Abstract
Theta frequency (4–8 Hz) fluctuations of the local field potential have long been implicated in learning and memory. Human studies of episodic memory, however, have provided mixed evidence for theta’s role in successful learning and remembering. Re-evaluating these conflicting findings leads us to conclude that (1) successful memory is associated both with increased narrow-band theta oscillations and a broad-band tilt of the power spectrum; that (2) theta oscillations specifically support associative memory, whereas the spectral tilt reflects a general index of activation; and that (3) different cognitive contrasts (generalized vs. specific to memory), recording techniques (invasive vs. non-invasive) and referencing schemes (local vs. global) alter the balance between the two phenomena to make one or the other more easily detectable.
Keywords: associative memory, theta, spectral tilt, SME, intracranial EEG
The theta hypothesis
Forming associations between different aspects of our sensory and cognitive experience allows us to remember specific events and abstract knowledge about the world that surrounds us. Our coworkers don’t query us each morning about who we are and where we’re from, since they’ve associated that information with the visual inputs corresponding to our faces. If they instead ask us how our weekend was, we can use that cue to remember our visit to the beach and tell them about our experience. We don’t need to consult a map to make it from our desks to the coffee machine, since we’ve associated those objects with locations in space. And we also don’t need to worry about our coffee being too hot, since we’ve associated the machine’s output with a reasonable temperature. It could have been a very bewildering and inefficient start to our day -- but thanks to associations, it was not.
The neural machinery responsible for the formation of associations between high-dimensional representations resides primarily in the medial temporal lobe (MTL), containing the hippocampus, entorhinal, perirhinal, and parahippocampal cortices. Communication and processing within these regions, and between these regions and neocortical association areas, has been linked to episodic memory [1] (see Glossary) and spatial navigation [2,3] -- both processes that involve associations either between the sensory and cognitive components that comprise an event memory, or between the features that mark specific locations in space. And potentially key to these functions is a physiological signature called the theta rhythm -- a 4–8 Hz oscillation in the local field potential (LFP) that was first characterized in rodents in the 1930s (see Box 1).
Box 1. Theta oscillations associated with movement and cognition.
Theta oscillations were first discovered in the rabbit hippocampus in 1938 [83], where they were found to occur both spontaneously and as a reaction to painful stimuli. A first link to memory was established, when researchers followed up this discovery with showing that the duration of cortical theta oscillations recorded in rats after an aversive foot shock correlates with later memory for that foot shock [84]. Even though many early studies in rodents were mainly focused on theta’s role in voluntary movement where theta oscillations can be observed reliably and with large amplitudes [85,86], some studies have noted that theta oscillations also occur during immobility [87]. The theta-memory link was later specifically strengthened by studies showing that synaptic plasticity is modulated by the phase of theta oscillations [88–90].
Whereas early studies in humans suggested that theta oscillations could have different behavioral correlates [91,92], later studies showed that theta oscillations occur in shorter episodes [58,93] and with a lower frequency compared to the movement related theta in rodents [94,95]. In humans, as in rodents, theta oscillations appear prominently during movement [2], but they can also be observed in a variety of cognitive tasks [96]. In addition to theta’s proposed role in declarative memory (see Box 2), theta oscillations may support working memory [97–100] and cognitive control [101], as well as rhythmic shifts of spatial attention [102].
In subsequent decades, a vast theoretical and empirical enterprise has grown around the theta rhythm. It appears in several mammalian species, including humans, and is most commonly observed during active exploration. Theta phase has been linked to the firing of MTL neurons that represent specific locations in space. The finding that hippocampal place cells fire at progressively earlier phases of the theta rhythm as an animal traverses a cell’s firing field [4] inspired a powerful conceptualization of MTL function [5]. In this framework, the ongoing theta rhythm forms a consistent reference for distributed cellular activity, allowing for coding of information not only in firing rates but also in spike-phase relations. Furthermore, the systematic co-activation of sequentially visited places allows for spike-timing dependent plasticity (STDP) to strengthen associations between sequentially activated cells with a bias for forward-associations (see Box 2 and Figure 1). This mechanism does not have to be specific to spatial memory but may be important for establishing temporal associations between arbitrary stimuli. And indeed, it can be related to two hallmark findings of episodic free recall: temporal contiguity, the tendency to successively remember items experienced in temporal proximity; and forward-asymmetry, a bias for forward-transitions [6]. In essence, the stream of sensory inputs to the brain is compressed by the theta rhythm, allowing MTL circuitry to form associations between sequential inputs. Over time and repeated encounters of the same stimuli in different sequences, this mechanism may lead to the formation of “cognitive maps” that reflect long-standing associations between all kinds of stimuli (such as places or concepts; see Box 2) [5,7].
Box 2. Mechanistic accounts of theta’s role in memory.
Theorists have proposed several mechanistic accounts for theta’s role in memory. Here we consider two such accounts: (1) the SPEAR model of Hasselmo and colleagues [103,104] and (2) the temporal encoding model of Buzsaki [5].
The SPEAR (separate phases of encoding and retrieval) model posits that different phases of the theta rhythm are associated with a bias of the CA1 region of the hippocampus to preferentially process input from the entorhinal cortex or from area CA3. This bias along with phasic changes in long-term potentiation (LTP) and depression (LTD) is thought to separate encoding (entorhinal cortex, LTP) and retrieval (CA3, LTD) processes in the hippocampal circuit to the peak and the trough of theta, respectively. The model is consistent with theta phase reset in response to behaviorally relevant stimuli [39,105], as well as with theta phase precession of place cells [4,39]. In this framework, phase precession arises from retrieved/anticipated activity and stimulus-driven activity that occur at different theta phases.
Buzsaki’s temporal encoding model sees theta as critical for establishing temporal associations between stimuli [5,106]. Phase precession of place cells produces a time-compressed sequence of firing representing sequentially visited places within each theta cycle (see Figure 1). Due to this temporal compression, cells fire at delays short enough to enable STDP to strengthen associations between sequentially visited places, preferentially in the forward direction. This mechanism may be at play during navigation, as well as when experiencing sequences of arbitrary stimuli such as words in a free-recall task. It would thereby explain subjects’ tendency to successively recall items experienced in succession (temporal contiguity effects) and in the forward direction (asymmetry effect) [6]. Experiencing the same places or items in different sequential order would ultimately lead to the generation of spatial or semantic maps, which reflect the long-standing temporal co-occurrence of places or concepts.
Figure 1. Formation of associative memories via theta oscillations.
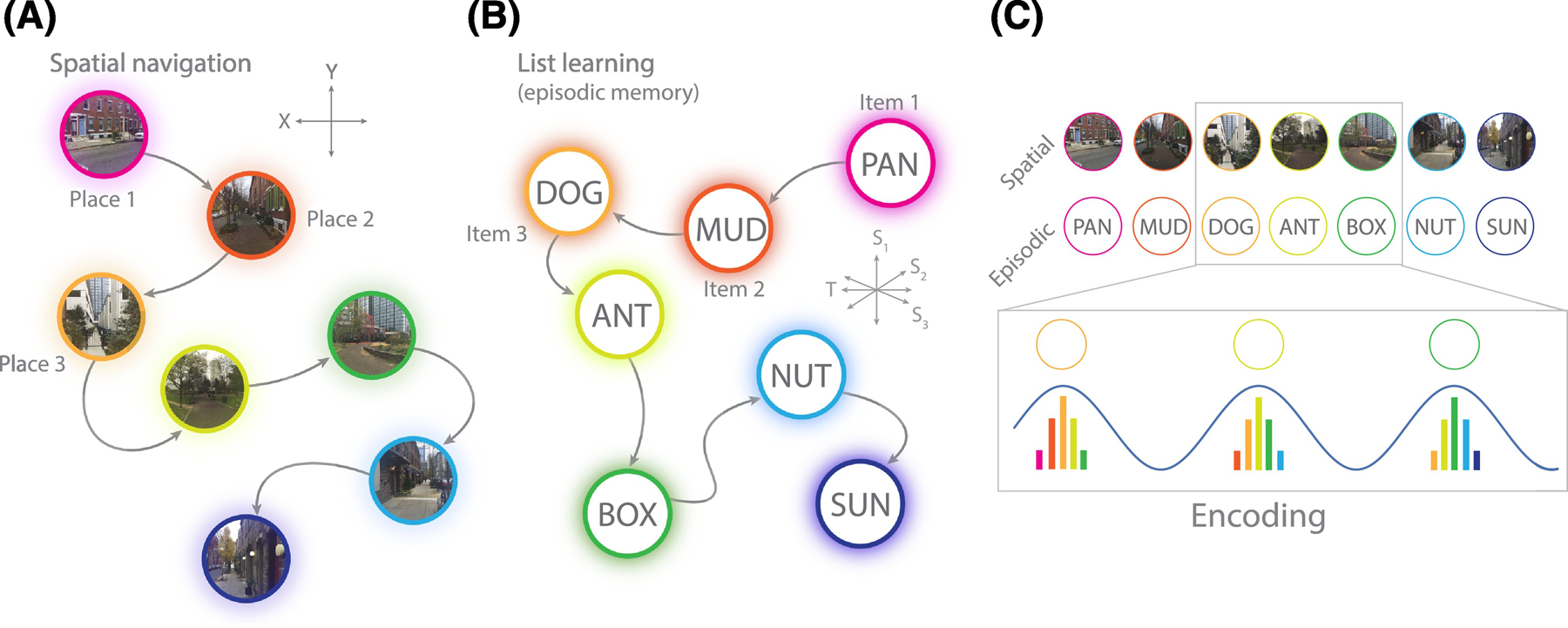
A. Spatial navigation constitutes a sequential set of sensory inputs that correspond to locations in space. Neural representations of a location become stronger the closer the observer is to that location. Locations exist as 2 (or 3) dimensional coordinates. B. Episodic memory, such as learning a list of nouns, consists of a sequential set of inputs that correspond to semantic and temporal features of encountered words. Words exist as locations in a multidimensional semantic/temporal feature space. C. Theta oscillations serve to organize sequential inputs, by representing multiple locations or items within a single theta cycle [5]. The current location or item is represented most strongly, but prior and forthcoming representations are also activated, though to a lesser degree (Panel C adapted from [2]). See Box 2 and for further details.
But if the theta rhythm truly supports effective encoding of episodic associations, it is surprising that the brain does not always show it. Whereas electrophysiological studies in humans have replicated increases in MTL and hippocampal theta power during spatial navigation [8–12], recordings during the formation of episodic memories have revealed an inconsistent relationship between theta and successful memory encoding. In some experiments, increases in MTL and neocortical theta power are predictive of good episodic memory. But over a dozen recent studies have shown exactly the opposite: widespread decreases in theta power during successful episodic encoding and retrieval. These findings undercut the idea that the theta rhythm is a general-purpose mechanism that links episodic memory and spatial navigation under the umbrella of cognitive mapping. If the MTL is agnostic to the type of information it is acting upon, why should theta power increase during spatial navigation but decrease during the formation of event memories?
Here, we seek to reconcile the conflicting literature regarding theta’s role in human episodic memory. First, we will review electrophysiological studies which report increases, decreases, or mixed effects of theta activity during episodic memory tasks. In doing so, we will address how the recording methods as well as particular experimental and analytical methods may be responsible for reported increases or decreases in theta power during successful memory encoding and retrieval. We specifically highlight how contrasts that compare activity for remembered and forgotten stimuli, though commonly used in the literature, inherently confound associational memory processes with a diverse array of other cognitive functions that support successful task completion. These confounds make it difficult to interpret the results and are a key factor behind the conflicting findings. To that end, we will offer a defense for the prevailing theta hypothesis, explaining how the existing body of literature in spatial and episodic domains supports the idea that the theta rhythm underlies associative processing in the brain.
