Abstract
The therapeutic significance of timing of decompression in acute traumatic central cord syndrome (ATCCS) caused by spinal stenosis remains unsettled. We retrospectively examined a homogenous cohort of patients with ATCCS and magnetic resonance imaging (MRI) evidence of post-treatment spinal cord decompression to determine whether timing of decompression played a significant role in American Spinal Injury Association (ASIA) motor score (AMS) 6 months following trauma. We used the t test, analysis of variance, Pearson correlation coefficient, and multiple regression for statistical analysis. During a 19-year period, 101 patients with ATCCS, admission ASIA Impairment Scale (AIS) grades C and D, and an admission AMS of ≤95 were surgically decompressed. Twenty-four of 101 patients had an AIS grade C injury. Eighty-two patients were males, the mean age of patients was 57.9 years, and 69 patients had had a fall. AMS at admission was 68.3 (standard deviation [SD] 23.4); upper extremities (UE) 28.6 (SD 14.7), and lower extremities (LE) 41.0 (SD 12.7). AMS at the latest follow-up was 93.1 (SD 12.8), UE 45.4 (SD 7.6), and LE 47.9 (SD 6.6). Mean number of stenotic segments was 2.8, mean canal compromise was 38.6% (SD 8.7%), and mean intramedullary lesion length (IMLL) was 23 mm (SD 11). Thirty-six of 101 patients had decompression within 24 h, 38 patients had decompression between 25 and 72 h, and 27 patients had decompression >72 h after injury. Demographics, etiology, AMS, AIS grade, morphometry, lesion length, surgical technique, steroid protocol, and follow-up AMS were not statistically different between groups treated at different times. We analyzed the effect size of timing of decompression categorically and in a continuous fashion. There was no significant effect of the timing of decompression on follow-up AMS. Only AMS at admission determined AMS at follow-up (coefficient = 0.31; 95% confidence interval [CI]:0.21; p = 0.001). We conclude that timing of decompression in ATCCS caused by spinal stenosis has little bearing on ultimate AMS at follow-up.
Keywords: decompression, outcome, spinal cord injury, timing, trauma
Introduction
In 1951, Schneider first described the clinical syndrome of acute traumatic central cord syndrome (ATCCS) caused by spinal stenosis.1 At the time, investigators advocated against surgical decompression, favoring instead a natural recovery of function and avoidance of surgical complications.2–4 Over the ensuing two decades, non-surgical management dominated as being the standard of care for these patients, but subsequently, surgeons increasingly adopted surgical decompression.5 Over time, surgery gradually emerged as the standard of care. Numerous investigations of ATCCS that focused on its epidemiology, pathophysiology, imaging characteristics, and optimal operative strategies have advanced understanding of this condition.6–12 Nevertheless, questions remain regarding best management, especially regarding surgical timing, operative technique, and extent of spinal cord decompression.12–17 At present, one of the most pressing questions is the timing of surgical intervention for ATCCS.6 8,10,11
Longitudinal epidemiological studies from around the world including the National Spinal Cord Injury Model Systems, Spinal Cord Injury Ontario, the University of Maryland, and Spain indicate that over the last several decades traumatic spinal cord injury (SCI) patients as a whole tend to be older and have less severe injuries and are more likely to have sustained a ground-level fall.16,18–24 At the same time, there has been a decline in the incidence of fracture-dislocations and catastrophic injuries, especially in younger patients.16 At present, nearly one half of patients with ATCCS have spinal stenosis-discosteophyte complex.25
Modern imaging, neuroanatomical, and biophysiological research indicate that the pathogenetic mechanisms of ATCCS differ from what Schneider and colleagues initially hypothesized based on the then-known anatomy of the spinal cord.1,3,4 Studies in non-human primates by Bucy and colleagues,26,27 and Pappas and colleagues28,29 have shown that the topographic differentiation of the corticospinal tracts becomes less discrete within and distal to the medullary pyramids, suggesting that the fibers primarily involved with the C8 and T1 motor neuron pool are not in fact medial to the lower extremity fibers. Correlative studies of magnetic resonance imaging (MRI) and post-mortem examinations generally have not shown the presence of central cord bleeding, indicating that axonal disruption and swelling within the posterolateral funiculi is the primary pathological hallmark of ATCCS and the foundation of its intramedullary lesion and its length.14,15,17,30,31 Reports from Levi and colleagues32 have confirmed that in human and non-human primates the corticospinal tracts are not discretely separated in layers with respect to one another, and that the majority of the corticospinal fibers traversing the cervical spinal cord are devoted to hand motor function rather than bipedal locomotion. As a result of these findings, it has been concluded that ATCCS-type injuries will primarily affect upper more than lower extremity function, regardless of the segmental level of traumatic involvement.17,33
Numerous pre-clinical studies, although heterogeneous in methodology, have supported the role of surgical decompression as a means of neuroprotection following traumatic spinal cord injuries.34–43 In American Spinal Injury Association Impairment Scale (AIS) grades A and B patients, spinal cord decompression not only reduces pulmonary complications and the length of in-hospital stay,44 but also enhances long-term neurological recovery.45–48 Recent evidence indicates that demonstrably complete spinal cord decompression may promote long-term upward AIS grade conversion.49 Similarly, some centers recommend intraspinal pressure monitoring and expansile duraplasty to enhance spinal cord perfusion pressure and to ensure complete spinal cord decompression.50–54
In recent years, there has been increasing interest in surgical decompression of ATCCS,5 but the therapeutic effectiveness of the timing, extent, and operative technique of decompression in ATCCS have not been extensively studied. Available retrospective studies are heterogeneous, consisting primarily of small case series with unadjusted independent variables.8,11,17,55–60 Here, based on our experience with 101 patients with ATCCS, we tested the hypothesis that, among those who undergo decompressive surgery and who have post-operative MRI confirmation of de facto spinal cord decompression, the timing of operative intervention does not affect the long-term neurological outcome.
Methods
A homogenous cohort of patients with ATCCS caused by spinal stenosis was admitted to the R Adams Cowley Shock Trauma Center over a 19-year period and was selected to define the effect of timing of decompression (Specific Aim # 1) on long-term neurological outcome. The study was approved by the Human Research Protection Office (HRPO) of the University of Maryland School of Medicine (IRB HP-00078395).
Design
This study was designed as a retrospective cohort study.
Primary outcome measure
The primary outcome measure was the ASIA motor score at least 6 months following traumatic cervical SCI (tCSCI).
Secondary outcome measure
There was no secondary outcome measure.
Cohort
From 2000 to 2018, 2372 tCSCI patients were admitted to The R Adams Cowley Shock Trauma Center, of whom 2061 patients had sustained fracture dislocations and were excluded from the study. From the cohort, 311 patients had the clinical picture of ATCCS and were without mechanical instability on imaging studies (computed tomography [CT] and MRI).
Inclusion criteria were
-
1.
Blunt tCSCI
-
2.
Presented as ATCCS
-
3.
ASIA motor score (AMS) ≤95
-
4.
AIS grades C and D
-
5.
No mechanical instability on CT scan
-
6.
Presence of high intensity signal change on T2 weighted image (WI) or short T1 inversion recovery (STIR) images indicating evidence of tCSCI
-
7.
Presence of spinal stenosis/disc osteophyte complex
-
8.
No evidence of discoligamentous injury on MRI except for minor extension distraction
-
9.
Underwent surgery for spinal cord decompression
-
10.
Post-operative MRI indicated presence of cerebrospinal fluid (CSF) interface between spinal cord and dura indicating complete decompression
-
11.
Followed for at least 6 months after acute care discharge, in order to determine long-term AMS
Exclusion criteria were
-
1.
AMS 96–100 (16 patients)
-
2.
Inadequate follow-up: no follow-up (46 patients); follow-ups <6 months (103 patients)
-
3.
Inadequate decompression on postoperative MRI (24 patients)
-
4.
No postoperative MRI (12 patients)
-
5.
Death during their acute care in the hospital (6 patients)
-
6.
No medical records (3 patients)
Sample size
This investigation was a retrospective cohort study and all the eligible 101 patients were included in the investigation.
Primary and secondary survey, resuscitation, and clinical examination
Patients were transferred to The R Adams Cowley Shock Trauma Center by emergency medical technicians (EMTs)61 supine on a rigid backboard with the head and neck secured by a hard collar and chin strap. Primary and secondary examinations were performed by trauma surgeons upon arrival. Once the patients were deemed medically stable following initial resuscitation, members of the neurosurgical team (senior resident or nurse practitioners) conducted a complete neurological assessment and then presented the case to the attending neurosurgeon. Admission AMS and AIS grades were determined according to the International Standards for Neurological Classification of Spinal Cord Injury (ISNCSCI).62
CT and MRI
Preoperative multiplanar cervical spine CT was performed within 2–3 h of trauma,63 and multi-sequence multiplanar T2-weighted and STIR sequences were acquired within 5–7 h following trauma.17,64 We defined the morphometric measures of spinal stenosis including the number of stenotic skeletal segments, the segmental level of maximum spinal cord compression, and the maximum canal compromise (MCC). Intramedullary lesion length was measured on sagittal T2 and/or STIR MRI. Sagittal and axial T2 and STIR MRI sequences were also used to determine the extent of spinal cord decompression following surgery.65 Post-operative MRI studies were performed within a median of 53 h (mean 75 h) from injury. Decompression was defined as the presence of a CSF interface between the spinal cord and dura (either ventral, dorsal, or both) continuously from the foramen magnum to the first thoracic vertebra. Two spine fellowship-trained neurosurgeons (C.A.S. and K.M.C.) and two neurosurgeons (G.T.S. and B.A.) validated the extent of spinal cord decompression following surgery. Discrepancies were adjudicated by a consensus conference of the four neurosurgeons.64, 66 An attending trauma neuroradiologist (K.S.) and the principal investigator (PI) independently measured the intramedullary lesion length (IMLL), and the mean value was taken for statistical analysis.64
Methylprednisolone protocol and blood pressure augmentation
From 2000 to 2009, 37 of the study patients were administered methylprednisolone following SCI; 30 mg/kg within the first hour and 5.4 mg/kg/h for the next 23 h. From 2010 on, the use of steroids for SCI was discontinued (64 patients). Patients' mean arterial blood pressure (MAP) was maintained between 85 to 90 mm Hg for 7 days following trauma when medically feasible.67,68
Surgical technique and timing of decompression
Twelve neurosurgeons including five spine fellowship-trained neurosurgeons performed the surgeries. Surgeries included anterior cervical discectomy and fusion (ACDF), anterior cervical corpectomy and fusion (ACCF), ACDF plus laminectomy and posterior spinal fusion (PSF), and laminectomy with PSF or laminoplasty. Post-operative CT and MRI were performed to confirm adequate hardware placement and to verify spinal cord decompression, respectively.
