Abstract
Cultures of the mussel Mytilus galloprovincialis are frequently affected by accumulation of the amnesic shellfish poisoning toxin domoic acid (DA). This species is characterized by a fast uptake and release of the toxin. In this work, the main characteristics of the uptake mechanism have been studied by incubation of digestive gland thin slices in media with different composition and DA concentration. DA uptake seems to follow Michaelis–Menten kinetics, with a very high estimated KM (1722 µg DA mL−1) and a Vmax of 71.9 µg DA g−1 h−1, which is similar to those found for other amino acids in invertebrates. Replacement of NaCl from the incubation media by Cl-choline (Na+-free medium) did not significantly reduce the uptake, but replacement by sorbitol (Na+-free and Cl−-depleted medium) did. A new experiment replacing all chlorides with their equivalent gluconates (Na+- and Cl−-free medium) showed an important reduction in the uptake that should be attributed to the absence of chloride, pointing to a Na+-independent, Cl− (or anion-) dependent transporter. In media with Na+ and Cl−, neither decreasing the pH nor adding cyanide (a metabolic inhibitor) had significant effect on DA uptake, suggesting that the transport mechanism is not H+- or ATP-dependent. In a chloride depleted medium, lowering pH or adding CN increased the uptake, suggesting that other anions could, at least partially, substitute chloride.
Keywords: membrane transporter, uptake velocity, sodium-independent, chloride-dependent, accumulation, maximum levels, cyanide, pH, Pseudo-nitzschia cell quota
1. Introduction
Domoic acid is a tricarboxylic amino acid first isolated from the red alga Chondria armata [1]. In 1987, it was identified as the main substance responsible [2] for a number of cases of intoxication caused by consumption of mussels grown in Prince Edward Island, Canada [3,4]. The syndrome associated with the intoxication was characterized mainly by the loss of recent memory, which led to it being named Amnesic Shellfish Poisoning (ASP) [5]. Two years after the first intoxication, the source of domoic acid in the plankton was identified as the pennate diatom Nitzschia pungens (currently Pseudo-nitzschia multiseries) [6]. Since then, populations of toxic Pseudo-nitzschia and the presence of domoic acid in bivalves have been reported worldwide [7,8,9,10,11,12,13,14,15,16,17].
Domoic acid accumulation in bivalves varies substantially between species. It is known that this variability is in part due to differences in depuration velocity. Some bivalves depurate most of the toxin very quickly, as is the case for the mussels M. galloprovincialis [18], M. edulis [19,20], Mesodesma donacium [21], and the scallop Argopecten purpuratus [22], while other species can retain it for very long periods of time, such as the razor clam Siliqua patula [23,24,25] or the king scallop Pecten maximus [26,27,28]. Mafra et al. [29,30] showed that the reduction in the amount of toxic cells ingested by pre-ingestive selection of the phytoplankton species can partially explain the observed differences between bivalve species, but the role of toxin absorption mechanisms in accumulation (acting after toxin ingestion) has not been sufficiently studied.
The presence of domoic acid in marine organisms, and especially in bivalves, produces important losses to fisheries and aquaculture because harvesting and marketing is banned when the DA concentrations exceed the established maximum allowable level, which in European Union and most countries is currently 20 mg kg−1 of soft tissues [31]. Furthermore, the high risk of persistent DA contamination discourages attempts to culture certain species of high commercial interest, such as Pecten maximus, because of the high risk of not being able to market it for months or even years if they are exposed to a toxic Pseudo-nitzschia bloom [26,32].
Developing methods to reduce the DA absorption, by chemical or biological treatments, genetic selection, or others, would be very useful for minimizing the consequences of Pseudo-nitzschia blooms. To effectively develop those methods, it is necessary to know the mechanism by which the toxin is taken up by the digestive gland of the bivalves (the main organ by which DA is incorporated from particles [20]), which is not currently known. Furthermore, very little information about DA absorption by bivalves exists.
In the digestive gland of the mussel M. edulis, the absorption has been suggested to take place by a cellular membrane transporter characterized by a marginal dependence on ATP and by the competitive inhibition of domoic acid intake by some structurally related amino acids, like kainic and glutamic acids, or proline [33].
In this work, we attempted to characterize in detail the mechanism of absorption of DA in the digestive gland of the mussel M. galloprovincialis. In a series of experiments, the implications of a transporter, sodium, chloride, pH, and energy have been checked in order to confirm that a transporter is involved, to contribute to its identification, and to ascertain the uptake kinetics.
2. Results
2.1. Domoic Acid Uptake Velocity and Saturation of the Transport
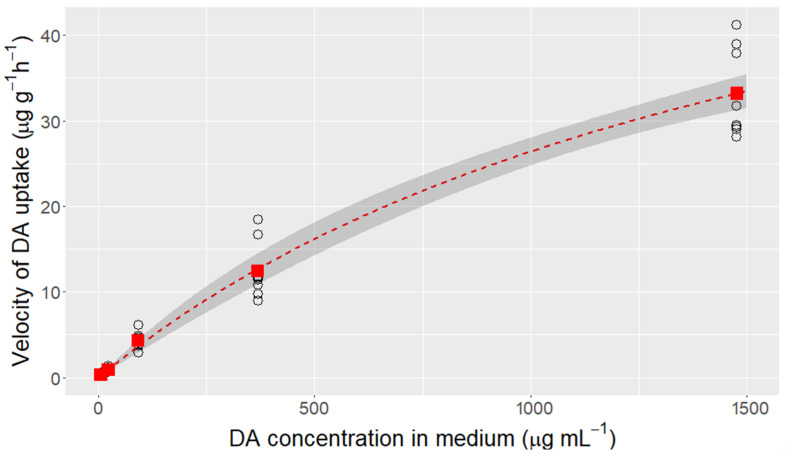
The uptake of domoic acid from seawater by the mussel’s digestive gland followed Michaelis–Menten kinetics (at least in its initial part) but the maximum concentration used in this study was still well below the saturation level. The estimated Vmax and Km were 71.9 µg DA g−1 h−1 and 1722 µg DA mL−1, respectively (Figure 1).
