Abstract
The clinical trials of the COVID-19 vaccines that are authorized in the European Union have revealed high efficacy in preventing symptomatic infections. However, during vaccination campaigns, some vaccine recipients, including those partially and fully vaccinated, will experience severe COVID-19, requiring hospitalization. This may particularly concern patients with a diminished immune response to the vaccine, as well as non-responders. This work has retrospectively analyzed the 92 cases of patients who were hospitalized between 27 December 2020 and 31 May 2021 in four Polish healthcare units due to COVID-19, and who have previously received the COVID-19 vaccine (54.3% ≤ 14 days after the first dose, 26.1% > 14 days after the first dose, 7.6% ≤ 14 days after the second dose, and 12% > 14 days after the second dose). These patients represented a minute fraction (1.2%) of all the COVID-19 patients who were hospitalized during the same period in the same healthcare institutions. No significant differences in white blood count, absolute lymphocyte count nadir, C-reactive protein, interleukin-6, procalcitonin, oxygen saturation, lung involvement, and fever frequency were found between the recipients of the first and second vaccine dose. A total of 15 deaths were noted (1.1% of all fatal COVID-19 cases in the considered period and healthcare units), including six in patients who received the second dose (five > 14 days after the second dose)—three of these subjects were using immunosuppressive medicines, and two were confirmed to be vaccine non-responders. The study reassures that severe COVID-19 and deaths are not common in vaccinated individuals, highlights that the clinical course in such patients may not reveal any distinctive features, and advocates for close monitoring of those at a higher risk of vaccine failure.
Keywords: vaccination, breakthrough infection, severe COVID-19, SARS-CoV-2
1. Introduction
The start of the rollout of COVID-19 vaccines at the turn of 2020–2021 brought new hope to the fight against the pandemic [1,2]. The vaccines that are authorized in the European Union have been proven to have a good safety profile, be immunogenic, and provide a high efficacy against symptomatic SARS-CoV-2 infection [3,4,5,6]. The COVID-19 vaccination is, however, met with several challenges, encompassing logistics, dosage shortages, vaccine hesitancy, media coverage, and the emergence of novel SARS-CoV-2 variants [1,7,8,9,10].
Three out of four vaccines that are authorized in the European Union require two doses, separated by specific intervals outlined in the summary of product characteristics (SmPC): BNT162b2 (BioNTech/Pfizer) by 21 days, mRNA-1273 (Moderna) by 28 days, and AZD1222 (Oxford/AstraZeneca) by 4–12 weeks. According to the clinical data, the partial protection against symptomatic infection begins around two-to-three weeks after the first dose, when the anti-spike IgG antibodies emerge. However, receiving the second dose of these vaccines is necessary and pivotal to ensuring optimal protection against COVID-19 [11,12,13]. Since the vaccine administration is conducted during the high spread of SARS-CoV-2 in the population, the risk of infections among vaccinated individuals, especially those who received only one dose, must be considered.
Additionally, reduced immunogenicity after a single dose and after the full vaccination regime must be taken into account in the case of some elderly subjects, cancer patients, and other immunocompromised individuals [14,15,16]. Importantly, some of these vaccine recipients may not produce any antibodies and may remain highly susceptible to the SARS-CoV-2 infection [17]. The exact percentage of individuals falling into the non-responders category is unknown. At the same time, administration of the first dose of the vaccine may lead to false conviction of full protection, and subsequent belittling of precaution and safety measures. Although most SARS-CoV-2 infections in partially or fully vaccinated individuals will be mild, and likely accompanied by decreased viral load and replication, some subjects may still experience severe disease, requiring hospitalization.
This study aimed to analyze cases of COVID-19 hospitalization of partially and fully vaccinated individuals in selected hospitals in Poland. The demographic, clinical and laboratory characteristics, and the course of disease, were considered. These observations add to real-world data on severe cases of post-vaccination SARS-CoV-2 infections, breakthrough infections in individuals more than 14 days after completing all recommended COVID-19 vaccine doses, and associated deaths.
2. Material and Methods
2.1. Data Extraction
The clinical data on patients who received at least one dose of the COVID-19 vaccine, later hospitalized due to COVID-19 between 27 December 2020 (the first day of the COVID-19 vaccine rollout in Poland) and 31 May 2021 in four Polish healthcare units in Wrocław, Białystok, Kielce and Poznań were extracted and retrospectively analyzed. During the same period, 7552 COVID-19 patients were hospitalized in these medical units, among which 1413 were fatal (18.7%), while the number of newly diagnosed SARS-CoV-2 infections in Poland rose by 1,614,484. The percentage of individuals who received at least a single dose at the end of January 2021 was 2.6%, February—5.7%, March—11.8%, April—23.6%, and reached 36.1% (13,707,350 individuals) at the end of May 2021. The share of fully vaccinated subjects reached 18.5% in the period considered in the study.
The study only included patients who reported the first COVID-19 symptoms following the vaccination and were discharged from the hospital as convalescent or died. The investigated subjects were divided into the following four subgroups: (1) recipients of the first dose with symptoms onset within 14 days since vaccination, (2) recipients of the first dose with symptoms onset >14 days after vaccination, (3) recipients of two doses with symptoms onset within 14 days from the second vaccine dose, (4) recipients of two doses with symptoms onset >14 days after the second vaccine dose (i.e., breakthrough infections). The first subgroup (1) cannot be unequivocally treated as infected after vaccination if one considers that the incubation time of SARS-CoV-2 can extend to 2 weeks [18].
