Abstract
The development of new diagnostic methods resulted in the discovery of novel hepaciviruses in wild populations of the bank vole (Myodes glareolus, syn. Clethrionomys glareolus). The naturally infected voles demonstrate signs of hepatitis similar to those induced by hepatitis C virus (HCV) in humans. The aim of the present research was to investigate the geographical distribution of bank vole-associated hepaciviruses (BvHVs) and their genetic diversity in Europe. Real-time reverse transcription polymerase chain reaction (RT-qPCR) screening revealed BvHV RNA in 442 out of 1838 (24.0%) bank voles from nine European countries and in one of seven northern red-backed voles (Myodes rutilus, syn. Clethrionomys rutilus). BvHV RNA was not found in any other small mammal species (n = 23) tested here. Phylogenetic and isolation-by-distance analyses confirmed the occurrence of both BvHV species (Hepacivirus F and Hepacivirus J) and their sympatric occurrence at several trapping sites in two countries. The broad geographical distribution of BvHVs across Europe was associated with their presence in bank voles of different evolutionary lineages. The extensive geographical distribution and high levels of genetic diversity of BvHVs, as well as the high population fluctuations of bank voles and occasional commensalism in some parts of Europe warrant future studies on the zoonotic potential of BvHVs.
Keywords: bank vole hepaciviruses, HCV, Hepacivirus F, Hepacivirus J, rodent-borne pathogen, Europe, emerging virus
1. Introduction
The genus Hepacivirus, family Flaviviridae, comprises 14 species (International Committee on Taxonomy of Viruses, 2020, [1]) of enveloped viruses with an icosahedral nucleocapsid and a diameter of 50–65 nm. The single-stranded, positive-sense RNA genome with a length of approximately 9600 nucleotides (nt) encodes, in a single open reading frame, a polyprotein that is cleaved into ten proteins [2,3,4,5].
One of these hepaciviruses, the human hepatitis C virus (HCV), is one of the leading causes of liver cirrhosis and hepatocellular carcinoma [6]. The fact that this virus does not infect small mammals makes it challenging to find a suitable animal model [7,8]. However, the recent development of new diagnostic technologies has led to the identification of several new hepaciviruses in rodents, horses, cattle, dogs, and bats [9,10,11,12,13,14,15].
The bank vole is a rodent species inhabiting forests in large parts of Europe and southern parts of Western Siberia [16,17]. This species often shows multiannual fluctuations in population densities with peaks approximately every 3–4 years [18,19]. Phylogenetic analyses of bank voles (based on the mitochondrial cytochrome b gene, cyt b) revealed several evolutionary lineages, including Western, Eastern, Carpathian, Ural, and Italian, with different geographical distributions, likely caused by isolation of subpopulations in refugia during the last glacial period [20,21,22]. Interestingly, the current distribution of Puumala orthohantavirus (PUUV) largely follows this differentiation. In Central and Western Europe, PUUV is limited to the Western evolutionary lineage of the bank vole with few detections in Carpathian and Eastern lineage individuals in regions of sympatric occurrence with the Western lineage [23].
Molecular screening of rodents has resulted in the identification of two bank vole-associated hepacivirus (BvHV) clades [9]. The high genetic divergence of BvHV clade 1 and clade 2 led to their classification as distinct species within the genus Hepacivirus, namely, Hepacivirus J and Hepacivirus F, respectively. These viruses can be differentiated by real-time reverse transcription polymerase chain reaction (RT-qPCR) (Figure S1). The successful experimental infection of bank voles with BvHV has been proposed as the basis for the development of a small animal model for evaluation of human HCV infection [24].The objective of this study was to investigate the occurrence, geographical distribution, and genetic diversity of BvHV in bank voles and several other small mammal species from different geographic regions in Europe.
2. Materials and Methods
2.1. Collection of Small Mammals and Species Identification
Rodents and other small mammals were sampled in 14 European countries within the EU FP7 project EDENext and several national research projects [23,25,26,27,28,29] (Table 1; Figure 1). Rodent necropsy and tissue samplings followed previously established standard protocols. Morphological species determination for selected animals was confirmed by PCR and partial sequencing of the mitochondrial cyt b gene [30]. For evolutionary lineage identification of BvHV-infected bank voles, we selected 23 viral RNA-positive animals. Consensus sequences of a 764 nt fragment of cyt b gene were generated and included in a phylogenetic tree with reference sequences from different lineages of the bank vole [31]. The phylogenetic tree was constructed with MrBayes v3.2.7 [32], using a Markov chain Monte Carlo algorithm and the GTR+I+G substitution model, as determined using jModelTest v2.1.10 [33], and run for 4 × 106 generations. Results were visualized using FigTree v1.4.4 (http://tree.bio.ed.ac.uk/software/figtree/) (Figure S2).
Table 1.
Overview of mammal species screened for presence of BvHV RNA and RT-qPCR results.
| Order | Family | Species | No. of Hepacivirus-Positive/Tested Animals | % RT-qPCR Positive | Country (Total Number of Animals) |
|---|---|---|---|---|---|
| Rodentia | Cricetidae | Lemmus lemmus | 0/21 | 0 | FIN (21) |
| Cricetidae | Microtus agrestis | 0/8 | 0 | GER (8) | |
| Cricetidae | Microtus arvalis | 0/129 | 0 | GER (129) | |
| Cricetidae |
Microtus oeconomus (syn. Alexandromys oeconomus) |
0/2 | 0 | FIN (2) | |
| Cricetidae | Myodes glareolus (syn. Clethrionomys glareolus) | 442/1838 | 24.0 | GBR (61), GER (1297), FRA (99), ITA (25), NED (20), AUT (33), POL (15), SWE (223), SVK (65) | |
| Cricetidae | Myodes rutilus (syn. Clethrionomys rutilus) | 1/7 | 14.3 | FIN (7) | |
| Cricetidae | Myopus schisticolor | 0/9 | 0 | FIN (9) | |
| Muridae | Apodemus spp. | 0/31 | 0 | CRO (30), ITA (1) | |
| Muridae | Apodemus agrarius | 0/5 | 0 | CRO (2), GER (3) | |
| Muridae | Apodemus flavicollis | 0/209 | 0 | GER (206), ITA (3) | |
| Muridae | Apodemus sylvaticus | 0/39 | 0 | GER (39) | |
| Muridae | Micromys minutus | 0/1 | 0 | GER (1) | |
| Muridae | Mus musculus | 0/22 | 0 | GER (1), EST (8), FIN (3), LAT (8), LTU (2) |
|
| Muridae | Mus spp. | 0/2 | 0 | CRO (2) | |
| Muridae | Rattus spp. | 0/4 | 0 | CRO (4) | |
| Carnivora | Mustelidae | Mustela nivalis | 0/1 | 0 | ITA (1) |
| Eulipotyphla | Erinaceidae | Erinaceus europaeus | 0/1 | 0 | GER (1) |
| Soricidae | Crocidura leucodon | 0/1 | 0 | ITA (1) | |
| Soricidae | Crocidura russula | 0/1 | 0 | GER (1) | |
| Soricidae | Neomys fodiens | 0/1 | 0 | ITA (1) | |
| Soricidae | Sorex araneus | 0/37 | 0 | GER (37) | |
| Soricidae | Sorex alpinus | 0/1 | 0 | ITA (1) | |
| Soricidae | Sorex antinorii | 0/7 | 0 | ITA (7) | |
| Soricidae | Sorex coronatus | 0/26 | 0 | GER (26) | |
| Soricidae | Sorex minutus | 0/25 | 0 | GER (25) | |
| Total | 443/2428 | 18.2% |
AUT, Austria; CRO, Croatia; EST, Estonia; FIN, Finland; FRA, France; GBR, Great Britain; GER, Germany; ITA, Italy; LAT, Latvia; LTU, Lithuania; NED, The Netherlands; POL, Poland; SVK, Slovakia; SWE, Sweden.
