Abstract
Nowadays, the tendency in pharmaceutical and food industries is to replace synthetic antioxidants with the natural ones. For this reason, there is a growing interest in analyzing natural, healthy and non-toxic additives as potential antioxidants. Some plants, which contain high levels of phenolic compounds, present an increasing interest for medicine due to their ability to scavenge free radicals, along with other pharmacological activities, such as antibacterial activity, wound healing and anti-inflammatory effect, to mention only a few. The aim of this review is to explore the therapeutic potential of Ocimum basilicum and Trifolium pratense in relation with their phytochemical profile and to highlight the pharmacological activity of aqueous or ethanol extracts. Special attention was devoted to the dermal pathology and wound healing effects, in the context of multiple skin conditions such as acne, eczema boils, psoriasis and rashes. Additionally, both extracts (Trifolium sp. and Ocimum sp.) are characterized by high content of antioxidant compounds, which are responsible for the radiance and resistance of the skin and slowing down of the aging process by maintaining estrogen levels. Moreover, the potential combined effect of the mixed extract is pointed out in terms of future applications for wound healing, based on some preliminary results obtained from a “scratch tests” assay performed with respect to human dermal fibroblasts.
Keywords: Ocimum basilicum sp., Trifolium pratense sp., phytochemistry, antioxidant capacity, antibacterial activity, wound healing, anti-inflammatory
1. Introduction
Plants have been sources of minerals, vitamins and bioactive compounds since ancient times that are used in traditional medicine to treat various diseases. The biologically active compounds from the plants have been the natural elements from which allopathic treatments and synthetic medicinal substances have developed over time and they are currently used successfully in the treatment of multiple diseases [1]. However, the World Health Organization has estimated that 80% of the population prefer plants-based treatments to treat respiratory and dermatological diseases, cancer, oxidative stress, diabetes, etc [2]. Plants are rich sources of biochemical compounds such as phenols, fatty acids, saponins, essential oils or alkaloids which have proven therapeutic properties but are less studied and valued [3].
Ocimum and Trifolium species are plants that have also not been valued in the past, but there is an increase interest in these two plants species in recent years [4,5]. Ocimum species is characterized by an abundance of compounds such as phenolic acids, but also volatile oils, and Trifolium species have been shown to be rich in biologically active compounds such as isoflavones [6,7].
Ocimum sp. belongs to the Lamiaceae family, one of the largest plant families comprising about 220 genera and almost 4000 species. The most important representatives of Ocimum sp. are: Ocimum basilicum, Ocimum sanctum, Ocimum gratissimum, Ocimum canum, Ocimum kilimandscharicum, Ocimum americanum and Ocimumm icranthum [8,9,10].
Ocimum basilicum L., called sweet basil, is botanically described as a branched plant that grows between 0.3 and 1.3 m height, with light green silky leaves in opposite directions and containing many oily glands that store essentials oils [11]. The basil flowers are colored from white to purple and arranged in a terminal spike [11].
The members of genus Ocimum are very important for their therapeutic potentials being used against abdominal cramps, gastroenteritis, dysentery, and diarrhea. The leaves extract was used in the treatment of wounds, acne, and vitiligo. Additionally, basil has been utilized traditionally for curing health issues such as: anxiousness, stings, strong aching, gripe, pyrexia, infective diseases, headaches, coughs, constipation, warts, worms and kidney malfunction [12,13,14]. It was also used as a deodorant, being considered to be an aphrodisiac [15].
The genus Trifolium is one of the most important genus in the Leguminosae family comprising over 240 species, being remarkable for its therapeutic effects such as: expectorant, analgesic, antioxidant and anti-inflammatory [16]. Some representatives species are: Trifolium repens (white clover), Trifolium pratense (red clover), Trifolium fragiferum and Trifolium hybridum. The Mediterranean region is very rich in Trifolium species, represented by 103 species [17].
The purpose of this paper is to concentrate the information from the literature about the two plants mentioned before (Trifolium sp. and Ocimum sp.). The plants characteristics are described in terms of chemical composition and therapeutic activity of different types of extracts.
This review also considers the combination of the two species, based on the common bioactive compounds identified in their composition, along with their similar therapeutic activities. One of these similar biological activities is the healing of wounds. Some preliminary results confirm the high therapeutic potential combining the extracts from both plants, working synergistically with great benefits.
2. Common Therapeutic Activities of Ocimum and Trifolium Species
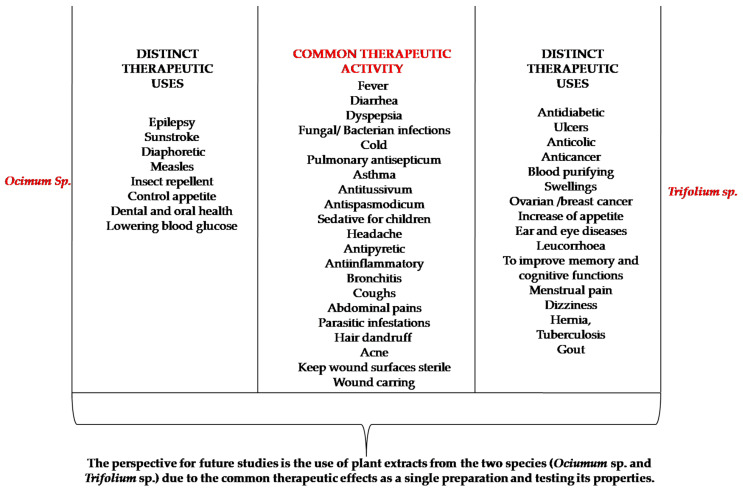
Ocimum species have been shown to have multiple therapeutic effects in the area of respiratory diseases, wound treatment, bacterial or fungal infections, headaches, and gastrointestinal disorders. Meanwhile, Trifolium species have been demonstrated to present multiple therapeutic effects in the field of respiratory diseases, wound treatment, bacterial or fungal infections, gastrointestinal disorders, menstrual pain, anticancer, antidiabetic, tuberculosis, to mention only a few [5,7,18]. So, there are some common therapeutic activities, but also some distinct therapeutic effects of the two species as presented in Figure 1.
Figure 1.
Common and distinct therapeutic effects of Ocimum and Trifolium species.
However, in skin wounds, including acute wounds and chronic wounds, the application of different formulations based on plants extracts for wound healing involve a dynamic and complex process for recovering tissue integrity and homeostasis: inflammation, reepithelization, granulated tissue formation, neovascularization, wound contraction and remodeling of the extracellular matrix. Hence, the potential of Ocimum and Trifolium species to enhance the healing process is far from being completely explored and new therapeutic options with fewer adverse effects, low cost and reduced healing time are still required for clinical or alternative treatments.
3. Phytochemistry
3.1. Phytochemical Profile of Ocimum Species
Ocimum is noted for its pungency and flavor apart from its aroma. The aroma in this genus is due to the essential oil, its contents ranging from 0.3% to 3.6% dry weight. The minor components in this genus, most of which are sesquiterpenes, are found to vary amongst species [11]. The chemical composition of Ocimum basilicum essential oil has been studied in various parts of the world [19]. Many authors isolated the essential oil from Ocimum basilicum reporting various volatile constituents. The main constituents are: linalool, 1,8-cineol, eugenol, methyl cinnamate, camphor, methyl eugenol, methyl chavicol, β-elemene, β-ocimene, camphene, carvacrol, α-bergamotene, α-cadinol and geranial [4,11,19,20].
Basil has been classified according to different geographical origins. There are many chemotypes such as: the European chemotype from Italy, France, Bulgaria, Romania, Egypt, and South Africa, having linalool and methyl chavicol as main components; the tropical chemotype from India, Pakistan and Guatemala, being rich in methyl cinnamate; and the Reunion chemotype from Thailand, Madagascar and Vietnam, being characterized by high concentration of methyl chavicol. There is also a eugenol-rich chemotype from North Africa and Russia [11,13]. Other chemotypes were reported in recent studies such as ß-caryophyllene in O. sanctum and Ocimum micranthum, citral in Ocimum citriodorium and Ocimum canum, ethyl cinnamate in Ocimum gratissimum, 1,8-cineole in Ocimum micranthum, thymol in Ocimum gratissimum, p-cymene in Ocimum gratissimum, geranyl acetate in Ocimum minimum, and camphor in Ocimum canum [9,21,22].
Recently, the hydroalcoholic extract of Ocimum basilicum harvested from Romania has been characterized in terms of total polyphenolic compounds, identifying cinnamic acid, caffeic acid, ferulic acid, syringic acid, catechin, rutin and chlorogenic acid [23]. The main antioxidant compounds in basil extracts are chlorogenic, p-hydroxybenzoic, caffeic, vanillic and rosmarinic acids, as well as apigenin, quercetin and rutin [20]. Ferulic acid were also identified by other authors [24]. These compounds were also identified in Ocimum basilicum by Dhama et al., pointing out its huge therapeutic potential [25].
3.2. Phytochemical Profile of Trifolium Species
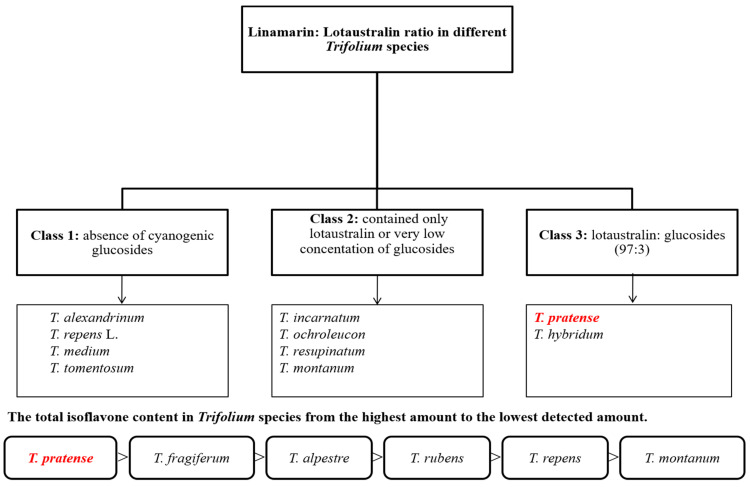
Until 2012, studies on Trifolium plants phytochemistry were mostly focused on Trifolium pratense or Trifolium repens [26]. Recent years have provided a noticeable growth in research on different clovers as a source of bioactive substances. Muzashvili et al. [27] have studied the composition of 88 species of Trifolium highlighting compounds such as linamarin and lotaustraline, which are part of the class of cyanogenic glycosides [27]. It has been shown by the phytochemical characterization that the ratio between the two glycosides is different from one species to another, depending on the harvest period. Plants harvested in the early period of the flowering phase presented the highest content of glycosides. Thus, Trifolium species can be grouped into three major classes, taking into account the amount of cyanogenic glycosides and their ratio in the extract of the aerial part of the plant [27,28,29] (Figure 2).