Figure 1.
Domoic acid uptake rate by mussel digestive gland slices as a function of the DA concentration in the incubation medium. Circles are the actual values for each slice, solid squares are the means for each concentration and the dotted line is the fitted Michaelis–Menten kinetics. The shadowed area represents the 95% confidence interval for the fitted kinetics.
2.2. Effect of Environmental Sodium
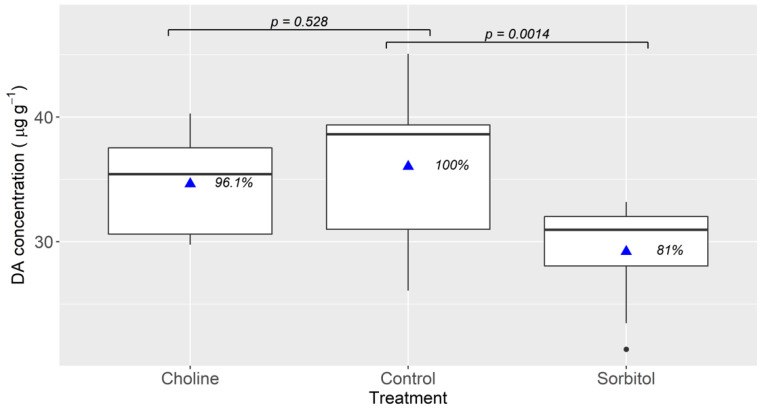
The results obtained when the incubation media did not contain Na were different depending on the compound which was used to replace NaCl. When choline (added to the medium as Cl-choline) was used, no significant reduction in the uptake was observed, while when sorbitol was used it was significantly reduced (Figure 2).
Figure 2.
Accumulation of domoic acid in mussel digestive gland tissues after 3 hours of incubation in seawater (control) and in two other media in which NaCl was replaced by choline chloride and sorbitol. The upper and lower limits of the boxes are the quartiles, the middle horizontal line is the median, the extremes of the vertical lines are the upper and lower limits of the observations, the dots are the outliers (values that deviate from the median more than 1.5 times the interquartile range), the triangles are the mean and the numbers to the right of the triangles are the percentages of the control treatment. The horizontal segments with associated probability values indicate the significance of the difference between the treatments at the extremes of the segments (paired Student t-test).
2.3. Effects of Cyanide in a Chloride-Depleted Environment and pH
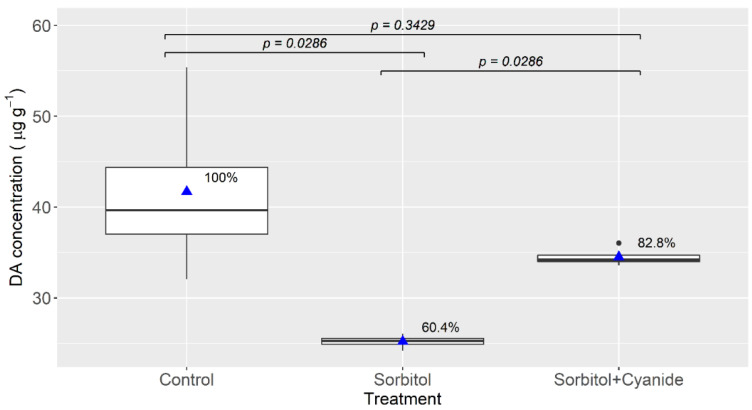
When cyanide was added to the sorbitol medium (chloride-depleted), a significant increase in the DA uptake, compared to the sorbitol medium alone, took place. The final DA concentration in the slices incubated in this medium was nearly 83% of the one in the control (seawater without cyanide) (Figure 3).
Figure 3.
Accumulation of domoic acid in mussel digestive gland tissues after 3 hours of incubation in seawater (control), and in two other media with NaCl replaced by sorbitol, one of which was supplemented with sodium cyanide. The upper and lower limits of the boxes are the quartiles, the middle horizontal line is the median, the extremes of the vertical lines are the upper and lower limits of the observations, the dots are the outliers, the triangles are the mean, and the numbers to the right of the triangles are the percentages of the control treatment. The horizontal segments with associated probability values indicate the significance of the difference between the treatments at the extremes of the segments (Wilcoxon test).
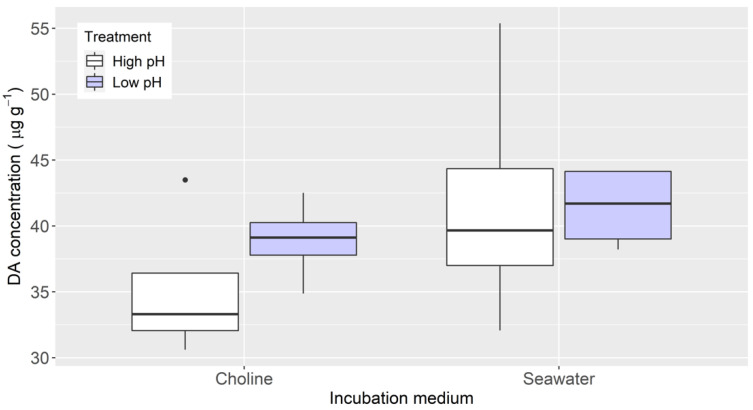
The pH changes did not significantly affect the DA uptake, neither in the “choline” nor in the “seawater” medium. In both cases, and especially in the “choline” medium, the slices absorbed more DA when the pH was lower (with H3PO4 added) (Figure 4).
Figure 4.
Accumulation of domoic acid in mussel digestive gland tissue after 3 hours of incubation in seawater with high and low pH, and a medium in which NaCl was replaced by choline chloride, also with high and low pH. The upper and lower limits of the boxes are the quartiles, the middle horizontal line is the median, the extremes of the vertical lines are the upper and lower limits of the observations, the dots are the outliers.
