Abstract
A total of 22 patients who had developed an adverse cutaneous reaction to the Moderna or Pfizer vaccine underwent biopsies. Each patient was assessed light microscopically, and, in select biopsies, spike glycoprotein and cytokine assessment were also conducted. The patients developed self-limited cutaneous reactions often described clinically as urticarial or eczematous within 1 day to 4 weeks after receiving the first or second dose of the Pfizer or Moderna vaccine. Classic clinical and morphologic depictions of type IV cutaneous hypersensitivity with features of eczematous dermatitis, interface dermatitis, granulomatous inflammation, and/or lymphocytic vasculitic component were observed. Clinical and/or histologic features of perniosis, pityriasis rosea, pityriasis rubra pilaris, and guttate psoriasis were seen in select cases. In 2 cases the dominant picture was urticarial vasculitis, possibly reflective of an Arthus type III immune complex action. The biopsy specimens of normal skin post vaccine and of skin affected by the post-vaccine eruption showed rare deep microvessels positive for spike glycoprotein with no complement deposition contrasting with greater vascular deposition of spike protein and complement in skin biopsies from patients experiencing severe coronavirus disease 2019 (COVID-19). It is concluded that self-limited hypersensitivity reactions to the vaccine occur possibly owing to a substance found in the vaccine vehicle (eg, polyethylene glycol). An immune response that is directed against human-manufactured spike has to be considered because some of the reactions clinically and or histologically closely resemble mild COVID-19. Finally, vaccine-associated immune enhancement largely attributable to the adjuvant properties of the vaccine may unmask certain inflammatory milieus operational in psoriasis, atopic dermatitis, and subclinical hypersensitivity.
Introduction
More than 176 million cases of coronavirus disease 2019 (COVID-19), with more than 3.8 million deaths worldwide, have been reported. COVID-19 is a pandemic that has resulted in sweeping social changes but, at the same time, has drawn a unified front globally to bring its end to fruition. With the advent of a number of effective vaccines, worldwide herd immunity is predicted in the near future. Each COVID-19 vaccine has as its epicenter of functionality an immune response to the spike glycoprotein, the critical viral capsid protein that binds to angiotensin converting enzyme 2 (ACE2), obligatory for viral entry.1, 2, 3
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccines do not prevent infection. They do however thwart serious forms of COVID-19 by eliciting an effective T-cell–mediated and humoral- (antibody-) mediated immunity response. The two most commonly administered vaccines in the United States are the Moderna vaccine, which is a nucleoside modified messenger RNA that encodes SARS-CoV-2 spike protein (mRNA-1273), and the Pfizer-BioNTech mRNA, which encodes the same protein (BNT162b2).1 , 4 , 5 Also available is the Johnson & Johnson vaccine (Ad26.COV2.S), which is viral-vector based and uses a modified version of adenovirus 26 to encode the spike protein. The vaccines focus on the receptor binding protein of SARS-CoV-2, namely the spike glycoprotein, engineering our cells to produce this foreign protein and hence promoting an immune response that is effective in preventing significant disease complications from COVID-19. Paradoxically it is the spike glycoprotein that likely plays an important role in the pathogenesis of severe and critical COVID-19 and has been postulated as being at the crux of the vascular complications of COVID-19.
We have demonstrated in earlier studies that the SARS-CoV-2 spike glycoprotein attaches to endothelium via ACE2 and results in complement-mediated microvascular injury in the lung and in other microvascular fields where endothelia have high ACE2+ expression such as the skin and the brain.6 The basis of the complement activation is that the spike glycoprotein has specific sugar moieties that are recognized by mannan-binding lectin, leading to the activation of MASP-2, ultimately resulting in the formation of C5b-9, which then damages the cell membranes of the endothelium.7
Data have suggested that, in sites other than the lung and the nasopharynx, the spike glycoprotein engagement with endothelium is without intact virus, as revealed by the lack of viral particles on electron microscopy and the absence of any detectable viral RNA in situ.7 Similarly, mice injected with large doses of the S1 subunit (but not S2 subunit) of the spike protein developed neurologic signs associated with central nervous system vascular injury; the spike protein was evident in their damaged central nervous system microvessels.8 Thus, one could postulate that the spike glycoprotein that is synthesized by the myocytes after receiving the mRNA-based vaccine could disseminate to select ACE2+ microvessels. Based on the millions of people already vaccinated without incident, if this microvascular dissemination occurs, it appears to be at a level that is not clinically significant.
To date, no adverse microvascular or larger vessel thrombotic events that resemble severe and critical COVID-19 have occurred with either the Moderna or the Pfizer vaccine. Catastrophic thrombotic complications involving the sagittal sinus and splenic veins with the adenovirus vector DNA vaccines manufactured by AstraZeneca and Johnson & Johnson have been reported. The exact basis is unknown, but a clinical parallel has been made with heparin-induced thrombocytopenia in which patients develop antibodies to heparin and platelet 4 complex. They have recently shown that human platelets express ACE2 and TMPRSS2, the surface serine protease for spike protein priming. They went on to demonstrate that SARS-CoV-2 spike protein directly enhanced platelet aggregation and clot retraction in vitro, and thereby spike protein resulted in thrombus formation in wild-type mice transfused with hACE2 transgenic platelets. Furthermore, recombinant human ACE2 protein and anti-spike monoclonal antibody-inhibited SARS-CoV-2 spike protein-induced platelet activation.9
We encountered 22 patients who developed cutaneous reactions temporally associated with the vaccine administration. All the patients were biopsied, and the samples were sent to our laboratories for diagnostic evaluation. We studied the nature of the adverse immune response to the vaccine and assessed for any evidence of cutaneous viral spike protein localization and microvascular complement pathway activation and cytokine expression within the cutaneous microvessels in select cases.10 Because a biopsy of normal skin can document systemic complement activation and the localization of spike glycoprotein in cutaneous microvessels in the setting of severe and critical COVID-19, 2 of the authors underwent a biopsy of unremarkable deltoid skin a few weeks after receiving the first dose of Pfizer vaccine (before receiving the second vaccination) and 1 of the authors underwent a biopsy after receiving the Johnson & Johnson vaccine. Our intent was to explore whether vaccine-derived spike glycoprotein could localize as pseudovirions to the cutaneous microvessels and, if so, whether it could have the same potential effect on endothelium that we observe in the cutaneous ACE2+ microvessels of patients with severe and critical COVID-19.7 , 10 , 11
Materials and methods
The skin biopsy specimens of 22 patients who developed cutaneous eruptions after receiving the COVID vaccine in which the clinical diagnosis was one of a vaccine triggered hypersensitivity reaction were studied. Deltoid skin biopsies from 3 people who died of COVID-19 and 5 pre–COVID-19 skin biopsy specimens were also included. Using a previously published protocol6 , 7 blinded to the clinical information, we tested tissues for the viral spike protein (a cocktail that can detect the S1, S2, and receptor-binding domain subunits), the SARS-CoV-2 membrane and envelope proteins, interleukin (IL)-6, caspase 3, ACE2, tumor necrosis factor α (TNFα), C3d, C4d, MASP-2, and C5b-9. Coexpression analysis was used with the Nuance system (Nuance, Burlington, MA).6 , 7 We examined deltoid skin biopsy specimens from 3 physician authors of this report who did not have COVID-19 infection and who had received the mRNA-based COVID-19 vaccine at days 10 and 14, and at 10 days with the Johnson & Johnson vaccine, respectively, post-vaccination. To evaluate for evidence of systemic complement pathway activation, a fourth deltoid biopsy specimen from the normal skin of a 21-year-old woman was tested to evaluate for evidence of systemic complement pathway activation. She developed myocardial insufficiency temporally associated with the administration of the Moderna vaccine. The study is covered under the institutional review board protocol 20-02021524.
Results
Post–COVID-19 vaccine cutaneous eruptions in patients (22 cases)
Skin biopsies were encountered in our routine diagnostic dermatopathology practices to evaluate generalized skin eruptions that had developed after patients had received the COVID-19–associated vaccines (Table 1 ). The patient population was represented by 10 women, and 12 men ranging in age from 23 to 96 years, with a median age of 53 years. In all cases, the onset of the eruption was temporally associated with the administration of the vaccine and was characterized by a generalized papulovesicular, eczematous dermatitis, and/or urticarial eruption in most cases (Figures 1 A, 2 A, 3 A, 4 A and B, 5 A, 6 A, 7 A, 8 , 9 A, 10 ). One patient developed Grover disease 1 week after receiving the Moderna vaccine. Another patient's symptoms were consistent with guttate psoriasis, presenting with red scaly macules all over the body for 2 weeks after the second dose of the Pfizer vaccine (Figure 8A). A vasculitic presentation was noted in four patients, including two patients who had acral lesions resembling perniosis and two patients who had urticarial vasculitis. After receiving the Moderna vaccine, 11 patients developed symptoms. After receiving the Pfizer vaccine, seven patients developed symptoms. In four patients the vaccine administered was not known. The reactions occurred after the first dose in five patients and after the second dose in nine patients. In eight patients it was not known whether the eruption occurred after the first or second dose. The reactions developed 1 day to 4 weeks after receiving the vaccine. In 17 patients a more precise timing between vaccine administration and eruption development was known. In eight patients the eruption developed within 1 week after receiving either the first or second dose of the vaccine, including five patients where the eruption developed within 48 hours after receiving the vaccine. In nine patients the reaction was more delayed, developing 8 days, 9 days, 10 days, 12 days, 2 weeks, 3 weeks, and 4 weeks after receiving the vaccine. Some degree of arthralgias and fever were common. Joint swelling was observed in two patients including one patient who had a folliculocentric vesiculopustular eruption (Figure 9A) and another who had thrombocytopenia and hemolysis. One patient had peripheral blood eosinophilia.
Table 1.
