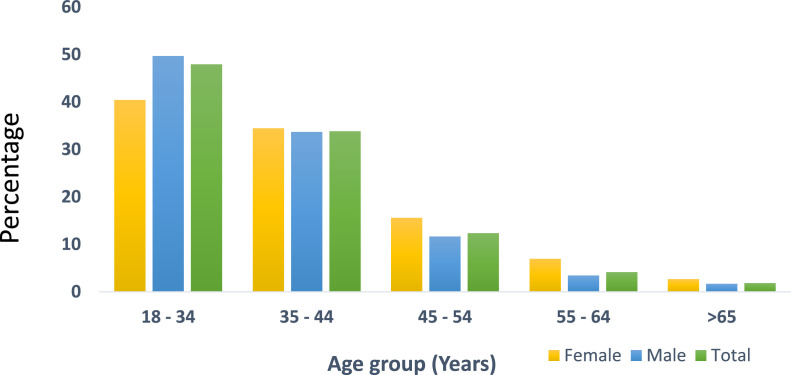Abstract
Introduction
The Kingdom of Saudi Arabia was one of the first countries to implement a COVID-19 vaccination program. This study estimated the safety and reactogenicity of the ChAdOx1-S vaccine after the first dose administered to adults.
Methods
This cross-sectional study included 1592 randomly selected vaccinees from April to May 2021. A questionnaire was delivered to the vaccinees via phone calls 7 and 21 days after the first vaccine dose.
Results
Of the 1592 vaccinees who had the first dose, the mean age was 37.4 (± 9.6) years and 81% were males. Of all the vaccinees, 553 (34.7%) reported an adverse reaction on the first telephone call. The most common symptoms were: pain at the site of injection (485, 30.5%), musculoskeletal symptoms (438, 27.5%), skin rash (307, 19.2%), gastrointestinal symptoms (379, 23.8%) and fever (498, 31.3%). Men were more likely to report fever (76.9% vs. 23.1%; P = 0.005), skin rash (81.1% vs. 18.9%, P = 0.005) and pain at the injection site (77.3% vs. 22.7%, P < 0.0001). Post-vaccine COVID-19 infection was 0.5% and there were no hospitalizations.
Conclusion
This study observed no major side effects of the ChAdOx1-S vaccine and no reported breakthrough infection during the observation period.
Keywords: ChAdOx1-S, COVID-19 vaccine, SARS-CoV-2, Oxford-AstraZeneca vaccine
Introduction
Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) caused the current Coronavirus Disease 2019 (COVID-19) pandemic. The pandemic has resulted in a significant disruption in social lives and has had a major economic impact. Countries around the globe have taken extraordinary measures to combat the disease. The results from phase III clinical trials have shown that both the Pfizer-BioNTech messenger RNA (mRNA) vaccine (BNT162b2) and the Oxford-AstraZeneca adenovirus vector vaccine ChAdOx1-S, also known as Ad26.COV2.S, are very effective in decreasing disease and mortality with a planned two-dose schedule (Polack et al., 2020). In addition, the ChAdOx1-S trial proposed that increasing the dosing interval between the first and the second dose provides increased protection (Voysey et al., 2021). The efficacy of a single dose of the BNT162b2 trial data suggests that a single dose of this vaccine has an efficacy of 92.6% in the early post-vaccination period (Vergnes, 2021). It had been also shown that extending the interval between the first and the second dose results in an enhanced immune response (Ledgerwood et al., 2013). The emergence of SARS-CoV-2 variants of concerns such as the alpha, beta and delta variants has caused significant attention, as a few of these variants such as the alpha variant are 50% more infectious (Davies et al., 2020). This variant within the B.1.1.7 lineage has several mutations; the most significant is N501Y within the S protein. The BNT162b2 vaccine has been shown to neutralize the N501 and Y501 viruses in vitro (Xie et al., 2021).
