Abstract
Our objective was to track and quantify the natural course of serological markers over the 1st year following spinal cord injury. For that purpose, data on serological markers, demographics, and injury characteristics were extracted from medical records of a clinical trial (Sygen) and an ongoing observational cohort study (Murnau study). The primary outcomes were concentration/levels/amount of commonly collected serological markers at multiple time points. Two-way analysis of variance (ANOVA) and mixed-effects regression techniques were used to account for the longitudinal data and adjust for potential confounders. Trajectories of serological markers contained in both data sources were compared using the slope of progression. Our results show that, at baseline (≤ 2 weeks post-injury), most serological markers were at pathological levels, but returned to normal values over the course of 6–12 months post-injury. The baseline levels and longitudinal trajectories were dependent on injury severity. More complete injuries were associated with more pathological values (e.g., hematocrit, ANOVA test; χ2 = 68.93, df = 3, adjusted p value <0.001, and χ2 = 73.80, df = 3, adjusted p value <0.001, in the Sygen and Murnau studies, respectively). Comparing the two databases revealed some differences in the serological markers, which are likely attributable to differences in study design, sample size, and standard of care. We conclude that because of trauma-induced physiological perturbations, serological markers undergo marked changes over the course of recovery, from initial pathological levels that normalize within a year. The findings from this study are important, as they provide a benchmark for clinical decision making and prospective clinical trials. All results can be interactively explored on the Haemosurveillance web site (https://jutzelec.shinyapps.io/Haemosurveillance/) and GitHub repository (https://github.com/jutzca/Systemic-effects-of-Spinal-Cord-Injury).
Keywords: clinical trial safety, cohort studies, serological markers, spinal cord trauma, systemic effects
Introduction
Because of its crucial role in the coordination of bodily functions, damage to the spinal cord can lead to severe dysfunction or failure in single or multiple organs, including the heart, kidney, and liver.1 As a consequence of altered functions, levels or concentration of biomarkers derived from conventional serological tests are modified.2,3 Their readiness and straightforward collection make these serological markers, which encompass both hematological (complete blood count [CBC]) and biochemical indices, ideally suited for evaluating the trauma-induced systemic perturbations. Laboratory tests are routinely conducted in the acute phase of injury to assess the initial magnitude of systemic damage and to monitor the bodily functions. However, little is known about how the systemic effects and their respective serological markers progress as a function of time. This paucity of knowledge is even more surprising, considering that these serological markers have the potential to guide the design (patient stratification) and implementation of clinical trials (safety assessment of trialed drug).3–5 To address this knowledge gap, the aim of this study was to determine the natural progression of serological markers following a spinal cord injury. We hypothesized that, by disruption of normal innervation of vital organs after a traumatic spinal cord injury, there will be time-dependent and injury-specific alterations in serological markers characterized by an initial pathological change that normalizes over time (i.e., reaches norm values of healthy able-bodied people). Lastly, we provide the scientific and medical community with a first-of-its-kind surveillance tool “Haemosurveillance,” which aims to generate novel research questions as well as to inform clinical decision making and clinical trial design.
Methods
Study design and data source
To determine the natural progression of serological markers following spinal cord injury, we performed an observational study of prospectively collected data. Therefore, we analyzed two different data sources, one each from the United States of America and Germany. The first data source was a prospective phase III, placebo-controlled, multi-center study assessing the efficacy of gangliosidosis-1 (GM-1) ganglioside therapy in acute traumatic spinal cord injury.6,7 Running from 1992 to 1998, the Sygen trial failed to demonstrate a superior treatment effect of GM-1 over placebo treatment. Full design, recruitment, and enrollment details of the Sygen trial have been described previously.8 A total of 797 patients across the United States were included in the randomization. Within the framework of this United States Food and Drug Administration (FDA) regulated trial, detailed information concerning neurological scores and blood chemistry were meticulously collected. The second data source was an observational cohort study conducted at the over-regional level-I trauma center in Murnau, Germany (hereafter referred to as the Murnau study). Between 2004 and 2017, 363 patients were enrolled and followed up for 1-year post-injury. All patients enrolled in the Murnau study received standard rehabilitation care.
Ethics approval
The study was performed in accordance with the Declaration of Helsinki. Approval for the secondary analysis of the Sygen trial was received by an institutional ethical standards committee on human experimentation at the University of British Columbia. The original Sygen clinical trial (results published elsewhere) also received ethical approval, but was conducted before clinical trials were required to be registered.7–9 The data received from the original clinical trial were de-identified. The Murnau study was approved by the Bavarian Medical Chamber (#2018-077).
Cohort definition: Inclusion and exclusion criteria
To be included in our study, patients needed to have blood values at three different time points as well as information on sex, age, and injury characteristics (i.e., injury severity, injury level, and baseline motor and sensory scores). Baseline was defined as the first 72 h after injury for the Sygen trial and the first 2 weeks post-injury for the Murnau study. Patients were excluded if any of these data were missing or if they had sustained a non-traumatic injury (e.g., a tumor), or had decided to withdraw their data over the course of the study.
