Abstract
Human adipose-derived stem cells (hADSCs) are widely used in regenerative medicine and affected by many biochemical and biophysical stimuli in vivo. Betaine has been reported to be a type of osteogenic stimulating biochemical factor. This study aimed to investigate the effects of betaine; on osteogenic differentiation of cultured hADSCs in osteogenesis differentiation medium. Mesenchymal stem cells were extracted from women undergoing liposuction after obtaining written consent and cultured in vitro. The cells at passage 4 were confirmed by flow cytometry and differentiated into osteocytes and adipocytes. Experimental groups were the cells cultured in osteogenesis differentiation medium (control), cultured in α-MEM and 10% serum-containing Betaine (BET) ,and cultured in osteogenesis differentiation medium containing 10 mM Betaine (OD+BET). After 14 and 21 days of treatment, osteogenic differentiation and the expression of RUNX2 and OCN genes were assessed by qualitative and quantitative Alizarin red staining and real-time PCR. There were significant increases in the calcium matrix deposits, alkaline phosphatase activity ,and expression of RUNX2 and OCN genes in the OD+BET group compared to the BET group. At the end of day 14, the calcium matrix formation was significantly decreased the in BET group compared to the control. Treatment of hADSCs with Betaine, and osteogenesis differentiation medium leads to increased alkaline phosphatase activity, matrix calcium deposits and expression of RUNX2 and OCN genes and finally stimulated osteogenesis. This kind of treatment could be used to support bone regeneration in the future of tissue engineering.
Key Words: Human adipose, derived stem cells, Betaine, Osteogenesis, In vitro
INTRODUCTION
The use of stem cells in tissue engineering is the basis for the emergence of regenerative medicine. Two broad types of stem cells, including embryonic and adult stem cells, have so far been identified [1-3]. The differentiation potentials and therapeutic potentials of embryonic stem cells are very high. The use of these cells in tissue engineering and regenerative medicine is ethically and legally controversial [4-6]. Researchers have focused more on the use of adult stem cells. Adipose tissue is a rich source of adult stem cells [1].
One of the ideal characteristics of stem cells extracted from adipose tissue is that they retain their differentiation potential in long-term culture or successive passages. Aging has more negligible effect on adipose tissue-derived stem cells (ADSCs) than on bone marrow-derived stem cells (BMSCs) [4, 7, 8]. There is also no significant difference in cell adhesion, aging, differentiation capacity, and gene transfer between ADSCs and BMSCs [9]. Tissue regeneration using human adipose-derived stem cells (hADSCs) also has significant potential in the treatment of bone and joint disorders [10]. Clinical transplantation of ADSCs is currently the most promising field for bone regeneration in the laboratory [3].
From 2013 onwards, roughly 3000 journals have been investigated the effectiveness and safety of ADSCs in regenerative medicine [11]. The findings suggested the in vitro osteogenic ability of ADSCs post-induction and their potential therapeutic applications in bone defects [12]. In 2003, Lee et al. observed the first evidence of bone formation in living organisms after differentiation of ADSCs into osteoblasts in the laboratory [4]. Because of these benefits, hADSCs have been used in this study.
Growth factors, biomolecules, or small inorganic molecules are used as chemical compounds to stimulate bone tissue regeneration. Betaine is a biochemical stimulant (a dietary supplement) that stimulates the proliferation and differentiation of osteoblasts. The main physiological role of betaine is as an osmolyte and donor of a methyl group. As an osmolyte, betaine by betaine/GABA transporter-1 (BGT1) protected from the cells, proteins, and enzymes against environmental stress (dehydration, high salinity, or high temperature) [13-17]. It also maintains fluid balance. As a methyl donor, it converts homocysteine to methionine by the enzyme betaine-homocysteine, and also produces N, N-dimethylglycine [13, 14, 18, 19].
Betaine is an effective and safe treatment for patients with genetic homocystinuria, alcoholic and non-alcoholic fatty liver diseases [20-23]. It has been reported that there is inverse association between betaine dietary intakes and lung, colon ,and breast cancers in humans [24]. In humans, betaine has been reported to improve athletic performance. Recent research has shown that betaine supplementation can increase muscle strength and function. Betaine may also increase muscle mass in trained men, but its mechanisms have not been identified [25]. Evidence suggests that betaine, as a trimethylglycine derivative, regulates several vital biological processes such as oxidative stress, inflammatory, osteoblast differentiation, and cellular apoptosis [26-28].
Therefore, considering the role of betaine in osteoblast differentiation, this study aimed to investigate the effects of betaine on osteogenic differentiation of human adipose-derived stem cells.
MATERIALS AND METHODS
Isolation and culture of human ADSCs: To isolate hADSCs, adipose tissue samples were collected from women (mean age 40±5) undergoing liposuction. Informed consent was obtained from the participants and approval of the local Ethics Committee at Velayat Hospital (Damghan, Iran). The study was carried out following the guidelines of the Medical Ethics Committee, Ministry of Health of Iran. Firstly, the sample was cut into very small pieces and then 0.2% collagenase (Gibco, 17100-017) was added, and incubated at 37°C for 2 h. To stop digestion, DMEM (Invitrogen, USA) containing 10% FBS was added to the suspension and centrifuged at 1200 rpm for 5 min at 37°C. Finally, the isolated cells were transferred and cultured in a 25 cm2 flask, and incubated at 37°C in 5% CO2 for 72 h. hADSCs adhered to the bottom of the flask, while floating blood cells washed away by replacing the medium with a fresh one. Cells were subcultured and used for experiments at passage 4 [4].
