | 2 (40) | 4.48 | 0 (0) | - |
| By-products d | 4 | 2 (50) | 0 (0) | - | - | - | - | 2 (50) | 2.22 | 0 (0) | - |
| Pastures | 3 | 1 (33) | 0 (0) | - | - | - | - | 1 (33) | 2.04 | 0 (0) | - |
| Feed in feed trough | 34 | 28 (82) | 10 (29) | IIa, IVb | 18, 20, 37, 194, 451 | 5.15 | 22 (65) | 5.22 | 2 (6) | 5.00 | |
| Hay | 21 | 12 (57) | 6 (29) | IIa | 18, 37 | 18, 37 | 4.37 | 6 (29) | 2.2 | 4 (19) | 1.83 |
| Grains and flour e | 23 | 14 (61) | 2 (9) | IIa | 451 | 11 | 2.31 | 11 (48) | 3.74 | 1 (4) | 4.00 |
| Silage | 50 | 11 (22) | 2 (4) | ND | ND | ND | 4.65 | 7 (14) | 5.77 | 4 (8) | 6.06 |
| Feeding Table | 4 | 2 (50) | 0 (0) | - | - | - | - | 2 (50) | 3.54 | 0 (0) | - |
| Silage pit | 5 | 0 (0) | 0 (0) | - | - | - | - | 0 (0) | - | 0 (0) | - |
| Heap | 2 | 0 (0) | 0 (0) | - | - | - | - | 0 (0) | - | 0 (0) | |
| Haylage bales | 19 | 8 (42) | 1 (5) | - | - | - | 2.6 | 4 (50) | 6.00 | 4 (50) | 6.06 |
| Trench | 9 | 0 (0) | 0 (0) | - | - | - | - | 0 (0) | - | 0 (0) | - |
| Tunnel | 1 | 0 (0) | 0 (0) | - | - | - | - | 0 (0) | - | 0 (0) | - |
| Other | 9 | 1 (11) | 0 (0) | - | - | - | - | 1 (11) | 5.15 | 0 (0) | - |
| Straw | 1 | 0 (0) | 0 (0) | - | - | - | - | 0 (0) | - | 0 (0) | - |
| Total | 141 | 71 (50) | 21 (15) | 3.46 f | 51 (36) | 4.04 f | 15 (11) | 4.59 f | |||
a CC—Clonal complexes; b ST—Sequence type; c MR—Total mixed ration; d By-products—rapeseed meal (2), potatoes (1), brewer’s spent grains (1); e L. fleishmanii—one grain sample positive with 10 cfu/g; f The significant differences between the mean counts of L. monocytogenes, L. innocua and L. seeligeri in different types of feeds were not identified (p > 0.05); ND—not identified.
The highest prevalence of L. monocytogenes of 93% was identified in soil near to the manure storage, but the lowest of 33% near to the pond (p < 0.001). The highest prevalence of L. innocua (67%) was found near to the manure storage, while the lowest prevalence of L. innocua of 17% was at the territory of farms. The highest prevalence of L. seeligeri of 20% was found near to manure storage, but the lowest prevalence of 5% was found at the pasture (Table 3). In feed, the highest prevalence of L. monocytogenes (24%), L. innocua (51%) and L. seeligeri (8%) was identified in feed from feed tables and troughs, while the lowest prevalence was found at the pastures—0%, 33% and 0%, respectively. In water, the highest prevalence of L. monocytogenes of 48% was identified in water bodies at pasture, but the lowest of 10% was in drinking troughs. The highest prevalence of L. innocua of 60% was found in drinking bowls, while the lowest of 12% was in other sources (ditch). The highest prevalence of L. seeligeri of 28% was found in water bodies, but the lowest of 8% was in drinking bowls. Other Listeria species associated with water from the farm environment were L. welshimeri and L. ivanovii (1.5%) isolated from the river and the farm water.
Table 3.
Prevalence of Listeria spp. in the soil, feed and water samples from cattle farms.
