Abstract
In 2017–2018, extensive symptoms of sudden decline and fruit rot were observed on date palms in southern Tunisia. Samples of diseased plants were randomly collected in six localities. Based on morphological identification, Fusarium was the most frequent fungal genus detected. A sequencing of translation elongation factor, calmodulin, and second largest subunit of RNA polymerase II genes was used to identify 63 representative Fusarium strains at species level and investigate their phylogenetic relationships. The main species detected was Fusarium proliferatum, and at a much lesser extent, Fusarium brachygibbosum, Fusarium caatingaense, Fusarium clavum, Fusarium incarnatum, and Fusarium solani. Pathogenicity on the Deglet Nour variety plantlets and the capability to produce mycotoxins were also assessed. All Fusarium species were pathogenic complying Koch’s postulates. Fusarium proliferatum strains produced mainly fumonisins (FBs), beauvericin (BEA), and, to a lesser extent, enniatins (ENNs) and moniliformin (MON). All F. brachygibbosum strains produced low levels of BEA, diacetoxyscirpenol, and neosolaniol; two strains produced also T-2 toxin, and a single strain produced HT-2 toxin. Fusarium caatingaense, F. clavum, F. incarnatum produced only BEA. Fusarium solani strains produced MON, BEA, and ENNs. This work reports for the first time a comprehensive multidisciplinary study of Fusarium species on date palms, concerning both phytopathological and food safety issues.
Keywords: Fusarium prolferatum, Fusarium brachygibbosum, Fusarium solani, Fusarium Equiseti Incarnatum species complex, fumonisins
1. Introduction
Date palm (Phoenix dactylifera L.) crop covers an area of about 1.1 million hectares worldwide, with a production of approximately 8,500,000 tons [1]. The main date-producing regions are Asia and Africa, with 56% and 43% of the world’s harvest, respectively. In Tunisia, the total production of dates has reached the highest level ever in 2018, with about 305 thousand tons of fruit produced, half of which destined to the export [1]. Therefore, in Tunisia, the date palm occupies a strategic place in the socio-economic stability of the oasis agro-system in desert regions and provides the main financial resource of the oasis. Indeed, an analysis on the competitive advantage of the date industry in the all Mediterranean area countries and Iran showed that Tunisia is the main supplier of dates to the EU, with about 10% of the Tunisian population depending on this crop [2].
In all cultivated areas worldwide, date palms, under suitable climatic conditions, are susceptible to various fungal pathogens, especially to Fusarium species [3,4]. In particular, the most severe pathogen is F. oxysporum, F. albedinis that causes Fusarium wilt of date palm trees, the Bayoud disease [4,5] and has been responsible of the death of more than 15,000,000 trees in Morocco and Algeria in the past [6]. Currently, strict phytosanitary rules are applied at the borders of date-palm countries that remain free of Bayoud, being this disease mostly spread in Northern Africa [5]. However, other Fusarium species have also been reported as causal agents of sudden the decline syndrome of date palms. F. proliferatum in particular is reported to be highly pathogenic in many countries where date palms are grown. In both Saudi Arabia and Spain, several authors have isolated this species from symptomatic young plants, leaves, or roots [3,7,8], or rotten fruit, as in Israel and Iran [9,10]. Another Fusarium species that has been significantly reported as a serious pathogen of date palms is F. solani, isolated from date palm wilted leaves and roots, in several countries such as Iran, Egypt, Oman, Pakistan, Saudi Arabia, United Arab Emirates, and Qatar [3,11,12,13,14,15,16]. In addition, a single strain of F. brachygibbosum and F. verticillioides, isolated from symptomatic date palm roots, were reported by Saleh et al. [3], while Abbas et al. [17] reported the identification of F. equiseti isolated from date palm roots in Iraq. Nishad and Ahmed [16] isolated the same species from wilted leaves in Qatar. Finally, the latter authors reported also a significant occurrence of F. brachygibbosum on wilted leaves and roots of date palms in Qatar [16].
A wide biodiversity characterizes the Fusarium genus, which species occur on several different crops and geographical areas, and their frequency is related to both climatic conditions and cropping practices [5,18]. Fusarium species cannot only be devastating plant pathogens or secondary invaders, colonizing plants throughout the whole growth cycle, but they can also produce and accumulate several secondary toxic metabolites, the mycotoxins, in plant tissues. Therefore, they also pose a serious risk to food safety due to the consumption of mycotoxin-contaminated crop products [19]. Fusarium species can produce mycotoxins under the influence of environmental factors, such as moisture, temperature, carbon dioxide, oxygen, substrate composition, and agronomic factors such as pesticides used, and plant variety susceptibility [20,21]. Moreover, Fusarium species are characterized by a wide inter- and intraspecific genetic diversity that can explain the dramatic variability of their mycotoxin profile that often is species-specific. The range of mycotoxins produced by this fungal genus is wide and includes trichothecenes, potent inhibitors of protein synthesis, zearalenone (ZEA), related to estrogenic disorders in human and animals, the carcinogenic fumonisins (FBs), classified by the International Agency of Cancer Research as group 2B [22,23], and other mycotoxins of lower concern for their toxicity such as moniliformin (MON), beauvericin (BEA), and enniatins (ENNs), but often reported at high amounts in crop products [19]. All mycotoxins cited above can be produced by a single or multiple Fusarium species reported to occur on date palm plants worldwide. Therefore, establishing the mycotoxin profile for the species occurring on date palm plants is an important step for a correct risk assessment related to Fusarium-mycotoxins contamination of date palm.
Due to the appearance of different disease symptoms on date palm leaves in South Tunisia, in 2017 (Figure 1), mainly wilt and dieback, a collection of date palm plant samples, from symptomatic roots and leaflets, was collected in both 2017 and 2018. The isolation from the samples led to the morphological identification of the fungal strains as mainly belonging to the Fusarium genus.
Figure 1.
A group of date palm trees showing symptoms of sudden decline syndrome.
The aims of this study were (i) identifying the Fusarium species isolated from symptomatic date palm plants; (ii) assessing their pathogenicity on date palm plantlets of the most common Tunisian variety Deglet Nour; (iii) establishing phylogenetic relationships of the Fusarium species isolated; (iv) describing the mycotoxin profile of the main Fusarium species identified on date palm plants in Tunisia.
2. Results
2.1. Phylogenetic Analyses
The evolutionary history of 63 Fusarium strains was studied at the genetic level by amplifying fragments of three different genes: calmodulin (CAL1), second largest subunit of RNA polymerase II (RPB2), and translation elongation factor (TEF1).
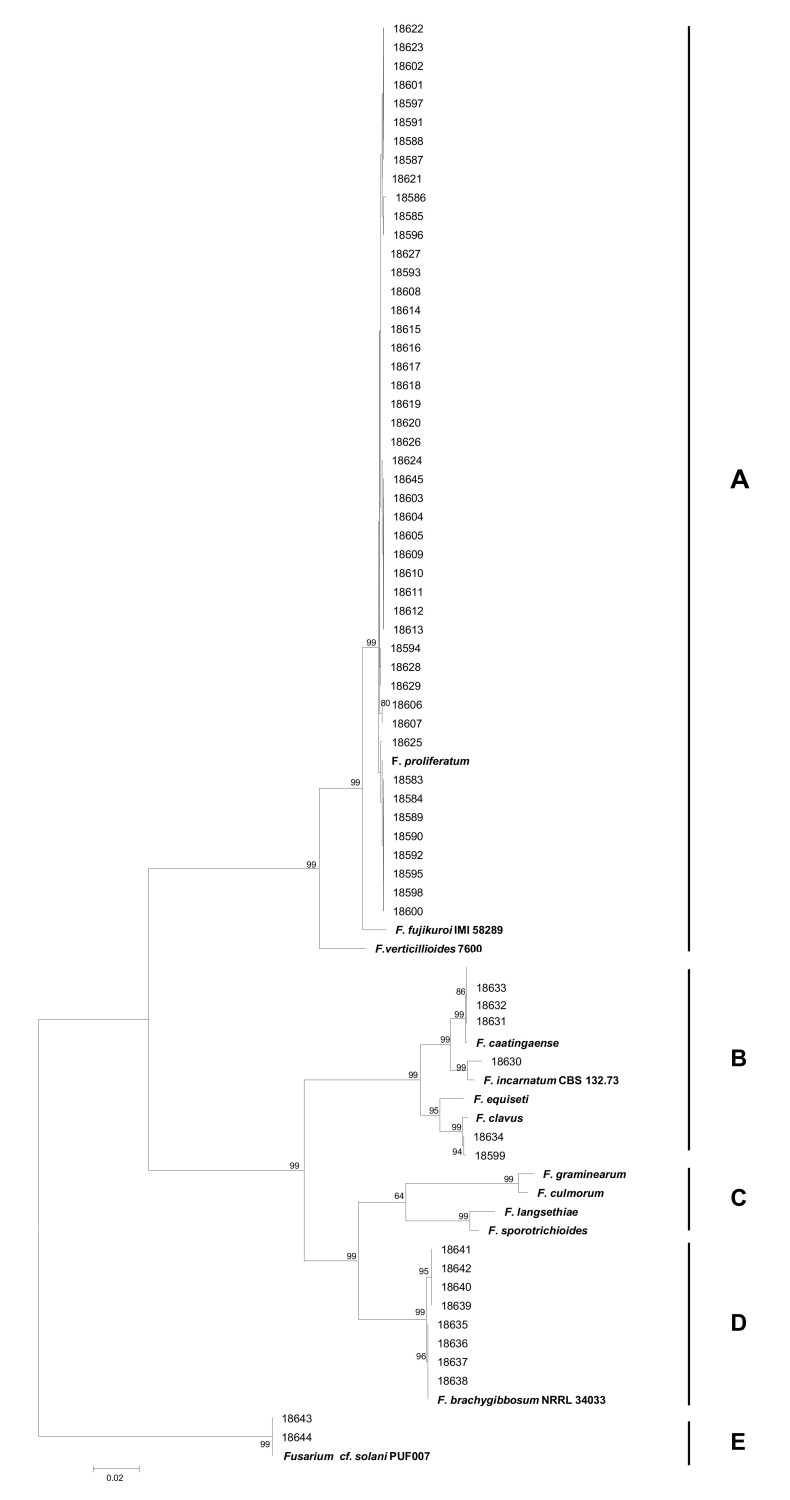
To further solve the identity of the Fusarium strains, the three gene sequences CAL1, RPB2, and TEF1 were concatenated and analyzed simultaneously with species reference strains (Table 1). In particular, common fragments around 590 bp (640 bp in F. solani strains) 870 bp, and 550 bp, respectively, were considered. A phylogenetic analysis of the concatenated sequences of about 2000 nt was carried out. The phylogenetic tree (Figure 2), generated by the Mega7 software using the Maximum Likelihood method, allowed us to define five well-separated clades (supported by high bootstrap values of 99), corresponding to Fusarium fujikuroi species complex (FFSC, A), Fusarium incarnatum equiseti species complex (FIESC, B), Fusarium sambucinum species complex (FSAMSC, C), F. brachygibossum (D), and Fusarium solani species complex (FSSC, E), as determined using reference strains downloaded from NCBI (Table 1, Figure 2).