Flow cytometry analysis: Flow cytometric analyses were performed on a FACSCalibur (BD biosciences, USA). Passage 4 of hADSCs was trypsinized, and then centrifuged at 1200 rpm for 3 min at RT and resuspended in FACS buffer (PBS, 2% FBS) and incubated on ice for 10 min. Then fluorescence antibodies against CD73-PE (BD Biosciences, cat. No. 562817), CD105-PE (Exbio, cat. No. 1P-298-T025), CD166-FITC (Exbio, cat. No. 1F-652-100T), CD45-FITC (BD Biosciences, cat. No. 560976) and CD34-PE (Exbio, cat. No. 1P-664-T025) were added and incubated with rotation at 4°C for 30 min. After removing of non-conjugated antibodies by three washes, the cells were resuspended in PBS. The expression of each surface marker was assessed [29].
Induction of osteoblast differentiation: The isolated hADSCs at passage four (P4) were cultured in 12- well plates, and medium were replaced with Osteogenesis Differentiation Medium (StemPro® Osteogenesis Differentiation Kit, A10072-01, Invitrogen); about 21 days later, the cells were stained with Alizarin red method. Briefly, cells were fixed with 4% formaldehyde for 10 min at 4°C and then incubated with Alizarin Red for 2 min. Then cells were washed with PBS and observed by inverted microscope (E600-Eclipse Nikon) equipped with a digital camera (DXM 1200 Camera Nikon Digital) [30].
Induction of adipocyte differentiation:The isolated hADSCs at P4 were subcultured in 12- well plates containing Adipogenesis Differentiation Medium (StemPro®Adipogenesis Differentiation Kit, A10070-01, Invitrogen). After 21 days, the cells were fixed and stained with Oil Red-O. Briefly, cells were washed with PBS two times and then fixed with 4% formaldehyde for 1 h at 4°C. Then the cells were washed with 70% ethanol for 10-15 min, and then stained with Oil Red. Finally, cells were washed with 70% ethanol about three times and microscopic observation was done to check the results [7].
Experimental groups: The studied groups were: cultured P4- cells in Osteogenesis Differentiation Medium (ODM), cultured P4-cells in α-MEM (α-Minimal Essential Medium, Invitrogen USA) containing 10% FBS and 10 mM of betaine( (20, 26, 31) (Betain Cat No: K1311, a product of Kawsar Biotech Company in Iran) and cultured P4- cells in ODM and 10 mM of betaine (OD+BET)
Alizarin Red staining and calcium deposit quantification: To identify the mineralized matrix of differentiated cells, Alizarin Red (069K1639, Sigma-Aldrich) staining was used on day 21. After removing the medium, the samples were washed with cold PBS and fixed in cold 4% paraformaldehyde (Merck, Germany) for 20 min at 4˚C. Fixative was removed ,and cells were washed twice with PBS. The fixed cells were stained with 400µl Alizarin Red at pH 7.2 for 5-10 min. Finally, the cells were washed again with PBS for three times and observed by inverted microscope [32]. For calcium quantification, 300 µl of 10% acetic acid was added to the cells stained with Alizarin Red and incubated for 30 min at RT with agitation. Cells were scraped off, transferred to microtubes, vortexed ,and incubated. Samples were centrifuged for 30 min at 2000 rpm ,and then 200 µl of the supernatant was transferred to another microtube, 22.5 µl of 10% ammonium hydroxide was added to neutralize the acid and mixed. Finally, the absorbance was measured at 405 nm by the BioTek device [33].
Alkaline Phosphatase activity: To measure alkaline phosphatase (ALP) activity, the total protein of the cells at days 7, 14 post-induction, were lysed with 30 μl of Triton X-100 lysis buffer. The lysate was centrifuged at 2000 rpm at 4°C for 10 min and the ALP activity of supernatant was measured using para-nitrophenyl phosphate (pNPP) as a phosphatase substrate (ALP Kit, Iran) at 405 nm by a microplate reader (BioTek Instruments, USA). Finally, the enzyme activity level (IU) was normalized against the total protein [34].
Calcium content assay: To evaluate the amount of calcium deposits of induced cells on day 21, the samples were washed with PBS and homogenized in 500 μl 0.6 N HCl (Merck, Germany), which were followed by shaking for 4 h at 4°C. Then, a reagent (Calcium Content Kit, Iran) was added to calcium solutions, and the optical density of samples was measured at 575 nm, by a microplate reader (BioTek Instruments, USA). In the end, the value of calcium contents was extracted from the standard curve of OD against a serial dilution of calcium concentrations [35].
Gene expression analyses using RT-qPCR: Total RNA was extracted using RNX-plus reagent (Sinaclon, Iran) according to the manufacturer’s protocol. The cDNA was synthesized using the PrimeScript™ 1st strand cDNA Synthesis Kit (Takara, Japan). Real-time PCR was done by Rotor-Gene 6000 PCR system, using RealQ Plus Master Mix Green (Amplicon, Denmark). The final volume of the reaction solution was 10μl and the program set as, denaturation at 95°C for 15min followed by 50 cycles at 95°C for 15s and annealing/extension for 45s at 60°C [36, 37]. Primer sequences were designed by AlleleID software version 7.5 (Premierbiosoft, USA) as shown in Table 1. The relative expression of target genes was calculated through the 2-ddCt formula, and the RPL13a gene was selected as an endogenous control.
