Abstract
Objective
To validate and implement an optimized screening method for the detection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) RNA combining use of self-collected raw saliva samples, single-step heat-treated virus inactivation and RNA extraction, and direct RT-qPCR.
Methods
This was a three-phase study conducted in Barcelona (Spain) during June to October, 2020. The three phases were (1) analytical validation against standard RT-qPCR in saliva samples; (2) diagnostic validation against standard RT-qPCR using paired saliva–nasopharyngeal samples obtained from asymptomatic teenagers and adults in a sports academy; and (3) pilot screening of asymptomatic health workers in a tertiary hospital.
Results
In phase 1, the detection yield of the new method was comparable to that of standard RT-qPCR. In phase 2, the diagnostic sensitivity and specificity values in 303 self-collected saliva samples were 95.7% (95% confidence interval 79.0–99.2%) and 100.0% (95% confidence interval 98.6–100.0%), respectively. In phase 3, only 17 (0.6%) of the saliva samples self-collected by 2709 participants without supervision were invalid. The rapid analytical workflow with the new method (up to 384 batched samples could be processed in less than 2 hours) yielded 24 (0.9%) positive results in the remaining 2692 saliva samples. Paired nasopharyngeal specimens were all positive by standard RT-qPCR.
Conclusions
Direct RT-qPCR on self-collected raw saliva is a simple, rapid, and accurate method with potential to be scaled up for enhanced SARS-CoV-2 community-wide screening.
KEYWORDS: SARS-CoV-2, COVID-19, PCR, Saliva, Screening, Surveillance
1. Introduction
The burden and health, educational, and economic implications of the coronavirus disease 2019 (COVID-19) pandemic have underlined an urgent need for rapid and accurate diagnostics for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (ECDC, 2020; CDC, 2021). Early identification of SARS-CoV-2 is challenging, since a proportion of infected individuals can show few or no symptoms for an indeterminate period of time (Li et al., 2020; Furukawa et al., 2020). Noticeable rates of asymptomatic and pre-symptomatic infection ranging from 3% to 67% have been reported previously (Buitrago-Garcia et al., 2020). Asymptomatic or pre-symptomatic individuals are nevertheless likely to be infectious (Bai et al., 2020; Tindale et al., 2020).
Upper respiratory tract (URT) samples are the specimens currently recommended for the diagnosis of COVID-19 (WHO, 2021). Reverse transcription real-time PCR (RT-qPCR) is the preferred method for the detection of SARS-CoV-2, given its high sensitivity and specificity (Tang et al., 2020). The accuracy of RT-qPCR may vary depending on URT sample quality and the time elapsed since virus acquisition (Kucirka et al., 2020). Standard RT-qPCR protocols for SARS-CoV-2 typically follow three sequential phases: (1) URT sample swabbing and sample transportation in viral inactivation transport medium (VITM) to the laboratory for analysis or, alternatively, sample transportation in viral transport medium (VTM) and inactivation in the laboratory; (2) RNA extraction, purification, and concentration with the use of targeted reagents and automated robots; and (3) viral RNA amplification and detection in thermal cyclers. RNA extraction, purification, and concentration are slow and cumbersome activities that take from 40 minutes to 3 hours, depending on the type of RNA extraction robot utilized and the number of samples batched together. During the first pandemic wave, a shortage of personal protective equipment (PPE), swabs, VITM, and RT-PCR reagent supplies created serious bottlenecks in the diagnostic workflow of clinical and epidemiological surveillance laboratories (Ranney et al., 2020).
Saliva appears to be a promising URT specimen type for screening, diagnosis, follow-up, and infection control of SARS-CoV-2. Diverse studies have reported consistent detection of SARS-CoV-2 RNA in the saliva of symptomatic COVID-19 patients and sensitivities of saliva-based RT-qPCR ranging from 84% to100% compared to paired positive nasopharyngeal (NP) samples (To et al., 2020; Procop et al., 2020; Pasomsub et al., 2020). While the collection of NP or oropharyngeal samples is inconvenient for patients and exposes healthcare workers to an infection risk, saliva specimens can be repeatedly collected or self-collected in a simple, safe, and inexpensive manner without specific training or the use of PPE. In addition, good saliva stability at room temperature can simplify sample transportation, avoiding the maintenance of cold-chain conditions (Ott et al., 2020). Recently, the US Food and Drug Administration granted accelerated emergency use authorization for the use of saliva, in addition to other respiratory specimen types, to facilitate mass screening for SARS-CoV-2 (US FDA, 2020). However, evidence on the implementation of saliva-based screening approaches to identify asymptomatic subjects is scarce.
We have developed a novel screening method for SARS-CoV-2 that combines the use of self-collected raw saliva samples, heat-treated virus inactivation, and RNA extraction in a single step, and RT-qPCR, herein referred to as direct RT-qPCR. This simple, safe, and rapid method circumvents the use of collection swabs, VITM, and RNA extraction reagents, as well as RNA purification and concentration steps, allows the utilization of different commercial RT-qPCR kits, and minimizes dependence on the supply chain of reagents and consumables. The objective of this study was to validate and implement direct RT-qPCR on self-collected saliva for first-line screening of SARS-CoV-2 infection.
2. Methods
2.1. Study design and participants
The study was conducted in three successive phases, in the Molecular Microbiology Department of Sant Joan de Déu Hospital (SJDH), a university reference maternal and child health medical centre located in Barcelona (Spain).
2.2. Phase 1: analytical validation
The SARS-CoV-2 RNA detection yield was assessed in saliva samples by direct RT-qPCR and a standard RT-qPCR protocol.
Samples required for analytical validation were voluntarily provided by healthy adult researchers involved in the study or obtained from the Biobank of SJDH, a research biorepository integrated into the Spanish Biobank Network of Instituto de Salud Carlos III. Positive saliva samples with known cycle threshold (Ct) values were used to produce quantified standard concentrations of SARS-CoV-2 RNA load for testing by direct and standard RT-qPCR. Additionally, volumes of 90 μl of SARS-CoV-2 RNA-negative saliva samples were spiked with 10 μl of positive NP samples to increase the range of standard concentrations available for analytical validation.