Table 1.
Fusarium reference strains used in this study for phylogenetic analysis.
| Fusarium species | Strain | GenBank Assembly Accession | Database * | References/Submitter | ||
|---|---|---|---|---|---|---|
| CAL1 | RPB2 | TEF1 | ||||
| F. brachygibbosum | NRRL 34033 | GQ505388 | GQ505482 | GQ505418 | NCBI | [24] |
| F. caatingaense | NRRL 66470 | GCA_013624355 | GCA_013624355 | GCA_013624355 | NCBI | USDA |
| F. clavum | NRRL_66337 | GCA_004367155 | GCA_004367155 | GCA_004367155 | NCBI | USDA |
| F. culmorum | FcUK99 | GCA_900074845 | GCA_900074845 | GCA_900074845 | NCBI | [25] |
| F. equiseti | D25-1 | GCA_003313175 | GCA_003313175 | GCA_003313175 | NCBI | [26] |
| F. fujikuroi | IMI 58289 | GCA_900079805 | GCA_900079805 | GCA_900079805 | NCBI | HMGU-IBIS |
| F. graminearum | PH-1 | GCA_900044135 | GCA_900044135 | GCA_900044135 | NCBI | [27] |
| F. langsethiae | Fl201059 | GCA_001292635 | GCA_001292635 | GCA_001292635 | NCBI | [28] |
| F. proliferatum | ET1 | GCA_900029915 | GCA_900029915 | GCA_900029915 | NCBI | HMGU-IBIS |
| F. solani | PUF007 | HQ412317 | HQ423201 | HQ165838 | NCBI | [29] |
| F. sporotrichioides | NRRL3299 | GCA_003012315 | GCA_003012315 | GCA_003012315 | NCBI | [30] |
| F. verticillioides | 7600 | GCA_000149555 | GCA_000149555 | GCA_000149555 | NCBI | [31] |
* NCBI = National Center for Biotechnology Information; USDA: US Department of Agriculture, Agriculture Research Service; HMGU-IBIS: Helmholtz Zentrum München—German Research Center for Environmental Health—Institute of Bioinformatics and Systems Biology.
Figure 2.
Phylogenetic tree generated by Maximum Likelihood method (bootstrap 1000 replicates) from combined DNA sequences of CAL1, RPB2, and TEF1 gene fragments of 63 Fusarium strains.
In clade A, where the reference strains of the main species belonging to FFSC, 47 Fusarium strains clustered with F. proliferatum reference sequences. In particular, eight Fusarium strains (ITEM18583, ITEM18584, ITEM18589, ITEM18590, ITEM18592, ITEM18595, ITEM18598, ITEM18600) showed a higher homology than other F. proliferatum field strains with F. proliferatum reference strain.
A high variability was observed in clade B in which six Fusarium strains grouped with FIESC references included in the analyses, F. caatingaense, F. incarnatum, F. equiseti, and F. clavum. In particular, the strains ITEM18631, ITEM18632, ITEM18633, with 100% of homology among them, showed a high homology with F. caatingaense reference species. Fusarium strain ITEM18630 was very similar to F. incrnatum and two strains (ITEM18634, ITEM18599) were identified as F. clavum. In clade C, only reference sequences of F. graminearum, F. culmorum, F. langhsethiae, and F. sporotrichioides were included, since no Fusarium species belonging to this species complex were isolated from date palm plants.
In clade D, eight Fusarium field strains clustered with the F. brachygibbosum reference strain. In particular, Fusarium ITEM18635, ITEM18636, ITEM18637, ITEM18638 strains showed 100% homology with reference sequences. The strains ITEM18639, ITEM18640, ITEM18641, and ITEM18642 showed 100% of homology among them and were very close to the other group (Figure 2).
Finally, two Fusarium strains (ITEM18643, ITEM18644), showing 100% of homology with F. solani PUF007 strain, were grouped together in a well-defined clade (E).
2.2. Pathogenicity Assay
Data on the pathogenicity are summarized in Table S1. Following inoculation with F. solani, and FIESC members, F. caatingaense, F. clavum, and F. incarnatum, the plantlets developed typical symptoms of sudden decline, such as yellowing leaves from the upper part to the lower leaflets, followed by total whitening that appeared at 20 days post inoculation (dpi) (Figure 3). Furthermore, the stems showed distinct brown or dark spots (Figure 3). In plants inoculated with F. proliferatum, the infection led to dryness and wilting leaflets after 20 dpi, with typical symptoms of leaf wilt (Figure 3). Finally, the infection by using F. brachygibbosum developed mild leaf yellowing symptoms (Figure 3).
Figure 3.
Symptoms of Fusarium infections on date palm plantlets cv Deglet Nour, showing general whitening and dryness of stem and leaf tissues and sudden decline, 20 days after inoculation with Fusarium proliferatum ITEM 18584 strain (A), Fusarium brachygibbosum ITEM 18635 strain (B) and Fusarium caatingaense ITEM18631 strain (C).
All strains tested were re-isolated and were identical to those isolated from leaflets infected in the open field, confirming Koch’s postulates. Control plants lacked symptoms.
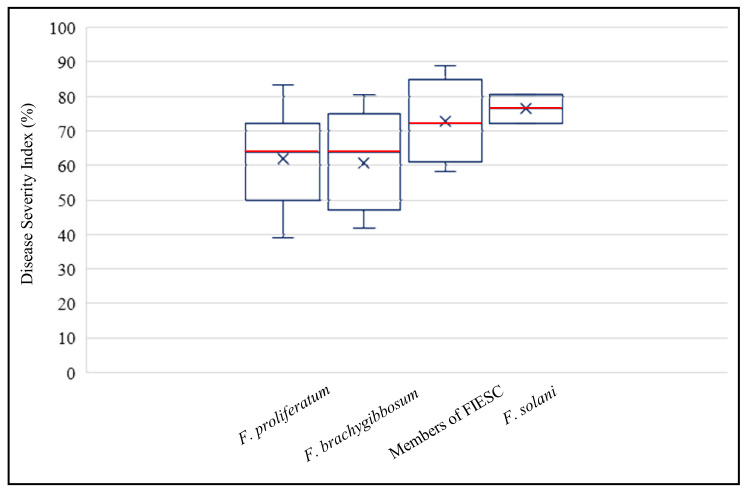
The DSI was calculated for all strains tested: F. proliferatum DSI varied from 38.9 to 83.3 among the 37 strains tested; the DSI of seven strains belonging to F. brachygibbosum varied from 41.7 to 80.6; DSI of strains belonging to FIESC varied from to 58.3 to 88.9; finally, the two strains of F. solani had a DSI of 72.2 and 80.6.
The analysis of variance revealed that there was not significant difference of pathogenicity rates among the Fusarium species and the distributions of DSI was not separated among the Fusarium species, as indicated by the box plots in Figure 4.
Figure 4.
Box plots (median, red line, upper, and lower 25th and 75th percentiles) of Disease Severity Index calculated for the different Fusarium species inoculated on date palm plantlets cv Deglet Nour.
In particular, for F. proliferatum, the species with biggest number of strains tested, the data were analyzed by comparing strains grouped based on the locality of origin. No significant differences in pathogenicity were recorded from groups of different regions (Table S1).
2.3. Mycotoxin Production
Data of mycotoxin production in vitro are reported in Table 2.
Table 2.
Mycotoxin production by Fusarium strains isolated from date palm in Tunisia (mg kg−1).