Intensive care unit (ICU) care and long-term follow-up
In the ICU, enoxaparin (Lovenox®, Sanofi, USA) was administered 30 mg twice daily starting within 24–48 h of trauma for deep vein thrombosis (DVT) prophylaxis, with screening for DVT by duplex ultrasound when clinically indicated. Early tracheostomy for ventilator support and percutaneous gastroenterostomy for nutrition were also routinely performed when indicated, as determined by the ICU and trauma team. During the entire in-hospital stay, ISNCSCI examinations, including digital rectal examination, were performed each day to track changes in AMS and AIS grade. After discharge, patients returned to the clinic at 6 weeks, 3 months, 6 months, and 12 months (or longer) for follow-up ISNCSCI examinations, which were performed by the staff of the Department of Neurosurgery as well as by certified neurologists and rehabilitation specialists in other facilities. Median follow-up was 12 months, and the interquartile range (IQR) was 9.5 months.
Statistical analysis
Descriptive statistics were used to characterize the patient population. We used a t test or analysis of variance (ANOVA) to assess the association between the outcome variable; that is, the final AMS, and the categorical explanatory variables. We also used a Pearson correlation coefficient to examine the association between the outcome variable and other continuous variables. Then, all variables that were significantly associated with the outcome variable in the univariate analysis were included in a multiple regression analysis to predict the outcome variable while adjusting for time-to-surgery (as ≤24 h, 25–72 h, and >72 h) and IMLL in the model. Statistical significance was set at a 0.05 level. The statistical program of Stata/SE version 16.1 (Stata Corporation, College Station, TX) was used for all analyses.
Results
Baseline characteristics
Descriptive data for the cohort of 101 patients who presented with ATCCS caused by spinal stenosis, including patient demographics, injury mechanism, clinical characteristics, neuroimaging biomarkers (CT and MRI), steroid protocol, and surgical technique, are presented in Table 1, stratified by the time elapsed between trauma and surgical decompression: early (≤ 24 h), late (25–72 h), and delayed (> 72 h).
Table 1.
Baseline Characteristics
| Category | ≤24 h Post-trauma | 25-72 h Post-trauma | >72 h Post-trauma | Total | p |
|---|---|---|---|---|---|
| Accident, n (%) | 0.23 | ||||
| Fall | 21 (30.4) | 29 (42.0) | 19 (27.6) | 69 (100) | |
| MVC | 6 (37.5) | 4 (25) | 6 (37.5) | 16 (100) | |
| Other | 9 (56.3) | 5 (31.2) | 2 (12.5) | 16 (100) | |
| Total | 36 (35.7) | 38 (37.6) | 27 (26.7) | 101) (100) | |
| Gender, n (%) | 0.55 | ||||
| Male | 31 (37.8) | 29 (35.4) | 22 (26.8) | 82 (100) | |
| Female | 5 (26.3) | 9 (47.4) | 5 (26.3) | 19 (16.7) | |
| Total | 36 (35.7) | 38 (37.6) | 27 (26.7) | 101 (100) | |
| Age (years), mean (SD) | 58.1 (11.3) | 57.4 (12.4) | 58.2 (11.8) | 57.9 (11.7) | 0.95 |
| AIS grade, n (%) | 0.19 | ||||
| AIS C | 10 (27.8) | 11 (28.9) | 3 (11.1) | 24(23.7) | |
| AIS D | 26 (72.2) | 27 (71.1) | 24 (88.9) | 77 (76.3) | |
| Admission ASIA motor score, mean (SD) | 62.9 (24.3) | 68.08 (24.7) | 75.9 (18.6) | 68.3 (23.4) | 0.08 |
| Follow-up ASIA motor score, mean (SD) | 91.1 (15.8) | 91.9 (13.4) | 97.5 (3.8) | 93.1 (12.8) | 0.11 |
| Neuroimaging biomarkers | |||||
| Morphometric measures | |||||
| Number of stenotic segments, n | 2.6 | 2.7 | 3.0 | 2.8 | 0.45 |
| Maximum canal compromise | 37.3 (7.8) | 39.1 (8.6) | 39.6 (10.1) | 38.6 (8.7) | 0.64 |
| Level of spinal cord compression, n (%) | 0.43 | ||||
| C3/C4 | 17 (32.1) | 22 (41.5) | 14 (26.4) | 53 | |
| C4/C5 | 9 (32.1) | 9 (32.1) | 10 (35.8) | 28 | |
| Other | 10 (50) | 7 (35) | 3 (15) | 20 | |
| MRI signal characteristics | |||||
| IMLL (mm) | 24.6 (12.2) | 24.0 (10.4) | 19.4 (9.7) | 23.0 (11.0) | 0.14 |
| Number of T2 signal intensity points | 1.19 (0.40) | 1.31 (0.52) | 1.29 (0.46) | 1.26 (0.46) | 0.69 |
| Methylprednisolone protocol | 0.15 | ||||
| Was infused | 11 (29.7) | 12 (32.4) | 14 (37.9) | 37 (36.6) | |
| Was not infused | 24 (37.5) | 26 (40.6)) | 14 (21.9) | 64 (64.4) | |
| Surgical technique, n (%) | 0.22 | ||||
| ACDF/ACCF | 13 (32.5) | 16 (40) | 11 (27.5) | 40 (100)) | |
| ACDF+laminectomy | 9 (56.3) | 6 (37.5) | 1 (6.2) | 16 (100) | |
| Laminectomy or expansive laminoplasty | 14 (31.1) | 16 (35.6) | 15 (33.3) | 45 (100) | |
| Total | 36 (35.7) | 38 (37.6) | 27 (26.7) | 101 (100) |
Descriptive data for the cohort of 101 patients who presented with ATCCS caused by spinal stenosis, including patient demographics, injury mechanism, clinical characteristics, neuroimaging biomarkers (CT and MRI), steroid protocol, and surgical technique. All patients were admitted to The R Adams Cowley Shock Trauma Center and are stratified here by the timing of surgical decompression from traumatic ictus: early (≤ 24 h), late (25–72 h), and delayed (>72 h).
ACDF/ACCF, anterior cervical discectomy and fusion/anterior cervical corpectomy and fusion; AIS, American Spinal Injury Association (ASIA) Impairment Scale; ATCCS, acute traumatic central cord syndrome; IMLL, intramedullary lesion length; MRI, magnetic resonance imaging; MVC, motor vehicle collision; SD, standard deviation.
Mechanism of injury
Nearly 70% of the ATCCS patients sustained ground level falls (Table 1), a finding that is consistent with previously reported systematic reviews and cohort studies.12,69–71
Glasgow Coma Scale (GCS) score, AMS, and AIS grade
GCS score at the time of admission in all patients was 14–15. The ISNCSCI AMS and AIS grades at admission were not statistically different between groups (Table 1).72 There was a trend (p = 0.08) toward later surgery in patients presenting with higher AMS.
CT and MRI morphology and morphometry
Morphological assessment of the cervical spine by multiplanar CT and multiplanar, multi-sequence MRI63,73,74 demonstrated no structurally destabilizing fractures, dislocations, or discoligamentous injuries. Minor distraction injuries to the anterior longitudinal ligament 17,75 were noted in a minority of patients. The length of the stenotic segments, the point of maximum spinal cord compression, and the MCC were not statistically different among the different surgical timing cohorts. We applied the formula suggested by Furlan and colleagues76 to measure MCC across multiple levels (Table 1). In our study, the mean Cobb angle was 11.7 degrees (standard deviation [SD] 11.9), and five patients experienced ossification of posterior longitudinal ligament (OPLL).
IMLL and the number of T2 signal changes
IMLL, as detected by T2-weighted or STIR MRI, was not as expansive as reported in more severe cervical spine injuries, in accordance with the study by Le and colleagues,31 which reported that the rate of IMLL expansion in AIS grade C and D patients was ∼300 μm/h, significantly less than the 900 μm/h recorded in AIS grades A and B patients.77 In the present study, mean IMLL for the entire cohort was 23 mm (SD 10). Intramedullary lesion morphology was classified as “discrete,” “patchy,” or “multiple.” In the group with early decompression, 29 patients had a single discrete spot of high intensity signal and 7 patients had two high intensity signals. In the group with late decompression, 27 patients had a single spot, 10 patients had two spots, and 1 patient had three spots. In the group with delayed decompression, 19 patients had one spot and 8 patients had two spots. IMLL and number of focal T2 signal changes were not statistically different between groups.