Outcome, predictor, and confounding variables
The primary outcomes were serological markers with data available for at least 50 patients at each time point. This threshold was chosen to ensure that the model output was interpretable, statistically powerful enough to make inferences, and clinically relevant. Independent variables were time points post-injury at which serological markers were collected. As an FDA requirement for the Sygen trial, detailed information regarding routine blood chemistry was collected at admission to the trauma center (hereinafter referred to as week 0), and at 1, 2, 4, 8, and 52 weeks post-injury. The laboratory analyses were all performed by SmithKline Beecham between February 1997 and April 1993 using the available clinical machines in this time period (Table S1). In the Murnau study, information on serological markers was collected upon the request of the attending physicians (i.e., not at standardized time points). As a consequence, different numbers of blood draws were collected for each patient on different days post-injury. All laboratory analyses were performed in-house at the BGU (Berufsgenossenschaftliche Unfallklinik) Murnau. Normal ranges for the serological markers were provided by the manufacturer of the analytic devices (Table S1). Normal ranges derived from the Murnau study were also applied to the analysis of the Sygen study. The rationale for that stems from the fact that the original upper and lower bound values in Sygen are not available anymore. Potential confounders included age, sex, injury completeness (at time of injury) according to the American Spinal Injury Association (ASIA) Impairment Scale (AIS),10 level of injury (at/above T6 vs below T6), and presence or absence of polytrauma. Polytrauma was defined as significant injuries of three or more points in two or more different anatomic regions in addition to the spinal cord injury.11 In the Sygen trial, the injury severity was assessed using the Frankel Scale, whereas in the Murnau study the AIS grading scale was employed. In order to facilitate a comparison between the two data sources, we recalculated the AIS grades for all patients enrolled in the Sygen trial using the European Multicenter Study on Human Spinal Cord Injury (EMSCI) International Standards for Neurological Classification of Spinal Cord Injury (ISNCSCI) calculator (https://ais.emsci.org/).
Statistical analyses
Two-way analysis of variance (ANOVA) and mixed-effects regression models were chosen for the primary analyses. These models were naturally suited to account for the longitudinal nature of the data as well as to adjust for potential confounders. Dependent variables were all serological markers that met our inclusion criteria. In the Murnau study, blood values were averaged per week, from week 0 to week 7 post-injury. In both studies, if, for a certain marker, patient, and time point, no data were available, the time point for this patient's marker was excluded. For analyses comparing both studies, we examined the percentage of deviation from the mean of the normal range, collected from the Murnau study. The rationale for this normalizing procedure was to make the data of the two cohorts comparable despite having different units. Independent variables were time post-injury, AIS grade, or level of injury, when examining data from the individual studies. When comparing the serological markers from both studies, we added the data sources as an independent variable. For mixed-effects regression models, pairwise comparisons of the different levels of the independent variable of interest were performed. Hence, significance levels were adjusted for multiple comparisons using Tukey's test, and p < 0.05 after adjustment, was regarded as statistical significance. For one-study two-way ANOVA tests, we applied Bonferroni correction for testing for six independent variables together. Thus, we adjusted p values, and p < 0.05 was regarded as statistical significance. In the same way, when comparing the two studies, no correction was applied, as only the data source was considered to be an independent variable. Thus, p < 0.05 was regarded as statistical significance. For all analyses, R Statistical Software, version 3.6.3 (running under: macOS Mojave 10.13.6), was used.
Data visualization
Using the R package Shiny and ShinyDashboard, we created an online interface to visualize the results of the current study and to interactively explore the data used for this study.
Data and code availability statement
Anonymized data used in this study will be made available upon request to the corresponding author and in compliance with the General Data Protection Regulation (EU GDPR). The code describing the analysis can be accessed on our GitHub repository (https://github.com/jutzca/Systemic-effects-of-Spinal-Cord-Injury).
Results
Cohort summary: Included patients
Subject and injury characteristics of both cohorts (Sygen: 679; Murnau: 239) are summarized in Table 1. A comparison revealed a comparable ratio of male and female patients (Pearson's χ2 test, χ2 = 0.07, df = 1, p = 0.786). However, significant differences were found in terms of age distribution (two-sided t test, t = 13.63, df = 322.55, p < 0.001, Figure S1) and injury severity distribution (Pearson's χ2 test, χ2 = 244.9, df = 3, p < 0.001).
Table 1.
Subject and Injury Characteristics of Patients Included in Our Analysis and Enrolled in the Sygen Trial and Murnau Study, Respectively
| Sygen trial | Murnau study | p value | |
|---|---|---|---|
| Subject characteristics | |||
| Total, n | 703 | 239 | |
| Sex, n (%) | 0.786 | ||
| Male | 560 (79.7) | 193 (80.8) | |
| Female | 143 (20.3) | 46 (19.2) | |
| Age in years at injury | < 0.001 | ||
| Mean ± SD | 33 ± 14 | 51 ± 19 | |
| Neurological/functional outcomes | |||
| Baseline ASIA impairment scalea, n (%) | < 0.001 | ||
| A | 446 (63.4) | 81 (33.9) | |
| B | 77 (11.0) | 22 (9.2) | |
| C | 149 (21.2) | 26 (10.9) | |
| D | 31 (4.4) | 110 (46.0) | |
| Lower extremity motor score, mean ± SD | |||
| Baseline | 2.82 ± 7.3 | 19.5 ± 19.9 | < 0.001 |
| After one year | 12.8 ± 19.3 | 28.1 ± 21.9 | < 0.001 |
| NA, n | 140 | 105 | |
| Serological markers, n | 47 | 39 | |
American Spinal Injury Association Impairment Scale (AIS): AIS-A, no sensory or motor function is preserved in the sacral segments S4–5. AIS-B, sensory but no motor function is preserved below the neurological level and includes the sacral segments S4–5 (light touch [LT] or pin prick [PP] at S4–5 or deep anal pressure [DAP]), and no motor function is preserved more than three levels below the motor level on either side of the body. AIS-C, motor function is preserved at the most caudal sacral segments for voluntary anal contraction or the patient meets the criteria for sensory incomplete status, and has some sparing of motor function more than three levels below the ipsilateral motor level on either side of the body. Fewer than half of key muscle functions below the single neurological level of injury (NLI) have a muscle grade ≥3. AIS-D, motor incomplete status as defined, with at least half (half or more) of key muscle functions below the single NLI having a muscle grade ≥3. AIS-E, if sensation and motor function as tested with the International Standards for Neurological Classification of Spinal Cord Injury (ISNCSCI) are graded as normal in all segments, and the patient had prior deficits, then the AIS grade is E. Someone without an initial SCI does not receive an AIS grade.
