Abstract
Background
The COVID-19 pandemic highlighted the need for evidence-based approaches to decontamination and reuse of N95 filtering facepiece respirators (FFRs). We sought to determine whether vapourized hydrogen peroxide (VHP) reduced SARS-CoV-2 bioburden on FFRs without compromising filtration efficiency. We also investigated coronavirus HCoV-229E as a surrogate for decontamination validation testing.
Methods
N95 FFRs were laced with SARS-CoV-2 or HCoV-229E and treated with VHP in a hospital reprocessing facility. After sterilization, viral burden was determined using viral outgrowth in a titration assay, and filtration efficiency of FFRs was tested against ATSM F2299 and NIOSH TEB-STP-APR-0059.
Results
Viable SARS-CoV-2 virus was not detected after VHP treatment. One replicate of the HCoV-229E laced FFRs yielded virus after processing. Unexpired N95 FFRs retained full filtration efficiency after VHP processing. Expired FFRs failed to meet design-specified filtration efficiency and therefore are unsuitable for reprocessing.
Discussion
In-hospital VHP is an effective decontaminant for SARS-CoV-2 on FFRs. Further, filtration efficiency of unexpired respirators is not affected by this decontamination process.
Conclusions
VHP is effective in inactivating SARS-CoV-2 on FFRs without compromising filtration efficiency. HCoV-229E is a suitable surrogate for SARS-CoV-2 for disinfection studies.
Key Words: Respiratory protection, Mask reprocessing, SARS-CoV-2, HCoV-229E, N95, COVID-19, Hydrogen peroxide vapor
INTRODUCTION
The COVID-19 pandemic has placed unprecedented strains on the production and supply chain of N95 filtering facepiece respirators (FFR) and other personal protective equipment (PPE). This has caused concern throughout the health care and laboratory sectors, as having sufficient respirators is critical to protect health care providers. The 2003 SARS-CoV-1 and 2009 H1N1 influenza pandemics highlighted the need for PPE1, 2, 3 and sparked research into reuse of certain equipment and supplies, including N95 FFR.4, 5, 6 To provide evidence-based approaches for future pandemic preparedness, including sustaining PPE supplies during shortages such as those seen in during the COVID-19 pandemic, comprehensive understanding of PPE reprocessing technology is crucial.
Vapourized hydrogen peroxide (VHP) is a widely available, effective decontamination technology for PPE reprocessing.6 Hospitals utilize this decontamination technology for medical device reprocessing, allowing for quick implementation for N95 respirators should it prove to be effective. Evidence for the use of VHP as an inactivating agent comes from studies on many pathogens,7 including related coronaviruses, such as SARS-CoV-1 and MERS-CoV.8 Recently published data support the ability of VHP to inactivate SARS-CoV-2 in the context of N95 respirator reprocessing.9 , 10 However, the safety and efficacy of this approach has not been rigorously established, and importantly, an evaluation of the effect of VHP on bioburden alongside filtration efficiency has been lacking. Establishing the impact of any decontamination approach on the filtration efficiency of N95 materials is crucial, as the protective capacity of the respirator must not be compromised in the attempt to reduce viral bioburden. This is of increasing importance given the emergence of more infectious viral variants.
There are various settings where N95 reprocessing could maintain supply of PPE (e.g., long-term care facilities, remote hospitals/communities, etc.). However, each VHP reprocessing system should be validated for that specific purpose. Given the logistical challenges of conducting validation studies with a high-risk pathogen, a low biorisk surrogate for process verification is needed in these settings.
In this study, we evaluated the ability of VHP to inactivate SARS-CoV-2 and HCoV-229E, as a less-pathogenic surrogate. Additionally, we investigated the effect of VHP processing on respirator filtration efficiency. The combined evaluation of these factors provides empirical justification for VHP reprocessing of N95 FFR in health care settings. Additionally, the validation of a low-pathogenicity surrogate for SARS-CoV-2 provides a means of implementing this verification platform to other settings where there is a need to extend N95 FFR beyond one-time use.