Electrophysiological studies of the theta rhythm in humans
The oscillatory correlates of human memory have been intensively studied for over 25 years using both non-invasive (scalp electroencephalography, EEG and magnetoencephalography, MEG) and invasive (intracranial EEG, iEEG or electrocorticography, ECoG) recording techniques (see Box 3 for a discussion on the origin of theta effects observed with both kinds of methods). These studies have shown variable evidence for theta oscillations during human episodic memory; some studies report memory-related theta increases while others report decreases. In many cases memory-related theta increases and decreases were found in the same study, depending on when and where in the brain oscillatory power was examined.
Box 3. Theta oscillations: A phenomenon of the hippocampus, neocortex, or both?
A wealth of recording methods have yielded a complicated picture of where theta is fundamentally generated. The original research focus on LFP recordings of hippocampal theta in rodents led to the discovery of a circuit that generates 3–8 Hz oscillations, centered on the MTL [107]. Volume conduction and projections from the MTL to neocortical areas could serve to entrain other brain regions to that MTL-derived rhythm, explaining how electrodes placed at the cortical surface (ECoG) or scalp (EEG) can detect theta rhythms during cognitive tasks [9]. However, subsequent research identified independent generators of the theta rhythm in the neocortex itself [108,109], which could more directly contribute to theta power detected by ECoG or scalp sensors. It is not known to what extent theta detected at the scalp or cortical surface reflect hippocampal vs neocortically-generated rhythms, though this is a key question in human electrophysiology.
In humans, simultaneous recording of cortical surface and MTL potentials via ECoG and depth electrodes, respectively, tend to demonstrate broadly similar patterns of electrical activity during cognition. As noted in this review (see Figure 2), intracranial SMEs tend to show decreases in theta power and increases in high-frequency activity at both the cortical surface and in hippocampus/MTL [46,49]. A high-powered study of spectral power in hippocampal subfields showed remarkably consistent SMEs with prior studies of ECoG potentials [46]. Furthermore, a broad set of neocortical areas become phase-synchronized with each other and with the MTL during successful memory formation and retrieval [51,110]. Another study found that electrical stimulation in the MTL could evoke oscillatory events in functionally-connected neocortical regions [111]. However, it remains unclear whether this synchronization is due to direct entrainment of the cortex by the hippocampus or induced synchronization between two independently generated rhythms.
In this review, we address a wide range of literature that considers electrical potentials derived from scalp EEG, MEG, iEEG depth electrodes, and ECoG. We directly discuss the differences between scalp EEG and intracranial recordings at the cortical surface (See “Why the scalp/invasive discrepancy?”). Establishing exactly how neocortical and hippocampal theta rhythms interact and differentially contribute to episodic memory is an important question for future study.
Beyond recording methods, studies of human theta activity also differ in their analysis schemes. During encoding, studies commonly compare patterns of spectral power between items that were subsequently remembered vs. not-remembered (the “subsequent memory effect,” or SME). During retrieval, studies compare activity surrounding the presentation of a cue or, in free recall, they compare correct recalls to time periods where no recall occurs. Such memory-success analyses may obscure neural dynamics that differentially support different kinds of successful memory encoding or retrieval. Therefore, the second-most common approach is to assess the correlation between neural activity and a specific measure of associative memory formation. For example, one study [13] contrasted activity relating to the accuracy of spatial recalls, while another study [14] contrasted activity relating to the recall of spatially-proximate vs. spatially-distant information. By only analyzing successful memory events but grouping activity by a measure of associative memory performance, these studies provide a more specific assay of memory-related neural activity.
Here we focus specifically on theta’s role in episodic memory. We restrict our focus to studies that employ some kind of episodic memory task (i.e. free recall, cued recall and recognition) and report effects on low frequency (~ 1–10 Hz) power or the prevalence of low frequency oscillations. To maintain a focus on theta effects in episodic memory, we exclude studies of working memory and those that only report measures of high-frequency activity. We treat studies of power and phase separately (see “What about theta phase?”), organizing our review according to recording method (invasive vs. noninvasive) and analytic approach (memory success contrast vs. associative memory contrast; see Figure 2).
Figure 2. Theta effects in human episodic memory.
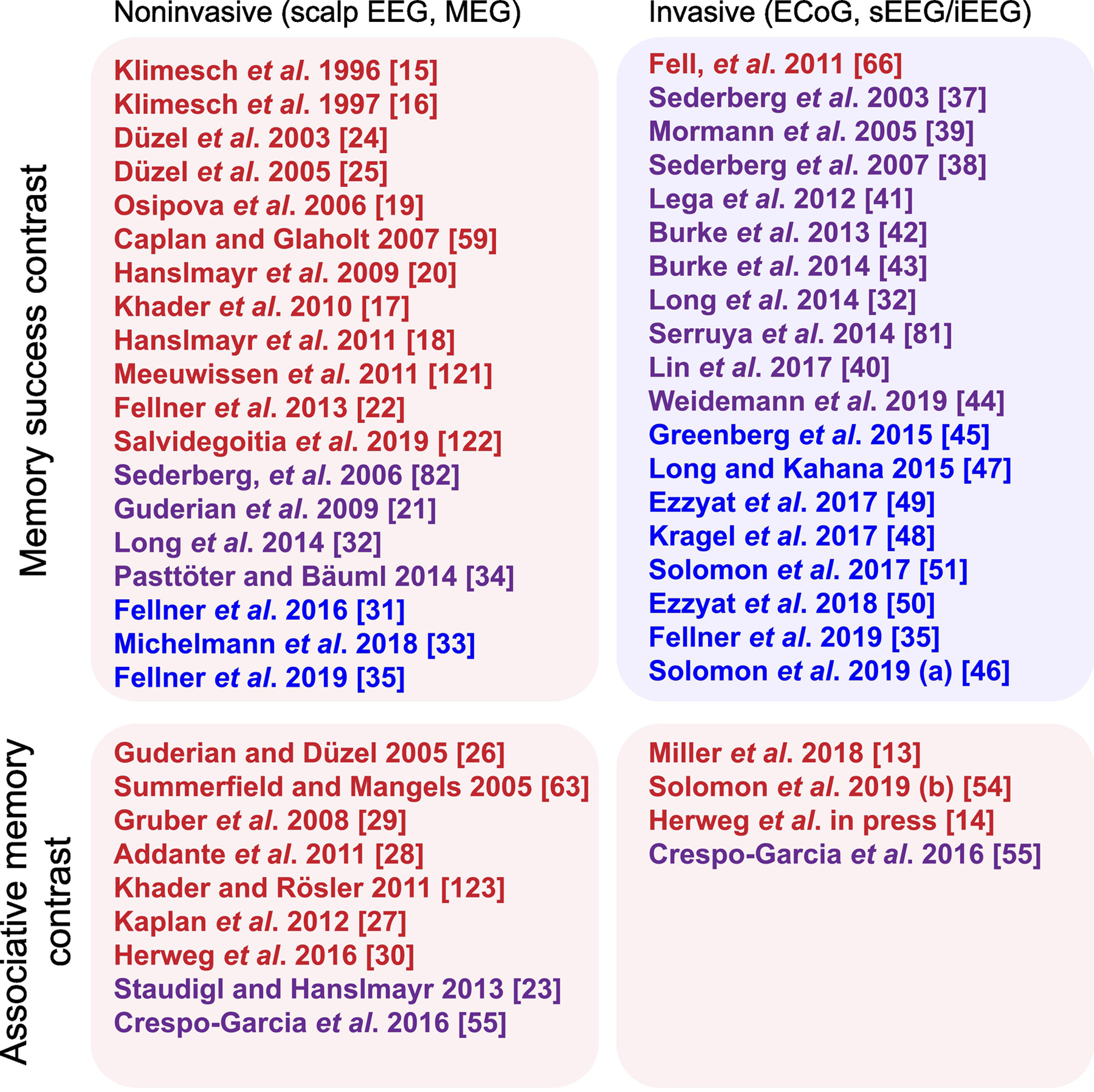
The matrix is organized by recording technique (invasive vs. non-invasive, columns) and analysis technique (memory success vs. measures of associative memory, rows). “Memory success” refers to encoding contrasts between subsequently remembered and forgotten items (subsequent memory effect, SME) or to retrieval contrasts between correct memory retrieval and events where no retrieval occurs (i.e. “deliberation” in free recall, misses or correct rejections in recognition tasks). “Associative memory contrast” refers to analytic methods that compare correct encoding or retrieval trials based on a measure of episodic association, such as the amount of retrieved/encoded context, memory accuracy, or vividness of recall. Red indicates the given study reported significant increases in theta power, blue means theta decreases, and purple means the study reported significant increases and decreases (see supplemental materials for a more detailed description of these studies). [13–15,17–35,37–51,54–56,59,63,81,82,120–123].
Evidence for theta oscillations in noninvasive studies of episodic memory
The prevailing theory is that episodic memory -- like spatial navigation -- is positively associated with theta oscillations. Though most models of memory function suggest the most prominent theta effects should be detectable in the hippocampus, hippocampal projections may drive theta activity in neocortical areas [9], which may be more easily picked up with non-invasive recordings (see also Box 3). From this perspective, theory gives us an eminently testable hypothesis: the MTL, and regions with which it communicates, should exhibit positive correlations between the theta rhythm and the formation and retrieval of memories.
In line with this prediction, many studies in scalp EEG and MEG have demonstrated positive theta effects during memory tasks. Two studies that inaugurated this line of work in the mid-1990s used scalp EEG to show that theta power during the encoding of word items was correlated with subsequent successful recall or recognition of those items, compared to forgotten items (i.e. an SME) [15,16]. Several later studies replicated this result, again showing higher theta power during [17–20] or prior to [21,22] encoding of subsequently recognized or recalled items, higher theta-power during successful item-in-context encoding [23], higher theta power for hits compared to correct rejections [24,25] and higher theta power during recollection or correct source memory retrieval [26–30].
We found only seven EEG/MEG studies that reported any decrease in theta power (including those that also show increases) for subsequently remembered items or during successful retrieval [21,31–35]. While most scalp EEG studies use either one or two common reference electrodes or average reference, [32] used a bipolar reference scheme. Since increases in theta power are often observed with a broad topography across the scalp, these effects might have been attenuated with a bipolar reference that acts as a spatial high-pass filter. Furthermore, task strategy and its interaction with effort or attention may also play a role; one study [31], for instance, explicitly instructed subjects to engage in certain mnemonic strategies (Loci or pegword method). We will return later to a more in-depth discussion of the potential relevance of these variables (see “Why the scalp/invasive discrepancy?” and “Low-frequency power decreases may be a general marker of activation”). In summary, evidence from non-invasive scalp EEG and MEG recordings seem to be broadly consistent with the hypothesis that theta oscillations facilitate successful memory operations (see Figure 2).
Evidence from intracranial recordings: Theta oscillations vs. spectral tilt?
Intracranial recordings have been obtained from patients undergoing monitoring for the treatment of drug-resistant epilepsy -- either from electrodes placed directly on the cortical surface (ECoG) or depth electrodes placed within the brain [36]. They provide a far more anatomically precise measure of neural activity at higher signal-to-noise ratios and they also enable direct recording from MTL structures. Do these recordings recapitulate findings of increased theta power from the noninvasive literature?