Significant p values are highlighted in bold.
SD, standard deviation.
Cohort summary: Excluded patients
A total of 94 and 124 patients in the Sygen trial and Murnau study, respectively, did not meet the inclusion criteria and were excluded. Reasons for exclusion comprised normal AIS score (AIS E, n = 5) and missing information on baseline AIS score (n = 192). Table S2 provides a detailed overview of the excluded cohorts. Excluded and included cohorts were significantly different in terms of age distribution (two-sided t test; t = 2.03, df = 124.56, p = 0.04, with excluded cohort younger than included cohort; and, t = -1.8852, df = 123.91, p = 0.06, with excluded cohort older than included cohort), in the Sygen trial and Murnau study, respectively. Excluded and included cohorts were comparable in terms of ratio of male and female patients (Pearson's χ2 test; χ2 = 3.43, df = 1, p = 0.06), in the Sygen trial, but significantly different in the Murnau study (Pearson's χ2 test; χ2 = 8.73, df = 1, p = 0.003).
Serological markers
A total of 32 and 28 routinely assessed blood markers were available in the Sygen trial and Murnau study, respectively. Among these, 14 and 8 blood markers, respectively, were part of the CBC, which is a test that evaluates the cells that circulate in blood. Notably, it includes counts of platelets, red and white blood cells, hemoglobin, and hematocrit. The remaining blood markers reflect renal function (5 and 4 markers in the Sygen trial and Murnau study, respectively), hepatic function (5 and 6 markers), pancreatic function (1 and 2 markers), and muscle damages (2 and 3 markers). Overall, 20 blood markers were shared among the two data sources. Table 2 provides an overview of all collected markers.
Table 2.
Serological Markers Collected in the Sygen Trial and Murnau Study
| Sygen trial | Murnau study | |
|---|---|---|
| Complete blood count | ||
| Erythrocytes | Erythrocytes | |
| Hemoglobin | Hemoglobin | |
| Hematocrit | Hematocrit | |
| MCHC | MCHC | |
| MCV | MCV | |
| Thrombocytes | Thrombocytes | |
| Leucocytes | Leucocytes | |
| Lymphocytes | Hemoglobin per erythrocyte | |
| Monocytes | ||
| Neutrophils | ||
| Eosinophils | ||
| Basophils | ||
| MCH | ||
| Total serum | ||
| Liver | ||
| Alkaline phosphatase | Alkaline phosphatase | |
| ASAT | ASAT | |
| ALAT | ALAT | |
| Total bilirubin | Total bilirubin | |
| Chloride | Gamma-GT | |
| Lactate dehydrogenase | ||
| Kidney | ||
| Calcium | Calcium | |
| Creatinine | Creatinine | |
| Albumin | Total proteins | |
| Blood urea nitrogen | Blood urea nitrogen | |
| Uric acid | ||
| Muscle | ||
| Potassium | Potassium | |
| Sodium | Sodium | |
| Cholinesterase | ||
| Pancreas | ||
| Amylase | Amylase | |
| Lipase | ||
| Others | ||
| Glucose | Glucose | |
| Prothrombin time | INR | |
| Cholesterol | Partial thromboplasmin time | |
| Triglycerides | CRP | |
| Carbon dioxide | Quick test | |
| Serological markers, n | 32 | 28 |
A total of 32 and 28 serological markers were available in the Sygen trial and Murnau study, respectively. Overall, 20 serological markers were collected in both studies (highlighted in bold).
MCHC, mean corpuscular hemoglobin concentration; MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin; ASAT, aspartate aminotransferase; ALAT, alanine aminotransferase; Gamma-GT, γ-glutamyl transferase; INR, international normalized ratio; CRP, C-reactive protein
Natural progression of serological markers post-injury
With the exception of amylase, γ-glutamyl transferase (GGT), glucose, lipase, and alanine aminotransferase (ALAT) in the Murnau study (p = 0.624, p = 1, p = 0.081, p = 1, p = 0.242, respectively) and alkaline phosphatase, potassium, and thrombocyte levels in the Sygen trial (p = 0.685, p = 1, p = 1, respectively), the concentrations of serological markers significantly changed as a function of time since injury (Tables S3 and S4). For 28 serological markers, these changes occurred within the normal range. The remaining 24 serological markers had baseline values outside the normal range, which normalized over the course of recovery (Figs 1 and 2). One serological marker (i.e., hematocrit) remained outside the normal range at 1 year post-injury.
