Abstract
Background:
The relationship between histologic disease activity in eosinophilic esophagitis (EoE) and generic measures of quality of life (QoL) is unclear.
Aims:
To determine differences in QoL in adults with EoE based on histologic activity and assess changes in QoL over time.
Methods:
We performed an analysis of prospectively collected data from patients in the University of North Carolina EoE Registry. Patients were categorized with histologically active (≥15 eosinophils per high-power field [eos/hpf]) or inactive (<15 eos/hpf) disease. Dysphagia severity was measured with a Likert scale. QoL was measured with 36-Item Short Form (SF-36), compared between active and inactive groups, and assessed longitudinally.
Results:
Of 147 EoE cases, those with inactive disease (n=56) reported less dysphagia severity (3.2 vs 1.9; p=0.003) and had lower endoscopic severity (3.8 vs. 1.0; p<0.001) than those with active disease (n=91). While SF-36 scores did not differ between active and inactive status, lower mental component scores (MCS) were seen in patients treated with empiric dietary elimination (44.9 vs 50.8; p=0.005). Dysphagia severity was negatively correlated with both physical component score (PCS) (r=−0.33; p<0.001) and MCS (r=−0.18; p=0.03). Despite more cases achieving histologic response over time, SF-36 scores did not improve on either raw or adjusted analyses.
Conclusion:
QoL measured by SF-36 in EoE was similar regardless of histologic disease activity and was in the range of population averages. General QoL metrics like the SF-36 do not appear to have substantial utility in EoE.
Keywords: Eosinophilic esophagitis, quality of life, treatment, outcomes, disease activity
Introduction
Eosinophilic Esophagitis (EoE) is a chronic inflammatory/allergic disease that is characterized by an eosinophilic infiltration of the esophageal mucosa and symptoms of esophageal dysfunction.[1] EoE gained recognition in the 1990s and has become increasingly prevalent since then.[2] Adults typically show symptoms of dysphagia and heartburn. In contrast, children can have abdominal pain, nausea, vomiting, heartburn/reflux, feeding intolerances, and failure to thrive.[3] EoE has a large burden of disease related to costs and procedure volumes for a disease that is still considered to be rare.[4,5]
While research advances have improved the identification, characterization, and treatment of EoE, the impact of disease on quality of life (QoL) is still incompletely understood. Existing literature suggests that EoE patients have lower mental health domains of QoL compared to healthy patients, partially due to disease duration and diagnostic delay.[6–8] Symptom severity also has an impact; patients with recurrent food impaction or who are receiving restrictive dietary interventions may have the largest decrement on QoL.[9,10] The role of endoscopic and histologic findings on QoL has not been extensively investigated, but in a prior study a significant association between histologic factors (eosinophil count) and QoL was not identified.[9] Preliminary studies investigating treatment impact on QoL demonstrated that patients using corticosteroids have improved QoL after treatment.[8,11–13] However, the relationship between QoL, disease activity, treatment response, and clinical outcomes is still not clear.
With treatment, EoE can transition between histologically active and inactive disease states. It is possible that QoL in EoE patients changes based on different factors including clinical manifestations and therapeutic approaches to control the disease. For example, as an individual with active symptomatic disease progresses to inactive asymptomatic disease, quality of life may improve due to fewer symptoms. However, EoE treatments may negatively impact quality of life depending on the complexity, inconvenience, and time requirements of regimens. Understanding the impact of symptoms and treatment on QoL will help optimize treatment regimens to maximize QoL in EoE patients. Therefore, this study aimed to determine how reported QoL varied by disease activity both at a single time point in a large cohort and within the same patients overtime.
Methods
Study design, data source, and subjects
This study was a secondary analysis of prospectively collected data from EoE patients in the UNC EoE Patient Registry, which was established in 2010. This registry is an IRB-approved longitudinal prospective cohort study. Research coordinators actively screened clinic and endoscopy schedules in order to determine which patients were eligible for enrollment. Eligible subjects (patients of any age with a confirmed diagnosis of EoE as per consensus guidelines)[14–16] were approached prior to endoscopy. After providing informed consent, which includes consent for future data use, patients were enrolled. Of note, the large majority of these patients were PPI non-responders, as required at the time that they were diagnosed.
Data were prospectively collected on comorbidities, medications and current EoE treatments, symptoms, and demographics. During the upper endoscopy, endoscopic data were collected, esophageal biopsies were obtained, and histologic findings were recorded. Dysphagia symptoms were measured on a 11 point Likert scale as previously described.[17] Endoscopic severity was measured using the validated EoE Endoscopic Reference Score (EREFS).[18] Additionally, we had patients complete the 36-Item Short Form (SF-36) to measure quality of life. When enrolled subjects returned for clinically indicated follow-up endoscopic visits, repeat measures with the same data collection instruments were obtained. Patients had a varying number of visits in this registry, with timing dependent on their clinical care. For the present study, all registry patients with at least one visit with full endoscopic, histologic, and QoL data were included.
