Abstract
Ever since the uncovering of the severe discrepancy of COVID-19 manifestations, irrespective of viral load, scientists have raced to locate and manage factors contributing to the genesis of a critical state. Recent evidence delineates the role of oral dysbiosis in the development of low-grade inflammation, characterized by the increase of inflammatory cytokines common to those fundamental to the development of severe COVID. Furthermore, high periodontopathic bacteria were recorded in severe acute respiratory syndrome in COVID patients, as well as its common provoking comorbidities such as diabetes and hypertension. This can be explained by the immigration and elimination of oral bacteria into the airways, which, in the context of an injured lung, allows for their preferential overgrowth familiar to that, causing the progression to advanced lung diseases. This is why we indicate the promising usage of oral microbiome transplantation as a treatment of oral microbial dysbiosis, not only associated with the worst outcomes of COVID-19 but also in other disorders of low-grade inflammation.
Keywords: COVID-19, low-grade inflammation, oral microbial transplantation-, periodontitis, vascular degeneration
Graphical abstract
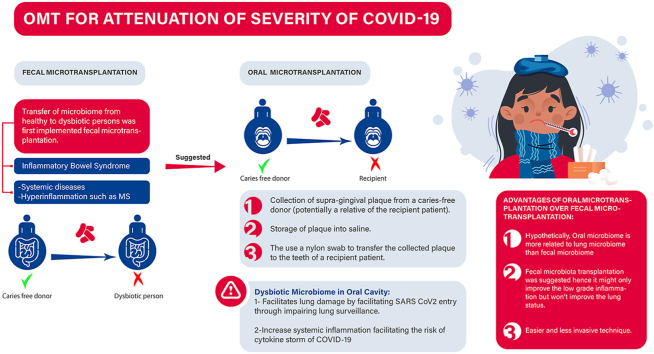
OMT for attenuation of severity of COVID-19. COVID-19: Coronavirus disease 2019, MS: Multiple Sclerosis, OMT: oral microbial transplantation, SARS-CoV-2: Severe acute respiratory syndrome coronavirus 2.
Background
Coronavirus 2019 Disease (COVID-19) is a contagious disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which was emerged in Hubei, the Chinese province in the Wuhan City, in December 2019 and rapidly showed severe spread worldwide [1]. Remarkably, patients with SARS-CoV-2 infection suffer from a wide range of symptoms. Most patients seem to have a mild form of the disease, and about 20% progress to severe disease, including pneumonia, respiratory failure, and even death [2]. Besides, the discrepancies of clinical manifestations seen in COVID-19 patients seem to be mediated by a differential immune response, not the differential viral load, as was recently claimed [3]. Even with the release of different vaccines to the market aiming for fighting the disease by building antibodies against it, unfortunately, there is still a great deal of doubt about its effectiveness, success, and its ability to offer a long-term immune cover.
Oral microbial contribution to Low-Grade Inflammation (LGI)
Low-grade inflammation (LGI), characterized by a chronic increase in inflammatory cytokines (IL-6(Interleukin), TNF (Tumour Necrosis Factor) α and IL-1-β), is the cornerstone of all the high-risk classifications for the development of severe COVID-19. Hyperglycemia, obesity and hypertension, all features of metabolic syndrome (MetS), are believed to increase the risk of cardiovascular disease and type 2 diabetes mellitus and are all participating agents of LGI. In severe acute respiratory syndrome (SARS-CoV-2) patients, target tissues, including the lungs, brain, gut, and kidneys, are infiltrated with proinflammatory macrophages and lymphocytes, leading to the hypercytokinaemia that supplements LGI in MetS patients. It has been found that patients with IL-6 levels of ≥80 pg/ml have a greater risk of developing respiratory failure by 22 times than patients with lower IL-6 levels [4].
Recent studies prove that oral dysbiosis contributes to chronic low-grade local and systemic inflammation. With that being said, we still have no concrete evidence of how oral dysbiosis causes systemic inflammation. Nevertheless, Socransky et al. outlined the possible mechanisms by which oral dysbiosis promotes systemic inflammation as follows: [5].
-
1
Immune system stimulation produces proinflammatory cytokines, mainly in the form of IL-6 and IL-8
-
2
Proteases secretion activate the complement system
-
3
Periodontal pathogen and their inflammatory products direct invasion resulting in inflammation. According to Socransky et al., Gram-positive aerobes showed an elevated presentation in healthy controls compared to periodontitis patients who showed around 85% of Gram-negative bacteria. The previously mentioned bacteria occupy the intact epithelium of a periodontal pocket and eventually gain access to the circulation leading to the systemic dissemination of bacterial products.