2.4. Effect of Environmental Chloride and Cyanide
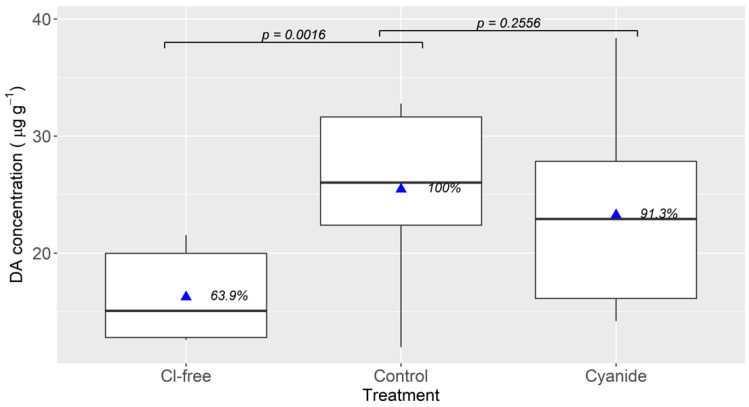
When all chlorides were omitted from the culture medium, DA uptake was significantly reduced, with the DA accumulated at the end of the experiment being 63.9% that of the control (Figure 5). This percentage was also lower than that obtained in the treatment with sorbitol in Section 4.3, in which some chlorides (but not NaCl) were present in the incubation medium.
Figure 5.
Accumulation of domoic acid in mussel digestive gland tissues after 3 hours of incubation in seawater (control), a medium in which all the chlorides were replaced by their corresponding gluconates (Cl-free), and in seawater to which the metabolic inhibitor sodium cyanide was added (cyanide). The upper and lower limits of the boxes are the quartiles, the middle horizontal line is the median, the extremes of the vertical lines are the upper and lower limits of the observations, the dots are the outliers, the triangles are the mean and the numbers to the right of the triangles are the percentages of the control treatment. The horizontal segments with associated probability values indicate the significance of the difference between the treatments at the extremes of the segments (paired Student t-test).
The addition of cyanide to the control medium had a minor, and not statistically significant, effect on DA uptake by the digestive gland slices.
3. Discussion
The uptake of DA by the mussel digestive gland is not linear with the concentration in the environment. The data obtained effectively follows Michaelis–Menten kinetics, suggesting that the uptake is carried out by a transporter protein of the cell membrane. This mechanism has already been suggested by Madhyastha et al. [33] in light of the effect that some structurally-related amino acids had on the uptake velocity. The uptake parameters have not been precisely estimated because the DA concentrations used were not high enough to approach the asymptote of the Michaelis–Menten curve, but it is very likely that they constitute a good approximation.
The uptake velocity is of the same order of magnitude as those found for other amino acids in invertebrates, such as glycine and alanine [34] in mussel M. edulis larvae, L-valine in the annelid Nereis virens [35], glutamic acid in several Nereis species [36], or in the crab Carcinus maenas [37], and others in Crassostrea gigas [38].
In extreme, but possible, conditions during a Pseudo-nitzschia bloom, the transporter could be saturated because the DA concentrations attained in the digestive system could be much higher than the estimated Km. For example, assuming bloom of P. australis (the most toxic Pseudo-nitzschia species), in which the cells have a volume of 750 µm3 and a DA content of 25 pg (as found in some cultures [39]), and also assuming that 90% of the digestive system is occupied by disrupted cells, the DA concentration in the digestive system would be 17,333 µg mL−1., that is, more than 10 times higher than the estimated Km for the DA uptake. Under these conditions, therefore, the uptake velocity would be maximal, and mussels could uptake DA at levels exceeding the regulatory limit in approximately 3 h (considering that the digestive gland of a mussel weighs approximately 1 g and represents 10% of the body weight, on average). Under more realistic conditions (using the highest estimates of P. australis biovolume (4084 µm3) [40], a DA cell quota of 4 pg cell−1, and a digestive system occupation of 50%), the uptake velocity would be approximately 16 µg DA g−1 h−1 and the mussels would take approximately 13 h to attain the regulatory limit concentration. These estimated times would be longer if some Pseudo-nitzschia cells pass through the digestive system intact, as happens with Alexandrium in the clam Mercenaria [41].
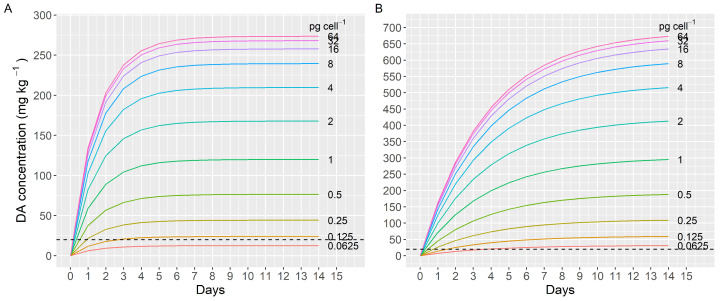
When longer periods of time are considered (14 days of continuous intoxication) and a depuration rate of 0.68 day−1 (recalculated from Blanco et al. [18]) is also taken into account, the simulation of the DA accumulation shows that the DA concentration in the mussels reaches a maximum, which increases very slowly with the toxin content of the Pseudo-nitzchia cells, of around 260 mg kg−1 (Figure 6). This value is consistent with the maximum value (248 mg kg−1) recorded in more than 25 years of monitoring in Galicia (data from Intecmar, publication in preparation). With toxin concentrations in the digestive system below those produced by a 100% occupation and DA per cell of approximately 0.1 pg, the toxin concentration in the mussel is not expected to reach the regulatory level (Figure 6A). It can be observed that an increase in cell toxin content above 16 pg has little effect on the maximum toxin accumulated by mussels with both depuration rates (Figure 6A,B). Notwithstanding this, those maximum values could be substantially affected by changes in the depuration rate.
Figure 6.
Simulated domoic acid accumulation in mussels, as a function of the Pseudo-nitzschia cell toxin content, assuming a precise cell volume, that all cells are completely filling the digestive system, and depuration rates of 0.68 (A), and 0.27 day−1 (B). The dashed line represents the regulatory level.
