Abstract
The genus Viburnum (Adoxaceae, Dipsacales) is of scientific interest due to the chemical components and diverse biological activities found across species of the genus, which includes more than 230 species of evergreen, semievergreen, or deciduous shrubs and small trees. Although frequently used as an ornament, the Viburnum species show biological properties with health-promoting effects. Fruits, flowers, and barks of certain species are used for pharmaceutical purposes or as cooking ingredients, hence containing biochemical compounds with health-promoting activity such are carotenoids, polyphenols, and flavonoids. However, its taxonomical determination is difficult, due to its wide distribution and frequent hybridizations; therefore, an objective classification would allow us to understand its biological activity based on its phytochemical components. More than sixty phytochemical compounds have been reported, where vibsanin-type diterpenes and their derivatives are the most prevalent. Leaves and twigs of V. dilatatum contain the largest number of phytochemicals among the genus. Through preclinical evidence, this study provides insight regarding antioxidant, antibacterial, anti-inflammatory, cytotoxic, and anticancer activities of genus Viburnum.
1. Introduction
The genus Viburnum (Adoxaceae, Dipsacales) is comprised of more than 230 species of evergreen, semievergreen, or deciduous shrubs and small trees distributed primarily within the temperate forest regions of the northern hemisphere but also in the mountains of Central and South America, Southeast Asia (Philippines, Malaysia), and southeastern Australia and Tasmania.
Although the species of the genus are generally well adapted to mesic forest environments, particular species have been reported to inhabit both, cold boreal forests, and tropical rainforests, as is the case of Viburnum edule and Viburnum amplificatum, respectively. The regions of highest species diversity are eastern Asia and Latin America [1–4]. Viburnum was established by Linnaeus, classified in the Caprifoliaceae family [5] and soon after reclassified in its own family Viburnaceae [6]. Based on the Dipsacales phylogeny research, Viburnum was subsequently listed in Adoxaceae, together with Adoxa, Sinoadoxa, Tetradoxa, and Sambucus [7] This classification is widely accepted today [1, 4, 8]. Nevertheless, certain plant classification systems, e.g., the one from Takhtajan [9], still recognize Viburnum as a member of the Viburnaceae family.
Leaves of the Viburnum species are simple, petiolate, and opposite and rarely whorled. The small hermaphroditic flowers form paniculate or umbellate inflorescences of 15-500 flowers each. Marginal flowers are sometimes sterile, and their purpose is to attract pollinators. The plants are self-incompatible. The androecium consists of five stamens. Filament bases are attached to corolla petals. The gynoecium is of three carpels. Two of the three inferior ovaries are aborted. The fruit is a red to purple black single-seeded drupe. Floral nectaries located at the gynoecium apex additionally differentiate viburnums from another Adoxaceae [10–14].
Taxonomical determination of the genus Viburnum is difficult, due to its wide distribution and frequent hybridizations, both natural and horticultural [15, 16]. Traditionally accepted classification is based on plant morphology (inflorescences, flowers, extrafloral nectaries, trichomes, and pollen grain exine) and recognizes the sections Solenotinus, Viburnum, Pseudotinus, Tomentosa, Tinus, Megalotinus, Lentago, Oreinotinus, Odontotinus, and Opulus Opulus ([17, 18]); however, several mostly regional-oriented section level revisions were made ([3] and references therein). Besides, plant morphology, taxonomical values of the phytochemical amentoflavone isolated from Viburnum leaves and branchlets [19], as well as anatomical characteristics of fruits, cork, assimilating parenchyma, and leaf epidermal cells [20, 21], were also assessed. More recently, assessments of Viburnum diversification have been made at a molecular level [3, 4, 8, 22, 23]. Clement et al. [24] proposed a Viburnum phylogenetic classification and provided formal phylogenetic definitions for 30 clades.
Many viburnums are of high ornamental value. Fruits, flowers, and barks of certain species are used for pharmaceutical purposes or as cooking ingredients, hence containing biochemical compounds with health-promoting activity such as carotenoids, polyphenols, and flavonoids. Many viburnums are of high ornamental value. Fruits, flowers, and barks of certain species are used for pharmaceutical purposes or as cooking ingredients, hence containing biochemical compounds with health-promoting activity such are carotenoids, polyphenols, and flavonoids. These biochemical components are responsible for the main biological activities of Viburnum plants, and although the antioxidant, antibacterial, anti-inflammatory, and cytotoxic activity have been well documented, some current research also links to the chemical components found in Viburnum exerting protection and treatment against diseases. Chronic diseases including diabetes [25], cancer [26], Alzheimer's [27], and all diseases that can be caused by a clot [28].
Substantial research has been carried out in relation to the mechanisms responsible for the synthesis, location, and accumulation of bioactive compounds. The most widely researched species are V. opulus, tinus, lantana, and orientale; although, various biochemical constituents have also been isolated from V. arboricolum, awabuki, ayavacense, betulifolium, cylindricum, davidii, dilatatum, erosum, furcatum, grandifolium, japonicum, jucundum, luzonicum, odoratissimum, phlebotrichum, pichinchense, prunifolium, rhytidophyllum, suspensum, urceolatum, and wrightii [21, 29–31]. Studies undertaken to assess within-species variability in terms of bioactive compounds profile and content [32–34] facilitate breeding and subsequent growing of viburnums as pharmaceutical crops.
2. Traditional Uses
Plants and humans are in a forever codependent relationship. Plants are considered the lungs of the earth but also provide food, shelter, timber, medicines, etc. for humans. Medicinal plants represent a rich reservoir of bioactive chemicals of therapeutic potential [35]. A review of the scientific literature reveals multiple applications from medicinal plants of the genus Viburnum (Table 1), implying the need for further research and documentation.
Table 1.
Ethnomedical use of genus Viburnum.
| S. no. | Species name | Part used | Medicinal applications | References |
|---|---|---|---|---|
| 1 | Viburnum grandiflorum | Wood, leaf, flower, bark | Digestive problems, purgative, abdominal pain, diuretic, antimalarial, respiratory diseases, toothaches, yphoid, whooping cough, anesthetic | ([35]; [36]; [37]; [38]; [39]) |
| 2 | Viburnum opulus | Fruit | Gall bladder, liver disease, diuretic; bleeding, heart disease, blood pressure, coughs, cold, neurosis, diabetes | ([40]; [41]; [42]; [43]; [44]; [45]; [34]) |
| 3 | Viburnum cylindricum | Leaves | Cough, diarrhea, rheumatoid arthritis, and tumefaction Cough, diarrhea, rheumatoid arthritis, and tumefaction Cough, diarrhea, rheumatoid arthritis, tumefaction, insecticide |
([46]; [30]; [47]) |
| 4 | Viburnum dilatatum | Fruits | Spice, pickles | [48] |
| 5 | Viburnum cotinifolium | Bark | Hepatic and digestive problems | [47] |
| 6 | Viburnum erubescens | Roots, stem, leaves | Cough, insecticide | ([49]; [47]) |
| 7 | Viburnum lantana | Bark | Rubefiant, analgesic | [41] |
| 8 | Viburnum foetens | Whole plant, bark | Purgative, sedative, cleaning teeth “miswak” | [50] |
| 9 | Viburnum punctatum | — | Fever, stomach disorders | [51] |
| 10 | Viburnum prunifolium | Roots | Dysmenorrhea, menstrual irregularities, convulsions, hysteria, fever, palpitation, heart diseases, hysterical fits, arthritis, heart tonic, improves blood circulation | ([52]; [51]) |
| 11 | Viburnum prunifolium | — | Sedatives, muscle relaxants, cardiotonics | [53] |
| 12 | Viburnum nervosum | Leaf, bark, root | Purification of blood, carminative, hemorrhage, uterine disorders, asthma, furunculosis, menorrhagia | ([54]; [55]; [52]) |
| 13 | Viburnum coriaceum | Root and bark | Antispasmodic, uterine relaxant | ([56]; [52]) |
| 14 | Viburnum foetidum | Leaves, aerial parts | Menorrhagia, hypothermic, cardiovascular; uterine disorders, skin disease, emmenetic | ([52]; [57]) |
| 15 | Viburnum jucundum | — | Cancer, gastrointestinal diseases | ([52]; [58]) |
3. Phytoconstituents
Although Viburnum initially diversified in East Asia, other regions such as eastern North America, the mountainous regions of Mexico, Central America, and northern South America are also viewed as centers of high diversity. Most of the American Viburnum species seem to have derived from Asian clades, while the species in Latin America appear to be directly related to those in the eastern United States [59]. In this way, the genus represents a classic pattern of disjunct distribution between the Old and the New World.
Roughly 200 species have been reported for the genus Viburnum, among shrubs and trees, distributed mainly on the Asian side, used primarily in traditional medicine for the treatment of diseases such as rheumatoid arthritis, cough, diarrhea, tumefaction, swelling, kidney cramps [60], antitumor, antimicrobial, antioxidant, antihyperglycemic, anti-inflammatory, and neuroprotective activities [61]. Leaves, flowers, and fruits are currently being used in Turkish folk medicine [62].
The study of Viburnum is of scientific interest due to the chemical components and diverse biological activities found across species of the genus. Although the highest number of chemical compounds has been found in leaves, the presence of phytochemicals has also been reported in fruits, roots, and seeds (Table 2). V. awabuki, V. dilatatum, V. fordiae Hance, V. odoratissimum, and V. opulus have the highest amount of extracted phytochemical constituents (diterpenes, triterpenes, iridioides, monoterpenes, sesquiterpenes, flavonoids, lignans, etc.) and are dependent on the specific part of the plant from which the extract is obtained, as well as the type of solvent used.
Table 2.
Main phytochemicals found in the species of the genus Viburnum.
| Viburnum spp. | Phytoconstituents | Type of extract | Part of plant | Biological activities | References |
|---|---|---|---|---|---|
| V. arboricolum | Viburolide | — | Leaves and twigs | Antitumor effects | [31] |
| V. ayavacense | 7,10,2′,3′-Tetraacetylsuspensolide F, 7,10,2′,3′-tetraacetylisosuspensolide F, 7,10,2′,6′-tetraacetylisosuspensolide F, 2′,3′-diacetylvalerosidate, 2′,3′-diacetylisovalerosidate, isoviburtinoside II, isoviburtinoside III, isosuspensolide E, isosuspensolide F. | — | Leaves and twigs | Antitumor effects | [31] |
| V. awabuki | 6-O-methyl-6,7-dihydroxyvibsanin B, 4-hydroxyvibsanin A, 14(R∗),15-epoxyneovibsanin B, 14(S∗),15-epoxyneovibsanin B, (8Z)-neovibsanin B, 18-O-methylvibsanin C, (8Z)-vibsanin E. | — | Leaves | — | [29] |
| Vibsanin G, vibsanin H, vibsanin K, vibsanin O, vibsanin P, vibsanin Q, vibsanin R, vibsanin S, vibsanin T, vibsanin U, vibsanin V, vibsanin W, furanovibsanin A, furanovibsanin B, furanovibsanin C, furanovibsanin D, furanovibsanin E, furanovibsanin F, furanovibsanin G, neovibsanin A, neovibsanin B, neovibsanin C, neovibsanin D, neovibsanin G, neovibsanin H, neovibsanin I, spirovibsanin A, 7-epineovibsanin D, 3-O-methylfuranovibsanin A, 7-epifuranovibsanin B, 15,18-di-O-methylvibsanin H, 18-O-methylvibsanin K, cyclovibsanin A, 15-O-methylcyclovibsanin A, 15-O-methylcyclovibsanin B, 3-hydroxy-15-O-methylcyclovibsanin A, 15-O-methylneovibsanin F, 15-O-methyl-14-epineovibsanin F, 15-O-methyl-18-oxoneovibsanin F, 2-O-methylneovibsanin H, 2-O-methylneovibsanin I, 14-epineovibsanin G, 5-epivibsanin C, 5-epivibsanin E, 5-epivibsanin H, 5-epivibsanin K, 18-O-methyl-5-epivibsanin K, 3-hydroxyvibsanin E, 3b,28-dihydroxyolean-12-en-1-one, 3b,28-dihydroxyolean-12-en-11-one, 13,28-epoxyolean-11-en-3-one, 6a-hydroxy-3-oxolup-20(29)-en-28-oic acid, Ψ-taraxasterol acetate, 6b-hydroxy-3,20-dioxo-30-norlupan-28-oic acid, 4,20-dihydroxy-3,4-secolupane 3,28-dioic acid 3-methyl ester, awabukinol, 3-hydroperoxyawabukinol, 4-hydroperoxyawabukinol, epicatechin, catechin, 7R-dihydrodehydrodiconferyl alcohol 4-O-β-D-glucopyranoside, 8R-dihydrodehydrodiconferyl alcohol 4-O-β-D-glucopyranoside, vibsanol, 9′-O-methylvibsanol, dihydrodehydrodiconiferyl alcohol, 3′,6'′-O-diacetylscopolin, 2′-O-acetylscopolin, 6′-O-acetylscopolin | — | Leaves and twigs | — | [31] | |
| V. betulifolium | Viburnalloside, decapetaloside | — | Leaves and twigs | Antitumor effects | [31] |
| V. chinshanence | Lignan, chinshanol A. | — | Roots | — | [63] |
| V. coriaceum | Phytosterols, triterpenoids, phenolics, phenolic glycosides | — | Roots | — | [64] |
| V. cotinifolium | Biflavonoid | Petrol, benzene and methanol | Leaves | — | [65] |
| V. cylindricum | 3-O-Caffeoylquinic acid methyl ester, 4-O-caffeoylquinic acid methyl ester, 5-O-caffeoylquinic acid methyl ester | — | Leaves and twigs | Antitumor effects | [31] |
| 2′-O-Acetylhenryoside, 2',3'-di-O-acetylhenryoside, 2′,6′-di-O-acetylhenryoside, 2′,3′,6′-tri-O-acetylhenryoside, 2′,3′,4′,6′-tetra-O-acetylhenryoside, 2-[(2,3-di-O-acetyl-beta-D-glucopyranosyl)oxy]-6-hydroxybenzoic acid, 6-hydroxy-2-[(2,3,4,6-tetra-O-acetyl-beta-D-glucopyranosyl)oxy]benzoic acid | Methanolic extract | Leaves and stems | — | [66] | |
| V. dilatatum | Viburnol A, viburnol B, viburnol C, viburnol D, viburnol E, viburnol F, viburnol G, viburnol H, viburnol I, viburnol J, viburnol K, viburnudienone B1 methyl ester, viburnudienone B2 methyl ester, viburnenone B1 methyl ester, viburnenone B2 methyl ester, viburnudienone H1, viburnudienone H2, 2,3,4-trihydroxybutyl 6-O-(E)-caffeoyl- β-D-glucopyranoside, 2,3,4,5-tetrahydroxyhexyl 6-O-(E)-caffeoyl-β-D-glucopyranoside, arbutin, furcatin, 4-allyl-2-methoxyphenyl 6-O-β-D-apiofuranosyl(1 → 6)-O-β-D-glucopyranoside, p-hydroxyphenyl 6-O-(E)-caffeoyl- β-D-glucopyranoside, p-hydroxyphenyl 6-O-(E)-caffeoyl- β-D -allopyranoside, salidroside, 3-O-caffeoylquinic acid, 4-O-caffeoylquinic acid, dilaspirolactone, kuromanin | — | Leaves and twigs | — | [31] |
| Jiamizioside E, jiamizioside A, jiamizioside B, jiamizioside C, jiamizioside D | — | Fruits | — | ([67]; [68]) | |
| Cyanidin 3-sambubioside, 5-caffeoyl quinic acid | — | Fruits | — | [69] | |
| Cyanidin 3-sambubioside, cyanidin 3-glucoside, quercetin, 5-O-caffeoyl-4-methoxyl quinic acid, chlorogenic acid. | — | Fruits | — | [3] | |
| Cyanidin 3-sambubioside, cyanidin 3-glucoside, 4-methoxy chlorogenic acid, chlorogenic acid, quercetin | — | Fruits | — | [70] | |
| 3Z-Hexenol, l-linalool | — | Flower | — | [71] | |
| 2-(-Glucopyranosyloxy)-benzyl 3-(-glucopyranosyloxy)-benzoate | — | Roots | — | [72] | |
| V. erosum | 7-O-Tigloylsecologanolic acid, 7-ketologanin, 7-O-benzoylsecologanolic acid, 7-ketologanin | Methanolic, ethyl acetate, n-butanol, water | Stems | — | [73] |
| Vibruresinol, (70 R,8S,80 S)-3,50-dimethoxy-30,4,80,90 -tetrahydroxy70,9-epoxy-8,80–lignan, (+)-syringaresinol, (+)-pinoresinol, (+)-pinoresinol-4-O-β-D-glucopyranoside, herpetol, vibsanol, (-)-dehydrodiconiferyl alcohol, icariside E4, (-)-dihydrodehydrodiconiferyl alcohol | Methanolic extract | Stems | Neuroprotective activity on glutamate-induced cell death in HT22 cells | [74] | |
| Loganic acid, sweroside, 7-O-tigloylsecologanol, 3,7-dihydroxy-8-methyl-cyclopenta[c] pyran-4-carboxylic acid, rel-(1S,5R,9S)-9-ethenyl-1-(beta-D-glucopyrinosyloxy)-5,9-dihydro-5-{2-[(2-methylbut-2-enoyl)oxy]ethyl}-1H-pyran-4-carboxylic acid, viburnin, epi-7-O-tigloylsecologanolic acid. | Methanolic extract | Stems | — | [75] | |
| V. erubescens | Phytosterols, triterpenoids, and phenolic compounds and their glycosides | — | Roots | — | [64] |
| Phytosterols, triterpenoids, glycosides (saponins), phenolic compounds (flavonoids and procyanidins) | Soxhlet method | Leaves and stems | — | [76] | |