Table 1.
Gene expression primer sequences and amplicons
| Gene | Accession number | Primer sequence (5 ' →3 ' ) | Amplicon size (bp) |
|---|---|---|---|
| RUNX2 | NM_001015051 | AATGCCTCTGCTGTTATGAA CTTCTGTCTGTGCCTTCTG |
192 |
| BGLAP | NM_199173 | ACCGAGACACCATGAGAG CCAGCCATTGATACAGGTAG |
183 |
| RPL13a | NM_012423 | GATAAGAAACCCTGCGACAAA AGAAATTGCCAGAAATGTTGATG |
193 |
Statistical Analysis: All data is shown as mean ± standard error. Data analysis was performed using SPSS software version 16. The differences between the experimental groups were analyzed using one-way ANOVA test followed by Tukey post-hoc test and P<0.05 was considered as a significant level.
RESULTS
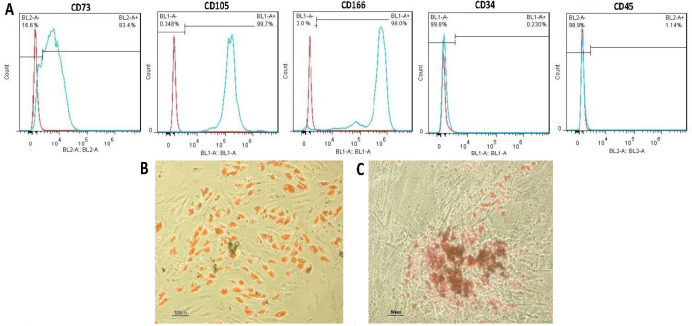
Purified MSCs from human adipose tissue could be characterized by the expression of surface markers. As shown in figure 1, about 83.4% of the cells responded positively to the CD73 marker, and about 98% of the cells responded positively to the CD166 marker. This rate reached about 99.7% for the CD105 marker. Isolated cells were negative in terms of CD34 and CD45 markers (Fig. 1A). Also, hADSCs demonstrated a strong capacity for differentiation into adipogenic and osteogenic lineages. Adipogenic differentiation was confirmed by the appearance of small lipid vesicles formed after treatment and stained with Oil Red (Fig. 1B). Osteogenic differentiation was confirmed by the production of calcium phosphate and mineralized extracellular matrix in induced cells, which were stained with Alizarin Red (Fig. 1C).
Figure 1.
Identity of isolated hADSCs. (A) The cells positively expressed CD73, CD166, CD105 markers, while being negative for CD34 and CD45. (B) Oil Red staining of adipogenic-induced cells shows intracellular lipid droplets. (C) Representative images of Alizarin Red staining at 21 days of osteogenic induction, mineralized calcium deposits are in red
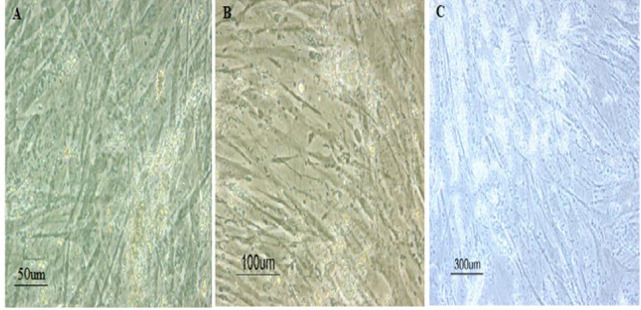
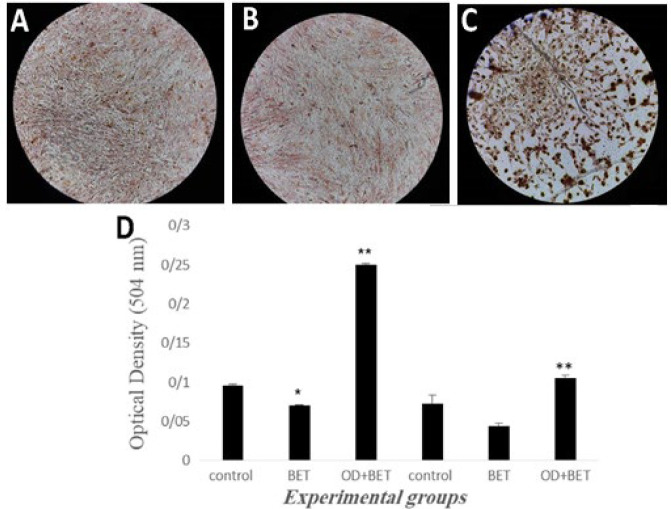
The cells appeared triangular or polygonal shaped after treatment with OD and betaine, the formation of calcium deposits and mineralized matrix was higher in the OD + BET group (Fig. 2C) compared to control and BET groups (Fig. 2A & B). Alizarin Red (red indicative of calcium deposition) staining showed more intense staining for mineral in the OD + BET group (Fig. 3C) compared to those of other groups, while there was no difference in the formation of calcium deposits between the control and BET groups (Fig. 3A & B).
Figure 2.
Morphology of the studied groups. A: control, B: BET, C: OD+BET
Figure 3.