In Prince Edward Island, Canada, where the first human intoxication by domoic acid was recorded, mussels of a closely related species, M. edulis, were found to contain 790 mg kg−1 of DA. Assuming that the characteristics of its DA transporter is similar to the one of M. galloprovincialis, and using the depuration rate for large starved mussels at 6 °C and salinity of 18 re-calculated from the data by Novaczek et al. [19], which is 0.46 day−1, the maximum attainable concentration would be around 400 mg kg−1. Those authors found a noticeable reduction in the depuration rate with temperature. From their data, we have estimated a rate of 0.27 day−1 for 3 °C, which is not unlikely for the surficial water of the area in November–December. In such a case, the maximum DA level that could be attained would be around 680 mg kg−1, which is also coherent, even than somewhat lower, with the existing data. In other species, as the king scallop P. maximus with much lower depuration rate, even if the transporter has the same characteristics, the accumulation would be expected to be substantially higher (Figure S1).
The transporter involved (or at least the main one) does not seem to be Na+-dependent, because the replacement of the salts containing Na by others containing K or choline chloride did not have any significant effect on DA uptake (Figure 2). The replacement of NaCl by sorbitol did reduce the uptake but, considering the results obtained when NaCl was replaced by choline chloride, the reduction should be attributed to the much smaller concentration of chloride in that medium (Cl− was still present in the medium because some salts containing Cl− were not replaced). Another experiment, carried out to confirm the effect of Cl−, in which Cl−-containing salts were replaced by their corresponding gluconates, showed that the uptake was significantly reduced, suggesting that the DA transport could be chloride-dependent, but not strictly dependent because the uptake in absence of Cl− was not completely suppressed. The involvement of several transporters, while possible, seems unlikely because the uptake follows Michaelis–Menten kinetics and it would require that all the transporters involved had the same uptake parameters.
The small effect that the addition of cyanide had in the DA uptake when the tissues were incubated in seawater (sodium- and chloride-replete media) suggests that the transport is not directly ATP-dependent. The lack of effect of the pH under the same conditions also suggests that it is not H+-dependent. Nevertheless, these two treatments increased the uptake in chloride-depleted media. This opens the possibility that other anions could replace chloride; this aspect, as well as the dependence of the uptake on the intensity of the chloride gradient, deserves additional study.
Most amino acid transporters are Na+-dependent [42,43,44,45,46,47]. Some Cl−-dependent amino acid transport systems have also been described, but most of them also require Na+ [42,48,49,50]. Na+-independent amino acid transporters exist [51] but are much less common, at least in mammals. Cl−-dependent glutamate transport has been described in the synaptic membrane [52]. It was hypothesized to be an exchange between glycine and glutamate and was induced by the energy shortage derived from ischemia, which would be consistent with the little effect that cyanide has on the domoic acid uptake. Sialin, the aspartate transporter described by Miyaji et al. [53], which is Na+-independent and Cl−-dependent, could be a possible candidate, but it was found to be overexpressed in mussel digestive gland tissue during domoic acid depuration [54], therefore, increasing its expression in the opposite direction to that expected if it was involved in the uptake. Another group of membrane proteins, the Organic Anion Transporters (OATs) which have some Cl−-dependent members, could be involved in the uptake of DA. The Organic Anion Transporter Proteins (OATPs), for example (that are involved in the hepatic function of mammals) can transport anions such as sulfobromophthalein (BSP) in a Na+-independent way but with important reduction in the rate when chloride is omitted from the incubation medium of rat hepatocytes [55,56]. The unequivocal identification of the transporter involved in DA uptake will require further study.
4. Materials and Methods
4.1. Biological Material and Sample Preparation
Mussels M. galloprovincialis from the Galician Rías were supplied by the Instituto Tecnolóxico para o Control do Medio Mariño (Intecmar, Vilagarcía de Arousa, Spain).
For all experiments, the mussels were opened, dissected to isolate the digestive gland, and then 3 to 6 thin (less than 1 mm) slices of approximately 80–100 mg were obtained from the central part of the digestive gland by mean of transversal cuts. The small part of stomach epithelium which remained was dissected and discarded, keeping only the hepatopancreas tissues, which are responsible for absorption and internal digestion. Each slice was weighed and placed into a culture plate with 24 wells filled with 2 mL of filter-sterilized seawater and very gently shaken in an orbital shaker (the minimum speed which produced an observable movement of the slices). Once all the needed slices were weighed and washed, they were then rinsed and washed again with seawater and transferred to other 24-well plates containing the incubation media corresponding to the treatments in each experiment. In Section 4.2, the slices were attributed to the treatments randomly. In all other experiments, slices of the same mussels were assigned to each treatment. Additionally, one slice of each mussel was extracted with 50% MeOH to quantify the initial DA concentration. The slices were incubated for 3 h, in the dark, at room temperature (20–25 °C), while subjected to gentle agitation using an orbital shaker.
At the end of the incubation period, the slices were placed in Eppendorf tubes filled with isotonic ammonium formate and centrifuged at 1000× g for 5 min, discarding the supernatant, twice, to remove the remains of the incubation medium. Finally, the DA in the slices was extracted by adding 0.3 mL of 50% MeOH and frozen at −80 °C. After 1–3 days, the samples were thawed and homogenized in an ultrasonic bath filled with a mixture of water and ice. Finally, the homogenates were clarified by centrifugation at 19,000× g, filtered through 0.22 µm syringe filters, and frozen at −80 °C until analysis.
4.2. Domoic Acid Uptake Velocity and Saturation of the Transport
This experiment was aimed at checking if the absorption of DA by the digestive gland is linearly dependent (typical of free passage through the cell membrane) or not (typical of the involvement of transporters or carriers) on the concentration of domoic acid in the environment.
The base of the incubation media in this experiment was filter-sterilized (0.22 µm) seawater (salinity = 34). The base medium was supplemented with domoic acid (ABCAM, Cambridge, UK) to obtain concentrations of 5.8, 23.1, 92.3, 369.1 and 1476 µg mL−1. Eight slices were incubated in each concentration, and DA extracted following the procedure described above, in this section.
4.3. Effect of Environmental Sodium
This experiment was designed to check if the uptake of DA by the digestive gland of the mussel is Na-dependent, as is the case for many amino acid transporters.