Figure 1.
Map of bank vole and northern red-backed vole (*) trapping sites and the detection of Hepacivirus J (black) and Hepacivirus F (grey) in this study. Empty circles represent the lack of RNA detection of both hepacivirus species. Only trapping sites where five or more animals were sampled are shown. Colored areas correspond to the approximate distribution of the evolutionary lineages of the bank vole according to Filipi et al. [34], and colored triangles indicate evidence of the corresponding lineage in a bank vole in this study.
2.2. Nucleic Acid Extraction and RT-PCR Analyses
RNA extraction was performed using a phenol–chloroform protocol for liver or lung tissue samples of a large part of the sample collection, as previously described [25]. Another part of liver samples was homogenized in 500 µL phosphate-buffered saline (PBS). Here, RNA was extracted using Microlab Star automate (Hamilton Robotics, Reno, NV, USA) in combination with the Nucleo Spin 96 Virus Core Kit (Macherey-Nagel, Düren, Germany) according to the manufacturer’s instructions. For molecular screening, two different RT-qPCR assays were used for individual samples or for pools of six individuals: rodHCVeur assay [9] for Hepacivirus J, and RHV-NS3-Line4 assay [24] for Hepacivirus F. A subset of RT-qPCR-positive samples were then analyzed by conventional RT-PCR, targeting the NS3 gene, and amplification products were sequenced by dideoxy chain termination method (for primers, see Table S1).
2.3. BvHV Sequence, Phylogenetic, and Isolation-by-Distance Analyses
A 472 nt fragment of the NS3 gene of the BvHV strains was aligned using Geneious Prime 2019.1.1 and MAFFT v7.388. A phylogenetic tree was constructed from the alignment using MrBayes v3.2.7 [35], with a Markov chain Monte Carlo algorithm, using the GTR+I+G substitution model, as determined using jModelTest v2.1.10 [33], run for 6 × 106 generations; results were visualized with FigTree v1.4.4 (http://tree.bio.ed.ac.uk/software/figtree/).
A test for isolation-by-distance was performed including all BvHV sequences described here. Isolation-by-distance is the result of the accumulation of mutations in viral strains in local populations and spatially limited dispersal, and manifests as a statistical association between genetic differences and geographic distance [36]. Genetic distances between pairs of partial NS3 gene sequences from the study sites were estimated as p-distance using MEGA X [37]. For the isolation-by-distance analysis, geographic distances between the trapping locations were measured with the package geosphere v1.5-10 in the R (version 4) software environment [38].
2.4. Statistical Analysis
Mantel tests, as implemented in the ade4 (v1.7-16) package [39] in R (version 4) [38], were used to determine the statistical significance of the association between geographic and genetic distances.
A subset of the data was used to estimate the potential drivers of BvHV circulation within its host populations. Spatially and temporally replicated data were available for four regions in four federal states in Germany: Jeeser (Mecklenburg-Western Pomerania 54°9.75′ N, 13°15.55′ E), Gotha (Thuringia, 50°57.38′ N, 10°39.13′ E), Billerbeck (North Rhine-Westphalia, 51°59.63′ N, 7°18.99′ E), and Weissach (Baden-Wuerttemberg, 48°49.88′ N, 8°57.71′ E). In a generalized linear mixed effect model (with binomial error distribution), the individual infection status was used as a dependent variable, while individual factors (mass (g), sex (male/female), reproductive status (yes/no)) and population level (abundance of bank voles and co-occurring yellow-necked field mice (Apodemus flavicollis) as individuals per 100 trap nights, trapping season (spring/summer/autumn), and trapping year (2010/2011)) were treated as independent variables. The year 2012 was excluded from the analysis, as only spring data were available. Trapping location was incorporated as a random factor to account for the spatial design of the study. A multimodal inference approach was used to determine the most parsimonious model. First, the dredge function (MuMIn package, version 1.43.17) was used to rank all combinations of independent variables according to their conditional AIC (AICc). Second, from all combinations within a ΔAIC of <2 of the best model, the respective coefficients were averaged using the model.avg function. All analyses were performed in R (version 4) [38].
3. Results
3.1. Detection of BvHV RNA in Small Mammals from European Countries
The initial RT-qPCR screening of small mammals resulted in the detection of BvHV RNA in 442 out of 1838 (24.0%) bank voles from nine European countries and in one of seven (14.3%) northern red-backed voles from Finland, but in no other small mammal species (Table 1, Table 2 and Table S2, and Figure 1). The highest site prevalence was detected in Vouzon (France) where 14 of 19 (73.7%) animals were positive in RT-qPCR (Table 2). Detailed temporal sampling of bank voles in the years 2010–2012 in four regions of Germany demonstrated the continuous circulation of BvHV in local populations (Table 3).
Table 2.
Results of RT-qPCR assays of bank voles and northern red-backed voles (*) at country and trapping site level. All samples were tested in both assays.
