Abstract
Gait, balance, and coordination are important in the development of chronic disease, but the ability to accurately assess these in the daily lives of patients may be limited by traditional biased assessment tools. Wearable sensors offer the possibility of minimizing the main limitations of traditional assessment tools by generating quantitative data on a regular basis, which can greatly improve the home monitoring of patients. However, these commercial sensors must be validated in this context with rigorous validation methods. This scoping review summarizes the state-of-the-art between 2010 and 2020 in terms of the use of commercial wearable devices for gait monitoring in patients. For this specific period, 10 databases were searched and 564 records were retrieved from the associated search. This scoping review included 70 studies investigating one or more wearable sensors used to automatically track patient gait in the field. The majority of studies (95%) utilized accelerometers either by itself (N = 17 of 70) or embedded into a device (N = 57 of 70) and/or gyroscopes (51%) to automatically monitor gait via wearable sensors. All of the studies (N = 70) used one or more validation methods in which “ground truth” data were reported. Regarding the validation of wearable sensors, studies using machine learning have become more numerous since 2010, at 17% of included studies. This scoping review highlights the current state of the ability of commercial sensors to enhance traditional methods of gait assessment by passively monitoring gait in daily life, over long periods of time, and with minimal user interaction. Considering our review of the last 10 years in this field, machine learning approaches are algorithms to be considered for the future. These are in fact data-based approaches which, as long as the data collected are numerous, annotated, and representative, allow for the training of an effective model. In this context, commercial wearable sensors allowing for increased data collection and good patient adherence through efforts of miniaturization, energy consumption, and comfort will contribute to its future success.
Keywords: gait, chronic pathology, tracker, wearable, validation, gold standard, machine learning, systematic review
1. Introduction
Human gait assessments study human movement and aim to quantify gait characteristics with various spatiotemporal parameters, such as stride speed and length, step length, cadence, standing, double support, and swing times [1]. Normal gait corresponds to an individual’s motion pattern, and deviation in gait from this normal pattern can indicate a change in health status. In this regard, recent works have demonstrated that gait could have a link to functional health and could be an indicator for the course of chronic disease and, hence, rehabilitation feedback [2]. For example, ref. [3] demonstrated the value of studying gait asymmetry in post-stroke patients, ref. [4] identified gait variability as a marker of balance in Parkinson’s disease, and ref. [5] described changes in gait and balance in the elderly. As a result, there is a move towards using gait analysis to aid in patient health assessment and monitoring.
Traditional methods for gait analysis in patients typically use walk tests as a standard assessment [6,7]. A walk test is an examination carried out over a fixed duration and/or distance in order to easily access speed measurements. The most commonly used walk test is the six-minute walk test (6MWT) [8], which assesses endurance at a comfortable speed for the subject by measuring the distance walked in 6 min along a straight corridor. Even though these tests are widely used to establish a link between the gait and physical state of the patient, important long-term gait longitudinal patterns or transition patterns from one daily activity to another are not measured and cannot be explored. The ability to explore these patterns, such as the transition from turning to sitting [9], frequency of falls [10], or freezing episodes [11] is important because recent literature suggests that they may be able to inform about a deterioration in the patient’s state of health and, therefore, of their chronic condition.
Emerging technologies offer the possibility to improve the evaluation of traditional methods by increasing the quality and the duration of the window of data acquisition by measuring gait in daily activities over long periods of time. Wearable devices with embedded sensors allow in particular for the passive collection of various data sets, which can then be used to develop algorithms to assess gait in real life conditions and over long periods of time [12,13]. This opens up many perspectives, especially in the case of chronic diseases where the disease profile varies for each individual and has fluctuating symptoms. Twenty-four hour home monitoring in a real environment is an ideal solution for an accurate diagnosis of symptoms as well as good patient compliance [14].
In the past decade, commercial wearable sensors have been used not only in the consumer market but also in research studies. In particular, wearable sensors are used in physical activity monitoring for measurements and goal setting [15]. More recently, a more specific use of these sensors was introduced in research studies in medicine and rehabilitation [16,17]. Wearable sensors for gait assessment have been primarily conducted in a lab and with controlled protocols [18], traducing that commercial sensors can be challenging to deploy and validate. More recently, the testing of the sensors in patient monitoring has expanded into real-life conditions. Previous research has shown significant differences in spatiotemporal gait parameters between similar in-lab and in-field studies [19], illustrating the importance of establishing commercial sensor validity for long-term patient monitoring and for detecting events and more particularly deviations from normal human gait.
There are already many reviews on the validation of commercial wearable sensors available in the literature, and most were interested in monitoring activity on healthy subjects [15,20,21,22] while others have taken a descriptive approach centered on a very specific medical application [18,23,24]. However, few studies focus on the validation methods, the ground truth used, and how the reference data are annotated. A common validation method is to use inferential statistics, such as a regression analysis to explore and model the relationship between sensor and ground truth data. These approaches typically assume that the relationship between sensor and ground truth data follows a linear pattern. Linear regression has the advantage of being simple to use and to interpret. In comparison with these linear methods, the nonlinear methods fit more types of data in terms of shape and are hence recognized as being more general. Some nonlinear approaches such as machine learning have the advantage of being less dependent on the assumption of the model and very recently produced promising results in sensor validation [25,26]. Nonlinearity seems particularly interesting in terms of patient monitoring in order to integrate networks of several sensors placed at different places on the patient [27,28] and for high-level tasks (such as the classification of patients into groups according to the evolution of a disease) [29,30], which requires the integration of various information on locomotion and control systems involved in complex gait regulation [31,32].
In this paper, our aim was to conduct a systematic review (i) to determine the statistical methods currently used for the validation of sensors and (ii) to determine to what extent machine learning (ML) is used as a statistical method for this validation step.
2. Methods
This scoping review is reported using the Preferred Reporting Items for Systematic reviews and Meta-Analyses Extension for Scoping Reviews (PRISMA-ScR) checklist [33].
2.1. Databases
We conducted a literature search of the PubMed, SCOPUS, ScienceDirect, Web of Science, IEEE Xplore, ACM Digital Library, Collection of Computer Science Bibliographies, Cochrane Library, DBLB, and Google Scholar (first 50 results) databases for all literature published between 2010 and 2020.
2.2. Literature Search
The literature search strategy included a combination of keywords to identify articles that addressed (i) gait assessment/detection, (ii) wearable and connected technology, (iii) chronic pathology monitoring, and (iv) validation. Keywords included “gait”, “walk”, “actigraphy”, “actimetry”; “smartphone”, “wearable”, “mobile device”, “IoT”; “chronic disease”, “rehabilitation”, “medicine”; “validity”, “validation”, “reliability”, and “reproductibility”. The full search term strategy that was used for each database is given in Table A1 of Appendix A.
2.3. Inclusion Criteria
Only peer-reviewed journals or conference papers were included in this review if they were published between January 2010 and December 2020 and were written in English. In addition, eligible articles had to complete all of the following criteria as part of the content given in the article:
The study must be centered on gait or posture analysis (e.g., detect stance and swing phases, detect the risk of falling, etc.). Studies focusing only on activities or step counting were excluded.
Given the application to remote monitoring in patients, only devices allowing wireless data flow wer considered. This flow had to have been conducted using bluetooth between the device and the smartphone to then send data by Wi-Fi to a remote server. Sensors that temporarily store the data locally and send the data a posteriori when a Wi-Fi connection is available were also included.
The devices had to have been used in a clinical setting for long-term follow-up or rehabilitation of a chronic pathology. Studies on young or healthy patients and on animals were excluded.
The validity of the sensor and the resulting indicators must have been assessed. Therefore, a ground truth must be proposed and the study must include at least one statistical measure (e.g., statistical test, correlation, and mean square error) or one evaluation metric (e.g., accuracy, F1-score, precision, and sensitivity) to indicate the performance of the sensor on detecting the associated gait feature.
Review articles, commentary articles, study protocol articles, and any other articles without reported results from empirical research were excluded.
2.4. Selection of Articles
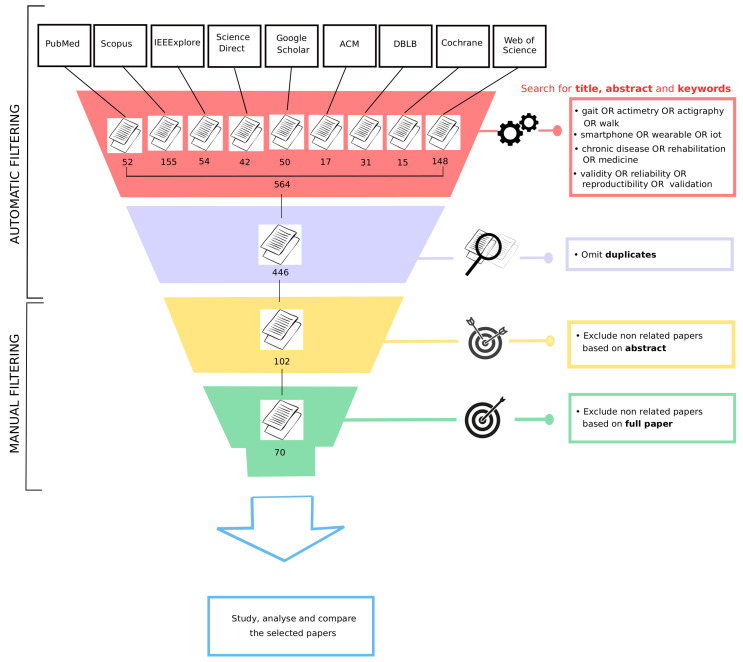
The records retrieved from the databases were gathered in CSV files. All duplicate articles were removed. First, we reviewed the titles and abstracts of all articles (Figure 1). During this first phase of selection, articles were excluded if they did not describe at least one wearable device used to automatically assess gait as part of the follow-up of a chronic pathology, with particular attention paid to the validation of the device. If this information could not be verified from the title and/or abstract, the article’s full text was reviewed in a further screening phase to determine whether it fit the eligibility criteria. Moreover, if the abstract indicated that the study was not peer-reviewed, was not written in English, was not accessible online, or corresponded to a study conducted on animals, it was excluded. After the initial title/abstract selection process, we evaluated the full text of the remaining articles. Articles were then excluded if they did not meet the eligibility criteria (Figure 1).
Figure 1.
Diagram of the article-selection process.
2.5. Data Extraction
Three research assistants independently extracted the following study characteristics from the final set of eligible studies using a custom-made data extraction worksheet. Here are the different characteristics identified for the analysis of identified papers in the context of our systematic review:
Sample size: the total number of participants for each study.
Pathology: the disease monitored in the study.
Duration of data collection: how long the participants wore the sensor(s) to collect data for the study.
Condition of data collection: specifies on whether the study was conducted in a laboratory or in free-living conditions.
Number of wearable devices: the total number of wearable devices in which the sensor’s signal data were used to study the patient’s gait. Any other equipment that was part of the acquisition system but did not provide data to evaluate the gait was not included in this count.
Type of sensor(s): the type of sensor embedded within the wearable device(s) used to assess gait.
Device brand(s) and model(s): the specific brand and model of the wearable device(s) used in the study.
Location of device(s): details specific to the placement/location of wearable device(s) on the patient’s body.
Gait indicators measured by the device(s): gait outcomes that were derived from the signal recorded on the device. In some studies, several gait indicators were extracted from the raw data.
Ground-truth method(s): the method that was used in the study to evaluate the performance of the device(s) to assess gait.
Evaluation metric(s) of the device(s): any evaluation metric, reported either in the text, a figure, or a table, that described the performance of the wearable device(s) on assessing gait. Only evaluation metrics that were exclusively used to study gait were included.
2.6. Summarizing Data and Categories
Mean and standard deviation were calculated for each extracted numerical variable (sample size, duration of data collection, and number of devices). Frequency tables were constructed for each extracted categorical variable (pathology, condition of data collection, sensor types, device brand and model, device location, ground-truth methods, gait features, and evaluation metrics). Regarding these categorical variables, here are the categories that we considered and their meanings. These categories are not exhaustive of all possible types of categories but correspond to those proposed in the context of the included studies.
The devices are categorized according to three types: (i) smartphone, (ii) inertial measurement unit (IMU), and (iii) single sensor.
The device location is categorized according to four levels: (i) superior, if the device was carried in the hands or on the arms; (ii) inferior, if the device was carried on the legs or feet; (iii) chest, if the device was carried on the chest or the trunk; and (iv) free location, if the device was in a pocket or more prone to moving around, or if the its location on the body was not distinguished.
The ground-truth methods are categorized according to six levels: (i) controls, where a group of subjects served as a reference; (ii) expert, where the data were analyzed with regard to annotations made by experts; (iii) med device, where the data were analyzed with regard to a portable device already used in clinical routine; (iv) medical, where the data were analyzed with regard to a medical examination/test or clinical score; (v) metrologic, where other high resolution equipment were used as a reference; and (vi) user annotations, where the data were analyzed with regard to annotations made by patients during the use of the device.
The gait features are categorized according to three levels: (i) low, where the analysis was conducted on raw signals without postprocessing; (ii) medium, where the analysis was based on statistical descriptors extracted from the signals (mainly statistical moments or common signal processing features); and (iii) high, where the analysis was based on descriptors at a high level of representation that disregards the technical characteristics of the equipment or methods used (e.g., step length, cadence, and number of steps).
Finally, the evaluation methods are categorized according to five levels: (i) descriptive stat, where evaluation was carried out through descriptive statistics only; (ii) descriptive stat + test, where evaluation was carried out through descriptive statistics with statistical tests; (iii) linear models + stat test, where evaluation was carried out through linear models with statistical tests; (iv) machine learning, where evaluation was carried out through machine learning only; and (v) machine learning + stat test, where evaluation was carried out through machine learning with statistical tests.
3. Results
In this section, we analyze the selected papers by categorizing them following different criteria in order to extract common patterns and trends.
3.1. Literature Search
Figure 1 details the entire process of paper selection for this review. The literature search (made from the queries given in Table A1 of Appendix A) produced 564 research articles, with 118 duplicates, resulting in 446 articles to be screened. After an initial screening, which consisted of reviewing all article titles and abstracts, the full content of 102 of these articles was screened in more detail for eligibility. After removing the articles that did not meet the inclusion criteria detailed in Section 2.3, 70 articles were deemed eligible for the review [34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103].
