Abstract
Cumulative burden of chronic health conditions and neurocognitive and physical function were examined among survivors of childhood acute myeloid leukemia (AML) treated with hematopoietic cell transplant (HCT; n=66) or conventional therapy (CT; n=67). Survivors and controls underwent a comprehensive clinical assessment, and health conditions were graded using a modified version of the Common Terminology Criteria for Adverse Events. By age 40 years, HCT and CT survivors had an average 17.4 (95% confidence interval [CI] 14.6–20.1) and 9.3 (7.7–11.1) grade 1–4 conditions versus 3.8 (3.3–4.2) in community controls. Compared to controls, HCT survivors had a higher prevalence of hypertriglyceridemia (45.5% vs. 18.3%), hypercholesterolemia (47.0% vs. 30.9%), hypothyroidism (27.3% vs. 4.0%), and primary hypogonadism (p<0.001). CT survivors had a higher prevalence of cardiomyopathy (11.9% vs. 2.7%) and hypertension (53.7% vs. 44.3%). Neurocognitive impairment was elevated across all domains compared to controls but did not differ by treatment modality. Compared to controls, a higher proportion of HCT survivors had impairments in strength and endurance; whereas flexibility and mobility impairments were noted among CT survivors. Despite successful advances in childhood AML therapy, many therapeutic exposures remain unchanged. These findings support ongoing investigations of novel therapies and strategies to ameliorate the risk of late morbidities.
Introduction:
Advancements in the treatment of childhood cancer and supportive care have contributed to improved 5-year survival rates for children diagnosed with a malignancy.(1) Trends in pediatric acute myeloid leukemia (AML) have mirrored this improvement with 70% of children living beyond five years.(2, 3) However, while the mortality rates have decreased, a significant proportion of cancer survivors remain at risk for late morbidity and mortality related to subsequent neoplasms (SNs) and other chronic health conditions.(4, 5) Recent reports suggest that survivors will have, on average, 17 chronic health conditions compared to nine in controls by the age of 50 years, with over a fourth of those being severe/ disabling, life-threatening, or fatal.(6) AML therapy, often more intense than that applied in other diagnoses, along with the need for allogeneic hematopoietic cell transplant (HCT) in a subset of patients, can have significant impacts on the long-term health of survivors. Studies reporting late health outcomes of AML survivors have often been restricted by a limited number of assessed outcomes, short post-therapy follow-up, and reliance on self-reported or registry-based data.(7–17) Using the well-characterized, clinically-assessed St. Jude Lifetime Cohort (SJLIFE), we comprehensively evaluated long-term health outcomes among adult survivors of childhood AML by cumulative burden and prevalence of chronic health conditions as well as neurocognitive and physical dysfunction.
Materials/ Subjects and Methods:
Study Population:
SJLIFE is a prospectively followed cohort of survivors of childhood cancer designed to facilitate longitudinal clinically-assessed health outcomes.(18, 19) This analysis included survivors of AML or myelodysplastic syndrome who were treated at St. Jude Children’s Research Hospital (SJCRH), ≥10 years from initial diagnosis, and ≥18 years of age at the time of evaluation, and who completed an on-campus health evaluation. Assessments included history and physical examination, a core laboratory battery, neurocognitive and physical function testing, as well as questionnaires detailing demographics, medical history, and self-reported health habits and quality of life. Medical records were abstracted for cumulative chemotherapy (anthracyclines in doxorubicin equivalent doses(20) and alkylating agents in cyclophosphamide equivalent doses (21)) and radiation dose exposures(22) as well as major medical events during and after therapy. A control group (n=450) was recruited from among friends and non-first-degree relatives of current or former SJCRH patients who might be accompanying or visiting a patient, SJCRH employees, or through advertising within the Memphis area, to avoid the possibility of selecting close relatives of SJLIFE participants. Informed consent was obtained from all participants and release of outside medical records to validate and grade health conditions diagnosed prior to the SJLIFE evaluation. The protocol was approved by the SJCRH Institutional Review Board.
Chronic Health Conditions:
A modified version of the National Cancer Institute’s Common Terminology Criteria for Adverse Events (CTCAE) v4.03 was used to define and grade the severity of 168 chronic health conditions grouped by organ systems.(23) Grades were defined as asymptomatic or mild (grade 1), moderate requiring minimal non-invasive intervention (grade 2), severe/ disabling (grade 3), or life-threatening requiring urgent intervention (grade 4).
Neurocognitive Impairment:
Neurocognitive assessments, performed by certified examiners under the general supervision of a board-certified neuropsychologist, measured attention, memory, processing speed, and executive function. Age-adjusted Z-scores ≤ −1 and > −2 (SD) were considered mildly impaired (grade 1), ≤ −2 and > −3 SD moderate (grade 2), and ≤ −3 SD severe (grade 3). Attention was assessed using Trail Making Test Part A (focused attention)(24) and Conners’ Continuous Performance Test-II (sustained/ variability attention).(25) Memory was assessed using Visual Selective Reminding (new visual learning)(26), California Verbal Learning Test-II (new verbal learning, short-term recall, long-term recall)(27), and Wechsler Digital Span Forward (span).(28) Grooved Pegboard Test(24) was used for motor processing speed and Wechsler Symbol Search and Digit Symbol (28) were used for visual and visual-motor processing speed assessment. Executive function was evaluated using Trail Making Test Part B (cognitive flexibility)(24), Controlled Oral Word Association Test (cognitive fluency)(24), and Wechsler Digit Span Backward (working memory).(28) (Supplementary Table S1)
Functional Impairment:
Functional status was assessed by an exercise physiologist in six domains: aerobic function (Six-Minute Walk(29), Physiologic Cost Index), mobility (Timed Up and Go(30)), strength (hand grip strength(31), knee extension at 60°/second(32)), endurance (knee extension at 300°/second(32)), flexibility (passive dorsiflexion, active dorsiflexion(33), Sit and Reach Test(34)), and balance (Sensory Organization Test(35), vestibular score) (Supplementary Table S2). Impairment in survivors was defined as >1.5 SD below the age-, gender-matched Z-score for controls.
Statistical Analysis:
Chi-square test and two-sample t-test were used as appropriate to compare baseline characteristics between survivor participants and non-participants and survivors treated with HCT, conventional therapy (CT), and controls.
Cumulative burden of grades 1–4 and 2–4 conditions was calculated using the mean cumulative count, accounting for competing risk and clinical differences in recurrency between different chronic conditions.(36, 37) Adjusted multinomial logistic regression was used to compare the prevalence of the most common conditions between survivors and community controls, namely cardiovascular (cardiomyopathy, hypertension, hypertriglyceridemia, hypercholesterolemia), endocrine/ metabolic (adult growth hormone deficiency, primary hypothyroidism, abnormal glucose metabolism, obesity), reproductive (Leydig cell insufficiency and abnormal sperm concentration in males, premature ovarian failure in females), gastrointestinal (liver fibrosis), musculoskeletal (low bone mineral density, osteonecrosis), neurologic (motor and sensory neuropathy), pulmonary (forced expiratory volume in one second [FEV1] <80% predicted, total lung capacity [TLC] <75% predicted, diffusion capacity of lung for carbon monoxide corrected for hemoglobin [DLCOcorr] <75% predicted), and ocular (cataract). Bootstrap percentile method was used to describe the grade 1–4 and grade 2–4 total and organ-system based cumulative burden and 95% confidence intervals (CI) in 5-year age increments for survivors and controls.(38)
Multivariable log-binomial models using a modified Poisson approach were conducted to compare survivors vs. community controls for relative risks (RR) of moderate to severe (grade 2–3) neurocognitive impairment, after adjusting for age at evaluation and sex. Age and sex referenced physical function impairments were compared between survivors and community controls using Chi-square test.
Standardized incidence ratios (SIRs) of observed to expected malignancies were calculated by using age-, gender-, and calendar year- specific cancer incidence from the National Cancer Institute (NCI) Surveillance, Epidemiology, and End Results (SEER) program. Nonmalignant meningiomas and nonmelanoma skin cancers were not included because these neoplasms are not registered in the SEER database. Person-years of follow-up were calculated from diagnosis to date of death or last contact. SAS version 9.4 and R version 3.4.3 were used for all analyses.
Code availability
Statistical code is available upon request. Study protocol is available at ClinicalTrials.gov (NCT00760656).
Results:
Study Participants:
Of 187 eligible AML survivors, 133 (71%) completed an on-campus clinical assessment as of June 30, 2017 and were included in the analysis (Supplementary Figure S1). Survivors who were lost to follow-up (n=6), declined participation (n=26) or agreed to participate by survey only (n=22) were excluded. Non-participating survivors were further from diagnosis (27.6 [standard deviation [SD] 8.2 vs. 22.4 years (SD 7.8), p<0.001) and more had received cranial radiation compared to participating survivors (33% vs. 10%, p<0.001) (Supplementary Table S3).