METHODS
Virus and cells: Vero-E6 cells (ATCC CRL-1586) and Huh-7 cells (JCRB 0403) were used to determine growth of SARS-CoV-2 and HCoV-229E, respectively. Cells were maintained in Dulbecco's Modified Eagle's Media (DMEM) supplemented with L-glutamine, 1% penicillin/streptomycin and 10% fetal bovine serum (FBS). SARS-CoV-2 virus (isolate SB3)11 was expanded using Vero E6 as previously described.11 HCoV-229E (ATCC VR-740) was outgrown on Huh-7 cells. Titres of viral stocks were determined by tissue culture infectious dose 50% (TCID50) assay as outlined below.
N95 respirator viral inoculation and recovery: Two models of N95 respirator (3M 8210 and 3M 9210+) were selected for this study as they are commonly used masks in health care settings in Ontario. For studies of SARS-CoV-2 and HCoV-229E inactivation, all direct manipulation of SARS-CoV-2 took place in the Combined CL3 Unit at the University of Toronto. Briefly, viral stock was diluted into tripartite soil suspension to simulate respiratory secretions (international standard ASTM E2197 matrix)12 and a viral inoculum of 105-106 TCID50 was layered onto the external face of the FFR in less than 100 µL. For each VHP processing cycle, equal numbers of 3M 9210+ (n=4) and 3M 8210 (n=3) were laced with each viral type. Virus-laced and unlaced respirators were placed into sterilization pouches and packaged appropriately for transport to the Medical Device Reprocessing Department at St. Michael's Hospital, Unity Health Toronto (Toronto, Canada). Control respirators (n=3) of each N95 model/virus combination were also prepared. These included negative controls (tripartite soil suspension alone, VHP processed), elution controls (virus laced; eluted off unprocessed respirators within 1 h), and time lapse controls (virus laced; eluted off unprocessed respirators when VHP processed respirators returned to the lab). The inoculated area was excised from the respirator and virus was recovered by elution in 1 mL of DMEM.
VHP Processing: Sterilization pouches (n=14) containing virus-laced or unlaced FFRs were placed into the processing basket and loaded into the V-PRO maX low temperature sterilizer (Steris, Mentor, Ohio). A non-lumen cycle was completed (28 min) and monitored by a biological indicator (Celerity 20 HP, Steris, SKU#LCB044). Each pouch was preloaded with a chemical indicator prior to treatment. For viral recovery assays, pouches were placed in an ordered fashion such that placement of N95 respirator/virus combination (e.g., 3M 8210 inoculated with SARS-CoV-2) was maintained between run dates. Three replicate cycles were completed on independent dates. For tests of filtration efficiency, respirators without viral inoculum were processed in the same manner and provided to the lab within a 24 h period after exposure to VHP.
Tissue Culture Infectious Dose 50 (TCID50) Assay: Samples eluted from VHP processed and control respirators were used to determine viral titres as previously described.11 Briefly, serial 10-fold dilutions of each sample were prepared and applied to monolayers of Vero-E6 cells (for SARS-CoV-2) or Huh-7 cells (for HCoV-229E) with DMEM (0.2×106 cells/mL) in flat bottom 96-well plates. Plates were incubated at 37°C and 5% CO2 for 1 h, with gentle shaking every 15 min to promote uniform distribution of the inoculum within the wells. After 1 h, the inoculum was removed, and the plate was then reconstituted with 200 µL of DMEM with 2% FBS. Cytopathic effects (CPE) were observed at 5 d post infection and reported as the median tissue culture infectivity dose (TCID50) in the 1 mL elution volume, as calculated by the Spearman-Kärber method.13 , 14 The limit of detection for the TCID50 assay was 20 TCID50.
Filtration efficiency testing: VHP processed unlaced FFRs were subjected to two filtration efficiency testing procedures: ASTM F2299 and NIOSH TEB-STP-APR-0059.