| Strain | Fumonisins | MON | BEA | Enniatins | Trichothecenes | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FB1 | FB2 | FB3 | ENN A1 | ENN B | ENN B1 | ENN H | ZEN | HT2 | T2 | |||
| Fusarium proliferatum | ||||||||||||
| 18583 | 240.2 | 31.8 | 413.7 | 2.4 | 17.7 | <LOD | - | - | - | |||
| 18584 | 22.1 | 7.1 | 803.1 | 21.7 | 75.9 | <LOD | - | - | - | |||
| 18585 | 133.9 | 362.3 | 18.7 | <LOD | 80.5 | <LOD | - | - | - | |||
| 18586 | 168.5 | 334.1 | 11.7 | <LOD | 52.2 | <LOD | - | - | - | |||
| 18587 | 1382.2 | 626.8 | 299.1 | <LOD | 39 | <LOD | - | - | - | |||
| 18588 | 32.1 | 14.03 | 287.9 | <LOD | 422.7 | <LOD | - | - | - | |||
| 18589 | 19.9 | 5.6 | 2.4 | 41.4 | 0.3 | <LOD | - | - | - | |||
| 18590 | 57.6 | 12 | 99.5 | <LOD | 0.8 | <LOD | - | - | - | |||
| 18591 | 45.3 | 7 | 65.2 | <LOD | 1.3 | <LOD | - | - | - | |||
| 18592 | 48 | 125.8 | 591.6 | 32.2 | 42.3 | <LOD | - | - | - | |||
| 18593 | 917.4 | 425.7 | 49.6 | 1.9 | 47 | <LOD | - | - | - | |||
| 18594 | 120.3 | 47.8 | 15 | <LOD | 1.8 | <LOD | - | - | - | |||
| 18595 | 461.1 | 121.6 | 544.2 | 17.2 | 29 | <LOD | - | - | - | |||
| 18596 | 719 | 140.2 | 35.2 | <LOD | 32.5 | <LOD | - | - | - | |||
| 18597 | 1472.3 | 808.9 | 290.8 | <LOD | 16 | <LOD | - | - | - | |||
| 18598 | 4.1 | 2.2 | 0.6 | <LOD | 0.1 | <LOD | - | - | - | |||
| 18600 | 1116.2 | 190.1 | <LOD | <LOD | 46 | <LOD | - | - | - | |||
| 18601 | 416.5 | 94.4 | 81 | <LOD | 7.2 | <LOD | - | - | - | |||
| 18602 | 8.7 | 6.2 | 2.2 | <LOD | 8.6 | <LOD | 0.01 | <LOD | <LOD | - | - | - |
| 18603 | 921.3 | 184.6 | 365.1 | <LOD | 97.7 | <LOD | - | - | - | |||
| 18604 | 763.3 | 104 | 273.7 | <LOD | 26.6 | <LOD | - | - | - | |||
| 18605 | 37 | 4.8 | 14.25 | <LOD | 20.8 | <LOD | - | - | - | |||
| 18606 | 1122.9 | 163.6 | 58 | <LOD | 77.5 | <LOD | - | - | - | |||
| 18607 | 55 | 36.7 | 60.7 | 1 | 84.6 | 112.40 | 48.6 | 33.7 | 51.1 | - | - | - |
| 18608 | 0.9 | 0.3 | <LOD | <LOD | 6 | <LOD | - | - | - | |||
| 18609 | <LOD | <LOD | <LOD | <LOD | 8.2 | <LOD | - | - | - | |||
| 18610 | 1649.2 | 5.9 | <LOD | <LOD | 40.5 | <LOD | - | - | - | |||
| 18611 | 1090.4 | 69.9 | 507 | <LOD | 260.2 | <LOD | - | - | - | |||
| 18612 | 1086.8 | 44.7 | 149.9 | <LOD | 336.6 | <LOD | - | - | - | |||
| 18613 | 2213.6 | 373.1 | 529.9 | <LOD | 420.6 | <LOD | - | - | - | |||
| 18614 | 4.2 | 1.8 | 1.4 | <LOD | 17.2 | <LOD | - | - | - | |||
| 18615 | 1.1 | 0.4 | <LOD | <LOD | 0.04 | <LOD | 0.1 | <LOD | 1.1 | - | - | - |
| 18616 | 0.8 | 0.3 | <LOD | <LOD | 12.5 | <LOD | - | - | - | |||
| 18617 | <LOD | <LOD | <LOD | <LOD | 10.4 | <LOD | - | - | - | |||
| 18618 | <LOD | <LOD | <LOD | <LOD | 210.5 | <LOD | - | - | - | |||
| 18619 | <LOD | <LOD | <LOD | <LOD | 211.7 | <LOD | - | - | - | |||
| 18620 | <LOD | <LOD | <LOD | <LOD | 33.4 | <LOD | - | - | - | |||
| 18621 | <LOD | <LOD | <LOD | <LOD | 437.4 | <LOD | - | - | - | |||
| 18622 | 1942.4 | 765.3 | 319.1 | <LOD | 290.6 | <LOD | - | - | - | |||
| 18623 | 319.2 | 44.2 | 49.8 | <LOD | 39.1 | <LOD | - | - | - | |||
| 18624 | 31 | 4.1 | 5 | 1 | 10 | <LOD | - | - | - | |||
| 18625 | 100.4 | 9.7 | 21.1 | <LOD | 46 | <LOD | - | - | - | |||
| 18626 | 9.8 | 1 | 2 | <LOD | 2.9 | <LOD | - | - | - | |||
| 18627 | 9 | 0.9 | 1.8 | <LOD | 3.9 | <LOD | - | - | - | |||
| 18628 | 11809.8 | 622.4 | 292.2 | <LOD | 8.6 | <LOD | - | - | - | |||
| 18629 | 1100.8 | 424.9 | 269.8 | 25 | 94.2 | <LOD | - | - | - | |||
| 18645 | 1222.2 | 234.6 | 425.3 | <LOD | 90.8 | <LOD | - | - | - | |||
| Fusarium brachygibbosum | ||||||||||||
| 18635 | - | - | - | <LOD | 1.2 | <LOD | 0.02 | <LOD | 1.9 | <LOD | 18.7 | <LOD |
| 18636 | - | - | - | <LOD | 0.4 | <LOD | <LOD | <LOD | 1.2 | |||
| 18637 | - | - | - | <LOD | 1.9 | <LOD | <LOD | |||||
| 18638 | - | - | - | <LOD | 1 | <LOD | <LOD | |||||
| 18639 | - | - | - | <LOD | 0.1 | <LOD | <LOD | |||||
| 18640 | - | - | - | <LOD | 0.6 | <LOD | <LOD | <LOD | 2.4 | |||
| Fusarium incarnatum-equiseti species complex | ||||||||||||
| 18630 F. incarnatum |
- | - | - | <LOD | 3.2 | <LOD | <LOD | |||||
| 18631 | - | - | - | <LOD | 0.5 | <LOD | <LOD | |||||
| 18632 | - | - | - | <LOD | 0.3 | <LOD | <LOD | |||||
| 18633 | - | - | - | <LOD | 0.9 | <LOD | <LOD | |||||
| F. caatingaense | ||||||||||||
| 18634 F. clavum |
- | - | - | <LOD | 423.1 | <LOD | <LOD | |||||
| Fusarium solani | ||||||||||||
| 18643 | - | - | - | 50.1 | 21.6 | 82.5 | <LOD | <LOD | 285 | <LOD | ||
| 18644 | - | - | - | 62.2 | 25.2 | 95.1 | 8.2 | <LOD | 224.1 | |||
Fusarium proliferatum: all strains were able to produce at least one mycotoxin. With regard to FBs, six strains did not produce FBs, while 41 strains produced FB1 in a range from 0.8 to 11809.8 mg kg−1 and FB2 in a range from 0.3 to 808.9 mg kg−1. Moreover, 37 strains produced FB3 in a range from 0.6 to 803.1 mg kg−1 Therefore, among the 47 F. proliferatum strains analyzed, four strains could produce FB1 and FB2 but were not able to produce FB3.
All strains were able to produce BEA in a range from 0.1 to 437.4 mg kg−1.
Only eight strains were able to produce MON, with values ranging between 1 and 41.4 mg kg−1.
Only a single strain was able to produce all ENNs: ENNA1 (112.4 mg kg−1) ENNB (48.6 mg kg−1), ENNB1 (33.7 mg kg−1), and ENNH (51.1 mg kg−1). A very low amount of ENNB was detected in two strains, with values of 0.1 and 0.06 mg kg−1, respectively. Only a single strain produced also ENNH, but at a low amount (1.1 mg kg−1).
Fusarium brachygibbosum: all strains produced low amount of BEA with values ranging between 0.1 and 1.9 mg kg−1. Only a single strain was able to produce a low amount of ENNB (0.02 mg kg−1) and ENNH (1.9 mg kg−1). Traces of DAS and NEO were found in all the strains, while a single strain was able to produce also HT2 toxin (18.7 mg kg−1). Also for T2 toxin production, a single strain synthetized the mycotoxin with a value of 1.2 mg kg−1.
FIESC: members of this complex were evaluated for their capability to produce MON, BEA, ENNs, and trichothecenes. All five strains analyzed were able to produce only BEA. In particular, with the exception of a strain that produced BEA at a high level (423.1 mg kg−1), all strains produced low amounts of BEA, with values ranging between 0.3 and 3.2 mg kg−1.
Fusarium solani: The two strains analyzed produced MON (50.1 and 62.2 mg kg−1), BEA (21.6 and 25.2 mg kg−1), ENNA1 (82.5 and 95.1 mg kg−1), and ENNH (285 and 224.1 mg kg−1). Only a single strain produced ENNB (8.2 mg kg−1).
3. Discussion
3.1. Species Identification
In this study, we report for the first time the occurrence of Fusarium species on date palm plants in Tunisia that show evident symptoms of disease. The prevalence was extensive in several places and the incidence was high. Among the Fusarium species, the most harmful pathogen F. oxysporum f. sp. albedinis, the causal agent of the destructive Bayoud Disease [5], was not detected, although this pathogen is currently found in Algeria, Morocco, and Mauritania. For this reason, F. oxysporum f. sp. albedinis is subject to strict phytosanitary regulations at the borders of neighboring countries that are free of Bayoud’s disease [32]. Based on phylogenetic analysis of three loci, we identified in this survey F. proliferatum, a member of FFSC, as the dominant species. To a lesser extent, also F. brachygibbosum, member of FSAMSC, and three members of FIESC, F. incarnatum, F. clavum, and F. caatingaense, were isolated and identified. Finally, rarely, also strains of F. solani, a species member of the FSSC, were identified. Our data confirm previous worldwide reports on the occurrence and pathogenicity of F. proliferatum on date palms [3,7,9,10,15]. Some of these reports attribute only a low or occasional frequency to this species [7,10,15], while in Israel [9] and Saudi Arabia [3] F. proliferatum occurred at high incidence in symptomatic plants in various localities. On the other hand, the occurrence of F. proliferatum has also been reported here for the first time also for the whole of northern Africa, while the previous reports were from Asia [3,7,9,10,15] and Europe [8].
3.2. Pathogenicity Test
In our pathogenicity test, carried out on the the Deglet Nour variety, which represents 80% of Tunisian production, F. proliferatum proved to be the least pathogenic of the Fusarium species tested, namely, moderately pathogenic, according to the scale that we used. However, the group of F. proliferatum strains isolated from Chabbat and Mides proved to have a similar mean level of pathogenicity as F. brachygibbosum, F. solani, and Fusarium species members of FIESC. Therefore, also within this species, strains with high pathogenic potential occurred which could provide a genetic source for increasing their pathogenicity on date palms. This confirms previous reports from other geographical areas on the high pathogenicity of F. proliferatum, assessed by different pathogenic bioassays [3,7,8,10]. On the other hand, although Alwashi et al. [15] isolated F. proliferatum at high frequency from date palm roots affected by Sudden Disease Syndrome in UAE, they reported that this species was not pathogenic on date palm plantlets. Further studies should be carried out to better assess the effective role played by F. proliferatum on the fungal diseases affecting date palm.