Surgical Intervention
Surgical decompression was performed within a median of 37.5 (IQR = 50) h (mean 65.2, SD 75.2 h). Thirty-six patients underwent surgery within 24 h, 38 patients within 25–72 h, and 27 patients in >72 h (range 79–439 h). Two patients excluded from the latter timing category underwent surgical decompression 90 and 300 days (2160 and 7200 h, respectively), and in a third patient, the exact timing of delayed decompression was unknown. De facto spinal cord decompression, confirmed by postoperative MRI, was achieved with anterior decompression alone (ACDF or ACCF) in 40 patients. Sixteen patients underwent both anterior and posterior decompression. The remaining 45 patients underwent posterior decompression only, using laminectomy or open-door laminoplasty. The surgical technique was similar among the three surgical timing groups.
AMS
The prognostic effects of 11 independent variables on follow-up AMS are shown in Table 2 based on univariate analysis. These variables included time to surgery, age, admission AMS, admission AIS grade, etiology, gender, surgical technique, IMLL, number of skeletal segments, number of signals on MRI, and MCC. Only admission AMS predicted follow-up AMS with strong significance (p < 0.0001).
Table 2.
Distribution of Follow-up Motor Score Based on 11 Independent Variables from Univariate Analysis
| Independent variable | n | Mean (SD) | Correlation coefficient | p value |
|---|---|---|---|---|
| Gender | - | 0.537 | ||
| Female | 19 | 94.8 (4.9) | ||
| Male | 82 | 92.8 (14.1) | ||
| Age | 101 | - | -0.111 | 0.270 |
| Time to surgery | - | 0.117 | ||
| ≤ 24 h | 36 | 91.1 (15.9) | ||
| 25-72 h | 38 | 91.9 (13.4) | ||
| > 72 h | 27 | 97.5 (3.8) | ||
| Mechanism of injury | - | 0.182 | ||
| Fall | 69 | 91.6 (14.9) | ||
| MVC | 16 | 97.4 (4.7) | ||
| Other | 16 | 95.6 (6.2) | ||
| Surgical technique | 0.099 | |||
| ACDF | 40 | 96.5 (6.1) | ||
| ACDF+Lami | 16 | 91.3 (9.5) | ||
| Lami | 45 | 90.8 (17.2) | ||
| Level of maximum pressure | - | 0.436 | ||
| C3/4 | 53 | 94.7 (6.8) | ||
| C4/5 | 28 | 91.1 (15.6) | ||
| Other | 20 | 91.9 (19.5) | ||
| Maximum canal compromise | 101 | - | 0.029 | 0.777 |
| Number of stenotic segments | 101 | - | -0.131 | 0.191 |
| IMLL | 101 | - | -0.058 | 0.564 |
| Number of signals | 101 | - | -0.195 | 0.051 |
| Admission AMS | 101 | - | 0.553 | <0.0001 |
SD, standard deviation; MVC, motor vehicle collision; ACDF, anterior cervical discectomy and fusion; ACDF+Lami, ACDF + laminectomy; IMLL, intramedullary lesion length; AMS, American Spinal Injury Association (ASIA) motor score.
Timing of decompression
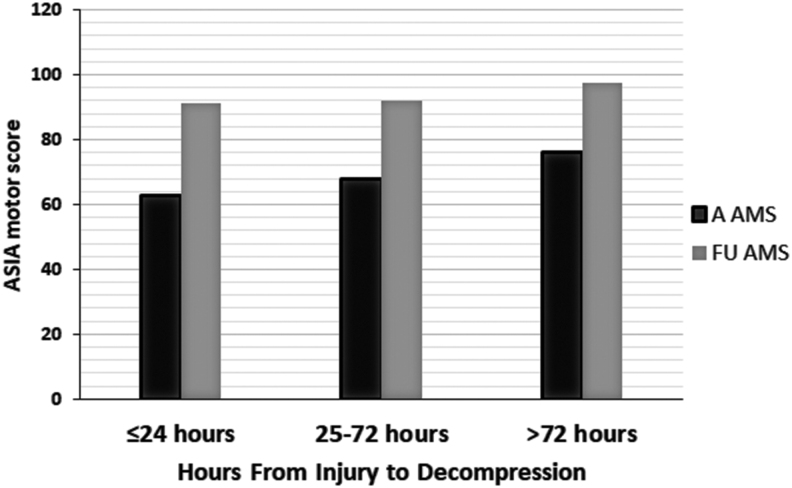
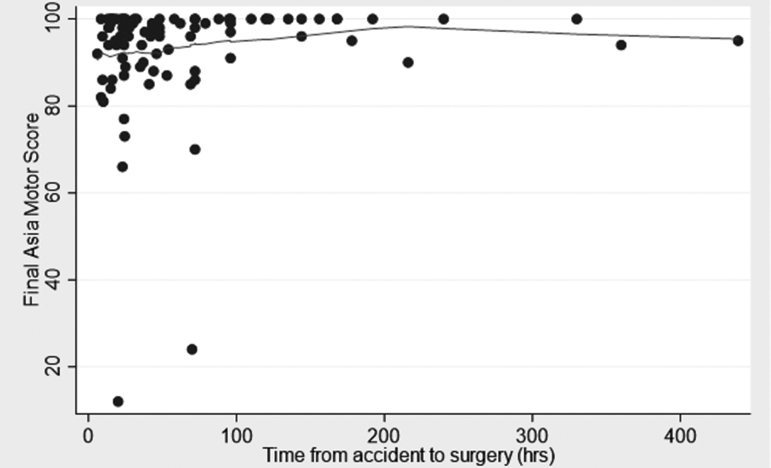
We analyzed the relationship between the timing of decompression and improvements in AMS after at least 6 months of follow-up (median 12 and range 6–138 months). The variable of time was taken categorically and continuously as suggested by the literature.12,47,48, 65,79,80 These categories were within 24 h (36 patients), from 25–72 h (38 patients), and from 73 to >400 h (17 patients) (Fig. 1). The element of time was also used as a continuous variable (Fig. 2). During the course of follow-up, the admission AMS increased from 68.3 (SD 21.4) to 93.1 (SD 12.8). There was no significant difference among the three categories of time to decompression and increases in AMS at the latest follow-up. Additionally, in multiple regression analyses, only AMS at admission was the most powerful determinant of AMS at the latest follow-up (Table 3).
FIG. 1.
Bar graph depicting the relationship between admission and follow-up American Spinal Injury Association (ASIA) motor score (AMS) when 101 patients with acute traumatic central cord syndrome (ATCCS) caused by spinal stenosis were fully decompressed within 24, 25–72, and >72 h following trauma.
FIG. 2.
Scatter graph of the final American Spinal Injury Association (ASIA) motor score (AMS) versus time to decompression in 101 patients with acute traumatic central cord syndrome (ATCCS) caused by spinal stenosis.
Table 3.
Stepwise Regression Analysis of 11 Independent Variables; Namely, Time from Injury to Surgery (Categorical), Age, Gender, Etiology, Admission AMS, Admission AIS Grade, Number of Stenosed Skeletal Segments, MCC, Point of Maximum Compression, Number of High Intensity Signals on MRI, and IMLL, and Their Prognostic Influence on Follow-up AMS
| Independent variable | Coefficient | p value | 95% confidence interval |
|---|---|---|---|
| Time to Surgery | |||
| ≤24 h | 0 (referent) | ||
| 25-72 h | -0.71 | 0.777 | (-5.71, 4.28) |
| >72 h | 3.00 | 0.293 | (-2.63, 8.62) |
| Admission AMS | 0.31 | <0.001 | (0.21, 0.40) |
| IMLL | 0.13 | 0.201 | (-0.07, 0.33) |
AMS, American Spinal Injury Association (ASIA) motor score; AIS, ASIA Impairment Scale; MCC, maximum canal compromise; MRI, magnetic resonance imaging; IMLL, intramedullary lesion length.
Discussion
Here, we present evidence that in ATCCS caused by spinal stenosis, when adequate decompression was obtained, the timing of decompression did not affect the outcome after adjustment for age, admission AMS, admission AIS grade, etiology, gender, surgical technique, IMLL, number of skeletal segments stenosed, segments of maximum compression, number of signals on MRI, and MCC. AMS at admission was the sole determinant of AMS at follow-up (p < 0.001).