The direct RT-qPCR workflow involved saliva incubation in a block heater for 15 minutes at 96°C to maximize virus inactivation and RNA extraction. RNA amplification was performed using two RT-qPCR kits (GeneFinder COVID-19 Plus RealAmp kit, Elitech, France; TaqPath COVID-19 RT-PCR kit, Thermo Fisher, USA) and two thermal cycler platforms (Applied Biosystems QuantStudio 7 and Applied Biosystems Prism 7500, Thermo Fisher, USA).
The standard RT-qPCR workflow included viral chemical inactivation and RNA extraction, purification, and concentration using the NucliSense easyMAG platform and reagents (bioMérieux, The Netherlands) or viral inactivation with 2 ml of sample preservation solution (Mole BioScience, China) and RNA extraction, purification, and concentration using an aliquot robot (Microlab STAR M, Hamilton Robotics, USA) and reagents (MagMAX Viral/Pathogen Nucleic Acid Isolation kit, Thermo Fisher, USA). RNA amplification was performed following the same procedure as for direct RT-qPCR.
A set of saliva specimens including one sample with a high SARS-CoV-2 RNA load, one sample with a low RNA load, one negative sample, and a negative control (water) were tested in triplicate in the same run to assess intra-assay precision. Three sets of saliva specimens including each of one SARS-CoV-2 high positive sample, one low positive sample, one negative sample, and a negative control were tested in different runs on different days to evaluate inter-assay precision.
The SARS-CoV-2 RNA detection yield by direct RT-qPCR was determined for different conditions of saliva storage: at room temperature for a maximum period of 24 hours, refrigerated at 4°C for 24 hours, or frozen at −80°C for longer than 24 hours.
2.3. Phase 2: diagnostic validation
The diagnostic validation was conducted using samples collected prospectively from participants in the ongoing “Kids Corona Study” of SARS-CoV-2 transmission at Football Club Barcelona Academy “La Masia”, run by SJDH. In brief, the Kids Corona Study entails the self-collection of saliva by teen and young adult soccer, basketball, handball, futsal, and roller hockey players, as well as adult accompanying coaches, teachers, physiotherapists, and staff residing at or attending the Football Club Barcelona Academy “La Masia” (Barcelona, Spain). A team of SJDH research nurses supervised the saliva self-collection by participants on site and simultaneously collected paired NP swabs from them for comparative testing.
Inclusion criteria for the diagnostic validation process were participant recruitment during August 2020 and follow-up for at least 9 weeks. Collected saliva and NP samples were transferred to sterile Eppendorf tubes (0.5 ml) and NP VITM tubes, respectively, and then labelled and transported by the nurses at ambient temperature to the SJDH Biobank for storage or to the SJDH Molecular Microbiology Department (NP samples) for standard RT-qPCR. Saliva was self-collected at baseline and on a weekly basis, whereas NP samples were collected at baseline and every second week. Serum-based enzyme-linked immunoassays (ELISA) were also performed at baseline. All baseline saliva, NP, and serum samples were tested at study start and any saliva and NP samples paired with ELISA-positive specimens were excluded from the validation. In the case of a positive RT-qPCR result in a NP sample, both the paired biobanked saliva sample collected at the same time point and the series of saliva samples obtained previously from the same participant were retrieved and analysed retrospectively by direct RT-qPCR using the GeneFinder COVID-19 Plus RealAmp kit. Results by any RT-PCR method were interpreted as positive if at least two target genes of SARS-CoV-2 were detected and the amplification curves were adequate; results were considered inconclusive if either only one gene was detected or the amplification curves were unusual.
2.4. Phase 3: pilot screening programme
Once validated, saliva-based direct RT-qPCR was deployed in SJDH to screen volunteer health workers and other staff. Planned outcomes were the rate of participation (as a proxy for pilot acceptance), identification of positive cases for the prevention of COVID-19 nosocomial outbreaks in the setting, and rates of inhibition due to unsupervised saliva self-collection by end-users.
Instructions were disseminated to participants so that they could collect their own saliva in an unsupervised but safe manner. Participants were recommended to collect their own saliva in the first morning hours or after a fasting period of 2 hours to avoid food remains, according to recent evidence (Hung et al., 2020). They were instructed to spit their saliva into tube collectors, transfer the samples to sterile Eppendorf tubes with disposable Pasteur pipettes, close the tubes with screw caps, decontaminate the external surfaces of the tubes with a hydroalcoholic solution, and identify them with heat-resistant barcode labels before delivery to the SJDH Molecular Microbiology Department. All of the information about the appropriate pre-analytical procedure was gathered in an explanatory video and a brochure. This training material was made accessible online to the participants through the SJDH intranet web site.
Eppendorf tubes received in the laboratory were not opened until the virus had been inactivated with heat, for safety reasons. A high productivity system was put into service for a rapid screening workflow utilizing an aliquot robot (Microlab STAR M, Hamilton Robotics, USA) and a thermal cycler (QuantStudio 7, Thermo Fisher, USA). Up to 384 batched RNA extracts, positive controls, and negative controls were dispensed by the aliquot robot onto the PCR plate of the thermal cycler for the performance of the direct RT-qPCR reaction with the TaqPath COVID-19 RT-PCR kit reagents. This process workflow can be completed in less than 2 hours. In the case of a positive detection of SARS-CoV-2 RNA in a saliva sample, a paired nasopharyngeal sample was obtained from the infected individual and a confirmatory standard RT-qPCR was performed within 24 hours.
