Abstract
Purpose:
Cardiorespiratory and skeletal muscle deconditioning occur following coronary artery bypass graft (CABG) surgery and hospitalization. Outpatient, phase 2 cardiac rehabilitation (CR) is designed to remediate this deconditioning, but typically does not begin until several weeks following hospital discharge. Although an exercise program between discharge and the start of CR could improve functional recovery, implementation of exercise at this time is complicated by post-operative physical limitations and restrictions. Our objective was to assess the utility of neuromuscular electrical stimulation (NMES) as an adjunct to current rehabilitative care following post-surgical discharge and prior to entry into CR on indices of physical function in patients undergoing CABG surgery.
Methods:
Patients were randomized to 4 wk of bilateral, NMES (5 d/wk) to their quadriceps muscles or no intervention (control). Physical function testing was performed at hospital discharge and 4-wk post-discharge using the Short Physical Performance Battery (SPPB) and the 6-min walk tests (6MWT). Data from 37 patients (19 control/18 NMES) who completed the trial were analyzed. The trial was registered at ClinicalTrials.gov (NCT03892460).
Results:
Physical function measures improved from discharge to 4-wk post-surgery across our entire cohort (P<.001). Patients randomized to NMES, however, showed greater improvements in 6MWT distance and power output compared to controls (P<.01).
Conclusion:
Our results provide evidence supporting the utility of NMES to accelerate recovery of physical function after CABG surgery.
Keywords: rehabilitation, 6-minute walk, disability
CONDENSED ABSTRACT
This study evaluated whether neuromuscular electrical stimulation can improve functional recovery following hospital discharge in patients undergoing coronary artery bypass graft surgery prior to cardiac rehabilitation. Neuromuscular electrical stimulation improved functional recovery, as measured by 6-min walk test performance.
Coronary artery bypass graft (CABG) surgery is one of the most common surgical procedures performed in the US 1 and is an important treatment option for coronary heart disease.2 Despite these benefits, cardiorespiratory and skeletal muscle deconditioning occur following surgery. Outpatient cardiac rehabilitation (CR) programs are designed to counter the deconditioning effects of medical and surgical interventions in cardiac patients and have well-accepted health and survival benefits.3 While CR is recommended to begin as soon as possible following discharge,4 enrollment typically occurs several weeks after hospital discharge,5, 6 creating a gap in rehabilitative care. Decreased physiological reserve resulting from surgery, hospitalization and the period of convalescence between discharge and CR may increase risk for post-operative complications, readmission and physical disability.7–9 While studies have examined the effects of non-rehabilitation-based transitional care initiatives in medical and surgical cardiac populations to improve clinical outcomes and minimize costs,10, 11 very few have focused on bridging this gap in rehabilitation care between hospital discharge and enrollment in outpatient CR.
One practical reason for this rehabilitative care gap is the difficulty of intervening in a post-surgical population, as pain, limited mobility, reduced functional capacity and activity restrictions prevent participation in classical exercise. 12, 13 Alternative modalities that confer an exercise training effect, but that do not have the physical requirements of classical exercise, may be beneficial. Neuromuscular electrical stimulation (NMES) may be one option, as it permits non-volitional initiation of muscle contractions that can mimic resistance- or aerobic-type exercise,14 producing a comparable training response.15 NMES improves muscle size, strength and performance in older adults with chronic disease,16 and counteracts muscle atrophy related to catabolic stimuli.17, 18 While NMES has received attention as an intervention in heart failure patients,19, to our knowledge, it has not been employed in CABG or other cardiac surgical populations.
Our objective was to evaluate whether NMES applied to the quadriceps musculature for 4 wk following discharge can improve functional recovery in patients undergoing CABG surgery. To accomplish this objective, we randomized patients receiving CABG surgery to bilateral NMES to their quadriceps muscle group or no intervention (control) directly following discharge from the hospital. Assessments were performed at hospital discharge and 4 wk post-discharge using the Short Physical Performance Battery (SPPB) and the 6-min walk test (6MWT), two objective tests of physical functional capacity that predict near- and long-term morbidity and mortality in cardiac surgical and non-surgical populations.7–9 Based on prior studies showing beneficial effects of NMES on muscle size and strength, as well as physical function,16–18 we hypothesized that NMES would lead to greater improvements in objective measures of physical function during the intervention period.
METHODS
This study was a single site, randomized, controlled trial (NCT03892460). Sample size calculations were based on SPPB score being 2.5 points greater in the NMES versus the control group, based on effects of NMES in both operative and non-operative older adult populations.16, 20 To detect this difference, we would need n=18/group with a power of 80% and an alpha of 0.05.