Intracranial studies of theta activity during episodic memory processing paint a complicated picture. Contrary to findings from scalp EEG/MEG, almost all intracranial studies of human memory report at least some decreases in theta power [32,37–44] associated with memory success, and many report exclusively decreases in theta power [35,45–51] (see Figure 2). These decreases are often accompanied by increases in high-frequency power (30+ Hz) so that the effect can be visualized as a “tilt” of the power spectrum during successful compared to unsuccessful memory operations (see Box 4 and Figure 3).The tilt is typically observed across widespread brain regions, including frontal, temporal, and medial temporal lobe. Furthermore, the tilt has been found during encoding and retrieval [48,51]. While this effect goes in the opposite direction of scalp studies, and undercuts the hypothesized role of theta in episodic memory formation, it is undeniable -- more than a dozen studies since 2007 have reported the spectral tilt during memory tasks, including high-powered studies with over 150 subjects [42,51]. We will later return to a discussion of differences between scalp and intracranial EEG that might explain these discordant findings (See “Why the scalp/invasive discrepancy?”).
Box 4. Theta effects: spectral tilt or narrow-band theta oscillations?
Spectral power provides an easy-to-calculate proxy for neural oscillations in a certain frequency band. However, a change in spectral power between two conditions can not only be driven by rhythmic narrow-band oscillations, but also by changes in the aperiodic background spectrum. These include both shifts (i.e. general increase or decrease in power across frequencies) and tilts (i.e. an increase/decrease in low-frequency power with a concomitant decrease/increase in high-frequency power) of the background spectrum. Such tilts of the spectrum have been observed in a diverse set of tasks and conditions [78,79], suggesting that spectral tilt may be a general marker of neural activation [53].
In the case of intracranial EEG data, where robust decreases in low-frequency power have been observed during successful compared to unsuccessful encoding and retrieval, it remains unknown whether this reduction in power is due to a change in oscillations or a change in the background spectrum (see Figure 3). As noted above, region-specific increases in high-frequency power are frequently observed alongside decreases in low-frequency power, across a range of tasks. This is suggestive of an underlying tilt in the power spectrum during successful cognition. A recent study challenges this account, presenting evidence that decreases in low-frequency power and increases in high-frequency power can be dissociated in time and space [35]. Consequently, this study rightly suggests that the spectral tilt alone cannot fully account for low-frequency power decreases in an SME contrast. Indeed, it is undoubtedly the case that isolated decreases in low-frequency power, unaccompanied by high-frequency increases, can occur in certain brain regions at certain times. More research will be needed to establish the different contributions of narrow-band oscillations, isolated reductions in low-frequency power, and broad-band tilts to theta power decreases in memory contrasts.
Figure 3. Narrow-band low frequency oscillations during memory processing.
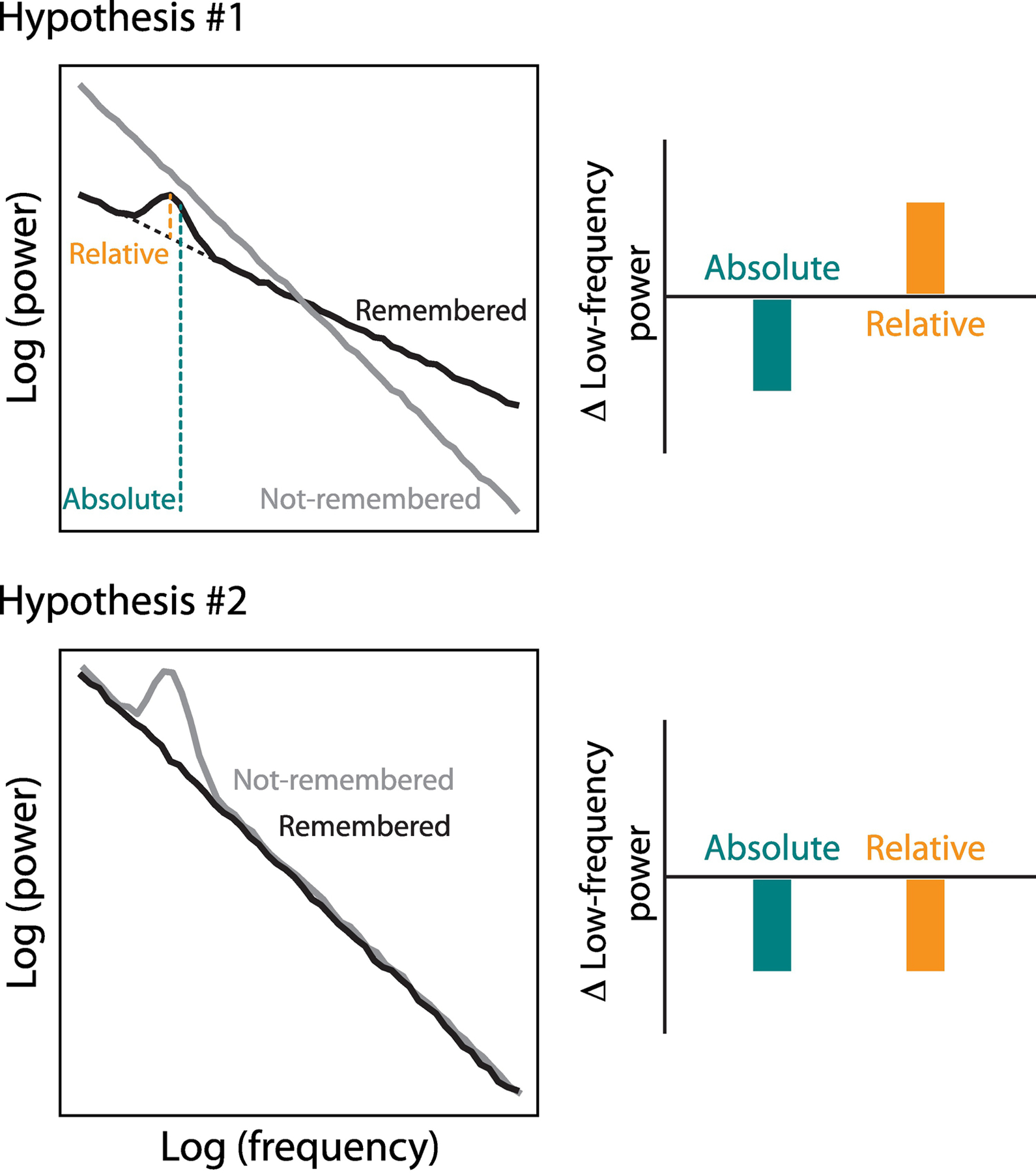
We describe two scenarios that could result in the observed low-frequency power decreases associated with successful memory processing: one in which the decrease is driven by a tilt of the background spectrum, which may be observed despite an increase in narrow-band oscillations in the successful compared to the unsuccessful condition (Hypothesis #1); and another one in which a decrease in power during successful vs. unsuccessful encoding occurs due to a true reduction in narrow-band oscillations (Hypothesis #2).
The spectral tilt has inspired significant debate [52,53]. Is the tilt pattern (1) a unitary broad-band effect with increases in high-frequency power and decreases in low-frequency power inherently yoked? Or, (2) do decreases in narrow-band low-frequency oscillations often -- but not exclusively -- co-occur with high-frequency increases due to overlapping but independent neural processes? If hypothesis (1) is true, underlying theta rhythms may exist on top of a tilt of the power spectrum, potentially resolving some of the discrepancy between invasive and non-invasive studies of human theta (see Box 4 and Figure 3). If hypothesis (2) is true, theta oscillations are reduced during successful memory formation, requiring us to rethink theta’s role in memory formation and retrieval.
Positive correlations between intracranial theta and memory
The intracranial studies we have discussed so far feature prominent theta power decreases, which are at odds with theta increases reported in many scalp EEG studies. There are however, several intracranial studies that show increases in theta power associated with successful encoding [13] or retrieval [14,54]. These intracranial studies found increases in theta by correlating power with a measure of the degree of associative memory rather than by comparing successful to unsuccessful trials. Below we discuss findings from three of these studies.
One study correlated MTL theta power during free recall of items encoded in a virtual environment with the virtual spatial distance between the encoding locations of successively recalled items [14]. They observed increased theta power for recalls that were followed by recall of an item encoded in spatial proximity. Assuming that ‘spatial clustering’ during recall indexes successful spatial context retrieval, this analysis specifically implicated theta in retrieving item-location associations. When applying the same rationale to the relation of theta power and temporal distances during encoding (i.e. indexing memory for temporal context), two studies found no effects of temporal clustering on theta power during encoding [47] or retrieval [14]. More recent results, however, suggest that hippocampal theta power does increase during temporally clustered recalls, but that this effect can only be observed on high-performance trials [54]. In free recall, temporally clustered recalls can reflect contextually mediated recall or other non-contextual processes, such as rehearsal of the first few or the last few items in a study list. Therefore, excluding lists that are likely to reflect significant rehearsal strategies may be crucial to uncover theta effects. The same study also observed a relation between theta power during retrieval with semantic distances between items, showing that increases in hippocampal theta power predicted greater clustering -- or contextual retrieval -- in semantic space [54]. Finally, a third study [13] asked subjects to retrieve the spatial location at which a cued item was presented, and compared more accurate to less accurate location retrievals, representing greater or lesser degrees of contextual reinstatement ([55] used a similar contrast to find mixed theta increases and decreases). Central to all of these studies was a comparison of “success to success,” with variability in neural processes relating solely to a specific measure of contextual reinstatement.
Other studies have examined the oscillatory correlates of associative memory, but their contrasts did not specifically control for non-associative contributions to memory success. For example, researchers assessed power differences between successful and unsuccessful word encoding in a verbal paired-associates paradigm, which could be considered a measure of associative memory [45]. However, the contrast employed in that study compared encoding related activity for successful retrievals against unsuccessful retrievals. As such, this study’s contrast is more akin to a classic SME, in that any neural processes which may result in a recollection failure -- associative or not -- are reflected in statistical power maps (see “Why is the SME not a test of associative memory?”).
Only one study [56] observed exclusive increases in hippocampal and rhinal theta power using an SME contrast. These increases occurred in the pre-stimulus interval; theta power appears to fall below baseline in the period after stimulus onset but statistics are not reported. Other studies found a mix of increases and decreases in memory success contrasts. Notably, several of these report positive effects in a “low theta” range, either using statistics at the electrode level [37,41] or specifically localized to the posterior hippocampus [40]. However, in almost all studies, theta decreases were far more widespread and stronger than reported increases. For example, [43] found significant theta power increases prior to item retrieval, but these effects were isolated to the right temporal pole and evolved to widespread, bilateral decreases shortly before and during item retrieval. A similar study [42] found patches of theta power increases in the SME, localized to prefrontal and lateral temporal cortices, which vanished amid strong theta decreases (including MTL) after 500 ms post-stimulus onset. Similarly, [32] found small but significant increases in frontal theta only within 500 ms of stimulus onset, raising the possibility that these relatively weak findings reflect spectral components of the ERP and not sustained oscillatory activity. Taken together, intracranial studies largely paint a picture of theta decreases in simple memory success contrasts, and where increases in theta are found they tend to be early and brief.
In short, findings of theta decreases associated with good memory are limited to intracranial studies of memory success. Findings from non-invasive studies as well as from intracranial studies that specifically assess associative memory suggest that theta power increases underlie the encoding and retrieval of episodic memories in humans. Moreover, the relationship between theta and memory is bolstered by a rich theoretical and animal literature that link theta oscillations to memory and spatial navigation, even at the scale of cellular ensembles (see Box 2).