Relationship between serological levels and injury characteristics
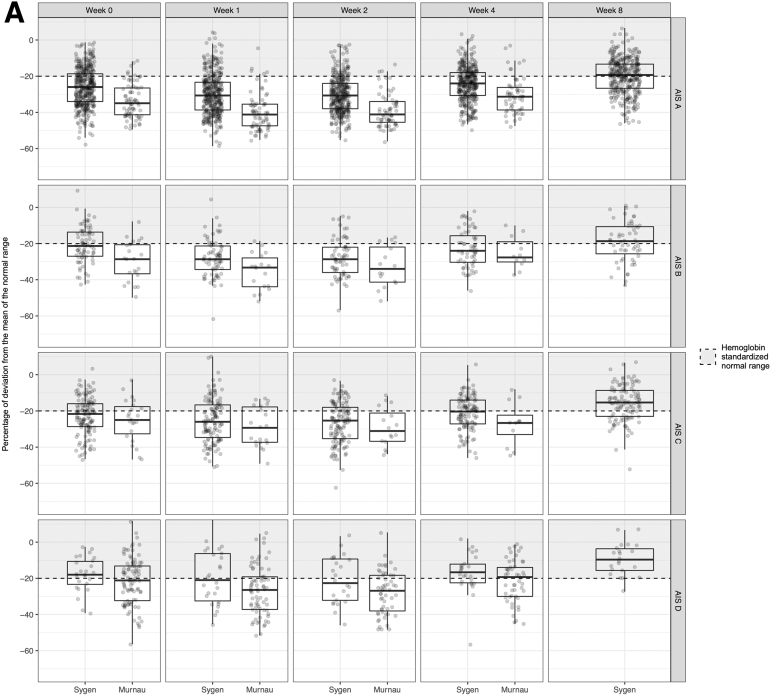
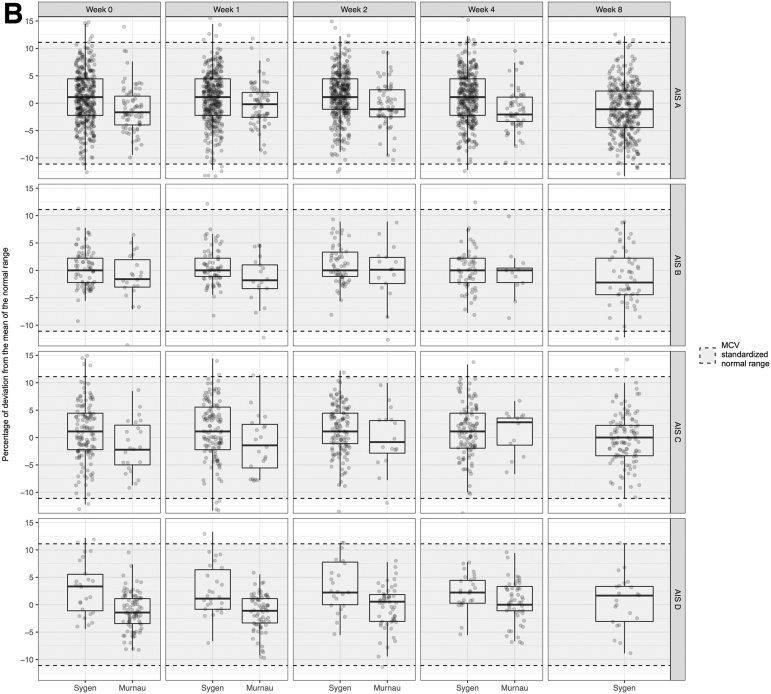
In line with our hypothesis, ANOVA revealed a global effect of injury severity (i.e., AIS score). Our post-hoc analysis revealed that the serological values were dependent on the AIS grades, calcium (p < 0.001 and p = 0.007), hematocrit (p < 0.001 and p < 0.001), hemoglobin (p < 0.001 and p < 0.001), erythrocytes count (p < 0.001 and p < 0.001), and total protein/albumin levels (p < 0.001 and p < 0.001), in both the Murnau study and the Sygen trial, respectively (Tables S3 and S4). The pairwise comparisons between the AIS grades yielded that calcium, hematocrit, hemoglobin, erythrocyte count, and total protein/albumin levels were significantly different between patients classified as AIS A and those classified as AIS D. In all cases, higher values for these markers, closer to the normal range, were associated with less severe injury (AIS D), as illustrated in Figure 1. Additionally, hematocrit, hemoglobin, erythrocyte count, and total protein/albumin were significantly different between patients classified as AIS A and those classifiefd as AIS B, C, and D. All results are reported in Tables S5 and S6 and illustrated in Figures S2–S7. In terms of injury level, we found no significant differences in serological values between patients with injuries at/above T6 and those with injuries below T6 in both the Murnau study and the Sygen trial (Tables S3 and S4). Lastly, the presence or absence of a polytrauma had a significant impact on some of the serological values (Tables S3 and S4).
