Abstract
We review knowledge about the roles of sex chromosomes in vertebrate hybridization and speciation, exploring a gradient of divergences with increasing reproductive isolation (speciation continuum). Under early divergence, well-differentiated sex chromosomes in meiotic hybrids may cause Haldane-effects and introgress less easily than autosomes. Undifferentiated sex chromosomes are more susceptible to introgression and form multiple (or new) sex chromosome systems with hardly predictable dominance hierarchies. Under increased divergence, most vertebrates reach complete intrinsic reproductive isolation. Slightly earlier, some hybrids (linked in ‘the extended speciation continuum') exhibit aberrant gametogenesis, leading towards female clonality. This facilitates the evolution of various allodiploid and allopolyploid clonal (‘asexual’) hybrid vertebrates, where ‘asexuality' might be a form of intrinsic reproductive isolation. A comprehensive list of ‘asexual' hybrid vertebrates shows that they all evolved from parents with divergences that were greater than at the intraspecific level (K2P-distances of greater than 5–22% based on mtDNA). These ‘asexual' taxa inherited genetic sex determination by mostly undifferentiated sex chromosomes. Among the few known sex-determining systems in hybrid ‘asexuals', female heterogamety (ZW) occurred about twice as often as male heterogamety (XY). We hypothesize that pre-/meiotic aberrations in all-female ZW-hybrids present Haldane-effects promoting their evolution. Understanding the preconditions to produce various clonal or meiotic allopolyploids appears crucial for insights into the evolution of sex, ‘asexuality' and polyploidy.
This article is part of the theme issue ‘Challenging the paradigm in sex chromosome evolution: empirical and theoretical insights with a focus on vertebrates (Part II)’.
Keywords: sex chromosomes, hybridization, evolution, clonal reproduction, speciation
1. Introduction
Our understanding of speciation has evolved from being regarded as a long and steady process, governed by natural selection in various forms [1–3], to a view that includes dynamic and/or reticulate and potentially fast processes [4–9]. Speciation may occur in parallel under similar ecological conditions [10]. In allopatry, incipient species accumulate subtle differences along the entire genome [11,12] with single speciation genes [13] being the first witnesses and perhaps sometimes the drivers of speciation.
In this paper, after a lead-in on intrinsic reproductive isolation and on sex chromosomes in speciation, we explore a gradient of divergences (the ‘speciation continuum' [14], detailed below) to review knowledge about the evolutionary impact of sex chromosomes under hybridization in vertebrates. We start our ‘evolutionary journey’ through speciation from the early onset of evolutionary divergence in near-panmictic populations that form meiotic hybrids. We then examine sex chromosomes by moving along various stages of increasing divergences and accumulating intrinsic reproductive isolation between hybridizing species (table 1) until a stage is reached, when hybrid vertebrates evolve that rarely exhibit so-called ‘asexual' (some forms of hybrid clonal and allopolyploid) reproductive modes (box 1 and figure 1). Our way of studying and thinking about sex chromosomes in (mostly allopatric) speciation may offer a useful framework (table 1). We discuss the current state of the field, focusing on available knowledge and major research gaps on sex chromosomes in various kinds of vertebrate hybrids.
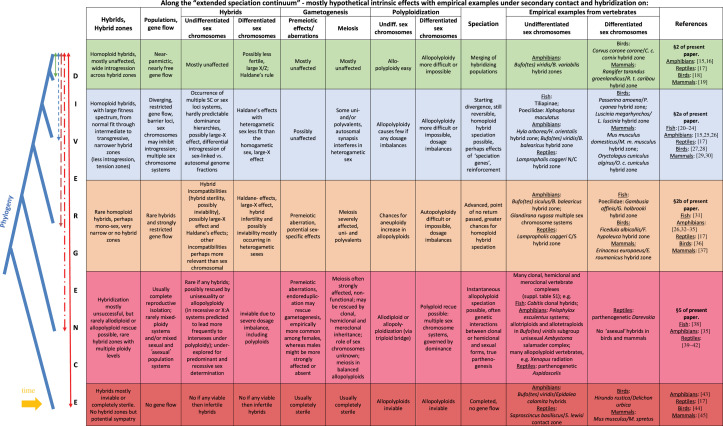
Table 1.
Hypothetical evolutionary stages with empirical examples along the ‘extended speciation continuum', with effects under secondary contact and hybridization, with special attention on undifferentiated and differentiated sex chromosomes. This table is supposed to show evolutionary tendencies as described in the text. Note that stages along the ‘extended speciation continuum' do not necessarily correspond to absolute divergence times as in some species, speciation proceeds more rapidly than in others, i.e. stages should be preferentially compared within a certain radiation of organisms. Evidence for Aspidoscelis lizards is equivocal since sex chromosomes are only known in very few species.
 |
Box 1. Glossary (definitions in part after Avise [46]).
Allospecific (=heterospecific): belonging to different taxonomic species.
Asexual reproduction: sensu stricto: Any form of reproduction that does not involve the fusion of sex cells (gametes); i.e. a reproductive mode, by which an organism passes on its genome clonally by circumventing the effects of recombination and meiotic reduction during gametogenesis; therefore, the genome is transmitted unaltered. This is achieved by different mechanisms. Some organisms transmit their genomes strictly asexually, i.e. in a completely clonal way (parthenogenesis, see below). In this paper, when we write ‘asexual’ (i.e. in quotation marks), we use the term sensu lato: some organisms transmit only parts of their genomes clonally, while the rest is eliminated and replaced in each generation by a sexually reproducing parental species (sexual host) (hybridogenesis). Many such organisms show a strong female bias (see: unisexual species). The literature uses the terms asexual and asexuality sometimes uncritically, causing scientific disputes over ‘asexual’ organisms, their evolution and long-term survival. Different mechanisms also exist with respect to the requirement (or not) for fertilization. True parthenogens are completely independent of sperm (and thus of males), while other types of ‘asexuals’, gynogens or sperm-dependent parthenogens, rely on insemination, usually, but not always, from closely related sexual species [47]. The sperm either only triggers embryogenesis while its genome gets eliminated after fertilization (gynogenesis, pseudogamy) (but it may also contribute genetically to the progeny either by subgenomic amounts, such as microchromosomes [48]), or the entire sperm genome may be incorporated into the progeny, resulting in ploidy elevation (genome addition); or elimination, after one generation—in the next round of gamete production (in some forms of hybridogenesis). Subgenomic amounts of sperm-DNA can occasionally also be incorporated into the egg and partly replace or perhaps recombine with the maternal genome (kleptogenesis); the paternal incorporation may serve to ‘purge’ deleterious mutations. See figure 1 for ‘asexual’ (sensu lato) reproductive modes in vertebrates.
Automixis: form of ‘asexual reproduction’ that includes the union of meiotic products of an individual (note: some authors use the term more broadly to encompass any form of uni-individual reproduction that includes meiosis or a meiosis-type process, including premeiotic endomitosis).
Bisexual: a population or species composed of male and female (=gonochoristic) individuals.
Clone: (noun) biological entity (e.g. gene, cell, or multicellular organism) that is genetically identical to another; alternatively, all genetically identical entities that have descended ‘asexually’ from a given ancestral entity; (verb) to produce such genetically identical entities or lineages.
Clonal: mode of inheritance by which the entire genome is transmitted unaltered (although rarely subgenomic amounts of DNA may be added or altered).
Conspecific: belonging to the same taxonomic species (opponyms: allospecific, heterospecific).
Premeiotic endoreplication (=endomitosis): chromosomal replication within a cell that does not divide.
Gamete: a mature reproductive cell (egg or sperm).
Gametogenesis: the process by which sex cells are produced.
Germline: the lineage of cells leading to an individual's gametes.
Gynogenesis (synonym: sperm-dependent parthenogenesis or pseudogamy): see figure 1.
Hemiclone: the portion (classically 50%) of a genome that is transmitted intact, without recombination in a hybridogenetic lineage.
Hemiclonal reproduction: mode of inheritance by which gamete production is partly (classically 50%) clonal, like in diploid hybridogenesis.
Heterogametic sex: the sex that produces gametes that each contain one of two different types of sex chromosomes.
Heterozygosity: the percentage of heterozygotes or loci in a heterozygous state in an organism or population.
Heterozygotes: a diploid organism possessing two different alleles at a specified genetic locus.
Homozygotes: a diploid organism possessing the same alleles at a specified genetic locus.
Homogametic sex: the sex that produces gametes that all contain the same type of sex chromosomes.
Hybridization: the successful mating of individuals belonging to genetically different populations, lineages, or species.
Hybridogenesis: see figure 1.
Intrinsic reproductive isolation: genetically caused post-zygotic mechanisms such as hybrid inviability, decreased fertility, sterility and hybrid breakdown that prevent sexual organisms from producing fully fertile multi-generation hybrids.
Introgression: the movement of genes (gene flow) between populations, lineages, or species via hybridization.
Kleptogenesis: see asexual reproduction and figure 1.
K2P-corrected distances: nucleotide-sequence divergences (here based on mitochondrial DNA) calculated using the Kimura-two-parameter (K2P) model, the best metric when genetic distances are low [74].
Meiosis: the cellular process whereby a germline cell divides to form gametes containing half the chromosomes of the parent cells (usually including crossing over and recombination).