The Kingdom of Saudi Arabia (KSA) was one of the first countries to implement several preventive measures to halt the spread of the disease (Al-Tawfiq et al., 2020; Al-Tawfiq and Memish, 2020). In addition, the KSA was one of the first countries to introduce COVID-19 vaccination programs following approval of the BNT162b2 vaccine (Assiri et al., 2021). The program then encompassed the ChAdOx1-S vaccine with scale-up of activities over time (Assiri et al., 2021). As part of the vaccine rollout, the KSA planned a phased approach to initially target the most vulnerable populations, including first responders, healthcare workers, individuals with comorbid illnesses, and the elderly. A second and third phase will follow, with the goal of vaccinating at least 70% of the whole population. The Ministry of Health requested that the population register to receive the vaccine via online platforms and smartphone applications, and provided a supportive digital infrastructure for doing so. Vaccines were distributed within major cities. Mass vaccination centers were erected across the country to meet the storage conditions of the vaccines (Assiri et al., 2021).
The vaccination campaign in the KSA was started on 17 December 2020 with the Pfizer- BioNTech vaccine and in February 2021 with the introduction of the ChAdOx1-S vaccine (Assiri et al., 2021). The KSA opened additional centers to accommodate and speed up the vaccination program (Assiri et al., 2021). The vaccination activity at the King Fahd Military complex started on 22 February 2021, and consisted of five clinics with an initial number of 1000 vaccinees per day. A total of > 50,000 vaccine doses were given by the end of April 2021. Later on, the clinics were also opened for all Saudi Arabian residents. This study presents the initial experience of vaccination and reports the reactogenicity among vaccinated individuals as well as the efficacy within 30 days of vaccination.
Materials and Methods
This was a phone call-based questionnaire and members of the study group called individuals who received the first dose of the ChAdOx1 (AZD1222) COVID-19 vaccine at the King Fahd Military complex between 10 April and 20 May 2021 when a total of 29,355 first dose vaccines were administered. The respondents who agreed to participate gave verbal consent and their responses were included anonymously. The study included a population who received their vaccine at King Fahad Military Medical complex, Dhahran. The questionnaire was designed and delivered to the candidates via phone calls (hospital Avaya System). One part of the survey covered demographics of the vaccinees, including: nationality, sex, age, and earlier infection with SARS-CoV-2. The second part of the study inquired about any reactions to the COVID-19 vaccine, and timing of these side-effects. The phone calls were performed twice: the first was 7 days and the second was 21 days after receiving the vaccine.
Results
This study included 1592 vaccinees who received a first dose of the ChAdOx1-S vaccine; the mean age (± SD) was 37.4 (± 9.6) (range 19-83) years (Figure 1 ) and 81% were males. Of all the vaccinees, 553 (34.7%) reported a reaction on the first call and none reported any reaction on the second call (Table 1 ). The most common symptoms were: pain at the site of injection (485, 30.5%), musculoskeletal symptoms (438, 27.5%), skin rash (307, 19.2%), gastrointestinal symptoms (379, 23.8%) and fever (498, 31.3%).
Figure 1.
Age distribution percentages of the total, female and male vaccinees.
Table 1.
Overall rate of reactogenicity to the ChAdOx1 (AZD1222) COVID-19 vaccine.
| Frequency | Percentage | |
|---|---|---|
| Any adverse event | 553 | 34.7 |
| Pain at the site of injection | 485 | 30.5 |
| Musculoskeletal (joint pain, myalgia) | 438 | 27.5 |
| Skin rash | 307 | 19.3 |
| Gastrointestinal (abdominal pain, diarrhea, vomiting) | 379 | 23.8 |
| Fever | 498 | 31.3 |
| Cardiac disorder (palpitation, chest pain) | 7 | 0.4 |
| Central nervous system | 3 | 0.2 |
| Blood disorder (anemia, bleeding, thrombosis) | 1 | 0.1 |
| Respiratory (shortness of breath) | 2 | 0.1 |
| Hemodynamic vasovagal attack | 2 | 0.1 |
| Lymphadenopathy | 0 | 0 |
| Admission to hospital | 0 | 0 |
Males were more likely to report fever (76.9% vs. 23.1%; P = 0.005), skin rash (81.1% vs. 18.9%, P = 0.005) and pain at the injection site (77.3% vs. 22.7%, P = 0.01). The rate of post-vaccine COVID-19 infection was 0.5% and there were no hospitalizations. Males had more reactions than females overall (76.7% vs. 23.3%; P = 0.001). In addition, there was a substantial difference in the rate of pain at the site of injection, skin rash and fever between males and females (Table 2 ). Males were more likely to report fever (76.9% vs. 23.1%; P = 0.005), skin rash (81.1% vs. 18.9%; P = 0.005) and pain at the injection site (77.3% vs. 22.7%; P < 0.0001).