-
4
Disturbances in the hypothalamic-pituitary-thyroid axis modify the periodontal status through immune system alteration [6].
Increased levels of systemic inflammatory markers are seen in individuals suffering from periodontitis, including C- Reactive Protein (CRP), IL-6, haptoglobin, and fibrinogen. According to Rai et al., there is a link between oral dysbiosis and increased interleukin 8 (IL8) levels, as well as interleukin 6 (IL-6), tumour necrosis factor (TNF-ɑ), granulocyte monocyte colony-stimulating factor (GM-CSF), and interferon γ (IFN- γ), besides increasing the susceptibility to developing oral cancer [7].
Oral microbiome predisposing to lung damage
The anatomic assembly between the oral cavity and the lungs offers plenty of opportunities for oral flora to impact lung flora in health and disease [8].
The lung is constantly subjected to a level of microbe immigration and elimination through the mucosal defence and mucociliary clearance. The level of microbes that migrates to the lungs is found in abundance in the upper respiratory tract and oropharynx, which has a direct form of association with the lungs [9].
Composition of lung microbiota and its link to the oral cavity
A theory supported by Charlson et al. states that the respiratory tract from the nasal and oral cavities to the upper and lower airways are contiguous, and the microbiota could be indistinguishable [10].
The lung microbiota displays vast variations between individuals and differences between sites in the lung resulting from waves of elimination/immigration and differences in distance from the mouth, which serves as the source of the community. In healthy individuals, Streptococcus, Neisseria, Haemophilus, and Fusobacterium are the most abundant genera in the lungs [12].
It has been established by Huffnagle et al. that a periodontopathic strain, namely Prevotella gingivalis, precipitates the pulmonary inflammation cascade via Th 17 in a mouse model [11].
A triple relationship between chronic inflammatory disorders-periodontopathic oral microbiota and worst outcomes of COVID-19
Prevotella gingivalis, predominating in the lung micro-environment of severe COVID-19 infection
Metagenomic investigations of patients withextreme intense respiratory disorder coronavirus recently detailed the expanded reads of periodontopathic microbes, especially Prevotella gingivalis. This demonstrates the concept of interaction between the oral microbiome and COVlD-19 complications. Evidence proposes that periodontopathic microbes are involved in the pathogenesis of respiratory illnesses, like those involved in COVlD- 19, and are related to chronic inflammatory systemic diseases such as hypertension, type 2 diabetes, and cardiovascular diseases. These diseases are, as often as possible, detailed comorbidities that lead to an expanded risk of fatal complications [13].
By comparing the microbial composition of healthy people with that encountered within the lung microenvironment of COVlD-19, it is obvious that the prevalence of two periodontopathic strains, specifically Prevotella and Veillonella. Furthermore, Prevotella has different roles within the pathogenesis of asthma, as well as in disorders of low-grade inflammation such as atherosclerosis [5].
Several mechanisms have been suggested to demonstrate how periodontopathic strains colonizing the lung facilitate SARS-CoV-2 entry and mediate damage, the most felicitous being the abolishing of the immune surveillance. The exposure of alveolar macrophages to lipopolysaccharide secreted by periodontopathic strains induces immune tolerance to Lipopolysaccharide (LPS) and similar endotoxins. This immune tolerance attenuates the subsequent immune response to similar pathogens and thus facilitates the escape of viruses such as SARS-CoV-2 from local immune cells within the lung milieu [[13], [14], [15], [16]].
Relationship of chronic inflammatory disorders such as diabetes to outcomes of COVID-19 and to the oral microbiome
Chronic inflammatory diseases such as diabetes and obesity have shown an explicit direct correlation with oral microbiome competence. Periodontal pathogenic bacteria, such as A. actinomycetemcomitans and P. gingivalis showed a spike in their flourishing rate, with uncontrolled glycaemic values and evident diabetes risk. Unfortunately, the accessibility of direct investigations emphasising the synergy between the oral microbiome and diabetes have been very inadequate, restricting the outcome of precise conclusions. A study was conducted, including 29 morbidly obese volunteers, involving 13 diabetes patients; the genus Bifidobacteria in the phylum Actinobacteria displayed a lower copiousness in diabetic patients. Further study, including 11 controls and 20 diabetic patients, two genera, Streptococci and Lactobacilli, in the phylum Firmicutes were found to be more evident in the diabetes patients. On the other hand, the biological specimens used in these two studies were assembled post disease diagnosis, which may have contributed to unreliable conclusions as disease condition and plan of treatment could lead to manipulating microbial profiles. Moreover, the sample size of the studies implemented was noticeably insufficient to reach a solid conclusion [17].