For two decades, 2001–2018, we have witnessed changing trends in the epidemiology of tSCI, including a progressive drop in the IMLL and an increase in AMS at admission.16 Going forward, surgeons may be challenged by milder incomplete tCSCI cases in older individuals presenting mostly with ATCCS.13,16,20–22,24 In the present study, the typical patient was 58 years old, was admitted to the trauma resuscitation unit after a ground level fall, and had an AMS of 65. Imaging parameters indicated an MCC of close to 40%, based on three skeletal segments of C3-C6, a Cobb angle of 11.7 degrees, and an IMLL of 23 mm.17
Biological rational, pre-clinical, and translational studies in tCSCI support decompression as a neuroprotective measure aimed at mitigating secondary injury and improving outcome.34–38,40,42,43,81–83 Whereas in ATCCS caused by fractures and disrupted discoligamentous injuries of the cervical spine, earlier surgery may help better and faster neurological recovery and more effective rehabilitation measures,47,78 in ATCCS caused by spinal stenosis disc/osteophyte complex (SSDOC), support for such evidence is much weaker.85,86 In a 2019, systematic review applying Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2009 guidelines, the Spinal Cord Society and Spine Trauma Study Group established a panel tasked reviewing management and prognosis of ATCCS. Out of 770 published articles, 37 dealt with management, complications, and prognosis, including 10 that scrutinized the timing of surgery.12,87 The consensus indicated that there was reasonable evidence that, in patients with ATCCS secondary to extension injury in stenotic cervical canal without fracture/dislocation/traumatic disc herniation/instability, there is a need for high-quality prospective randomized controlled trials to resolve the controversy regarding early surgery versus conservative management and delayed surgery if recovery plateaus or if there is a neurological deterioration. Further review of the 10 studies reported by Yelamarthy and coworkers12 indicated only four studies similar to our investigation (Table 4). Selection bias and inhomogeneity in these four level 3 studies precluded any firm conclusion about the effect size of timing of decompression and long-term outcome of patients with extension injuries caused by spinal stenosis disc/osteophyte complex.8,17,57,88 Until such investigations are completed, our retrospective cohort study, which was well-adjusted for multiple independent variables, can help spine surgeons with surgical planning and the timing of surgical decompression applying appropriate techniques.
Table 4.
Comparison of Published Studies on the Effects of Timing of Decompression on AMS at Least 6 Months after tCSCI in Patients with ATCCS Caused by SSDOC Adjusted for Morphometric Neuroimaging, Surgical Technique, and the Extent of Decompression
| Investigator year journal | D | Cohort | Outcome | SSDOC | NO SSS | MCC | Level of MSCC | # of MRI signal | Surg. Tech. | PO MRI | IMLL | Extent of DEC | F/U m | F/U m | Effect of timing |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Guest et al. 2002 J. Neurosurg. | R | 50 | PSIMFS | 24 | NM | NM | NM | NM | Y | NM | NM | NM | 13 | ≤24: 6 >24: 18 |
No effect |
| Lenehan et al. 2010 Spine (Phila PA 1976) | R | 73 | AMS | 73 | NM | NM | NM | NM | Y | NM | NM | NM | 6 | <24: 17 >24: 56 |
Early better |
| Aarabi et al. 2011 J. Neurosurg. Spine | R | 42 | AMS | 42 | M | Y | Y | NM | Y | Y | Y | NM | 12 | ≤24:9 24-48: 10 > 48: 23 | No effect |
| Anderson et al. 2012 Am. J. Orthop. (Belle Mead NJ) | R | 69 | AMS | 31 | NM | NM | NM | NM | Y | NM | NM | NM | ≥6 | ≤24: 14 24-48: 30 >48: 25 |
No effect |
| Aarabi et al. 2021 J. Neurotrauma | R | 101 | AMS | 101 | M | Y | Y | Y | Y | Y | Y | Y | ≥6 | ≤24: 36 25-72:38 > 72: 27 | No effect |
AMS, American Spinal Injury Association (ASIA) motor score; tCSCI, traumatic cervical spinal cord injury; ATCCS, acute traumatic central cord syndrome; D, design; DEC, decompression; F/U, follow-up; IMLL, intramedullary lesion length; m, months, M, mentioned, MCC, maximum canal compromise; MSCC, maximum spinal cord compression; MRI, magnetic resonance imaging; NM, not mentioned; NO, number; SSS, spinal stenosis segments; PO, post-operative, PSIMFS, Post-Spinal Injury Motor Function Scale; R, retrospective; Surg. Tech., surgical technique, SSDOC, spinal stenosis disc/osteophyte complex; Y, yes,
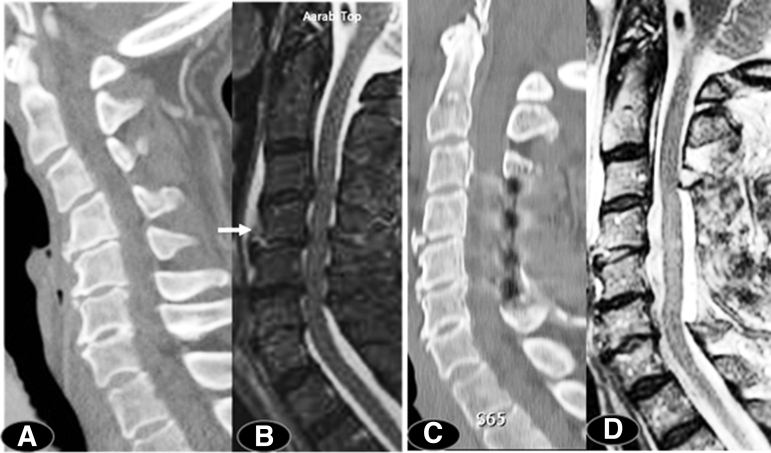
An important preoperative implication of the present study is the availability of adequate time for risk stratification, medical optimization,58 and planning for an appropriate surgical technique to address both decompression and deformity (Fig. 3). Consideration of the modified Frailty Index,89–92 comorbidities,92 and current medications including anticoagulants is important for reducing morbidity and hospital length of stay.93,94 Multiple levels of ACDF or corpectomies in older individuals can be complicated by dysphagia and swallowing difficulty.95–99
FIG. 3.
Following a ground-level fall, this 75-year-old woman had an American Spinal Injury Association (ASIA) motor score (AMS) of 52. Computed tomography (CT) scan indicated swan-neck deformity (panel A) with a Cobb angle of -13 degrees. Magnetic resonance imaging (MRI) indicated a four-segment spinal stenosis and evidence of mild extension distraction injury at C4/C5 skeletal segment (arrow) (panel B), maximum canal compromise (MCC) was 42.3% and intramedullary lesion length (IMLL) was 22.1 mm. Sixty-nine hours following her trauma, a laminectomy was performed (panels C,D). Her post-operative Cobb angle was 24 degrees. Twelve months after discharge her AMS was 85.
The most important imaging biological marker of tCSCI is the intramedullary lesion: the product of kinetic energy and molecular cascades immediately following trauma.100–103 In vivo MRI studies on intramedullary lesion in rodent models of tSCI have shown neuroprotective effects of hypertonic saline, glibenclamide, and S-nitrosoglutathion (GSNO), as evidenced by neurobehavioral improvement and reduced intramedullary lesion size.104–106 Similar studies on nonhuman primates have been modeled to reproduce lesions strikingly similar to ATCC.107 IMLL is predictive of neurological outcome in humans with severe tCSCI.65,108–111 By contrast, our study did not find admission IMLL to be predictive of long-term neurological outcome, although IMLL had a strong relationship with admission AMS (p = 0.007). This accords with the study by Le and colleagues31 that reported very slow expansion of IMLL in AIS grades C and D patients (∼300 μm/h). Knowing the IMLL following a timely total spinal cord decompression may make it possible to administer targeted neuroprotective therapies immediately following surgical intervention. A previous study of ATCCS caused by SSDOC reported that AMS at admission had a strong relationship with Functional Independent Measure (FIM) and hand dexterity at 12 months following injury.17 In a study by Noonan and colleagues,112 admission AMS had a significant effect on quality of life at follow-up.
Study limitations
This clinical study is a level 2/3 investigation and inherently prone to be confounded by bias. Although not statistically significant (Table 1, p = 0.08), a potential confounder was the fact that surgery was performed later on patients with a better AMS; however, this is unlikely to account for the study results (Figs 1 and 2). Similarly, the ceiling effect of adopting ≤95 as our AMS at the time of admission might be another confounder (Fig. 2). These issues need to be clarified by further studies. In addition, although statistically not significant, post-operative MRI indicated full decompression of the spinal cord following trauma, and the exact ideation, pre-planning, and surgical techniques used by multiple surgeons is unknown and may have had some confounding effect on the results.
Conclusion
Spine surgeons are gaining interest in ATCCS and its operative management, although the extent of such surgery and surgical technique remain to be further defined. In our study, following adjustment for multiple independent variables and with de facto spinal cord decompression demonstrated on post-operative MRI, the timing of surgery did not play a significant role in changing the final ASIA motor score at 6 month follow-up. With changing trends in demographics and markers of injury severity, we are likely to have increased numbers of patients with spinal stenosis and ATCCS. This study supports spinal cord decompression but does not identify a specific time frame for decompression. Considering the imaging complexities of the cervical spine in the elderly with ATCCS and added comorbidities, it is prudent to carefully pre-operatively decide on the surgical intervention in such a way as not only to decompress the spinal cord but also to address complicated spine morphometric deformities in a timely manner. Further research is needed to address questions such as the need for decompression, extent and timing of decompression, and the best surgical technique to achieve these important goals. In addition, further detailed investigations are needed, including defining the effect of age and comorbidities on the final long-term motor and functional outcome.
Acknowledgment
The investigators thank Dr. Patricia Stephens for her editorial assistance.
Authors' Contributions
B. Aarabi was responsible for the original concept, writing, data safety and control, oversight, and final review. Review of imaging markers was performed by K. Shanmuganathan, B. Aarabi, C. A. Sansur, G.T. Schwartzbauer, and K.M. Crandall. The statistical analysis was provided by N. Akhtar-Danesh. A critical review was provided by B. Aarabi, J.M. Simard, T. Chryssikos, G.T. Schwartzbauer, C.A. Sansur, K.M. Crandall, N. Akhtar-Danesh, J. Olexa, A.P. Wessell, G. Cannarsa, A. Sharma, C.D. Lomangino, J. Boulter, M. Scarboro, J. Oliver, A. Kareem Ahmed, N, Wenger, R. Serra, and P. Shea.