Instead of the seawater used in Section 4.2, three artificial seawaters were used. The first one, used as “control”, was prepared by adding 24.55 g of NaCl, 0.75 g of KCl, 4.07 g of MgSO4·7H2O, 1.47 g of CaCl2·2H2O, 6.04 g of MgCl2·6H2O, and 0.21 g of NaHCO3 to 1 L of Milli-Q water. The two others (labeled as “choline” and “sorbitol”) did not contain Na. In both of them, NaHCO3 was replaced by KHCO3, and NaCl was replaced by Choline-Cl (choline medium) and sorbitol (“sorbitol” medium). Domoic acid was added to all incubation media to a 500-µg mL−1 level.
Eight slices were incubated in each medium and extracted following the procedure described above, in this section.
4.4. Effects of Cyanide in a Chloride-Depleted Environment and pH
This experiment was carried out to check if the uptake of domoic acid was proton-dependent, and as a preliminary evaluation of the effect of cyanide. To evaluate the effect of pH, two aliquots of the incubation media “control” and “choline” of Section 4.3. were used. The pH of one aliquot of each medium was lowered by addition of approximately 25 µL of 85% H3PO4. Eight slices were incubated in each of the two incubation media (“control” and “choline”), four at each pH treatment (6.5 and 5.1 for the “control”, 7.2 and 5.4 for the “choline” medium). DA was extracted following the procedure described in Section 4.3. To preliminarily evaluate the effect of the metabolic inhibitor cyanide, two aliquots of the remaining treatment (sorbitol) of Section 4.3 were used. NaCN was added to one of them, to a final concentration of 5 mM. Four slices were incubated in each medium, with and without NaCN.
4.5. Effect of Environmental Chloride and Cyanide
The aim of this experiment was two-fold. First, to confirm that the transporter was chloride-dependent (discarding a possible effect of sorbitol and the combined effect of Na+ and Cl− depletion), and second, to evaluate the effect of cyanide, a metabolic inhibitor, on the uptake in Na+- and Cl−-sufficient media.
The control treatment was the same as in Section 4.3. For the Cl−-free treatment, NaCl was replaced by sodium gluconate (instead of sorbitol) and all other chlorides were replaced by their correspondent gluconates. For the cyanide treatment, a medium made of the same components as the control but with NaCN added to a 5 mM final concentration was used.
4.6. LC-MS/MS Analysis
The analysis of DA in the extracts obtained was carried out by LC-MS/MS using a Thermo Accela coupled chromatographic system, through a HESI-II electrospray interface, to a Thermo Quantum Access Max triple quadrupole mass spectrometer (Thermo Fisher Scientific, Waltham, MA, USA).
The chromatographic method used a Kinetex C18 (50 × 2.5 mm, 2.6 µm) reversed-phase chromatographic column (Phenomenex, Torrance, CA, USA), 0.2% formic acid as phase A, and 50% MeOH as phase B. The run started at 100% A, changing linearly to 45% A from min 2 to min 4, maintaining this proportion for 2 min, and then returning in 1.4 min to the initial conditions, which were maintained for 1.6 min to re-equilibrate the column before the next injection. The flow rate was 280 µL min−1 and the injection volume 5 µL.
The mass spectrometer was operated in positive ionization mode, with 3500 V of capillary voltage, 20 and 10 nominal units of sheath and auxiliary gas (nitrogen), respectively, 100 °C of capillary temperature, 250 °C of transfer tube temperature, and 1.5 mTorr of collision gas (argon) pressure. The transition 312.1 > 266.1 (collision energy = 15 V) was used for quantification and 312.1 > 248.1 (collision energy = 17) for confirmation.
The quantification was made by the external standard method using solution provided by CIFGA (Lugo, Spain) as a certified reference.
The LOQ (s/n = 10) and LOD (s/n = 3) of the method are 26.3 and 7.9 ng mL−1, respectively. They were computed by extrapolation of the s/n obtained for a 39.5 ng mL−1 solution, for the peak of the confirmation transition.
4.7. Statistical Analysis and Simulation
All statistical analysis and plotting has been carried out with R [57], using the base module and different packages. GGPLOT2 [58] was used for most plots, DRC package [59] for fitting the Michaelis–Menten model, MULTCOMP [60] for post hoc tests, R stats for ANOVA, Student t and Wilcoxon tests, and deSolve [61] for the accumulation simulations (Text S1).
Acknowledgments
We acknowledge the Department of Biotoxins of the Instituto Tecnolóxico para o Control de Medio Mariño de Galicia (Intecmar) for supplying us with the mussels used in this study and for permitting inclusion of the domoic acid analyses.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/toxins13070458/s1, Text S1—R code for the simulation of domoic acid accumulation. Figure S1—Simulated domoic acid accumulation in King Scallops, as a function of the Pseudo-nitzschia cell toxin content.