Table 2.
Univariate analysis of the local and systemic reactogenicity to ChAdOx1 (AZD1222) COVID-19 vaccine in relation to gender.
| Male |
Female |
Chi square | P-value | Confidence interval | |||
|---|---|---|---|---|---|---|---|
| Frequency | Percentage | Frequency | Percentage | ||||
| Fever | 383 | 76.9 | 115 | 23.1 | 111.54 | < 0.0001 | 44.20 – 61.62 |
| CNS | 2 | 66.7 | 1 | 33.3 | 0.20 | 0.65 | -43.12 – 75.53 |
| GI (abdominal pain, diarrhea, vomiting) | 314 | 82.8 | 65 | 17.2 | 113.54 | < 0.0001 | 53.75 – 73.79 |
| Blood disorder (anemia, bleeding, thrombosis) | 0 | 0 | 1 | 100 | - | - | - |
| Cardiac disorder (palpitation, chest pain) | 3 | 42.9 | 4 | 57.1 | 0.12 | 0.73 | -42.11 – 59.94 |
| Musculoskeletal (joint pain, myalgia) | 342 | 78.1 | 96 | 21.9 | 104.94 | < 0.0001 | 45.82 – 64.38 |
| Lymphadenopathy | - | - | - | - | |||
| Respiratory (shortness of breath) | 1 | 50 | 1 | 50 | 0.00 | 1.00 | -62.99 – 62.99 |
| Skin rash | 249 | 81.1 | 58 | 18.9 | 85.34 | < 0.0001 | 49.18 – 71.33 |
| Hemodynamic vasovagal attack | 0 | 0 | 2 | 100 | - | - | - |
| Pain at the site of injection | 375 | 77.3 | 110 | 22.7 | 111.10 | < 0.0001 | 44.83 – 62.49 |
| Any AEs | 424 | 76.7 | 129 | 23.3 | 122.56 | < 0.0001 | 44.34 – 60.88 |
CNS, central nervous system; AE, adverse event
Older individuals were more likely to report symptoms compared with the younger population (Table 3 ). However, based on the rate of reactogenicity in those aged 18-34 years as the comparison group, vaccinees aged => 65 years had an Odds Ratio of 1.105 (95% CI: 0.46-2.653; P = 0.823) and only those aged 45-54 years had an OR of 0.448 (95% CI: 0.211-0.95; P = 0.036), indicating fewer events. The overall rate of post-vaccine COVID-19 infection was 0.5% with no hospitalization during the study period.
Table 3.
Reactogenicity to ChAdOx1 (AZD1222) COVID-19 vaccine in relation to age group (based on age group 18-34 years as the comparison group).
| Adverse events | 95% CI |
||||
|---|---|---|---|---|---|
| n/N (%) | OR | Lower | Upper | P-value | |
| 18 - 34 | 288/759 (38) | ___ | ___ | ___ | ___ |
| 35 - 44 | 158/536 (29.5) | 0.655 | 0.312 | 1.377 | 0.265 |
| 45 - 54 | 60/196 (31) | 0.448 | 0.211 | 0.95 | 0.036* |
| 55 - 64 | 33/65 (51) | 0.473 | 0.215 | 1.041 | 0.063 |
| ≥ 65 | 14/29 (48) | 1.105 | 0.46 | 2.653 | 0.823 |
Discussion
This study evaluated local and systemic responses to the first dose of the ChAdOx1 nCoV-19 (AZD1222) vaccine. The data showed that the vaccine was well tolerated, with differences in the reactogenicity between males and females. There were no reported COVID-19 infections, hospital admissions or deaths in the follow-up period. However, prevalence of the different variants in the KSA was not reported. In an international randomized, double-blind, placebo-controlled, phase 3 clinical trial a single dose of the Ad26.COV2.S vaccine showed 67% efficacy in preventing moderate to severe–critical COVID-19, as evaluated 14-28 days after dose administration. The efficacy against severe–critical COVID-19 was 77-85%, as evaluated 14-28 days after administration (Sadoff et al., 2021b).