Funding Information
This study did not have a funding source except for the technical support of the Department of Neurosurgery, University of Maryland School of Medicine.
Author Disclosure Statement
No competing financial interests exist.
References
- 1. Schneider, R.C. (1951). A syndrome in acute cervical spine injuries for which early operation is indicated. J. Neurosurg. 8, 360–367 [DOI] [PubMed] [Google Scholar]
- 2. Schneider, R.C., Cherry, G., and Pantek, H. (1954). The syndrome of acute central cervical spinal cord injury. J. Neurosurg. 13, 546–577 [DOI] [PubMed] [Google Scholar]
- 3. Schneider, R.C., Crosby, E.C., Russo, R.H., and Gosch, H.H. (1973). Traumatic spinal cord syndromes and their management. Clin. Neurosurg. 20, 424–492 [DOI] [PubMed] [Google Scholar]
- 4. Schneider, R.C., Thompson, J.C., and Bebin, J. (1958). The syndrome of acute central cervical spinal cord injury. J. Neurol. Neurosurg. Psychiatry 21, 216–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yoshihara, H., and Yoneoka, D. (2013). Trends in the treatment for traumatic central cord syndrome without bone injury in the United States from 2000 to 2009. J. Trauma Acute Care Surg. 75, 453–458 [DOI] [PubMed] [Google Scholar]
- 6. Bose, B., Northrup, B.E., Osterholm, J., Cotler, J.M., and DiTunno, J.F. (1984). Reanalysis of central cervicalcord injury management. Neurosurgery 15, 367–372 [DOI] [PubMed] [Google Scholar]
- 7. Chen, T.Y., Lee, S.T., Lui, T.N., Wong, C.W., Yeh, Y.S., Tzaan, W.C., and Hung, S.Y. (1980). Efficacy of surgical treatment in traumatic central cord syndrome. Surg. Neurol. 48, 435–440 [DOI] [PubMed] [Google Scholar]
- 8. Guest, J., Eleraky, M.A., Apostolides, P.J., Dickman, C.A., and Sonntag, V.K. (2002). Traumatic central cord syndrome: results of surgical management. J. Neurosurg. 97, 25–32 [DOI] [PubMed] [Google Scholar]
- 9. Miranda, P., Gomez, P., and Alday, R. (2009). Acute traumatic central cord syndrome: analysis of clinical and radiological correlations. J. Neurosurg. Sci. 52, 107–112 [PubMed] [Google Scholar]
- 10. Uribe, J., Green, B.A., Vanni, S., Moza, K., Guest, J.D., and Levi, A.D. (2005). Acute traumatic central cord syndrome––experience using surgical decompression with open-door expansile cervical laminoplasty. Surg. Neurol. 63, 505–510 [DOI] [PubMed] [Google Scholar]
- 11. Yamazaki, T., Yanaka, K., Fujita, K., Kamezaki, T., Uemura, K., and Nose, T. (2005). Traumatic central cord syndrome: analysis of factors affecting the outcome. Surg. Neurol. 63, 95–99 [DOI] [PubMed] [Google Scholar]
- 12. Yelamarthy, P.K.K., Chhabra, H.S., Vaccaro, A., Vishwakarma, G., Kluger, P., Nanda, A., Abel, R., Tan, W.F., Gardner, B., Chandra, P.S., Chatterjee, S., Kahraman, S., Naderi, S., Basu, S., and Theron, F. (2019). Management and prognosis of acute traumatic cervical central cord syndrome: systematic review and Spinal Cord Society–Spine Trauma Study Group position statement. Eur. Spine J. 28, 2390–2407 [DOI] [PubMed] [Google Scholar]
- 13. Chen, Y., He, Y., and DeVivo, M.J. (2016). Changing demographics and injury profile of new traumatic spinal cord injuries in the United States, 1972–2014. Arch. Phys. Med. Rehabil. 97, 1610–1619 [DOI] [PubMed] [Google Scholar]
- 14. Martin, D., Schoenen, J., Lenelle, J., Reznik, M., and Moonen, G. (1992). MRI–pathological correlations in acute traumatic central cord syndrome: case report. Neuroradiology 34, 262–266 [DOI] [PubMed] [Google Scholar]
- 15. Jimenez, O., Marcillo, A., and Levi, A.D. (2000). A histopathological analysis of the human cervical spinal cord in patients with acute traumatic central cord syndrome. Spinal Cord 38, 532–537 [DOI] [PubMed] [Google Scholar]
- 16. Aarabi, B., Albrecht, J.S., Simard, J.M., Chryssikos, T. Schwartzbauer, G. , Sansur, C.A., Crandall, K., Gertner, M., Howie, B., Wessell, A., Cannarsa. G., Caffes, N., Oliver, J., Shanmuganathan, K., Olexa, J., Lomangino, C.D., and Scarboro, M. (2021). Trends in demographics and markers of injury severity in traumatic cervical spinal cord injury. J. Neurotrauma 38, 756–764 [DOI] [PubMed] [Google Scholar]
- 17. Aarabi, B., Alexander, M., Mirvis, S.E., Shanmuganathan, K., Chesler, D., Maulucci, C., Iguchi, M., Aresco, C., and Blacklock, T. (2011). Predictors of outcome in acute traumatic central cord syndrome due to spinal stenosis. J. Neurosurg. Spine 14, 122–130 [DOI] [PubMed] [Google Scholar]
- 18. Chen, Y., Devivo, M.J., and Jackson, A.B. (2005). Pressure ulcer prevalence in people with spinal cord injury: age–period–duration effects. Arch. Phys. Med. Rehabil. 86, 1208–1213 [DOI] [PubMed] [Google Scholar]
- 19. Chen, Y., DeVivo, M.J., Richards, J.S., and SanAgustin, T.B. (2016). Spinal cord injury model systems: review of program and national database from 1970 to 2015. Arch. Phys. Med. Rehabil. 97, 1797–1804 [DOI] [PubMed] [Google Scholar]
- 20. Chen, Y., Tang, Y., Allen, V., and DeVivo, M.J. (2016). Fall-induced spinal cord injury: External causes and implications for prevention. J. Spinal Cord Med. 39, 24–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. DeVivo, M.J. (2007). Sir Ludwig Guttmann Lecture: trends in spinal cord injury rehabilitation outcomes from model systems in the United States: 1973–2006. Spinal Cord 45, 713–721 [DOI] [PubMed] [Google Scholar]
- 22. Devivo, M.J. (2012). Epidemiology of traumatic spinal cord injury: trends and future implications. Spinal Cord 50, 365–372 [DOI] [PubMed] [Google Scholar]
- 23. Wilson, J.R., Cronin, S., Fehlings, M.G., Kwon, B.K., Badhiwala, J.H., Ginsberg, H.J., Witiw, C., and Jaglal, S. (2020). Epidemiology and impact of spinal cord injury in the elderly: results of a fifteen-year population-based cohort study. J. Neurotrauma 37, 1740–1751 [DOI] [PubMed] [Google Scholar]
- 24. Montoto-Marques, A., Ferreiro-Velasco, M.E., Salvador-de la Barrera, S., Balboa-Barreiro, V., Rodriguez-Sotillo, A., and Meijide-Failde, R. (2017). Epidemiology of traumatic spinal cord injury in Galicia, Spain: trends over a 20-year period. Spinal Cord 55, 588–594 [DOI] [PubMed] [Google Scholar]
- 25. Aarabi, B., Koltz, M., and and Ibrahimi, D. (2008). Hyperextension cervical spine injuries and traumatic central cord syndrome. Neurosurg. Focus 25, E9. [DOI] [PubMed] [Google Scholar]
- 26. Bucy, P.C. (1957). Is there a pyramidal tract. Brain 80, 376–392 [DOI] [PubMed] [Google Scholar]
- 27. Bucy, P.C., Keplinger, J.E., and Siqueira, E.B. (1964). Destruction of the “pyramidal tract” in man. J. Neurosurg. 21, 285–298 [PubMed] [Google Scholar]
- 28. Dickman, C.A., Hadley, M.N., Pappas, C.T., Sonntag, V.K., and Geisler, F.H. (1990). Cruciate paralysis: a clinical and radiographic analysis of injuries to the cervicomedullary junction. J. Neurosurg. 73, 850–858 [DOI] [PubMed] [Google Scholar]
- 29. Pappas, C.T., Gibson, A.R., and Sonntag, V.K. (1991). Decussation of hind-limb and fore-limb fibers in the monkey corticospinal tract: relevance to cruciate paralysis. J. Neurosurg. 75, 935–940 [DOI] [PubMed] [Google Scholar]
- 30. Quencer, R.M., Bunge, R.P., Egnor, M., Green, B.A., Puckett, W., Naidich, T.P., Post, M.J., and Norenberg, M. (1992). Acute traumatic central cord syndrome: MRI–pathological correlations. Neuroradiology 34, 85–94 [DOI] [PubMed] [Google Scholar]
- 31. Le, E., Aarabi, B., Hersh, D.S., Shanmuganathan, K., Diaz, C., Massetti, J., and Akhtar-Danesh, N. (2015). Predictors of intramedullary lesion expansion rate on MR images of patients with subaxial spinal cord injury. J. Neurosurg. Spine, 22, 611–621 [DOI] [PubMed] [Google Scholar]
- 32. Levi, A.D., Tator, C.H., and Bunge, R.P. (1996). Clinical syndromes associated with disproportionate weakness of the upper versus the lower extremities after cervical spinal cord injury. Neurosurgery 38, 179–183 [DOI] [PubMed] [Google Scholar]
- 33. Wallenberg, A. (1901). Anatomischer Befund in einem als “ acute Bulbarraffection (embolie der Art. cerebellar post. inf. sinsistr?)” beschrieben Falle. Arch Psychiat Nervenkr 34:923–959 [Google Scholar]