| Country | Trapping Site | rodHCVeur Assays (Hepacivirus J) | RHV-NS3-Line4 Assay (Hepacivirus F) | Both Assays (Hepacivirus F and Hepacivirus J) | Positive/Tested Per Trapping Site | Positive/Tested Per Country |
|---|---|---|---|---|---|---|
| Austria | Laa an der Thaya | 3 | 10 | 1 | 12/33 | 12/33 |
| Finland | Pallasjärvi | 0 * | 1 * | 0 * | 1/7 * | 1/7 * |
| France | Cormaranche-en-Bugey | 12 | 5 | 3 | 14/20 | 61/99 |
| La Venotiere | 10 | 7 | 4 | 13/20 | ||
| Mignovillard | 9 | 5 | 3 | 11/20 | ||
| Mont-sous-Vaudrey | 9 | 2 | 2 | 9/20 | ||
| Vouzon | 8 | 12 | 6 | 14/19 | ||
| Germany | Ahlhorn | 1 | 0 | 0 | 1/2 | 286/1297 |
| Bad Waldsee | 0 | 0 | 0 | 0/5 | ||
| Bierhütte | 0 | 0 | 0 | 0/2 | ||
| Billerbeck | 67 | 8 | 0 | 75/285 | ||
| Bogen | 0 | 0 | 0 | 0/1 | ||
| Bremerhaven | 3 | 0 | 0 | 3/19 | ||
| Falkenstein | 0 | 2 | 0 | 2/5 | ||
| Freyung | 0 | 0 | 0 | 0/5 | ||
| Geversdorf | 0 | 0 | 0 | 0/4 | ||
| Glashütte | 0 | 0 | 0 | 0/1 | ||
| Gotha | 56 | 61 | 51 | 66/319 | ||
| Hangenleithen | 0 | 3 | 0 | 3/6 | ||
| Jasnitz | 5 | 0 | 0 | 5/20 | ||
| Jeeser | 41 | 3 | 2 | 42/155 | ||
| Langenfurth | 0 | 0 | 0 | 0/1 | ||
| Lucka (bei Groitzsch) | 3 | 1 | 0 | 4/17 | ||
| Mühlberg, Spiegelau | 0 | 1 | 0 | 1/1 | ||
| Mutzenwinkel | 0 | 1 | 0 | 1/2 | ||
| Oberndorf (Hemmoor) | 0 | 2 | 0 | 2/8 | ||
| Osnabrück | 11 | 8 | 4 | 15/23 | ||
| Raimundsreuth | 0 | 3 | 0 | 3/14 | ||
| Reinberg | 0 | 0 | 0 | 0/1 | ||
| Rothemühl | 0 | 2 | 0 | 2/4 | ||
| Schrevendorf | 9 | 2 | 0 | 11/20 | ||
| Steinheim am Albuch | 0 | 0 | 0 | 0/20 | ||
| Treben (Altenburg) | 0 | 0 | 0 | 0/3 | ||
| Tussenhausen | 0 | 4 | 0 | 4/9 | ||
| Weissach | 41 | 4 | 1 | 44/332 | ||
| Wolbrechtshausen | 1 | 0 | 0 | 1/2 | ||
| Wolfertschlag | 0 | 1 | 0 | 1/5 | ||
| Zußdorf | 0 | 0 | 0 | 0/5 | ||
| Zwiesel | 0 | 0 | 0 | 0/1 | ||
| Great Britain | Cumbria | 0 | 2 | 0 | 2/51 | 5/61 |
| Pentland Hills | 0 | 3 | 0 | 5/10 | ||
| Italy | Brescia | 0 | 0 | 0 | 0/5 | 5/25 |
| Trento | 3 | 2 | 0 | 5/20 | ||
| The Netherlands | Nutter | 11 | 4 | 2 | 13/20 | 13/20 |
| Poland | Mikołajki | 0 | 0 | 0 | 0/5 | 1/15 |
| Dobskie island | 0 | 1 | 0 | 1/5 | ||
| Dejguny island | 0 | 0 | 0 | 0/5 | ||
| Slovakia | Bratislava | 8 | 9 | 1 | 16/22 | 42/65 |
| Fugelka | 10 | 7 | 3 | 14/23 | ||
| Rozhanovce | 6 | 10 | 4 | 12/20 | ||
| Sweden | Ammarnäs | 0 | 0 | 0 | 0/20 | 17/223 |
| Grimsö | 0 | 4 | 0 | 4/20 | ||
| Haparanda | 0 | 2 | 0 | 2/20 | ||
| Harads | 0 | 3 | 0 | 3/20 | ||
| Öster Malma | 0 | 1 | 0 | 1/20 | ||
| Umeå | 0 | 2 | 0 | 2/42 | ||
| Vålådalen | 0 | 0 | 0 | 0/20 | ||
| Västernorrland | 0 | 0 | 0 | 0/20 | ||
| Växjö | 0 | 4 | 0 | 4/21 | ||
| Vindeln | 0 | 1 | 0 | 1/20 | ||
| Total | 327 | 202 + 1 * | 87 |
Table 3.
Detection of BvHV RNA in bank voles collected at four sites in Germany from 2010 to 2012.
| Year | Season | Number of BvHV RNA-Positive/Total Number of Bank Voles | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Jeeser | Billerbeck | Gotha | Weissach | ||||||
| 2010 | spring | 5/11 | 26/82 | 24/84 | 57/242 | 8/48 | 23/218 | 15/131 | 24/262 |
| summer | 8/28 | 15/84 | 6/65 | 5/77 | |||||
| autumn | 13/43 | 18/74 | 9/105 | 4/54 | |||||
| 2011 | spring | 0/1 | 9/59 | 1/1 | 7/32 | 3/6 | 22/89 | 0/0 | 5/31 |
| summer | 0/23 | 3/17 | 10/45 | 5/23 | |||||
| autumn | 9/35 | 3/14 | 9/38 | 0/8 | |||||
| 2012 | spring | 6/15 | 3/11 | 10/15 | 10/39 | ||||
| Total | 41/156 | 67/285 | 55/322 | 39/332 | |||||
In total, 327 bank voles were positive in the rodHCVeur assay (Hepacivirus J), 202 bank voles and one northern red-backed vole were positive in the RHV-NS3-Line4 assay (Hepacivirus F), and 87 were positive in both assays (Table 2; for semiquantitative genome loads, see Table S2). For 96 bank vole samples, partial NS3-gene sequences could be amplified and sequenced by RT-PCR; for 50 of these 96 vole samples consensus sequences were generated (available under GenBank accession numbers MW822235–MW822284). We observed a high background in electropherograms, and RT-PCR amplification failed on some RT-qPCR-positive samples with a low threshold cycle (Ct) value (e.g., Mu08/1265 Ct-value 18.75).
3.2. Sequence Divergence of BvHV
The use of two different RT-qPCR assays, partial sequencing of the NS3 gene, and phylogenetic analysis resulted in the confirmed detection of both BvHV species in bank voles. Hepacivirus J RNA was detected in voles from six countries, and Hepacivirus F RNA in animals from nine (Table 2, Figure 1, and Table S2). Both BvHV species were detected by RT-qPCR in 87 bank voles from six countries (Table 2 and Figure 1); the occurrence of both BvHV species was confirmed by sequence and phylogenetic analysis in Germany and Slovakia (Figure 2).
Figure 2.
Phylogenetic relationships of partial BvHV NS3 sequences (472 nt). Sequences generated during this study are highlighted in bold. Circles at nodes indicate Bayesian posterior probabilities: black: probability exceeds 90%; white: probability exceeds 70%. The names of sequences obtained from GenBank include their accession numbers. Colored circles to the right of sequence names indicate the evolutionary lineage of the bank vole: yellow: Western lineage; red: Eastern lineage; green: Carpathian lineage. Asterisks mark type strains of both virus species.