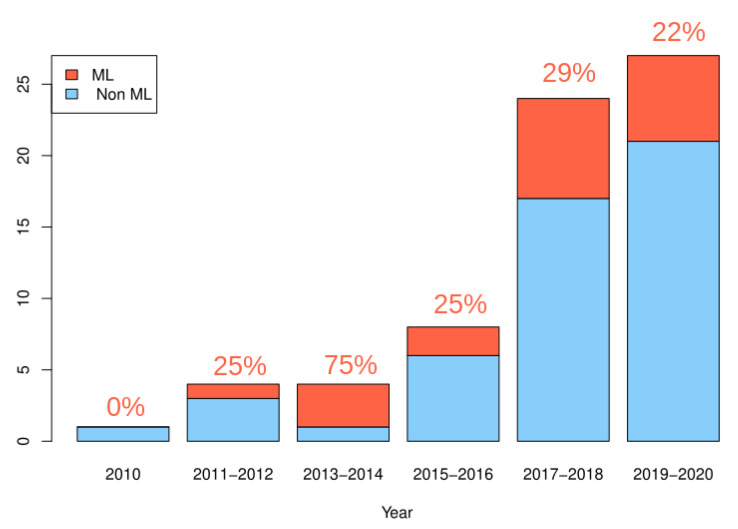
The number of studies related to the issue of validation on sensors used for patient monitoring has significantly increased since 2010, with a number of papers between 2017 and 2020, more than twice the number of papers between 2010 and 2017 (see Figure 2). Studies using machine learning as a validation method also became more numerous since 2010 [34,35,36,38,45,53,60,63,68,69,70,77,79,80,81,86,95,97], with a stable proportion compared to the total number of studies per year.
Figure 2.
Evolution of the number of papers considering the issue of validation for the use of commercial wearable devices in chronic disease monitoring, with a distinction between papers using machine learning (in red) or not (in blue). The percentages given in red represent the proportion of studies using machine learning.
3.2. Clinical Context
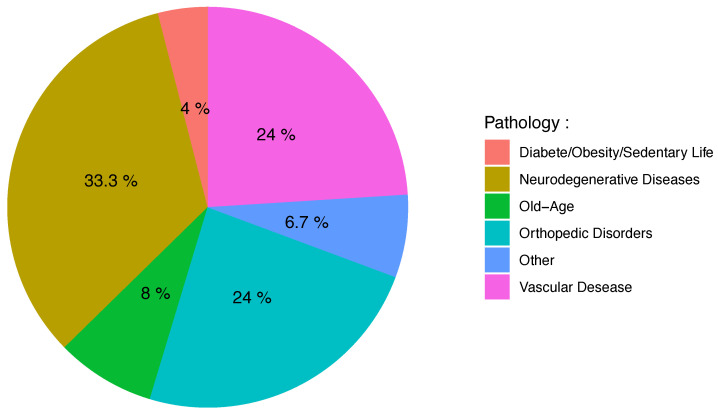
The sample size of the studies ranged from 1 to 130 participants, with a mean of 37.89 participants (SD = 30.68) per study. The duration of data collection in two different conditions (laboratory or free living) varied and was not always reported with an exact numerical value or unit. Therefore, in Table 1, we only report the ranges of acquisition times that go from hours to years. Among the selected studies, as displayed in Figure 3, 33% (N = 25) focused on neurodegenerative diseases [35,36,37,39,44,50,54,55,57,58,60,61,63,70,72,77,79,80,81,86,90,92,94,98,103], 24% (N = 18) focused on orthopedic disorders [34,47,52,59,65,71,73,75,76,78,83,85,89,91,96,97,99,101], 24% (N = 18) focused on diseases of vascular origin [40,43,45,48,49,51,52,53,62,64,67,68,69,87,91,95,99,102], 8% (N = 6) focused on aging and associated pathologies [38,56,66,88,91,100], and 4% (N = 3) focused on diseases associated with poor lifestyle [42,62,74]. Finally, five studies were classified as “others” [41,46,82,84,93] because they could not be grouped together in an existing group.
Table 1.
Frequency of studies according to conditions of data collection (laboratory or free living) and acquisition time t (from a few minutes to more than a year).
| Acquisition Time |
t < 1 h | 1 ≤ t < 24 h | 1 ≤ t < 7 d | 1 ≤ t < 4 w | 1 ≤ t < 12 m | t ≤ 1 y |
|---|---|---|---|---|---|---|
| Laboratory (N = 53) |
46 | 3 | 0 | 1 | 2 | 1 |
| Free Living (N = 17) |
1 | 1 | 1 | 8 | 3 | 3 |
Figure 3.
Pie chart representing the frequency of pathology types in included studies.
3.3. Wearable Sensor Types
As detailed in Table 2, the most frequently used type of wearable device is the Inertial Measurement Unit (IMU; N = 39) [34,37,44,46,52,54,55,56,57,58,60,61,62,63,66,71,72,73,75,78,79,81,82,83,84,87,88,90,91,92,93,94,95,97,98,99,100,101,102], and then, almost equally, the smartphone (N = 18) [38,39,40,41,42,43,45,47,51,64,68,69,70,76,77,86,89,103] and a single sensor (N = 17) [35,36,38,40,48,49,50,53,59,65,67,69,74,80,85,96,103]. The majority of studies (N = 56) [34,35,36,37,38,40,43,44,48,49,51,52,53,54,55,56,57,58,60,61,62,63,65,66,67,69,70,71,72,73,74,75,77,78,79,80,81,82,83,84,85,87,88,89,90,91,92,93,95,97,98,99,100,101,102,103] used multi-sensor systems (incorporating more than one sensor) to automatically assess gait in chronic pathologies. On average, 5.78 wearable sensors (SD = 8.43) were used in the studies, with a range of 1 to 64 sensors (see Table 2). As depicted in Table 3, the most commonly utilized sensor was an accelerometer (95%) either by itself (N = 17) or embedded into a device (N = 57). The second most frequently used sensor was a gyroscope (51%) followed by magnetometer (14%) and others (16%).
Table 2.
Criteria related to commercial wearable devices through the 70 selected papers. Abbreviations used in the column “No. of device(s)”: IMU (Inertial Motion Unit), S (Sensor), and SPHN (Smartphone). Abbreviations used in the column “Sensor Type(s)”: A (accelerometer), G (gyroscope), M (magnetometer), and O (others).
| Author | No. of Device(s) | Sensor Type(s) | Location of Device(s) | Sensor Model, Brand |
|---|---|---|---|---|
| Salarian et al. [90] | 7 (IMU) | A,G | Forearms, shanks, thighs, sternum | Physilogs, BioAGM |
| Dobkin et al. [53] | 2 (S) | A | Both ankles | GCDC, LLC |
| Kozey-Keadle et al. [74] | 2 (S) | A | Right leg, right side of the hip | activPAL, PALF GT3X, ActiGraph |
| Munguía-Izquierdo et al. [82] | 1 (IMU) | A,O | Arm | SenseWear, Bodymedia |
| Item-Glatthorn et al. [65] | 5 (S) | A | Chest, thigh, forefoot | MiniSun, IDEEA |
| Grimpampi et al. [61] | 1 (IMU) | A,G | Lumbar spine | Freesense, Sensorize |
| Schwenk et al. [92] | 1 (IMU) | A,G | Chest | Physilog, GaitUp |
| Juen et al. [68] | 1 (SPHN) | A | Pants pocket or fanny pack | Galaxy Ace, Samsung |
| Juen et al. [69] | 2 (SPHN and S) | A | L3 vertebra | Galaxy Ace/4, Samsung |
| Sprint et al. [95] | 3 (IMU) | A,G | Lumbar spine, shank | Shimmer3, Shimmer |
| Capela et al. [43] | 1 (SPHN) | A,G,M | Rear pocket | Z10, BlackBerry |
| Schwenk et al. [93] | 5 (IMU) | A,G,M | Shank, thigh, lower back | LegSys, BioSensic |
| Isho et al. [64] | 1 (SPHN) | A | Torso | Xperia Ray SO-03C, Sony |
| Wuest et al. [102] | 8 (IMU) | A,G | Wrists, shanks, trunk, feet, back | Physilog, GaitUp |
| Raknim et al. [86] | 1 (SPHN) | A | Free (pocket, during phone call, on the bag during walk) |
HTC and Samsung |
| Ferrari et al. [57] | 2 (IMU) | A,G | Shoes | EXLs1 and EXLs3, EXEL |
| Brinkløv et al. [42] | 1 (SPHN) | A | Pants pocket, jacket pocket | Iphone 5C, Apple |
| El-Gohary et al. [54] | 3 (IMU) | A,G | Lumbar vertebra, feet, ankles | Opal, APDM |
| Ilias et al. [63] | 4 (IMU) | A,G | Upper, lower limbs, wrists, legs | Shimmer3, Shimmer |
| Maqbool et al. [78] | 1 (IMU) | A,G | Shank | MPU 6050, InvenSense |
| Terrier et al. [96] | 1 (S) | A | Right hip | wGT3X-BT, ActiGraph |
| Rogan et al. [88] | 1 (IMU) | A,G | Lateral malleolus | RehaWatch, Hasomed |
| Chiu et al. [47] | 1 (SPHN) | A | Shin | Zenfone 2, ASUS |
| Cheng et al. [45] | 1 (SPHN) | A | Carried in fanny pack | Galaxy S5, Samsung Optimus Zone2, LG |
| Kobsar et al. [73] | 4 (IMU) | A,G | Foot, shank, thigh, lower back | iNEMO, STmicroelectronics |
| McGinnis et al. [79] | 5 (IMU) | A | Sacrum, thighs, shanks | BioStampRC, MC10 |
| Lipsmeier et al. [77] | 1 (SPHN) | A,G,M,O | Hand, trouser pocket, belt | Galaxy S3 mini, Samsung |
| Kleiner et al. [72] | 1 (IMU) | A,G,M | L5 verterbra | BTS G-walk, BTS G-Sensor |
| Carpinella et al. [44] | 1 (IMU) | A,G,M | Sternum | MTw, Xsens |
| Jayaraman et al. [67] | 4 (S) | A,O | Arm, waist, ankle | wGT3X-BT, ActiGraph Metria-IH1, Vandrico |
| Jang et al. [66] | 1 (IMU) | A,O | Wrist | Mi band 2, Xiaomi |
| Derungs et al. [52] | 6 (IMU) | A,G,M | Wrists, arms, thighs | Shimmer3, Shimmer |
| Mileti et al. [81] | 10 (IMU and S) | A,G,M,O | Feet | Mtw, MTw, Xsens |
| Aich et al. [35] | 2 (S) | A | Knees | Fit Meter, Fit.Life |
| Cheong et al. [46] | 1 (IMU) | A | Wrists | Urban S, Partron Co |
| Ata et al. [40] | 2 (SPHN and S) | A | Hand, hip | iPhones SE/6/7/7+, Apple GT9X, ActiGraph |
| Kim et al. [70] | 3 (SPHN) | A,G | Waist, pocket, ankle | Nexus 5, Google |
| Vadnerkar et al. [100] | 1 (IMU) | A,G | Feet | Shimmer 2r, Shimmer |
| Rosario et al. [51] | 1 (SPHN) | A,G | Trouser pocket | Galaxy S3, Samsung |
| Lemoyne et al. [76] | 1 (SPHN) | A | Malleolus | iPhone, Apple |
| Dasmahapatra et al. [50] | 1 (S) | A | Belt, pocket, or bra | Fitbit One, Fitbit |
| Schliessmann et al. [91] | 2 (IMU) | A,G,M | Feet | RehaGait, HASOMED GmbH |
| Ummels et al. [99] | 9 (IMU and S) | other | Leg, belt, wrist | UP24, Jawbone Lumoback, Lumo Bodytech Moves, ProtoGeo Oy Accupedo, Corusen LLC Walking Style X, Omron |
| Banky et al. [41] | 1 (SPHN) | G | Galaxy S5, Samsung | |
| Flachenecker et al. [58] | 2 (IMU) | A,G | Shoes | Shimmer 3, Shimmer |
| Gadaleta et al. [60] | 3 (IMU) | A,G,M | L5 lumbar vertebrae, ankles | Opal, APDM |
| Teufl et al. [97] | 7 (IMU) | A,G | Pelvis, both foot, both thighs | MTw Awinda, Xsens |
| Angelini et al. [37] | 3 (IMU) | A,G | L5 lumbar vertebra, ankles | MTw Xsens Opal, APDM |
| Antos et al. [38] | 2 (S and SPHN) | A,G | Waist, wrist | Nexus 5, Google wGT3X-BT, Actigraph |
| Compagnat et al. [48] | 9 (S) | A,O | Wrists, ankles, hip, arm, neck | GT3x, Actigraph Sensewear, Body Media |
| Newman et al. [84] | 1 (IMU) | A,G | Interclavicular notch | Opal, APDM |
| Ullrich et al. [98] | 3 IMU | A,G | Ankles, shoes | Shimmer2R, Shimmer |
| Wang et al. [101] | 2 (IMU) | A,G | Pectoralis major | BioStampRC, MC10 |
| Pavon et al. [85] | 2 (S) | A | Ankle | GT3x+, ActiGraph |
| Arcuria et al. [39] | 1 (SPHN) | A | Breastbone | Galaxy J3, Samsung |
| Erb et al. [55] | 7 to 16 (IMU) | A,G,M,O | Wrists, torso, thigh, feet | Shimmer, Shimmer |
| Aich et al. [36] | 2 (S) | A | Knees | Fit Meter, Fit. Life |
| Rubin et al. [89] | 1 (SPHN) | A,G | Pants pocket, belt | iPhone 6, Apple |
| Henriksen et al. [62] | 1 (IMU) | A,O | Wrist | M430 AT, Polar |
| Shema-Shiratzky et al. [94] | 1 (IMU) | A | Lower Back | Opal, APDM and AX3, Axivity |
| Abdollahi et al. [34] | 1 (IMU) | A,G | Sternum | 9DOF Razor IMU, Sparkfun |
| Kim et al. [71] | 2 (IMU) | A,G | Shoe, ankle | GT9X Link, ActiGraph |
| Lemay et al. [75] | 5 (IMU) | A,G,O | Feet, shanks, sacrum | Physilog, GaitUp |
| Meisel et al. [80] | 1 (S) | A,O | Wrist or ankle | E4, Empatica |
| Fantozzi et al. [56] | 5 (IMU) | A,G,M | Trunk, pelvis, thigh, shank, foot | Opal, APDM |
| Zhai et al. [103] | 2 (SPHN and S) | A | Wrist, pocket | Galaxy S4 mini, Samsung GT3X+, ActiGraph |
| Revi et al. [87] | 3 (IMU) | A | Shank, thigh, pelvis | MTw Awinda, Xsens |
| Compagnat et al. [49] | 2 (S) | A | Non-paretic hip | GT3x, ActiGraph |
| Furtado et al. [59] | 1 (S) | A | L5 lumbar vertebrae within the pocket of a belt |
AX3, Axivity |
| Na et al. [83] | 5 (IMU) | A,G | Femur, tibia, pelvis, sacral ridge | 3D Myomotion, Noraxon |
Table 3.