Patients were recruited from our Cardiothoracic Surgery unit between November 2017 and October 2019 and were eligible if they were 50–85 yr of age and scheduled to undergo either urgent or elective CABG with or without valve replacement. Patients were excluded if they had: 1) rheumatoid arthritis or other inflammatory/autoimmune diseases; 2) cancer, excluding non-melanoma skin cancer; 3) exercise-limiting peripheral vascular disease, neuromuscular disease or lower extremity neuromuscular dysfunction related to prior cerebrovascular event; 4) body mass index ≥38 kg/m2; 5) moderate or greater valvular heart disease that was not corrected surgically; or 6) an existing lower extremity blood clot or an implanted cardiac pacemaker or defibrillator, as both are contraindications for NMES.21 After enrollment, patients were withdrawn if they had post-operative complications requiring an extended hospital stay (>7 d) or if an implanted cardiac device was placed. The study was approved by the University of Vermont Committee on Research in the Medical Sciences and written informed consent obtained from each volunteer prior to surgery.
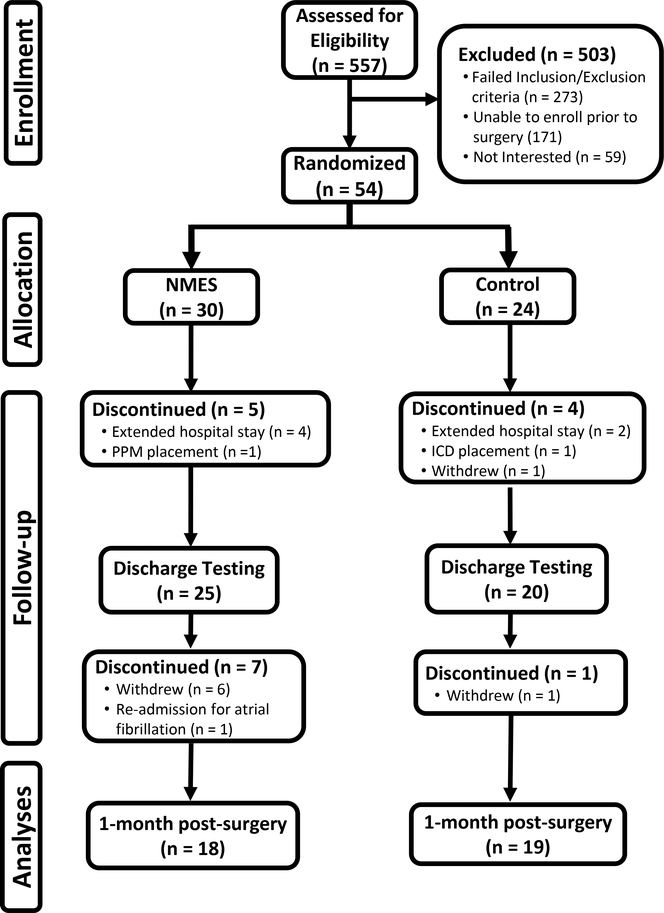
A total of 557 patients were screened from our Cardiothoracic Surgery service (Figure 1). Of these, 113 were eligible and 54 consented to enroll in the study. Following enrollment, patients were randomized (1:1) using a covariate adaptive approach to receive NMES or no intervention, with stratification for age and sex. Of those patients randomized, 8 patients were withdrawn prior to post-surgical hospital discharge because of extended hospital stays or placement of pacemakers or internal cardiac defibrillators and 1 volunteer withdrew. During the 4-wk intervention period, 1 volunteer was withdrawn because of re-admission for atrial fibrillation and 7 patients withdrew (6 cited time commitment and 1 general fatigue).
Figure 1.
Consolidated Standards for Reporting Trials (CONSORT) flow diagram. NMES, neuromuscular electrical stimulation; PPM, permanent pacemaker; ICD, implanted cardioverter defibrillator.
PROTOCOL and MEASURES
Patients were evaluated: pre-surgery, at hospital discharge and 4 wk following discharge. At all evaluations, patients completed the SPPB and Medical Outcomes Study 36-item short form questionnaire. Additionally, at discharge and 4-wk post-discharge testing, the 6MWT was performed. The 6MWT was not performed pre-surgery, as cardiac-related symptomology would limit patients ability to complete the test.22 Finally, at hospital discharge, patients randomized to NMES were trained in the proper use of the NMES device and all patients were provided an activity monitor to measure physical activity throughout the 4-wk intervention period. Volunteers demonstrated operational competency with the NMES device to obtain a solid tetanic contraction prior to discharge.