Upon admission, the body temperature, oxygen saturation (SpO2) and lung involvement based on computed tomography (CT) imaging were recorded. If available, laboratory data analysis included white blood cell (WBC), C-reactive protein (CRP), interleukin-6 (IL-6), procalcitonin (PCT) at admission. Absolute lymphocyte count (ALC) nadir during the hospital stay was also considered. Patients were defined as vaccine non-responders if they received at least one dose of vaccine >14 days before the onset of COVID-19 symptoms and had a negative result of the serological test (anti-SARS-CoV-2 spike protein IgG antibodies) at admission. The clinical improvement was assessed with the ordinal scale based on WHO recommendations, but modified to an 8-score version to fit the specificity of the national health care system and previously used in the SARSTer study [19]. The scores, given at baseline and after 7, 14, 21 and 28 days after hospitalization, were defined as follows: (1) not hospitalized, no activity restrictions; (2) not hospitalized, no activity restrictions and/or requiring oxygen supplementation at home; (3) hospitalized, does not require oxygen supplementation and does not require medical care; (4) hospitalized, requiring no oxygen supplementation, but requiring medical care; (5) hospitalized, requiring normal oxygen supplementation; (6) hospitalized, on non-invasive ventilation with high-flow oxygen equipment; (7) hospitalized, for invasive mechanical ventilation or extracorporeal membrane oxygenation; (8) death. Patients were diagnosed and treated according to the most recent national recommendations for the management of COVID-19 [20].
Due to the retrospective nature of the presented study, written consent by participants was not necessary.
2.2. Statistical Analysis
The statistical analysis was performed using Statistica v.13.1 (StatSoft Inc., Tulsa, OK, USA). Because the disease severity was reported in the ordinal scale, while other variables did not meet the assumption of Gaussian distribution (Shapiro–Wilk test; p < 0.05), non-parametric methods were employed. The differences between the sub-groups of patients were analyzed with the Kruskal–Wallis ANOVA with the post hoc Dunn’s test. Differences in data frequencies reported as nominal categorical variables were evaluated by running multiple Pearson’s χ2 tests between each sub-group of patients. A p-value < 0.05 was considered statistically significant.
3. Results
3.1. Demographic Characteristics
A total of 92 patients were considered in this analysis—they constituted 1.22% of all COVID-19 patients who were hospitalized during the same period (27 December 2020–31 May 2021) in the considered healthcare units. Their demographic characteristics are summarized in Table 1. The majority had received only a first vaccine dose (80.4%). The maximum number of days from the second dose to symptoms onset in the studied group was 90. The age of the studied patients was 32–93 years; the subjects aged <70 years constituted 66.5%. The most-frequent chronic comorbidities included cardiovascular diseases (82.6% of subjects), particularly hypertension (63%) and type 2 diabetes (31.5%). Hospitalized individuals who received a second dose more than 14 days before the onset of the symptoms were significantly older than those who displayed symptoms within two weeks from receiving the first dose—no individuals <50 years old were observed. Most of the patients had increased BMI, and no difference, in this regard, was noted between the considered sub-groups. Overall, seven patients were immunosuppressed, while four met the criteria of non-responders. For other patients, the status of anti-SARS-CoV-2 spike protein IgG antibodies was unknown, or the results of the test were positive (although it does not necessarily imply the good responsiveness to the vaccine, as the antibody levels were determined after hospitalization and could also be, at least partially, due to viral infection). None of the patients who were considered in the analysis underwent the documented SARS-CoV-2 infection prior to vaccination.
Table 1.
The demographic breakdown of COVID-19 patients who have previously received at least one dose of COVID-19 vaccine (n = 92). Different letters in each row indicate statistically significant differences between the sub-groups (p < 0.05) demonstrated with the post hoc Dunn’s test following the Kruskal–Wallis ANOVA or with the Pearson’s χ2 test.