Meroclonal: (mero-, Greek: ‘partial’) partly clonal gamete production of triploid (or other polyploid) organisms, first described from allotriploid water frogs.
Mitosis: the process of cell division that produces daughter cells with the same chromosomal constitution as the parental cells.
Oogenesis: the production of oocytes, egg cells or ova.
Parthenogenesis: see also asexual reproduction and figure 1; obligate parthenogenesis is a reproductive mode by which offspring (at least an embryo) is produced from an egg without genetic contribution of sperm; in vertebrates, this reproductive mode is mostly of hybrid origin, but see [75] for potential exceptions; some non-hybrid vertebrate clades (sharks, reptiles) can reproduce (occasionally) by so-called facultative parthenogenesis [76–78], which is neither of hybrid origin nor in the focus of this paper.
Paternal leakage: the occasional incorporation of a sperm or its mtDNA into an ovum of a gynogenetic organism and thereby into the resulting offspring.
Pseudo-bivalent: bivalent containing two identical (homozygous) chromosomes as a result of premeiotic endoreplication.
Sexual reproduction: prevailing mode of reproduction in metazoans, characterized by production of offspring via syngamy of meiotically produced gametes. Recombination and segregation of chromosomes (alleles) during meiosis result in genetically variable gametes and offspring.
Unisexual species: a species consisting exclusively of females or sometimes also applied to species with a strong female-bias.
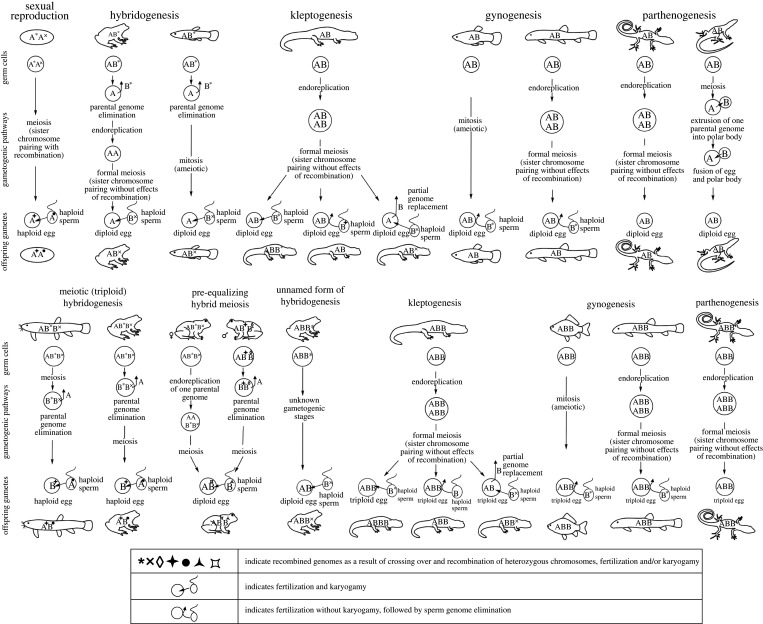
Figure 1.
Clonal, hemiclonal and meroclonal reproductive modes of hybrid vertebrates (diploids: upper row; triploids: lower row) in comparison with sexual reproduction (upper left). Each column shows parental individuals, gametogenic pathways with germ cells, gametes and offspring genome composition (expanded from Lamatsch & Stöck [49] and Stöck et al. [50]. Letters and symbols: A, B: genomes of parental species that formed the hybrid taxon/form; when lacking additional modifying symbols, the genomes are usually inherited and transmitted clonally; symbols are explained overleaf, below the figure. Description of reproductive modes: Sexual reproduction (upper row): oogonia enter a normal meiosis, which results in recombined haploid ova; after fertilization by haploid sperm from conspecific males, diploid offspring with recombined maternal and paternal genomes form diploid male or female offspring. Hybridogenesis (upper row): hemiclonal reproductive mode, during which the genome of one parental species is eliminated from the germ cells [51–56]; the genome of the other parental species is either endoreplicated and undergoes meiosis without effects of recombination (e.g. diploid water frog, Pelophylax esculentus [55,57,58]) or gametogenesis is ameiotic (e.g. the livebearing fish Poeciliopsis monacha-lucida [51,56]). Diploid hybrid offspring emerge after fertilization of the haploid ovum by recombined allospecific sperm, usually from a parental, sexual species. Unnamed form of hybridogenesis (lower row): Clonal diploid eggs are possibly formed by the elimination of one of the double copied genomes while the remaining genomes undergo endoreplication followed by meiosis without effects of recombination (P. esculentus example [59,60]). Kleptogenesis: (upper and lower rows): occurs in unisexual salamanders, Ambystoma [61]. The genome of germ cells is endoreplicated, undergoes meiosis without effects of recombination resulting in diploid eggs (above) or triploid eggs (below) [62,63]. Ova may either be activated by allospecific sperm without karyogamy, i.e. like in gynogenesis (middle), be truly fertilized, leading to ploidy elevation of offspring (left), or sperm may in part replace one of the maternal genomes in the egg, followed by its partial elimination (right) [54]. Gynogenesis (upper and lower rows): formation of clonal gametes by an ameiotic process (example: Poecilia formosa, upper row [47,64]; example: Carassius langsdorfii, lower row [65]) or endoreplication (example: diploid Cobitis elongatoides-taenia, upper row [66,67]; example: triploid Cobitis 1elongatoides-2taenia, lower row [66–68]) of genomes in germ cells followed by meiosis without effects of recombination. Diploid gametes (upper row) or triploid gametes (lower row) are fertilized without karyogamy, followed by sperm genome elimination. Parthenogenesis (upper and lower row): Clonal gametes form via endoreplication of genomes in germ cells followed by meiosis without effects of recombination (example: Aspidoscelis tesselatus, upper row [69]; example: Aspidoscelis uniparens, lower row [70]). Alternatively, the genome of one parental species is extruded into the polar body and then fuses with the egg, restoring diploidy (Darevskia unisexualis) [71]. Eggs develop without sperm/fertilization. Meiotic (triploid) hybridogenesis (lower row): recombined haploid gamete formation after meiotic (example: Misgurnus anguillicaudatus [72]) or premeiotic elimination of a single copied genome (example: P. esculentus [55]); offspring are diploid. Pre-equalizing hybrid meiosis (lower row): occurs in allotriploid Batura toads (Bufo(tes) baturae) [50,73] and presumably also in related taxa (§5c(iii)). In females (left), a single copy genome (A) is separately endoreplicated and enters meiosis as ‘pseudo-bivalents' (box 1), along with bivalents of heterozygous chromosomes from another parental species (BB′). The formally tetraploid meiosis results in diploid gametes. Males (right) eliminate the single-copy clonal genome (A), while the two remaining genomes (B) undergo a normal meiosis (BB′) and form haploid recombined sperm. Batura toads present the only known gonochoristic vertebrate taxon with simultaneous Mendelian (BB′) and clonal (A) genome transmission. Fertilization results in triploid offspring of both sexes.
(a) . The evolution of intrinsic reproductive isolation
Intrinsic postzygotic isolation (i.e. decreased fertility, sterility or even inviability of interspecific hybrids) is an important spectrum of mechanisms of reproductive isolation that prevents many related species from merging [4]. For more than 80 years, there has been a prevailing view that intrinsic postzygotic isolation arises as a result of accumulating (Bateson–)Dobzhansky–Muller (BDM) incompatibilities at individual genes that diverged between species to a degree preventing proper chromosome pairing or interaction of their protein products in hybrids [79–81]. The search for ‘speciation genes' involved in such incompatibilities led to the discovery of several candidates in various taxa [82,83]. Such candidate genes have common characteristics, defined by relatively fast evolution, often driven by positive selection and coevolutionary arms races (e.g. [84–86]). Nevertheless, the evolution of intrinsic postzygotic isolation is a complex process that, beyond incompatibilities between individual protein products as assumed by the original Dobzhansky–Muller model, includes additional mechanisms. For instance, it may be driven by overall divergence of noncoding DNA [87], as similarly predicted by Bateson [88], whose concept is analogous to a current chromosomal speciation model [89]. It predicts diverging lineages to accumulate mutually incompatible changes in karyotypes, causing problems in meiotic homology search, synapses and bivalent formation in hybrids, leading to aborted gametogenesis [90,91]. Reproductive isolation may also result from a disrupted regulatory cross-talk between merged genomes [92], which may, for example, result in the activation of transposable elements in hybrid genomes [93–96].
(b) . The prominent role of sex chromosomes in speciation
Sex chromosomes play key roles at the origin of intrinsic postzygotic reproductive isolation [97–99]. Research in many animals, including vertebrates, led to two more or less general ‘rules of speciation' involving sex chromosomes: (i) Haldane's rule, predicting increased sterility or inviability of the heterogametic sex (i.e. XY males or ZW females) [100,101] and (ii) the large-X effect ([102]; discussed in [103,104], assuming a disproportionately large effect of the X chromosome (or the Z chromosome in organisms with heterogametic females) on reduced hybrid fitness compared to autosomes. Both rules were generally attributed to recessive hybrid incompatibilities, manifested if present on the hemizygous parts of the X or Z chromosomes in the heterogametic sex. In addition, such incompatibility loci may be manifested if present on the non-pairing Y or W chromosomes—these, however, usually harbour relatively few genes and their role for speciation may thus be limited, even in strongly heteromorphic sex chromosomes like in mammals and birds [81].