Home-based, bilateral NMES (Empi Continuum; EMPI Inc.) was applied to the quadriceps of both legs 5 d/wk for 45 min/d. The NMES intensity was patient-selected, with the goal of obtaining maximal tetanic contractions within pain tolerance. The device was used passively (ie, without volitional contraction) and administered biphasic pulses (400 μs duration at 25 Hz), with a duty cycle of 25% (10 s on, 30 s off). Electrode pads (7.5 × 13.5 cm) were placed horizontally on the proximal and distal aspects of the quadriceps, with the legs immobilized at ~40º relative to full knee extension. Controls received no intervention. All patients were instructed by their surgeon to restrict heavy lifting to allow for surgical site healing and to gradually increase daily walking towards a target of 45–60 min/d by 4 wk post-discharge. Both groups were contacted on a weekly basis by the research coordinator to assess general health and, specific to the NMES group, progress and compliance with the intervention. Adherence to the NMES prescription was monitored covertly using the device software, which assesses total device use time, number of sessions and average stimulation intensity. The device will not emit current unless leads are plugged in and there is sufficient resistance across the electrode pads, making device use data a suitable index of intervention fidelity.
The SPPB was the primary outcome and was performed as described by us.23 The SPPB has been validated across a diverse range of older adult populations.24 Briefly, the SPPB is composed of three tasks assessing balance, gait speed, and ability to stand from a chair, with scores from of 0–4 for each for a total score of 0 to 12.
The 6MWT was the secondary outcome and was conducted as described previously.25 The 6MWT has been validated in cardiac surgical patients. 26 Because body weight could change during the intervention period,27 we also calculated power production as: 6MWT power (W) = body weight (kg) • 9.8 (m·s−2) • average gait speed (m·s−1), where 9.8 represents the acceleration of gravity.
The Medical Outcomes Study 36-item short form (MOS SF-36) was used to assess mental and physical health domains, as described,25 because of its broad use in clinical populations to assess physical function.
Physical activity was measured from step count data over the 4-wk intervention period using a wrist-worn accelerometer (Fitbit Flex 2; Fitbit, Inc.).
STATISTICS
Differences between groups in baseline and clinical variables were determined using unpaired t-tests and Fisher exact tests. Analyses of variance, with group and time as factors, was used to evaluate changes in functional and patient-reported outcomes. Effects sizes are reported as partial eta squared (ηp2). SPSS (version 23, IBM Co., Armonk, NY) was used for all analyses and data reported as mean ± SEM, unless otherwise specified, with significance at P<.05.
RESULTS
No differences were found in age, physical characteristics or time to various clinical and study-related milestones, except days between surgery and discharge, which was ~0.5 d longer in the control group (Table 1). All patients underwent sternotomy, and 35 received CABG only, while 2 received CABG and aortic valve replacement (both in control group). There was a time effect for body weight (P<.001) from hospital discharge to 4-wk post-surgery evaluation, with controls decreasing from 90.0 ± 3.1 to 86.2 ± 2.9 kg and NMES from 91.5 ± 3.2 to 88.3 ± 3.0 kg, with no group by time interaction (P=.477). Of the 54 patients enrolled, 8 were withdrawn prior to discharge because of complications that cause a stay of >7 d (n=6) or placement of a pacemaker or defibrillator (n=2), and one volunteer withdrew, leaving n=45 for randomization.
Table 1.
Physical characteristics and clinical and study milestones in control and NMES groupsa
| Control | NMES | P Value | |
|---|---|---|---|
| n, men/women | 19 (17/2) | 18 (16/2) | |
| Age, yr | 66.5 ± 1.6 | 66.2 ± 1.4 | .908 |
| Body weight, kg | 89.4 ± 2.7 | 90.9 ± 3.8 | .752 |
| Height, cm | 173 ± 1 | 176 ± 3 | .332 |
| Body mass index, kg/m2 | 29.7 ± 0.8 | 29.0 ± 0.8 | .558 |
| Total length of hospital stay, d | 8.8 ± 0.6 | 8.2 ± 0.7 | .490 |
| Time from surgery to discharge, d | 5.2 ± 0.2 | 4.6 ± 0.2 | .044 |
| Intervention period, d | 31 ± 1 | 31 ± 1 | .641 |
| Elective CABG | 42 | 44 | 1.000 |
| Exertional angina/dyspneab | 53 | 72 | .313 |
| Current smokers | 11 | 0 | .486 |
Abbreviations: CABG, coronary artery bypass graft.