Pathogenicity was tested also for other species identified in this survey that occurred to a lesser extent. The pathogenicity of the two strains of F. solani was the highest with a DSI of 3.06. Fusarium solani has been reported as highly pathogenic on date palms by Alwashi et al. [15] in a plantlet assay in UAE, and by Saleh et al. [3] in a detached leaflets assay in Saudi Arabia. On the contrary, Nishad and Ahmed [16], testing in Qatar the pathogenicity of F. solani on a detached leaf assay, reported that the strain tested was little virulent. In all papers mentioned above, the identification of F. solani was carried out by using different molecular markers. The FSSC species members cause foot and root rot of numerous crops, and the complex includes over 80 phylogenetically distinct species, including saprophytes, plant, animal, and human pathogens [33,34]. Since the previous reports on F. solani from date palms, no details were provided on which species member of the FSSC was tested for pathogenicity. The dramatic difference of F. solani pathogenicity, reported by several authors, could be related to different phylogenetic species of the complex tested in each paper. Our data confirm that members of FFSC can be highly pathogenic. Therefore, it is worrisome that this species can also occur on wilted date palm plants in Tunisia, as this species, together with F. proliferatum, is considered an increasing pathogen in palm date growing countries [3,15]. The three species of FIESC identified in this study and F. brachygibbosum, a species member of FSAMSC, proved to be highly pathogenic on date palms in our assay. Our data are in contrast with previous reports, since both FIESC members and F. brachygibbosum were evaluated as weakly or moderately pathogenic species [13,16]. In particular, both F. equiseti, a member of FIESC, and F. brachygibbosum, proved to be moderately pathogen in Qatar, in a date palm detached leaf assay [16], while F. brachygibbosum was reported to be a weak pathogen of roots in Oman [13]. It is important to underline that recent new taxonomic analyses of both FIESC and F. brachygibbosum have generated new phylogenetic species in FIESC [35] and split F. brachygibbosum in 12 phylogenetic new species grouped in F. brachygibbosum clade within FSAMSC [36]. Therefore, the different pathogenicity on date palm shown by FIESC members and F. brachygibbosum in different assays, reported in different papers, can be due to different phylogenetic species tested.
3.3. Mycotoxin Profile
In addition to their pathogenicity ability that causes loss of production, all Fusarium species identified in this survey are also reason of high concern since they can produce a wide range of mycotoxins [19]. Their occurrence on date palm plants indicates that a contamination of the fruit at the harvest in the field and its by-products in postharvest can occur. Since we proved a high presence of toxigenic Fusarium species in some organs of the date palm plants (roots and leaves), to monitor environmental conditions in the field suitable for mycotoxin production in planta by the Fusarium species is important to manage and avoid an eventual, although unlike, final contamination of fruits. Our molecular and phylogenetic results showed that most of the identified Fusarium strains belong to F. proliferatum, a species member of the FFSC, while only a very small set of strains belonged to other complexes (FSAMSC, FIESC, FSSC). A correct identification of Fusarium species is a key aspect, since many species have a specific own mycotoxin profile. Therefore, accurate risk assessment is strongly linked to the use of advanced diagnostic tools. Fusarium proliferatum is characterized by a distinct mycotoxin profile. The in vitro mycotoxin production by the strains tested was analyzed with respect to the main mycotoxins associated to this species: FBs, MON, BEA, and ENNs. The data showed that two different groups of strains occurred with respect to FBs production, within F. proliferatum. A group of six strains that were isolated from three different localities (Degueche, Hezoue, Mides) did not produce any of the three FBs analyzed, while a group of 41 strains produced all FBs. These data could be comforting since a ratio of non-producing strains reduces the risk related to the occurrence of such important FBs producing species in the date palm. Fusarium proliferatum is a polyphagus species able to colonize a very wide range of crops [37], with a mixed population including FBs producers and FBs-not producers. We have reported that among F. proliferatum strains isolated from some crops (e.g., fig, wheat), strains unable to produce FBs in laboratory conditions could co-exist with FB-producing strains, even though they keep the whole biosynthetic FB gene cluster, with the same syntheny observed in FBs producers [38]. Similarly, in Aspergillus genus, we identified in two FB2 producing species, Aspergillus niger and Aspergillus welwitschiae [39] both chemotypes: FB2 producing and non-producing strains [40]. Therefore, the cause of lack of FB production in some of F. proliferatum strains isolated from date palm remains to be evaluated, to determine whether it is a conserved trait, due to possible mutations of one or more genes in the FB biosynthetic gene cluster. Indeed, the phylogenetically closest species to F. proliferatum, Fusarium fujikuroi, considered a sibling species of F. proliferatum [41], has been shown to include two phylogenetic groups where all strains of the so-called G-group lacked to produce FBs [42]. Associated to this trait, the authors proved the occurrence of three mutations that involved three different FUM-cluster genes essential for FBs production, where each mutation alone could account for the lack of FBs production [43,44]. If these mutations could occur also in the F. proliferatum strains lacking the FBs production capability is a hypothesis under evaluation. On the other hand, the high occurrence of F. proliferatum on date palm in Tunisia and the high incidence of highly FBs producing strains is worrisome, since both FB1 and FB2 are considered by the IARC group 2B, meaning potentially carcinogenic. The reports on the occurrence of this species on date fruit bunch in Iran and Israel, where F. proliferatum strains caused fruit drying and dropping [9,10], confirm it the concern for human health related to this species occurrence on date palm. Moreover, all strains analyzed produced BEA, while a significant group of them produced also ENNs A and B. Altogether, these compounds are cyclic hexadepsipeptides and have been reported to be not only toxic to humans and animals but also to possess phytotoxic activity [45,46]. Beauvericin production has been reported in several species of the genus Fusarium including different formae speciales of F. oxysporum [47,48,49]. Beauvericin can act as virulence factor for phytopathogenic Fusarium species, as reported by Lopez-Berges et al. [50] that proved that BEA deficient mutants of F. oxysporum were attenuated in virulence on tomato plants. Moreover, BEA was shown to be an intracellular phytotoxin and an inhibitor of respiration in young maize leaves [51], while in tomato BEA acts as an intermediate of cell death signaling, by causing an imbalance in the ascorbate system [45]. On the other hand, also ENNs were proved to be phytotoxic to plants. Mutants of the cereal pathogen F. avenaceum lacking enniatin synthetase exhibited significantly reduced virulence in an infection assay on potato tubers [52]. Therefore, the large production of cyclic hexadepsipeptides by the F. proliferatum isolated from date palm shows that this species has a high potential of using these compounds to increase its virulence to the plants. The ability of producing BEA and, more rarely, ENNs, was proved also for the strains of the FIESC species, especially F. clavum, for F. solani, and, at a low level for F. brachygibbosum. This shows that all these species can have a different potential to increase their virulence on plants by using cyclic hexadepsipeptides. We also analyzed the ability of all species to produce MON, a mycotoxin that plays a role in various animal mycotoxicoses, especially in poultry fed contaminated maize [53]. More recently, however, EFSA considered MON a low risk for humans and animals [54]. On the other hand, MON was often reported to have phytotoxic effects, as it can inhibit plant growth by reducing the efficiency of photosynthetic pigments, and may inhibit leaf development and can also reduce the mass of wheat plantlets [55]. Moniliformin was produced in very low concentrations by only some strains of F. proliferatum, but at high levels by both strains of F. solani. Interestingly, the latter species, the most pathogenic in our assay and reported as highly pathogenic on date palm in previous reports [3,15], was able to produce in vitro high amounts of MON, BEA, and some of the tested ENNs (ENNA1 and ENNH). These are all metabolites that could increase the virulence of F. solani against date palm under field conditions. Finally, the production of ZEN and trichothecenes was analyzed for the strains of FIESC, FSAMSC, and FSSC. No strain produced in vitro ZEN, while, among trichothecenes, only T-2 and HT-2 were produced by three strains of F. brachygibbosum along with DAS and NEO in traces. To the best of our knowledge, this is the first report demonstrating that F. brachygibbosum is capable of producing these mycotoxins, which are considered to be the most toxic Fusarium mycotoxins for humans and animals [19]. Previous studies reported the production of type B trichothecenes fusarenon X and 4,15diacetoxy-nivalenol by F. brachygibbosum isolated from legume pastures in Australia [56] and soybean roots in Ethiopia [57]. On the other hand, more recently, Laraba et al. [36] reported that strains of F. brachygibbosum isolated from Virgin jungle soil in Thailand and pearl millet in Niger could produce the type A trichothecenes diacetoxyscirpenol and neosolaniol. Our report is consistent with Laraba et al. [36], since DAS and NEO are well-known precursors of T-2 and HT-2 type A trichothecenes. Only a single species of Fusarium has been reported to be able to produce both types of trichothecenes, Fusarium poae [19], therefore further studies are in progress, aimed to analyze the trichothecene gene cluster of F. brachygibbosum and to obtain more accurate information on the potential mycotoxin profile of this species. The occurrence of such toxigenic species in date palms is reason of further concern, also since its occurrence has been often detected in our survey, in combination with the FB producing F. proliferatum, and therefore the risk of multiple mycotoxins contamination of fruits increases the risk for human health.
4. Conclusions
To the best of our knowledge, currently in Tunisia, there is a lack of information on mycotoxin contamination of date fruits and related by-products. For the first time, we reported the occurrence of several Fusarium species on date palms grown in Tunisia. However, we could confirm that, although we detected a high incidence of Fusarium species on date palms, the quarantine pathogen F. oxysporum f. sp. albedinis is absent in all the considered samples. Among the Fusarium species identified, we reported a frequent occurrence of F. proliferatum as main species associated to date palm, confirming previous reports from Asia and Europe. This is a reason of serious concern, since this species produces the mycotoxins FBs, associated to a wide number of mycotoxicoses. We also provided new information on F. brachygibbosum, which was shown in our report for the first time to be able to produce T-2 and HT-2 toxins and on the additional potential contamination by cyclic hexadepsipeptides BEA and ENNs. Our data suggest the need of further investigations (i) for a better understanding of the possibility that Fusarium species occurring on date palm can produce mycotoxins in planta, (ii) for defining which environmental conditions would influence such production, useful for establishing proper management protocols to be adopted to minimize production loss, and (iii) to investigate whether Fusarium mycotoxins can actually occur in normal-looking fruits. Finally, our phylogenetic studies confirm that, also on date palm, the detection of multiple Fusarium species brings us, by using Monica Evans words, “into the invisible, indispensable world of microbial biodiversity”.
5. Materials and Methods
5.1. Origin of the Samples and Fungal Isolation
During 2017–2018, date-palm leaflets and roots showing fungal disease symptoms were randomly collected from 7 different Tunisian oases: Hazoua (33°43′49.81′′ N, 7°35′23.44′′ E), IBN Chabbat (33°55′53.34′′ N, 8° 4′57.07′′ E), Mides (34°24′31.97′′ N, 7°55′7.56′′ E), Tozeur (33°54′39.71′′ N, 8°8′29.90′′ E), Nafta (33°52′34.09′′ N, 7°52′44.55′′ E), and Degueche (33°59′32.39′′ N, 8°14′19.26′′ E), and El-Hamma (33°59′51.70′′ N, 8°9′36.31′′ E), located in Tozeur governorate.