| Parameter | All (n = 92) |
≤14 Days after 1st Dose (n = 50) |
>14 Days after 1st Dose (n = 24) |
≤14 Days after 2nd Dose (n = 7) |
>14 Days after 2nd Dose (n = 11) |
|---|---|---|---|---|---|
|
Age Mean ± SD, years Range, years >70 years, n (%) |
68.3 ± 11.9 32–93 40 (43.5) |
65.5 ± 11.5 a 32–89 16 (32.0) |
69.9 ± 10.2 ab 46–89 12 (50.0) |
73.4 ± 6.9 ab 67–85 4 (57.1) |
78.5 ± 14.3 b 52–93 8 (72.7) |
|
Gender Male, n (%) Female, n (%) |
58 (63.0) 34 (37.0) |
a 31 (62.0) 19 (38.0) |
a 15 (62.5) 9 (37.5) |
a 4 (57.1) 3 (42.9) |
a 8 (72.7) 3 (27.3) |
|
BMI Mean ± SD, kg/m2 Underweight Normal (18.5–24.9), n (%) Overweight (25.0–29.9), n (%) Obesity (≥30.0), n (%) |
28.7 ± 5.4 1 (1.1) 23 (25.0) 36 (39.1) 32 (34.8) |
28.6 ± 4.5 a 1 (2.0) 8 (16.0) 21 (42.0) 20 (40.0) |
29.5 ± 7.3 a 0 (0.0) 8 (33.3) 8(33.3) 8 (33.3) |
28.7 ± 3.8 a 0 (0.0) 2 (28.6) 3 (42.9) 2 (28.6) |
27.4 ± 6.3 a 0 (0.0) 5 (45.5) 4 (36.4) 2 (18.2) |
| Chronic comorbidities, n (%) | 82 (89.1) | 41 (82.0) a | 23 (95.8) a | 7 (100.0) a | 11 (100.0) a |
| Immunosuppression, n (%) | 7 (7.6) | 0 (0.0) | 3 (12.5) | 1 (14.3) | 3 (27.3) |
|
Vaccine BNT162b2, n (%) mRNA-1273, n (%) AZD1222, n (%) |
51 (55.4) 13 (14.1) 28 (30.4) |
a 22 (44.0) 9 (18.0) 19 (38.0) |
a 12 (50.0) 3 (12.5) 9 (37.5) |
b 6 (85.7) 1 (14.3) 0 (0.0) |
b 11 (100.0) 0 (0,0) 0 (0.0) |
| Confirmed vaccine non-responders, n (%) | 4 (4.4) | - | 2 (8.3) | 1 (14.3) | 1 (9.1) |
3.2. Clinical Characteristics
The clinical and laboratory data of each sub-group considered in the study are summarized in Table 2. The disease severity at baseline did not differ between these groups, similarly to other clinical data and laboratory parameters. However, the patients who were hospitalized >14 days after the second dose tended to have the highest median IL-6 and CRP levels, and the lowest frequency of fever >38 °C.
Table 2.
The clinical characteristics of COVID-19 patients who previously received at least one dose of COVID-19 vaccine (n = 92). Different letters in each row indicate statistically significant differences between the sub-groups (p < 0.05) demonstrated with the post hoc Dunn’s test following the Kruskal–Wallis ANOVA or with the Pearson’s χ2 test.
| Parameter | Group | |||
|---|---|---|---|---|
| ≤14 Days after 1st Dose (n = 50) |
>14 Days after 1st Dose (n = 24) |
≤14 Days after 2nd Dose (n = 7) |
>14 Days after 2nd Dose (n = 11) |
|
| Baseline WHO, median (IQR) | 4 (4-4) a | 4 (4-4) a | 4 (4-5) a | 5 (4-6) a |
| Fever > 38 °C, % | 62.1 a | 62.5 a | 57.1 a | 36.4 a |
| SpO2 < 90%, % | 30.0 a | 54.2 a | 28.6 a | 54.5 a |
| Lung involvement, % | 30 (20–40) a | 30 (25–47.5) a | 30 (10–50) a | 24 (15–70) a |
| WBC * (×103/µL), median (IQR) | 6.2 (5.1–8.2) a | 7.2 (5.1–9.3) a | 9.3 (5.7–12.9) a | 7.1 (5.4–13.3) a |
|
ALC nadir * (×103/µL, median (IQR) |
1.0 (0.8–1.3) a | 0.9 (0.6–2.0) a | 1.3 (0.9–2.5) a | 0.7 (0.6–1.0) a |
| IL-6 (pg/mL), median (IQR) | 63.3 (37.1–139.1) a | 47.8 (23.6–138.1) a | 35.6 (17.4–72.6) a | 112.5 (73.0–208.7) a |
| CRP (mg/L), median (IQR) | 62.8 (39.1–143.9) a | 76.8 (45.1–200.5) a | 82.0 (3.2–108.0) a | 113.0 (73.0–208.7) a |
| PCT (ng/mL), median (IQR) | 0.09 (0.05–0.14) a | 0.11 (0.05–0.31) a | 0.11 (0.05–0.16) a | 0.09 (0.05–0.18) a |
| Fatal outcome, n (%) | 3 (6.0) a | 6 (25) b | 1 (14.3) ab | 5 (45.5) b |
IQR—interquartile range; WBC—white blood count; ALC—absolute lymphocyte count; IL-6—interleukin-6; CRP—C-reactive protein; PCT—procalcitonin; *—the data for patients suffering from chronic lymphocytic leukemia (n = 2) were excluded from these statistics.