Why is the SME not a test of associative memory?
The SME is often thought of as a direct test of associative episodic memory, in that successful recall of an item must derive from successful item-to-context binding. To some extent, this is true -- part of the underlying neural activity related to successful encoding definitionally comes from the formation of episodic associations. However, successful encoding also relies on other neural processes responsible for (1) perception, because an item must be seen or heard to be encoded, (2) attention, because the brain must be oriented towards processing experimental stimuli, and (3) task engagement or intentionality, because the study subject must be trying to maximize task performance. The lines between these cognitive processes are undoubtedly blurry, and this list is by no means exhaustive. The broader message is that by comparing successful to unsuccessful encoding, the SME captures neural activity responsible for forming episodic associations and neural activity responsible for many other processes, and then averages the effects.
To illustrate this point with an extreme example, consider a study subject in a verbal free-recall task. The subject opens their eyes for two words per list and encodes them but closes their eyes for all others. The SME would likely show a dramatic effect in the occipital cortex, which is not wholly wrong; visual processing is a key step in parsing items and context. But the resulting statistical picture could obscure important effects happening elsewhere in the brain unrelated to vision but essential for episodic memory processing. If, for example, visual attention is associated with strong decreases in theta power, but memory is associated with mild increases, the SME in this toy example would guide a researcher to a misleading conclusion about how the brain encodes memories.
Contrasts which compare successful recall events to “deliberation” or “baseline” intervals in which no retrieval occurs [43] are similarly influenced by non-mnemonic factors. A subject’s disengagement from the task, or distraction with something else, could easily result in a lower rate of recall during that period. Therefore, a comparison of successful retrieval to such baseline intervals may not merely reflect cognitive processes related to memory retrieval. It remains therefore unclear, whether theta power decreases observed using this contrast [43,46,48,51,57] are linked to associative memory processes or the mentioned confounds.
How can associative memory be directly assessed? To measure neural processes related to episodic association, one should construct a contrast between degrees of association that controls for successful memory. As discussed earlier, several studies using scalp, MEG, and intracranial EEG have used this approach to demonstrate increases in theta power associated with episodic memory formation and retrieval. A common approach is to compare encoding or retrieval events in which subjects remember items with or without retrieving the correct contextual or associative information (as in [26]). Some studies use an explicit metric of association, such as spatial distances (during encoding) between successively recalled items [14] or semantic-temporal distances between remembered words [54]. Another study [23] assessed contextual memory by comparing recognition for items presented in matching or non-matching contexts. And yet another approach is to ask subjects for a subjective report of confidence, and compare correctly retrieved items based on their confidence ratings, under the assumption that high-confidence judgments tend to accompany retrieval of source or contextual information. Crucial to all of these studies is a contrast between groups of successful encoding or retrieval events, which merely differ in the degree of retrieved context. In setting up such contrasts, a confound with non-associative processes such as vision, attention, or intentionality can be reduced. Strikingly, no study using either scalp or intracranial EEG that has employed an associative memory contrast observed selective decreases in low-frequency power (see Figure 2).
Separating spectral tilt and oscillations analytically
We suggest that memory contrasts that specifically isolate associative mechanisms should reveal narrow-band low frequency oscillations, which are otherwise obscured by a spectral tilt (see Figure 3) or by a reduction in low-frequency power [35] that is related to less specific cognitive variables such as attention or task engagement. To the extent that the non-specific low-frequency power decrease is due to a tilt of the background spectrum, it can be characterized by specifically analyzing the shape of the power spectrum to separate the two phenomena. One such method is interchangeably referred to as BOSC (Better OSCillation detection) or Pepisode [58]. It searches for time periods during which there exists a narrow-band peak in the power spectrum that exceeds a certain amplitude relative to a fit of the background power spectrum for a certain duration (e.g. 3 cycles).
Studies using this method have shown that hippocampal theta oscillations during episodic memory encoding predominantly occur at the edges of the conventional 4–8 Hz theta band (i.e. “slow” 2.5– 5 Hz and “fast” 5.5–10 Hz theta) [41]. When counting the number of electrodes that exhibited a significant SME using (conventional) spectral power, negative effects outnumbered positive effects, but positive effects were observed in the same low theta range in which the authors also found oscillatory activity, as measured with BOSC. While this study did not directly compare the prevalence of theta oscillations during successful versus unsuccessful encoding, another (scalp EEG) study did and found that theta (4–8 Hz) oscillations were associated with successful encoding of word pairs in cued recall (i.e. a memory success contrast) [59]. If this finding can be confirmed with intracranial EEG, it would suggest that oscillation detection algorithms offer a complementary way to uncover the presence of rhythmic theta activity during successful memory operations, even in the presence of a broad-band tilt of the power spectrum. More recent methods, such as IRASA (irregular-resampling auto-spectral analysis) [60], FOOOF (Fitting oscillations & one-over f) [61], and bycycle [62] allow to estimate additional parameters, such as waveform symmetry or they more accurately fit the slope and offset of the background spectrum. Even though these methods have not yet been applied widely to the study of human memory, they seem to provide a powerful tool to advance our understanding of the differential contributions of narrow-band oscillations and broad-band tilts or shifts of the power spectrum to successful encoding and retrieval.
What about theta synchrony?
So far, we have focused on theta power. But at the heart of the study of oscillations is the idea that rhythmic activity coordinates the activity of single cells or populations of cells within and across different brain regions. The presence of oscillations (be it inferred from changes in power or via oscillation detection) is a prerequisite for these phenomena, but we can also investigate them more directly. Studies have mostly addressed (1) the synchronization of single-unit firing and faster gamma oscillations (30–100 Hz) with theta phase (see Box 5), as well as (2) the phase-phase synchronization of theta oscillations measured in different brain regions. Measures of neural synchrony may be less susceptible to concurring changes in broad-band power. If theta synchrony is enhanced during memory formation and retrieval, this finding would therefore strengthen the theta hypothesis while also allowing more direct insight into the mechanisms by which theta oscillations support memory.
Box 5. Spike-phase relations and theta-gamma coupling.
Single neuron recordings in neurosurgical patients provide a rare window on human memory processes at the cellular level. One study observed that single neurons in the hippocampus fire phase locked to 1–4 Hz oscillations (i.e. the frequency range in which intracranial studies observed positive SME effects in the hippocampus [40,41]) during virtual navigation [112]. Theta (4–8 Hz) phase locking, in turn, was observed in temporal and parietal cortices [112]. Another study showed that the preferred phase a neuron fires at codes for navigational goals [113]. But only one study has so far looked at differences in neuronal phase locking associated with successful memory: Phase locking of single neurons in the MTL to 3–7 Hz theta oscillations is predictive of subsequent recognition and also distinguishes high-confidence from low-confidence hits [114]. One potential mechanism by which phase locking might support memory formation is by coordinating synaptic inputs to a postsynaptic neuron to more reliably induce a postsynaptic spike [56]. Animal studies suggest that the precise timing of action potentials with respect to the ongoing theta rhythm also plays a role in organizing sequential information. As described earlier (Box 2), place cells in rodents fire at drifting phases of an ongoing theta rhythm. No study, however, has yet succeeded in documenting such “phase precession” in humans.
Studies in rodents have shown that place cells fire at a preferred gamma phase [115]. This has led to the proposal that successive gamma cycles within one theta cycle serve to discretize sequential information. A place (concept) cell may precess discretely from gamma to gamma cycle within a theta cycle [116]. If these time-compressed “sweeps” do not span an entire theta-cycle, a proxy for their detection is a modulation of gamma amplitude by theta phase. Using intracranial EEG, it has been shown that theta-gamma coupling in the hippocampus increases during successful memory formation [39,117] and that theta-gamma coupling between hippocampus and parahippocampal gyrus increases during retrieval of spatial context [14]. Using MEG, theta-gamma coupling has been linked to successful sequence encoding [118]. And with scalp EEG theta-gamma coupling has been observed between anterior and posterior areas during successful memory encoding [119]. These studies support the notion that theta oscillations organize and strengthen connections between sequentially presented items and they suggest that theta gamma coupling may not only synchronize neuronal spiking locally but also across different brain regions.
A role for oscillations in interregional communication has mostly been studied in terms of theta phase-phase relationships (i.e. phase locking or spectral coherence). Using scalp EEG, investigators have observed increased theta coherence between frontal and posterior electrodes during successful encoding of item-context pairs [63] and working memory [64] -- for a review, see [65]. Similarly, using iEEG, several studies have observed increased theta phase locking between a large set of brain regions during successful encoding [42,46,51,66] and retrieval [46,51] in free recall, as well as during encoding or retrieval of spatial or temporal relations between landmarks in a navigation task [55,67]. Furthermore, synchronized entrainment of theta oscillations in visual and auditory cortex using rhythmically modulated visual and auditory stimuli has been shown to improve performance in subsequent cued recall [68,69].
Increases in long-range theta phase synchrony support the hypothesis that episodic memory in humans relies on theta oscillations to coordinate cellular activity across disparate regions. But these findings also raise an important question: Why are there many reports of increased memory-related theta phase synchrony using intracranial measures, even as almost every intracranial study reports predominantly decreased theta power? If theta oscillations coordinate activity across distant brain regions, we would expect changes in local power and inter-regional synchronization to occur in tandem, not in opposite directions.
We propose two hypotheses for the divergence of theta power and phase synchrony. Under the first model, power and synchrony increase simultaneously on the same recording contacts. However, on a background of decreased power and negligible change in synchrony amongst other contacts, averaging over time and space may obscure power increases while retaining inter-regional synchronization effects (Figure 4A, upper panel). A second possibility is that increases in theta synchrony come hand in hand with concomitant decreases in power on the same recording contacts, even as the level of narrow-band theta power is relatively increased (Figure 4A, lower panel). In a memory success contrast, both hypotheses are compatible with an observed decrease in theta power (Figure 4B) accompanying an increase in synchrony.
Figure 4. Opposing directions of theta power and phase synchrony.