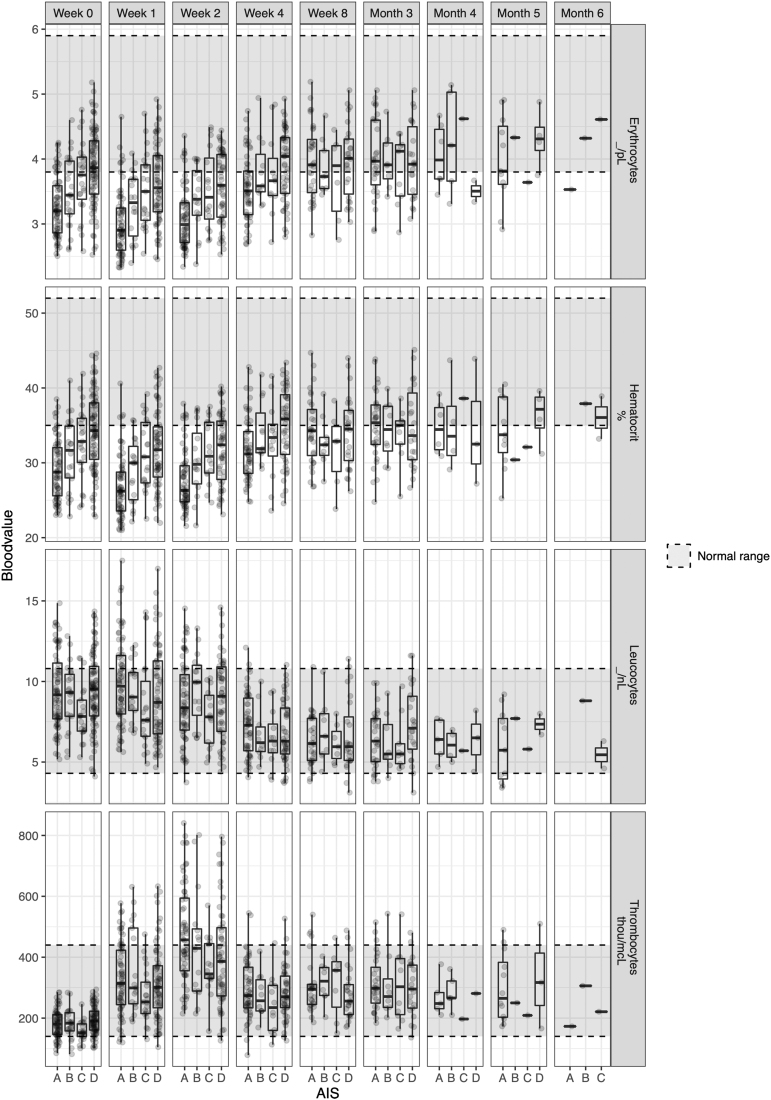
Fig. 1.
Natural progression of the complete blood count in patients with spinal cord injury who were enrolled in the Sygen trial. Three different patterns of progression were observed. First, the blood markers, such as thrombocytes, remained constant and within the range of able-bodied people. Second, blood markers were pathological immediately after the trauma, but recovered over the course of a year and reached the normal range. Erythrocytes, hemoglobin, and leucocytes are characterized by such a course. Third, values were initially within the normal range, but as a function of time they became pathological when compared with those of able-bodied people. Hematocrit is one such example (not shown here). For clinical decision making as well as the design and implementation of clinical trials, it is of utmost importance to know the temporal progression of these blood markers. For further exploration of the data, please refer to the web application Haemosurveillance (https://jutzelec.shinyapps.io/Haemosurveillance/).
Comparison between historical and contemporary cohort
As described, the Murnau study and Sygen trial have a number of major differences in their design. As illustrated in Figure 3, there were significant differences in the serological markers and their progression (Table S7), with the exception of amylase (p = 0.114), alkaline phosphatase (p = 0.409), mean corpuscular hemoglobin concentration (MCHC) (p = 0.053), sodium (p = 0.476), and ALAT levels (p = 0.746).
Fig. 3.
Comparison of the natural progression of hemoglobin (A) and mean corpuscular volume (B) in patients with spinal cord injury enrolled in the Sygen trial and the Murnau study, respectively.
Data visualization
All results can be explored interactively on the Haemosurveillance web site (https://jutzelec.shinyapps.io/Haemosurveillance/). Information is presented in separate tabs for patients enrolled in the Sygen and Murnau studies, respectively. The interactive interface also allows visualization of the data stratified by demographics (sex and age group) and injury characteristics (i.e., injury severity and type of plegia). Additionally, the interface facilitates a direct comparison of the two data sources.
Discussion
The present study describes the natural progression of serological parameters that are routinely assessed on admission and in the days to weeks following acute spinal cord injury. Consistent with our first hypothesis, we found trauma-induced changes in routinely collected serological markers (e.g., hemoglobin, glucose). By and large, most of the markers normalized at 1 year post-injury (i.e., reached the normal values of healthy able-body people). Our second hypothesis was also confirmed, insofar as the observed changes in markers were dependent on age at injury, sex, and injury severity, but not injury level. This suggests that these changes, in addition to reflecting the polytrauma and the consequent recovery process, are also capturing the severity of the spinal cord injury. Additionally, age at injury can be considered as a potential confounder for both the serological levels and the injury severity, which, itself impacts significantly the observed changes in serological markers. Collectively, this study provides new insights that will aid the design and implementation of clinical trials.
Natural progression and the relationship between serological levels and injury severity
In the present study, the majority of the serological markers reach pathological level shortly after the traumatic event and then normalize within a year post-injury. At baseline (within 2 weeks post-injury), the degree of alterations in the serological markers was associated with the injury severity, in such a way that patients with complete injuries exhibited more pronounced abnormalities in serological markers than those with incomplete injuries. This relationship between serological markers and degree of injury severity underpins the notion that serological markers may be utilized as measurable indicators of the severity. As such, they bear the potential to aid the diagnosis of spinal cord injury severity, particularly in cases in which standard neurological examination is not possible (e.g., intoxicated or unresponsive patients).3 Moreover, abnormalities in certain serological markers (e.g., albumin)2,12 may also induce further damage or delay the recovery process and, therefore, need to be addressed. In a recent study, Tong and colleagues detected that patients with prolonged hypoalbuminemia recovered to a lesser degree than those patients with normal albumin levels.2,12 Timely substitution of albumin might have beneficial effects on the functional and neurological recovery of the patient, as suggested by findings from animal studies.13 Although the return to normal serological levels occurs along the same timeline as the neurological and functional recovery, for many serological markers there is no longer an association between serological levels and injury severity. This lack of association in the chronic phase of injury suggests that the serological markers are more representative of the initial polytrauma and the recovery from it as opposed to being specific indices of the spinal cord injury.
