Abstract
Background: Cancer pain can remain refractory despite escalating opioids and adjuvants. Systemic Lidocaine is an option, but current approaches are hospital centered. While advantageous in advanced cancer, evidence is lacking for parenteral Lidocaine use in community-based care.
Objectives: Review evidence for parenteral lidocaine in complex cancer pain outside the hospital setting.
Design: Systematic review of peer-reviewed articles of any study design, including reviews. Search in four databases used keyword variations of “cancer,” “pain,” “Lidocaine,” and “parenteral.” Search was extended through reference lists of full texts assessed. Abstracted data from articles screened and selected were synthesized narratively by a palliative care clinician in Singapore.
Results: Eight hundred eighty-three articles identified were screened by title and abstract. Twenty-eight full texts were assessed. Seven articles fulfilled criteria for synthesis of findings. A total of 73 patients received parenteral Lidocaine for mixed pains, reported collectively in 1 retrospective chart review, 3 practice guidelines, 2 case series, and 1 case study. Intravenous or subcutaneous Lidocaine was commenced in hospital or hospice and continued at home. Dosages and administration schedules varied, involving slow bolus with continuous infusion or the latter alone, for up to 240 days. All produced positive outcomes, with no severe adverse events. Monitoring included routine vital signs and conscious levels; electrocardiogram, liver, and renal function tests were uncommon. Lidocaine levels were not consistently assessed.
Conclusion: Parenteral Lidocaine can be effective and safe in the community setting. More empirical studies are needed to inform patient selection and treatment protocol, and to validate expected outcomes.
Keywords: cancer pain, home, hospice, palliative, parenteral Lidocaine
Introduction
Up to 60% of patients with advanced cancer experience pain from progressive illness, with prevalence increasing to 80% at terminal stages of disease.1,2 While distress associated with cancer pain may be alleviated adhering to the World Health Organization cancer pain guidelines,3 some patients continue to suffer persistent symptoms, from unrelieved pain or adverse effects of treatments prescribed.1,4–8 Their symptoms are described as intractable or refractory.9,10 By that time, these pains are frequently unresponsive to escalating opioids.4,7,11 Change in routes of administration, opioid rotations, adjuvants such as antidepressants and anticonvulsants, steroids, and antihyperalgesic therapies such as ketamine might have been tried, with unsatisfactory outcomes.4,10,12,13 Anesthetic options such as nerve blocks, epidural, and intrathecal infusions were most likely considered or have failed. Otherwise, the patients could have declined or were too vulnerable for any invasive intervention.4,14 Lidocaine appears promising as one of several multimodal approaches in the management of complex cancer pain.13,15,16
Lidocaine is an amide local anesthetic and class 1B antiarrhythmic agent.17,18 Systemic Lidocaine,14,19 administered either intravenously (IV) or subcutaneously (SC) as opposed to local injections, epidural, or intrathecal routes, is the focus of this review. Its pharmacological effects are achieved through nonselective blockage of voltage and frequency dependent sodium channels on nerve membranes. The exact mechanism by which Lidocaine produces analgesia when administered parenterally is believed to be more than minimizing neuronal sensitivity.7,13 Apart from direct sodium channel blockade, findings from molecular, animal, and clinical studies indicate additional antihyperalgesic and anti-inflammatory actions.1,13,17,20 Increasingly, clinicians are managing complex cancer pains using parenteral Lidocaine, particularly those with mixed or neuropathic typologies that failed first- and second-tier treatments.17 A systematic review of randomized controlled trials investigating parenteral Lidocaine for cancer pain in adults confirmed its utility over placebo in refractory pain that had failed conventional treatments using opioids and different adjuvants.2
Pain and palliative physicians have administered Lidocaine in multiple ways—including slow bolus (single or repeated), continuous infusion (over varying periods), or a combination of both (the initial bolus often a “challenge” dose that determines if an infusion follows).12,21,22 Acknowledging a steep dose-response curve,17,23 a minimum threshold drug level before benefits are observed,1,15 and a narrow therapeutic index with risks of neuromuscular and cardiac toxicity,13,14 almost all initiations of parenteral Lidocaine are performed in the institutional setting under close monitoring. This may include a baseline electrocardiogram (ECG), laboratory tests (cardiorespiratory screening, liver, and renal panels), and continuous nursing observation throughout drug administration. In addition, serum Lidocaine levels are assayed at various points. These requirements precluded patients from being treated outside the hospital, until recently. This review aims to synthesize available literature, with a view to highlight interim progress, lessons learnt, and research gaps in this specific context. The review question conceptualized was as follows: “How is parenteral Lidocaine used to manage pain in patients with advanced cancer of any age, outside the hospital, when first and second lines of analgesic treatments have failed or become unsuitable?”