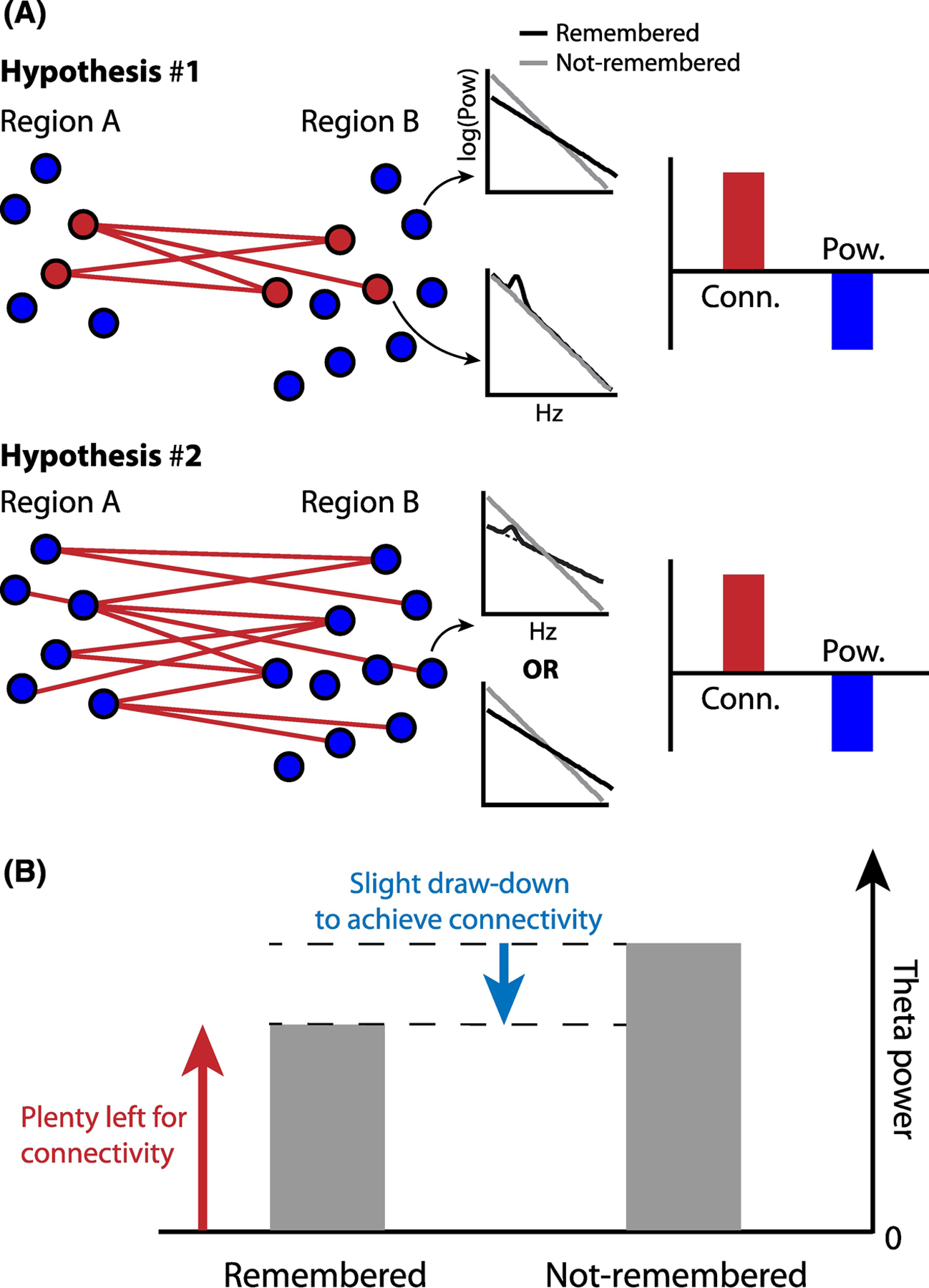
A. Top: Local and narrowband increases in theta power, as detected by a subset of intracranial electrodes (red circles), specifically underlie increases in long-range phase synchronization. Surrounding electrodes exhibit decreases in power due to tilt-related factors and show no significant change in their long-range synchrony with other regions. Averaged across electrodes, an increase in phase synchronization and decrease in power is observed. Bottom: All electrodes show a decrease in power, driven either by spectral tilt alone or narrowband increases in theta that are overwhelmed by the strength of the tilt. Due to increases in narrowband theta, or another mechanism entirely, such decreases in power co-occur with increases in long-range phase synchronization. B. In the case of Hypothesis #2, decreases in power can occur alongside increases in connectivity so long as the overall level of theta power is still sufficient to achieve inter-regional phase locking.
These two possibilities can be resolved with current methods. The most straightforward approach would be to examine changes in theta power and phase synchrony at local scales in time and space, by assessing these time-resolved metrics at the level of individual electrodes. Indeed, as we have noted earlier, a focus on local dynamics has revealed more subtle increases in theta power against a background of broad decreases. Separating broadband tilts from narrowband oscillations could be particularly useful in such analyses (see “Separating spectral tilt and oscillations analytically”).
Why the scalp/invasive discrepancy?
Noninvasive studies of episodic memory generally support the theta hypothesis. Since the 1990s, scalp EEG and MEG studies have uncovered increases in theta during successful memory (see Fig. 2). With the assumption that hippocampal projections to the neocortex drive theta responses detectable by scalp EEG and MEG (see Box 3), authors have generally concluded that these results support the hypothesis of theta unifying spatial navigation and episodic memory. However, noninvasive measures of neural activity are limited in their capacity to precisely localize the source of a neural oscillation and capture blurred neural signals as they are filtered through skull, muscle, and skin.
Why do noninvasive studies of memory success generally show theta increases if the most prominent finding in intracranial analyses is a theta decrease? We cannot confidently answer this question without simultaneous invasive/noninvasive recordings in the same set of subjects. However, we hypothesize that these discordant results stem from differing scales of spatial resolution between scalp EEG and iEEG/ECoG.
Specifically, we propose that two mechanisms contribute to the observed discrepancies between scalp EEG and intracranial EEG. First, studies in non-human primates have simultaneously recorded scalp EEG and LFPs and show that spectral power in scalp EEG represents a linear combination of LFP power and inter-electrode synchrony. In fact, increases in synchrony can produce positively modulated spectral power at the scalp even in the presence of a negative LFP modulation [70–72]. As schematized in Figure 5A, intracranial electrodes record relative decreases in theta power (blue shading), but the oscillation itself is highly correlated across electrodes (red lines). Accordingly, scalp electrodes detect this synchronization as a relative increase in theta power (red shading). In view of these findings, the predominantly positive effects in theta phase locking associated with successful memory that we have described above might be observed as positive power modulations in scalp EEG, even in situations where local activity measured intracranially shows decreases in power. It is less clear how this analysis would generalize to MEG. Studies that simultaneously measured MEG and scalp EEG have shown that MEG may be less sensitive to frontal theta oscillations than scalp EEG [35,73], possibly explaining why some MEG studies have observed negative theta effects in retrieval success contrasts [21,35].
Figure 5. Physiological origins of the scalp vs. intracranial theta discrepancy.
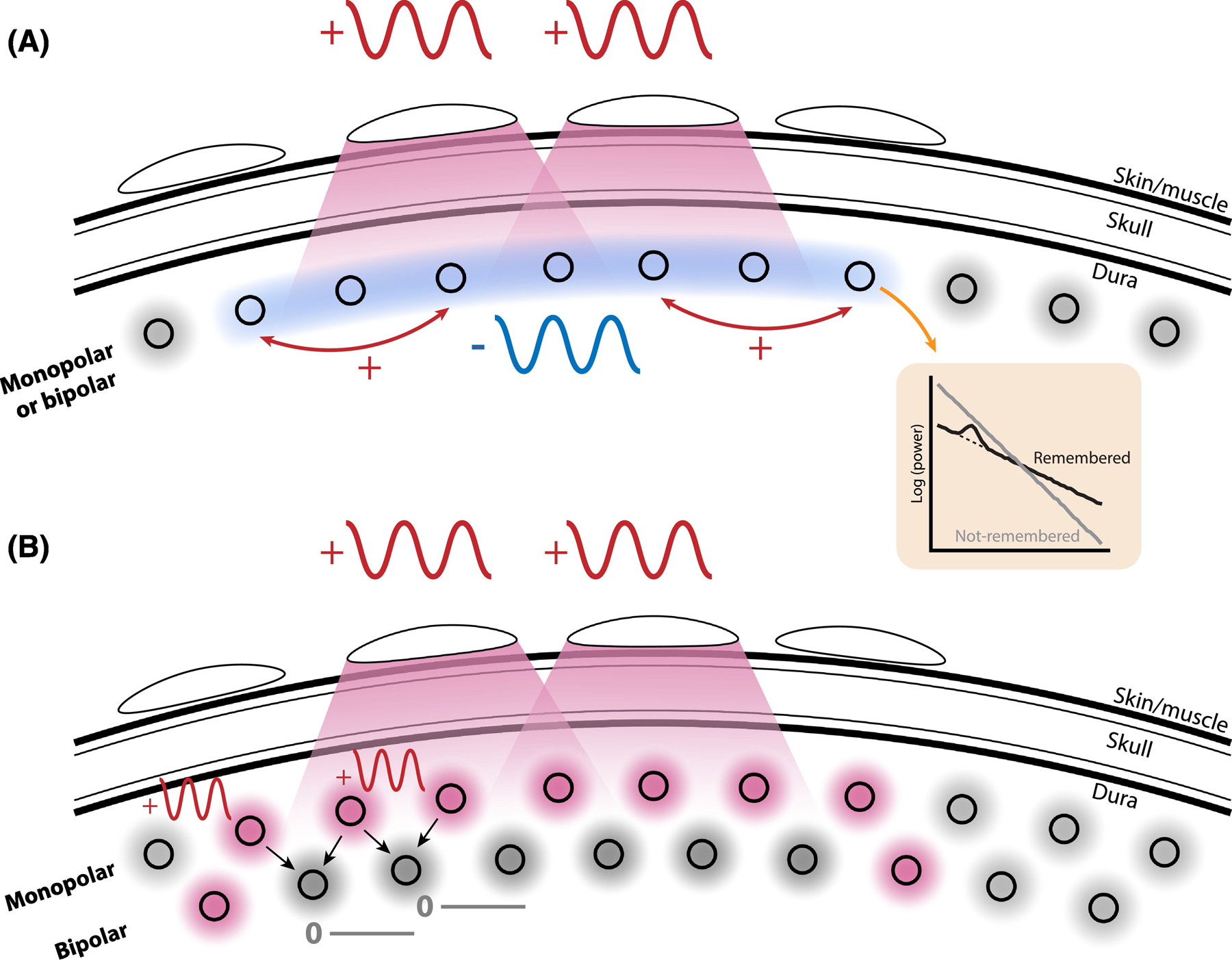
A. A possible contribution to the dissociation between theta findings between scalp and intracranial EEG is the tendency for scalp EEG to integrate signals over much larger areas of cortex, and thereby reflect not only theta amplitude but also the degree of large-scale theta synchronization. Under this model, theta amplitude can decrease in good memory states (blue-colored circles), but long-range synchronization may increase (red lines). Intracranial measurements reflect highly local activity and therefore exhibit decreases, while scalp EEG reflects larger-scale synchronization and shows increases. Notably, increases in long-range synchronization occur due to increases in narrowband theta power that are obscured in the average by a spectral tilt (orange box). B. Electrode referencing schemes can also affect measurements of theta power. In a “monopolar” style referencing scheme, such as the common average, intracranial electrodes may detect local increases in theta power (red circles). Similarly, scalp EEG electrodes sum these increases over wide areas of cortex. The high-pass spatial filtering properties of the bipolar reference tend to reduce or abolish this effect, potentially obscuring findings of memory-related theta increases.
Second, we posit that discordant theta findings may be exacerbated by the referencing schemes that researchers preferentially use to with scalp EEG vs. intracranial EEG. Whereas scalp EEG most commonly uses an average reference scheme or a single electrode as a common reference (e.g. on the mastoid), intracranial EEG is mostly referenced with a bipolar scheme to eliminate local noise that similarly affects neighboring electrodes. This choice of reference amplifies the difference in spatial resolution between scalp EEG and intracranial EEG, since bipolar referencing acts as a spatial high-pass filter by selectively removing correlated measurements across spatially close electrodes. These correlated measurements include both spatially distributed noise and spatially distributed signals. We know that low frequency activity (where low-frequency refers to the temporal domain) typically is observed with a low spatial frequency, meaning that it is correlated across large areas of cortex [71,72]. A bipolar referencing scheme, as commonly used with intracranial data, should therefore eliminate spatially distributed low-frequency effects in the majority of electrodes, and only reveal those effects at the bipolar channels that border the true effect (i.e. at channels that combine activity from one electrode that exhibits a positive theta effect and one that does not; see Figure 5B). Accordingly, negative theta effects have been observed in scalp EEG when using a bipolar reference scheme [32]. A common average referencing scheme, as commonly used with scalp EEG, on the other hand, should preserve spatially distributed positive effects in the majority of electrodes.