Cases of COVID-19 vaccine–induced changes in the skin
| Sex | Age (y) | Clinical history | Biopsy site | Histology | Outcome | Spike protein | Cytokines | |
|---|---|---|---|---|---|---|---|---|
| Case 1 | F | 38 | After receiving the COVID-19 Pfizer vaccine on February 15, 2021, patient developed redness and swelling in the suprapubic area on February 16,2021 and then a full-blown rash on the back and abdomen 2 weeks later. | Superior back | Lymphocyte-mediated interface dermatitis with prominent dyskeratosis; interstitial granulomatous features. Prominent type I interferon signal. | Recovered | Rare positive cells only in deep dermis | IL-6 and TNFα rare positive endothelial cells |
| Case 2 | F | 90 | Patient developed acute onset generalized erythema and pustules with transaminitis and eosinophilia (abs eos 11). Occurred 2 days after patient received first dose of Moderna COVID-19 vaccine. | Left chest | Eczematous and interface dermatitis with tissue eosinophilia | Recovered | Rare positive cells in deep dermal microvessels | Rare positive cells for IL-6 and caspase 3 |
| Case 3 | F | 34 | Patient has a pruritic rash all over the body, which developed after her second dose of the Moderna vaccine on February 14, 2021. | Left abdomen: | Subtle eczematous changes and interface dermatitis, low-grade lymphocytic vasculitis, focal tissue eosinophilia | Recovered | One positive blood vessel | A few positive microvessels for IL-6 and caspase 3 |
| Case 4 | M | 66 | Patient developed a fixed urticarial and purpuric papular rash 9 days after receiving the first Moderna vaccine on February 22, 2021. | Right upper arm | Mixed interstitial lymphocytic, neutrophilic, and eosinophilic infiltrate with leukocytoclasia and hemorrhage consistent with urticarial vasculitis. Focal vascular C5b-9 (8 positive vessels) | Recovered | Rare positive blood vessels | Focal microvascular staining for IL-6 and caspase 3 |
| Case 5 | M | 67 | Patient received the Moderna vaccine on January 15, 2021, and on February 16, 2021. Patient developed rash within 1 day of receiving the second dose of the vaccine. The rash had a diffuse macular morbilliform appearance. | Left back and right anterior thigh | Interface dermatitis, low-grade lymphocytic vasculitis, focal tissue eosinophilia | Recovered | Rare deep microvessels positive for spike | Caspase 3 with 2 positive and occasional positive vessels for IL-6 |
| Case 6 | M | 34 | The patient developed a generalized erythematous papulovesicular eruption 1 week following the Moderna vaccine. | Right arm | Eczematous and interface dermatitis with tissue eosinophilia | Recovered | 1 positive microvessel | IL-6 negative |
| Case 7 | F | 73 | Patient presented with purpura with hives on the thighs 10 days after a COVID-19 vaccine. | Left thigh | Interstitial neutrophilia and leukocytoclasia with hemorrhage | Recovered | Rare positive endothelial cells deep | N/A |
| Case 8 | F | 54 | Patient developed purple acral nodules 9 days after receiving the first dose of the Moderna vaccine. | Left finger | Lymphocytic vascular reaction and lymphocytic eccrine hidradenitis with papillary dermal edema and focal hemorrhage consistent with perniosis | Recovered | N/A | N/A |
| Case 9 | M | 66 | Patient developed itchy red papules on the abdomen 7 days after a COVID-19 vaccine. | RUQ | acantholytic dyskeratosis with suprabasilar clefting consistent with Grover disease. | Unknown | Spike negative | IL-6 negative |
| Case 10 | M | 72 | Patient presented with urticarial plaques on both arms and legs that developed 3 weeks after the Moderna vaccine. | Right arm | acanthosis with spongiosis and Langerhans cell–rich microvesiculation. | Recovered | Spike negative | IL-6 negative |
| Case 11 | F | 38 | Patient developed widespread itchy papules with blisters 4 days after receiving the second dose of the Moderna vaccine. | Left arm | an interface dermatitis with interstitial granulomatous features | Recovered | N/A | N/A |
| Case 12 | F | 66 | Patient developed an eczematous rash on the thighs 8 days after receiving the Pfizer vaccine | Right thigh | acanthosis with spongiosis and Langerhans cell–rich vesiculation | Recovered | N/A | N/A |
| Case 13 | M | 96 | Patient developed an eczematous dermatitis 4 weeks after receiving the second dose of the Pfizer vaccine | Back | acanthosis with spongiosis and Langerhans cell–rich vesiculation. | Recovered | N/A | N/A |
| Case 14 | M | 72 | Patient developed a papulovesicular rash developed 4 days after receiving the second dose of the Moderna vaccine. | Back | acantholytic dyskeratosis with suprabasilar clefting consistent with Grover disease. | Persists | N/A | N/A |
| Case 15 | F | 27 | Patient who received the Pfizer vaccine on March 14, 2021 and April 4, 2021, began developing a very striking vesicular pustular rash initially on the chest, 2 weeks after the first dose. | Lower back | a very striking necrotizing neutrophilic and granulomatous folliculitis. | Recovered | Rare deep vessels positive for spike | N/A |
| Case 16 | M | 37 | Patient developed a rash that began on the elbows in February of 2021 and subsequently spread to the knees. | Left arm | eczematoid alterations as characterized by spongiosis with lymphocytic exocytosis along with Langerhans cell–rich microvesiculation. | Recovered | Rare deep vessels positive for spike | N/A |
| Case 17 | M | 58 | Patient presented with red spots all over the body for 2 weeks after the second dose of the Pfizer vaccine on March 30, 2021. | Left arm | a mild psoriasiform epidermal hyperplasia. Granular cell layer loss was noted very focally with overlying lenticular-shaped parakeratosis. | Recovered | N/A | N/A |
| Case 18 | F | 24 | Patient developed a rash on feet and hands shortly after receiving the second dose of the Moderna vaccine in March of 2021. | Right dorsal second toe | a lymphocyte-mediated interface dermatitis with papillary dermal edema and an accompanying brisk perivascular interstitial lymphocytic infiltrate. | Recovered | N/A | N/A |
| Case 19 | M | 64 | Patient developed an eczematous reaction almost immediately after the first dose of the Pfizer vaccine. The eruption progressed such that nummular plaques involved 10% of the body. | Left upper back | a mild psoriasiform epidermal hyperplasia. There is spongiosis with lymphocytic exocytosis. Overlying areas of lenticular-shaped parakeratosis are identified. |
Recovered | N/A | N/A |
| Case 20 | M | 27 | Patient is a 27 year old male presented with rashes on the arms, legs, around the nipple and mucosal lip area one month after the second dose of the Moderna vaccine. | Left forearm | eosinophil-enriched subacute eczematous dermatitis with a pustular component as revealed by neutrophil-imbued parakeratosis. Biopsy demonstrated a psoriasiform epidermal hyperplasia. There was spongiosis with intercellular edema. Serum and neutrophil-imbued parakeratosis were noted. | Improved | N/A | N/A |
| Case 21 | M | 23 | Patient experienced petechial macules on hands and pink blanching macules and papule on arms, chest, and legs that developed 12 days after receiving the COVID-19 vaccine. | Right arm | interface dermatitis with dermal edema and a superficial lymphocytic and granulomatous vasculitis. | Recovered | Rare deep vessels were positive for spike | Caspase 3 and IL-6 positive in rare deep vessels |
| Case 22 | F | 34 | Patient developed a rash on the face, trunk and extremities 1 week after the second dose of the Moderna vaccine. | Left posterior shoulder | eczematous dermatitis with interstitial granulomatous features. | Recovered | N/A | N/A |
ACE2, angiotensin converting enzyme 2; COVID-19, coronavirus disease 2019; F, female; IL, interleukin; M, male; N/A, not applicable; RUQ, right upper quadrant; TNFα, tumor necrosis factor α.
Fig. 1.
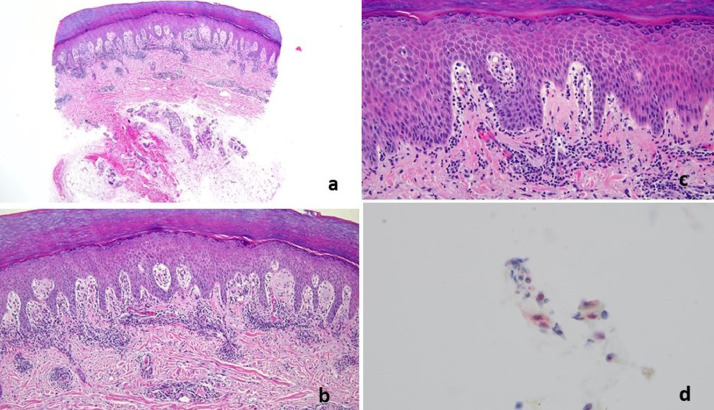
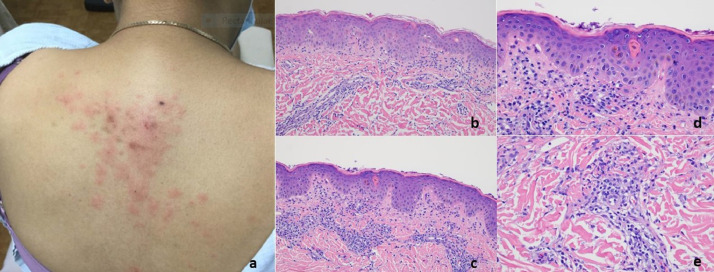
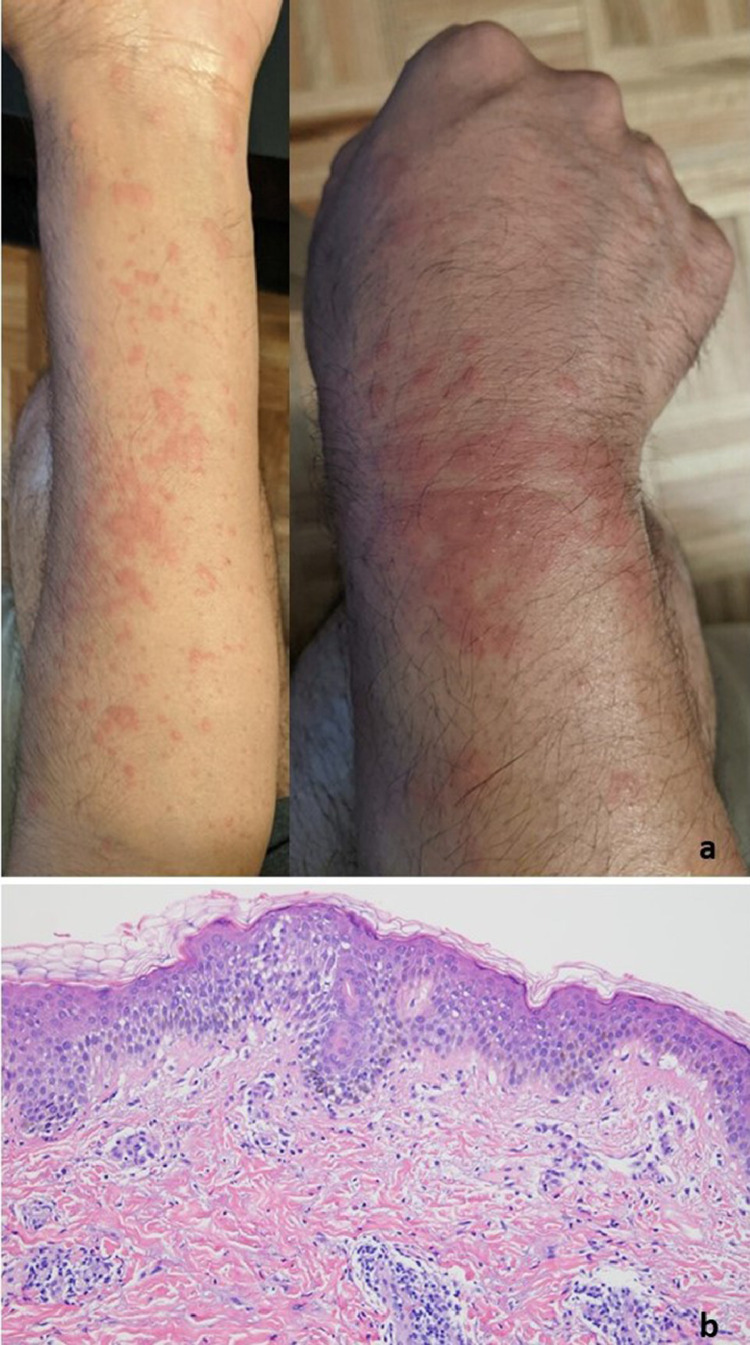
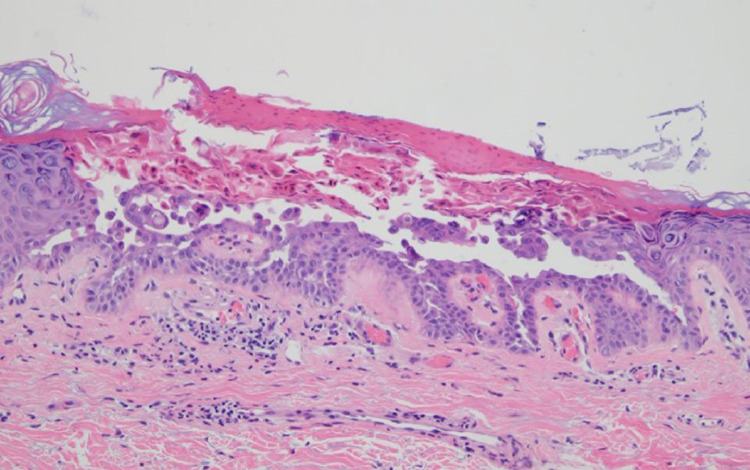
T- cell response with features of lymphocytic vasculitis (case 8). Nine days after receiving the first does of a COVID-19 vaccine, a 54-year-old woman developed perniosis on the fingers, characterized by indurated plaques and nodules on the hands. (A-C) A punch biopsy specimen of acral skin demonstrated a lymphocytic interface dermatitis with marked papillary dermal edema accompanied by vertical strands of fibrin in the dermis and a superficial and deep perivascular and peri-adnexal mononuclear cell infiltrate. The histology exactly recapitulated perniosis including COVID-19–associated perniosis. (D) Deep dermal vessels positive for spike glycoprotein and caspase 3 were rare. There is one microvessel in the deep dermis demonstrating spike/interleukin 6/Casp3.The microvessels in the papillary dermis—where the inflammation is observed—have no spike glycoprotein. COVID-19, coronavirus disease 2019.