Extracellular matrix calcium deposits were visualized with Alizarin Red staining. A: control, B: BET and C: OD+BET groups. Magnification 100 .×D: Diagram of the amount of calcium phosphate through quantitative staining of Alizarin Red. Control (cultured cells in Osteogenesis Differentiation Medium); BET (cultured cells in α-MEM containing 10% FBS and Betaine); OD+BET (cultured cells in Osteogenesis Differentiation Medium containing Betaine). *Significant difference versus control, **Significant difference versus BET group
The quantitative analysis of Alizarin Red staining after 14 and 21 days of induction was shown in fig. 3D. On day 14, BET group (0.05) showed a significant decrease of calcium deposits, compared to the control group (0.095±0.002) (P<0.05). But it was significantly higher in OD+BET group (0.250±0.001) compared to the BET group (P<0.05). On day 21, no significant difference of calcium deposits could be detected among the control (0.070±0.010) and BET groups (0.044±0.003). In contrast, OD+BET group (0.105±0.003) showed a significant increase of calcium deposits, compared to the BET group (P<0.05).
The ALP activity of the experimental groups was measured on days 14 and 21 post-induction (Fig. 4). After 14 days, the ALP activity of the BET group (0.573±0.008) showed no difference from that of the control (0.575±0.022). Whereas ALP activity of the OD+BET group (1.248±0.007) was significantly increased, compared to the BET group. After 21 days, no significant difference in ALP activity could be detected among the control (0.349±0.002) and BET (0.329±0.010) groups. While OD+BET group (0.451±0.001) showed a significant increase in ALP activity, compared to BET groups.
Figure 4.
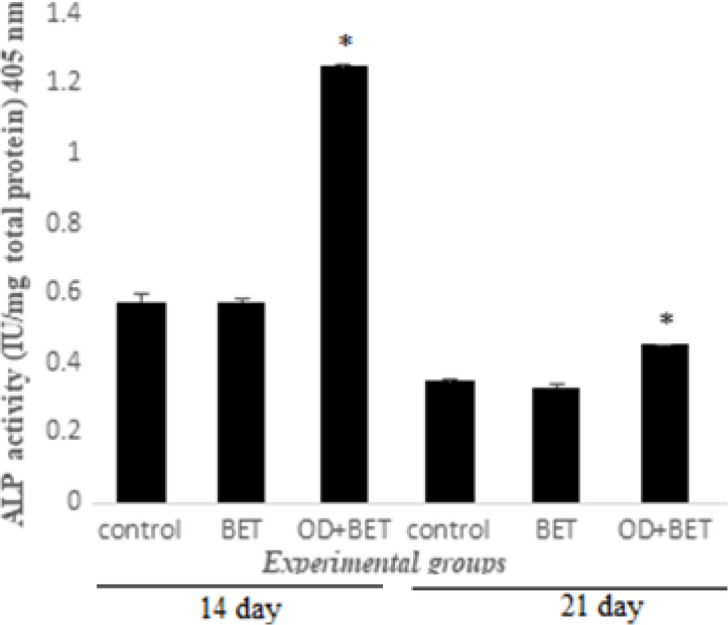
ALP expression rate of the experimental groups on day 14 and 21. * significant increase versus BET group
The amount of calcium deposition of experimental groups was evaluated on day 21 (Fig. 5). There was a significant decrease in calcium deposition in the BET group (0.350) compared to that of the control group (1.187±0.116). There was a significant increase in calcium deposition in OD+BET (23.330±0.120) group as compared to that of the BET group (P<0.05).
Figure 5.
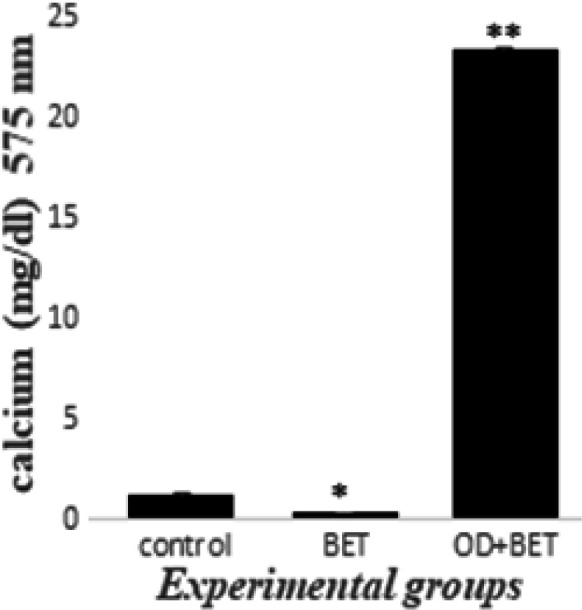
The amount of calcium deposits in experimental groups on day 21.*Significant decrease versus control group, **Significant increase versus BET group
According to Figure 6, after 21 days no significant difference in the RUNX2 mRNA expression level, could be detected among the control (1.003±0.0788) and BET (0.613±0.0233) groups. While there was a significant increase in the mRNA level of the RUNX2 gene in the OD+BET group (2.10±0.251) compared to the BET group. Also, after 21 days no significant difference in the OCN mRNA expression level, could be detected among the control (1±0.060) and BET (0.276±0.050) groups. While there was a significant increase in the mRNA level of the OCN in the OD+BET group (2.04±30.230) compared to the BET group.
Figure 6.