| Sample | No. of Samples | Listeria spp. | L. monocytogenes | L. innocua | L. seeligeri | |||
|---|---|---|---|---|---|---|---|---|
| No. of Positive Sample (%) | Serogroup | ST | CC | No. of Positive Samples (%) | ||||
| Soil | ||||||||
| Near to pond | 3 | 1 (33) | 1 (33) | IIa | 37 | 37 | 0 (0) | 1 (33) |
| Near to farm | 7 | 4 (57) | 3 (43) | IIa | 7, 37 | 7, 37 | 3 (43) | 0 (0) |
| Near to manure a | 15 | 14 (93) | 3 (20) | IIa | 451 | 11 | 10 (67) | 3 (20) |
| Manure | 26 | 19 (73) | 5 (19) | IIa | 7 | 7 | 15 (58) | 2 (8) |
| Pasture | 21 | 14 (67) | 4 (19) | IIa, | 18, 37, 1085 | 18, 37, 1085 | 12 (46) | 1 (4) |
| Near feed storage | 22 | 15 (68) | 2 (9) | IIa | 8, 403 | 8, 403 | 13 (59) | 3 (14) |
| Bedding at farm | 25 | 16 (64) | 5 (20) | IIa | 18, 20, 21, 37 | 18, 20, 21, 37 | 11 (44) | 3 (12) |
| Straw and bedding in storage | 14 | 8 (57) | 1 (7) | IIa | 7 | 7 | 7 (50) | 1 (7) |
| Feed a | ||||||||
| Feeding table | 59 | 40 (68) | 14 (24) | IIa, IVb | 31,518, 20, 37, 194, 451 | 11, 18, 20, 27 | 5 (8) | |
| Pasture | 3 | 1 (33) | 0 (0) | - | - | - | 1 (33) | 0 (0) |
| Storage | 79 | 29 (37) | 7 (9) | IIa | 18, 37, 451 | 11, 18, 371 | 18 (23) | 6 (8) |
| Water b | ||||||||
| Farm | ||||||||
| -drinking bowl | 60 | 45 (75) | 13 (22) | IIa | 8, 37, 451, 1482 | 8, 11, 37, 1482 | 36 (60) | 5 (8) |
| -drinking trough | 32 | 22 (69) | 9 (28) | IIa | 8, 37, 451, 1482 | 8, 14, 37, 1482 | 13 (41) | 3 (9) |
| -other | 15 | 0 (0) | 0 (0) | 0 | - | - | 0 (0) | 0 (0) |
| Pasture | ||||||||
| -drinking trough | 10 | 3 (33) | 1 (10) | ND | - | - | 2 (20) | 0 (0) |
| -water body | 7 | 7 (100) | 3 (43) | IIa | 18, 37 | 18,37 | 1 (14) | 2 (28) |
| Other | 8 | 1 (12) | 0 (0) | - | - | - | 1 (12) | 0 (0) |
| Feces | 111 | 65 (59) | 28 (25) | IIa, IIc, IVb | 4, 8, 9, 18, 29, 37, 451 | 4, 8, 9, 11, 18, 29, 37 | 37 (33) | 17 (15) |
ST—sequence type; CC—clonal complexes; a The prevalence of L. monocytogenes in soil samples near to manure storage (93%) was significantly higher (p < 0.001); b Differences were not significant differences between the inside and outside environment of the farms for water and soil samples (p > 0.05) excluding feed in feed troughs (p < 0.05); ND—not identified.
2.2. Molecular Serotyping, Clonal Complexes (CCs) and Genetic Diversity of Listeria Species Isolated from the Farm Environment
At least one L. monocytogenes representative for each farm and each source type was selected for sequencing and, after quality control, 67 sequences were included in further characterization. The majority of the sequenced L. monocytogenes isolates were of the IIa serogroup (64 out of 67), two isolates were IVb and one isolate IIc. The serogroup IIa was detected in various sources—soil, feed, water and animal feces—while IVb was in water and feces, but IIc was only in feces (Table 3).
Altogether 15 STs/15 CCs were detected in L. monocytogenes isolates with multilocus sequence typing. In total, the most abundant STs (CCs) were ST37 (CC37) (30%, 20/67), ST451 (CC11) (20%, 13/67) and ST18 (CC18) (17%, 11/67) (Table 4).
Table 4.
Genetic diversity of Listeria monocytogenes isolates in the environmental and animal samples.
| Serogroup | ST/CC | Faeces | Feed | Soil | Water | Total |
|---|---|---|---|---|---|---|
| No. of Isolates | ||||||
| IIa | ST7/CC7 | 0 | 0 | 4 | 0 | 4 |
| ST8/CC8 | 2 | 0 | 2 | 2 | 6 | |
| ST451/CC11 | 4 | 4 | 2 | 3 | 13 | |
| ST399/CC14 | 0 | 0 | 0 | 1 | 1 | |
| ST18/CC18 | 2 | 4 | 3 | 1 | 11 | |
| ST20/CC20 | 0 | 1 | 1 | 0 | 2 | |
| ST21/CC21 | 0 | 0 | 1 | 0 | 1 | |
| ST29/CC29 | 1 | 0 | 0 | 1 | 2 | |
| ST37/CC37 | 2 | 4 | 7 | 7 | 20 | |
| ST403/CC403 | 0 | 0 | 1 | 0 | 1 | |
| ST1482/CC1482 | 0 | 0 | 0 | 2 | 2 | |
| ST1085/CC1085 | 0 | 0 | 1 | 0 | 1 | |
| IIc | ST9/CC9 | 1 | 0 | 0 | 0 | 0 |
| IVb | ST4/CC4 | 1 | 0 | 0 | 0 | 0 |
| ST194/CC315 | 0 | 1 | 0 | 0 | 0 | |
ST—sequence type; CC—clonal complex.
Between the analyzed L. monocytogenes isolates, the predominant STs (CCs) at case farms were ST18 (CC18) (24%, 11/33), ST37 (CC37) (21%, 7/33) and ST (CC8) (18%, 6/33), but at control farms they were ST37 (CC37) (38%, 13/34), ST451 (CC11) (24%, 8/34) and ST18 (CC18) (9%, 3/34) (Table 5). There were not significant differences in the prevalence of the most abundant STs/CCs between the case and control farms (p > 0.05).
Table 5.
Listeria monocytogenes clonal complexes isolated from case and control cattle farms.
| Clonal Complex | Case Farm | Control Farm | Total |
|---|---|---|---|
| No. of Isolates | |||
| CC4 | 1 | 0 | 1 |
| CC7 | 2 | 2 | 4 |
| CC8 | 6 | 0 | 6 |
| CC9 | 1 | 0 | 1 |
| CC11 | 5 | 8 | 13 |
| CC14 | 1 | 0 | 1 |
| CC18 | 8 | 3 | 11 |
| CC20 | 0 | 2 | 2 |
| CC21 | 1 | 0 | 1 |
| CC29 | 0 | 2 | 2 |
| CC37 | 7 | 13 | 20 |
| CC315 | 1 | 0 | 1 |
| CC403 | 0 | 1 | 1 |
| CC1085 | 0 | 1 | 1 |
| CC1482 | 0 | 1 | 1 |
CC—clonal complexes.