In addition, Chakraborty did not detail in his report the comorbidities in severe COVID19 patients whose BAL samples demonstrated periodontopathic stains [18].
This means that we cannot conclude whether he coexistence of periodontopathic strains as P. gingivalis in severe COVID-19 patients' lung microenvironment is only a coincidence due to their underlying proinflammatory comorbidities as DM, or these strains directly induce the lung damage observed in such patients or a mixture of both theories.
We, thereby, hypothesize that the replacement of oral microbiome by taxa from healthy individuals can be an efficient adjuvant therapy in patients with LGI in general, specifically COVID-19 patients, to mitigate its complications.
Evaluation and implications
Faecal microbial transplantation (FMT), a promising strategy for regression of systemic and intestinal inflammation, but with major setbacks
Currently, FMT is an established technique, which involves transfer of faecal or stool matter from a healthy donor and its insertion in the GI tract of patients to correct dysbiosis and restore healthy conditions.
FMT has already been widely used for the treatment of local intestinal inflammatory disorders such as traditional antibiotic therapy-resistant recurrent Clostridium difficile infection (rCDI), with an efficacy of >90%. For this reason, it is now considered, as well in other intestinal disorders such as Inflammatory Bowel Disease (IBD), as an experimental treatment and labelled as New Drug Development by the Food and Drug Administration in 2016 [19,20].
Initial clinical studies were implemented to follow up patients that had ulcerative colitis and Crohn's disease, illustrating a long-term alleviation of their clinical manifestations, along with a noticeable histological and endoscopic improvement in some patients. Furthermore, a meta-analysis was conducted, including nine studies showing an abatement rate of 36.2%; however, the outcomes depend on various factors as age, route of administration whether via nasojejunal tube, colonoscopy or enema, dose and the preparation of the donor's faeces. In particular, age is a factor that showed noticeable results, as it was proven that younger patients, with ages between 7 and 20 years old, had significantly high remission rates. Moreover, according to certain statistics, faecal microbiota transplant showed more promising results in Crohn's disease than in ulcerative colitis with remission rates of 60.5% and 22%, respectively. Interestingly, the latter statistic can be taken in a reversed manner, as a study was previously done involving 15 patients with steroid-dependent ulcerative colitis that received faecal microbiota transplant via colonoscopy, presenting with a preserved remission rate in 57% of the cases [21].
The promising local effects of faecal transplantation have encouraged its use for diseases of systemic inflammation, particularly multiple sclerosis. Borody and colleagues reported a reversal of significant neurological symptoms in three MS patients after FMT. These findings suggest that FMT can reverse MS-like symptoms, probably through the reversal of the low-grade inflammation underlying its pathogenesis [22].
Table 1 highlights the ongoing clinical trial of the use of FMT for the management of disorders of LGI.
Table 1.
Review of Clinical trials involving faecal microbial transplantation in treating extra-intestinal inflammatory disorders
| Clinical trial identifier | Disease/condition | Trial status | Sponsor | Country |
|---|---|---|---|---|
| NCT02741518 | Obesity | Phase 1 | Brigham and Women's Hospital | USA |
| NCT02530385 | Obesity | Phase 2 | Massachusetts General Hospital | USA |
| NCT03926286 | Sjogren's Syndrome | Phase 1 | University of Miami | USA |
| NCT02255617 | Hepatic Encephalopathy | Phase 1 | University of Alberta | Canada |
| NCT02960074 | Peanut allergy | Phase 1 | Boston Children's Hospital | USA |
| NCT03594487 | Relapsing-remitting MS | Phase 1 | University of California, San Francisco | USA |
| NCT03998423 | Alzheimer's disease (AD) | Phase 1 | University of Wisconsin, Madison | USA |
| NCT03353402 | Metastatic Melanoma Patients Who Failed Immunotherapy | Phase 1 | Sheba Medical Center | USA |
| NCT03341143 | Melanoma | Melanoma | University of Pittsburgh | USA |
| NCT04014413 | a variety of dysbiosis-associated disorder | N/A | Chinese University of Hong Kong | China |
| NCT04251767 | COVID-19 Complicated with Refractory Intestinal Infections | N/A | The Second Hospital of Nanjing Medical University | China |
| NCT03058900 | Psoriatic Arthritis | Completed | Odense University Hospital | Denmark |
| NCT03279224 | Bipolar Depression | Phase 2: Allogenic Phase 3: Autologous |
Women's College Hospital | Canada |
| NCT03281044 | Major Depressive Disorder | Melanoma | Psychiatric Hospital of the University of Basel | Switzerland |
Abbreviations: USA: United States of America.