The ASTM F2299 test used an apparatus closely conforming to the description of Nicholson.15 Specifically, a suspension of 20 ppm (w/v) polystyrene latex microspheres (100± 10 nm diameter; Polysciences Inc., Warrington, Pennsylvania) was aerosolized using a 1-jet Collison nebulizer (CH Technologies, Westwood, New Jersey) and delivered into a ∼20 L/min flow of dry, HEPA filtered air which was passed through an aerosol mixing chamber into a 5 cm diameter stainless steel duct. A 4.5 cm diameter coupon of FFR was hermetically clamped inside the duct 50 cm downstream of the mixing chamber outlet. The outlet of the duct located 50 cm further downstream was connected through a HEPA filter to an air pump operating at 20 L/min (combined flow of 23.8 L/min including nebulized microsphere contribution), resulting in an airflow velocity of 25 cm/s through the FFR filtration medium. Air samples were collected through pitot ports located upstream and downstream of the filter sample using a TSI SMPS Model 3034 (TSI, Shoreview Minnesota). Per the ASTM F2299 method, the filtration efficiency of the filter was expressed as a percentage (difference in particle count at ∼100 nm measured upstream and downstream of the filter, divided by the upstream count).
In the NIOSH test, masks were sealed to a testing buck with a central hole through which air was drawn at a flow rate of 85 L/min. These were placed in an atmosphere of polydisperse dry salt (NaCl) aerosol with a target Count Median Diameter (CMD) of 75 nm +/- 20 nm and Mass Median Diameter (MMD) of 238 nm, generated using a 6-jet Collison nebulizer. Particles were neutralized to a Boltzmann distribution by passage through a krypton-85 charge neutralizer (370 MBq at time of testing) (TSI 3012A). Total particles in the size range of 10-470 nm were counted on both sides of the FFR in 54 size categories using a TSI SMPS Model 3034. Per the NIOSH procedure, the filtration efficiency was expressed as a percentage of total calculated particle mass excluded by the FFR material (difference between mass measured upstream and downstream of the filter, divided by the upstream mass).
RESULTS
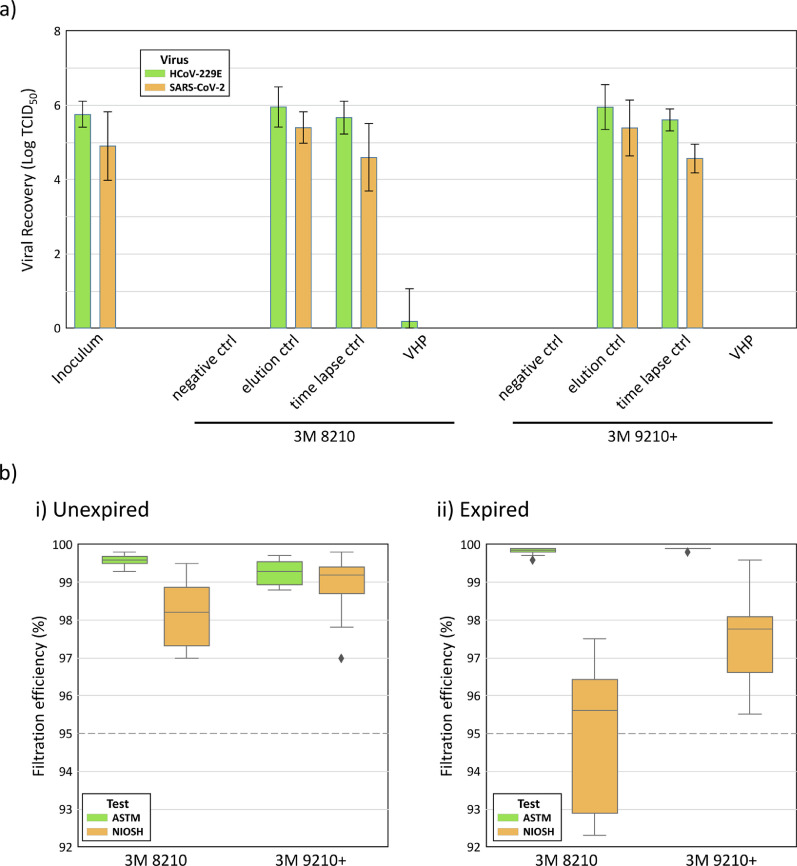
Viral recovery: Over the course of 3 independent cycle runs, a total of 42 FFRs laced with either SARS-CoV-2 or HCoV-229E were exposed to VHP using the V-PRO maX system. Viable virus was not recovered from any SARS-CoV-2-laced FFRs after VHP processing. HCoV-229E was isolated from one of 21 FFRs after processing (Fig 1 a). In contrast, both viruses were easily recoverable from the N95 FFR filtration media if no VHP processing took place and viral recovery occurred within an hour (HCoV-229E, mean = 106.0 TCID50; SARS-CoV-2, mean = 105.4 TCID50). FFRs that were maintained at room temperature until VHP processed samples were returned to the lab, but were not themselves exposed to VHP, showed a small decrease in recovery of viable virus (HCoV-229E, mean = 105.6 TCID50; SARS-CoV-2, mean = 104.6 TCID50) compared to recovery controls. Based on these controls, a conservative estimate of reduction of bioburden indicates that VHP was efficient in providing at least a 4-log reduction of SARS-CoV-2 and 5-log reduction in HCoV-229E.