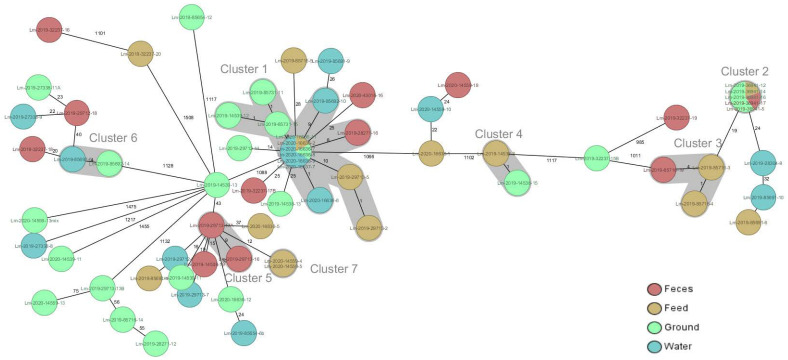
Between the isolates limited to one farm from one up to six different STs (CCs) were observed. Most of the STs (CCs) were not associated with certain sources, except for ST7 (CC7) that was significantly associated with soil and was observed only between soil isolates from four different farms (p > 0.05) (Table 4, Figure 1). Comparing isolates at a cgMLST level (Figure 1), a total of seven clusters were observed with six of them (clusters 2, 3, 4, 5, 6, 7) were limited to a single farm including two to five isolates from various sources. The distances between isolates within clusters were 0–9 alleles. Cluster 1 of ST37 (CC37) included 14 isolates from seven farms. Within Cluster 1, the distance between the isolates limited to one farm was smaller (0–1 allele) than between different farms (0–10 alleles) (Figure 1).
Figure 1.
Ridom SeqSphere + Minimum spanning tree of 67 L. monocytogenes isolates from cattle farms based on L. monocytogenes 1701 cgMLST loci pairwise ignoring missing values. For each isolated farm number and ST that is indicated, the source of isolate is represented by color code and the number on lines represent the distance between isolates in an absolute number of loci. Cluster alert distance—10 loci.
2.3. Factors Associated with L. monocytogenes within-Farm Prevalence
A lack of cleaning and disinfection of the feeding tables was associated with an increased prevalence of L. monocytogenes in soil samples of case farms (odds ratio: 3.89, 95% credibility interval: 1.11–37.31) and control farms (odds ratio: 2.56, 95% credibility interval: 1.09–9.98). The contamination of water samples with L. monocytogenes was associated with the type of production—beef farms had an increased prevalence of L. monocytogenes (odds ratio: 3.29, 95% credibility interval: 1.12–9.87). In this study, there were no significant differences in the association for all the other models.
3. Discussion
Listeria spp. was isolated from the farm environment where the highest prevalence of L. monocytogenes was found in cattle feces (25%). Overall, the prevalence of L. monocytogenes was comparable with previous studies confirming that the cattle may serve as a significant reservoir of L. monocytogenes [8,25]. L. monocytogenes was identified in farms without listeriosis records, which indicates that animals may shed the pathogen asymptomatically and excrete L. monocytogenes in the farm environment, as was proposed previously [14,16,18]. The higher prevalence of L. monocytogenes in cattle from case farms than in control farms was in agreement with previous reports [14,26].
Hay and animal feed in feed bunks were found to be the most contaminated with L. monocytogenes (29%), which was higher than that reported by Fox et al. [27]. In contrast, the identified prevalence of L. monocytogenes in silage (4%), which is supposed to be the main source of the pathogen for cattle, was lower than the 6.2–30% reported previously [8,13,28,29]. Non-L. monocytogenes species were more abundant in baled silage in comparison with L. monocytogenes, which is in line with previous reports [13,30]. Although in case farms baled silage was used, L. monocytogenes was rarely isolated. Silage that suffered aerobic spoilage may harbor Listeria species due to the favorable conditions for the survival and growth of Listeria spp., including pathogenic ones [30]. The low density of silage, the high pH and the presence of oxygen due to bag damage or an insufficient amount of the plastic were linked to the growth of L. monocytogenes in baled silage [12,31].
L. monocytogenes was also isolated from soil, water and feed samples, which indicates that animals can be exposed to different sources of contamination. The farm environment was found to be contaminated with L. monocytogenes due to close contact with the animals; however, the significant differences between the inside and outside environment of the farms were not identified in the water and soil samples (p > 0.05) with the exception of contaminated feed in feed troughs (p < 0.05). Within the individual sampling site, the highest prevalence of L. monocytogenes was found in soil near to the farm (38%), the feed trough and the drinking trough (28%). Our results are in accordance with published studies on the ecology of L. monocytogenes in the farm environment [8,13]. The high prevalence and colonization of feed bunks and water troughs with L. monocytogenes could contribute to the exposure of cattle to the pathogen [8,13]. The fecal shedding and following spread of L. monocytogenes to the surrounding environment could be the main source of infection for animals at farms.
Several L. monocytogenes clones were observed in all the studied farms. The multitude of different contamination sources with L. monocytogenes in the farm environment may lead to the higher diversity of STs (CCs) identified in the cattle fecal isolates due to the exposure to the pathogen from in-farm contamination sites [32]. Widespread distribution of the same CCs in the internal and external environment of the cattle farms supports the hypothesis that cattle act as an important reservoir of L. monocytogenes. Thus, some specific measures, including animal and environmental hygiene, have to be considered to minimize unnecessary Listeria contamination and following growth in the farm environment.