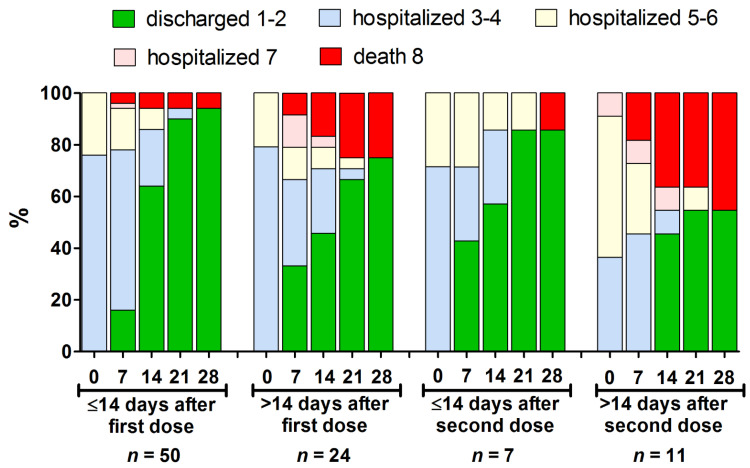
The clinical course of the disease, from the first to the 28th day of hospitalization, is presented in Figure 1. Overall, 15 cases (16.3%) were fatal—they constituted 1.06% of all the deaths to COVID-19 patients that were recorded during the same period in the considered healthcare units (Table 3). Among those, six occurred in the group that received a one vaccine dose more than 14 days before the onset of the symptoms, one case was noted in the group with symptoms onset ≤14 days after the second dose, and five in the group with symptoms onset >14 following the second dose (Table 2). There was one fatal case of a non-responder (female, aged 80, normal BMI, no immunosuppression) among the recipients of the first dose with symptoms onset >14 days after vaccination. Among those who received both the vaccine doses (regardless of the time prior to the symptoms onset), three fatal subjects were using immunosuppressive medicines; one was a kidney transplant recipient (male, aged 55, normal BMI), and the second suffered from chronic lymphocytic leukemia (male, aged 74, normal BMI), and both had no detectable levels of anti-S IgG antibodies upon admission. The third patient had rheumatoid arthritis (male, aged 83, normal BMI), but his seroconversion status after vaccination was not established. The detailed characteristics of fatal COVID-19 patients in each sub-group are presented in Table 3. Three out of four (75%) confirmed vaccine non-responders in the entire group considered in the present study died to COVID-19. The fatal COVID-19 patients that had previously received two vaccine doses at least 14 days before the onset of the symptoms, revealed the highest disease severity at the baseline, all had a fever > 38 °C, and none had SpO2 < 90% (Table 4).
Figure 1.
The clinical course presented using an eight-score disease severity scale [19] from baseline to day 28 of hospitalization in the different subsets of hospitalized patients who received at least one vaccine dose (n = 92). Further, 1–2 indicates not hospitalized patients; 3–4—hospitalized requiring no oxygen supplementation; 5–6—hospitalized, requiring normal oxygen supplementation or non-invasive ventilation; 7—hospitalized, requiring invasive mechanical ventilation or extracorporeal membrane oxygenation; 8—death.
Table 3.
The share of patients who received at least one dose of COVID-19 vaccine and were later hospitalized and fatal due to COVID-19 between 27 December 2020 and 31 May 2021 in the Polish healthcare units considered in the present study.
| Patients | Group | All | |||
|---|---|---|---|---|---|
| ≤14 Days after 1st Dose |
>14 Days after 1st Dose |
≤14 Days after 2nd Dose |
>14 Days after 2nd Dose |
||
| Hospitalized | 50 | 24 | 7 | 11 | 92 |
|
% of all hospitalized (n = 7552) |
0.66 | 0.32 | 0.09 | 0.15 | 1.22 |
| Fatal cases | 3 | 6 | 1 | 5 | 15 |
|
% of all fatal cases (n = 1413) |
0.21 | 0.42 | 0.07 | 0.35 | 1.06 |
Table 4.
The clinical and laboratory characteristics of patients with fatal COVID-19 who have received at least one dose of COVID-19 vaccine.
| Parameter | Group | |||
|---|---|---|---|---|
| ≤14 Days after 1st Dose (n = 3) |
>14 Days after 1st Dose (n = 6) |
≤14 Days after 2nd Dose (n = 1) |
>14 Days after 2nd Dose (n = 5) |
|
|
Age, mean ± SD, years >70 years, n (%) |
71.7 ± 3.2 2 (66.6) |
77.0 ± 8.7 5 (83.3) |
74 1 (100) |
77.4 ± 14.3 4 (80.0) |
|
Gender Male, n (%) / Female, n (%) |
1 (33.3)/2 (66.6) |
3 (50.0)/3 (50.0) |
1 (100.0)/0 (0.0) |
4 (80.0)/1 (20.0) |
| BMI, mean ± SD, kg/m2 Normal (18.5–24.9), n (%) Overweight (25.0–29.9), n (%) Obesity (≥30.0), n (%) |
31.7 ± 3.2 0 (0) 1 (33.3) 2 (66.6) |
24.7 ± 3.4 4 (66.6) 2 (33.3) 0 (0) |
21.7 1 (100) 0 (0) 0 (0) |
24.5 ± 0.75 3 (60.0) 2 (40.0) 0 (0) |
|
Chronic comorbidities, n (%) CVD, n (%) Diabetes, n (%) |
100 (100) 100 (100) 100 (100) |
5 (83.3) 4 (66.6) 1 (16.6) |
100 (100) 0 (0) 0 (0) |
100 (100) 4 (80) 3 (60) |
| Immunosuppression, n (%) | 0 (0) | 0 (0) | 1 (100) | 2 (40) |
|
Confirmed vaccine non-responders, n (%) |
- | 1 (16.7) | 1 (100) | 1 (20.0) |
| Baseline WHO, median (IQR) | 4 (4-4) | 4 (4-5) | 3 | 6 (5-6) |
| Fever >38 °C, % | 2 (66.6) | 3 (50.0) | 1 (100.0) | 0 (0.0) |
| SpO2 <90%, % | 2 (66.6) | 4 (66.6) | 0 (0) | 5 (100.0) |
| Lung involvement, % | 40 (30–40) | 55 (40–60) | 60 | 70 (50–70) |
| WBC (×103/µL),median (IQR) | 6.0 (5.9–11.9) | 7.3 (3.5–15.4) | 19.5 * | 8.8 (7.1–14.5) |
|
ALC nadir (×103/µL), median (IQR) |
1 (0.4–1.4) | 0.6 (0.3–2.0) | 15.0 * | 0.6 (0.5–0.7) |
| IL-6 (pg/mL),median (IQR) | n.a. | 138 (134–142) | 40.8 | 122 (103–228) |
| CRP (mg/L), median (IQR) | 207 (61–226) | 136 (43–238) | 82 | 125 (22–199) |
| PCT (ng/mL), median (IQR) | 0.3 (0.06–0.6) | 0.1 (0.1–0.6) | 0.12 | 0.2 (0.2–0.2) |
CVD—cardiovascular diseases; * patient suffering from chronic lymphocytic leukemia; n.a.—data not available.