Fig 1.
Reduction of viral burden and retention of filtration efficiency of unexpired N95 FFR after VHP processing a) Viable virus recovery of SARS-CoV-2 and HCoV-229E from 3M 8210 and 3M 9210+ respirators: negative ctrl: no virus laced on FFR; elution ctrl: immediate recovery of virus; time lapse ctrl: viral recovery alongside processed FFR; VHP: viral recovery from processed FFR. 4-log reduction of SARS-CoV-2 titre and 5-log reduction of HCoV-229E titre was observed after VHP processing b) Filtration efficiency of 3M 8210 and 3M 9210+ FFRs after 1 cycle VHP processing by the NIOSH and ATSM testing protocols; Dashed line indicates design standard of 95% filtration efficiency (n=10 for all tests except unexpired 9210+ where n=9). i) Unexpired FFRs showed consistent filtration efficiencies >95% using both testing standards ii) Expired FFRs revealed failures by the NIOSH testing after 1 cycle of VHP decontamination with 4 of 10 FFRs showing <95% efficiency according to the NIOSH test.
Filtration efficiency
For the NIOSH test, the measured Count Median Diameter (CMD) and Mass Median Diameter (MMD) of salt particles were within range (65.0 nm and 221.7 nm, respectively). The CMD geometric standard deviation was 1.896, slightly higher than the NIOSH target of 1.860. Following one cycle of VHP treatment, the average filtration efficiency of unexpired samples of both FFR types remained above 95% as determined by ASTM F2299 and NIOSH testing at 85 L/min (Fig 1bi). All filter materials demonstrated above 97% filtration efficiency according to the ASTM test; however, the absence of a charge neutralizer in our ASTM testing apparatus may have artefactually increased observed filtration efficiency); Of the expired FFRs, all of the 3M 9210+ samples exhibited a filtration efficiency greater than 95% per the NIOSH test; however, only 6 of 10 of the 3M 8210 FFRs passed NIOSH filtration criteria (Fig 1bii).
Interpretation
The data we have presented here shows that SARS-CoV-2 and HCoV-229E are both inactivated on N95 FFR filtration media during the process of VHP decontamination administered by the in-hospital Steris device, with the consistent ability to show a 4 to 5 log reduction in infectious viral load which meets Health Canada guidelines for successful decontamination.16 The reliable parallel of inactivation data between the two viruses indicates that HCoV-229E can be used as a low-pathogenic surrogate for SARS-CoV-2 in efforts to validate VHP processing platforms for N95 reprocessing during the COVID-19 pandemic. Further, our results confirm that this one-time decontamination process is not disruptive to the filtration efficiency of unexpired N95 FFRs, and as such, that the FFRs could still function as effective PPE, providing equivalent protection before and after reprocessing. By contrast, expired FFRs failed to consistently maintain filtration efficiency greater than 95% according to the NIOSH test, and would therefore be unsuitable for reuse after reprocessing. As the filtration efficiency of some of the expired FFRs may have degraded over time, it is unknown whether the expired FFRs that failed the NIOSH test requirement after reprocessing would also have failed prior to reprocessing.
For these studies, a very large infectious inoculum, upwards of 105 TCID50, was placed on a small, localized area of the N95 surface. This is expected to vastly exceed the viral burden that would be present on N95 FFRs used in a clinical setting.17 Further, a small patch of the respirator material was sufficiently saturated with the inoculum solution to be visibly soiled, again deviating from a representative scenario for real-world use, since such a respirator would be discarded and not selected for reprocessing. Our results demonstrate that VHP is effective at reducing the viral burden even in the context of a soiled FFR. This study therefore sets a very high threshold for establishment of decontamination efficiency, by showing reduction of bioburden in excess of what would be required during regular reprocessing of N95 FFRs.