Other explanations of Haldane's rule and the large X-effect may include generally faster rates of molecular evolution on the X and Z chromosomes [105,106], rapid coevolutionary arms races between sex-linked segregation distorters and their suppressors [107] or failure of epigenetic inactivation of sex chromosomes during meiosis [108,109]. A possible activation of endogenous retroviruses on the W chromosome may also explain Haldane's rule in birds with highly heteromorphic sex chromosomes [96]. Filatov ([110] and citations therein) recently concluded that haploid expression and species-specific Y-degeneration need more attention regarding their roles in speciation. Thus, both major rules of speciation may represent composite phenomena, resulting from different causes active in different contexts [111]. Until recently, undifferentiated sex chromosomes have been hardly accessible by genetics for many species, and empirical sex chromosomal sequence data are just becoming available through chromosome-scale genomics.
(c) . The speciation continuum of diploid lineages
Reproductive isolation of diploid lineages tends to increase with genetic distance [87,112], and thus with divergence time [15,17,113], usually as a series of ‘small steps rather than a single genetic revolution' [114]. In this ‘speciation continuum' [14,115,116], we witness diverging evolutionary lineages anywhere between near-panmictic populations along various levels of partial separation up to complete reproductive isolation, causing many of the controversies over ‘what is a species?' [117,118]. Diverging lineages often show permeable boundaries across some parts of the genome, while loci underlying reproductive isolation resist introgression, resulting in a highly heterogeneous differentiation landscape across the genome. This includes regions with low differentiation as well as genome parts that are considerably differentiated (differentiation islands), potentially corresponding to loci resistant to introgression [119–121]. Proportions of such differentiated regions may expand with divergence time and accumulate reproductive isolation. This also allows measuring the speciation stage for a given pair of species [122] (table 1).
Usually, when the divergence between incipient species increases, so does the amount of incompatibilities, negatively affecting the fitness of interspecific hybrids [11,123,124]. Along this speciation continuum [14], hybrid fitness may in some cases even increase (hybrid vigor), potentially facilitating introgression. Nevertheless, at later stages, hybrids' fitness inevitably decreases (see 3.1), often first being affected by impaired gametogenesis and other adverse effects. These include impairments of the ability to reproduce, often initially affecting the heterogametic hybrids [100,125,126], and subsequently by reaching complete reproductive isolation (complete infertility or inviability of hybrids). This trajectory suggests that pre-meiotic and meiotic gametogenetic processes may be more vulnerable to intergenomic incompatibilities than traits related to the viability of hybrids (see §3a).
2. Sex chromosomes in hybrids along the speciation continuum
(a) . Sex chromosomes of hybrids in early stages of divergence: introgression, genetic interaction and/or dominance and multiplication
Under secondary contact of diverging lineages, introgression in hybrid zones into the parental gene pools requires that some of the hybrids are fertile and can backcross with the parental lineages. Multi-generation backcrosses only occur between incipient species, i.e. under incomplete reproductive isolation.
Generally, in such situations, X and Z chromosomes introgress less across the hybrid zones than do autosomes in many vertebrates, including fish [127], birds [27,28,36] and mammals [29,30]. Most of these taxa feature heteromorphic sex chromosomes, suggesting that greater heteromorphy and thus hemizygosity (i.e. unequal gene content causing potential dosage imbalances) increase the chances for sex chromosome dosage imbalances and postzygotic hybrid incompatibilities (Haldane effects). This was also supported by simulations [128]. In fruit flies (Drosophila) with large-sized sex chromosomes, intrinsic postzygotic isolation evolved relatively earlier than in species possessing smaller sex chromosomes [129].
So far, only some empirical population genetic studies have been accomplished in hybrid zones with undifferentiated sex chromosomes, comparing introgression at sex-linked versus autosomal markers. Data from amphibians with homomorphic sex chromosomes pointed to large X-effects in hylid frogs [25] or apparent absence of such effects in bufonid toads [26]. A metastudy of interspecies crosses suggested that higher levels of sex chromosome heteromorphism were associated with stronger reproductive isolation [130]. Taken together, among closely related lineages, sex chromosome introgression appears to be easier the less differentiated these sex chromosomes are.
Several examples from teleosts suggest that introgression of sex chromosomes in an early stage of divergence of evolutionary lineages may not only result in interactions among parental sex chromosomes (e.g. in hybrid zones), but even in the evolution of multiple sex chromosome systems or new sex-determining systems (table 2). Namely, certain platyfish (Xiphophorus maculatus) populations possess multiple sex chromosomes (X, Y, W; [20]), where Y is dominant over X, and W over Y, so that YY- and XY-individuals develop into males, while XW-, XX- and WY-individuals become females [20,21]. Pure WY versus XY populations had been described by Kallman [20], who also showed that the Y that co-occurs with the W, is homologous to the Y, found in the northern populations with the X, which therefore cannot be deemed Z. Whether this system stems from secondary contacts of incipient species and hybridization still remains unexplored (M. Schartl 2020, personal communication) but it could explain the occurrence of multiple sex chromosomes.
Table 2.
Expected sexual genotypes and phenotypes in the F1 of interspecies crosses at hybridization of an XX/XY and a ZZ/ZW sex determination system, with dominant Y or W versus recessive y or w. While all ZY/Zy genotypes, irrespective of the dominance of the Y, presumably develop into males, all XW/Xw probably become female, whereas XZ phenotypes are hardly predictable, as they depend on the unknown XZ dominance/recessiveness, which may cause ♂ male, ♀ female or ⚥ intersex F1-phenotypes.
| parents, genotype, phenotype | XY, dominant Y, ♂ | XY, recessive y, ♂ | any XY, ♂ | any XY, ♂ | XX ♀ |
|---|---|---|---|---|---|
| ZW, dominant W, ♀ | ZY: presumably ♂ | Zy: presumably ♂ | XW: presumably ♀ | XZ: ♂,⚥,♀ | — |
| ZW, recessive w, ♀ | ZY: presumably ♂ | Zy: presumably ♂ | Xw: presumably ♀ | XZ: ♂,⚥,♀ | — |
| ZZ, males, ♂ | — | — | — | — | XZ: ♂,⚥,♀ |
Multiple different sex chromosomes of questionable hybrid origin are also known in anurans. Roco et al. [131] showed the coexistence of three sex chromosomes (Z, Y, W) in the clawed frog, Xenopus tropicalis, in which no master sex determination gene is known [132]. In laboratory triploids, ZZW genotypes developed as females, but YWW into males, showing the Y is a much stronger male determiner than the Z; while the Z of X. tropicalis can determine maleness only in the absence of W [131]. Importantly, commenting on the relative ‘strength' of sex chromosomes, Schartl [133] concluded that this hierarchy in multiple sex-chromosome systems is context-dependent and can vary in different organisms. Recently, nucleotide polymorphisms of expressed transcripts suggested genetic degeneration on the W chromosome, emergence of a new Y chromosome from an ancestral Z chromosome, and natural co-occurrence of the W, Z and Y chromosomes in the same X. tropicalis population [134]. Again, a hybrid origin seems likely but is pending confirmation.
Few if any empirical data are available for hybridization of female (ZZ/ZW) and male (XX/XY) heterogametic systems with dominant versus recessive sex chromosomes; table 2 shows the assumed phenotypes under such conditions. Importantly, while all ZY-genotypes may develop as males and XW into females, irrespective of the dominance, XZ phenotypes are hardly predictable, since they depend on the unknown XZ dominance/recessiveness, which may cause male, intersex or female F1-phenotypes (table 2).
In Tiliapinae fish, male-heterogamety (XY) on linkage group 1 (LG1) coexists with a female-heterogametic system (ZW) on LG3, sometimes within the same species or populations (e.g. Oreochromis aureus, O. mossambicus; [22,23]), where W is dominant over Y, resulting in ZWXY females. Also, in Haplochrominae, a male-heterogamety (XY) on LG7 co-occurs with female-heterogamety (ZW) on LG5, intraspecifically or in populations (e.g. Metriaclima pyrsonotus [24]). Again, W dominates over Y, causing ZWXY to be females. The latter authors speculate that interspecific hybrids with different sex-determining systems may produce intersexes with reduced viability or fertility, directly contributing to postzygotic isolation [24]. This suggests that even in early stages of divergence, undifferentiated, in this case non-homologous, sex chromosomes may over-proportionately contribute to the onset of emerging reproductive isolation [135].
Another well-examined teleost example involving, however, heteromorphic sex chromosomes under relatively early divergence, comprises the Central American mosquito fish (Gambusia holbrooki, G. affinis), with a divergence time of ca. 2–7 Ma ([31] and citations therein). Here, the heteromorphic ZW sex chromosomes of G. affinis females and the homomorphic XY of G. holbrooki males present different linkage groups and evolved independently from separate autosomes. In interspecific laboratory hybrids, the Y is dominant over the W chromosome, and X is dominant over Z, in agreement with nonlinear gene flow in a hybrid zone between both species [136].
