Abstract
Background
: COVID-19 seroprevalence studies use serum/plasma samples to detect SARS-CoV-2 IgG. Data supporting alternate specimen types and freeze-thaw antibody stability is lacking. The stability of IgG and other immunoglobulins in multiple blood sample types stored in differing conditions and multiple freeze-thaw cycles (FTCs) was evaluated.
Materials and methods
: Serum, plasma, and heparinized-plasma samples were collected from COVID-19 recovered individuals. Samples underwent testing for SARS-CoV-2 antibodies upon collection, after each of 10–12 FTCs, and storage at -70°C, -20°C, 4°C, and room-temperature for 10–12 days using four high-throughput commercial assays, two rapid-test cassettes, a manual ELISA, and a surrogate neutralization assay.
Results
: All three specimen types were collected from 34 COVID-19 recovered seropositive individuals (≥21 days post-symptoms). Using the Architect and Liaison assays, a positive qualitative SARS-CoV-2 IgG result was detected daily up to 12 FTCs and up to 10 days of storage at different temperatures. An additional 25 plasma samples consistently demonstrated detection of SARS-CoV-2 antibodies daily after 12 FTCs and storage at -20°C using two rapid test cassette assays (SD Biosensor and Hangzhou All Test), manual (Beijing Wantai) and surrogate neutralization (GenScript) ELISAs, and two high-throughput assays (Roche Elecsys nucleocapsid and spike). IgM antibodies were less frequently detected by one of the rapid test cassette assays.
Conclusions
: Serum, plasma, and heparinized-plasma constitute reliable samples for SARS-CoV-2 antibody detection. In particular, the IgG response was stable and reliably detected after multiple FTCs and storage at common laboratory conditions. IgM detection was variable due to the labile nature of this antibody class.
Keywords: SARS-CoV-2 IgG, Stability, Lithium heparin, Plasma, Serum, Freeze-thaw cycle
Abbreviations: FTC, freeze-thaw cycle; DSO, date of symptom-onset; CBS, Canadian Blood Services; PRNT, plaque reduction neutralization test; NML, National Microbiology Laboratory; SVNT, surrogate virus neutralization test
Introduction
The principal utility of SARS-CoV-2 IgG high throughput assays is conducting seroprevalence studies of different populations. Most surveys use serum, plasma, or heparinized-plasma [1]. While it is presumed these three specimen types are likely equivalent, some studies have demonstrated that the yield of SARS-CoV-2 IgG results from these specimen types can differ based on the assay [2].
A number of studies have documented the stability of IgG antibodies produced to other viruses (eg. herpes simplex, varicella zoster, measles, mumps, rubella) when blood samples are stored or exposed to a variety of laboratory conditions [3], [4], [5]. Such conditions include multiple FTCs, varying storage temperatures, and prolonged storage over time. Variables in these studies that limit comparison include use or non-use of storage matrix (such as bovine-serum albumin or phosphate-buffered saline), and type of assay being used to measure antibody level (such as immunofluorescence assays versus ELISA versus large automated high-throughput systems). To-date however, there are a paucity of studies evaluating the stability of SARS-CoV-2 antibodies, including IgG, in different laboratory conditions and its overall effect on the quality of the result generated.
In addition to serum, we aimed to evaluate the utility of plasma and heparinized-plasma samples as alternate sample types for SARS-CoV-2 antibody detection and assess the stability of these types across different temperatures and FTCs.
Materials and methods
Participant sample collection
Serum and plasma blood samples were collected simultaneously from a group of 37 COVID-19 recovered individuals with laboratory-confirmed SARS-CoV-2 infection, diagnosed using molecular assays [6, 7]. Samples were collected ≥21 days post date of symptom onset (DSO). A subset (34 of 37) additionally submitted heparinized-plasma blood samples.