Methods
The target population are patients (of any age) in advanced stages of cancer suffering from pain that has failed conventional analgesic therapy. Parenteral Lidocaine (intravenous or subcutaneous) refers to systemic administration of the drug, remote from its sites of action within the peripheral or central nervous system. Outcomes of interest include protocol recommendations and clinical experience in the use of systemic Lidocaine. The setting is contextualized to that outside the hospital, where patients in later phases of disease trajectories often wish to spend most of their remaining time.24 This could be in their own homes, supported by a hospital outreach service or community hospice; otherwise, treatment is received within a dedicated facility where palliative care is rendered, most commonly an inpatient hospice.
Early scoping review revealed only observational studies. Hence, the literature search was kept broad and inclusive, with the purpose of locating any article that documented therapeutic approaches using parenteral Lidocaine to manage complex cancer pain within the community setting. A search strategy was constructed by the second author using variation of key words “Cancer,” “Pain,” “Lidocaine,” and “Parenteral.” It was run across four databases (PubMed, Embase, Cochrane Library, and Google Scholar), chosen to provide the best yield of articles, without limits set on level of evidence or study design. Given that parenteral Lidocaine is still a contemporary intervention, a date filter (from 1991 to present) was set to trace articles published only in the last 30 years. A sample search string used on PubMed is available in Supplementary Table S1.
After duplicates were removed, titles and abstracts of all articles identified were screened for eligibility. Full texts of shortlisted articles were obtained, and the literature search extended through individual reference lists. Assessment criteria include: (i) not trial protocols or broad reviews of analgesic interventions that only partially discussed parenteral Lidocaine, (ii) not studies on post-op pain, postchemotherapy pain, or chronic pains from noncancer conditions, and (iii) only English articles with full texts available were obtained. Quality assessment was not performed for the purpose of this review.
A data extraction template was created, focusing on collating modes of drug administration and their treatment outcomes. Abstracted findings from selected articles were tabulated to facilitate data analysis and synthesis. A narrative method of data integration was applied, as it became evident that articles found were predominantly uncontrolled observational or case studies and summative reviews.
In this qualitative review, the PRISMA guidelines25 were referenced during planning, execution, and reporting. As this was a systematic review of published literature, IRB review was not sought.
Results
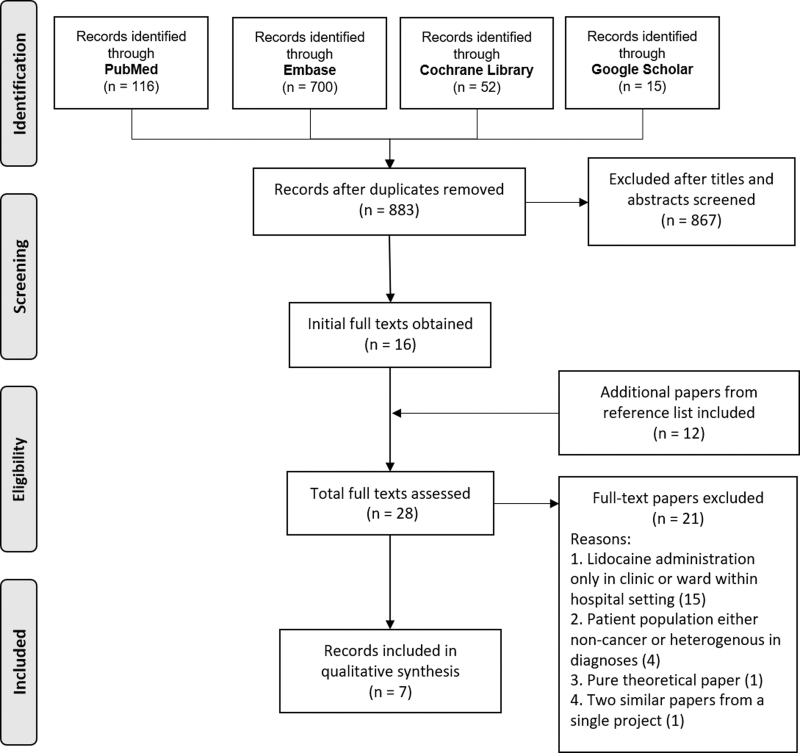
The search was run on July 14, 2020. A total of 883 unique articles were found. Titles and abstracts, and later full texts were screened by the first author for eligibility. The PRISMA diagram in Figure 1 details the process.
