Abstract
The authors present the case of a patient who underwent the removal of a small bluish lesion of the cheek. After discharge, the patient presented with profuse bleeding and hematoma of the cheek. Blood tests revealed severe secondary immune thrombocytopenia (SITP). SITP was probably triggered by the anti-SARS-CoV-2 Pfizer vaccine, which was inoculated to the patient 3 days before the lesion appeared and 12 days before surgery. The authors' aim is to inform colleagues about this possible, rare, adverse effect of the vaccine. In all patients who have recently undergone the COVID-19 vaccine and who present lesions suspected to be due to blood extravasation of the oral mucosa or unjustified gingival bleeding it is advisable to request a blood count before surgery.
Keywords: SARS-CoV-2, Coronavirus, Vaccine, Thrombocytopenia, Secondary thrombocytopenia, Oral surgery, COVID-19
1. Introduction
Healthcare systems around the world are currently committed in vaccinating the population against severe acute respiratory syndrome 2 (SARS-CoV-2) infection. In the face of high efficacy in preventing infection, safety studies have found a low frequency of side effects which are generally benign and temporary [1].
Secondary immune thrombocytopenia (SITP) was not reported among these adverse events. SITP is characterized by reduced platelet production or increased platelet destruction that occurred from secondary causes associated with chronic disorders or with disturbed immune function due to medications, infections, lymphoproliferative and myeloproliferative disorders or autoimmune diseases [2].
In the past, several cases of SIPT have been associated with measles-mumps-rubella and varicella / zoster vaccines [3]. A recent analysis of the literature and of adverse events reported to drug agencies around the world found only 20 cases SITP related to the administration of SARS-CoV-2 vaccines [4].
We report the case of a patient who presented to the Maxillofacial Surgery Unit of the University Hospital of Sassari to perform an excisional biopsy of a small lesion of the cheek, which appeared three days after the administration of the second dose of the Pfizer vaccine.
2. Case report
An 81-year-old man reported the sudden appearance of a bluish cheek lesion. This lesion grew in a few days until it reached the size of 22 × 9 mm. On the advice of the dentist, the patient referred to the Maxillofacial Surgery Unit of the University Hospital of Sassari.
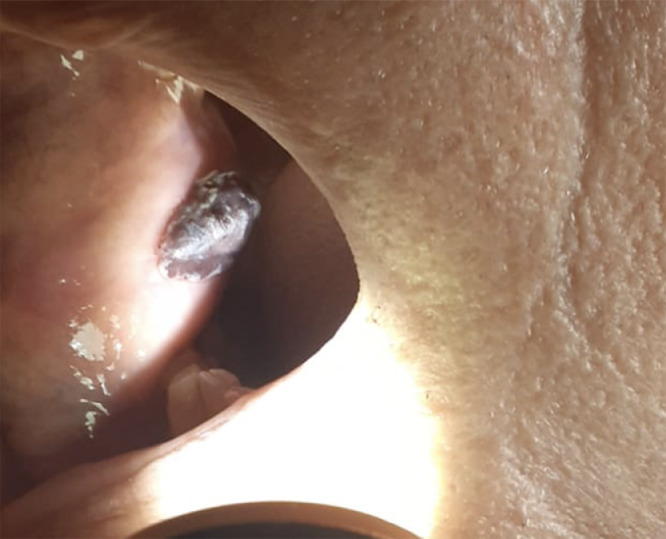
At the time of the visit, the patient presented a bluish lesion of the right cheek mucosa, 22 × 9 mm in diameter, soft consistency, painless and mobile on the underlying plane (Fig. 1 ). The patient presented with stage III chronic renal failure and hypercholesterolemia and had no personal or family history of bleeding or autoimmune disease. In July 2020 he underwent left hemicolectomy for an adenocarcinoma. The patient was being treated with the following therapy: allopurinol 300 mg/day, omeprazole 20 mg/day and atorvastatin 40 mg/day. The patient had completed the anti-SARS-CoV-2 vaccination protocol with Pfizer vaccine: first dose on 03/20/2021 (COMIRNATY mRNA, lot No. ET3620) and second dose on 04/12/2021 (COMIRNATY mRNA, lot No. EW2246). The second dose was given 3 days before the lesion appeared and 18 days before surgery. The patient had a complete blood count performed on 03/22/2021, between the two doses of the vaccine, which was normal (platelet count: 156 × 103/μL). Suspecting a benign lesion, an excisional biopsy was performed under local anesthesia. The operation did not present any complications and after haemostasias with electrocautery the wound was sutured and the patient was discharged.