A parallel study was conducted on plasma samples collected by Canadian Blood Services (CBS) from donors who self-identified as having recovered from COVID-19. Samples were previously tested by a SARS-CoV-2 plaque reduction neutralization test (PRNT). In total, 25 samples with PRNT titers ranging from 1:40–1:640 were selected for this study. Clinical information and demographics were not available for this cohort of patients.
SARS-CoV-2 antibody stability
Samples were initially stored at −20°C and underwent one FTC prior to aliquoting. SARS-CoV-2 IgG detection was performed using the Architect SARS-CoV-2 IgG and Liaison SARS-CoV-2 S1/S2 IgG qualitative assays (Table 1 ) [1]. One 600 µL sample of each specimen type (serum, plasma, or heparinized-plasma) was stored at −70°C and underwent repeated FTCs over 10 consecutive days, while single-use 200 µL aliquots from the same patient were stored at three different temperatures (−70°C, 4°C, and room temperature (21–25°C)) and thawed only on the day of testing to assess the stability of SARS-CoV-2 IgG. In situations of insufficient volume for a single sample to make all 10 aliquots, several patient specimens of the same sample type were pooled at the onset. Testing of these initial samples was carried out at the Public Health Laboratory, Alberta Precision Laboratories (Edmonton, Alberta, Canada).
Table 1.
Serological assays used and their commercial information.
| Assay | Qualitative/Quantitative | Antibody component measured | Index Value | Manufacturer |
|---|---|---|---|---|
| AllTest COVID-19 IgG Rapid Test Cassette | Qualitative | IgG | No | Hangzhou Alltest Biotech, Hangzhou, China |
| Architect SARS-CoV-2 IgG | Qualitative | IgG(target: nucleocapsid protein) | Yes;S/C ≥ 1.40 considered positive | Abbott Laboratories, Chicago, USA |
| cPass Surrogate Virus Neutralization Test (sVNT) Kit | Quantitative | Neutralizing antibodies | Yes;% inhibition | GenScript Biotech, Piscataway, USA |
| Elecsys Anti-SARS-CoV-2 | Qualitative | Total (IgM+IgG)(target: nucleocapsid protein) | Yes;COI ≥1.0 considered positive | Roche, Basel, Switzerland |
| Elecsys Anti-SARS-CoV-2 S | Quantitative | Total(IgM+IgG)(target: spike protein) | Yes; ≥ 0.8 U/mL reactive | Roche, Basel, Switzerland |
| Liaison SARS-CoV-2 S1/S2 IgG | Qualitative | IgG(target: spike protein) | Yes;≥15.0 AU/mL considered positive. | Diasorin, Saluggia, Italy |
| Standard Q COVID-19 IgM/IgG Combo | Qualitative | IgM + IgG | No | SD Biosensor, Suwon-si, South Kora |
| Wantai SARS-COV-2 Ab ELISA | Qualitative | Total (IgM+IgG) | Yes;A/CO ≥1.0 considered positive. | Beijing Wantai Biological, Beijing, China |
Abbreviations: A/CO – absorbance over cut-off; AU/mL – arbitrary units per mL; COI – cut-off index; S/C – signal/cut-off; U/mL – units per mL.
Similar procedures were followed for 12 FTCs on the PRNT-positive plasma samples. Plasma units from CBS were initially stored at −80°C and thawed to remove 15 mL for this study. Two 500 µL aliquots of each sample (one for each lab conducting testing) were removed, labeled (FTC #1) and stored at −20°C. This process was repeated for an additional 11 FTCs over three consecutive days. All aliquots were thawed on the day of testing and the stability of SARS-CoV-2 antibodies was assessed by all diagnostic platforms with the exception of the Roche Elecsys Anti-SARS-CoV-2 S and the AllTest rapid test cassettes. For these assays, samples were held at 4°C for 16 days post-thaw prior to testing.