| V. fordiae Hance | (7S,8R)-4-Hydroxy-3,3′,5′-trimethoxy-8′,9′-dinor-8,4′-oxyneolignan-7,7′,9-triol, (7R,8R)-4-hydroxy-3,3',5'-trimethoxy-8′,9′-dinor-8,4′-oxyneolignan-7,7′,9-triol, (7R,8R)-4-hydroxy-3,3′,5′-trimethoxy-8,4′-oxyneolignan-7,9,9′-triol-7′-one, γ-lactone, 3-(3,4-dihydroxyphenyl)-4-pentanolide, uvaol, 28-nor-urs-12-ene-3b,17b-diol, 2,3-O-isopropylidenyl-2a,3a,19a-trihydroxyurs-12- en-28-oic acid, erythrodiol, oleanolic acid, lupeol, megastigmadien-3,9-dione, loliolide, dehydrololiolide, 2a-hydroxycineole, (+)-isolariciresinol, umbelliferone, 3-(4- hydroxy-3-methoxyphenyl)propane-1,2-diol, 1-(4-hydroxy-3-methoxyphenyl)-1- methoxypropan-2-ol, coniferyl aldehyde, p-hydroxylcinnamaldehyde, (+)-2-hydroxy-1-(4-hydroxy-3- methoxypheny) propan-1-one, syringaldehyde, protocatechuate, 3,4-dihydroxybenzoic acid methyl ester, vanillin, p-hydroxybenzaldehyde, salicylic acid, benzyl alcohol, hydroquinone | — | Aerial parts | — | ([77]; [78]) |
| 7,8-bis-O-Isopropylidene-dihydroeugenol | — | Air-dried plants | — | [79] | |
| Fordioside, alangilignoside D, salicin, rhapontigenin | — | Leaves | — | [67] | |
| C-13-Norisoprenoid, alangionoside C, pisumionoside, koaburaside, 3,5-dimethoxy-benzyl alcohol 4-O-β-D-glucopyranoside, 3,4,5-trimethoxybenzyl- β-D -glucopyranoside, arbutin, salidroside, (3R,9R)-3-hydroxy-7,8-didehydro-β-ionyl 9-O-α-D-arabinopyranosyl-(1 → 6)-β-D-glucopyranoside, 2-(4-O-β-D-glucopyranosyl) syringylpropane-1,3-diol | — | Stems | — | [80] | |
| Norneolignan glycoside, 7-noraryl-4′,7-epoxy-8,5′-neolignan glycoside, (7R,8R)-guaiacylglycerol 4-O-β-D -(6-O-vanilloyl) glucopyranoside, (7S,8S)-guaiacylglycerol 4-O- β-D-(6-O-vanilloyl) glucopyranoside, (7S,8R)-guaiacylglycerol 4-O-β-D-(6-O-vanilloyl) glucopyranoside, coniferyl alcohol 4-O-[6-O- (4-O-β-D-glucopyranosyl)vanilloyl]-β-D-glucopyranoside | — | Stems | — | ([77]; [78]) | |
| Viburfordoside A, viburfordoside B, viburfordoside C, viburfordoside D, viburfordoside E, viburfordoside F, viburfordoside G, viburfordoside H, viburfordoside I. Fordiane A, fordiane B |
— | Fruits | — | [81] | |
| V. formosanum | Dioxatricyclodecane | Methanolic extract, ethyl acetate | Leaves | — | [82] |
| V. furcatum | Furcatoside A, furcatoside B, furcatoside C, isoquercitroside, kaempferol 3-O-b-d-glucopyranosyl-7-O-a-l-rhamnoside, furcatin, | — | Leaves and twigs | Antitumor effects | [31] |
| V. grandifolium | Luteolin, 3′-O-b-d-xylopyranosyl (1 → 2)-O-b-d-glucopyranoside | — | Leaves and twigs | Antitumor effects | [31] |
| V. japonicum | 2′,3′-O-Diacetylfurcatoside C, chavicol | — | Leaves and twigs | Antitumor effects | [31] |
| V. lantana | 2′-Acetyldihydropenstemide, 2′-acetylpatrinoside, 3′-acetylpatrinoside, lantanoside, dihydropenstemide, betulalbuside A. | — | Leaves and twigs | Antitumor effects | [31] |
| 2-Heptanone, n-heptanal, benzaldehyde, 1-octen-3-ol, 6-methyl-5-hepten-2-one, 2-pentylfuran, 2, 4 heptadienal, n-octanal, limonene, n-octanol, cis-linalool oxide, L-linalool, n-nonanal, α-terpineol, methyl salicylate, n-decanal, 2E, 4E-nonadienal, 2E-decanal, cinnamaldehyde, 2E, 4Z-decadienal, 2E, 4E-decadienal, α-cubebene, α-copaene, β-bourbonene, E-caryophyllene, β-copaene, geranyl acetone, γ-murolene, α-amorphene, germacrene D, β-ionone, α-muurolene, γ-cadinene, Δ-cadinene, trans-cadina-1, 4-diene, α-calacorene, occidentalol, E-nerolidol, spathulenol, caryophyllene oxide, salvial-4(14)-en-1-one, γ-eudesmol, α-muurolol, β-eudesmol, α-cadinol, occidenol, eudesma-4(15,7-dien-1-β-ol, pentadecanal, 6, 10, 14-trimethyl-2-pentadecanone, nonadecane, n-heneicosane, n-docosane, n-tricosane | Hydrodistillation | Air-dried whole plants | Antimicrobial activity | [83] | |
| V. luzonicum | Luzonial A, luzonial B, luzonoside A, luzonoside B, luzonidial A, luzonidial B, luzonoside C, luzonoside D, luzonoid A, luzonoid B, luzonoid C, luzonoid D, luzonoid E, luzonoid F, luzonoid G, | — | Leaves and twigs | Antitumor effects | [31] |
| V. macrocephalum | Methyl (2-α-L-rhamnopyranosyloxy)acetate, methyl (2R-3-α-L-rhamnopyranosyloxy)glycerate, methyl (3R-4-α-L-rhamnopyranosyloxy-3-hydroxy)butanoate, bridelionoside B (4), (6S,7E,9R)-roseoside, linarionoside A, 3,7,11-trimethyl-1,6-dodecadien-3,10,11-triol, (+)-8-hydroxylinalool, beta-sitosterol and daucosterol | Ethanolic extract | Branch | Insecticidal and antimicrobial activities | [61] |
| Apigenin-7-O-[6-O-(5-methoxy-3-hydroxy-3-methyl-5-oxovaleryl)]-beta-D-glucopyranoside, kaempferol-3-O-(6″-O-acetyl)- β-D-glucopyranoside, kaempferol-3-O-(6″-O-crotonyl)- β-D-glucopyranoside, kaempferol 4′-O-α-L-rhamnopyranoside, (+)-naringenin-7-O-β-D-glucopyranoside, (–)-naringenin-7-O-β-D-glucopyranoside, afzelin, apigenin-7-O-β-D-glucopyranoside | Ethanolic extract | Branch | — | [84] | |
| V. melanocarpum | (7R,8S)-Guaiacylglycerol4-O-β-D-(6-O-vanilloyl) glucopyranoside. | Ethanolic extract | Fruits | Intestinal alpha-glucosidase inhibitory activity | [85] |
| (−)-(7R,7′R,8S,8′S)-Pinoresinol 4′-O-β-dglucopyranosyl-4-O-(6-O-vanilloyl)-β-d-glucopyranoside, (7′E,7S,8R)-7,9,9′-trihydroxy-3,3′,5′-trimethoxy-8-O-4′-neolignan-4-O-[6-O-(4-O-β-D-glucopyranosyl)vanilloyl]-β-d-glucopyranoside, pinoresinol 4,4′-O-β-dglucopyranoside, pinoresinol 4′-O-β-d-glucoside, syringaresinol 4′-O-β-d-glucopyranoside, pinoresinol 4-O-β-d-glucopyranosyl-(1→6)-β-d-glucopyranoside | Ethanolic extract | Stems | Inhibitory activity against alpha-glucosidase | [86] | |
| (7R,8S)-Syringylglycerol 8-O--D-allopyranoside, (7S,8S)-syringylglycerol 8-O--D-allopyranoside. | Ethanolic extract | Stems | Radical scavenging and glucosidase inhibitory activities | [87] | |
| V. odoratissimum | Vibsane, vibsanol I, 15-hydroperoxyvibsanol A, 14-hydroperoxyvibsanol B, 15-O-methylvibsanin U, 5,6-dihydrovibsanin B, 14,18-O-diacetyl-15-O-methylvibsanin U, vibsanin K | — | Leaves | — | [88] |
| Vibsane, vibsanin B, vibsanin F, neovibsanin B, neovibsanin | — | — | — | [89] | |
| Vibsanin I, vibsanin L, 14-hydroxyvibsanin F, 14R∗,15-epoxyvibsanin C, 14S∗,15-epoxyvibsanin C | — | Leaves | — | [90] | |
| Vibsanol C, vibsanol D, vibsanol E, vibsanol F, vibsanol G, vibsanol H, vibsanin X | — | Leaves and twigs | — | [91] | |
| Vibsanin A, vibsanin B, vibsanin C, vibsanin D, vibsanin E, vibsanin F, vibsanin I, vibsanin L, vibsanin M, aldovibsanin A, aldovibsanin B, aldovibsanin C, 7-epialdovibsanin A, 5-epivibsanin G, 18-O-methylvibsanin G, 14-hydroxyvibsanin F, (14R∗)-14,15-epoxyvibsanin C, (14S∗)-14,15-epoxyvibsanin C, vibsanol A, vibsanol B, 6β-hydroxy-3-oxolup-20(29)-ene-27,28-dioic acid, 6α-hydroxy-3-oxolup-20(29)-ene-27,28-dioic acid, quercetine. | — | Leaves and twigs | — | [31] | |
| Vibsanin C, vibsanin H, dehydrovibsanin G, vibsanol, 9-aldehydevibsanol, (+)-9′-O-senecioyllariciresinol, (8Z)-10-epi-vibsanin C, (+)-9′-O-isovaleryllariciresinol | — | Leaves and branch | — | [92] | |
| 5-epi-Vibsanin G, 18-O-methylvibsanin G, vibsanin M, aldovibsanin C | — | Leaves and flowers | — | [93] | |
| Vibsanin B, vibsanin E, vibsanol A, vibsanol B, 6β-hydroxylup-20(29)-en-3-oxo-27,28-dioic acid, 6α-hydroxylup-20-(29)-en-3-oxo-27,28-dioic acid, 6α-hydroxylup-20(29)-en-3-oxo-28-oic acid | — | Leaves and flowers | — | [94] | |
| Benzaldehyde, exo-2-methylnorbornane, cis-linalool oxide (furanoid), linalool, nonanal, isophorone, 4-oxoisophorone, trans-linalool oxide (pyranoid), methyl salicylate, decanal, methyl nonanoate, eucarvone, 1-[2-(1-hydroxy-1-methylethyl)cyclopropyl]-ethanone, nonanoic acid, methyl geranate, methyl o-anisate, α-ionone, geranyl acetone, pentadecane, hexadecane, β-eudesmol, heptadecane, methyl eudesmate, octadecane, hexahydrofarnesyl acetone, phthalic acid, decyl isobutyl ester, methyl palmitate, methyl linoleate, methyl linolenate, heneicosane, docosane | — | Flowers | — | [95] | |
| β-Amyrin, α-amyrin, stigmasta-4-en-3-one, ergosta-4,6,8(14),22-tetraen-3-one, Olean-12-en-3-one, lupeol, 3-hydroxyolean-12-en-1-one, 3-acetoxyolean-12-en-28-ol, 3-acetoxyolean-12-en-28-oic acid, 3,28-dihydroxyolean-12-ene, 3,28-dihydroxyurs-12-ene, 28-hydroxyolean-12-en-3-one, trans-phytol, betulin | — | Roots | — | [96] | |
| V. opulus | Ascorbic acid, total phenolics, total anthocyanin | — | Leaves | — | [97] |
| Methyl pentanoate, 3Z -Hexen-1-ol, n-heptanal, 2-pentylfuran, phenyl acetaldehyde, linalool oxide, terpinolene, L-linalool, n-nonanal, 2E, 6Z-nonadienal, 4-terpineol, α-terpineol, methyl salicylate, myrtenol, n-decanal, trans-carveol, geraniol, 2E-decanal, 2E, 4Z–decadienal, 2E, 4E-decadienal, α-copaene, rans-β-damascenone, trans-α-ambrinol, α-amorphene, germacrene D, β-ionone, γ-cadinene, Δ-cadinene, trans-cadina-1, 4-diene, α-calacorene, ledol, tetradecanal, α-muurolol, α-cadinol, pentadecanal, manool, n-heneicosane, phytol, n-docosane, n-tricosane | — | Air-dried whole plants | — | [83] | |
| Chlorogenic acid | — | Fruits | — | [98] | |
| Gallic acid, procyanidin B1, (+)-catechin, procyanidin B2, (−)-epicatechin, neochlorogenic acid, chlorogenic acid, rutin, isorhamnetin, isorhamnetin 3-O-rutinoside, quercetin, anthocyanins, cyanidin-3-O-sambubioside, cyanidin-3-O-glucoside, cyanidin-3-O-rutinoside | — | Fruits | [99] | ||
| Coumaroyl-quinic acid, chlorogenic acid dimer, procyanidin B2, catechin, procyanidin trimer epicatechin, proanthocyanidin dimer monoglycoside, quercetin-hexose + pentose, rutin, quercetin-hexose, quercetin-deoxyhexose | — | Fruits | — | [100] | |
| Quinic acid, catechin dimer, catechin, chlorogenic acid (3-O-caffeoylquinic acid), procyanidin C1, epicatechin, neochlorogenic acid (5-O-caffeoylquinic acid) | — | Fruits | — | [101] | |
| Ethyl alcohol, 1-propano, 2-butanone, acetic acid, ethyl acetate, isobutanol, 2-pentanone, 3-methyl-1-butano, 2-methyl-1-butanol, 1-pentanol, 2-hexanone, 2-hexanol, hexanal, 3-methyl-butanoic acid, 2-methyl-butanoic acid, 3-hexen-1-ol (Z), 1-hexanol, 2-heptanone, 2-heptanol, heptanal, 3-methyl-pentanoic acid, 1-heptanol, 1-octen-3-ol, 6-methyl-5-hepten-2-one, 2-octanone, ethyl hexanoate, 2-octano, octanal, hexyl acetate, a-terpinene, p-cymene, limonene, 1,8-cineole, trans-linalool oxide (furanoid), 2-nonanone, linalool L, nonanal, dill ether, α-terpineol, ethyl decanoate, β-caryophyllene (E) | — | Fruits | — | [101] | |
| Gallic acid, ascorbic acid, vitamin C | — | Fruits | — | [102] | |
| L-Malic acid, L-ascorbic acid, oxalic acid | — | Fruits | — | [103] | |
| Chlorogenic acid, (+)-catechin, (-)-epicatechin, cyanidin-3-glucoside, cyanidin-3-rutinoside and six different glucosides of quercetin. | — | Fruits | — | [104] | |
| Chlorogenic acid, oxalic acid, citric acid, tartaric acid, malic acid, quinic acid, succinic acid, fumaric acid, procyanidin B2, (−)-epicatechin, p-coumaric acid, isorhamnetin 3-O-rutinoside, isorhamnetin 3-O-glucoside, quercetin 3-O-glucoside. | — | Fruits, flowers, and bark | — | [34] | |
| β-Sitosterol, stigmasterol, colesterol, α-amyrin-urs-12-en-3-β-ol, β-amyrin-Olean-12-en-3-β-ol, lupeol, 3-keto-urs-12-ene, 3-keto-Olean-12-ene, A:D-neoolean-12,14-diene, A-neoolean-5,12-diene | — | Seeds | — | [105] | |
| V. orientale | Chlorogenic acid. | Methanolic extract and aqueous | Fruit, leaf, and branch | Enzyme inhibitory and antioxidant effect | [106] |
| Betulalbuside A, anatolioside E, betulalbuside B, anatolioside, anatolioside A, anatolioside B, anatolioside C, anatolioside D, | — | Leaves and twigs | Antitumor effects | [31] | |
| V. phlebotrichum | Phlebotrichin, p-hydroquinone, arbutin, | — | Leaves and twigs | Antitumor effects | [31] |
| V. plicatum | Dideoxyplicatumoside A, erythro-syringylglycerol-β-O-4′-(+)-isoeucommin A 4″-O-β-D-glucopyranoside, | — | Leaves | — | [107] |
| Plicatumoside A, (+)-neomedioresinol 4,4′-di-O-β-D-glucopyranoside, (+)-neomedioresinol 4,40-O-di-β-D-glucopyranoside | — | Leaves | — | [108] | |
| 7-O-Tigloylsecologanol, 7-O-tigloylsecologanolic anolic acid, 3′-O-[(2S)-2-methylbutanoyl]henryoside, (4R)-α-terpineol O-β-D-glucopyranoside2), (7S,8R)-dihydrodehydrodiconiferyl alcohol 9-O-β-d-glucopyranoside2), (7R,8S)-dihydrodehydrodiconiferyl alcohol 9-O-β-d-glucopyranoside2), quercetin 3-O-robinobioside2), quercetin 3-O-rutinoside2), kaempferol 3-O-robinobioside2), kaempferol 3-orutinoside2). | Methanolic extract, chloroform, ethyl acetate, butanol, water | Leaves | — | [109] | |
| V. propinquum | (3,4,2′,4′-tetrahydroxy-trans-chalcone), (3,4,2′,4′-tetrahydroxy-trans-chalcone-2′-O-β-D-glucoside), quercetin, (+)-dihydroquercetin, eriodictyol, taraxerol, β-sitosterol, stigmasterol, 3β,28-dihydroxy-12-ursene, ursolic acid, daucosterol, 4,2′,4′-trihydroxy-dihydrochalcone, 4,2′,4′-trihydroxy-dihydrochalcone-2′-O-β-D-glucoside. | — | Leaves and stems | Antioxidant activity | [110] |
| V. prunifolium | Scopoletin | — | Haw | Antispasmodics | [111] |
| 2′-Acetyldihydropenstemide, 2′-acetylpatrinoside, patrinoside, 2′-(E)-p-coumaroyldihydropenstemide | — | Leaves and twigs | Antitumor effects | [31] | |
| V. punctatum | Phytosterols, triterpenoids, and phenolic compounds and their glycosides | — | Roots | — | [64] |
| V. rhytidophyllum | Ursolic acid, 7,10,2′-triacetylpatrinoside, 7-p-coumaroylpatrinoside, 10-acetylpatrinoside, catechin, arbutin, henryoside, salicin, viburnine | — | Leaves and twigs | Antitumor effects | [31] |
| V. sargentii | (-)-Epicatechin, 5,7,4-trihydroxy-flavonoid-8-C--d-glucopyranoside, 1-(4-hydroxy-3-methoxyphenyl)-2-[4-(3--l-rhamnopyranoxypropyl)-2-methoxyphenoxy]-1,3-propane-diol (erythro), 1-(4-hydroxy-3-methoxyphenyl)-2-[4-(3--l-rhamnopyranoxypropyl)-2-methoxyphenoxy]-1,3-propanediol (threo), (R)-4-hydroxylphenol O-(6-O-oleuropeoyl)--d-glucopyranoside, (R)-3-methoxy-4-hydroxylphenolO-(6-O-oleuropeoyl)--d-glucopyranoside, quercetin-3-O-rutinoside. | Ethanolic extract and aqueous | Fruits | Antioxidant activity | [112] |
| V. suspensum | Neovibsanin, vibsanins B, vibsanins F, vibsanin F, neovibsanin B. | — | — | — | [89] |
| Neovibsanin F, gomojoside A, gomojoside B, gomojoside C, gomojoside D, gomojoside E, gomojoside F, gomojoside G, gomojoside H, gomojoside I, gomojoside J, gomojoside K, gomojoside L, gomojoside M, gomojoside N, gomojoside O, gomojoside P, gomojoside Q, 3-oxooleana-11,13(18)-dien-28-oic acid, 24-hydroxy-3-oxooleana-11,13(18)-dien-28-oic acid, 6β-hydroxy-3-oxooleana-11,13(18)-dien-28-oic acid, 2′,6′-O-diacetylscopolin. | — | Leaves and twigs | Antitumor effects | [31] | |
| V. tinus | 3-O-β-D-Galactopyranosyl-(1 → 2)-O-β-D-glucuronopyranosideoleanolic acid 28-O-β-D-glucopyranosyl ester, 3-O-(β-D-glucuronopyranosyl)oleanolic acid, 28-O-β-D-glucopyranosyl ester, oleanolic acid, viburtinoside A, viburtinoside B, viburtinoside I, viburtinoside II, viburtinoside III, viburtinoside IV, viburtinoside V, suspensolide F, suspensolide A, isoquercitroside, kaempferol 3-O-β-D-galactopyranoside, quercetine, nobiletin, rutin, afzelin, scopoletin 7-O-sophoroside, 2,6-Di-C-methylnicotinic acid 3,5-diethyl ester | — | Leaves and twigs | Antitumor effects | [31] |
| V. urceolatum | α-Amyrin palmitate, lupeol palmitate, β-amyrin acetate, ursolic acid, urceolatoside A, urceolatoside B, urceolatoside C, urceolatoside D, urceolide, | — | Leaves and twigs | Antitumor effects | [31] |
| V. wrightii | α-Amyrin palmitate, ursolic acid, astragalin, kaempferol 3-O-β-D-galactopyranoside, kaempferol 3-O-rutinoside, apigenin 7-O-β-D-glucoside, arbutin, p-hydroxypheny β-D-allopyranoside, 6-O-acetylarbutin, 4′-hydroxycinnamic acid, viburnolides A, viburnolides B, viburnolides C | — | Leaves and twigs | Antitumor effects | [31] |
In the case of V. awabuki, few studies have been found regarding the description of its phytochemical constituents (Table 2). The number of chemical compounds reported amounts to more than sixty, where vibsanin-type diterpenes and their derivatives are the most prevalent (Figure 1). These phytochemicals can also be present in species such as V. odoratissimum and V. suspensum. These diterpenoids can chemically be eleven-membered ring, seven-membered ring, and rearranged types, represented by vibsanine B, vibsanine C, and neovibsanine A, respectively. Some vibsane-type diterpenoids have exhibited increasing biological activities, and their challenging structures combined with attractive neurotrophs have drawn synthetic attention [29]. Table 2 reports the compounds found in V. awabuki corresponding to methanolic extracts of leaves and twigs of the plant [29, 31]. Other compounds such as triterpenoid derivatives, sesquiterpenes (such as awabukinol), specific flavonoids of the catechin type, coumarin derivatives, and lignans have also been observed.