We have proposed two possible mechanisms that contribute to differences in measured theta power between scalp and intracranial EEG. Of the two, we believe the first to be the most prominent. Though referencing schemes likely contribute to observed theta effects, there are notable counterexamples: [46], for example, used an intracranial average reference to find decreases in theta power during successful memory encoding and retrieval. A full list of the referencing scheme for each study reviewed here can be found in the Supplement (Supplemental Table 1).
In addition to the spatial scale, differences also exist between the populations being studied with intracranial (patients with epilepsy) vs. scalp EEG/MEG. Although differences in study population could theoretically contribute to observed differences in the neural correlates of memory performance, this appears unlikely for several reasons: First, the memory contrasts reveal within-subject differences that should control for differences between subjects that are independent of memory processes. Second, the neural correlates of memory in these subjects appear in tissue far from the epileptic focus and absent any evidence of interictal spikes or sharp waves [41]. Nevertheless, more studies are needed that provide direct comparisons between epileptic and non-epileptic patients [32,35] or that record intracranial and scalp EEG in the same set of patients.
Low-frequency power decreases may be a general marker of activation
In this review, we sought to reconcile conflicting findings on the presence of theta activity during memory processing. We noted that theta power decreases are commonly observed in intracranial recordings, often associated with an overall tilt of the power spectrum. And though we have discussed how this tilt effect can obscure true underlying increases in oscillations, the tilt remains a widespread phenomenon that could play an important role in brain function. Indeed, beyond studies of human memory, the tilt has also been observed during mathematical computation and sensory processing (see Box 2).
Why does the spectral tilt manifest during successful cognition and perception? A common thread through all studies is an association with increased attention, effort, or task engagement in conditions where the tilt emerges -- whether it be successful memory encoding, audiovisual processing, or arithmetic. The meaning of “attention” or “effort” can be difficult to define, and these processes blur together with the nature of the task at hand. For the sake of this review, we will define “attention” as an upregulation of mechanisms which downweigh noisy inputs and upweight inputs with high task relevance. Indeed, it has previously been suggested that decreases in low-frequency power facilitate increases in neural coding capacity, by decreasing synchronized unit activity, or increasing firing rates, and thereby increasing represented information content [45,52,74].
Decreases in low-frequency power -- and accompanying increases in cortical information representation -- could more fundamentally reflect modulations in attention. For example, active whisking in rodents causes a decrease in low-frequency power in stimulated barrel cortex [75,76]. In primates, directed attention to a visual receptive field tends to decrease low-frequency power, suggesting a link between attention, power, and reduced neural noise correlations [77]. Attention-related decreases in low-frequency power would also be consistent with studies that have found the spectral tilt during successful states in non-mnemonic tasks, such as audiovisual judgment or mathematical problem solving [78–80].
Two human studies have found that viewing early items in an encoding list are associated with greater low-frequency power decreases than viewing later items [81,82], and one of these studies also found that successful memory for earlier items is associated with greater low-frequency power decreases [81]. In other words, the successful encoding of early items is associated with stronger power decreases than the successful encoding of late items. Considered with the classic “primacy effect” finding that early list items are recalled more frequently -- perhaps due to an upregulation of attentional mechanisms -- this evidence further suggests that heightened attention is associated with low-frequency power decreases, in the setting of a memory task.
Broad decreases in low-frequency power that accompany increased attention must coexist with the band-limited increases in theta oscillatory power that underlie successful episodic and spatial memory. This is precisely why theta oscillations are detectable during high-attention states in memory tasks (i.e. successful encoding/retrieval), either via analytic corrections for the tilt or with contrasts that compare one high-attention state to another high-attention state. Moreover, the presence of memory-related, band-limited theta power yields detectable increases in long-range theta connectivity even as overall measures of spectral power decrease.
To summarize, successful memory relies on two cognitive processes. First, a deployment of attention causes generalized decreases in low-frequency power, potentially decreasing neural noise correlations and allowing the brain to represent higher information content [52,74]. These neural dynamics are found in memory and non-memory tasks, because attention is critical to both. Second, memory-related increases in theta power manifests as a peak in the power spectrum and increased inter-regional connectivity. The theta peak is detectable so long as a correction is made for the underlying tilt in the power spectrum.
Concluding remarks
Cognitive neuroscientists have long grappled with a fundamental question: Does human declarative memory rely on the same neural machinery as spatial navigation? If so, does the theta oscillation observed in the hippocampus and in connected cortical brain regions during navigation also emerge during memory encoding and retrieval? Evidence from scalp EEG and MEG has generally suggested “yes”; theta oscillations support episodic memory. But many intracranial EEG studies suggest “no”; theta power actually decreases during successful memory encoding and retrieval. At stake is our understanding of a core brain structure -- does the MTL have a domain-general function that uses the same neural elements to process associations between arbitrary inputs, or is it functionally segmented to process spatial navigation in one way, and memory in an entirely different way?
We approached this question by reconsidering the past 25 years of work in the electrophysiology of human memory. We categorized studies by whether they employed invasive or non-invasive methods, and whether they used a memory success contrast or a specific measure of associative memory. We discovered that findings of theta power decreases were mostly isolated to intracranial studies analyzing memory success. Otherwise, theta power increases were found in studies of scalp EEG, MEG, and intracranial EEG that utilized associational contrasts.
Accordingly, we posited that memory success effects are cognitively nonspecific in that they not only contrast successful with unsuccessful memory but are also sensitive to other cognitive and perceptual processes such as attention and task engagement. Likewise, theta power decreases and concurrent high frequency power increases (i.e. spectral tilt) are not only found in memory success contrasts, but also occur during audio-visual perception, hand movements, and arithmetic problem solving -- further suggesting that they represent a general biomarker of task engagement or attention.
In short, the classic SME as a scientific tool is a blunt instrument. Much trailblazing work in the electrophysiology of human memory has used the SME, but its time has come to an end. Instead, we urge memory scientists to adopt contrasts that specifically measure associative memory, ideally between conditions where attention is matched. For example, a straightforward way to do this is to identify trials of successful memory that differ in the degree of encoded contextual detail, the vividness of retrieval, or the amount of retrieved information. Additionally, oscillation detection algorithms that separately assess spectral tilt and narrow-band oscillations can help detect the presence of oscillations despite co-occurring broad-band effects.
Studies which assess long-range theta synchronization reveal increased connectivity during successful memory, even in relatively nonspecific memory success contrasts. One possible explanation is that while averaging across electrodes washes out local increases in power, it retains inter-regional connectivity. Alternatively, decreases in power could be mechanistically related to increases in inter-regional synchrony -- further research is necessary to distinguish between these possibilities (see Outstanding Questions). Relatedly, the fact that scalp EEG measures both changes in local power and changes in synchrony between nearby areas could explain some of the discrepancies between invasive and non-invasive studies. Scalp EEG studies may more reliably report increases in theta power associated with good memory because power measured at the scalp reflects large-scale neural synchrony, even as local theta amplitude is diminished.
Outstanding questions:
Which theoretical models of theta function are supported by evidence from human electrophysiology? In this review, we highlighted two conceptualizations of theta’s role in memory. One hypothesis focused on the idea that theta oscillations (potentially with nested gamma cycles) serve to organize ensemble-level representations of sequential inputs and facilitate STDP. A second hypothesis highlighted the potential role of theta in segregating phases of encoding and retrieval. Partly due to the rarity of single-unit recordings in humans, neither of these theories are robustly supported by human studies. Using intracranial techniques to validate theoretical models of MTL function remains a key goal in cognitive neuroscience.
Can algorithmic approaches to oscillation detection expand our understanding of theta’s role in memory? Analytic approaches to identifying theta oscillations have been proposed for over a decade, but to date, only a handful of studies have employed these methods to study the electrophysiology of human memory. Do these methods identify theta oscillations on a background of spectral tilt? Furthermore, do oscillation detection methods replicate recent findings of band-specific theta increase in associative memory contrasts?
Are changes in local theta power and inter-regional connectivity inherently linked? Here we reviewed a collection of studies that generally found broad decreases in low-frequency power but increases in low-frequency connectivity. However, existing work is insufficient to disambiguate two possible origins of this phenomenon. Can connectivity increase even as spectral power decreases, or is enhanced connectivity actually correlated with local and transient increases in power?
Intracranial reports of decreased theta power during memory tasks have been discordant with an otherwise large body of human and animal literature supporting the role of theta in episodic memory. Here, we argue that when considerations are made for methodological details, the field is not as discordant as it seems. The body of literature supports the original theoretically motivated hypothesis: theta oscillations in the human MTL support the formation and retrieval of episodic memories.
Supplementary Material
Highlights.
Influential theories state that human declarative memory relies on the same neural machinery as spatial navigation and specifically implicate the theta rhythm in memory formation for associations between sequentially visited places and experiences events.
Electrophysiological studies in humans, however, paint a complicated picture of theta’s role in episodic memory.
Whereas some studies observe increases in theta power associated with successful memory, other studies observe a spectral tilt of the power spectrum with increased high frequency and decreased low frequency power during successful memory formation or retrieval.
Here we reconcile these findings by considering the distinction between narrow-band theta oscillations and co-occurring broad-band effects. We show how recording methods as well as analytical choices may alter the balance between the two phenomena.
Acknowledgements
We thank Nicholas Diamond, Daniel Rubinstein and Daniel Schonhaut for helpful comments on a previous version of the manuscript. This work was supported by DARPA grant N66001–14–2–4032 and NIH grant MH55687 to MJK as well as by DFG grant HE 8302/1–1 to NAH.
Glossary
- Broad-band effect
Using Fourier transform or related methods, the potentials measured with EEG can be decomposed into their constituent frequencies. The resulting power spectrum exhibits a 1/f shape with higher power at lower frequencies; Changes in slope and offset of this background spectrum are referred to as broad-band effects or, more specifically, as “tilt” and “shift”. These should be distinguished from narrow-band oscillations, which can be detected as a peak in the power spectrum that deviates from the 1/f background spectrum.
- Electroencephalography (EEG)
Method to record electrical potentials generated by neuronal activity using electrodes placed on the scalp (scalp EEG), on the cortical surface (ECoG) or within the brain (depth electrodes); The latter two are jointly referred to as intracranial EEG (iEEG).
- Episodic memory
Memory for personally experienced events that are associated with a particular time and space.
- Local field potential (LFP)
Electrical potential generated by changes in ion concentrations as a result of neuronal activity. LFPs can be recorded by placing electrodes in the extracellular space.
- Magnetoencephalography (MEG)
Method to record electro-magnetic fields generated by neuronal activity with sensors surrounding the head.
- Oscillation
Rhythmic fluctuation of the LFP at a particular frequency. At each time point, oscillations can be characterized by instantaneous amplitude and phase of the oscillation. In the frequency domain, oscillations should be detectable as a peak relative to the background power spectrum.
- Place cells
Neurons in the medial temporal lobe that fire when an animal is located in a particular location in space -- the cell’s place field.
- Phase synchronization
Constant phase offset across trials or time-points between oscillations at different recording electrodes.
