Abstract
Genome size is one of the fundamental cytogenetic features of a species, which is critical for the design and initiation of any genome sequencing projects and can provide essential insights in studying taxonomy, cytogenetics, phylogenesis, and evolutionary studies. However, this key cytogenetic information is almost lacking in the endemic species Reseda pentagyna and the locally rare species Reseda lutea in Saudi Arabia. Therefore, genome size was analyzed by propidium iodide PI flow cytometry and compared to k-mer analysis methods. The standard method for genome size measures (flow cytometry) estimated the genome size of R. lutea and R. pentagyna with nuclei isolation MB01 buffer were found to be 1.91 ± 0.02 and 2.09 ± 0.03 pg/2 °C, respectively, which corresponded approximately to a haploid genome size of 934 and 1.022 Mbp, respectively. For validation, K-mer analysis was performed on both species’ Illumina paired-end sequencing data from both species. Five k-mer analysis approaches were examined for biocomputational estimation of genome size: A general formula and four well-known programs (CovEST, Kmergenie, FindGSE, and GenomeScope). The parameter preferences had a significant impact on GenomeScope and Kmergenie estimates. While the general formula estimations did not differ considerably, with an average genome size of 867.7 and 896. Mbp. The differences across flow cytometry and biocomputational predictions may be due to the high repeat content, particularly long repetitive regions in both genomes, 71% and 57%, which interfered with k-mer analysis. GenomeScope allowed quantification of high heterozygosity levels (1.04 and 1.37%) of R. lutea and R. pentagyna genomes, respectively. Based on our observations, R. lutea may have a tetraploid genome or higher. Our results revealed fundamental cytogenetic information for R. lutea and R. pentagyna, which should be used in future taxonomic studies and whole-genome sequencing.
Keywords: flow cytometry, endemic, ploidy, rare, Reseda, genome size, k-mer
1. Introduction
The development of advanced genomic technologies, and the subsequent storm of data from next-generation sequencing (NGS), has been a great asset to genomic research. However, many fundamental issues concerning genomes remain mostly unresolved. One such issue is the largely unexplored amount of DNA (C-value) in most of the higher clades of life. The amount of DNA (C-value) in the haploid gametic nucleus is referred to as genome size [1], which is often quantified in picograms (pg) or megabase pairs (1 pg = 978 Mbp) [2] and is typically broadly constant within an organism [3,4]. Besides external characteristics, genome size is a key value for research on taxonomy, ecology, and evolution [5,6]. Variations significant enough to differentiate a population into distinct species may still be difficult to discern employing classic morphological or DNA sequence; nevertheless, such variations may become more obvious when genome size is investigated along with other proofs [7,8]. Moreover, a precise calculation of genome size is a prerequisite in the age of high-throughput sequencing technologies for sequencing projects [7], since it influences the budget plan for anticipated sequencing depths and offers an approximate figure for estimating genome assembly completeness.
As a result, there is a great demand for reliable and easy-to-use methods for calculating genome sizes throughout a wide range of eukaryotic taxa [9]. There are two main methods for calculating genome size: Laboratory and computational. The Feulgen microdensitometer and flow cytometry are fairly tested and often used laboratory approaches [10]. Flow cytometry is a low-cost, relatively reliable, and quick laboratory technique for estimating plant genome size. It is an appealing alternative to microspectrophotometry in that it involves the calculation of DNA quantities based on the staining of undamaged nuclei with a fluorochrome that quantitatively adheres to the DNA. Moreover, for the analysis, only a small amount of tissue is needed, which is important in the case of valuable and/or protected specimens [10,11]. These methods, however, rely on living, adequately fixed, or frozen tissues with substantially intact cells, thereby limiting research to lifeforms that can be cultivated in the lab or easily obtained in the field and transferred to the lab [12]. Furthermore, considering that the significant amounts of phenolic compounds can create stoichiometric errors, the flow cytometry approach must be tailored to each plant species.
Meanwhile, with the explosive growth of next-generation sequencing technology, a computational technique arose through k-mer (distinct subsequences of a given length, k, derived from a longer DNA sequence) approaches. A k-mer frequency distribution could be generated by plotting the coverage distribution over all k-mers in a sequence. This k-mer distribution should resemble a Poisson distribution when the created k-mers from genomic sequencing reads possess minuscule amounts of sequence defects (repetitions, sequencing errors, or coverage bias). The distribution peak will be centered on the average sequencing depth for the genome [13]. K-mer based genome size estimates were accurately employed in many genome projects due to their feasibility and rationale [14,15,16,17]. Researchers can utilize many available programs to estimate genome size using sequencing data as well as the popular equation, i.e., the quotient of the k-mers total number and the peak frequency distribution. However, the accuracy and efficiency of these strategies have not been thoroughly investigated.
The Resedaceae is a relatively small family with only six genera (i.e., Reseda, Randonia, Sesamoides, Oligomeris, Ochradenus, and Caylusea) and about 85 species [18]. Genus Reseda contains approximately 65 species throughout the world, mostly restricted to the Mediterranean basin. Several of its species flourish on soils under arid environments, while others are ruderal weeds and only a few are available in high mountains [19]. Pharmacological studies of various Reseda species showed antimicrobial [20], anti-inflammatory [21], and antioxidant [22] activities. In Saudi Arabia, seven species of the genus Reseda were recorded, viz. R. alba, R. arabica, R. aucheri, R. lutea, R. muricata, R. pentagyna, and R. sphenocleoides [23]. Among these, R. pentagyna is endemic and native to Saudi Arabia and has been observed in northeastern region in Tabuk, Wadi Sawawin, and Northern Hijaz Mountain range [24]. R. pentagyna is an annual sparsely branched herbaceous plant well adapted to hard sand and low rocky hills with stems erect up to 30 cm, distinguished from the other Reseda species via its five to six-toothed capsule [25]. While R. lutea L. is locally rare and restricted only to a single gathering in the mountainous region of Abha, Saudi Arabia [26]. R. lutea L. is a deep-rooted biennial or perennial herbaceous plant that can grow up to 80 cm high and well adapted to fallow fields, rocky slopes, and roadsides. It is distributed and spread throughout many temperate zones of the world [27]. This study aimed to determine the genome size focusing on the endemic species R. pentagyna and the locally rare species R. lutea both experimentally using flow cytometry and computationally using the k-mer approach through a combination of short-read sequencing with bioinformatics tools.
2. Material and Methods
2.1. Plant Material
Seeds from adult plants of both the endemic species R. pentagyna and the rare species R. lutea were collected from Abha, Saudi Arabia, for in vitro plant propagation. The identification was confirmed through morphological features coupled with the assistance of Flora of Saudi Arabia [28] and protologue [29], and a voucher specimen (SBSN00015 and SBSN00016) was deposited at the Seed Bank Herbarium, College of Sciences, King Saud University, KSA. The intact seeds were surface-sterilized with 0.3% sodium hypochlorite for 2 to 3 min, then washed 3 to 4 times with double-sterilized water. The seeds were germinated on 2% agar then inoculated on Murashige and Skoog (MS) medium [30].
2.2. Genomic DNA Extraction
A leaf sample from germinated seeds was detached from the medium and directly used for DNA isolation (Figure 1). Total genomic DNA was isolated from R. lutea and R. pentagyna leaves using the DNeasy Plant Mini Kit (Qiagen, Valencia, CA, USA) according to the manufacturer’s instructions. The NanoDrop2000 spectrophotometer was used to evaluate the purity and amount of DNA (Thermo Fisher Scientific, Waltham, MA, USA). DNA integrity was determined using a 1% (w/v) agarose gel electrophoresis. The nuclear ITS region (internal transcribed spacer sequences) was amplified on an AB Veriti 96 well Thermal cycler (Applied Biosystems, Waltham, MA, USA) using PuReTaq Ready-To-Go PCR Beads (GE Healthcare, Little Chalfont, Buckinghamshire, UK). Universal ITS primers were used for amplification and cycle sequencing (ITS1 and ITS4 [31,32]) using the following conditions: Initial denaturation at 94 °C for 5 min, 25 cycles of denaturation for 30 s at 94 °C, annealing at 48 °C for 30 s, extension at 72 °C for 1 min, and a final extension at 72 °C for 7 min. PCR reactions were examined on a 1.2% (w/v) agarose gel to confirm the concentration and size of the PCR products. Following standard procedures, Macrogen Inc. (Geumchun-gu, Seoul, South Korea) used a 96-capillary ABI 3730xl DNA analyzer (Applied Biosystems, Foster City, CA, USA) to sequence the amplicons bidirectionally.
Figure 1.
Shoots raised on MS medium (a) Reseda lutea; (b) Reseda pentagyna.