FIG. 1.
PRISMA diagram.
Seven articles fulfilled inclusion criteria for final review. They included a total of 73 patients, with 61 patients from a single study alone, which is a retrospective review of a patient cohort admitted into an adult inpatient hospice for management of opioid refractory pain. Three among the rest were a blend of literature reviews and practice guidelines. Two case series reported parenteral Lidocaine use in the inpatient hospice in adult patients. Finally, there is a single-case study of a terminally ill pediatric patient with refractory cancer pain. Two case studies additionally described how parenteral Lidocaine was continued in the home setting until patients' demise. Articles selected for final synthesis were all published from year 2000 onwards. Table 1 below provides other relevant findings.
Table 1.
Summary of Articles Reviewed
| Full original title (author/year of publication) | Study design | Setting | Sample | Intervention/s | Findings | Remarks |
|---|---|---|---|---|---|---|
| Continuous Lidocaine infusion for the relief of refractory malignant pain in a terminally III pediatric cancer patient (Massey et al., 2002)8 | Single-case study | Lidocaine started in hospital and discharged home, care of a home care nurse. | Five-year-old girl (17.2 kg) with metastatic retinoblastoma. | No bolus. Continuous IV Lidocaine infusion started at 35 mcg/kg/min (36 mg/h). Escalated at home to 63 mcg/kg/min (65 mg/h). Continued till demise two months later. | Good response. “Excellent pain relief” without associated lethargy from high dose opioids. No cardiac or neuroexcitatory adverse effects observed. | Before commencing Lidocaine, intensive monitoring in hospital provided, including ECG. Lidocaine levels NOT measured. Only routine monitoring by parents at home. Managed successfully until the child's death two months later. |
| Parenteral Lidocaine for severe intractable pain in six hospice patients continued at home (Ferrini 2000)15 | Case series | Patients receiving hospice support at home. Lidocaine either started in hospital, then continued at home or initiated at home. | Six adult patients with severe intractable pain (mixed and neuropathic). Near end-of-life. | IV/SC Lidocaine 50–100 mg administered over 10–30 minutes (1.5–1.75 mg/kg). Continuous infusion IV/SC 10–80 mg/h for varying periods (till demise—24 to 240 days). Lidocaine bolus sometimes ordered (at two times the hourly doses). | All with good outcomes. Two of six patients reported light headedness that improved with Lidocaine dose reduction. | Prior workup not mentioned. Lidocaine levels measured (one patient declined). Ongoing monitoring only involved routine vital signs and conscious levels. |
| Parenteral Lignocaine in cancer neuropathic pain: A series of case reports (Chia et al., 2014)1 | Case series | Inpatient hospice | Four patients with cancer neuropathic pain | SC bolus Lidocaine 50 mg over 1 hour as a challenge dose. Followed by continuous infusion of 800–1500 mg/day for 2–13 days. | Good response. All showed objective response to challenge dose. MEDD of background opioids remained stable throughout. No documented adverse effects, including phlebitis. | No ECG performed. No measurement of drug levels. Hemodynamic monitoring performed four hourly, except in one patient who was dying. |
| Intravenous Lidocaine relieves severe pain: Results of an inpatient hospice chart review (Thomas et al., 2004)4 | Retrospective cohort study | Inpatient hospice | Sixty-one patients with opioid refractory pain admitted for hospice and palliative care. | Majority received IV bolus of Lidocaine 1–2 mg/kg over 15–20 minutes followed by continuous infusion 1 mg/kg/h. A small group only received continuous infusion. | Eighty-two percent had major pain relief. Ten percent no change. Thirty percent experienced some adverse effect (three in four experienced lethargy or drowsiness, only 3% needed to have Lidocaine stopped). | Prior workup (if done) not reported. Some had and some did NOT have Lidocaine levels measured. Details about monitoring of parameters within inpatient hospice not provided. |