Author Contributions
Conceptualization, J.B.; methodology, J.B., A.E.R., C.M. and H.M.; software, J.B.; investigation, J.B., A.E.R., C.M. and H.M.; writing—original draft preparation, J.B.; writing—review and editing, J.B., A.E.R. and G.Á.; project administration, C.M.; funding acquisition, J.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Ministerio de Economía y Competitividad, grant number AGL2012-39972-C02-02.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Key Contribution
The membrane transporter responsible for uptake of domoic acid by the digestive gland of mussels has been partially characterized. It seems to be Na+-independent and Cl−- (or anion-) dependent, and to have a high capability to transport domoic acid without being directly dependent on an energy source.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Takemoto T., Daigo K. Constituents of Chondria armata. Chem. Pharm. Bull. 1958;6:578–580. doi: 10.1248/cpb.6.578b. [DOI] [PubMed] [Google Scholar]
- 2.Wright J.L.C., Boyd R.K., Freitas A.S.W., Falk M., Foxall R.A., Jamieson W.D., Laycock M.V., McCulloch A.W., McInnes A.G., Odense P. Identification of domoic acid, a neuroexcitatory amino acid, in toxic mussels from eastern Prince Edward Island. Can. J. Chem. 1989;67:481–490. doi: 10.1139/v89-075. [DOI] [Google Scholar]
- 3.Perl T.M., Bedard L., Kosatsky T., Hockin J.C., Todd E.C., McNutt L.A., Remis R.S. Amnesic shellfish poisoning: A new clinical syndrome due to domoic acid. Can. Dis. Wkly. Rep. 1990;16:7–8. [PubMed] [Google Scholar]
- 4.Perl T.M., Bédard L., Kosatsky T., Hockin J.C., Todd E.C., Remis R.S. An outbreak of toxic encephalopathy caused by eating mussels contaminated with domoic acid. N. Engl. J. Med. 1990;322:1775–1780. doi: 10.1056/NEJM199006213222504. [DOI] [PubMed] [Google Scholar]
- 5.Pulido O.M. Domoic Acid Toxicologic Pathology: A Review. Mar. Drugs. 2008;6:180–219. doi: 10.3390/md6020180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bates S., Bird C., Defreitas A., Foxall R., Gilgan M., Hanic L., Johnson G., McCulloch A., Odense P., Pocklington R., et al. Pennate diatom Nitzschia pungens as the primary source of domoic acid, a toxin in shellfish from eastern Prince Edward Island, Canada. Can. J. Fish. Aquat. Sci. 1989;46:1203–1215. doi: 10.1139/f89-156. [DOI] [Google Scholar]
- 7.Bates S.S. Domoic-acid-producing diatoms: Another genus added. J. Phycol. 2000;36:978–983. doi: 10.1046/j.1529-8817.2000.03661.x. [DOI] [Google Scholar]
- 8.Bates S.S. Amnesic shellfish poisoning: Domoic acid production by Pseudo-nitzschia diatoms. Aqua Info Aquac. Notes. 2004;16:4. [Google Scholar]
- 9.Bates S.S., Hubbard K.A., Lundholm N., Montresor M., Leaw C.P. Pseudo-nitzschia, Nitzschia, and domoic acid: New research since 2011. Harmful Algae. 2018;79:3–43. doi: 10.1016/j.hal.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 10.Fehling J., Green D.H., Davidson K., Bolch C.J., Bates S.S. Domoic acid production by Pseudo-nitzschia seriata (Bacillariophyceae) in Scottish waters. J. Phycol. 2004;40:622–630. doi: 10.1111/j.1529-8817.2004.03200.x. [DOI] [Google Scholar]
- 11.Gallacher S., Howard G., Hess P., MacDonald E., Kelly M.C., Bates L.A., Brown N., Mackenzie M., Gillibrand P., Turrell W.R. The occurrence of Amnesic Shellfish poison in shellfish from Scottish waters. In: Hallegraeff G.M., Blackburn S.I., Bolch C.J., Lewis R.J., editors. Harmful Algal Blooms 2000. IOC of UNESCO; Paris, French: 2001. pp. 30–33. [Google Scholar]
- 12.Lelong A., Hegaret H., Soudant P., Bates S.S. Pseudo-nitzschia (Bacillariophyceae) species, domoic acid and amnesic shellfish poisoning: Revisiting previous paradigms. Phycologia. 2012;51:168–216. doi: 10.2216/11-37.1. [DOI] [Google Scholar]
- 13.Sahraoui I., Bates S.S., Bouchouicha D., Mabrouk H.H., Hlaili A.S. Toxicity of Pseudo-nitzschia populations from Bizerte Lagoon, Tunisia, southwest Mediterranean, and first report of domoic acid production by P. brasiliana. Diatom Res. 2011;26:293–303. doi: 10.1080/0269249X.2011.597990. [DOI] [Google Scholar]
- 14.Tan S.N., Teng S.T., Lim H.C., Kotaki Y., Bates S.S., Leaw C.P., Lim P.T. Diatom Nitzschia navis-varingica (Bacillariophyceae) and its domoic acid production from the mangrove environments of Malaysia. Harmful Algae. 2016;60:139–149. doi: 10.1016/j.hal.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 15.Trainer V.L., Bates S.S., Lundholm N., Thessen A.E., Cochlan W.P., Adams N.G., Trick C.G. Pseudo-nitzschia physiological ecology, phylogeny, toxicity, monitoring and impacts on ecosystem health. Harmful Algae. 2012;14:271–300. doi: 10.1016/j.hal.2011.10.025. [DOI] [Google Scholar]
- 16.Dao H.V., Takata Y., Sato S., Fukuyo Y., Kodama M. Domoic acid in a bivalve Spondylus cruentus Nha Trang Bay Khanh Hoa Province, Vietnam. Coast. Mar. Sci. 2006;30:130–132. [Google Scholar]
- 17.Álvarez G., Uribe E., Quijano-Scheggia S., López-Rivera A., Mariño C., Blanco J. Domoic acid production by Pseudo-nitzschia australis and Pseudo-nitzschia calliantha isolated from North Chile. Harmful Algae. 2009;8:938–945. doi: 10.1016/j.hal.2009.05.005. [DOI] [Google Scholar]
- 18.Blanco J., Bermúdez de la Puente M., Arévalo F., Salgado C., Moroño A. Depuration of mussels (Mytilus galloprovincialis) contaminated with domoic acid. Aquat. Living Resour. 2002;15:53–60. doi: 10.1016/S0990-7440(01)01139-1. [DOI] [Google Scholar]