Hybridization and introgression thus seem to lead to sex chromosome interactions in hardly predictable dominance hierarchies, which either cause ‘evolutionary melting pots' or ‘Darwinian laboratories' with multiple contacts and interactions [137], containing multiple sex loci and/or chromosomes and hypothetically may drive diversification and potentially reinforce the speciation process [135]. More generally, sex-biased introgression and recombination may lead to sex-specific consequences of hybridization and thereby fuel speciation [138].
(b) . Sex chromosomes of hybrids in early stages of divergence: hybrid origin of sex chromosomes and evolution of new sex determination systems
While the systems described above (§2(a)) exemplify that genetic and thus evolutionary interactions by hybridization between incipient or even further separated species may result in hardly predictable outcomes, they nevertheless demonstrate considerable evolutionary impact of sex chromosomes during early divergence. Their introgression may even lead to the establishment of new sex chromosomes and thus sex determination systems. A well-characterized example from teleosts is the Y chromosome in the stickleback, Pungitius pungitius. This Y arose by introgression from P. sinensis [139], although current hybrid F1-males are sterile, females are fertile [140], suggesting that the Y-introgression happened in an early/-ier stage of divergence [139].
An intensely studied anuran hybrid sex chromosome system is that of the Japanese frog Glandirana (previously Rana) rugosa, with five genetic lineages. The West-Japan and East-Japan lineages feature undifferentiated, yet unidentified XX/XY-chromosomes, while the eastern XY-group shows differentiated male heterogamety of chromosome 7. This chromosome bears a ZW sex determination system in northwestern Japan, while a Neo-ZW system occurs in western Central Japan [32,141,142]. The Neo-ZW group, which has a different origin from the ZW-group, shares mitochondrial haplotypes with the geographically proximate XY-group. Nuclear single nucleotide polymorphisms (SNPs) showed the Neo-ZW2 genome to share alleles with the XY-group and partly the Neo-ZW1 group, indicating a hybrid origin of Neo-ZW2. Its sex-linked SNPs on the W stemmed mostly from X chromosomes (XY-group), while alleles on the Z originated from the Z (Neo-ZW1) as well as from Y chromosomes (XY group), suggesting that hybridization of two opposite sex-chromosome systems led to a female heterogametic system by recycling the existing X chromosomes into new W chromosomes. Thus, a new sex-chromosome system evolved by reusing genomic material from ancestral sex chromosomes [33,143]. Populations of G. rugosa at the SW-edge of the Neo-ZW group exhibit homomorphic XY-sex chromosomes, but shared mitochondrial haplotypes with the heteromorphic XY-group to the east of its range. Ogata et al. [34] concluded that the heteromorphic sex chromosome systems independently reversed back to or were turned over to a homomorphic system at the edges of the Neo-ZW group through hybridization with the West-Japan group, bearing homomorphic sex chromosomes.
Taken together, in relatively earlier stages of divergence, hybridization and introgression of sex chromosomes into foreign gene pools may even lead to the evolution of intermediate or new multilocus sex determination systems. From the examples at hand, this seems much easier in closely related species with undifferentiated sex chromosomes than in more diverged lineages with differentiated sex chromosomes (table 1; cf. [144]). When closely related species differ in their sex determination systems, the outcomes might be more complex than in cases with the same or similar sex determination systems (table 2).
3. The ‘extended speciation continuum'
(a) . A new term
Historically, the botanist Alfred Ernst [145] noted that the divergence between parental species predetermines the type of gametogenesis in hybrids—which supposedly follows a continuum from sexual reproduction—when closely related lineages hybridize, through obligately ‘asexual’ hybrid seed production at intermediately distant species, to purely vegetative reproduction in hybrids of distant parents. Focusing on vertebrates, Wetherington et al. [146] considered a similar concept, which later was developed by Moritz et al. [147] into the ‘balance hypothesis’. It states that the formation of ‘asexually' reproducing hybrids (box 1) is particularly likely when the genetic divergence between parental genomes is large enough to distort hybrid gametogenesis towards producing a high proportion of unreduced gametes, but not too large to significantly affect hybrid viability or fertility. Discussing the balance hypothesis, Stöck et al. ([148], supported by [149,150]), also emphasized that ‘asexual' vertebrates are very rarely formed (e.g. 0.5% of reptile species [39,151,152]) since both sufficient divergence and generally complex genetic preconditions are necessary to naturally produce viable and fertile clonal genomes and phenotypes (‘rare formation hypothesis' [148]).
However, once a window of favourable genetic divergences among hybridizing species occurs, the stage is temporally set for specific combinations of their genomes, potentially allowing repeated origins of natural ‘asexual' lineages. These in turn may promote the formation of allopolyploid lineages/species, either immediately or by incorporation of additional genomes upon fertilization of their unreduced gametes (i.e. the ‘genome addition hypothesis', e.g. [35,153]; §3b). Such shifts in hybrid reproduction [46,154] as well as the triggers for allopolyploidization [155,156] have traditionally been examined separately from classical research on speciation, but as we would like to point out, there is a great overlap between both phenomena.
At the molecular level, the mechanisms underlying hybrid sterility and hybrid ‘asexuality' remain elusive but several independently proposed concepts share interesting parallels. For example, Moritz et al. [147] proposed that gametogenic aberrations leading to hybrid asexuality arise as a consequence of accumulated gene-to-gene incompatibilities between hybridizing genomes, which conceptually matches the Dobzhansky–Muller genic view on speciation. De Storme & Mason [157] rather proposed that unreduced gametes may be formed in response to decreased homology, preventing proper pairing of orthologous chromosomes, which is analogous to Bateson's [88] non-genic model, currently considered in chromosomal speciation models [158]. Alternatively, Carman [159] suggested that gametogenesis in ‘asexuals' is a consequence of a hampered cross-talk between diverged regulatory programs, combined by hybridization, which exemplifies the important role of postzygotic trans-regulatory incompatibility, recently also considered in speciation research (e.g. [92]).
Hybrid sterility and inviability on the one hand, and a shift in hybrid reproduction to clonality on the other, may both be considered as forms of (partial) postzygotic isolation, evolving along the speciation continuum [38], because the production of clonal gametes by hybrids also reduces the frequency of interspecific introgression by backcrossing into the parental sexual gene pool. As discussed by Janko et al. [38], hybrid clonality could thereby contribute to speciation (table 1) before the parental lineages reach complete reproductive incompatibility.
Thus, a century after the seminal works by Bateson [88] and Ernst [145], it appears that the research in the fields of speciation and on hybrid clonal, hemiclonal, meroclonal (‘asexual’) and allopolyploid vertebrates would greatly benefit from greater synergy. To provide a framework for such a synergy and to link the evolution of hemiclonal, clonal or meroclonal ‘asexual’ inheritance mechanisms in allodiploid and allopolyploid species to the concept of the ‘speciation continuum’, we here coin the term ‘extended speciation continuum’.
This new term frames three conceptual steps: profound divergence [147] between two lineages (i) first causes pre-meiotic or meiotic, potentially sex-specific, intrinsic hybrid incompatibilities in gametogenesis ([101, cf. [38]), and (ii) leads to increased potential production of unreduced gametes (e.g. by emergence of endo-duplication) that may rarely either directly lead to the establishment of an ‘asexual' allodiploid lineage/species and/or (iii) at the same time strongly increase the chances of producing unbalanced, meroclonal triploids or directly (or via this ‘triploid bridge' [160]) evolve balanced allotetraploids (cf. [35]). While we develop the concept for vertebrates, future research should evaluate its relevance beyond this group.
(b) . Cytological mechanisms of ‘asexual' reproduction of hybrid vertebrates and link to polyploidy
With few potential exceptions ([75], box 1), all hemi-, mero- and clonally (asexually) reproducing vertebrates are of hybrid origin [144,161], and hemiclonally or clonally reproducing F1 progenies have also been obtained upon experimental crossing of certain sexual species ([162] and citations therein). Hybridization thus may affect pre-meiotic processes and/or hybrid meiosis, leading to the production of unreduced gametes with hemiclonal or clonal transmission of (at least parts of) the hybrids' genome [49,163,164]. These forms of ‘asexuality' (sensu lato, box 1) in vertebrates, are cytologically characterized by a wide spectrum of gametogenetic mechanisms that range from completely ameiotic processes (apomixis), via hemiclonal mechanisms (classical hybridogenesis) to those involving more or less aberrant meiotic divisions (automixis [165–167], box 1 and figure 1). One gametogenic pathway commonly evolved by ‘asexual' vertebrates is premeiotic endoreplication (figure 1), during which the proliferating germ cells auto-duplicate their chromosome sets, so that identical homozygous copies pair during the subsequent meiotic division, which results in unreduced gametes and a lack of variability among offspring [62,69].