Outcomes and Variables of Interest
The primary outcome was histologic response. Active EoE was defined as ≥15 eosinophils per high-power field (eos/hpf; hpf size = 0.24mm2), and inactive EoE (i.e. disease in remission) was defined as <15 eos/hpf.[19,20] Other outcomes included endoscopic severity (EREFS) and dysphagia symptom severity, which was measured by using an 11-point Likert scale (scores from 0 to 10).[17]
The main variable of interest was QoL, measured using the SF-36. The SF-36 is a patient-completed questionnaire containing 36 questions that represent eight domains: physical functioning, role limitations due to physical health, bodily pain, general health, vitality, social functioning, role limitations due to emotional functioning, and mental health.[8,21,22] Physical function refers to the extent to which health affects physical activities; a sample question is “Does your health now limit your ability to climb stairs?”[22] Role limitations due to physical health considers the impact of physical health on daily activities. Bodily pain reflects the subject’s reported pain level; a sample question is, “How much has bodily pain interfered with normal daily activities?”[22] General health considers the subject’s rating of their personal health. Vitality indicates the subject’s perceived fatigue and energy level. Social functioning refers to the extent to which a subject’s physical and emotional problems affect baseline social activities; a sample question is, “How much have your physical and emotional problems affected your social activities?”[22] Role limitations due to emotional functioning addresses the impact of emotional health on daily activities. Mental health represents the subject’s rating of their mental health (i.e. depressed vs. happy). The questions can be grouped together to give scores representing patient perceptions of the different quality of life domains. Patient responses are also compiled to create a physical component summary score (PCS) comprised of the first 4 listed domains and a mental component summary score (MCS) comprised of the last 4 listed domains. SF-36 scores range from 0 to 100 with a higher score indicating better QoL.[8,21,22] Notably, at the time the UNC EoE Registry was created, EoE disease-specific metrics to assess quality of life did not exist, which prompted the use of the SF-36. Similarly, validated symptom measures for EoE also were not available and could not be included with the current cohort.
Data Analysis
Demographic and clinical features of the study population were described with summary statistics. To assess QoL based on disease activity, we used cross-sectional data from the first Registry visit for all included patients. Each patient was classified either as histologically active (≥15 eos/hpf) or inactive (<15 eos/hpf) based on their initial histologic status; a patient was only in one of the groups so the groups were independent. The mean SF-36 scores in the active and inactive groups were compared using a two-sample t-test. Similarly, we performed secondary analyses for each of the sub-domains of the SF-36 using the same method, and we repeated this analysis with a more stringent threshold for histologic response (<1 eos/hpf). We also examined overall QoL by treatment types (topical steroids or dietary elimination) and performed correlational analysis (Pearson’s correlation) between SF-36 scores and disease outcome measures. We performed multiple linear regression analyses to assess for independent predictors of PCS and MCS. We selected predictors based on clinical, endoscopic, and histologic features, as well as treatments, that were felt to potentially impact outcomes, and therefore quality of life. To measure changes in QoL over time among individual EoE patients, we required patients included in this analysis to have at least 2 Registry visits with corresponding SF-36 data. Thus, a subset of the overall study population was included in the longitudinal analyses. Mean SF-36 scores overtime were compared with a paired t-test.
Results
Patient Characteristics
Of 291 patients in the EoE Registry overall, 147 met inclusion criteria in this study with full data available. Of these, 91 (62%) had active disease and 56 (38%) had inactive disease. The groups were similar in terms of demographics, rates of atopic disorders, and symptom length prior to diagnosis (Table 1). Most patients (80%) were on EoE treatment at the time of Registry enrollment. As would be expected, patients with histologically inactive disease reported less dysphagia severity (1.9 vs 3.2; p=0.003) and had a significantly lower mean EREFS score (1.0 vs 3.8; p<0.001, Table 1) compared to those with active disease. The mean peak eosinophil count was 64.6 eos/hpf in the active group and 2.9 in the inactive group.
Table 1.
Clinical, endoscopic, and histologic features between histologically active and inactive EoE study subjects
| Active EoE ≥15 eos/hpf (n = 91) | Inactive EoE <15 eos/hpf (n = 56) | p* | |
|---|---|---|---|
|
|
|
||
| Age (mean years ± SD) | 36.1 ± 13.4 | 38.5 ± 12.3 | 0.247 |
| Male (n, %) | 52 (57) | 31 (55) | 0.83 |
| White (n, %) | 87 (96) | 54 (96) | 0.81 |
| College education of higher (n, %) | 45 (60) | 32 (61) | 0.72 |
| Atopy (n, %) | |||
| Asthma | 24 (32) | 21 (47) | 0.26 |
| Eczema | 21 (28) | 10 (22) | 0.19 |
| Food allergy | 44 (59) | 26 (59) | 0.88 |
| Allergic rhinitis | 52 (69) | 34 (76) | 0.66 |
| Symptom length >5 years prior to dx (n, %) | 57 (63) | 35 (63) | 0.64 |
| Dysphagia severity (mean Likert score ± SD) | 3.2 ± 2.7 | 1.9 ± 2.3 | 0.003 |
| Current treatments (n, %) | |||
| Any PPI** | 35 (40) | 35 (67) | 0.002 |
| Oral viscous budesonide | 30 (34) | 21 (38) | 0.68 |
| Fluticasone | 5 (6) | 10 (18) | 0.02 |
| Targeted elimination | 17 (19) | 9 (16) | 0.67 |
| Empiric elimination | 20 (22) | 13 (23) | 0.89 |
| Foods eliminated (mean number ± SD) | 4.6 ± 2.2 | 5.6 ± 2.7 | 0.16 |
| No treatment (not counting PPI) | 21 (23) | 8 (14) | 0.18 |
| Endoscopic features (n, %) | |||
| Exudates | 57 (63) | 6 (11) | < 0.001 |
| Rings | 71 (78) | 28 (50) | < 0.001 |
| Edema | 52 (57) | 2 (4) | < 0.001 |
| Furrows | 78 (86) | 7 (13) | < 0.001 |
| Stricture | 44 (48) | 16 (29) | 0.02 |
| Diameter (mean mm ± SD) | 10.4 ± 5.0 | 13.5 ± 4.8 | 0.04 |
| Narrowing | 32 (35) | 13 (23) | 0.13 |
| Crepe-paper | 4 (4) | 1 (2) | 0.40 |
| Dilation performed | 46 (51) | 23 (42) | 0.31 |
| Total EREFS score (mean ± SD) | 3.8 ± 2.1 | 1.0 ± 1.0 | < 0.001 |
| Inflammatory EREFS scores | 2.3 ± 1.4 | 0.3 ± 0.5 | < 0.001 |
| Fibrosis EREFS score | 1.7 ± 1.1 | 0.8 ± 0.9 | < 0.001 |
| Peak eosinophil count (mean eos/hpf ± SD) | 64.6 ± 47.8 | 2.9 ± 4.0 | -- |
Means compared with a two-sample t-test and proportions compared with a chi square test.