Figure 2.
Grouping the different species of Trifolium according to the amount of cyanogenic glycosides and isoflavones and identifying the species with rich chemical composition.
Several species of clovers were also a subject of previous study [30] focused on the distribution of three isoflavones (formononetin, daidzein and genistein) in Trifolium species. Total isoflavones were quantified from clover leaves, stems and flowers.
Figure 2 shown the Trifolium species that contain amounts of isoflavones in descending order. Among the Trifolium species the richest in isoflavones is T. pratense [27,31,32].
Trifolium pratense is a rich source of isoflavonoids (biochanin A, daidzein, formononetin, afrormosin, orobol, genistein, pratensein, trifoside) and flavonoids (quercetin and kaempferol) [7,30,31,33]. Other constituents include medicagol, coumestrol, coumarin. The components found abundantly in the roots are biochanin A, afrormosin, daidzein, genistein, methyl orobol, irilin and irilone, meanwhile the components from the leaves are formononetin, biochanin A, soyasaponins, clovamides and flavonoids [7,27,31,33].
Antonescu et al. [23] have identified in the T. pratense extract the following phenolic compounds: cinnamic acid, caffeic acid, ferulic acid, syringic acid, catechin, rutin and chlorogenic acid [23].
4. Pharmacological Activities
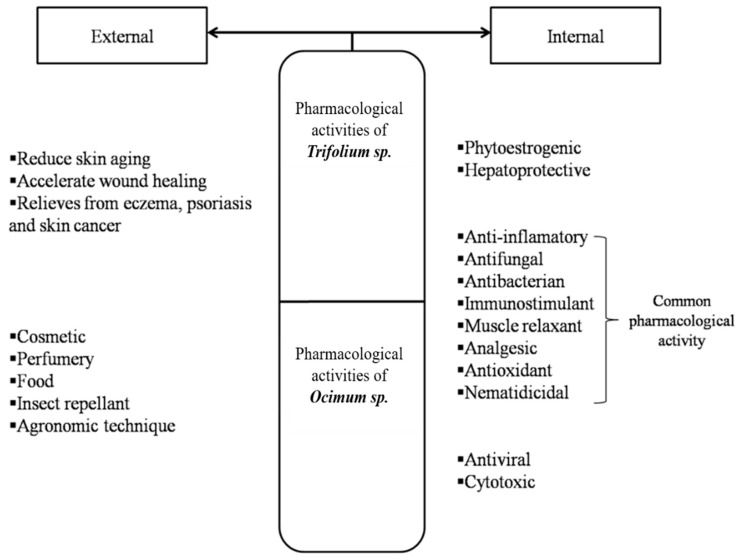
Due to some common compounds the extracts from the plants T. pratense and O. basilicum have been shown to have similar therapeutic effects [34,35,36]. The biological activities specific to the T. Pratense and O. basilicum, but also its common biological effects are presented in Figure 3 [11,28].
Figure 3.
Pharmacological activities of extracts from Trifolium and Ocimum species after internal and external administration.
The chemical structures of the major compounds of the Ocimum and Trifolium species and the specific therapeutic activities are presented in Table S1 (for Ocimum sp.) and Table S2 (for Trifollium sp.).
4.1. Antioxidant Capacity of Ocimum and Trifolium Species
Nowadays, the tendency in pharmaceutical and food industries is to replace synthetic antioxidants with the natural ones. For these reasons there is a growing interest in analyzing natural, healthy and non-toxic additives as potential antioxidants [28,37,38]. Some plants, which contain high level of phenolic compounds, present an increasing interest in medicine due to their ability to scavenge free radicals [39,40,41]. It has been noticed a direct correlation between the antioxidant activity and the phenols concentration. Phenols are very important bioactive compounds because they are acting as scavengers of intermediate peroxyl and alkoxyl radicals, and chelating agents for metal ions which are of major importance for the initiation stage of radical reactions [42].
Ocimum and Trifolium sp. contain many antioxidant compounds which contribute to their intense antiradical activity [43,44] and could have potential human health benefits [21]. Due to the strong antioxidant capacity, basil acts as a protector to prevent heart diseases, reduce inflammation, lower the incidence of cancers and diabetes [40]. A very strong correlation was demonstrated between the some phenolic compounds and antioxidant capacity of medicinal plants [45,46]. Ocimum basilicum extracts possess a higher total phenolic acid content and greater antioxidant activity.
The aqueous extract of basil is a superoxide and hydroxyl radical scavenger [43,47]. The antioxidant capacity of this extract has been attributed to its polar phenolic compounds. The total phenolic content of water and ethanol extracts of basil was reported to be similar in the linoleic acid peroxidation (94.8% and 97.5%) [48]. Hinneburg et al. [49] reported that hydrodistilled extracts from basil had the highest antioxidant capacity in comparison with several herbs like laurel, parsley, juniper, aniseed, fennel, cumin, cardamom, and ginger, but not the greatest iron chelation ability [49]. Rosmarinic acid has been identified as the primary phenolic compound in basil leaves and stems [36]. Chicoric acid has also been identified in substantial quantities [36].
Extracts of Trifolium pratense are becoming increasingly popular, primarily for the treatment of menopausal symptoms [47,50,51]. Furthermore, phytoestrogens present in T. pratense are also effective antioxidants and may have tyrosine kinase inhibitory activity. The antioxidant properties of genistein and other phytoestrogens have been demonstrated in several models such as protection from phorbol ester-induced singlet oxygen or peroxide formation and particularly from UV-radiation-induced oxidative damage to DNA in vitro [52,53]. Dietary genistein has been shown during the in vivo experiments in mice to stimulate the endogenous antioxidants, SOD (superoxide dismutase), GSHP (glutathione peroxidase) and glutathione S-transferase, with the effects found mainly in small intestine and the skin [54,55,56]. Sanja Vlaisavljevic et al. [57] studied the antioxidant capacity of the extracts by using tests that are based on electron transfer (neutralization of DPPH radical), neutralization of free radical species (capacity of scavenging O2, OH and NO radicals) and the potential to inhibit lipid peroxidation [36,57,58].
The highest concentration of phenolic and flavonoid substances was found in methanol extract isolated from plants cultivated in vivo condition which displayed the highest reducing ABTS radical scavenging and chelating abilities [23]. However, the most effective scavenger of DPPH radical, superoxide anion and hydrogen peroxide, was the chloroform fraction of red clover grown in vivo. The current state of understanding the antioxidant actions of clover species is still mostly based on in vitro experimental systems [58,59].
Besides phenols, some volatile compounds were demonstrated to possess antioxidant activity. Araujo Couto et al. [6] indicated that the major essential oil compound (eugenol) has been corelated with high antioxidant capacity. Necar and Tansi have highlighted linalool as a major compound in Greek basil [60]. Linalool, epi-α-cadinol, and α-bergamotene (7.4% to 9.2%) and γ-cadinene have been identified as the most common compounds in basil essential oil. Basil essential oil strongly inhibits lipid peroxidation whether induced by Fe2+/ascorbate or by Fe2+/H2O2.
Al-Maskria et al. have demonstrated that the essential oil content and antioxidant capacity varied in function of the season when it was harvested (the antioxidant capacity was the highest in spring) [61]. Politeo et al. investigated antioxidant activity measured by DPPH revealing that free volatile compounds (eugenol, chavicol, linalool and α-terpineol) possess good antioxidant properties comparable with that of the essential oil and well-known synthetic antioxidant butylated hydroxytoluene (BHT), but less than pure eugenol [37].
The recent literature evidenced antioxidant properties of several Trifolium species (Trifolium angustifolium, Trifolium balansae, Trifolium stellatum, Trifolium nigrescens, Trifolium constantinopolitanum, Trifolium pallidum and Trifolium resupinatum), but only the antioxidant action of Trifolium pratense was examined in vivo [26,29]. Recently, antioxidant action has become one of the most studied properties of clovers (after the estrogenic effect) [28,29]. The antioxidant activity of glycosidically bound volatile compounds in clover essential oil has been reported to be significantly greater than that of the volatile aglycones [62]. The glycosides can undergo enzymatic hydrolysis releasing their aglycones, therefore, they may be considered as potential antioxidant precursors [63,64].
4.2. Antimicrobial, Antiviral and Antifungal Activity of Ocimum and Trifolium Species
Essential oil present in most of the Ocimum species is responsible for its antifungal, antibacterial and antiviral properties. The essential oils of various Ocimum species have been shown, in vitro, to have antibacterial activity against Staphylococcus aureus, Salmonella enteritidis, Escherichia coli, Proteus vulgaris, Bacillus subtilis, Salmonella typhi, Shigella sonnei, Shigella boydii, Pseudomonas aeruginosa and Salmonella paratyphi [8,57,58].
The Ocimum basilicum oil was tested also against pathogenic fungi such as: Aspergillus niger, Aspergillus fumigatus, Penicillium italicum and Rhizopus stolonifera by using a disc diffusion method, and by determination of minimum inhibitory concentration. Surprisingly, high antifungal effect were found highlighting the potential of Ocimum species as a preservative in food and medical industries [61].
Studies have shown Ocimum basilicum act as a strong antiviral agent against DNA viruses (herpes simplex viruses, adenoviruses and hepatitis B virus) and RNA viruses (coxsackievirus and enterovirus). Ocimum tenuiflorum has been also reported to have antiviral activity against bovine herpesvirus 1 [65,66,67]. Another study investigated the influence of honey and some surfactants (cationic, anionic) on the antibacterial activity of Ocimum gratissimum essential oil pointing out that honey was more efficient than a macrogol bend due to which it could be suitable for the infected wounds treatment [63].