Frequency of devices and sensor types in included studies. The device is the tracker used by the patient (first column), which may include different sensors that are detailed in the second column. Note that, since a device can use several sensors, the total number of occurrences in the second column is much greater than that of the first column.
| Device Type | Sensor Type | ||
|---|---|---|---|
| IMU | 39 | Accelerometer | 39 (100%) |
| Gyroscope | 30 (77%) | ||
| Magnetometer | 8 (20%) | ||
| Others | 7 (18%) | ||
| Sensors | 17 | Accelerometer | 14 (82%) |
| Gyroscope | 1 (0.7%) | ||
| Magnetometer | 1 (0.7%) | ||
| Others | 4 (3%) | ||
| Smartphones | 18 | Accelerometer | 17 (94%) |
| Gyroscope | 7 (38%) | ||
| Magnetometer | 2 (11%) | ||
| Others | 1 (5%) | ||
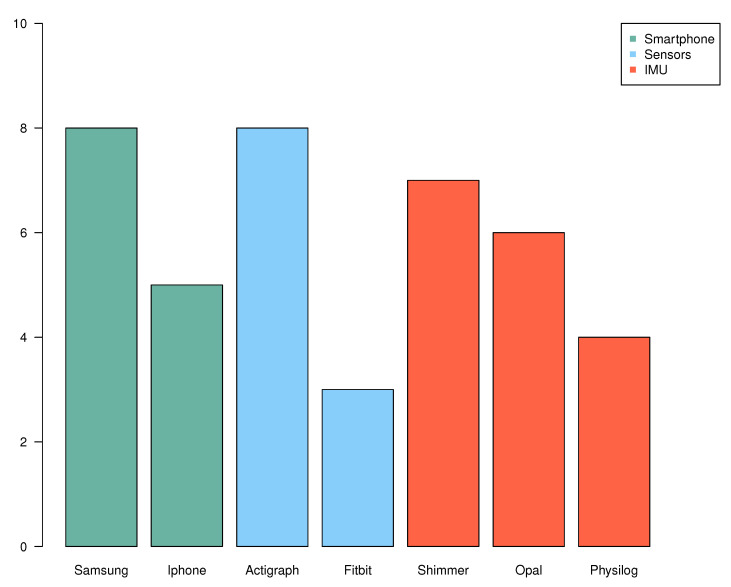
Figure 4 reports the different brands used for smartphones, sensors, and IMUs. Regarding smartphones, Samsung [41,45,51,68,69,77,86,103] and iPhone [40,42,69,76,89] are the most represented, certainly because of their health applications made for gait recording. Actigraph is the most commonly used brand for sensors [38,40,48,49,67,71,74,85,96,103]. Regarding the different brands in IMU, there is no particular brand that stands out.
Figure 4.
Frequencies of the most used brands (number of occurrences > 3) by type of device (smartphone, sensor, and IMU). Among smartphones, seven papers used Samsung and five used iPhone (bars in green). Among sensors, eight papers used Actigraph and three used Fitbit (bars in blue). Finally, among IMUs, seven papers used Shimmer, six papers used Opal, and four used Physiolog (bars in red).
3.4. Data-Acquisition Conditions
Most of the papers collected their data in laboratory conditions (N = 53) [34,35,36,37,38,39,40,41,42,43,44,45,47,48,49,54,56,57,58,60,61,63,64,65,67,68,69,70,71,72,73,75,76,78,79,80,81,82,83,84,85,87,88,89,90,91,92,95,97,99,100,101,102], while a smaller part collected data in free living conditions (N = 17) [46,50,51,59,77,85,86,96,103] (see Table 1).
Regarding the positioning of sensors and/or devices (Table 4), 60% of the studies placed them on an inferior part of the body [35,36,37,40,47,48,49,52,53,54,55,56,57,58,60,62,63,67,70,71,73,74,75,76,78,79,80,81,83,85,87,88,90,91,92,95,96,97,98,99,100,102,103], generally on the feet (N = 14) or on the hips (N = 6). The chest was also widely used (49%) [34,37,38,39,44,48,50,54,55,56,59,60,61,64,65,67,70,72,73,75,77,79,83,84,89,90,92,93,94,95,97,99,101,102]; 17% of the studies carried out sensor positioning on the hands and arms [38,40,46,48,52,63,66,67,77,80,82,90,102], while the other 17% used a trouser or jacket pocket [42,43,45,50,51,59,68,70,77,86,89,103]
Table 4.
Frequency of sensor locations reported on the patient from the included studies. These different locations were classified into the four categories described in Section 2.6.
| Superior | Inferior | Chest | Free |
|---|---|---|---|
| 12 | 42 | 34 | 12 |
3.5. Gait Indicators
The majority (70%) of studies (see Table 5) used high-level features for gait analysis [35,36,37,39,40,43,44,45,46,48,49,50,51,54,55,56,57,58,59,62,65,66,67,71,72,74,75,76,77,78,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,99,102], which can be correlated to the high use of smartphones (in the studies reviewed; see Table 3) that already compute this type of features on the device.
Table 5.
Frequency of features extracted from sensor signal reported from the included studies. These different features were classified into the three categories described in Section 2.6.
| Low Level | Medium Level | High Level | |||
|---|---|---|---|---|---|
| Total | 6 | Total | 20 | Total | 49 |
| Magnitude mean | 11 | Step length | 20 | ||
| Magnitude standard deviation | 10 | Number of steps | 18 | ||
| Peak frequency | 9 | Cadence | 15 | ||
| Mean crossing rate | 5 | Speed | 11 |
A significative part of the studies (28%) used medium-level features [34,38,42,45,47,52,53,59,61,63,64,68,69,70,73,79,98,101,103], while low-level features (raw data) are much less exploited (8%) [41,60,61,80,81,100].
3.6. Ground Truth
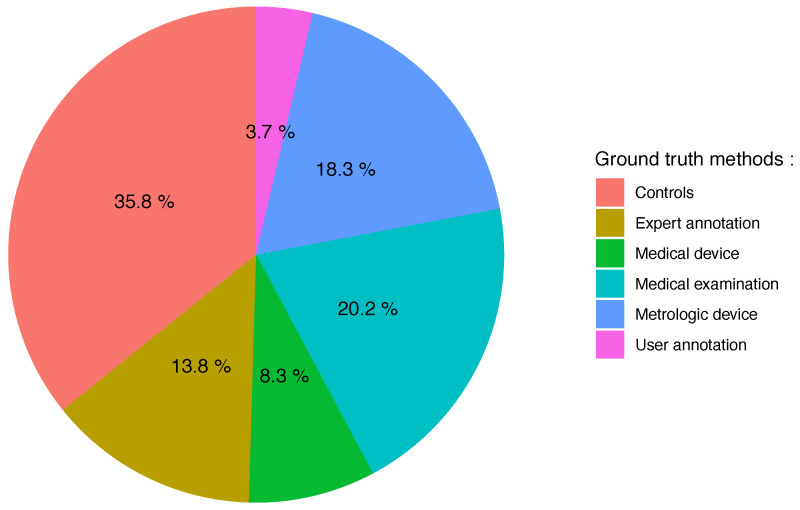
To evaluate the validity of commercial wearable sensors for gait monitoring in patients, all of the studies (N = 70) used one or more validation methods in which the “ground truth” data were reported. As illustrated in Figure 5, about half of the studies (53.3%) use annotations and the other half (46.7%) use a reference to validate the results from the sensors. Regarding annotations, most studies use labeling according to two or more groups of subjects (the vast majority of the time, a group of patients and healthy controls) [35,36,37,38,39,41,44,46,47,50,51,53,54,56,58,59,64,66,71,75,76,77,78,79,81,83,84,85,86,90,91,92,93,94,96,97,101,102,103], others use annotations made by experts on data from videos or measurements during the experiment [37,38,40,43,48,52,55,63,67,70,74,80,94,98,100], and four studies [55,64,92,93] had participants self-report via a log or diary. With regard to the reference with which the studies compare the data from the sensors, it concerns a metrological device (18.3%) [35,36,39,41,49,53,54,57,60,61,65,67,72,78,79,83,87,96,97,99] or a medical examination (20.2%) [34,36,39,44,45,50,51,58,59,68,72,73,75,77,81,84,85,90,95,96,102,103] in equal parts and, to a lesser extent (8.3%), a third-party portable medical device [40,42,45,49,62,69,82,89,103].
Figure 5.
Pie chart representing the frequency of different ground-truth methods identified among the 70 selected papers. These different levels correspond to the categories described in Section 2.6.
3.7. Evaluation Methods and Metrics
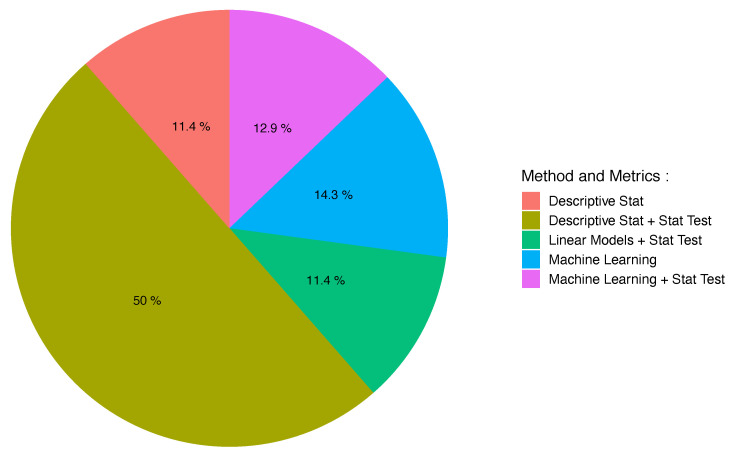
The studies often reported multiple and varied evaluation metrics. All reported evaluation outcomes and their corresponding evaluation method are included in Table 6 and depicted in Figure 6. The most common evaluation method was descriptive statistics (61.4%) including or not statistical tests [37,39,40,41,44,46,48,49,51,54,55,58,59,61,62,65,66,67,71,72,74,76,78,82,83,84,85,87,88,89,90,91,92,94,98,99,101,102,103] where correlations, mean errors, or p-values are most commonly reported. The other evaluation methods present models either as a linear model (11.4%) [42,50,52,56,57,73,75,93,96,100] or as a machine learning model (17.2%) [34,35,36,38,45,53,60,63,68,69,70,77,79,80,81,86,95,97]. Due to the lack of a standardized evaluation metric across studies, we do not summarize (calculate mean, standard deviation, etc.) the reported metrics. However, evaluation metric values—as given in the abstract or the conclusion of the associated studies—are available in Table 6.
Table 6.
Evaluation criteria through the 70 selected papers. Abbreviations used in the column “Evaluation method”: stats (descriptive statistics), stats + test (descriptive statistics + statistical tests), LM + test (linear models + statistical tests), ML (machine learning), and ML+test (machine learning + statistical tests). Abbreviations used in the column “Evaluation outcomes”: r (correlation coefficient), (coefficient of determination), ICC (intraclass correlation coefficient), AUC (area under curve, sen (sensitivity), spe (specificity), IQR (interquartile range), FN (false negatives), FP (false positives), and acc (accuracy).