PPI treatment status information available for n=132 subjects.
Quality of Life in Active and Inactive Disease
The difference in quality of life in active and inactive disease was not statistically significant based on SF-36 domain scores and PCS/MCS. For example, the PCS was 51.0 in the active group and 51.4 in the inactive group (p=0.80) and the MCS scores were 48.8 and 50.1 in active and inactive, respectively (p=0.49) (Table 2). Results were similar using the complete response disease activity threshold of <1 eos/hpf in the 25 subjects who achieved this result.
Table 2.
Quality of life, as measured by SF-36, between histologically active and inactive EoE, using two different thresholds (<15 eos/hpf and <1 eos/hpf) to define histologically inactive disease. P-values indicate comparisons between the active EoE group with each inactive EoE group.
| Active EoE ≥15 eos/hpf (n = 91) | Inactive EoE <15 eos/hpf (n = 56) | p* | Inactive EoE <1 eos/hpf (n = 25) | p* | |
|---|---|---|---|---|---|
|
|
|||||
| SF-36 metrics (mean ± SD) | |||||
| Physical component score (PCS) | 51.0 ± 10.1 | 51.4 ± 9.3 | 0.80 | 54.5 ± 7.5 | 0.11 |
| General health (GH) | 69.4 ± 24.3 | 70.0 ± 22.0 | 0.89 | 75.3 ± 19.9 | 0.27 |
| Physical function (PF) | 91.2 ± 18.2 | 92.8 ± 15.7 | 0.57 | 94.1 ± 11.7 | 0.45 |
| Role limitations – physical (RP) | 81.5 ± 36.5 | 86.4 ± 30.3 | 0.39 | 97.0 ± 15.0 | 0.04 |
| Bodily pain (BP) | 76.2 ± 25.0 | 77.9 ± 19.8 | 0.65 | 83.8 ± 16.7 | 0.15 |
| Mental component score (MCS) | 48.8 ± 11.5 | 50.1 ± 9.5 | 0.49 | 49.6 ± 10.3 | 0.76 |
| Vitality (VT) | 58.5 ± 23.7 | 55.7 ± 23.8 | 0.50 | 59.0 ± 22.3 | 0.92 |
| Social functioning (SF) | 83.9 ± 25.6 | 89.0 ± 19.1 | 0.19 | 91.0 ± 19.3 | 0.20 |
| Role limitations – emotional (RE) | 80.6 ± 36.2 | 89.5 ± 27.6 | 0.12 | 92.0 ± 24.1 | 0.14 |
| Mental health (MH) | 74.7 ± 18.7 | 75.3 ± 17.0 | 0.85 | 72.9 ± 19.9 | 0.67 |
Means compared with a two-sample t-test.
Quality of Life and Disease Features
Dysphagia severity was mildly negatively correlated with PCS (R=−0.33, p<0.001) and MCS (R=−0.18, p=0.03). There was no significant correlation between PCS and MCS and disease outcome metrics including peak eosinophil count, EREFS score, and stricture diameter (Table 3). Mean PCS was higher in patients with stricture on endoscopy (p=0.03) and when dilation was performed (p=0.03). Patients using oral viscous budesonide (OVB) as their EoE treatment were also noted to have higher PCS than those not on OVB (53.6 vs 50.2; p=0.04). Presence of other endoscopic findings and EoE treatments did not yield statistically significant results with regard to PCS (Figure 1A). Patients on empiric food elimination had lower mean MCS scores (44.9 vs 50.8; p=0.005). No other differences in mean MCS based on endoscopic findings or EoE treatments were noted (Figure 1B).
Table 3.
Correlations between the physical and mental component scores (PCS and MCS) and peak eosinophil count, endoscopic findings, and dysphagia severity.
| PCS (r; p) | MCS (r; p) | |
|---|---|---|
|
|
||
| Peak eosinophil count | 0.01; 0.87 | −0.09; 0.30 |
| Total EREFS | 0.05; 0.62 | 0.08; 0.41 |
| Inflammatory EREFS | −0.07; 0.48 | 0.04; 0.66 |
| Fibrostenotic EREFS | 0.17; 0.09 | 0.09; 0.38 |
| Stricture diameter | −0.004; 0.98 | −0.24; 0.07 |
| Dysphagia severity (Likert) | −0.33; < 0.001 | −0.18; 0.03 |
Correlation coefficient (r) calculated with Pearson’s correlation.
Figure 1.