Data are presented as mean ± SEM or (%).
Exertional angina/dyspnea was classified as reporting exertional symptoms for > 1 wk prior to CABG consultation.
No differences between groups were found in total SPPB score, its components or 6MWT (discharge only) measured pre-surgery (range of P-values: .19 – .61) or at post-surgery discharge (range of P-values: .21 – .56). Similarly, no differences between groups were found in any MOS-SF36 domain or composite score pre-surgery (range of P-values: .09 – .73) or at post-surgery discharge (range of P-values: .14 – .77).
No adverse events were observed related to the NMES intervention. One patient experienced pleuritic chest pain during discharge testing, but no other procedure-related events occurred. Adherence to the prescribed number of NMES sessions was 94 ± 3% (range: 47–100%) and to the total amount of NMES device use time was 89 ± 3% (range: 42–100%). The average NMES intensity level was 45 ± 3 (range: 31–69) and was similar between legs. NMES adherence data represent n=17 patients, as a technical problem prevented device use tracking in one volunteer. Following randomization, 7 patients withdrew from the study (n=6 in NMES group and n=1 in control) and one was withdrawn because of hospital readmission for atrial fibrillation (NMES group).
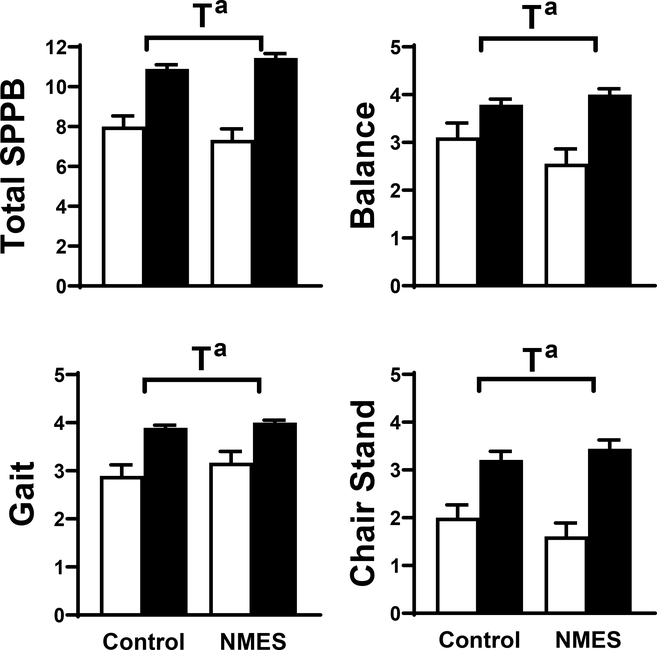
Time effects for total and individual component SPPB scores (all P<.001; Figure 2) were found. Group by time effects did not reach significance for balance (P=.09; ηp2=.081; change from discharge to 4-wk post-discharge in control: 0.7 ± 0.3 vs. NMES: 1.4 ± 0.3 units) and chair stand (P=.07; ηp2=.091; change from discharge to 4-wk post-discharge in control: 1.2 ± 0.2 vs. NMES: 1.8 ± 0.3 units). Finally, the interaction effect for total SPPB score was not significant (P=.11; ηp2=.073; change from discharge to 4-wk post-discharge in control: 2.89 ± 0.50 vs. NMES: 4.11 ± 0.54 units).
Figure 2:
Short physical performance battery (SPPB) test results, including total score and balance, gait and chair stand component scores for volunteers randomized to control (n=19) or NMES (n=18) interventions at hospital discharge (open bar) and 1-mo post-discharge (filled bar). Data represent mean ± SE. T, time effect; G x T, group by time interaction effect. a, P<.001.