Fig. 2.
T-cell response with features of interface dermatitis accompanied by lymphocytic and granulomatous vasculitis (case 21). (A) The biopsy shows an interface dermatitis mediated by lymphocytes with concomitant papillary dermal edema and a subjacent necrotizing lymphocytic and granulomatous vasculitis with evidence of vascular compromise as characterized by red cell extravasation (hematoxylin and eosin [H and E], 200 ×). Higher power magnification demonstrates the extent and nature of the vascular injury pattern. It is one that involves the capillaries and venules largely confined to the superficial corium whereby inflammatory cells course through the vessel wall, and it is associated with fibrin along with red cell extravasation. (B) The infiltrate is predominated by lymphocytes and histiocytes defining a hybrid lymphocytic and granulomatous vasculitis, but there is also a smattering of neutrophils (H and E, 400 ×). (C, D) A microvessel is visible in the deep dermis, demonstrating spike and interleukin 6. The microvessels in the papillary dermis, where the inflammation is has no spike glycoprotein.
Fig. 3.
T-cell–mediated cytotoxic interface dermatitis (case 5). A 67-year-old man presented with a 2-week history of pruritic eruption on the trunk and extremities. The patient received the first and second doses of the Moderna vaccine on January 15, 2021 and February 16, 2021, respectively. (A) He developed an itchy eruption after the second dose (reproduced with permission from Dr. Silvia Mancebo, New York, NY). (B, C) The histologic findings are those of a classic morbilliform type IV hypersensitivity reaction combining delayed dermal hypersensitivity with a very mild cytotoxic interface dermatitis.
Fig. 4.
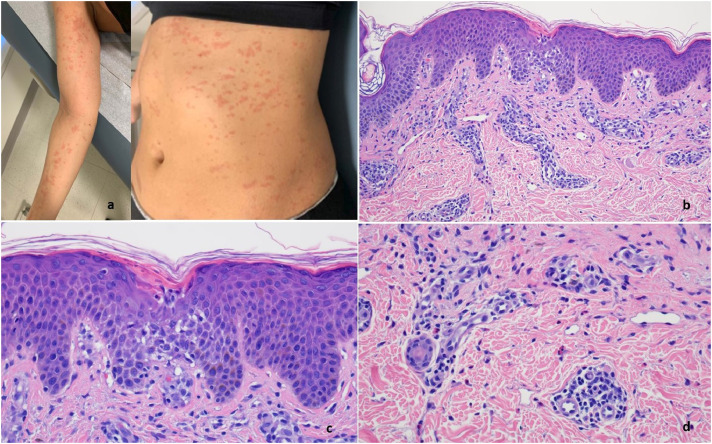
The biopsy shows a psoriasiform epidermal hyperplasia (case 20). Intercellular edema is observable within the epidermis. The suprapapillary plates are thickened. There is focal exocytosis of lymphocytes into the epidermis. (A) A perivascular and intersitital lymphohistiocytic and eosinophilic infiltrate is noted in the dermis (hematoxylin and eosin [H and E], 100 ×). Higher power magnification shows the composition and architectural disposition of the dermal inflammatory cell infiltrate whereby it is both interstitial and perivascular. The degree of interstitial histiocytic infiltration imparts a subtle interstitial granulomatous quality to the infiltrate. (B) Note the significant tissue eosinophilia (H and E, 200 ×)
Fig. 5.
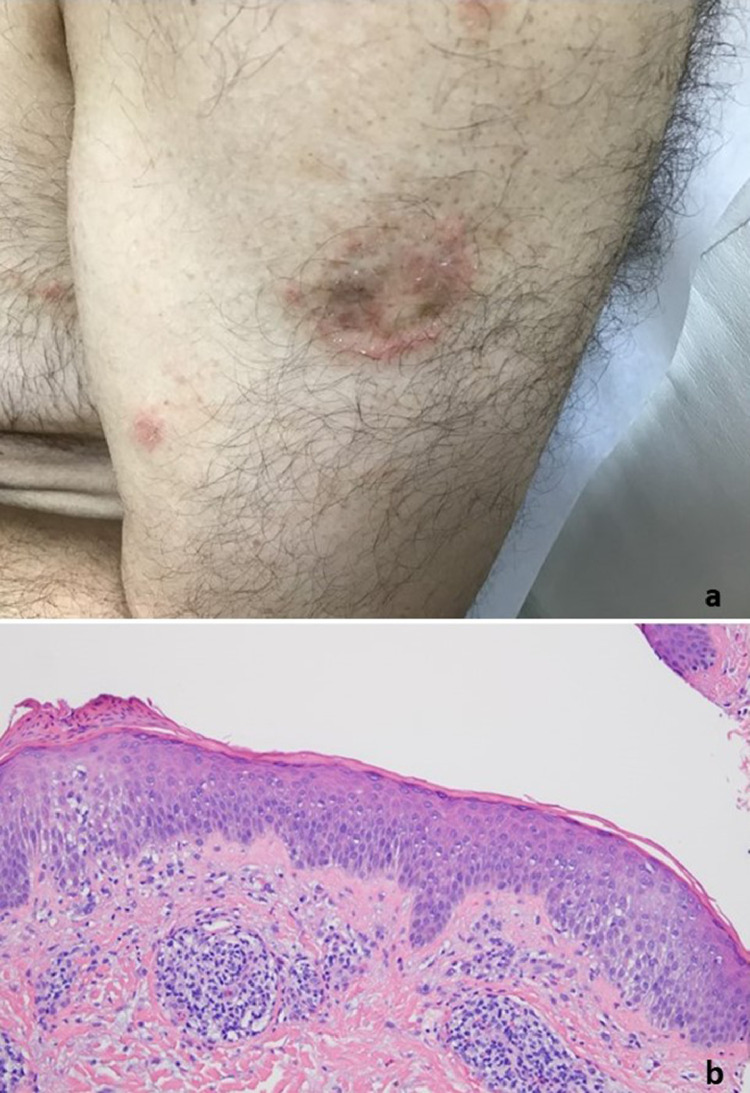
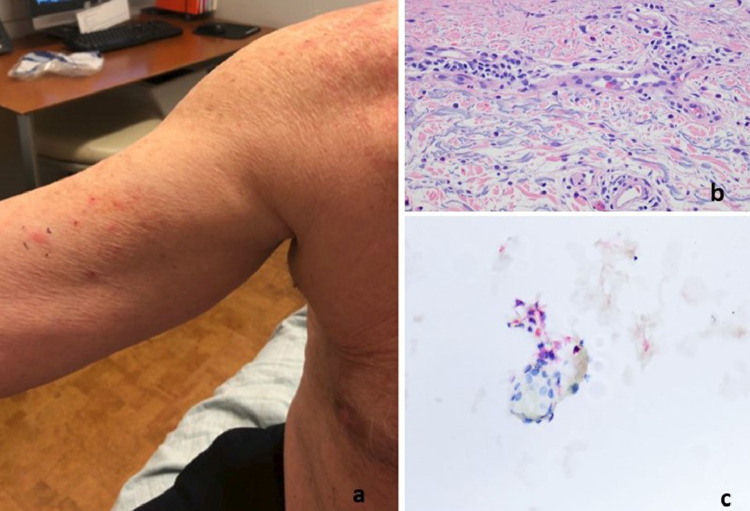
T-cell response with features of interface dermatitis and interstitial granulomatous inflammation (case 1). The patient was a 38-year-old woman who presented with blanchable erythematous papules (reproduced with permission from Dr. Henry J. Lee, New York, NY). The patient had received a COVID-19 vaccine, either the Moderna or the Pfizer, on February 15, 2021. The patient developed redness and swelling in the suprapubic area on February 16, 2021. (B, C) The biopsy showed a lymphocyte-mediated interface dermatitis associated with focal areas of epidermal attenuation. Lymphocyte satellitosis is visible around injured keratinocytes. (D) Focal areas of interstitial granulomatous inflammation accompanied by some degree of mesenchymal mucin deposition are observed. COVID-19, coronavirus disease 2019.(E)
Fig. 6.
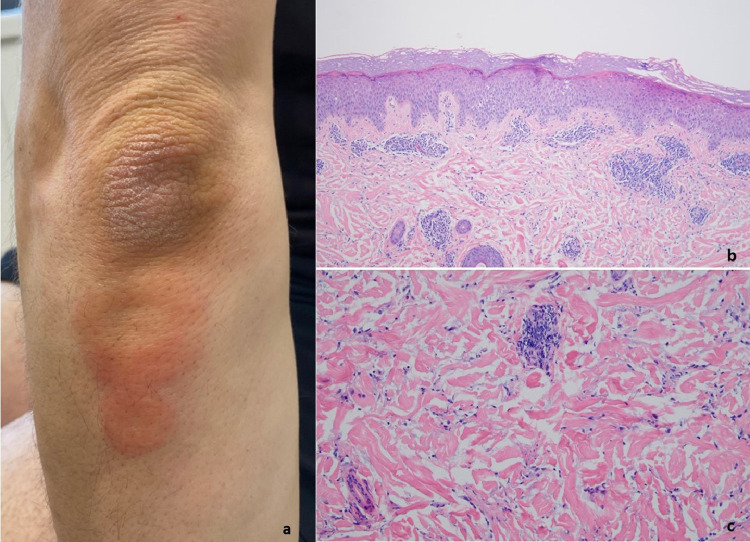
T-cell–mediated reaction with eczematous features (case 6). (A) A 34-year-old woman had a pruritic erythematous eruption all over the body that developed after her second Moderna COVID-19 vaccine on February 14, 2021, accompanied by body aches and chills (reproduced with permission from Dr. Eva Kerby, New York, NY). On the third day, she developed the eruption at the site of the vaccine that later spread to the trunk, arms, and proximal thighs. (B, C) The biopsy demonstrated a spongiotic eczematous picture along with a subtle interface dermatitis accompanied by a lymphohistiocytic and eosinophilic purpuric vascular reaction. COVID-19, coronavirus disease 2019.