In this study, the majority of CCs from case farms belonged to CC 8, CC11, CC18 and CC37 (all serogroup IIa). CC8, CC9 and CC11 were associated with food and persistence in food-processing environments, and were involved in listeriosis outbreaks [33,34]. CC8 and CC9 were previously identified in meat samples from broad geographical regions [35,36]. CC8 (IIa serogroup) was associated with a higher fatality rate and invasive human listeriosis cases in Poland and was described for the first time in association with the Canadian listeriosis outbreak in 1990–2010s [37,38].
The widespread distribution of L. monocytogenes CC18 and CC37 in control and case farms without significant differences in their prevalence (p > 0.05) may be associated with their environmental origin. In previous studies, ST37 (CC37) was associated with ruminants, ruminant farms and wildlife environments [21,39]. CC37 was prevalent among clinical isolates from ruminant farms [40]. Significantly, a higher prevalence of CC18 and CC37 clones was associated with milk products [33,41]. An abundance of CC37 and CC18 clones may indicate their adaptation and persistence in the cattle farm environment, [33,41]. CC7’s association with foodborne outbreaks, prevalence in foods and animals was established in previous studies [34,40].
CC4 was the only hypervirulent clone that has been isolated in the present study from one case farm. Within lineage I, isolates from CC1 have been reported as a significant cause of listeriosis and rhombencephalitis in ruminants in central Europe that might be related to the hypervirulence of CC1 [21]. The prevalence of hypervirulent clones CC4 and CC6 was reported in clinical isolates from ruminant farms; that is similar to the CC reported in clinical isolates in humans [40,41]. CC6 was associated with abortion in ruminants [40]. The present study reveals the genetic diversity of L. monocytogenes isolates associated with case farms without the predominance of hypervirulent clones in the cattle and farm environments. The high prevalence of environment adapted clones, which were associated with milk and milk products, highlights the importance of the in-farm occurrence of L. monocytogenes for the possible transmission of STs (CCs) in the cattle farms and dairy producing chain. Improper hygienic practices, access to pasture with contaminated soil and shedding of L. monocytogenes were factors associated with the prevalence of L. monocytogenes. Hygienic practice and the disinfection of the feeding table was a significant factor associated with the increased prevalence of L. monocytogenes at control and case farms. In a study by Castro et al. [8], the surfaces of the feeding tables were among the most frequently contaminated with persistent L. monocytogenes that may facilitate the oral intake of the pathogen with food and water. Infrequent cleaning of the feeding bunk was significant for the increased prevalence of L. monocytogenes in milk tanks [42].
The association between the distribution of L. monocytogenes in soil and insufficient disinfection alongside with the widespread distribution of environmental CC indicates that the farm and outside environment may significantly influence the presence of L. monocytogenes at the farm. Significantly, the higher prevalence of L. monocytogenes at beef cattle farms could be attributed to free range production and contamination of the surrounding environment, including the farm and pastures.
4. Materials and Methods
4.1. Sampling
Altogether 521 samples were collected from 27 cattle farms from March 2019 till August 2020 in Latvia. Farms were defined as case farms (n = 9) where ruminant listeriosis was reported within the last three years (2016–2019) and control farms (n = 18) without listeriosis history in the last three years. Overall, 15 to 21 samples were collected from each farm, including on-site samples from the farm’s inside and outside environment, forage and animals. Sampling sites of animal feed (n = 141) included silage and other forage at the storage site and in the feed trough. Water samples (n = 136) were obtained from drinking bowls, troughs and taps at the farm or/and from drinking troughs or water bodies at the pasture. Soil samples (n = 133) were collected from pastures, farm territory, bedding and manure. Samples from animals (n = 111) were collected from cows at farms. Samples were aseptically collected in sample transportation bags with an amount up to 200 g for each individual feed, water and soil sample. Animal samples were collected from rectum with sterile gloves.
4.2. Microbiological Testing and Confirmation of Listeria spp.
The isolation of Listeria spp. from samples was performed according to ISO-11290-1 (2017). For the isolation procedure, an amount of 25 g/mL of sample was enriched in ½ Frazer broth (Biolife, Monza, Italy) and incubated at 30 °C for 24 h. Then, 0.1 mL of enriched suspension was transferred into 10 mL of Frazer broth (all microbiological media—Biolife, Monza, Italy) and incubated at 37 °C for 24 h. A 10-microliter loop of sample suspension in Frazer broth was plated out onto two selective Listeria agars according to Ottaaviani and Agosti (ALOA) and OXFORD formulation (OXFORD agar), and incubated at 37 °C. The presence of characteristic colonies of Listeria spp. was checked after 24–48 h of incubation and typical colonies were small and round (0.5–1 mm in diameter) colonies in blue, green color or blue-green color, with/without opaque halo on ALOA medium and black, brown or olive color colonies on OXFORD medium. Presumptive colonies were subcultured onto sheep blood agar overnight and confirmed and identified with MALDI-TOF Biotyper (Bruker, Bremen, Germany).