- 19.Novaczek I., Madhyastha M.S., Ablett R.F., Donald A., Johnson G., Nijjar M.S., Sims D.E. Depuration of domoic acid from live blue mussels (Mytilus edulis) Can. J. Fish. Aquat. Sci. 1992;49:312–318. doi: 10.1139/f92-035. [DOI] [Google Scholar]
- 20.Novaczek I., Madhyastha M.S., Ablett R.F., Johnson G., Nijjar M.S., Sims D.E. Uptake, disposition and depuration of domoic acid by blue mussels (Mytilus edulis) Aquat. Toxicol. 1991;21:103–118. doi: 10.1016/0166-445X(91)90009-X. [DOI] [Google Scholar]
- 21.Álvarez G., Uribe E., Regueiro J., Martin H., Gajardo T., Jara L., Blanco J. Depuration and anatomical distribution of domoic acid in the surf clam Mesodesma donacium. Toxicon. 2015;102:1–7. doi: 10.1016/j.toxicon.2015.05.011. [DOI] [PubMed] [Google Scholar]
- 22.Alvarez G., Rengel J., Araya M., Alvarez F., Pino R., Uribe E., Diaz P.A., Rossignoli A.E., Lopez-Rivera A., Blanco J. Rapid Domoic Acid Depuration in the Scallop Argopecten purpuratus and Its Transfer from the Digestive Gland to Other Organs. Toxins. 2020;12:698. doi: 10.3390/toxins12110698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Drum A.S., Siebens T.L., Crecelius E.A., Elston R.A. Domoic acid in the Pacific razor clam Siliqua patula (Dixon, 1789) J. Shellfish Res. 1993;12:443–450. [Google Scholar]
- 24.Horner R.A., Kusske M.B., Moynihan B.P., Skinner R.N., Wekell J.C. Retention of domoic acid by Pacific razor clams, Siliqua patula (Dixon, 1789): Preliminary study. J. Shellfish Res. 1993;12:451–456. [Google Scholar]
- 25.Trainer V.L., Bill B.D. Characterization of a domoic acid binding site from Pacific razor clam. Aquat. Toxicol. 2004;69:125–132. doi: 10.1016/j.aquatox.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 26.Blanco J., Acosta C.P., Bermúdez de la Puente M., Salgado C. Depuration and anatomical distribution of the amnesic shellfish poisoning (ASP) toxin domoic acid in the king scallop Pecten maximus. Aquat. Toxicol. 2002;60:111–121. doi: 10.1016/S0166-445X(01)00274-0. [DOI] [PubMed] [Google Scholar]
- 27.Blanco J., Acosta C.P., Mariño C., Muñíz S., Martín H., Moroño A., Correa J., Arévalo F., Salgado C. Depuration of domoic acid from different body compartments of the King Scallop Pecten maximus grown in raft culture and natural bed. Aquat. Living Resour. 2006;19:257–265. doi: 10.1051/alr:2006026. [DOI] [Google Scholar]
- 28.Bogan Y.M., Kennedy D., Harkin A.L., Gillespie J., Hess P., Slater J.W. Comparison of domoic acid concentration in king scallops, Pecten maximus from seabed and suspended culture systems. J. Shellfish Res. 2006;25:129–135. doi: 10.2983/0730-8000(2006)25[129:CODACI]2.0.CO;2. [DOI] [Google Scholar]
- 29.Mafra L.L., Bricelj V.M., Ouellette C., Bates S.S. Feeding mechanics as the basis for differential uptake of the neurotoxin domoic acid by oysters, Crassostrea virginica, and mussels, Mytilus edulis. Aquat. Toxicol. 2010;97:160–171. doi: 10.1016/j.aquatox.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 30.Mafra L., Jr., Bricelj V., Ward J. Mechanisms contributing to low domoic acid uptake by oysters feeding on Pseudo-nitzschia cells. II. Selective rejection. Aquat. Biol. 2009;6:213–226. doi: 10.3354/ab00122. [DOI] [Google Scholar]
- 31.EFSA Panel on Contaminants in the Food Chain Marine biotoxins in shellfish–Summary on regulated marine biotoxins. EFSA J. 2009;7 doi: 10.2903/j.efsa.2009.1306. [DOI] [Google Scholar]
- 32.Arévalo F., Bermúdez de la Puente M., Salgado C. Seguimiento de biotoxinas marinas en las Rías Gallegas: Control y evolución durante los años 1995–1996. In: Vieites J.M., Leira F., editors. Proceedings of V Reunión Ibérica de Fitoplancton Tóxico y Biotoxinas, Actas de la Reunión. Anfaco-Cecopesca; Vigo, Spain: 1997. pp. 90–101. [Google Scholar]
- 33.Madhyastha M.S., Novaczek I., Ablett R.F., Johnson G., Nijjar M.S., Sims D.E. In vitro study of domoic acid uptake by digestive gland tissue of blue mussel (Mytilus edulis L.) Aquat. Toxicol. 1991;20:73–82. doi: 10.1016/0166-445X(91)90042-8. [DOI] [Google Scholar]
- 34.Manahan D.T. The uptake and metabolism of dissolved amino acids by bivalve larvae. Biol. Bull. 1983;164:236–250. doi: 10.2307/1541142. [DOI] [Google Scholar]
- 35.Jørgensen N.O.G. Uptake of L-valine and other amino acids by the polychaete Nereis virens. Mar. Biol. 1979;52:45–52. doi: 10.1007/BF00386856. [DOI] [Google Scholar]
- 36.Jørgensen N., Kristensen E. Uptake of Amino Acids by Three Species of Nereis (Annelida: Polychaeta). I. Transport Kinetics and Net Uptake from Natural Concentrations. Mar. Ecol. Prog. Ser. 1980;3:329–340. doi: 10.3354/meps003329. [DOI] [Google Scholar]
- 37.Blewett T.A., Goss G.G. A novel pathway of nutrient absorption in crustaceans: Branchial amino acid uptake in the green shore crab (Carcinus maenas) Proc. Biol. Sci. 2017;284 doi: 10.1098/rspb.2017.1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rice M.A., Stephens G.C. Uptake and internal distribution of exogenously supplied amino acids in the Pacific oyster, Crassostrea gigas (Thunberg) Aquaculture. 1987;66:19–31. doi: 10.1016/0044-8486(87)90280-8. [DOI] [Google Scholar]