- Recall
Memory task in which subjects encode lists of items (e.g. words) and subsequently recall those items. In “Free recall” subjects recall the items in any order they come to mind. In “Cued recall” subjects encode pairs of items, so that during recall one of the items serves as a cue and the other one has to be recalled.
- Recognition
Memory task in which subjects encode lists of items and subsequently are presented with mixed lists of previously encoded and new items. For each item subjects have to indicate whether they recognize the item as old or whether they think it is a new item.
- Reference
EEG records potentials between pairs of electrodes. During recording, all electrodes are commonly paired with a single reference electrode. For offline analysis, data can be re-referenced; This can for instance be done by subtracting from each electrodes’ time series the time series of its closest neighboring electrode (i.e. bipolar referencing scheme) or the average time series of all electrodes (i.e. average referencing scheme).
- Spike-timing dependent plasticity (STDP)
Process by which the strength of synaptic connections between neurons are strengthened or weakened based on the relative timing of action potentials (spikes) of the neurons. If a neuron tends to receive a certain input before it spikes, that connection will be strengthened, if it tends to receive a certain input after it spikes, that connection will be weakened.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mayes A et al. (2007) Associative memory and the medial temporal lobes. Trends Cogn. Sci 11, 126–135 [DOI] [PubMed] [Google Scholar]
- 2.Herweg NA and Kahana MJ (2018) Spatial Representations in the Human Brain. Front. Hum. Neurosci 12, 297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moser EI et al. (2008) Place Cells, Grid Cells, and the Brain’s Spatial Representation System. Annual Review of Neuroscience 31, 69–89 [DOI] [PubMed] [Google Scholar]
- 4.O’Keefe J and Recce ML (1993) Phase relationship between hippocampal place units and the EEG theta rhythm. Hippocampus 3, 317–330 [DOI] [PubMed] [Google Scholar]
- 5.Buzsáki G (2005) Theta rhythm of navigation: link between path integration and landmark navigation, episodic and semantic memory. Hippocampus 15, 827–840 [DOI] [PubMed] [Google Scholar]
- 6.Kahana MJ (1996) Associative retrieval processes in free recall. Mem. Cognit 24, 103–109 [DOI] [PubMed] [Google Scholar]
- 7.Tolman EC (1948) Cognitive maps in rats and men. Psychol. Rev 55, 189–208 [DOI] [PubMed] [Google Scholar]
- 8.Kahana MJ et al. (1999) Human theta oscillations exhibit task dependence during virtual maze navigation. Nature 399, 781–784 [DOI] [PubMed] [Google Scholar]
- 9.Ekstrom AD et al. (2005) Human hippocampal theta activity during virtual navigation. Hippocampus 15, 881–889 [DOI] [PubMed] [Google Scholar]
- 10.Bohbot VD et al. (2017) Low-frequency theta oscillations in the human hippocampus during real-world and virtual navigation. Nat. Commun 8, 14415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bush D et al. (2017) Human hippocampal theta power indicates movement onset and distance travelled. Proc. Natl. Acad. Sci. U. S. A 114, 12297–12302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aghajan ZM et al. (2017) Theta Oscillations in the Human Medial Temporal Lobe during Real-World Ambulatory Movement. Curr. Biol 27, 3743–3751.e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miller J et al. (2018) Lateralized hippocampal oscillations underlie distinct aspects of human spatial memory and navigation. Nat. Commun 9, 2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herweg NA et al. (in press) Reactivated spatial context guides episodic recall. J. Neurosci [DOI] [PMC free article] [PubMed]
- 15.Klimesch W et al. (1996) Theta band power in the human scalp EEG and the encoding of new information. Neuroreport 7, 1235–1240 [DOI] [PubMed] [Google Scholar]
- 16.Klimesch W et al. (1997) Brain oscillations and human memory: EEG correlates in the upper alpha and theta band. Neurosci. Lett 238, 9–12 [DOI] [PubMed] [Google Scholar]
- 17.Khader PH et al. (2010) Theta and alpha oscillations during working-memory maintenance predict successful long-term memory encoding. Neurosci. Lett 468, 339–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hanslmayr S et al. (2011) The relationship between brain oscillations and BOLD signal during memory formation: a combined EEG-fMRI study. J. Neurosci 31, 15674–15680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Osipova D et al. (2006) Theta and gamma oscillations predict encoding and retrieval of declarative memory. J. Neurosci 26, 7523–7531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hanslmayr S et al. (2009) Brain oscillations dissociate between semantic and nonsemantic encoding of episodic memories. Cereb. Cortex 19, 1631–1640 [DOI] [PubMed] [Google Scholar]
- 21.Guderian S et al. (2009) Medial temporal theta state before an event predicts episodic encoding success in humans. Proc. Natl. Acad. Sci. U. S. A 106, 5365–5370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fellner M-C et al. (2013) Brain oscillatory subsequent memory effects differ in power and long-range synchronization between semantic and survival processing. Neuroimage 79, 361–370 [DOI] [PubMed] [Google Scholar]
- 23.Staudigl T and Hanslmayr S (2013) Theta oscillations at encoding mediate the context-dependent nature of human episodic memory. Curr. Biol 23, 1101–1106 [DOI] [PubMed] [Google Scholar]
- 24.Düzel E et al. (2003) A multivariate, spatiotemporal analysis of electromagnetic time-frequency data of recognition memory. NeuroImage 18, 185–197 [DOI] [PubMed] [Google Scholar]
- 25.Düzel E et al. (2005) The oscillatory dynamics of recognition memory and its relationship to event-related responses. Cereb. Cortex 15, 1992–2002 [DOI] [PubMed] [Google Scholar]
- 26.Guderian S and Düzel E (2005) Induced theta oscillations mediate large-scale synchrony with mediotemporal areas during recollection in humans. Hippocampus 15, 901–912 [DOI] [PubMed] [Google Scholar]
- 27.Kaplan R et al. (2012) Movement-related theta rhythm in humans: coordinating self-directed hippocampal learning. PLoS Biol 10, e1001267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Addante RJ et al. (2011) Prestimulus theta activity predicts correct source memory retrieval. Proc. Natl. Acad. Sci. U. S. A 108, 10702–10707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gruber T et al. (2008) Induced electroencephalogram oscillations during source memory: familiarity is reflected in the gamma band, recollection in the theta band. J. Cogn. Neurosci 20, 1043–1053 [DOI] [PubMed] [Google Scholar]
- 30.Herweg NA et al. (2016) Theta-Alpha Oscillations Bind the Hippocampus, Prefrontal Cortex, and Striatum during Recollection: Evidence from Simultaneous EEG-fMRI. J. Neurosci 36, 3579–3587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fellner M-C et al. (2016) Spatial Mnemonic Encoding: Theta Power Decreases and Medial Temporal Lobe BOLD Increases Co-Occur during the Usage of the Method of Loci. eNeuro 3, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Long NM et al. (2014) Subsequent memory effect in intracranial and scalp EEG. Neuroimage 84, 488–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Michelmann S et al. (2018) Replay of Stimulus-specific Temporal Patterns during Associative Memory Formation. J. Cogn. Neurosci 30, 1577–1589 [DOI] [PubMed] [Google Scholar]
- 34.Pastötter B and Bäuml K-HT (2014) Distinct slow and fast cortical theta dynamics in episodic memory retrieval. Neuroimage 94, 155–161 [DOI] [PubMed] [Google Scholar]
- 35.Fellner M-C et al. (2019) Spectral fingerprints or spectral tilt? Evidence for distinct oscillatory signatures of memory formation. PLoS biology 17, e3000403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Parvizi J and Kastner S (2018) Promises and limitations of human intracranial electroencephalography. Nat. Neurosci 21, 474–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sederberg PB et al. (2003) Theta and Gamma Oscillations during Encoding Predict Subsequent Recall. J. Neurosci 23, 10809–10814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sederberg PB et al. (2007) Hippocampal and neocortical gamma oscillations predict memory formation in humans. Cereb. Cortex 17, 1190–1196 [DOI] [PubMed] [Google Scholar]
- 39.Mormann F et al. (2005) Phase/amplitude reset and theta-gamma interaction in the human medial temporal lobe during a continuous word recognition memory task. Hippocampus 15, 890–900 [DOI] [PubMed] [Google Scholar]
- 40.Lin J-J et al. (2017) Theta band power increases in the posterior hippocampus predict successful episodic memory encoding in humans. Hippocampus 27, 1040–1053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lega BC et al. (2012) Human hippocampal theta oscillations and the formation of episodic memories. Hippocampus 22, 748–761 [DOI] [PubMed] [Google Scholar]
- 42.Burke JF et al. (2013) Synchronous and asynchronous theta and gamma activity during episodic memory formation. J. Neurosci 33, 292–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Burke JF et al. (2014) Theta and high-frequency activity mark spontaneous recall of episodic memories. J. Neurosci 34, 11355–11365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weidemann CT et al. (2019) Neural activity reveals interactions between episodic and semantic memory systems during retrieval. J. Exp. Psychol. Gen 148, 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Greenberg JA et al. (2015) Decreases in theta and increases in high frequency activity underlie associative memory encoding. Neuroimage 114, 257–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Solomon EA et al. (2019) Dynamic Theta Networks in the Human Medial Temporal Lobe Support Episodic Memory. Curr. Biol 29, 1100–1111.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Long NM and Kahana MJ (2015) Successful memory formation is driven by contextual encoding in the core memory network. Neuroimage 119, 332–337 [DOI] [PubMed] [Google Scholar]
- 48.Kragel JE et al. (2017) Similar patterns of neural activity predict memory function during encoding and retrieval. Neuroimage 155, 60–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ezzyat Y et al. (2017) Direct Brain Stimulation Modulates Encoding States and Memory Performance in Humans. Curr. Biol 27, 1251–1258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ezzyat Y et al. (2018) Closed-loop stimulation of temporal cortex rescues functional networks and improves memory. Nat. Commun 9, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Solomon EA et al. (2017) Widespread theta synchrony and high-frequency desynchronization underlies enhanced cognition. Nat. Commun 8, 1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hanslmayr S et al. (2016) Oscillations and Episodic Memory: Addressing the Synchronization/Desynchronization Conundrum. Trends Neurosci 39, 16–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Burke JF et al. (2015) Human intracranial high-frequency activity during memory processing: neural oscillations or stochastic volatility? Curr. Opin. Neurobiol 31, 104–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Solomon EA et al. (2019) Hippocampal theta codes for distances in semantic and temporal spaces. Proc. Natl. Acad. Sci. U. S. A DOI: 10.1073/pnas.1906729116 [DOI] [PMC free article] [PubMed]
- 55.Crespo-García M et al. (2016) Slow-theta power decreases during item-place encoding predict spatial accuracy of subsequent context recall. Neuroimage 142, 533–543 [DOI] [PubMed] [Google Scholar]
- 56.Fell J et al. (2011) Medial temporal theta/alpha power enhancement precedes successful memory encoding: evidence based on intracranial EEG. J. Neurosci 31, 5392–5397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Long NM et al. (2017) Contextually Mediated Spontaneous Retrieval Is Specific to the Hippocampus. Curr. Biol 27, 1074–1079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Caplan JB et al. (2001) Distinct patterns of brain oscillations underlie two basic parameters of human maze learning. J. Neurophysiol 86, 368–380 [DOI] [PubMed] [Google Scholar]