Despite its promising results, there are multiple concerns about the generalization of faecal transplantation. One of the major concerns in the FMT is the uniformity of its delivery to patients. Several methods have been tried, the commonest of which are via nasojejunal tube and colonoscopy. When FMT was delivered via the nasojejunal tube, patients experienced high fever and C-reactive protein elevation. Moreover, cases of perforation, bleeding, and symptoms associated with anaesthesia have been documented while using colonoscopy [23].
As mentioned by Nejadghaderi, the role of the faecal microbiome in mediating low-grade inflammation can incite the use of FMT as a potential strategy in critical patients with COVID-19. The latter strategy was proposed in a clinical trial ‘NCT04251767,’ although it has been halted probably due to the fear of the previously mentioned complications of FMT. [24].
Why oral microbial transplantation (OMT) can be a better strategy
Due to its reassuring outcomes, the recent utilization of oral microbiome transplantation has garnered notice over that of the faecal microbiome, which has exhibited impediments and side effects, as stated before.
Aided by system-level studies of oral microbiota that identify novel molecular targets for the bacteria, OMT displays the potential of being a distinguished tool of probiotic therapies essential for the treatment of the array of human diseases affiliated with microbial dysbiosis [25].
Pozkhitov approach
Pozhitkov first proposed OMT by listing the following theoretical steps [26].
-
1)
The acquirement of supra-gingival plaque from a caries-free donor (preferably related to the recipient),
-
2)
The preservation of the collected plaque in saline, and
-
3)
The transplantation of the collected plaque by use of a nylon swab to the teeth of the recipient.
Nascimento adjustments of the former approach
Just as the transfer of faecal microbiome from healthy subject, was first indicated for the treatment of bowel disorders, in 2017, Nascimento et al. advocated for the transplantation of oral biofilms from healthy subjects, known as OMT, for the treatment of dental disorders such as periodontitis [27].
He adjusted the former technique by adding the following prerequisites:
-
-
To evade the complications of cariogenic bacteria such as streptococcus mutans, the donor (irrespective of the relation to the recipient) should have a healthy oral microbiome void of cariogenic bacteria.
-
-
The transplanted bacteria must exhibit the ability to colonize the diseased microbiome by reacting to it through the secretion of bacteriocin and hydrogen peroxide, impeding its growth and thus effectively restoring and conserving local and systemic immune functions [27].
Promising results in periodontitis
Pozhitkov et al. have conducted a study, including 16 healthy white adults with clinical manifestations of periodontitis, illustrating how OMT can be a forthcoming line of therapy. The process is quite schematized, starting with the assembling of sub and supra-gingival microbiota from healthy donors, with a further screening of the samples harvested in an in-vitro antimicrobial protocol to be safely used in the recipient's oral cavity. This is followed by extensive cleaning, root planing, and administering a wide-spectrum antimicrobial agent in the form of sodium hypochlorite (NaOCl) to the recipient patient. The NaOCl sample is then neutralized by a sodium ascorbate buffer and inundated with the microbial specimen previously collected from the healthy donor with periodontitis in the regression phase.
Recently, NaOCl can be used with a moderate concentration range cautiously for the preliminary elimination of the oral microbiota prototype. The prior concentration rates of NaOCl can be deliberately neutralized before the transplantation process for safer results. Hereby, the outcomes concluded through the previous study demonstrates that the specific oral microbiome specimens found in the healthy donors with periodontitis can be affirmatively transplanted to the diseased patients [26].
Advantages of OMT over FMT
This generated the opportunity for a challenge between OMT and FMT due to the cost-effectiveness, simplicity, and genetic stability afforded by the transplanted biofilms and the stability of the oral microbiome compared to other body niches such as the gut.