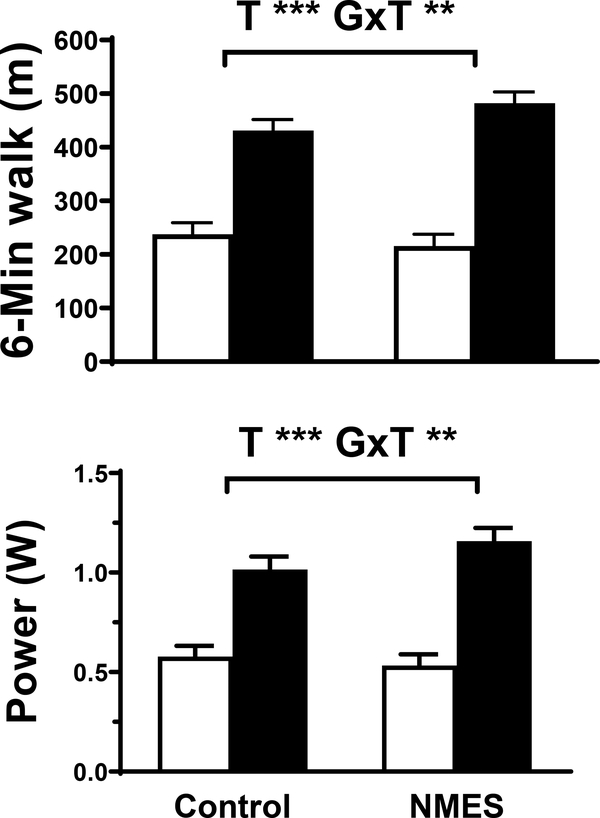
Time effects for 6MWT distance and power (P<.001; Figure 3) were found. Additionally, there were group by time interaction effects for 6MWT distance (P<.01; ηp2=.207; change from discharge to 4-wk post-discharge in control: 194 ± 18 vs. NMES: 267 ± 16 m) and 6MWT power output (P=.01; ηp2=.168; change from discharge to 4-wk post-discharge in control: 0.4 ± 0.1 vs. NMES: 0.6 ± 0.1 W; P=.01).
Figure 3.
Six-minute walk test (6MWT) results, including walk distance and power output for volunteers randomized to control (n=19) or NMES (n=18) interventions at hospital discharge (open bar) and 1-mo post-discharge (filled bar). Data represent mean ± SE. T, time effect; G x T, group by time interaction effect. a, P<.001; b, P≤.01.
There were time effects for physical function, general health and mental health domains, as well as physical composite score (SDC 1), but group by time interaction effects were non-significant (range of P-values: .29 to .87).
The trajectory for recovery of step counts during the 1-mo, post-discharge period was similar between groups (SDC 2), suggesting that group differences in improvement in physical function is not due to differences in ambulation between groups during the intervention period.
DISCUSSION
Our study found that bilateral NMES of the quadriceps muscles improved functional recovery during the first month post-discharge following CABG, as indicated by improvements in 6MWT performance. We used two objective measures of physical function to test our hypothesis: SPPB and 6MWT, both of which have strengths and weaknesses and practically measure different aspects of physical function.
SPPB is easy to perform and is widely used to characterize physical function in disabled older adult populations.28 We chose SPPB because of its widespread use and simplicity, but also because we anticipated all patients would be able to complete the test at hospital discharge. However, SPPB suffers ceiling effects,29 which may hinder its ability to detect improvements in function with NMES. This was likely the case in our study, as 46% (17/37 total; 6/19 control and 11/18 NMES) of patients had maximal SPPB scores of 12 at 4-wk post-discharge evaluation. Despite this, the net difference in the change between groups for total SPPB score (1.2 units), while not statistically different, is clinically meaningful (change of 0.5 units).30
The 6MWT test is widely used as an index of cardiorespiratory fitness in cardiac populations31 and, in contrast to the SPPB, is less prone to ceiling effects.29 Using the 6MWT, we found group by time interaction effects, with a net improvement in 6MWT distance for the NMES group of 73 m more than controls. This 73 m improvement far exceeds most estimates for clinically significant differences (14 – 30 m32) and persisted when 6MWT data were expressed as power output, To put the magnitude of this improvement into context, it is equivalent to the average improvement observed during outpatient CR.33 Thus, functional capacity of patients in the NMES group at enrollment into CR is equivalent to what patients in the control group might expect to achieve by the end of CR. As reduced functional capacity increases risks for complications and mortality after a variety of cardiac procedures, including cardiac surgery, 7–9 NMES-induced adaptations in 6MWT performance may have implications for improving long-term clinical outcomes.34
We included patient-reported indices of physical function because they are commonly used to define physical function and are important for defining patient perception of their functional capacity. However, it is unclear whether they reliably detect improvements in physiological capacity. In the present study, this was not the case, as no group by time interaction effects for any subjective index of health or function were found, congruent with prior studies from our lab.25 This likely reflects the fact that interventions improve physiological capacity, but that patients do not perceive this improvement and/or are reluctant to undertake certain tasks/activities. Collectively, these results underscore the need to conduct objective measurements of physical function to identify physiological improvements with NMES.