In total, 300 aliquots of the CBS plasma samples generated in the 12 FTCs on 25 samples were tested by the Roche Elecsys Anti-SARS-CoV-2 and Elecsys Anti-SARS-CoV-2 S, SD BioSensor Standard Q COVID-19 IgM/IgG Combo and Hangzhou AllTest IgG rapid test cassettes, and Beijing Wantai SARS-COV-2 Ab ELISA. Aliquots (n = 100) of plasma collected from FTCs 1, 6, and 12 were also tested by the GenScript cPass™ SARS-CoV-2 Neutralization Antibody Detection Kit (Table 1).
Testing of the PRNT-positive plasma samples from CBS was carried out at the National Microbiology Laboratory (NML), Public Health Agency of Canada (Winnipeg, Manitoba, Canada) [8].
Statistical analysis
To assess stability of SARS-CoV-2 IgG qualitative detection between samples, temperatures, or FTCs, assay index values (Table 1) were compared. Evaluation for IgG stability of the Abbott and Diasorin assays for specimen type (lithium heparin, plasma, serum) was compared using a one-way ANOVA while comparison of the three different temperatures evaluated were compared using a multivariate ANOVA. Evaluation of antibody stability for the GenScript, Wantai, and Roche assays was conducted using a one-way ANOVA. Significance was set at p<0.05. All statistical analysis was conducted using Stata (version 14.1; StataCorp, College Station, USA). Graphs were constructed using GraphPad Prism version 9.12 for Windows.
Results
Convalescent patient cohort: SARS-CoV-2 IgG stability with repeated freeze-thaw cycles and storage at different temperatures
Convalescent serum, plasma, and heparinized-plasma samples were collected from 34 patients at a median of 67 days post DSO (median age 57.5 years; 55.9% female) (Supplementary Table 1). Of these, 6 (17.6%) required hospitalization. All 34 patients had detectable SARS-CoV-2 IgG detectable at baseline.
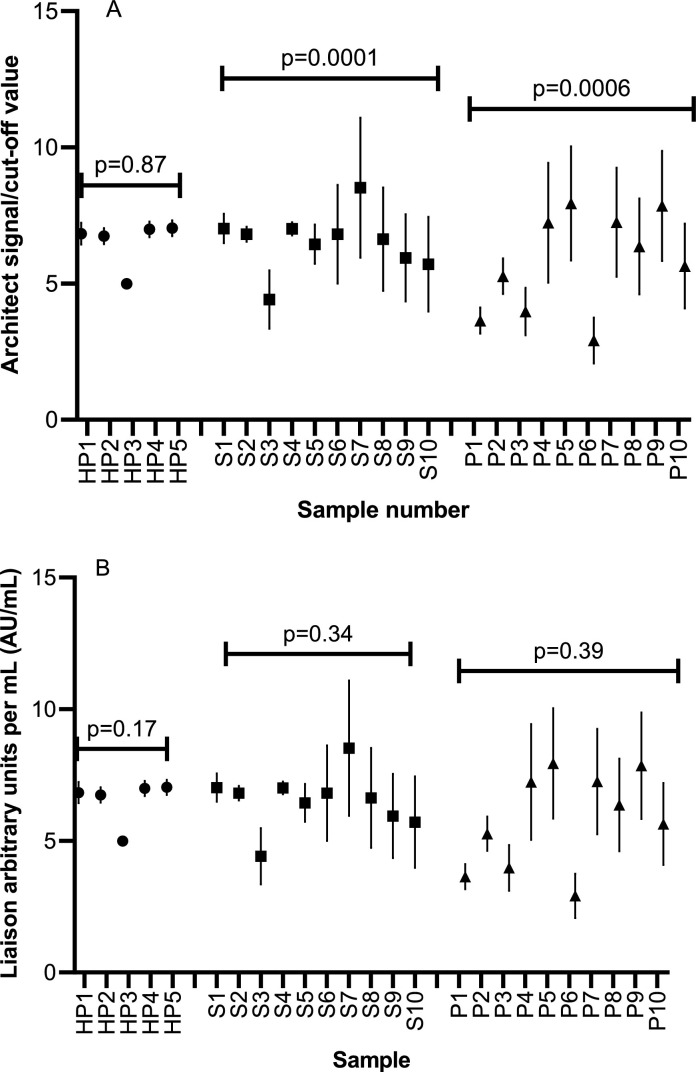
Ten serum, ten plasma, and five heparinized-plasma specimens (total 25 specimens) from the 34 patients underwent repeated daily FTCs (storage at −70°C) over 10 days. Due to an instrument error, five serum and seven plasma specimens could not undergo testing on days 9 and 10 on the Liaison assay. Thus, samples were run on both assays for an additional two days (days 11 and 12). Samples from all specimen types were positive for SARS-CoV-2 IgG with 100% concordance between the Architect and Liaison assays (Supplementary Table 2). For repeated freeze-thaw runs, the assay index values for heparinized plasma did not differ significantly for the Architect (p = 0.87) and Liaison assays (p = 0.17) (Fig. 1 ). Similarly, no significant difference was seen for serum and plasma samples run on the Liaison. However, comparison of index values for serum and plasma samples run on the Architect assay showed significant differences (p = 0.0001 and p = 0.0006, respectively) (Fig. 1). Despite these significant differences in the index values however, the qualitative results remained consistent with the assay being able to detect antibody with repeated freeze-thaws, which is normally how the assay would be reported (positive/negative) (Table 1; Supplementary Table 2). Post-hoc testing demonstrated higher index values of the Architect on day 10 for serum (standard deviation of 3.1 vs standard deviation range of 0.9–2.2 on the other days) and plasma (standard deviation of 4.2 vs standard deviation range of 1.4–2.3 on the other days).
Fig. 1.
Comparison of mean quantitative index values from Architect (1A) and Liaison (1B) assays after repeated freeze-thaws for heparinized-plasma (HP), serum (S), and plasma (P) samples. Each mean value represents daily runs over twelve consecutive days.
Due to limited volume, three samples in the serum group, ten in the plasma group, and three in the heparinized-plasma group consisted of pooled specimens (representing samples from all 34 patients). All samples were positive for SARS-CoV-2 IgG prior to pooling and all specimens showed 100% concordance by assay and storage temperature (Supplementary Table 3). Index value comparison for each sample type across the temperatures and sample sets between temperatures on both assays revealed no significant differences (Supplementary Fig. S1 and Fig. S2) (p>0.05 for all comparisons).
Positive and negative instrument controls (for the Architect and Liaison) gave expected qualitative results for each run (for repeated freeze-thaws and temperature storage). Quantitative index values (signal/cut-off or arbitrary units/mL) were not available for the controls.
Testing of PRNT-positive CBS plasma samples
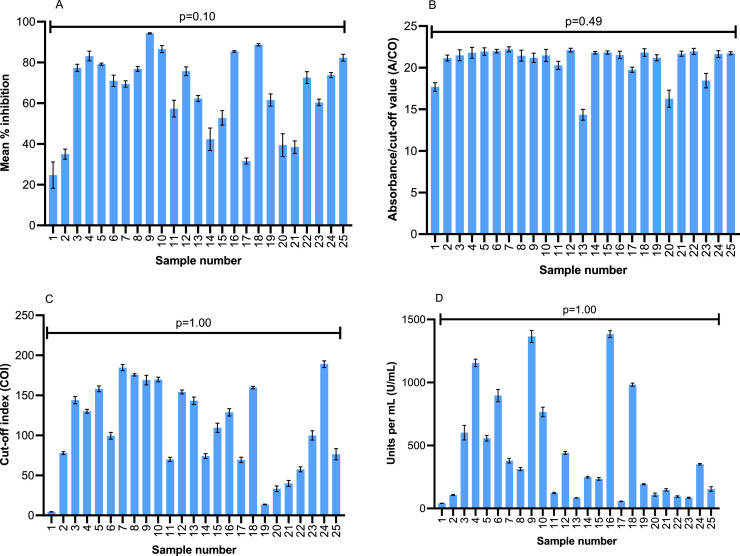
All assays at the NML were able to consistently detect SARS-CoV-2 antibodies across 12 FTCs (Supplementary Table 4). The effects of multiple FTCs on 25 plasma specimens were negligible based on the results of the Elecsys Anti-SARS-CoV-2, Elecsys Anti-SARS-CoV-2 S, and the Wantai SARS-CoV-2 Ab manual ELISA, where all samples were strongly positive for total antibody. The GenScript c-Pass™ assay was able to detect antibodies on most samples with 1, 6, and 12 freeze-thaw cycles with the exception of Sample #1 that had a low PRNT titer of 1:40. Little variability was observed per sample in numerical outputs generated across the 12 freeze-thaw cycles (Fig. 2 ; all p-values >0.05 for the four assays). All but one sample was also positive for IgG antibody using the SD Biosensor Standard Q IgG/IgM Combo rapid test cassettes; however, there was considerable variability in the detection of IgM antibody in this assay (Supplementary Table 5). The AllTest rapid cassette assay, which detects only IgG antibodies, was positive on 100% of the samples, regardless of the number of freeze-thaw cycles.