The production of unreduced gametes may consequently pave evolutionary pathways to animal polyploidy, leading to triploid hybrids and then, by further genome addition, to allotetraploids, e.g. by the so-called ‘triploid bridge' ([160,168]; citations in [153]). It has also been proposed that clonal reproduction may facilitate initial establishment of new rare polyploids [35,153,169,170], which may become instantly reproductively isolated from their diploid ancestors and avoid back-crossing producing triploid or aneuploid, potentially infertile progeny. However, empirical data from plants [171] and animals show many exceptions of fertile triploids [50,172,173]. In their balance hypothesis, Moritz et al. [147] had also proposed that incorporation of additional genomes into a diploid ‘asexual' hybrid would affect fecundity and viability of allopolyploids by shifts in genome dosages in the hybrids. Such ploidy shifts may cause dosage imbalances between the gene products, potentially causing ‘asexuality'. Indeed, while many triploid hybrid vertebrates with ‘imbalanced’ genomes (e.g. AA'B or AB'B genome-types) usually reproduce by clonal or meroclonal (i.e. ‘partially clonal', box 1) reproductive modes [46], polyploids with ‘balanced' genome configurations, like AA'BB' tetraploids, often reproduce meiotically (e.g. [35,174]), i.e. possibly even facilitating the formation of novel tetraploid species [175]. This suggests that genomic imbalance and divergence are causal for maintenance of clonal reproductive modes [35,147].
Cytogenetically, one may think of these phenomena as follows: Under a certain divergence of hybridizing lineages (cf. [147]), multivalents and thus mis-segregation and chromosome rearrangements during meiosis are expected, posing obstacles to polyploid evolution owing to resulting aneuploidy [35,176,177]. By contrast, fewer inter-lineage multivalents (i.e. of orthologous) may occur when hybridizing lineages exhibit an even greater divergence and genome differentiation [176], i.e. when orthologous chromosomes of the parental lineages no longer match (find) chromosomes in hybrid meiosis, so that new allodiploid ([35,149,157] and citations therein) and especially allopolyploid hybrid lineages [178] may evolve immediately. Indeed, genetic divergence is greater for parents of allopolyploid than of homoploid plant hybrids [179]. Production and/or occasional fertilization of unreduced gametes owing to disturbed premeiotic or meiotic processes in hybrids offers several, in part identical pathways to the evolution of allopolyploid taxa [35,153,179], another evolutionary pathway to overcome hybrid infertility (table 1).
At least in vertebrates, natural allodiploid and allopolyploid, hemiclonally or clonally reproducing taxa, or even allotetraploid meiotic species, arise mostly at relatively similar divergences between their parental lineages (figure 2). Probably as a consequence, also the likelihood of allopolyploid establishment scales with the genetic divergence between hybridizing lineages [179].
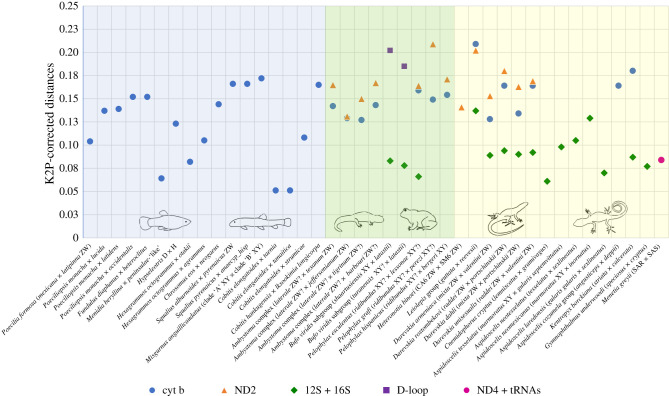
Figure 2.
Distribution of the K2P-corrected distances (box 1) between parental taxa of ‘asexual' hybrids in teleost fish, amphibians and reptiles, calculated from different available mitochondrial DNA sequence data. Sequences of cytochrome b (cyt b), NADH dehydrogenase subunit 2 (ND2), NADH dehydrogenase subunit 4 and adjacent tRNAs (ND4 + tRNAs), 12S and 16S rDNA (12S + 16S) and the mitochondrial D-loop (D-loop) were analysed; for details on species names: electronic supplementary material, table S1; for sex determination of parental species: electronic supplementary material, table S2; for data and methodology: electronic supplementary material, file S2. Abbreviations: XY, parental species is male heterogametic XX/XY; ZW, parental species is female heterogametic ZZ/ZW; species names without XY or ZW addition, sex determination in parental species is unknown; ? after XY or ZW indicates that sex determination was inferred (e.g. from crosses or based on apparent evolutionary conservation in the complex).
Beyond comprising a potential form of reproductive isolation, ‘asexual' reproduction and evolutionary shifts to allopolyploidy can also present ‘evolutionary escape routes' for hybrids from complete sterility. Indeed, interspecific hybridization may induce alterations of gametogenetic pathways, sometimes giving the hybrid a possibility to alleviate the problems of improper orthologous pairing (e.g. inverted meiosis in butterflies [180]). Likewise, clonal gametogenic pathways, as premeiotic endoreplication, may also enable hybrids to successfully pass meiotic checkpoints [66] and to transmit at least parts of their genomes, despite the problems they experience with postzygotic incompatibilities [35]. Processes involving some type of hybrid-origin clonality allow the existence of hybrid vertebrates in the ‘extended speciation continuum’.
(c) . Empirical support for the concept of the ‘extended speciation continuum’
The assumption that ‘asexual' reproduction may arise as a consequence of accumulating incompatibilities was supported by two meta-studies in hybrid lizards [181] and fish [38] that compared the occurrence of reproductive anomalies in hybrids with the genetic divergence of their sexual parental species, approximated by their mtDNA sequence divergence. The genetic divergence between parental species of these parthenogenetic lizards or gynogenetic fish was significantly higher than between species producing viable gonochoristic/sexual hybrids. Species pairs producing ‘asexual' hybrids were also less diverged than those producing sterile fish hybrids [38]. Similarly, in Palearctic green toads (Bufo or Bufotes viridis subgroup), the parental lineages of diploid sexually reproducing hybrids at secondary contact zones [15,26] are much more closely related than two deeply diverged nuclear clades (6 Ma) that formed the maternal and paternal ancestors of all meroclonal allotriploid and meiotic allotetraploid taxa [35].
Of note, the production of ‘asexuals' coincides with the formation of sterile hybrids in certain species/hybrid complexes, like e.g. Cobitis loaches [38], killifish, Fundulus [182] or medaka, Oryzias [183]. Natural hybridization between the loaches Cobitis elongatoides and C. taenia, diverged approximately 9 Ma, yields sterile diploid males with improper chromosome pairing and bivalent formation during the first meiotic division. In diploid hybrid females, gonial cells undergo premeiotic endo-duplication of chromosomes, form bivalents and clonal progeny (figure 1) [38,66]. Hence, both reproductive isolating mechanisms (hybrid sterility and ‘asexuality') may occur simultaneously and some ‘asexual' pathways may not only serve as reproductive barrier but also as at least temporal ‘remedy' preventing sterility.
In addition, we have compiled or calculated the K2P-corrected distances between parental taxa of 41 ‘asexual' hybrids in fish, amphibians and reptiles, analysed from available mitochondrial DNA sequence data (electronic supplementary material, table S1, files S2 and S3). Parental K2P-distance data for lineages of 17 teleost fish, 9 amphibians and 15 reptiles (figure 2) show them all to be greater than approximately 5% and to reach up to approximately 22%. While our data can only be a rough approximation, and part of the observed variation stems from different mitochondrial markers (figure 2), they show that divergences between parental lineages are larger than intraspecific mitochondrial variation in gonochoristic taxa, which typically reach K2P distances of approximately 1–4% in fish (e.g. [184–186]), approximately 1–5% in amphibians (e.g. [187–191]) and approximately 1–3% in reptiles (e.g. [189,191]). Our data suggest that a genetic distance exceeding (most) intraspecific levels presents a major precondition to evolve a natural hybrid ‘asexual' vertebrate.
4. Sex chromosomes in hybrids in the extended speciation continuum
(a) . Sex-specific differences of cytogenetic mechanisms, gametogenesis and reproductive modes of hybrid clonal, hemiclonal and meroclonal vertebrates
There is another important aspect of the evolution of ‘asexual’ and several allopolyploid hybrids, which has an apparent analogy to the accumulation of postzygotic reproductive incompatibilities, i.e. the tendency to arise asymmetrically in both sexes. In particular, most ‘asexual' vertebrates exhibit strongly female-biased sex ratios, which is why they have also been referred to as ‘unisexual' or ‘all-female' species [49,164,192,193].
Such a female bias might result from the simple fact that (hemi-)clonal males cannot generate progeny on their own, since their reproduction requires ova; even in cases like androgenesis [194,195], where clonal sperm replaces egg nuclei from related females. This reliance on eggs could explain why hybrid males are often absent in ‘asexual' vertebrate taxa, even if they would be able to produce fertile (hemi-)clonal gametes.
However, there might be more fundamental differences between male and female hybrids in terms of their ability to undergo ‘asexual' gametogenesis. Although studies that compared sex-specific gametogenesis in ‘asexual' vertebrate complexes are scarce, they consistently suggest that hybrid females may reproduce ‘asexually', while males often cannot generate functional sperm [66,196–198]. For instance, research in loaches refers to the basis for different sex-specific outcomes. Hybrid males faced problems with pairing of homeologous/orthologous chromosomes and thus failed to pass meiotic checkpoints. By contrast, hybrid females of unknown genetic sex pre-meiotically endo-reduplicated their chromosomes in the oogonia and formed bivalents, formally recombining between self-duplicated sister chromosomes (auto-copies), which allowed successful accomplishment of oogenesis but yielded no variability among offspring (figure 1). Thus, despite completing the meiotic divisions, females reproduced clonally, while males were sterile [66,198,199]. Another type of asymmetries has been documented in medaka fish (Oryzias latipes x O. curvinotus), in which female hybrids yielded clonal ova by premeiotic endoreplication, while hybrid males skipped meiosis and generated a single unreduced diploid spermatozoid from each spermatogonium [183,200].