Fig. 7.
T-cell response with features of an eczematous dermatitis (case 19). The patient developed an eczematous reaction almost immediately after receiving the COVID-19 vaccine. (A) Nummular plaques involved 20% of the body (reproduced with permission from Dr. Scott Sanders, New City, NY). The biopsy showed an eczematous dermatitis characterized by acanthosis, intercellular edema, and exocytosis of lymphocytes and monocytes into the epidermis. (B) A subtle cell-poor–interface dermatitis is also observed. A lymphocytic purpuric vascular reaction is also noted (hematoxylin and eosin, 200 ×). COVID-19, coronavirus disease 2019.
Fig. 8.
T-cell response predominated by eczematous features (case 16). (A) The patient is a 37-year-old man who developed an eruption that began on the elbows in February of 2021 and then subsequently spread to the knees (reproduced with permission from Dr. Andrew Avarbock, New York, NY). The eruption on the elbows and knees improved, but subsequently spread to the lower legs. (B) The epidermal changes are predominated by eczematoid alterations but a subtle interface dermatitis is also noted. (C) The dermal component exhibits features of delayed dermal hypersensitivity characterized by vasocentric lymphocytic and eosinophilic infiltrates along with an interstitial granulomatous component.
Fig. 9.
T-cell response predominated by eczematous features (case 6). (A) A 34-year-old man presented with papulovesicular eruptions on the extremities, hands, and palms 1 week after receiving the Moderna vaccine (reproduced with permission from Dr. Paul Dantzig, New York, NY). The patient was treated with prednisone 60 mg daily, and the eruption cleared 3 days later. The biopsy showed intercellular edema within the epidermis along with lymphocytic exocytosis. A concomitant interface dermatitis was identified as evidenced by basilar vacuolar change with a few lymphocytes present along the dermal-epidermal junction. (B) Scattered eosinophils are noted.
Fig. 10.
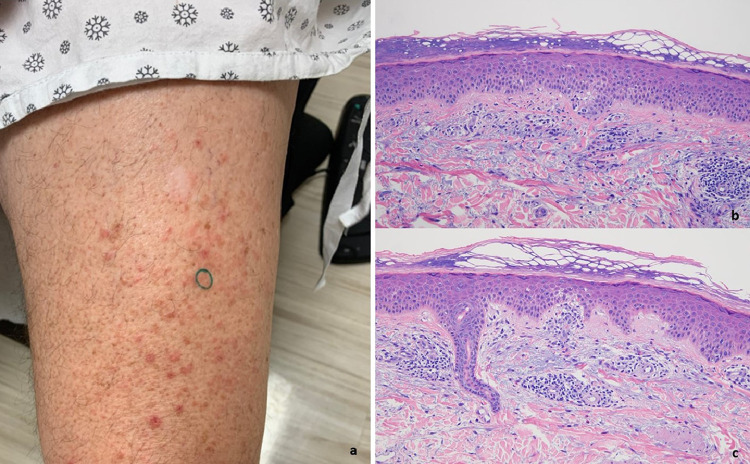
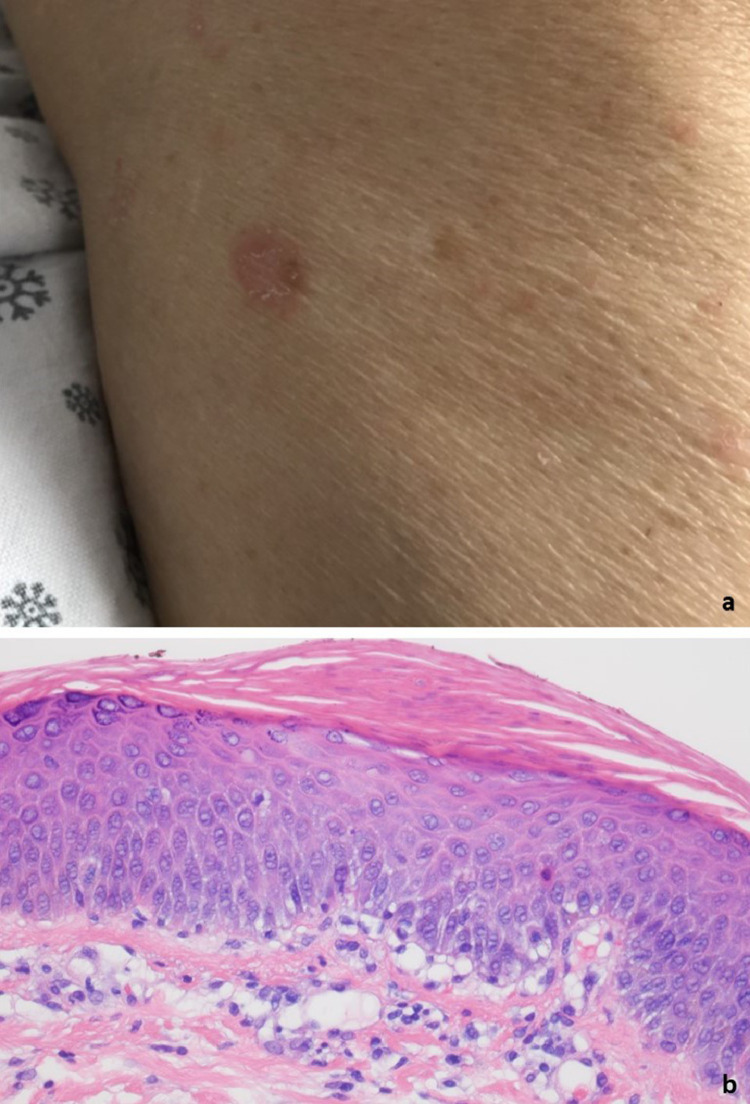
(A) The patient (case 17) developed a generalized guttate eruption shortly after receiving the second dose of the Pfizer vaccine on March 30, 2021 (reproduced with permission from Dr. JeanYoung Kim, New York, NY). The eruption occurred roughly 2 weeks later. The biopsy demonstrated focal areas of lenticular-shaped parakeratosis with subjacent granular cell layer loss. Very focally the capillaries within the dermal papillae are juxtaposed to the basal layer of the epidermis. (B) The findings suggest eruptive guttate psoriasis temporally associated with the COVID-19 vaccine (hematoxylin and eosin, 400 ×). COVID-19, coronavirus disease 2019.
Light microscopy
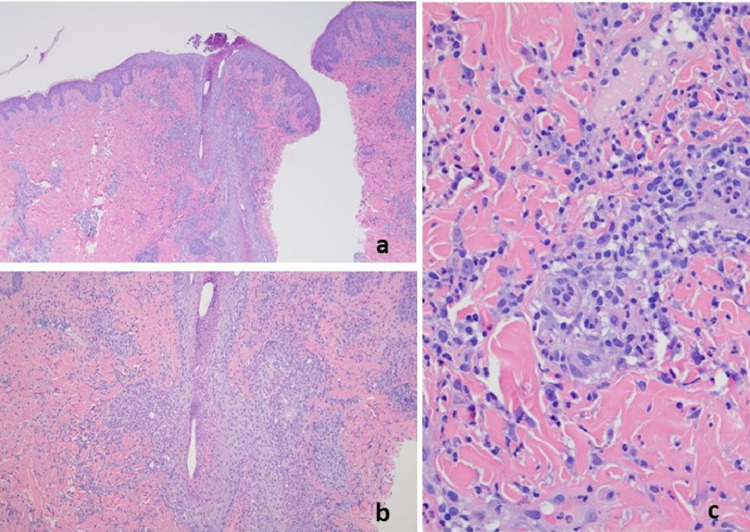
The dominant histologic patterns included eczematous dermatitis (10 cases) (Figures 2, 4C-E, 5B, 6B, 7B), interface dermatitis (13 cases) (Figures 1B and C, 2B and C, 3B and C, 4, 5, 7B, 11A), urticaria (one case), lymphocytic vasculitis (three cases) including two cases of perniosis (Figure 12A-C), Grover disease (two cases) (Figure 13), urticarial vasculitis (two cases) (Figures 10B, 14A and B), and granulomatous inflammation (three cases) (Figures 3, 6C, 11 A and B), with one exhibiting an interstitial pattern and the other a folliculocentric neutrophilic and granulomatous one reminiscent of vesiculopustular pyoderma gangrenosum (Figures 9B-D).12 In addition, in 1 case there were pustules noted clinically although there was no histologic documentation of a pustular diathesis. The clinical impression was acute generalized exanthematous pustulosis. Another case presented a photo-distributed papular eruption on an erythematous base. In most cases there were other overlapping morphologic reaction patterns defining a hybrid dermatitis, including cases showing combined eczematous, interface, vasculitic, and interstitial granulomatous features. The most common pattern was concurrent interface and eczematous dermatitis identified in six cases. Tissue eosinophilia was common. In all cases the clinical impression was congruous with the histologic findings, and the eruptions resolved either spontaneously or with topical or systemic steroid therapy except the case in which the eruption had persisted for 4.5 months. The patient was then given a trial of ustekinumab. One case was compatible with guttate psoriasis, another T-cell–mediated process in which it has been established that an exogenous antigenic trigger is frequently implicated albeit typically in the context of streptococcal antigen (Figure 8B). In another case an unusual picture of interface dermatitis with concomitant features of pityriasis rubra pilaris was observed. The type I interferon signature was upregulated in four out of five cases tested including 1 case of perniosis.
Fig. 12.
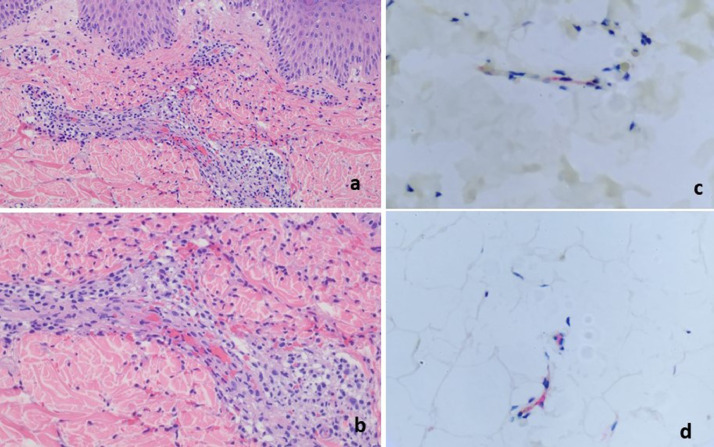
The patient was a 66-year-old man who developed a urticarial vasculitic process on the right arm and right flank 9 days after receiving the first Moderna COVID-19 vaccine on February 22, 2021 (case 4). (A) Numerous erythematous papules are visible on the arms, thighs, and back (reproduced with permission from Dr. Jalong Gaan, New York, NY). The biopsy demonstrated an inflammatory process within the dermis, exhibiting urticarial-like features. In particular, there is a mixed infiltrate that is interstitial and perivascular, composed of lymphocytes, monocytes, and neutrophils along with a few eosinophils with concomitant dermal edema. (A) Leukocytoclasia and red cell extravasation are present, although with no evidence of mural and or luminal fibrin deposition. (C) A few microvessels in the deep dermis were interleukin 6 positive. COVID-19, coronavirus disease 2019.