4. Discussion
The present paper documents the clinical characteristics of Polish patients who were hospitalized due to COVID-19, who received at least a single dose of the vaccine. The data extracted from selected hospitals demonstrate that the highest number of more-severe infections after vaccination can occur after the first dose, particularly within the first two weeks. During this period, most the vaccinated subjects will not reach a threshold of the immune response that would ensure partial protection, which begins around two-to-three weeks after first dose, when the anti-spike IgG antibodies emerge [11,12,13]. Therefore, they need to be considered as fully vulnerable to contracting the virus. Moreover, the median incubation time of SARS-CoV-2 is 4–5 days, but it can extend through two weeks [18,21]. Therefore, it cannot be excluded that the number of infections with COVID-19 symptoms onset within 14 days from the first vaccine dose, is due to exposure to SARS-CoV-2 prior to vaccination. Nevertheless, the vaccinated patients who received at least one dose of the vaccine constituted a minute fraction of all the subjects who were hospitalized due to COVID-19, in the considered four healthcare institutions during the same period, i.e., 0.98 and 0.24% in the case of the first and second dose recipients, respectively. During the same period, the share of partially and fully vaccinated individuals in the Polish population reached 36.1 and 18.5%, respectively. This further reassures the high efficacy of the COVID-19 vaccines, in preventing severe disease, and, in line with large real-world observations, indicates that, to date, severe breakthrough infections (i.e., identified >14 days after receipt of all recommended doses) are sporadic [22,23].
Most of the investigated subjects in the present study were >60 years old, male, had increased BMI, and suffered from comorbidities. This is consistent with the risk factors for severe COVID-19 [24,25]. However, the patients hospitalized more than 14 days after receiving the second dose tended to be older. Moreover, all the subjects requiring hospitalization after receiving two doses were at least 52 years old, while in the group of single-dose receivers, individuals aged 30–50 were also present. Moreover, the majority of fatal cases occurred in subjects ≥70 years. Experiences with previous vaccines demonstrate that, in elderly subjects, the immune response to vaccination is often less potent, which is the phenomenon attributed to adaptive immunosenescence [26]. This has already been observed in small investigations that were conducted after authorization of the COVID-19 vaccines, e.g., one study reported that more than 30% of vaccinated subjects aged >80 years did not attain neutralizing antibody responses [14].
The present study did not find any distinct clinical or laboratory features for sub-groups that were divided on the basis of the vaccination status of patients (number of doses, days after the last dose). This implies that individuals who suffer from severe COVID-19, despite partial or complete vaccination, will likely undergo a similar clinical course of the disease, and should likely be subject to indifferent treatment. However, one should note that the present study did not compare a clinical course of vaccinated and unvaccinated subjects.
Important observations of this retrospective study include the cases of patients using immunosuppressive medicines. As suggested by different authorities (e.g., UK Joint Committee on Vaccination and Immunisation), individuals with immunosuppression should be prioritized for the COVID-19 vaccination [27]. However, on the other hand, these individuals may reveal a diminished response to the vaccine, while some, as confirmed for two cases reported in our study, could be subject to vaccine failure [17]. Therefore, it is pivotal to advise these patients to continue to adhere to preventive measures, particularly at a time of a high number of infections in the population, and to test the serum level of anti-SARS-CoV-2 IgG antibodies following the vaccination. This is particularly important if one considers that fully vaccinated individuals may be subject to an increasing number of various benefits, including lifting the face mask and physical distancing recommendations. Otherwise, some of these individuals may falsely assume the protection from the adaptive immunity and put themselves in high-risk situations. Notably, in the present study, three out of four (75%) confirmed vaccine non-responders died due to COVID-19.
It should also be noted that most of the breakthrough infections that are reported in the present study cannot be explained by immunosuppression or vaccine failure. Whether this is due to the emergence and spread of selected SARS-CoV-2 variants of concern (VOC), requires further studies [28,29]. As shown using the neutralization assays employing the sera of vaccinated individuals and pseudotyped virus, some variants, e.g., those possessing the E484K mutation in the receptor-binding domain, can potentially evade, to some extent, the immunity provided by the vaccines [30]. A recent study in Washington State indicated that VOC are overrepresented among post-vaccination breakthrough infections that do not require hospitalization [31]. As shown, mutations present in VOC, such as B.1.1.7 and B.1.351, do not escape the T-cell-mediated immunity that is elicited through vaccination [32]. Therefore, it is urgently needed to not rely solely on the antibody status level, but also to assess the cellular responses in vaccinated patients with severe breakthrough infections.