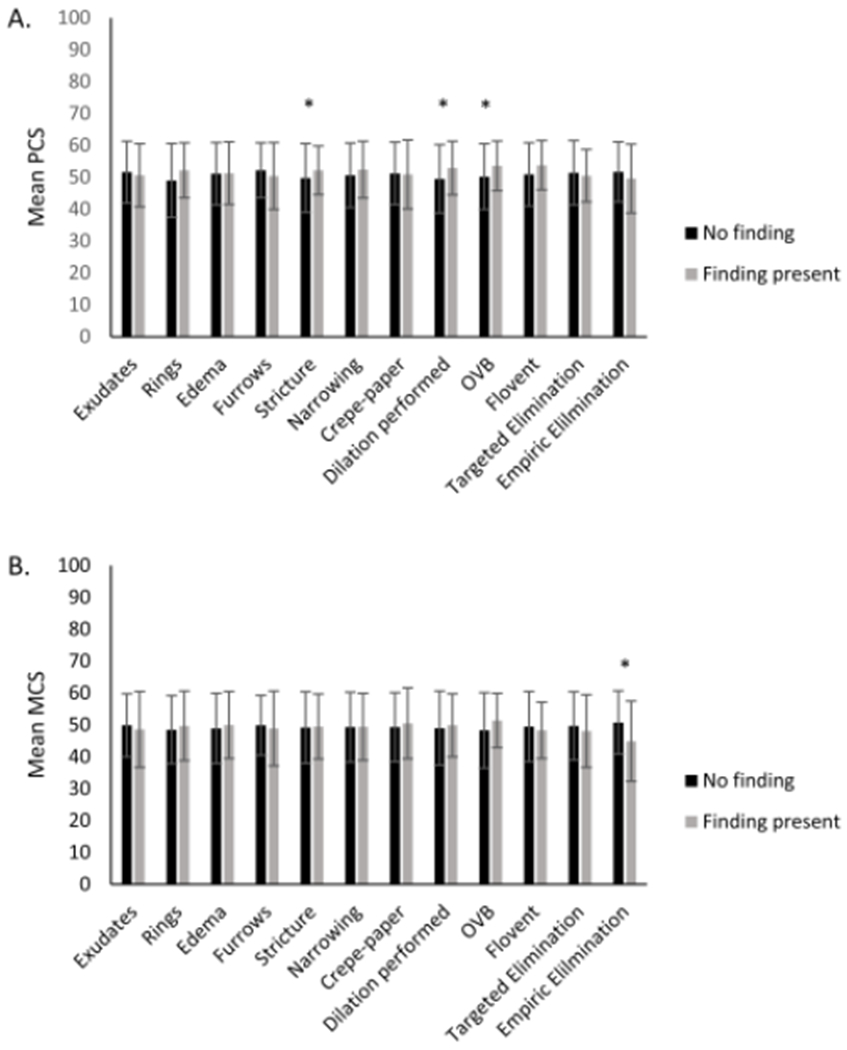
Associations between the physical component scores (A) and mental component scores (B) and endoscopic findings and treatment status. The black bars indicate that the finding/treatment is not present, and the gray bars indicate that the finding/treatment is present. (* indicates p<0.05)
When assessing endoscopic findings and SF-36 subdomains, bodily pain was found to be significantly worse in the absence of rings (69.4 vs. 80.4; p=0.006), absence of stricture (73.5 vs. 81.8; p=0.03), and if dilation was not performed (72.3 vs. 81.6; p=0.01). Vitality was worse without esophageal rings (50.6 vs. 60.7; p=0.01) (Figure 2A). Patients on an empiric elimination diet reported worse general health (p=0.03), physical role limitations (p=0.05), vitality (p=0.008), social functioning (p=0.02), emotional role limitations (p=0.007), and mental health (p=0.03) (Figure 2B).
Figure 2.
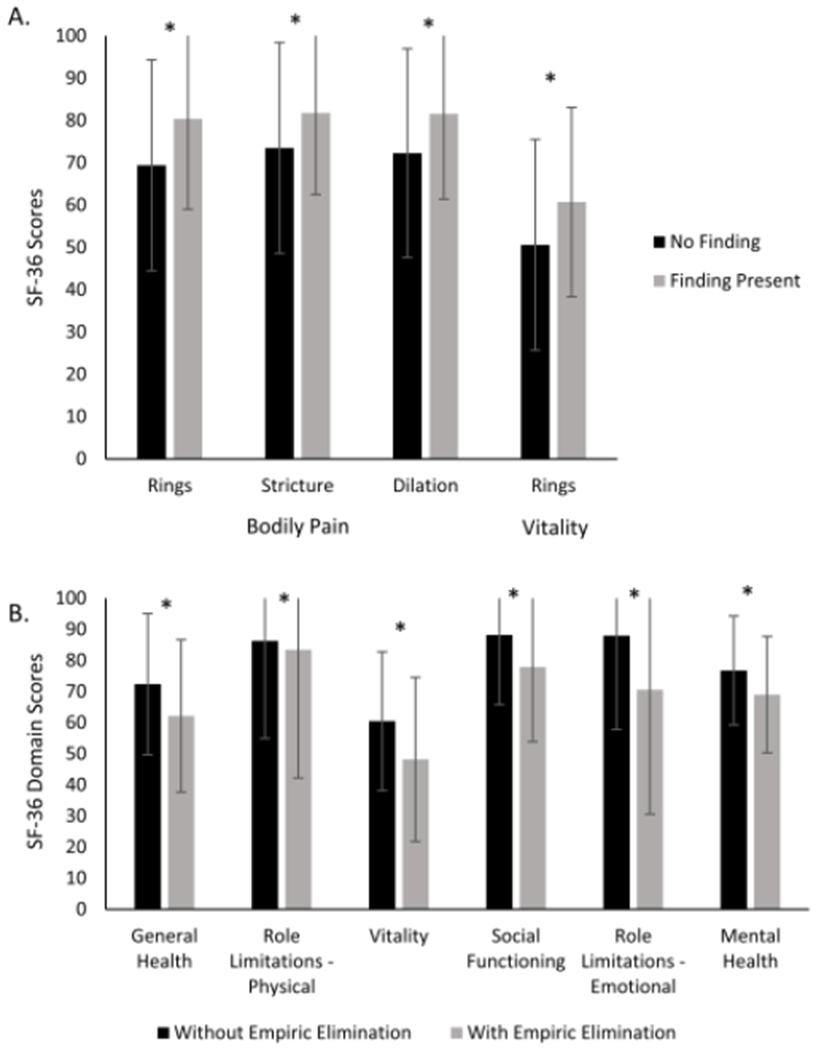
(A) Associations detected between endoscopic findings and either the bodily pain (BP) or vitality (VT) domains of the SF-36. Black bars indicate BP scores and gray bars indicate VT scores. (B) Associations detected between selected SF-36 subscores in patients with and without empiric elimination dietary therapy. Black bars indicate no diet elimination and gray bars indicate current empiric elimination.