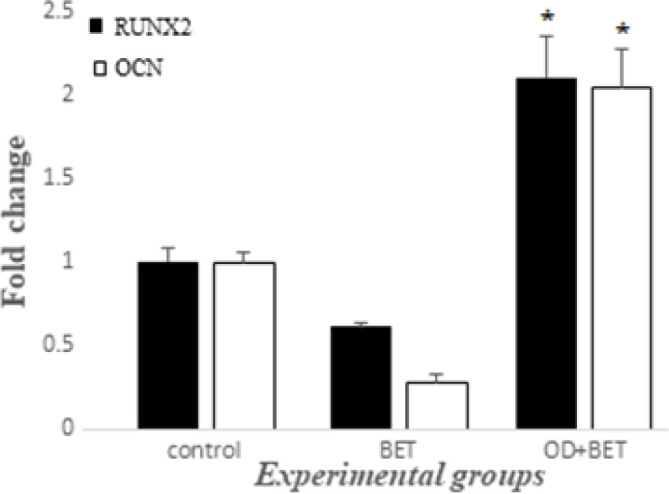
The expression of RUNX2 and OCN genes in hADSCs, 21 days post-induction in experimental groups. *Significant increase versus BET group
DISCUSSION
The importance physiological function of betaine as an osmoprotectant and methyl group donor indicating its deficiency associated with adverse consequences. Betaine is added to animal diets to improve meat quality (fat loss and muscle gain) [38]. Betaine has been reported as a promising therapeutic agent against sarcopenia and osteoporosis (a major cause of bone weakness and mortality) [20]. Betaine has been previously reported to induce stimulatory effects on human osteoblasts and myoblasts by synergistic activation of IGF-1 production. It leads to the expression of genes and ultimately the production of matrix proteins [20, 31, 39, 40]. It has also been shown that betaine supplements may be important in bone and muscle disorders in the elderly by acting on bone and muscle cells through common pathways [41]. Therefore, the aim of this study was to investigate the effects of betaine on the osteogenic differentiation of hADSCs.
Evidence suggests that betaine depolarizes osteoblast plasma membranes through the VGCC type L channel, thus exerts osteogenic effects. Betaine has been reported to affect osteoblasts by increasing intracellular Ca2 + levels, ECM production, insulin-like growth factor (IGF-2), and a series of events leading to osteogenesis in vitro. IGF-I has been reported to stimulate the RUNX2 gene expression during osteogenesis. The RUNX2 gene is the major transcription factor of the osteogenesis process.
RUNX2 can stimulate other osteoblast-specific transcription factors such as Osterix (OSX) followed by the activation of BSP and OPN (two non-collagen proteins). These proteins are multifunctional, having roles in matrix mineralization, bone formation, and regeneration [20, 42-47].
The cells exhibited a polygonal or triangular morphology. The calcium deposits stained by Alizarin Red were observed in OD + BET with the high amount and BET groups (two main characteristics during the osteogenic differentiation). Thus, it can be argued that the betaine/ODM enhanced osteogenic activity of hADSCs. Similar to this study, Villa observed changes in the morphology of human osteoblast-like cells (hOBs) post induced by betaine and osteogenic medium. He also suggested that betaine increases the osteogenic differentiation of hOBs [20].
hADSCs are detected by the expression of surface antigens such as CD73, CD166 and CD105, and by the lack of hematopoietic antigens such as CD34, CD45 [48-50]. The efficacy of hADSCs studied in this study was confirmed by examining these markers and the results were by Zhu reports [51]. The results showed that hADSCs differentiate into osteoblasts and adipocytes, similar to Tapp's and Locke's findings [4, 52].
Cultured hADSCs in ODM for 14 to 28 days, produced the mineralized calcium phosphate in the extracellular matrix stained by Alizarin Red [3]. Analysis of the quantification of Alizarin Red staining after 14 and 21days, showed the increased osteogenic potential of hADSCs post induced by betaine/ODM. These results are in line with those of Villa study. He reported that the co-treatment of betaine ,and the osteogenic medium through the RUNX2/OSX axis leads to the activation of bone formation markers such as type-1 collagen, BSP and OPN. Expression and activation of these proteins lead to matrix mineralization, bone formation and regeneration. Treatment with betaine alone did not induce osteogenesis of hADSCs.
The present study showed that after the end of days 14 and 21, co-treatment of these cells with ODM and betaine increased the ALP activity. These findings are similar to Villa's results. He observed a significant increase in ALP activity and osteocalcin gene expression after treatment of hOBS with betaine (10 mM) and ODM. Treatment of hADSCs alone with betaine exhibited a significant reduction of alkaline phosphatase activity at 14 and 21 days. In fact, it can be claimed that betaine alone does not affect the osteogenic differentiation potential of hADSCs. After 21 days of cell incubation and betaine treatment, the stimulatory effects of betaine /ODM on osteogenesis, similar to the Villa study were determined. He reported that betaine (10 mM) increased intracellular calcium by the flow of calcium ions through L-type VGCC channels. Also, treatment with betaine alone had inhibitory effects on osteogenesis of hADSC.
Betaine has been reported to be effective in the proliferation and differentiation of osteoblasts in the laboratory by stimulating DNA synthesis and the expression of osteogenesis-related genes during differentiation and matrix mineralization [20]. At the end of days 14 and 21, we found the stimulatory effect of ODM betaine on the expression of RUNX2 and OCN genes. Data analysis also indicates that betaine alone may have no affect on the expression of RUNX2 and OCN genes.
Acknowledgements:
This study was financially supported by the Biological School of Damghan University.
Conflict of Interest:
There is no conflict of interest in this article.