The study limitations must be stressed. Firstly, this is a retrospective analysis from four hospitals in Poland, and cannot draw a general conclusion on the frequency of hospitalizations and deaths due to COVID-19 among vaccinated Poles. However, the present study demonstrates a trend according to which SARS-CoV-2 infections among vaccinated individuals requiring hospitalization are expected more often after the first dose, particularly within 14 days, during which the vaccinated subjects remain fully vulnerable to the virus and may also contract it before vaccine administration. Secondly, no conclusions should be drawn on the differences in the frequency of COVID-19 hospitalizations after a particular vaccine, as the rate of their administration in Poland was related to the availability, and was also different among various occupational and age groups. Moreover, as recommended by the Polish Ministry of Health since 8 March 2021, a second dose of the AZD1222 vaccine was given after 12 weeks, resulting in a lower share of individuals being fully vaccinated with this vaccine as compared to BNT162b2. Thirdly, the considered group of patients was relatively small, and the demographic, clinical, and laboratory characteristics require further confirmations on larger cohorts. Although the data on immunosuppressed individuals are of interest, one should bear in mind that these patients constituted only 7.6% of the studied group. More specific research on immunocompromised subjects is needed in order to understand their risk of vaccine failure. Moreover, the present study included patients identified as non-responders, based on the negative result of the anti-SARS-CoV-2 spike protein IgG antibodies status. However, this does not imply that every other patient should unambiguously be considered as a vaccine responder, since the antibody status was assessed after hospital admission, not enabling the vaccine- and infection-induced adaptive immune response to be distinguished. Last, but not least, the variants that caused infections in the studied individuals were not identified. According to the genomic surveillance of SARS-CoV-2 in Poland, infections with the B.1.1.7 variant were increasingly dominant during the studied period, from approx. 15% at the beginning of 2021, to over 90% in May 2021 [33]. Although the COVID-19 vaccines used in Poland retain high efficacy against this variant, the immunoprotection after the first dose appears to be decreased [34,35]. Other variants of concern, such as B.1.351 that has a more significant effect on vaccine efficacy [36,37], were only sporadically identified [33]. Nevertheless, one should note that the data presented in this manuscript may not apply to all SARS-CoV-2 variants, including B.1.617.2, which was first detected in Poland in May 2021.
5. Conclusions
The study indicates that, for the considered period and healthcare institutions, COVID-19 patients who received at least one dose of the COVID-19 vaccine before hospitalization, constituted a minute fraction of all COVID-19 patients, and were represented mainly by those who received only one dose ≤14 days before first symptoms onset. Therefore, this indirectly reassures the high efficacy of vaccines in preventing severe disease. As shown, the demographic and clinical characteristics of hospitalized subjects that are partially or fully vaccinated, may not vary significantly. The fatal patients were mostly >70-year-old men, and had increased BMI and comorbidities, which were mostly cardiovascular diseases. The study findings also suggest that fully vaccinated individuals with immunosuppression should first be screened for their adaptive response, to avoid the potential risks of severe infection in the case of vaccine failure. Further studies are warranted in order to elucidate the more severe cases of breakthrough infections in the vaccine-responding individuals.
Author Contributions
Conceptualization, P.R., M.C. and R.F.; methodology, P.R., M.C. and R.F.; investigation, P.R., M.P.-S., K.S., T.Ł., B.S., D.Z.-M., M.C., I.M.-L. and R.F.; data curation, P.R.; writing—original draft preparation, P.R. and R.F.; supervision—P.R. and R.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Medical Research Agency in Poland, grant number 2020/ABM/COVID19/PTEILCHZ.
Institutional Review Board Statement
Ethical review and approval were waived for this study, due to the study design not meeting the requirements of the medical experiment according to the Polish law.
Informed Consent Statement
Patient consent was not necessary due to the retrospective nature of the study and the research not meeting the requirements of the medical experiment according to Polish law.