We performed multiple linear regression analyses to assess for factors independently associated with PCS and the MCS. Clinical factors of interest, histologic response, and other factors that differed between histologically active and inactive EoE patients were included in the model. Only the presence of esophageal rings on endoscopy (PCS score difference of 3.8, 95% CI: 0.4-7.2) and pain with swallowing (PCS score difference of 4.5, 95% CI: 1.6-7.4) were independently associated with an increased PCS score, though the direction of this trend is opposite of what would be expected (Table 4). Being on an elimination diet was the only factor independently associated with decreased MCS score (MCS score difference of −7.2, 95% CI: −12.5 - −1.9) (Table 4).
Table 4.
Multivariate analysis of the physical and mental component scores (PCS and MCS).
| PCS |
MCS |
|||
|---|---|---|---|---|
| Characteristic | β value | 95% CI | β value | 95% CI |
|
|
|
|||
| Histologic response | −1.7 | −6.2-2.7 | 3.3 | −2.3-9.0 |
| Exudates | 0.2 | −3.9-4.3 | −1.6 | −6.7-3.6 |
| Rings | 3.8 | 0.3-7.2 | −0.2 | −5.6-4.2 |
| Edema | 2.5 | −1.7-6.7 | 4.4 | −1.0-9.7 |
| Furrows | −1.4 | −5.9-3.2 | 1.0 | −4.8-6.8 |
| Stricture | 2.8 | −1.5-7.2 | −1.2 | −6.7-4.3 |
| Dilation performed | −2.5 | −6.7-1.7 | 1.2 | −4.1-6.5 |
| Budesonide therapy | 3.4 | −0.5-7.3 | 0.1 | −4.8-5.1 |
| Fluticasone therapy | 4.4 | −1.1-9.8 | −5.5 | −12.5-1.5 |
| Targeted diet | −0.1 | −4.1-3.9 | −0.9 | −6.0-4.2 |
| Empiric diet | −0.2 | −4.4-3.9 | −7.2 | −12.5- −1.9 |
| PPI therapy | 0.1 | −3.2-3.5 | 1.4 | −2.9-5.6 |
| Dysphagia Likert | −0.3 | −0.9-0.4 | −0.4 | −1.2-0.4 |
| Pain with swallowing | 4.5 | 1.6-7.4 | 3.4 | −0.2-7.0 |
Longitudinal Analysis of Quality of Life
Fifty-five patients completed the SF-36 at their initial EoE visit and their next subsequent visit separated by a median of 98 days (IQR: 63-245). Twenty-six of those patients also completed it at another subsequent visit separated from their initial visit with a median of 245 days (IQR:141-434). Overall, 20% of patients in these groups were in histologic remission (inactive disease at 15 eos/hpf) at their initial visit, 44% at their subsequent visit, and 45% at their next subsequent visit. Despite this, there was not a significant difference in SF-36 scores between the initial visit and the first subsequent visit nor between the initial visit and the next subsequent visit, suggesting that QoL did not change over time (Figure 3). Of note, while dysphagia severity measured by the Likert numerically decreased between visit 1 and 3, this was not significant (3.2 ± 3.7 vs 2.2 ± 3.0; p=0.11). To explore QoL over time in more detail, a model with general estimating equations was performed accounting for Registry visit and histologic disease activity, and no change in QoL metrics over time was observed (data not shown).
Figure 3.
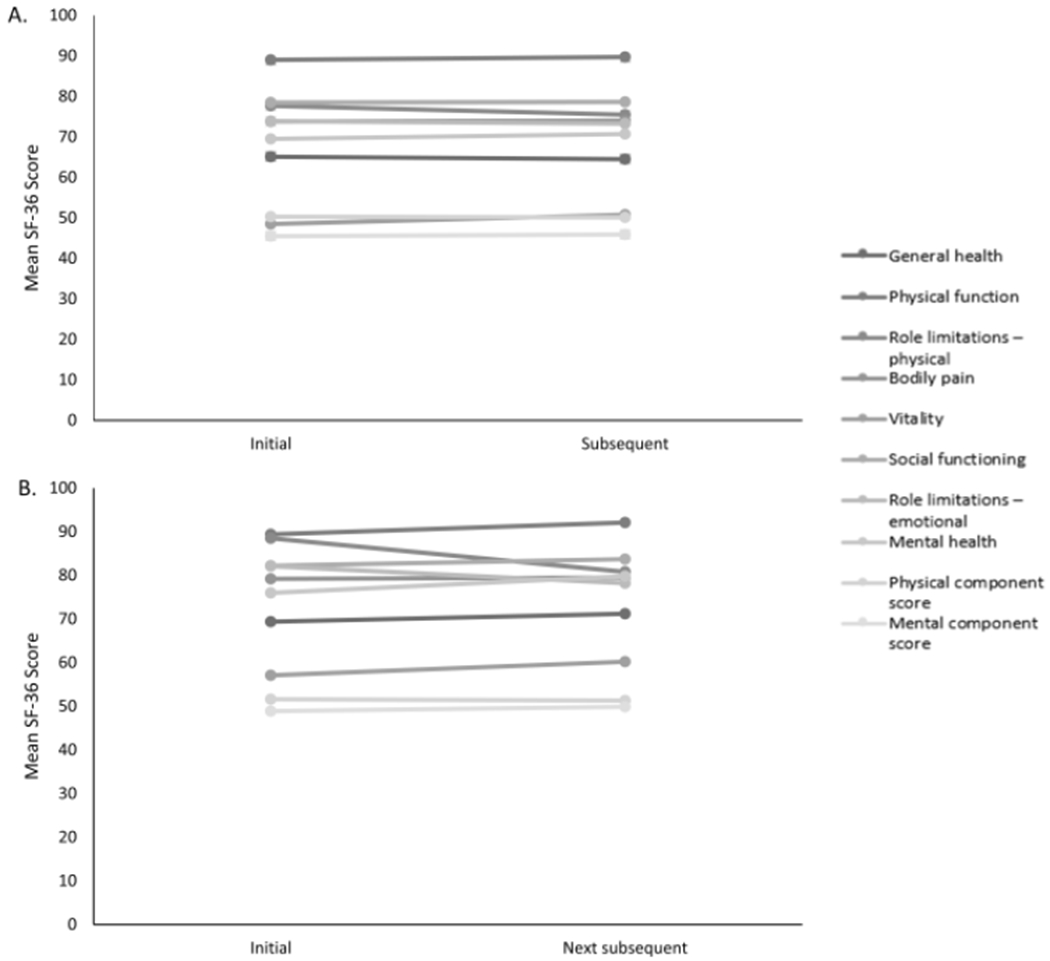
Longitudinal analysis of mean SF-36 scores. (A) Comparison of SF-36 scores between initial visit and subsequent visit (median follow-up time of 98 days) in the Registry. (B) Comparison of SF-36 scores between initial visit and next subsequent visit (median follow-up time of 245 days) in the Registry.
Discussion
A key issue in EoE is understanding how the disease and related factors affect reported QoL so that clinicians can better care for this patient population. The goal of our study was to characterize QoL in EoE based on histologic disease activity. Overall, we found that QoL as measured by the SF-36 was not different in EoE patients with histologically active and inactive disease. Dysphagia severity was lower for inactive EoE at baseline, and as dysphagia severity increased there was also a mild correlation with worse PCS and MCS. Similarly, patients who were endoscopically dilated also reported higher PCS. Looking at different EoE treatments, we found that patients on oral viscous budesonide reported higher PCS, while those on empiric food elimination diets reported lower MCS. Surprisingly, our longitudinal analysis indicated that QoL did not change in patients over time, even with increasing rates of histologic response. While our study did not find a difference in QoL between active and inactive EoE when we expected to find one, there are several possible explanations for this. The SF-36 may still provide an accurate representation of general QoL in EoE patients given that these patients may not experience chronic bodily pain, mental health issues, or other health issues addressed by the questionnaire. In this case, QoL may not change in EoE patients because these specific factors contributing to patients’ QoL as measured by the SF-36 are not changing with EoE treatment or disease activity. Alternatively, it may be that improving clinical markers of disease does not improve QoL, although this is less likely given that dysphagia, a major marker of impairment in EoE, generally improved in our cohort. The SF-36 also does not address EoE-specific aspects of QoL, which may also explain the lack of change in QoL with disease activity. It may be that other factors not accounted for by the SF-36 such as treatment burden and complexity of patients contribute to reported QoL. Thus, the SF-36 as a non-disease-specific metric may be too generalized of a survey to assess disease-specific changes in QoL in EoE and disease-specific measures may be more appropriate to study QoL.