References
- 1.Cheng KH, Kuo TL, Kuo KK, Hsiao GM. Human adipose-derived stem cells: Isolation, characterization and current application in regeneration medicine. Genomic Medicine, Biomarker and Health Society. 2011;3:53–62. [Google Scholar]
- 2.Roobrouck VD, Ulloa-Montoya F, Verfaillie CMJEcr. Self-renewal and differentiation capacity of young and aged stem cells. Exp Cell Res. 2008;314:1937–1944. doi: 10.1016/j.yexcr.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 3.Ciuffi S, Zonefrati R, Brandi ML. Adipose stem cells for bone tissue repair. Clin Cases Miner Bone Metab. 2017;14:217–226. doi: 10.11138/ccmbm/2017.14.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Locke M, Windsor J, Dunbar PR. Human adipose-derived stem cells: isolation, characterization and applications in surgery. ANZ J Surg. 2009;79:235–244. doi: 10.1111/j.1445-2197.2009.04852.x. [DOI] [PubMed] [Google Scholar]
- 5.Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stamatopoulos A, Stamatopoulos T, Gamie Z, Kenanidis E, Ribeiro RDC, Rankin KS, Gerrand C, Dalgarno K, Tsiridis E. Mesenchymal stromal cells for bone sarcoma treatment: Roadmap to clinical practice. J Bone Oncol . 2019::100231. doi: 10.1016/j.jbo.2019.100231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Safwani WKZW, Makpol S, Sathapan S, Chua K. Impact of adipogenic differentiation on stemness and osteogenic gene expression in extensive culture of human adipose-derived stem cells. Arch Med Sci. 2014;10:597–606. doi: 10.5114/aoms.2014.43753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen HT, Lee MJ, Chen CH, Chuang SC, Chang LF, Ho ML, Hung SH, Fu YC, Wang YH, Wang HI, Wang GJ, Kang L, Cheng JK. Proliferation and differentiation potential of human adipose‐derived mesenchymal stem cells isolated from elderly patients with osteoporotic fractures. J Cell Mol Med. 2012;16:582–593. doi: 10.1111/j.1582-4934.2011.01335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hattori H, Masuoka K, Sato M, Ishihara M, Asazuma T, Takase B, Kikuchi M, Nemoto K, Ishihara M. Bone formation using human adipose tissue-derived stromal cells and a biodegradable scaffold. J Biomed Mater Res B Appl Biomater. 2006;76:230–239. doi: 10.1002/jbm.b.30357. [DOI] [PubMed] [Google Scholar]
- 10.Marędziak M, Marycz K, Tomaszewski KA, Kornicka K, Henry BM. The influence of aging on the regenerative potential of human adipose derived mesenchymal stem cells. Stem Cells Int. 2016:1–15. doi: 10.1155/2016/2152435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barba M, Cicione C, Bernardini C, Michetti F, Lattanzi W. Adipose-derived mesenchymal cells for bone regereneration: state of the art. Biomed Res Int . 2013:2013. doi: 10.1155/2013/416391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li X, Yao J, Wu L, Jing W, Tang W, Lin Y, Tian W, Liu L. Osteogenic induction of adipose‐derived stromal cells: not a requirement for bone formation in vivo. Artif Organs. 2010;34:46–54. doi: 10.1111/j.1525-1594.2009.00795.x. [DOI] [PubMed] [Google Scholar]
- 13.Lever M, Slow S. The clinical significance of betaine, an osmolyte with a key role in methyl group metabolism. Clin biochem. 2010;43:732–744. doi: 10.1016/j.clinbiochem.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 14.Craig SA. Betaine in human nutrition. Am J Clin Nutr. 2004;80:539–549. doi: 10.1093/ajcn/80.3.539. [DOI] [PubMed] [Google Scholar]
- 15.Parikh NR, Vaughn CL, Williams LL, Kempson SA. Acute activation of the renal betaine/GABA transporter in response to a decrease in extracellular calcium. ISRN Physiol . 2012:2013. [Google Scholar]
- 16.Waditee R, Hibino T, Tanaka Y, Nakamura T, Incharoensakdi A, Hayakawa S, Suzuki S, Futsuhara Y, Kawamitsu Y, Takabe T, Takabe T. Functional characterization of betaine/proline transporters in betaine-accumulating mangrove. J Biol Chem. 2002;277:18373–18382. doi: 10.1074/jbc.M112012200. [DOI] [PubMed] [Google Scholar]
- 17.Zhou Y, Holmseth S, Hua R, Lehre AC, Olofsson AM, Poblete-Naredo I, Kempson SA, Danbolt NC. The betaine-GABA transporter (BGT1, slc6a12) is predominantly expressed in the liver and at lower levels in the kidneys and at the brain surface. Am J Physiol Renal Physiol. 2011;302:F316–F328. doi: 10.1152/ajprenal.00464.2011. [DOI] [PubMed] [Google Scholar]
- 18.Slow S, Lever M, Chambers ST, George PM. Plasma dependent and independent accumulation of betaine in male and female rat tissues. Physiol Res. 2009;58:403–410. doi: 10.33549/physiolres.931569. [DOI] [PubMed] [Google Scholar]
- 19.Kharbanda KK, Rogers II DD, Mailliard ME, Siford GL, Barak AJ, Beckenhauer HC, Sorrell MF, Tuma DJ. Role of elevated S-adenosylhomocysteine in rat hepatocyte apoptosis: protection by betaine. Biochem Pharmacol. 2005;70:1883–1890. doi: 10.1016/j.bcp.2005.09.021. [DOI] [PubMed] [Google Scholar]