A phylogenetic analysis confirmed the previously described [9] separation of clade 1/species Hepacivirus J and clade 2/species Hepacivirus F (Figure 2). The pairwise sequence variability at the nt level reached 21.2% and 24.3% within Hepacivirus J and Hepacivirus F, respectively, and 48.2% between species. Amino acid sequence variability reached 8.6% and 12.6% within species, respectively, and 44.4% between species. Within trapping sites, nucleotide sequence divergences between BvHV strains ranged between 0 and 16.8% within species and 42.5% and 44.9 % between species (Table S3). Both BvHV species independently showed very similar patterns of isolation-by-distance across their distribution ranges in Europe (Figure 3; Mantel tests; both p < 0.001). Pairwise genetic distances between sequences increased steeply within each BvHV species over relatively short geographic distances between trapping sites, and the increase levelled off for comparisons over larger geographic distances (Figure 3).
Figure 3.
Relationships between pairwise genetic distance (p) and geographic distance between BvHV NS3 nucleotide sequences from bank voles collected across Europe. The virus species showed independently significant isolation-by-distance relationships (Mantel tests; both p < 0.001).
3.3. BvHV Association with Bank Vole Evolutionary Lineages
Phylogenetic analysis of cyt b sequences of infected bank voles indicated that at least four evolutionary lineages are susceptible to BvHV. Both virus species were detected in bank voles of the Western and Carpathian evolutionary lineages, and Hepacivirus F was also found in the Eastern lineage (Figure 2). BvHV RNA was also detected in a bank vole of the Ural lineage (trapping site: Umeå, Sweden). However, we were not able to generate a consensus BvHV sequence from this sample.
3.4. BvHV–Host Dynamics
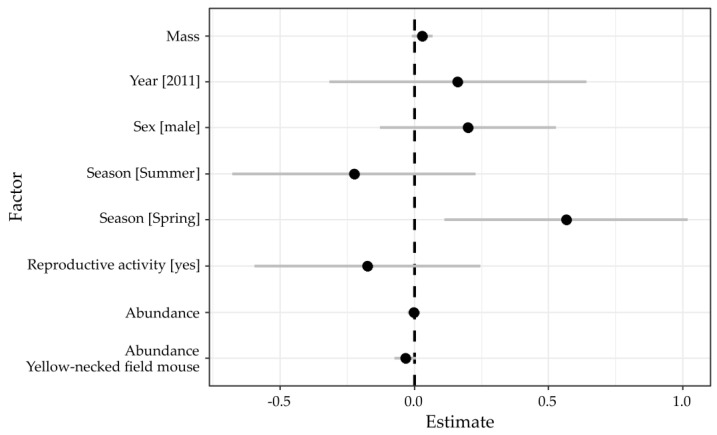
Candidate models generated to evaluate BvHV circulation in bank voles as well as their respective AICc and model weights are presented in Table 4. A total of 14 factor combinations were within ΔAIC of <2 and considered for further model averaging. Relative importance (RI) of each factor can be approximated by the relative proportion of model weights including the respective factor (ranging from 0 (no importance) to 1 (very important)). The most important factor was “season” being selected in all candidate models (RI = 1), followed by “abundance of yellow-necked field mouse” (RI = 0.69), “mass” (RI = 0.46), “sex” (RI = 0.34), “reproductive activity” (RI = 0.18), “year” (RI = 0.10), and “abundance of bank voles” (RI = 0.05).
Table 4.
Binomial generalized linear models explaining the probability of BvHV infections in bank voles. Presence of a factor within a candidate model is indicated either by a + for categorical factors or the estimate for continuous factors. If a factor is not included in a candidate model, the cell remains empty. Models with ΔAIC > 2 were excluded from subsequent model averaging. AIC of the null model was 958.7 and was therefore not included in the Table below. df = degrees of freedom; logLik = log-likelihood value.
| Model | Factors | Model Statistics | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Population | Individual | |||||||||||
| Abundance (Bank Vole) |
Abundance (Yellow-Necked Field Mouse) |
Season | Year | Reproductive Activity | Sex | Mass | df | logLik | AICc | ΔAICc | Model Weight | |
| 1 | −0.0339 | + | 5 | −468.021 | 946.1 | 0 | 0.06 | |||||
| 2 | −0.0327 | + | 0.0248 | 6 | −467.137 | 946.4 | 0.26 | 0.053 | ||||
| 3 | −0.0335 | + | + | 0.0270 | 7 | −466.347 | 946.8 | 0.7 | 0.042 | |||
| 4 | −0.0346 | + | + | 6 | −467.379 | 946.8 | 0.74 | 0.041 | ||||
| 5 | + | 4 | −469.513 | 947.1 | 0.96 | 0.037 | ||||||
| 6 | + | 0.0263 | 5 | −468.525 | 947.1 | 1.01 | 0.036 | |||||
| 7 | −0.0350 | + | + | 0.0349 | 7 | −466.604 | 947.3 | 1.22 | 0.033 | |||
| 8 | −0.0360 | + | + | + | 0.0378 | 8 | −465.743 | 947.6 | 1.53 | 0.028 | ||
| 9 | + | + | 0.0284 | 6 | −467.808 | 947.7 | 1.6 | 0.027 | ||||
| 10 | + | + | 5 | −468.927 | 947.9 | 1.81 | 0.024 | |||||
| 11 | + | + | 5 | −468.936 | 947.9 | 1.83 | 0.024 | |||||
| 12 | −0.0306 | + | + | 6 | −467.967 | 948 | 1.92 | 0.023 | ||||
| 13 | −0.0346 | + | + | 6 | −467.978 | 948 | 1.94 | 0.023 | ||||
| 14 | −0.0021 | −0.0320 | + | 6 | −468.004 | 948.1 | 1.99 | 0.022 | ||||
| 15 | −0.0101 | + | 5 | −469.044 | 948.1 | 2.05 | 0.022 | |||||
Averaged parameter estimates and their 95% confidence intervals are shown in Figure 4, where a factor can be considered significant when confidence intervals do not cross zero. Results indicate that “sex” or “reproductive activity” did not influence the infection status significantly. Although older individuals (as inferred from the “mass” factor) tended to have a higher infection risk (only positive estimates, Table 4), the effect size appeared to be small. The most dominant factor was “season”, with individuals caught in spring more likely to be infected. There was no significant effect of “bank vole abundance” on the infection risk, ruling out direct density-dependent transmission of BvHV. However, increasing abundance of yellow-necked field mouse tended to decrease individual infection risk in bank voles (only negative estimates, Table 4) though averaged confidence intervals still included zero (Figure 4).
Figure 4.