After a surface-disinfection with 2% sodium hypochlorite solution for 2 min and two washings with distilled sterilized water for 1 min, leaf and roots portions, taken from the margin of the symptomatic tissues, were cut into small pieces (5 mm in diameter) with a sterilized scalpel and transferred on Potato Dextrose Agar (PDA) amended with 100 mg L−1 of streptomycin sulphate salt and 50 mg L−1 of neomycin. Petri dishes were incubated at 25 ± 1 °C for 7 days under an alternating light/darkness cycle of 12 h photoperiod. After incubation, fungal colonies originated from plant tissues and morphologically identified as Fusarium species, were transferred on new PDA plates and then purified by using the single spore isolation technique. All representative 63 monoconidial Fusarium strains (Table 3), 54 isolated from leaves, and 9 isolated from roots, were stored at −80 °C in 10% glycerol, as suspensions of conidia and mycelium, for further analyses.
Table 3.
List of Fusarium strains considered in this study.
| Strain (ITEM *) |
Fusarium species | Part of Plant | Origin | Years of Sampling |
|---|---|---|---|---|
| 18618 | F. proliferatum | Leaflets | Degueche | 2017 |
| 18619 | F. proliferatum | Leaflets | Degueche | 2017 |
| 18583 | F. proliferatum | Leaflets | El-Hamma | 2017 |
| 18584 | F. proliferatum | Leaflets | El-Hamma | 2017 |
| 18592 | F. proliferatum | Leaflets | El-Hamma | 2017 |
| 18595 | F. proliferatum | Leaflets | El-Hamma | 2017 |
| 18585 | F. proliferatum | Leaflets | Hezoua | 2017 |
| 18586 | F. proliferatum | Leaflets | Hezoua | 2017 |
| 18590 | F. proliferatum | Leaflets | Hezoua | 2017 |
| 18591 | F. proliferatum | Leaflets | Hezoua | 2017 |
| 18605 | F. proliferatum | Leaflets | Hezoua | 2017 |
| 18611 | F. proliferatum | Leaflets | IBN Chabbat | 2017 |
| 18612 | F. proliferatum | Leaflets | IBN Chabbat | 2017 |
| 18613 | F. proliferatum | Leaflets | IBN Chabbat | 2017 |
| 18589 | F. proliferatum | Leaflets | Mides | 2017 |
| 18624 | F. proliferatum | Leaflets | Mides | 2017 |
| 18627 | F. proliferatum | Leaflets | Mides | 2017 |
| 18606 | F. proliferatum | Leaflets | Tozeur | 2017 |
| 18607 | F. proliferatum | Leaflets | Tozeur | 2017 |
| 18610 | F. proliferatum | Leaflets | Tozeur | 2017 |
| 18623 | F. proliferatum | Leaflets | Tozeur | 2017 |
| 18602 | F. proliferatum | Roots | Mides | 2017 |
| 18616 | F. proliferatum | Roots | Mides | 2017 |
| 18636 | F. brachygibbosum | Leaflets | IBN Chabbat | 2017 |
| 18639 | F. brachygibbosum | Leaflets | IBN Chabbat | 2017 |
| 18640 | F. brachygibbosum | Leaflets | IBN Chabbat | 2017 |
| 18635 | F. brachygibbosum | Leaflets | Mides | 2017 |
| 18599 | F. clavum | Leaflets | Mides | 2017 |
| 18631 | F. caatingaense | Leaflets | Tozeur | 2017 |
| 18632 | F. caatingaense | Leaflets | Tozeur | 2017 |
| 18633 | F. caatingaense | Roots | Tozeur | 2017 |
| 18596 | F. proliferatum | Leaflets | Degueche | 2018 |
| 18621 | F. proliferatum | Leaflets | Degueche | 2018 |
| 18594 | F. proliferatum | Leaflets | Hezoua | 2018 |
| 18617 | F. proliferatum | Leaflets | Hezoua | 2018 |
| 18620 | F. proliferatum | Leaflets | Hezoua | 2018 |
| 18593 | F. proliferatum | Leaflets | IBN Chabbat | 2018 |
| 18597 | F. proliferatum | Leaflets | IBN Chabbat | 2018 |
| 18600 | F. proliferatum | Leaflets | IBN Chabbat | 2018 |
| 18601 | F. proliferatum | Leaflets | IBN Chabbat | 2018 |
| 18603 | F. proliferatum | Leaflets | IBN Chabbat | 2018 |
| 18622 | F. proliferatum | Leaflets | IBN Chabbat | 2018 |
| 18615 | F. proliferatum | Leaflets | Mides | 2018 |
| 18626 | F. proliferatum | Leaflets | Mides | 2018 |
| 18645 | F. proliferatum | Leaflets | Mides | 2018 |
| 18608 | F. proliferatum | Leaflets | Mides | 2018 |
| 18598 | F. proliferatum | Leaflets | Mides | 2018 |
| 18609 | F. proliferatum | Leaflets | Mides | 2018 |
| 18614 | F. proliferatum | Leaflets | Mides | 2018 |
| 18604 | F. proliferatum | Leaflets | Nafta | 2018 |
| 18628 | F. proliferatum | Leaflets | Nafta | 2018 |
| 18629 | F. proliferatum | Leaflets | Nafta | 2018 |
| 18625 | F. proliferatum | Leaflets | Tozeur | 2018 |
| 18588 | F. proliferatum | Roots | IBN Chabbat | 2018 |
| 18587 | F. proliferatum | Roots | IBN Chabbat | 2018 |
| 18641 | F. brachygibbosum | Leaflets | El-Hamma | 2018 |
| 18637 | F. brachygibbosum | Leaflets | Mides | 2018 |
| 18638 | F. brachygibbosum | Roots | Hezoua | 2018 |
| 18642 | F. brachygibbosum | Roots | El-Hamma | 2018 |
| 18634 | F. clavum | Leaflets | IBN Chabbat | 2018 |
| 18630 | F. incarnatum | Roots | Mides | 2018 |
| 18643 | F. solani | Roots | IBN Chabbat | 2018 |
| 18644 | F. solani | Leaflets | Mides | 2018 |
* = ITEM, Agri-Food Toxigenic Fungi Culture Collection, ISPA, Bari. http://www.ispa.cnr.it/Collection (accessed on 3 May 2021).
5.2. DNA Extraction and Molecular Analyses
Sixty-three Fusarium monoconidial strains were firstly cultured on PDA medium, and after 3 days of incubation, 5 mycelial plugs from the margins of actively growing colonies were transferred on cellophane disks overlaid on PDA plates. Pure cultures were further incubated at 25 °C for 3 days and then mycelium of each strain was collected by scraping and lyophilized. DNAs of single pure cultures was isolated and purified from powdered lyophilized mycelium (10–15 mg) using the “Wizard Magnetic DNA Purification System for Food” kit (Promega Corporation, Madison, WI, USA), according to the manufacturer’s protocol. Integrity of DNA was checked by electrophoretic analysis on 0.8% agarose gel and by comparison with a standard DNA (1 kb DNA Ladder, Fermentas GmbH, St. Leon-Rot, Germany); quantity was evaluated by Thermo-Scientific Nanodrop (LabX, Midland, ON, Canada). Translation elongation factor, CAL1 and RPB2 were selected among the most informative housekeeping genes for the molecular characterization and for building phylogenetic relationships at inter- and intra-specific levels of Fusarium strains, using a multi-locus sequence approach. Polymerase chain reaction (PCR) reactions were set up using the following specific primer pairs and related amplification protocols: EF1/EF2 [58], CL1/CL2 [59], 5F/7CR [60]. PCR mixture (15 μL) containing approximately 15–20 ng of DNA template, 1.5 μL (10X) PCR solution buffer, 0.45 μL each primer (10 mM), 1.2 μL dNTPs (2.5 mM), and 0.125 μL of Hot Start Taq DNA Polymerase (1 U/μL; Fisher Molecular Biology, Trevose, PA, USA). For each reaction, a not template control was included to ascertain the absence of contamination. The PCR products, stained with GelRed® (GelRed® Nucleic Acid Gel Stain, 10,000X, Biotium Inc., Fremont, CA, USA), were visualized with UV after electrophoretic separation in 1X TAE buffer, on 1.5% agarose gel and sized by comparison with 100 bp DNA Ladder (Invitrogen, Thermo Fisher Scientific, Carlsbad, CA, USA). For DNA sequencing, PCR residual primers were removed using the enzymatic mixture EXO/FastAP (ExonucleaseI, FastAP thermosensitive alkaline phosphatase, Thermo Scientific, Vilnius, Lithuania) and PCR ampliocs were labelled using the BigDye Terminator v3.1 Cycle Sequencing Ready Reaction Kit (Applied Biosystems, Foster City, CA, USA), according to the manufacturer’s recommendations. Reaction of both strands were purified by filtration through Sephadex G-50 (5%) (Sigma-Aldrich, Saint Louis, MO, USA) and analyzed using “ABI PRISM 3730 Genetic Analyzer” (Applied Biosystems, Foster City, CA, USA). Partial FASTA sequences were analyzed and assembled using the BioNumerics v. 5.1 software (Applied Maths, Kortrijk, Belgium). DNA sequences of Fusarium species reference strains (Table 2) were downloaded through the National Center for Biotechnology Information (NCBI). All sequences of the 3 genes (TEF1, CAL1, RPB2) were aligned using the ClustalW algorithm [61] and the phylogenetic relationships were built using the maximum likelihood method with the MEGA software version 7 [62]. The bootstrap analysis [63] was conducted to determine the confidence of internal nodes using a heuristic search with 1000 replicates, removing gaps.
5.3. Pathogenicity Assay
Pathogenicity assays were carried out by using 51 strains, representing all species identified in this study (Table S1). The strains were grown for 7–10 days at 25 °C on PDA under a light/darkness photoperiod of 12 h. For each strain, the inoculum was prepared by flooding the agar plate surface with 10 mL of sterile distilled water (SDW) and scraping with a spatula. Conidial suspensions were filtered through four layers of cheese cloth and were diluted with SDW for obtaining a final concentration of 105 conidia mL−1 [64].