- 20.Villa I, Senesi P, Montesano A, Ferraretto A, Vacante F, Spinello A, Bottani M, Bolamperti S, Rubinacci A, Luzi L, Terruzzi I. Betaine promotes cell differentiation of human osteoblasts in primary culture. J Transl Med. 2017;15 doi: 10.1186/s12967-017-1233-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee MS, Kim MS, Park SY, Kang CW. Effects of betaine on ethanol-stimulated secretion of IGF-I and IGFBP-1 in rat primary hepatocytes: involvement of p42/44 MAPK activation. World J Gastroenterol. 2006;12:1718–1722. doi: 10.3748/wjg.v12.i11.1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Song Z, Deaciuc I, Zhou Z, Song M, Chen T, Hill D, McClain CJ. Involvement of AMP-activated protein kinase in beneficial effects of betaine on high-sucrose diet-induced hepatic steatosis. Am J Physiol Gastrointest Liver Physiol. 2007;293:G894–G902. doi: 10.1152/ajpgi.00133.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Z, Yao T, Pini M, Zhou Z, Fantuzzi G, Song Z. Betaine improved adipose tissue function in mice fed a high-fat diet: a mechanism for hepatoprotective effect of betaine in nonalcoholic fatty liver disease. Am J Physiol Gastrointest Liver Physiol. 2010;298:G634–G642. doi: 10.1152/ajpgi.00249.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee I. Betaine is a positive regulator of mitochondrial respiration. Biochem Biophys Res Commun. 2015;456:621–625. doi: 10.1016/j.bbrc.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 25.Apicella JM, Lee EC, Bailey BL, Saenz C, Anderson JM, Craig SA, Kraemer WJ, Volek JS, Maresh CM. Betaine supplementation enhances anabolic endocrine and Akt signaling in response to acute bouts of exercise. Eur J Appl Physiol. 2013;113:793–802. doi: 10.1007/s00421-012-2492-8. [DOI] [PubMed] [Google Scholar]
- 26.Yang Q, Yin W, Chen Y, Zhu D, Yin J, Zhang C, Gao Y. Betaine alleviates alcohol-induced osteonecrosis of the femoral head via mTOR signaling pathway regulation. Biomed Pharmacother. 2019;120:109486. doi: 10.1016/j.biopha.2019.109486. [DOI] [PubMed] [Google Scholar]
- 27.Veskovic M, Mladenovic D, Milenkovic M, Tosic J, Borozan S, Gopcevic K, Labudovic-Borovic M, Dragutinovic V, Vucevic D, Jorgacevic B, Isakovic A, Trajkovic V, Radosavljevic T. Betaine modulates oxidative stress, inflammation, apoptosis, autophagy, and Akt/mTOR signaling in methionine-choline deficiency-induced fatty liver disease. Eur J Pharmacol. 2019;848:39–48. doi: 10.1016/j.ejphar.2019.01.043. [DOI] [PubMed] [Google Scholar]
- 28.Li C, Wang Y, Li L, Han Z, Mao S, Wang G. Betaine protects against heat exposure–induced oxidative stress and apoptosis in bovine mammary epithelial cells via regulation of ROS production. Cell Stress Chaperones. 2019;24:453–460. doi: 10.1007/s12192-019-00982-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hosseinpur Z, Hashemi SM, Salehi E, Ghazanfari T. Comparison of TGF-β1 and NO production by mesenchymal stem cells isolated from murine lung and adipose tissues. Immunopharmacol Immunotoxicol. 2016;38:214–220. doi: 10.3109/08923973.2016.1168434. [DOI] [PubMed] [Google Scholar]
- 30.Wen L, Wang Y, Wang H, Kong L, Zhang L, Chen X, Ding Y. L-type calcium channels play a crucial role in the proliferation and osteogenic differentiation of bone marrow mesenchymal stem cells. Biochem Biophys Res Commun. 2012;424:439–445. doi: 10.1016/j.bbrc.2012.06.128. [DOI] [PubMed] [Google Scholar]
- 31.Senesi P, Luzi L, Montesano A, Mazzocchi N, Terruzzi I. Betaine supplement enhances skeletal muscle differentiation in murine myoblasts via IGF-1 signaling activation. J Transl Med. 2013;1:174. doi: 10.1186/1479-5876-11-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Palumbo P, Lombardi F, Siragusa G, Cifone MG, Cinque B, Giuliani M. Methods of isolation, characterization and expansion of human adipose-derived stem cells (ASCs): an overview. Int J Mol Sci. 2018;19:1897. doi: 10.3390/ijms19071897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gregory CA, Gunn WG, Peister A, Prockop DJ. An Alizarin red-based assay of mineralization by adherent cells in culture: comparison with cetylpyridinium chloride extraction. Anal Biochem. 2004;329:77–84. doi: 10.1016/j.ab.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 34.Seyedjafari E, Soleimani M, Ghaemi N, Shabani I. Nanohydroxyapatite-coated electrospun poly (l-lactide) nanofibers enhance osteogenic differentiation of stem cells and induce ectopic bone formation. Biomacromolecules. 2010;11:3118–3125. doi: 10.1021/bm1009238. [DOI] [PubMed] [Google Scholar]