- 34. Carlson, G.D., Gorden, C.D., Nakazowa, S., Wada, E., Warden, K., and LaManna, J.C. (2000). Perfusion-limited recovery of evoked potential function after spinal cord injury. Spine 25, 1218–1226 [DOI] [PubMed] [Google Scholar]
- 35. Carlson, G.D., Gorden, C.D., Wada, E., Nakazawa, S., Biro, C., and La Manna, J. (1998). Vascular re-perfusion and neurol preservation after spinal cord injury. J. Neurotrauma 15, 860 [Google Scholar]
- 36. Carlson, G.D., Gordon, C.D., Oliff, H.S., Pillai, J.J. and Lamanna, J.C. (2003). Sustained spinal cord compression: part I: time-dependent effect on long-term pathophysiology. J. Bone Joint Surg. Am. 85-A, 86–94 [PubMed] [Google Scholar]
- 37. Carlson, G.D., Minato, Y., Okada, A., Gorden, C.D., Warden, K.E., Barbeau, J.M., Biro, C.L., Bahnuik, E., Bohlman, H.H., and Lamanna, J.C. (1997). Early time-dependent decompression for spinal cord injury: vascular mechanisms of recovery. J. Neurotrauma 14 , 951–962 [DOI] [PubMed] [Google Scholar]
- 38. Carlson, G.D., Warden, K.E., Barbeau, J.M., Bahniuk, E., Kutina-Nelson, K.L., Biro, C.L., Bohlman, H.H., and LaManna, J.C. (1997). Viscoelastic relaxation and regional blood flow response to spinal cord compression and decompression. Spine 22, 1285–1291 [DOI] [PubMed] [Google Scholar]
- 39. Tarlov, I.M., and Herz, E. (1954). Spinal cord compression studies. IV. Outlook with complete paralysis in man. AMA Arch. Neurol. Psychiatry 72, 43–59 [PubMed] [Google Scholar]
- 40. Tarlov, I.M. and Klinger, H. (1954). Spinal cord compression studies. II. Time limits for recovery after acute compression in dogs. AMA Arch. Neurol. Psychiatry 71, 271–290 [PubMed] [Google Scholar]
- 41. Furlan, J.C., Noonan, V., Cadotte, D.W., and Fehlings, M.G. (2011). Timing of decompressive surgery of spinal cord after traumatic spinal cord injury: an evidence-based examination of pre-clinical and clinical studies. J. Neurotrauma 28, 1371–1399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kobrine, A.I., Evans, D.E., and Rizzoli, H. (1978). Correlation of spinal cord blood flow and function in experimental compression. Surg. Neurol. 10, 54–59 [PubMed] [Google Scholar]
- 43. Kobrine, A.I., Evans, D.E., and Rizzoli, H.V. (1979). Experimental acute balloon compression of the spinal cord. Factors affecting disappearance and return of the spinal evoked response. J. Neurosurg. 51, 841–845 [DOI] [PubMed] [Google Scholar]
- 44. McKinley, W., Meade, M.A., Kirshblum, S., and Barnard, B. (2004). Outcomes of early surgical management versus late or no surgical intervention after acute spinal cord injury. Arch. Phys. Med. Rehabil. 85, 1818–1825 [DOI] [PubMed] [Google Scholar]
- 45. Papadopoulos, S.M., Selden, N.R., Quint, D.J., Patel, N., Gillespie, B., and Grube, S. (2002). Immediate spinal cord decompression for cervical spinal cord injury: feasibility and outcome. J. Trauma 52, 323–332 [DOI] [PubMed] [Google Scholar]
- 46. Sapkas, G., Papadakis, S., Katonis, P., Roidis, N., and Kontakis, G. (1999). Operative treatment of unstable injuries of the cervicothoracic junction. Eur. Spine J. 8, 279–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Fehlings, M.G., Vaccaro, A., Wilson, J.R., Singh, A., D, W.C., Harrop, J.S., Aarabi, B., Shaffrey, C., Dvorak, M., Fisher, C., Arnold, P., Massicotte, E.M., Lewis, S., and Rampersaud, R. (2012). Early versus delayed decompression for traumatic cervical spinal cord injury: results of the Surgical Timing in Acute Spinal Cord Injury Study (STASCIS). PLoS One 7, e32037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Badhiwala, J.H., Wilson, J.R., Witiw, C.D., Harrop, J.S., Vaccaro, A.R., Aarabi, B., Grossman, R.G., Geisler, F.H., and Fehlings, M.G. (2021). The influence of timing of surgical decompression for acute spinal cord injury: a pooled analysis of individual patient data. Lancet Neurol. 20, 117–126 [DOI] [PubMed] [Google Scholar]
- 49. Aarabi, B., Olexa, J., Chryssikos, T., Galvagno, S.M., Hersh, D.S., Wessell, A., Sansur, C., Schwartzbauer, G., Crandall, K., Shanmuganathan, K., Simard, J.M., Mushlin, H., Kole, M., Le, E., Pratt, N., Cannarsa, G., Lomangino, C.D., Scarboro, M., Aresco, C., and Curry, B. (2019). Extent of spinal cord decompression in motor complete (American Spinal Injury Association Impairment Scale Grades A and B) traumatic spinal cord injury patients: post-operative magnetic resonance imaging analysis of standard operative approaches. J. Neurotrauma 36, 862–876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hogg, F.R.A., Gallagher, M.J., Kearney, S., Zoumprouli, A., Papadopoulos, M.C., and Saadoun, S. (2020). Acute spinal cord injury: monitoring lumbar cerebrospinal fluid provides limited information about the injury site. J. Neurotrauma 37, 1156–1164 [DOI] [PubMed] [Google Scholar]
- 51. Phang, I., Werndle, M.C., Saadoun, S., Varsos, G., Czosnyka, M., Zoumprouli, A., and Papadopoulos, M.C. (2015). Expansion duroplasty improves intraspinal pressure, spinal cord perfusion pressure, and vascular pressure reactivity index in patients with traumatic spinal cord injury: injured spinal cord pressure evaluation study. J. Neurotrauma 32, 865–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Saadoun, S., Chen, S., and Papadopoulos, M.C. (2017). Intraspinal pressure and spinal cord perfusion pressure predict neurological outcome after traumatic spinal cord injury. J. Neurol. Neurosurg. Psychiatry 88, 452–453 [DOI] [PubMed] [Google Scholar]
- 53. Saadoun, S., and Papadopoulos, M.C. (2020). Targeted perfusion therapy in spinal cord trauma. Neurotherapeutics 17, 511–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Werndle, M.C., Saadoun, S., Phang, I., Czosnyka, M., Varsos, G.V., Czosnyka, Z.H., Smielewski, P., Jamous, A., Bell, B.A., Zoumprouli, A., and Papadopoulos, M.C. (2014). Monitoring of spinal cord perfusion pressure in acute spinal cord injury: initial findings of the injured spinal cord pressure evaluation study. Crit. Care Med. 42, 646–655 [DOI] [PubMed] [Google Scholar]
- 55. Dvorak, M.F., Noonan, V.K., Fallah, N., Fisher, C.G., Finkelstein, J., Kwon, B.K., Rivers, C.S., Ahn, H., Paquet, J., Tsai, E.C., Townson, A., Attabib, N., Bailey, C.S., Christie, S.D., Drew, B., Fourney, D.R., Fox, R., Hurlbert, R.J., Johnson, M.G., Linassi, A.G., Parent, S., and Fehlings, M.G. (2015). The influence of time from injury to surgery on motor recovery and length of hospital stay in acute traumatic spinal cord injury: an observational Canadian cohort study. J. Neurotrauma 32, 645–654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Godzik, J., Dalton, J., Hemphill, C., Walker, C., Chapple, K., Cook, A., Uribe, J.S., and Turner, J.D. (2019). Early surgical intervention among patients with acute central cord syndrome is not associated with higher mortality and morbidity. J. Spine Surg. (Hong Kong) 5, 466–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lenehan, B., Fisher, C.G., Vaccaro, A., Fehlings, M., Aarabi, B., and Dvorak, M.F. (2010). The urgency of surgical decompression in acute central cord injuries with spondylosis and without instability. Spine (Phila Pa 1976) 35 (21 Suppl.), S180–S186 [DOI] [PubMed] [Google Scholar]
- 58. Samuel, A.M., Grant, R.A., Bohl, D.D., Basques, B.A., Webb, M.L., Lukasiewicz, A.M., Diaz-Collado, P.J., and Grauer, J.N. (2015). Delayed surgery after acute traumatic central cord syndrome is associated with reduced mortality. Spine (Phila Pa 1976) 40, 349–356 [DOI] [PubMed] [Google Scholar]
- 59. Stevens, E.A., Marsh, R., Wilson, J.A., Sweasey, T.A., Branch, C.L.Jr., and Powers, A.K. (2010). A review of surgical intervention in the setting of traumatic central cord syndrome. Spine J. 10, 874–880 [DOI] [PubMed] [Google Scholar]