2.3. Molecular Identification
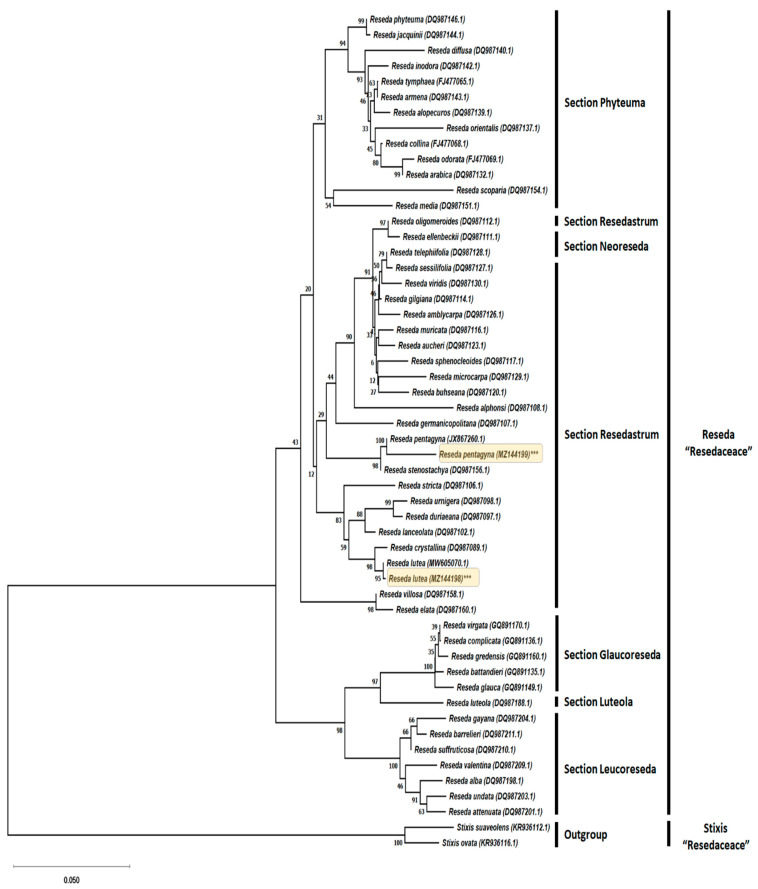
For molecular identification and phylogenetic assessment, ITS sequences from 50 related Reseda species (including representatives from each of the six sections, namely Resedastrum, Phyteuma, Neoreseda, Luteola, Leucoreseda, and Glaucoreseda [18]) were acquired from GenBank (Figure 2). Sequences of two species from the genus Stixis (Resedaceae) were selected and retrieved from GenBank as the outgroup in the phylogenetic analyses (Figure 2). All analyses were implemented in MEGA X [33]. Sequence alignments were performed using Clustal W within the MEGA X windows interface, with manual adjustments. The Neighbor-Joining (NJ) method was utilized for phylogenetic analysis, and the model test was employed to identify the best-fit model for the NJ analysis (Kimura 2-parameter model with a discontinuous Gamma distribution K2 + G). The NJ method was selected for the construction of the phylogenetic tree because it has demonstrated advantages over distance and parsimony approaches to analyze the process of sequence evolution [34]. To obtain statistical support for every internal and external branch, a bootstrap test with 2000 replication was run concurrently for all analyses.
Figure 2.
Molecular identification of Reseda lutea and Reseda pentagyna (highlighted) was conducted via evolutionary analyses in MEGA X and inferred from the analysis of internal transcribed spacer (ITS) sequence using the Neighbor-Joining algorithm. Next to the branches is the percentage of replicate trees around which the linked taxa grouped with each other in the bootstrap test (2000 replicates). The Kimura 2-parameter model was used to calculate the evolutionary distances (NCBI accession numbers between brackets).
2.4. Flow Cytometric Genome Size
The young leaves from multiple shoots raised on MS media were used for the extraction of nuclei. Dr. Jaroslav Dolezel (Laboratory of Molecular Cytogenetics and Cytometry, Institute of Experimental Botany, Sokolorakrá 6, Olomouc, Czech Republic) kindly offered the seeds of external reference Solanum lycopersicum cv. Stupicke (2C = 1.96 pg) [35]. MB01 buffer [36] was used for the estimation of 2C DNA content of Reseda lutea and Reseda pentagyna (2.5 mM Na2EDTA; 20 mM MOPS; 0.2% (v/v) Triton X-100; 80 mM KCl; 0.7 mM Spermine tetrahydrochloride; 20 mM NaCl; pH 7.4). In addition, antioxidants including 1% PVP and 0.5% β-mercaptoethanol were freshly prepared and added for extraction of pure nuclei.
All experiment steps of nuclei extraction were performed on ice (4 °C). The young leaves (30 mg) were chopped with a sharp razor blade into 0.3–0.6 mm size in a petri dish containing ice-cold 500 µL MB01 nuclei isolation buffer. The suspension was mixed by pipetting and filtered through a 20 µM double nylon mesh. After filtration, the nuclei suspension was stained for 10 min with 50 µg/mL of PI (Propidium iodide, Sigma, St. Louis, MO, USA) under dark refrigeration, and the samples were stored on ice prior to analysis.
The fluorescence of a minimum of 5000 propidium iodide-stained nuclei was estimated using a flow cytometer Muse cell analyzer (Merck Millipore, Burlington, MA, USA). The flow rate of the capillary was set at 0.12 µL/s, which is very low. Propidium iodide was measured at 585 nm to read the 2C nuclei DNA content of the sample. The obtained histograms were computerized by Muse cell analyzer software package (Muse 1.8 analyses, Burlington, MA, USA). The sample 2C DNA content was calculated according to the formula [37]:
| (1) |
The number of base pairs per haploid genome was determined using the formula 1 pg DNA = 978 Mbp [2,38]. Three replicate measurements were taken for each plant species independently. The fluorescence histograms were resolved into G0/G1 (2C), S, and G2/M (4C) cell-cycle compartments. The fluorescence mean intensity was taken for the calculation of the 2C DNA content of Reseda species. To improve accuracy, the genome size was determined for each sample as the mean of two technical and three biological replicates, enabling the standard error to be calculated.
2.5. Whole-Genome Sequencing and Filtering Contaminated Reads
Macrogen Inc. prepared the DNA libraries for genome sequencing (S. Korea). Using the TruSeq Nano DNA kit, a paired-end 350 bp insert size library was created for the two species (Illumina, San Diego, California , USA). The libraries were then sequenced using 2 × 151 bp paired-end sequencing on the Illumina NovaSeq6000 platform using standard Illumina operating protocol, yielding a minimum of 90 Gb of raw data. The run’s primary data processing was completed with the manufacturer’s program Real-Time Analysis (RTA 1.18.66.3), followed by the construction of FASTQ sequence files with the Illumina tool bcl2fastq. The raw sequencing reads were deposited in the GenBank database under the BioProject accession PRJNA733338. FastQC v0.11.9 [39] was used to visually examine the raw read quality. Trimmomatic (v0.38) [40] was used to delete the remaining adapter sequences, leading and trailing nucleotides with a Phred score of less than 25, and reads less than 50 bp. SOAPec v2.03 [41] was used to fix the errors in filtered reads. FastUniq v1.1 [42] was used to remove duplicated read pairs. All reads were filtered of potential contaminants by mapping via the BBDuk module (BBMap v38.9 [43]) against a contamination database that included chloroplast, mitochondrial, bacterial, and viral sequences, etc., detected with FastQ Screen [44] keeping only unmapped reads and subsequently assessed again using FastQC.
2.6. K-Mer Based Genome Size
Even though the genome size can be calculated by tallying the k-mer frequency of the read data, the k-mer must be high enough to differentiate most of the genome. The optimal k-mer length for genome size estimation has not been extensively tested. The k-mer value varies amongst investigations, whereas values between 17 and 35 are prominent [45,46]. At least 17 are commonly employed in most eukaryotic genomes to prevent palindromic sequences and the effect of excessively repetitive DNA sequences. For analysis, first the frequency distribution of three k-mers (i.e., 21, 31, and 41) was generated using Jellyfish v2.3.0 [47]. Second, four k-mer analysis-based methods were evaluated for computational genome size estimation, including the most recent dedicated tools (Kmergenie v1.7 [48], GenomeScope v1 [49], FindGSE v1.94 [50], and CovEST-repeat [51]) and the commonly used formula for the calculations of genome size sourced from the equation (M = N × (L – K + 1)/L) proposed by the M.S. Waterman group, where (M) the reads k-mer frequency peak is associated with (N) the actual sequencing depth, (K) kmer length, and (L) read length [13,52,53]. Third, the ploidy structure was estimated with Smudgeplot v0.2.3 [54]. Finally, GenomeScope v1 was run using k-mer length (k = 21) and analyzed the histograms to estimate the complexity of the genome (heterozygosity and repeats) with maximal k-mer coverage = − 1.