Another advantage is the strong evidence of the interplay between the oral microbiome and SARS-CoV-2's ability to invade the lung mucosal barrier. Replacing the strains associated with lung predisposition to COVID-19, such as Prevotella, can minimize the ability of COVID-19 to cause significant lung damage, while the gut-lung microbial axis remains largely hypothetical.
However, further studies, clinical trials, and long-term follow up are needed to understand oral microbiota dynamics and provide new insights on how a dysbiotic microbiota can be successfully replaced by a health-beneficial flora [26,27].
Advantages of OMT to the classic treatment of periodontitis
Chen et al. compared in a recent study the microbial diversity of patients with periodontitis before and after treatment to healthy controls. They found that despite the improvement of the oral microbiome profile after four weeks of treatment, and the enhancement of microbial species that have a role in regulating inflammation, the microbiota profile of healthy controls still differed significantly when compared to patients after treatment. This finding shows how hypothetical OMT can be of greater efficacy when compared to classic treatment of periodontitis:
-
-
It will ‘clone’ the microbiota profile of healthy controls, and this will create a microbial niche more efficient in antagonizing low-grade inflammation
-
-
It will achieve this goal within a few hours or days compared to a minimum of four weeks in the classic treatment of periodontitis [28].
Choosing the suitable COVID-19 candidates and the suitable donors for OMT in COVID-19 patients
When to transplant, early in the course of the disease or late in established critical patients
Owing to the predominance of a paucisymptomatic presentation in the majority of SARS-CoV-2 patients, we suspect that OMT will implement the most beneficiary effects in patients with pre-existing chronic inflammatory disorders such as diabetes or obesity. These individuals are amongst a minority of COVID patients, often experiencing a detrimental delayed inflammatory response due to an ineffective unregulated early response. Moreover, their oral microbial profiles are presumed to correspond to the respiratory periodontopathic strains of critical COVID-19 patients. Ideally, this procedure should be performed before the development of complications to halt the incriminated unregulated immune process and minimize the need for hospitalization [29].
Choice of donors
Several clinical trials, as well as evidence from other healthcare applications, studying the efficiency of FMT through recurrent CDI treatment, for instance, has shown disparate responses owing to a multitude of variables. Expressly, the role of microbial diversity and composition of the transplanted stools is notable. This generated the term ‘super-donors,’ alluding to the donors guaranteeing a more favourable impact in comparison to other faecal donations. Furthermore, some oral microbiota categorically improves the lung milieu of COVID-19 patients, such as Streptococcus mutans and Eubacteria. Therefore, donor supra-gingival plaques ought to be screened for predicted favourable organisms, as well for the absence of Prevotella gingivalis, associated with the worst outcome in the lung micro-environment of COVID-19 patients. Moreover, the absence of SARS-CoV-2 antigens should be ensured in the donor's saliva to prevent intensifying the recipient viral load [30].
Conclusion
In conclusion, OMT shows very promising results, and upon clinical trials, it can prove that its benefits can outweigh faecal transplant due to the factors stated above and that it can help in the treatment of systemic diseases and in taming the low-grade inflammation of COVID-19. This statement will remain largely speculative until clinical trials prove its feasibility and success.
Transparency declaration
None.
Acknowledgements
As first author, I wanted to offer a tribute to all physicians and nurses around the world who are offering themselves to revive the hope of many patients and their families. I wanted also to thank one of my best mentors in research, Prof. Lamis Ragab, the vice-head of New Giza University. I also wanted to thank all the families of my students who co-authored this work, they raised their scientific knowledge and and capabilities, and this will make able to reshape medical science, one day. I wanted also to offer a warm thank you note to Reem Husseiny and Meryam El shershaby, two of the best members of our research team, whose work and perseverance was greatly missed during the conception of this work. Finally, yet importantly, I wanted to thank our dearest colleagues, Menna Ayed Habib, Nirvana Ashraf, Maha Shulqamy, Mariam Khaled-Ibn-ElWalid, Esraa Farahat, Hend Hesham, Aisha AbdelAzeam who participated in presenting part of this topic in the annual meeting of Pediatrics' department in 2021 and 2019.