| Intravenous Lidocaine: An outdated or underutilized Treatment for Pain? (McCleane 2007)12 | Review | Either outpatient clinic in a hospital or home | Not applicable | IV Lidocaine 1000 mg over 7–9 hours or 1200 mg over 30 hours. May be administered at home. | Long-term relief from short-term administration. Little tolerance and may be repeated. Potential beneficial effect on visceral pain. Short-term adverse effects that improves after infusion completed. Thrombophlebitis occasionally noted. | Prior workup not reported. No mention of measurement of drug levels or monitoring of parameters. |
| Parenteral Lidocaine for neuropathic pain #180 (Thomas 2009)19 | Review/Guideline | Not applicable | Not applicable | IV bolus Lidocaine 1–5 mg/kg over 15–60 minutes. If initial response noted, can continue IV/SC continuous infusion over days to months. | Weak, uncontrolled evidence for benefits in cancer pain (neuropathic and opioid-refractory). Not used as first line. Aim for target serum levels of 2–5 mg/L. Adverse effects are dose related. | Suggests liver and renal function assessments before. Measurement of drug levels recommended. No mention about monitoring parameters. |
| Lidocaine infusions and other options for opioid-resistant pain due to pediatric advanced cancer (Berde et al., 2016)14 | Review/Guideline | Not applicable | Pediatric cancer population | IV/SC. Narrow therapeutic index. Keep to below 2 mg/kg/h (serum levels below 6 mg/L) to reduce risk of seizures. | “Pain interruption”—analgesia duration that outlasts clearance of lidocaine or its metabolites. Good response in neuropathic pain yet not good enough for movement related or incident pain. Adverse cognitive and neurological effects close to the therapeutic range. | No definite recommendations for prior workup. Measurement of drug levels and close hemodynamic monitoring recommended. |
ECG, electrocardiogram; IV, intravenously; SC, subcutaneously.
Typologies of cancer pains include nociceptive, neuropathic, or mixed. Lidocaine was administered IV (n = 3) or SC (n = 1) or both (n = 3). It could be commenced within the inpatient hospice or started in hospital and then continued at home. None reported parenteral Lidocaine being administered first within the home setting.
Similar to how systemic Lidocaine is variably used in the acute hospital settings by clinicians from different specialities, a variety of administration schedules were found in the community setting of hospice or home. Two articles reported the use of continuous infusion of Lidocaine without a prior bolus dose: one article reported using 1200 mg of parenteral Lidocaine infusion over 30 hours in the adult setting12; the second is a single-case study of a pediatric patient that received between 35 mcg/kg/min (36 mg/h or 864 mg/day) and 63 mcg/kg/min (65 mg/h or 1560 mg/day) of continuous infusion of Lidocaine.8
In contrast to hospital-based studies that use “pulsed” boluses of parenteral Lidocaine (administered over minutes to short hours under tight hemodynamic monitoring) given intermittently over weeks, articles reviewed described a process of slow bolus Lidocaine administration, followed by continuous infusion. One article described the bolus both as a loading and breakthrough dose,15 while three others specifically used the initial bolus as a “challenging dose” to assess individual patient response to Lidocaine that determined if continuous infusion should proceed.1,4,19 Dosing regimens were charted in a variety of ways by different authors, but some patterns are discerned. Boluses were delivered at fixed doses of 50–100 mg or 1–5 mg/kg, over 10 to 60 minutes. When used specifically as a breakthrough, two times the hourly dose was used. Continuous infusions were administered 1–2 mg/kg/h, 10–80 mg/h or 800–1500 mg/day. These continuous infusions were administered for varying periods, including cases where they were repeated when symptoms recurred. Most were continued until the patient died and the longest duration reported was 240 days.