Zahran et al., also emphasized the complex composition of Ocimum species extract, which determines its strongly anti-inflammatory, antibacterial and antiviral activity. The authors also indicated a classification of the most powerful therapeutic species of Ociumum, which are: Ocimum basilicum, Ocimum sanctum and Ociumum gratissiumum [68].
The literature about the antimicrobial activity of clover species contains the evaluation of the efficiency of plant extracts from Trifolium species [69,70,71]. Testing of the antimicrobial and antifungal activity of the extracts of these species was performed on gram-positive bacteria (Streptococcus pyogenes and Staphylococcus aureus), gram-negative bacteria (Pseudomonas aeruginosa, Escherichia coli) and fungi (Candida albicans). According to the literature, the extraction solvent of the active principles has a major importance on the antibacterial and antifungal activity of the Trifolium species [72]. Studies on the antimicrobial properties of Trifolium pratense included a comparison of the actions of different extracts (using solvents such as ethanol, methanol, water, ether), all pathogens examined were inhibited by the extract made in methanol, which was declared to have the highest antibacterial and antifungal activity [46,69].
4.3. Anti-Inflammatory Effect of Ocimum Species
Up to date, basil extract has been experimented on rats to reduce acute inflammation. Basil alcohol extract has been shown to have a slight effect on nitrogen oxide synthesis, but it has reduced the number of leukocytes and monocytes, as well as significantly activated circulating phagocytes. The highlighting of the anti-inflammatory effect of basil species extract was compared with diclofenac, the extract presenting less intense activity compared to it [24].
The oils of different species of Ocimum (Ocimum sanctum, Ocimum basilicum, Ocimum americanum), showed a different response against edema. Ocimum bazilicum oils possess the highest percentages of linolenic acid (21.0%) and provided maximum inhibition of edema (72.42%) [73]. The oil can inhibit increased vascular permeability and leukocyte migration, as evidenced by the inflammatory stimulus [73,74].
The anti-inflammatory effect of Ocimum species has also been studied in the ear [75]. According to this study, it was found that when applying 50 μg extract in the ear, the inflammation and edema at this level is significantly reduced by 80% due to its local anti-inflammatory effect. The effects were comparable to 100 μg hydrocortisone as a control, showing an inhibition of 54.8%.
In another study was investigated the immunomodulatory effect of Ocimum sanctum seed oil on immunological parameters in both stress-free and stressed animals and assessed that this oil appears to modulate both the humoral immune reaction, as well as the immediate one and these immunomodulatory effects can be mediated by the GABA-ergic pathway [76].
4.4. Estrogenic and Anticancer Activity of Trifolium Species
4.4.1. Estrogenic Action
Lately, much emphasis has been placed on the estrogenic effects of different types of isoflavonic compounds of Trifolium species. In general, research on the estrogenic properties of Trifolium pratense has recently been extended by more detailed examinations of the pharmacological role of individual isoflavones, which is a new issue in investigations of the phytoestrogenic action of this plant. It has been shown in in vivo studies that daidzein and genistein are the main isoflavonoid compounds that are present in blood plasma after administration of Trifolium pratense extracts. However, recent studies have shown that the bioavailability of several isoflavones present in Trifolium pratense is increased, these being irilone, prunetine and pseudobaptigenin [77].
In addition, it is suggested that after consuming a red clover dietary supplement, irilone may be the second most abundant isoflavone in human plasma, along with daidzein. The estrogenic action of irilone and daidzein was evaluated compared to different estrogens [78]. Irilone has been shown to significantly increase alkaline phosphatase activity, as well as induce mRNA for this enzyme, progesterone receptors and androgen receptor mRNA levels. Experiments performed on cells showed that irilone significantly induced their proliferation [78]. The studies conducted by Spagnuolo P et al. showed that the estrogenic effect of Trifolium species extracts is dependent and increasing with the administered dose [79].
4.4.2. Anticancer Activity
The anticancer activity of Trifolium pratense is given by the extract’s ability to determine cell regeneration [59]. The active ingredients in Trifolium pratense have been shown to be used as an adjunct in the treatment of cancer in combination with other medicinal plants in the form of an internal infusion or tincture [59,80]. The 95% ethyl alcohol extract of Trifolium pratense significantly inhibited the metabolism of cancer cells and decreased the level of binding of benzopyrene to DNA by 30 to 40% [80]. Biochanin A has also been shown to be an isolated isoflavone and identified as a major active compound of Trifolium pratense extract. The ability of this isoflavone to inhibit carcinogen activation in culture cells suggests that in vivo studies of this compound as a potential chemopreventive agent are warranted [42,80]. Up to date, no anticancer activity has been found on breast cancer (including estrogenic activity) and hepatocellular carcinoma, but promising anticancer activity of aqueous Trifolium pratense extract on gastric or colon cancer has been demonstrated [81].
4.5. Dermal Pathology and Wound Healing Effects of Ocimum and Trifolium Species
Recent literature has shown the beneficial effects of Trifolium and Ocimum species extracts on skin health. Various concentrations of Ocimum gratissimum oil were tested compared to benzoyl peroxide 10% and a placebo over a four-week period to reduce the acne lesions in a predominantly student population. Ocimum gratissimum oil in different concentrations of 0.5%, 1%, 2% and 5% v/v were incorporated in various topical formulations. This study showed that preparations containing 2% and 5% Ocimum oil in alcohol and 5% in ketomacrogol were significantly more active than benzoyl peroxide [82].
Another clinical study was performed using a combination of Ocimum gratissimum and Aloe vera gel. Aloe vera gel has been found to improve the anti-acne properties of Ocimum oil. The oil or its combination with Aloe vera gel has been shown to be more effective than 1% clindamycin in the treatment of acne vulgaris [83]. In another study by Pansanga et al. it was found that a microemulsion of Ocimum species 3% should be safe and well tolerated on human skin [84].
Renda et al. described the in vivo wound healing effects of aqueous-methanolic extracts of 13 species of Trifolium [85]. The effects of Trifolium extracts in animals were compared with the reference medicine Madecassol, whose activity was assumed to be 100%. The most effective wound healing properties were found for Trifolium canescens, the second was Trifolium pratense extract [85].
Both extracts (Trifolium and Ocimum) are characterized by high content of antioxidants compounds, which are also responsible for the radiance and resistance of the skin and the slowing down of the aging process by maintaining estrogen levels [86]. Additionally, due to the existence of isoflavone-like compounds, the extracts of these plants quickly heal wounds and burns and reduce the chances of skin cancer [69,86].
These extracts can be used in multiple skin conditions such as acne, eczema boils, psoriasis and rashes because they help regenerate cells and have anti-inflammatory properties [59,87]. External applications are also beneficial to heal wounds, but they are less studied.
5. Therapeutic Activities and Mechanisms of Action for Ocimum sp. and Trifolium sp. Depending on the Type of Extraction Performed
The phytochemical profile of plant differs depending on the extraction method and solvents used to obtain their extracts [88]. Thus, organic solvents (ethanol, methanol) or hydroalcoholic mixtures are most commonly used for the extraction of phenol, flavonoid compounds [89]. To obtain volatile compounds steam distillation or cold pressing is most often used [90]. Thus, depending on the type and method of extraction, different compounds are extracted which will determine differentiated therapeutic effects [88]. The type of extract made on Trifolium pratense and Ocimum basilicum, respectively the therapeutic effects demonstrated in the specialized literature and their mechanism of action are presented in Table 1 and Table 2.
Table 1.
Biomedical activities of Ocimum basilicum in different extracts types.
| Extract Type | Therapeutic Effect | Mechanism of Action | Ref. |
|---|---|---|---|
| Methanol extract | Wound healing effect | Angiogenesis stimulation (by cytokine activity modulation (TNF- α); Antimicrobial activity and antifungal activity; Antioxidative properties (Ferullic and Chlorogenic Acid). |
[91,92,93,94] |
| Hepatoprotection | Modulatory effect in hepatocytes comparable to oleanolic and ursolic acids | [67,74] | |
| Ethanol extract | Dermatological effects | Strong antiviral activity against DNA viruses and RNA viruses; Effect on lipid accumulation in human macrophage |
[95] |
| Anti-cancer | Cytotoxic effect: increase of Glutathione S-Transferase (GST) activity and protection in carcinogenicity or toxicity (antioxidant activity, antiproliferative effect). | [96,97,98] | |
| Hypocholesterolemia | Lowering the lipid accumulation in human macrophage. | [99] | |
| Hydroalcoholic extract | Adjuvant in diabetes treatment | Anti-hyperglycemic effect (antioxidant activity and inhibition of α-glucosidase and α-amylase activities). | [93] |
| Vasorelaxant/anti-platelet effect | Anti-thrombotic effect (inhibits ADP and thrombin induced platelet aggregation) | [24,100,101,102] | |
| Neuro-psycho effects | Anxiolytic and sedative effect (action of malic, caffeic, kaempferol and oleanolic acids) | [101] | |
| Antiosteoporotic effect | Bone protection against osteoporosis induced by glucocorticoids. | [103] | |
| Anti-inflammatory effect | slight effect on Nitrogen Oxide synthesis, reduced leukocytes and monocytes, activation of phagocytes circulation |
[104] | |
| Essential oils | Treatment of different skin pathologies/antiaging | Enhancing the skin penetration in vitro animal experiments. Antioxidant capacity (major oil compounds: linalool, isoanethole, eugenol) comparable to tocopherol. |
[105,106] |
| Complementary with antibiotics | Synergic effect of Basil with some antibiotics for the treatment of certain bacterial infection (ex. Propionibacterium acne) | [107] | |
| Antitumoral effect | Cytotoxic activity (higher inhibition of the viability of Ehrlich ascites carcinoma cells due to linalool) | [108] | |
| Anti-Colitis treatment | Protective effect against colitis induced by acetic acid (significant decrease of myeloperoxidase). | [109] |
Table 2.