4.3. Whole Genome Sequencing
At least one isolate of each source type per farm was selected for WGS analysis. DNA from fresh culture was extracted with QIAamp DNA Mini Kit (Qiagen, Hilden, Germany), according to the manufacturer’s protocol and used for WGS. Nextera XT Library construction kit (Illumina) and Illumina MiSeq with 300 bp paired-end reads were used for the preparation of libraries and sequencing, respectively. Sequencing adapters and low-quality bases were trimmed from raw reads using Trimmomatic v0.38 [43]. De novo assembly of the trimmed reads was performed with SPAdes v3.14.0 [44]. For L. monocytogenes isolate characterization, in general, serotyping in silico and ST/CC with a seven gene multi-locus scheme developed by Ragon et al. [45] were used. For detailed genome characterization, a cgMLST typing scheme based on 1701 gene-by-gene comparison was used [46]. For serotyping, ST and cgMLST determination allele calling were performed by SeqSphere+ (Ridom, Münster, Germany) [47]. The newly identified cgMLST alleles were submitted to the nomenclature server (www.cgmlst.org) maintained by Ridom. After quality control (N50 > 10,000, genome size, average coverage > 30), a total of 67 L. monocytogenes sequence isolates were selected for further data analysis.
4.4. Questionnaire
Case and control farms were included in the study after their consent. During the visit of farms, a set of samples were collected, and the questionnaire was filled in by interviewing the farm owner, farm manager or farm veterinarian (included in Supplementary Data). The questions covered by the questionnaire included type of production and farm characteristics (size, type, number of animals, origin if animals), management (access to pasture, bedding material, drinking and feeding regimes, manure) with an assessment of pasture and in-farm holding condition, origin of feed and feeding regimen and biosecurity (access of farm and surrounding environment, cleaning and disinfection procedures, control of rodents, pests and wildlife, other production and companion animal at holding). Additionally, the information about Listeria-infected animals, including clinical symptoms, was collected at case farms (Table S1).
4.5. Data Analysis
Differences in the prevalence of Listeria species, L. monocytogenes and CCs in feed, water, soil and animal samples were calculated with Fisher’s exact test (p < 0.05). Bayesian binary logistic generalized the linear mixed effects models, as implemented in software R 4.0.4. [48], and library brms [49] were used to test the associations between contamination of the environment (soil, feed, water and feces) (response variable), the farm status (case or control cattle farm) (independent variable) and farm characteristics/management (independent variable). There were multiple models developed where each contained contamination of the tested environment as a dependent variable, and status and the interaction between the farms’ characteristic variables as independent variables. As there were multiple observations per farm and the farm ID was used as a random factor in the model, for the Bayesian models the number of iterations was set to 2000 for each of the four chains. The Rhat values (all values were close to 1.00) were used to assess the convergence of the model. If there was a significant interaction effect in the model, Tukey’s adjusted pairwise comparison of estimated marginal means, as implemented in R library emmeans, [50] was used to compare groups.
5. Conclusions
Our study confirms that despite the widespread occurrence of Listeria spp. and L. monocytogenes in the farm environment, the highest prevalence was associated with animals. The contamination rates and genetic characterization of L. monocytogenes indicates that animals may be the most important source of L. monocytogenes, causing the circulation of L. monocytogenes in the farm and outside environments with further exposure of animals to the pathogen. Improper hygienic practices were strongly associated with the case farms indicating the importance of hygiene measures for the prevention of the on-farm spread of L. monocytogenes.
Acknowledgments
We thank Lelde Tītmane and Aīda Vanaga from the Faculty of Veterinary Medicine of Latvia University of Life Sciences and Technologies for their contribution in sampling and data collection.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/pathogens10070851/s1, Table S1: Questionnaire. Information about the farm.
Author Contributions
Conceptualization, A.B., Ž.Š. and I.M.; methodology, A.B., I.M., Ž.Š., M.T., J.A., M.S., L.A. and D.E.; software, D.E. and J.Ķ.; validation, I.M., Ž.Š., M.T., D.E. and J.Ķ.; formal analysis, M.T., I.M., Ž.Š., D.E. and J.Ķ.; investigation, J.A., L.A., S.G., M.S., I.M., Ž.Š., M.T., D.E. and A.B.; resources, Ž.Š., I.M., J.A., L.A. and M.S.; data curation, M.T., I.M., S.G. and Ž.S.; writing—original draft preparation, M.T.; writing—review and editing, M.T., Ž.Š., I.M., D.E., J.A., M.S., S.G., L.A., J.Ķ. and A.B.; visualization, I.M. and J.Ķ.; supervision, I.M., Ž.Š. and A.B., project administration, I.M. and A.B.; funding acquisition, I.M., Ž.Š. and A.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Latvia Council of Science, grant number Izp-2018/2-0361 “Whole genome-based characterization of environmental Listeria spp. and their role in ruminants listeriosis and public health”.
Institutional Review Board Statement
Not applicable as all fecal samples were collected during the herd health visits performed by the farm veterinarian.
Informed Consent Statement
Informed consent was obtained from the farms owners or representatives involved in the study before they completed the questionnaire and prior to sampling at the farms.