The reactogenicity of the Ad26.COV2.S vaccine was short-lived, acceptable, transient, and lower in those who were relatively old (Sadoff et al., 2021a). In the current study, the first AstraZeneca-Oxford vaccine dose was associated with 34.7% of any type of reactogenicity symtoms and the incidence of pain at the local site of injection was 30.5%. In a phase 3 clinical trial, injection site pain was observed in 48.6% of the participants (Sadoff et al., 2021b). Pain at the injection site was commonly reported as the local reaction in the different COVID-19 vaccines (McDonald et al., 2021).
The most prevalent systemic symptoms included: fever, chills, headache, myalgia, and fatigue, as documented in a meta-analysis of all COVID-19 vaccines (McDonald et al., 2021). The administration of COVID-19 vaccination could be homologous (the first and second doses are of the same vaccine) or heterologous (different vaccines for the first and the second doses). The occurrence of post-COVID-19 symptoms was more pronounced if AstraZeneca and Pfizer vaccines were used sequentially, with a report of 41% having symptoms in those having Pfizer-BioNTech followed by the AstraZeneca-Oxford vaccines as compared with the 21% rate in the Pfizer-BioNTech for both doses (Shaw et al., 2021). However, the current study also observed that side effects were more common among males than females. The reactions and immunogenicity towards vaccinations are probably related to the immune response among the gender differences (Flanagan et al., 2017). The current study observed lower reactogenicity among those aged 45-54 years, with an OR of 0.448 (95% CI: 0.211-0.95; P = 0.036) indicating lower events compared to younger people. In a clinical trial, reactogenic symptoms were less common in those aged ≥ 56 years (Ramasamy et al., 2020). The variances might be secondary to the differences between the included population in the two reports. Severe reactogenicity (grade ≥ 3) and the need for hospitalization were not reported by the included vaccinees in this study. There were no reported anaphylactic reactions among AstraZeneca Oxford COVID-19 vaccine recipients (Moghimi, 2021). Previous studies have shown occurrence of anaphylactic reactions after the AstraZeneca COVID-19 vaccine and one study reported 28 thrombotic events at the time when 17 million people were vaccinated (Tobaiqy et al., 2021).
There were several limitations of the current study. The study was based on phone calls to vaccinees. The follow-up period was short and a longer duration is required to verify the prevention of SARS-CoV-2 infections. The study had a relatively small sample size and thus further studies with larger sample sizes are required to ascertain the safety profile. In addition, a multi-center evaluation of the vaccine from the KSA is needed to examine the reactogenicity profile of the introduced SARS-CoV-2 vaccines. The study was also predominated by males and a better male to female ratio is needed to confirm gender predominance.
In conclusion, this study observed no major side effects of the ChAdOx1-S vaccine and no reported breakthrough infection during the observation period. There was a difference in the reported symptoms between males and females and across the age groups.
Acknowledgments
Conflict of Interest
All authors have no conflict of interest to declare.
Funding Source
None.
Ethical Approval
The IRB of the King Fahad Military Medical Complex approved the study (AFHER-IRB-2021-011).