The pathogenicity tests were performed on healthy date palm plantlets of the most common Tunisian cultivar Deglet Nour. The plantlets used were regenerated from direct somatic embryogenesis, derived from shoot-tip explants. Therefore, they were acclimatized in greenhouse under controlled conditions, for 6-months. The plants were of 25–30 cm length, having 4–5 leaves, and have grown in large pots (12 cm diameter and 18 cm height filled with planting medium peat/perlite 2:1 (v/v)). Each plantlet was inoculated by injecting 2 mL of conidial suspension into the plantlet crown area, using a sterile hypodermic syringe. Three plantlets per treatment were considered, and each experiment was performed in triplicate. Date palm plantlets injected only with SDW were used as negative control. To favor fungal development, all plantlets were watered and covered with plastic bags for 24 h, in order to ascertain high moisture content. Plantlets were growth in greenhouse at 25 ± 2 °C, and 80% RH, 12 h light/darkness photoperiod. After inoculation, disease symptoms were assessed weekly for 3 months.
Pathogenicity was evaluated by assessing the degree of disease symptoms in the plantlets, according with Alwahshi et al. [15], partially adapted. Each plantlet was rated on a scale of 0–4, where:
0 indicated a healthy plantlet with a well-developed foliar apparatus that exhibited negligible symptoms;
1 indicated plants with up to 25% of disease symptoms in crown area, exhibiting necrotic lesions from 5 to 10 mm and beginning of whitening;
2 indicated plants with symptoms from 25% to 50% of disease symptoms, exhibiting necrosis and wilting lesions from 11 to 30 mm;
3 indicated plants with symptoms from 50 to 75% exhibiting extensive necrotic lesions (30–70 mm);
4 indicated plants with symptoms more than 75% of crown area, including also necrosis and whitening of leaves and root rot symptoms.
To confirm Koch’s postulates, pieces of leaves, where symptoms of the disease appeared, were sterilized on the surface and re-isolated. Plates were incubated at 25 ± 2 °C and subsequent growth was recorded.
Disease severity index (DSI) was calculated using the Townsend–Heuberger formula [65]: DSI = Σ [(n × c) ⁄ (V × N)] × 100, where n is number of palm plantlets per class, c is the numerical value of each class, V is the highest class value, and N is the total number of plantlets. Data of pathogenicity assays were subjected to analysis of variance (ANOVA) and Duncan’s multiple range test, by using Statistical Package for the Social Sciences (SPSS) v. 27.0 software, with significance level (P) of 0.05.
5.4. Mycotoxin Analyses
5.4.1. Sample Preparation
Sixty Fusarium strains were analyzed for the in vitro mycotoxins production (Table 2). In detail, 47 F. proliferatum strains were tested for the capability to produce MON, BEA, FBs (FB1, FB2, FB3), and ENNs (EnnA1, EnnB, EnnB1, EnnH); 6 F. brachygibbosum strains, 5 Fusarium strains of FIESC, and 2 F. solani strains were tested for the production of MON, BEA, FBs, ENNs, ZEA, and trichothecenes.
Each strain was grown on plates containing PDA and then mycelial plugs of these cultures were used to inoculate 30 g of autoclaved rice, previously kept to 45% of moisture for one night. Cultures were incubated at 25 °C in darkness for 3 weeks, then dried at 65 °C for 24 h and ground to a fine powder and used for chemical analyses. Not inoculated rice, treated in the same way, was used as negative control. Samples were prepared according to Malachová et al. [66] procedure. Briefly, 0.5 g of ground cereal was stirred for 90 min at 200 strokes/min on a shaker with 2 mL of acetonitrile/water (80/20, v/v) mixture acidified with 0.2% of formic acid and then centrifuged for 10 min at 14,000 rpm. A total of 1 µL of supernatant was injected into LC-MS.
5.4.2. Chemicals and Reagents
Mycotoxin standard solutions (in acetonitrile) were obtained from Romer Labs (Tulln, Austria).
LC-MS grade methanol and acetonitrile were purchased from Scharlab Italia srl (Milan, Italy); bidistilled water was obtained using Milli-Q System (Millipore, Bedford, MA, USA). MS-grade ammonium acetate, acetic acid, and formic acid from Fisher Chemical (Thermo Fisher Scientific Inc., San Jose, CA, USA) were also used.
5.4.3. Targeted UHPLC-MS/MS Analysis of MON
UHPLC Dionex Ultimate 3000 separation system coupled to a triple quadrupole mass spectrometer (TSQ Vantage; Thermo Fisher Scientific Inc., San Jose, CA, USA) equipped with an electrospray source (ESI) was employed.
For the chromatographic separation, XBridge Amide BEH column (Waters, Wilmslow, UK) with 2.10 × 100 mm and a particle size of 2.6 µm heated to 40 °C was used. A total of 2 μL of sample extract was injected into the system; the flow rate was 0.4 mL/min.
Gradient elution was performed by using acetonitrile (eluent A), water acidified with 0.2% formic acid (eluent B) and ammonium formate 20 mM 1% formic acid (eluent C). The elution gradient started from 0% of B, 5% of C and 95% of A and, after an initial isocratic step of 2 min, B increased at 60% in 3 min and this composition is kept for 2.5 min. At 8 min, the initial conditions were restored and kept for 5 min. The total run time was 13 min.
Moniliformin was monitored in negative ionization mode; spray voltage 3500 V, capillary temperature at 270 °C, vaporizer temperature was kept at 200 °C, sheath gas flow was set at 50 units, and the auxiliary gas flow at 5 units. S-Lens RF amplitude value and collision energies (CE) were optimized during infusion of analyte standard solutions (1000 ng mL−1, in methanol) employing an automatic function of the Xcalibur software (Thermo Fisher Scientific, San Jose, CA, USA). Detection was performed using multiple reaction monitoring (MRM) mode. The following optimized transitions were used for the quantification: m/z 97→41 (CE 16 eV); m/z 97→80 (CE 23 eV); m/z 97→63 (CE 45 eV). Quantification of target analytes was performed using matrix-matched calibration standards (range 0.001–2 ppm).
5.4.4. UHPLC-TWIMS-QTOF Screening of Mycotoxins
ACQUITY I-Class UPLC separation system coupled to a VION IMS QTOF mass spectrometer (Waters, Wilmslow, UK) equipped with an electrospray ionization (ESI) interface was employed for mycotoxins screening. Samples were injected (1 µL) and chromatographically separated using a reversed-phase C18 BEH ACQUITY column 2.1 × 100 mm, 1.7 µm particle size (Waters, Milford, MA, USA). A gradient profile was applied using water 1 mM ammonium acetate (eluent A) and methanol (eluent B) both acidified with 0.5% acetic acid as mobile phases. Initial conditions were set at 5% B, after 0.7 min of isocratic step, a linear change to 50% B in 5.8 min. Moreover, 100% B was achieved in 3 min and holding for 3 min to allow for column washing before returning to initial conditions. Column recondition was achieved over 1.5 min, providing a total run time of 14 min. The column was maintained at 40 °C and a flow rate of 0.4 mL/min used.
Mass spectrometry data were collected in positive electrospray mode over the mass range of m/z 50−1000. Source settings were maintained using a capillary voltage, 1.0 kV; source temperature, 150 °C; desolvation temperature, 600 °C and desolvation gas flow, 1000 L/h. The TOF analyzer was operated in sensitivity mode and data acquired using HDMSE, which is a data independent approach (DIA) coupled with ion mobility. The optimized ion mobility settings included: nitrogen flow rate, 90 mL/min (3.2 mbar); wave velocity 650 m/s and wave height, 40 V. Device within the Vion was calibrated using the Major Mix IMS calibration kit (Waters, Wilmslow, UK) to allow for CCS values to be determined in nitrogen. The calibration covered the CCS range from 130–306 Å2. The TOF was also calibrated prior to data acquisition and covered the mass range from 151 Da to 1013 Da. TOF and CCS calibrations were performed for both positive and negative ion mode. Data acquisition was conducted using UNIFI 1.8 (Waters, Wilmslow, UK).
Mycotoxins identification was performed by comparison of rt, fragmentation pattern and collision cross-sections with the standard collect in our UNIFI library, created by running a mix of standards with the same analytical method. Quantification of target analytes was performed using calibration standards (range 0.1–2 ppm).
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/toxins13070463/s1, Table S1—Pathogenicity of 51 Fusarium strains selected for pathogenicity assay. For each experiment, 3 replications consisting of 3 plants per replicate were considered. Data were analyzed using one-way ANOVA, followed by means separation using Duncan test at p ≤ 0.05.
Author Contributions
Conceptualization, A.N., A.M., and M.M.; data curation, A.R. and M.M.; formal analysis, A.R., C.D., L.R., A.S., R.G., S.P.O.W., and M.M.; funding acquisition, A.F.L.; investigation, A.R., C.D., L.R., A.N., R.G., S.P.O.W., and M.M.; methodology, A.R., C.D., A.S., A.N., and M.M.; project administration, S.P.O.W. and A.M.; resources, C.D., L.R., A.N., S.P.O.W., A.M., and M.M.; supervision, A.N., S.P.O.W., and A.M.; validation, A.R., C.D., A.S., A.N., A.M., and M.M.; visualization, A.R., A.M., and M.M.; writing—original draft, A.R., C.D., A.M., and M.M.; writing—review & editing, C.D., A.S., A.F.L., A.M., and M.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in Rabaaoui, A.; Dall’Asta, C.; Righetti, L.; Susca, A.; Logrieco, A.F.; Namsi, A.; Gdoura, R.; Werbrouck, S.P.O.; Moretti, A.; Masiello, M. Phylogeny and Mycotoxin Profile of Pathogenic Fusarium species Isolated from Sudden Decline Syndrome and Leaf Wilt Symptoms, on Date Palm (Phoenix dactylifera), in Tunisia. Toxins 2021, 13, 463. doi: 10.3390/toxins13070463.
Conflicts of Interest
The authors declare no conflict of interest.
Key Contribution
We detected a high incidence of Fusarium species on date palms in Tunisia for the first time; however, the quarantine pathogen F. oxysporum f. sp. albedinis is absent in all the considered samples. Among the Fusarium species identified, the extended occurrence of F. proliferatum as main species associated to the date palm is worrisome since it produces the mycotoxins FBs, associated to a wide number of mycotoxicoses. There is a need of defining which environmental conditions would influence the production, which would be useful for establishing proper management protocols to be adopted to minimize production loss.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Food and Agriculture Organization Statistics Division (FAOSTAT) [(accessed on 3 May 2021)]; Available online: www.fao.org/faostat.