Fig. 13.
A 73-year-old woman presented with purpura and hives on the thighs 10 days after receiving a COVID-19 vaccine (case 7). The biopsy showed a superficial to mid-dermal vasocentric infiltrate predominated by neutrophils. Attendant endothelial swelling, leukocytoclasia, and mild dermal hemorrhage were observed. (A, B) No fibrinoid necrosis is present. Interstital eosinophils are a prominent finding. COVID-19, coronavirus disease 2019.
Fig. 14.
A 66-year-old man patient developed a pruritic papular eruption 1 week after receiving the COVID-19 vaccine (case 9). The biopsy specimen showed dyskeratosis characterized by suprabasal clefting. The epidermal surface is focally eroded and associated with subepithelial neutrophilia. The histologic findings are characteristic for Grover disease/transient acantholytic dermatosis. COVID-19, coronavirus disease 2019.
Fig. 11.
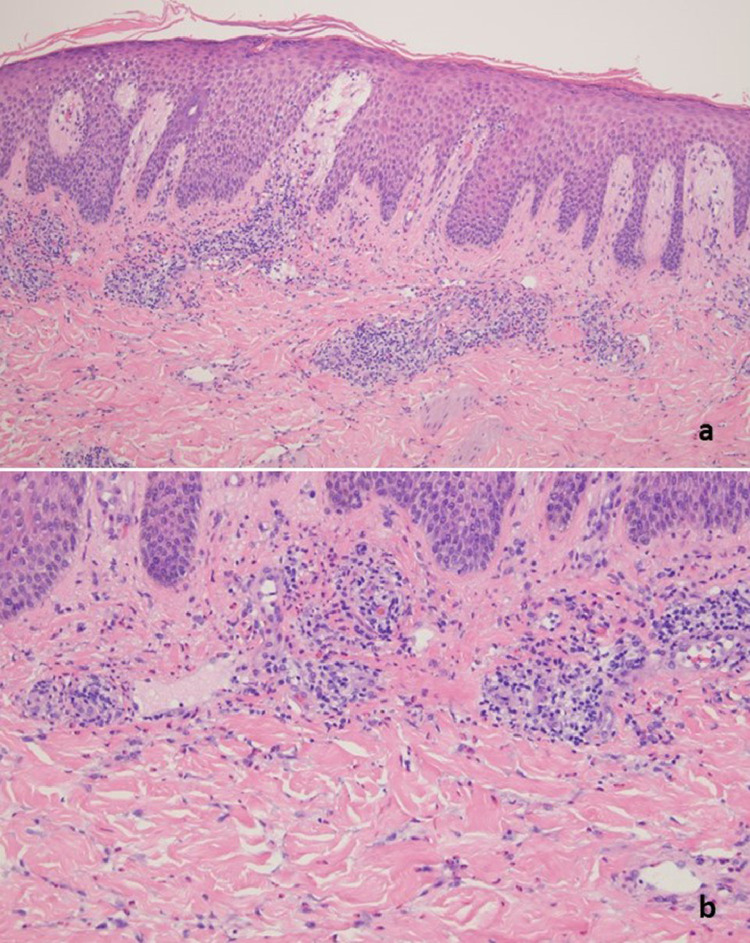
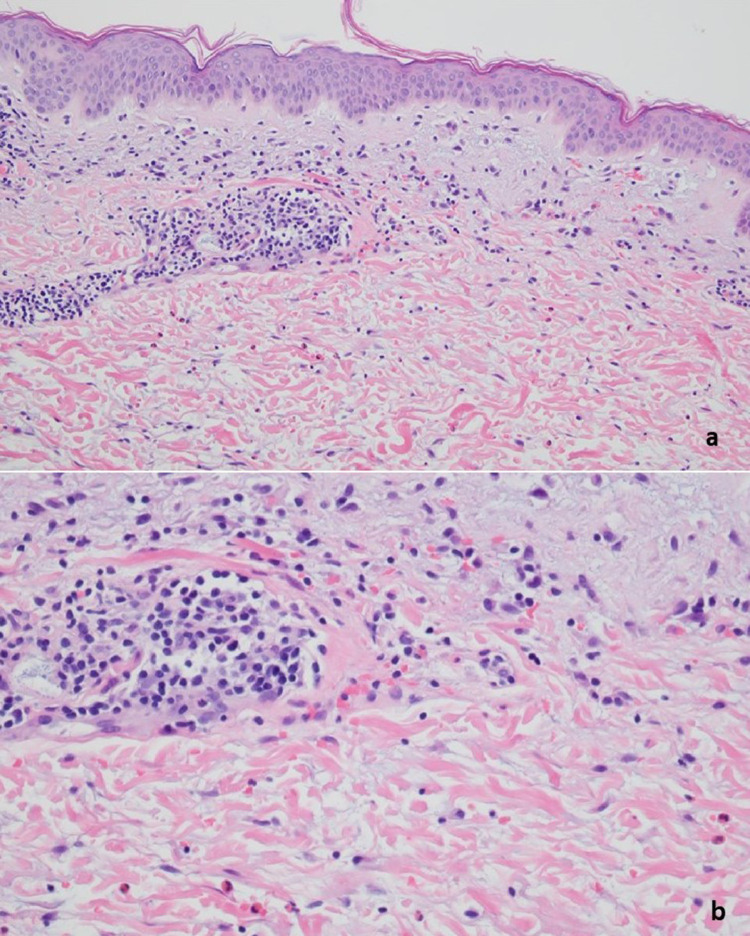
The patient was a 27-year-old woman with a history of mixed connective tissue disease received the Pfizer vaccine on March 14, 2021, and on April 4, 2021, (case 15). The patient began to feel unwell about 2 weeks after receiving her first dose of the Pfizer vaccine, heralded by pain and swelling in many of her joints. The patient then developed a very striking vesicular pustular eruption. (A) The biopsy demonstrated a very striking necrotizing neutrophilic and granulomatous folliculitis. (B) Extensive infiltration of the outer root sheath epithelium by a mixed inflammatory cell infiltrate with disruption of the wall and adjacent perifollicular inflammation with accentuation around vessels is visible. (C) A significant component of the infiltrate is lymphocytic and granulomatous in nature.
Immunohistochemical assessment for spike glycoprotein, complement deposition, and endothelial cytokine expression (IL-6, caspase 3, and TNFα)
The immunohistochemical stain to assess for spike glycoprotein was conducted on 12 of the cases. The basis for doing the stain was to document evidence of human synthesis and establish the ability of spike glycoprotein to dock to ACE2+ vessels as a pseudovirion, a hypothesis proffered as the basis of systemic complement activation in the setting of severe and critical COVID-19. We were able to document spike glycoprotein in the cutaneous microvasculature in 10 cases tested. In particular, there were rare deep-seated vessels in the reticular dermis and subcutaneous fat that showed focal detection of spike glycoprotein in endothelium reflecting the preferential expression of ACE2 in the deeper microvessels. There were typically only one or at most a few positive staining vessels (fewer than 5) (Figures 11C and D, 12 D). A similar distribution was observed for IL-6, caspase 3, and/or TNFα, but with no significant microvascular complement deposition (Figure 10C). The overall amount of microvascular spike glycoprotein and ACE2 expression was much less than that observed in the setting of thrombotic retiform purpura of severe and critical COVID-19.
Post-vaccine normal deltoid biopsies in patients without symptoms
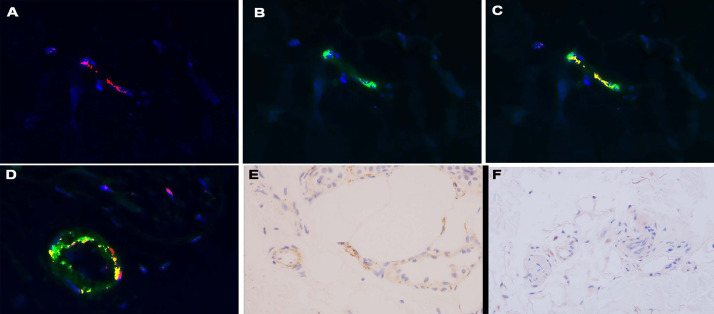
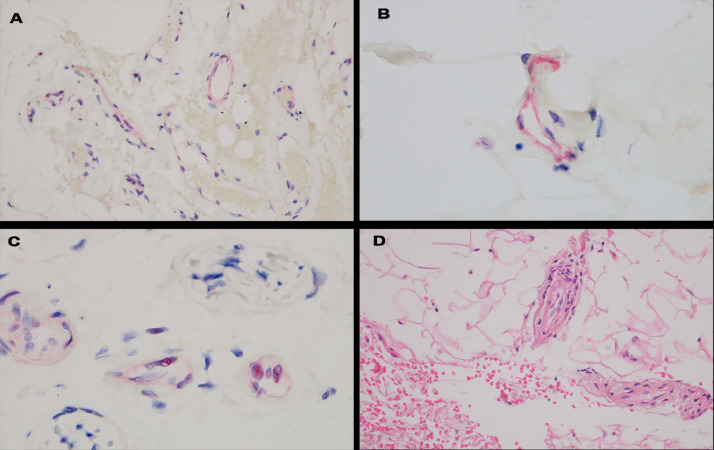
Serial sections of each of the two normal post-vaccine skin biopsy specimens demonstrated a lymphocytic vascular reaction localized to a single vessel in the subcutaneous fat in one biopsy specimen. In both biopsies there was expression of ACE2 in endothelium in deeper dermal and subcutaneous microvessels. The deltoid skin biopsy specimens from patients who died of COVID-19 showed significant microvascular endothelial cell localization of spike glycoprotein, whereas the pre–COVID-19 skin samples were negative (Figure 15 ). In the two normal biopsy specimens taken after the patients had received the Pfizer vaccine, occasional endothelial cells in the microvessels of the deep dermis and subcutis were positive for spike glycoprotein (fewer than 5 microvessels with some degree of positivity in the endothelial lining cells). Unlike the deltoid skin biopsy specimens from the patients with severe COVID-19 that also contained the envelope and membrane viral proteins within the endothelium, these additional viral capsid proteins were absent in the biopsy specimens taken after the patients had received the Pfizer vaccine (data not presented) (Figure 16 ). Complement studies to assess for C3d, C4d, and C5b-9 deposition were conducted on both cases, and only rare vessels showed any evidence of complement deposition and hence did not support a diagnosis of systemic complement activation. Nuance software (Nuance) coexpression analysis showed coexpression of spike glycoprotein with caspase 3, TNFα, and IL-6 (Figure 16). An additional deltoid biopsy specimen was procured from another physician author who received the Johnson & Johnson vaccine. Spike glycoprotein was not identified Figure 17 .