Demographic and treatment-related characteristics of study participants are shown in Table 1. Half underwent HCT, 25 (18.8%) of whom were autologous and 47 (35.3%) allogeneic HCT (6 survivors received both). No differences were noted between HCT and CT survivors on sex (p=0.09), race (p=0.40), age at diagnosis (p=0.84), education (p=0.97), employment (p=0.65), annual household income (p=0.07), insurance (p=0.91), or marital status (p=0.73). CT survivors were further from diagnosis (mean 24.1 years [standard deviation [SD] 9.3] than HCT survivors (20.6 [SD 5.4], p=0.011). Years of diagnosis are shown in Supplementary Figure S2. Two thirds of HCT survivors received total body irradiation (TBI) (median dose 1 200 cGy [1 150–1 400]) and 15% of CT survivors had a history of cranial radiation (median dose 2 400 cGy [1 800–2 600]). Anthracycline and alkylator exposures were significantly higher in the CT group (median 376 [152–734] mg/m2 and 7 031 [1 956–20 273] mg/m2) compared to HCT (181 [88–477] mg/m2 and 4 185 [961–24 241] mg/m2). Controls were older (mean 35.0 [SD 10.2] years) compared to HCT survivors (mean 30.4 [SD 7.6] years, p<0.001) but not CT survivors (mean 33.6 [SD 9.1] years, p=0.27).
Table 1:
Characteristics of study participants stratified by type of therapy received (hematopoietic cell transplant [HCT] and conventional therapy [CT]) and controls
| Survivors (N=133) | HCT Survivors* (N=66) | CT Survivors (N=67) | Community Control (n=450) | HCT vs. CT Survivors | HCT Survivors vs. Controls | CT Survivors vs. Controls | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | P-value* | P-value* | P-value* | |
| Gender | |||||||||||
| Female | 74 | (55.6) | 32 | (48.5) | 42 | (62.7) | 250 | (55.6) | 0.10 | 0.28 | 0.27 |
| Male | 59 | (44.4) | 34 | (51.5) | 25 | (37.3) | 200 | (44.4) | |||
| Race | |||||||||||
| White | 109 | (82.0) | 54 | (81.8) | 55 | (82.1) | 393 | (87.3) | 0.40 | 0.44 | 0.16 |
| Black | 17 | (12.8) | 7 | (10.6) | 10 | (14.9) | 36 | (8.0) | |||
| Others | 7 | (5.3) | 5 | (7.6) | 2 | (3.0) | 21 | (4.7) | |||
| Age at diagnosis (years) | N/A | N/A | N/A | ||||||||
| Mean (SD) | 9.6 (6.0) | 9.7 (5.8) | 9.5 (6.2) | 0.84 | |||||||
| 0–4 | 41 | (30.8) | 19 | (28.8) | 22 | (32.8) | 0.62 | ||||
| 5–9 | 26 | (19.5) | 13 | (19.7) | 13 | (19.4) | |||||
| 10–14 | 34 | (25.6) | 20 | (30.3) | 14 | (20.9) | |||||
| ≥15 | 32 | (24.1) | 14 | (21.2) | 18 | (26.9) | |||||
| Time since diagnosis (years) | |||||||||||
| Mean (SD) | 22.4 (7.8) | 20.6 (5.4) | 24.1 (9.3) | 0.01 | |||||||
| 10–19 | 61 | (45.9) | 37 | (56.1) | 24 | (35.8) | 0.01 | ||||
| 20 – 29 | 47 | (35.3) | 23 | (34.8) | 24 | (35.8) | |||||
| ≥30 | 25 | (18.8) | 6 | (9.1) | 19 | (28.4) | |||||
| Age at evaluation (years) | |||||||||||
| Mean (SD) | 32.0 (8.5) | 30.4 (7.6) | 33.6 (9.1) | 35.0 (10.2) | 0.03 | < 0.001 | 0.27 | ||||
| 18 – 29 | 60 | (45.1) | 29 | (43.9) | 31 | (46.3) | 153 | (34.0) | 0.005 | 0.004 | 0.09 |
| 30–39 | 46 | (34.6) | 30 | (45.5) | 16 | (23.9) | 161 | (35.7) | |||
| ≥40 | 27 | (20.3) | 7 | (10.6) | 20 | (29.9) | 136 | (30.2) | |||
| Diagnosis | |||||||||||
| Acute myeloid leukemia | 122 | (91.7) | 55 | (83.3) | 67 | (100) | NA | <0.001 | NA | NA | |
| Myelodysplastic syndrome | 11 | (8.3) | 11 | (16.7) | 0 | (0) | |||||
| Body mass index (kg/m2) | |||||||||||
| Normal/ Underweight (<25) | 51 | (38.3) | 34 | (51.5) | 17 | (25.4) | 169 | (37.5) | 0.002 | 0.02 | 0.15 |
| Overweight (25–29) | 42 | (31.6) | 20 | (30.3) | 22 | (32.8) | 121 | (26.9) | |||
| Obese (≥30) | 40 | (30.1) | 12 | (18.2) | 28 | (41.8) | 160 | (35.5) | |||
| Education | |||||||||||
| Less than high school | 15 | (11.3) | 7 | (10.6) | 8 | (11.9) | 14 | (3.1) | 0.97 | 0.002 | <0.001 |
| Completed high school/GED | 28 | (21.1) | 14 | (21.2) | 14 | (20.9) | 50 | (11.1) | |||
| Vocational training/Some college | 36 | (27.1) | 17 | (25.8) | 19 | (28.4) | 139 | (30.9) | |||
| College graduate and higher | 54 | (40.6) | 28 | (42.4) | 26 | (38.8) | 247 | (54.9) | |||
| Employment | |||||||||||
| Full-time | 77 | (57.9) | 37 | (56.1) | 40 | (59.7) | 295 | (65.6) | 0.65 | 0.002 | 0.02 |
| Part-time | 15 | (11.3) | 6 | (9.1) | 9 | (13.4) | 64 | (14.2) | |||
| Not employed | 35 | (26.3) | 19 | (28.8) | 16 | (23.9) | 51 | (11.3) | |||
| Student/Homemaker/Retired | 6 | (4.5) | 4 | (6.1) | 2 | (3.0) | 40 | (8.9) | |||
| Annual household income | |||||||||||
| <$19 999 | 22 | (16.5) | 10 | (15.2) | 12 | (17.9) | 32 | (7.1) | 0.07 | 0.002 | 0.001 |
| $20 000–$59 999 | 43 | (32.3) | 22 | (33.3) | 21 | (31.3) | 139 | (30.8) | |||
| $60 000–$99 999 | 28 | (21.1) | 19 | (28.8) | 9 | (13.4) | 123 | (27.3) | |||
| ≥$100 000 | 17 | (12.8) | 4 | (6.1) | 13 | (19.4) | 115 | (25.6) | |||
| Donť know | 23 | (17.3) | 11 | (16.7) | 12 | (17.9) | 41 | (9.1) | |||
| Insurance status | |||||||||||
| Yes | 109 | (82.0) | 55 | (83.3) | 54 | (80.6) | 396 | (88.0) | 0.91 | 0.21 | 0.10 |
| No | 22 | (16.5) | 10 | (15.2) | 12 | (17.9) | 53 | (11.8) | |||
| Non-U.S. resident/citizen | 2 | (1.5) | 1 | (1.5) | 1 | (1.5) | 1 | (0.2) | |||
| Marital status | |||||||||||
| Single, never married | 62 | (46.6) | 33 | (50.0) | 29 | (43.3) | 102 | (22.7) | 0.73 | <0.001 | <0.001 |
| Married, living as married | 53 | (39.8) | 25 | (37.9) | 28 | (41.8) | 304 | (67.6) | |||
| Widowed/Divorced/ Separated | 18 | (13.5) | 8 | (12.1) | 10 | (14.9) | 44 | (9.8) | |||
| Smoking | |||||||||||
| Current smoker | 20 | (15.0) | 8 | (12.1) | 12 | (17.9) | 52 | (11.6) | 0.60 | 0.45 | 0.25 |
| Ever smoker | 17 | (12.8) | 8 | (12.1) | 9 | (13.4) | 83 | (18.4) | |||
| Nonsmoker | 96 | (72.2) | 50 | (75.8) | 46 | (68.7) | 315 | (70.0) | |||
| Relapse | N/A | N/A | N/A | ||||||||
| None | 108 | (81.2) | 43 | (65.2) | 65 | (97.0) | <0.001 | ||||
| Yes | 25 | (18.8) | 23 | (34.8) | 2 | (3.0) | |||||
| Hematopoietic cell transplant b | NA | ||||||||||
| None | 67 | (50.4) | |||||||||
| Autologous | 25 | (18.8) | |||||||||
| Allogeneic | 47 | (35.3) | |||||||||
| Anthracyclines (mg/m2) a | |||||||||||
| Median dose (range) | 294.0 (87.0–734.0) |
181.1 (87.9–477.2) |
376.1 (152.3–734.2) |
||||||||
| None | 16 | (9.0) | 12 | (18.2) | 4 | (6.0) | <0.001 | ||||
| <250 | 56 | (42.1) | 42 | (63.6) | 14 | (20.9) | |||||