Figure 1.
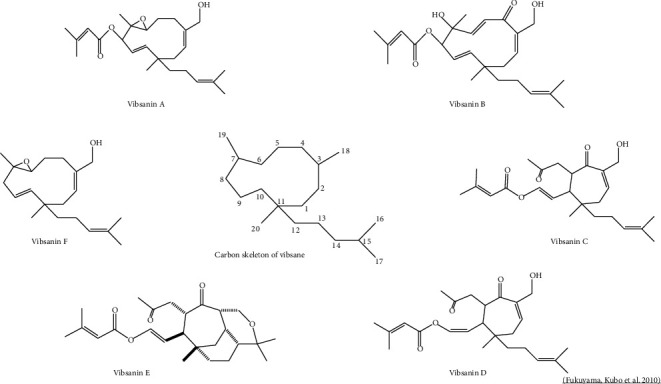
Chemical structure of vibsane-type diterpenoids components reported on the genus Viburnum.
Leaves and twigs of V. dilatatum contain the largest number of phytochemical constituents within the genus (Table 2). The triterpenoids viburnols (Viburnol A, B, C, D, E, F, G, H, I, J, and K), viburnudienone, and viburnenone are present in leaves, as well as, flavonoids, phenolic, and lactone type compounds [31]. The main compounds in the essential oils of V. dilatatum flowers are phenethyl alcohol, 3Z-hexenol, and l-linalool [71]. Glycosylated phenolic compounds of the jiamizioside type (A, B, C, and D) and anthocyanidins and quercetin flavonoids have also been found in methanolic and squeezed juice extracts of fruits. Compounds derived from phenylpropanoids such as 5-O-caffeoyl-4-methoxyl quinic acid and polyphenolic esters (chlorogenic acid) were also reported in the fruits, along with cyanidin 3-sambubioside, 5-caffeoyl quinic acid [3, 69, 70], cyanidin 3-glucoside, 4-methoxy chlorogenic acid, chlorogenic acid, and quercetin [3, 70]. These extracts show a significant antioxidant activity related to the described compounds [113]. Only one study reports the presence of the compound, 2-(-glucopyranosyloxy)-benzyl 3-(-glucopyranosyloxy)-benzoate, in the methanolic extract of roots [72].
This field of research is relatively novel and phytochemicals in V. fordiae have been found in leaves, branches, and fruits (Table 2) [61, 79]. First reports of the phytochemical compounds in V. fordiae were made in methanolic extracts of leaves. Compounds such us glycosylated phenolic type (fordioside), lignan glucoside (alangilignoside D), alcoholic β-glucoside (salicin), sand tilbenoid (rhapontigenin) were reported [67]. Recent studies describe essential oils [114], terpenoids, neolignans [81], and 52 phenolics [67] in stems, leaves, and roots. These compounds have shown to exhibit weak or moderate antioxidant, anti-inflammatory, and α-glucosidase inhibition properties [78, 81]. Neolignan glycosides, viburfordosides A–I, neolignans, fordianes A and B (Figure 2), and analogues present in the ethanolic fruit extract have been described to serve as functional foods and for the prevention and treatment of type 2 diabetes (T2D) [81].
Figure 2.
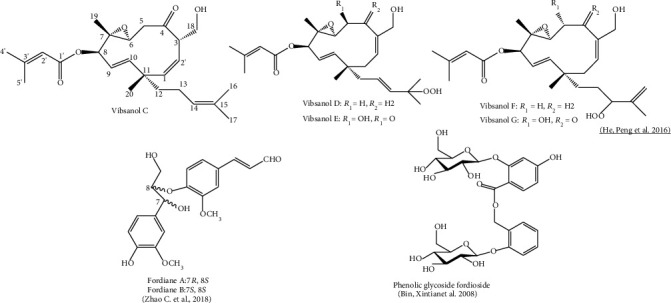
Chemical structure of the visanin components reported on the genus Viburnum.
Studies have described a wide range of phytochemical constituents for members of the genus Viburnum, such as diterpene, triterpene, and flavonoid type for V. odoratissimum. vibsanin, vibsane [89], and vibsanol, and their derivates present in leaves (Figure 2) [88, 90, 93, 94], twigs [91], and branches [92], have been found using methanol and ethanol as primary extraction solvents (Table 2). Triterpenes 6β-hydroxy-3-oxolup-20(29)-ene-27, 28-dioic acid, and 6α-hydroxy-3-oxolup-20(29)-ene-27, 28-dioic acid [31, 94] have been reported in leaves. Volatiles in V. odoratissimum flowers consist of esters, alkanes, ketones, alcohols, aldehydes, and acids. The main compounds in flowers were methyl o-anisate, heneicosane, methyl salicylate, 1-[2-(1-hydroxy-1-methylethyl) cyclopropyl] ethanone, linalool, nonanal, and methyl palmitate [95]. Compounds such as triterpenes and trans-phytol fatty alcohol have been detected in the root [96].
Studies carried out on fruit juice [100, 103, 104], aqueous methanolic extracts [98], acidic mixtures of water/methanol [102], and mixtures of methanol/acetone/water [99] have been assessed in V. opulus (Table 2). It is one of the few species where the volatile compounds of its fruits are described [115]. Mass spectrometry analysis has identified nine components in V. opulus juice, using ultra high-performance liquid chromatography (UPLC) coupled to quadruple time-of-flight mass spectrometers (QTOF-MS) [100]. Viburnum fruits have been reported to contain lipids, pectins, proteins, lipid compounds (carotenoids, essential oils, steroids, and saponins), tannins, flavonoids, and anthocyanins-type polyphenols. V. opulus fruits have a higher content of carotenoids, polyphenols, flavonoids, steroids, and pectins than V. lantana; the latter species being characterized by a higher content of proteins, saponins, and essential oils. Metabolites in V. opulus fruits have been found in different layers of the pericarp with greater amounts in the skin [21]. Phenolic compounds have been reported in fruit juice via hydrochloric acid analysis [101] while triterpenic compounds have been obtained from seeds after triterpene esters hydrolysis (terpenes of the sterols-I type, triterpenyl alcohols and their derivatives-II) [105]. In a review published in 2010, it was reported that only 3-O-caffeoylquinic acid and 5-O-caffeoylquinic acid were isolated from the V. opulus [31]. The presence of phenolic compounds, anthocyanins, and others constituents (Table 2) have been reported in leaves and hydrodistillation extracts of dried V. opulus plants [83, 97].
In this context, the genus Viburnum contains chemical compounds grouped in diterpenes, triterpenes, iridoids, monoterpenes, sesquiterhytopenes, flavonoids, lignans, phenols, coumarins, lactones, and alkaloids. Among the chemical contents of Viburnum, vibsane-type diterpenoids are characteristic of the genus, as have not been found in other higher plants. Compounds of the type vibsanin A-F, vibsanol (C-F), phenolic glycoside fordioside, fordiane A and B, and their derivatives have also been highlighted (Figure 2). The base structure of the visanin (A-F) compound group corresponds to a vibsane carbon skeleton, with a 20-carbon structure (Figure 2).
Another key aspect is the type of solvent used for the phytochemical extraction, where water, methanol, ethanol, and their mixture are used in higher proportion. In some cases, less polar solvents such as ethyl acetate or n-butanol are used, from branches and leaves. Chloroform has been used in leaves, while benzene or petroleum ether for the extraction of phytochemicals from branches.
Table 2 shows the main phytochemicals found in the genus Viburnum, as well as the type of extract obtained, the part of the plant used and the biological activity.
4. Biological Activities
4.1. Antioxidant Activities of Viburnum Plants (In Vitro Studies/In Vivo Studies)
Many traditionally used medicinal herbs exert a beneficial impact on human health thanks to their antioxidant potential. Phenolic compounds, commonly found in plants, are the largest group of natural antioxidants. Plants produce them to protect their cells from oxidative damage caused by oxygen radicals and molecular excitation [116, 117]. Viburnum plant species have been extensively studied in vitro and in vivo assays. Most antioxidant studies relate to Viburnum fruits and juices, being V. opulus the most investigated plant species. According to the numerous published results, it can be said that Viburnum species and their products are exceptional antioxidants finding their place as naturally safe agents.
4.1.1. In Vitro Studies
The fruit juice of V. opulus (from the Eastern Black Sea Region, Turkey) had a prominent activity in the 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) and ferric reducing antioxidant power (FRAP) system in comparison to methanol, acetonitrile, and aqueous extracts of fruits skin and seeds, where the seed extract contained a higher number and quantity of antioxidant compounds. Coumaroyl-quinic acid, chlorogenic acid, procyanidin B2, and procyanidin trimer were dominant in the juice [100]. Turkish V. opulus fruit, flesh, and seeds were analysed by the 2,2-diphenyl-1-picrylhydrazyl (DPPH) method. Fruits and seeds expressed a more potent effect with EC50 of 2.35 mg/mg DPPH, as compared to EC50 of 24.56 mg/mg DPPH found in the flesh [103]. Antiradical activity tests in DPPH and ABTS, and antilipoperoxidation in the β-carotene/linoleic acid bleaching, were applied in aqueous and methanol extracts of the same species. Methanol extract of dried fruits had a greater performance in the DPPH test with IC50 of 0.104 mg/ml, while that of fresh fruits scavenged more ABTS radicals. All extracts inhibited the linoleic acid peroxidation, thus protecting the loss of β-carotene. The methanol extract reduced the ferric (III) to the ferro (II) form more effectively than the aqueous extract [118]. Sagdic et al. [119] also tested the fruits' methanol extract of Turkish V. opulus using the phosphomolybdenum complex method and found a value of 315.50 ± 8.2 mg/g in relation to the ascorbic acid. The antioxidant capacities of aqueous extracts of commercially available fruits, bark, and flowers of V. opulus from Poland were assessed by ABTS, hydroxyl radical scavenging, and peroxyl radical scavenging (ORAC) and FRAP techniques. The bark aqueous extract displayed the highest antioxidant capacity, followed by flowers and fruits. Strong correlations were found between total phenolic, flavanol, and proanthocyanidin contents with these assays [34]. The same authors investigated the antioxidant capacities of V. opulus fruits, flowers, and bark ethanol extracts by measuring of ABTS-, hydroxyl- (HORS), peroxyl- (ORAC), and superoxide- (SORS) free radicals scavenging and reducing power (FRAP). The antioxidant activity of different parts of the herb was in the following order for HORS, SORS, and ORAC tests: bark>flowers>fruits, and for ABTS and FRAP tests: bark>fruits>flowers. The dominant compound in V. opulus bark was (+)-catechin, while chlorogenic acid was dominant in flowers and fruits [34].
Andreeva et al. [120] determined the antioxidant potential of Russian V. opulus bark extracts using the cathode voltammetry method measuring the relative decrease in the oxygen electroreduction. Results showed that the ethyl acetate fraction of the 70% ethanol extract expressed a higher antioxidant potential than chloroform and aqueous fractions, 70% and 30% ethanol extract, respectively. Bubulica et al. [121] conducted an antioxidant effects screening across several extracts of in vitro assays of Romanian plants. The authors found that the V. opulus methanol bark extract produced an antiradical effect against DPPH radicals with IC50 of 0.918 ± 0.46 mg/ml, as well as ferrous ion chelating effects with IC50 of 1.865 ± 0.05 mg/ml. Additionally, the antioxidant activity of the ethanol extract of the fruits was determined by the ABTS test. After 24 h of refrigerating, a 16% decrease in the antioxidant effect was recorded, followed by a 22% increase in the next 24 h, showing no correlation between the total the antioxidant potential and the phenol content in the extract during storage [122]. Paşayeva et al. [123] suggests that Turkish V. opulus fruits could be used as a neuroprotective agent. Antioxidant properties of both the decoction ethanol extract and fruit juice were used against hydrogen peroxide-induced oxidative stress in human SH-SY5Y neuronal cells. The Polish V. opulus fresh juice and extracts acted as scavenging potential agents toward ABTS and peroxyl radical cations estimated by ABTS and ORAC assays, expressed using Trolox equivalents (TE). The phenolic-rich fraction from fresh juice was compared to the methanol-acetone extract from pomace. The former was the most active in all identified phenol compounds (flavanols, falvonols, hydroxycinnamic acids, and anthocyanins) with values within the range of 2619.59 ± 123.1 and 7810.29 ± 342.3 μg TE/g. Also, the V. opulus extracts had powerful chemopreventive effects against oxidative stress in Caco-2 cells induced by tert-butylhydroperoxide and against DNA damage through the repair induction after cell exposure to hydrogen peroxide and methylnitronitrosoguanidine [99].
Studies have compared the antioxidant effects of different V. opulus genotypes and cultivars. Kraujalytė et al. [101] reported that V. opulus var. sargentii had the highest radical scavenging capacity (77.5%), followed by V. opulus var. americanum, and V. opulus P3. The strongest activity in a FRAP test system was observed in V. opulus var. sargentii juice while the weakest was expressed by the ‘Shukshinskaya' cultivar. The V. opulus var. sargentii juice was most effective in the ORAC method with the highest TEAC (Trolox equivalent antioxidant capacity) value, while ‘Shukshinskaya' was powerless as observed in the previous test. Moskalets et al. [124] assessed the antioxidant capacity of Ukrainian V. opulus fruit varieties using a Blisar A analyser. The antioxidant activity was expressed through gallic acid in a range between 387 and 540 mg%. The cultivar with the maximal tested effect was ЕF 3-10-2010. Furthermore, approximately 10 genotypes of Turkish V. opulus fruits showed antioxidant potential, marked SIV 1-10 by a FRAP test system. SIV genotypes in the forms of acetone extracts displayed effects in a narrow range from 21.02 ± 2.6 to 34.90 ± 4.5 μmol TE/g, where SIV-10 had the greatest capacity, suggesting the higher synthesis or accumulations of phenolics and others antioxidant compounds in fruits [33]. Scavenging effects of fruit porridge (25%) of V. opulus var. edule cultivars Leningradskaya otbornaya, Souzga, and Taezny rubiny on nitric oxide, superoxide anion, hydroxyl radical, and lipid peroxidation inhibition, were moderate from 11.20 to 28.50%. Fruits' total antioxidant activity was determined by the ABTS and DPPH methods, where Taezny rubiny was most prominent. A high correlation between total phenolics and ascorbic acid content, and antioxidant activity was reported [102]. V. opulus (Latvia) fruits and pomace were subjected to supercritical carbon dioxide extraction (SFE-CO2) with different conditions to obtain the optimal lipophilic fraction. The antioxidant potential of V. opulus SFE-CO2 extracts was assessed by the ORAC method. The antioxidant capacity values of the washed pomace, unwashed pomace, and dried berry extracts, at the highest yield (optimal SFE-CO2 conditions), were 65.3 ± 1.8, 74.3 ± 2.2, and 142.4 ± 3.6 μmol TE/g, respectively. It can be concluded that the dried berry extracts were twice as higher in the antioxidant activity expression in comparison to the pomace extracts [125].
Çanga and Dudak [126] implemented cellulose acetate/gum Arabic fibers loaded with a V. opulus (Turkey) fruit extract. Within the examination of the materials, they tested the antioxidant activity of the loaded fibers and observed high values of DPPH radical inhibitions ranging 56-59% and 55–58% at 4°C and 25°C, respectively.
These free radical inhibitions were more effective than the free V. opulus fruit extract (40% and 34%, respectively). Barak et al. [98] studied the difference in the antioxidant potential of Turkish V. opulus methanol, and water fruit extracts before and after in vitro gastrointestinal human digestion. Antioxidant effects of the extracts were assessed in the following phases: nondigested, postgastric, colon available, and serum available, by different methods (N,N-dimethyl-p-phenylendiamine-DMPD, cupric reducing antioxidant capacity (CUPRAC), DPPH, FRAP, and total antioxidant capacity). The methanol extract was superior in antioxidant expression than the aqueous one, and the antioxidant effect decreased during the in vitro digestion. An interesting investigation was conducted with V. opulus (Turkey) fruit pomace in wheat flower cakes at concentrations of 0, 5, 10, and 15%. Cakes' total phenolic content and antiradical activity (DPPH) increased in correlation to the level of V. opulus fruit pomace addition (from 10.26 ± 1.5 to 76.83 ± 4.5%) [127]. The V. opulus (guelder rose) fruit concentrate (65%) decreased the thiobarbituric acid reactive substance (TBARS) levels in turkey meat samples during storage at concentrations of 5% and 10%, particularly after 10, 15, and 30 days. The addition of 10% concentrate to the meat significantly reduced the TBARS in comparison to control and butylated hydroxytoluene (p < 0.05) both under aerobic and anaerobic conditions [128].