Fig. 2.
Comparison of mean index values of plasma samples from Canadian Blood Services cohort run on GenScript C-Pass ELISA (2A)a; Wantai SARS-CoV-2 Ab ELISA (2B)b; Elecsys Anti-SARS-CoV-2 (NP) (2C)b; and the Elecsys Anti-SARS-CoV-2 S (RBD) (2D)b assays.c Error bars represent standard deviation of multiple runs for each sample. Abbreviations: NP – nucleoprotein; RBD – receptor-binding domain; S – spike. aBased-on values at 1, 6 and 12 freeze-thaw cycles. bBased on values at all 12 freeze-thaw cycles. cSee Table 1 for details of assays used.
The coefficient of variation (CoV) of index values over the FTCs was found to be >10% for plasma and serum samples run on the Architect as well as all three specimen types (heparinized-plasma, serum, and plasma) on the Liaison (Supplementary Table 6). The index value CoVs were found to be <5% for all testing carried out using the GenScript, Wantai, Elecsys NP, and Elecsys S assays (Table 1) on the CBS cohort samples.
Discussion
Detection of SARS-CoV-2 antibodies across four commercial high throughput assays with different epitope targets, two rapid test cassettes, a manual ELISA, and a surrogate neutralization assay was shown to be robust and stable in common blood specimen types for up to 12 FTCs and varied storage temperatures.
Few studies have examined the utility of heparinized-plasma [2], with most large-scale seroprevalence studies focusing on serum or plasma samples [9, 10]. One study showed a difference in concordance of negative results between serum and heparinized-plasma sample types using Euroimmun, Roche, and Epitope Diagnostics assays [2]. Using the Abbott and DiaSorin assays, we found results from heparinized-plasma to be in concordance with serum and plasma samples. Use of heparinized-plasma as an additional sample type is advantageous in expanding the sample types large-scale seroprevalence studies can access.
We could not find other studies evaluating stability of SARS-CoV-2 IgG in recovered patients across multiple serology assay types. Studies using multiple FTCs have demonstrated stability for other IgG markers including hepatitis C IgG (5 cycles) [11], herpes simplex/varicella IgG (12 cycles) [3], HIV (15 cycles) [12], influenza (14 cycles) [13], and measles/mumps/rubella (10 cycles) [5]. There are presently no guidelines outlining optimal storage conditions for SARS-CoV-2 IgG. Based on our findings, it is likely that long-term storage (at −70 or −20 °C is optimal with short term storage at 4 °C; although sample storage at 25 °C does not appear to impact antibody stability, storage at this temperature is only recommended when other options do not exist).