Studies have examined NMES in patients with chronic disease, including those with heart failure and chronic obstructive pulmonary disease, providing evidence that NMES improves 6MWT distance,35 similar to our results. While most studies have applied NMES to the quadriceps, the NMES stimulation parameters, session length, frequency of use and overall intervention length and intensity vary widely across studies.35 Because of this, there is insufficient data to develop recommendations for an NMES regimen that produces optimal functional improvements. In fact, our study is unique in utilizing NMES in a surgical population early, post-discharge, which may account for the robust improvements observed 6MWT. Studies have demonstrated the utility of early, post-surgical use of NMES to improve physical function following orthopedic surgery,20 but none have extended its use into non-orthopedic surgical populations.
There were several limitations to our study. First, patients were not blinded to treatment status because NMES elicits skeletal muscle contraction and any sham treatment would not. Moreover, any sham that increased muscle loading may confound comparisons, as mechanical stress and strain likely mediate the benefits of NMES.36 Second, study personnel were not blinded, as resources were insufficient for unblinded and blinded personnel. Functional outcome testing, however, followed strict protocols and performance was likely more impacted by physiological capacity and motivation than assessor bias. Third, we did not analyze data using an intent-to-treat approach, which may have overestimated the effect of NMES. Fourth, our study included few women because fewer women received CABG surgery (19%), as enrollment and rates of patients declining enrollment or dropping out was similar between sexes. This ratio of men to women receiving CABG at our institution is (~4:1), which agrees with nationwide trends.37 Whether men and women respond similarly to NMES is unclear, but our recent meta-analytical findings38 show that they benefit similarly from resistive-type exercise training, which is the type of exercise that our NMES emulates. Finally, we designed our exclusion criteria to exclude the possibility that confounding factors would bias our results in this relatively small pilot trial. Moreover, our small sample size may have limited our ability to detect significant effects of NMES, such as with SPPB. Studies in larger, more diverse cohorts will be required to assess the efficacy of NMES in the broader cardiac surgical population.
CONCLUSION
To our knowledge, this is the first study to use NMES to bridge rehabilitative care for cardiac surgical patients from hospital discharge until entry into outpatient CR. Our results suggest that NMES has marked functional benefits in CABG patients, as revealed by greater improvements in 6MWT performance. Further studies need to determine if these benefits of NMES translate to improved outcomes during CR and beyond and if NMES may benefit other cardiac populations.
Supplementary Material
ACKNOWLEDGEMENTS
We thank all the volunteers who dedicated their valuable time. The study was funded by an internal, departmental pilot grant award (to P.A.A. and M.J.T.)
Funding: This study was funded by the University of Vermont College of Medicine, Department of Medicine.
Footnotes
Conflicts of interest: none
REFERENCES
- 1.Hall MJ, DeFrances CJ, Williams SN, Goloshinskiy A, Schwartzman A. National Hospital Discharge Survey: 2007 summary. Natl Health Stat Report 2010;29:1–20. [PubMed] [Google Scholar]
- 2.Hillis LD, Smith PK, Anderson JL, Bittl JA, Bridges CR, Byrne JG, et al. 2011 ACCF/AHA Guideline for Coronary Artery Bypass Graft Surgery: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines Developed in Collaboration With the American Association for Thoracic Surgery, Society of Cardiovascular Anesthesiologists, and Society of Thoracic Surgeons. J Am Coll Cardiol 2011;58:e123–e210. [DOI] [PubMed] [Google Scholar]
- 3.Ades PA. Cardiac rehabilitation and secondary prevention of doronary heart disease. N Engl J Med 2001;345:892–902. [DOI] [PubMed] [Google Scholar]