Fig. 15.
Patients with normal deltoid skin after vaccination shows endothelial cells activated by the spike protein. (A) Using the Nuance software, a microvessel positive for spike glycoprotein using a red chromagen gives a red signal localized to endothelium (red chromagen, 1000 ×). (B) The same microvessels show a green signal in a similar distribution highlighting interleukin 6, using a diaminobenzidine stain (diaminobenzidine, 1000 ×). (C) The combined signal fluoresces yellow, proving colocalization of spike glycoprotein and interleukin 6 in the biopsy of a person post-vaccination (1000 ×). (D) Panel demonstrates a similar strong coexpression between the spike protein and caspase 3 in a post-vaccine biopsy specimen (1000 ×). (C) An extensive microvascular deposition of C5b-9 is visible in a case of severe COVID-19 (diaminobenzidine, 400 ×). (F) In contrast, the post-vaccine sample does not show significant complement deposition (diaminobenzidine, 400 ×). COVID-19, coronavirus disease 2019.
Fig. 16.
Normal deltoid skin in patients with fatal COVID-19 versus healthy patients after vaccination. (A) In both deltoid skin samples from patients with fatal COVID-19 versus healthy patients after vaccination, there are a relatively greater number of positive staining vessels for ACE2 in the deeper dermis and in subcutaneous fat compared with the microvessels present superficially (red chromagen, 200 ×). The ACE2 distribution pattern mirrors spike glycoprotein endothelial cell localization. (B) Granular deposition within the endothelium for spike glycoprotein was present in the setting of fatal COVID-19 (red chromagen, 1000 ×). (C) A similar pattern of endothelial cell staining for spike glycoprotein was noted in the post-vaccine biopsy (red chromagen, 1000 ×). (D) A microvessel after vaccine in which a mononuclear cell response is evident (hematoxylin and eosin, 1000 ×). ACE2, angiotensin converting enzyme 2; COVID-19, coronavirus disease 2019.
Fig. 17.
One of the authors underwent the deltoid biopsy after receiving the Johnson & Johnson COVID-19 vaccine. No localization of spike in the microvessels was detected. COVID-19, coronavirus disease 2019.
Post-vaccine normal deltoid biopsy in a patient with post-vaccine acute myocardial insufficiency
A deltoid biopsy specimen was procured from a 21-year-old patient who went to the emergency room on March 12, 2021, with chest pain radiating into her left arm 2 days after she received her second Moderna vaccination. The skin biopsy specimen exhibited a minimal perivascular lymphocytic infiltrate. The C5b-9 studies showed roughly 8 positive staining vessels including capillaries, venules, and a single arteriole, and hence did not meet criteria for evidence of systemic complement activation. A single deep dermal vessel showed spike glycoprotein, TNFα, and caspase 3 positivity.
.
Discussion
We have presented a series of adverse cutaneous responses temporally associated with the administration of the first or second dose of the Pfizer or Moderna COVID-19 vaccines. Patients from all age ranges could develop the vaccine reaction with either the Pfizer or Moderna. The eruptions were typically generalized and had an urticarial or eczematous appearance. In our series, reactions were more commonly observed with the Moderna vaccine rather than with the Pfizer vaccine. In addition, the adverse reactions could develop either after the first dose or the second dose, although it was almost twice as common after the second dose compared with the first dose. The reaction could develop as quickly as 48 hours after the vaccine was administered or could be delayed for as long as 4 weeks after the vaccine was administrated, with almost half of the cases developing within a week of administration of the vaccine.
Histologic patterns resembling hypersensitivity
The more common histologic patterns were eczematous dermatitis, interface dermatitis, interstitial granulomatous dermatitis, and lymphocytic vasculitis including two cases of perniosis. One of the hallmarks of the vaccine reactions that we encountered was a hybrid inflammatory pattern. A case could show a mixed pattern best exemplified by cases of interface and eczematous dermatitis with an accompanying mild lymphocytic vasculitis. These specific histologic patterns are commonly reflective of underlying type IV hypersensitivity. One case associated with a striking pattern of interface dermatitis had concomitant pityriasis rubra pilaris–like changes. This patient has had a persistent severe generalized skin eruption for at least 4 months refractory to prednisone and has begun ustekinumab therapy. One biopsy specimen showed a distinctive folliculocentric immune reaction that falls under the category of sterile neutrophilic folliculitis with folliculocentric vascular injury.13 Pathogenetically, this type of sterile neutrophilic follicular reaction has been hypothesized to represent a TH1 dominant type IV immune response in which the cytokine milieu is conducive to a neutrophilic influx into the skin.13 , 14 A minor subset of cases demonstrated an urticarial vasculitis, a morphologic subset of leukocytoclastic vasculitis characteristically triggered by immune complex deposition but other proinflammatory pathways can be implicated. One might consider a scenario in which antibodies bound to an undefined foreign protein introduced by the vaccine could be deposited in microvessels as an immune complex and trigger the classic complement pathway to result in a neutrophil-rich inflammatory reaction.
Post-vaccine reactions resembling a dermatosis with an underlying genetic predisposition
One patient developed eruptive psoriasis, expanding the clinical and morphologic spectrum of type IV T-cell immune responses.
Based on certain COVID-19 vaccine trials cutaneous reactions are becoming increasingly recognized and while psoriasis was not originally described in some of the initial trials, the influenza vaccine has been recognized as a trigger for guttate psoriasis.15, 16, 17 – 18 Molecular mimicry between streptococcal antigens and keratins in the epidermis of patients with eruptive psoriasis underlies the association between streptococcal pharyngitis and guttate psoriasis. There is structural homology between spike glycoprotein and M6 protein implicated in guttate psoriasis although not specifically the spike glycoprotein of SARS-CoV-2. Vaccine-associated immune enhancement, however, may play a role in unmasking psoriasis in a genetically predisposed patient. Two patients also showed changes of Grover disease histologically although mechanistically its basis is unclear.
Post-vaccine cutaneous reactions literature review
Other authors have described cutaneous eruptions in patients who have received the Pfizer or Moderna COVID-19 vaccine, both in the context of generalized eruptions as well as in erythema at the vaccination site. Farinazzo et al. described the first registered cases of cutaneous adverse reactions in North-East Italy after patients had received the Comirnaty-BioNTech/Pfizer mRNA COVID-19 vaccine in Trieste.19 They reported one or more cutaneous adverse effects in 0.22% of all vaccinated individuals and 16.54% of communicated adverse effects in all vaccinated individuals. The reactions were divided into those at the vaccine site and those that were discontiguous and more generalized. The reactions included urticaria, malar erythema, a hand eruption, pityriasis rosea, and a reaction that resembled a fixed drug eruption. Reactions were seen in patient who had received the first dose or the second dose of the vaccine in the absence of a reaction to the first dose and could appear as quickly as 60 hours from the time of the initial vaccination. In trying to establish the trigger, it was suggested that polyethylene-glycol 200, which is a known cause of immediate type I hypersensitivity as well as delayed reactions, could be responsible. Other terms have been used to describe the polymers such as macrogol, oxyethylene polymer, and laureth. In a second review that examined more than 400 adverse cutaneous reactions collected from a US-based database, more than 80% of cases were seen in association with the Moderna vaccine.17 Less than 20% of the reactions were associated with the Pfizer vaccine.17 There was a predominance of the adverse vaccine reaction in women. The adverse reaction could occur after the patients received the first or second dose of the vaccine, and if it occurred after the first dose there was a 40% likelihood that it would occur again after the second dose. There were two basic time frames: an immediate one that occurred at 1 to 3 days and a delayed one developing 6 to 7 days after the vaccine. The reactions included swelling at the vaccine site, morbilliform eruptions, pityriasis rosea-like, and chilblains/perniosis. We have seen cases of post-vaccination erythema at the site of the vaccine including one case with concomitant lymphangitis but with no biopsy to confirm the nature of the inflammatory response. Authors have suggested certain agents used in the vaccine vehicle as the putative trigger such as polyethylene glycol.17
Pathogenesis
Role of a product in the vaccine vehicle as an allergen
When one considers vaccines in general and not specifically in regard to the SARS-CoV-2 mRNA vaccines, the common constituents found in diverse vaccines have elicited a variety of systemic allergic contact dermatitis reactions. The most common culprits are antibiotics (eg, neomycin) used as certain key preservatives such as formaldehyde, propylene glycol, and sorbic acid. In each patient that develops an adverse reaction, one could consider patch testing to determine which component elicits the immune response.
The cutaneous reactions associated with the COVID-19 vaccine appear to be self-limited. The histology suggests that two common limbs of hypersensitivity observed with other exogenous antigens could be the basis, although unproven. The dominant pattern is one that would be consistent with a systemic eczematoid hypersensitivity reaction. In our series and consistent with other reported studies, the most common pattern is one resembling type IV hypersensitivity characterized by an eczematous dermatitis and or a concomitant cytotoxic interface dermatitis. One might suggest that the antigen could be a substance in the vehicle used to administer the vaccine, although a T-cell and/or humoral reaction to the myocyte-manufactured spike glycoprotein emerges as a putative antigenic trigger, especially given its localization to the cutaneous microvessels. An antigen unrelated to the vaccine, but in which an immune response to the antigen becoming unmasked owing to vaccine-associated immune enhancement, is possible.
Without testing the various components of the vaccines, it would be difficult to elucidate the antigenic trigger. The Moderna COVID-19 vaccine contains the following: mRNA, lipids (SM-102, polyethylene glycol 2000 dimyristoyl glycerol, cholesterol, and 1,2-distearoyl-sn-glycero-3-phosphocholine), tromethamine, tromethamine hydrochloride, acetic acid, sodium acetate trihydrate, and sucrose. The Pfizer vaccine contains the following: mRNA, lipids including (4-hydroxybutyl) azanediyl)bis(hexane-6,1-diyl)bis(2-hexyldecanoate), 2 [(polyethylene glycol)-2000]-N,N-ditetradecylacetamide, 1,2-distearoyl-sn-glycero-3-phosphocholine, and cholesterol) potassium chloride, monobasic potassium phosphate, sodium chloride, dibasic sodium phosphate dehydrate and sucrose. A significant emphasis has been placed on polyethylene glycol as a putative antigenic trigger.