Erdogan-Orhan et al. [129] observed the antioxidant effects of Turkish V. opulus and V. lantana ethyl acetate, methanol, and aqueous extracts from branches, leaves, and fruits. The most powerful antioxidant agents in the ferrous ion chelating capacity test were the V. opulus ethyl acetate leaf extract (44.62 ± 0.02% of inhibition; 2000 μg/ml) and the V. lantana ethyl acetate fruit extract (58.72 ± 1.00% of inhibition; 2000 μg/ml). V. opulus aqueous extracts from branches and V. lantana methanol leaf extracts exhibited the highest effects (3.396 ± 0.01 and 3.401 ± 0.02; 2000 μg/ml, respectively) using the FRAP method with chlorogenic acid as a reference. In the β-carotene bleaching assay, V. opulus ethyl acetate fruit extracts and V. lantana methanol leaf extracts were significant antioxidant agents with coefficients of 60.5 ± 1.36 and 79.50 ± 1.76, respectively, at 2000 μg/ml. The authors noticed that a higher total phenolic content in the tested extracts usually indicates greater antioxidant effects [129]. Both Viburnum species were also studied by Altun et al. [41]. The antioxidant effects of different water extracts (branches, fruits, and leaves) were assessed using the DPPH and superoxide anion scavenging methods. Branch extracts successfully reduced the effects of the superoxide anion (IC50 = 3.7 and 3.1 mg/ml, respectively). On the contrary, the extracts produced a positive antiradical effect on the DPPH radical with various inhibition values, especially the V. opulus branch extract with IC50 values at 0.014 mg/ml [41].
Interestingly, during autumn migration birds select quality fruits rich in anthocyanins, phenolic profile, and strong antioxidant activity. Bolser et al. [130] revealed that birds preferred V. recognitum and V. dentatum fruits which have the highest total antioxidant content. Serteser et al. [131] investigated the antioxidant properties of selected wild-growing plants in Turkey. Among them, the V. lantana methanol fruit extract proved to be a moderate antioxidant agent in the DPPH method (EC50 = 1.523 mg/mg DPPH), the Fe+2 chelating assays (39.43 ± 2.69%), and through H2O2 inhibition (43.37 ± 2.86%). V. lanata buds, macerated in a glycerin-ethanol solution (as part of a gemmotherapy preparation for respiratory diseases), showed the weakest antiradical effect in the DPPH test (30.08 ± 2.14 μg/ml) in comparison to other herbs (buds of Betula pubescens, Ribes nigrum, Carpinus betulus, and offshoots of Rosa canina). V. lanata buds were the poorest in total phenolic and flavonoid, caffeic, and chlorogenic acid contents [132]. Iranian V. lantana methanol leaf extract was evaluated by the DPPH method and IC50 value of 52 μg/ml, demonstrating a great antioxidant capacity. This extract contained two chalcone glycosides (trans-3-ethoxy-4-O-(glucopyranoside)-2′, 3′, 4′, 5′, 6′-pentahydroxy chalcone and trans-3-methoxy-4-O-(glucopyranoside)-2′, 3′, 4′, 5′, 6′-pentahydroxy chalcone) isolated for the first time from V. lantana leaves [133].
The GC-MS data for the methanol soluble fractions of V. sargentii extract (originating from the Republic of Korea) highlights the presence of guanosine, levoglucosan, vitamin E, stigmast-5-en-3-ol, and stigmata-5,24(28)-dien-3-ol. Patil et al. [134] showed a significant antioxidant potential of the extract in a concentration-dependent manner for all three methods. The extract produced a strong antiradical effect in the DPPH assay with an IC50 value of 15.33 ± 0.58 μg/ml. For both remaining methods, the extract had an electron-donating capacity which reflected its reducing power to change the ferric (Fe3+) to the ferrous (Fe2+) form and Mo (VI) to Mo (V), respectively [134]. V. nervosum roots, essential oil, and extracts showed strong antiradical effects in DPPH system. Methanol and ethanol extracts (100% and 80%) were more potent with IC50 = 22.97 ± 0.38-25.65 ± 1.02 μg/ml than essential oil (IC50 = 33.32 ± 0.67 μg/ml). Additionally, the inhibition capacity of the extracts was greater in a linoleic acid system, where lipoperoxidation inhibition ranged from 47.67 ± 0.87 to 69.25 ± 1.67% in comparison to oil 44.03 ± 0.96% where the dominant compounds were α-eudesmol, caryophyllene oxide, linalool, spathulenol, and ledene Awan et al. [135]. Fu et al. [136] conducted an antioxidant potential screening of edible fruits from South China. They reported that V. sempervirens (in particular V. fordiae) fruits produced great total antioxidant effects estimated by FRAP and TEAC. However, it is important to note that the V. sempervirens nonpolar fruit fraction showed greater effects as compared to V. fordiae, which polar fruit fraction was more active. The authors also noticed strong correlations of antioxidant expression with total phenols [136]. Leiner et al. [137] evaluated the antioxidant capacity of Alaskan berries by the ORAC method and revealed an excellent effect of V. edule fruits (117 mmol of TE/g), after Vaccinium vitis-idaea. Antioxidant potential was also confirmed for V. dilatatum leaves and fruits [138, 139]. Iwai et al. [138] from Japan examined V. dilatatum juice (fruit squeezing solution) claiming its strong antioxidant activity. The in vitro antioxidant potential of the V. dilatatum sample was measured by the XYZ-dish and electron spin resonance (ESR) method. In the XYZ-dish technique, the tested sample expressed effective activity against OH· (10.163 ± 2.376 units/ml), as opposed to its antiperoxide effect (H2O2) (0.529 ± 0.127 units/ml). The activity of the V. dilatatum juice to scavenge OH· radicals, measured by the ESR method and represented as DMSO equivalent concentration, was also strong (0.937 ± 0.176 mM DMSO eq/ml) [138]. Three Indian Viburnum species and their methanol leaf extracts were investigated in a DPPH test, a nitric oxide (NO) scavenging test, and an assay of reduced glutathione and ferrous sulphate-induced lipid peroxidation. Ponnudurai et al. [140] concluded that these extracts could be effective antioxidants in the following order: V. erubescens>V. coriaceum>V. punctatum. V. awabuki (originating from China) and its extracts exhibited exceptional activities. The ethyl acetate-soluble fraction of the crude methanol-chloroform extract was dominant in antioxidant effects in DPPH radical inhibition (1000 μg/ml = 61.88 ± 0.23%) and reducing power activity (1000 μg/ml = absorbance of 0.287 ± 0.006), while the basic crude extract was prominent in the hydroxyl radical-scavenging activity test (100 μg/ml = 71.26 ± 0.38%). On the contrary, other V. awabuki extracts: petroleum ether-soluble fraction, n-butanol-soluble fraction, and aqueous residue, were significantly weaker [141]. Abbasi [142] revealed that V. foetens fruit from the Himalayan region-Pakistan represented a rich source of natural antioxidants possessing significant antioxidant effects. Compared with the water extract, the acetone extract showed higher levels of the total antioxidant capacity (84.67 ± 0.48 μM AAE/100 g), DPPH antiradical capacity (84.62 ± 0.63%), and hydroxyl radical scavenging capacity (75.53 ± 0.95%). They also found significant correlations between the ascorbic acid, phenols, and metal contents with free radical scavenging activity [142]. Nine V. tinus extracts from Turkey were screened in detail for their antioxidant potential. Antioxidant capacities of the ethyl acetate, methanol, and water extracts of leaves, branches, and fruits were tested against DPPH, DMPD, superoxide, and NO radicals. The methanol extracts of leaves, branches, and fruits and leaves' aqueous extract exhibited a remarkable DPPH antiradical activity, over 89%. The fruits' methanol extract was prominent against the DMPD radicals (67.1 ± 0.33%), the branches' aqueous extract in NO radical quenching (near 80%), and the fruits' ethyl acetate extract was the only active in the superoxide radical scavenging test (38.4 ± 1.01%). The fruits' methanol extract displayed the strongest activity in the FRAP and phosphomolybdenum-reducing antioxidant power (PRAP) tests. In the metal-chelation capacity test, the aqueous extracts were predominant with over 60% of the activity [143]. V. tinus from Tunisia and its leaves' acetone extract also produced strong antiradical effects in a DPPH test with high total phenolic, flavonoid, and tannin content [144]. The Indian V. punctatum leaf ethanol extract was tested for its scavenging effects in a DPPH and ABTS system and total antioxidant capacity (reducing power activity) in a phosphomolybdenum assay. The extract proved to be an efficient scavenging agent with IC50 values of 83.29 μg/ml and 92.04 μg/ml, respectively, with excellent reduction effects of 65.67 ± 0.15% with the maximal concentration of 100 μg/ml [145]. V. grandiflorum, among other wild berries of the Northwestern Himalayan region, demonstrated promising antioxidant properties. Namely, the methanol fruit and leaf extract produced an antiradical effect in a DPPH system with IC50 values of 294.5 and 125.82 μg/ml, respectively, as well as effective chelating and reducing power [146]. Fruit, leaf, and branch extracts from V. orientale (traditionally used in Turkey -Anatolia) were studied for their antioxidant and neurobiological effects. The fruit and branch methanol extracts (characterized by the highest total phenolic contents) showed the highest inhibition capacity against DPPH radicals and in FRAP and phosphomolibdenum reducing antioxidant power assays. The leaf aqueous extract showed the greatest NO scavenging (75.00 ± 1.22%), DMPD scavenging (33.70 ± 1.13%), and metal-chelating (54.66 ± 3.56%) at the concentration of 2500 μg/ml [106]. The aqueous extract of the aerial parts of V. punctatum from India showed a significant antiradical effect in a DPPH test system. The extract inhibited from 44.33 ± 0.21% to 93.65 ± 0.56% of free radicals at the concentrations from 20 to 100 μg/ml Susmitha et al. [147]. The antioxidant effects of V. odoratissimum seed extract, from China, were analysed in superoxide radical scavenging, reducing power, and lipid peroxidation inhibition assays. The butanol-soluble fraction of the methanol-chloroform crude extract was dominant in the first two tests, which was in accordance with the total phenol and flavonoid contents. The authors observed that the petrol ether-soluble fraction had the highest antilipoperoxidant activity and that the high temperature was not effective for the lipid peroxidation inhibition [148]. V. mullaha (India) acetone fruit extracts showed prominent antioxidant activities in vitro assays: ABTS, DPPH, superoxide anion, and linoleate peroxyl radicals scavenging and ferric reducing except ferrous metal chelating activity. The HR-LC-MS analysis detected 15 phenolic compounds: chlorogenic acid, acetyl salicylic acid, dihydroquercetin, dihydrorobinetin, dihydromyricetin, 2-isoprenylemodin, rutin, cosmosiin hexaacetate, pectolinarin, eriodictyol, iriginol hexaacetate, theaflavin, epicatechin pentaacetate, lomatin, and peucenin [149]. Methanol, ethyl acetate, and water extracts made of V. grandiflorum (Pakistan) stem exhibited antiradical activity in a DPPH assay. The water extract had the highest potential (IC50 = 255 μg/ml) followed by ethyl acetate (IC50 = 322 μg/ml) and methanol (IC50 = 742 μg/ml) extracts [39].
4.1.2. In Vivo Studies
In vivo studies of the antioxidant effects of Viburnum species are not as extensive as in vitro studies. The literature refers to several experimental studies which mainly included V. opulus and V. dilatatum.
The V. opulus (Turkey) fruit methanol extract showed protective effects against ischemia/reperfusion- (I/R-) induced oxidative stress during lung transplantation in rats, presumably due to its antioxidant effects and ability to neutralize free radicals. Namely, the treatment with the extract (200 mg/kg, intraperitoneally) significantly increased the levels of the antioxidant system (superoxide dismutase (SOD), glutathione peroxidase (GPx), catalase (CAT), and total glutathione) and repaired the total antioxidant plasma status of rats (69.59 ± 8.9 μmol Trolox eq/mg protein) compared to the untreated group (43.02 ± 4.75 μmol Trolox eq/mg protein). In addition, the extract reduced the malonyldialdehyde (MDA) and protein carbonyl levels. It is considered that malic, caffeic, quinic, coumaroyl-quinic, and chlorogenic acid, as well as particular caffeic acid, derivatives are responsible for the antioxidant effects previously described [150]. The impact of V. opulus water fruit extract (also originating from Turkey) on testicular and epididymal rats tissue treated by i.p. injection of taxane-based chemotherapeutics was investigated by measuring the lipid peroxidation level and antioxidant activities. Docetaxel and paclitaxel imbalanced an oxidant/antioxidant system, which was repaired with an oral dose of 100 mg/kg of the extract. MDA levels were significantly lower in rats' testis and epididymis while the levels of superoxide dismutase, glutathione peroxidase, and catalase were increased. The authors identified several compounds in the extract by GC-MS (α- and β-pinene, butanoic acid, DL-limonene, α-terpineol, and germacrene D) [151]. Furthermore, the lyophilized V. opulus juice and the commercial lyophilized V. opulus juice showed a significant antiurolithiatic activity in rats (100 mg/kg) compared to the Cystone standard. Ilhan et al. [152] attributed this effect to their antioxidant and diuretic activity and the inhibitory effects on the oxalate levels. The antioxidant action was estimated by measuring TBARS, total thiols, and glutathione in kidney tissues. TBARS levels were significantly reduced, after administration of V. opulus juices, with increased levels of total thiols and glutathione [152]. V. opulus proanthocyanidins produced gastroduodenoprotective effects against water immersion and restraint stress in rats, improving the levels of antioxidant enzymes' superoxide dismutase, catalase, and gluthatione peroxidase and decreasing the MDA content. Proanthocyanidins, as V. opulus extract, were intragastrically administered in three doses at 25, 50, and 75 mg/kg body weight [40].
V. dilatatum juice showed an inhibitory effect on gastric ulcer formation and oxidative damage caused by water immersion restraint stress in rats. The concentrations of lipid peroxides, assessed by TBARS, in the plasma, liver, and stomach were significantly lower compared with the group treated with water [138]. The same Japanese researchers tested the V. dilatatum crude fruit extract, proving ineffective for preventing oxidative injury induced by water immersion restraint stress. The extract improved the lipid peroxidation in the plasma, liver, and stomach but had weak effects on the enzymatic activities (superoxide dismutase, catalase, and gluthatione peroxidase). However, ferrous ascorbate-induced oxidation in hepatic homogenate of rats was inhibited. According to the results, V. dilatatum fruits cannot induce antioxidant enzymes and its absorbed antioxidant components have a direct effect on the oxidative injury in the body [153]. In addition, the V. dilatatum crude fruit extract was subjected to the experiment with streptozotocin-induced diabetic rats. The animals received V. dilatatum extract solution (16.8 mg/ml) for 10 weeks. TBARS levels in the plasma, erythrocytes, liver, kidney, and pancreas were significantly reduced together with plasma glucose levels. The extract contained two cyanidin glycosides, two chlorogenic acids, and quercetin. It is suggested that cyanidin 3-sambubioside is crucial for the physiological effects of V. dilatatum fruit, given the strong antioxidant nature of the compound [70, 154].
The leaf methanol extract of V. tinus from Egypt produced a significant effect on serum lipid peroxides (measuring of thiobarbituric acid-reactive substance) and nitric oxide levels (Griess reaction) with the dose of 50 mg/kg i.p. on CCl4-induced hepatotoxicity in rats, although not at lower doses of 25 mg/kg [155].
4.2. Antimicrobial Activities of Viburnum Plants (In Vitro Studies)
During the last two decades, antimicrobial activity of plant species of the genus Viburnum L. has been extensively studied. Antimicrobial activity of the essential oils from the air-dried whole plants of V. opulus, V. lantana, and V. orientala were tested against the bacteria Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, Enterococcus faecalis, Staphylococcus aureus, Bacillus cereus, and the fungus Candida tropicalis. The oils were at a maximum concentration of 250, 500, and 1000 μg/ml in hexane, respectively. The activity was tested using the agar dilution MIC assay. The oils of V. lantana and V. opulus showed no activity against the microorganisms tested. The essential oil of V. orientale showed a weak antibacterial activity against Gram-positive bacteria such as E. faecalis, S. aureus, and B. cereus [83]. In another study, the essential oil of V. betulifolium was analysed and tested for antimicrobial activity using microdilution assay of human pathogenic bacteria and yeast. V. betulifolium is an evergreen shrub widely distributed throughout the Yunnan Province and southwestern parts of China. The main essential oil constituents of the species are phytol (9.8%), trans-b-damascenone (5.9%), α-cadinol (5.7%), γ-cadinene (5.6%), Δ-cadinene (5.3%), methyl pentanoate (4.6%), and tetradecanal (3.8%). The oil showed strong antimicrobial activity against both Gram-positive and Gram-negative bacteria and the yeast, the effect being more significant against Gram-positive than Gram-negative bacteria. Positive inhibitory activity was shown by Pseudomonas aeruginosa (MIC 125 μg ml−1) and Candida albicans (MIC 62.5 μg ml−1) [156]. Awan et al. [135] investigated antimicrobial activities of Viburnum nervosum root essential oil and several methanolic and ethanolic root extracts. V. nervosum is a large deciduous precocious shrub, 2-3 m tall with stiff stout branches. In Kashmir's traditional medicine, it is used as an astringent and emmenagogue, as well as for the treatment of acute furunculosis. Bergenin, a trihydroxybenzoic acid glycoside, was isolated from its roots. This glycoside is also useful in the treatment of hypercholestraemia, kidney stones, fever, diarrhea, and pulmonary infection. The essential oil of the root possesses carminative, insecticidal, antiseptic, and disinfectant properties. The main compounds of the root oil are eudesmol (30.3%), caryophyllene oxide (17.0%), spathulenol (10.7%), and linalool (12.65%). The essential oil and root extracts of V. nervosum were tested against Gram-positive bacteria (Staphylococcus aureus and Bacillus subtilis), Gram-negative bacteria (Escherichia coli and Pasteurella multocida), and pathogenic fungi (Aspergillus niger, Aspergillus flavus, Fusarium solani, and Rhizopus solani) with the disc diffusion method. The oil showed maximum activity against B. subtils, A. niger, and R. solani. However, no activity was observed by the extracts Awan et al. [135]. Nonetheless, the chemical composition and antimicrobial activity of Viburnum species essential oils were examined, neither were the antimicrobial activities of the dominant compounds investigated.
Bibi et al. [157] tested the antibacterial activity of the methanolic dry extract of Viburnum foetens (20 mg/ml), with an agar well diffusion method, against Bacillus subtilis, Micrococcus leuteus, Salmonella setubal, Salmonella aureus, and Pseudomonas picketii. All bacteria tested were sensitive to the extract. S. setubal was the most sensitive bacteria. The extracts of V. foetens were subject of another study. Awan et al. [158] examined four different extracts of V. nervosum and V. foetens leaves for antibacterial activities against eight different bacteria (Staphylococcus aureus, Bacillus subtilis, Salmonella typhi, Pseudomonas aeruginosa, Klebsiela pneumoniae, Proteus vulgaris, Citrobacter freundii, and Streptococcus pneumoniae) using the disc diffusion method. The study concluded that the ethanolic extract was the most effective, contrary to that of the petroleum ether extract. The ethanolic and methanolic extracts showed prominent activities against all tested bacteria, in comparison to the chloroform extract which had moderate activity. Turker et al. [159] used the same antibacterial activity evaluation method by analysing the antimicrobial activity of the V. lantana extracts obtained from dry and fresh fruits (water and ethanol) against Gram-positive bacteria (Streptococcus pyogenes, Staphylococcus aureus, and Staphylococcus epidermidis) and Gram-negative bacteria (Escherichia coli, Pseudomonas aeruginosa, Salmonella typhimurium, Serratia marcescens, Proteus vulgaris, Enterobacter cloacae, and Klebsiella pneumoniae). Gram-positive bacteria were more susceptible to the inhibitory effects of the plant extracts than the Gram-negative bacteria. The extracts of V. lantana fresh fruits exhibited antibacterial activities. The inhibition capacity of the hot ethanolic extract was greater than the cold ethanolic extract against S. aureus, S. epidermidis, and S. pyogenes. Both S. marcescens and P. aeruginosa were resistant to all examined fruit extracts.