Due to the high-impact of the current pandemic pathogen, SARS-CoV-2, all sites conducting reprocessing of PPE would desire initial independent validation data to show efficacy of their particular decontamination device/technology. Implementing these tests at disparate sites, many with no containment handling protocols for direct manipulation of pathogens, would be challenging. With the data provided here, we suggest that HCoV-229E can be utilized as a surrogate organism with confidence in these settings to validate VHP-based technologies and procedures for the decontamination of SARS-CoV-2. In fact, our data point to the possibility that HCoV-229E may be slightly more resilient than SARS-CoV-2, as we were able to detect viral growth from 1 of the 21 FFRs laced with HCoV-229E, but none with SARS-CoV-2, after VHP exposure. This study supports the applicability of HCoV-229E as a surrogate for pathogenic coronaviruses in the context of evaluating various decontamination devices/technologies.
In terms of testing of filtration efficiency, we have limited our analysis to results after one cycle of VHP processing. The tests we have conducted provide an accurate evaluation thereof, but it should be noted they do so in a somewhat idealized setting. One of the most dynamic elements, respirator-to-face seal or the fit of the N95 FFR onto the wearer's face, is not accounted for. However, there is guidance18 and data19 suggesting that the face seal can be maintained for up to 5 donning/doffing events with no visible deformation or soiling. Further studies are needed to determine whether filtration efficiency is retained after repeated VHP processing runs. We believe it is important to highlight that implementation of even one reprocessing cycle would allow for health care institutions to double the lifespan of FFR resources, extending supplies significantly and to confidently protect front-line workers.
For any N95 FFR reprocessing methodology to be successfully implemented, the infectious agent must be rendered inactive within the context of the N95 material and the filtration efficiency of the FFR must be maintained such that it can be safely reused. Although many reprocessing technologies are already in place in hospitals and research institutions, the variability of these platforms necessitates validation to ensure that sterilization performance is consistent. To this end, the establishment of comparative low-risk biological surrogates for highly pathogenic agents (such as SARS-CoV-2) is essential for widespread testing of systems that could be used in N95 reprocessing efforts. Our results support the use of HCoV-229E for this purpose and provide strong evidence that a single cycle of standardized VHP reprocessing will inactivate SARS-CoV-2 without compromising the filtration efficiency of unexpired N95 FFRs; however, future work should include comparative testing data with untreated FFRs to determine if there is an impact of these treatments on filtration efficiency.
Acknowledgements
The authors would like to thank Health Canada and the Public Health Agency of Canada for their guidance during the conception of this study. This research was funded in part by support from the University of Toronto's Temerty Faculty of Medicine to enhance capacity and operations of the Toronto Combined Containment Level 3 Facility during the COVID-19 pandemic, the Toronto COVID-19 Action Fund, and the Ontario Together Fund through the Ontario Ministry of Economic Development, Job Creation, and Trade.
References
- 1.US Centers for Disease Control and Prevention [Internet], Atlanta: interim guidance on infection control measures for 2009 H1N1 influenza in healthcare settings, including protection of healthcare personnel; 2009. Available at: https://www.cdc.gov/h1n1flu/guidelines_infection_control.htm. Accessed August 24, 2021.
- 2.World Health Organization [Internet], Geneva: Infection prevention and control of epidemic- and pandemic-prone acute respiratory infections in healthcare, 2014. Available at: https://www.who.int/publications/i/item/infection-prevention-and-control-of-epidemic-and-pandemic-prone-acute-respiratory-infections-in-health-care. Accessed August 24, 2021. [PubMed]
- 3.Campbell A., The SARS commission. [Internet] Toronto; 2007. Available at: http://www.archives.gov.on.ca/en/e_records/sars/index.html. Accessed August 24, 2021.