3. Result and Discussions
3.1. Molecular Identification
Internal transcribed spacer ITS sequences of nuclear ribosomal DNA have received a lot of attention over the last two decades, not only because of their effectiveness in performing plant phylogeny at a lower taxonomic level, but also because they are regarded as far more reliable markers available for plant DNA barcoding. Due to the highly intriguing morphological similarities reported across Reseda species [28,29], molecular identification and phylogenetic analysis with ITS were implemented to determine the species designation of R. lutea and R. pentagyna.
To validate the morphology-based taxonomic identification of R. pentagyna and R. lutea, the ITS region was sequenced and aligned to 50 Reseda species with ITS sequences currently available at NCBI (including the ones for both R. lutea and R. pentagyna). The combined length of ITS region for the two plants comprised 699 and 707 nucleotides, respectively. A BLAST screening of R. pentagyna’s ITS query sequence revealed the highest sequence identity and similarity to previously published R. pentagyna ITS sequences JX867260.1 97.95% and similarly R. lutea 99.86% for itself KR936125.1.
The Neighbor-Joining algorithm was used to infer the evolutionary phylogram tree with the lowest BIC (Bayesian Information Criterion) score of 9855.053 based on the Kimura 2-parameter model to estimate a matrix of pairwise distances. The evolutionary rate differences between sites were modeled using a discrete Gamma distribution (5 categories). The tree is depicted to scale, and branch lengths are calculated by counting the number of substitutions for each site. The tree was rooted with the help of Stixis suaveolens (KR936112.1) and Stixis ovata (KR936116.1) as an outgroup. Bootstrap supports (%) with a value greater than 50% are displayed above branches.
The Neighbor-Joining tree derived from the analysis of ITS sequences is in line with previous phylogenetic analyses and revealed grouping of Reseda species consistent with established taxonomic sections of the genus, R. pentagyna showed proximity with R. stenostachya (98% bootstrap support), while R. lutea showed proximity with R. crystallina (99% bootstrap support) nested within the clade of section Resedastrum (Figure 2). The research concluded that Reseda species were grouped and consistent with preexisting taxonomic sections [18]. As a result, our ITS analysis validated the taxonomic identification and classification of the examined plants based on morphology.
3.2. C-Value Determination via Flow cytometry
Due to the development of flow cytometry, the study of genome size and its significance has dramatically increased in recent years not just as a taxonomic marker, but also for assessing how it corresponds to environmental, ecological, and phenotypic variables [55,56,57,58]. Furthermore, before determining the nucleotide sequence of a plant’s DNA, it is necessary to understand how large the genome is [59]. According to a large-scale analysis of plant genome sizes, large genomes are less resistant to environmental pressures like drought or pollution, and are less capable of adjusting, making them more vulnerable to extinction [60,61]. Consequently, the genome size evolution heads toward small genomes [59]. Therefore, knowledge of the genome size of the two species of Reseda under study could be used for the prediction of the threat of extinction particularly the rare species R. lutea [60].
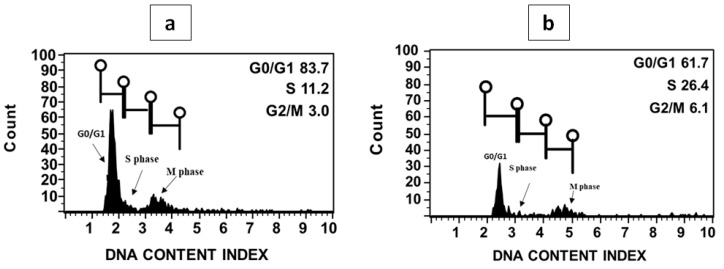
Preliminary testing revealed the success of flow cytometry analyses with both Reseda species forming peaks in the histograms. The 2C peaks in the histograms for fresh plant materials were suitable for genome size estimation (Figure 3). The nuclear DNA content of the two species of Reseda was evaluated by flow cytometry using tomato (2C = 1.96 pg) as an external reference standard, which was later determined to be the most appropriate standard for Reseda samples due to their proximate DNA content. The genome size for R. lutea and R. pentagyna showed a narrow range and was estimated to be 1.91 ± 0.02 and 2.09 ± 0.03, respectively (Table 1). Our estimations for R. lutea and R. pentagyna constitute one of the highest values so far for this genus Reseda (0.92–2.86 pg/2C). For R. lutea, whose genome size had previously been assessed, there was a clear agreement with earlier findings (Table 2). The slight difference in DNA content could occur due to the type of laser lamp equipped in the flow cytometer [62]. According to Soltis et al. [63] classification, both Reseda species genomes belong to the category of plants with a smaller genome. The genome of R. pentagyna is around the same size as that of R. lutea, an octoploid species [18]. Furthermore, its genome is nearly twice as large as that of R. suffruticosa, which possesses a tetraploid genome [18,64].
Figure 3.
Histogram of fluorescence intensity for genome size assessments in R. lutea (a) and R. pentagyna (b) nuclei stained with propidium iodide prepared from shoot tissues. The two major phases of the cell cycle (interphase G0, G1, S, G2 and the mitotic phase M).
Table 1.
C-value comparison (flowcytometry vs. k-mer) genome size and G0/G1 phases of the cell cycle in Reseda lutea and Reseda pentagyna. Genome size (mean ± standard deviation). The number of individuals analyzed for genome size (n).
| n | G0/G1 (%) | 2C DNA Content (pg) | 1C DNA Content (pg) | 1C DNA Content (Mbp) | 2C K-Mer (pg) | 1C K-Mer (pg) | 1C K-Mer (Mbp) | |
|---|---|---|---|---|---|---|---|---|
| Reseda lutea | 3 | 86.53 ± 2.20 | 1.91 ± 0.02 | 0.955 ± 0.01 | 973.11 | 1.78 | 0.89 | 867.7 |
| Reseda pentagyna | 3 | 80.96 ± 0.83 | 2.09 ± 0.029 | 1.045 ± 0.015 | 1026.9 | 1.84 | 0.92 | 896.3 |
* The external reference standard [Solanum lycopersicum (2C = 1.96 pg)] * c-values with the two methods are significantly different at p < 0.01 (one-sample t-test).
Table 2.
| Species | 2C (pg) | Chromosome Number | Ploidy Level | Basic Chromosome Number | Section | |
|---|---|---|---|---|---|---|
| 1 | R. lutea | 2.06 | 24, 48 | 4-8 | 6 | Resedastrum |
| 2 | R. stricta | 2.86 | 24 | 4 | 6 | |
| 3 | R. lanceolata | 1.70 | 24 | 4 | 6 | |
| 4 | R. odorata | 0.96 | 12 | 2 | 6 | Phyteuma |
| 5 | R. phyteuma | 1.34 | 24 | 4 | 6 | |
| 6 | R. media | 2.09 | 12 | 2 | 6 | |
| 7 | R. undata | 1.22 | 20 | 4 | 5 | Leucoreseda |
| 8 | R. barrelieri | 1.68 | 20 | 4 | 5 | |
| 9 | R. suffruticosa | 0.92 | 20 | 4 | 5 | |
| 10 | R. alba | 1.45 | 40 | 8 | 5 | |
| 11 | R. luteola | 1.75 | 24 | 4 | 6 | Luteola |
| 12 | R. glauca | 2.11 | 28 | 4 | 7 | Glaucoreseda |
| 13 | R. complicata | 1.71 | 28 | 4 | 7 | |
| 14 | R. virgata | 1.44 | 28 | 4 | 7 | |
| 15 | R. gredensis | 2.63 | 28 | 4 | 7 |
3.3. Whole-Genome Sequencing
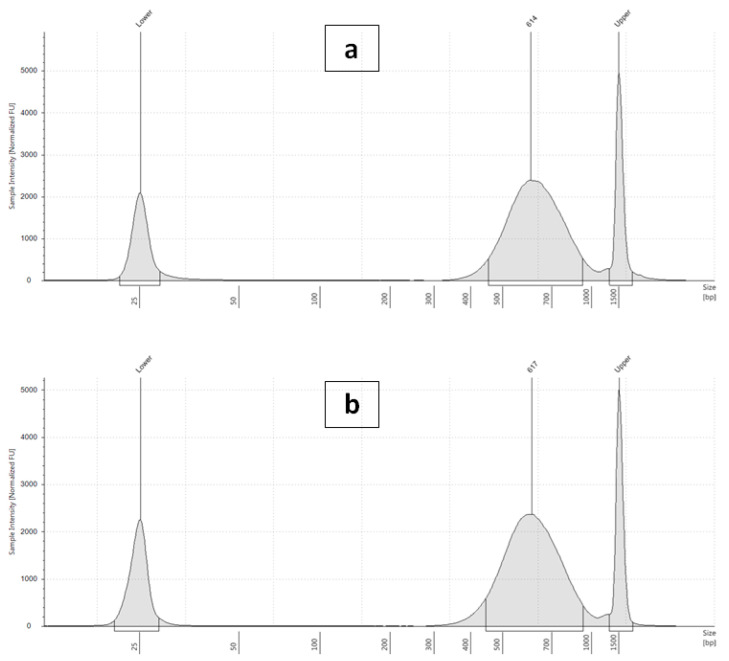
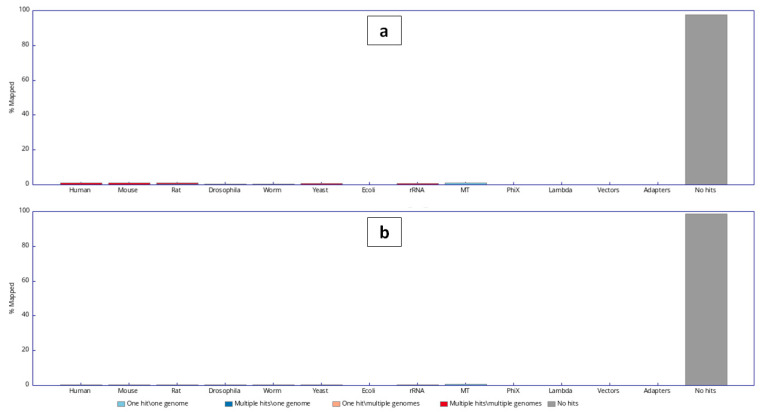
The development of improved sequencing technology capable of producing considerable amounts of sequence data at a low cost, combined with enhanced assembling procedures, has expanded both model and non-model plants genome sequencing [66]. The paired-end 350 bp insert size libraries (Figure 4) of R. lutea and R. pentagyna were sequenced using the HiSeq 2500 Illumina sequencing platform, which produced 358.2 and 352.4 million pairs of 151bp reads, accounting for a total of 108.2 Gb and 106.4 Gb of sequence, respectively. Based on the flow cytometry estimates of genome size, the sequence data represented more than 100× coverage of both genomes (Table 3). Tools for estimating genome size employing k-mer distributions perform much better whenever the average coverage is higher than 10× [67]. Quality filtering (removing bases with a Phred score of less than 25 and reads shorter than 50 bp) did not significantly decrease the dataset. Approximately 0.6–1.5% of the reads identified by FastQ Screen (Figure 5) as contaminants (chloroplast, mitochondrial, bacterial, and viral sequences, etc.), which in turn were used to map the clean reads with bbduk2, leaving between 637.9 Mbp and 632.3 Mbp unmapped reads for further processing (Table 3 and Figure 6). After the quality filtering was established, the raw data mean read length was 148bp.