Previous studies have attempted to better understand QoL in EoE.[6–13] In the pediatric sphere, one study, like ours, suggested that generic QoL surveys do not account for EoE-specific disease aspects. That study identified some of those categories to be EoE symptoms, food and eating, impact on activities and school, treatment, worry about disease and symptoms, and communication about disease.[23] In contrast to our results, another study found that healthcare-related QoL and EoE symptom severity in children improved over time with treatment, particularly in patients with low baseline EoE symptom severity.[13] Furthermore, proximal esophageal findings and eosinophil counts have been found to correlate inversely with QoL in pediatric patients.[24] The impact of disease on QoL may also extend to caregivers of pediatric patients, who tend to be more worried about child breathing and choking during feeding, the child’s general health, and the child never being able to eat like healthy children.[25]
In adults, key short-term and long-term therapeutic goals for EoE patients include symptom and QoL improvement, though asymptomatic adult patients considered histologic remission to also be important.[26] A study of adult Dutch EoE patients found that QoL measured by SF-36 was worse in young adult EoE patients, especially in the vitality and general health domains of the SF-36, and that mental health-related QoL was affected by disease duration.[7] The finding that QoL in mental health domains is worse in EoE patients was confirmed in a study conducted in UK adults that used the SF-36 and this was validated by correlational analysis between MCS and the EoE-QoL-A, a disease-specific QoL metric in EoE.[8] However, these results have not been consistently replicated.[8,27] Because our study was only in EoE cases, we do not have comparable data for controls, though PCS and MCS scores for our cohort were similar to population-defined averages (PCS = 49.22, MCS = 53.78).[28] Furthermore, the patients in our study scored similarly or slightly higher compared to other patients with chronic conditions like obesity and asthma evaluated by SF-36.[29,30] Similar to the findings in pediatric EoE patients and to our findings with the SF-36, symptom severity in adult EoE patients is correlated to EoE-specific QoL.[9,27]
A few studies have been conducted specifically to analyze the impact of different EoE treatments on QoL. One such study compared former and active six food elimination diet (SFED) users and found that patients who formerly were treated with SFED and stopped treatment had more anxiety and difficulty with the adherence, while active users spent more time planning meals and had more concern about finding safe foods they could eat.[31] Another study published as an abstract evaluated the four food elimination diet and reported improvement of endoscopic findings and EoE symptoms but no change in QoL metrics.[32] Studies conducted to evaluate mometasone furoate as an EoE treatment found no change in SF-36 scores from baseline but patients reported symptom improvement.[11,12]
While our study has limitations, it also has strengths. One limitation is that our study population came from a single medical center and only consisted of adults. However, the demographic and disease characteristics of our cohort is similar to what would be expected in most general EoE patient populations. We could also only include the subset of all Registry patients with full data on outcomes available, though analysis of those who were and were not included did not show substantial differences in baseline features (data not shown). Additionally, while our study population was heterogenous in terms of disease activity and treatment types, this variety may make the results more generalizable to clinical practice. However, we acknowledge that the large majority of the patients in the active group had ongoing evidence of disease despite therapy, suggesting that this was a somewhat treatment-resistant population. Given that our primary analysis was the comparison for PCS and MCS and all other analyses were secondary, a method to assess for familywise error rate and protect against alpha inflation was not included. Were a multiple correction method to be used, p-values of 0.01 or less should be considered significant and results interpreted in that light. A strength of our study is the use of a generalized QoL survey, the SF-36, instead of a disease-specific measure of QoL, which allowed us to determine whether generalized QoL assays are able to capture changes in QoL in EoE. The generalized nature of the SF-36 could explain our finding that QoL is not different in histologically active and inactive disease since generalized surveys may not capture disease-specific aspects that contribute to QoL. Thus, it may not be useful to use generalized QoL assays when studying QoL in EoE. While EoE-specific QoL measurement instruments like the EoE-QoL-A now exist, they are relatively new and have only recently been implemented in studies.