Serological markers in the design and implementation of clinical trials
Our study provides an important framework for the implementation of serological markers in the design and conduction of clinical trials. Conventionally, the safety and tolerability of trialed treatments are assessed by means of specific abnormalities of routinely collected serological and cerebrospinal fluid (CSF) markers.14 As the majority of drugs, including the currently trialed riluzole15,16 and minocycline,17,18 are metabolized and cleared by the liver and kidney, respectively, regulatory agencies released guidelines for the assessment of risk surrounding drug-induced liver injuries (DILI)19,20 and nephrotoxicity21 in clinical trials. Multiple scheduled blood draws facilitate the early detection, tracking, and management of drug-induced organ damage. Typically, any deviation from the norm values of healthy able-bodied people would alert the investigators. In spinal cord injury, however, baseline values of numerous serological markers are pathological (Figs. 1 and 2), which, when ignored or unknown, can substantially bias assumptions on drug safety. Our haemosurveillance tool offers a first-of-its-kind platform to accurately disentangle drug-induced from trauma-driven perturbations in routinely collected serological markers. This tool is particularly useful for (1) clinical trials without a control group (i.e., placebo) and (2) clinical trials with a control group that is not being managed by a standard of care. In the former situation, historical data can aid evaluation of the safety of the trialed drug, whereas in the latter situation, the effect of the deviation from the standard of care can be measured. For example, in the ongoing Nogo Inhibition in Spinal Cord Injury (NISCI) trial (https://nisci-2020.eu/index.php?id=1449), all enrolled patients are subject to repeated lumbar puncture regardless of their allocation. As repeated lumbar puncture is not a standard of care, historical data can be leveraged to assess their impact on health (e.g., rate of infections).
Fig. 2.
Natural progression of the complete blood count in patients with spinal cord injury who were enrolled in the Murnau study.
In addition to providing guidance on drug safety and tolerability, serological markers bear the potential to refine the stratification of patients and increase the likelihood of detecting a significant treatment effect.22,23 A major barrier to detecting small treatment effects in clinical trials is the extensive heterogeneity of the neurological recovery and the scarcity of reliable predictors, such as the initial damage to the spinal cord (i.e., AIS scores), that can fully capture the extent of the injury. Therefore, utilizing a biological correlate (e.g., blood or central nervous system [CNS] marker) is potentially advantageous and informative because of its representation of the trauma and indirect involvement in the CNS.
Differences between data sources
In the current study, we analyzed data from two different data sources to validate our findings regarding temporal trajectories of the serological markers. Overall, these trajectories show comparable trends. However, some differences were uncovered that are likely attributable to differences in the study design, study period, standard of care, population structure, and sample size. The Sygen trial, our first data source, was conducted in the 1990s and had five pre-defined time points of blood collection. Moreover, as part of the standard of care at the time, all patients sustaining a spinal cord injury received methylprednisolone, a corticosteroid, to reduce inflammation and secondary damage.24,25 Corticosteroids have been reported to alter the concentration of certain serological markers, including bilirubin, albumin, and leukocytes.26–28 Patients enrolled in the Sygen trial exhibited reduced bilirubin levels and leukocytosis (i.e., an increase in the number of white cells in the blood) compared with the patients in the Murnau study, who did not receive acute treatment with methylprednisolone. Moreover, the time points of blood draw could have contributed to the differences observed. Whereas the Sygen trial collected blood samples at pre-defined time points, the patients in the observational Murnau study were subject to blood draws when indicated by the treating physician. Lastly, it is well known that organ function declines with age and is correlated with changes in laboratory values. A larger proportion of elderly patients was enrolled in the Murnau study (Supplementary Fig. S1), which could have contributed to the divergent findings.29,30
Limitations
The primary limitation of the current study is that we utilized nearly 20-year-old retrospective data, collected in clinical trial conditions, which might compromise the translation of our results to the current clinical context. We partially address this limitation by prospectively collecting contemporary data in the framework of the Murnau study. Potential bias introduced by changes in standards of care over the last decades can be, at least in part, mitigated. However, time points of data collection were not standardized in the Murnau study. As a consequence, the time-varying sample size complicated the analyses. For example, the chosen cutoff of 50 patients for the analyses was largely driven by the sample size. Future studies with larger and more consistent sample sizes at each time point of data collection are warranted to validate our findings and provide the optimal cutoff values in a data-driven fashion. The small sample size further prevented a meaningful subgroup analysis stratified by sex and age, considering that many serological markers have different normal ranges for women and men as well as being subject to age-related changes. It should also be noted that excluding patients because of missing AIS grade (e.g., because the patient was unconscious at baseline) represents a loss of information and introduces a potential bias toward patients with slightly less severe injuries. Studies with large sample sizes at baseline and follow-up time points are warranted to address this in further detail. Additionally, our study is focused on correlations at the population level, which does not guarantee the translation of our findings at the individual level. Further investigations are needed to assess the potential of serological markers in individual recovery prediction. Moreover, we did not account for any of the medications that were administered to the patients to treat secondary complications associated with spinal cord injury.31,32 Some medications (e.g., corticosteroids and nonsteroidal anti-inflammatory [NSAID] medication) can affect the concentration of the serological markers. Future studies should also address the impact of medication on the serological markers, particularly in the acute phase of injury.
Conclusion
To our best knowledge, this is the first study to comprehensively investigate the natural progression of serological markers in patients with a traumatic spinal cord injury. As a consequence of the sustained trauma, numerous routinely collected serological markers are altered in their concentration. The majority of these markers return to a normal range after 6–12 months post-injury. The current study provides a first step toward establishing a benchmark for serological markers and their natural course, which can inform clinical decision making and prospective clinical trials. Our online surveillance platform (Haemosurveillance) provides a tool for the spinal cord injury community, researchers, authorities, and policy makers to interactively exploit the natural progression of serological markers and compare different data sets with each other. The platform is configured such that existing or newly generated data sets can be added if they comply with GDPR.