| ≥250 | 61 | (45.8) | 12 | (18.2) | 49 | (73.1) | |||||
| Intrathecal cytarabine | |||||||||||
| None | 57 | (42.9) | 24 | (36.4) | 33 | (49.3) | 0.13 | ||||
| Yes | 76 | (57.1) | 42 | (63.6) | 34 | (50.7) | |||||
| High dose cytarabine | |||||||||||
| None | 71 | (53.4) | 28 | (42.4) | 43 | (64.2) | 0.01 | ||||
| Yes | 62 | (46.6) | 38 | (57.6) | 24 | (35.8) | |||||
| Alkylating Agents (mg/m2) a | |||||||||||
| Median dose (range) | 6 807 (961–24 240) |
4 185 (961–24 241) |
7 031 (1 956–20 273) |
||||||||
| None | 45 | (33.8) | 1 | (1.5) | 44 | (65.7) | <0.001 | ||||
| <4 000 | 36 | (27.1) | 32 | (48.5) | 4 | (6.0) | |||||
| >4 000–8 000 | 20 | (15.0) | 8 | (12.1) | 12 | (17.9) | |||||
| >8 000 | 32 | (24.1) | 25 | (37.9) | 7 | (10.4) | |||||
| Total body irradiation a | |||||||||||
| Median dose (range) (cGy) | 1 200 (1 150–1 400) |
1 200 (1 150–1 400) |
N/A | ||||||||
| None | 88 | (66.2) | 21 | (31.8) | 67 | (100) | <0.001 | ||||
| Yes | 45 | (33.8) | 45 | (68.2) | 0 | (0) | |||||
| Cranial radiation a | |||||||||||
| None | 120 | (90.2) | 63 | (95.5) | 57 | (85.1) | 0.04 | ||||
| Yes | 13 | (9.8) | 3 | (4.5) | 10 | (14.9) | |||||
| Median dose (range) (cGy) | 2 400 (600–2 600) |
1 000 (600–2 400) |
2 400 (1 800–2 600) |
||||||||
P-values were based on two-sample t-test on continuous variables and Chi-Square test on categorical variables
Median (Range) and Mean (SD) were summarized cumulative doses among survivors with dose>0; SD=Standard Deviation
47 survivors received allogeneic HCT, 25 survivors received autologous HCT (6 survivors received both autologous and allogeneic HCTs)
Chronic Conditions and Cumulative Burden:
Survivors had a higher cumulative burden of grade 1–4 and 2–4 chronic health conditions compared to controls (Figure 1). By age 40, HCT survivors had on average 17.4 (95% CI 14.6–20.1) grade 1–4 chronic health conditions and 10.1 (95% CI 8.3–11.9) grade 2–4 conditions, and CT survivors had 9.3 (95% CI 7.7–11.1) and 5.5 (95% CI 4.4–6.8), respectively, compared to 3.8 (95% CI 3.3–4.2) and 1.9 (95% CI 1.7–2.2) among controls. The highest burden of grade 2–4 conditions among HCT survivors was in the endocrine (2.1 95% CI 1.7–2.6), cardiovascular (1.7 95% CI 1.1–2.3), and pulmonary (1.7 95% CI 1.2–2.1) organ systems. For CT survivors the highest burden was in the gastrointestinal (0.9 95% CI 0.5–1.4), endocrine (0.9 95% CI 0.7–1.1), and cardiovascular (0.8 95% CI 0.5–1.2) systems (Supplementary Table S4A, S4B).
Figure 1.

Cumulative burden and 95% confidence intervals of grade 1–4 and 2–4 overall chronic health conditions among survivors of AML treated with allogeneic hematopoietic cell transplant (HCT) or conventional therapy (non-HCT) and community controls
At least one chronic health condition was identified in nearly all the AML survivors (98.5% grade 1–4 and 90% grade 2–4) regardless of treatment modality (HCT: 97% grade 1–4, 91% grade 2–4 and CT: 100% grade 1–4, 88% grade 2–4). The prevalence of the most common conditions is reported in Table 2. After adjusting for age, sex, and race, HCT survivors had a higher prevalence of endocrine/ reproductive and metabolic conditions compared to controls: primary hypothyroidism (27.3% vs. 4.0%, p<0.001), primary hypogonadism (Leydig cell insufficiency in males: 64.7% vs. 7.0%; Premature ovarian failure in females: 43.8% vs. 0.4%; p<0.001), abnormal glucose metabolism (28.8% vs. 16.0%, p<0.001), hypertriglyceridemia (45.5% vs. 18.3%, p<0.001), and hypercholesterolemia (47.0% vs. 30.9%, p=0.001). Additionally, among 21 male HCT survivors who consented to semen analysis, 14 (67%) were azoospermic. Notably, no difference in the prevalence of obesity was noted between HCT survivors and controls (48.5% vs. 61.8%, p=0.23). Pulmonary deficits were more prevalent among HCT survivors compared to controls, with 53% having an abnormal FEV1, 45.5% an abnormal DLCOcorr, and 33.3% an abnormal TLC, compared to 8.2%, 4.2%, and 2.3%, respectively, in controls (p<0.001). CT survivors had a significantly higher prevalence of cardiomyopathy (11.9% vs. 2.7%, p=0.001), hypertension (53.7% vs. 44.3%, p=0.05), and abnormal DLCOcorr (9.0% vs. 4.2%, p=0.01) compared to controls. The prevalence of endocrine/ reproductive and metabolic conditions was comparable between CT survivors and controls. Both HCT and CT survivors were noted to have significantly higher prevalence of sensory neuropathy compared to controls (18.2%, 28.4%, and 7.7%, respectively).
Subsequent neoplasms:
At a mean of 22.6 years since diagnosis (SD 8.1), eighteen (13.5%) survivors, including 11 with a history of radiation exposure, developed 23 SNs (Supplementary Table S5). Three of these 18 developed basal cell carcinomas. Survivors had a seventeen-fold higher risk of developing SNs compared to the general population (SIR 17.7 95% CI 11.2–26.6). Risks were highest for thyroid cancer (SIR 44.0 95% CI 18.9–86.6), breast cancer (SIR 31.7 95% CI 11.6–69.0), and central nervous system tumors (SIR 26.3 95% CI 3.0–94.9). Six of the eight thyroid malignancies occurred in patients with a history of radiation exposure to the head or neck region. Three of six breast cancers occurred in survivors with a history of radiation exposure, while the other three had a history of >250 mg/m2 of anthracycline exposure.
Neurocognitive impairment:
One hundred and twenty-six survivors (94.7%) underwent comprehensive neurocognitive assessments. Both HCT and CT survivors had a higher prevalence of neurocognitive impairment in all four tested domains. Grade 2–3 executive function impairment was noted in a fourth of HCT (26.5%) and CT (25.8%) survivors, compared to 10.5% of controls. Similarly, moderate to severe impairment in processing speed and memory was noted in 9.7% and 28.2% of HCT survivors and 6.6% and 29.0% of CT survivors, respectively. Twenty two percent of both HCT and CT survivors had grade 2–3 deficits in attention. Survivors were most likely to develop moderate to severe impairments in processing speed (HCT: RR 13.8 95% CI 3.1–61.6; CT: 10.1 95% CI 2.2–46.2) and attention (HCT: RR 3.6 95% CI 2.2–6.1; CT: 3.4 95% CI 2.0–5.6) when compared to age- and sex-adjusted controls (Figure 2, Figure 3).