The degree of workup before commencement of treatment and extent of hemodynamic monitoring during drug administration are different from those where parenteral Lidocaine was given in the hospital setting.5,11,17,20,22 Assessment of liver and renal function before Lidocaine administration was only explicitly recommended in one article.19 Similarly, an ECG was performed only in one pediatric patient before Lidocaine infusion was commenced.8 In the home setting, only routine vital signs and conscious levels were monitored.8,15 In the inpatient hospice, hemodynamic monitoring was performed four hourly and in one dying patient, this was omitted altogether.1,4 Only one out of the three practice guidelines mentioned the need for close monitoring during drug administration outside the hospital context.14
Serum Lidocaine levels were only assayed in two studies where there were empirical trials of treatment and even then, not consistently.4,15 It was nonetheless recommended in two practice guideline articles for the purpose of titrating to efficacy and preventing toxicity.14,19
All articles reported positive outcomes for analgesia in the management of complex cancer pain. None, however, had used validated outcome measures for pain. A single retrospective cohort study with the largest sample demonstrated that 82% of patients had obtained “major response” in relieving severe pain.4 A single-case report of a five-year-old girl with painful metastatic retinoblastoma experienced “excellent pain relief,” regaining lost cognitive function when her opioids were rapidly escalated previously.8 Another study documented stable mean equivalent daily dose of morphine, despite cancer progression in all four patients with uncontrolled neuropathic cancer pain complicated by delirium and drowsiness.1 Adverse effects were universally uncommon despite patient frailty, apart from lethargy or light-headedness that improved with reduction in Lidocaine doses. In the largest study cohort mentioned previously, only 3% of patients required termination of drug infusion.4 The authors further qualified that no significant adverse effects could be clearly attributed to Lidocaine.
Discussion
This review of largely observational studies shows parenteral Lidocaine has been used successfully without severe adverse events within the community, whether in an inpatient hospice or home, and was recommended as second- or third-line intervention to manage different types of complex cancer pain in patients of all ages. Where empirical treatments using parenteral Lidocaine were reported, patients were able to achieve symptom control and eventually died outside the acute hospital setting. In some cases, they were cared for varying periods in their preferred place of death like home.8,15 These are encouraging findings for the group of patients that this review is focused on. Where conventional therapies such as opioids and adjuvants fail, and aggressive interventions are not appropriate, systemic Lidocaine appears to be a feasible, effective, and safe option. These recent reports highlighted yet another possibility—hope for a “good death” in a place of choice.24,26–29
Findings here add to the systematic review by Lee et al.2 of parenteral Lidocaine for cancer pain in adults. Their meta-analysis of pooled data from randomized controlled trials confirmed that Lidocaine administered 4 to 5 mg/kg IV over 30 to 80 minutes is superior to placebo for more than 50% reduction in cancer pain of various typologies.11,30 However, study participants were all adult patients who were administered Lidocaine only IV, and solely within the hospital or clinic outpatient setting. Only one regimen of administration was examined, that of “slow” bolus alone, rather than continuous infusion over days or weeks commonly practiced in end-of-life care.1,4,8,15 Hence, there is a need to locate other evidence, if parenteral Lidocaine is perceived a viable therapeutic option for patients with complex cancer pain residing outside the hospital system.
Systemic Lidocaine, whether administered IV or SC through slow bolus or continuous infusion, has inherent benefits in cancer pain insufficiently controlled with opioids or adjuvants. With a half-life of 90 to 120 minutes, it is rapidly distributed systemically before hepatic metabolism and renal excretion.22 While half-life can increase with infusion beyond 24 hours, drug levels decrease equally rapidly after administration is stopped (less than an hour after continuous infusion for 3 days).13,20 Efficacy is noted precipitously once the drug level reaches a “break point,” with adverse effects noticed soon after, as another threshold is crossed—characteristic of its steep dose-response curve and narrow therapeutic index mentioned before.1,4,14,15,17,23 Rapid onset of action not only brings prompt relief in a pain crisis,11,31 but it is also critical when a patient's prognosis is short.4 Dying patients may become drowsy, delirious, or dysphagic. A parenteral drug that maintains or augments analgesia would be most helpful at this time.1,18,32 Others have reported Lidocaine's putative benefits in severe headache from progressive brain tumor33 and difficult visceral pains in abdominal malignancies.12,34 Quite remarkably, Lidocaine demonstrates a potential for durable analgesia after “bursts” of systemic drug administration beyond its limited half-life. This implies drug-free periods without round the clock medications,34 or minimally opioid or adjuvant drug sparing,21 with adverse effects lessened while comfort is preserved. Without tolerance encountered or anticipated, infusions may be repeated as needed to extend benefits further.12