Biomedical activities of Trifolium pratense in different extracts types.
| Extract Type | Therapeutic Effect | Mechanism of Action | Ref. |
|---|---|---|---|
| Methanolic extract | Wound healing effect | The genistein present in the extract stimulates angiogenesis by activating the beta estrogen receptor, both by mechanisms dependent on this receptor and by independent mechanisms regulating wound healing. Antioxidant (isoflavones: triterpene saponins and flavonoids). Anti-inflammatory: genistein achieved by the downregulation of proinflammatory mediator activity (inactivation of nuclear factor-κB (NF-κB) and reduction in the expression levels of TNF-α Antimicrobial Antifungal effect against: Aspergillus niger, C. albicans and Fusarium verticillioides. |
[28,110,111,112,113,114] |
| Antiaging | high concentration of phenolic compounds and flavonoids have the ability to reduce and neutralize free radicals in the skin | [115] | |
| Antiplatelet aggregation | activates the antiplatelet factor nitric oxide synthesis in the cells | [116] | |
| Ethanolic extract | Antispasmodic | in laryngitis, whooping cough, bronchitis and tuberculosis causes relaxation of the smooth muscles of the airways with relief of spasms | [112] |
| Hydroalcoholic extract | Hepatoprotective | increases the level of methionine in hepatic steatosis | [100] |
| Anti-diabetic | Ferulic acid inhibits the enzymes involved in the digestion of carbohydrates (α-amylase and α-glucosidase) and has anti-lipase activity. | [63,117] | |
| Anticancer | The dimeric alkaloids vinblastine and vincristine have anticancer properties due to their activity in destroying cancer cells. Polyphenolic compounds have a protective role and induce a reduction in the number of human tumor cells or an increase in them. |
[57,118] |
6. In Vitro Wound Healing Effect of the Mixture of Trifolium pratense and Ocimum basilicum Extracts
Due to the wound healing properties of both Trifolium and Ocimum species highlighted in the literature and mentioned in this paper, the future perspectives refer to the possible combinate effect of the two species extracts. The ability to promote wound healing by synergic effect of Trifolium Pratense and Ocimum basilicum mixt extract has not been studied yet, being the central point for future studies. So far, the anti-inflammatory, antimicrobial, antifungal and anticancer properties have been demonstrated for each extract individually, obtaining promising results, and for these reasons, in the future, the mixture of both extracts are of great interest to be studied, expecting for a potential synergistic effect.
It is known that antioxidant enzymes play a key role in wound healing [119,120], due to which we assume that the mixture of Trifolium pretense and Ocimum basilicum extracts would have an increased antioxidant potential leading to in vitro wound healing. Another aspect that led to future studies and applications of the extract mixture of Trifolium pretense and Ocimum basilicum is that in the wound healing process also intervenes the inflammatory phase, the mixture of both extracts having anti-inflammatory effect.
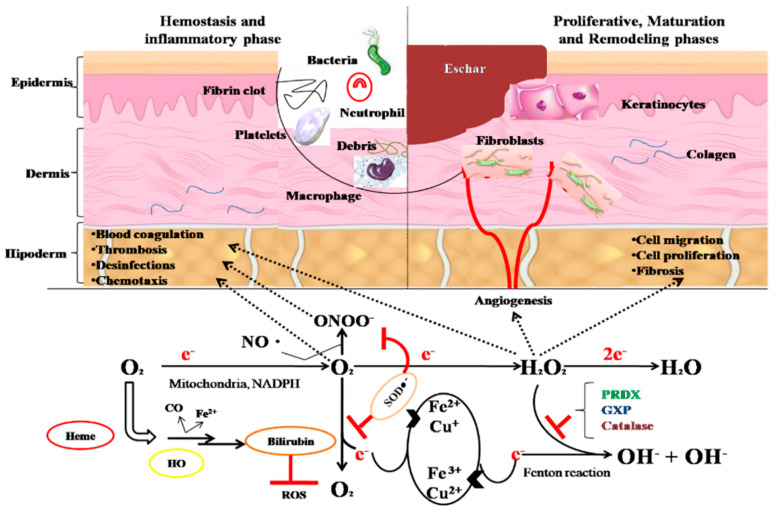
According to Figure 4, it is highlighted that the wound healing phases are reproduced through the processes of homeostasis, inflammation, blood coagulation with thrombus formation and natural disinfection of the wound. After these phases, healing stages are observed, represented by the migration and proliferation of dermal fibroblasts. All these steps are based on the biochemical reactions shown in Figure 4, which are strongly influenced and catalyzed by enzymes such as NADPH oxidase present in immune cells, superoxide dismutase (SOD) which catalyzes the reaction between superoxide and nitric oxide resulting in peroxynitrite (antibacterial) and the reaction of formation of hydrogen peroxide and molecular oxygen. Hydrogen peroxide formed by the reaction catalyzed by SOD is the key element that dictates the beginning of all stages of healing and re-epithelialization of the damaged area. Hydrogen peroxide must also be maintained at an optimal level to dictate the migration and proliferation of fibroblasts inside the wound and this level is maintained by a series of enzymes such as catalase, glutathione peroxidase (GXP) and peroxyredoxin (PRDX).
Figure 4.
Stages of the wound healing process (adapted from [121]).
To the best of our knowledge, there are no studies in the literature dealing with any in vitro tests of the mixture Trifolium pretense and Ocimum basilicum extracts. Our research group performed a preliminary study using the “scratch test” assay on human fibroblasts, by applying the extract mixture in different concentrations on fibroblasts culture, in order to evaluate the optimum concentration to promote the stimulation and proliferation of the cells. Within this test, which is an in vitro model of wound healing, human fibroblasts were primarly grown to a confluent monolayer, and then was scraped in a straight line with a pipette tip, in order to simulate a wound. The fibroblasts migration into the wound area was monitored during 48 h incubation in the presence of different concentrations of mixed plant extracts along with the control (no treatment).
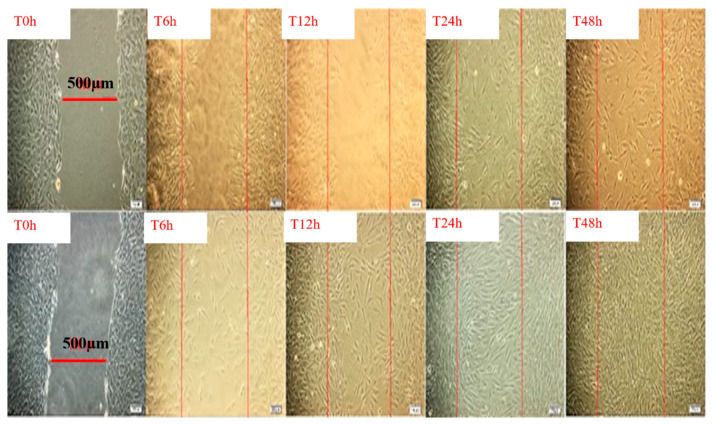
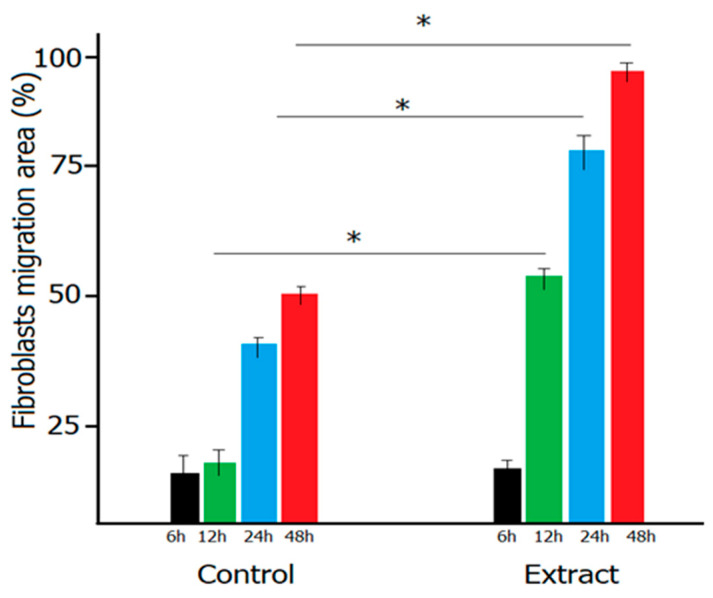
In Figure 5, the spontaneous migration of dermal fibroblasts is evidenced under light microscopy, along with the control samples, showing the progressive covering of the pseudo-wound monitored at different times intervals. The percent of wound closure, expressed as migration of fibroblasts to cover the scratch area, is evidenced in Figure 6.
Figure 5.
Migration of dermal fibroblasts after treatment with mixed extract of Trifolium pretense and Ocimum basilicum (lower line) compared to the control (upper line) monitored after different times intervals under light microscopy (objective 20×). Scale bar: 100 μm. The edge of initial pseudo-wound area is labeled in red, in order to emphasize the progressive covering of the area, during 48 h incubation (unpublished results).
Figure 6.
Wound healing percent express as fibroblast migration to cover the scratched area. Values are expressed as mean value of three independent measurements ± standard deviation. Statistically significant difference were considered for p < 0.05 (unpublished results).
At the end of the monitoring period, a 100% coverage was achieved for the treated samples, compared to 52% for the control. These results are very promising, indicating that the mixture Trifolium pretense and Ocimum basilicum extract presents favorable biological activity to improve dermal regenerative process, being a good candidate to be used in both cosmetic and therapeutic formulations.
7. Conclusions
The antioxidant, antimicrobial, antiviral, antifungal and anti-inflammatory activity of Ocimum and Trifolium species are summarized in this review in order to explore the therapeutic potential of Ocimum basilicum and Trifolium pretense in relation with their phytochemical profile and to highlight the pharmacological activity of aqueous or ethanol extracts. Special attention was devoted to the dermal pathology and wound healing effects, in the context of multiple skin conditions such as acne, eczema boils, psoriasis and rashes. Both extracts (Trifolium sp. and Ocimum sp.) are characterized by high content of antioxidant compounds, which are also responsible for the radiance and resistance of the skin and the slowing down of the aging process by maintaining estrogen levels. Moreover, the potential combined effect of the mixed extract is pointed out in terms of future applications for wound healing, based on some preliminary results obtained from a “scratch tests” assay performed with respect to human dermal fibroblasts.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/plants10071390/s1, Table S1: The chemical structures and therapeutic activities of the major compounds identified for Ocicum sp., Table S2: The chemical structures and therapeutic activities of the major compounds identified for Trifolium sp.