Figure 4.
Quality check electropherogram of post-library construction on the Agilent 2200 TapeStation (a) Reseda lutea and (b) Reseda pentagyna.
Table 3.
Summary statistics for Reseda lutea and Reseda pentagyna genome sequences.
| Sample ID | Fragment Length (bp) | Read Length (bp) | Total Reads | Clean Unmapped Reads a | GC(%) | AT(%) | Q20(%) | Q30(%) |
|---|---|---|---|---|---|---|---|---|
| Reseda lutea | 614 | 2 × 151 | 716,375,240 | 637,861,144 | 45.01 | 54.99 | 95.83 | 90.59 |
| Reseda pentagyna | 617 | 2 × 151 | 704,839,182 | 632,346,592 | 52.83 | 47.17 | 97.18 | 92.59 |
a Clean reads: The number of reads that have survived after quality trimming and contamination removal.
Figure 5.
The plot shows the frequency distribution of the contaminating taxa for (a) Reseda lutea and (b) Reseda pentagyna.
Figure 6.
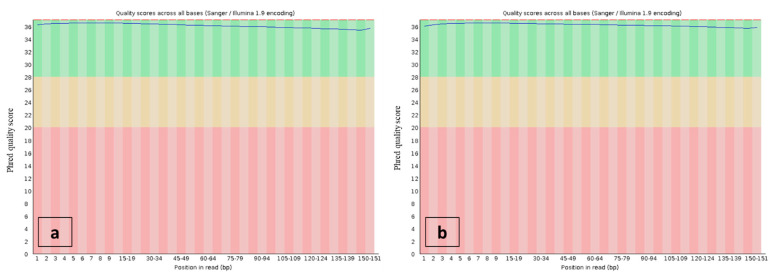
Phred quality scores throughout the read bases—dataset ready for genome size estimation—Reseda lutea (a) and Reseda pentagyna (b).
3.4. K-Mer Based Genome Size and Complexity
Accurate genome size measurement is crucial for genome research projects [1], and it provides data for analyzing variation in genome size over a wide taxonomic group [68]. Nevertheless, calculating genome size effectively with flow cytometry demands the elimination of potential erroneous sources [12,69,70]. Flow cytometry analysis may exaggerate the measured values due to the impact of various plant metabolites on stain binding. Consequently, k-mer analysis was carried out to validate the flow cytometry findings. Although estimates based on k-mer analysis may vary depending on the program’s parameter choices, the quality of the sequencing data may also hold a role. Hence, four methods were investigated for computational genome size prediction using k-mer analysis, including the most notable trusted programs (CovEST-repeat, kmergenie, GenomeScope, and FindGSE) and the widely used equation for genome size calculations sourced from the formulas proposed by M.S. Waterman group. The GenomeScope authors suggested k-mer 21 as an acceptable compromise between both computation accuracy and speed [49], while k-mers ranging from 17–27 have been employed in other research [45,50,71]. In this study, all k-mer evaluations were executed with k values ranging from 21–41 to ensure that the k length had no effect on the estimations. The impacts of k-mer size (21-mer, 31-mer, and 41-mer) and raw vs. quality processed data were explored for each program (Table 4). The differences between raw and quality processed datasets were minor and skewed in favor of processed data.
Table 4.
K-mer estimations of genome size (Mbp) utilizing raw (R) and quality processed (P) sequencing data for Reseda lutea and Reseda pentagyna.
| Reseda lutea | |||||||
| Genome Estimation Software | K21 | K31 | K41 |
Average
Processed data (SD) |
|||
| R | P | R | P | R | P | ||
| General Formula | 845 | 851 | 860 | 868 | 876 | 884 | 867.7 (16.5) |
| FindGSE | 864 | 876 | 972 | 988 | 1077 | 1078 | 980.7 (101.2) |
| Covest-Repeat | 826 | 772 | 885 | 958 | 1123 | 1209 | 979.67 (219.3) |
| Kmergenie | 391 | 401 | 471 | 483 | 542 | 559 | 447.7 (132.6) |
| GenomeScope V1 | 584 | 591 | 652 | 665 | 788 | 796 | 684 (103.8) |
| Reseda pentagyna | |||||||
| Genome Estimation Software | K21 | K31 | K41 |
Average
Processed data (SD) |
|||
| R | P | R | P | R | P | ||
| General Formula | 871 | 880 | 874 | 882 | 931 | 927 | 896.3 (26.6) |
| FindGSE | 768 | 781 | 825 | 848 | 935 | 971 | 866.7 (96.4) |
| Covest-Repeat | 817 | 825 | 1010 | 1067 | 1249 | 1318 | 1070 (246.51) |
| Kmergenie | 484 | 486 | 582 | 591 | 611 | 614 | 552.3 (66.4) |
| GenomeScope V1 | 515 | 524 | 602 | 619 | 723 | 748 | 630.3 (112.4) |
(K21) (K31) (K41) k-mer sizes; (SD) Standard Deviation.
According to our findings, the behavior of kmergenie and GenomeSope performance was drastically affected by increasing k-mer. The GenomeSope genome size estimates in processed data varied from 591 Mbp to 796 Mbp in R. lutea and from 524 Mbp to 748 Mbp in R. pentagyna. A closer examination of the kmergenie results revealed that the predicted genome was roughly half the output expected for both species’ haploid genomes, resulting in an underestimated genome size. This was also demonstrated in investigations with cane toad [72], vanilla [73], and Pacific oyster [49,74], where k-mer-based GenomeScope estimations of genome sizes were barely half of those derived by flow cytometry and far smaller than those achieved after genome assembly. The discrepancy demonstrates that these strategies might be unreliable in some instances. The genome size estimates from the other k-mer methods were generally slightly low compared to flow cytometry estimates, but different from CovEST “repeat” estimates, which were higher on average than the size suggested by the flow cytometry measurements. Such a basic pattern was also detected while matching whole-genome assemblies to flow cytometry and Feulgen staining [75].
GSE predicted R. lutea genome size of average 980.7 ± 101.2Mbp while 886.7 ± 96.4 Mbp in R. pentagyna. For both genomes with the General Formula prediction, the effects of using different kmer sizes were small (<0.016 Gbps). With this Formula, the genome size estimates for R. lutea and R. pentagyna of 907 ± 16.5 Mbp and 896.3 ± 26.6 Mbp. In general, the haploid genome size estimation of R. lutea based on k-mer distributions of the Illumina sequence reads ranged from 447.7Mbp (kmergenie), over 680 Mbp (GenomeScope), over 860 Mbp (General Formula), to over 950 Mbp (FindGSE) while R. pentagyna ranged from 552.3Mbp (kmergenie), over 630.3 Mbp (GenomeScope) to around 900 Mbp (FindGSE, General Formula) (Table 4).