- 39.Cusack C.K., Bates S.S., Quilliam M.A., Patching J.W., Raine R. Confirmation of domoic acid production by Pseudo-nitzschia australis (Bacillariophyceae) isolated from Irish waters. J. Phycol. 2002;38:1106–1112. doi: 10.1046/j.1529-8817.2002.01054.x. [DOI] [Google Scholar]
- 40.Walz P.M., Garrison D.L., Graham W.M., Cattey M.A., Tjeerdema R.S., Silver M.W. Domoic acid-producing diatom blooms in Monterey Bay, California: 1991–1993. Nat. Toxins. 1994;2:271–279. doi: 10.1002/nt.2620020505. [DOI] [PubMed] [Google Scholar]
- 41.Bricelj V.M., Shumway S.E. Paralytic Shellfish Toxins in Bivalve Molluscs: Occurrence, Transfer Kinetics, and Biotransformation. Rev. Fish. Sci. 1998;6:315–383. doi: 10.1080/10641269891314294. [DOI] [Google Scholar]
- 42.Seal R.P., Amara S.G. EXCITATORY AMINO ACID TRANSPORTERS: A Family in Flux. Annu. Rev. Pharmacol. Toxicol. 1999;39:431–456. doi: 10.1146/annurev.pharmtox.39.1.431. [DOI] [PubMed] [Google Scholar]
- 43.Kristensen A.S., Andersen J., Jørgensen T.N., Sørensen L., Eriksen J., Loland C.J., Strømgaard K., Gether U. SLC6 Neurotransmitter Transporters: Structure, Function, and Regulation. Pharmacol. Rev. 2011;63:585–640. doi: 10.1124/pr.108.000869. [DOI] [PubMed] [Google Scholar]
- 44.Boudko D.Y. Ancestry and progeny of nutrient amino acid transporters. Proc. Natl. Acad. Sci. USA. 2005;102:1360–1365. doi: 10.1073/pnas.0405183101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hyde R., Taylor P.M., Hundal H.S. Amino acid transporters: Roles in amino acid sensing and signalling in animal cells. Biochem. J. 2003;373:1. doi: 10.1042/bj20030405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Preston R.L. Transport of amino acids by marine invertebrates. J. Exp. Zool. 1993;265:410–421. doi: 10.1002/jez.1402650410. [DOI] [Google Scholar]
- 47.Bröer S. Amino Acid Transport Across Mammalian Intestinal and Renal Epithelia. Physiol. Rev. 2008;88:249–286. doi: 10.1152/physrev.00018.2006. [DOI] [PubMed] [Google Scholar]
- 48.Bröer S. The SLC6 orphans are forming a family of amino acid transporters. Neurochem. Int. 2006;48:559–567. doi: 10.1016/j.neuint.2005.11.021. [DOI] [PubMed] [Google Scholar]
- 49.Chen N.-H., Reith M.E.A., Quick M.W. Synaptic uptake and beyond: The sodium- and chloride-dependent neurotransmitter transporter family SLC6. Pflügers Arch. Eur. J. Physiol. 2004;447:519–531. doi: 10.1007/s00424-003-1064-5. [DOI] [PubMed] [Google Scholar]
- 50.Hatanaka T., Haramura M., Fei Y.-J., Miyauchi S., Bridges C.C., Ganapathy P.S., Smith S.B., Ganapathy V., Ganapathy M.E. Transport of Amino Acid-Based Prodrugs by the Na+-and Cl−-Coupled Amino Acid Transporter ATB 0,+ and Expression of the Transporter in Tissues Amenable for Drug Delivery. J. Pharmacol. Exp. Ther. 2004;308:1138–1147. doi: 10.1124/jpet.103.057109. [DOI] [PubMed] [Google Scholar]
- 51.Shaffer P.L., Goehring A., Shankaranarayanan A., Gouaux E. Structure and mechanism of a Na+-independent amino acid transporter. Science. 2009;325:1010–1014. doi: 10.1126/science.1176088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Koyama Y., Ishibashi T., Baba A. Increase in Chloride-Dependent l-Glutamate Transport Activity in Synaptic Membrane After In Vitro Ischemic Treatment. J. Neurochem. 2002;65:1798–1804. doi: 10.1046/j.1471-4159.1995.65041798.x. [DOI] [PubMed] [Google Scholar]
- 53.Miyaji T., Echigo N., Hiasa M., Senoh S., Omote H., Moriyama Y. Identification of a vesicular aspartate transporter. Proc. Natl. Acad. Sci. USA. 2008;105:11720–11724. doi: 10.1073/pnas.0804015105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pazos A.J., Ventoso P., Martínez-Escauriaza R., Pérez-Paralle M.L., Blanco J., Trivino J.C., Sanchez J.L. Transcriptional response after exposure to domoic acid-producing Pseudo-nitzschia in the digestive gland of the mussel Mytilus galloprovincialis. Toxicon. 2017;140:60–71. doi: 10.1016/j.toxicon.2017.10.002. [DOI] [PubMed] [Google Scholar]
- 55.Wolkoff A.W., Samuelson A.C., Johansen K.L., Nakata R., Withers D.M., Sosiak A. Influence of Cl− on organic anion transport in short-term cultured rat hepatocytes and isolated perfused rat liver. J. Clin. Investig. 1987;79:1259–1268. doi: 10.1172/JCI112946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Anwer M.S., Wolkoff A.W. Basolateral Plasma Membrane Organic Anion Transporters. In: Arias I.M., Alter H.J., Boyer J.L., Cohen D.E., Shafritz D.A., Thorgeirsson S.S., Wolkoff A.W., editors. The Liver. 1st ed. Wiley; New York, NY, USA: 2020. pp. 327–336. [DOI] [Google Scholar]
- 57.R Core Team . R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna, Austria: 2019. [Google Scholar]
- 58.Wickham H. ggplot2: Elegant Graphics for Data Analysis. Springer; Berlin, Germany: 2016. [DOI] [Google Scholar]
- 59.Ritz C., Baty F., Streibig J.C., Gerhard D. Dose-Response Analysis Using R. PLoS ONE. 2015;10:e0146021. doi: 10.1371/journal.pone.0146021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hothorn T., Bretz F., Westfall P. Simultaneous inference in general parametric models. Biom. J. 2008;50:346–363. doi: 10.1002/bimj.200810425. [DOI] [PubMed] [Google Scholar]
- 61.Soetaert K., Petzoldt T., Setzer R.W. Solving Differential Equations in R: Package deSolve. J. Stat. Softw. 2010;33 doi: 10.18637/jss.v033.i09. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.