Figure 2:

Neurocognitive impairment among HCT survivors and controls by domains (A. Executive function, B. Processing speed, C. Memory, D. Attention)
Figure 3:
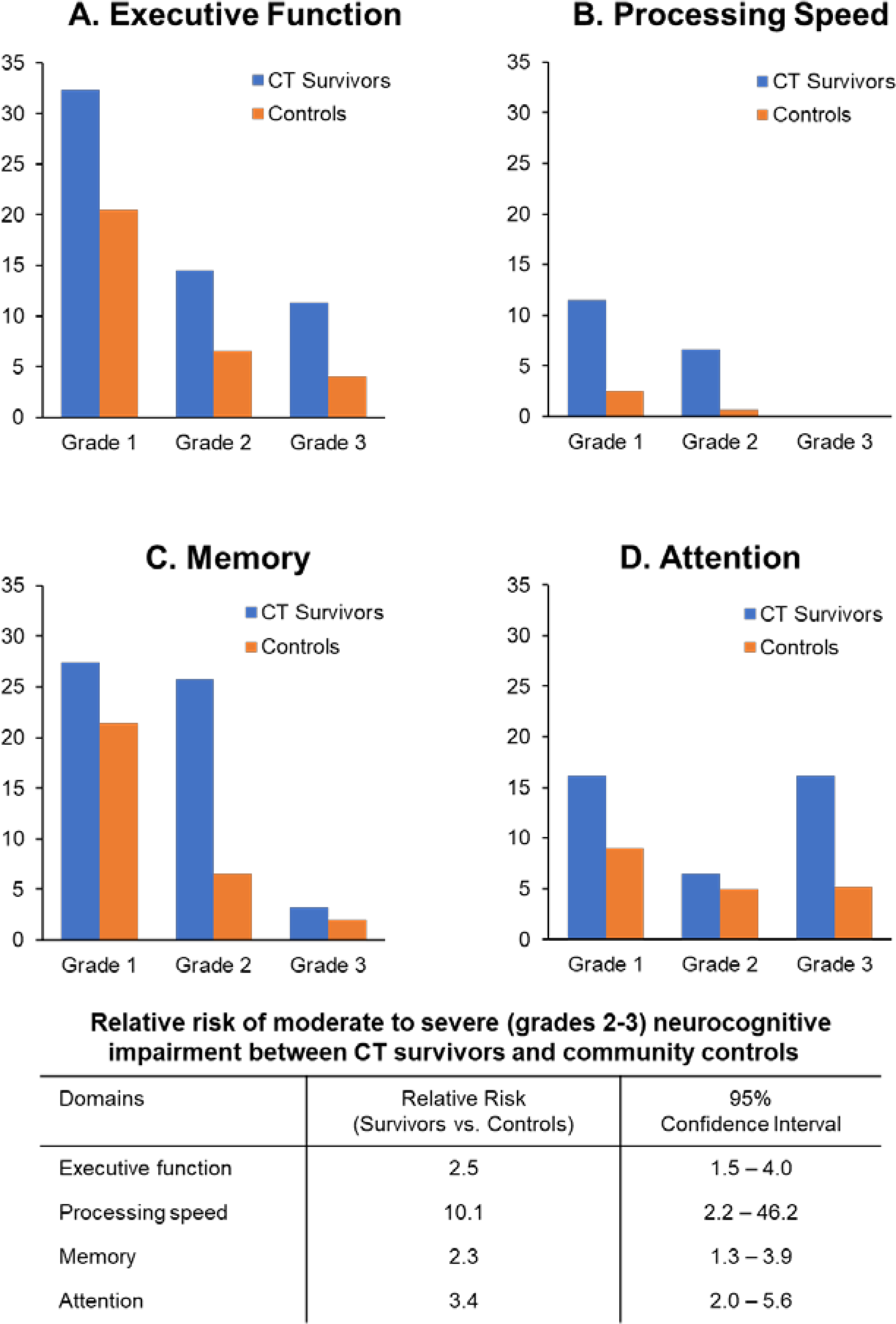
Neurocognitive impairment among CT survivors and controls by domains (A. Executive function, B. Processing speed, C. Memory, D. Attention)
Physical function impairment:
Survivors had a higher prevalence of physical function impairment compared to controls across all domains, except balance (Figure 4 A/B). The highest prevalence of impairment was in strength (knee extension at 60°/sec 44.7%; grip strength 23.1%) and endurance (knee extension at 300°/sec 32.6%) among HCT survivors. Impaired flexibility (Sit and Reach test 20.6%, passive dorsiflexion 18.7%, active dorsiflexion 15.6%), aerobic function (Six-Minute Walk Test 20%, Physiologic Cost Index 13.3%), and mobility (Timed Up and Go 16.9%) were also more prevalent compared to controls. Among CT survivors, the highest prevalence of impairment was in flexibility (active dorsiflexion 20.6%, passive dorsiflexion 18.7%, Sit and Reach test 14.5%), mobility (Timed Up and Go 18.7%), aerobic function (Six-Minute Walk Test 16.1%), and strength (grip strength 12.5%). Compared to survivors treated with CT, those treated with HCT had higher prevalence of impaired strength (grip strength: 32.6% vs. 9.6%; knee extension at 60°/sec: 60% vs. 10.9%), endurance (knee extension at 300°/sec: 41.3% vs. 9.5%), and flexibility (percentage impaired for Sit and Reach: 27.2% vs. 12.3%) (Supplementary Figure S3).
Figure 4:

A:Prevalence of physical function impairments among survivors of AML treated with HCT
B:Prevalence of physical function impairments among survivors of AML treated with CT
Discussion:
While lagging behind other pediatric cancers, survival rates for children diagnosed with AML, a rare but aggressive pediatric malignancy, have consistently improved over the last four decades.(39–43) Intensified chemotherapeutic protocols, HCT, and improved supportive care measures have all contributed to these gains, however, not without substantial long-term toxicity and chronic sequelae. In this study of clinically evaluated long-term survivors of AML, we provide a comprehensive characterization of treatment-related multi-morbidity relevant to survivors treated on historic SJCRH protocols which are comparable to contemporary regimens as they still uniformly rely on high cumulative anthracycline doses and, in cases with high-risk biology, HCT. While survivors of HCT experienced the majority of clinically actionable (grades 2–4) endocrine/ reproductive, metabolic, and pulmonary conditions, those treated with CT had a higher prevalence of cardiomyopathy. Moreover, both HCT and CT survivors had elevated neurocognitive and physical function impairments not previously reported in this population. Our analysis identified risk profiles for morbidity that may aid current therapeutic decision making as well as help identify survivors who may benefit from early intervention and/or increased surveillance.
Our study reports a higher burden and prevalence of chronic health conditions among AML survivors overall and by treatment modality compared to previously published estimates. In a report of 272 AML survivors (median 21.4 years [5–33] from diagnosis) treated with conventional therapy alone, participating in the Childhood Cancer Survivor Study (CCSS), only half self-reported having a chronic health condition at a median age of 27 years (10–49).(12) Similarly, Schultz et al. reported long-term complications among 180 AML survivors treated on legacy Children’s Oncology Group (COG) trials (1979–1995) and found that 44% of CT survivors (median 15 [7–21] years from diagnosis) self-reported at least one chronic health condition.(13) Nearly one fourth of the HCT survivors did not report having been diagnosed with a chronic health condition. Survivors in our investigation were on average 10 years older at evaluation and survival time was comparable to these prior studies with the exception of HCT survivors in the COG analysis who were only 12.5 (6–22) years from diagnosis. Similarly, using questionnaires to assess 95 AML survivors treated with HCT on Nordic Society of Pediatric Hematology and Oncology protocols (NOPHO-AML 84, 88, 93, and 2004), no differences in the need for gonadal hormone supplementation, medication for diabetes, or bronchodilator usage were noted between AML survivors and sibling controls.(15) These differences between self-reported outcomes and our estimates suggest that aging survivors may have a substantial number of unrecognized health impairments.
Comprehensive clinical assessments increased our ability to identify late effects irrespective of prior treatment exposures. Leung et al. previously described long-term sequelae in a historical cohort of AML survivors (N=77) followed at SJCRH.(9) Late complications were ascertained by medical record review, however, unlike our study, chronic condition grading was not applied nor was a control population available. As in our study, infertility, hypogonadism, and cataracts were common late complications among HCT survivors, however, pulmonary dysfunction and metabolic abnormalities were not documented as significant concerns. While there may be potential overlap with some current survivors in the SJLIFE cohort, our study had longer follow-up and the comprehensive SJLIFE assessment likely identified dysfunction in organ systems overlooked by risk-based surveillance. Systematic grading and application of the cumulative burden metric compared to a community control group permitted more precise estimation of the overall health status of these survivors.
The cumulative burden of late cardiovascular morbidity was high in both HCT and CT survivors, however, with a different distribution. The prevalence of dyslipidemia was elevated among HCT survivors while a higher prevalence of cardiomyopathy and hypertension was only seen among CT treated survivors. This prevalence of cardiomyopathy after chemotherapy only is similar to that reported by Jarfelt et al., in a study of survivors (median time from diagnosis 11 years [4–25]) treated on NOPHO-AML regimens.(8) Ten of 98 (10.2%) survivors who underwent echocardiography had an LVEF <55%. Leung and colleagues also reported a 9% incidence of cardiomyopathy in their historic cohort of AML survivors treated with chemotherapy only.(9) The anthracycline dose-response relationship with cardio-toxicity is well described, with patients exposed to ≥250 mg/m2 having worse outcomes.(44) In our study, 94% of the CT survivors were exposed to anthracyclines, with nearly three-fourths receiving doses ≥250 mg/m2. It should be noted, however, that the HCT group included survivors of MDS (n=11), not typically exposed to upfront anthracyclines, which may have contributed to the lower cumulative anthracycline exposure in this group. Our findings align with and support recent studies designed to minimize cardiotoxic exposures for children with AML, such as liposomal preparations of daunorubicin and cytarabine (45) or remission induction with clofarabine (39), or the expanded use of dexrazoxane for cardioprotection.(46) Importantly, pre-hypertension (grade 1) or hypertension requiring medication management (grade 2–3) was identified in over half of these CT only survivors, and 15% of HCT survivors had elevated fasting triglycerides, 12% high cholesterol, and over 13% impaired glucose metabolism. Modifiable risk factors combined with therapeutic exposures are known to amplify the risk for cardiotoxicity(47) and provide potential targets for intervention.