Studies reviewed showed a mix of both IV and SC Lidocaine administration. Although never specifically investigated, we assume that responses do not differ as many authors reported their use interchangeably without mention of dose adjustments. The latter would be preferred in continuous infusion over days to weeks, compared to slow bolus over minutes to hours.7,35,36 It is anticipated to be safer and more feasible maintaining a SC line for long periods outside the hospital, especially within the home setting.5,15 Moderate diversity is noted in drug administration schedules, indicating a lack of consensus. Given that all included articles had reported positive outcomes, it is unclear which regimen should be supported. A recent randomized, double-blind, placebo controlled crossover trial investigating the efficacy of weekly SC Lidocaine 10 mg/kg administered over 5.5 hours in patients with cancer pain was terminated early as it failed to show better results than placebo.5 The authors posited that their cohort of patients was perhaps “too well” and wondered if a titrated-to-effect regimen instead of a weight-based protocol could have produced different outcomes. Comparing results from two groups of investigators that used a similar design (retrospective chart review) to separately study “challenge before continuous infusion”4 and “immediate continuous infusion”35 in patients with refractory cancer pain yields further insight. Eighty-two percent of patients in the former showed “major response” while 68% in the latter obtained “significant decrease in pain scores.” This is not surprising though, with prior selection using a test dose in the first group, but the fact is more than half or at least one in two patients would respond either way. Like in the choice of IV or SC, pragmatic considerations unique to local circumstances could be the answer, at least till more evidence become available.
Toxicities emerge sequentially along a well described course corresponding to drug levels, and are apparent in alert patients who can then get timely medical attention.4,13 Two groups that reported empirical Lidocaine use had not monitored drug levels.1,8 Reasons include inappropriateness (patients were terminally ill), choice (from parent of a pediatric patient), and perceived safety (established protocols with standard dosing adopted).1,8 None of their patients experienced significant adverse effects. Local side effects such as thrombophlebitis reported elsewhere7 were also not observed among patients included in this review. Before starting Lidocaine infusions, liver and renal function tests and baseline ECG were rarely performed or recommended. Ongoing monitoring, particularly in the home setting, involves only routine indicators such as vital signs and conscious levels.
Findings from this review serve to guide future crafting of pain management protocols by individual community providers, should parenteral Lidocaine be added into their treatment armamentarium. When patients are offered this line of therapy, emergency room visits in pain crises may be reduced,37 with concomitant decrease in hospital admissions and greater chances of dying at home.38 More importantly, the goals of palliative care in reducing suffering and improving quality-of-life become possible.12,18 Although only a single-case study and one pediatric review article are included, relevant guidance is now made available to those caring for young patients.
The process of review was structured and informed by the PRISMA guidelines conceptually.25 Owing to sparse and heterogenous evidence in the area of interest, quality criteria were not set and an integrative approach to data synthesis was taken. Review findings are limited by the same issues raised—lack of robust evidence in topic area. Insights obtained here should be qualified with this understanding. Gray literature or articles published in other languages were not included for practical reasons. This could impact data selection, abstraction, and synthesis. The steps involved and all outputs are made explicit, however, in study procedures and data presented here, including Supplementary Table S1.
Controlled trials outside the institutional setting are needed to generate findings that are coherent with patients' preference, explore the optimal drug regimen (if a challenge dose is required and how), and confirm the efficacy of continuous Lidocaine infusion. Given level one evidence from previous systematic reviews on the use of Lidocaine for various types of pain, active controls instead of placebo could be considered. Although serious adverse effects are uncommon and preventable, they should be evaluated as secondary outcomes.
Conclusion
Clinicians have used parenteral Lidocaine outside the hospital to manage difficult cancer pain when other treatment strategies are not satisfactory or inappropriate. This has allowed patients to remain comfortable despite advanced progressive cancer and be cared for in a place of their choice. More studies are needed to define the best regimen for drug administration, both to maximize treatment effectiveness and reduce care burden and risks involved.
Supplementary Material
Authors' Contributions
P.H.C. was the main author and analysed the articles. Z.Z.Y. contributed to the design of the search strategy, extracted the search results, and produced the list of titles for analysis. Both authors contributed to the editing of the article.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
No funding was received for this article.