- 4.Thomas RJ, Balady G, Banka G, Beckie TM, Chiu J, Gokak S, et al. 2018 ACC/AHA Clinical Performance and Quality Measures for Cardiac Rehabilitation: A report of the American College of Cardiology/American Heart Association Task Force on Performance Measures. J Am Coll Cardiol 2018;71:1814–1837. [DOI] [PubMed] [Google Scholar]
- 5.Savage PD, Rengo JL, Menzies KE, Ades PA. Cardiac rehabilitation after heart valve surgery: comparison with coronary artery bypass graft patients. J Cardiopulm Rehabil Prev 2015;35:231–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnson DA, Sacrinty MT, Gomadam PS, Mehta HJ, Brady MM, Douglas CJ, et al. Effect of early enrollment on outcomes in cardiac rehabilitation. Am J Cardiol 2014;114:1908–1911. [DOI] [PubMed] [Google Scholar]
- 7.Afilalo J, Kim S, O’Brien S, Brennan JM, Edwards FH, Mack MJ, et al. Gait speed and operative mortality in older adults following cardiac surgery. JAMA Cardiol 2016;1:314–321. [DOI] [PubMed] [Google Scholar]
- 8.Lytwyn J, Stammers AN, Kehler DS, Jung P, Alexander B, Hiebert BM, et al. The impact of frailty on functional survival in patients 1 year after cardiac surgery. J Thor Cardiovasc Surg 2017;154:1990–1999. [DOI] [PubMed] [Google Scholar]
- 9.Afilalo J, Eisenberg MJ, Morin J-F, Bergman H, Monette J, Noiseux N, et al. Gait speed as an incremental predictor of mortality and major morbidity in elderly patients undergoing cardiac surgery. J Am Coll Cardiol 2010;56:1668–1676. [DOI] [PubMed] [Google Scholar]
- 10.Jones CE, Hollis RH, Wahl TS, Oriel BS, Itani KMF, Morris MS, et al. Transitional care interventions and hospital readmissions in surgical populations: a systematic review. Am J Surg 2016;212:327–335. [DOI] [PubMed] [Google Scholar]
- 11.Bettger JP, Alexander KP, Dolor RJ, Olson DM, Kendrick AS, Wing L, et al. Transitional care after hospitalization for acute stroke or myocardial infarction: a systematic review. Ann Intern Med 2012;157:407–416. [DOI] [PubMed] [Google Scholar]
- 12.Sethares KA, Chin E, Costa I. Pain intensity, interference and patient pain management strategies the first 12weeks after coronary artery bypass graft surgery. Appl Nurs Res 2013;26:174–179. [DOI] [PubMed] [Google Scholar]
- 13.Tse L, Bowering JB, Schwarz SKW, Moore RL, Sztramko R, Barr AM. Incidence and risk factors for impaired mobility in older cardiac surgery patients during the early postoperative period. Geriatr Gerontol Int 2015;15:276–281. [DOI] [PubMed] [Google Scholar]
- 14.Atherton PJ, Babraj JA, Smith K, Singh J, Rennie MJ, Wackerhage H. Selective activation of AMPK-PGC-1α or PKB-TSC2-mTOR signaling can explain specific adaptive responses to endurance or resistance training-like electrical muscle stimulation. FASEB J 2005;19:786–788. [DOI] [PubMed] [Google Scholar]
- 15.Harris S, LeMaitre JP, Mackenzie G, Fox KAA, Denvir MA. A randomised study of home-based electrical stimulation of the legs and conventional bicycle exercise training for patients with chronic heart failure. Eur Heart J 2003;24:871–878. [DOI] [PubMed] [Google Scholar]
- 16.Maddocks M, Gao W, Higginson IJ, Wilcock A. Neuromuscular electrical stimulation for muscle weakness in adults with advanced disease. Cochrane Database Syst Rev 2013;1:1465–1858. [DOI] [PubMed] [Google Scholar]
- 17.Martin TP, Gundersen LA, Blevins FT, Coutts RD. The influence of functional electrical stimulation on the properties of the vastus lateralis fibers following total knee arthroplasty. Scand J Rehabil Med 1991;23:207–210. [PubMed] [Google Scholar]
- 18.Gerovasili V, Stefanidis K, Vitzilaios K, Karatzanos E, Politis P, Koroneos A, et al. Electrical muscle stimulation preserves the muscle mass of critically ill patients: a randomized study. Crit Care 2009;13:R161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karavidas A, Arapi S, Pyrgakis V, Adamopoulos S. Functional electrical stimulation of lower limbs in patients with chronic heart failure. Heart Fail Rev 2010;15:563–579. [DOI] [PubMed] [Google Scholar]
- 20.Stevens-Lapsley JE, Balter JE, Wolfe P, Eckhoff DG, Kohrt WM. Early neuromuscular electrical stimulation to improve quadriceps muscle strength after total knee arthroplasty: a randomized controlled trial. Phys Ther 2012;92:210–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Houghton PE, Nussbaum EL, Hoens AM. Electrophysical agents. Contraindications and precautions: an evidence-based approach to clinical decision making in physical therapy. Physiother Can 2010;62:1–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Enright PL, McBurnie MA, Bittner V, Tracy RP, McNamara R, Arnold A, et al. The 6-min walk test: a quick measure of functional status in elderly adults. Chest 2003;123:387–398. [DOI] [PubMed] [Google Scholar]