Endocrine and reproductive outcomes varied by treatment modality. The prevalence was significantly elevated compared to controls among HCT survivors but did not differ among those treated with CT. This is consistent with a previous report assessing pubertal and fertility status among 102 (56 female and 46 male) AML survivors, a median of 10.7 years (4.4–25.0) from diagnosis treated with chemotherapy only. At a median age of 16 years (5–36), survivors were either prepubertal, in puberty, or had progressed normally through puberty. No hormonal abnormalities were detected except for decreased anti-Mullerian hormone (≤ 2.5 percentile) in five of 40 post-pubertal females.(11) Furthermore, no differences were noted between survivors who reported having or siring a pregnancy and their siblings. Recently, the Center for International Blood and Marrow Transplant Research assessed TBI and non-TBI conditioning regimens in pediatric AML patients.(48) From 2008–2016, 199 patients were treated on TBI-based conditioning and 425 received chemotherapy based conditioning. While there was no difference in overall survival rates between the two groups, the incidence of gonadal and growth hormone deficiencies was significantly higher among those exposed to TBI (24% vs. 8%, p<0.001). Our data support ongoing efforts for radiation-free transplant regimens to reduce late effects in addition to the importance of pre-HCT fertility counseling.
Neurocognitive compromise in survivors of childhood cancer has been well described(49), yet formal assessments specifically among AML survivors are lacking. Based on questionnaires and psychology reports, Leung et al. reported 14% (n=11) with academic difficulties, but among nine who had psychologic evaluations no particular pattern of learning disability was identified. However, formal psychometric testing on seven of the nine identified decreases in measures of intelligence and academic achievement.(9) To our knowledge, this is the first study to perform detailed neurocognitive evaluations among survivors of AML. Deficits were identified in all domains tested (memory, attention, processing speed, executive function) compared to community controls. However, the risk did not differ by treatment modality and appeared to be highest in processing speed and attention compared to controls. Recently, using the CCSS Neurocognitive Questionnaire (50, 51), Stefanski and colleagues reported a higher risk for neurocognitive dysfunction among AML survivors (median age at evaluation 30 years, 18–49) compared to a sibling comparison group (RR 2.03 95% CI 1.47–2.79), but no significant differences between those treated with HCT (n=183) versus CT (n=299).(52) While HCT appears to have a substantial impact on late chronic health conditions, this does not seem to be the case for late neurocognitive findings.
Physical function, impaired across nearly all domains tested, has not been previously uniformly measured in AML survivors. Wilhelmsson and colleagues compared self-reported activity limitations among survivors treated with HCT (N=95) compared to a historical chemotherapy-only group (N=101) and siblings (N=53).(15) Transplanted survivors reported significantly more limitations with vigorous (39%) and moderate (18%) physical activities than both the CT (7% and 1%, respectively) and sibling (9% and 0%) groups, respectively. Limitations were associated with chronic graft-vs-host disease, being underweight, and any grade ≥3 chronic health condition. The prevalence of functional limitations, elevated for all AML survivors in our cohort, appeared particularly elevated in the HCT group and was significantly different from CT on strength and endurance measures. Poor physical function has been associated with late mortality and psychological distress in childhood cancer survivors(53, 54), suggesting that AML survivors may benefit from interventions to optimize function during and following completion of therapy. Such strategies are underway.(55, 56)
Despite the comprehensive clinical assessments performed on the St. Jude campus, some study limitations should be noted. Nearly 30% of survivors did not return for a medical evaluation and were not included in our analysis. Survivors may have been unable to return due to health limitations, death, or other commitments, potentially under- or over-estimating our estimates. Importantly, no significant major demographic differences were found between participants and non-participants. Participation in the SJLIFE cohort is voluntary, potentially raising concern about the overall representativeness of the cohort. However, there was a 71% participation rate and no significant demographic, disease, or neighborhood level characteristics have been identified between participating survivors and the SJLIFE source population.(57) Given limited sample size and the low number of some outcomes, our ability to perform detailed multivariable modeling was limited.
Our comprehensive study of late health, neurocognitive, and physical outcomes among AML survivors identified not only a high cumulative burden of late effects in this intensively treated population but also better characterized outcomes across historical treatment modalities. While not only confirming a need for long-term surveillance and health promotion, these findings may also help guide treatment discussions for patients and providers confronting many of the same treatment options today.
Supplementary Material
Table 2A:
Prevalence of chronic health conditions of survivors of AML treated with hematopoietic cell transplant (n=66) and community controls (N=450) *
| Cardiovascular | Cardiomyopathy | Hypertension | Hypertriglyceridemia | High Cholesterol | |||||||||||||||||||
| Survivors | Controls | Survivors | Controls | Survivors | Controls | Survivors | Controls | ||||||||||||||||
| N | % | N | % | P | N | % | N | % | P | N | % | N | % | p | N | % | N | % | p | ||||
| Normal | 62 | 93.9 | 438 | 97.3 | 0.10 | 34 | 51.5 | 251 | 55.7 | 0.22 | 36 | 54.5 | 368 | 81.7 | <0.001 | 35 | 53.0 | 311 | 69.1 | 0.001 | |||
| Grade 1 | - | - | - | - | 22 | 33.3 | 145 | 32.2 | 20 | 30.3 | 68 | 15.1 | 23 | 34.8 | 120 | 26.6 | |||||||
| Grade 2 | 2 | 3.0 | 10 | 2.2 | 9 | 13.6 | 43 | 9.5 | 6 | 9.1 | 12 | 2.7 | 6 | 9.1 | 19 | 4.2 | |||||||
| Grade 3 | 2 | 3.0 | 2 | 0.4 | 1 | 1.5 | 11 | 2.4 | 2 | 3.0 | 2 | 0.4 | 2 | 3.0 | 0 | 0 | |||||||
| Grade 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 3.0 | 0 | 0 | 0 | 0 | 0 | 0 | |||||||
| Endocrine/ Metabolic | Growth Hormone Deficiency | Primary Hypothyroidism | Abnormal Glucose Metabolism | Obesity | |||||||||||||||||||
| Survivors | Controls | Survivors | Controls | Survivors | Controls | Survivors | Controls | ||||||||||||||||
| N | % | N | % | N | % | N | % | N | % | N | % | N | % | N | % | 0.23 | |||||||
| Normal | 59 | 89.4 | 442 | 98.2 | 0.39 | 48 | 72.7 | 432 | 96.0 | <0.001 | 47 | 71.2 | 378 | 84.0 | <0.001 | 34 | 51.5 | 172 | 38.2 | ||||
| Grade 1 | 3 | 4.5 | 8 | 1.7 | 1 | 1.5 | 1 | 0.2 | 10 | 15.1 | 57 | 12.6 | - | - | - | - | |||||||
| Grade 2 | 4 | 6.1 | 0 | 0 | 17 | 25.7 | 17 | 3.7 | 2 | 3.0 | 13 | 2.8 | 20 | 30.3 | 119 | 26.4 | |||||||
| Grade 3 | - | - | - | - | 0 | 0 | 0 | 0 | 7 | 10.6 | 2 | 0.4 | 11 | 16.7 | 121 | 26.8 | |||||||
| Grade 4 | - | - | - | - | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1.5 | 38 | 8.4 | |||||||
| Reproductive | Leydig Cell Insufficiency (Male) | Premature Ovarian Failure (Female) | Abnormal Sperm Concentration‡ | Liver Fibrosis | |||||||||||||||||||