Graphical representation of the model averaging from all candidate models within an AIC of 2 of the best model (Models 1 to 14 in Table 4). Averaged mean estimates for all factors (black circle) and their 95% confidence interval (+/− grey line) are presented on the x-axis. For categorical factors, the reference categories are female (Sex), autumn (Season), the year 2010 (Year), and reproductive inactivity (No). Here, a factor can be considered significant if the confidence intervals do not include zero (dashed line).
4. Discussion
Two BvHV clades corresponding to species Hepacivirus J and Hepacivirus F, first described in 2013, were previously detected only in Germany and the Netherlands and later in the Ukraine [9,40]. In this study, we show a very wide geographical distribution of these viruses in Europe based on the detection of BvHV RNA in 442 bank voles from nine countries and one northern red-backed vole from Finland (Figure 1). The prevalence of BvHV in bank voles in many regions and the lack of detected viral RNA in the 23 other small mammal species suggest that the bank vole is one of the main reservoir hosts of these viruses. However, the sample size of many species was low, and future studies have to further investigate the possible susceptibility of these rodent species as potential hosts of the virus. The reservoir role of northern red-backed voles should be examined with additional samples from a broader region. These future studies might profit from adapted RT-qPCR protocols; the mismatches between the primer and probe sequences and the homologous BvHV species prototype strain might have caused a slightly reduced sensitivity of the RT-qPCR in our study (Figure S1).
We detected, for both BvHV species, a positive association between genetic differences and spatial distance. These isolation-by-distance patterns are similar to the patterns observed in bank vole-associated PUUV [36] or common vole-associated Tula orthohantavirus [41]. These associations can be explained by host dispersal occurring only at a local scale and the restriction of the accumulation of mutations in viral strains within local vole populations [42,43]. Over larger geographic distances, virus populations in each BvHV species thus evolve independently. However, in contrast to European hantaviruses [41,44,45], we detected both BvHV species in the same host populations in several regions of Europe, which suggests long-term co-existence of Hepacivirus J and Hepacivirus F in their host species.
The high genetic divergence between both hepacivirus species and their sympatric occurrence at multiple sites confirm their taxonomic classification as two distinct species. The nucleotide/amino sequence divergence within the NS3 gene/protein reached between both BvHV species a maximum level of 48.2%/12.6%. As this initial analysis described here is based on a short fragment of the NS3 gene, further studies are needed to examine the relationship of both BvHV species in the entire NS3 coding sequence/protein, several coding sequences/proteins, or the entire genome/polyprotein. In this study, we found that bank voles of four investigated evolutionary lineages, namely, Western, Carpathian, Eastern, and Ural, are susceptible to BvHV infection. Interestingly, both viruses were detected in the Western and Carpathian lineages of the bank vole, whereas Hepacivirus F can also infect the Eastern lineage (Figure 2). However, these initial findings, particularly for the Eastern and Ural lineages, are based on a small number of animals, and further studies need to be conducted to assess the relevance of each lineage.
The evaluation of individual and population-based factors did not show evidence for the influence of age, sex, or bank vole abundance on BvHV prevalence. This is in contrast to results obtained for PUUV and Leptospira prevalence in the same populations [26,45], where individual demographics can significantly determine individual infection risk. Our study did however find indications that increasing densities for non-hosts such as yellow-necked field mouse can potentially dilute BvHV in bank voles, similar to PUUV, for example, in Belgium [46].
High prevalences in spring have been reported for PUUV in Central Europe [47] and Scandinavia [48] and can be attributed to the contact of animals in their burrows during winter. This process entails that mainly older individuals in spring are infected and potentially new cohorts are still protected by transient immunity through maternal antibodies. The lack of age as a key factor in BvHV circulation might suggest a diverging transmission route of BvHV compared to PUUV. To our knowledge, there is no information on intraspecific transmission of hepaciviruses within wild rodent populations. In well-studied equine hepaciviruses, cases of vertical transmission during parturition have been described [49]. Theoretically, vertical transmission through blood transfer during pregnancy and/or parturition could explain why individual factors were not detected, which are often found associated in demographic analyses of more indirect, horizontally transmitted pathogens, like PUUV. This finding may also suggest an additional similarity of BvHV to human HCV, which is transmitted by blood, blood products and sexual contact or perinatally [50].
5. Conclusions
This study shows a broad geographical distribution of BvHV in bank voles across Europe. This is also reflected in the detection of BvHV RNA in bank voles of different evolutionary lineages. The high genetic divergence between both hepacivirus species and their sympatric occurrence in host populations at multiple sites clearly support their taxonomic classification as two distinct species, Hepacivirus J and Hepacivirus F. The influence of season on BvHV prevalence, but not of age, may suggest a varying transmission route (by vertical or direct contact) compared to other well-studied pathogens circulating in bank voles. Future investigations need to evaluate the role of additional lineages in the rodent host, for example, the Italian or Spanish ones, and the role of other Myodes vole species as possible reservoirs of BvHV, e.g., M. rufocanus and M. centralis in Asia or M. gapperi and M. californicus in North America. Finally, the multiannual fluctuation of bank vole populations warrants future investigations regarding the potential spillover of BvHV to other mammals and humans.
Acknowledgments
The support of Friederike Spitzenberger (Vienna), Edyta T. Sadowska, Magdalena Mikowska, Pawel Koteja (all Krakow), Jaroslav Piálek (Studenec), Anton Labutin (Bern), Cornelia Silaghi (Munich, now Greifswald-Insel Riems), Nathalie Charbonnel (Montferrier sur Lez Cedex), Sandra Blome, Christian Korthase, Stephan Drewes (all Greifswald-Insel Riems), Lisa Schröder (Stralsund), Marion Saathoff, Jona Freise (both Wardenburg), Jörg Thiel (Gotha), Margrit Bemmann, Heiko Schulz (both Schwerin), Peter Borkenhagen (Probsteierhagen), Kati Sevke, Thomas Büchner, and Daniel Masur (all Greifswald) is kindly acknowledged.
Supplementary Materials
The following Supplementary Materials are available online at https://www.mdpi.com/article/10.3390/v13071258/s1, Figure S1: Primer and probe sequences of the two RT-qPCR assays rodHCVeur (A) and RHV-NS3-Line4 (B) used for BvHV detection compared to corresponding homologous and heterologous prototype strains of the Hepacivirus J and Hepacivirus F species, Figure S2: Phylogenetic relationships of partial cyt b gene sequences (764 nt), Table S1: Sequences of primers and probes used in this study, Table S2: Semiquantitative genome loads of all BvHV RNA-positive animals, Table S3: Nucleotide (nt) and amino acid (aa) sequence identity of BvHV sequences to sequences of the same trapping site and to reference sequences of species Hepacivirus J and Hepacivirus F.