Author Contributions
Conceptualization, A.-I.A., F.M. and A.A.; investigation, A.-I.A., F.M. and L.F.; resources, M.G., M.Z., L.D. and A.A.; writing—review and editing, S.C. and S.I.V.; visualization, F.M., R.K.S. and F.B.; supervision, S.C., S.I.V. and A.A.; project administration, A.-I.A. and F.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data available in a publicly accessible repository.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Tünde J., Vicas L., Tóth I., Braun M., Marian E., Teuşdea A., Vicaş S., Mureșan M. Mineral Elements Profile, Bioactive Compounds and Antioxidant Capacity of Wild Blueberry and of Pharmaceutical Preparations from Blueberry (Vaccinium Myrtillus) Farmacia. 2016;64:581–587. [Google Scholar]
- 2.WHO|Global Status Report on Noncommunicable Diseases. [(accessed on 31 May 2021)];2014 Available online: http://www.who.int/nmh/publications/ncd-status-report-2014/en/
- 3.Fajemiroye J.O., da Silva D.M., de Oliveira D.R., Costa E.A. Treatment of Anxiety and Depression: Medicinal Plants in Retrospect. Fundam. Clin. Pharmacol. 2016;30:198–215. doi: 10.1111/fcp.12186. [DOI] [PubMed] [Google Scholar]
- 4.Rezzoug M., Bakchiche B., Gherib A., Roberta A., Guido F., Kilinçarslan Ö., Mammadov R., Bardaweel S.K. Chemical Composition and Bioactivity of Essential Oils and Ethanolic Extracts of Ocimum Basilicum L. and Thymus Algeriensis Boiss. & Reut. from the Algerian Saharan Atlas. BMC Complement. Altern. Med. 2019;19:146. doi: 10.1186/s12906-019-2556-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Egan L.M., Hofmann R.W., Seguin P., Ghamkhar K., Hoyos-Villegas V. Pedigree Analysis of Pre-Breeding Efforts in Trifolium spp. Germplasm in New Zealand. BMC Genet. 2020;21:104. doi: 10.1186/s12863-020-00912-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Araújo Couto H.G.S., Blank A.F., de Oliveira e Silva A.M., de Lima Nogueira P.C., de Fátima Arrigoni-Blank M., de Castro Nizio D.A., de Oliveira Pinto J.A. Essential Oils of Basil Chemotypes: Major Compounds, Binary Mixtures, and Antioxidant Activity. Food Chem. 2019;293:446–454. doi: 10.1016/j.foodchem.2019.04.078. [DOI] [PubMed] [Google Scholar]
- 7.Myers S.P., Vigar V. Effects of a Standardised Extract of Trifolium Pratense (Promensil) at a Dosage of 80 mg in the Treatment of Menopausal Hot Flushes: A Systematic Review and Meta-Analysis. Phytomedicine. 2017;24:141–147. doi: 10.1016/j.phymed.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 8.Darrah H.H. The Cultivated Basils. Buckeye Print Co.; Buckeye, AZ, USA: 1980. [Google Scholar]
- 9.Silva M.G.V., Vieira I.G.P., Mendes F.N.P., Albuquerque I.L., dos Santos R.N., Silva F.O., Morais S.M. Variation of Ursolic Acid Content in Eight Ocimum Species from Northeastern Brazil. Molecules. 2008;13:2482–2487. doi: 10.3390/molecules13102482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zheljazkov V.D., Callahan A., Cantrell C.L. Yield and Oil Composition of 38 Basil (Ocimum Basilicum L.) Accessions Grown in Mississippi. J. Agric. Food Chem. 2008;56:241–245. doi: 10.1021/jf072447y. [DOI] [PubMed] [Google Scholar]
- 11.Sestili P., Ismail T., Calcabrini C., Guescini M., Catanzaro E., Turrini E., Layla A., Akhtar S., Fimognari C. The Potential Effects of Ocimum Basilicum on Health: A Review of Pharmacological and Toxicological Studies. Expert Opin. Drug Metab. Toxicol. 2018;14:679–692. doi: 10.1080/17425255.2018.1484450. [DOI] [PubMed] [Google Scholar]
- 12.Akgül A. Volatile Oil Composition of Sweet Basil (Ocimum Basilicum L.) Cultivating in Turkey (Short Communication) Food Nahr. 1989;33:87–88. doi: 10.1002/food.19890330129. [DOI] [Google Scholar]
- 13.Simon J.E., Quinn J., Murray R.G. Basil: A Source of Essential Oils. Advances in new crops; Proceedings of the First National Symposium “New Crops: Research, Development, Economics”; Indianapolis, IN, USA. 23–26 October 1988; pp. 484–489. [Google Scholar]
- 14.Lachowicz K.J., Jones G.P., Briggs D.R., Bienvenu F.E., Palmer M.V., Ting S.S.T., Hunter M. Characteristics of Essential Oil from Basil (Ocimum Basilicum L.) Grown in Australia. J. Agric. Food Chem. 1996;44:877–881. doi: 10.1021/jf9405214. [DOI] [Google Scholar]
- 15.Handbook of Arabian Medicinal Plants. [(accessed on 28 April 2021)]; Available online: https://www.routledge.com/Handbook-of-Arabian-Medicinal-Plants/Ghazanfar/p/book/9780849305399.
- 16.Booth N.L., Overk C.R., Yao P., Burdette J.E., Nikolic D., Chen S.-N., Bolton J.L., van Breemen R.B., Pauli G.F., Farnsworth N.R. The Chemical and Biological Profile of a Red Clover (Trifolium Pratense) Phase II Clinical Extract. J. Altern. Complement. Med. 2006;12:133–139. doi: 10.1089/acm.2006.12.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zohary M., Heller D. The Genus Trifolium. Israel Academy of Sciences and Humanities; Jerusalem, Israel: 1984. [Google Scholar]
- 18.Shang H., Li R., Wu H., Sun Z. Polysaccharides from Trifolium Repens L. Extracted by Different Methods and Extraction Condition Optimization. Sci. Rep. 2019;9:6353. doi: 10.1038/s41598-019-42877-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sundararajan B., Moola A.K., Vivek K., Kumari B.D.R. Formulation of Nanoemulsion from Leaves Essential Oil of Ocimum Basilicum L. and Its Antibacterial, Antioxidant and Larvicidal Activities (Culex Quinquefasciatus) Microb. Pathog. 2018;125:475–485. doi: 10.1016/j.micpath.2018.10.017. [DOI] [PubMed] [Google Scholar]
- 20.Makri O., Kintzios S. Ocimum sp. (Basil): Botany, Cultivation, Pharmaceutical Properties, and Biotechnology. J. Herbs Spices Med. Plants. 2008;13:123–150. doi: 10.1300/J044v13n03_10. [DOI] [Google Scholar]
- 21.Flanigan P.M., Niemeyer E.D. Effect of Cultivar on Phenolic Levels, Anthocyanin Composition, and Antioxidant Properties in Purple Basil (Ocimum Basilicum L.) Food Chem. 2014;164:518–526. doi: 10.1016/j.foodchem.2014.05.061. [DOI] [PubMed] [Google Scholar]
- 22.Teofilović B., Grujić-Letić N., Goločorbin-Kon S., Stojanović S., Vastag G., Gadžurić S. Experimental and Chemometric Study of Antioxidant Capacity of Basil (Ocimum Basilicum) Extracts. Ind. Crops Prod. 2017;100:176–182. doi: 10.1016/j.indcrop.2017.02.039. [DOI] [Google Scholar]
- 23.Antonescu A.I., Jurca T., Gligor F., Craciun I., Fritea L., Patay E.B., Muresan M., Udeanu D.I., Ioniță C.A., Antonescu A., et al. Comparative phytochemical and antioxidative characterization of Trifolium pratense L. and Ocimum basilicum L. Farmacia. 2019;67 doi: 10.31925/farmacia.2019.1.20. [DOI] [Google Scholar]
- 24.Benedec D., Pârvu A.E., Oniga I., Toiu A., Tiperciuc B. Effects of Ocimum Basilicum L. Extract on Experimental Acute Inflammation. Rev. Med. Chir. Soc. Med. Nat. Iasi. 2007;111:1065–1069. [PubMed] [Google Scholar]
- 25.Dhama K., Sharun K., Gugjoo M.B., Tiwari R., Alagawany M., Yatoo M.I., Thakur P., Iqbal H.M.N., Chaicumpa W., Michalak I., et al. A Comprehensive Review on Chemical Profile and Pharmacological Activities of Ocimum Basilicum. Food Rev. Int. 2021 doi: 10.1080/87559129.2021.1900230. [DOI] [Google Scholar]
- 26.Kolodziejczyk-Czepas J. Trifolium Species-Derived Substances and Extracts—Biological Activity and Prospects for Medicinal Applications. J. Ethnopharmacol. 2012;143:14–23. doi: 10.1016/j.jep.2012.06.048. [DOI] [PubMed] [Google Scholar]
- 27.Muzashvili T., Moniuszko-Szajwaj B., Pecio L., Oleszek W., Stochmal A. Ultraperformance Liquid Chromatography Tandem Mass Spectrometry Determination of Cyanogenic Glucosides in Trifolium Species. J. Agric. Food Chem. 2014;62:1777–1782. doi: 10.1021/jf4056659. [DOI] [PubMed] [Google Scholar]
- 28.Kolodziejczyk-Czepas J. Trifolium Species—The Latest Findings on Chemical Profile, Ethnomedicinal Use and Pharmacological Properties. J. Pharm. Pharmacol. 2016;68:845–861. doi: 10.1111/jphp.12568. [DOI] [PubMed] [Google Scholar]
- 29.Ahmad S., Zeb A. Phytochemical Profile and Pharmacological Properties of Trifolium Repens. J. Basic Clin. Physiol. Pharmacol. 2020 doi: 10.1515/jbcpp-2020-0015. [DOI] [PubMed] [Google Scholar]