Data Availability Statement
The data presented in this study are available from the corresponding authors on reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rzymski P., Borkowski L., Drąg M., Flisiak R., Jemielity J., Krajewski J., Mastalerz-Migas A., Matyja A., Pyrć K., Simon K., et al. The Strategies to Support the COVID-19 Vaccination with Evidence-Based Communication and Tackling Misinformation. Vaccines. 2021;9:109. doi: 10.3390/vaccines9020109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kashte S., Gulbake A., El-Amin Iii S.F., Gupta A. COVID-19 vaccines: Rapid development, implications, challenges and future prospects. Hum. Cell. 2021;34:711–733. doi: 10.1007/s13577-021-00512-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., Perez J.L., Pérez Marc G., Moreira E.D., Zerbini C., et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sadoff J., Le Gars M., Shukarev G., Heerwegh D., Truyers C., de Groot A.M., Stoop J., Tete S., Van Damme W., Leroux-Roels I., et al. Interim Results of a Phase 1-2a Trial of Ad26.COV2.S Covid-19 Vaccine. N. Engl. J. Med. 2021 doi: 10.1056/NEJMoa2034201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Voysey M., Clemens S.A.C., Madhi S.A., Weckx L.Y., Folegatti P.M., Aley P.K., Angus B., Baillie V.L., Barnabas S.L., Bhorat Q.E., et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., Diemert D., Spector S.A., Rouphael N., Creech C.B., et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2020;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rzymski P., Zeyland J., Poniedziałek B., Małecka I., Wysocki J. The Perception and Attitudes toward COVID-19 Vaccines: A Cross-Sectional Study in Poland. Vaccines. 2021;9:382. doi: 10.3390/vaccines9040382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Forman R., Shah S., Jeurissen P., Jit M., Mossialos E. COVID-19 vaccine challenges: What have we learned so far and what remains to be done? Health Policy. 2021;125:553–567. doi: 10.1016/j.healthpol.2021.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen J., Lu H. New challenges to fighting COVID-19: Virus variants, potential vaccines, and development of antivirals. Biosci. Trends. 2021;15:126–128. doi: 10.5582/bst.2021.01092. [DOI] [PubMed] [Google Scholar]
- 10.Rzymski P., Perek B., Flisiak R. Thrombotic Thrombocytopenia after COVID-19 Vaccination: In Search of the Underlying Mechanism. Vaccines. 2021;9:559. doi: 10.3390/vaccines9060559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walsh E.E., Frenck R.W., Jr., Falsey A.R., Kitchin N., Absalon J., Gurtman A., Lockhart S., Neuzil K., Mulligan M.J., Bailey R., et al. Safety and Immunogenicity of Two RNA-Based Covid-19 Vaccine Candidates. N. Engl. J. Med. 2020;383:2439–2450. doi: 10.1056/NEJMoa2027906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barrett J.R., Belij-Rammerstorfer S., Dold C., Ewer K.J., Folegatti P.M., Gilbride C., Halkerston R., Hill J., Jenkin D., Stockdale L., et al. Phase 1/2 trial of SARS-CoV-2 vaccine ChAdOx1 nCoV-19 with a booster dose induces multifunctional antibody responses. Nat. Med. 2021;27:279–288. doi: 10.1038/s41591-020-01179-4. [DOI] [PubMed] [Google Scholar]
- 13.Anderson E.J., Rouphael N.G., Widge A.T., Jackson L.A., Roberts P.C., Makhene M., Chappell J.D., Denison M.R., Stevens L.J., Pruijssers A.J., et al. Safety and Immunogenicity of SARS-CoV-2 mRNA-1273 Vaccine in Older Adults. N. Engl. J. Med. 2020;383:2427–2438. doi: 10.1056/NEJMoa2028436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Müller L., Andrée M., Moskorz W., Drexler I., Walotka L., Grothmann R., Ptok J., Hillebrandt J., Ritchie A., Rabl D., et al. Age-dependent immune response to the Biontech/Pfizer BNT162b2 COVID-19 vaccination. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2021 doi: 10.1093/cid/ciab381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Monin L., Laing A.G., Muñoz-Ruiz M., McKenzie D.R., Del Molino Del Barrio I., Alaguthurai T., Domingo-Vila C., Hayday T.S., Graham C., Seow J., et al. Safety and immunogenicity of one versus two doses of the COVID-19 vaccine BNT162b2 for patients with cancer: Interim analysis of a prospective observational study. Lancet Oncol. 2021 doi: 10.1016/S1470-2045(21)00213-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boyarsky B.J., Werbel W.A., Avery R.K., Tobian A.A.R., Massie A.B., Segev D.L., Garonzik-Wang J.M. Immunogenicity of a Single Dose of SARS-CoV-2 Messenger RNA Vaccine in Solid Organ Transplant Recipients. JAMA. 2021;325:1784–1786. doi: 10.1001/jama.2021.4385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Agha M., Blake M., Chilleo C., Wells A., Haidar G. Suboptimal response to COVID-19 mRNA vaccines in hematologic malignancies patients. medRxiv. 2021 doi: 10.1101/2021.04.06.21254949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lauer S.A., Grantz K.H., Bi Q., Jones F.K., Zheng Q., Meredith H.R., Azman A.S., Reich N.G., Lessler J. The Incubation Period of Coronavirus Disease 2019 (COVID-19) From Publicly Reported Confirmed Cases: Estimation and Application. Ann. Intern. Med. 2020;172:577–582. doi: 10.7326/M20-0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flisiak R., Jaroszewicz J., Rogalska M., Łapiński T., Berkan-Kawińska A., Bolewska B., Tudrujek-Zdunek M., Kozielewicz D., Rorat M., Leszczyński P., et al. Tocilizumab Improves the Prognosis of COVID-19 in Patients with High IL-6. J. Clin. Med. 2021;10:1583. doi: 10.3390/jcm10081583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Flisiak R., Horban A., Jaroszewicz J., Kozielewicz D., Pawłowska M., Parczewski M., Piekarska A., Simon K., Tomasiewicz K., Zarębska-Michaluk D. Management of SARS-CoV-2 infection: Recommendations of the Polish Association of Epidemiologists and Infectiologists as of 31 March 2020. Pol. Arch. Intern. Med. 2020;130:352–357. doi: 10.20452/pamw.15270. [DOI] [PubMed] [Google Scholar]