- 35.Arjmand M, Ardeshirylajimi A, Maghsoudi H, Azadian E. Osteogenic differentiation potential of mesenchymal stem cells cultured on nanofibrous scaffold improved in the presence of pulsed electromagnetic field. J Cell Physiol. 2018;233:1061–1070. doi: 10.1002/jcp.25962. [DOI] [PubMed] [Google Scholar]
- 36.Su CY, Fang T, Fang HW. Effects of Electrostatic Field on Osteoblast Cells for Bone Regeneration Applications. Biomed Res Int . 2017;2017:7124817. doi: 10.1155/2017/7124817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tsai MT, Li WJ, Tuan RS, Chang WH. Modulation of osteogenesis in human mesenchymal stem cells by specific pulsed electromagnetic field stimulation. J Orthop Res. 2009;27:1169–1174. doi: 10.1002/jor.20862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martins JM, Neves JA, Freitas A, Tirapicos JL. Effect of long-term betaine supplementation on chemical and physical characteristics of three muscles from the Alentejano pig. J Sci Food Agric. 2012;92:2122–2127. doi: 10.1002/jsfa.5595. [DOI] [PubMed] [Google Scholar]
- 39.Devol DL, Rotwein P, Sadow JL, Novakofski J, Bechtel PJ. Activation of insulin-like growth factor gene expression during work-induced skeletal muscle growth. Am J Physiol. 1990;259:E89–E95. doi: 10.1152/ajpendo.1990.259.1.E89. [DOI] [PubMed] [Google Scholar]
- 40.Conejo R, Lorenzo M. Insulin signaling leading to proliferation, survival, and membrane ruffling in C2C12 myoblasts. J Cell Physiol. 2001;187:96–108. doi: 10.1002/1097-4652(2001)9999:9999<::AID-JCP1058>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 41.Burattini S, Ferri P, Battistelli M, Curci R, Luchetti F, Falcieri E. C2C12 murine myoblasts as a model of skeletal muscle development: morpho-functional characterization. Eur J Histochem . 2004:223–234. [PubMed] [Google Scholar]
- 42.Zahanich I, Graf EM, Heubach JF, Hempel U, Boxberger S, Ravens U. Molecular and functional expression of voltage‐operated calcium channels during osteogenic differentiation of human mesenchymal stem cells. J Bone Miner Res. 2005;20:1637–1646. doi: 10.1359/JBMR.050521. [DOI] [PubMed] [Google Scholar]
- 43.Yang S, Xu H, Yu S, Cao H, Fan J, Ge C, Fransceschi RT, Dong HH, Xiao G. Foxo1 mediates insulin-like growth factor 1 (IGF1)/insulin regulation of osteocalcin expression by antagonizing Runx2 in osteoblasts. J Biol Chem. 2011;286:19149–19158. doi: 10.1074/jbc.M110.197905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Qiao M, Shapiro P, Kumar R, Passaniti A. Insulin-like growth factor-1 regulates endogenous RUNX2 activity in endothelial cells through a phosphatidylinositol 3-kinase/ERK-dependent and Akt-independent signaling pathway. J Biol Chem . 2004;279:42709–42718. doi: 10.1074/jbc.M404480200. [DOI] [PubMed] [Google Scholar]
- 45.Xue P, Wu X, Zhou L, Ma H, Wang Y, Liu Y, Ma J, Li Y. IGF1 promotes osteogenic differentiation of mesenchymal stem cells derived from rat bone marrow by increasing TAZ expression. Biochem Biophys Res Commun. 2013;433:226–231. doi: 10.1016/j.bbrc.2013.02.088. [DOI] [PubMed] [Google Scholar]
- 46.Chen J, Yuan K, Mao X, Miano JM, Wu H, Chen Y. Serum response factor regulates bone formation via IGF‐1 and Runx2 signals. J Bone Miner Res. 2012;27:1659–1668. doi: 10.1002/jbmr.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang M, Xuan S, Bouxsein ML, Von Stechow D, Akeno N, Faugere MC, Malluche H, Zhao G, Rosen CJ, Efstratiadis A, Clemens TL. Osteoblast-specific knockout of the insulin-like growth factor (IGF) receptor gene reveals an essential role of IGF signaling in bone matrix mineralization. J Biol Chem. 2002;277:44005–44012. doi: 10.1074/jbc.M208265200. [DOI] [PubMed] [Google Scholar]
- 48.Yarak S, Okamoto OK. Human adipose-derived stem cells: current challenges and clinical perspectives. An Bras Dermatol . 2010;85:647–656. doi: 10.1590/s0365-05962010000500008. [DOI] [PubMed] [Google Scholar]
- 49.Takemitsu H, Zhao D, Yamamoto I, Harada Y, Michishita M, Arai T. Comparison of bone marrow and adipose tissue-derived canine mesenchymal stem cells. BMC Vet Res. 2012;8:150. doi: 10.1186/1746-6148-8-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Varma MJO, Breuls RG, Schouten TE, Jurgens WJ, Bontkes HJ, Schuurhuis GJ, Ham Van SM, Milligen FJ. Phenotypical and functional characterization of freshly isolated adipose tissue-derived stem cells. Stem Cells Dev. 2007;16:91–104. doi: 10.1089/scd.2006.0026. [DOI] [PubMed] [Google Scholar]
- 51.Zhu Y, Liu T, Song K, Fan X, Ma X, Cui Z. Adipose-derived stem cell: a better stem cell than BMSC. Cell Biochem Funct. 2008;26:664–675. doi: 10.1002/cbf.1488. [DOI] [PubMed] [Google Scholar]
- 52.Tapp H, Hanley Jr EN, Patt JC, Gruber HE. medicine. Adipose-derived stem cells: characterization and current application in orthopaedic tissue repair. Exp Biol Med. 2009;234:1–9. doi: 10.3181/0805/MR-170. [DOI] [PubMed] [Google Scholar]