Data Availability Statement
All raw sequence reads generated were submitted to the European Nucleotide Archive (http://www.ebi.ac.uk/ena/) under the study accession number PRJEB45227.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Quereda J.J., Leclercq A., Moura A., Vales G., Gómez-Martín A., García-Muñoz A., Thouvenot P., Tessaud-Rita N., Bracq-Dieye H., Lecuit M. Listeria valentina sp. nov., isolated from a water trough and the faeces of healthy sheep. Int. J. Syst. Evol. Microbiol. 2020;70:5868–5879. doi: 10.1099/ijsem.0.004494. [DOI] [PubMed] [Google Scholar]
- 2.Engelbrecht F., Dominguez-Bernal G., Hess J., Dickneite C., Greiffenberg L., Lampidis R., Raffalsbauer D., Daniels J.J.D., Kreft J., Kaufmann S.H.E., et al. A novel PrfA-regulated chromosomal locus, which is specific for Listeria ivanovii, encodes two small, secreted internalins and contributes to virulence in mice. Mol. Microbiol. 1998;30:405–417. doi: 10.1046/j.1365-2958.1998.01076.x. [DOI] [PubMed] [Google Scholar]
- 3.Doganay M. Listeriosis: Clinical presentation. FEMS Immunol. Med. Microbiol. 2003;35:173–175. doi: 10.1016/S0928-8244(02)00467-4. [DOI] [PubMed] [Google Scholar]
- 4.Bērziņš A., Terentjeva M., Korkeala H. Prevalence and genetic diversity of Listeria monocytogenes in vacuum-packaged ready-to-eat meat products at retail markets in Latvia. J. Food. Prot. 2009;72:1283–1287. doi: 10.4315/0362-028X-72.6.1283. [DOI] [PubMed] [Google Scholar]
- 5.Desai A.N., Anyoha A., Madoff L.C., Lassmann B. Changing epidemiology of Listeria monocytogenes outbreaks, sporadic cases, and recalls globally: A review of ProMED reports from 1996 to 2018. Int. J. Infect. Dis. 2019;84:48–53. doi: 10.1016/j.ijid.2019.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koskar J., Kramarenko T., Meremäe K., Kuningas M., Sõgel J., Mäesaar M., Anton D., Lillenberg M., Roasto M. Prevalence and numbers of Listeria monocytogenes in various ready-to-eat foods over a 5-year period in Estonia. J. Food. Prot. 2019;82:597–604. doi: 10.4315/0362-028X.JFP-18-383. [DOI] [PubMed] [Google Scholar]
- 7.Stea E.C., Purdue L.M., Jamieson R.C., Yost C.K., Hansen L.T. Comparison of the prevalence and diversities of Listeria species and Listeria monocytogenes in an Urban and a Rural Agricultural Watershed. Appl. Environ. Microbiol. 2015;81:3812–3822. doi: 10.1128/AEM.00416-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Castro H., Jaakkonen A., Hakkinen M., Korkeala H., Lindström M. Occurrence, persistence, and contamination routes of Listeria monocytogenes genotypes on three Finnish dairy cattle farms: A Longitudinal study. Appl. Environ. Microbiol. 2018;84:e02000-17. doi: 10.1128/AEM.02000-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oevermann A., Zurbriggen A., Vandevelde M. Rhombencephalitis caused by Listeria monocytogenes in humans and ruminants: A zoonosis on the rise? Interdiscip. Perspect. Infect. Dis. 2010;2010:632513. doi: 10.1155/2010/632513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Whitman K.J., Bono J.L., Clawson M.L., Loy J.D., Sosilevac J.M., Arthur T.M., Ondrak J.D. Genomic-based identification of environmental and clinical Listeria monocytogenes strains associated with an abortion outbreak in beef heifers. BMC Vet. Res. 2020;16:70. doi: 10.1186/s12917-020-2276-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hellström S., Kiviniemi K., Autio T., Korkeala H. Listeria monocytogenes is common in wild birds in Helsinki region and genotypes are frequently similar with those found along the food chain. J. Appl. Microbiol. 2008;104:883–888. doi: 10.1111/j.1365-2672.2007.03604.x. [DOI] [PubMed] [Google Scholar]
- 12.Queiroz O.C.M., Ogunade I.M., Weinberg Z., Adesogan A.T. Silage review: Foodborne pathogens in silage and their mitigation by silage additives. J. Dairy Sci. 2018;101:4132–4142. doi: 10.3168/jds.2017-13901. [DOI] [PubMed] [Google Scholar]
- 13.Mohammed H.O., Stipetic K., McDonough P.L., Ginzalez R.N., Nydam D.V., Atwill E.R. Identification of potential on-farm sources of Listeria monocytogenes in herds of dairy cattle. Am. J. Vet. Res. 2009;70:383–388. doi: 10.2460/ajvr.70.3.383. [DOI] [PubMed] [Google Scholar]
- 14.Nightingale K.K., Schukken Y.H., Nightingale C.R., Fortes E.D., Ho A.J., Her Z., Grohn Y.T., McDonough P.L., Wiedmann M. Ecology and transmission of Listeria monocytogenes infecting ruminants and in the farm environment. Appl. Environ. Microbiol. 2004;70:4458–4467. doi: 10.1128/AEM.70.8.4458-4467.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ho A.J., Ivanek R., Gröhn Y.T., Nightingale K.K., Wiedmann M. Listeria monocytogenes fecal shedding in dairy cattle shows high levels of day-to-day variation and includes outbreaks and sporadic cases of shedding of specific L. monocytogenes subtypes. Prev. Vet. Med. 2007;80:287–305. doi: 10.1016/j.prevetmed.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 16.Esteban J.I., Oporto B., Aduriz G., Juste R.A., Hurtado A. Faecal shedding and strain diversity of Listeria monocytogenes in healthy ruminants and swine in Northern Spain. BMC Vet. Res. 2009;5:2. doi: 10.1186/1746-6148-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rocha P.R., Lomonaco S., Bottero M.T., Dalmasso A., Dondo A., Grattarola C., Zuccon F., Iulini B., Knabel S.J., Capucchio M.T., et al. Ruminant rhombencephalitis-associated Listeria monocytogenes strains constitute a genetically homogeneous group related to human outbreak strains. Appl. Environ. Microbiol. 2013;79:3059–3066. doi: 10.1128/AEM.00219-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haley B.J., Sonnier J., Schukken Y.H., Karns J.S., Van Kessel J.A. Diversity of Listeria monocytogenes within a U.S. dairy herd, 2004–2010. Foodborne Pathog. Dis. 2015;12:844–850. doi: 10.1089/fpd.2014.1886. [DOI] [PubMed] [Google Scholar]