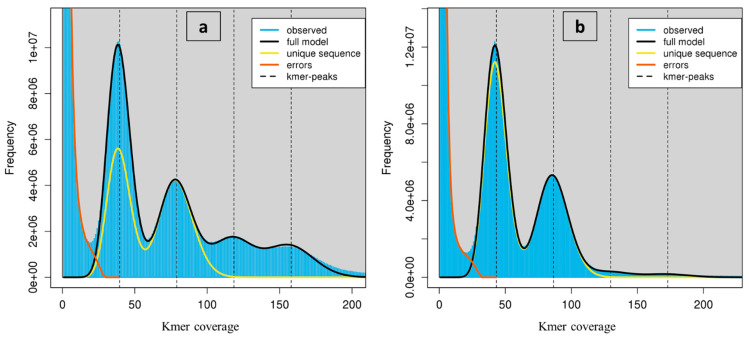
The k-mers depth distribution histograms (Figure 7) revealed a unique bimodal profile in both species with a high peak around 40× coverage and a shorter peak around 80×. This could be evidence of a highly heterogeneous genome [49]. Additionally, GenomeScope estimated that all genomes consisted of high repetitive sequences. Values of lower k-mers yielded much lower genome size estimates than suggested by flow cytometry, while larger k values produced estimates that were more consistent. The C-value determined by the General Formula k-mer analysis average value for R. lutea was 1.78 pg/2C, which is 0.13 pg lower than the C-value determined by flow cytometry. The proportion of repetitive sequences was determined to be approximately 71.26% based on the distribution of k-mers, while heterozygosity was approximately 1.04% (Table 5). The proportion of repetitive sequences and heterozygosity in R. pentagyna were approximately 56.77% and 1.37%, respectively. The C-value based on k-mer analysis was 1.84 pg/2C, which is 0.25 pg lower than that predicted from flow cytometry (Table 1). Similar inconsistencies have been documented for Arabidopsis thaliana, as well as European eels, and were attributed to chemical compounds interference in stoichiometric DNA content estimations in flow cytometry analysis [50,76].
Figure 7.
K-mer profile (k = 41) spectrum analysis to estimate genome size in Reseda lutea (a) and Reseda pentagyna (b) generated from sequence data using GenomeScope V1. The high peak at quite low depths, induced by sequencing errors, has been trimmed to empower visualization.
Table 5.
Genome properties of Reseda lutea and Reseda pentagyna.
| Genome size Property * | Reseda lutea | Reseda pentagyna | ||
|---|---|---|---|---|
| min | max | min | max | |
| Homozygous (%) | 98.96 | 98.96 | 98.63 | 98.63 |
| Heterozygous (%) | 1.04 | 1.04 | 1.37 | 1.37 |
| Genome Haploid Length (bp) | 789,888,133 | 796,236,693 | 747,545,978 | 747,661,754 |
| Genome Repeat Length (bp) | 562,888,521 | 566,147,654 | 424,413,611 | 424,480,553 |
| Genome Unique Length (bp) | 226,999,612 | 230,089,039 | 323,132,368 | 323,181,201 |
| Model Fit (%) | 91.35 | 97.38 | 95.13 | 98.3 |
| Read Error Rate (%) | 0.06 | 0.06 | 0.12 | 0.12 |
| Repeats (%) | 71.26 | 71.1 | 56.77 | 56.77 |
* Estimated from processed reads by GenomeScope v1 with k = 41.
However, the observed slight difference in genome size estimated for R. lutea and R. pentagyna when determined through using k-mer and flow cytometry methods could be attributable to the comparatively low sequencing depth as well as the relatively significant proportion of complex or long repetitive elements (>short reads) in these species’ nuclear genomes (Table 5) that were not recovered in the sequencing [77]. According to Kidwell [78], there is a close association between repetitive DNA sequences and genome size, and the link was demonstrated by Li et al. [79]. Once they account for a high fraction of the genome, repetitive elements are known to restrict genome size estimates downwards [80].
In maize, repeats account for approximately 80% [81] of the genome, with a sophisticated structure that complicates whole-genome sequencing [82]. These constraints could be quickly overcome with further participation of deep sequencing from third-generation sequencing technology. Additionally, the substantial genome size estimated for R. lutea and R. pentagyna (≈1 Gbp) indicates that constructing a high-quality (i.e., chromosomal level) genome will most likely require a combination of short and long reads (i.e., ONT, PacBio). Long reads with lengths of ~10–20 kbp [83] can allow clarification of repetitive genomic zones, while short reads, in turn, increase assembly accuracy since their error rate is relatively lower than long reads ones [84,85].
The kmer length had a significant impact on predicted genome size in both species (p-value >0.01—one-way ANOVA).
3.5. Ploidy Level Estimation
Detailed bibliographic research on the documented basic chromosome number and ploidy levels of the examined taxa was performed to determine the DNA ploidy level. In terms of chromosomal numbers and ploidy level (mostly from the following online databases and bibliography: Plant DNA C-values Database [86], Chromosome Counts Database (CCDB) [87], and Index of Plant Chromosome Numbers [88]).
In these studies, the basic chromosome number was proposed to be (× = 6) within the Resedastrum section (Table 2) with two ploidy levels (terta-, octoploid) [64,89], and species possessing chromosome counts n = 24 or more were proposed to have evolved from interspecific hybridization and the generation of reproductive plants through hybrid genome doubling [65]. Previously reported chromosome counts for R. lutea have been inconsistent [90] with most reports determining its chromosome number to be 2n = 48 [25,64] whereas few studies identified the chromosome numbers to be 24 [25]. Considering the documented chromosomal counts in R. lutea and its unavailability in R. pentagyna and depending on the comparable C value among both species, we hypothesize that these species possess the same number of chromosomes, 48.
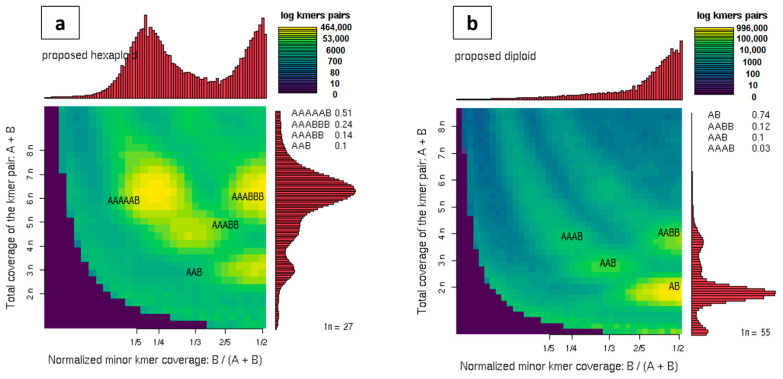
Moreover, the wide range of DNA content between species (0.92–2.86 pg/2C) in the genus Reseda usually supports changes in ploidy, hence Illumina reads were used to assess the ploidy level via Smudgeplot, which uses the ratio of heterozygous k-mer pairs to estimate ploidy. Analysis of R. lutea sequence data provided hints that a polyploid genome, from analysis with a k-mer size of 21 with the most abundant k-mer pairs, is the hexaploid heterozygous (AAAAAB) form of R. lutea (Figure 8). To our knowledge, this is the first report of a hexaploid form of R. lutea. The probability of R. lutea possessing a polyploid genome has been implied based on the genome size expansion and the increase in the basic chromosome numbers.
Figure 8.
Two-dimensional heat maps were constructed to depict the prediction of ploidy from clean reads using Smudgeplot (k = 21). (a) Reseda lutea and (b) Reseda pentagyna. The color intensity corresponds to the approximate amount of k-mers per bin, ranging from purple (weak) to yellow (strong). Estimated ploidies are shown in the upper left corner of each graph, with the likelihood of various ploidies shown on the right.
Meanwhile, R. pentagyna Smudgeplot analysis supported a diploid heterozygous genome (AB) and not polyploidy, which may be the result of the occurrence of a strict uncommon autopolyploid phenomenon that has been revealed in some species [91] and the analysis tool could not interpret. Smudgeplot is designed to predict high heterozygous species and therefore fails to interpret a totally homozygous polyploid genome [54]. However, more cytological studies should be carried out to confirm the chromosome number and verify the ploidy type. Furthermore, because there is a good association between DNA content and ploidy within a species, population-size studies using flow cytometry could be undertaken in the future to differentiate ploidy levels within a species.