| Survivors | Controls | Survivors | Controls | Survivors | Controls† | Gastrointestinal | Survivors | Controls† | |||||||||||||||
| N | % | N | % | N | % | N | % | N | % | N | % | N | % | N | % | ||||||||
| Normal | 12 | 35.3 | 186 | 93.0 | <0.001 | 18 | 56.2 | 249 | 99.6 | <0.001 | 4 | 19.0 | - | - | 64 | 97.0 | - | - | |||||
| Grade 1 | 12 | 35.3 | 10 | 5.0 | - | - | - | - | - | - | - | - | 1 | 1.5 | - | - | |||||||
| Grade 2 | 10 | 29.4 | 4 | 2.0 | - | - | - | - | 3 | 14.3 | - | - | 0 | 0 | - | - | |||||||
| Grade 3 | 0 | 0 | 0 | 0 | 14 | 43.7 | 1 | 0.4 | 14 | 66.7 | - | - | 1 | 1.5 | - | - | |||||||
| Grade 4 | - | - | - | - | - | - | - | - | - | - | - | - | 0 | 0 | - | - | |||||||
| Abnormal FEV1 | Abnormal DLCOcorr | Abnormal TLC | Cataract | ||||||||||||||||||||
| Survivors | Controls | Survivors | Controls | Survivors | Controls | Survivors | Controls | ||||||||||||||||
| Pulmonary | N | % | N | % | N | % | N | % | N | % | N | % | N | % | N | % | |||||||
| Normal | 31 | 47.0 | 413 | 91.8 | 36 | 54.5 | 431 | 95.8 | <0.001 | 44 | 66.7 | 440 | 97.7 | <0.001 | Ocular | 43 | 65.1 | 428 | 95.1 | 0.002 | |||
| Grade 1 | 17 | 25.7 | 22 | 4.9 | 9 | 13.6 | 17 | 3.8 | 6 | 9.1 | 9 | 2.0 | 19 | 28.8 | 19 | 4.2 | |||||||
| Grade 2 | 11 | 16.7 | 11 | 2.4 | <0.001 | 16 | 24.2 | 1 | 0.2 | 8 | 12.1 | 1 | 0.2 | 1 | 1.5 | 2 | 0.4 | ||||||
| Grade 3 | 2 | 3.0 | 3 | 0.7 | 5 | 7.6 | 1 | 0.2 | 8 | 12.1 | 0 | 0 | 1 | 1.5 | 1 | 0.2 | |||||||
| Grade 4 | 5 | 7.6 | 1 | 0.2 | - | - | - | - | - | - | - | - | 2 | 3.0 | 0 | 0 | |||||||
| Low Bone Mineral Density | Osteonecrosis | Motor Neuropathy | Sensory Neuropathy | ||||||||||||||||||||
| Survivors | Controls† | Survivors | Controls† | Survivors | Controls | Survivors | Controls | ||||||||||||||||
| Bone Health | N | % | N | % | N | % | N | % | Neurology | N | % | N | % | N | % | N | % | ||||||
| Normal | 23 | 34.8 | - | - | 60 | 90.9 | - | - | 61 | 92.4 | 450 | 100 | 0.98 | 54 | 81.8 | 415 | 92.2 | 0.005 | |||||
| Grade 1 | 27 | 40.9 | - | - | 3 | 4.5 | - | - | 1 | 1.5 | 0 | 0 | 11 | 16.7 | 30 | 6.6 | |||||||
| Grade 2 | 16 | 24.2 | - | - | 3 | 4.5 | - | - | 3 | 4.5 | 0 | 0 | 1 | 1.5 | 5 | 1.1 | |||||||
| Grade 3 | 0 | 0 | - | - | 0 | 0 | - | - | 1 | 1.5 | 0 | 0 | 0 | 0 | 0 | 0 | |||||||
| Grade 4 | - | - | - | - | 0 | 0 | - | - | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||||||
Adjusted for age, sex, race (White vs. others)
FEV1= forced expiratory volume in one second, DLCOcorr= diffusion capacity of lungs for carbon monoxide corrected for hemoglobin, TLC= total lung capacity
Not assessed among controls
Assessed among survivors who consented to semen analysis (n=25)
Table 2B:
Prevalence of chronic health conditions of survivors of AML treated with conventional therapy (n=67) and community controls (N=450) *
| Cardiovascular | Cardiomyopathy | Hypertension | Hypertriglyceridemia | High Cholesterol | |||||||||||||||||||
| Survivors | Controls | Survivors | Controls | Survivors | Controls | Survivors | Controls | ||||||||||||||||
| N | % | N | % | P | N | % | N | % | P | N | % | N | % | p | N | % | N | % | p | ||||
| Normal | 59 | 88.1 | 438 | 97.3 | 0.001 | 31 | 46.3 | 251 | 55.7 | 0.05 | 56 | 83.6 | 368 | 81.7 | 0.57 | 51 | 76.1 | 311 | 69.1 | 0.69 | |||
| Grade 1 | - | - | - | - | 22 | 32.8 | 145 | 32.2 | 9 | 13.4 | 68 | 15.1 | 14 | 20.9 | 120 | 26.6 | |||||||
| Grade 2 | 6 | 8.9 | 10 | 2.2 | 10 | 14.9 | 43 | 9.5 | 1 | 1.5 | 12 | 2.6 | 2 | 3.0 | 19 | 4.2 | |||||||
| Grade 3 | 2 | 3.0 | 2 | 0.4 | 4 | 6.0 | 11 | 2.4 | 1 | 1.5 | 2 | 0.4 | 0 | 0 | 0 | 0 | |||||||
| Grade 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||||||
| Endocrine/ Metabolic | Growth Hormone Deficiency | Primary Hypothyroidism | Abnormal Glucose Metabolism | Obesity | |||||||||||||||||||
| Survivors | Controls | Survivors | Controls | Survivors | Controls | Survivors | Controls | ||||||||||||||||
| N | % | N | % | N | % | N | % | N | % | N | % | N | % | N | % | 0.16 | |||||||
| Normal | 65 | 97.0 | 442 | 98.2 | 0.50 | 63 | 94.0 | 432 | 96.0 | 0.67 | 54 | 80.6 | 378 | 84.0 | 0.35 | 18 | 26.8 | 172 | 38.2 | ||||
| Grade 1 | 2 | 3.0 | 8 | 1.7 | 0 | 0 | 1 | 0.2 | 8 | 11.9 | 57 | 12.6 | - | - | - | - | |||||||
| Grade 2 | 0 | 0 | 0 | 0 | 4 | 6.0 | 17 | 3.7 | 4 | 6.0 | 13 | 2.8 | 21 | 31.3 | 119 | 26.4 | |||||||
| Grade 3 | - | - | - | - | 0 | 0 | 0 | 0 | 1 | 1.5 | 2 | 0.4 | 22 | 32.8 | 121 | 26.8 | |||||||
| Grade 4 | - | - | - | - | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 6 | 8.9 | 38 | 8.4 | |||||||
| Reproductive | Leydig Cell Insufficiency (Male) | Premature Ovarian Failure (Female) | Abnormal Sperm Concentration‡ | Liver Fibrosis | |||||||||||||||||||
| Survivors | Controls | Survivors | Controls | Survivors | Controls† | Gastrointestinal | Survivors | Controls† | |||||||||||||||
| N | % | N | % | N | % | N | % | N | % | N | % | N | % | N | % | ||||||||
| Normal | 25 | 100 | 186 | 93.0 | 0.10 | 42 | 100 | 249 | 99.6 | 0.97 | 2 | 66.7 | - | - | 59 | 88.0 | - | - | |||||
| Grade 1 | 0 | 0 | 10 | 5.0 | - | - | - | - | - | - | - | - | 1 | 1.5 | - | - | |||||||
| Grade 2 | 0 | 0 | 4 | 2.0 | - | - | - | - | 0 | 0 | - | - | 0 | 0 | - | - | |||||||
| Grade 3 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.4 | 1 | 33.3 | - | - | 6 | 8.9 | - | - | |||||||
| Grade 4 | - | - | - | - | - | - | - | - | - | - | - | - | 1 | 1.5 | - | - | |||||||
| Abnormal FEV1 | Abnormal DLCOcorr | Abnormal TLC | Cataract | ||||||||||||||||||||
| Survivors | Controls | Survivors | Controls | Survivors | Controls | Survivors | Controls | ||||||||||||||||
| Pulmonary | N | % | N | % | N | % | N | % | N | % | N | % | Ocular | N | % | N | % | ||||||
| Normal | 60 | 89.5 | 413 | 91.8 | 61 | 91.0 | 431 | 95.8 | 0.01 | 66 | 98.5 | 440 | 97.7 | 0.99 | 63 | 94.0 | 428 | 95.1 | 0.86 | ||||
| Grade 1 | 6 | 8.9 | 22 | 4.9 | 1 | 1.5 | 17 | 3.8 | 1 | 1.5 | 9 | 2.0 | 4 | 6.0 | 19 | 4.2 | |||||||
| Grade 2 | 0 | 0 | 11 | 2.4 | 0.61 | 5 | 7.5 | 1 | 0.2 | 0 | 0 | 1 | 0.2 | 0 | 0 | 2 | 0.4 | ||||||
| Grade 3 | 1 | 1.5 | 3 | 0.7 | 0 | 0 | 1 | 0.2 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.2 | |||||||
| Grade 4 | 0 | 0 | 1 | 0.2 | - | - | - | - | - | - | - | - | 0 | 0 | 0 | 0 | |||||||
| Low Bone Mineral Density | Osteonecrosis | Motor Neuropathy | Sensory Neuropathy | ||||||||||||||||||||
| Survivors | Controls† | Survivors | Controls† | Survivors | Controls | Survivors | Controls | ||||||||||||||||
| Bone Health | N | % | N | % | N | % | N | % | Neurology | N | % | N | % | N | % | N | % | ||||||
| Normal | 38 | 56.7 | - | - | 66 | 98.5 | - | - | 61 | 91.0 | 450 | 100 | 0.10 | 48 | 71.6 | 415 | 92.2 | <0.001 | |||||
| Grade 1 | 22 | 32.8 | - | - | 1 | 1.5 | - | - | 2 | 3.0 | 0 | 0 | 16 | 23.9 | 30 | 6.6 | |||||||
| Grade 2 | 7 | 10.4 | - | - | 0 | 0 | - | - | 3 | 4.5 | 0 | 0 | 2 | 3.0 | 5 | 1.1 | |||||||
| Grade 3 | 0 | 0 | - | - | 0 | 0 | - | - | 1 | 1.5 | 0 | 0 | 1 | 1.5 | 0 | 0 | |||||||
| Grade 4 | - | - | - | - | 0 | 0 | - | - | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||||||
Adjusted for age, sex, race (White vs. others)
FEV1= forced expiratory volume in one second, DLCOcorr= diffusion capacity of lungs for carbon monoxide corrected for hemoglobin, TLC= total lung capacity
Not assessed among controls
Assessed among survivors who consented to semen analysis (n=25)
Acknowledgements:
Funding: This study was supported by the National Cancer Institute: Cancer Center Support (CORE) Grant (CA21765) to St. Jude Children’s Research Hospital (PI: Dr. Charles W. Roberts) and U01 CA195547 (MPI: Drs. Melissa M. Hudson and Leslie L. Robison) and the American Lebanese Syrian Associate Charities (ALSAC), Memphis, TN.