- 2.Ben-Amor R., Aguayo E., de Miguel-Gomez M.D. The competitive advantage of the Tunisian palm date sector in the Mediterranean region. Span. J. Agric. Res. 2015;13:10. doi: 10.5424/sjar/2015132-6390. [DOI] [Google Scholar]
- 3.Saleh A.A., Sharafaddin A.H., El Komy M.H., Ibrahim Y.E., Hamad Y.K., Molan Y.Y. Fusarium species associated with date palm in Saudi Arabia. Eur. J. Plant Pathol. 2017;148:367–377. doi: 10.1007/s10658-016-1095-3. [DOI] [Google Scholar]
- 4.Sedra M., Lazrek B.H. Fusarium oxysporum f. sp. albedinis Toxin Characterization and use for selection of resistant date palm to Bayoud Disease. In: Jain S., Al-Khayri J., Johnson D., editors. Date Palm Biotechnology. Springer; Dordrecht, The Netherlands: 2011. [DOI] [Google Scholar]
- 5.Zaid A., De Wet P.F., Djerbi M., Oihabi A. Chapter XII diseases and pests of date palm. In: Zaid A., editor. Date Palm Cultivation. Food and Agriculture Organization of the United Nations; Rome, Italy: 2002. pp. 223–287. [Google Scholar]
- 6.Djerbi M. Bayoudh disease in North Africa: History distribution, diagnosis and control. Date Palm J. 1982;1:153–197. [Google Scholar]
- 7.Abdalla M.Y., Al-Rokibah A., Moretti A., Mulè G. Pathogenicity of toxigenic Fusarium proliferatum from Date Palm in Saudi Arabia. Plant Dis. 2000;84:321–324. doi: 10.1094/PDIS.2000.84.3.321. [DOI] [PubMed] [Google Scholar]
- 8.Armengol J., Moretti A., Perrone G., Vicent A., Bengoechea J.A., García-Jiménez J. Identification, incidence and characterization of Fusarium proliferatum on ornamental palms in Spain. Eur. J. Plant Pathol. 2005;112:123–131. doi: 10.1007/s10658-005-2552-6. [DOI] [Google Scholar]
- 9.Cohen Y., Freeman S., Zveibil A., Ben Zvi R., Nakache Y., Biton S., Soroker V. Reevaluation of factors affecting bunch drop in date palm. Hort. Sci. 2010;45:887–893. doi: 10.21273/HORTSCI.45.6.887. [DOI] [Google Scholar]
- 10.Mansoori B. Fusarium proliferatum induces gum in xylem vessels as the cause of Date Bunch Fading in Iran. J. Agric. Sci. Tech. 2012;14:1133–1140. [Google Scholar]
- 11.Mansoori B., Kord H. Yellow Death: A Disease of Date Palm in Iran caused by Fusarium solani. J. Phytopathol. 2006;154:125–127. doi: 10.1111/j.1439-0434.2006.01067.x. [DOI] [Google Scholar]
- 12.Alkahtani M., EL-Naggar M.A., Omer S.A., Abdel-Kareem E.M., Ammar M.F. Effect of toxic Fusarium moniliforme on some biochemical component of some date palm cultivars. Am. J. Food Technol. 2011;6:730–741. doi: 10.3923/ajft.2011.730.741. [DOI] [Google Scholar]
- 13.Al-Sadi A.M., Al-Jabri A.H., Al-Mazroui S.S., AlMahmooli I.H. Characterization and pathogenicity of fungi and oomycetes associated with root diseases of date palms in Oman. Crop Prot. 2012;37:1–6. doi: 10.1016/j.cropro.2012.02.011. [DOI] [Google Scholar]
- 14.Maitlo W.A., Markhand G.S., Abul-Soad A.A., Lodhi A.M., Jatoi M.A. Fungi associated with sudden decline disease of date palm (Phoenix dactylifera L.) and its incidence at Khairpur, Pakistan. Pak. J. Phytopathol. 2014;26:67–73. [Google Scholar]
- 15.Alwahshi K.J., Saeed E.E., Sham A., Alblooshi A.A., Alblooshi M.M., El-Tarabily K.A., Abu-Qamar S.F. Molecular Identification and Disease Management of Date Palm Sudden Decline Syndrome in the United Arab Emirates. Int. J. Mol. Sci. 2019;20:923. doi: 10.3390/ijms20040923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nishad R., Ahmed T.A. Survey and Identification of Date Palm Pathogens and Indigenous Biocontrol Agents. Plant Dis. 2020;104:2498–2508. doi: 10.1094/PDIS-12-19-2556-RE. [DOI] [PubMed] [Google Scholar]
- 17.Abbas I.H., Mouhi M.N., Al-Roubaie J.T., Hama N.N., El-Bahadli A.H. Phomopsis phoenicola and Fusarium equiseti, new pathogens on date palm in Iraq. Mycol. Res. 1991;95:509. doi: 10.1016/S0953-7562(09)80857-5. [DOI] [Google Scholar]
- 18.Haq I.U., Khan N.A. Fungal Diseases of Date Palm (Phoenix dactylifera): Etiology and Management. In: Ul Haq I., Ijaz S., editors. Etiology and Integrated Management of Economically Important Fungal Diseases of Ornamental Palms. Sustainability in Plant and Crop Protection, 16. Springer; Cham, Switzerland: 2020. [DOI] [Google Scholar]
- 19.Desjardins A.E. Fusarium Mycotoxins: Chemistry, Genetics, and Biology. American Phytopathological Society (APS Press); Saint Paul, MN, USA: 2006. [Google Scholar]
- 20.Blandino M., Reyneri A., Vanara F., Tamietti G., Pietri A. Influence of agricultural practices on Fusarium infection, fumonisin and deoxynivalenol contamination of maize kernels. World Mycotoxin J. 2009;2:409–418. doi: 10.3920/WMJ2008.1098. [DOI] [Google Scholar]
- 21.Drakopoulos D., Sulyok M., Jenny E., Kägi A., Bänziger I., Logrieco A.F., Krska R., Vogelgsang S. Fusarium Head Blight and Associated Mycotoxins in Grains and Straw of Barley: Influence of Agricultural Practices. Agronomy. 2021;11:801. doi: 10.3390/agronomy11040801. [DOI] [Google Scholar]
- 22.IARC . IARC Monographs on the Evaluation of Carcinogenic Risks to Human. Volume 56. IARC; Lyon, France: 1993. Some naturally occurring substances: Food ITEMs and constituents, heterocyclic aromatic amines and mycotoxins; pp. 245–395. [Google Scholar]
- 23.IARC . IARC Monographs on the Evaluation of Carcinogenic Risks to Human. Volume 82. IARC; Lyon, France: 2002. Some traditional herbal medicines, some mycotoxins, naphthalene and styrene; pp. 1–556. [PMC free article] [PubMed] [Google Scholar]
- 24.O’Donnell K., Sutton D.A., Rinaldi M.G., Sarver B.A., Balajee S.A., Schroers H.J., Summerbell R.C., Robert V.A., Crous P.W., Zhang N., et al. Internet-accessible DNA sequence database for identifying fusaria from human and animal infections. J. Clin. Microbiol. 2010;48:3708–3718. doi: 10.1128/JCM.00989-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Urban M., King R., Andongabo A., Maheswari U., Pedro H., Kersey P., Hammond-Kosack K. First Draft Genome Sequence of a UK Strain (UK99) of Fusarium culmorum. Genome Announc. 2016;4:e00771-16. doi: 10.1128/genomeA.00771-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li X.P., Li J.H., Qi Y.H., Guo C., Li X., Li M.Q. Determination and structural analysis of whole genome sequence of Fusarium equiseti D25-1. Acta Phytopathol. Sin. 2019;49:474–487. doi: 10.13926/j.cnki.apps.000449. [DOI] [Google Scholar]
- 27.King R., Urban M., Hammond-Kosack M.C.U., Hassani-Pak K., Hammond-Kosack K.E. The completed genome sequence of the pathogenic ascomycete fungus Fusarium graminearum. BMC Genom. 2015;16:544. doi: 10.1186/s12864-015-1756-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lysøe E., Frandsen R.J.N., Divon H.H., Terzi V., Orrù L., Lamontanara A., Kolseth A.K., Nielsen K.F., Thrane U. Draft genome sequence and chemical profiling of Fusarium langsethiae, an emerging producer of type A trichothecenes. Int. J. Food Microbiol. 2016;221:29–36. doi: 10.1016/j.ijfoodmicro.2016.01.008. [DOI] [PubMed] [Google Scholar]
- 29.Wang H., Xiao M., Kong F., Chen S., Dou H.T., Sorrell T., Li R.Y., Xu Y.C. Accurate and practical identification of 20 Fusarium species by seven-locus sequence analysis and reverse line blot hybridization, and an in vitro antifungal susceptibility study. J. Clin. Microbiol. 2011;49:1890–1898. doi: 10.1128/JCM.02415-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Proctor R.H., McCormick S.P., Kim H.S., Cardoza R.E., Stanley A.M., Lindo L., Kelly A., Brown D.W., Lee T., Vaughan M.M., et al. Evolution of structural diversity of trichothecenes, a family of toxins produced by plant pathogenic and entomopathogenic fungi. PLoS Pathog. 2018;14:e1006946. doi: 10.1371/journal.ppat.1006946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ma L.J., van der Does H.C., Borkovich K.A., Coleman J.J., Daboussi M.J., Di Pietro A., Dufresne M., Freitag M., Grabherr M., Henrissat B., et al. Comparative genomics reveals mobile pathogenicity chromosomes in Fusarium. Nature. 2010;464:367–373. doi: 10.1038/nature08850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jeger M., Bragard C., Caffier D., Candresse T., Chatzivassiliou E., Dehnen-Schmutz K., Gilioli G., Gregoire J.C., Miret J.A.J., MacLeod A., et al. Pest categorisation of Fusarium oxysporum f. sp. Albedinis. EFSA J. 2018;16:e05183. doi: 10.2903/j.efsa.2018.5183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang N., O’Donnell K., Sutton D.A., Nalim F.A., Summerbell R.C., Padhye A.A., Geiser D.M. Members of the Fusarium solani species complex that cause infections in both humans and plants are common in the environment. J. Clin. Microb. 2006;44:2186–2190. doi: 10.1128/JCM.00120-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Coleman J.J. The Fusarium Solani Species Complex: Ubiquitous pathogens of agricultural importance. Mol. Plant Pathol. 2016;17:146–158. doi: 10.1111/mpp.12289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O’Donnell K., Sutton D.A., Rinaldi M.G., Gueidan C., Crous P.W., Geiser D.M. Novel multilocus sequence typing scheme reveals high genetic diversity of human pathogenic members of the Fusarium incarnatum-equiseti and F. chlamydosporum species complexes within the United States. J. Clin. Microbiol. 2009;47:3851–3861. doi: 10.1128/JCM.01616-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Laraba I., McCormick S.P., Vaughan M.M., Geiser D.M., O’Donnell K. Phylogenetic diversity, trichothecene potential, and pathogenicity within Fusarium sambucinum species complex. PLoS ONE. 2021;16:e0245037. doi: 10.1371/journal.pone.0245037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Proctor H.R., Desjardins A.E., Moretti A. Biology and chemical complexity of Fusarium proliferatum. In: Strange R.N., Gullino M.L., editors. The Role of Plant Pathology in Food Safety and Food Security, Plant Pathology in the 21st Century 3. Springer; Berlin/Heidelberg, Germany: 2010. [Google Scholar]