Eryilmaz et al. [160] also studied the antimicrobial activity of V. lantana, together with Viburnum opulus L., V. orientale Pallas, and V. tinus L. against Staphylococcus aureus, Bacillus subtilis, Escherichia coli, Pseudomonas aeruginosa, Klebsiella pneumoniae, and Candida albicans. The disc diffusion and tube dilution techniques were used to determine the activities of the extracts. Ethanolic and water plant extracts of leaf, stem, and fruit were used in the experiment. Ethanolic extracts from all analysed species showed antimicrobial activity against all the tested microbes. Water extracts were either weak or not effective against tested microorganisms. The antimicrobial activity of the dried fruit methanolic extract of V. opulus was also analysed by another study with an agar diffusion method [119]. The study analysed ten microorganisms (Aeromonas hydrophila, Bacillus cereus, Enterobacter aerogenes, Escherichia coli, Klebsiella pneumoniae, Proteus vulgaris, Pseudomonas aeruginosa, Salmonella typhimurium, Staphylococcus aureus, and Yersinia enterocolitica). A 15% concentration extract completely inhibited the growth of all analysed bacteria. The same plant species have shown the capacity to reduce the potential of Staphylococcus aureus and S. epidermidis to colonize inert substratum and form biofilms [121]. Similarly, fruit juices and ethanolic extracts of V. opulus genotypes were tested against ten Gram-positive and Gram-negative bacterial cultures and nine yeast strains. The fruit juices showed greater antibacterial activity compared to the ethanol extracts. The most effective antibacterial activity was exhibited by the juices against Salmonella typhimurium, Salmonella agona, and Listeria monocytogenes. The fruit juices and ethanol extracts showed weak or no activity on the yeast strains [161]. Antimicrobial activities of fruit juices of six V. opulus genotypes were evaluated by Česoniene et al., [42], using the agar well diffusion method against ten Gram-positive and Gram-negative bacteria and seven strains of yeast. The juices strongly inhibited the growth of Gram-negative (S. typhimurium and S. agona) and Gram-positive (S. aureus, L. monocytogenes, and Enterococcus faecalis) bacteria. As previously reported, the effect of the juices on the yeast was low or lacking [42]. Up to Česonienė's studies, most Viburnum plant extracts had presented the highest effect on Gram-positive bacteria strains and some yeasts. However, antimicrobial analysis of the juices has shown that they can be used to fight Gram-negative microorganisms. Differences in antimicrobial activity are most likely due to the chemical composition of the extracts and juices. Therefore, further research of Viburnum species should connect the chemical composition with the antimicrobial activity.
Paulauskas et al. [162] went one step further. They analysed the antimicrobial activity of unripe mashed berries and ripe berry juice of V. trilobum Marshall, V. sargentii Koehne, and V. opulus cultivar “Leningradskaya Otbornaja.” The unripe berry mass and ripe berry juices both significantly influenced the bacteria. The unripe berry mass manifested greater antibacterial activity, similarly, on both the Gram-positive and Gram-negative bacteria. Micrococcus sp. and S. aureus were the most sensitive bacteria to the mashed berries and all analysed juices. Viburnum juice impacted the microscopic fungi the least.
Methanolic extract of V. cotinifolium leaves from Pakistan was tested against four Gram-positive bacteria, five Gram-negative bacteria, and ten fungal strains with the agar diffusion method. The extract demonstrated maximum activity against Aspergilus flavus and A. fumigates. The extract also showed positive antimicrobial activity against A. niger. The V. cotinifolium extract showed the most effective activity against Enterococcus faecalis and Enterobacter coccus. As can be observed, the extract of Viburnum species was also effective against Gram-negative bacteria [163]. This indicates that V. cotinifolium leaf methanolic extract has great potential as a natural antimicrobial agent. Hence, chemical analyses of the extract should be carried out and associated with antimicrobial activity. This connection is supported by the research carried out by Roy [164], which proves that the methanolic extract (and fraction) from the whole plant of V. foetidum exhibit a significant antimicrobial activity against Gram-positive and Gram-negative bacteria strains, as well as significant antifungal activity. The ethyl acetate (EA) fraction from the methanolic extract exhibited the highest antimicrobial potential. Agrobacterium species were most susceptible to the EA fraction of the extract, and clearly, the EA fraction differs in its composition from its counterparts. Unfortunately, detailed chemical analyses of the extracts and/or fractions have currently not been carried out.
4.3. Anti-Inflammatory Activities of Plant Species of the Genus Viburnum L. (In Vitro Studies/In Vivo Studies)
Inflammatory diseases are usually treated by steroid drugs, nonsteroidal anti-inflammatory drugs, and immunosuppressant. Although the effects of these drugs have been proven, their side effects are not negligible. The usage of these drugs is often associated with bleeding gastrointestinal and peptic ulcers [165]. In search for new harmless drugs, scientists are once again turning to medicinal plants. Among these plants, the species of the genus Viburnum are of interest. For that purpose, anti-inflammatory activities of V. lantana, V. trilobum, V. pichinchense, V. sargentii, V. fordiae, and V. opulus were investigated [78, 166–170].
The bark of this species has been used in Turkish traditional medicine as a rubefacient and analgesic [41]. Viburnum lantana L. leaf water extract was investigated for anti-inflammatory activity, in rats with a carrageenan-induced rat paw edema test. The anti-inflammatory activity of the extract at doses of 100 and 200 mg/kg has been low as compared to indomethacin [169].
Viburnum trilobum Marshall (American highbush cranberry) is widely used in traditional medicine as it displays an anti-inflammatory and antidiabetic effect, sometimes used to improve lipid metabolism. The bark can act as a sedative and pain reliever. Due to the high content of ursolic acid, which expresses anti-inflammatory properties, the bark acts as an anti-inflammatory agent. This effect is proven in a RAW 264.7 macrophage cell system. All fractions of the V. trilobum ethanolic extract significantly inhibited the levels of IL-1β, IL-6, and TNFα [168].
Viburnum pichinchense Benth also displays anti-inflammatory properties. The anti-inflammatory effects of the methanol extract were demonstrated using LPS-stimulated macrophages and HCl/EtOH-induced gastritis model mice. The extract expresses anti-inflammatory activity by targeting NF-κB and caspase-11 noncanonical inflammasome pathways in macrophage-mediated inflammatory responses [167].
Leaves, stems, and fruits of the plants have been used in traditional folk medicines as therapeutic agents, as styptics and analgesics, to treat boils, rheumatoid arthritis, traumatic injuries, ringworm, skin itching, and coughs [170]. In the last two decades analgesic, anti-inflammatory, and hepatoprotective activities of its methanol extract were confirmed. The butanol fraction of the methanolic extract showed the highest activity on inflammatory reactions [170].
Viburnum fordiae Hance, a small tree widely distributed in the south of China, has been used in traditional Chinese medicine for centuries to treat rheumatic arthralgia and allergic dermatitis. Recent studies have reported a new, unusual ɣ-lactone, obtained from the aerial parts of these plants, capable of expressing an in vitro anti-inflammatory effect vitro [78].
V. opulus is well known as a medicinal and horticultural plant with a dietary value. Its fruits have been used in traditional medicine to cure pulmonary, stomach, cardiovascular, and kidney diseases, as well as for the treatment of cramps, diabetes, bleeding, coughs, and colds. Arginase activity and arterial vasodilation of the plant extract have also been proven [100, 101, 104, 171–173]. The anti-inflammatory activity of V. opulus water leaf extract was conducted in rats by a carrageenan-induced rat paw edema, test at doses of 50, 100, and 200 mg/kg, i.p., proving the extract had no anti-inflammatory effect at these doses [166].
4.4. Cytotoxic Activities of Viburnum Plants (In Vitro Studies)
For centuries, herbs and plants have had a role in the treatment of various forms of tumors as have also shown to reduce the risk of cancer development or serve as a treatment for different types of cancer [174]. Viburnum species and their products have been extensively studied for their cytotoxic properties, being promising anticancer agents. To date, the most studied species in this regard is V. opulus.
Sauter and Wolfensberger [175] were the first to report the cytotoxic activity of Viburnum extracts. Aqueous fruit extracts of V. opulus and V. lantana, from Switzerland, showed no cytotoxic activity on BT 20 breast cancer cells within 72 h of incubation. Similarly, further research revealed no cytotoxicity of V. opulus extracts. An Indian aqueous bark extract was tested for its cytotoxic effect using a simple bioassay, brine shrimp lethality test, but no remarkable effect was observed [3]. The same findings were reported for V. opulus seed extracts by Cantrell et al. [176]. Russian V. opulus fruits and its ethanol extract exhibited low cytotoxicity, suppressing cell growth at concentrations above 200 μg/ml [3].
On the contrary, some authors consider V. opulus as an effective cytotoxic agent. Laux et al. [177] investigated the aldehyde fraction of the V. opulus chloroform-methanol fruit extract (Canada) for the cytotoxic effect on human gastric carcinoma cells. The fraction containing (E) 2-hexenal, (Z) 2-decenal, 2,4–decenal, (E) 2-octenal, and 2-undecenal produced a direct antiproliferative effect on the growth of the carcinoma cells with death at a concentration of 27 μM [177]. The methanol and acetone extract, juice, and juice after extraction to the solid phase of V. opulus from Poland showed cytotoxic activity against human breast (MCF-7) and cervical (HeLa) cancer cell lines. The strongest toxic agent towards both cell lines was observed for the juice obtained after purification with IC50 values of 63.541 and 19.380 μg/ml for HeLa and MCF cell lines, respectively [178]. According to IC50 values (250-450 μg/ml), the cytotoxicity of the same origin V. opulus extracts against Caco-2 cells, measured with the PrestoBlue assay, were in the following order: phenoli- rich fraction from fresh juice>methanol-acetone extract from pomace>acetone extract from pomace>fresh juice. This effect could be attributed to the highest content of phenolic compounds (flavanols, falvonols, hydroxycinnamic acids, and anthocyanins) [99]. The commercial V. opulus juice from Turkey produced a cytotoxic effect against Caco-2 (human colon adenocarcinoma) and HeLa cells but was not active in a test using A549 (human type II lung epithelium) cells over a 72-hour period with the concentrations of 10-80 μg/ml. Futhermore, V. opulus juice caused no significant decrease in the viability of MDCK (Madin Darby Canine Kidney) and HUVEC (Human umbilical vein endothelial cells) normal cell lines [179].
Antitumor activities of water and ethanol extracts prepared using the hot and cold procedures of fresh and dried V. opulus and V. lantana fruits (Turkey) were tested with the potato disc tumor induction method. V. opulus was more active with the inhibition of 61.9-100%. Both water extracts from dried fruits were the most effective in the assay. Among V. lantana extracts, hot water and ethanol extracts were the most effective, 90.5% and 95.2%, respectively [159]. The same technique was used to assess the antitumor activity of aqueous, ethanol, and methanol V. lantana (Turkey) leaf and fruit extracts. The methanol extract was the most effective with 100% of tumor inhibition, followed by the ethanol (90.9%) and the aqueous one (86.4%) [180].
The researchers from Pakistan, Shah et al. [37], conducted an identical assay for cytotoxicity with V. grandiflorum methanol extract and its n-hexane, chloroform, ethyl acetate, and n-butanol fractions. The chloroform extract was more active, expressing a strong ability to kill brine shrimp (EC50 = 107.45 μg/ml). The methanol extract of V. grandiflorum from China exerted a strong impact on lung cancer cells H1650, HCC827, and H1299 by decreasing their viability in a concentration- and time-dependent manner. The viability of the H1650, HCC827, and H1299 cells decreased to 34%, 31%, and 29%, respectively, after three days of treatment with the extract. A detailed analysis found that the viability of cells was inhibited by the apoptosis activation through a caspase-dependent pathway [181].
V. punctatum from India was tested in several studies. A V. punctatum methanol extract made from aerial parts displayed cytotoxic activity against human liver cancer cells (HepG2) with CTC50 (cytotoxicity 50%) values of 205.8 ± 1.92 μg/ml by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) test and against human laryngeal epithelial carcinoma (Hep2) with CTC50 value 197.3 ± 2.89 μg/ml [182]. In vitro anticancer activity of aerial parts of V. punctatum was tested using chloroform and methanol extracts and the HCT 15 cell line (human colon carcinoma). Maximal concentrations of the extracts (400 μg/ml) inhibited 63.93 ± 2.76% and 80.16 ± 2.13% of cell viability, respectively [183]. V. punctatum chloroform and methanol extracts expressed a hepatoprotective activity in vitro protecting the Chang liver cells against CCl4-induced toxicity in the MTT test. The cell viability ranged from 62% to 84% at concentrations of 200-400 μg/ml, with the methanol extract being more effective [184]. The ethanol extract of V. punctatum leaves showed anticancer activity against MCF-7 in MTT test with IC50 of 56.73 μg/ml [185].
The V. foetens (Pakistan) methanol crude extract and fractions showed a significant anticancer effect against the breast cancer cell line MCF-7. The crude extract was active 90.5% at 200 μg/ml, while the fractions were less effective at the same concentrations. The highest MCF-7 cell line inhibition percentage was reported for the methanol (83%), followed by the chloroform 55.5%, the hexane 25.11%, and water (2%) fractions [157]. The crude methanol extract and fractions of V. foetens (Pakistan) were evaluated against MCF-7, MDA-MB-468, and Caco-2 cancer cell lines by the MTT test and NRU (neutral red uptake) assay. The crude extract inhibited the cancerous cell growth in a dose-dependent manner. The ethyl acetate fraction significantly reduced Caco-2 cells (93.44%) growth in the MTT test. The methanol and ethyl acetate fractions decreased 99% and 96% cell growth of MCF-7 and Caco-2 cell lines, respectively, in the NRU assay. Also, ethyl acetate fraction of the V. foetens extract exhibited a considerable inhibition of MDA-MB-468 cells in both used assays. Other fractions (chloroform, hexane, and aqueous) produced a weaker effect on cancer cell proliferation [186]. Methanol leaf extract of V. dilatatum (Korea) produced a cytotoxic effect on MCF-7 human breast cancer cells with IC50 of 139 ± 16 μg/ml. The authors reported that this effect could not be considered strong, according to the screening program of the National Cancer Institute, USA, which recommends IC50 under 20 μg/ml to be the effective cytotoxic agent [139]. Roy [164] reported significant lethality in a brine shrimp cytotoxicity assay for the V. foetidum crude methanol extract and its petroleum ether and n-hexan fractions with LC50 of 39.81, 25, and 25 μg/ml, respectively, while LC50 for standard vincrinstine sulphate was 10.44 μg/ml. The Colombian V. cornifolium leaf dichlormetan extract showed high cytoxicity tested on the V79 cell line (Chinese hamster lung fibroblasts) with IC50 of 25 μg/ml [187]. Ponnudurai et al. [188] (India) tested methanol leaf and chloroform root extracts of V. coriaceum and V. erubescens for their bacterial strain-based cytotoxicity (E. coli AB 1157 strain), using the MTT method (MCF-7 breast cancer cell lines and HeLa cervical cell lines). All extracts, except the chloroform root extract of V. erubescens, showed an effect on the bacterial strain-based carcinogenicity. IC50 values in the MTT test, only determined for the V. coriaceum extracts, indicated a moderate anticancer activity (over 500 μg/ml for MCF-7 cells and 300 μg/ml HeLa cells) [188]. Calderón-Montaño et al. [189] reported cytotoxic effects for the Spanish V. tinus water fruit and leaf extracts in the MTT test. The fruit extract was more potent in the inhibition of proliferation of A549-human lung adenocarcinoma cells and MRC-5-human lung fibroblastic cells with IC50 of 26.6 ± 6.5 and 65.4 ± 8.6 μg/ml, respectively. Methanol extracts of Chinese V. odoratissimum wood and bark successfully inhibited melanin biosynthesis and cell proliferation of B16 melanoma cells at 100 and 50 μg/ml, respectively [190].
4.5. Anticancer Effects of Viburnum Plants In Vivo
The anticancer effect of V. opulus juice was previously reported on Ehrlich ascites carcinoma cells [26]. However, Ceylan et al. [191] from Turkey investigated V. opulus juice for its antitumor potential in an in vivo experiment with experimental Balb/c mice. To implement tumors to mice, they applied Ehrlich ascite carcinoma (EAC) 1 × 106 cells i.p. and lyophilized V. opulus juice at a dose of 1000, 2000, and 4000 mg/kg. The tumor weight significantly decreased in mouse groups treated with the juice compared to the control group. The survival rate of Ehrlich ascites tumor cells was reported to be 88.72%, 69.02%, and 51.87%, respectively. The results of the in vitro assay indicated the cytotoxicity of the juice with the IC50 value of 199.58 μg/ml [191]. Also, the same authors reported that gilaburu fractions below and above 50 kDa can stop the cell cycle at the G0/G1 stage and slow the cell division of the Ehrlich ascites tumor [123].
Ulger et al. [192] (Turkey) experimented with Balb-c male mice to study the effect of V. opulus juice on colon tumorogenesis induced with 1,2-dimethylhydrazine (DMH). All groups treated with DMH developed colon tumors as observed by histogenesis. However, mice that received the juice showed a reduced number of tumor lesions, as well as the incidence of invasive carcinoma, as compared to untreated mice. The authors concluded that V. opulus juice could be useful at the initiation stage and prevention of colon cancer.
4.6. Other Health-Promoting Effects
A 1000 mg Viburnum opulus dose and diclofenac on-demand were administered, orally, to 53 patients with urethral stones < 10 mm, in comparison to 50 patients receiving only diclofenac on-demand. It was observed that the expulsion of the stones was greater, and the passage time was faster in the treatment with V. opulus. The demand for diclofenac was lower compared to patients who received only diclofenac [193]. There are no clinical trial reports for the premenstrual syndrome [111], only the folk medicine use, as antispasmodic, in menstrual cramps, dysmenorrhea, and miscarriage prevention [194]. No side effects or reports regarding safety were found [194, 195].
5. Conclusions
The genus Viburnum includes about 200 species, distributed mainly on the Asian side Fruits, flowers, and barks of certain species are used in traditional medicine for the treatment of diseases, such as rheumatoid arthritis, cough, diarrhea. They contain a plethora of biochemical compounds with health-promoting activity, including carotenoids, polyphenols, and flavonoids, which can explain the high antioxidant activity as shown by in vitro studies. Preclinical evidence supports antibacterial, anti-inflammatory, cytotoxic, and anticancer properties of certain species, such as V. opulus.
Contributor Information
Javad Sharifi-Rad, Email: javad.sharifirad@gmail.com.
Dusanka Kitic, Email: kitic@msu.edu.
Jesús Herrera-Bravo, Email: jehebra2@gmail.com.