Supplementary Material
Acknowledgments
The authors acknowledge the participating centers in the EMSCI network (http://emsci.org/members) that were involved in the patient care and collection of data necessary for this study.
Authors' Contributions
Lucie Bourguignon was responsible for data cleaning, data analyses, interpretation of data, and drafting the manuscript. Anh Kho Vo was responsible for data analyses, interpretation of data, and revising the manuscript for intellectual content. Bobo Tong was responsible for data cleaning, interpretation of data, and revising the manuscript for intellectual content. Fred Geisler was responsible for primary data collection, interpretation of data, and revising the manuscript for intellectual content. Orpheus Mach was responsible for primary data collection, and revising the manuscript for intellectual content. Doris Maier was responsible for primary data collection, and revising the manuscript for intellectual content. John L.K. Kramer was responsible for study concept/design, interpretation of data, and revising the manuscript for intellectual content. Lukas Grassner was responsible for primary data collection, interpretation of data, and revising the manuscript for intellectual content. Catherine R. Jutzeler was responsible for data entry, data cleaning, data analyses, interpretation of data, and drafting the manuscript. Statistical analyses were completed by Lucie Bourguignon and Catherine R. Jutzeler (Swiss Federal Institute of Technology, ETH Zurich).
Funding Information
This study was funded by research grants from the Swiss National Science Foundation (Ambizione Grant #PZ00P3_186101, Dr. Jutzeler) and Wings for Life Research Foundation (#2017_044, Drs, Jutzeler and Kramer).
Author Disclosure Statement
No competing financial interests exist.
Supplementary Material
References
- 1. Stein, D.M., Menaker, J., McQuillan, K., Handley, C., Aarabi, B., and Scalea T.M. (2010). Risk factors for organ dysfunction and failure in patients with acute traumatic cervical spinal cord injury. Neurocrit. Care 13, 29–39 [DOI] [PubMed] [Google Scholar]
- 2. Tong, B., Jutzeler, C.R., Cragg, J.J., Grassner, L., Casha, S., Geisler, F., and Kramer, J.L.K. (2018). Serum albumin predicts long-term neurological outcomes after acute spinal cord injury. Neurorehabil. Neural Repair 32, 7–17 [DOI] [PubMed] [Google Scholar]
- 3. Kwon, B.K., Bloom, O., Wanner, I.B., Curt, A., Schwab, J.M., Fawcett, J., and Wang, K.K. (2019). Neurochemical biomarkers in spinal cord injury. Spinal Cord 57, 819–831 [DOI] [PubMed] [Google Scholar]
- 4. Kwon, B.K., Streijger, F., Fallah, N., Noonan, V.K., Belanger, L.M., Ritchie, L.M., Paquette, S.J., Ailon, T., Boyd, M.C., Street, J., Fisher, C.J., and Dvorak, M.F. (2017). Cerebrospinal fluid biomarkers to stratify injury severity and predict outcome in human traumatic spinal cord injury. J. Neurotrauma 34, 567–580 [DOI] [PubMed] [Google Scholar]
- 5. Badhiwala, J.H., Wilson, J.R., Kwon, B.K., Casha, S., and Fehlings, M.G. (2018). A review of clinical trials in spinal cord injury including biomarkers. J. Neurotrauma 35, 1906–1917 [DOI] [PubMed] [Google Scholar]
- 6. Geisler, F.H., Coleman, W.P., Grieco, G., and Poonian, D. (2001). The Sygen multicenter acute spinal cord injury study. Spine (Phila Pa 1976) 26, S87–98 [DOI] [PubMed] [Google Scholar]
- 7. Geisler, F.H., Dorsey, F.C., and Coleman, W.P. (1991). Recovery of motor function after spinal-cord injury—a randomized, placebo-controlled trial with GM-1 ganglioside. N. Engl. J. Med. 24, 1829–1838 [DOI] [PubMed] [Google Scholar]
- 8. Geisler, F.H., Coleman, W.P., Grieco, G., and Poonian, D. (2001). Recruitment and early treatment in a multicenter study of acute spinal cord injury. Spine (Phila Pa 1976) 26, S58–S67 [DOI] [PubMed] [Google Scholar]
- 9. Geisler, F.H., Coleman, W.P., Grieco, G., et al. (2001). Measurements and recovery patterns in a multicenter study of acute spinal cord injury. Spine (Phila Pa 1976) 26, S68–86 [DOI] [PubMed] [Google Scholar]
- 10. Kirshblum, S.C., Waring, W., Biering-Sorensen, F., et al. (2011). Reference for the 2011 revision of the international standards for neurological classification of spinal cord injury. J. Spinal Cord Med. 34, 547–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pape, H.-C., Lefering, R., Butcher, N., et al. (2014). The definition of polytrauma revisited: an international consensus process and proposal of the new 'Berlin Definition'. J. Trauma Acute Care Surg. 77, 780–786 [DOI] [PubMed] [Google Scholar]
- 12. Vo AK, Geisler F, Grassner L, Schwab, J., Whiteneck, G., Jutzeler, C., and Kramer, J.L.K. (2021). Serum albumin as a predictor of neurological recovery after spinal cord injury: a replication study. Spinal Cord 59, 282–290 [DOI] [PubMed] [Google Scholar]