- 38.Moretti A., Susca A., De Boevre M., De Saeger S., Di Mavungu J.D., Villani A., Haidukowski M., Somma S., van der Lee T., Waalwijk C., et al. The mycotoxigenic Fusarium proliferatum: A perfect example of the Great Beauty of fungal biodiversity; Proceedings of the 2nd MycoKey International Conference “Integrated Solutions for Mycotoxin Management”; Wuhan, China. 16–18 September 2018. [Google Scholar]
- 39.Susca A., Proctor R.H., Butchko R.A.E., Haidukowski M., Stea G., Logrieco A.F., Moretti A. Variation in the fumonisin biosynthetic gene cluster in fumonisin-producing and nonproducing black aspergilli. Fungal Genet. Biol. 2014;73:39–52. doi: 10.1016/j.fgb.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 40.Susca A., Proctor R.H., Morelli M., Haidukowski M., Gallo A., Logrieco A.F., Moretti A. Variation in Fumonisin and Ochratoxin Production Associated with Differences in Biosynthetic Gene Content in Aspergillus niger and A. welwitschiae Isolates from Multiple Crop and Geographic Origins. Front. Microbiol. 2016;7:1412. doi: 10.3389/fmicb.2016.01412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leslie J.F., Summerell B.A. The Fusarium Laboratory Manual. Blackwell Publishing; Hoboken, NY, USA: 2006. [Google Scholar]
- 42.Suga H., Arai M., Fukasawa E., Motohashi K., Nakagawa H., Tateishi H., Fuji S.I., Shimizu M., Kageyama K., Hyakumachi M. Genetic differentiation associated with fumonisin and gibberellin production in Japanese Fusarium fujikuroi. Appl. Environ. Microbiol. 2019;85:e02414-18. doi: 10.1128/AEM.02414-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sultana S., Kitajima M., Kobayashi H., Nakagawa H., Shimizu M., Kageyama K., Suga H. A Natural Variation of Fumonisin Gene Cluster Associated with Fumonisin Production Difference in Fusarium fujikuroi. Toxins. 2019;11:200. doi: 10.3390/toxins11040200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sultana S., Bao W.X., Shimizu M., Kageyama K., Suga H. Frequency of three mutations in the fumonisin biosynthetic gene cluster of Fusarium fujikuroi that are predicted to block fumonisin production. World Mycotoxin J. 2021;14:49–59. doi: 10.3920/WMJ2020.2572. [DOI] [Google Scholar]
- 45.Paciolla C., Dipierro N., Mulè G., Logrieco A., Dipierro S. The mycotoxins beauvericin and T-2 induce cell death and alteration to the ascorbate metabolism in tomato protoplasts. Physiol. Mol. Plant Pathol. 2004;65:49–56. doi: 10.1016/j.pmpp.2004.07.006. [DOI] [Google Scholar]
- 46.Firakova S., Proksa B., Sturdikova M. Biosynthesis and biological activity of enniatins. Die Pharm. 2007;62:563–568. doi: 10.1691/ph.2007.8.7600. [DOI] [PubMed] [Google Scholar]
- 47.Song H.H., Lee H.S., Jeong J.H., Park H.S., Lee C. Diversity in Beauvericin and Enniatins H, I, and MK1688 by Fusarium oxysporum isolated from potato. Int. J. Food Microbiol. 2008;122:296–301. doi: 10.1016/j.ijfoodmicro.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 48.Logrieco A., Moretti A., Castella G., Kostecki M., Golinski P., Ritieni A., Chelkowski J. Beauvericin production by Fusarium species. Appl. Environ. Microbiol. 1998;64:3084–3088. doi: 10.1128/AEM.64.8.3084-3088.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moretti A., Belisario A., Tafuri A., Ritieni A., Corazza L., Logrieco A. Production of Beauvericin by Different Races of Fusarium Oxysporum F. sp. Melonis, The Fusarium Wilt Agent of Muskmelon. Eur. J. Plant Pathol. 2002;108:661–666. doi: 10.1023/A:1020674929084. [DOI] [Google Scholar]
- 50.López-Berges M.S., Capilla J., Turrà D., Schafferer L., Matthijs S., Jöchl C., Cornelis P., Guarro J., Haas H., Di Pietro A. HapX-mediated iron homeostasis is essential for rhizosphere competence and virulence of the soilborne pathogen Fusarium oxysporum. Plant Cell. 2012;24:3805–3822. doi: 10.1105/tpc.112.098624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pavlovkin J., Mistríková I., Jašková K., Tamás L. Impact of beauvericin on membrane properties of young initial leaves of maize with different susceptibility to Fusarium. Plant Soil Environ. 2012;58:205–210. doi: 10.17221/432/2011-PSE. [DOI] [Google Scholar]
- 52.Herrmann M., Zocher R., Haese A. Effect of disruption of the enniatin synthetase gene on the virulence of Fusarium avenaceum. Mol. Plant Microbe Interact. 1996;9:226–232. doi: 10.1094/MPMI-9-0226. [DOI] [PubMed] [Google Scholar]
- 53.Jestoi M. Emerging Fusarium Mycotoxins Fusaproliferin, Beauvericin, Enniatins, and Moniliformin—A Review. Crit. Rev. Food Sci. Nutr. 2008;48:21–49. doi: 10.1080/10408390601062021. [DOI] [PubMed] [Google Scholar]
- 54.Knutsen H.K., Alexander J., Barregård L., Bignami M., Brüschweiler B., Ceccatelli S., Cottrill B., Dinovi M., Grasl-Kraupp B., Hogstrand C., et al. Risks to human and animal health related to the presence of moniliformin in food and feed. EFSA J. 2018;16:e05082. doi: 10.2903/j.efsa.2018.5082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Perincherry L., Lalak-Kańczugowska J., Stępień Ł. Fusarium-Produced Mycotoxins in Plant-Pathogen Interactions. Toxins. 2019;11:664. doi: 10.3390/toxins11110664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tan D.C., Flematti G.R., Ghisalberti E.L., Sivasithamparam K., Barbetti M.J. Toxigenicity of enniatins from Western Australian Fusarium species to brine shrimp (Artemia franciscana) Toxicon. 2011;57:817–825. doi: 10.1016/j.toxicon.2011.02.019. [DOI] [PubMed] [Google Scholar]
- 57.Hartman G.L., McCormick S.P., O’Donnell K. Trichothecene-Producing Fusarium Species Isolated from Soybean Roots in Ethiopia and Ghana and their Pathogenicity on Soybean. Plant Dis. 2019;103:2070–2075. doi: 10.1094/PDIS-12-18-2286-RE. [DOI] [PubMed] [Google Scholar]
- 58.O’Donnell K., Cigelnik E., Nirenberg H.I. Molecular systematics and phylogeography of the Gibberella fujikuroi species complex. Mycologia. 1998;90:465–493. doi: 10.1080/00275514.1998.12026933. [DOI] [Google Scholar]
- 59.O’Donnell K., Kistler H.C., Tacke B.K., Casper H.H. Gene genealogies reveal global phylogeographic structure and reproductive isolation among lineages of Fusarium graminearum, the fungus causing wheat scab. Proc. Natl Acad. Sci. USA. 2000;97:7905–7910. doi: 10.1073/pnas.130193297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu Y.L., Whelen S., Hall B.D. Phylogenetic relationships among ascomycetes: Evidence from an RNA polymerase II subunit. Mol. Biol. Evol. 1999;16:1799–1808. doi: 10.1093/oxfordjournals.molbev.a026092. [DOI] [PubMed] [Google Scholar]
- 61.Thompson J.D., Higgins D.G., Gibson T.J. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kumar S., Stecher G., Tamura K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Felsenstein J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution. 1985;39:783–791. doi: 10.1111/j.1558-5646.1985.tb00420.x. [DOI] [PubMed] [Google Scholar]
- 64.Namsi A., Rabaoui A., Masiello M., Moretti A., Othmani A., Gargouri S., Gdoura R., Werbrouck S. First Report of Leaf Wilt Caused by Fusarium proliferatum and F. brachygibbosum on Date Palm (Phoenix dactylifera) in Tunisia. Plant Dis. 2021;105:1217. doi: 10.1094/PDIS-08-20-1791-PDN. [DOI] [Google Scholar]
- 65.Townsend G.R., Heuberger J.W. Methods for estimating losses caused by diseases in fungicide experi ments. Plant Dis. Report. 1943;27:340–343. [Google Scholar]
- 66.Malachová A., Sulyok M., Beltrán E., Berthiller F., Krska R. Optimization and validation of a quantitative liquid chromatography–tandem mass spectrometric method covering 295 bacterial and fungal metabolites including all regulated mycotoxins in four model food matrices. J. Chromatogr. 2014;1362:145–156. doi: 10.1016/j.chroma.2014.08.037. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available in Rabaaoui, A.; Dall’Asta, C.; Righetti, L.; Susca, A.; Logrieco, A.F.; Namsi, A.; Gdoura, R.; Werbrouck, S.P.O.; Moretti, A.; Masiello, M. Phylogeny and Mycotoxin Profile of Pathogenic Fusarium species Isolated from Sudden Decline Syndrome and Leaf Wilt Symptoms, on Date Palm (Phoenix dactylifera), in Tunisia. Toxins 2021, 13, 463. doi: 10.3390/toxins13070463.