[9,33] The patients in the UNC EoE Registry used for this study did not complete those questionnaires since they did not exist at the time the Registry was initiated. Other strengths of our study include its prospective nature, rigorous data collection with detailed characterization of subjects in the UNC EoE Registry, clear treatment response definitions, and methods used for data analysis.
In conclusion, our study showed that while EoE patients with histologically inactive disease also had less severe dysphagia and endoscopic findings, QoL as measured by SF-36 did not correlate with histologic disease activity. Additionally, QoL did not change in individual patients with treatment over time, even though more patients achieved histologic remission over time. These findings suggest that the SF-36 does not have a high level of utility in EoE, and future studies should favor use of EoE-specific QoL measures. However, further studies are needed to determine whether these EoE-specific measures can better characterize changes in QoL related to EoE disease activity and over time.
Acknowledgments
Funding: This research was supported by NIH awards T35 DK007386 (NC) and R01 DK101856 (ESD).
Financial disclosures: Dr. Dellon is a consultant for Abbott, Adare, Aimmune, Allakos, Arena, AstraZeneca, Biorasi, Calypso, Eli Lilly, EsoCap, Gossamer Bio, GSK, Receptos/Celegene, Regeneron, Robarts, Salix, and Shire/Takeda, receives research funding from Adare, Allakos, GSK, Meritage, Miraca, Nutricia, Receptos/Celgene, Regeneron, and Shire/Takeda, and has received an educational grant from Allakos, Banner, and Holoclara. None of the other authors report and potential conflicts of interest with this study.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- 1.Dellon ES, Liacouras CA, Molina-lnfante J, et al. Updated International Consensus Diagnostic Criteria for Eosinophilic Esophagitis: Proceedings of the AGREE Conference. Gastroenterology. 2018; 155:1022–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dellon ES, Hirano I. Epidemiology and Natural History of Eosinophilic Esophagitis. Gastroenterology. 2018; 154:319–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O’Shea KM, Aceves SS, Dellon ES, et al. Pathophysiology of Eosinophilic Esophagitis. Gastroenterology. 2018;154:333–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jensen ET, Kappelman MD, Martin CF, Dellon ES. Health-care utilization, costs, and the burden of disease related to eosinophilic esophagitis in the United States. Am J Gastroenterol. 2015; 110:626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hiremath G, Kodroff E, Strobel MJ, et al. Individuals affected by eosinophilic gastrointestinal disorders have complex unmet needs and frequently experience unique barriers to care. Clin Res Hepatol Gastroenterol. 2018;42:483–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mukkada V, Falk GW, Eichinger CS, King D, Todorova L, Shaheen NJ. Health-Related Quality of Life and Costs Associated With Eosinophilic Esophagitis: A Systematic Review. Clin Gastroenterol Hepatol. 2018;16:495–503. [DOI] [PubMed] [Google Scholar]
- 7.Van Rhijn BD, Smout AJPM, Bredenoord AJ. Disease duration determines health-related quality of life in adult eosinophilic esophagitis patients. Neurogastroenterol Motil. 2014;26:772–778. [DOI] [PubMed] [Google Scholar]
- 8.Hewett R, Alexakis C, Farmer AD, et al. Effects of eosinophilic oesophagitis on quality of life in an adult UK population: A case control study. Dis Esophagus. 2017;30:1–7. [DOI] [PubMed] [Google Scholar]
- 9.Safroneeva E, Coslovsky M, Kuehni CE, et al. Eosinophilic oesophagitis: Relationship of quality of life with clinical, endoscopic and histological activity. Aliment Pharmacol Ther. 2015;42:1000–1010. [DOI] [PubMed] [Google Scholar]
- 10.Lucendo AJ, Arias-González L, Molina-Infante J, Arias Á. Determinant factors of quality of life in adult patients with eosinophilic esophagitis. United Eur Gastroenterol J. 2018;6:38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Larsson H, Bergman K, Finizia C, Johansson L, Bove M, Bergquist H. Dysphagia and health-related quality of life in patients with eosinophilic esophagitis: a long-term follow-up. Eur Arch Oto-Rhino-Laryngology. 2015;272:3833–3839. [DOI] [PubMed] [Google Scholar]
- 12.Bergquist H, Larsson H, Johansson L, Bove M. Dysphagia and quality of life may improve with mometasone treatment in patients with eosinophilic esophagitis: A pilot study. Otolaryngol - Head Neck Surg. 2011;145:551–556. [DOI] [PubMed] [Google Scholar]