Supplementary Material
References
- 1. Chia SC, Hum A, Ong WY, et al. : Parenteral lignocaine in cancer neuropathic pain: A series of case reports. Prog Palliat Care 2014;22:253–257 [Google Scholar]
- 2. Lee JT, Sanderson CR, Xuan W, et al. : Lidocaine for cancer pain in adults: A systematic review and meta-analysis. J Palliat Med 2019;22:326–334 [DOI] [PubMed] [Google Scholar]
- 3. World Health Organization: WHO Guidelines for the Pharmacological and Radiotherapeutic Management of Cancer Pain in Adults and Adolescents. Geneva: World Health Organization, 2018 [PubMed] [Google Scholar]
- 4. Thomas J, Kronenberg R, Cox MC, et al. : Intravenous lidocaine relieves severe pain: Results of an inpatient hospice chart review. J Palliat Med 2004;7:660–667 [DOI] [PubMed] [Google Scholar]
- 5. Hawley P, Fyles G, Jefferys SG: Subcutaneous Lidocaine for cancer-related pain. J Palliat Med 2020;23:1357–1364 [DOI] [PubMed] [Google Scholar]
- 6. Fainsinger RL, Nekolaichuk CL: A “TNM” classification system for cancer pain: The Edmonton Classification System for Cancer Pain (ECS-CP). Support Care Cancer 2008;16:547–555 [DOI] [PubMed] [Google Scholar]
- 7. Brose WG, Cousins MJ: Subcutaneous lidocaine for treatment of neuropathic cancer pain. Pain 1991;45:145–148 [DOI] [PubMed] [Google Scholar]
- 8. Massey GV, Pedigo S, Dunn NL, et al. : Continuous lidocaine infusion for the relief of refractory malignant pain in a terminally III pediatric cancer patient. J Pediatr Hematol Oncol 2002;24:566–568 [DOI] [PubMed] [Google Scholar]
- 9. Currow DC, Spruyt O, Hardy J: Defining refractory pain in cancer for clinicians and researchers. J Palliat Med 2012;15:5–6 [DOI] [PubMed] [Google Scholar]
- 10. Afsharimani B, Kindl K, Good P, et al. : Pharmacological options for the management of refractory cancer pain—What is the evidence? Support Care Cancer 2015;23:1473–1481 [DOI] [PubMed] [Google Scholar]
- 11. Sharma S, Rajagopal M, Palat G, et al. : A phase II pilot study to evaluate use of intravenous lidocaine for opioid-refractory pain in cancer patients. J Pain Symptom Manage 2009;37:85–93 [DOI] [PubMed] [Google Scholar]
- 12. McCleane G: Intravenous lidocaine: An outdated or underutilized treatment for pain? J Palliat Med 2007;10:798–805 [DOI] [PubMed] [Google Scholar]
- 13. Eipe N, Gupta S, Penning J: Intravenous lidocaine for acute pain: An evidence-based clinical update. BJA Educ 2016;16:292–298 [Google Scholar]
- 14. Berde C, Koka A, Donado-Rincon C: Lidocaine infusions and other options for opioid-resistant pain due to pediatric advanced cancer. Pediatr Blood Cancer 2016;63:1141–1143 [DOI] [PubMed] [Google Scholar]
- 15. Ferrini R: Parenteral lidocaine for severe intractable pain in six hospice patients continued at home. J Palliat Med 2000;3:193–200 [DOI] [PubMed] [Google Scholar]
- 16. Peixoto RDA, Hawley P: Intravenous lidocaine for cancer pain without electrocardiographic monitoring: A retrospective review. J Palliat Med 2015;18:373–377 [DOI] [PubMed] [Google Scholar]
- 17. Gibbons K, DeMonbrun A, Beckman EJ, et al. : Continuous Lidocaine infusions to manage opioid-refractory pain in a series of cancer patients in a pediatric hospital. Pediatr Blood Cancer 2016;63:1168–1174 [DOI] [PubMed] [Google Scholar]
- 18. Peixoto RD, Hawley P: Intravenous lidocaine for cancer pain without electrocardiographic monitoring: A retrospective review. J Palliat Med 2015;18:373–377 [DOI] [PubMed] [Google Scholar]
- 19. Thomas J: Parenteral lidocaine for neuropathic pain #180. J Palliat Med 2009;12:188–189 [DOI] [PubMed] [Google Scholar]