4. Conclusions
The significance of the genome size trait is self-evident, as it not only determines plant community configurations at the ecological level, but also impacts plant genome evolution. In this study, the first published flow cytometry estimate for R. pentagyna and a confirmation of the previously reported estimate for R. lutea were presented alongside the validation and comparison against the estimates via the exploitation of short-read sequence data k-mer analysis. However, some k-mer-based tools demonstrated consistency with flow cytometry estimates. Unfortunately, k-mer analysis remains problematic since its estimates fluctuate based on the tool parameter choices as well as coverage and quality of reads. When fresh material and enough resources are available, flow cytometry should be the preferable method for determining genome size, and kmer should be used solely to provide an approximate estimate. Furthermore, the substantial proportion of repeated elements identified in both species could imply that the expanded genome resulted from repetitive element amplification along with polyploidization. Based on our results and the rise in chromosome number, we hypothesize that R. lutea has a tetraploid genome or higher. More research is needed, however, to validate the ploidy type. The information acquired from this study should provide a basis for future phylogenetic and evolutionary studies, as well as the initiation of genome sequencing projects at the chromosome level.
Author Contributions
Conceptualization, F.A.-Q. and A.-R.Z.G.; methodology, A.-R.Z.G. and S.K.; software, A.-R.Z.G.; validation, A.-R.Z.G., S.K. and A.M.A.; formal analysis, A.-R.Z.G.; investigation, A.-R.Z.G. and S.K.; resources, S.A., M.T. and N.S.A.; data curation, A.-R.Z.G.; writing—original draft preparation, A.-R.Z.G. and S.K.; writing—review and editing, A.-R.Z.G.; visualization, A.M.A.; supervision, F.A.-Q.; project administration, M.N. and N.B.A.; funding acquisition, F.A.-Q. All authors have read and agreed to the published version of the manuscript.
Funding
This Project was funded by the National Plan for Science, Technology, and Innovation (MAARIFAH), King Abdulaziz City for Science and Technology, Kingdom of Saudi Arabia, Award Number (BIO724).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data which support the results of this study are available in GenBank at the NCBI (https://www.ncbi.nlm.nih.gov, accessed on 3 July 2021) under the BioProject accession PRJNA733338. NCBI accession numbers for all species in the molecular identification analysis are available in Figure 2.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Dolezel J., Bartos J. Plant DNA flow cytometry and estimation of nuclear genome size. Ann. Bot. 2005;95:99–110. doi: 10.1093/aob/mci005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dolezel J., Bartos J., Voglmayr H., Greilhuber J. Nuclear DNA content and genome size of trout and human. Cytom. Part A. 2003;51:127–128. doi: 10.1002/cyto.a.10013. [DOI] [PubMed] [Google Scholar]
- 3.Greilhuber J., Doležel J., Lysak M.A., Bennett M.D. The origin, evolution and proposed stabilization of the terms ‘genome size’ and ‘C-value’ to describe nuclear DNA contents. Ann. Bot. 2005;95:255–260. doi: 10.1093/aob/mci019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Swift H. The constancy of desoxyribose nucleic acid in plant nuclei. Proc. Natl. Acad. Sci. USA. 1950;36:643–654. doi: 10.1073/pnas.36.11.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fay M.F., Cowan R.S., Leitch I.J. The effects of nuclear, DNA content (C-value) on the quality and utility of AFLP fingerprints. Ann. Bot. 2005;95:237–246. doi: 10.1093/aob/mci017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leitch I.J., Bennett M.D. Flow Cytometry with Plant Cells: Analysis of Genes, Chromosomes and Genomes. Wiley-VCH; Weinheim, Germany: 2007. Genome size and its uses: The impact of flow cytometry; pp. 153–176. [Google Scholar]
- 7.Gregory T.R. The Evolution of the Genome. Elsevier; Amsterdam, The Netherlands: 2005. Genome size evolution in animals; pp. 3–87. [Google Scholar]
- 8.Leong-Škorničková J., Šída O., Jarolímová V., Sabu M., Fér T., Trávníček P., Suda J. Chromosome numbers and genome size variation in Indian species of Curcuma (Zingiberaceae) Ann. Bot. 2007;100:505–526. doi: 10.1093/aob/mcm144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kelly L.J., Leitch A.R., Fay M.F., Renny-Byfield S., Pellicer J., Macas J., Leitch I.J. Why size really matters when sequencing plant genomes. Plant. Ecol. Divers. 2012;5:415–425. doi: 10.1080/17550874.2012.716868. [DOI] [Google Scholar]
- 10.Doležel J., Greilhuber J., Suda J. Estimation of nuclear DNA content in plants using flow cytometry. Nat. Protoc. 2007;2:2233. doi: 10.1038/nprot.2007.310. [DOI] [PubMed] [Google Scholar]
- 11.Sliwinska E. Flow cytometry—A modern method for exploring genome size and nuclear DNA synthesis in horticultural and medicinal plant species. Folia Hortic. 2018;30:103–128. doi: 10.2478/fhort-2018-0011. [DOI] [Google Scholar]
- 12.Hanrahan S.J., Johnston J.S. New genome size estimates of 134 species of arthropods. Chromosome Res. 2011;19:809–823. doi: 10.1007/s10577-011-9231-6. [DOI] [PubMed] [Google Scholar]
- 13.Li X., Waterman M.S. Estimating the repeat structure and length of DNA sequences using ℓ-tuples. Genome Res. 2003;13:1916–1922. doi: 10.1101/gr.1251803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guo L.T., Wang S.L., Wu Q.J., Zhou X.G., Xie W., Zhang Y.J. Flow cytometry and K-mer analysis estimates of the genome sizes of Bemisia tabaci B and Q (Hemiptera: Aleyrodidae) Front. Physiol. 2015;6:144. doi: 10.3389/fphys.2015.00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He K., Lin K., Wang G., Li F. Genome Sizes of Nine Insect Species Determined by Flow Cytometry and k-mer Analysis. Front. Physiol. 2016;7:569. doi: 10.3389/fphys.2016.00569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li R., Fan W., Tian G., Zhu H., He L., Cai J., Huang Q., Cai Q., Li B., Bai Y., et al. The sequence and de novo assembly of the giant panda genome. Nature. 2010;463:311–317. doi: 10.1038/nature08696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Potato Genome Sequencing Consortium Genome sequence and analysis of the tuber crop potato. Nature. 2011;475:189. doi: 10.1038/nature10158. [DOI] [PubMed] [Google Scholar]
- 18.Martin-Bravo S., Meimberg H., Luceno M., Markl W., Valcarcel V., Brauchler C., Vargas P., Heubl G. Molecular systematics and biogeography of Resedaceae based on ITS and trnL-F sequences. Mol. Phylogenet. Evol. 2007;44:1105–1120. doi: 10.1016/j.ympev.2006.12.016. [DOI] [PubMed] [Google Scholar]
- 19.Yildirim H., Şenol S.G. Reseda malatyana (Resedaceae), a new chasmophytic species from eastern Anatolia, Turkey. Turk. J. Bot. 2014;38:1013–1021. doi: 10.3906/bot-1311-19. [DOI] [Google Scholar]
- 20.Sales A., Bagherizadeh Y., Malekzadeh P., Ahmadi B., Bonab F. Evaluation of the antimicrobial effects of essential oil of reseda lutea L on pathogenic bacteria: Staphylococcus aureus, Staphylococcus epidermidis, and Escherichia coli. Arch. Clin. Microbiol. 2017;8:1–6. doi: 10.4172/1989-8436.100041. [DOI] [Google Scholar]
- 21.Casetti F., Jung W., Wolfle U., Reuter J., Neumann K., Gilb B., Wahling A., Wagner S., Merfort I., Schempp C.M. Topical application of solubilized Reseda luteola extract reduces ultraviolet B-induced inflammation in vivo. J. Photochem. Photobiol. B. 2009;96:260–265. doi: 10.1016/j.jphotobiol.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 22.Berrehal D., Khalfallah A., Bencharif-Betina S., Kabouche Z., Kacem N., Kabouche A., Calliste C.-A., Duroux J.-L. Comparative antioxidant activity of two Algerian Reseda species. Chem. Nat. Compd. 2010;46:456–458. doi: 10.1007/s10600-010-9643-0. [DOI] [Google Scholar]
- 23.Ali M.A., Al-Hemaid F.M., Choudhary R.K., Lee J., Kim S.-Y., Rub M. Status of Reseda pentagyna Abdallah & AG Miller (Resedaceae) inferred from combined nuclear ribosomal and chloroplast sequence data. Bangladesh J. Plant. Taxon. 2013;20:233–238. [Google Scholar]