Abbreviations
- COVID-19
Coronavirus disease 2019
- GM-CSF
Granulocyte Monocyte colony-stimulating factor
- FMT
Faecal microbial transplantation
- IL
Interleukin
- MS
Multiple sclerosis
- OMT
Oral microbial transplantation
- SARS-CoV-2
Severe Acute Respiratory Syndrome coronaviridae-2
References
- 1.Prasad R. COVID-19: current status, challenges and future perspectives. Indian J Clin Biochem. 2020;35(4):383–384. doi: 10.1007/s12291-020-00922-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.AbdelMassih A.F., Mahrous R., Taha A., Saud A., Osman A., Kamel B. The potential use of ABO blood group system for risk stratification of COVID-19. Med Hypotheses. 2020 Dec;145:110343. doi: 10.1016/j.mehy.2020.110343. https://linkinghub.elsevier.com/retrieve/pii/S030698772032627X [Internet] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.AbdelMassih A., Yacoub E., Husseiny R.J., Kamel A., Hozaien R., El Shershaby M. Hypoxia-inducible factor (HIF): the link between obesity and COVID-19. Obes Med. 2020:100317. doi: 10.1016/j.obmed.2020.100317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fakhry A., Kamel A., Mishriky F., Ismail H., El L., Malak L. 2020. Is it infection or rather vascular inflammation ? Game-changer insights and recommendations from patterns of multi-organ involvement and affected subgroups in COVID-19; pp. 15–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Socransky S.S., Haffajee A.D. Dental biofilms: difficult therapeutic targets. Periodontol 2000. 2002 Jan;28(1):12–55. doi: 10.1034/j.1600-0757.2002.280102.x. [Internet] [DOI] [PubMed] [Google Scholar]
- 6.AbdelMassih A., Hassan A.A., Abou-Zeid A.S., Hassan A., Hussein E., Gadalla M. Salivary markers and coronavirus disease 2019. Cardiovasc Endocrinol Metab. 2021 doi: 10.1097/XCE.0000000000000242. [Internet] Ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rai A.K., Panda M., Das A.K., Rahman T., Das R., Das K. Dysbiosis of salivary microbiome and cytokines influence oral squamous cell carcinoma through inflammation. Arch Microbiol. 2021 doi: 10.1007/s00203-020-02011-w. Ahead of print. [DOI] [PubMed] [Google Scholar]
- 8.Mammen M.J., Scannapieco F.A., Sethi S. Oral-lung microbiome interactions in lung diseases. Periodontol 2000. 2020;83(1):234–241. doi: 10.1111/prd.12301. [DOI] [PubMed] [Google Scholar]
- 9.O’Dwyer D.N., Dickson R.P., Moore B.B. The lung microbiome, immunity, and the pathogenesis of chronic lung disease. J Immunol. 2016;196(12):4839–4847. doi: 10.4049/jimmunol.1600279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Charlson E.S., Bittinger K., Haas A.R., Fitzgerald A.S., Frank I., Yadav A. Topographical continuity of bacterial populations in the healthy human respiratory tract. Am J Respir Crit Care Med. 2011 Oct 15;184(8):957–963. doi: 10.1164/rccm.201104-0655OC. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huffnagle G.B., Dickson R.P., Lukacs N.W. The respiratory tract microbiome and lung inflammation: a two-way street. Mucosal Immunol. 2017;10(2):299–306. doi: 10.1038/mi.2016.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mathieu E., Escribano-Vazquez U., Descamps D., Cherbuy C., Langella P., Riffault S. Paradigms of lung microbiota functions in health and disease, particularly, in asthma. Front Physiol. 2018;9(AUG):1–11. doi: 10.3389/fphys.2018.01168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chakraborty S. 2020. SARS-Cov2 enables anaerobic bacteria (Prevotella, et al) to colonize the lungs disrupting homeostasis, causing long-drawn chronic symptoms, and acute severe symptoms (ARDS, septic shock, clots, arterial stroke) which finds resonance, with key differences. Ahead of print. [Google Scholar]
- 14.Lopes M.P., Cruz Á.A., Xavier M.T., Stöcker A., Carvalho-Filho P., Miranda P.M. Prevotella intermedia and periodontitis are associated with severe asthma. J Periodontol. 2020 Jan 16;91(1):46–54. doi: 10.1002/JPER.19-0065. [Internet] [DOI] [PubMed] [Google Scholar]
- 15.Ono E., Taniguchi M., Higashi N., Mita H., Yamaguchi H., Tatsuno S. Increase in salivary cysteinyl-leukotriene concentration in patients with aspirin-intolerant asthma. Allergol Int [Internet] 2011;60(1):37–43. doi: 10.2332/allergolint.09-OA-0166. [DOI] [PubMed] [Google Scholar]