2.5. Statistical analysis
SARS-CoV-2 detection yields in saliva by direct and standard RT-qPCR, measured in cycle threshold (Ct) values, were compared using the Student t-test or the Mann–Whitney U-test. Ct values obtained for the SARS-CoV-2 genes targeted by the two commercial RT-PCR kits across samples were summarized as the mean and standard deviation or median and interquartile range (IQR) values. Differences between Ct values obtained for SARS-CoV-2 targeted genes in different replicates and runs were analysed to assess precision and the effect of saliva storage conditions. Diagnostic sensitivity and specificity values were determined as reported elsewhere (Altman and Bland, 1994). Statistical significance was set at a P-value of <0.05 and confidence intervals (CI) at the 95% level. All statistical analyses were performed using Stata v.15 software (Stata Corp., Texas, USA).
2.6. Ethics statement
The study was approved by the Ethics Committee of SJDH prior to the beginning of activities (Ref. PIC-240-20). Use of the samples collected from the participants in the “Kids Corona Study” of SARS-CoV-2 transmission at Football Club Barcelona Academy “La Masia” for the present study and future studies was covered in the informed consent process and approval of that study (Ref. PIC-200-20).
3. Results
3.1. Phase 1: analytical validation
A non-significantly higher median SARS-CoV-2 Ct value was obtained in saliva using GeneFinder amplification reagents for direct RT-qPCR (28.3, IQR 26.2–30.2) when compared to standard RT-qPCR (26.6, IQR 24.3–28.4) (P = 0.14). The median Ct values yielded by direct RT-qPCR when utilizing TaqPath reagents were lower for direct RT-qPCR (23.0, IQR 19.7–24.5) than for standard RT-qPCR (23.7, IQR 22.1–26.8), but not significantly (P = 0.33). A significant difference was observed in median Ct values yielded by direct RT-qPCR depending on the commercial reagents used (GeneFinder, 28.3; TaqPath, 23.0; P < 0.01). The difference in median Ct values for standard RT-qPCR did not vary significantly by commercial kit (GeneFinder, 26.6; TaqPath, 23.7; P = 0.19) (Table 1 ). Saliva-based direct RT-qPCR showed differences in Ct value in a range of −0.99 to 2.84 within a run of replicates (Table 2 ) and in a range of −5.57 to 4.28 between different runs (Table 3 ).
Table 1.
Cycle threshold values of direct RT-qPCR and standard RT-qPCR in saliva
| GeneFinder COVID-19 Plus RealAmp kit |
TaqPath COVID-19 RT-PCR kit |
|||||||
|---|---|---|---|---|---|---|---|---|
| SARS-CoV-2 gene | Direct RT-qPCR (A) | Standard RT-qPCRa (B) | Difference (A − B) | SARS-CoV-2 gene | Direct RT-qPCR (C) | Standard RT-qPCRb (D) | Difference (C − D) | |
| Sample 1 | IC (RnasaP) | 29.30 | 26.63 | 2.67 | IC (MS2) | - | 32.57 | - |
| E | 20.25 | 15.09 | 5.16 | S | 11.62 | 11.99 | −0.37 | |
| N | 18.22 | 15.23 | 2.99 | N | 13.36 | 11.52 | 1.84 | |
| R | 20.35 | 15.58 | 4.77 | ORF1ab (R) | 11.23 | 11.10 | 0.13 | |
| Sample 2 | IC (RnasaP) | 28.73 | 25.94 | 2.79 | IC (MS2) | 23.95 | 24.09 | −0.14 |
| E | 30.15 | 26.56 | 3.59 | S | 23.04 | 23.74 | −0.70 | |
| N | 28.28 | 26.21 | 2.07 | N | 24.73 | 24.83 | −0.10 | |
| R | 29.09 | 26.83 | 2.26 | ORF1ab (R) | 22.71 | 23.02 | −0.31 | |
| Sample 3 | IC (RnasaP) | 27.97 | 25.35 | 2.62 | IC (MS2) | 28.44 | 24.01 | 4.43 |
| E | 27.20 | 24.27 | 2.93 | S | 22.46 | 22.05 | 0.41 | |
| N | 26.15 | 24.25 | 1.90 | N | 23.67 | 23.53 | 0.14 | |
| R | 26.60 | 24.73 | 1.87 | ORF1ab (R) | 23.75 | 22.24 | 1.51 | |
| Sample 4 | IC (RnasaP) | 29.53 | 25.81 | 3.72 | IC (MS2) | 34.70 | 23.70 | 11.00 |
| E | 28.71 | 27.58 | 1.13 | S | 19.74 | 26.30 | −6.56 | |
| N | 27.82 | 27.18 | 0.64 | N | 23.59 | 26.75 | −3.16 | |
| R | 28.33 | 28.39 | −0.06 | ORF1ab (R) | 22.17 | 25.60 | −3.43 | |
| Sample 5 | IC (RnasaP) | 26.87 | 24.76 | 2.11 | IC (MS2) | 27.67 | 23.71 | 3.96 |
| E | 41.24 | 31.32 | 9.92 | S | 24.54 | 29.97 | −5.43 | |
| N | 34.26 | 29.18 | 5.08 | N | 28.15 | 30.01 | −1.86 | |
| R | 36.05 | 31.00 | 5.05 | ORF1ab (R) | 26.21 | 29.28 | −3.07 | |
SARS-CoV-2 RNA inactivation, extraction, and amplification by NucliSense easyMAG reagents and platform.
SARS-CoV-2 RNA preservation by Mole Bioscience, RNA extraction by MagMAX Viral/Pathogen Nucleic Acid Isolation and Microlab STAR M platform, and RNA amplification by TaqPath COVID-19 reagents and Thermo Fisher thermal cycler.
Table 2.