Footnotes
Competing Interests:
The authors declare no competing financial interest
Data availability:
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.
References:
- 1.Ward E, DeSantis C, Robbins A, Kohler B, Jemal A. Childhood and adolescent cancer statistics, 2014. CA Cancer J Clin. 2014;64(2):83–103. [DOI] [PubMed] [Google Scholar]
- 2.Nea Howlader. SEER Cancer Statistics Review, 1975–2012, National Cancer Institute. 2015. [Google Scholar]
- 3.Creutzig U, van den Heuvel-Eibrink MM, Gibson B, Dworzak MN, Adachi S, de Bont E, et al. Diagnosis and management of acute myeloid leukemia in children and adolescents: recommendations from an international expert panel. Blood. 2012;120(16):3187–205. [DOI] [PubMed] [Google Scholar]
- 4.Armstrong GT, Chen Y, Yasui Y, Leisenring W, Gibson TM, Mertens AC, et al. Reduction in Late Mortality among 5-Year Survivors of Childhood Cancer. N Engl J Med. 2016;374(9):833–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gibson TM, Mostoufi-Moab S, Stratton KL, Leisenring WM, Barnea D, Chow EJ, et al. Temporal patterns in the risk of chronic health conditions in survivors of childhood cancer diagnosed 1970–99: a report from the Childhood Cancer Survivor Study cohort. Lancet Oncol. 2018;19(12):1590–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhakta N, Liu Q, Ness KK, Baassiri M, Eissa H, Yeo F, et al. The cumulative burden of surviving childhood cancer: an initial report from the St Jude Lifetime Cohort Study (SJLIFE). The Lancet. 2017;390(10112):2569–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barlogis V, Auquier P, Bertrand Y, Chastagner P, Plantaz D, Poiree M, et al. Late cardiomyopathy in childhood acute myeloid leukemia survivors: a study from the L.E.A. program. Haematologica. 2015;100(5):e186–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jarfelt M, Andersen NH, Glosli H, Jahnukainen K, Jonmundsson GK, Malmros J, et al. Cardiac function in survivors of childhood acute myeloid leukemia treated with chemotherapy only: a NOPHO-AML study. Eur J Haematol. 2016;97(1):55–62. [DOI] [PubMed] [Google Scholar]
- 9.Leung W, Hudson MM, Strickland DK, Phipps S, Srivastava DK, Ribeiro RC, et al. Late effects of treatment in survivors of childhood acute myeloid leukemia. J Clin Oncol. 2000;18(18):3273–9. [DOI] [PubMed] [Google Scholar]
- 10.Molgaard-Hansen L, Glosli H, Jahnukainen K, Jarfelt M, Jonmundsson GK, Malmros-Svennilson J, et al. Quality of health in survivors of childhood acute myeloid leukemia treated with chemotherapy only: a NOPHO-AML study. Pediatr Blood Cancer. 2011;57(7):1222–9. [DOI] [PubMed] [Google Scholar]
- 11.Molgaard-Hansen L, Skou AS, Juul A, Glosli H, Jahnukainen K, Jarfelt M, et al. Pubertal development and fertility in survivors of childhood acute myeloid leukemia treated with chemotherapy only: a NOPHO-AML study. Pediatr Blood Cancer. 2013;60(12):1988–95. [DOI] [PubMed] [Google Scholar]
- 12.Mulrooney DA, Dover DC, Li S, Yasui Y, Ness KK, Mertens AC, et al. Twenty years of follow-up among survivors of childhood and young adult acute myeloid leukemia: a report from the Childhood Cancer Survivor Study. Cancer. 2008;112(9):2071–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schultz KA, Chen L, Chen Z, Kawashima T, Oeffinger KC, Woods WG, et al. Health conditions and quality of life in survivors of childhood acute myeloid leukemia comparing post remission chemotherapy to BMT: a report from the children’s oncology group. Pediatr Blood Cancer. 2014;61(4):729–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Skou AS, Glosli H, Jahnukainen K, Jarfelt M, Jonmundsson GK, Malmros-Svennilson J, et al. Renal, gastrointestinal, and hepatic late effects in survivors of childhood acute myeloid leukemia treated with chemotherapy only--a NOPHO-AML study. Pediatr Blood Cancer. 2014;61(9):1638–43. [DOI] [PubMed] [Google Scholar]
- 15.Wilhelmsson M, Glosli H, Ifversen M, Abrahamsson J, Winiarski J, Jahnukainen K, et al. Long-term health outcomes in survivors of childhood AML treated with allogeneic HSCT: a NOPHO-AML Study. Bone Marrow Transplant. 2019;54(5):726–36. [DOI] [PubMed] [Google Scholar]
- 16.Liesner RJ, Leiper AD, Hann IM, Chessells JM. Late effects of intensive treatment for acute myeloid leukemia and myelodysplasia in childhood. J Clin Oncol. 1994;12(5):916–24. [DOI] [PubMed] [Google Scholar]
- 17.Leahey AM, Teunissen H, Friedman DL, Moshang T, Lange BJ, Meadows AT. Late effects of chemotherapy compared to bone marrow transplantation in the treatment of pediatric acute myeloid leukemia and myelodysplasia. Med Pediatr Oncol. 1999;32(3):163–9. [DOI] [PubMed] [Google Scholar]
- 18.Hudson MM, Ness KK, Nolan VG, Armstrong GT, Green DM, Morris EB, et al. Prospective medical assessment of adults surviving childhood cancer: study design, cohort characteristics, and feasibility of the St. Jude Lifetime Cohort study. Pediatr Blood Cancer. 2011;56(5):825–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Howell C, Bjornard K, Ness K, Alberts N, Armstrong G, Bhakta N, et al. Cohort profile: the St. Jude Lifetime Cohort Study (SJLIFE) for pediatric cancer survivors Int J Epidemiol 2020;in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feijen EA, Leisenring WM, Stratton KL, Ness KK, van der Pal HJ, Caron HN, et al. Equivalence Ratio for Daunorubicin to Doxorubicin in Relation to Late Heart Failure in Survivors of Childhood Cancer. J Clin Oncol. 2015;33(32):3774–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Green DM, Nolan VG, Goodman PJ, Whitton JA, Srivastava D, Leisenring WM, et al. The cyclophosphamide equivalent dose as an approach for quantifying alkylating agent exposure: a report from the Childhood Cancer Survivor Study. Pediatr Blood Cancer. 2014;61(1):53–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Howell RM, Smith SA, Weathers RE, Kry SF, Stovall M. Adaptations to a Generalized Radiation Dose Reconstruction Methodology for Use in Epidemiologic Studies: An Update from the MD Anderson Late Effect Group. Radiat Res. 2019;192(2):169–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hudson MM, Ehrhardt MJ, Bhakta N, Baassiri M, Eissa H, Chemaitilly W, et al. Approach for Classification and Severity Grading of Long-term and Late-Onset Health Events among Childhood Cancer Survivors in the St. Jude Lifetime Cohort. Cancer Epidemiol Biomarkers Prev. 2017;26(5):666–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Strauss E, Sherman EM, Spreen O. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary. Third ed. New York, NY: Oxford University Press; 2006. [Google Scholar]
- 25.Conners CK. Conners’ Continuous Performance Test II. North Tonawanda, NY: Multi-Health Systems; 2001. [Google Scholar]
- 26.Reynolds C, Voress JK. Test of Memory and Learning. Second ed. Austin, TX: PRO-ED; 2007. [Google Scholar]
- 27.Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test. Second ed. San Antonio, TX: The Psychological Corporation; 2000. [Google Scholar]
- 28.Wechsler D Wechsler Adult Intelligence Scale. Third ed. San Antonio, TX: Psychological Corporation; 1997. [Google Scholar]
- 29.Lipkin DP, Scriven AJ, Crake T, Poole-Wilson PA. Six minute walking test for assessing exercise capacity in chronic heart failure. Br Med J (Clin Res Ed). 1986;292(6521):653–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39(2):142–8. [DOI] [PubMed] [Google Scholar]