- 24.Llewellyn O., Hall M., Miller A., Al-Abbasi T., Al-Wetaid A., Al-Harbi R., Al-Shammari K., Al-Farhan A. Important Plant Areas in the Arabian Peninsula: 1. Jabal Qaraqir. Edinb. J. Bot. 2010;67:37. doi: 10.1017/S0960428609990229. [DOI] [Google Scholar]
- 25.Abdallah M.S. The Resedaceae: A Taxonomical Revision of the Family. De Landbouwhogeschool te Wageningen; Wageningen, Germany: 1967. [Google Scholar]
- 26.Alwadi H., Moustafa F. Altitudinal impact on the weeds species distribution in the semi-arid mountainous region of Abha, Saudi Arabia. J. Crop. Weed. 2016;12:87–95. [Google Scholar]
- 27.Pagnotta E., Montaut S., Matteo R., Rollin P., Nuzillard J.-M., Lazzeri L., Bagatta M. Glucosinolates in Reseda lutea L: Distribution in plant tissues during flowering time. Biochem. Syst. Ecol. 2020;90:104043. doi: 10.1016/j.bse.2020.104043. [DOI] [Google Scholar]
- 28.Chaudhary S. Resedaceae. In: Chaudhary S., editor. Flora of the Kingdom of Saudi Arabia. Ministry of Agriculture and Water, National Herbarium, National Agriculture and Water Research Center; Riyadh, Saudi Arabia: 1999. pp. 536–543. [Google Scholar]
- 29.Miller A., Nyberg J. Studies in the Flora of Arabia: XXVII. Some new taxa from the Arabian Peninsula. Edinb. J. Bot. 1994;51:33–47. doi: 10.1017/S0960428600001694. [DOI] [Google Scholar]
- 30.Murashige T., Skoog F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962;15:473–497. doi: 10.1111/j.1399-3054.1962.tb08052.x. [DOI] [Google Scholar]
- 31.White T.J., Bruns T., Lee S., Taylor J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protoc. A Guide Methods Appl. 1990;18:315–322. [Google Scholar]
- 32.Gardes M., Bruns T.D. ITS primers with enhanced specificity for basidiomycetes-application to the identification of mycorrhizae and rusts. Mol. Ecol. 1993;2:113–118. doi: 10.1111/j.1365-294X.1993.tb00005.x. [DOI] [PubMed] [Google Scholar]
- 33.Kumar S., Stecher G., Li M., Knyaz C., Tamura K., Mega X. Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018;35:1547–1549. doi: 10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kumar S., Gadagkar S.R. Efficiency of the neighbor-joining method in reconstructing deep and shallow evolutionary relationships in large phylogenies. J. Mol. Evol. 2000;51:544–553. doi: 10.1007/s002390010118. [DOI] [PubMed] [Google Scholar]
- 35.Doležel J., Sgorbati S., Lucretti S. Comparison of three DNA fluorochromes for flow cytometric estimation of nuclear DNA content in plants. Physiol. Plant. 1992;85:625–631. doi: 10.1111/j.1399-3054.1992.tb04764.x. [DOI] [Google Scholar]
- 36.Sadhu A., Bhadra S., Bandyopadhyay M. Novel nuclei isolation buffer for flow cytometric genome size estimation of Zingiberaceae: A comparison with common isolation buffers. Ann. Bot. 2016;118:1057–1070. doi: 10.1093/aob/mcw173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yan J., Zhang J., Sun K., Chang D., Bai S., Shen Y., Huang L., Zhang J., Zhang Y., Dong Y. Ploidy level and DNA content of Erianthus arundinaceus as determined by flow cytometry and the association with biological characteristics. PloS ONE. 2016;11:e0151948. doi: 10.1371/journal.pone.0151948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bennett M.D., Bhandol P., Leitch I.J. Nuclear DNA amounts in angiosperms and their modern uses—807 new estimates. Ann. Bot. 2000;86:859–909. doi: 10.1006/anbo.2000.1253. [DOI] [Google Scholar]
- 39.Andrews S. Babraham Bioinformatics. Babraham Institute; Cambridge, UK: 2019. [(accessed on 10 April 2021)]. FastQC V0.11.9: A quality control tool for high throughput sequence data. Available online: www.bioinformatics.babraham.ac.uk/projects/fastqc. [Google Scholar]
- 40.Bolger A.M., Lohse M., Usadel B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Luo R., Liu B., Xie Y., Li Z., Huang W., Yuan J., He G., Chen Y., Pan Q., Liu Y., et al. SOAPdenovo2: An empirically improved memory-efficient short-read de novo assembler. Gigascience. 2012;1:18. doi: 10.1186/2047-217X-1-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu H., Luo X., Qian J., Pang X., Song J., Qian G., Chen J., Chen S. FastUniq: A fast de novo duplicates removal tool for paired short reads. PLoS ONE. 2012;7:e52249. doi: 10.1371/journal.pone.0052249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bushnell B. BBMap: A fast, accurate, splice-aware aligner; Proceedings of the 9th Annual, Genomics of Energy & Environment Meeting; Walnut Creek, CA, USA. 17–20 March 2014; Berkeley, CA, USA: Lawrence Berkeley National Lab; 2014. [Google Scholar]
- 44.Wingett S.W., Andrews S. FastQ Screen: A tool for multi-genome mapping and quality control. F1000Research. 2018;7:1338. doi: 10.12688/f1000research.15931.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen W., Hasegawa D.K., Arumuganathan K., Simmons A.M., Wintermantel W.M., Fei Z., Ling K.S. Estimation of the Whitefly Bemisia tabaci Genome Size Based on k-mer and Flow Cytometric Analyses. Insects. 2015;6:704–715. doi: 10.3390/insects6030704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu B., Shi Y., Yuan J., Hu X., Zhang H., Li N., Li Z., Chen Y., Mu D., Fan W. Estimation of genomic characteristics by analyzing k-mer frequency in de novo genome projects. arXiv. 20131308.2012 [Google Scholar]
- 47.Marcais G., Kingsford C. A fast, lock-free approach for efficient parallel counting of occurrences of k-mers. Bioinformatics. 2011;27:764–770. doi: 10.1093/bioinformatics/btr011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chikhi R., Medvedev P. Informed and automated k-mer size selection for genome assembly. Bioinformatics. 2014;30:31–37. doi: 10.1093/bioinformatics/btt310. [DOI] [PubMed] [Google Scholar]
- 49.Vurture G.W., Sedlazeck F.J., Nattestad M., Underwood C.J., Fang H., Gurtowski J., Schatz M.C. GenomeScope: Fast reference-free genome profiling from short reads. Bioinformatics. 2017;33:2202–2204. doi: 10.1093/bioinformatics/btx153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sun H., Ding J., Piednoël M., Schneeberger K. findGSE: Estimating genome size variation within human and Arabidopsis using k-mer frequencies. Bioinformatics. 2018;34:550–557. doi: 10.1093/bioinformatics/btx637. [DOI] [PubMed] [Google Scholar]
- 51.Hozza M., Vinař T., Brejová B. International Symposium on String Processing and Information Retrieval: 2015. Springer; Berlin/Heidelberg, Germany: 2015. How big is that genome? Estimating genome size and coverage from k-mer abundance spectra; pp. 199–209. [Google Scholar]
- 52.Lander E.S., Waterman M.S. Genomic mapping by fingerprinting random clones: A mathematical analysis. Genomics. 1988;2:231–239. doi: 10.1016/0888-7543(88)90007-9. [DOI] [PubMed] [Google Scholar]
- 53.Ryan J. Estimate_Genome_Size. Pl (Version 0.03) [Computer Software]. Sars International Centre for Marine Molecular Biology, Bergen, Norway 2013. [(accessed on 10 April 2021)]; Available online: http://josephryan.github.com/estimate_genome_size.pl/
- 54.Ranallo-Benavidez T.R., Jaron K.S., Schatz M.C. GenomeScope 2.0 and Smudgeplot for reference-free profiling of polyploid genomes. Nat. Commun. 2020;11:1432. doi: 10.1038/s41467-020-14998-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Loureiro J., Doležel J., Greilhuber J., Santos C., Suda J. Plant Flow Cytometry—Far beyond the Stone Age. Cytom. Part A. 2008;73a:579–580. doi: 10.1002/cyto.a.20578. [DOI] [PubMed] [Google Scholar]
- 56.Knight C.A., Ackerly D.D. Variation in nuclear DNA content across environmental gradients: A quantile regression analysis. Ecol. Lett. 2002;5:66–76. doi: 10.1046/j.1461-0248.2002.00283.x. [DOI] [Google Scholar]
- 57.Beaulieu J.M., Moles A.T., Leitch I.J., Bennett M.D., Dickie J.B., Knight C.A. Correlated evolution of genome size and seed mass. New Phytol. 2007;173:422–437. doi: 10.1111/j.1469-8137.2006.01919.x. [DOI] [PubMed] [Google Scholar]