| Author | Ground-Truth Method | Gait Descriptors | # of Descriptors | Evaluation Method | Evaluation Outcomes |
|---|---|---|---|---|---|
| Salarian et al. [90] | controls, medical | high | 20 | stats + test | p-value < 0.023 |
| Dobkin et al. [53] | controls, metrologic | medium | 8 | ML + test | r = 0.98 |
| Kozey-Keadle et al. [74] | expert | high | 3 | stats | = 0.94 |
| Munguía-Izquierdo et al. [82] | med device | high | 1 | stats + test | r = 0.87–0.99 |
| Item-Glatthorn et al. [65] | metrologic | high | 6 | stats + test | = 0.815–0.997 |
| Grimpampi et al. [61] | metrologic | low, medium | 3 | stats + test | r = 0.74–0.87 |
| Schwenk et al. [92] | controls, user | high | 9 | stats + test | = 0.77, sen/spe = 72%/76% |
| Juen et al. [68] | medical | medium | 8 | ML | acc = 89.22–94.13% |
| Juen et al. [69] | med device | medium | 9 | ML | error < 10.2% |
| Sprint et al. [95] | medical | medium,high | 18 | ML + test | r = 0.97 |
| Capela et al. [43] | expert | high | 10 | stats | time difference = 0.014 s |
| Schwenk et al. [93] | controls, user | high | 6 | LM + test | p-value < 0.022 |
| Isho et al. [64] | controls, user | medium | 3 | ML + test | = 0.745 |
| Wuest et al. [102] | controls, medical | high | 13 | stats + test | p-value < 0.02 |
| Raknim et al. [86] | controls | high | 2 | ML | acc = 94% |
| Ferrari et al. [57] | metrologic | high | 4 | LM + test | error = 2.9% |
| Brinkløv et al. [42] | med device | medium | 6 | LM + test | = 0.45–0.60 |
| El-Gohary et al. [54] | metrologic, controls | high | 7 | stats + test | r = 0.592–0.992 |
| Ilias et al. [63] | expert | medium | 152 | ML + test | r = 0.78–0.79 |
| Maqbool et al. [78] | metrologic, controls | high | 1 | stats | time difference = 50 ms |
| Terrier et al. [96] | controls, medical | high | 4 | LM + stats | = 0.44 |
| Rogan et al. [88] | metrologic | high | 6 | stats + test | p-value < 0.05 |
| Chiu et al. [47] | controls | medium | 1 | stats + test | p-value < 0.027 |
| Cheng et al. [45] | med device, medical | medium,high | 10 | ML | NA |
| Kobsar et al. [73] | medical | medium | 38 | LM + test | acc = 74–81.7% |
| McGinnis et al. [79] | metrologic, controls | medium | 32 | ML + test | speed difference = 0.12–0.16 m/s |
| Lipsmeier et al. [77] | controls, medical | high | 6 | ML + test | p-value < 0.055 |
| Kleiner et al. [72] | metrologic, medical | high | 1 | stats | time difference = 0.585 s |
| Carpinella et al. [44] | medical, controls | high | 5 | stats + test | r = −0.367–0.536 |
| Jayaraman et al. [67] | expert, metrologic | high | 3 | stats + test | p-value < 0.05 |
| Jang et al. [66] | controls | high | 5 | stats + test | p-value < 0.02 |
| Derungs et al. [52] | expert | medium | 8 | LM + test | sen/spe = 80%/94% |
| Mileti et al. [81] | controls, medical | low | 3 | ML + test | = 0.48–0.98 |
| Aich et al. [35] | metrologic, controls | high | 28 | ML | acc = 88% |
| Cheong et al. [46] | controls | high | 1 | stats + test | p-value < 0.04 |
| Ata et al. [40] | expert, med device | high | 3 | stats | = 0.9–0.92 |
| Kim et al. [70] | expert | medium | 8 | ML | sen/spe = 93.8%/90.1% |
| Vadnerkar et al. [100] | expert | low | 1 | LM + test | acc = 84%, sen/spe = 75.9%/95.9% |
| Rosario et al. [51] | controls, medical | high | 2 | stats + test | r = 0.472 |
| Lemoyne et al. [76] | controls | high | 5 | stats + test | p-value < 0.05 |
| Dasmahapatra et al. [50] | controls, medical | high | 6 | LM + test | p-value < 0.05 |
| Schliessmann et al. [91] | controls | high | 4 | stats + test | p-value < 0.05 |
| Ummels et al. [99] | metrologic | high | 1 | stats + test | r = −0.02–0.33 |
| Banky et al. [41] | metrologic, controls | low | 3 | stats + test | r=0.8 |
| Flachenecker et al. [58] | controls, medical | high | 8 | stats + test | r = −0.583–0.668 |
| Gadaleta et al. [60] | metrologic | low | 24 | ML | bias = −0.012–0.000, IQR = 0.004–0.032 |
| Teufl et al. [97] | metrologic, controls | high | 10 | ML + test | acc = 0.87–0.97 |
| Angelini et al. [37] | expert, controls | high | 14 | stats + test | p-value < 0.05 |
| Antos et al. [38] | expert, controls | medium | 56 | ML + test | acc = 0.90–0.95 |
| Compagnat et al. [48] | expert | high | 2 | stats + test | p-value < 0.05 |
| Newman et al. [84] | controls, medical | high | 9 | stats + test | p-value < 0.05 |
| Ullrich et al. [98] | expert | medium | 7 | stats + test | sen/spe = 98%/96% |
| Wang et al. [101] | controls | medium | 1 | stats + test | p-value < 0.05 |
| Pavon et al. [85] | controls, medical | high | 3 | stats + test | p-value < 0.16 |
| Arcuria et al. [39] | metrologic, controls, medical | high | 1 | stats + test | r = −0.72–0.91 |
| Erb et al. [55] | user, expert | high | 2 | stats + test | FN = 35%, FP = 15% |
| Aich et al. [36] | metrologic, controls, medical | high | 5 | ML | acc = 88.46% |
| Rubin et al. [89] | med device | high | 1 | stats + test | = 0.72 |
| Henriksen et al. [62] | med device | high | 4 | stats | r = 0.446–0.925 |
| Shema-Shiratzky et al. [94] | controls, expert | high | 5 | stats + test | p-value < 0.05 |
| Abdollahi et al. [34] | medical | medium | 920 | ML | acc = 60–75% |
| Kim et al. [71] | controls | high | 5 | stats + test | p < 0.05 |
| Lemay et al. [75] | medical, controls | high | 6 | LM + test | r = −0.49–0.498 |
| Meisel et al. [80] | expert | low | 6 | ML + test | acc = 43% |
| Fantozzi et al. [56] | controls | high | 14 | LM + test | NA |
| Zhai et al. [103] | med device, controls, medical | medium | 14 | stats + test | r = 0.43–0.605 |
| Revi et al. [87] | metrologic | high | 8 | stats | = 0.90–0.93 |
| Compagnat et al. [49] | med device | high | 1 | stats + test | r = 0.44–0.87 |
| Furtado et al. [59] | metrologic, controls, medical | medium,high | 10 | stats + test | p-value < 0.024 |
| Na et al. [83] | metrologic, controls | high | 6 | stats + test | p-value < 0.04 |
Figure 6.
Pie chart representing the percentage of papers using different levels of evaluation identified among the 70 selected papers. These different levels correspond to the categories described in Section 2.6.
A closer look at the studies using ML highlights that machine learning-based approaches are often used for high-level validation tasks (see Table 7), such as distinguishing between different groups of patients or stages of disease progression [34,35,36,45,68,70,80,86,97]. This is an important point because ML aims to generalize a model to patients not included in the initial data set. Another point to emphasize, as illustrated in Table 8, is that studies using machine learning as a validation method incorporate a large number of variables (the complete raw signal or a collection of different sensors) [34,60,63,70,77,80,81]. This is not the case in studies using statistical methods that work with a few dozen variables at the maximum and often in a uni-variate way two by two [37,56,59,90,102,103].
Table 7.
Selection of papers that use machine learning methods in validation. Abbreviations used in the column “Model type”: SVM (support vector machine), GPR (gaussian process regression), NN (neural network), RF (random forest), LSTM (long short time memory), HMM (hidden markov model), kNN (k-nearest neighbors), CNN (convolutional neural network), ROC (receiver operating characteristic), and LDA (linear discriminant analysis). Abbreviations used in the column “Outcome”: r (correlation coefficient), NRMSE (normalized root mean square error), RMSE (root mean square error), AUC (area under curve), sens (sensitivity), spe (specificity), and IQR (interquartile range). Studies that use raw data as input have a number of descriptors that correspond to the number of sensors and/or axes multiplied by the length of the recorded data. This is noted (*n) in the table.
| Author | Task | Model Type | Training Size | # of Descriptors | Outcome |
|---|---|---|---|---|---|
| Dobkin et al. [53] | Speed prediction | Naive Bayes | NA | 24 | r = 0.98 |
| Juen et al. [68] | Healthy/patient | SVM | 10–20 | 8 | accuracy = 89.22–94.13% |
| Juen et al. [69] | Speed prediction Distance prediction |
GPR NN SVM |
24 | 60 | error rate = 2.51% error rate = 10.2% |
| Sprint et al. [95] | FIM motor score prediction | SVM RF |
19 | 18 | NRMSE = 10–30% |
| Raknim et al. [86] | Step length estimation Before/after PD |
SVM | 1 | 2 | accuracy = 98% accuracy = 94% |
| Ilias et al. [63] | Motor function prediction | SVM | 6 | 152 | RMSE = 0.46-0.70 r = 0.78–0.79 |
| Cheng et al. [45] | 3 pulmonary severity stages | SVM | 22–25 | 10 | NA |
| McGinnis et al. [79] | Walking speed | SVM | 16 | 32 | RMSE = 10–20% |
| Lipsmeier et al. [77] | Activities | LSTM | 44 | 6 (*n) | accuracy = 98% |
| Mileti et al. [81] | 4 gait phases | HMM | 1–11 | 3 (*n) | = 0.48–0.98 sens= 80–100% spe = 70–90% |
| goodness Index = 10–40% | |||||
| Aich et al. [35] | Healthy/patient | SVM Decision tree Naive Bayes kNN | 36 | 28 | accuracy=91.42% sens/spe = 90.9%/91.2% |
| Kim et al. [70] | Walking/freezing | CNN | 29 | 8 (*n) | f1-score = 91.8 sen/spe = 93.8%/90.1% |
| Vadnerkar et al. [100] | Gait quality | ROC decision boundary | 8 | 1 | accuracy = 84% sen/spe = 75.9%/95.9% |
| Gadaleta et al. [60] | Right/left foot events | CNN | 138 | 24 (*n) | bias = −0.012–0.000 IQR = 0.004–0.032 |
| Teufl et al. [97] | Healthy/patient | SVM | 40 | 10 | accuracy = 87–97% |
| Antos et al. [38] | With/without assistance | RF SVM Naive Bayes Logistic regression LDA | 1–13 | 56 | accuracy = 90–95% |
| Aich et al. [36] | Healthy/patient | kNN SVM Naive Bayes Decision tree | 62 | 10 | accuracy = 88.5% sens/spe = 92.9%/90.9% |
| Abdollahi et al. [34] | Risk of disability | SVM Perceptron | 93 | 920 | accuracy = 60–75% |
| Meisel et al. [80] | Seizure/healthy | LSTM | 68 | 6 (*n) | accuracy = 43% |
Table 8.
Frequency of studies using less than 10 descriptors, between 10 and 100 descriptors and more than 100 descriptors for the validation of both statistical and ML methods.
| Number of Studies | <10 | 10–100 | >100 |
|---|---|---|---|
| Statistical | 43 | 8 | 0 |
| ML | 3 | 9 | 7 |
3.8. Summary of Key Findings
This scoping review included 70 studies related to the validation of commercial wearable sensors to automatically monitor gait in patients published between 2010 and 2020. The majority of studies (95%) used accelerometers either by itself (N = 17 of 70) or embedded into a device (N = 57 of 70), and/or gyroscopes (51%) to automatically monitor gait via wearable sensors. Labeling according to two groups (group of patients and healthy controls) was the most frequently used method (N = 39 of 70) for annotating ground-truth gait data, followed by annotations made by experts on data from videos or measurements during the experiment (N = 15 of 70) and patient self-reports (N = 4 of 70). The references against which the sensor data were compared were a metrological device and a medical examination in equal parts and, to a lesser extent, a third-party portable medical device. Finally, studies using machine learning as a validation method have become more numerous since 2010, at 17% of included studies.
4. Discussion
Gait monitoring of patients during daily life using commercial wearable sensors is a growing field and offers novel opportunities for future public health research. However, despite their rapid expansion, the use of commercial wearable sensors remains contested in the medical community: objections concern the quality of the data collected as well as the reliability of the technologies in a clinical context where the pathologies are diverse and sometimes combined [104]. Previous literature reviews on the validation of wearable sensors were interested in monitoring activity on healthy subjects [15,20,21,22] or have often placed a focus on a very specific medical application [18,23,24]. No review to date has focused on studies using wearable devices in a very general way to automatically detect gait in patients in their daily life and via machine learning, which is an approach increasingly used to learn a recognition task from data. By examining the validation methods and performances of wearable devices and sensors that automatically monitor patient gait, several major trends and challenges can be identified.
4.1. Trends and Challenges
Acquisition context. Most of the first studies were restricted to the laboratory environment and over short acquisition times (of the order of a few minutes). The first papers to report sensor validation in a free living environment were in 2011 [53,74]. As seen in Table 9, from 2017, studies of this type become more frequent [46,50,51,52,55,59,62,66,77,86,94,96,98,103] due to changes in the sensors, which are detailed in the following section.
Table 9.
Data acquisition criteria through the 70 selected papers. Abbreviations used in the column “Duration of data collection”: min (t <1 h), hours (1 ≤ t < 24 h), days (1 ≤t< 7 days), weeks (1 ≤ t < 4 weeks), months (1 ≤ t < 12 months), and year (t ≥ 1 year). Finally, the cohort size is given as the number of patients.