- 60. Stevenson, C.M., Dargan, D.P., Warnock, J., Sloan, S., Espey, R., Maguire, S., and Eames, N. (2016). Traumatic central cord syndrome: neurological and functional outcome at 3 years. Spinal Cord 54, 1010–1015 [DOI] [PubMed] [Google Scholar]
- 61. MIEMSS (2015). Maryland Institute for Emergency Medical Services Systems. https://www.miemss.org/home/Portals/0/Docs/AnnualReports/Ann-Report-2018-WEB.pdf?ver=2018-10-25-091023-133
- 62. American Spinal Injury Association (1992). International Standards for Neurological and Functional Classification of Spinal Cord Injury. American Spinal Injury Association/International Medical Society of Paraplegia: Chicago [Google Scholar]
- 63. Vaccaro, A.R., Koerner, J.D., Radcliff, K.E., Oner, F.C., Reinhold, M., Schnake, K.J., Kandziora, F., Fehlings, M.G., Dvorak, M.F., Aarabi, B., Rajasekaran, S., Schroeder, G.D., Kepler, C.K., and Vialle, L.R. (2016). AOSpine subaxial cervical spine injury classification system. Eur. Spine J. 25, 2173–2184 [DOI] [PubMed] [Google Scholar]
- 64. Aarabi, B., Sansur, C.A., Ibrahimi, D.M., Simard, J.M., Hersh, D.S., Le, E., Diaz, C., Massetti, J., and Akhtar-Danesh, N. (2017). Intramedullary lesion length on postoperative magnetic resonance imaging is a strong predictor of ASIA Impairment Scale grade conversion following decompressive surgery in cervical spinal cord injury. Neurosurgery 80, 610–620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Aarabi, B., Akhtar-Danesh, N., Chryssikos, T., Shanmuganathan, K., Schwartzbauer, G.T., Simard, J.M., Olexa, J., Sansur, C.A., Crandall, K.M., Mushlin, H., Kole, M.J., Le, E.J., Wessell, A.P., Pratt, N., Cannarsa, G., Lomangino, C., Scarboro, M., Aresco, C., Oliver, J., Caffes, N., Carbine, S., and Mori, K. (2020). Efficacy of ultra-early (< 12 h), early (12–24 h), and late (>24–138.5 h) surgery with magnetic resonance imaging-confirmed decompression in American Spinal Injury Association Impairment Scale grades A, B, and C cervical spinal cord injury. J. Neurotrauma 37, 448–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Aarabi, B., Olexa, J., Chryssikos, T., Galvagno, S.M., Hersh, D.S., Wessell, A., Sansur, C., Schwartzbauer, G., Crandall, K., Shanmuganathan, K., Simard, J.M., Mushlin, H., Kole, M., Le, E., Pratt, N., Cannarsa, G., Lomangino, C.D., Scarboro, M., Aresco, C., and Curry, B. (2019). Extent of spinal cord decompression in motor complete (American Spinal Injury Association Impairment Scale Grades A and B) traumatic spinal cord injury patients: post-operative magnetic resonance imaging analysis of standard operative approaches. J. Neurotrauma 36, 862–876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Hadley, M.N., Walters, B.C., Grabb, P.A., Oyesiku, N.M., Prezybylski, G.J., Resnick, D.K., Ryken, T.C., and Mielke, D.H. (2002). Guidelines for the management of acute cervical spine and spinal cord injuries. Neurosurgery 50, S1–S199 [DOI] [PubMed] [Google Scholar]
- 68. Walters, B.C., Hadley, M.N., Hurlbert, R.J., Aarabi, B., Dhall, S.S., Gelb, D.E., Harrigan, M.R., Rozelle, C.J., Ryken, T.C., and Theodore, N. (2013). Guidelines for the management of acute cervical spine and spinal cord injuries: 2013 update. Neurosurgery 60, Suppl. 1, 82–91 [DOI] [PubMed] [Google Scholar]
- 69. Fehlings, M.G., Tetreault, L.A., Wilson, J.R., Aarabi, B., Anderson, P., Arnold, P.M., Brodke, D.S., Burns, A.S., Chiba, K., Dettori, J.R., Furlan, J.C., Hawryluk, G., Holly, L.T., Howley, S., Jeji, T., Kalsi-Ryan, S., Kotter, M., Kurpad, S., Marino, R.J., Martin, A.R., Massicotte, E., Merli, G., Middleton, J.W., Nakashima, H., Nagoshi, N., Palmieri, K., Singh, A., Skelly, A.C., Tsai, E.C., Vaccaro, A., Yee, A. and Harrop, J.S. (2017). A clinical practice guideline for the management of patients with acute spinal cord injury and central cord syndrome: recommendations on the timing (≤24 hours versus >24 hours) of decompressive surgery. Global Spine J. 7, 195s–202s [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Brodell, D.W., Jain, A., Elfar, J.C., and Mesfin, A. (2015). National trends in the management of central cord syndrome: an analysis of 16,134 patients. Spine J. 15, 435–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Dvorak, M.F., Fisher, C.G., Hoekema, J., Boyd, M., Noonan, V., Wing, P.C., and Kwon, B.K. (2005). Factors predicting motor recovery and functional outcome after traumatic central cord syndrome: a long-term follow-up. Spine 30 (20), 2303–2311 [DOI] [PubMed] [Google Scholar]
- 72. Ditunno, J.F.Jr. (1994). American spinal injury standards for neurological and functional classification of spinal cord injury: past, present and future. 1992 Heiner Sell Lecture of the American Spinal Injury Association. J. Am. Paraplegia Soc. 17, 7–11 [DOI] [PubMed] [Google Scholar]
- 73. Allen, B.L.Jr., Ferguson, R.L., Lehmann, T.R., and O'Brien, R.P. (1982). A mechanistic classification of closed, indirect fractures and dislocations of the lower cervical spine. Spine 7, 1–27 [DOI] [PubMed] [Google Scholar]
- 74. Harris, J.H.Jr., Edeiken-Monroe, B. and Kopaniky, D.R. (1986). A practical classification of acute cervical spine injuries. Orthop. Clin. North Am. 1, 15–30 [PubMed] [Google Scholar]
- 75. White, A.A., 3rd., and Panjabi, M.M. (1978). The basic kinematics of the human spine. A review of past and current knowledge. Spine (Phila Pa 1976) 3, 12–20 [DOI] [PubMed] [Google Scholar]
- 76. Furlan, J.C., Kailaya-Vasan, A., Aarabi, B., and Fehlings, M.G. (2011). A novel approach to quantitatively assess posttraumatic cervical spinal canal compromise and spinal cord compression: a multicenter responsiveness study. Spine (Phila Pa 1976) 36, 784–793 [DOI] [PubMed] [Google Scholar]
- 77. Aarabi, B., Simard, J.M., Kufera, J.A., Alexander, M., Zacherl, K.M., Mirvis, S.E., Shanmuganathan, K., Schwartzbauer, G., Maulucci, C.M., Slavin, J., Ali, K., Massetti, J., and Eisenberg, H.M. (2012). Intramedullary lesion expansion on magnetic resonance imaging in patients with motor complete cervical spinal cord injury. J. Neurosurg. Spine 17, 243–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Burke, J.F., Yue, J.K., Ngwenya, L.B., Winkler, E.A., Talbott, J.F., Pan, J.Z., Ferguson, A.R., Beattie, M.S., Bresnahan, J.C., Haefeli, J., Whetstone, W.D., Suen, C.G., Huang, M.C., Manley, G.T., Tarapore, P.E., and Dhall, S.S. (2019). Ultra-early (<12 hours) surgery correlates with higher rate of American Spinal Injury Association Impairment Scale conversion after cervical spinal cord injury. Neurosurgery 85, 199–203 [DOI] [PubMed] [Google Scholar]
- 79. Dahdaleh, N.S., Lawton, C.D., El Ahmadieh, T.Y., Nixon, A.T., El Tecle, N.E., Oh, S., Fessler, R.G., and Smith, Z.A. (2013). Evidence-based management of central cord syndrome. Neurosurg. Focus 35, E6. [DOI] [PubMed] [Google Scholar]
- 80. Sapkas, G.S., and Papadakis, S.A. (2007). Neurological outcome following early versus delayed lower cervical spine surgery. J. Orthop. Surg. (Hong Kong) 15, 183–186 [DOI] [PubMed] [Google Scholar]
- 81. Tator Ch, F.M. (1999). Review of clinical trials of neuroprotection in acute spinal cord injury. Neurosurg. Focus 6, e8. [PubMed] [Google Scholar]
- 82. Tator, C.H., and and Koyanagi, I. (1997). Vascular mechanisms in the pathophysiology of human spinal cord injury. J. Neurosurg. 86, 483–492 [DOI] [PubMed] [Google Scholar]
- 83. Tator, C.H. (1996). Experimental and clinical studies of the pathophysiology and management of acute spinal cord injury. J. Spinal Cord Med. 19, 206–214 [DOI] [PubMed] [Google Scholar]
- 84. Tator, C.H. (1991). Review of experimental spinal cord injury with emphasis on the local and systemic circulatory effects. Neurochirurgie 37, 291–302 [PubMed] [Google Scholar]
- 85. Bosch, A., Stauffer, E.S., and Nickel, V.L. (1971). Incomplete traumatic quadriplegia: a ten-year review. JAMA 216, 473–478 [PubMed] [Google Scholar]
- 86. Fehlings, M.G., Rabin, D., Sears, W., Cadotte, D.W., and Aarabi, B. (2010). Current practice in the timing of surgical intervention in spinal cord injury. Spine (Phila Pa 1976) 35, S166–173 [DOI] [PubMed] [Google Scholar]