- 20. Daykin H: The efficacy and safety of intravenous lidocaine for analgesia in the older adult: A literature review. Br J Pain 2017;11:23–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Buchanan DD, Maclvor FJ: A role for intravenous lidocaine in severe cancer-related neuropathic pain at the end-of-life. Support Care Cancer 2010;18:899–901 [DOI] [PubMed] [Google Scholar]
- 22. Kintzel PE, Knol JD, Roe G: Intravenous Lidocaine administered as twice daily bolus and continuous infusion for intractable cancer pain and wound care pain. J Palliat Med 2019;22:343–347 [DOI] [PubMed] [Google Scholar]
- 23. Ferrante FM, Paggioli J, Cherukuri S, et al. : The analgesic response to intravenous lidocaine in the treatment of neuropathic pain. Anesth Analg 1996;82:91–97 [DOI] [PubMed] [Google Scholar]
- 24. Gomes B, Calanzani N, Gysels M, et al. : Heterogeneity and changes in preferences for dying at home: A systematic review. BMC Palliat Care 2013;12:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Moher D, Liberati A, Tetzlaff J, et al. : Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cottrell L, Duggleby W: The” good death”: An integrative literature review. Palliat Support Care 2016;14:686. [DOI] [PubMed] [Google Scholar]
- 27. Meier EA, Gallegos JV, Thomas LPM, et al. : Defining a good death (successful dying): Literature review and a call for research and public dialogue. Am J Geriatr Psychiatry 2016;24:261–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Granda-Cameron C, Houldin A: Concept analysis of good death in terminally ill patients. Am J Hosp Palliat Care 2012;29:632–639 [DOI] [PubMed] [Google Scholar]
- 29. Borgstrom E: What is a good death? A critical discourse policy analysis. BMJ Support Palliat Care 2020. [Epub ahead of print]; DOI: 10.1136/bmjspcare-2019-002173 [DOI] [PubMed] [Google Scholar]
- 30. Joad ASK, Burad J, Mehta C: Intravenous lignocaine infusion for neuropathic pain in cancer patients—A preliminary study. Indian J. Anaesth 2002;46:360–364 [Google Scholar]
- 31. Ellemann K, Sjögren P, Banning AM, et al. : Trial of intravenous lidocaine on painful neuropathy in cancer patients. Clin J Pain 1989;5:291–294 [DOI] [PubMed] [Google Scholar]
- 32. Lee J, Sanderson C, Xuan W, et al. : Systematic review and meta-analysis of systemic lignocaine infusion for cancer pain in adults. Palliat Med 2018;32:47. [DOI] [PubMed] [Google Scholar]
- 33. Viderman D, Nurpeissov A, Bilotta F: Intravenous lidocaine in the management of severe brain tumor-associated headache. J Clin Anesth 2019;55:67–68 [DOI] [PubMed] [Google Scholar]
- 34. Seah DS, Herschtal A, Tran H, et al. : Subcutaneous lidocaine infusion for pain in patients with cancer. J Palliat Med 2017;20:667–671 [DOI] [PubMed] [Google Scholar]
- 35. Tagami K, Miura T, Suzuki M, et al. : Analgesic effectiveness of systemic lidocaine administration for abdominal cancer pain caused by peritoneal carcinomatosis: A case series of 10 patients. J Palliat Med 2016;19:1247–1248 [DOI] [PubMed] [Google Scholar]
- 36. Devulder J, Ghys L, Dhondt W, et al. : Neuropathic pain in a cancer patient responding to subcutaneously administered lignocaine. Clin J Pain 1993;9:220–223 [DOI] [PubMed] [Google Scholar]
- 37. Alsirafy SA, Raheem AA, Al-Zahrani AS, et al. : Emergency department visits at the end of life of patients with terminal cancer: Pattern, causes, and avoidability. Am J Hosp Palliat Care 2016;33:658–662 [DOI] [PubMed] [Google Scholar]
- 38. Lee YS, Akhileswaran R, Ong EHM, et al. : Clinical and socio-demographic predictors of home hospice patients dying at home: A retrospective analysis of hospice care association's database in Singapore. J Pain Symptom Manage 2017;53:1035–1041 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.