| Author | Year | Pathology | Cohort Size | Duration of Data Collection | Condition Data Collection |
|---|---|---|---|---|---|
| Salarian et al. [90] | 2010 | Parkinson | 12 | min | Laboratory |
| Dobkin et al. [53] | 2011 | Stroke | 12 | min (Lab), days (FL) |
Both |
| Kozey-Keadle et al. [74] | 2011 | Obesity | 20 | hours | Free living |
| Munguía-Izquierdo et al. [82] | 2012 | Fibromyalgia | 25 | min | Laboratory |
| Item-Glatthorn et al. [65] | 2012 | Osteoarthritis | 26 | min | Laboratory |
| Grimpampi et al. [61] | 2013 | Hemiplegia/Parkinson | 24 | min | Laboratory |
| Schwenk et al. [92] | 2014 | Dementia | 77 | days | Free living |
| Juen et al. [68] | 2014 | Lung disease | 30 | min | Laboratory |
| Juen et al. [69] | 2014 | Lung disease | 25 | min | Laboratory |
| Sprint et al. [95] | 2015 | Diverse | 20 | min | Laboratory |
| Capela et al. [43] | 2015 | Lung disease | 15 | min | laboratory |
| Schwenk et al. [93] | 2016 | Cancer | 22 | hours | laboratory |
| Isho et al. [64] | 2015 | Stroke | 24 | min | Laboratory |
| Wuest et al. [102] | 2016 | Stroke | 26 | min | Laboratory |
| Raknim et al. [86] | 2016 | Parkinson | 1 | years | Free living |
| Ferrari et al. [57] | 2016 | Parkinson | 14 | min | Laboratory |
| Brinkløv et al. [42] | 2016 | Diabete | 27 | min | Laboratory |
| El-Gohary et al. [54] | 2017 | Multiple sclerosis | 52 | min | Laboratory |
| Ilias et al. [63] | 2017 | Parkinson | 19 | min | Laboratory |
| Maqbool et al. [78] | 2017 | Amputee | 2 | min | Laboratory |
| Terrier et al. [96] | 2017 | Chronic Pain | 66 | weeks | Both |
| Rogan et al. [88] | 2017 | Old Age | 23 | min | Laboratory |
| Chiu et al. [47] | 2017 | Ankle instability | 15 | min | Laboratory |
| Cheng et al. [45] | 2017 | Cardiopulmonary disease | 25 | min | Laboratory |
| Kobsar et al. [73] | 2017 | Osteoarthritis | 39 | months | Laboratory |
| McGinnis et al. [79] | 2017 | Multiple sclerosis | 30 | min | Laboratory |
| Lipsmeier et al. [77] | 2018 | Parkinson | 44 | months | Free living |
| Kleiner et al. [72] | 2018 | Parkinson | 30 | min | Laboratory |
| Carpinella et al. [44] | 2018 | Diverse | 30 | min | Laboratory |
| Jayaraman et al. [67] | 2018 | Spinal Cord Injury | 18 | hours | Laboratory |
| Jang et al. [66] | 2018 | Old Age | 22 | years | Free living |
| Derungs et al. [52] | 2018 | Hemiparesis | 11 | weeks | Free living |
| Mileti et al. [81] | 2018 | Parkinson | 26 | min | Laboratory |
| Aich et al. [35] | 2018 | Parkinson | 51 | min | Laboratory |
| Cheong et al. [46] | 2018 | Cancer | 102 | months | Free living |
| Ata et al. [40] | 2018 | Artery disease | 114 | min | Laboratory |
| Kim et al. [70] | 2018 | Parkinson | 32 | min | Laboratory |
| Vadnerkar et al. [100] | 2018 | Old Age | 16 | min | Laboratory |
| Rosario et al. [51] | 2018 | Cardiac disease | 66 | months | Free living |
| Lemoyne et al. [76] | 2018 | Hemiplegia | 1 | min | Laboratory |
| Dasmahapatra et al. [50] | 2018 | Multiple Sclerosis | 114 | weeks | Free living |
| Schliessmann et al. [91] | 2018 | Diverse | 41 | min | Laboratory |
| Ummels et al. [99] | 2018 | Diverse | 130 | years | Laboratory |
| Banky et al. [41] | 2019 | Diverse | 35 | hours | Laboratory |
| Flachenecker et al. [58] | 2019 | Multiple sclerosis | 102 | min | Laboratory |
| Gadaleta et al. [60] | 2019 | Parkinson | 71 | min | Laboratory |
| Teufl et al. [97] | 2019 | Arthroplasty | 20 | min | Laboratory |
| Angelini et al. [37] | 2019 | Multiple sclerosis | 26 | min | Laboratory |
| Antos et al. [38] | 2019 | Old Age | 20 | min | Laboratory |
| Compagnat et al. [48] | 2019 | Stroke | 35 | min | Laboratory |
| Newman et al. [84] | 2020 | Brain injury | 12 | min | Laboratory |
| Ullrich et al. [98] | 2020 | Parkinson | 128 | min | Both |
| Wang et al. [101] | 2020 | Post Sternotomy | 22 | min | Laboratory |
| Pavon et al. [85] | 2020 | Disability | 46 | days | Laboratory |
| Arcuria et al. [39] | 2020 | Cerebellar ataxia | 40 | min | Laboratory |
| Erb et al. [55] | 2020 | Parkinson | 34 | weeks | Free Living |
| Aich et al. [36] | 2020 | Parkinson | 48 | min | Laboratory |
| Rubin et al. [89] | 2020 | Diverse | 78 | min | Laboratory |
| Henriksen et al. [62] | 2020 | Obesity | 16 | years | Free living |
| Shema-Shiratzky et al. [94] | 2020 | Multiple Sclerosis | 44 | min | Both |
| Abdollahi et al. [34] | 2020 | Chronic pain | 94 | min | Laboratory |
| Kim et al. [71] | 2020 | Amputation | 17 | min | Laboratory |
| Lemay et al. [75] | 2020 | Spinal cord injury | 18 | min | Laboratory |
| Meisel et al. [80] | 2020 | Epilepsy | 69 | months | Laboratory |
| Fantozzi et al. [56] | 2020 | Old Age | 9 | min | Laboratory |
| Zhai et al. [103] | 2020 | Multiple Sclerosis | 67 | min (Lab), weeks (FL) |
Both |
| Revi et al. [87] | 2020 | Stroke | 5 | min | Laboratory |
| Compagnat et al. [49] | 2020 | Stroke | 26 | min | Laboratory |
| Furtado et al. [59] | 2020 | Amputation | 34 | hours (Lab), weeks (FL) |
Both |
| Na et al. [83] | 2020 | Osteoarthritis | 39 | min | Laboratory |
Sensors. In this review, we observe that early research efforts attempted to find improvements for gait monitoring in patients by experimenting with new sensor types and/or sensor locations. The first paper to report the validation of a wearable sensor for monitoring gait in patients was in 2010 [90], but it did not become more prevalent until 2017, during which nine other papers on this subject were published [45,47,54,63,73,78,79,88,96]. Over time, research efforts have focused on refining validation protocols, whether in terms of the number of sensors or their locations, with emphasis on two major criteria: the ability of sensors to capture gait patterns and the practicality of everyday life. As seen in Table 2 and Table 3, the majority of studies (95%) used accelerometers and/or gyroscopes, typically embedded within an IMU or smartphone. This observation highlights the emergence of commercial wearable devices as a practical and user-friendly modality for gait monitoring in daily life. In addition to user adoption, commercial wearable devices also have engineering advantages, such as a compact format with suitable computing and power resources. If it is a single sensor, it is usually worn near the center of gravity, in a pocket [42,43,45,50,51,77,86], or on the chest [39,44,64,84,92] or pelvis [59,61,65,72,94,96].
Another trend that emerges from Table 2 is the fact that several sensors were used together and generally at various on-body locations [37,48,52,54,55,56,60,63,65,67,70,73,75,79,83,87,90,93,95,97,98,99,102]. However, using a multi-sensor system introduces several challenges, including the integration of different sampling rates and signal amplitudes, and how to align signals from multiple devices and, therefore, different clock times. Despite these challenges, the multi-sensor approach offers high potential for the real-time monitoring of gait, where multi-sensor fusion can provide context-awareness (e.g., if the patient stays mainly at home or leaves home from time to time) and can contribute to the optimization of power (e.g., a low-power sensor can trigger a higher-power sensor only when necessary).
Ground truth. Our review indicates that 53% of the included studies use annotations. As seen in Figure 5, there is still a strong reliance on annotations by groups of individuals (56%; mainly a group of patients versus a group of healthy subjects) followed by annotations made by experts on data from videos or measurements during the experiment (21%) and patient self-report (0.05%). These last two annotation methods are surely less numerous because they can be very costly and time-intensive and are also of questionable quality because maintaining logs is a process that is very burdensome to the participant and ultimately relies on their memory. This fact has namely led to the emergence of initiatives in terms of intelligent annotation [105].
Another trend in ground-truth validation is increasingly in favor of using a reference (46%) because of the confidence established from visually confirming the gait pattern being detected: this can be a metrological device (18%), a medical examination (20%), or a third-party portable medical device (8%). However, in this case, the data are not annotated and therefore do not allow for the use of conventional machine learning approaches. At best, the medical examination allows for a regression task to be carried out, which however, from a machine learning point of view, is more difficult. In general, comparisons are limited to traditional statistical tools such as correlations or difference tests [35,39,40,41,42,49,53,54,59,61,62,65,67,72,77,78,79,82,83,84,87,88,89,90,95,97,99,103].
Machine learning. The combination of machine learning algorithms and wearable sensors for gait analysis has shown promising results in validating the extraction of complex gait patterns [34,35,36,38,45,53,60,63,68,69,70,77,79,80,81,86,95,97,100].
As seen in Table 7, researchers have used machine learning on sensor data for different tasks: regression for continuous labelled data (speed, step length, or distance) [53,69,79,86] and classification of discrete labelled data such as groups of patients [35,36,38,45,68,80,86,97] or medical functional scores [34,45,63,95,100]. Classification, less commonly used for the validation of sensors, aims for higher-level analyses, namely to identify a robust methodology able to monitor patients in time while at the same time discriminating between a pathological and physiological gait, or the evolution of the disease studied on the basis of gait movements.
The types of machine learning algorithm families have evolved over time, with standard approaches being used before 2017 and the appearance of deep learning approaches with automatic feature extraction without human intervention for the first time in 2018 [77], which are unlike most traditional machine learning algorithms. It should be noted that, in the context of the papers studied in this review [60,70,77,80], these approaches concern studies with a significant number of patients (≥30) or/and relatively long acquisition times [77,80] in order to guarantee a sufficiently representative and realistic sample. Other studies based on machine learning preferred more standard approaches with a small number of expert features if their samples were more limited regarding the number of patients [38,63,68,69,79,81,86,95,100] or the acquisition time [34,35,36,45,97]. Comparing the results of the different studies in terms of performance seems, at this stage, to be a difficult task because, as stated previously, it depends on the complexity of the task to be performed and on the complexity of the machine learning algorithm implemented.
Finally, it should be mentioned that machine learning also has drawbacks, with the first being the computational time required to train a model [106]. This is justified for complex analysis tasks such as classification or significant performance increases for a regression task. Moreover, ML may require the adjustment of hyperparameters that may demand theoretical knowledge in optimization. Finally, ML tends to be more difficult to interpret for a clinician who looks for the most relevant parameters to analyze the gait patterns of patients. However, it should be noted that recent initiatives have been carried out to demystify these two points [107,108].
4.2. Recommendations
Advanced inertial sensors, including accelerometers and gyroscopes, are commonly integrated into smartphones and smart devices nowadays. Therefore, it is very convenient and cheap to collect inertial gait data to achieve gait monitoring with high accuracy. Most existing validation methods ask the person to walk along a specified road (e.g., a straight lounge) and/or at a normal speed. Obviously, such strict requirements heavily limit its wide application, which motivates us to give some recommendations for future work in this context.
Data acquisition. A first step would be to precisely define validation protocols—by consulting the medical staff—adapted to the study of chronic pathologies. Indeed, many studies only validate sensors for a given medical application without having tested them outside the laboratory, on a very limited number of patients, and over a relatively short time window (at most a few hours). The protocol to be defined should therefore impose experimentation constraints closer to the daily life of patients, namely the data should be acquired at home, on a sufficient number of patients, and over a sufficiently long acquisition period (several weeks or even months).
It would also be necessary to define within the protocol which types of sensors would be more suitable according to the studied pathology, how many sensors would be necessary, and where to place them on the patient [18]. There is a clear trade-off between the accuracy of the recorded data and the invasiveness of the portable system: the greater the number of sensors and the more varied they are placed on different parts of the patient’s body, the more accurate the measurements will be, but this is at the expense of a practical, accommodating, and portable use.
Data collection and processing. Today, most sensors record a lot of data about their users. However, most wearable devices do not have the memory and computing power to process and analyze all of the recorded signals. Faced with this problem, two solutions are generally considered: either the system uses only a part of the recorded data to provide accurate indicators (throwing away a massive amount of potentially interesting data) [109,110] or the system stores and analyzes all raw data on the cloud [111,112]. The latter option is often problematic because the traditional architecture is centralized and offers little protection against potential cyber attacks. Centralizing raw data on a server poses some risk, especially if the data is sent to an external server, as it facilitates access to malicious attackers. A more reliable and secure alternative regarding the collection and processing of data would therefore be to process the raw inertial signal on the user’s smartphone and to transfer only relevant features unlinked to the identity of users to the cloud [113,114]. Finally, the mobile clients associated with wearable devices have to send a lot of data to a centralized server for training and model inference. This is especially difficult due to user billing plans and user privacy. Thus, very recently, decentralized architectures dedicated to machine learning have emerged [115].
Validation. It is mandatory to ensure that sensor recordings are accurate and sensitive enough for medical diagnosis and prognosis. This is crucial to ensure not only the generalizability of a sensor within a target population but also its ability to measure day-to-day variability data, which can be corroborated with disease symptoms. To this end, data acquired by commercial wearable sensors should be systematically compared to data acquired by reference medical devices (i.e., reliable gold standard systems, medical scores, or groups of subjects). Machine learning approaches make it possible to loosen the strict framework of acquisition protocols but must ensure that the data set collected for training is large, labelled, and realistic. Deep approaches, which automatically select features from data, offer very interesting perspectives given that feature extraction is a task that can take teams of data scientists years to accomplish. It augments the powers of small expert teams, which by their nature do not scale.
Statistical models versus ML. Statistical models are designed for inference about the relationships between variables within the data and are designed for data with a few dozen input variables and small sample sizes. On the other hand, machine learning models are designed to make the most accurate predictions possible. Statistical models can make predictions, but predictive accuracy is not their strength. Indeed, no training and test sets are necessary. Furthermore, machine learning aims to build a model that can make repeatable predictions in a high-dimensional space without formulating a hypothesis on the underlying data generation mechanism. ML methods are particularly useful when the number of input variables exceeds the number of samples [116]. Hence, using machine learning in a validation task highly depends on the purpose of the study. To prove that a sensor is able to respond to a certain kind of stimuli (such as a walking speed), a statistical model should be used. Conversely, to predict from a collection of different sensors whether a patient is affected by a certain grade of a disease affecting the musculoskeletal system, machine learning is probably the best approach. Indeed, this multi-dimensional space (one or more for each sensor) is in fact difficult to interpret and therefore to analyze. The ML model would then probably be a neural network or a random forest in order to take into account the nonlinearities resulting from the complex relationship between the physical sensors and the classification output.
5. Conclusions
The field of gait monitoring in patients is still emerging, and the accuracy of commercial wearable sensors still depends on careful constraints during data acquisition. Collecting data in daily life is considerably more challenging than conducting research in a laboratory. In free-living conditions, continuous control of the sensors, participants, and hardware or software is lost. Therefore, successful sensor deployment requires really robust algorithms. If the objective is to be able to monitor the gait completely freely over a long period of time, precision must be valued. Considering this review of the last 10 years in the field, validation takes an increasingly important place in the literature, with the number of studies having gradually increased since 2010. In these studies, a significant part of the validation was based on traditional statistical approaches (75%) with a stable contribution of machine learning-based approaches (25%). Machine learning approaches are algorithms that should be considered for the future. These are in fact data-based approaches, which, as long as the data collected are numerous, annotated, and representative, allow for the training of an effective model. It should be noted that commercial wearable sensors allowing for increased data collection and good patient adherence through efforts of miniaturization, energy consumption, and comfort will contribute to its future success.