Author Contributions
Conceptualization, R.G.U.; sample collection: C.F., F.E., B.H. (Birger Hörnfeldt), M.M., G.E.O., A.R., V.T., M.C., C.R., E.B., M.K., M.S., T.A.W., A.O., A.M., S.E., H.H., J.J., H.C.H., C.I. and D.R.; methodology, J.F.D., C.I., B.H. (Bernd Hoffmann) and S.F.; validation, S.F., C.I. and G.H.; formal analysis, J.S.; investigation, J.S., B.H. (Bernd Hoffmann), M.L.S., S.F., C.I. and G.H.; resources, G.H. and C.I.; data curation, J.S., R.G.U., S.F., C.I. and G.H.; writing—original draft preparation, J.S., C.I., G.H. and R.G.U.; writing—review and editing, J.S., C.I., H.H., H.C.H., G.H. and R.G.U.; visualization, J.S., C.I. and G.H.; supervision, M.B., G.H., R.G.U. and J.J.; project administration, H.H. and R.G.U.; funding acquisition, J.J., G.H., R.G.U., B.H. (Birger Hörnfeldt), F.E., H.C.H. and H.H. All authors have read and agreed to the published version of the manuscript.
Funding
The investigations were partly funded by EU grant FP7-261504 EDENext (awarded to R.G.U., A.R., V.T., H.C.H. and H.H.), by the German Federal Ministry of Education and Research (BMBF) through the German Research Platform for Zoonoses (“NaÜPa-net”, FKZ 01KI1018 and 01KI1303) and partly commissioned and funded by the Federal Environment Agency (UBA) within the Environment Research Plan of the German Federal Ministry for the Environment, Nature Conservation and Nuclear Safety (BMU) (grant number 3709 41 401 and grant number 3713 48 401 awarded to J.J.). Sampling of bank voles in Sweden was mainly funded by grants to B.H. (Birger Hörnfeldt) and F.E. from the Swedish Environmental Protection Agency (via the National Environmental Monitoring Programme), Alvins’ fund, Stiftelsen Oscar och Lili Lamms Minne, VINNOVA (P32060-1), and the Swedish Research Council Formas (2007-107). G.H. was supported by the Swiss National Science Foundation (grant 31003A-176209). Sampling in Italy was supported by the Fondazione E. Mach (C.F., A.R., V.T., H.C.H.). The contents of this publication are the sole responsibility of the authors and do not necessarily reflect the views of the European Commission.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Ethical Permits
Small mammal trapping was performed with permission from the ethical committees in the respective countries according to their national laws [25]: Czech Republic: authorized in the protocol PP 27/2007 (institutional and state committees of the Czech Academy of Sciences in 2007); France: authorized under French and European regulations on care and protection of laboratory animals: French Law 2001-486 issued on 6 June 2001 and Directive 2010/63/EU issued on 22 September 2010; all animal procedures (trapping and euthanasia) were preapproved by the Direction des Services Vétérinaires of the Herault Department under Agreement B 34-169-1; Finland: snap trapping does not require ethical permits under the Finnish Act on Animal Experimentation 62/2006 and by the decision of Finnish Animal Experiment Board 16 May 2007. A permit (23/5713/2001) for capturing protected species (Sorex spp., Myodes rufocanus and Myopus schisticolor) was granted by the Finnish Ministry of the Environment; Germany: Small mammals were trapped using Sherman©live animal traps according to relevant legislation (H. B. Sherman Traps Inc., Tallahassee, FL, USA) (official permit Site R1: Regierung der Oberpfalz 55.1-8646.4-140, Site T: Regierung von Schwaben 55.1-8646-2/30, Site S: AZ 36.11-36.45.12/4/12-001). Additional sample collection was authorized according to relevant legislation and by permission of the federal authorities (permits Regierungspräsidium Stuttgart 35-9185.82/0261, Landesamt für Natur, Umwelt und Verbraucherschutz Nordrhein-Westfalen 8.87-51.05.20.09.210, Landesamt für Landwirtschaft, Lebensmittelsicherheit und Fischerei Mecklenburg-Vorpommern 7221.3-030/09, Thüringer Landesamt für Lebensmittelsicherheit und Verbraucherschutz 22-2684-04-15-107/09; Italy: authorized by the ‘Comitato Faunistico Provinciale della Provincia di Trento’, protocol n° 595, issued on 4 May 2011; The Netherlands: authorized in compliance with Dutch laws on animal handling and welfare: RIVM/DEC permits 200700119, 200800053, 200800113 and 20100139; Poland: approved by the First Local Bioethical Committee in Krakow (decision # 48/2007); Slovakia: authorized according to current laws of the Slovak Republic, approved by the Ministry of Environment of the Slovak Republic, license numbers 297/108/06-3.1, 6743/2008-2.1 and ZPO-594/2012-SAB; Slovenia: authorized by the Ministry of Culture of the Republic of Croatia (No. 532–08–01-01/1–11-03) and the Veterinary Administration of the Republic of Slovenia (No. 34401–36/2012/9; Sweden: authorized under the Animal Ethics Committees of Umeå: A 44-08, A 61-11, and A 121-11, the Swedish Board of Agriculture: A 135-12 and Dnr A78-08, and the Swedish Environmental Protection Agency: Dnr 412-2635-05, Dnr 412-4009-10, Nv 02939-11). A Home Office Project License (PPL 60/3652) under the Animals (Scientific Procedures) Act 1986 was obtained, to authorize restraint, anesthesia, and blood sampling of live wild rodents in Great Britain. The sampling was also approved by the local (Royal (Dick) School of Veterinary Studies) and institutional (University of Edinburgh) Ethical Review Process. Additional bank voles were collected in Germany and Austria by pet cats, during pest rodent control, or by forest institutions due to their official duties that did not require a special permit.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study, in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.ICTV. [(accessed on 1 December 2020)]; Available online: https://talk.ictvonline.org/ictv-reports/ictv_online_report/positive-sense-rna-viruses/w/flaviviridae/362/genus-hepacivirus.