William C. Cho, Email: chocs@ha.org.hk.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- 1.Choi Y. G., Oh S. H. A comparative morphological study of Viburnum (Adoxaceae) in Korea. Korean Journal of Plant Taxonomy. 2019;49(2):107–117. doi: 10.11110/kjpt.2019.49.2.107. [DOI] [Google Scholar]
- 2.Donoghue M. J. A preliminary analysis of phylogenetic relationships in Viburnum (Caprifoliaceae s.1.) Systematic Botany. 1983;8(1):p. 45. doi: 10.2307/2418562. [DOI] [Google Scholar]
- 3.Krishnaraju A. V., Rao T. V., Sundararaju D., Vanisree M., Tsay H. S., Subbaraju G. V. Assessment of bioactivity of Indian medicinal plants using brine shrimp (Artemia salina) lethality assay. International Journal of Applied Science and Engineering. 2005;3(2):125–134. [Google Scholar]
- 4.Spriggs E. L., Clement W. L., Sweeney P. W., Madriñán S., Edwards E. J., Donoghue M. J. Temperate radiations and dying embers of a tropical past: the diversification of Viburnum. New Phytologist. 2015;207(2):340–354. doi: 10.1111/nph.13305. [DOI] [PubMed] [Google Scholar]
- 5.Jussieu A. L. Genera Plantarum. Paris: Herissant; 1789. [Google Scholar]
- 6.Rafinesque C. S. Monographie des coquilles bivalves fluviatiles de la rivière Ohio, contenant douze genre et soixantehuit espèces. Annales Générales des Sciences Physiques. 1820;5:287–322. [Google Scholar]
- 7.Angiosperm Phylogeny Group. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG IV. Botanical Journal of the Linnean Society. 2016;181(1):1–20. doi: 10.1111/boj.12385. [DOI] [Google Scholar]
- 8.Clement W. L., Donoghue M. J. Dissolution of Viburnum section Megalotinus (Adoxaceae) of Southeast Asia and its implications for morphological evolution and biogeography. International Journal of Plant Sciences. 2011;172(4):559–573. doi: 10.1086/658927. [DOI] [Google Scholar]
- 9.Takhtajan A. Flowering Plants. 2nd. Springer; 2009. [DOI] [Google Scholar]
- 10.Donoghue M. Flowering times in Viburnum. Arnoldia. 1980;40:2–22. [Google Scholar]
- 11.Donoghue M. J., Bell C. D., Winkworth R. C. The evolution of reproductive characters in Dipsacales. International Journal of Plant Sciences. 2003;164(Supplement 5):S453–S464. doi: 10.1086/376874. [DOI] [Google Scholar]
- 12.Jin B. Observations on the anatomy of reproductive organs and the pollinators of Viburnum macrocephalum f. keteleeri (Caprifoliaceae) Acta Phytotaxonomica Sinica. 2007;45(6):p. 753. doi: 10.1360/aps06018. [DOI] [Google Scholar]
- 13.Konarska A. Comparative micromorphology and anatomy of flowers and floral secretory structures in two Viburnum species. Protoplasma. 2017;254(1):523–537. doi: 10.1007/s00709-016-0972-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilkinson A. M. Floral anatomy and morphology of some species of the genus Viburnum of the Caprifoliaceae. American Journal of Botany. 1948;35(8):p. 455. doi: 10.2307/2438162. [DOI] [Google Scholar]
- 15.Hoch W. A., Zeldin E. L., Nienhuis J., McCown B. H. Generation and identification of new Viburnum hybrids. Journal of Environmental Horticulture. 1995;13(4):193–195. doi: 10.24266/0738-2898-13.4.193. [DOI] [Google Scholar]
- 16.Lobstein A., Weniger B., Malécot V., Um B. H., Alzate F., Anton R. Polyphenolic content of two Colombian Viburnum species (Caprifoliaceae) Biochemical Systematics and Ecology. 2003;31(1):95–97. doi: 10.1016/S0305-1978(02)00078-9. [DOI] [Google Scholar]
- 17.Nicolson D. H., Hara H. A revision of Caprifoliaceae of Japan with reference to allied plants in other districts and the Adoxaceae. Brittonia. 1983;35(2):p. 184. doi: 10.2307/2805964. [DOI] [Google Scholar]
- 18.Oersted A. S. Til belysning af slaegten Viburnume. Videnskabelige Meddelelser fra Dansk Naturhistorisk Forening i Kjobenhavn. 1861;13:267–305. [Google Scholar]
- 19.Lobstein A., Haan-Archipoff G., Englert J., Kuhry J. G., Anton R. Chemotaxonomical investigation in the genus Viburnum. Phytochemistry. 1999;50(7):1175–1180. doi: 10.1016/S0031-9422(98)00681-5. [DOI] [Google Scholar]
- 20.Beyazoğlu O., Coşkunçelebı K., Odabaş H. Anatomical properties of wild Turkish Viburnum (Caprifoliaceae) species. Phytologia Balcanica. 2008;14(1):103–110. [Google Scholar]
- 21.Konarska A., Domaciuk M. Differences in the fruit structure and the location and content of bioactive substances in Viburnum opulus and Viburnum lantana fruits. Protoplasma. 2018;255(1):25–41. doi: 10.1007/s00709-017-1130-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barish S., Arakaki M., Edwards E. J., Donoghue M. J., Clement W. L. Characterization of 16 microsatellite markers for the Oreinotinus clade of Viburnum (Adoxaceae) Applications in Plant Sciences. 2016;4(12) doi: 10.3732/apps.1600103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moore B. R., Donoghue M. J. A Bayesian approach for evaluating the impact of historical events on rates of diversification. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(11):4307–4312. doi: 10.1073/pnas.0807230106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clement W. L., Arakaki M., Sweeney P. W., Edwards E. J., Donoghue M. J. A chloroplast tree for Viburnum (Adoxaceae) and its implications for phylogenetic classification and character evolution. American Journal of Botany. 2014;101(6):1029–1049. doi: 10.3732/ajb.1400015. [DOI] [PubMed] [Google Scholar]
- 25.Zakłos-Szyda M., Kowalska-Baron A., Pietrzyk N., Drzazga A., Podsędek A. Evaluation of Viburnum opulus L. fruit phenolics cytoprotective potential on insulinoma MIN6 cells relevant for diabetes mellitus and obesity. Antioxidants. 2020;9(5):p. 433. doi: 10.3390/antiox9050433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Al Ö., Ülger H., Eetekin T., et al. The effect of gilaburu (Viburnum opulus) juice on Ehrlich ascites tumor (EAT) cell culture. Proceedings. 2017;1(10):p. 1051. doi: 10.3390/proceedings1101051. [DOI] [Google Scholar]
- 27.Tomassini L., Ventrone A., Frezza C., et al. Phytochemical analysis of Viburnum davidii Franch. and cholinesterase inhibitory activity of its dihydrochalcones. Natural Product Research. 2020;23:1–7. doi: 10.1080/14786419.2020.1837814. [DOI] [PubMed] [Google Scholar]
- 28.Roy S., Rahman A. A., Ali H., Sayeed M. A., Ali S. Evaluation of thrombolytic activity of different fractions of Viburnum foetidum L. World Journal of Medical Sciences. 2015;12:349–353. [Google Scholar]
- 29.Kubo M., Nakai M., Harada K., Fukuyama Y. Structure of seven new vibsane-type diterpenoids from Viburnum awabuki. Tetrahedron. 2019;75(16):2379–2384. doi: 10.1016/j.tet.2019.02.049. [DOI] [Google Scholar]
- 30.Wang L. Q., Chen Y. G., Xu J. J., Liu Y., Li X. M., Zhao Y. Compounds from Viburnum species and their biological activities. Chemistry and Biodiversity. 2008;5(9):1879–1899. doi: 10.1002/cbdv.200890175. [DOI] [PubMed] [Google Scholar]
- 31.Wang X. Y., Shi H. M., Li X. B. Chemical constituents of plants from the genus viburnum. Chemistry and Biodiversity. 2010;7(3):567–593. doi: 10.1002/cbdv.200800254. [DOI] [PubMed] [Google Scholar]
- 32.Česonienė L., Daubaras R., Venclovienė J., Viškelis P. Biochemical and agro-biological diversity of Viburnum opulus genotypes. Central European Journal of Biology. 2010;5(6):864–871. doi: 10.2478/s11535-010-0088-z. [DOI] [Google Scholar]
- 33.Ersoy N., Ercisli S., Gundogdu M. Evaluation of European Cranberrybush (Viburnum opulus L.) genotypes for agro-morphological, biochemical and bioactive characteristics in Turkey. Folia Horticulturae. 2017;29(2):181–188. doi: 10.1515/fhort-2017-0017. [DOI] [Google Scholar]
- 34.Polka D., Podsędek A., Koziołkiewicz M. Comparison of chemical composition and antioxidant capacity of fruit, flower and bark of Viburnum opulus. Plant Foods for Human Nutrition. 2019;74(3):436–442. doi: 10.1007/s11130-019-00759-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Latif A., Shinwari Z. K., Hussain J., Murtaza S. NTFPS: an alternative to forest logging in Minadam and Sultanar Valley Swat. Lyonia. 2006;11(2):15–21. [Google Scholar]
- 36.Kumar M., Paul Y., Anand V. K. An ethnobotanical study of medicinal plants used by the locals in Kishtwar, Jammu and Kashmir, India. Journal of Ethnobiology and Ethnomedicine. 2009;2009(10) [Google Scholar]
- 37.Shah Z., Ali F., Ullah H., et al. Biological screening and chemical constituents of Viburnum grandiflorum. Journal of the Chemical Society of Pakistan. 2014;36 [Google Scholar]
- 38.Amjad M. S., Arshad M., Qureshi R. Ethnobotanical inventory and folk uses of indigenous plants from Pir Nasoora National Park, Azad Jammu and Kashmir. Asian Pacific Journal of Tropical Biomedicine. 2015;5(3):234–241. doi: 10.1016/S2221-1691(15)30011-3. [DOI] [Google Scholar]
- 39.Suleman M., Nouren S., Hassan S., et al. Vitality and implication of natural products from viburnum grandiflorum: an eco-friendly approach. Polish Journal of Environmental Studies. 2018;27(3):1407–1411. doi: 10.15244/pjoes/76798. [DOI] [Google Scholar]
- 40.Zayachkivska O. S., Gzhegotsky M. R., Terletska O. I., Lutsyk D. A., Yaschenko A. M., Dzhura O. R. Influence of viburnum opulus proanthocyanidins on stress-induced gastrointestinal mucosal damage. Journal of Physiology and Pharmacology. 2006;57 [PubMed] [Google Scholar]
- 41.Levent Altun M., Saltan Çitoğlu G., Sever Yilmaz B., Çoban T. Antioxidant properties of Viburnum opulus and Viburnum lantana growing in Turkey. International Journal of Food Sciences and Nutrition. 2008;59(3):175–180. doi: 10.1080/09637480701381648. [DOI] [PubMed] [Google Scholar]
- 42.Česonienė L., Daubaras R., Viškelis P., Šarkinas A. Determination of the total phenolic and anthocyanin contents and antimicrobial activity of Viburnum opulus fruit juice. Plant Foods for Human Nutrition. 2012;67(3):256–261. doi: 10.1007/s11130-012-0303-3. [DOI] [PubMed] [Google Scholar]
- 43.Akgül G., Yılmaz N., Celep A., Celep F., Çakılcıoğlu U. Ethnobotanical purposes of plants sold by herbalists and folk bazaars in the center of cappadocica (Nevşehir, Turkey) Indian Journal of Traditional Knowledge. 2016;15(1):103–108. [Google Scholar]
- 44.Erdem G., Kesik V., Honca T., et al. Antinephrolithiatic activity of Persea americana (avocado) and Viburnum opulus (guelder rose) against ethylene glycol-induced nephrolithiasis in rats. African Journal of Traditional, Complementary and Alternative Medicines. 2016;13(2) doi: 10.4314/ajtcam.v13i2.14. [DOI] [Google Scholar]
- 45.Shikov A. N., Tsitsilin A. N., Pozharitskaya O. N., Makarov V. G., Heinrich M. Traditional and current food use of wild plants listed in the Russian Pharmacopoeia. Frontiers in Pharmacology. 2017;8 doi: 10.3389/fphar.2017.00841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhu X., Dong X., Wang Y., Ju P., Luo S. Phenolic compounds from Viburnum cylindricum. Helvetica Chimica Acta. 2005;88(2):339–342. doi: 10.1002/hlca.200590017. [DOI] [Google Scholar]
- 47.Singh S., Bhat J. A., Malik Z. A., Youssouf M., Bussmann R. W., Kunwar R. M. Sacred groves in Western Himalaya, India: community-managed nature refuges for conservation of biodiversity and culture. Ethnobotany Research and Applications. 2019;18 doi: 10.32859/era.18.15.1-21. [DOI] [Google Scholar]
- 48.Machida K., Nakano Y., Kikuchi M. Phenolic glycosides from Viburnum dilatatum. Phytochemistry. 1991;30(6):2013–2014. doi: 10.1016/0031-9422(91)85058-8. [DOI] [Google Scholar]
- 49.Chhetri P., Gudade B. A., Bhattarai N. K., et al. Common shade trees of large cardamom and its ethno botanical studies in Sikkim and darjeeling, India. Ecology, Environment and Conservation. 2014;20 [Google Scholar]
- 50.Qureshi R. A., Ghufran M. A., Gilani S. A., Yousaf Z., Abbas G., Batool A. Indigenous medicinal plants used by local women in southern Himalayan regions of Pakistan. Pakistan Journal of Botany. 2009;41 [Google Scholar]
- 51.Özbilgin S., Ergene B., Altun M. L., Sever Yılmaz B., Saltan G., Yüksel E. HPLC method for the analysis of chlorogenic acid of Viburnum tinus L. and Viburnum orientale Pallas. Turkish Journal of Pharmaceutical Sciences. 2015;12(2):130–136. doi: 10.5505/tjps.2015.54264. [DOI] [Google Scholar]
- 52.Zahid Iqbal Awan Z. I. A. Ethnobotanical importance of some highly medicinal plants of district Muzaffarabad, Pakistan with special reference to the species of the genus Viburnum. IOSR Journal of Pharmacy and Biological Sciences. 2013;6(2):53–66. doi: 10.9790/3008-0625366. [DOI] [Google Scholar]
- 53.Nobre J., Santos C., Romano A. Micropropagation of the Mediterranean species Viburnum tinus. Plant Cell, Tissue and Organ Culture. 2000;60(1):75–78. doi: 10.1023/A:1006405700121. [DOI] [Google Scholar]
- 54.Tiwari B. K., Khosa R. Studies on Viburnum nervosum Hook: chemistry and spectroscopy of bergenin and its derivatives. East and Central African Journal of Pharmaceutical Sciences. 2009;12 [Google Scholar]
- 55.Bhat J. A., Kumar M., Bussmann R. W. Ecological status and traditional knowledge of medicinal plants in Kedarnath Wildlife Sanctuary of Garhwal Himalaya, India. Journal of Ethnobiology and Ethnomedicine. 2013;9(1):p. 1. doi: 10.1186/1746-4269-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Prabhu K., Ponnudurai K., Hemalatha S., Karar P. K. Pharmacognostic investigations on the leaves of Viburnum coriaceum Blume. Natural Product Radiance. 2009;8 [Google Scholar]
- 57.Rymbai H., Roy A. R., Deshmukh N. A., et al. Analysis study on potential underutilized edible fruit genetic resources of the foothills track of eastern Himalayas, India. Genetic Resources and Crop Evolution. 2016;63(1):125–139. doi: 10.1007/s10722-015-0342-3. [DOI] [Google Scholar]
- 58.Jacobo-Herrera N. J., Jacobo-Herrera F. E., Zentella-Dehesa A., Andrade-Cetto A., Heinrich M., Pérez-Plasencia C. Medicinal plants used in Mexican traditional medicine for the treatment of colorectal cancer. Journal of Ethnopharmacology. 2016;179:391–402. doi: 10.1016/j.jep.2015.12.042. [DOI] [PubMed] [Google Scholar]
- 59.Villarreal-Quintanilla J. Á., Estrada-Castillón A. E. Revisión taxonómica del género Viburnum (Adoxaceae) para México. Botanical Sciences. 2014;92(4):p. 493. doi: 10.17129/botsci.103. [DOI] [Google Scholar]
- 60.Wang D., Yang Y., Shi X., et al. Viburnumfocesides A - D, 1- O -isovaleroylated iridoid 11- O -alloside derivatives from Viburnum foetidum var. ceanothoides. Fitoterapia. 2020;143, article 104601 doi: 10.1016/j.fitote.2020.104601. [DOI] [PubMed] [Google Scholar]
- 61.Shao J. H., Chen J., Zhao C. C., et al. Insecticidal and α-glucosidase inhibitory activities of chemical constituents from Viburnum fordiae Hance. Natural Product Research. 2019;33(18):2662–2667. doi: 10.1080/14786419.2018.1466130. [DOI] [PubMed] [Google Scholar]
- 62.Zarifikhosroshahi M., Tugba Murathan Z., Kafkas E., Okatan V. Variation in volatile and fatty acid contents among Viburnum opulus L. fruits growing different locations. Scientia Horticulturae. 2020;264, article 109160 doi: 10.1016/j.scienta.2019.109160. [DOI] [Google Scholar]
- 63.Zhang H., Liu Y., Zhang H., Chen J., Wang K. New tetrahydrofuran type lignan from viburnum chinshanense. Natural Product Communications. 2018;13(7) doi: 10.1177/1934578x1801300718. [DOI] [Google Scholar]
- 64.Prabhu K., Karar P. K., Hemalatha S., Ponnudurai K. Comparative micromorphological and phytochemical studies on the roots of three Viburnum (Caprifoliaceae) species. Turkish Journal of Botany. 2011;35 doi: 10.3906/bot-1006-7. [DOI] [Google Scholar]
- 65.Muhaisen H. M. H., Ilyas M., Mushfiq M., Parveen M., Basudan O. A. Flavonoid from Viburnum cotinifolium. Journal of Chemical Research. 2002;2002(10):480–481. doi: 10.3184/030823402103170655. [DOI] [Google Scholar]
- 66.Tu L., Xu G., Zhao Y., et al. Seven new phenolic glucosides from Viburnum cylindricum. Helvetica Chimica Acta. 2009;92(7):1324–1332. doi: 10.1002/hlca.200800420. [DOI] [Google Scholar]
- 67.Wu B., Zheng X., Qu H., Cheng Y. Phenolic glycosides from Viburnum fordiae Hance and their antioxidant activities. Letters in Organic Chemistry. 2008;5(4):324–327. doi: 10.2174/157017808784049407. [DOI] [Google Scholar]
- 68.Wu B., Zeng X., Zhang Y. New metabolite from Viburnum dilatatum. Natural Product Communications. 2010;5(7) doi: 10.1177/1934578x1000500723. [DOI] [PubMed] [Google Scholar]
- 69.Iwai K., Kim M. Y., Onodera A., Matsue H. α-Glucosidase inhibitory and antihyperglycemic effects of polyphenols in the fruit of Viburnum dilatatum Thunb. Journal of Agricultural and Food Chemistry. 2006;54(13):4588–4592. doi: 10.1021/jf0606353. [DOI] [PubMed] [Google Scholar]