- 21.Leung C. The Incubation Period of COVID-19: Current Understanding and Modeling Technique. Adv. Exp. Med. Biol. 2021;1318:81–90. doi: 10.1007/978-3-030-63761-3_5. [DOI] [PubMed] [Google Scholar]
- 22.CDC COVID-19 Vaccine Breakthrough Case Investigations Team COVID-19 Vaccine Breakthrough Infections Reported to CDC—United States, 1 January–30 April 2021. MMWR. Morb. Mortal. Wkly. Rep. 2021;70:792–793. doi: 10.15585/mmwr.mm7021e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pritchard E., Matthews P.C., Stoesser N., Eyre D.W., Gethings O., Vihta K.D., Jones J., House T., VanSteenHouse H., Bell I., et al. Impact of Vaccination on New SARS-CoV-2 Infections in the United Kingdom. Nat. Med. 2021 doi: 10.1038/s41591-021-01410-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Booth A., Reed A.B., Ponzo S., Yassaee A., Aral M., Plans D., Labrique A., Mohan D. Population risk factors for severe disease and mortality in COVID-19: A global systematic review and meta-analysis. PLoS ONE. 2021;16:e0247461. doi: 10.1371/journal.pone.0247461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hobbs A.L.V., Turner N., Omer I., Walker M.K., Beaulieu R.M., Sheikh M., Spires S.S., Fiske C.T., Dare R., Goorha S., et al. Risk Factors for Mortality and Progression to Severe COVID-19 Disease in the Southeast United States (US): A Report from the SEUS Study Group. Infect. Control Hosp. Epidemiol. 2021:1–33. doi: 10.1017/ice.2020.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crooke S.N., Ovsyannikova I.G., Poland G.A., Kennedy R.B. Immunosenescence and human vaccine immune responses. Immun. Ageing. 2019;16:25. doi: 10.1186/s12979-019-0164-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mahase E. Covid vaccine: Prioritise over 16s living with immunosuppressed adults, says JCVI. BMJ. 2021;372:n852. doi: 10.1136/bmj.n852. [DOI] [PubMed] [Google Scholar]
- 28.Charkiewicz R., Nikliński J., Biecek P., Kiśluk J., Pancewicz S., Moniuszko-Malinowska A.M., Flisiak R., Krętowski A.J., Dzięcioł J., Moniuszko M., et al. The first SARS-CoV-2 genetic variants of concern (VOC) in Poland: The concept of a comprehensive approach to monitoring and surveillance of emerging variants. Adv. Med Sci. 2021;66:237–245. doi: 10.1016/j.advms.2021.03.005. [DOI] [PubMed] [Google Scholar]
- 29.Hacisuleyman E., Hale C., Saito Y., Blachere N.E., Bergh M., Conlon E.G., Schaefer-Babajew D.J., DaSilva J., Muecksch F., Gaebler C., et al. Vaccine Breakthrough Infections with SARS-CoV-2 Variants. N. Engl. J. Med. 2021;384:2212–2218. doi: 10.1056/NEJMoa2105000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou D., Dejnirattisai W., Supasa P., Liu C., Mentzer A.J., Ginn H.M., Zhao Y., Duyvesteyn H.M.E., Tuekprakhon A., Nutalai R., et al. Evidence of escape of SARS-CoV-2 variant B.1.351 from natural and vaccine-induced sera. Cell. 2021;184:2348–2361.e6. doi: 10.1016/j.cell.2021.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McEwen A.E., Cohen S., Bryson-Cahn C., Liu C., Pergam S.A., Lynch J., Schippers A., Strand K., Whimbey E., Mani N.S., et al. Variants of concern are overrepresented among post-vaccination breakthrough infections of SARS-CoV-2 in Washington State. medRxiv. 2021 doi: 10.1101/2021.05.23.21257679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Geers D., Shamier M.C., Bogers S., den Hartog G., Gommers L., Nieuwkoop N.N., Schmitz K.S., Rijsbergen L.C., van Osch J.A.T., Dijkhuizen E., et al. SARS-CoV-2 variants of concern partially escape humoral but not T-cell responses in COVID-19 convalescent donors and vaccinees. Sci. Immunol. 2021;6:eabj1750. doi: 10.1126/sciimmunol.abj1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.SARS-CoV-2 w Polsce Mapa Wariantów (Map of Variants) [(accessed on 22 June 2021)]; Available online: https://sarswpolsce.pl/?page_id=7.
- 34.Haas E.J., Angulo F.J., McLaughlin J.M., Anis E., Singer S.R., Khan F., Brooks N., Smaja M., Mircus G., Pan K., et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: An observational study using national surveillance data. Lancet. 2021;397:1819–1829. doi: 10.1016/S0140-6736(21)00947-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Iacobucci G. Covid-19: Single vaccine dose is 33% effective against variant from India, data show. BMJ. 2021;373:n1346. doi: 10.1136/bmj.n1346. [DOI] [PubMed] [Google Scholar]
- 36.Madhi S.A., Baillie V., Cutland C.L., Voysey M., Koen A.L., Fairlie L., Padayachee S.D., Dheda K., Barnabas S.L., Bhorat Q.E., et al. Efficacy of the ChAdOx1 nCoV-19 Covid-19 Vaccine against the B.1.351 Variant. N. Engl. J. Med. 2021;384:1885–1898. doi: 10.1056/NEJMoa2102214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abu-Raddad L.J., Chemaitelly H., Butt A.A. Effectiveness of the BNT162b2 Covid-19 Vaccine against the B.1.1.7 and B.1.351 Variants. N. Engl. J. Med. 2021 doi: 10.1056/NEJMc2104974. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available from the corresponding authors on reasonable request.