All COVID-19-vaccine reactions were observed in patients after they received the Moderna vaccine or the Pfizer vaccine. The Johnson & Johnson vaccine is a viral vector vaccine using a replication-incompetent recombinant adenovirus vector that expresses the SARS-COV-2 spike protein in a stabilized conformation. The stabilized version includes two mutations in which amino acids are replaced with prolines. In addition, the vaccine has inactive ingredients like citric acid monohydrate but does not include polyethylene glycol. The combination of these factors may potentially define the basis for why the Johnson & Johnson vaccine has not been associated with these hypersensitivity reactions.18, 19, 20
Mechanism of systemic contact dermatitis could be implicated in the post-vaccine reactions
Prior studies on systemic contact dermatitis21 , 22 have suggested that cross reactivity between the systemically administered antigen and a topical agent to which the patient has been sensitized could underlie the pathophysiology of the systemic eczematoid reaction. Antigenically primed memory T cells along with Langerhans cells are involved,21 with recruitment of memory T cells to sites on the skin where the topical agent was previously applied. Discontiguous sites could become inflamed analogous to the cutaneous interface dermatitis reaction occurring at cutaneous sites distant from the primary dermatosis. The allergens, rather than being topical, are travelling through the circulation by varying routes such as ingestion, intravenous administration or intramuscular injection such as through a vaccine. The clinical presentation is somewhat diverse—among the cutaneous manifestations are an eruption at the prior site of allergic contact dermatitis, an eruption at a site of a prior patch test, vesicular hand dermatitis, and pruritic papules on the elbows and knees, erythroderma, and small vessel vasculitis like lesions. After metabolism of the causative systemic antigen, such as a drug in the skin where it functions as a hapten-carrier complex, the antigen is processed by antigen-presenting cells and leads to clonal expansion of T cells in the local lymph node, which can then migrate to the skin and elicit a type IV immune response. There is a preferential sequestration of memory T cells expressing cutaneous lymphocyte antigen into the skin, leading to a relative decrease in memory T cells in the peripheral blood. Another mechanism is the so-called p-1 concept whereby the drugs are bound directly to a T-cell receptor. There is no direct presentation with the major histocompatibility complex and there is no prior metabolism. Regardless of the exact mechanisms, one can explain at least some of the vaccine-associated cutaneous reactions through the mechanisms that have been advanced for systemic contact dermatitis because the well-known cutaneous sensitizer—polyethylene glycol—is found in both Pfizer and Moderna COVID-19 vaccine preparations.21
Spike glycoprotein as the potential stimulus to the post-vaccine reaction
Given that some of the reactions resemble classic cutaneous manifestations encountered in mild COVID-19, such as pityriasis rosea (ie, those cases showing a hybrid interface and eczematous dermatitis with a concomitant low-grade lymphocytic vascular injury), interstitial granulomatous inflammation, small vessel vasculitis, and chilblains/perniosis, one has to consider that an additional potential candidate for this vaccine reaction could be the novel protein manufactured by the genetically altered myocytes.
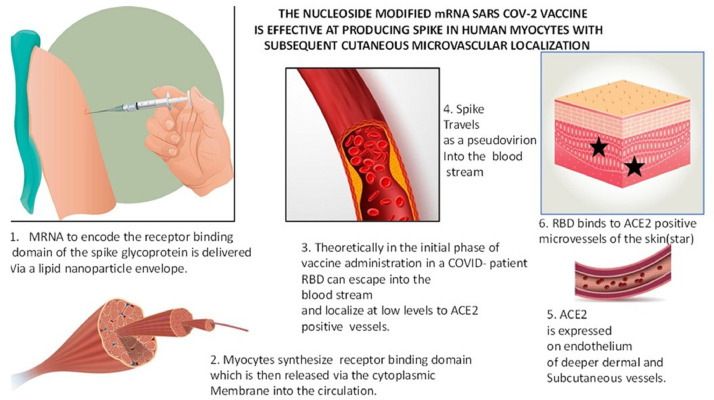
Another question is whether the localization of the hypersensitivity in any way relates to the in situ cutaneous localization of spike glycoprotein because of the relatively high level of ACE2 expression in the cutaneous microvessels relative to other organ sites. Indeed, the immunohistochemical stain to assess for spike glycoprotein showed consistent reproducible findings in all samples tested. The basis for doing the stain was to document evidence of human synthesis and to establish the ability of spike glycoprotein to dock to ACE2+ vessels as a pseudovirion, a hypothesis offered as the basis of systemic complement activation in the setting of severe and critical COVID-19. We were able to show spike glycoprotein in the cutaneous microvasculature in all cases tested. In particular, there were occasional deep-seated vessels in the deep reticular dermis and fat that showed focal expression of spike glycoprotein in endothelium, which is not surprising as it reflects the preferential expression of ACE2 in deeper dermal and subcutaneous microvessels. There were only rare positive-staining vessels, however. A similar distribution was observed for IL-6, caspase 3, and/or TNFα, but with no significant microvascular complement deposition observed. The overall amount of spike glycoprotein in the microvessels was much less than what we observed in the setting of thrombotic retiform purpura of severe and critical COVID-19. Even though the spike is produced for the lifetime of the muscle cell (ie, 10 to 16 years), the neutralizing antibody in response to the manufactured spike would likely prevent spike glycoprotein localization to distant ACE2 positive microvascular beds after humoral immunity is achieved; however, during that nascent period during which complete adaptive immunity has not been reached to neutralize spike glycoprotein binding, an immune response to circulating pseudovirions is very possible (Figure 18).
Fig. 18.
The nucleoside modified mRNA severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccine is effective at producing spike protein in human myocytes with subsequent cutaneous microvascular localization.
1. The mRNA to encode spike protein is delivered via a lipid nanoparticle envelope.
2. Myocytes synthesize spike protein, which is then released into the circulation.
3. Spike travels as a pseudovirion into the bloodstream.
4. Angiotensin converting enzyme 2 (ACE2) on endothelium of deeper dermal/subcutaneous vessels (red chromagen highlighting ACE2 positive vessels, blue arrow).
5. Spike binds to the ACE2-positive microvessels of the skin (red chromagen highlighting spike in endothelium, blue arrow). In the initial phase of vaccine administration spike protein can escape into the blood stream and localize at low levels to ACE2 positive vessels.
Post–COVID-19 vaccine reaction is largely a cutaneous confined reaction but not every case
The lack of systemic and/or multiorgan parenchymal dysfunction implies that the skin is selectively targeted in these adverse cutaneous reactions. In many cases, the cutaneous reaction is not part of a multiorgan adverse hypersensitivity response triggered by the vaccine but rather that inflammation appears to be limited to the skin in most cases.
A few outliers developed in which the reaction was characterized by more severe joint swelling and a folliculocentric vasculitic process in a 27-year-old woman and a 23-year-old man who developed fever, hemolysis, thrombocytopenia, and cutaneous lymphocytic and granulomatous vasculitis. Cases of hemolysis occurred after the COVID-19 vaccine was administered to patients with antecedent histories of hemolysis attributable to paroxysmal nocturnal hemoglobinuria where it has been postulated that the inflammatory response associated with the vaccine is a trigger to complement pathway activation as opposed to the direct effect of spike glycoprotein on causing red cell hemolysis.23 In this particular case, although the skin eruption was typical for a COVID-19–vaccine reaction given the combination of interface dermatitis along with lymphocytic and granulomatous vasculitis, there was also evidence of systemic complement pathway activation. The patient was not known to have any prior disease associated with hemolysis such as atypical hemolytic uremic syndrome. Another 21-year-old patient developed myocardial dysfunction, and imaging studies suggested myocarditis. The deltoid skin biopsy did not disclose any evidence of excessive type I interferon signaling nor was there evidence of systemic complement pathway activation unlike the pattern of excessive complement vascular deposition we see in the normal deltoid skin biopsies of patients with severe COVID-19, including a previously reported patient who developed significant myocardial disease likely representing a small vessel vasculitic variant of myocarditis in the setting of severe COVID-19.7 In addition, the amount of spike glycoprotein localized to the microvessels and the endothelial based cytokine response was similar to the two healthy adults who underwent deltoid biopsy after receiving the vaccine. There have been 226 cases of myocarditis or pericarditis in people aged 30 years and younger who have received an mRNA COVID-19 vaccine, occurring more commonly after the second dose and with a higher incidence occurring in male individuals. Symptoms include chest pain, elevated cardiac enzymes, ST- or T-wave changes, dyspnea, and abnormal echocardiography/imaging. The patients typically make a full recovery.24 The myocardium is rich in ACE2 positive microvessels. We have already demonstrated that human synthesized spike glycoprotein localizes to ACE2 positive vessels of the deeper dermis and fat. We would expect a similarly low level of localization to other organs in which the microvessels express ACE2 as does the heart. As T-cell and B-cell responses are invariably elicited to the human spike glycoprotein, some degree of inflammation could be occurring in the heart, reflective of the localization of the antigenic target.
Role of the vaccine as an adjuvant in unmasking an adaptive immune response in a predisposed host
All vaccines have adjuvants that are added to enhance the adaptive and innate immune response. For example, the bacille Calmette-Guerin enhances the tumoricidal capacity of the autoreactive T cells targeting bladder cancer. The adjuvant plays a role in activating molecules involved in antigen presentation and other pro-inflammatory cytokines and therefore kick-start the immune system. The adjuvants in mRNA vaccines are lipid or polymer-based nanoparticles that protect and stabilize the fragile mRNA and improve its uptake by our immune cells. The mRNA nucleic acid itself is an inherent immunostimulatory molecule owing to its recognition by a variety of innate immune receptors localized at the cell surface, endosome, and cytoplasm. It is logical that our innate immune system would be hardwired to recognize foreign nucleic acid as a threat.25
Because of the high levels of proinflammatory cytokines associated with the adaptive TH1 or TH2 immune response, a microenvironment conducive to the influx of inflammatory cells associated with either TH1 or TH2 immune polarization could be operational in some cases. If there is a genetic tendency for a psoriatic diathesis or atopic dermatitis, the vaccine could trigger the inflammatory cascade that could eventuate in a particular dermatosis such as dermatitis. Conversely, a subclinical hypersensitivity reaction could become unmasked.26 , 27
Owing to the lack of data on safety of novel mRNA COVID-19 vaccines, concern regarding its impact on patients suffering from inflammatory diseases has been raised. In general, vaccination is an uncommon factor triggering psoriasis flares. The association of vaccination with the new development or exacerbation of this skin disease has been reported. The mechanisms responsible for psoriasis exacerbation after vaccination are yet to be understood. It is possible that, similar to influenza vaccines, this mechanism may be caused by both dysregulation of immune system due to viral components and vaccine adjuvants.16 , 28
Human-manufactured spike glycoprotein does not result in systemic complement pathway activation and vascular injury
Despite microvascular localization of spike glycoprotein, significant microvascular sequelae do not appear to occur, reflecting the low burden of manufactured spike glycoprotein in the systemic circulation and the progressive neutralization of human synthesized spike protein by antibodies produced by the host. The data show successful production of the spike glycoprotein post-mRNA vaccination. During that nascent period before the adaptive immune response to neutralize the spike glycoprotein, it is not surprising that circulating spike protein localizes to ACE2 positive endothelium situated in the deeper skin vessels. The human-derived spike glycoprotein that was endocytosed through the endothelium resulted in an endothelial cell response, given the focal expression of caspase 3, IL-6, and TNFα; however, it was not associated with activation of the complement pathway—the critical pathway that is triggered in severe and critical COVID-19—and contributes significantly to the microvascular and procoagulant complications that characterize severe and critical COVID-19. The basis for the lack of complement activation is unclear but the amount of the protein localized to the receptor binding site might be a factor, as could be a difference in the glycosylation pattern of the wild-type spike protein versus the protein synthesized by vaccinated myocytes. In the young woman who developed myocardial insufficiency after receiving the Moderna COVID-19 vaccine, there were no thrombotic changes and no evidence of complement pathway activation. In addition, the amount of spike glycoprotein deposition was minimal and consistent with the extent of deposition observed in the other cases. A summary of the pathogenetic events that underlie the localization of human-manufactured spike glycoprotein to distant microvascular beds is highlighted in Figure 18 .