Cycle threshold values of direct RT-qPCR in saliva replicates
| Direct RT-qPCR resulta,b | SARS-CoV-2 gene | Replicate 1 (A) | Replicate 2 (B) | Replicate 3 (C) | Difference (A − B) | Difference (A − C) | Difference (B − C) |
|---|---|---|---|---|---|---|---|
| Negative | IC (RnasaP) | 23.40 | 23.35 | 23.40 | 0.05 | 0.00 | −0.05 |
| E | - | - | - | - | - | - | |
| N | - | - | - | - | - | - | |
| R | - | - | - | - | - | - | |
| Low positive | IC (RnasaP) | 23.44 | 23.45 | 23.40 | −0.01 | 0.04 | 0.05 |
| E | 37.93 | 37.10 | 35.09 | 0.83 | 2.84 | 2.01 | |
| N | 31.84 | 32.83 | 31.58 | −0.99 | 0.26 | 1.25 | |
| R | 32.23 | 32.89 | 32.03 | −0.66 | 0.20 | 0.86 | |
| High positive | IC (RnasaP) | 23.21 | 23.31 | 23.29 | −0.10 | −0.08 | 0.02 |
| E | 23.36 | 23.31 | 23.67 | 0.05 | −0.31 | −0.36 | |
| N | 23.01 | 22.91 | 23.23 | 0.10 | −0.22 | −0.32 | |
| R | 23.77 | 23.58 | 24.01 | 0.19 | −0.24 | −0.43 |
Direct-qPCR was performed after heat treatment using GeneFinder COVID-19 Plus RealAmp reagents and Thermo Fisher thermal cycler.
The three replicates yielded negative results for the negative controls.
Table 3.
Cycle threshold values of direct RT-qPCR in saliva runs
| Direct RT-qPCR resulta,b | SARS-CoV-2 gene | Run 1 (A) | Run 2 (B) | Run 3 (C) | Difference (A − B) | Difference (A − C) | Difference (B − C) |
|---|---|---|---|---|---|---|---|
| Negative | IC (RnasaP) | 23.40 | 24.73 | 23.44 | −1.33 | −0.04 | 1.29 |
| E | - | - | - | - | - | - | |
| N | - | - | - | - | - | - | |
| R | - | - | - | - | - | - | |
| Low positive | IC (RnasaP) | 23.44 | 24.89 | 24.58 | −1.45 | −1.14 | 0.31 |
| E | 37.93 | - | - | - | - | - | |
| N | 31.84 | 32.99 | 31.92 | −1.15 | −0.08 | 1.07 | |
| R | 32.23 | 33.78 | 33.41 | −1.55 | −1.18 | 0.37 | |
| High positive | IC (RnasaP) | 23.21 | 24.82 | 23.35 | −1.61 | −0.14 | 1.47 |
| E | 23.36 | 25.09 | 26.61 | −1.73 | −3.25 | −1.52 | |
| N | 23.01 | 25.45 | 24.27 | −2.44 | −1.26 | 1.18 | |
| R | 23.77 | 29.34 | 25.06 | −5.57 | −1.29 | 4.28 |
Direct-qPCR was performed after heat treatment using GeneFinder COVID-19 Plus RealAmp reagents and Thermo Fisher thermal cycler.
The three replicates yielded negative results for the negative control.
Minor differences were found between Ct values using GeneFinder SARS-CoV-2 gene targets for samples stored at room temperature for 24 hours compared to preserved in the refrigerator for 24 hours (range −1.11 to 0.78) or frozen at −80°C (range −0.41 to 0.76). Ct value differences using the TaqPath kit were more noticeable (24-hour room temperature vs 24-hour refrigerator preservation, range −2.59 to 3.90; 24-hour room temperature vs freezing at −80°C, range −2.37 to 1.06) (Table 4 ).
Table 4.
Cycle threshold values of direct RT-qPCR in saliva according to storage conditions
| GeneFinder COVID-19 Plus RealAmp kit |
TaqPath COVID-19 RT-PCR kit |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SARS-CoV-2 gene | Room temperature, 24 hours (A) | Refrigeration at 4°C, 24 hours (B) | Freezing at −80°C, >24 hours (C) | Difference (A − B) | Difference (A − C) | Difference (B − C) | SARS-CoV-2 gene | Room temperature, 24 hours (A) | Refrigeration at 4°C, 24 hours (B) | Freezing at −80°C, >24 hours (C) | Difference (A − B) | Difference (A − C) | Difference (B − C) |
| IC (RnasaP) | 29.70 | 31.65 | 31.68 | −1.95 | −1.98 | −0.03 | IC (MS2)a | - | - | - | - | - | - |
| E | 20.25 | 19.57 | 20.44 | 0.68 | −0.19 | −0.87 | S | 11.62 | 13.67 | 13.21 | −2.05 | −1.59 | 0.46 |
| N | 18.22 | 19.33 | 18.22 | −1.11 | 0.00 | 1.11 | N | 13.36 | 13.66 | 12.9 | −0.30 | 0.46 | 0.76 |
| R | 20.35 | 19.57 | 20.37 | 0.78 | −0.02 | −0.80 | ORF1ab (R) | 11.23 | 13.33 | 13.11 | −2.10 | −1.88 | 0.22 |
| IC (RnasaP) | 28.73 | 28.03 | 27.97 | 0.70 | 0.76 | 0.06 | IC (MS2)a | - | - | - | - | - | - |
| E | 30.15 | 30.50 | 30.56 | −0.35 | −0.41 | −0.06 | S | 23.04 | 24.33 | 24.6 | −1.29 | −1.56 | −0.27 |
| N | 28.28 | 28.54 | 28.51 | −0.26 | −0.23 | 0.03 | N | 24.73 | 25.47 | 25.51 | −0.74 | −0.78 | −0.04 |
| R | 29.09 | 29.31 | 29.33 | −0.22 | −0.24 | −0.02 | ORF1ab (R) | 22.71 | 25.3 | 25.08 | −2.59 | −2.37 | 0.22 |
| IC (RnasaP) | 27.97 | 27.77 | 27.81 | 0.20 | 0.16 | −0.04 | IC (MS2)a | - | - | - | - | - | - |
| E | 27.20 | 27.57 | 27.53 | −0.37 | −0.33 | 0.04 | S | 22.46 | 22.97 | 22.17 | −0.51 | 0.29 | 0.80 |
| N | 26.15 | 26.38 | 26.42 | −0.23 | −0.27 | −0.04 | N | 23.67 | 23.35 | 22.72 | 0.32 | 0.95 | 0.63 |
| R | 26.60 | 26.90 | 26.96 | −0.30 | −0.36 | −0.06 | ORF1ab (R) | 23.75 | 19.85 | 22.69 | 3.90 | 1.06 | −2.84 |
The internal control of the TaqPath COVID-19 RT-PCR kit was not tested because it is included directly in the RT-qPCR reagent mix.