Comparison of serum and plasma samples for the Alberta group of patients found a significant difference across assay index values over twelve days of FTCs on the Architect SARS-CoV-2 IgG assay. The cause of the higher S/C values in these two sample types is not clear, but suspected due to a potential blip in assay performance around day ten of testing upon post-hoc analysis. The CoV of both these specimen types was approximately 4–5-fold higher than that compared to heparinized-plasma (19.35–24.18 versus 4.33). While the Liaison demonstrated CoV values >10%, this was observed all three specimen types. Both assays used (Architect and Liaison) are meant however, to be reported out only qualitatively, which was not affected by the changes in index values seen over FTCs, as a SARS-CoV-2 IgG was consistently detected for all FTC runs.
A recent publication raised concern that antibody detection by the Architect SARS-CoV-2 IgG assay may decline ≥100 post-DSO [14]. Interestingly, 9/59 (15.3%) of our samples were drawn ≥100 days post-DSO (max range 172 days; Table 2). In each case, SARS-CoV-2 IgG was positive in all specimen types. While we did not observe antibody waning in our post-100-day samples, it is an important observation which is likely related to individual assay performance rather than storage or freeze-thaw conditions.
The Alberta portion of the study was limited by the use of pooled patient samples in some instances. It is possible that IgG from a single specimen in the pool may have deteriorated, yet the overall IgG result remained positive. A major strength of this study is the demonstration of consistent SARS-CoV-2 antibodies across numerous freeze-thaw cycles on multiple assays and labs independently. Furthermore, all convalescent sera (from the Alberta cohort of patients) were collected ≥21 days post-DSO, which has been shown to help optimize detection of a serologic response.
In summary, SARS-CoV-2 IgG can be detected reliably from serum, plasma, and heparinized-plasma. Antibody remains stable and detectable using several qualitative commercial COVID-19 serologic assays for at least 10–12 freeze-thaw cycles across 12 days at −70 °C, −20 °C, 4 °C, or room temperature; and two quantitative assays at −20 °C. Ongoing evaluation to assess the stability of antibodies over more prolonged periods of time is important, and may play a role in other SARS-CoV-2 immunologic studies including the evaluation of vaccine-induced antibodies.
Funding
This study was investigator-initiated and required no funding support. This work did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Research ethics approval
The Alberta component of this study was approved by the Human Research Ethics Board at the University of Alberta (study identifier Pro00099818); while the NML study protocol was approved by the Public Health Agency of Canada research ethics board (REB 2020–004P).
Author credit statement
Jamil N Kanji – Conceptualization, resources, data curation, writing – original draft, writing – review and editing.
Ashley Bailey – Investigation, validation, data curation, writing – review and editing.
Jayne Fenton – Investigation, validation, writing – review and editing
L. Robbin Lindsay – Conceptualization, methodology, resources, investigation, validation, data curation, writing – review and editing.
Antonia Dibernardo – Conceptualization, methodology, Investigation, validation, data curation, writing – review and editing.
Niki Toledo – Methodology, investigation, data curation, writing - review and editing
Brooks Waitt – Investigation, data curation, writing – review and editing
Nadine Lecocq – Investigation, data curation, review and editing
Carla Osiowy - Conceptualization, methodology, resources, investigation, validation, data curation, writing – review and editing.
Elizabeth Giles - Investigation, data curation, review and editing.
Jacqueline Day - Investigation, data curation, review and editing.
William Stokes - Validation, data curation, formal analysis, writing – review and editing.
Clayton MacDonald - Investigation, validation, data curation, writing – review and editing.
LeeAnn Turnbull - Investigation, writing – review and editing.
Carmen Charlton - Conceptualization, methodology, validation, formal analysis, project administration, resources, writing – original draft, writing – review and editing.
Declaration of Competing Interest
None of the authors declare any competing interests with regards to this publication. All authors have completed an ICMJE form. The authors affirm that this manuscript is an honest, accurate, and transparent account of the study being reported. No important aspects of this study have been omitted. Any discrepancies from this study as originally planned have been fully explained.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jcv.2021.104933.