Not surprisingly, based on our identification of spike glycoprotein of presumptive human myocyte origin in cutaneous microvessels in vaccinated patients, the spike protein product of the mRNA vaccine is detectable in peripheral blood. A prospective study of 13 Boston healthcare workers who had received the Moderna mRNA-1273 vaccine showed the S1 spike subunit in plasma beginning on day 1, peaking at day 525. Although the S1 subunit became undetectable by day 14 as antibody levels rose, intact spike protein persisted much longer in a few of the healthcare workers. There is evidence that the mRNA vaccines themselves may distribute widely through the body. In one manufacturer's report to Japanese regulators, the biodistribution of lipid nanoparticles in injected rats showed that up to 75% of the inoculum escaped the injection site and was found circulating in the blood and pooled in the spleen, liver, bone marrow, adrenal glands, ovaries, and other tissues.29 , 30
Conclusions
In summation, the data demonstrate that the basis of most post-COVID-19–vaccine skin eruptions suggest a type IV hypersensitivity reaction and, less commonly, immune-complex–mediated hypersensitivity. The exact antigenic trigger is not yet established, but the possibilities include an exogenous agent found within the vaccine vehicle, namely polyethylene glycol versus the foreign spike protein manufactured by human myocytes functioning as a systemic antigen capable of eliciting classic forms of hypersensitivity, especially type IV but also antibody-mediated reactions resulting in immune complex deposition. An unrelated antigen or an underlying genetic predisposition for an inflammatory dermatosis like psoriasis, which becomes unmasked owing to immune-enhancing properties of the vaccine, has to be considered as well. The exceptionally small amount of spike glycoprotein that disseminates to ACE2+ microvascular never results in a disease process that resembles severe or critical COVID-19 and hence, not surprisingly, complement pathway activation is not observed, and thrombotic vascular complications, although rarely reported after administration of the COVID-19 vaccine, have not been proven to be caused by the vaccine.
Acknowledgments
Acknowledgments
We are grateful to Dr. Jonathan Zippin for reviewing our manuscript and offering valuable suggestions. We also thank the following clinicians and pathologists for contributing cases to our study: Andrew Alexis, MD, WCMC Dermatology, New York, NY; Andrew Averbock, MD, PhD, WCMC Dermatology, New York, NY; Brett Fisher, MD, Division of Hospital Medicine, New York, NY; Otobia G. Dimson, MD, Oklahoma City, OK; Elisha Singer, MD, NYP Dermatology, New York, NY; Eva Kerby, MD, WCMC Dermatology, New York, NY; Henry J. Lee, MD, WCMC Dermatology, New York, NY; Jalong Gaan, MD, WCMC Dermatology, New York, NY; Jeanyoung Kim, MD, WCMC Dermatology, New York, NY; Jeffrey Endsley, DO, Muskogee, OK; Kelli Lovelace, MD, Tulsa Dermatology Clinic, Tulsa, OK; Melvin Van Boven, DO, Southwest Pathology, Tulsa, OK; Patrick Safo, MD, MMG-Dermatology, Stevens Point, WI; Paul Dantzig, MD, New York, NY; Ryanne Waage, PAC, Aberdeen Dermatology, Aberdeen, SD; Scott Sanders, MD, New City, NY; Silvia Mancebo, MD, WCMC Dermatology, New York, NY; and Sylvana Tuur-Saunders, MD, HCT Dermatology Services, Baltimore, MD.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Chilamakuri R, Agarwal S. COVID-19: characteristics and therapeutics. Cells. 2021;10:206. doi: 10.3390/cells10020206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murphy KR, Patel NC, Ein D, et al. Insights from American College of Allergy, Asthma, and Immunology COVID-19 Vaccine Task Force: allergic reactions to mRNA SARS-CoV-2 vaccines. Ann Allergy Asthma Immunol. 2021;126:319–320. doi: 10.1016/j.anai.2021.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Forni G, Mantovani A. COVID-19 Commission of Accademia Nazionale dei Lincei, Rome. COVID-19 vaccines: where we stand and challenges ahead. Cell Death Differ. 2021;28:626–639. doi: 10.1038/s41418-020-00720-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sahin U, Muik A, Derhovanessian E, et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature. 2020;586:594–599. doi: 10.1038/s41586-020-2814-7. [DOI] [PubMed] [Google Scholar]
- 5.Jackson LA, Anderson EJ, Rouphael NG, et al. An mRNA vaccine against SARS-CoV-2—preliminary report. N Engl J Med. 2020;383:1920–1931. doi: 10.1056/NEJMoa2022483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Magro CM, Mulvey J, Kubiak J, et al. Severe COVID-19: a multifaceted viral vasculopathy syndrome. Ann Diagn Pathol. 2020;50 doi: 10.1016/j.anndiagpath.2020.151645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 2020;220:1–13. doi: 10.1016/j.trsl.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nuovo GJ, Magro C, Shaffer T, et al. Endothelial cell damage is the central part of COVID-19 and a mouse model induced by injection of the S1 subunit of the spike protein. Ann Diagn Pathol. 2020;51 doi: 10.1016/j.anndiagpath.2020.151682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang S, Liu Y, Wang X, et al. SARS-CoV-2 binds platelet ACE2 to enhance thrombosis in COVID-19. J Hematol Oncol. 2020;13:120. doi: 10.1186/s13045-020-00954-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Magro CM, Momtahen S, Mulvey JJ, Yassin AH, Kaplan RB, Laurence JC. Role of the skin biopsy in the diagnosis of atypical hemolytic uremic syndrome. Am J Dermatopathol. 2015;37:349–356. doi: 10.1097/DAD.0000000000000234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mulvey JJ, Laurence J, Seshan S, et al. Docked severe acute respiratory syndrome coronavirus 2 proteins within the cutaneous and subcutaneous microvasculature and their role in the pathogenesis of severe coronavirus disease 2019. Hum Pathol. 2020;106:106–116. doi: 10.1016/j.humpath.2020.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Magro CM, Crowson AN. A distinctive vesiculopustular eruption associated with hepatobiliary disease. Int J Dermatol. 1997;36:837–844. doi: 10.1046/j.1365-4362.1997.00010.x. [DOI] [PubMed] [Google Scholar]
- 13.Magro CM, Crowson AN. Sterile neutrophilic folliculitis with perifollicular vasculopathy: a distinctive cutaneous reaction pattern reflecting systemic disease. J Cutan Pathol. 1998;25:215–221. doi: 10.1111/j.1600-0560.1998.tb01722.x. [DOI] [PubMed] [Google Scholar]
- 14.Crowson AN, Mihm MC, Jr, Magro C. Pyoderma gangrenosum: a review. J Cutan Pathol. 2003;37:97–107. doi: 10.1034/j.1600-0560.2003.00024.x. [DOI] [PubMed] [Google Scholar]
- 15.Janssen Vaccines & Prevention B.V. Clinical Trials. A randomized, double-blind, placebo-controlled phase 3 study to assess the efficacy and safety of Ad26. COV2. S for the prevention of SARS-CoV-2-mediated COVID-19 in adults aged 18 years and older. https//clinicaltrials.gov/ct2/show/NCT04505722. Accessed August 3, 2021.
- 16.Gunes AT, Fetil E, Akarsu S, Ozbagcivan O, Babayeva L. Possible triggering effect of influenza vaccination on psoriasis. J Immunol Res. 2015;2015 doi: 10.1155/2015/258430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McMahon DE, Amerson E, Rosenbach M, et al. Cutaneous reactions reported after Moderna and Pfizer COVID-19 vaccination: a registry-based study of 414 cases. J Am Acad Dermatol. 2021;85:46–55. doi: 10.1016/j.jaad.2021.03.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Food and Drug Administration. FDA briefing document: Janssen Ad26. COV2. S vaccine for the prevention of COVID-19. Vaccines and Related Biological Products Advisory Committee Meeting. https//fda.gov/media//146217/download. Accessed August 3, 2021.
- 19.Farinazzo E, Ponis G, Zelin E, et al. Cutaneous adverse reactions after m-RNA COVID-19 vaccine: early reports from Northeast Italy [e-pub ahead of print]. J Eur Acad Dermatol Venereol. doi:10.1111/jdv.17343, accessed May 22, 2021. [DOI] [PMC free article] [PubMed]
- 20.Cross R. The tiny tweak behind COVID-19 vaccines. Chemical Engineering News. https//cen.acs.org/pharmaceuticals/vaccines/tiny-tweak-behind-COVID-19/98/i38. Accessed August 3, 2021.
- 21.Usatine RP, Riojas M. Diagnosis and management of contact dermatitis. Am Fam Physician. 2010;82:249–255. [PubMed] [Google Scholar]
- 22.Andersen KE, Hjorth N, Menné T. The baboon syndrome: systemically-induced allergic contact dermatitis. Contact Dermatitis. 1984;10:97–100. doi: 10.1111/j.1600-0536.1984.tb00343.x. [DOI] [PubMed] [Google Scholar]
- 23.Gerber GF, Yuan X, Yu J, et al. COVID-19 vaccines induce severe hemolysis in paroxysmal nocturnal hemoglobinuria. Blood. 2021;137:3670–3673. doi: 10.1182/blood.2021011548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Centers for Disease Control and Prevention. Myocarditis and pericarditis following mRNA COVID-19 vaccination. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/myocarditis.html. Accessed August 3, 2021.
- 25.Huang Q, Zeng J, Yan J. Covid-19 mRNA vaccines. J Genet Genomics. 2021;48:107–114. doi: 10.1016/j.jgg.2021.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Magro CM, Scheck L, Soleymani AD. Unmasking of a TH1-mediated vitiligo-like tendency in the setting of dupilumab therapy for adult atopic dermatitis. The Dermatologist. https://www.hmpgloballearningnetwork.com/site/thederm/article/unmasking-th1-mediated-vitiligo-tendency-setting-dupilumab-therapy-adult-atopic-dermatitis. Accessed August 3, 2021.
- 27.Dalton SJ, Haeney MR, Patel L, David TJ. Exacerbation of atopic dermatitis after bacillus Calmette-Guérin vaccination. J R Soc Med. 1998;91:133–134. doi: 10.1177/014107689809100305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krajewski PK, Matusiak Ł, Szepietowski JC. Psoriasis flare-up associated with second dose of Pfizer-BioNTech BNT16B2b2 COVID-19 mRNA vaccine [e-pub ahead of print]. J Eur Acad Dermatol Venereol. doi:10.1111/jdv.17449, accessed 16 Jun, 2021. [DOI] [PMC free article] [PubMed]
- 29.Ogata AF, Cheng C, Desjardins M, et al. Circulating SARS-CoV-2 vaccine antigen detected in the plasma of mRNA-1273 vaccine recipients [e-pub ahead of print]. Clin Inf Dis. doi:10.1093/cid/ciab465, accessed 20 May, 2021. [DOI] [PMC free article] [PubMed]
- 30.Doshi P. Covid-19 vaccines: in the rush for regulatory approval, do we need more data? BMJ. 2021;373:n1244. doi: 10.1136/bmj.n1244. [DOI] [PubMed] [Google Scholar]