- 19.Moura A., Criscuolo A., Pouseele H., Maury M.M., Leclercq A., Tarr C., Björkman J.T., Dallman T., Reimer A., Enouf V., et al. Whole-genome-based population biology and epidemiological surveillance of Listeria monocytogeens. Nat. Microbiol. 2016;2:16185. doi: 10.1038/nmicrobiol.2016.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maury M.M., Tsai Y.H., Charlier C., Touchom M., Chenal-Francisque V., Leclercq A., Criscuolo A., Gaultier C., Roussel S., Brisabois A., et al. Uncovering Listeria monocytogenes hypervirulence by harnessing its biodiversity. Nat. Genet. 2016;48:308–313. doi: 10.1038/ng.3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dreyer M., Aguilar-Bultet L., Rupp S., Guldimann C., Stephan R., Schock A., Otter A., Schupbach G., Brisse S., Lecuit M., et al. Listeria monocytogenes sequence type 1 is predominant in ruminant rhombencephalitis. Sci. Rep. 2016;6:1–11. doi: 10.1038/srep36419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Papic B., Golob M., Kušar D., Pate M., Zdovc I. Source tracking on a dairy farm reveals a high occurrence of subclinical mastitis due to hypervirulent Listeria monocytogenes clonal complexes. J. Appl. Microbiol. 2019;127:1349–1361. doi: 10.1111/jam.14418. [DOI] [PubMed] [Google Scholar]
- 23.Ortiz S., Lopez-Alonso V., Rodriguez P., Martinez-Suarez V. The connection between persistent, disinfectant-resistant Listeria monocytogenes strains from two geographically separate Iberian pork processing plants: Evidence from comparative genome analysis. Appl. Environ. Microbiol. 2016;82:308–317. doi: 10.1128/AEM.02824-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cabal A., Pietzka A., Huhulescu S., Allelberger F., Ruppitsch W., Schmid D. Isolate-based surveillance of Listeria monocytogenes by Whole Genome Sequencing in Austria. Front. Microbiol. 2019;10:2282. doi: 10.3389/fmicb.2019.02282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bandelj P., Jamnikar-Ciglenecki U., Ocepek M., Blagus R., Vengust M. Risk factors associated with fecal shedding of Listeria monocytogenes by dairy cows and calves. J. Vet. Intern. Med. 2018;32:1773–1779. doi: 10.1111/jvim.15234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Borucki M.K., Gay C.C., Reynolds J., McElwain K.L., Kim S.H., Call D.R., Knowles D.P. Genetic diversity of Listeria monocytogenes strains from a high-prevalence dairy farm. Appl. Environ. Microbiol. 2005;71:5893–5899. doi: 10.1128/AEM.71.10.5893-5899.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fox E., O’Mahony T., Clancy M., Dempsey R., O’Brien M., Jordan K. Listeria monocytogenes in the Irish dairy farm environment. J. Food Prot. 2009;72:1450–1456. doi: 10.4315/0362-028X-72.7.1450. [DOI] [PubMed] [Google Scholar]
- 28.Murinda S.E., Nguyen L.T., Nam H.M., Almeida R.A., Headrick S.J., Oliver S.P. Detection of sorbitol-negative and sorbitol-positive Shiga toxin-producing Escherichia coli, Listeria monocytogenes, Campylobacter jejuni, and Salmonella spp. in dairy farm environmental samples. Foodborne Pathog. Dis. 2004;1:97–104. doi: 10.1089/153531404323143611. [DOI] [PubMed] [Google Scholar]
- 29.Vilar M.J., Yus E., Sanjuán M.L., Diéguez F.J., Rodríguez-Otero J.L. Prevalence of and risk factors for Listeria species on dairy farms. J. Dairy Sci. 2007;90:5083–5088. doi: 10.3168/jds.2007-0213. [DOI] [PubMed] [Google Scholar]
- 30.Nucera D.M., Grassi M.A., Morra P., Piano S., Tabacco E., Borreani G. Detection, identification, and typing of Listeria species from baled silages fed to dairy cows. J. Dairy Sci. 2016;99:6121–6133. doi: 10.3168/jds.2016-10928. [DOI] [PubMed] [Google Scholar]
- 31.McDonald P., Henderson N., Heron S. The Biochemistry of Silage. 2nd ed. Chalcombe Publications; Bucks, UK: 1991. p. 340. [Google Scholar]