- 87. Karthik Yelamarthy, P.K., Chhabra, H.S., Vaccaro, A., Vishwakarma, G., Kluger, P., Nanda, A., Abel, R., Tan, W.F., Gardner, B., Sarat Chandra, P., Chatterjee, S., Kahraman, S., Naderi, S., Basu, S., and Theron, F. (2021). Correction to: Management and prognosis of acute traumatic cervical central cord syndrome: systematic review and Spinal Cord Society–Spine Trauma Study Group position statement. Eur. Spine J. 30, 232–233 [DOI] [PubMed] [Google Scholar]
- 88. Anderson, D.G., Sayadipour, A., Limthongkul, W., Martin, N.D., Vaccaro, A., and Harrop, J.S. (2012). Traumatic central cord syndrome: neurologic recovery after surgical management. Am. J. Orthop. (Belle Mead NJ) 41, E104–108 [PubMed] [Google Scholar]
- 89. Alas, H., Segreto, F.A., Chan, H.Y., Brown, A.E., Pierce, K.E., Bortz, C.A., Horn, S.R., Varlotta, C.G., Baker, J.F., and Passias, P.G. (2019). Association between frailty status and odontoid fractures after traumatic falls: investigation of varying injury mechanisms among 70 elderly odontoid fracture patients. J. Orthop. Trauma 33, e484–e488 [DOI] [PubMed] [Google Scholar]
- 90. Banaszek, D., Inglis, T., Marion, T.E., Charest-Morin, R., Moskven, E., Rivers, C.S., Kurban, D., Flexman, A.M., Ailon, T., Dea, N., Kwon, B.K., Paquette, S., Fisher, C.G., Dvorak, M.F., and Street, J.T. (2020). Effect of frailty on outcome after traumatic spinal cord injury. J. Neurotrauma 37, 839–845 [DOI] [PubMed] [Google Scholar]
- 91. Yang, F., and Gu, D. (2016). Predictability of frailty index and its components on mortality in older adults in China. BMC Geriatr. 16, 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Charlson, M.E., Pompei, P., Ales, K.L., and MacKenzie, C.R. (1987). A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J. Chronic Dis. 40, 373–383 [DOI] [PubMed] [Google Scholar]
- 93. Grymonprez, M., Steurbaut, S., De Backer, T.L., Petrovic, M. and Lahousse, L. (2020). Effectiveness and safety of oral anticoagulants in older patients with atrial fibrillation: a systematic review and meta-analysis. Front. Pharmacol. 11, 583311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Steib, A., Hadjiat, F., Skibba, W., and Steib, J.P. (2011). Focus on perioperative management of anticoagulants and antiplatelet agents in spine surgery. Orthop. Traumatol. Surg. Res. 97, S102–106 [DOI] [PubMed] [Google Scholar]
- 95. Chung, W.F., Liu, S.W., Huang, L.C., Chang, H.K., Wu, J.C., Chen, L.F., Chen, Y.C., Huang, W.C., Cheng, H., and Lo, S.S. (2020). Serious dysphagia following anterior cervical discectomy and fusion: long-term incidence in a national cohort. J. Neurosurg. Sci. 64, 231–237 [DOI] [PubMed] [Google Scholar]
- 96. Ebot, J., Domingo, R., and Nottmeier, E. (2020). Post-operative dysphagia in patients undergoing a four level anterior cervical discectomy and fusion (ACDF). J. Clin. Neurosci. 72, 211–213 [DOI] [PubMed] [Google Scholar]
- 97. Ian Dhar, S., Wegner, A.M., Rodnoi, P., Wuellner, J.C., Mehdizadeh, O.B., Shen, S.C., Nachalon, Y., Nativ-Zeltzer, N., Belafsky, P.C., and Klineberg, E.O. (2020). Fluoroscopic swallowing abnormalities in dysphagic patients following anterior cervcal spine surgery. Ann. Otol. Rhinol. Laryngol. 129, 1101–1109 [DOI] [PubMed] [Google Scholar]
- 98. Oh, L.J., Ong, S., Ghozy, S., Dmytriw, A.A., Zuccato, J., Mobbs, R., Phan, K., Dibas, M., and Faulkner, H. (2020). Dysphagia rates in single- and multiple-level anterior cervical discectomy and fusion surgery: a meta-analysis. J. Spine Surg. 6, 581–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Vaishnav, A.S., Saville, P., McAnany, S., Haws, B., Singh, K., Iyer, S., Albert, T., Gang, C.H., and Qureshi, S.A. (2020). Is the likelihood of dysphagia different in patients undergoing one-level versus two-level anterior cervical discectomy and fusion? Spine J. 20, 737–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Bilgen, M., Abbe, R., Liu, S.J., and Narayana, P.A. (2000). Spatial and temporal evolution of hemorrhage in the hyperacute phase of experimental spinal cord injury: in vivo magnetic resonance imaging. Magn. Reson. Med. 43, 594–600 [DOI] [PubMed] [Google Scholar]
- 101. Hackney, D.B., Asato, R., Joseph, P.M., Carvlin, M.J., McGrath, J.T., Grossman, R.I., Kassab, E.A., and DeSimone, D. (1986). Hemorrhage and edema in acute spinal cord compression: demonstration by MR imaging. Radiology 161, 387–390 [DOI] [PubMed] [Google Scholar]
- 102. Simard, J.M., Tsymbalyuk, O., Ivanov, A., Ivanova, S., Bhatta, S., Geng, Z., Woo, S.K., and Gerzanich, V. (2007). Endothelial sulfonylurea receptor 1-regulated NC Ca-ATP channels mediate progressive hemorrhagic necrosis following spinal cord injury. J. Clin. Invest. 117, 2105–2113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Mihai, G., Nout, Y.S., Tovar, C.A., Miller, B.A., Schmalbrock, P., Bresnahan, J.C., and Beattie, M.S. (2008). Longitudinal comparison of two severities of unilateral cervical spinal cord injury using magnetic resonance imaging in rats. J. Neurotrauma 25, 1–18 [DOI] [PubMed] [Google Scholar]
- 104. Nout, Y.S., Mihai, G., Tovar, C.A., Schmalbrock, P., Bresnahan, J.C., and Beattie, M.S. (2009). Hypertonic saline attenuates cord swelling and edema in experimental spinal cord injury: a study utilizing magnetic resonance imaging. Crit. Care Med. 37, 2160–2166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Simard, J.M., Popovich, P.G., Tsymbalyuk, O., Caridi, J., Gullapalli, R.P., Kilbourne, M.J., and Gerzanich, V. (2013). MRI evidence that glibenclamide reduces acute lesion expansion in a rat model of spinal cord injury. Spinal Cord 51, 823–827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Chou, P.C., Shunmugavel, A., El Sayed, H., Desouki, M.M., Nguyen, S.A., Khan, M., Singh, I., and Bilgen, M. (2011). Preclinical use of longitudinal MRI for screening the efficacy of S–nitrosoglutathione in treating spinal cord injury. J. Magn. Reson. Imaging 33, 1301–1311 [DOI] [PubMed] [Google Scholar]
- 107. Salegio, E.A., Bresnahan, J.C., Sparrey, C.J., Camisa, W., Fischer, J., Leasure, J., Buckley, J., Nout-Lomas, Y.S., Rosenzweig, E.S., Moseanko, R., Strand, S., Hawbecker, S., Lemoy, M.J., Haefeli, J., Ma, X., Nielson, J.L., Edgerton, V.R., Ferguson, A.R., Tuszynski, M.H., and Beattie, M.S. (2016). A unilateral cervical spinal cord contusion injury model in non-human primates (Macaca mulatta). J. Neurotrauma 33, 439–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Miyanji, F., Furlan, J.C., Aarabi, B., Arnold, P.M., and Fehlings, M.G. (2007). Acute cervical traumatic spinal cord injury: MR imaging findings correlated with neurologic outcome––prospective study with 100 consecutive patients. Radiology 243, 820–827 [DOI] [PubMed] [Google Scholar]
- 109. Boldin, C., Raith, J., Fankhauser, F., Haunschmid, C., Schwantzer, G., and Schweighofer, F. (2006). Predicting neurologic recovery in cervical spinal cord injury with postoperative MR imaging. Spine (Phila Pa 1976) 31, 554–559 [DOI] [PubMed] [Google Scholar]
- 110. Freund, P., Seif, M., Weiskopf, N., Friston, K., Fehlings, M.G., Thompson, A.J., and Curt, A. (2019). MRI in traumatic spinal cord injury: from clinical assessment to neuroimaging biomarkers. Lancet Neurol. 18, 1123–1135 [DOI] [PubMed] [Google Scholar]
- 111. Kukreja, S., Schwab, J. and Farhadi, F. (2016). 168 impact of initial clinical and imaging parameters on long-term neurological outcomes in acute traumatic cervical spinal cord injury. Neurosurgery 63, Suppl. 1, 167 [Google Scholar]
- 112. Noonan, V.K., Kopec, J.A., Zhang, H., and Dvorak, M.F. (2008). Impact of associated conditions resulting from spinal cord injury on health status and quality of life in people with traumatic central cord syndrome. Arch. Phys. Med. Rehabil. 89, 1074–1082 [DOI] [PubMed] [Google Scholar]