- 30.Dabkevičienė G., Butkutė B., Lemežienė N., Jakštas V., Vilčinskas E., Janulis V. Distribution of Formononetin, Daidzein and Genistein in Trifolium Species and Their Aerial Plant Parts. Chemija. 2012;23:306–311. [Google Scholar]
- 31.Yokoyama S.-I., Kodera M., Hirai A., Nakada M., Ueno Y., Osawa T. Red Clover (Trifolium Pratense L.) Sprout Prevents Metabolic Syndrome. J. Nutr. Sci. Vitaminol. 2020;66:48–53. doi: 10.3177/jnsv.66.48. [DOI] [PubMed] [Google Scholar]
- 32.Zgonc Škulj A., Poljšak N., Kočevar Glavač N., Kreft S. Herbal Preparations for the Treatment of Hair Loss. Arch. Dermatol. Res. 2020;312:395–406. doi: 10.1007/s00403-019-02003-x. [DOI] [PubMed] [Google Scholar]
- 33.Kubes J., Skalicky M., Tumova L., Martin J., Hejnak V., Martinkova J. Vanadium Elicitation of Trifolium Pratense L. Cell Culture and Possible Pathways of Produced Isoflavones Transport across the Plasma Membrane. Plant Cell Rep. 2019;38:657–671. doi: 10.1007/s00299-019-02397-y. [DOI] [PubMed] [Google Scholar]
- 34.Wu Z., Xu B., Yu Z., He Q., Hu Z., Zhou S., Chen M., Zhu L. Trifolium Flavonoids Overcome Gefitinib Resistance of Non-Small-Cell Lung Cancer Cell by Suppressing ERK and STAT3 Signaling Pathways. Biomed. Res. Int. 2020;2020:2491304. doi: 10.1155/2020/2491304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Masuda T., Ino Y., Hirai A., Okamura A., Ishikawa H., Yokoyama S.-I., Osawa T. Effects of Isoflavone-Rich Red Clover Extract on Blood Glucose Level: A Randomized, Double-Blind, Placebo-Controlled Trial. J. Food Sci. 2021;86:1393–1399. doi: 10.1111/1750-3841.15672. [DOI] [PubMed] [Google Scholar]
- 36.Lee J., Scagel C.F. Chicoric Acid Levels in Commercial Basil (Ocimum Basilicum) and Echinacea Purpurea Products. J. Funct. Foods. 2010;2:77–84. doi: 10.1016/j.jff.2009.11.004. [DOI] [Google Scholar]
- 37.Politeo O., Jukic M., Milos M. Chemical Composition and Antioxidant Capacity of Free Volatile Aglycones from Basil (Ocimum Basilicum L.) Compared with Its Essential Oil. Food Chem. 2007;101:379–385. doi: 10.1016/j.foodchem.2006.01.045. [DOI] [Google Scholar]
- 38.Fritea L., Banica F., Costea T.O., Moldovan L., Iovan C., Cavalu S. A gold nanoparticles—Graphene based electrochemical sensor for sensitive determination of nitrazepam. J. Electroanal. Chem. 2018;830–831:63–71. doi: 10.1016/j.jelechem.2018.10.015. [DOI] [Google Scholar]
- 39.Miere F., Teusdea A.C., Laslo V., Fritea L., Moldovan L., Costea T., Uivarosan D., Vicas S.I., Pallag A. Natural Polymeric Beads for Encapsulation of Stellaria Media Extract with Antioxidant Properties. Mater. Plast. 2019;56:671–679. doi: 10.37358/MP.19.4.5252. [DOI] [Google Scholar]
- 40.Mastaneh M., Ahamd M., Taher N., Mehrdad H. Antioxidant Effect of Purple Basil (Lamiaceae) Phenolics. Orient. J. Chem. 2014;30:1965–1969. doi: 10.13005/ojc/300459. [DOI] [Google Scholar]
- 41.Dobjanschi L., Luminita F., Patay E., Tamas M. Comparative Study of the Morphological and Phytochemical Characterization of Romanian Solidago Species. Pak. J. Pharm. Sci. 2019;32:1571–1579. [PubMed] [Google Scholar]
- 42.Miere F., Fritea L., Cavalu S., Vicaș S.I. Formulation, characterization, and advantages of using liposomes in multiple therapies. Pharmacophore. 2020;11:1–12. [Google Scholar]
- 43.Kwee E.M., Niemeyer E.D. Variations in Phenolic Composition and Antioxidant Properties among 15 Basil (Ocimum Basilicum L.) Cultivars. Food Chem. 2011;128:1044–1050. doi: 10.1016/j.foodchem.2011.04.011. [DOI] [Google Scholar]
- 44.Oza M.J., Kulkarni Y.A. Trifolium Pratense (Red Clover) Improve SIRT1 Expression and Glycogen Content in High Fat Diet-Streptozotocin Induced Type 2 Diabetes in Rats. Chem. Biodivers. 2020;17:e2000019. doi: 10.1002/cbdv.202000019. [DOI] [PubMed] [Google Scholar]
- 45.Bahcesular B., Yildirim E.D., Karaçocuk M., Kulak M., Karaman S. Seed Priming with Melatonin Effects on Growth, Essential Oil Compounds and Antioxidant Activity of Basil (Ocimum Basilicum L.) under Salinity Stress. Ind. Crops Prod. 2020;146:112165. doi: 10.1016/j.indcrop.2020.112165. [DOI] [Google Scholar]
- 46.Miere F., Vicas S.I., Timar A.V., Ganea M., Zdrinca M., Cavalu S., Fritea L., Vicas L., Muresan M., Pallag A., et al. Preparation and Characterization of Two Different Liposomal Formulations with Bioactive Natural Extract for Multiple Applications. Processes. 2021;9:432. doi: 10.3390/pr9030432. [DOI] [Google Scholar]
- 47.Bakkali F., Averbeck S., Averbeck D., Idaomar M. Biological Effects of Essential Oils—A Review. Food Chem. Toxicol. 2008;46:446–475. doi: 10.1016/j.fct.2007.09.106. [DOI] [PubMed] [Google Scholar]
- 48.Gülçin I., Elmastaş M., Aboul-Enein H.Y. Determination of Antioxidant and Radical Scavenging Activity of Basil (Ocimum Basilicum L. Family Lamiaceae) Assayed by Different Methodologies. Phytother. Res. 2007;21:354–361. doi: 10.1002/ptr.2069. [DOI] [PubMed] [Google Scholar]
- 49.Hinneburg I., Damien Dorman H.J., Hiltunen R. Antioxidant Activities of Extracts from Selected Culinary Herbs and Spices. Food Chem. 2006;97:122–129. doi: 10.1016/j.foodchem.2005.03.028. [DOI] [Google Scholar]
- 50.Pichersky E., Gershenzon J. The Formation and Function of Plant Volatiles: Perfumes for Pollinator Attraction and Defense. Curr. Opin. Plant Biol. 2002;5:237–243. doi: 10.1016/S1369-5266(02)00251-0. [DOI] [PubMed] [Google Scholar]
- 51.Salas J.J., Sánchez C., García-González D.L., Aparicio R. Impact of the Suppression of Lipoxygenase and Hydroperoxide Lyase on the Quality of the Green Odor in Green Leaves. J. Agric. Food Chem. 2005;53:1648–1655. doi: 10.1021/jf040331l. [DOI] [PubMed] [Google Scholar]
- 52.Dudareva N., Pichersky E., Gershenzon J. Biochemistry of Plant Volatiles. Plant Physiol. 2004;135:1893–1902. doi: 10.1104/pp.104.049981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vuorinen T., Reddy G.V.P., Nerg A.-M., Holopainen J.K. Monoterpene and Herbivore-Induced Emissions from Cabbage Plants Grown at Elevated Atmospheric CO2 Concentration. Atmos. Environ. 2004;38:675–682. doi: 10.1016/j.atmosenv.2003.10.029. [DOI] [Google Scholar]
- 54.Vianna E., Ebeler S.E. Monitoring Ester Formation in Grape Juice Fermentations Using Solid Phase Microextraction Coupled with Gas Chromatography-Mass Spectrometry. J. Agric. Food Chem. 2001;49:589–595. doi: 10.1021/jf000907g. [DOI] [PubMed] [Google Scholar]
- 55.Pedraza-Chaverri J., Cárdenas-Rodríguez N., Orozco-Ibarra M., Pérez-Rojas J.M. Medicinal Properties of Mangosteen (Garcinia Mangostana) Food Chem. Toxicol. 2008;46:3227–3239. doi: 10.1016/j.fct.2008.07.024. [DOI] [PubMed] [Google Scholar]
- 56.Jurca T., Baldea I., Filip G.A., Olteanu D., Clichici S., Pallag A., Vicas L., Marian E., Micle O., Muresan M. The Effect of Tropaeolum Majus L. on Bacterial Infections and in Vitro Efficacy on Apoptosis and DNA Lesions in Hyperosmotic Stress. J. Physiol. Pharmacol. 2018;69 doi: 10.26402/jpp.2018.3.06. [DOI] [PubMed] [Google Scholar]
- 57.Vlaisavljevic S., Kaurinovic B., Popovic M., Djurendic-Brenesel M., Vasiljevic B., Cvetkovic D., Vasiljevic S. Trifolium Pratense L. as a Potential Natural Antioxidant. Molecules. 2014;19:713–725. doi: 10.3390/molecules19010713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reis A., Boutet-Mercey S., Massot S., Ratet P., Zuanazzi J.A.S. Isoflavone Production in Hairy Root Cultures and Plantlets of Trifolium Pratense. Biotechnol. Lett. 2019;41:427–442. doi: 10.1007/s10529-018-02640-8. [DOI] [PubMed] [Google Scholar]
- 59.Chauhan P. Skin Cancer and Role of Herbal Medicines. Asian J. Pharm. Pharmacol. 2018;4:404–412. doi: 10.31024/ajpp.2018.4.4.5. [DOI] [Google Scholar]
- 60.Nacar S., Tansi S. Chemical Components of Different Basil (Ocimum Basilicum L.) Cultivars Grown in Mediterranean Regions in Turkey. Isr. J. Plant Sci. 2000;48:109–112. doi: 10.1560/3TKC-W098-BGBU-4358. [DOI] [Google Scholar]
- 61.Al-Maskri A.Y., Hanif M.A., Al-Maskari M.Y., Abraham A.S., Al-Sabahi J.N., Al-Mantheri O. Essential Oil from Ocimum Basilicum (Omani Basil): A Desert Crop. Nat. Prod. Commun. 2011;6:1487–1490. [PubMed] [Google Scholar]