Differences between sexes exist also in ‘asexuals'' with genome elimination. For instance, in hybridogenetic water frogs (Pelophylax esculentus; see below), male and female hybrids typically eliminate the L(lessonae)-genome and produce hemiclonal gametes with only the R(ridibundus)-genome [201] (figure 1). However, some male hybrids produce the ‘opposite type' of gametes by eliminating the R-genome, while females do not show this genome elimination [202–204] (see below). Similarly, triploid hybrid bisexual Batura-toads (Bufo(tes) baturae; see §5c(iii)) exhibit sex-specific differences in elimination of one genome in males and its separate endoreplication in females (figure 1) [50,73].
Differences in gametogenesis and reproductive modes between male and female hybrids of many clonal, hemiclonal and meroclonal taxa may reflect complex patterns and depend, among others, on hybrid's ploidy and genome dosage. In some cases, diploid and triploid hybrids of the same sex that arose from the same parental species may differ in gametogenesis and/or reproductive modes. For instance, all-female diploid Poeciliopsis monacha-lucida hybrids, with an estimated divergence between the parental lineages of 5–6 Ma [205,206], are hybridogenetic (figure 1) [163], while all-female triploid Poeciliopsis hybrids reproduce clonally by gynogenesis [51]. Inverse patterns were revealed in the Cobitis hankugensis × Iksookimia longicorpa hybrid complex, with diploid hybrids reproducing gynogenetically and thus clonally, while triploid hybrids eliminate the single genome and do not undergo endoreplication [196].
Crossing experiments in loaches (Cobitis, Misgurnus), livebearers (Poeciliopsis) and whiptail lizards (Aspidoscelis) also demonstrated that the origins of female hybrid (asexuality) and male sterility are directly linked to their hybrid origin since both patterns immediately co-occurred in F1-hybrids [51,66,162,198,207]. Moreover, when Yoshikawa et al. [208] sex-reverted clonal diploid Misgurnus female hybrids into males, such sex-reversed males differed from sterile natural male hybrids by producing unreduced spermatozoa via endoreplication. This suggests that ‘asexual’ gametogenesis may depend on genetic rather than phenotypic sex determination (see §5a), making it tempting to speculate that emergence of ‘asexual' vertebrates could be linked to the evolution of sex chromosomes.
(b) . Sex chromosomes, Haldane's rule and Darwin's corollary at the establishment of hybrid clonal, hemiclonal, meroclonal and allopolyploid vertebrates
When the parental species of an ‘asexual' (or allopolyploid) species exhibits genetic sex determination, it can be assumed that at their initial (F1) hybridization Haldane's rule [100,209] could play a role. Importantly, most hybrid vertebrates feature homomorphic (presumably also molecularly undifferentiated) sex chromosomes (electronic supplementary material, table S1), and the question is how much Haldane's rule applies to them at all (§1b). However, if applicable, two hypotheses can be established: (i) ‘asexual' hybrids could be expected to evolve more easily in male heterogametic systems (XX/XY), with hybrid XX females being fitter but the heterogametic XY hybrids (males) being less fit, infertile or even absent. (ii) Alternatively, if ‘asexuality' of hybrid females arises similarly to hybrid sterility or inviability as a by-product of gene-to-gene incompatibilities (§3a), we may expect its preferential occurrence in female heterogametic systems (ZZ/ZW), because recessive incompatibilities first appear in heterogametic females (ZW). Premeiotic or meiotic aberrations, enabling the evolution of ‘asexuals’, would thus present Haldane effects. Intriguingly, the absence of ZZ males (predicted to be fitter) could arise owing to their inability to produce offspring on their own or by counterselection through backcrosses with the parental lineages.
To shed some light on these hypotheses and generally to infer whether sex determination systems play a role at the establishment of an ‘asexual' vertebrate complex, we have compiled the available evidence for sex-determining systems of the parental forms (electronic supplementary material, tables S1 and S2). Assuming that ‘asexuals', which share their parental genomes and just differ by ploidy and quantitative composition (e.g. AB, ABB or AAB), have a common hybrid origin (AB), out of 144 ‘asexual' vertebrate forms, we have chosen 52 complexes (with ancestry information: electronic supplementary material, table S1) that may be traceable to a single separate hybridization event. In 36 cases, out of these 52 complexes, we have no information about parental sex chromosomes/sex determination. In five ‘asexual' complexes, the information about genetic sex is available for only one parental species (2 ZZ/ZW, 3 XX/XY), and from eight ‘asexual' complexes sexual genotypes are known from both parents: 5 with a ZZ/ZW, and 3 XX/XY. Polyploid complexes with multiple (3 or 4) genome donors come exclusively from 3 female heterogametic (ZW) systems. Taken together, among 52 ‘asexual' taxa with known ancestry, for the vast majority of 36, information on sex chromosomes is entirely missing, 10 parental species possess ZW and 6 have XY sex determination systems. This suggests that it could be easier to evolve an ‘asexual vertebrate' in a female heterogametic system (hypothesis ii).
Other reasons underlying the different reproductive capacities of ‘asexual’ F1-females and their F1-brothers (§4a) at the basal hybridization of an ‘asexual' complex, however, may not be caused by genetic sex determination (only). For instance, Darwin's corollary [103,210] refers to asymmetric fitness in hybrids of reciprocal crosses [111] and Bateson-Dobzhansky–Muller-interactions between autosomal and uniparentally inherited factors, like cytoplasmic elements, maternal transcripts or sex chromosomes in heterogametic hybrids, which depend on the direction of hybridization, thus contributing to asymmetric reproductive isolation between parental lineages. This implies that randomness (i.e. which species is by chance the maternal and which is the paternal ancestor) regarding the direction of initial crosses could also be causal of whether this F1 may or may not give rise to a unisexual or allopolyploid lineage. Indeed, the maternal (mitochondrial) ancestors of multiple allopolyploid green toads stem always from the same clades [35], supporting such asymmetry.
A related hypothesis, testable in longer term, is whether hybrid vertebrate complexes with female-biased sex ratios (all-female species) may evolve owing to (or be influenced by) the dominance hierarchy of different (homologous or non-homologous) sex-determining loci of the parental species, e.g. similar to the sex determination systems in platyfish or some cichlids (see §2a).
(c) . Evolutionary expectations for sex chromosomes in polyploids
Except for some of the lizards, most hybrid-origin ‘asexual’ and allopolyploid vertebrates (see also §5d) feature undifferentiated sex chromosomes. This fits theoretical assumptions about the evolution of polyploids and sex chromosomes in general as Muller [211] attributed the rarity of polyploid animals to the disruption of sex determination under polyploidization. Duplication of degenerated sex chromosomes may imbalance sex versus autosomal gene expression [212], implying the rarity of polyploid animals with degenerate Y (or W). Therefore, Otto & Whitton [213] assumed polyploids to occur in animals with: (a) ‘asexual’ and hermaphroditic reproduction, (b) sex determination based on a Y-linked sex determiner rather than an X : A ratio, and (c) non-degenerate sex chromosomes and absence of dosage compensation (e.g. amphibians). Mable [214] and later similarly Wertheim et al. [215] excluded a single common explanation for the relative rarity of polyploid animals compared to plants. Using phylogenetic analyses, Evans et al. [216] concluded that soon after inferred sex chromosome turnovers in the amphibian phylogeny, polyploidization might evolve more easily and thus more frequently.
Muller [211] drew his conclusions from research on fruit flies, Drosophila, in which the X : A(=autosomes)-ratio is disrupted under polyploidy. Wertheim et al. [215] predicted the various sexual phenotypes resulting from polyploidization events under male (XY) or female heterogamety (ZW) of diploid parents with either a dominant male (Y) determiner or a dominant female-determining (W) locus (as well as sex chromosomes to autosomes ratios, unknown to play a sex-determining role in vertebrates). Under a dominant Y, the sex ratio is expected to be biased towards the heterogametic sex so that new tetraploids (XXXY, XXYY, XYYY) individuals will likely develop into males and only XXXX-individuals into females. However, strong sex-ratio selection should quickly restore the balance in natural populations [111,213]. By contrast, in female-heterogametic (ZZ/ZW) systems with a dominant W, where three-quarters of progeny (ZZZW, ZZWW, ZWWW) would be female, sex-ratio selection might be weaker. Polyploids would thus arise more easily in ZW-systems (which is, for example, in accordance with ZW-systems of clawed frogs, Xenopus; see §5c(i)) than in XY-systems under dominant drivers [215].
However, Wertheim et al. [215] did not discuss polyploid hybrids governed by varying numbers and thus dosages of sex chromosomes (table 3; for example, with a recessive Y: XY = male, XXY = intersex, XXXY = female; or with recessive W: ZW = female, ZZW = intersex, ZZZW = male), hybrids with multiple sex chromosomes resulting from allopolyploidy, or hybrids with more complicated dominance hierarchies (e.g. XZW or YZW triploids; XXZW or XYZW tetraploids etc.; for multiple sex loci in diploid hybrids: see §2a). Clawed frogs, Xenopus (see §5c(i)), may even have evolved a new master sex-determining gene in response to allotetraploidization [217], suggesting allopolyploidy may also de novo-generate a sex determination system.