Appendix. Supplementary materials
References
- 1.United States Food and Drug Administration. In Vitro Diagnostics EUAs. 2020.
- 2.Haselmann V., Kittel M., Gerhards C., Thiaucourt M., Eichner R., Costina V., et al. Comparison of test performance of commercial anti-SARS-CoV-2 immunoassays in serum and plasma samples. Clin. Chim. Acta. 2020;510:73–78. doi: 10.1016/j.cca.2020.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hart J., Miller C., Tang X., Vafai A. Stability of varicella-zoster virus and herpes simplex virus IgG monoclonal antibodies. J. Immunoassay Immunochem. 2009;30:180–185. doi: 10.1080/15321810902782871. [DOI] [PubMed] [Google Scholar]
- 4.Wong S.J., Seligman S.J. Long-term stability of West Nile virus IgM and IgG antibodies in diluted sera stored at 4 °C. Ann. N Y Acad. Sci. 2001;951:369–372. doi: 10.1111/j.1749-6632.2001.tb02721.x. [DOI] [PubMed] [Google Scholar]
- 5.Pinsky N.A., Huddleston J.M., Jacobson R.M., Wollan P.C., Poland G.A. Effect of multiple freeze-thaw cycles on detection of measles, mumps, and rubella virus antibodies. Clin. Diagn. Lab. Immunol. 2003;10:19–21. doi: 10.1128/CDLI.10.1.19-21.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pabbaraju K., Wong A.A., Douesnard M., Ma R., Gill K., Dieu P., et al. Development and validation of RT-PCR assays for testing for SARS-CoV-2. Off. J. Assoc. Med. Microbiol. Inf. Dis. Canada. 2021;0 doi: 10.3138/jammi-2020-0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roche Diagnostics. cobas(R) SARS-CoV-2 Test. 2020.
- 8.Drews S.J., Devine D.V., McManus J., Mendoza E., Manguiat K., Wood H., et al. A trend of dropping anti-SARS-CoV-2 plaque reduction neutralization test titers over time in Canadian convalescent plasma donors. Transfusion. 2021;61:1440–1446. doi: 10.1111/trf.16364. [DOI] [PubMed] [Google Scholar]
- 9.Phipps W.S., SoRelle J.A., Li Q.-.Z., Mahimainathan L., Araj E., Markantonis J., et al. SARS-CoV-2 antibody responses do not predict COVID-19 disease severity. Am. J. Clin. Pathol. 2020;154:459–465. doi: 10.1093/ajcp/aqaa123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anand S., Montez-Rath M., Han J., Bozeman J., Kerschmann R., Beyer P., et al. Prevalence of SARS-CoV-2 antibodies in a large nationwide sample of patients on dialysis in the USA: a cross-sectional study. Lancet North Am. Ed. 2020;396:1335–1344. doi: 10.1016/S0140-6736(20)32009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Filomena A., Göpfert J.C., Duffy D., Pol S., Abdel-Hamid M., Esmat G., et al. Study of the humoral immune response towards HCV genotype 4 using a bead-based multiplex serological assay. High Throughput. 2017;6 doi: 10.3390/ht6040015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laeyendecker O., Latimore A., Eshleman S.H., Summerton J., Oliver A.E., Gamiel J., et al. The effect of sample handling on cross sectional HIV incidence testing results. PLoS ONE. 2011;6:e25899. doi: 10.1371/journal.pone.0025899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Torelli A., Gianchecchi E., Monti M., Piu P., Barneschi I., Bonifazi C., et al. Effect of repeated freeze–thaw cycles on influenza virus antibodies. Vaccines (Basel) 2021;9:267. doi: 10.3390/vaccines9030267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muecksch F., Wise H., Batchelor B., Squires M., Semple E., Richardson C., et al. Longitudinal analysis of serology and neutralizing antibody levels in COVID19 convalescents. J. Infect. Dis. 2020 doi: 10.1093/infdis/jiaa659. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.