3.2. Phase 2: diagnostic validation
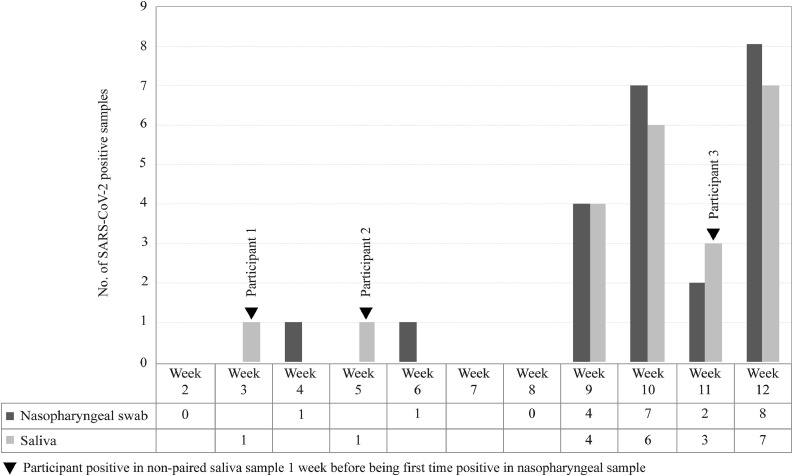
A total of 183 out of 230 participants in the “Kids Corona Study” of SARS-CoV-2 transmission at Barça (185 teens and young adults, 45 older adults) met the inclusion criteria and were followed up from August to October 2020. Ten participants were excluded from the validation process because they were positive for SARS-CoV-2 antibodies by ELISA at baseline. The remaining 173 participants yielded negative results in both paired saliva and NP samples at baseline and were followed up during 9 to 12 weeks. Seven NP samples had inconclusive results by standard RT-qPCR within the follow-up period, including six negatives and one positive in paired saliva, and were excluded from the analysis together with their saliva pairs. A total of 100 paired serial saliva–NP samples were found negative during follow-up, whereas a positive NP sample was detected in 23 participants, in weeks 4 (n = 1), 6 (n = 1), 9 (n = 4), 10 (n = 7), 11 (n = 2), and 12 (n = 8). SARS-CoV-2 positivity was confirmed by direct RT-qPCR in 22 paired saliva samples and one was inconclusive. Of note, viral RNA was detected in the saliva specimens of three participants 1 week earlier than being detected for the first time in NP specimens (Figure 1 ). Sensitivity and specificity values were 95.7% (95% CI 79.0–99.2%) and 100.0% (95% CI 98.6–100.0%), respectively, corresponding to 22 positives detected in saliva among 23 positives detected in paired NP samples and to a total of 273 negatives detected in both saliva and NP sample pairs at baseline and during follow-up (Table 5 ).
Figure 1.
Time distribution of the first positive SARS-CoV-2 result by sample type in the diagnostic validation phase.
Table 5.
Diagnostic accuracy of saliva-based direct RT-PCR versus standard RT-PCR on nasopharyngeal swab
| Saliva-based direct RT-qPCR |
||||
|---|---|---|---|---|
| Positive | Negative | Inconclusive | Total | |
| Standard RT-qPCR in NP | ||||
| Positive | 22 | 0 | 1 | 23 (7.6%) |
| Negative | 0 | 273 | 0 | 273 (90.1%) |
| Inconclusive | 1 | 6 | 0 | 7 (2.3%) |
| Total | 23 (7.6%) | 279 (92.1%) | 1 (0.3%) | 303 (100.0%) |
3.3. Phase 3: pilot screening programme
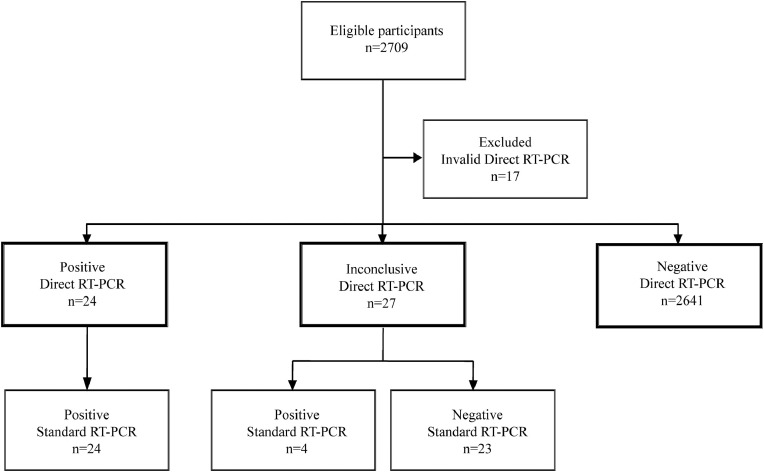
A total of 2709 symptomless participants voluntarily engaged in the SARS-CoV-2 pilot screening programme in SJDH in the second half of October 2020, including 2076 (83.4%) out of 2489 total health workers, 203 students, 23 aid volunteers, and another 407 professionals. Seventeen (0.6%) saliva samples provided by participants yielded invalid results by direct RT-qPCR. Among the remaining 2692 saliva specimens, direct RT-qPCR was positive in 24 (0.9%) and inconclusive in 27 (1.0%). NP swabs were collected from participants with positive or inconclusive saliva results and tested by standard RT-PCR. All 24 (100.0%) participants with saliva-positive results were also found positive by standard RT-qPCR in NP swab. Four (14.8%) out of 27 participants with inconclusive saliva results were positive by RT-qPCR in NP swab and 23 were negative (Figure 2 ).