- 4.Viscusi D.J., Bergman M.S., Eimer B.C., Shaffer R.E. Evaluation of five decontamination methods for filtering facepiece respirators. Ann Occup Hyg. 2009;53:815–827. doi: 10.1093/annhyg/mep070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heimbuch B.K., Wallace W.H., Kinney K., Lumley A.E., Wu C.Y., Woo M.H. A pandemic influenza preparedness study: use of energetic methods to decontaminate filtering facepiece respirators contaminated with H1N1 aerosols and droplets. Am J Infect Control. 2011;39:e1–e9. doi: 10.1016/j.ajic.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 6.Rodriguez-Martinez C.E., Sossa-Briceño M.P., Cortés J.A. Decontamination and reuse of N95 filtering facemask respirators: a systematic review of the literature. Am J Infect Control. 2020;48:1520–1532. doi: 10.1016/j.ajic.2020.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goyal S.M., Chander Y., Yezli S., Otter J.A. Evaluating the virucidal efficacy of hydrogen peroxide vapour. J Hosp Infect. 2014;86:255–259. doi: 10.1016/j.jhin.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kampf G., Todt D., Pfaender S., Steinmann E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J Hosp Infect. 2020;104:246–251. doi: 10.1016/j.jhin.2020.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fischer R.J., Morris D.H., van Doremalen N., Sarchette S., Matson M.J., Bushmaker T. Effectiveness of N95 respirator decontamination and reuse against SARS-CoV-2 virus. Emerg Infect Dis. 2020;26:2253–2255. doi: 10.3201/eid2609.201524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kumar A., Kasloff S.B., Leung A., Cutts T., Strong J.E., Hills K. Decontamination of N95 masks for re-use employing 7 widely available sterilization methods. PLoS One. 2020;15 doi: 10.1371/journal.pone.0243965. e0243965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Banerjee A., Nasir J.A., Budylowski P., Yip L., Aftanas P., Christie N. Isolation, sequence, infectivity, and replication kinetics of severe acute respiratory syndrome coronavirus 2. Emerg Infect Dis. 2020;26:2054–2063. doi: 10.3201/eid2609.201495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sattar S.A., Springthorpe V.S., Adegbunrin O., Zafer A.A., Busa M. A disc-based quantitative carrier test method to assess the virucidal activity of chemical germicides. J Virol Methods. 2003;112:3–12. doi: 10.1016/s0166-0934(03)00192-7. [DOI] [PubMed] [Google Scholar]
- 13.Hamilton M., Russo R., Thurston R. Trimmed Spearman–Karber method for estimating median lethal concentrations in toxicity bioassays. Environ Sci Technol. 1977;11:714–719. [Google Scholar]
- 14.Spearman C. The method of ‘right and wrong cases’ (‘constant stimuli’) without Gauss's formulae. Br J Psychol. 1908;2:227–242. 1904–1920. [Google Scholar]
- 15.Nicholson R.M. In: Fluid filtration: Gas, Volume I, ASTM STP 975. Raber RR, editor. American Society for Testing and Materials; Philadelphia: 1986. Standard method for initial efficiency measurements on flatsheet media; p. p.2986. editor. [Google Scholar]
- 16.Health Canada [Internet] Ottawa, Important Regulatory Considerations for the Reprocessing of Single Use N95 Respirators during the COVID-19 Response: Notice; 2020. Available at: https://www.canada.ca/en/health-canada/services/drugs-health-products/medical-devices/activities/announcements/covid19-notice-reprocessing-n95-respirators.html. Accessed August 24, 2021.
- 17.Wölfel R., Corman V.M., Guggemos W., Seilmaier M., Zange S., Müller M.A. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581:465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- 18.US Centers for Disease Control and Prevention [Internet], Atlanta: recommended guidance for extended use and limited reuse of N95 filtering facepiece respirators in healthcare settings; 2020. Available at: https://www.cdc.gov/niosh/topics/hcwcontrols/recommendedguidanceextuse.html. Accessed August 24, 2021.
- 19.Duncan S., Bodurtha P., Bourgeois C., Dickson E., Jensen C., Naqvi S. The impact of extreme reuse and extended wear conditions on protection provided by a surgical-style N95 filtering facepiece respirator. J Occup Environ Hyg. 2020;17:546–559. doi: 10.1080/15459624.2020.1829633. [DOI] [PubMed] [Google Scholar]