Table 3.
Sex chromosomal genotypes and assumed sexual phenotypes of diploids and polyploids of crosses resulting from XX/XY and ZZ/ZW genotypic ancestors in vertebrates under dominant or recessive Y or W chromosomal sex determination. Symbols: ♂ male, ♀ female, ⚥ intersex and ? unclear.
| ploidy | sex chromosomes | dominant W | recessive w | sex chromosomes | dominant Y | recessive y |
|---|---|---|---|---|---|---|
| diploid | ZW | ♀ | ♀ | XY | ♂ | ♂ |
| triploid | ZZW | ♀ | ⚥ ? | XXY | ♂ | ⚥ ? |
| tetraploid | ZZZW | ♀ | ♂ | XXXY | ♂ | ♀ |
The complex implications from §§4a–c suggest that the type of hybrid gametogenesis and the sex-specific differences in many clonal, hemiclonal and meroclonal taxa may not only reflect a combination of particular parental genomes and, possibly, sex determination systems, but also their dosage. Whether and how sex-specific cytogenetic mechanisms and reproductive modes are linked to the sex chromosomal genotypes remains an open question.
5. Examples of sex chromosomes in hybrid clonal, hemiclonal, meroclonal (‘asexual’) and meiotic allopolyploid vertebrates
According to Neaves & Baumann [161] female-bias is found in about 80 vertebrates, while some form of hybrid clonality (asexuality) has been confirmed in approximately 140 forms of fish, amphibians and reptiles (electronic supplementary material, table S1). While there are major empirical knowledge gaps, here we provide examples for hybrid diploid/polyploid vertebrate complexes, most of which exhibit clonal, hemiclonal or meroclonal reproduction, and the current level of understanding about their sex chromosomal situations (electronic supplementary material, table S1). We focus on examples from fish, amphibians and reptiles that are relatively well-examined and exhibit a variety of sex-determination systems and reproductive modes.
(a) . Teleost fishes
(i) . Cobitidae
‘Asexuality' is frequently observed in this teleost family. Spined loaches represent a monophyletic, yet deeply divergent group with multiple independent hybridization events, resulting in more than 20 hybrid combinations varying in ploidy levels and reproductive modes, including both gynogenesis and hybridogenesis [72,218–223]. Hybrid females and males notably differ in their ability to reproduce; while diploid and triploid hybrid males are always sterile [196,224–226], hybrid females maintain fertility and reproduce either via gynogenesis or meiotic hybridogenesis (figure 1) [218,220–223]. Male sterility is evident by aberrant pairing of homeologous chromosomes resulting in the failure of meiosis and formation of aneuploid sperm [66,198]. On the other hand, hybrid females show premeiotic endoreplication of chromosomes, allowing normal pairing and meiotic progression with recombining identical copies of chromosomes (figure 1) [66,68,72,199,221]. In dojo loaches (Misgurnus anguillicaudatus), sex reversal of females by hormone treatment revealed that such males were able to produce unreduced spermatozoa via endoreplication like hybrid females [208]. This suggests that clonal gametogenesis is linked to female genetic sex and may depend rather on genotypic than on phenotypic sex. Therefore, the question arises whether the hybrid sex chromosomal configuration contributes to the evolution of ‘asexuality' and/or whether the sex-specific outcomes of inter-lineage hybridizations may be other Haldane-effects (§4b). The results suggest that genetic but not phenotypic sex determination controls the endoreplication ability in diploid hybrids. Male heterogametic sex determination was suggested in both dojo (Misgurnus) and spined (Cobitis) loaches, with the latter genus putatively possessing multiple sex chromosome systems [227–231]. Nevertheless, these reports for Cobitis involved individuals of uncertain genetic composition, with the possibility of their hybrid origin, as they had 49 chromosomes and were sampled from isolated populations [228,229]. In other sexual and hybrid species, the analysis of mitotic and meiotic chromosomes did not reveal any morphological differences between sex chromosomes and autosomes [232–234], requiring genomics to reveal potential sex-linked molecular differences.
(ii) . Poeciliidae
Poecilia formosa, the allodiploid hybrid approximately 100 ka-old all-female Amazon molly, produces clonal gametes by apomixis and reproduces by gynogenesis [64] (figure 1), in a system traceable back to a very few initial hybridization events [148,149]. Cytogenetic methods could not clarify the sex-determining system of its maternal (mitochondrial) ancestor [235], P. mexicana [236], while its paternal ancestor, P. latipinna, exhibits female heterogamety and heteromorphy [235]. Laboratory hybrids between the ancestral species (P. mexicana x P. latipinna) showed automictic gametogenesis [237] involving the random fusion of meiotic products after the second meiotic division. Masculinized diploid P. formosa, obtained by hormonal treatments [238], were examined regarding their sexual phenotype and behaviour, but whether their spermatogenesis is apomictic, like P. formosa oogenesis, has not been examined (M. Schartl 2020, personal communication). Natural triploid P. formosa are usually female [239,240], while unusual triploid males, possessing supernumerary microchromosomes, showed aberrant spermatogenesis, resulting in aneuploid sperm [149,241]. Genomics showed that genes that serve organs or processes that are no longer in use in the all-female fish, such as spermatogenesis, male development and meiosis genes, are not corrupted [149]. Genomic approaches should in the longer term also allow identification of the sex chromosomes in P. mexicana and their elucidation in the allodiploid P. formosa, in which most recent transcriptomic analyses of transcriptional divergence between different clonal lineages suggest that functional P. formosa allelic expression patterns do not simply reflect the ancestral situation of an F1-hybrid but potentially result from long-term selection of transcriptional fitness [242].
(b) . Amphibia, Urodela
The unisexual Ambystoma salamander complex comprises at least 24 hybrid combinations of diploid to pentaploid forms [243], involving nuclear genomes of two to five species [63,243–245].
Mostly triploid hybrid females (e.g. LLJ or JJL) undergo a premeiotic endoreplication (endomitosis) leading to hexaploid oocytes. Meiosis produces triploid oocytes that can be activated by sperm from gonochoristic species [245] (figure 1). Female hybrids obtain (steal) this sperm from five bisexual congeneric species, used only to trigger egg development by gynogenesis (sperm-dependent parthenogenesis), or for incorporation into the zygote to elevate the ploidy level (tetraploid to pentaploid), or to replace one of the female's haploid genomes, a reproductive mode in summary called ‘kleptogenesis' [243] (figure 1).
The complex likely arose from an ancient hybridization event of a female close to Ambystoma barbouri (providing its mtDNA [61,246]), and a dated phylogeny based on complete mitochondrial genomes [247] suggested the complex to be ca 5 Myr old. None of the unisexuals can be considered hybrids between any contemporary species. Although all unisexual combinations of Ambystoma include at least one A. laterale (L) genome [192], this does not represent the most ancestral hybrid, since the maternal A. barbouri ancestry implies that neither A. laterale nor A. jeffersonianum could have been the female that gave rise to the complex. Instead, the A. laterale genome present in all hybrids, as well as those genomes of all other sperm donors in the complex, are considered to come from males (likely containing a Z-chromosome).
Sessions [248] cytogenetically identified a ZZ/ZW sex chromosome heteromorphism in the diploid nuclear A. laterale (LL), and concluded that its Z (Lz) formed a diploid ancestral hybrid female (JwLz). The genome of A. jeffersonianum including its dominant W (Jw) appeared thus important to maintain all-female clones, and explained female triploids as JwJzLz and JwLzLz-genotypes [248]. This explanation, however, is in conflict with the later-identified maternal ancestry by A. barbouri that provided its mtDNA [61,246], and, if so, should have also contributed a W-chromosome (Bw in figure 3) to the F1-hybrid (e.g. BwLz). Since male sperm donors probably always add Z-chromosomes to the complex that are also considered to have replaced the ancestral nuclear A. barbouri genome [251], and thus its W, it remains unknown how a female condition could have evolved or be maintained in the complex. Robertson et al. [250] hypothesized that inter-genomic chromosome recombination [249] and translocations [252], which demonstrate that crossovers have occurred between homeologous chromosomes, and not only the sister (endoduplicated) chromosomes, could also have affected the sex chromosomes, and a translocated female, perhaps A. barbouri (W)-locus on an A. laterale chromosome, might thus explain the femaleness of the kleptogens [250]. Owing to the enormous genome size, genetic information on the sex chromosomes is still missing in the complex but by using genomic approaches female heterogamety (ZW) has also been shown in other Ambystoma [253], and generally, a dominant W could sufficiently explain the overwhelming unisexuality in the complex. However, a very few ‘unisexual' males (11 of 962 unisexuals) have been found in the complex; discussed and male meiotic figures provided by Bogart [246]. Molecular details of the sex chromosome evolution, function and interactions remain to be elucidated in the unisexual Ambystoma complex.