- 2.Kim C.W., Chang K.-M. Hepatitis C virus: Virology and life cycle. Clin. Mol. Hepatol. 2013;19:17–25. doi: 10.3350/cmh.2013.19.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaito M., Watanabe S., Tsukiyama-Kohara K., Yamaguchi K., Kobayashi Y., Konishi M., Yokoi M., Ishida S., Suzuki S., Kohara M. Hepatitis C virus particle detected by immunoelectron microscopic study. Pt 7J. Gen. Virol. 1994;75:1755–1760. doi: 10.1099/0022-1317-75-7-1755. [DOI] [PubMed] [Google Scholar]
- 4.Shimizu Y.K., Feinstone S.M., Kohara M., Purcell R.H., Yoshikura H. Hepatitis C virus: Detection of intracellular virus particles by electron microscopy. Hepatology. 1996;23:205–209. doi: 10.1002/hep.510230202. [DOI] [PubMed] [Google Scholar]
- 5.Penin F. Structural biology of hepatitis C virus. Clin. Liver Dis. 2003;7:1–21. doi: 10.1016/S1089-3261(02)00066-1. [DOI] [PubMed] [Google Scholar]
- 6.Perz J.F., Armstrong G.L., Farrington L.A., Hutin Y.J., Bell B.P. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J. Hepatol. 2006;45:529–538. doi: 10.1016/j.jhep.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 7.Bukh J. Animal models for the study of hepatitis C virus infection and related liver disease. Gastroenterology. 2012;142:1279–1287.e3. doi: 10.1053/j.gastro.2012.02.016. [DOI] [PubMed] [Google Scholar]
- 8.McGivern D.R., Lemon S.M. Model systems for hepatitis C research: The cup half empty? Gastroenterology. 2011;141:806–809. doi: 10.1053/j.gastro.2011.07.028. [DOI] [PubMed] [Google Scholar]
- 9.Drexler J.F., Corman V.M., Müller M.A., Lukashev A., Gmyl A., Coutard B., Adam A., Ritz D., Leijten L.M., Van Riel D., et al. Evidence for novel hepaciviruses in rodents. PLoS Pathog. 2013;9:e1003438. doi: 10.1371/journal.ppat.1003438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Firth C., Bhat M., Firth M.A., Williams S.H., Frye M.J., Simmonds P., Conte J.M., Ng J., Garcia J., Bhuva N.P., et al. Detection of zoonotic pathogens and characterization of novel viruses carried by commensal Rattus norvegicus in New York City. mBio. 2014;5:e01933-14. doi: 10.1128/mBio.01933-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramsay J.D., Evanoff R., Mealey R.H. Hepacivirus A Infection in Horses Defines Distinct Envelope Hypervariable Regions and Elucidates Potential Roles of Viral Strain and Adaptive Immune Status in Determining Envelope Diversity and Infection Outcome. J. Virol. 2018;92:e00314-18. doi: 10.1128/JVI.00314-18. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12.Baechlein C., Fischer N., Grundhoff A., Alawi M., Indenbirken D., Postel A., Baron A.L., Offinger J., Becker K., Beineke A., et al. Identification of a Novel Hepacivirus in Domestic Cattle from Germany. J. Virol. 2015;89:7007–7015. doi: 10.1128/JVI.00534-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.El-Attar L., Mitchell J.A., Brownlie H.B., Priestnall S.L., Brownlie J. Detection of non-primate hepaciviruses in UK dogs. Virology. 2015;484:93–102. doi: 10.1016/j.virol.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quan P.-L., Firth C., Conte J.M., Williams S.H., Zambrana-Torrelio C.M., Anthony S.J., Ellison J.A., Gilbert A.T., Kuzmin I.V., Niezgoda M., et al. Bats are a major natural reservoir for hepaciviruses and pegiviruses. Proc. Natl. Acad. Sci. USA. 2013;110:8194–8199. doi: 10.1073/pnas.1303037110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moreira-Soto A., Arroyo-Murillo F., Sander A.-L., Rasche A., Corman V., Tegtmeyer B., Steinmann E., Corrales-Aguilar E., Wieseke N., Avey-Arroyo J., et al. Cross-order host switches of hepatitis C-related viruses illustrated by a novel hepacivirus from sloths. Virus Evol. 2020;6:veaa033. doi: 10.1093/ve/veaa033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilson D.E., Reeder D.M. Mammal Species of The World: A Taxonomic and Geographic Reference. 3rd ed. The Johns Hopkins University Press; Baltimore, MD, USA: 2005. [Google Scholar]
- 17.Burgin C.J., Wilson D.E., Mittermeier R.A., Rylands A.B., Lacher T.E., Sechrest W. Illustrated Checklist of the Mammals of the World. Lynx Edicions; Barcelona, Spain: 2020. [Google Scholar]
- 18.Voutilainen L., Kallio E.R., Niemimaa J., Vapalahti O., Henttonen H. Temporal dynamics of Puumala hantavirus infection in cyclic populations of bank voles. Sci. Rep. 2016;6:21323. doi: 10.1038/srep21323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krebs C.J., Myers J.H. Population cycles in small mammals. Adv. Ecol. Res. 1974;8:267–399. [Google Scholar]
- 20.Ledevin R., Michaux J.R., Deffontaine V., Henttonen H., Renaud S. Evolutionary history of the bank vole Myodes glareolus: A morphometric perspective. Biol. J. Linn. Soc. 2010;100:681–694. doi: 10.1111/j.1095-8312.2010.01445.x. [DOI] [Google Scholar]
- 21.Wójcik J.M., Kawałko S., Marková S., Searle J.B., Kotlík P. Phylogeographic signatures of northward post-glacial colonization from high-latitude refugia: A case study of bank voles using museum specimens. J. Zool. 2010;281:249–262. doi: 10.1111/j.1469-7998.2010.00699.x. [DOI] [Google Scholar]
- 22.Marková S., Horníková M., Lanier H.C., Henttonen H., Searle J.B., Weider L.J., Kotlík P. High genomic diversity in the bank vole at the northern apex of a range expansion: The role of multiple colonizations and end-glacial refugia. Mol. Ecol. 2020;29:1730–1744. doi: 10.1111/mec.15427. [DOI] [PubMed] [Google Scholar]
- 23.Drewes S., Ali H.S., Saxenhofer M., Rosenfeld U.M., Binder F., Cuypers F., Schlegel M., Röhrs S., Heckel G., Ulrich R.G. Host-Associated Absence of Human Puumala Virus Infections in Northern and Eastern Germany. Emerg. Infect. Dis. 2017;23:83–86. doi: 10.3201/eid2301.160224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Röhrs S., Begeman L., Straub B.K., Boadella M., Hanke D., Drewes S., Hoffmann B., Keller M., Drexler J.F., Drosten C., et al. The bank vole (Clethrionomys glareolus)—Small animal model for hepacivirus infection. 2021 doi: 10.3390/v13122421. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fevola C., Rossi C., Rosso F., Girardi M., Rosà R., Manica M., Delucchi L., Rocchini D., Garzon-Lopez C.X., Arnoldi D., et al. Geographical Distribution of Ljungan Virus in Small Mammals in Europe. Vector-Borne Zoonotic Dis. 2020;20:692–702. doi: 10.1089/vbz.2019.2542. [DOI] [PubMed] [Google Scholar]