- 23.Ades PA, Savage PD, Cress ME, Brochu M, Lee NM, Poehlman ET. Resistance training on physical performance in disabled older female cardiac patients. Med Sci Sports Exerc 2003;35:1265–1270. [DOI] [PubMed] [Google Scholar]
- 24.Freire AN, Guerra RO, Alvarado B, Guralnik JM, Zunzunegui MV. Validity and reliability of the Short Physical Performance Battery in two diverse older adult populations in Quebec and Brazil. J Aging Health 2012;24:863–878. [DOI] [PubMed] [Google Scholar]
- 25.Brochu M, Savage P, Lee M, Dee J, Cress ME, Poehlman ET, et al. Effects of resistance training on physical function in older disabled women with coronary heart disease. J Appl Physiol 2002;92:672–678. [DOI] [PubMed] [Google Scholar]
- 26.Chen Y-C, Chen K-C, Lu L-H, Wu Y-L, Lai T-J, Wang C-H. Validating the 6-minute walk test as an indicator of recovery in patients undergoing cardiac surgery: A prospective cohort study. Medicine (Baltimore) 2018;97:e12925–e12925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Savage PD, Lakoski SG, Ades PA. Course of body weight from hospitalization to exit from cardiac rehabilitation. J Cardiopulm Rehabil Prev 2013;33:274–280. [DOI] [PubMed] [Google Scholar]
- 28.Fisher S, Ottenbacher KJ, Goodwin JS, Graham JE, Ostir GV. Short Physical Performance Battery in hospitalized older adults. Aging Clin Exp Res 2009;21:445–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sayers SP, Guralnik JM, Newman AB, Brach JS, Fielding RA. Concordance and discordance between two measures of lower extremity function: 400 meter self-paced walk and SPPB. Aging Clin Exp Res 2006;18:100–106. [DOI] [PubMed] [Google Scholar]
- 30.Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc 2006;54:743–749. [DOI] [PubMed] [Google Scholar]
- 31.Guyatt GH, Sullivan MJ, Thompson PJ, Fallen EL, Pugsley SO, Taylor DW, et al. The 6-minute walk: a new measure of exercise capacity in patients with chronic heart failure. Can Med Assoc J 1985;132:919–923. [PMC free article] [PubMed] [Google Scholar]
- 32.Bohannon RW, Crouch R. Minimal clinically important difference for change in 6-minute walk test distance of adults with pathology: a systematic review. J Eval Clin Pract 2017;23:377–381. [DOI] [PubMed] [Google Scholar]
- 33.Gremeaux V, Troisgros O, Benaïm S, Hannequin A, Laurent Y, Casillas J-M, et al. Determining the minimal clinically important difference for the six-minute walk test and the 200-meter fast-walk test during cardiac rehabilitation program in coronary artery disease patients after acute coronary syndrome. Arch Phys Med Rehabil 2011;92:611–619. [DOI] [PubMed] [Google Scholar]
- 34.Keteyian SJ, Brawner CA, Savage PD, Ehrman JK, Schairer J, Divine G, et al. Peak aerobic capacity predicts prognosis in patients with coronary heart disease. Am Heart J 2008;156:292–300. [DOI] [PubMed] [Google Scholar]
- 35.Jones S, Man WDC, Gao W, Higginson IJ, Wilcock A, Maddocks M. Neuromuscular electrical stimulation for muscle weakness in adults with advanced disease. Cochrane Database Syst Rev 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guigni BA, Fix DJ, Bivona JJ, Palmer BM, Carson JA, Toth MJ. Electrical stimulation prevents doxorubicin-induced atrophy and mitochondrial loss in cultured myotubes. Am J Physiol Cell Physiol 2019;317:C1213–C1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim LK, Looser P, Swaminathan RV, Minutello RM, Wong SC, Girardi L, et al. Outcomes in patients undergoing coronary artery bypass graft surgery in the United States based on hospital volume, 2007 to 2011. J Thor Cardiovasc Surg 2016;151:1686–1692. [DOI] [PubMed] [Google Scholar]
- 38.Straight CR, Fedewa MV, Toth MJ, Miller MS. Improvements in skeletal muscle fiber size with resistance training are age-dependent in older adults: a systematic review and meta-analysis. J Appl Physiol 2020;129:392–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.