- 16.Bao L., Zhang C., Dong J., Zhao L., Li Y., Sun J. Oral microbiome and SARS-CoV-2: beware of lung Co-infection. Front Microbiol. 2020 doi: 10.3389/fmicb.2020.01840. [Internet] Ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Daniel R., Gokulanathan S., Shanmugasundaram N., Lakshmigandhan M., Kavin T. Diabetes and periodontal disease. J Pharm Bioallied Sci. 2012;4(6):280. doi: 10.4103/0975-7406.100251. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chakraborty S. 2020. Metagenome of SARS-Cov2 patients in Shenzhen with travel to Wuhan shows a wide range of species - Lautropia, Cutibacterium, Haemophilus being most abundant - and Campylobacter explaining diarrhea. Ahead of print. [Google Scholar]
- 19.Rohlke F., Stollman N. Fecal microbiota transplantation in relapsing Clostridium difficile infection. Therap Adv Gastroenterol. 2012 Nov 19;5(6):403–420. doi: 10.1177/1756283X12453637. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Caldeira L.F., Borba H.H., Tonin F.S., Wiens A., Fernandez-Llimos F., Pontarolo R. In: PLoS One. Singh U.P., editor. 2020. Fecal microbiota transplantation in inflammatory bowel disease patients: a systematic review and meta-analysis. [Internet] Ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Browne A.S., Kelly C.R. Fecal transplant in inflammatory bowel disease. Gastroenterol Clin North Am [Internet] 2017 Dec;46(4):825–837. doi: 10.1016/j.gtc.2017.08.005. [DOI] [PubMed] [Google Scholar]
- 22.Walsh A., Mabee J., Trivedi K. Inflammatory bowel disease. Prim Care - Clin Off Pract. 2011;38(3):415–432. doi: 10.1016/j.pop.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 23.Dailey F.E., Turse E.P., Daglilar E., Tahan V. The dirty aspects of fecal microbiota transplantation: a review of its adverse effects and complications. Curr Opin Pharmacol [Internet. 2019 Dec;49:29–33. doi: 10.1016/j.coph.2019.04.008. [DOI] [PubMed] [Google Scholar]
- 24.Nejadghaderi S.A., Nazemalhosseini-Mojarad E., Asadzadeh Aghdaei H. Fecal microbiota transplantation for COVID-19; a potential emerging treatment strategy. Med Hypotheses. 2021 doi: 10.1016/j.mehy.2020.110476. [Internet] Ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pozhitkov A.E., Leroux B.G., Randolph T.W., Beikler T., Flemmig T.F., Noble P.A. Towards microbiome transplant as a therapy for periodontitis : an exploratory study of periodontitis microbial signature contrasted by oral health , caries and edentulism. BMC Oral Health. 2015:1–11. doi: 10.1186/s12903-015-0109-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pozhitkov A.E., Leroux B.G., Randolph T.W., Beikler T., Flemmig T.F., Noble P.A. Towards microbiome transplant as a therapy for periodontitis: an exploratory study of periodontitis microbial signature contrasted by oral health, caries and edentulism. BMC Oral Health. 2015 Dec 14;15(1):125. doi: 10.1186/s12903-015-0109-4. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nascimento M.M. Oral microbiota transplant: a potential new therapy for oral diseases. J Calif Dent Assoc. 2017;45(10):565–568. [Internet] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen C., Hemme C., Beleno J., Shi Z.J., Ning D., Qin Y. Oral microbiota of periodontal health and disease and their changes after nonsurgical periodontal therapy. ISME J. 2018 May 16;12(5):1210–1224. doi: 10.1038/s41396-017-0037-1. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.AbdelMassih A.F., Kamel A., Mishriky F., Ismail H., El L., Malak L. 2020. Is it infection or rather vascular inflammation ? Game-changer insights and recommendations from patterns of multi-organ involvement and affected subgroups in COVID-19; pp. 15–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bibbò S., Settanni C.R., Porcari S., Bocchino E., Ianiro G., Cammarota G., Gasbarrini A. Fecal microbiota transplantation: screening and selection to choose the optimal donor. J Clin Med. 2020 Jun 5;9(6):1757. doi: 10.3390/jcm9061757. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]