- 70.Iwai K., Kim M. Y., Onodera A., Matsue H. Physiological effects and active ingredients of Viburnum dilatatum Thunb fruits on oxidative stress. BioFactors. 2004;21(1-4):273–275. doi: 10.1002/biof.552210153. [DOI] [PubMed] [Google Scholar]
- 71.Miyazawa M., Hashidume S., Takahashi T., Kikuchi T. Aroma evaluation of gamazumi (Viburnum dilatatum) by aroma extract dilution analysis and odour activity value. Phytochemical Analysis. 2012;23(3):208–213. doi: 10.1002/pca.1344. [DOI] [PubMed] [Google Scholar]
- 72.Lu D., Yao S. Phenolic glycoside from the roots of Viburnum dilatatum. Natural Product Communications. 2009;4(7) doi: 10.1177/1934578x0900400714. [DOI] [PubMed] [Google Scholar]
- 73.In S. J., Seo K. H., Kim H. G., Song N. Y., Baek N. I. New iridoid from the stems of Viburnum erosum. Chemistry of Natural Compounds. 2017;53(2):265–268. doi: 10.1007/s10600-017-1967-6. [DOI] [Google Scholar]
- 74.In S. J., Seo K. H., Song N. Y., Lee D. S., Kim Y. C., Baek N. I. Lignans and neolignans from the stems of Vibrunum erosum and their neuroprotective and anti-inflammatory activity. Archives of Pharmacal Research. 2015;38(1):26–34. doi: 10.1007/s12272-014-0358-9. [DOI] [PubMed] [Google Scholar]
- 75.In S. J., Seo K. H., Song N. Y., Song M. C., An E. M., Baek N. I. Iridoids from the stems of Viburnum erosum. Holzforschung. 2014;68(7):761–767. doi: 10.1515/hf-2013-0227. [DOI] [Google Scholar]
- 76.Prabhu K., Karar P. K., Ponnudurai K., Hemalatha S. Pharmacognostic investigation of the leaves and stems of Viburnum erubescens Wall.ex DC. Tropical Journal of Pharmaceutical Research. 2009;8(6) doi: 10.4314/tjpr.v8i6.49404. [DOI] [Google Scholar]
- 77.Chen J., Shao J., Zhao C., et al. A novel norneolignan glycoside and four new phenolic glycosides from the stems of Viburnum fordiae Hance. Holzforschung. 2018;72(4):259–266. doi: 10.1515/hf-2017-0151. [DOI] [Google Scholar]
- 78.Chen J., Shao J., Zhao C., et al. Chemical constituents from Viburnum fordiae Hance and their anti-inflammatory and antioxidant activities. Archives of Pharmacal Research. 2018;41(6):625–632. doi: 10.1007/s12272-018-1026-2. [DOI] [PubMed] [Google Scholar]
- 79.Chen J., Huang X. H., Shao J. H., Xu X. Q., Zhang F. M., Zhao C. C. A new phenolic compound with antifungal activity from Viburnum fordiae. Chemistry of Natural Compounds. 2016;52(2):222–223. doi: 10.1007/s10600-016-1599-2. [DOI] [Google Scholar]
- 80.Shao J. H., Chen J., Xu X. Q., et al. Chemical constituents and biological activities of Viburnum macrocephalum f. keteleeri. Natural Product Research. 2019;33(11):1612–1616. doi: 10.1080/14786419.2018.1428593. [DOI] [PubMed] [Google Scholar]
- 81.Zhao C., Chen J., Shao J., et al. Neolignan constituents with potential beneficial effects in prevention of type 2 diabetes from Viburnum fordiae Hance fruits. Journal of Agricultural and Food Chemistry. 2018;66(40):10421–10430. doi: 10.1021/acs.jafc.8b03772. [DOI] [PubMed] [Google Scholar]
- 82.Yang G., Ye M., Hu C., Xiong J., Hu J. New iridoids possessing a dioxatricyclodecane skeleton from the leaves of viburnum formosanum hayata subsp. leiogynum hsu. Chinese Journal of Organic Chemistry. 2015;35(2):p. 428. doi: 10.6023/cjoc201409033. [DOI] [Google Scholar]
- 83.Yilmaz N., Yayli N., Misir G., Karaoglu S., Yayli N. Chemical composition and antimicrobial activities of the essential oils of Viburnum opulus, Viburnum lantana and Viburnum orientala. Asian Journal of Chemistry. 2008;20 [Google Scholar]
- 84.Xu X. Q., Huang X. H., Chen J., Shao J. H., Zhao C. C. A new flavonoid glycoside from Viburnum macrocephalum f. keteleeri. Chemistry of Natural Compounds. 2017;53(6):1035–1037. doi: 10.1007/s10600-017-2196-8. [DOI] [Google Scholar]
- 85.Zhao Z. Y., Chen J., Shen J., Shao J. H., Huang X. H., Zhao C. C. A new phenolic glycoside from Viburnum melanocarpum fruits and its α-glucosidase inhibitory activity. Chemistry of Natural Compounds. 2020;56(2):246–248. doi: 10.1007/s10600-020-02998-0. [DOI] [Google Scholar]
- 86.Zhao C., Chen J., Shao J., et al. Lignan glycosides from the stems of Viburnum melanocarpum and their α-glucosidase inhibitory activity. Holzforschung. 2019;74(1):88–93. doi: 10.1515/hf-2019-0159. [DOI] [Google Scholar]
- 87.Shen J., Shao J. H., Chen J., Liu W. Y., Zhao C. C. Two new phenolic glycosides from Viburnum melanocarpum. Chemistry of Natural Compounds. 2019;55(1):25–27. doi: 10.1007/s10600-019-02607-9. [DOI] [Google Scholar]
- 88.Zhu Q. F., Qi Y. Y., Zhang Z. J., et al. Vibsane-type diterpenoids from Viburnum odoratissimum and their cytotoxic and HSP90 inhibitory activities. Chemistry and Biodiversity. 2018;15(5) doi: 10.1002/cbdv.201800049. [DOI] [PubMed] [Google Scholar]
- 89.Fukuyama Y., Kubo M., Esumi T., Harada K., Hioki H. ChemInform abstract: chemistry and biological activities of vibsane-type diterpenoids. ChemInform. 2010;41(49) doi: 10.1002/chin.201049237. [DOI] [Google Scholar]
- 90.Kubo M., Chen I. S., Fukuyama Y. Vibsane-type diterpenes from Taiwanese Viburnum odoratissimum. Chemical and Pharmaceutical Bulletin. 2001;49(2):242–245. doi: 10.1248/cpb.49.242. [DOI] [PubMed] [Google Scholar]
- 91.He J., Peng L. Y., Tu L., et al. Vibsane-type diterpenes from leaves and twigs of Viburnum odoratissimum. Fitoterapia. 2016;109:224–229. doi: 10.1016/j.fitote.2016.01.014. [DOI] [PubMed] [Google Scholar]
- 92.Li F. J., Yu J. H., Wang G. C., Zhang H., Yue J. M. Diterpenes and lignans from Viburnum odoratissimum var. odoratissimum. Journal of Asian Natural Products Research. 2015;17(5):475–481. doi: 10.1080/10286020.2015.1041934. [DOI] [PubMed] [Google Scholar]
- 93.Shen Y. C., Lin C. L., Chien S. C., Khalil A. T., Ko C. L., Wang C. H. Vibsane diterpenoids from the leaves and flowers of Viburnum odoratissimum. Journal of Natural Products. 2004;67(1):74–77. doi: 10.1021/np030173c. [DOI] [PubMed] [Google Scholar]
- 94.Shen Y. C., Prakash C. V. S., Wang L. T., Chien C. T., Hung M. C. New vibsane diterpenes and lupane triterpenes from Viburnum odoratissimum. Journal of Natural Products. 2002;65(7):1052–1055. doi: 10.1021/np020007p. [DOI] [PubMed] [Google Scholar]
- 95.Wei J. F., Yin Z. H., Kang W. Y. Volatiles in flowers of Viburnum odoratissimum. Chemistry of Natural Compounds. 2013;49(1):154–155. doi: 10.1007/s10600-013-0541-0. [DOI] [Google Scholar]
- 96.Ge Y. C., Zhang H. J., Lei J. X., Wang K. W. Chemical constituents of Viburnum odoratissimum and their cytotoxic activities. Chemistry of Natural Compounds. 2018;54(3):600–602. doi: 10.1007/s10600-018-2422-z. [DOI] [Google Scholar]
- 97.Akbulut M., Calisir S., Marakoglu T., Coklar H. Chemical and technological properties of European cranberrybush (Viburnum opulus L.) fruits. Asian Journal of Chemistry. 2008;20 [Google Scholar]
- 98.Barak T. H., Celep E., İnan Y., Yesilada E. Influence of in vitro human digestion on the bioavailability of phenolic content and antioxidant activity of Viburnum opulus L. (European cranberry) fruit extracts. Industrial Crops and Products. 2019;131:62–69. doi: 10.1016/j.indcrop.2019.01.037. [DOI] [Google Scholar]
- 99.Zakłos-Szyda M., Pawlik N., Polka D., Nowak A., Koziołkiewicz M., Podsędek A. Viburnum opulus fruit phenolic compounds as cytoprotective agents able to decrease free fatty acids and glucose uptake by Caco-2 cells. Antioxidants. 2019;8(8):p. 262. doi: 10.3390/antiox8080262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Karaçelik A. A., Küçük M., İskefiyeli Z., et al. Antioxidant components of Viburnum opulus L. determined by on-line HPLC -UV- ABTS radical scavenging and LC-UV-ESI-MS methods. Food Chemistry. 2015;175:106–114. doi: 10.1016/j.foodchem.2014.11.085. [DOI] [PubMed] [Google Scholar]
- 101.Kraujalytė V., Venskutonis P. R., Pukalskas A., Česonienė L., Daubaras R. Antioxidant properties and polyphenolic compositions of fruits from different European cranberrybush (Viburnum opulus L.) genotypes. Food Chemistry. 2013;141(4):3695–3702. doi: 10.1016/j.foodchem.2013.06.054. [DOI] [PubMed] [Google Scholar]
- 102.Rop O., Reznicek V., Valsikova M., Jurikova T., Mlcek J., Kramarova D. Antioxidant properties of European cranberrybush fruit (Viburnum opulus var.edule) Molecules. 2010;15(6):4467–4477. doi: 10.3390/molecules15064467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Çam M., Hişil Y. Comparison of chemical characteristics of fresh and pasteurised juice of gilaburu (Viburnum opulus L.) Acta Alimentaria. 2007;36(3):381–385. doi: 10.1556/AAlim.36.2007.3.10. [DOI] [Google Scholar]
- 104.Sedat Velioglu Y., Ekici L., Poyrazoglu E. S. Phenolic composition of European cranberrybush (Viburnum opulus L.) berries and astringency removal of its commercial juice. International Journal of Food Science and Technology. 2006;41(9):1011–1015. doi: 10.1111/j.1365-2621.2006.01142.x. [DOI] [Google Scholar]
- 105.Karimova A. R., Yunusova S. G., Maslennikov S. I., et al. Lipids, lipophilic components, and biologically active fractions of Viburnum opulus L. seeds. Chemistry of Natural Compounds. 2000;36(6):560–564. doi: 10.1023/A:1017551306132. [DOI] [Google Scholar]
- 106.Orhan I. E., Senol F. S., Yılmaz B. S., et al. Neuroprotective potential of Viburnum orientale Pallas through enzyme inhibition and antioxidant activity assays. South African Journal of Botany. 2018;114:126–131. doi: 10.1016/j.sajb.2017.10.023. [DOI] [Google Scholar]
- 107.Katagiri S., Watanabe Y., Yaoita Y., Kikuchi M., Machida K. Two new phenolic glycosides from Viburnum plicatum var. plicatum f. plicatum. Natural Product Communications. 2011;6(12) doi: 10.1177/1934578x1100601227. [DOI] [PubMed] [Google Scholar]
- 108.Kikuchi M., Onoguchi R., Yaoita Y., Machida K. Two new glycosides from Viburnum plicatum Thunb. ex Murray var. plicatum f. plicatum. Journal of Natural Medicines. 2011;65(1):202–205. doi: 10.1007/s11418-010-0455-0. [DOI] [PubMed] [Google Scholar]
- 109.Machida K., Sagawa H., Onoguchi R., Kikuchi M. Three new glycosides from Viburnum plicatum THUNB. var. tomentosum MIQ. Helvetica Chimica Acta. 2010;93(2):290–297. doi: 10.1002/hlca.200900232. [DOI] [Google Scholar]
- 110.Wang X. Y., Shi H. M., Zhang L., Li X. B. A new chalcone glycoside, a new tetrahydrofuranoid lignan, and antioxidative constituents from the stems and leaves of viburnum propinquum. Planta Medica. 2009;75(11):1262–1265. doi: 10.1055/s-0029-1185523. [DOI] [PubMed] [Google Scholar]
- 111.Dietz B. M., Hajirahimkhan A., Dunlap T. L., Bolton J. L. Botanicals and their bioactive phytochemicals for women’s health. Pharmacological Reviews. 2016;68(4):1026–1073. doi: 10.1124/pr.115.010843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Xie Y., Wang J., Geng Y. M., Zhang Z., Qu Y. F., Wang G. S. Pheonolic compounds from the fruits of Viburnum sargentii Koehne. Molecules. 2015;20(8):14377–14385. doi: 10.3390/molecules200814377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Iwai K., Onodera A., Iwai K., Morinaga Y., Matsue H. Antihyperglycemic and antioxidant effects of flesh and peel from pomace of Viburnum dilatatum on normal and diabetic mice. Nippon Shokuhin Kagaku Kogaku Kaishi. 2011;58(9):413–420. doi: 10.3136/nskkk.58.413. [DOI] [Google Scholar]
- 114.Zhu X. Y., Lu R. M., Lu G. Z., Zhao H. Y. Analysis of chemical constituents of essential oils from Viburnum fordiae by GC-MS. Lishizhen Medicine and Materia Medica Research. 2011;22:2101–2102. [Google Scholar]
- 115.Kraujalytė V., Leitner E., Venskutonis P. R. Chemical and sensory characterisation of aroma of Viburnum opulus fruits by solid phase microextraction-gas chromatography -olfactometry. Food Chemistry. 2012;132(2):717–723. doi: 10.1016/j.foodchem.2011.11.007. [DOI] [Google Scholar]
- 116.Paur I., Carlsen M. H., Halvorsen B. L., Blomhoff R. Herbal Medicine: Biomolecular and Clinical Aspects. 2nd. Press/Taylor & Francis; 2011. Antioxidants in herbs and spices: roles in oxidative stress and redox signaling. [PubMed] [Google Scholar]
- 117.Wojdyło A., Oszmiański J., Czemerys R. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chemistry. 2007;105(3):940–949. doi: 10.1016/j.foodchem.2007.04.038. [DOI] [Google Scholar]
- 118.Koşar M., Orakçı E., Şeker Karatoprak G. Antioxidant properties and phenolic composition of Viburnum opulus from Turkey. Planta Medica. 2011;77(12) doi: 10.1055/s-0031-1282688. [DOI] [Google Scholar]
- 119.Sagdic O., Aksoy A., Ozkan G. Evaluation of the antibacterial and antioxidant potentials of cranberry (gilaburu, Viburnum opulus L.) fruit extract. Acta Alimentaria. 2006;35(4):487–492. doi: 10.1556/AAlim.35.2006.4.12. [DOI] [Google Scholar]
- 120.Andreeva T. I., Komarova E. N., Yusubov M. S., Korotkova E. I. Medicinal plants: antioxidant activity of cranberry tree (Viburnum opulus L.) bark extract. Pharmaceutical Chemistry Journal. 2004;38(10):548–550. doi: 10.1007/s11094-005-0007-1. [DOI] [Google Scholar]
- 121.Bubulica M. V., Anghel I., Grumezescu A. M., et al. In vitro evaluation of bactericidal and antibiofilm activity of Lonicera tatarica and Viburnum opulus plant extracts on Staphylococcus strains. Farmacia. 2012;60 [Google Scholar]
- 122.Moldovan B., Ghic O., David L., Chisbora C. The influence of storage on the total phenols content and antioxidant activity of the cranberrybush (Viburnum opulus L.) fruits extract. Revista de Chimie. 2012;63 [Google Scholar]
- 123.Al Ö. The effects of different fractions of gilaburu Viburnum opulus juice on the experimentally induced cancer in mice. International Journal of Experimental & Clinical Anatomy. 2019;13 [Google Scholar]
- 124.Moskalets T. Z., Moskalets V. V., Vovkohon А. H., Knyazyuk О. V. Fruits of new selection forms and varieties of snowball tree for manufacture of products of therapeutic and prophylactic purpose. Regulatory Mechanisms in Biosystems. 2019;10 doi: 10.15421/021964. [DOI] [Google Scholar]
- 125.Kraujalis P., Kraujalienė V., Kazernavičiūtė R., Venskutonis P. R. Supercritical carbon dioxide and pressurized liquid extraction of valuable ingredients from Viburnum opulus pomace and berries and evaluation of product characteristics. Journal of Supercritical Fluids. 2017;122:99–108. doi: 10.1016/j.supflu.2016.12.008. [DOI] [Google Scholar]
- 126.Çanga E. M., Dudak F. C. Characterization of cellulose acetate/gum Arabic fibers loaded with extract of Viburnum opulus L. fruit. LWT. 2019;110:247–254. doi: 10.1016/j.lwt.2019.04.085. [DOI] [Google Scholar]
- 127.Şeker I. T., Ertop M. H., Hayta M. Physicochemical and bioactive properties of cakes incorporated with gilaburu fruit (Viburnum opulus) pomace. Quality Assurance and Safety of Crops & Foods. 2016;8(2):261–266. doi: 10.3920/QAS2014.0542. [DOI] [Google Scholar]
- 128.Cemtekin B., Kilinc E., Karabacak L., et al. Aa evaluationof guelder rose (Viburnum opulus L.) and hawthorn (Crataegus monogyna) concentrates as alternative antioxidant sources to BHT and nitrite in poultry meat model system. Scientific Papers: Series D, Animal Science-The International Session of Scientific Communications of the Faculty of Animal Science. 2019;62 [Google Scholar]
- 129.Erdogan-Orhan I., Altun M. L., Sever-Yilmaz B., Saltan G. Anti-acetylcholinesterase and antioxidant assets of the major components (salicin, amentoflavone, and chlorogenic acid) and the extracts of Viburnum opulus and Viburnum lantana and their total phenol and flavonoid contents. Journal of Medicinal Food. 2011;14(4):434–440. doi: 10.1089/jmf.2010.0053. [DOI] [PubMed] [Google Scholar]
- 130.Bolser J. A., Alan R. R., Smith A. D., Li L., Seeram N. P., McWilliams S. R. Birds select fruits with more anthocyanins and phenolic compounds during autumn migration. The Wilson Journal of Ornithology. 2013;125(1):97–108. doi: 10.1676/12-057.1. [DOI] [Google Scholar]
- 131.Serteser A., Kargıoğlu M., Gök V., Bağci Y., Özcan M. M., Arslan D. Antioxidant properties of some plants growing wild in Turkey. Grasas y Aceites. 2009;60(2):147–154. doi: 10.3989/gya.086208. [DOI] [Google Scholar]
- 132.Orodan M., Vodnar D. C., Toiu A. M., et al. Phytochemical analysis, antimicrobial and antioxidant effect of some gemmotherapic remedies used in respiratory diseases. Farmacia. 2016;64 [Google Scholar]
- 133.Shafaghat A., Shafaghatlonbar M. Two new chalcone glycoside compounds from Viburnum lantana (family Caprifoliaceae) and antioxidant activity of its hydroalcoholic extract. Letters in Organic Chemistry. 2019;16(2):93–98. doi: 10.2174/1570178615666180925125213. [DOI] [Google Scholar]