- 32.Kim S.W., Haendiges J., Keller E.N., Myers R., Kim A., Lombard J.E., Karns J.S., Van Kessel J.A.S., Haley B.J. Genetic diversity and virulence of Listeria monocytogenes recovered from bulk tank milk, milk filters, and milking equipment from dairies in the United States (2002 to 2014) PLoS ONE. 2018;13:e0197053. doi: 10.1371/journal.pone.0197053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen Y., Gonzalez-Escalona N., Hammack T.S., Allard M.W., Strain E.A., Brown E.W. Core genome multilocus sequence typing for identification of globally distributed clonal groups and differentiations of outbreaks of strains of Listeria monocytogenes. Appl. Environ. Microbiol. 2016;82:6258–6272. doi: 10.1128/AEM.01532-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Knudsen G.M., Nielsen J.B., Marvig R.L., Ng R.L., Worning P., Westh H., Gram L. Genome-wide-analyses of Listeria monocytogenes from food-processing plants reveal clonal diversity and date the emergence of persisting sequence types. Environ. Microbiol. Rep. 2017;9:428–440. doi: 10.1111/1758-2229.12552. [DOI] [PubMed] [Google Scholar]
- 35.Rychli K., Stresl B., Szakmary-Brändle A., Strauß A., Wagner M., Schoder D. Listeria monocytogenes isolated from illegally imported food products into the European Union harbor different virulence factor variants. Genes. 2018;9:428. doi: 10.3390/genes9090428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen M., Cheng J., Zhang J., Chen Y., Zeng H., Xue L., Lei T., Pang R., Wu S., Wu H., et al. Isolation, potential virulence, and population diversity of Listeria monocytogenes from meat and meat products in China. Front. Microbiol. 2019;10:946. doi: 10.3389/fmicb.2019.00946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Knabel S.J., Reimer A., Verghese B., Lok M., Ziegler J., Farber J., Pagotto F., Graham M., Nadon C.A., Gilmour M.W. Sequence typing confirms that a predominant Listeria monocytogenes clone caused human listeriosis cases and outbreaks in Canada from 1988 to 2010. J. Clin. Microbiol. 2012;50:1748–1751. doi: 10.1128/JCM.06185-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kuch A., Goc A., Belkiewicz K., Filipello V., Ronkiewicz P., Golebiewska A., Wrobel I., Kiedrowska M., Wasko I., Hryniewicz W., et al. Molecular diversity and antimicrobial susceptibility of Listeria monocytogenes isolates from invasive infections in Poland (1997–2013) Sci. Rep. 2018;8:1–11. doi: 10.1038/s41598-018-32574-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Raschle S., Stephan R., Stevens M.J.A., Cernela N., Zurfluh K., Muchaamba F., Nüesch-Inderbinen M. Environmental dissemination of pahogenic Listeria monocytogenes in flowing surface waters in Switzerland. Sci. Rep. 2021;11:1–11. doi: 10.1038/s41598-021-88514-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Papić B., Pate M., Félix B., Kušar D. Genetic diversity of Listeria monocytogenes strains in ruminant abortion and rhomenchephalitis cases in comparison with the natural environment. BMC Microbiol. 2019;19:299. doi: 10.1186/s12866-019-1676-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maury M.M., Bracq-Dieye H., Huang L., Vales G., Lavina M., Thouvenot P., Disson O., Leclercq A., Brisse S., Lecuit M. Hypervirulent Listeria monocytogenes clones’ adaption to mammalian gut accounts for their association with dairy products. Nat. Commun. 2019;10:1–13. doi: 10.1038/s41467-019-10380-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hassan L., Guard C.L., Mohammed H.O. Feeding practices associated with the presence of Listeria monocytogenes: A case control study in New York state diaries. Dairy Food Environ. Sanit. 2002;22:326–332. [Google Scholar]
- 43.Bolger A.M., Lohse M., Usadel B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Prjibelski A., Antipov D., Meleshko D., Lapidus A., Korobeynikov A. Using SPAdes de novo assembler. Curr. Protoc. Bioinform. 2020;70:e102. doi: 10.1002/cpbi.102. [DOI] [PubMed] [Google Scholar]
- 45.Ragon M., Wirth T., Hollandt F., Lavenir R., Lecuit M., Le Monnier A., Brisse S. A new perspective on Listeria monocytogenes evolution. PLoS Pathog. 2008;4:e1000146. doi: 10.1371/journal.ppat.1000146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ruppitsch W., Pietzka A., Prior K., Bletz S., Fernandez H.L., Allerberger F., Harmsen D., Mellmann A. Defining and evaluating a core genome multilocus sequence typing scheme for whole-genome sequence-based typing of Listeria monocytogenes. J. Clin. Microbiol. 2015;53:2869–2876. doi: 10.1128/JCM.01193-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jünemann S., Sedlazeck F.J., Prior K., Albersmeier A., John U., Kalinowski J., Mellmann A., Goesmann A., von Haeseler A., Stoye J., et al. Updating benchtop sequencing performance comparison. Nat. Biotechnol. 2013;31:294–296. doi: 10.1038/nbt.2522. [DOI] [PubMed] [Google Scholar]
- 48.R Core Team . R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna, Austria: 2021. [(accessed on 15 April 2021)]. Available online: https://www.R-project.org/ [Google Scholar]
- 49.Bürkner P.C. brms: An R package for Bayesian multilevel models using Stan. J. Stat. Softw. 2017;80:1–28. doi: 10.18637/jss.v080.i01. [DOI] [Google Scholar]
- 50.Lenth R.V. Emmeans: Estimated Marginal Means, aka Least-Squares Means. R Package Version 1.5.5-1. [(accessed on 20 April 2021)];2021 Available online: https://CRAN.R-project.org/package=emmeans.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All raw sequence reads generated were submitted to the European Nucleotide Archive (http://www.ebi.ac.uk/ena/) under the study accession number PRJEB45227.