Fig. 1.
The patient presented a bluish lesion of the right cheek mucosa.
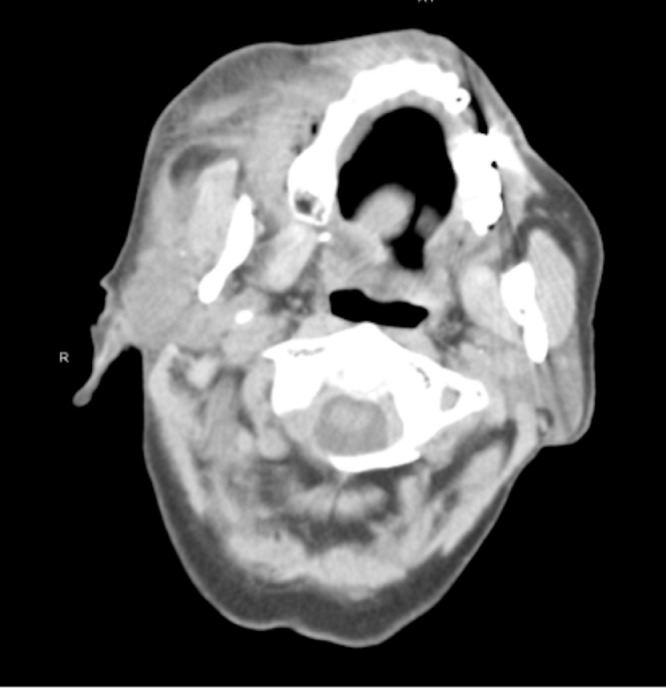
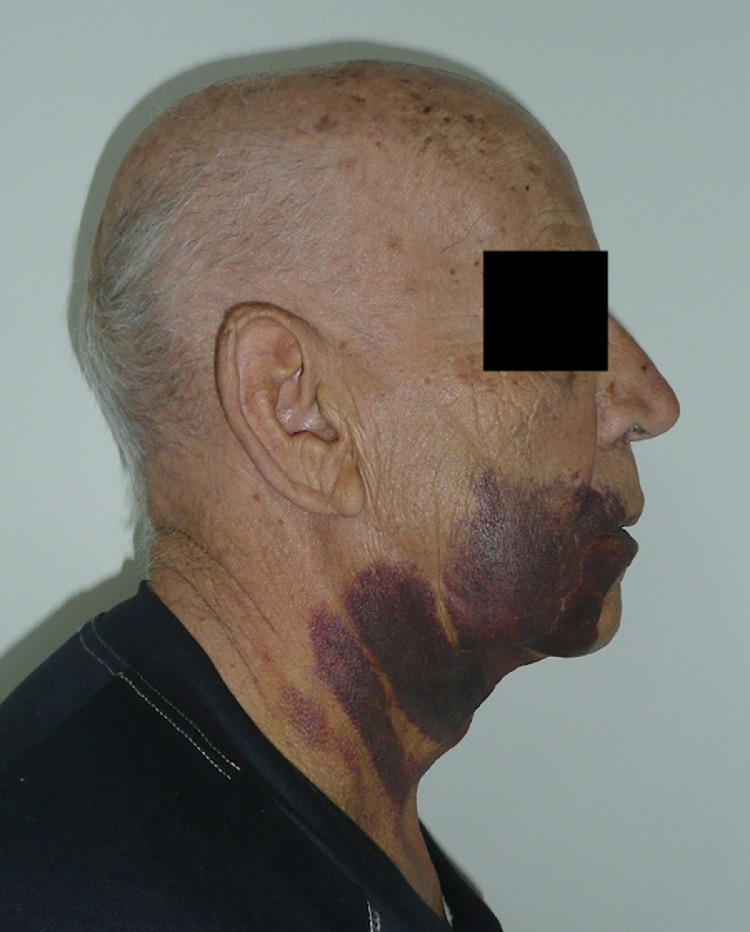
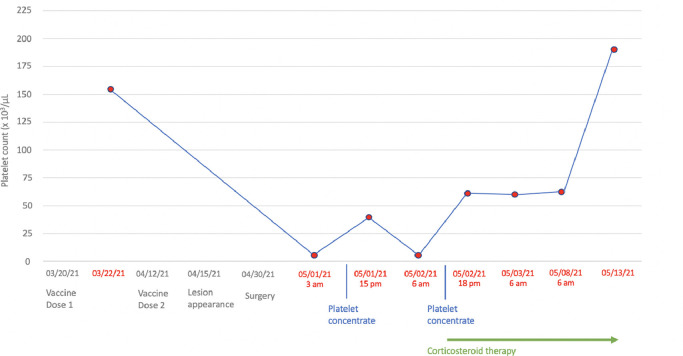
Approximately 8 h after surgery, the patient referred to the emergency department due to copious bleeding through the surgical wound and massive hematoma and ecchymosis of the right cheek (Figs. 2 and 3 ). Blood tests revealed severe thrombocytopenia (platelet count: 4 × 103/μL), normal white blood cells (8.81 × 103/μL), mild anemia (hemoglobin: 11.6 g/dL, red blood cell count: 3.85 × 103/μL), and normal coagulation profile (PT: 11.4 s, aPTT 21.0 s, INR 1.07). Upon admission to the ward, in the suspicion of consumption thrombocytopenia, the patient was administered a platelet pool which allowed an increase in the platelet count up to 40 × 103/μL. However, the subsequent 12-hour follow-up revealed a recurrence of severe thrombocytopenia (5 × 103/μL). For this reason, SITP was suspected and confirmed by the analysis of the bone marrow aspirate showing increased megakaryocytes without dysplastic changes. On this basis, the patient started steroid therapy with methylprednisolone 80 mg / day and underwent a new infusion of platelet pool resulting in an increase in platelet count up to 56 × 103/μL. The patient was discharged on the seven day after with a platelet count of 56 × 103/μL and clinical improvement of the right cheek hematoma which appeared completely resolved at 15 days clinical follow-up. Five days later he performed hematological reevaluation and blood tests which found a platelet count of 188 × 103/μL. Fig. 4 graphically summarizes the trend of the platelet count over time (Fig. 4). The histological examination on the surgical specimen resulted in an organized hematoma due to blood extravasation.
Fig. 2.
Maxillo-facial CT scan of the patient upon admission to the emergency department.
Fig. 3.
The patient upon admission to the maxillofacial surgery ward.
Fig. 4.
Platelet count trend.
3. Discussion
Vaccines are the main weapon with which the whole world is trying to defeat SARS-CoV-2. Their effectiveness and safeness have been proven by rigorous clinical trials, our report cannot question their safety by reporting a possible adverse effect which, although already detected in a few other cases, is to be considered as very rare and certainly tolerable in a risk/benefit analysis.
As hypothesized for some cases of SITP during coronavirus disease 2019 (COVID-19) [5], the pathogenesis of SITP following the vaccine could be linked to the possibility that some patients have pre-formed antibodies directed against polyethylene glycol or other components of the outer lipid membrane of the nanoparticles. It has been hypothesized that these antibodies, directed against a new antigen formed by the attachment of vaccine particles on a small number of platelets, would then trigger a reaction involving all platelets [4].