Abbreviations
The following abbreviations are used in this manuscript:
| 6MWT | Six-minute walk test |
| ML | Machine learning |
| SD | Standard deviation |
| IMU | Inertial Measurement Unit |
Appendix A. Extraction from Databases
Table A1.
Search term strategy.
| Database | Search String | Records |
|---|---|---|
| ACM | [[Abstract: gait] OR [Abstract: actimetry] OR [Abstract: actigraphy] | 17 |
| OR [Abstract: walk]] AND [[[Abstract: smartphone] OR [Abstract: wearable] | ||
| OR [Abstract: iot]] AND [[Abstract: “chronic disease”] OR [Abstract: rehabilitation] | ||
| OR [Abstract: medicine]] AND [[Abstract: validity] OR [Abstract: reliability] | ||
| OR [Abstract: reproductibility or validation] OR [Publication Title: gait] | ||
| OR [Publication Title: actimetry] OR [Publication Title: actigraphy] | ||
| OR [Publication Title: walk]] AND [[Publication Title: smartphone] | ||
| OR [Publication Title: wearable] OR [Publication Title: iot] | ||
| AND [Publication Title: “chronic disease”] OR [Publication Title: rehabilitation] | ||
| OR [Publication Title: medicine]] AND [[Publication Title: validity] | ||
| OR [Publication Title: reliability] OR [Publication Title: reproductibility or validation]] | ||
| AND [Publication Date: (01 January 2010 TO 31 October 2020)] | ||
| Cochrane | ((gait OR actimetry OR actigraphy OR walk) AND (smartphone OR wearable OR iot) AND | 15 |
| (“chronic disease” OR rehabilitation OR medicine) AND (validity OR reliability OR | ||
| reproductibility OR validation)) in Title Abstract Keyword—between Jan 2010 and October 2020 | ||
| DBLB | (gait | walk | actimetry) (smartphone | device | iot) (valid | rehabilitation) | 31 |
| IEEE Xplore | ((gait OR actimetry OR actigraphy OR walk) AND (smartphone OR wearable OR iot) | 54 |
| AND (“chronic disease” OR rehabilitation OR medicine) AND (validity | ||
| OR reliability OR reproductibility or validation)) | ||
| PubMed | ((gait OR actimetry OR actigraphy OR walk) | 52 |
| AND (smartphone OR wearable OR iot) AND | ||
| (“chronic disease” OR rehabilitation OR medicine) AND | ||
| (validity OR reliability OR reproductibility or validation)) | ||
| Filters: from 2010–2020 | ||
| Scholar | title:(gait smartphone “wearable device” rehabilitation validity) | 1010 |
| ScienceDirect | ((gait OR actimetry) AND (smartphone OR iot) AND | 3 |
| #1 | (“chronic disease” OR medicine) AND | |
| (validity OR validation)) | ||
| ScienceDirect | ((gait OR walk) AND (smartphone OR wearable) AND | 10 |
| #2 | (rehabilitation OR medicine) AND | |
| (validity OR reliability)) | ||
| ScienceDirect | ((gait OR walk) AND (smartphone OR iot) AND | 1 |
| #3 | AND (“chronic disease” OR medicine) AND | |
| (validity OR validation)) | ||
| ScienceDirect | ((gait OR walk) AND (smartphone OR wearable) AND | 16 |
| #4 | AND (rehabilitation OR medicine) AND | |
| (validity OR validation)) | ||
| ScienceDirect | ((gait OR actimetry OR walk) AND | 12 |
| #5 | (smartphone OR wearable OR iot) AND | |
| rehabilitation AND validation) | ||
| SCOPUS | TITLE-ABS-KEY((( gait OR actimetry OR actigraphy OR walk ) | 155 |
| AND ( smartphone OR wearable OR iot ) AND | ||
| ( “chronic disease” OR rehabilitation OR medicine ) AND | ||
| ( validity OR reliability OR reproductibility OR validation))) | ||
| AND PUBYEAR ≥ 2010 AND PUBYEAR ≤ 2020 | ||
| Web of Science | (TS = ((gait OR actimetry OR actigraphy OR walk) | 148 |
| AND (smartphone OR wearable OR iot) AND (“chronic disease” OR | ||
| rehabilitation OR medicine) AND (validity OR reliability OR | ||
| reproductibility OR validation))) AND LANGUAGE: (English) | ||
| AND DOCUMENT TYPES: (Article) Indexes=SCI-EXPANDED, | ||
| SSCI, A&HCI, CPCI-S, CPCI-SSH, ESCI, | ||
| CCR-EXPANDED, IC Timespan=2010-2020 |
Author Contributions
Conceptualization, T.J., N.D., and C.F.; methodology, T.J., N.D., and C.F.; validation, C.F.; formal analysis, T.J., N.D., and C.F.; investigation, T.J., N.D., and C.F.; writing—original draft preparation, T.J., N.D., and C.F.; supervision, C.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded in part by the French national research agency (ANR) projects PMR (ANR-20-CE23-0013).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Roberts M., Mongeon D., Prince F. Biomechanical parameters for gait analysis: A systematic review of healthy human gait. Phys. Ther. Rehabil. 2017;4:6. doi: 10.7243/2055-2386-4-6. [DOI] [Google Scholar]
- 2.Middleton A., Fritz S.L., Lusardi M. Walking speed: The functional vital sign. J. Aging Phys. Act. 2015;23:314–322. doi: 10.1123/japa.2013-0236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lewek M.D., Bradley C.E., Wutzke C.J., Zinder S.M. The relationship between spatiotemporal gait asymmetry and balance in individuals with chronic stroke. J. Appl. Biomech. 2014;30:31–36. doi: 10.1123/jab.2012-0208. [DOI] [PubMed] [Google Scholar]
- 4.Galna B., Lord S., Rochester L. Is gait variability reliable in older adults and Parkinson’s disease? Towards an optimal testing protocol. Gait Posture. 2013;37:580–585. doi: 10.1016/j.gaitpost.2012.09.025. [DOI] [PubMed] [Google Scholar]
- 5.Cruz-Jimenez M. Normal changes in gait and mobility problems in the elderly. Phys. Med. Rehabil. Clin. 2017;28:713–725. doi: 10.1016/j.pmr.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 6.Uszko-Lencer N.H., Mesquita R., Janssen E., Werter C., Brunner-La Rocca H.P., Pitta F., Wouters E.F., Spruit M.A. Reliability, construct validity and determinants of 6-minute walk test performance in patients with chronic heart failure. Int. J. Cardiol. 2017;240:285–290. doi: 10.1016/j.ijcard.2017.02.109. [DOI] [PubMed] [Google Scholar]
- 7.DePew Z.S., Karpman C., Novotny P.J., Benzo R.P. Correlations between gait speed, 6-minute walk distance, physical activity, and self-efficacy in patients with severe chronic lung disease. Respir. Care. 2013;58:2113–2119. doi: 10.4187/respcare.02471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holland A.E., Spruit M.A., Troosters T., Puhan M.A., Pepin V., Saey D., McCormack M.C., Carlin B.W., Sciurba F.C., Pitta F., et al. An official European Respiratory Society/American Thoracic Society technical standard: Field walking tests in chronic respiratory disease. Eur. Respir. J. 2014;44:1428–1446. doi: 10.1183/09031936.00150314. [DOI] [PubMed] [Google Scholar]
- 9.Weiss A., Herman T., Mirelman A., Shiratzky S.S., Giladi N., Barnes L.L., Bennett D.A., Buchman A.S., Hausdorff J.M. The transition between turning and sitting in patients with Parkinson’s disease: A wearable device detects an unexpected sequence of events. Gait Posture. 2019;67:224–229. doi: 10.1016/j.gaitpost.2018.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cuevas-Trisan R. Balance problems and fall risks in the elderly. Phys. Med. Rehabil. Clin. 2017;28:727–737. doi: 10.1016/j.pmr.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 11.Shine J., Handojoseno A., Nguyen T., Tran Y., Naismith S., Nguyen H., Lewis S. Abnormal patterns of theta frequency oscillations during the temporal evolution of freezing of gait in Parkinson’s disease. Clin. Neurophysiol. 2014;125:569–576. doi: 10.1016/j.clinph.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 12.Majumder S., Mondal T., Deen M.J. Wearable sensors for remote health monitoring. Sensors. 2017;17:130. doi: 10.3390/s17010130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dias D., Paulo Silva Cunha J. Wearable health devices—vital sign monitoring, systems and technologies. Sensors. 2018;18:2414. doi: 10.3390/s18082414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Botros A., Schütz N., Camenzind M., Urwyler P., Bolliger D., Vanbellingen T., Kistler R., Bohlhalter S., Müri R.M., Mosimann U.P., et al. Long-term home-monitoring sensor technology in patients with Parkinson’s disease—Acceptance and adherence. Sensors. 2019;19:5169. doi: 10.3390/s19235169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Evenson K.R., Goto M.M., Furberg R.D. Systematic review of the validity and reliability of consumer-wearable activity trackers. Int. J. Behav. Nutr. Phys. Act. 2015;12:159. doi: 10.1186/s12966-015-0314-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Appelboom G., Yang A.H., Christophe B.R., Bruce E.M., Slomian J., Bruyère O., Bruce S.S., Zacharia B.E., Reginster J.Y., Connolly E.S., Jr. The promise of wearable activity sensors to define patient recovery. J. Clin. Neurosci. 2014;21:1089–1093. doi: 10.1016/j.jocn.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 17.Sprint G., Cook D., Weeks D., Dahmen J., La Fleur A. Analyzing sensor-based time series data to track changes in physical activity during inpatient rehabilitation. Sensors. 2017;17:2219. doi: 10.3390/s17102219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vienne A., Barrois R.P., Buffat S., Ricard D., Vidal P.P. Inertial sensors to assess gait quality in patients with neurological disorders: A systematic review of technical and analytical challenges. Front. Psychol. 2017;8:817. doi: 10.3389/fpsyg.2017.00817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carcreff L., Gerber C.N., Paraschiv-Ionescu A., De Coulon G., Newman C.J., Aminian K., Armand S. Comparison of gait characteristics between clinical and daily life settings in children with cerebral palsy. Sci. Rep. 2020;10:2091. doi: 10.1038/s41598-020-59002-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feehan L.M., Geldman J., Sayre E.C., Park C., Ezzat A.M., Yoo J.Y., Hamilton C.B., Li L.C. Accuracy of Fitbit devices: Systematic review and narrative syntheses of quantitative data. JMIR mHealth uHealth. 2018;6:e10527. doi: 10.2196/10527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Düking P., Fuss F.K., Holmberg H.C., Sperlich B. Recommendations for assessment of the reliability, sensitivity, and validity of data provided by wearable sensors designed for monitoring physical activity. JMIR mHealth uHealth. 2018;6:e102. doi: 10.2196/mhealth.9341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kobsar D., Charlton J.M., Tse C.T., Esculier J.F., Graffos A., Krowchuk N.M., Thatcher D., Hunt M.A. Validity and reliability of wearable inertial sensors in healthy adult walking: A systematic review and meta-analysis. J. Neuroeng. Rehabil. 2020;17:1–21. doi: 10.1186/s12984-020-00685-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Poitras I., Dupuis F., Bielmann M., Campeau-Lecours A., Mercier C., Bouyer L.J., Roy J.S. Validity and reliability of wearable sensors for joint angle estimation: A systematic review. Sensors. 2019;19:1555. doi: 10.3390/s19071555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Straiton N., Alharbi M., Bauman A., Neubeck L., Gullick J., Bhindi R., Gallagher R. The validity and reliability of consumer-grade activity trackers in older, community-dwelling adults: A systematic review. Maturitas. 2018;112:85–93. doi: 10.1016/j.maturitas.2018.03.016. [DOI] [PubMed] [Google Scholar]
- 25.Loy-Benitez J., Heo S., Yoo C. Soft sensor validation for monitoring and resilient control of sequential subway indoor air quality through memory-gated recurrent neural networks-based autoencoders. Control Eng. Pract. 2020;97:104330. doi: 10.1016/j.conengprac.2020.104330. [DOI] [Google Scholar]
- 26.Seibert V., Araújo R., McElligott R. Sensor Validation for Indoor Air Quality using Machine Learning; Proceedings of the Anais do XVII Encontro Nacional de Inteligência Artificial e Computacional (ENIAC); Rio Grande, Brazil. 20–23 October 2020; pp. 730–739. [Google Scholar]
- 27.Bergamini E., Iosa M., Belluscio V., Morone G., Tramontano M., Vannozzi G. Multi-sensor assessment of dynamic balance during gait in patients with subacute stroke. J. Biomech. 2017;61:208–215. doi: 10.1016/j.jbiomech.2017.07.034. [DOI] [PubMed] [Google Scholar]
- 28.Qiu S., Liu L., Zhao H., Wang Z., Jiang Y. MEMS inertial sensors based gait analysis for rehabilitation assessment via multi-sensor fusion. Micromachines. 2018;9:442. doi: 10.3390/mi9090442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nukala B.T., Nakano T., Rodriguez A., Tsay J., Lopez J., Nguyen T.Q., Zupancic S., Lie D.Y. Real-time classification of patients with balance disorders vs. normal subjects using a low-cost small wireless wearable gait sensor. Biosensors. 2016;6:58. doi: 10.3390/bios6040058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Altilio R., Rossetti A., Fang Q., Gu X., Panella M. A comparison of machine learning classifiers for smartphone-based gait analysis. Med Biol. Eng. Comput. 2021;59:535–546. doi: 10.1007/s11517-020-02295-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goshvarpour A., Goshvarpour A. Nonlinear Analysis of Human Gait Signals. Int. J. Inf. Eng. Electron. Bus. 2012;4:15–21. doi: 10.5815/ijieeb.2012.02.03. [DOI] [Google Scholar]
- 32.Pérez-Toro P., Vásquez-Correa J., Arias-Vergara T., Nöth E., Orozco-Arroyave J. Nonlinear dynamics and Poincaré sections to model gait impairments in different stages of Parkinson’s disease. Nonlinear Dyn. 2020;100:3253–3276. doi: 10.1007/s11071-020-05691-7. [DOI] [Google Scholar]