Figure 2.
Participant flow diagram in the pilot screening phase.
4. Discussion
There is a lack of evidence on the feasibility and usefulness of saliva-based RT-qPCR protocols for early SARS-CoV-2 infection. This study reports the results of validation and subsequent implementation of a direct RT-qPCR method based on end-user self-collection of raw saliva. Despite bypassing the use of VITM and RNA extraction reagents, this method achieved high accuracy for screening asymptomatic individuals. The sensitivity (95.7%) and specificity values (100.0%) validated in a diverse cohort of teenagers and young and older adults without symptoms were comparable to those of standard RT-qPCR protocols that use NP samples for clinical diagnosis. Of note, the only saliva result discrepant from a positive result in the NP sample was inconclusive. Thus direct RT-qPCR in saliva flagged the need for confirmatory testing for the individual with this inconclusive saliva result and fulfilled its screening purpose. Interestingly, three subjects in the validation cohort who were positive in saliva 1 week before giving a positive result in the NP sample were identified. Since subjects were screened in saliva weekly and in the nasopharynx every second week, this finding suggests that serial screening for SARS-CoV-2 should not consider intervals of longer than 1 week between successive tests to be effective.
When the method was implemented for pilot screening of SARS-CoV-2 in a reference hospital, all saliva-positive results (0.9%) agreed with positive results in the paired NP samples. In addition, a few inconclusive results in saliva (1.0%) raised the need for confirmatory testing and uncovered a minor proportion of additional NP positive samples. Overall, these results indicate that the proposed method performs adequately in a real-life scenario for its intended use of screening. It is worth highlighting that no significant usability issues occurred during the pre-analytical phase, as shown by the negligible proportion of invalid results obtained in saliva (0.6%). Moreover, pilot screening gained high participation among health workers at the study site, suggesting their willingness to self-collect and dispense saliva samples according to a simple set of instructions. In operational terms, use of a high productivity system allowed a fast analytical workflow for close surveillance and timely control of potential SARS-CoV-2 nosocomial infection in the setting. We speculate that method implementation may result in savings both in consumables (swabs, PPE, VITM, RNA extraction reagents) and health workforce before the RNA amplification step.
Research on SARS-CoV-2 RNA detection in pre-heated URT specimens other than saliva has been addressed by diverse groups, with a primary focus on the diagnosis of symptomatic patients (Bruce et al., 2020; Alcoba-Florez et al., 2020; Smyrlaki et al., 2020). A preprint study specifically compared the accuracy of direct RT-qPCR on saliva against standard RT-PCR on NP or oropharyngeal swabs of adult patients with SARS-CoV-2 symptoms (Fernández-Pittol et al., 2020). While we observed 95.7% sensitivity and 100% specificity of saliva-based direct RT-qPCR in asymptomatic individuals, the previous group reported sensitivity and specificity values of 90.0% and 87.5%, respectively, in a cohort of adults who had experienced symptom onset within the preceding 9 days. As the protocols of heat treatment were identical in the two studies, our hypothesis is that after SARS-CoV-2 acquisition, the viral load in saliva may decrease progressively over the pre-symptomatic stage, a declining trend that could continue after the onset of symptoms, as already observed in symptomatic patients (Wyllie et al., 2020; Williams et al., 2020).
Few studies have analysed the performance of saliva-based direct RT-qPCR on individuals without SARS-CoV-2 symptoms. In this regard, the results of the present study were consistent with those of a study of 495 asymptomatic health workers tested with a RT-qPCR protocol that included the use of VTM and previous RNA extraction (Wyllie et al., 2020). That study found 13 saliva samples positive, as well as all of their paired NP samples. In contrast, Williams et al., in their study of ambulatory patients attending a screening clinic, reported a lower saliva performance in 33 (84.6%) out of 39 self-collected saliva samples paired with 39 NP-positive samples (Williams et al., 2020). They used a RT-qPCR protocol preceded by RNA extraction from saliva diluted in Amiens medium and did not specify the proportion of asymptomatic infection in recruited outpatients. Similarly, a preprint study determined SARS-CoV-2 RNA positivity rates of 79% in saliva and 85% in NP samples among a group of symptomatic and asymptomatic individuals (approximate ratio 1:1) who tested positive by at least one of various URT samples (Kojima et al., 2020). Their RT-qPCR workflow included saliva collection swabs, VTM use, and RNA extraction. Differences in pre-analytical steps prior to RT-qPCR and in proportions of asymptomatic individuals studied derived from different epidemiological contexts could explain the different performance of saliva-based RT-qPCR reported in our study and in those of William et al. and Kojima et al.
A number of studies available in preprint have addressed the development and clinical validation of saliva-based direct RT-PCR methods for SARS-CoV-2 screening, yet the results of their implementation are unknown. Ranoa et al. developed a method that includes heat inactivation at 65°C for 30 minutes and the use of sample stabilizing buffers (Tris–EDTA and Tris–borate–EDTA) and additives (Tween 20) to enhance detection (Ranoa et al., 2020). This group reported high sensitivity (88.9%) and specificity (98.9%) values in nine paired positive and 91 paired negative saliva–NP samples. Similarly, Vogels et al. developed a protocol that consists of mixing saliva without preservative buffers with a proteinase K before performing heat inactivation at 95°C for 5 minutes and a duplex RT-qPCR (Vogels et al., 2020). High positive (97.1%) as well as negative agreement (100.0%) was found in 37 paired positive and 91 paired negative saliva–NP samples. Comparatively, our optimized method did not require the addition of specific buffers to saliva for optimal performance while maintaining a process workflow as safe and simple as possible.