- 13. Avila-Martin, G., Galan-Arriero, I., Gómez-Soriano, J., and Taylor, J. (2011). Treatment of rat spinal cord injury with the neurotrophic factor Albumin-Oleic acid: translational application for paralysis, spasticity and pain. PLoS One 6, e26107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Casha, S., Rice, T., Stirling, D.P., Silva, C., Gnanapavan, S., Giovannoni, G., Hurlburt, R.J., Yong, V.W. (2018). Cerebrospinal fluid biomarkers in human spinal cord injury from a phase ii minocycline trial. J. Neurotrauma 35, 1918–1928 [DOI] [PubMed] [Google Scholar]
- 15. Ajroud-Driss., S, Saeed, M., Khan, H., et al. (2007). Riluzole metabolism and CYP1A1/2 polymorphisms in patients with ALS. Amyotroph Lateral Scler. 8, 305–309 [DOI] [PubMed] [Google Scholar]
- 16. Bruno, R., Vivier, N., Montay, G,., Liboux, A.L., Powe, L.K., Delumeau, J.C., and Rhodes, G.R. (1997). Population pharmacokinetics of riluzole in patients with amyotrophic lateral sclerosis. Clin. Pharmacol Ther. 62, 518–526 [DOI] [PubMed] [Google Scholar]
- 17. Nelis, H.J.C.F., and De Leenheer, A.P. (1982). Metabolism of minocycline in humans. Drug Metab Dispos. 10, 142–146 [PubMed] [Google Scholar]
- 18. Saivin, S., and Houin, G. (1988). Clinical pharmacokinetics of doxycycline and minocycline. Clin. Pharmacokinet. 15, 355–366 [DOI] [PubMed] [Google Scholar]
- 19. FDA Center for Drug Evaluation and Research, Center for Biologics Evaluation and Research FDA-2008-D-0128.
- 20. Watkins, P.B., Merz, M., Avigan, M.I., Kaplowitz, N., Regev, A., and Senior, J.R. (2014). The Clinical Liver Safety Assessment Best Practices Workshop: rationale, goals, accomplishments and the future. Drug Saf. 37, Suppl. 1, S1–S7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. FDA Center for Drug Evaluation and Research Center for Biologics Evaluation and Research FDA-2004-D-0121. Guidance for industry premarketing risk assessment (I). Pharm Care Res.
- 22. Hulme, C.H., Brown, S.J., Fuller, H.R., Riddell, J., Osman, Chowdhury, J., Kumar, N., Johnson, W.E., and Wright, K. T. (2017). The developing landscape of diagnostic and prognostic biomarkers for spinal cord injury in cerebrospinal fluid and blood. Spinal Cord 55, 114–125 [DOI] [PubMed] [Google Scholar]
- 23. Burns, A.S., Lee, B.S., Ditunno, J.F., and Tessler, A. (2003). Patient selection for clinical trials: the reliability of the early spinal cord injury examination. J. Neurotrauma 20, 477–482 [DOI] [PubMed] [Google Scholar]
- 24. Bracken, M.B., Collins, W.F., Freeman, D.F., et al. (1984). Efficacy of methylprednisolone in acute spinal cord injury. JAMA 251, 45–52 [PubMed] [Google Scholar]
- 25. Bracken, M.B., Shepard, M.J., Holford, T.R., et al. (1997). Administration of methylprednisolone for 24 or 48 hours or tirilazad mesylate for 48 hours in the treatment of acute spinal cord injury: results of the Third National Acute Spinal Cord Injury randomized controlled trial. JAMA 277, 1597–1604 [PubMed] [Google Scholar]
- 26. Gutkowski, K., Chwist, A., and Hartleb, M. (2011). Liver injury induced by high-dose methylprednisolone therapy: a case report and brief review of the literature. Hepat. Mon. 11, 656–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kadle, M.A.H., and Mazurchik, N.V. (2016). Hepatotoxicity induced by high dose of methylprednisolone therapy in a patient with multiple sclerosis: a case report and brief review of literature. Open J. Gastroenterol. 6, 146–150 [Google Scholar]
- 28. Shoenfeld, Y., Gurewich, Y., Gallant, L.A., and Pinkhas, J. (1981). Prednisone-induced leukocytosis. Influence of dosage, method and duration of administration on the degree of leukocytosis. Am J Med. 71, 773–778 [DOI] [PubMed] [Google Scholar]
- 29. Vásárhelyi, B., Debreczeni, L.A. (2017). Lab test findings in the elderly. EJIFCC 28, 328–332 [PMC free article] [PubMed] [Google Scholar]
- 30. Fraser, C.G. (1993). Age-related changes in laboratory test results: clinical implications. Drugs Aging 3, 246–257 [DOI] [PubMed] [Google Scholar]
- 31. Glennie, R.A., Noonan, V.K., Fallah, N., Park, S.E., Thorogood, N.P., Cheung, A., Fisher, C.G., Dvorak, M.F., Street, J.T. (2014). Reliability of the spine adverse events severity system (SAVES) for individuals with traumatic spinal cord injury. Spinal Cord 52, 758–763 [DOI] [PubMed] [Google Scholar]
- 32. Marion, T.E., Rivers, C.S., Kurban, D., et al. (2017). Previously identified common post-injury adverse events in traumatic spinal cord injury - validation of existing literature and relation to selected potentially modifiable comorbidities: a prospective Canadian cohort study. J. Neurotrauma 34, 2883–2891 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Anonymized data used in this study will be made available upon request to the corresponding author and in compliance with the General Data Protection Regulation (EU GDPR). The code describing the analysis can be accessed on our GitHub repository (https://github.com/jutzca/Systemic-effects-of-Spinal-Cord-Injury).