However, it should also be disclosed that the diagnosis of SITP may have been purely coincidental and unrelated to vaccination. Distinguishing vaccine-induced SITP from coincidental SITP presenting soon after vaccination is impossible at this time, but this report, along with the other rare cases already reported [4,[6], [7], [8]], suggests the need for additional surveillance to determine the true incidence of thrombocytopenia following COVID-19 vaccination. Moreover, it allows us to give some recommendation to colleagues who deal with oral surgery.
First, in all patients who have recently undergone the COVID-19 vaccine and who present lesions suspected to be due to blood extravasation of the oral mucosa or unjustified gingival bleeding it is advisable to request a blood count before surgery. This attention is even more important in patients presenting subclinical forms of primary thrombocytopenia, even if they have recent but pre-vaccination blood analysis with normal values of platelet count.
Second, in case of major post-surgical bleeding, especially if not related to the extent of the surgery, in patients with recent vaccination, it is essential to perform a blood count to establish an adequate diagnostic-therapeutic process. In the reported case, given the stability of the hematoma, its drainage and the surgical haemostasias were not carried out, pending the results of the blood tests.
Third, from a therapeutic point of view, the administration of platelet pools has a temporary benefit. On the contrary, the use of high-dose corticosteroids has proved effective in extinguishing the immune reaction and allowing the improvement of laboratory indices with consequent spontaneous clinical recovery. Obviously, given the rarity of this adverse event, it is not possible to draw definitive conclusions on which therapy is the most effective. However, the efficacy of corticosteroid therapy, already established for other SITP [2], is in line with what was found in the other cases of SITP related to COVID-19 vaccination [4,[6], [7], [8]].
Compliance with ethical standards
Funding
None declared.
Ethical approval
This study did not required Ethical Committee approval.
Informed consent
Written consent was obtained to publish clinical photographs.
Declaration of Competing Interest
None of the authors has a financial interest in any of the products, devices or drugs mentioned in this manuscript.
Acknowledgement
None.
References
- 1.Polack F.B., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., CA4591001 Clinical Trial Group Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2021;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cines D.B., Liebman H., Stasi R. Pathobiology of secondary immune thrombocytopenia. Semin Hematol. 2009;46:S2–S14. doi: 10.1053/j.seminhematol.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grimaldi-Bensoyda L., Michel M., Aubrun E., Leighton P., Viallard J.F., Adoue D., et al. A case-control study to assess the risk of immune thrombocytopenia associated with vaccines. Blood. 2012;120:4938–4944. doi: 10.1182/blood-2012-05-431098. [DOI] [PubMed] [Google Scholar]
- 4.Lee E.J., Cines D.B., Gernsheimer T., Kessler C., Michel M., Tarantino M.D., et al. Thrombocytopenia following Pfizer and Moderna SARS-CoV-2 vaccination. Am J Hematol. 2021;96:534–537. doi: 10.1002/ajh.26132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mahevas M., Moulis G., Andres E., Riviere E., Garzaro M., Cricks E., et al. Clinical characteristics, management and outcome of Covid-19-associated immune thrombocytopenia. A French multicenter series. Br J Haematol. 2020;190:e224–e229. doi: 10.1111/bjh.17024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Candelli M., Rossi E., Valletta F., De Stefano V., Francesci F. Immune thrombocytopenic purpura after SARS-CoV-2 vaccine. Br J Haematol. 2021 doi: 10.1111/bjh.17508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Helms J.M., Ansteatt K.T., Roberts J.C., Kamatam S., Goong K.S., Labayog J.E., et al. Severe, refractory immune thrombocytopenia occurring after SARS-CoV-2 vaccine. J Blood Med. 2021;12:221–224. doi: 10.2147/JBM.S307047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tarawneh O., Tarawneh H. Immune thrombocytopenia in a 22-year-old post Covid-19 vaccine. Am J Hematol. 2021;96:E133–E134. doi: 10.1002/ajh.26106. [DOI] [PMC free article] [PubMed] [Google Scholar]