The main strengths of this study are the diagnostic validation of the proposed method in a diverse cohort of asymptomatic teenagers and young and older adults, as well as extensive method implementation for screening SARS-CoV-2 in a hospital environment. Some limitations for generalization of the results need to be noted. First, the number of samples tested for analytical accuracy was limited. Second, significant differences in Ct values were observed for direct RT-qPCR depending on the use of GeneFinder or TaqPath amplification reagents in the analytical validation process. To be noted, the GeneFinder kit is designed for the performance of 45 amplification cycles, whereas the TaqPath kit entails 40 cycles, and each of them sets different threshold values for a positive result (GeneFinder, 40; TaqPath, 37). Therefore, we were not able to provide insights into the significance of saliva viral load or Ct values obtained from these two commercial reagent kits. Differences in Ct values between kits may suggest limited usefulness of the SARS-CoV-2 RNA load as a potential marker of active and transmissible infection, since the definition of a cut-off value with adequate discriminatory power appears to be highly dependent on the specific reagent used for viral detection. Third, the optimal sensitivity (100%) of the direct RT-qPCR in the pilot screening was determined on a relatively low number of direct saliva positive samples (n = 24). Fourth, adequate performance of direct RT-qPCR on saliva was achieved by engaging participants to collect their samples in the first hours of the morning or after a fasting period of 2 hours. We cannot assume that similar results would be obtained under other sampling conditions. Fasting before self-sampling certainly represents a minor inconvenience, but in our view this inconvenience is clearly outweighed by an improved user experience, as shown during the pilot implementation.
In conclusion, this study showed that a novel direct RT-qPCR on self-collected raw saliva is a simple, safe, and accurate method for first-line screening of SARS-CoV-2. The high throughput pilot implementation proved to be feasible, allowed a fast analytical workflow, and gained high levels of voluntary participation in a sensitive hospital scenario. Self-collection of saliva by end-users had negligible effects on the validity of the results. Evidence generated by this study supports the potential scale-up of self-collected, saliva-based direct RT-qPCR for enhanced community-wide screening of SARS-CoV-2.
Acknowledgments
Acknowledgements
We are indebted to the SJDH Biobank (Cristina Jou and Anna Codina), Occupational Health and Prevention Department (Pilar Subirats and Olga Nadal), Clinical Laboratory Department (Cristina Esteva, Assumpta Fasanella, Manuel Monsonis, and Ana Valls), and the Kids Corona Study Group (Mariona F. de Sevilla, Claudia Fortuny, Maria Mele, Maria Rios, Laia Alsina, Elisenda Bonet, Aleix Garcia-Miguel, Iris Uribesalgo, Cristina Jou, Anna Codina, Maite Miranda, Felipe Perez-Soler, and Marta Cubells) of SJDH. We also thank Dr Jordi Vila from Hospital Clinic Barcelona and Dr Dirk Egging and Dr Adam Meijer from the National Institute for Public Health and the Environment of The Netherlands for sharing their experience of RT-PCR on saliva.
Declarations
Funding: This work was supported by the Kids Corona Project promoted by SJDH, which received donations from the Stavros Niarchos Foundation and Banco de Santander. The funding sources had no role in the writing up of the manuscript or in the decision to submit for publication.
Conflict of interest: CMA reports past grants to her organization from BioMérieux, Roche Diagnostics, Qiagen, BioFire Diagnostics, Alere, and Genomica, outside the submitted work, and personal fees from BioMérieux, Roche Diagnostics, and Qiagen for presentations in satellite symposiums outside the submitted work. PB reports personal fees from Roche Diagnostics for a presentation in a satellite symposium outside the submitted work. The rest of the authors declare no conflicts of interest.
Author contributions
PB and CMA designed the study and wrote the paper. APA and JS performed the experiments and collected data. PB, CL, FT, MPS, VF, EG, IJ, QB, and CMA analysed and interpreted the data. JC, JGG, IJ, and GR managed participant recruitment. CMA supervised the study. All authors discussed the results and critically reviewed, discussed, and accepted the final version of the manuscript. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
References
- Alcoba-Florez J, Gonzalez-Montelongo R, Iñigo-Campos A, de Artola DG, Gil-Campesino H, The Microbiology Technical Support Team Fast SARS-CoV-2 detection by RT-qPCR in preheated nasopharyngeal swab samples. Int J Infect Dis. 2020;97:66–68. doi: 10.1016/j.ijid.2020.05.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman DG, Bland JM. Diagnostic tests. 1: Sensitivity and specificity". BMJ. 1994;308:1552. doi: 10.1136/bmj.308.6943.1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai Y, Yao L, Wei T, Tian F, Jin DY, Chen L, et al. Presumed asymptomatic carrier transmission of COVID-19. JAMA. 2020;323:1406–1407. doi: 10.1001/jama.2020.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce EA, Huang M-L, Perchetti GA, Tighe S, Laaguiby P, Hoffman JJ, et al. Direct RTqPCR detection of SARS-CoV-2 RNA from patient nasopharyngeal swabs without an RNA extraction step. PLoS Biol. 2020;18 doi: 10.1371/journal.pbio.3000896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buitrago-Garcia D, Egli-Gany D, Counotte MJ, Hossmann S, Imeri H, Ipekci AM, et al. Occurrence and transmission potential of asymptomatic and presymptomatic SARS-CoV-2 infections: A living systematic review and meta-analysis. PLoS Med. 2020;17 doi: 10.1371/journal.pmed.1003346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Overview of testing for SARS-CoV-2 (COVID-19). Available from: https//www.cdc.gov/coronavirus/2019-ncov/hcp/testing-overview.html. 2021