- 59.Caplan JB and Glaholt MG (2007) The roles of EEG oscillations in learning relational information. Neuroimage 38, 604–616 [DOI] [PubMed] [Google Scholar]
- 60.Wen H and Liu Z (2016) Separating Fractal and Oscillatory Components in the Power Spectrum of Neurophysiological Signal. Brain Topogr 29, 13–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Haller M, Parameterizing neural power spectra. [DOI] [PMC free article] [PubMed]
- 62.Cole SR and Voytek B (2019) Cycle-by-cycle analysis of neural oscillations. Journal of Neurophysiology 122, 849–861 [DOI] [PubMed] [Google Scholar]
- 63.Summerfield C and Mangels JA (2005) Coherent theta-band EEG activity predicts item-context binding during encoding. Neuroimage 24, 692–703 [DOI] [PubMed] [Google Scholar]
- 64.Sarnthein J et al. (1998) Synchronization between prefrontal and posterior association cortex during human working memory. Proc. Natl. Acad. Sci. U. S. A 95, 7092–7096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fell J and Axmacher N (2011) The role of phase synchronization in memory processes. Nat. Rev. Neurosci 12, 105–118 [DOI] [PubMed] [Google Scholar]
- 66.Fell J et al. (2003) Rhinal-hippocampal theta coherence during declarative memory formation: interaction with gamma synchronization? Eur. J. Neurosci 17, 1082–1088 [DOI] [PubMed] [Google Scholar]
- 67.Watrous AJ et al. (2013) Frequency-specific network connectivity increases underlie accurate spatiotemporal memory retrieval. Nat. Neurosci 16, 349–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Clouter A et al. (2017) Theta Phase Synchronization Is the Glue that Binds Human Associative Memory. Curr. Biol 27, 3143–3148.e6 [DOI] [PubMed] [Google Scholar]
- 69.Wang D et al. (2018) Single-Trial Phase Entrainment of Theta Oscillations in Sensory Regions Predicts Human Associative Memory Performance. J. Neurosci 38, 6299–6309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Musall S et al. (2014) Effects of neural synchrony on surface EEG. Cereb. Cortex 24, 1045–1053 [DOI] [PubMed] [Google Scholar]
- 71.Nunez PL and Srinivasan R (2010) Scale and frequency chauvinism in brain dynamics: too much emphasis on γ band oscillations. Brain Struct. Funct 215, 67–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nunez PL and Srinivasan R (2006) Electric Fields of the Brain: The Neurophysics of EEG, Oxford University Press, USA. [Google Scholar]
- 73.Srinivasan R et al. (2006) Source analysis of EEG oscillations using high-resolution EEG and MEG. Prog. Brain Res 159, 29–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hanslmayr S et al. (2012) Oscillatory power decreases and long-term memory: the information via desynchronization hypothesis. Front. Hum. Neurosci 6, 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Poulet JFA and Petersen CCH (2008) Internal brain state regulates membrane potential synchrony in barrel cortex of behaving mice. Nature 454, 881–885 [DOI] [PubMed] [Google Scholar]
- 76.Poulet JFA et al. Thalamic control of cortical states. , Nature Neuroscience, 15. (2012) , 370–372 [DOI] [PubMed] [Google Scholar]
- 77.Harris KD and Thiele A (2011) Cortical state and attention. Nat. Rev. Neurosci 12, 509–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ramot M et al. (2012) A widely distributed spectral signature of task-negative electrocorticography responses revealed during a visuomotor task in the human cortex. J. Neurosci 32, 10458–10469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Podvalny E et al. (2015) A unifying principle underlying the extracellular field potential spectral responses in the human cortex. J. Neurophysiol 114, 505–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Randazzo MJ et al. (2019) Spectral tilt underlies mathematical problem solving. bioRxiv DOI: 10.1101/601880 [DOI]
- 81.Serruya MD et al. (2014) Power shifts track serial position and modulate encoding in human episodic memory. Cereb. Cortex 24, 403–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sederberg PB et al. (2006) Oscillatory correlates of the primacy effect in episodic memory. Neuroimage 32, 1422–1431 [DOI] [PubMed] [Google Scholar]
- 83.Jung R and Kornmüller AE (1938) Eine Methodik der Ableitung Iokalisierter Potentialschwankungen aus subcorticalen Hirngebieten. Archiv für Psychiatrie und Nervenkrankheiten 109, 1–30 [Google Scholar]
- 84.Landfield PW et al. (1972) Theta rhythm: a temporal correlate of memory storage processes in the rat. Science 175, 87–89 [DOI] [PubMed] [Google Scholar]
- 85.Vanderwolf CH (1969) Hippocampal electrical activity and voluntary movement in the rat. Electroencephalogr. Clin. Neurophysiol 26, 407–418 [DOI] [PubMed] [Google Scholar]
- 86.Vanderwolf CH (1988) Cerebral activity and behavior: control by central cholinergic and serotonergic systems. Int. Rev. Neurobiol 30, 225–340 [DOI] [PubMed] [Google Scholar]
- 87.Kramis R et al. (1975) Two types of hippocampal rhythmical slow activity in both the rabbit and the rat: relations to behavior and effects of atropine, diethyl ether, urethane, and pentobarbital. Exp. Neurol 49, 58–85 [DOI] [PubMed] [Google Scholar]
- 88.Hölscher C et al. (1997) Stimulation on the Positive Phase of Hippocampal Theta Rhythm Induces Long-Term Potentiation That Can Be Depotentiated by Stimulation on the Negative Phase in Area CA1In Vivo. J. Neurosci 17, 6470–6477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Buzsáki G (2002) Theta oscillations in the hippocampus. Neuron 33, 325–340 [DOI] [PubMed] [Google Scholar]
- 90.Huerta PT and Lisman JE (1995) Bidirectional synaptic plasticity induced by a single burst during cholinergic theta oscillation in CA1 in vitro. Neuron 15, 1053–1063 [DOI] [PubMed] [Google Scholar]
- 91.Halgren E et al. (1978) Human hippocampal formation EEG desynchronizes during attentiveness and movement. Electroencephalography and Clinical Neurophysiology 44, 778–781 [DOI] [PubMed] [Google Scholar]
- 92.Arnolds DEAT et al. (1980) The spectral properties of hippocampal EEG related to behaviour in man. Electroencephalography and Clinical Neurophysiology 50, 324–328 [DOI] [PubMed] [Google Scholar]
- 93.Caplan JB et al. (2003) Human theta oscillations related to sensorimotor integration and spatial learning. J. Neurosci 23, 4726–4736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Watrous AJ et al. (2013) A comparative study of human and rat hippocampal low-frequency oscillations during spatial navigation. Hippocampus 23, 656–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jacobs J (2014) Hippocampal theta oscillations are slower in humans than in rodents: implications for models of spatial navigation and memory. Philos. Trans. R. Soc. Lond. B Biol. Sci 369, 20130304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jacobs J and Kahana MJ (2010) Direct brain recordings fuel advances in cognitive electrophysiology. Trends Cogn. Sci 14, 162–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Roux F and Uhlhaas PJ (2014) Working memory and neural oscillations: alpha–gamma versus theta–gamma codes for distinct WM information? Trends Cogn. Sci 18, 16–25 [DOI] [PubMed] [Google Scholar]
- 98.Raghavachari S et al. (2001) Gating of human theta oscillations by a working memory task. J. Neurosci 21, 3175–3183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tesche CD and Karhu J (2000) Theta oscillations index human hippocampal activation during a working memory task. Proc. Natl. Acad. Sci. U. S. A 97, 919–924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jensen O and Lisman JE (1998) An Oscillatory Short-Term Memory Buffer Model Can Account for Data on the Sternberg Task. J. Neurosci 18, 10688–10699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cavanagh JF and Frank MJ (2014) Frontal theta as a mechanism for cognitive control. Trends Cogn. Sci 18, 414–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fiebelkorn IC and Kastner S (2019) A Rhythmic Theory of Attention. Trends Cogn. Sci 23, 87–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hasselmo ME et al. (2002) A proposed function for hippocampal theta rhythm: separate phases of encoding and retrieval enhance reversal of prior learning. Neural Comput 14, 793–817 [DOI] [PubMed] [Google Scholar]
- 104.Hasselmo ME and Stern CE (2014) Theta rhythm and the encoding and retrieval of space and time. Neuroimage 85 Pt 2, 656–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rizzuto DS et al. (2003) Reset of human neocortical oscillations during a working memory task. Proc. Natl. Acad. Sci. U. S. A 100, 7931–7936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Buzsáki G and Moser EI (2013) Memory, navigation and theta rhythm in the hippocampal-entorhinal system. Nat. Neurosci 16, 130–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Vinogradova OS (1995) Expression, control, and probable functional significance of the neuronal theta-rhythm. Progress in Neurobiology 45, 523–583 [DOI] [PubMed] [Google Scholar]
- 108.Raghavachari S et al. (2006) Theta oscillations in human cortex during a working-memory task: evidence for local generators. J. Neurophysiol 95, 1630–1638 [DOI] [PubMed] [Google Scholar]
- 109.Cantero JL et al. (2003) Sleep-dependent theta oscillations in the human hippocampus and neocortex. J. Neurosci 23, 10897–10903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Schonhaut DR, Solomon EA, Herweg NA, Phan TD & Kahana MJ (2019) , Human cortical neurons phase-lock to hippocampal theta , presented at the Society for Neuroscience, Chicago, IL, pp. Program No. 195.09. [Google Scholar]
- 111.Solomon EA et al. (2018) Medial temporal lobe functional connectivity predicts stimulation-induced theta power. Nat. Commun 9, 4437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Jacobs J et al. (2007) Brain oscillations control timing of single-neuron activity in humans. J. Neurosci 27, 3839–3844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Watrous AJ et al. (2018) Phase-tuned neuronal firing encodes human contextual representations for navigational goals. Elife 7, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rutishauser U et al. (2010) Human memory strength is predicted by theta-frequency phase-locking of single neurons. Nature 464, 903–907 [DOI] [PubMed] [Google Scholar]
- 115.Senior TJ et al. (2008) Gamma oscillatory firing reveals distinct populations of pyramidal cells in the CA1 region of the hippocampus. J. Neurosci 28, 2274–2286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lisman JE and Jensen O (2013) The θ-γ neural code. Neuron 77, 1002–1016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lega B et al. (2016) Slow-Theta-to-Gamma Phase-Amplitude Coupling in Human Hippocampus Supports the Formation of New Episodic Memories. Cereb. Cortex 26, 268–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Heusser AC et al. (2016) Episodic sequence memory is supported by a theta-gamma phase code. Nat. Neurosci 19, 1374–1380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Friese U et al. (2013) Successful memory encoding is associated with increased cross-frequency coupling between frontal theta and posterior gamma oscillations in human scalp-recorded EEG. Neuroimage 66, 642–647 [DOI] [PubMed] [Google Scholar]
- 120.Klimesch W et al. (1997) Theta synchronization and alpha desynchronization in a memory task. Psychophysiology 34, 169–176 [DOI] [PubMed] [Google Scholar]
- 121.Meeuwissen EB et al. (2011) Increase in posterior alpha activity during rehearsal predicts successful long-term memory formation of word sequences. Hum. Brain Mapp 32, 2045–2053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Salvidegoitia MP et al. (2019) Out and about: Subsequent memory effect captured in a natural outdoor environment with smartphone EEG. Psychophysiology 56, e13331. [DOI] [PubMed] [Google Scholar]
- 123.Khader PH and Rösler F (2011) EEG power changes reflect distinct mechanisms during long-term memory retrieval. Psychophysiology 48, 362–369 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