- 33.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Moher D. Updating guidance for reporting systematic reviews: Development of the PRISMA 2020 statement. J. Clin. Epidemiol. 2021;134:103–112. doi: 10.1016/j.jclinepi.2021.02.003. [DOI] [PubMed] [Google Scholar]
- 34.Abdollahi M., Ashouri S., Abedi M., Azadeh-Fard N., Parnianpour M., Khalaf K., Rashedi E. Using a Motion Sensor to Categorize Nonspecific Low Back Pain Patients: A Machine Learning Approach. Sensors. 2020;20:3600. doi: 10.3390/s20123600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aich S., Pradhan P.M., Park J., Sethi N., Vathsa V.S.S., Kim H.C. A validation study of freezing of gait (FoG) detection and machine-learning-based FoG prediction using estimated gait characteristics with a wearable accelerometer. Sensors. 2018;18:3287. doi: 10.3390/s18103287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aich S., Pradhan P.M., Chakraborty S., Kim H.C., Kim H.T., Lee H.G., Kim I.H., Joo M.i., Jong Seong S., Park J. Design of a Machine Learning-Assisted Wearable Accelerometer-Based Automated System for Studying the Effect of Dopaminergic Medicine on Gait Characteristics of Parkinson’s Patients. J. Healthc. Eng. 2020;2020:1823268. doi: 10.1155/2020/1823268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Angelini L., Carpinella I., Cattaneo D., Ferrarin M., Gervasoni E., Sharrack B., Paling D., Nair K.P.S., Mazzà C. Is a wearable sensor-based characterisation of gait robust enough to overcome differences between measurement protocols? A multi-centric pragmatic study in patients with multiple sclerosis. Sensors. 2020;20:79. doi: 10.3390/s20010079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Antos S.A., Danilovich M.K., Eisenstein A.R., Gordon K.E., Kording K.P. Smartwatches can detect walker and cane use in older adults. Innov. Aging. 2019;3:igz008. doi: 10.1093/geroni/igz008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Arcuria G., Marcotulli C., Amuso R., Dattilo G., Galasso C., Pierelli F., Casali C. Developing a smartphone application, triaxial accelerometer-based, to quantify static and dynamic balance deficits in patients with cerebellar ataxias. J. Neurol. 2020;267:625–639. doi: 10.1007/s00415-019-09570-z. [DOI] [PubMed] [Google Scholar]
- 40.Ata R., Gandhi N., Rasmussen H., El-Gabalawy O., Gutierrez S., Ahmad A., Suresh S., Ravi R., Rothenberg K., Aalami O. Clinical validation of smartphone-based activity tracking in peripheral artery disease patients. NPJ Digit. Med. 2018;1:66. doi: 10.1038/s41746-018-0073-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Banky M., Clark R.A., Mentiplay B.F., Olver J.H., Kahn M.B., Williams G. Toward accurate clinical spasticity assessment: Validation of movement speed and joint angle assessments using Smartphones and camera tracking. Arch. Phys. Med. Rehabil. 2019;100:1482–1491. doi: 10.1016/j.apmr.2018.11.026. [DOI] [PubMed] [Google Scholar]
- 42.Brinkløv C.F., Thorsen I.K., Karstoft K., Brøns C., Valentiner L., Langberg H., Vaag A.A., Nielsen J.S., Pedersen B.K., Ried-Larsen M. Criterion validity and reliability of a smartphone delivered sub-maximal fitness test for people with type 2 diabetes. BMC Sport. Sci. Med. Rehabil. 2016;8:31. doi: 10.1186/s13102-016-0056-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Capela N.A., Lemaire E.D., Baddour N. Novel algorithm for a smartphone-based 6-minute walk test application: Algorithm, application development, and evaluation. J. Neuroeng. Rehabil. 2015;12:19. doi: 10.1186/s12984-015-0013-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carpinella I., Gervasoni E., Anastasi D., Lencioni T., Cattaneo D., Ferrarin M. Instrumental assessment of stair ascent in people with multiple sclerosis, stroke, and Parkinson’s disease: A wearable-sensor-based approach. IEEE Trans. Neural Syst. Rehabil. Eng. 2018;26:2324–2332. doi: 10.1109/TNSRE.2018.2881324. [DOI] [PubMed] [Google Scholar]
- 45.Cheng Q., Juen J., Bellam S., Fulara N., Close D., Silverstein J.C., Schatz B. Predicting pulmonary function from phone sensors. Telemed. e-Health. 2017;23:913–919. doi: 10.1089/tmj.2017.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cheong I.Y., An S.Y., Cha W.C., Rha M.Y., Kim S.T., Chang D.K., Hwang J.H. Efficacy of mobile health care application and wearable device in improvement of physical performance in colorectal cancer patients undergoing chemotherapy. Clin. Color. Cancer. 2018;17:e353–e362. doi: 10.1016/j.clcc.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 47.Chiu Y.L., Tsai Y.J., Lin C.H., Hou Y.R., Sung W.H. Evaluation of a smartphone-based assessment system in subjects with chronic ankle instability. Comput. Methods Programs Biomed. 2017;139:191–195. doi: 10.1016/j.cmpb.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 48.Compagnat M., Batcho C.S., David R., Vuillerme N., Salle J.Y., Daviet J.C., Mandigout S. Validity of the walked distance estimated by wearable devices in stroke individuals. Sensors. 2019;19:2497. doi: 10.3390/s19112497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Compagnat M., Mandigout S., Batcho C., Vuillerme N., Salle J., David R., Daviet J. Validity of wearable actimeter computation of total energy expenditure during walking in post-stroke individuals. Ann. Phys. Rehabil. Med. 2020;63:209–215. doi: 10.1016/j.rehab.2019.07.002. [DOI] [PubMed] [Google Scholar]
- 50.DasMahapatra P., Chiauzzi E., Bhalerao R., Rhodes J. Free-living physical activity monitoring in adult US patients with multiple sclerosis using a consumer wearable device. Digit. Biomarkers. 2018;2:47–63. doi: 10.1159/000488040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Del Rosario M.B., Lovell N.H., Fildes J., Holgate K., Yu J., Ferry C., Schreier G., Ooi S.Y., Redmond S.J. Evaluation of an mHealth-based adjunct to outpatient cardiac rehabilitation. IEEE J. Biomed. Health Informa. 2017;22:1938–1948. doi: 10.1109/JBHI.2017.2782209. [DOI] [PubMed] [Google Scholar]
- 52.Derungs A., Schuster-Amft C., Amft O. Longitudinal walking analysis in hemiparetic patients using wearable motion sensors: Is there convergence between body sides? Front. Bioeng. Biotechnol. 2018;6:57. doi: 10.3389/fbioe.2018.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dobkin B.H., Xu X., Batalin M., Thomas S., Kaiser W. Reliability and validity of bilateral ankle accelerometer algorithms for activity recognition and walking speed after stroke. Stroke. 2011;42:2246–2250. doi: 10.1161/STROKEAHA.110.611095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.El-Gohary M., Peterson D., Gera G., Horak F.B., Huisinga J.M. Validity of the instrumented push and release test to quantify postural responses in persons with multiple sclerosis. Arch. Phys. Med. Rehabil. 2017;98:1325–1331. doi: 10.1016/j.apmr.2017.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Erb M.K., Karlin D.R., Ho B.K., Thomas K.C., Parisi F., Vergara-Diaz G.P., Daneault J.F., Wacnik P.W., Zhang H., Kangarloo T., et al. mHealth and wearable technology should replace motor diaries to track motor fluctuations in Parkinson’s disease. NPJ Digit. Med. 2020;3:6. doi: 10.1038/s41746-019-0214-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fantozzi S., Cortesi M., Giovanardi A., Borra D., Di Michele R., Gatta G. Effect of walking speed during gait in water of healthy elderly. Gait Posture. 2020;82:6–13. doi: 10.1016/j.gaitpost.2020.08.112. [DOI] [PubMed] [Google Scholar]
- 57.Ferrari A., Ginis P., Hardegger M., Casamassima F., Rocchi L., Chiari L. A mobile Kalman-filter based solution for the real-time estimation of spatio-temporal gait parameters. IEEE Trans. Neural Syst. Rehabil. Eng. 2015;24:764–773. doi: 10.1109/TNSRE.2015.2457511. [DOI] [PubMed] [Google Scholar]
- 58.Flachenecker F., Gaßner H., Hannik J., Lee D.H., Flachenecker P., Winkler J., Eskofier B., Linker R.A., Klucken J. Objective sensor-based gait measures reflect motor impairment in multiple sclerosis patients: Reliability and clinical validation of a wearable sensor device. Mult. Scler. Relat. Disord. 2020;39:101903. doi: 10.1016/j.msard.2019.101903. [DOI] [PubMed] [Google Scholar]
- 59.Furtado S., Godfrey A., Del Din S., Rochester L., Gerrand C. Are Accelerometer-based Functional Outcome Assessments Feasible and Valid After Treatment for Lower Extremity Sarcomas? Clin. Orthop. Relat. Res. 2020;478:482–503. doi: 10.1097/CORR.0000000000000883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gadaleta M., Cisotto G., Rossi M., Rehman R.Z.U., Rochester L., Del Din S. Deep learning techniques for improving digital gait segmentation; Proceedings of the 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC); Berlin, Germany. 23–27 July 2019; pp. 1834–1837. [DOI] [PubMed] [Google Scholar]
- 61.Grimpampi E., Bonnet V., Taviani A., Mazzà C. Estimate of lower trunk angles in pathological gaits using gyroscope data. Gait Posture. 2013;38:523–527. doi: 10.1016/j.gaitpost.2013.01.031. [DOI] [PubMed] [Google Scholar]
- 62.Henriksen A., Sand A.S., Deraas T., Grimsgaard S., Hartvigsen G., Hopstock L. Succeeding with prolonged usage of consumer-based activity trackers in clinical studies: A mixed methods approach. BMC Public Health. 2020;20:1300. doi: 10.1186/s12889-020-09406-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ilias T., Filip B., Radu C., Dag N., Marina S., Mevludin M. Using measurements from wearable sensors for automatic scoring of Parkinson’s disease motor states: Results from 7 patients; Proceedings of the 2017 39th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC); Jeju, Korea. 11–15 July 2017; pp. 131–134. [DOI] [PubMed] [Google Scholar]
- 64.Isho T., Tashiro H., Usuda S. Accelerometry-based gait characteristics evaluated using a smartphone and their association with fall risk in people with chronic stroke. J. Stroke Cerebrovasc. Dis. 2015;24:1305–1311. doi: 10.1016/j.jstrokecerebrovasdis.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 65.Item-Glatthorn J.F., Casartelli N.C., Petrich-Munzinger J., Munzinger U.K., Maffiuletti N.A. Validity of the intelligent device for energy expenditure and activity accelerometry system for quantitative gait analysis in patients with hip osteoarthritis. Arch. Phys. Med. Rehabil. 2012;93:2090–2093. doi: 10.1016/j.apmr.2012.06.018. [DOI] [PubMed] [Google Scholar]
- 66.Jang I.Y., Kim H.R., Lee E., Jung H.W., Park H., Cheon S.H., Lee Y.S., Park Y.R. Impact of a wearable device-based walking programs in rural older adults on physical activity and health outcomes: Cohort study. JMIR mHealth uHealth. 2018;6:e11335. doi: 10.2196/11335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jayaraman C., Mummidisetty C.K., Mannix-Slobig A., Koch L.M., Jayaraman A. Variables influencing wearable sensor outcome estimates in individuals with stroke and incomplete spinal cord injury: A pilot investigation validating two research grade sensors. J. Neuroeng. Rehabil. 2018;15:19. doi: 10.1186/s12984-018-0358-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Juen J., Cheng Q., Prieto-Centurion V., Krishnan J.A., Schatz B. Health monitors for chronic disease by gait analysis with mobile phones. Telemed. e-Health. 2014;20:1035–1041. doi: 10.1089/tmj.2014.0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Juen J., Cheng Q., Schatz B. Towards a natural walking monitor for pulmonary patients using simple smart phones; Proceedings of the 5th ACM Conference on Bioinformatics, Computational Biology, and Health Informatics; Newport Beach, CA, USA. 20–23 September 2014; pp. 53–62. [Google Scholar]
- 70.Kim H.B., Lee H.J., Lee W.W., Kim S.K., Jeon H.S., Park H.Y., Shin C.W., Yi W.J., Jeon B., Park K.S. Validation of freezing-of-gait monitoring using smartphone. Telemed. e-Health. 2018;24:899–907. doi: 10.1089/tmj.2017.0215. [DOI] [PubMed] [Google Scholar]
- 71.Kim J., Colabianchi N., Wensman J., Gates D.H. Wearable Sensors Quantify Mobility in People With Lower Limb Amputation During Daily Life. IEEE Trans. Neural Syst. Rehabil. Eng. 2020;28:1282–1291. doi: 10.1109/TNSRE.2020.2990824. [DOI] [PubMed] [Google Scholar]
- 72.Kleiner A.F.R., Pacifici I., Vagnini A., Camerota F., Celletti C., Stocchi F., De Pandis M.F., Galli M. Timed up and go evaluation with wearable devices: Validation in Parkinson’s disease. J. Bodyw. Mov. Ther. 2018;22:390–395. doi: 10.1016/j.jbmt.2017.07.006. [DOI] [PubMed] [Google Scholar]
- 73.Kobsar D., Osis S.T., Boyd J.E., Hettinga B.A., Ferber R. Wearable sensors to predict improvement following an exercise intervention in patients with knee osteoarthritis. J. Neuroeng. Rehabil. 2017;14:94. doi: 10.1186/s12984-017-0309-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kozey-Keadle S., Libertine A., Lyden K., Staudenmayer J., Freedson P.S. Validation of wearable monitors for assessing sedentary behavior. Med. Sci. Sport. Exerc. 2011;43:1561–1567. doi: 10.1249/MSS.0b013e31820ce174. [DOI] [PubMed] [Google Scholar]