- European Centre for Disease Prevention and Control . ECDC; Stockholm: 2020. An overview of the rapid test situation for COVID-19 diagnosis in the EU/EEA. 1 April 2020. [Google Scholar]
- Fernández-Pittol M, Hurtado JC, Moreno-García E, Rubio E, Navarro M, Valiente M, et al. Assessment of the use and quick preparation of saliva for rapid microbiological diagnosis of COVID-19. bioRxiv. 2020 https://www.biorxiv.org/content/10.1101/2020.06.25.172734v1 2020.06.25.172734 [Preprint]Available from: [Google Scholar]
- Furukawa NW, Brooks JT, Sobel J. Evidence supporting transmission of severe acute respiratory syndrome coronavirus 2 while presymptomatic or asymptomatic. Emerg Infect Dis. 2020;26 doi: 10.3201/eid2607.201595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung DL, Li X, Chiu KH, Yip CC, To KK, Chan JF, et al. Early-morning vs spot posterior oropharyngeal saliva for diagnosis of SARS-CoV-2 infection: implication of timing of specimen collection for community-wide screening. Open Forum Infect Dis. 2020;7:ofaa210. doi: 10.1093/ofid/ofaa210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima N, Turner F, Slepnev V, Bacelar A, Deming L, Kodeboyina S, et al. Self-collected oral fluidand nasal swabs demonstrate comparable sensitivity to cliniciancollected nasopharyngeal swabs for Covid-19 detection. medRxiv. 2020 doi: 10.1093/cid/ciaa1589. https://www.medrxiv.org/content/10.1101/2020.04.11.20062372v1 2020.04.11.20062372 [Preprint]Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucirka LM, Lauer SA, Laeyendecker O, Boon D, Lessler J. Variation in false-negative rate of reverse transcriptase polymerase chain reaction-based SARS-CoV-2 tests by time since exposure. Ann Intern Med. 2020;173:262–267. doi: 10.7326/M20-1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Pei S, Chen B, Song Y, Zhang T, Yang W, et al. Substantial undocumented infection facilitates the rapid dissemination of novel coronavirus (SARS-CoV-2) Science. 2020;368:489–493. doi: 10.1126/science.abb3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott IM, Strine MS, Watkins AE, Boot M, Kalinich CC, Harden CA, et al. Simply saliva: stability of SARS-CoV-2 detection negates the need for expensive collection devices. medRxiv. 2020 https://www.medrxiv.org/content/10.1101/2020.08.03.20165233v1 2020.08.03.20165233 [Preprint]Available from: [Google Scholar]
- Pasomsub E, Watcharananan SP, Boonyawat K, Janchompoo P, Wongtabtim G, Suksuwan W, et al. Saliva sample as a non-invasive specimen for the diagnosis of coronavirus disease 2019: a cross-sectional study. Clin Microbiol Infect. 2020 doi: 10.1016/j.cmi.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Procop GW, Shrestha NK, Vogel S, Van Sickle K, Harrington S, Rhoads DD, et al. A direct comparison of enhanced saliva to nasopharyngeal swab for the detection of SARS-CoV-2 in symptomatic patients. J Clin Microbiol. 2020;58 doi: 10.1128/JCM.01946-20. e01946–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranney ML, Griffeth V, Jha AK. Critical supply shortages - The need for ventilators and personal protective equipment during the Covid-19 pandemic. N Engl J Med. 2020;382:e41. doi: 10.1056/NEJMp2006141. [DOI] [PubMed] [Google Scholar]
- Ranoa DRE, Holland RL, Alnaji FG, Green KJ, Wang L, Brooke CB, et al. Saliva-based molecular testing for SARS-CoV-2 that bypasses RNA extraction. bioRxiv. 2020 https://www.biorxiv.org/content/10.1101/2020.06.18.159434v1 2020.06.18.159434 [Preprint]Available from: [Google Scholar]
- Smyrlaki I, Ekman M, Lentini A, Rufino de Sousa N, Papanicolaou N, Vondracek M, et al. Massive and rapid COVID-19 testing is feasible by extraction-free SARS-CoV-2 RT-PCR. Nat Commun. 2020;11:4812. doi: 10.1038/s41467-020-18611-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y-W, Schmitz JE, Persing DH, Stratton CW. Laboratory diagnosis of COVID-19: current issues and challenges. J Clin Microbiol. 2020;58 doi: 10.1128/JCM.00512-20. e00512–e520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tindale LC, Stockdale JE, Coombe M, Garlock ES, Lau WYV, Saraswat M, et al. Evidence for transmission of COVID-19 prior to symptom onset. Elife. 2020;9:e57149. doi: 10.7554/eLife.57149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- To KK, Tsang OT, Yip CC, Chan KH, Wu TC, Chan JM, et al. Consistent detection of 2019 novel coronavirus in saliva. Clin Infect Dis. 2020;71:841–843. doi: 10.1093/cid/ciaa149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Food and Drug Administration. Accelerated emergency use authorization (EUA) summary: SARS-CoV-2 assay, 2020. Available from: https://www.fda.gov/media/136875/download.
- Vogels CB, Watkins AE, Harden CA, Brackney D, Shafer J, Wang J, et al. SalivaDirect: A simplified and flexible platform to enhance SARS-CoV-2 testing capacity. medRxiv. 2020 doi: 10.1016/j.medj.2020.12.010. https://www.medrxiv.org/content/10.1101/2020.08.03.20167791v2 2020.08.03.20167791 [Preprint]Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams E, Bond K, Zhang B, Campbell M, Tokuyama M, Vijayakumar P, et al. Saliva as a noninvasive specimen for detection of SARS-CoV-2. J Clin Microbiol. 2020;58 doi: 10.1128/JCM.00776-20. e00776–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. Laboratory testing for 2019 novel coronavirus disease (2019-nCOVID) in suspected human cases. Available from: https://www.who.int/publications-detail/laboratory-testing-for-2019-novel-coronavirus-in-suspected-human-cases-20200117. 2021
- Wyllie AL, Fournier J, Casanovas-Massana A, et al. Saliva or nasopharyngeal swab specimens for detection of SARS-CoV-2. N Engl J Med. 2020;383:1283–1286. doi: 10.1056/NEJMc2016359. [DOI] [PMC free article] [PubMed] [Google Scholar]