- 13.Klinnert MD, Silveira L, Harris R, et al. Health-related quality of life over time in children with eosinophilic esophagitis and their families. J Pediatr Gastroenterol Nutr. 2014;59:308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Furuta GT, Liacouras CA, Collins MH, et al. Eosinophilic Esophagitis in Children and Adults: A Systematic Review and Consensus Recommendations for Diagnosis and Treatment. Gastroenterology. 2007;133:1342–1363. [DOI] [PubMed] [Google Scholar]
- 15.Liacouras CA, Furuta GT, Hirano I, et al. Eosinophilic esophagitis: Updated consensus recommendations for children and adults. J Allergy Clin Immunol. 2011;128:3–20. [DOI] [PubMed] [Google Scholar]
- 16.Dellon ES, Gonsalves N, Hirano I, Furuta GT, Liacouras CA, Katzka DA. ACG clinical guideline: Evidenced based approach to the diagnosis and management of esophageal eosinophilia and eosinophilic esophagitis (EoE). Am J Gastroenterol. 2013;108:679–692. [DOI] [PubMed] [Google Scholar]
- 17.Reed CC, Wolf WA, Cotton CC, Dellon ES. A visual analogue scale and a Likert scale are simple and responsive tools for assessing dysphagia in eosinophilic oesophagitis. Aliment Pharmacol Ther. 2017;45:1443–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hirano I, Moy N, Heckman MG, Thomas CS, Gonsalves N, Achem SR. Endoscopic assessment of the oesophageal features of eosinophilic oesophagitis: Validation of a novel classification and grading system. Gut. 2013;62:489–495. [DOI] [PubMed] [Google Scholar]
- 19.Wolf WA, Cotton CC, Green DJ, et al. Evaluation of histologic cutpoints for treatment response in eosinophilic esophagitis. J Gastroenterol Hepatol Res. 2015;4:1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reed CC, Wolf WA, Cotton CC, et al. Optimal Histologic Cutpoints for Treatment Response in Patients With Eosinophilic Esophagitis: Analysis of Data From a Prospective Cohort Study. Clin Gastroenterol Hepatol. 2018;16:226–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ware JE, Kosinski M, Bayliss MS, McHorney CA, Rogers WH, Raczek AE. Comparison of methods for the scoring and statistical analysis of SF-36 health profile and summary measures: summary of results from the Medical Outcomes Study. Med Care. 1995;33:AS264–AS279. [PubMed] [Google Scholar]
- 22.Ware JEJ, Sherbourne CD. The MOS 36-item short-form health survey (SF-36): I. Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 23.Franciosi JP, Hommel KA, Debrosse CW, et al. Quality of life in paediatric eosinophilic oesophagitis: What is important to patients? Child Care Health Dev. 2012;38:477–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aceves SS, King E, Collins MH, et al. Alignment of parent- and child-reported outcomes and histology in eosinophilic esophagitis across multiple CEGIR sites. J Allergy Clin Immunol. 2018;142:130–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hiremath G, Rogers E, Kennedy E, Hemler J, Acra S. A Comparative Analysis of Eating Behavior of School-Aged Children with Eosinophilic Esophagitis and Their Caregivers’ Quality of Life: Perspectives of Caregivers. Dysphagia. 2019;34:567–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Safroneeva E, Balsiger L, Hafner D, et al. Adults with eosinophilic oesophagitis identify symptoms and quality of life as the most important outcomes. Aliment Pharmacol Ther. 2018;48:1082–1090. [DOI] [PubMed] [Google Scholar]
- 27.Stern E, Taft T, Zalewski A, Gonsalves N, Hirano I. Prospective assessment of disease-specific quality of life in adults with eosinophilic esophagitis. Dis Esophagus. 2018;31:dox128. [DOI] [PubMed] [Google Scholar]
- 28.Maglinte GA, Hays RD, Kaplan RM. US general population norms for telephone administration of the SF-36v2. J Clin Epidemiol. Published online2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Doll HA, Petersen SEK, Stewart-Brown SL. Obesity and physical and emotional well-being: Associations between body mass index, chronic illness, and the physical and mental components of the SF-36 questionnaire. Obes Res. Published online2000. [DOI] [PubMed] [Google Scholar]
- 30.Goldney RD, Ruffin R, Fisher LJ, Wilson DH. Asthma symptoms associated with depression and lower quality of life: A population survey. Med J Aust. Published online2003. [DOI] [PubMed] [Google Scholar]
- 31.Wang R, Hirano I, Doerfler B, Zalewski A, Gonsalves N, Taft T. Assessing Adherence and Barriers to Long-Term Elimination Diet Therapy in Adults with Eosinophilic Esophagitis. Dig Dis Sci. 2018;63:1756–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gonsalves N, Doerfler B, Schwartz S, et al. Prospective Trial of Four Food Elimination Diet Demonstrates Comparable Effectiveness in the Treatment of Adult and Pediatric Eosinophilic Esophagitis. [Abstract]. Gastroenterology. 2013;144(5):S–154. [Google Scholar]
- 33.Schoepfer AM, Straumann A, Panczak R, et al. Development and validation of a symptom-based activity index for adults with eosinophilic esophagitis. Gastroenterology. 2014;147:1255–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]