- 62.Politeo O., Jukic M., Milos M. Comparison of Chemical Composition and Antioxidant Activity of Glycosidically Bound and Free Volatiles from Clove (Eugenia Caryophyllata Thunb.) J. Food Biochem. 2010;34:129–141. doi: 10.1111/j.1745-4514.2009.00269.x. [DOI] [Google Scholar]
- 63.Sullivan M.L., Quesenberry K.H. Clover, Red (Trifolium Pratense) Methods Mol. Biol. 2015;1223:237–254. doi: 10.1007/978-1-4939-1695-5_19. [DOI] [PubMed] [Google Scholar]
- 64.Mouradov A., Panter S., Labandera M., Ludlow E., Emmerling M., Spangenberg G. Clovers (Trifolium spp.) Methods Mol. Biol. 2006;343:325–335. doi: 10.1385/1-59745-130-4:325. [DOI] [PubMed] [Google Scholar]
- 65.Sakkas H., Papadopoulou C. Antimicrobial Activity of Basil, Oregano, and Thyme Essential Oils. J. Microbiol. Biotechnol. 2017;27:429–438. doi: 10.4014/jmb.1608.08024. [DOI] [PubMed] [Google Scholar]
- 66.Araújo Silva V., Pereira da Sousa J., de Luna Freire Pessôa H., Fernanda Ramos de Freitas A., Douglas Melo Coutinho H., Beuttenmuller Nogueira Alves L., Oliveira Lima E. Ocimum Basilicum: Antibacterial Activity and Association Study with Antibiotics against Bacteria of Clinical Importance. Pharm. Biol. 2016;54:863–867. doi: 10.3109/13880209.2015.1088551. [DOI] [PubMed] [Google Scholar]
- 67.Chiang L.-C., Ng L.-T., Cheng P.-W., Chiang W., Lin C.-C. Antiviral Activities of Extracts and Selected Pure Constituents of Ocimum Basilicum. Clin. Exp. Pharmacol. Physiol. 2005;32:811–816. doi: 10.1111/j.1440-1681.2005.04270.x. [DOI] [PubMed] [Google Scholar]
- 68.Zahran E., Abdelmohsen U., Khalil H., Desoukey S., Ahmed M., Kamel M. Diversity, Phytochemical and Medicinal Potential of the Genus Ocimum L. (Lamiaceae) Phytochem. Rev. 2020;19 doi: 10.1007/s11101-020-09690-9. [DOI] [Google Scholar]
- 69.Loing E., Lachance R., Ollier V., Hocquaux M. A New Strategy to Modulate Alopecia Using a Combination of Two Specific and Unique Ingredients. J. Cosmet. Sci. 2013;64:45–58. [PubMed] [Google Scholar]
- 70.Ghitea T.C., El-Kharoubi A., Ganea M., Bimbo-Szuhai E., Nemeth T.S., Ciavoi G., Foghis M., Dobjanschi L., Pallag A., Micle O. The Antimicrobial Activity of Origanum Vulgare L. Correlated with the Gastrointestinal Perturbation in Patients with Metabolic Syndrome. Molecules. 2021;26:283. doi: 10.3390/molecules26020283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Marian E., Vicas L.G., Jurca T., Muresan M., Pallag A., Stan R.L., Sevastre B., Diaconeasa Z., Ionescu C.M.L., Hangan A.C. Salivia Officinalis L. and Verbascum Phlomoides L. Chemical, Antimicrobial, Antioxidant and Antitumor Investigations. Rev. Chim. 2018;69:365–370. doi: 10.37358/RC.18.2.6108. [DOI] [Google Scholar]
- 72.Harlow B.E., Flythe M.D., Kagan I.A., Goodman J.P., Klotz J.L., Aiken G.E. Isoflavone Supplementation, via Red Clover Hay, Alters the Rumen Microbial Community and Promotes Weight Gain of Steers Grazing Mixed Grass Pastures. PLoS ONE. 2020;15:e0229200. doi: 10.1371/journal.pone.0229200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Singh S., Majumdar D.K. Evaluation of Antiinflammatory Activity of Fatty Acids of Ocimum Sanctum Fixed Oil. Indian J. Exp. Biol. 1997;35:380–383. [PubMed] [Google Scholar]
- 74.Singh S., Taneja M., Majumdar D.K. Biological Activities of Ocimum Sanctum L. Fixed Oil—An Overview. Indian J. Exp. Biol. 2007;45:403–412. [PubMed] [Google Scholar]
- 75.Okoye F.B.C., Obonga W.O., Onyegbule F.A., Ndu O.O., Ihekwereme C.P. Chemical composition and anti-inflammatory activity of essential oils from the leaves of Ocimum basilicum L. and Ocimum gratissimum L. (Lamiaceae) Int. J. Pharm. Sci. Res. 2014;5:2174–2180. [Google Scholar]
- 76.Mediratta P.K., Sharma K.K., Singh S. Evaluation of Immunomodulatory Potential of Ocimum Sanctum Seed Oil and Its Possible Mechanism of Action. J. Ethnopharmacol. 2002;80:15–20. doi: 10.1016/S0378-8741(01)00373-7. [DOI] [PubMed] [Google Scholar]
- 77.Maul R., Kulling S.E. Absorption of Red Clover Isoflavones in Human Subjects: Results from a Pilot Study. Br. J. Nutr. 2010;103:1569–1572. doi: 10.1017/S0007114509993564. [DOI] [PubMed] [Google Scholar]
- 78.Lutter S., Schmalbach K., Esch H.L., Lehmann L. The Isoflavone Irilone Contributes to the Estrogenic Potential of Dietary Supplements Containing Red Clover. Arch. Toxicol. 2014;88:309–321. doi: 10.1007/s00204-013-1114-5. [DOI] [PubMed] [Google Scholar]
- 79.Spagnuolo P., Rasini E., Luini A., Legnaro M., Luzzani M., Casareto E., Carreri M., Paracchini S., Marino F., Cosentino M. Isoflavone Content and Estrogenic Activity of Different Batches of Red Clover (Trifolium Pratense L.) Extracts: An in Vitro Study in MCF-7 Cells. Fitoterapia. 2014;94:62–69. doi: 10.1016/j.fitote.2014.01.027. [DOI] [PubMed] [Google Scholar]
- 80.Finley J.W. Proposed Criteria for Assessing the Efficacy of Cancer Reduction by Plant Foods Enriched in Carotenoids, Glucosinolates, Polyphenols and Selenocompounds. Ann. Bot. 2005;95:1075–1096. doi: 10.1093/aob/mci123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Karakaş F., Yildirim A., Bayram R., Yavuz M., Gepdiremen A., Turker A. Antiproliferative Activity of Some Medicinal Plants on Human Breast and Hepatocellular Carcinoma Cell Lines and Their Phenolic Contents. Trop. J. Pharm. Res. 2015;14:1787. doi: 10.4314/tjpr.v14i10.8. [DOI] [Google Scholar]
- 82.Orafidiya L.O., Agbani E.O., Oyedele A.O., Babalola O.O., Onayemi O. Preliminary Clinical Tests on Topical Preparations of Ocimum Gratissimum Linn Leaf Essential Oil for the Treatment of Acne Vulgaris. Clin. Drug Investig. 2002 doi: 10.2165/00044011-200222050-00005. [DOI] [Google Scholar]
- 83.Orafidiya L.O., Agbani E.O., Adelusola K.A., Iwalewa E.O., Adebanji O.A., Adediran E.A.F., Agbani N.T. A Study on the Effect of the Leaf Essential Oil of Ocimum Gratissimum Linn. on Cyclophosphamide-Induced Hair Loss. Int. J. Aromather. 2004;14:119–128. doi: 10.1016/j.ijat.2004.06.006. [DOI] [Google Scholar]
- 84.Pansang S., Maphanta S., Tuntijarukorn P., Viyoch J. Skin Irritation Test of a Microemulsion Containing Essential Oil Isolated from Ocimum Basilicum. ScienceAsia. 2010;36:355–358. doi: 10.2306/scienceasia1513-1874.2010.36.355. [DOI] [Google Scholar]
- 85.Renda G., Yalçın F.N., Nemutlu E., Akkol E.K., Süntar I., Keleş H., Ina H., Çalış I., Ersöz T. Comparative Assessment of Dermal Wound Healing Potentials of Various Trifolium L. Extracts and Determination of Their Isoflavone Contents as Potential Active Ingredients. J. Ethnopharmacol. 2013;148:423–432. doi: 10.1016/j.jep.2013.04.031. [DOI] [PubMed] [Google Scholar]
- 86.Lipovac M., Chedraui P., Gruenhut C., Gocan A., Kurz C., Neuber B., Imhof M. Effect of Red Clover Isoflavones over Skin, Appendages, and Mucosal Status in Postmenopausal Women. Obstet. Gynecol. Int. 2011;2011 doi: 10.1155/2011/949302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dweck A.C. The Internal and External Use of Medicinal Plants. Clin. Dermatol. 2009;27:148–158. doi: 10.1016/j.clindermatol.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 88.Zhan Y., An X., Wang S., Sun M., Zhou H. Basil Polysaccharides: A Review on Extraction, Bioactivities and Pharmacological Applications. Bioorg. Med. Chem. 2020;28:115179. doi: 10.1016/j.bmc.2019.115179. [DOI] [PubMed] [Google Scholar]
- 89.Shahrajabian M.H., Sun W., Cheng Q. Chemical Components and Pharmacological Benefits of Basil (Ocimum Basilicum): A Review. Int. J. Food Prop. 2020;23:1961–1970. doi: 10.1080/10942912.2020.1828456. [DOI] [Google Scholar]
- 90.Hussain A.I., Anwar F., Hussain Sherazi S.T., Przybylski R. Chemical Composition, Antioxidant and Antimicrobial Activities of Basil (Ocimum Basilicum) Essential Oils Depends on Seasonal Variations. Food Chem. 2008;108:986–995. doi: 10.1016/j.foodchem.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 91.Goel A., Kumar S., Singh D.K., Bhatia A.K. Wound Healing Potential of Ocimum Sanctum Linn. with Induction of Tumor Necrosis Factor-Alpha. Indian J. Exp. Biol. 2010;48:402–406. [PubMed] [Google Scholar]