- 58.Knight C.A., Beaulieu J.M. Genome size scaling through phenotype space. Ann. Bot. 2008;101:759–766. doi: 10.1093/aob/mcm321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Knight C.A., Molinari N.A., Petrov D.A. The large genome constraint hypothesis: Evolution, ecology and phenotype. Ann. Bot. 2005;95:177–190. doi: 10.1093/aob/mci011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vinogradov A.E. Selfish DNA is maladaptive: Evidence from the plant Red List. Trends Genet. 2003;19:609–614. doi: 10.1016/j.tig.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 61.Vidic T., Greilhuber J., Vilhar B., Dermastia M. Selective significance of genome size in a plant community with heavy metal pollution. Ecol. Appl. 2009;19:1515–1521. doi: 10.1890/08-1798.1. [DOI] [PubMed] [Google Scholar]
- 62.Doležel J., Greilhuber J., Lucretti S., Meister A., Lysák M., Nardi L., Obermayer R. Plant genome size estimation by flow cytometry: Inter-laboratory comparison. Ann. Bot. 1998;82(Suppl. 1):17–26. doi: 10.1093/oxfordjournals.aob.a010312. [DOI] [Google Scholar]
- 63.Soltis D.E., Soltis P.S., Bennett M.D., Leitch I.J. Evolution of genome size in the angiosperms. Am. J. Bot. 2003;90:1596–1603. doi: 10.3732/ajb.90.11.1596. [DOI] [PubMed] [Google Scholar]
- 64.González-Aguilera J., Fernández-Peralta A.M. Phylogenetic relationships in the family Resedaceae L. Genetica. 1984;64:185–197. doi: 10.1007/BF00115343. [DOI] [Google Scholar]
- 65.Eigsti O.J. Cytological studies in the Resedaceae. Bot. Gaz. 1936;98:363–369. doi: 10.1086/334645. [DOI] [Google Scholar]
- 66.Michael T.P., VanBuren R. Progress, challenges and the future of crop genomes. Curr. Opin. Plant. Biol. 2015;24:71–81. doi: 10.1016/j.pbi.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 67.Williams D., Trimble W.L., Shilts M., Meyer F., Ochman H. Rapid quantification of sequence repeats to resolve the size, structure and contents of bacterial genomes. BMC Genom. 2013;14:1–11. doi: 10.1186/1471-2164-14-537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gregory T.R., Nathwani P., Bonnett T.R., Huber D.P. Sizing up arthropod genomes: An evaluation of the impact of environmental variation on genome size estimates by flow cytometry and the use of qPCR as a method of estimation. Genome. 2013;56:505–510. doi: 10.1139/gen-2013-0044. [DOI] [PubMed] [Google Scholar]
- 69.DeSalle R., Gregory T.R., Johnston J.S. Preparation of samples for comparative studies of arthropod chromosomes: Visualization, in situ hybridization, and genome size estimation. Methods Enzym. 2005;395:460–488. doi: 10.1016/S0076-6879(05)95025-8. [DOI] [PubMed] [Google Scholar]
- 70.Hardie D.C., Gregory T.R., Hebert P.D. From pixels to picograms: A beginners’ guide to genome quantification by Feulgen image analysis densitometry. J. Histochem. Cytochem. 2002;50:735–749. doi: 10.1177/002215540205000601. [DOI] [PubMed] [Google Scholar]
- 71.Zhang G., Fang X., Guo X., Li L., Luo R., Xu F., Yang P., Zhang L., Wang X., Qi H., et al. The oyster genome reveals stress adaptation and complexity of shell formation. Nature. 2012;490:49–54. doi: 10.1038/nature11413. [DOI] [PubMed] [Google Scholar]
- 72.Edwards R.J., Tuipulotu D.E., Amos T.G., O’Meally D., Richardson M.F., Russell T.L., Vallinoto M., Carneiro M., Ferrand N., Wilkins M.R., et al. Draft genome assembly of the invasive cane toad, Rhinella marina. Gigascience. 2018;7:giy095. doi: 10.1093/gigascience/giy095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hu Y., Resende M.F., Bombarely A., Brym M., Bassil E., Chambers A.H. Genomics-based diversity analysis of Vanilla species using a Vanilla planifolia draft genome and Genotyping-By-Sequencing. Sci. Rep. 2019;9:1–16. doi: 10.1038/s41598-019-40144-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hedgecock D., Gaffney P.M., Goulletquer P., Guo X., Reece K., Warr G.W. The case for sequencing the Pacific oyster genome. J. Shellfish. Res. 2005;24:429–441. [Google Scholar]
- 75.Elliott T.A., Gregory T.R. What’s in a genome? The C-value enigma and the evolution of eukaryotic genome content. Philos. Trans. R. Soc. B Biol. Sci. 2015;370:20140331. doi: 10.1098/rstb.2014.0331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jansen H.J., Liem M., Jong-Raadsen S.A., Dufour S., Weltzien F.A., Swinkels W., Koelewijn A., Palstra A.P., Pelster B., Spaink H.P., et al. Rapid de novo assembly of the European eel genome from nanopore sequencing reads. Sci. Rep. 2017;7:7213. doi: 10.1038/s41598-017-07650-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jimenez A.G., Kinsey S.T., Dillaman R.M., Kapraun D.F. Nuclear DNA content variation associated with muscle fiber hypertrophic growth in decapod crustaceans. Genome. 2010;53:161–171. doi: 10.1139/G09-095. [DOI] [PubMed] [Google Scholar]
- 78.Kidwell M.G. Transposable elements and the evolution of genome size in eukaryotes. Genetica. 2002;115:49–63. doi: 10.1023/A:1016072014259. [DOI] [PubMed] [Google Scholar]
- 79.Li S.F., Su T., Cheng G.Q., Wang B.X., Li X., Deng C.L., Gao W.J. Chromosome Evolution in Connection with Repetitive Sequences and Epigenetics in Plants. Genes. 2017;8:290. doi: 10.3390/genes8100290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Baeza J.A., MacManes M. De novo assembly and functional annotation of the heart + hemolymph transcriptome in the Caribbean spiny lobster Panulirus argus. Mar. Genom. 2020;54:100783. doi: 10.1016/j.margen.2020.100783. [DOI] [PubMed] [Google Scholar]
- 81.SanMiguel P., Tikhonov A., Jin Y.K., Motchoulskaia N., Zakharov D., Melake-Berhan A., Springer P.S., Edwards K.J., Lee M., Avramova Z., et al. Nested retrotransposons in the intergenic regions of the maize genome. Science. 1996;274:765–768. doi: 10.1126/science.274.5288.765. [DOI] [PubMed] [Google Scholar]
- 82.Chandler V.L., Brendel V. The Maize Genome Sequencing Project. Plant. Physiol. 2002;130:1594–1597. doi: 10.1104/pp.015594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jain M., Koren S., Miga K.H., Quick J., Rand A.C., Sasani T.A., Tyson J.R., Beggs A.D., Dilthey A.T., Fiddes I.T., et al. Nanopore sequencing and assembly of a human genome with ultra-long reads. Nat. Biotechnol. 2018;36:338–345. doi: 10.1038/nbt.4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rhoads A., Au K.F. PacBio Sequencing and Its Applications. Genom. Proteom. Bioinform. 2015;13:278–289. doi: 10.1016/j.gpb.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rang F.J., Kloosterman W.P., de Ridder J. From squiggle to basepair: Computational approaches for improving nanopore sequencing read accuracy. Genome Biol. 2018;19:90. doi: 10.1186/s13059-018-1462-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pellicer J., Leitch I.J. The Plant DNA C-values database (release 7.1): An updated online repository of plant genome size data for comparative studies. New Phytol. 2020;226:301–305. doi: 10.1111/nph.16261. [DOI] [PubMed] [Google Scholar]
- 87.Rice A., Glick L., Abadi S., Einhorn M., Kopelman N.M., Salman-Minkov A., Mayzel J., Chay O., Mayrose I. The Chromosome Counts Database (CCDB)—A community resource of plant chromosome numbers. New Phytol. 2015;206:19–26. doi: 10.1111/nph.13191. [DOI] [PubMed] [Google Scholar]
- 88.Goldblatt P., Johnson D. Index to Plant Chromosome Numbers (ICPN Reports) Missouri Botanical Garden; St. Louis, Missouri: 2015. [Google Scholar]
- 89.Gonzhlez-Aguilera J., FernCtndez-Peralta A., Safiudo A. Cytogenetic and evolutionary studies on the Spanish species of the Family Resedaceae L: Sections Phyteuma L and Resedastrum Duby. Bol. Soc. Brot. Ser. 1980;2:519–536. [Google Scholar]
- 90.Pustahija F., Brown S.C., Bogunić F., Bašić N., Muratović E., Ollier S., Hidalgo O., Bourge M., Stevanović V., Siljak-Yakovlev S. Small genomes dominate in plants growing on serpentine soils in West Balkans, an exhaustive study of 8 habitats covering 308 taxa. Plant. Soil. 2013;373:427–453. doi: 10.1007/s11104-013-1794-x. [DOI] [Google Scholar]
- 91.Sañudo A., Rejon R. Sobre la Naturaleza Autoploide de Algunas Plantas Silvestre. An. Inst. Bot. Cavanilles. 1975;32:633–648. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data which support the results of this study are available in GenBank at the NCBI (https://www.ncbi.nlm.nih.gov, accessed on 3 July 2021) under the BioProject accession PRJNA733338. NCBI accession numbers for all species in the molecular identification analysis are available in Figure 2.