- 75.Lemay J.F., Noamani A., Unger J., Houston D.J., Rouhani H., Musselmann K.E. Using wearable sensors to characterize gait after spinal cord injury: Evaluation of test—retest reliability and construct validity. Spinal Cord. 2020;59:675–683. doi: 10.1038/s41393-020-00559-4. [DOI] [PubMed] [Google Scholar]
- 76.Lemoyne R., Mastroianni T. Implementation of a smartphone as a wireless accelerometer platform for quantifying hemiplegic gait disparity in a functionally autonomous context. J. Mech. Med. Biol. 2018;18:1850005. doi: 10.1142/S0219519418500057. [DOI] [Google Scholar]
- 77.Lipsmeier F., Taylor K.I., Kilchenmann T., Wolf D., Scotland A., Schjodt-Eriksen J., Cheng W.Y., Fernandez-Garcia I., Siebourg-Polster J., Jin L., et al. Evaluation of smartphone-based testing to generate exploratory outcome measures in a phase 1 Parkinson’s disease clinical trial. Mov. Disord. 2018;33:1287–1297. doi: 10.1002/mds.27376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Maqbool H.F., Husman M.A.B., Awad M.I., Abouhossein A., Iqbal N., Dehghani-Sanij A.A. A real-time gait event detection for lower limb prosthesis control and evaluation. IEEE Trans. Neural Syst. Rehabil. Eng. 2016;25:1500–1509. doi: 10.1109/TNSRE.2016.2636367. [DOI] [PubMed] [Google Scholar]
- 79.McGinnis R.S., Mahadevan N., Moon Y., Seagers K., Sheth N., Wright J.A., Jr., DiCristofaro S., Silva I., Jortberg E., Ceruolo M., et al. A machine learning approach for gait speed estimation using skin-mounted wearable sensors: From healthy controls to individuals with multiple sclerosis. PLoS ONE. 2017;12:e0178366. doi: 10.1371/journal.pone.0178366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Meisel C., El Atrache R., Jackson M., Schubach S., Ufongene C., Loddenkemper T. Machine learning from wristband sensor data for wearable, noninvasive seizure forecasting. Epilepsia. 2020;61:2653–2666. doi: 10.1111/epi.16719. [DOI] [PubMed] [Google Scholar]
- 81.Mileti I., Germanotta M., Di Sipio E., Imbimbo I., Pacilli A., Erra C., Petracca M., Rossi S., Del Prete Z., Bentivoglio A.R., et al. Measuring gait quality in Parkinson’s disease through real-time gait phase recognition. Sensors. 2018;18:919. doi: 10.3390/s18030919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Munguía-Izquierdo D., Santalla A., Legaz-Arrese A. Evaluation of a wearable body monitoring device during treadmill walking and jogging in patients with fibromyalgia syndrome. Arch. Phys. Med. Rehabil. 2012;93:115–122. doi: 10.1016/j.apmr.2011.08.021. [DOI] [PubMed] [Google Scholar]
- 83.Na A., Buchanan T.S. Validating wearable sensors using self-reported instability among patients with knee osteoarthritis. PM&R. 2021;13:119–127. doi: 10.1002/pmrj.12393. [DOI] [PubMed] [Google Scholar]
- 84.Newman M.A., Hirsch M.A., Peindl R.D., Habet N.A., Tsai T.J., Runyon M.S., Huynh T., Phillips C., Zheng N., Group C.T.N.R., et al. Use of an instrumented dual-task timed up and go test in children with traumatic brain injury. Gait Posture. 2020;76:193–197. doi: 10.1016/j.gaitpost.2019.12.001. [DOI] [PubMed] [Google Scholar]
- 85.Pavon J.M., Sloane R.J., Pieper C.F., Colón-Emeric C.S., Cohen H.J., Gallagher D., Hall K.S., Morey M.C., McCarty M., Hastings S.N. Accelerometer-Measured Hospital Physical Activity and Hospital-Acquired Disability in Older Adults. J. Am. Geriatr. Soc. 2020;68:261–265. doi: 10.1111/jgs.16231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Raknim P., Lan K.C. Gait monitoring for early neurological disorder detection using sensors in a smartphone: Validation and a case study of parkinsonism. Telemed. e-Health. 2016;22:75–81. doi: 10.1089/tmj.2015.0005. [DOI] [PubMed] [Google Scholar]
- 87.Revi D.A., Alvarez A.M., Walsh C.J., De Rossi S.M., Awad L.N. Indirect measurement of anterior-posterior ground reaction forces using a minimal set of wearable inertial sensors: From healthy to hemiparetic walking. J. Neuroeng. Rehabil. 2020;17:82. doi: 10.1186/s12984-020-00700-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rogan S., de Bie R., de Bruin E.D. Sensor-based foot-mounted wearable system and pressure sensitive gait analysis. Z. Gerontol. Geriatr. 2017;50:488–497. doi: 10.1007/s00391-016-1124-z. [DOI] [PubMed] [Google Scholar]
- 89.Rubin D.S., Dalton A., Tank A., Berkowitz M., Arnolds D.E., Liao C., Gerlach R.M. Development and pilot study of an iOS smartphone application for perioperative functional capacity assessment. Anesth. Analg. 2020;131:830–839. doi: 10.1213/ANE.0000000000004440. [DOI] [PubMed] [Google Scholar]
- 90.Salarian A., Horak F.B., Zampieri C., Carlson-Kuhta P., Nutt J.G., Aminian K. iTUG, a sensitive and reliable measure of mobility. IEEE Trans. Neural Syst. Rehabil. Eng. 2010;18:303–310. doi: 10.1109/TNSRE.2010.2047606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Schließmann D., Nisser M., Schuld C., Gladow T., Derlien S., Heutehaus L., Weidner N., Smolenski U., Rupp R. Trainer in a pocket-proof-of-concept of mobile, real-time, foot kinematics feedback for gait pattern normalization in individuals after stroke, incomplete spinal cord injury and elderly patients. J. Neuroeng. Rehabil. 2018;15:44. doi: 10.1186/s12984-018-0389-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Schwenk M., Hauer K., Zieschang T., Englert S., Mohler J., Najafi B. Sensor-derived physical activity parameters can predict future falls in people with dementia. Gerontology. 2014;60:483–492. doi: 10.1159/000363136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Schwenk M., Grewal G.S., Holloway D., Muchna A., Garland L., Najafi B. Interactive sensor-based balance training in older cancer patients with chemotherapy-induced peripheral neuropathy: A randomized controlled trial. Gerontology. 2016;62:553–563. doi: 10.1159/000442253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shema-Shiratzky S., Hillel I., Mirelman A., Regev K., Hsieh K.L., Karni A., Devos H., Sosnoff J.J., Hausdorff J.M. A wearable sensor identifies alterations in community ambulation in multiple sclerosis: Contributors to real-world gait quality and physical activity. J. Neurol. 2020;26:1912–1921. doi: 10.1007/s00415-020-09759-7. [DOI] [PubMed] [Google Scholar]
- 95.Sprint G., Cook D.J., Weeks D.L., Borisov V. Predicting functional independence measure scores during rehabilitation with wearable inertial sensors. IEEE Access. 2015;3:1350–1366. doi: 10.1109/ACCESS.2015.2468213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Terrier P., Le Carré J., Connaissa M.L., Léger B., Luthi F. Monitoring of gait quality in patients with chronic pain of lower limbs. IEEE Trans. Neural Syst. Rehabil. Eng. 2017;25:1843–1852. doi: 10.1109/TNSRE.2017.2688485. [DOI] [PubMed] [Google Scholar]
- 97.Teufl W., Taetz B., Miezal M., Lorenz M., Pietschmann J., Jöllenbeck T., Fröhlich M., Bleser G. Towards an inertial sensor-based wearable feedback system for patients after total hip arthroplasty: Validity and applicability for gait classification with gait kinematics-based features. Sensors. 2019;19:5006. doi: 10.3390/s19225006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ullrich M., Küderle A., Hannink J., Del Din S., Gaßner H., Marxreiter F., Klucken J., Eskofier B.M., Kluge F. Detection of gait from continuous inertial sensor data using harmonic frequencies. IEEE J. Biomed. Health Inform. 2020;24:1869–1878. doi: 10.1109/JBHI.2020.2975361. [DOI] [PubMed] [Google Scholar]
- 99.Ummels D., Beekman E., Theunissen K., Braun S., Beurskens A.J. Counting steps in activities of daily living in people with a chronic disease using nine commercially available fitness trackers: Cross-sectional validity study. JMIR mHealth uHealth. 2018;6:e70. doi: 10.2196/mhealth.8524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Vadnerkar A., Figueiredo S., Mayo N.E., Kearney R.E. Design and validation of a biofeedback device to improve heel-to-toe gait in seniors. IEEE J. Biomed. Health Informatics. 2017;22:140–146. doi: 10.1109/JBHI.2017.2665519. [DOI] [PubMed] [Google Scholar]
- 101.Wang C., Goel R., Noun M., Ghanta R.K., Najafi B. Wearable Sensor-Based Digital Biomarker to Estimate Chest Expansion During Sit-to-Stand Transitions–A Practical Tool to Improve Sternal Precautions in Patients Undergoing Median Sternotomy. IEEE Trans. Neural Syst. Rehabil. Eng. 2019;28:165–173. doi: 10.1109/TNSRE.2019.2952076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wüest S., Masse F., Aminian K., Gonzenbach R., De Bruin E.D. Reliability and validity of the inertial sensor-based Timed “Up and Go” test in individuals affected by stroke. J. Rehabil. Res. Dev. 2016;53:599–610. doi: 10.1682/JRRD.2015.04.0065. [DOI] [PubMed] [Google Scholar]
- 103.Zhai Y., Nasseri N., Pöttgen J., Gezhelbash E., Heesen C., Stellmann J.P. Smartphone accelerometry: A smart and reliable measurement of real-life physical activity in multiple sclerosis and healthy individuals. Front. Neurol. 2020;11:688. doi: 10.3389/fneur.2020.00688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Keogh A., Taraldsen K., Caulfield B., Vereijken B. It’s not about the capture, it’s about what we can learn”: A qualitative study of experts’ opinions and experiences regarding the use of wearable sensors to measure gait and physical activity. J. Neuroeng. Rehabil. 2021;18:78. doi: 10.1186/s12984-021-00874-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Martindale C.F., Roth N., Hannink J., Sprager S., Eskofier B.M. Smart annotation tool for multi-sensor gait-based daily activity data; Proceedings of the 2018 IEEE International Conference on Pervasive Computing and Communications Workshops (PerCom Workshops); Athens, Greece. 19–23 March 2018; pp. 549–554. [Google Scholar]
- 106.Ordóñez F.J., Roggen D. Deep convolutional and lstm recurrent neural networks for multimodal wearable activity recognition. Sensors. 2016;16:115. doi: 10.3390/s16010115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Truong A., Walters A., Goodsitt J., Hines K., Bruss C.B., Farivar R. Towards automated machine learning: Evaluation and comparison of AutoML approaches and tools; Proceedings of the 2019 IEEE 31st International Conference on Tools with Artificial Intelligence (ICTAI); Portland, OR, USA. 4–6 November 2019; pp. 1471–1479. [Google Scholar]
- 108.Lundberg S.M., Erion G., Chen H., DeGrave A., Prutkin J.M., Nair B., Katz R., Himmelfarb J., Bansal N., Lee S.I. From local explanations to global understanding with explainable AI for trees. Nat. Mach. Intell. 2020;2:2522–5839. doi: 10.1038/s42256-019-0138-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rawassizadeh R., Pierson T.J., Peterson R., Kotz D. NoCloud: Exploring network disconnection through on-device data analysis. IEEE Pervasive Comput. 2018;17:64–74. doi: 10.1109/MPRV.2018.011591063. [DOI] [Google Scholar]
- 110.Dobbins C., Rawassizadeh R. Towards clustering of mobile and smartwatch accelerometer data for physical activity recognition. Informatics. 2018;5:29. doi: 10.3390/informatics5020029. [DOI] [Google Scholar]
- 111.Vallati C., Virdis A., Gesi M., Carbonaro N., Tognetti A. ePhysio: A wearables-enabled platform for the remote management of musculoskeletal diseases. Sensors. 2019;19:2. doi: 10.3390/s19010002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Park S.J., Hussain I., Hong S., Kim D., Park H., Benjamin H.C.M. Real-time Gait Monitoring System for Consumer Stroke Prediction Service; Proceedings of the 2020 IEEE International Conference on Consumer Electronics (ICCE); Las Vegas, NV, USA. 4–6 January 2020; pp. 1–4. [Google Scholar]
- 113.Jourdan T., Boutet A., Frindel C. Toward privacy in IoT mobile devices for activity recognition; Proceedings of the 15th EAI International Conference on Mobile and Ubiquitous Systems: Computing, Networking and Services (MobiQuitous); New York, NY, USA. 5–7 November 2018; pp. 155–165. [Google Scholar]
- 114.Debs N., Jourdan T., Moukadem A., Boutet A., Frindel C. Motion sensor data anonymization by time-frequency filtering; Proceedings of the 2020 28th European Signal Processing Conference (EUSIPCO); Amsterdam, The Netherlands. 18–21 January 2021; pp. 1707–1711. [Google Scholar]
- 115.Sozinov K., Vlassov V., Girdzijauskas S. Human activity recognition using federated learning; Proceedings of the 2018 IEEE Intl Conf on Parallel & Distributed Processing with Applications, Ubiquitous Computing & Communications, Big Data & Cloud Computing, Social Computing & Networking, Sustainable Computing & Communications (ISPA/IUCC/BDCloud/SocialCom/SustainCom); Melbourne, Australia. 11–13 December 2018; pp. 1103–1111. [Google Scholar]
- 116.Bzdok D., Altman N., Krzywinski M. Points of Significance: Statistics Versus Machine Learning. Nat. Methods. 2018;15:233–234. doi: 10.1038/nmeth.4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data is contained within the article.