References
- Al-Tawfiq JA, Memish ZA. COVID-19 in the Eastern Mediterranean Region and Saudi Arabia: prevention and therapeutic strategies. Int J Antimicrob Agents. 2020;55 doi: 10.1016/j.ijantimicag.2020.105968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Tawfiq JA, Sattar A, Al-Khadra H, Al-Qahtani S, Al-Mulhim M, Al-Omoush O, et al. Incidence of COVID-19 among returning travelers in quarantine facilities: A longitudinal study and lessons learned. Travel Med Infect Dis. 2020;38 doi: 10.1016/j.tmaid.2020.101901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assiri A, Al-Tawfiq JA, Alkhalifa M, Al Duhailan H, Al Qahtani S, Dawas RA, et al. Launching COVID-19 vaccination in Saudi Arabia: lessons learned, and the way forward. Travel Med Infect Dis. 2021;43 doi: 10.1016/j.tmaid.2021.102119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies NG, Barnard RC, Jarvis CI, Kucharski AJ, Munday J, Pearson CAB, et al. Estimated transmissibility and severity of novel SARS-CoV-2 Variant of Concern 202012/01 in England. MedRxiv. 2020 doi: 10.1101/2020.12.24.20248822. 2020.12.24.20248822. [DOI] [Google Scholar]
- Flanagan KL, Fink AL, Plebanski M, Klein SL. Sex and gender differences in the outcomes of vaccination over the life course. Annu Rev Cell Dev Biol. 2017;33:577–599. doi: 10.1146/annurev-cellbio-100616-060718. [DOI] [PubMed] [Google Scholar]
- Ledgerwood JE, Zephir K, Hu Z, Wei CJ, Chang L, Enama ME, et al. Prime-boost interval matters: A randomized phase 1 study to identify the minimum interval necessary to observe the h5 dna influenza vaccine priming effect. J Infect Dis. 2013;208:418–422. doi: 10.1093/infdis/jit180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald I, Murray SM, Reynolds CJ, Altmann DM, Boyton RJ. Comparative systematic review and meta-analysis of reactogenicity, immunogenicity and efficacy of vaccines against SARS-CoV-2. Npj Vaccines. 2021;6:1–14. doi: 10.1038/s41541-021-00336-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghimi SM. Allergic Reactions and Anaphylaxis to LNP-Based COVID-19 Vaccines. Mol Ther. 2021;29:898–900. doi: 10.1016/j.ymthe.2021.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/nejmoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramasamy MN, Minassian AM, Ewer KJ, Flaxman AL, Folegatti PM, Owens DR, et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial. Lancet. 2020;396:1979–1993. doi: 10.1016/S0140-6736(20)32466-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadoff J, Le Gars M, Shukarev G, Heerwegh D, Truyers C, de Groot AM, et al. Interim Results of a Phase 1–2a Trial of Ad26.COV2.S Covid-19 Vaccine. N Engl J Med. 2021;384:1824–1835. doi: 10.1056/nejmoa2034201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadoff J, Gray G, Vandebosch A, Cárdenas V, Shukarev G, Grinsztejn B, et al. Safety and Efficacy of Single-Dose Ad26.COV2.S Vaccine against Covid-19. N Engl J Med. 2021;384:2187–2201. doi: 10.1056/nejmoa2101544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw RH, Stuart A, Greenland M, Liu X, Van-Tam JSN, Snape MD. Heterologous prime-boost COVID-19 vaccination: initial reactogenicity data. Lancet. 2021;397 doi: 10.1016/s0140-6736(21)01115-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobaiqy M, Elkout H, Maclure K. Analysis of thrombotic adverse reactions of COVID-19 AstraZeneca vaccine reported to EudraVigilance database. Vaccines. 2021;9 doi: 10.3390/vaccines9040393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergnes J-N. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2021;384:1577. doi: 10.1056/NEJMc2036242. [DOI] [PubMed] [Google Scholar]
- Voysey M, Clemens SAC, Madhi SA, Weckx LY, Folegatti PM, Aley PK, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X, Zou J, Fontes-Garfias CR, Xia H, Swanson KA, Cutler M, et al. Neutralization of N501Y mutant SARS-CoV-2 by BNT162b2 vaccine-elicited sera. BioRxiv Prepr Serv Biol. 2021 doi: 10.1101/2021.01.07.425740. [DOI] [PubMed] [Google Scholar]