- 134.Patil M. P., Seong Y., Kang M. J., et al. Antibacterial and antioxidant potential of methanol extract of Viburnum sargentii seeds. Journal of Life Science. 2019;29 doi: 10.5352/JLS.2019.29.6.671. [DOI] [Google Scholar]
- 135.Awan Z. I., Minhas F. A., Awan A. A., Khan M. N. Antioxidant and antimicrobial activities of essential oil and extracts of Viburnum nervosum growing wild in the state of Jammu and Kashmir. International Journal of Innovative Research and Development. 2013;2(5):1881–1895. [Google Scholar]
- 136.Fu L., Xu B. T., Xu X. R., Qin X. S., Gan R. Y., Li H. B. Antioxidant capacities and total phenolic contents of 56 wild fruits from South China. Molecules. 2010;15(12):8602–8617. doi: 10.3390/molecules15128602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Leiner R. H., Holloway P. S., Neal D. B. Antioxidant capacity and quercetin levels in Alaska wild berries. International Journal of Fruit Science. 2006;6(1):83–91. doi: 10.1300/J492v06n01_06. [DOI] [Google Scholar]
- 138.Iwai K., Onodera A., Matsue H. Antioxidant activity and inhibitory effect of Gamazumi (Viburnum dilatatum THUNB.) on oxidative damage induced by water immersion restraint stress in rats. International Journal of Food Sciences and Nutrition. 2001;52(5):443–451. doi: 10.1080/09637480120078339. [DOI] [PubMed] [Google Scholar]
- 139.Woo Y., Lee H., Jeong Y. S., et al. Antioxidant potential of selected Korean edible plant extracts. BioMed Research International. 2017;2017:9. doi: 10.1155/2017/7695605.7695605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ponnudurai K., Prabhu K., Murthy M. S., Rani S. S. Antioxidant potentials of 80% methanolic leaf fractions of Viburnum punctatum, Viburnum coriaceum and Viburnum erubescens. International Journal of Pharmacy and Pharmaceutical Analysis. 2016;1(1):33–41. [Google Scholar]
- 141.Huang H. L., Qiu S. M., Wang H. Y., Wang Z. H. Antioxidative principals of extracts from the fruit of Viburnum awabuki. Advanced Materials Research. 2013;709:875–878. doi: 10.4028/www.scientific.net/AMR.709.875. [DOI] [Google Scholar]
- 142.Abbasi A. M., Shah M. H. Assessment of phenolic contents, essential/toxic metals and antioxidant capacity of fruits of Viburnum foetens decne. Biointerface Research in Applied Chemistry. 2018;8(3):3178–3186. [Google Scholar]
- 143.Yılmaz B. S., Altun M. L., Orhan I. E., Ergene B., Citoglu G. S. Enzyme inhibitory and antioxidant activities of Viburnum tinus L. relevant to its neuroprotective potential. Food Chemistry. 2013;141(1):582–588. doi: 10.1016/j.foodchem.2013.03.020. [DOI] [PubMed] [Google Scholar]
- 144.Elaloui M., Ennajah A., Ghazghazi H., et al. A comparative phytochemical and biological study between different solvent extracts of leaves and stems extracts of Erica arborea L. and Viburnum tinus L. plants growing in Tunisia. Current Bioactive Compounds. 2019;15(6):686–691. doi: 10.2174/1573407214666180730110232. [DOI] [Google Scholar]
- 145.Jayasheela D. Evaluation of in vitro antioxidant potential of Viburnum punctatum. International Journal of Innovative Pharmaceutical Sciences and Research. 2016;4(7):817–831. [Google Scholar]
- 146.Bhagat M., Jamwal V. S., Choudhary P., Kaul A., Singh J. Immunomodulatory, antioxidant potential and phytochemical study of some wild berries of NorthWestern Himalayan region: a comparative study. European Journal of Biotechnology and Bioscience. 2015;3(12):50–56. [Google Scholar]
- 147.Susmitha P. V., Arunlal V. B., Biju C. R., Babu G. Phytochemical screening and antioxidant activity of aqueous extract of the aerial parts of Viburnum punctatum Buch.-Ham. ex. D. Don. International Journal of Pharmaceutical Sciences and Research. 2013;4(6):2322–2326. [Google Scholar]
- 148.Qiu S. M., Huang H. L., Wang H. Y., Wang Z. H. Quantification of total flavonoids, total phenolic compounds and antioxidant activity of extracts from the seed of Viburnum odoratissimum. Advanced Materials Research. 2013;709:879–882. doi: 10.4028/www.scientific.net/AMR.709.879. [DOI] [Google Scholar]
- 149.Singh H., Lily M. K., Dangwal K. Viburnum mullaha D. DON fruit (Indian cranberry): a potential source of polyphenol with rich antioxidant, anti-elastase, anti-collagenase, and anti-tyrosinase activities. International Journal of Food Properties. 2017;20(8):1729–1739. doi: 10.1080/10942912.2016.1217878. [DOI] [Google Scholar]
- 150.Eken A., Yücel O., Boşgelmez İ. İ., et al. Ratlarda Akciğer Transplantasyonda Iskemi/Reperfüzyonun İndüklediği Oksidatif Hasara Karşı Viburnum opulus Meyve Ekstresinin Koruyucu Etkilerinin Araştırılması. Kafkas Universitesi Veteriner Fakultesi Dergisi. 2017;23 doi: 10.9775/kvfd.2016.16964. [DOI] [Google Scholar]
- 151.Sarıözkan S., Türk G., Eken A., Bayram L. Ç., Baldemir A., Doğan G. Gilaburu (Viburnum opulus L.) fruit extract alleviates testis and sperm damages induced by taxane-based chemotherapeutics. Biomedicine and Pharmacotherapy. 2017;95:1284–1294. doi: 10.1016/j.biopha.2017.09.057. [DOI] [PubMed] [Google Scholar]
- 152.İlhan M., Ergene B., Süntar I., et al. Preclinical evaluation of antiurolithiatic activity of viburnum opulus L. on sodium oxalate-induced urolithiasis rat model. Evidence-based Complementary and Alternative Medicine. 2014;2014:10. doi: 10.1155/2014/578103.578103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Iwai K., Onodera A., Matsue H. Mechanism of preventive action of Viburnum dilatatum Thunb (gamazumi) crude extract on oxidative damage in rats subjected to stress. Journal of the Science of Food and Agriculture. 2003;83(15):1593–1599. doi: 10.1002/jsfa.1580. [DOI] [Google Scholar]
- 154.Iwai K., Onodera A., Matsue H. Inhibitory effects of Viburnum dilatatum Thunb. (Gamazumi) on oxidation and hyperglycemia in rats with streptozotocin-induced diabetes. Journal of Agricultural and Food Chemistry. 2004;52(4):1002–1007. doi: 10.1021/jf0302557. [DOI] [PubMed] [Google Scholar]
- 155.Mohamed M. A., Marzouk M. S. A., Moharram F. A., El-Sayed M. M., Baiuomy A. R. Phytochemical constituents and hepatoprotective activity of Viburnum tinus. Phytochemistry. 2005;66(23):2780–2786. doi: 10.1016/j.phytochem.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 156.Pal M., Singh V., Agrawal J., Tewari S. K., Zhao Q. S. Chemical composition and antimicrobial activity of essential oil of Viburnum betulifolium. Journal of Medical Sciences. 2013;13(1):72–75. doi: 10.3923/jms.2013.72.75. [DOI] [Google Scholar]
- 157.Bibi Y., Nisa S., Waheed A., et al. Evaluation of Viburnum foetens for anticancer and antibacterial potential and phytochemical analysis. African Journal of Biotechnology. 2010;9 doi: 10.5897/AJB09.1867. [DOI] [Google Scholar]
- 158.Yasin A., Minhas F. A., Zubair M. Antibacterial screening of leaves of wild Viburnum nervosum and Viburnum foetens of Azad Kashmir. International Journal of Pharmaceutical Science Invention. 2013;2 [Google Scholar]
- 159.Turker A. U., Yildirim A. B., Karakas F. P. Antibacterial and antitumor activities of some wild fruits grown in Turkey. Biotechnology and Biotechnological Equipment. 2012;26(1):2765–2772. doi: 10.5504/bbeq.2011.0156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Eryilmaz M., Ozbilgin S., Ergene B., Yilmaz B., Altun M., Saltan G. Antimicrobial activity of Turkish Viurnum species. Bangladesh Journal of Botany. 2014;42(2):355–360. doi: 10.3329/bjb.v42i2.18044. [DOI] [Google Scholar]
- 161.Česonienė L., Daubaras R., Kraujalytė V., Venskutonis P. R., Šarkinas A. Antimicrobial activity of Viburnum opulus fruit juices and extracts. Journal für Verbraucherschutz und Lebensmittelsicherheit. 2014;9(2):129–132. doi: 10.1007/s00003-014-0864-1. [DOI] [Google Scholar]
- 162.Paulauskas A., Žukauskienė J., Žiaukienė D., et al. Differentiation of Viburnum accessions according to their molecular, biochemical, genotoxic and microbiological features of importance to selection. Academia Journal of Agricultural Research. 2015;3(6):81–93. [Google Scholar]
- 163.Khan A. M., Qureshi R. A., Gilani S. A., Ullah F. Antimicrobial activity of selected medicinal plants of Margalla hills, Islamabad, Pakistan. Journal of Medicinal Plant Research. 2011;5 [Google Scholar]
- 164.Roy S., Khatun R., Rahman M. A. A. In vitro antimicrobial and cytotoxic activities of various methanolic fractions of Viburnum foetidum L. (Adoxaceae) Journal of Pharmacognosy and Phytochemistry. 2017;6(5):183–186. [Google Scholar]
- 165.Corley D. A., Kerlikowske K., Verma R., Buffler P. Protective association of aspirin/NSAIDs and esophageal cancer: a systematic review and meta-analysis. Gastroenterology. 2003;124(1):47–56. doi: 10.1053/gast.2003.50008. [DOI] [PubMed] [Google Scholar]
- 166.Altun M. L., Saltan Çitoğlu G., Sever Yılmaz B., Özbek H. Antinociceptive and anti-inflammatory activities of Viburnum opulus. Pharmaceutical Biology. 2009;47(7):653–658. doi: 10.1080/13880200902918345. [DOI] [Google Scholar]
- 167.Hwang S. H., Lorz L. R., Yi D. K., Noh J. K., Yi Y. S., Cho J. Y. Viburnum pichinchense methanol extract exerts anti-inflammatory effects via targeting the NF- κB and caspase-11 non-canonical inflammasome pathways in macrophages. Journal of Ethnopharmacology. 2019;245:p. 112161. doi: 10.1016/j.jep.2019.112161. [DOI] [PubMed] [Google Scholar]
- 168.Van Q., Nayak B. N., Reimer M., Jones P. J. H., Fulcher R. G., Rempel C. B. Anti-inflammatory effect of Inonotus obliquus, Polygala senega L., and Viburnum trilobum in a cell screening assay. Journal of Ethnopharmacology. 2009;125(3):487–493. doi: 10.1016/j.jep.2009.06.026. [DOI] [PubMed] [Google Scholar]
- 169.Yılmaz B. S., Çitoğlu G. S., Altun M. L., Özbek H. Antinociceptive and anti-inflammatory activities of Viburnum lantana. Pharmaceutical Biology. 2007;45(3):241–245. doi: 10.1080/13880200701213187. [DOI] [Google Scholar]
- 170.Huh Y. K., Kang J. H., Lee S. Y., Yim D. S. Antiinflammatory, analgesic and hepatoprotective effects of aerial part of Viburum sargentii for. sterile. Korean Journal of Pharmacognosy. 2007;38 [Google Scholar]
- 171.Bujor A., Miron A., Luca S. V., et al. Metabolite profiling, arginase inhibition and vasorelaxant activity of Cornus mas, Sorbus aucuparia and Viburnum opulus fruit extracts. Food and Chemical Toxicology. 2019;133:p. 110764. doi: 10.1016/j.fct.2019.110764. [DOI] [PubMed] [Google Scholar]
- 172.Česonienė L., Daubaras R., Viškelis P. Evaluation of productivity and biochemical components in fruit of different Viburnum accessions. Biologija. 2008;54(2):93–96. doi: 10.2478/v10054-008-0018-4. [DOI] [Google Scholar]
- 173.Dennehy C. E. The use of herbs and dietary supplements in gynecology: an evidence-based review. Journal of Midwifery and Women’s Health. 2006;51(6):402–409. doi: 10.1016/j.jmwh.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 174.Tayarani-Najaran Z., Ahmad S. Current Cancer Treatment-Novel Beyond Conventional Approaches. IntechOpen; 2011. Cytotoxic plants: potential uses in prevention and treatment of cancer. [DOI] [Google Scholar]
- 175.Sauter C., Wolfensberger C. Anticancer activities as well as antiviral and virus-enhancing properties of aqueous fruit extracts from fifty-six European plant species. European Journal of Cancer and Clinical Oncology. 1989;25(6):987–990. doi: 10.1016/0277-5379(89)90159-4. [DOI] [PubMed] [Google Scholar]
- 176.Cantrell C. L., Berhow M. A., Phillips B. S., Duval S. M., Weisleder D., Vaughn S. F. Bioactive crude plant seed extracts from the NCAUR oilseed repository. Phytomedicine. 2003;10(4):325–333. doi: 10.1078/094471103322004820. [DOI] [PubMed] [Google Scholar]
- 177.Laux M. T., Aregullin M., Rodriguez E. Inhibition of Helicobacter pylori and gastric cancer cells by lipid aldehydes from Viburnum opulus (Adoxaceae) Natural Product Communications. 2007;2(10) doi: 10.1177/1934578x0700201009. [DOI] [Google Scholar]
- 178.Zakłos-Szyda M., Pawlik N. The influence of Viburnum opulus polyphenolic compounds on metabolic activity and migration of HeLa and MCF cells. Acta Innovations. 2019;2(31):33–42. doi: 10.32933/actainnovations.31.4. [DOI] [Google Scholar]
- 179.Korapal A. T. In vitro evaluation of Gilaburu (Viburnum Opulus L.) juice on different cell lines. Anadolu Journal of Educational Sciences International. 2019;9:549–571. doi: 10.18039/ajesi.577253. [DOI] [Google Scholar]
- 180.Yildirim A. B., Karakas F. P., Turker A. U. In vitro antibacterial and antitumor activities of some medicinal plant extracts, growing in Turkey. Asian Pacific Journal of Tropical Medicine. 2013;6(8):616–624. doi: 10.1016/S1995-7645(13)60106-6. [DOI] [PubMed] [Google Scholar]
- 181.Han B., Wu J., Huang L. Induction of apoptosis in lung cancer cells by Viburnum grandiflorum via mitochondrial pathway. Medical Science Monitor. 2020;26, article 920265 doi: 10.12659/MSM.920265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Alex A. R., Ilango K. Cytotoxicity effect of methanolic extract of aerial parts of viburnum punctatum buch-ham. Ex D. Don. Asian Journal of Chemistry. 2011;23 [Google Scholar]
- 183.Alex A. R., Ilango K. In vitro cytotoxic activity of isolated compounds from Viburnum punctatum buch-ham EX D. Don. International Journal of Current Pharmaceutical Research. 2016;9(1) doi: 10.22159/ijcpr.2017v9i1.16622. [DOI] [Google Scholar]
- 184.Ranjith A. A., Ilango K., Vishwanath A. B., Ganeshan S. In vitro hepatoprotective activity of extracts of Viburnum Punctatum buch-ham EX D. DON against carbon tetrachloride induced toxicity. International Journal of Pharmacy and Pharmaceutical Sciences. 2014;6 [Google Scholar]
- 185.Jayasheela D., Dhivya A., Sivakumar R. In vitro cytotoxic studies of Viburnum punctatum Buch.-Ham ex D. Don ethanolic leaf extract against MCF-7 breast cancer cell lines. International Journal of Innovative Pharmaceutical Sciences and Research. 2018;6(3):55–64. [Google Scholar]
- 186.Waheed A., Bibi Y., Nisa S., Chaudhary F. M., Sahreen S., Zia M. Inhibition of human breast and colorectal cancer cells by Viburnum foetens L. extracts in vitro. Asian Pacific Journal of Tropical Disease. 2013;3(1):32–36. doi: 10.1016/S2222-1808(13)60007-9. [DOI] [Google Scholar]
- 187.De Cerain A. L., Pinzón R., Calle J., Marín A., Monge A. Cytotoxic activities of Colombian plant extracts on Chinese hamster lung fibroblasts. Phytotherapy Research. 1996;10(5):431–432. doi: 10.1002/(SICI)1099-1573(199608)10:5<431::AID-PTR863>3.0.CO;2-M. [DOI] [Google Scholar]
- 188.Ponnudurai K., Prabhu K., Rani S. S., Murthy M. S. E. Coli AB 1157 susceptibility test, MTT assay on MCF-7 and HeLa cell lines of root and leaf fractions of Viburnum Linn. species. Indian Journal of Traditional Knowledge. 2019;18 [Google Scholar]
- 189.Calderón-Montaño J. M., Martínez-Sánchez S. M., Burgos-Morón E., et al. Screening for selective anticancer activity of 65 extracts of plants collected in Western Andalusia, Spain. 2018. [DOI] [PMC free article] [PubMed]
- 190.Shimizu K., Fukunaga S., Yoshikawa K., Kondo R. Screening of extracts of Japanese woods for melanin biosynthesis inhibition. Journal of Wood Science. 2007;53(2):153–160. doi: 10.1007/s10086-006-0824-1. [DOI] [Google Scholar]
- 191.Ceylan D., Aksoy A., Ertekin T., et al. The effects of gilaburu (Viburnum opulus) juice on experimentally induced Ehrlich ascites tumor in mice. Journal of Cancer Research and Therapeutics. 2018;14(2):314–320. doi: 10.4103/0973-1482.181173. [DOI] [PubMed] [Google Scholar]
- 192.Ulger H., Ertekin T., Karaca O., et al. Influence of gilaburu (Viburnum opulus) juice on 1,2-dimethylhydrazine (DMH)-induced colon cancer. Toxicology and Industrial Health. 2013;29(9):824–829. doi: 10.1177/0748233712445049. [DOI] [PubMed] [Google Scholar]
- 193.Liu J. R., Wang S. Y., Chen M. J., Yueh P. Y., Lin C. W. The anti-allergenic properties of milk kefir and soymilk kefir and their beneficial effects on the intestinal microflora. Journal of the Science of Food and Agriculture. 2006;86:2527–2533. doi: 10.1002/jsfa.2649. [DOI] [Google Scholar]
- 194.Edwards S. E., Rocha I. . C., Williamson E. M., Heinrich M. Phytopharmacy: An Evidence-Based Guide to Herbal Medicinal Products. Wiley-Blackwell; 2015. [DOI] [Google Scholar]
- 195.Kizilay F., Ulker V., Celik O., et al. The evaluation of the effectiveness of gilaburu (Viburnum opulus l.) extract in the medical expulsive treatment of distal ureteral stones. Turkish Journal of Urology. 2019;45(1):63–69. doi: 10.5152/tud.2019.23463. [DOI] [PMC free article] [PubMed] [Google Scholar]