- 92.Manosroi J., Dhumtanom P., Manosroi A. Anti-Proliferative Activity of Essential Oil Extracted from Thai Medicinal Plants on KB and P388 Cell Lines. Cancer Lett. 2006;235:114–120. doi: 10.1016/j.canlet.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 93.Huang X., Sun J., Chen G., Niu C., Wang Y., Zhao C., Sun J., Huang H., Huang S., Liang Y., et al. Resveratrol Promotes Diabetic Wound Healing via SIRT1-FOXO1-c-Myc Signaling Pathway-Mediated Angiogenesis. Front. Pharmacol. 2019;10 doi: 10.3389/fphar.2019.00421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Saha S., Mukhopadhyay M.K., Ghosh P.D., Nath D. Effect of Methanolic Leaf Extract of Ocimum Basilicum L. on Benzene-Induced Hematotoxicity in Mice. Evid. Based Complement. Altern. Med. 2012;2012:e176385. doi: 10.1155/2012/176385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Monga J., Sharma M., Tailor N., Ganesh N. Antimelanoma and Radioprotective Activity of Alcoholic Aqueous Extract of Different Species of Ocimum in C(57)BL Mice. Pharm. Biol. 2011;49:428–436. doi: 10.3109/13880209.2010.521513. [DOI] [PubMed] [Google Scholar]
- 96.Rastogi S., Shukla Y., Paul B.N., Chowdhuri D.K., Khanna S.K., Das M. Protective Effect of Ocimum Sanctum on 3-Methylcholanthrene, 7,12-Dimethylbenz(a)Anthracene and Aflatoxin B1 Induced Skin Tumorigenesis in Mice. Toxicol. Appl. Pharmacol. 2007;224:228–240. doi: 10.1016/j.taap.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 97.Bravo E., Amrani S., Aziz M., Harnafi H., Napolitano M. Ocimum Basilicum Ethanolic Extract Decreases Cholesterol Synthesis and Lipid Accumulation in Human Macrophages. Fitoterapia. 2008;79:515–523. doi: 10.1016/j.fitote.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 98.Agrawal P., Rai V., Singh R.B. Randomized Placebo-Controlled, Single Blind Trial of Holy Basil Leaves in Patients with Noninsulin-Dependent Diabetes Mellitus. Int. J. Clin. Pharmacol. Ther. 1996;34:406–409. [PubMed] [Google Scholar]
- 99.Amrani S., Harnafi H., Gadi D., Mekhfi H., Legssyer A., Aziz M., Martin-Nizard F., Bosca L. Vasorelaxant and Anti-Platelet Aggregation Effects of Aqueous Ocimum Basilicum Extract. J. Ethnopharmacol. 2009;125:157–162. doi: 10.1016/j.jep.2009.05.043. [DOI] [PubMed] [Google Scholar]
- 100.Alipour G., Dashti S., Hosseinzadeh H. Review of Pharmacological Effects of Myrtus Communis L. and Its Active Constituents. Phytother. Res. 2014;28:1125–1136. doi: 10.1002/ptr.5122. [DOI] [PubMed] [Google Scholar]
- 101.Tchimene M.K., Okoli C.O., Iwu M.M. Antidiabetic Property of Some Nigerian Medicinal Plants. J. Med. Plants Res. 2016;10:139–148. doi: 10.5897/JMPR2015.5895. [DOI] [Google Scholar]
- 102.Jain R., Aqil M., Ahad A., Ali A., Khar R.K. Basil Oil Is a Promising Skin Penetration Enhancer for Transdermal Delivery of Labetolol Hydrochloride. Drug Dev. Ind. Pharm. 2008;34:384–389. doi: 10.1080/03639040701657958. [DOI] [PubMed] [Google Scholar]
- 103.Hozayen W.G., El-Desouky M.A., Soliman H.A., Ahmed R.R., Khaliefa A.K. Antiosteoporotic Effect of Petroselinum Crispum, Ocimum Basilicum and Cichorium Intybus L. in Glucocorticoid-Induced Osteoporosis in Rats. BMC Complement. Altern. Med. 2016;16:165. doi: 10.1186/s12906-016-1140-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nweze E.I., Eze E.E. Justification for the Use of Ocimum Gratissimum L. in Herbal Medicine and Its Interaction with Disc Antibiotics. BMC Complement. Altern. Med. 2009;9:37. doi: 10.1186/1472-6882-9-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Viyoch J., Pisutthanan N., Faikreua A., Nupangta K., Wangtorpol K., Ngokkuen J. Evaluation of in Vitro Antimicrobial Activity of Thai Basil Oils and Their Micro-Emulsion Formulas against Propionibacterium Acnes. Int. J. Cosmet. Sci. 2006;28:125–133. doi: 10.1111/j.1467-2494.2006.00308.x. [DOI] [PubMed] [Google Scholar]
- 106.Rashidian A., Roohi P., Mehrzadi S., Ghannadi A.R., Minaiyan M. Protective Effect of Ocimum Basilicum Essential Oil Against Acetic Acid–Induced Colitis in Rats. J. Evid. Based Complement. Altern. Med. 2016;21:NP36–NP42. doi: 10.1177/2156587215616550. [DOI] [PubMed] [Google Scholar]
- 107.Taie H.A.A., Salama Z.A.E.-R., Samir R. Potential Activity of Basil Plants as a Source of Antioxidants and Anticancer Agents as Affected by Organic and Bio-Organic Fertilization. Not. Bot. Horti Agrobot. Cluj Napoca. 2010;38:119–127. doi: 10.15835/nbha3813534. [DOI] [Google Scholar]
- 108.Nguyen P.M., Niemeyer E.D. Effects of Nitrogen Fertilization on the Phenolic Composition and Antioxidant Properties of Basil (Ocimum Basilicum L.) J. Agric. Food Chem. 2008;56:8685–8691. doi: 10.1021/jf801485u. [DOI] [PubMed] [Google Scholar]
- 109.Chokechaijaroenporn O., Bunyapraphatsara N., Kongchuensin S. Mosquito Repellent Activities of Ocimum Volatile Oils. Phytomedicine. 1994;1:135–139. doi: 10.1016/S0944-7113(11)80031-0. [DOI] [PubMed] [Google Scholar]
- 110.Ueda-Nakamura T., Mendonça-Filho R.R., Morgado-Díaz J.A., Korehisa Maza P., Prado Dias Filho B., Aparício Garcia Cortez D., Alviano D.S., do Socorro M.S.R., Lopes A.H.C.S., Alviano C.S., et al. Antileishmanial Activity of Eugenol-Rich Essential Oil from Ocimum Gratissimum. Parasitol. Int. 2006;55:99–105. doi: 10.1016/j.parint.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 111.Wagay N. Medicinal Flora and Ethno-Botanical Knowledge of Baramulla Tehsil in Jammu and Kashmir, India. Int. J. Adv. Biotechnol. Res. 2014;5:539–546. [Google Scholar]
- 112.Chen T., Zhong F.-J., Hong Y.-M., Su W.-J., Zhuang L.-L., Qiu L.-X. Effect of Trifolium Pratense Extract on Methionine-Choline-Deficient Diet-Induced Steatohepatitis in C57BL/6 Mice. Chin. J. Nat. Med. 2014;12:194–198. doi: 10.1016/S1875-5364(14)60032-7. [DOI] [PubMed] [Google Scholar]
- 113.Tundis R., Marrelli M., Conforti F., Tenuta M., Bonesi M., Menichini F., Loizzo M. Trifolium Pratense and T. Repens (Leguminosae): Edible Flower Extracts as Functional Ingredients. Foods. 2015;4:338–348. doi: 10.3390/foods4030338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Jung E.H., Kim S.R., Hwang I.K., Ha T.Y. Hypoglycemic Effects of a Phenolic Acid Fraction of Rice Bran and Ferulic Acid in C57BL/KsJ-Db/Db Mice. J. Agric. Food Chem. 2007;55:9800–9804. doi: 10.1021/jf0714463. [DOI] [PubMed] [Google Scholar]
- 115.Circosta C., De Pasquale R., Palumbo D.R., Samperi S., Occhiuto F. Effects of Isoflavones from Red Clover (Trifolium Pratense) on Skin Changes Induced by Ovariectomy in Rats. Phytother. Res. 2006;20:1096–1099. doi: 10.1002/ptr.2017. [DOI] [PubMed] [Google Scholar]
- 116.Ijaz F., Iqbal Z., Alam J., Khan S.M., Afzal A., Rahman I.U., Afzal M., Islam M. Ethno Medicinal Study upon Folk Recipes Against Various Human Diseases in Sarban Hills, Abbottabad, Pakistan. World J. Zool. 2015;10:41–46. [Google Scholar]
- 117.Ghazanfarpour M., Sadeghi R., Latifnejad Roudsari R., Mirzaii Najmabadi K., Mousavi Bazaz M., Abdolahian S., Khadivzadeh T. Effects of Red Clover on Hot Flash and Circulating Hormone Concentrations in Menopausal Women: A Systematic Review and Meta-Analysis. Avicenna J. Phytomed. 2015;5:498–511. [PMC free article] [PubMed] [Google Scholar]
- 118.Cegieła U., Folwarczna J., Pytlik M., Zgórka G. Effects of Extracts from Trifolium Medium L. and Trifolium Pratense L. on Development of Estrogen Deficiency-Induced Osteoporosis in Rats. Evid. Based Complement. Altern. Med. 2012;2012:e921684. doi: 10.1155/2012/921684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ali Khan B., Ullah S., Khan M.K., Alshahrani S.M., Braga V.A. Formulation and Evaluation of Ocimum Basilicum-Based Emulgel for Wound Healing Using Animal Model. Saudi Pharm. J. 2020;28:1842–1850. doi: 10.1016/j.jsps.2020.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Habibi Zadeh S.K., Farahpour M.-R., Kar H.H. The Effect of Topical Administration of an Ointment Prepared from Trifolium Repens Hydroethanolic Extract on the Acceleration of Excisional Cutaneous Wound Healing. Wounds. 2020;32:253–261. doi: 10.25270/wnds/2020.253261. [DOI] [PubMed] [Google Scholar]
- 121.Kurahashi T., Fujii J. Roles of Antioxidative Enzymes in Wound Healing. J. Dev. Biol. 2015;3:57–70. doi: 10.3390/jdb3020057. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data available in a publicly accessible repository.