- 31.Mathiowetz V, Kashman N, Volland G, Weber K, Dowe M, Rogers S. Grip and pinch strength: normative data for adults. Arch Phys Med Rehabil. 1985;66(2):69–74. [PubMed] [Google Scholar]
- 32.Muscular weakness assessment: use of normal isometric strength data. The National Isometric Muscle Strength (NIMS) Database Consortium. Arch Phys Med Rehabil. 1996;77(12):1251–5. [DOI] [PubMed] [Google Scholar]
- 33.Moseley AM, Crosbie J, Adams R. Normative data for passive ankle plantarflexion--dorsiflexion flexibility. Clin Biomech (Bristol, Avon). 2001;16(6):514–21. [DOI] [PubMed] [Google Scholar]
- 34.Shephard RJ, Berridge M, Montelpare W. On the generality of the “sit and reach” test: an analysis of flexibility data for an aging population. Res Q Exerc Sport. 1990;61(4):326–30. [DOI] [PubMed] [Google Scholar]
- 35.Nashner LM, Peters JF. Dynamic posturography in the diagnosis and management of dizziness and balance disorders. Neurol Clin. 1990;8(2):331–49. [PubMed] [Google Scholar]
- 36.Dong H, Robison LL, Leisenring WM, Martin LJ, Armstrong GT, Yasui Y. Estimating the burden of recurrent events in the presence of competing risks: the method of mean cumulative count. Am J Epidemiol. 2015;181(7):532–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bhakta N, Liu Q, Ness KK, Baassiri M, Eissa H, Yeo F, et al. The cumulative burden of surviving childhood cancer: an initial report from the St Jude Lifetime Cohort Study (SJLIFE). Lancet. 2017;390(10112):2569–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Geskus RB. Cause-specific cumulative incidence estimation and the fine and gray model under both left truncation and right censoring. Biometrics. 2011;67(1):39–49. [DOI] [PubMed] [Google Scholar]
- 39.Rubnitz JE, Lacayo NJ, Inaba H, Heym K, Ribeiro RC, Taub J, et al. Clofarabine Can Replace Anthracyclines and Etoposide in Remission Induction Therapy for Childhood Acute Myeloid Leukemia: The AML08 Multicenter, Randomized Phase III Trial. J Clin Oncol. 2019;37(23):2072–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rubnitz JE, Inaba H, Dahl G, Ribeiro RC, Bowman WP, Taub J, et al. Minimal residual disease-directed therapy for childhood acute myeloid leukaemia: results of the AML02 multicentre trial. Lancet Oncol. 2010;11(6):543–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dluzniewska A, Balwierz W, Armata J, Balcerska A, Chybicka A, Kowalczyk J, et al. Twenty years of Polish experience with three consecutive protocols for treatment of childhood acute myelogenous leukemia. Leukemia. 2005;19(12):2117–24. [DOI] [PubMed] [Google Scholar]
- 42.Alexander TB, Wang L, Inaba H, Triplett BM, Pounds S, Ribeiro RC, et al. Decreased relapsed rate and treatment-related mortality contribute to improved outcomes for pediatric acute myeloid leukemia in successive clinical trials. Cancer. 2017;123(19):3791–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Creutzig U, Zimmermann M, Lehrnbecher T, Graf N, Hermann J, Niemeyer CM, et al. Less toxicity by optimizing chemotherapy, but not by addition of granulocyte colony-stimulating factor in children and adolescents with acute myeloid leukemia: results of AML-BFM 98. J Clin Oncol. 2006;24(27):4499–506. [DOI] [PubMed] [Google Scholar]
- 44.Mulrooney DA, Hyun G, Ness KK, Ehrhardt MJ, Yasui Y, Duprez D, et al. Major cardiac events for adult survivors of childhood cancer diagnosed between 1970 and 1999: report from the Childhood Cancer Survivor Study cohort. BMJ. 2020;368:l6794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cooper TM, Absalon MJ, Alonzo TA, Gerbing RB, Leger KJ, Hirsch BA, et al. Phase I/II Study of CPX-351 Followed by Fludarabine, Cytarabine, and Granulocyte-Colony Stimulating Factor for Children With Relapsed Acute Myeloid Leukemia: A Report From the Children’s Oncology Group. J Clin Oncol. 2020;38(19):2170–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Getz KD, Sung L, Alonzo TA, Leger KJ, Gerbing RB, Pollard JA, et al. Effect of Dexrazoxane on Left Ventricular Systolic Function and Treatment Outcomes in Patients With Acute Myeloid Leukemia: A Report From the Children’s Oncology Group. J Clin Oncol. 2020;38(21):2398–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Armstrong GT, Oeffinger KC, Chen Y, Kawashima T, Yasui Y, Leisenring W, et al. Modifiable risk factors and major cardiac events among adult survivors of childhood cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013;31(29):3673–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dandoy CE, Davies SM, Ahn KW, He Y, Kolb AE, Levine J, et al. Comparison of total body irradiation versus non- total body irradiation containing regimens for de novo acute myeloid leukemia in children. Haematologica. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Phillips SM, Padgett LS, Leisenring WM, Stratton KK, Bishop K, Krull KR, et al. Survivors of childhood cancer in the United States: prevalence and burden of morbidity. Cancer Epidemiol Biomarkers Prev. 2015;24(4):653–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Krull KR, Gioia G, Ness KK, Ellenberg L, Recklitis C, Leisenring W, et al. Reliability and validity of the Childhood Cancer Survivor Study Neurocognitive Questionnaire. Cancer. 2008;113(8):2188–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kenzik KM, Huang IC, Brinkman TM, Baughman B, Ness KK, Shenkman EA, et al. The Childhood Cancer Survivor Study-Neurocognitive Questionnaire (CCSS-NCQ) revised: item response analysis and concurrent validity. Neuropsychology. 2015;29(1):31–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stefanski KJ, Anixt JS, Goodman P, Bowers K, Leisenring W, Baker KS, et al. Long-Term Neurocognitive and Psychosocial Outcomes After Acute Myeloid Leukemia: A Childhood Cancer Survivor Study Report J Natl Cancer Inst. 2020;in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Scott JM, Li N, Liu Q, Yasui Y, Leisenring W, Nathan PC, et al. Association of Exercise With Mortality in Adult Survivors of Childhood Cancer. JAMA Oncol. 2018;4(10):1352–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tonorezos ES, Ford JS, Wang L, Ness KK, Yasui Y, Leisenring W, et al. Impact of exercise on psychological burden in adult survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. Cancer. 2019;125(17):3059–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Howell CR, Krull KR, Partin RE, Kadan-Lottick NS, Robison LL, Hudson MM, et al. Randomized web-based physical activity intervention in adolescent survivors of childhood cancer. Pediatr Blood Cancer. 2018;65(8):e27216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mendoza JA, Baker KS, Moreno MA, Whitlock K, Abbey-Lambertz M, Waite A, et al. A Fitbit and Facebook mHealth intervention for promoting physical activity among adolescent and young adult childhood cancer survivors: A pilot study. Pediatr Blood Cancer. 2017;64(12). [DOI] [PubMed] [Google Scholar]
- 57.Ojha RP, Oancea SC, Ness KK, Lanctot JQ, Srivastava DK, Robison LL, et al. Assessment of potential bias from non-participation in a dynamic clinical cohort of long-term childhood cancer survivors: results from the St. Jude Lifetime Cohort Study. Pediatr Blood Cancer. 2013;60(5):856–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