- 26.Fischer S., Mayer-Scholl A., Imholt C., Spierling N.G., Heuser E., Schmidt S., Reil D., Rosenfeld U.M., Jacob J., Nöckler K., et al. Leptospira Genomospecies and Sequence Type Prevalence in Small Mammal Populations in Germany. Vector-Borne Zoonotic Dis. 2018;18:188–199. doi: 10.1089/vbz.2017.2140. [DOI] [PubMed] [Google Scholar]
- 27.Schmidt S., Essbauer S.S., Mayer-Scholl A., Poppert S., Schmidt-Chanasit J., Klempa B., Henning K., Schares G., Groschup M.H., Spitzenberger F., et al. Multiple infections of rodents with zoonotic pathogens in Austria. Vector-Borne Zoonotic Dis. 2014;14:467–475. doi: 10.1089/vbz.2013.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ali H.S., Drewes S., Sadowska E.T., Mikowska M., Groschup M.H., Heckel G., Koteja P., Ulrich R.G. First molecular evidence for Puumala hantavirus in Poland. Viruses. 2014;6:340–353. doi: 10.3390/v6010340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alexander N., Allepuz A., Alten B., Bødker R., Bonnet S., Carpenter S., Cetre-Sossah C., Chirouze E., Depaquit J., Dressel K., et al. The Impact of a Decade of Research on Vector Borne Disease. CIRAD; Montpellier, France: 2015. [Google Scholar]
- 30.Schlegel M., Ali H.S., Stieger N., Groschup M.H., Wolf R., Ulrich R.G. Molecular identification of small mammal species using novel cytochrome B gene-derived degenerated primers. Biochem. Genet. 2011;50:440–447. doi: 10.1007/s10528-011-9487-8. [DOI] [PubMed] [Google Scholar]
- 31.Braaker S., Heckel G. Transalpine colonisation and partial phylogeographic erosion by dispersal in the common vole (Microtus arvalis) Mol. Ecol. 2009;18:2518–2531. doi: 10.1111/j.1365-294X.2009.04189.x. [DOI] [PubMed] [Google Scholar]
- 32.Ronquist F., Teslenko M., Van Der Mark P., Ayres D.L., Darling A., Hoehna S., Larget B., Liu L., Suchard M.A., Huelsenbeck J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012;61:539–542. doi: 10.1093/sysbio/sys029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Posada D. jModelTest: Phylogenetic model averaging. Mol. Biol. Evol. 2008;25:1253–1256. doi: 10.1093/molbev/msn083. [DOI] [PubMed] [Google Scholar]
- 34.Filipi K., Marková S., Searle J., Kotlík P. Mitogenomic phylogenetics of the bank vole Clethrionomys glareolus, a model system for studying end-glacial colonization of Europe. Pt AMol. Phylogenet. Evol. 2015;82:245–257. doi: 10.1016/j.ympev.2014.10.016. [DOI] [PubMed] [Google Scholar]
- 35.Ronquist F., Huelsenbeck J.P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19:1572–1574. doi: 10.1093/bioinformatics/btg180. [DOI] [PubMed] [Google Scholar]
- 36.Saxenhofer M., de Melo V.W., Ulrich R.G., Heckel G. Revised time scales of RNA virus evolution based on spatial information. Proc. R. Soc. B Boil. Sci. 2017;284:20170857. doi: 10.1098/rspb.2017.0857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kumar S., Stecher G., Li M., Knyaz C., Tamura K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018;35:1547–1549. doi: 10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.R Core Team . R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna, Austria: 2020. [Google Scholar]
- 39.Dray S., Dufour A.-B. The ade4 Package: Implementing the Duality Diagram for Ecologist. J. Stat. Softw. 2007;22:1–20. doi: 10.18637/jss.v022.i04. [DOI] [Google Scholar]
- 40.Kesäniemi J., Lavrinienko A., Tukalenko E., Mappes T., Watts P.C., Jurvansuu J. Infection Load and Prevalence of Novel Viruses Identified from the Bank Vole Do Not Associate with Exposure to Environmental Radioactivity. Viruses. 2019;12:44. doi: 10.3390/v12010044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saxenhofer M., Schmidt S., Ulrich R.G., Heckel G. Secondary contact between diverged host lineages entails ecological speciation in a European hantavirus. PLoS Biol. 2019;17:e3000142. doi: 10.1371/journal.pbio.3000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.De Melo V.W., Ali H.S., Freise J., Kühnert D., Essbauer S., Mertens M., Wanka K.M., Drewes S., Ulrich R.G., Heckel G. Spatiotemporal dynamics of Puumala hantavirus associated with its rodent host, Myodes glareolus. Evol. Appl. 2015;8:545–559. doi: 10.1111/eva.12263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hiltbrunner M., Heckel G. Assessing Genome-Wide Diversity in European Hantaviruses through Sequence Capture from Natural Host Samples. Viruses. 2020;12:749. doi: 10.3390/v12070749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schmidt S., Saxenhofer M., Drewes S., Schlegel M., Wanka K.M., Frank R., Klimpel S., Von Blanckenhagen F., Maaz D., Herden C., et al. High genetic structuring of Tula hantavirus. Arch. Virol. 2016;161:1135–1149. doi: 10.1007/s00705-016-2762-6. [DOI] [PubMed] [Google Scholar]
- 45.Binder F., Ryll R., Drewes S., Jagdmann S., Reil D., Hiltbrunner M., Rosenfeld U.M., Imholt C., Jacob J., Heckel G., et al. Spatial and Temporal Evolutionary Patterns in Puumala Orthohantavirus (PUUV) S Segment. Pathogens. 2020;9:548. doi: 10.3390/pathogens9070548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tersago K., Schreurs A., Linard C., Verhagen R., Van Dongen S., Leirs H. Population, environmental, and community effects on local bank vole (Myodes glareolus) Puumala virus infection in an area with low human incidence. Vector-Borne Zoonotic Dis. 2008;8:235–244. doi: 10.1089/vbz.2007.0160. [DOI] [PubMed] [Google Scholar]
- 47.Reil D., Rosenfeld U.M., Imholt C., Schmidt S., Ulrich R.G., Eccard J.A., Jacob J. Puumala hantavirus infections in bank vole populations: Host and virus dynamics in Central Europe. BMC Ecol. 2017;17:9. doi: 10.1186/s12898-017-0118-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Khalil H., Ecke F., Evander M., Bucht G., Hörnfeldt B. Population Dynamics of Bank Voles Predicts Human Puumala Hantavirus Risk. EcoHealth. 2019;16:545–557. doi: 10.1007/s10393-019-01424-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gather T., Walter S., Todt D., Pfaender S., Brown R.J.P., Postel A., Becher P., Moritz A., Hansmann F., Baumgaertner W., et al. Vertical transmission of hepatitis C virus-like non-primate hepacivirus in horses. J. Gen. Virol. 2016;97:2540–2551. doi: 10.1099/jgv.0.000561. [DOI] [PubMed] [Google Scholar]
- 50.Preciado M.V., Valva P., Escobar-Gutierrez A., Rahal P., Ruiz-Tovar K., Yamasaki L., Chacon C.A.V., Martinez-Guarneros A., Carpio-Pedroza J.C., Fonseca-Coronado S., et al. Hepatitis C virus molecular evolution: Transmission, disease progression and antiviral therapy. World J. Gastroenterol. 2014;20:15992. doi: 10.3748/wjg.v20.i43.15992. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.