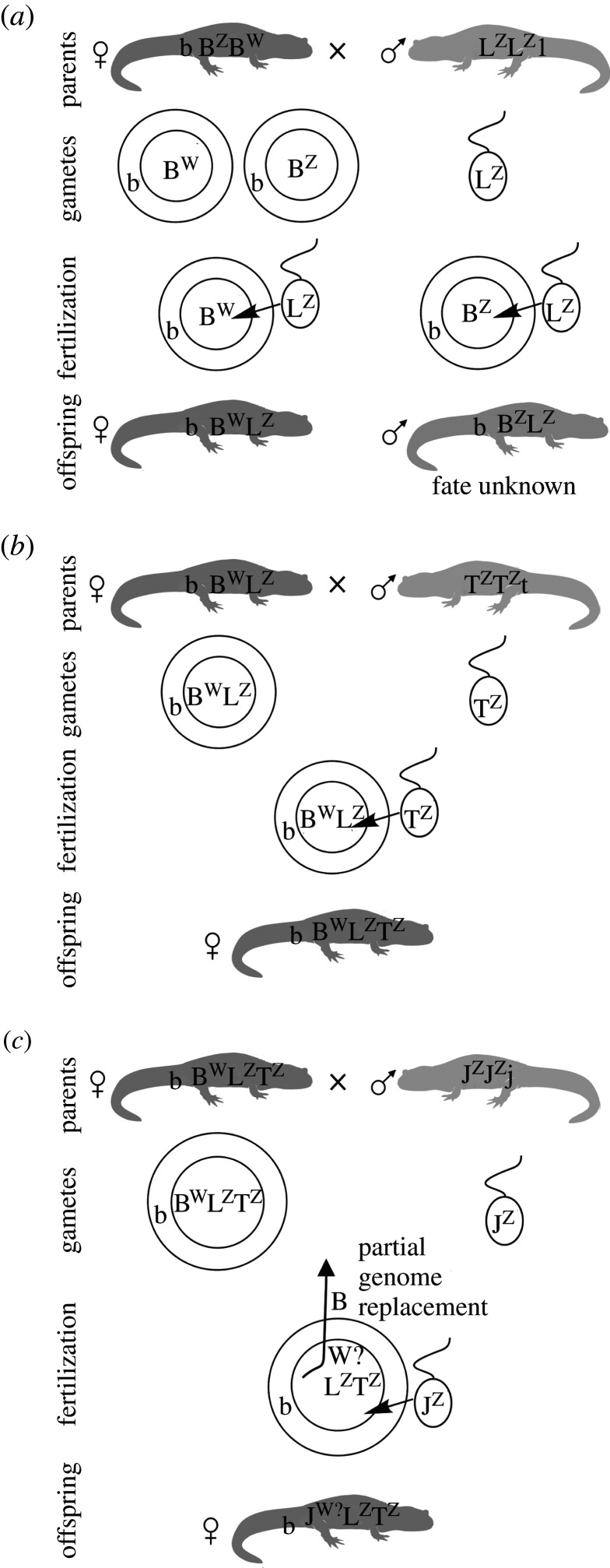
Figure 3.
Inferred primary hybridization event at the origin of the kleptogenetic mole salamanders (unisexual Ambystoma complex) and hypothetical sex chromosome transmission within this complex. Kleptogenetic unisexual Ambystoma (figure 1 and box 1), their gametes and the resulting offspring. (a) Mating of a diploid female as the maternal sexual ancestor (A. barbouri (BwBz)) with zW sex chromosomes and a A. laterale male (LzLz) with zz sex chromosomes resulted in a diploid clonal BwLz female F1-hybrid (left) and possibly a diploid BzLz hybrid F1-male. (b) Cross of the female F1-hybrid (BwLz) with an A. texanum (LzLz) male, sperm incorporation and thus ploidy elevation result in a triploid BwLzTz-female. (c) Kleptogenetic reproduction of a triploid (unisexual) BwLzTz-female and an A. jeffersonianum (JzJz) male, resulting in the replacement of the A. barbouri (B) genome by a paternal J-genome. The female-determining factor on the W chromosome of A. barbouri is hypothetically translocated (possibly by intergenomic recombination, as well known in the complex; [249]) to the J-genome and thus might have caused the emergence of JwLzTz females. W is the inferred dominant female-determining factor; z indicates recessive male-determining factors; b, j, l, t symbolize A. barbouri, A. jeffersonianum, A. laterale and A. texanum mitochondrial DNAs, respectively; silhouettes symbolize A. jeffersonianum: dark grey; A. laterale: light grey; their diploid, triploid and tetraploid hybrids: intermediate grey. Drawn according to the discussion in Robertson et al. [250].
(c) . Amphibia, Anura
Polyploidy evolved frequently in Amphibia (e.g. [156,214]) with 50 anuran and six salamander species [216], including many allopolyploids. All known polyploid anurans feature poorly differentiated (homomorphic) sex chromosomes. Here, we focus on an example of a polyploid complex of allopolyploids with even ploidies (Xenopus), a hybridogenetic complex involving triploids (Pelophylax) and on diploid and tetraploid meiotic but meroclonal triploid hybrids (Bufo).
(i) . Pipidae
Clawed frogs (Xenopus) comprise the largest ploidy-range known in an anuran radiation, reaching from diploid to do-decaploid (12n), all of which appear to be of hybrid origin [254]. Diploid X. tropicalis features W, Z and Y sex chromosomes (discussed §2(a)). Subgenome evolution in allopolyploids has only recently been studied in Xenopus laevis [255,256]. Its female-determining gene Dm-W is situated on the undifferentiated chromosome (2 L) and presents the only well-characterized anuran master sex determiner, a paralog of Dmrt1 [142,257], and arose after (and perhaps in response to) tetraploidization [217,258,259]. It is also found in some related Xenopus [258–260] but not in the entire radiation. Allotetraploid Xenopus borealis lost Dm-W and evolved new sex chromosomes on chromosome 8 L (chr8 [134,261]). Song et al. [261] summarized the variance in recombination suppression around the sex-linked portions to be very small in X. tropicalis and X. laevis but almost half the sex chromosomes in X. borealis, the other half presenting a pseudoautosomal region [260]. Although all polyploids are of hybrid origin, to our knowledge, no clonal or hemiclonal forms are known in Xenopus but only gonochoristic meiotic lineages with even ploidies. The elucidation of sex evolution and its role in this anuran radiation will continue to provide major insights into the links between sex determination and allopolyploidy in vertebrates.
(ii) . Ranidae
The Western Palearctic water frogs of the Pelophylax esculentus (previously Rana esculenta) complex include two parental species, Pelophylax ridibundus (RR) and Pelophylax lessonae (LL), and their natural hybrid forms P. esculentus, which are either allodiploid hybridogenetic (RL) or allotriploid (LLR or LRR) (figure 1); other hybridogenetic forms include additional parental species (electronic supplementary material, table S1). A striking feature of esculentus-hybrids that distinguishes them from most other clonal and hemiclonal vertebrates is the frequent incidence of males [262]. According to the comprehensive reviews by Günther [262] and Plötner [263], about 15 population systems occur, in which unisexual (either male or female) or bisexual (male and female) diploid and/or triploid esculentus hybrids coexist with either parental gonochoristic species. This complex comprises at least P. lessonae (five L-e-systems) or P. ridibundus (seven R-e-systems) or both (two L-R-e-systems). Uniquely, so-called ‘all-hybrid populations' (e-system) occur, composed of diploid (RL) and triploid (RLL, RRL) esculentus hybrids that genetically interact and depend on their specific gamete contributions for successful reproduction, as therein, the parental genotypes P. lessonae (LL) and P. ridibundus (RR) are absent among adults [59,262]. At least two additional diploid European hybridogenetic forms exist, Pelophylax grafi (RG), an allodiploid hybrid between P. ridibundus and Pelophylax perezi [264], and Pelophylax hispanicus (RB), an allodiploid hybrid between Pelophylax ridibundus and Pelophylax bergeri [265,266]. Importantly, all hybridogens contain at least one ridibundus (R)-genome. Various forms of hemiclonal inheritance have been described from allodiploid RL-hybrids, with either L-elimination and clonal inheritance of R or vice versa or even diploid RL, LL and RR gametes (figure 1) [60,262,263,267]. Triploid hybrids usually eliminate the genome, which is single (RRL: L; RLL: R), but also produce occasional RL, LL and RR gametes (figure 1) [172]. The karyotypes of the parental P. ridibundus and P. lessonae can be distinguished by few cytogenetic markers [268] but sex chromosomes were indistinguishable [269]. Like many ranid frogs [270], water frogs have an XX/XY sex determination system. This is suggested mostly from crossing experiments, involving water frogs from many parts of Central and Eastern Europe ([271] and citations therein), by inheritance patterns of allozymes for P. lessonae [272], and assumed for diploid hybrid P. esculentus [273], but the latter presenting a potential misinterpretation of the hybrid RL-karyotype. In all-hybrid populations, XX/XY-sex determination involves a dominant Y, exclusively on the L-genome [172,201,274], which is either LX or LY, while all R-genomes are RX [59]. Therefore, LLR and LR genotypes can be male (LXLYRX; LYRX) or female (LXRX; LXLXRX), but most LRR are females [275]. Based on microsatellite analysis of parents and offspring (sexed by dissection) from crossing experiments, Christiansen [172] confirmed sex determination as XX/XY with the Y confined to the L-genome. From crossings, gamete frequencies could be deduced. A model explained genetic interactions of di- and triploid hybrid frogs in self-sustaining populations (figure 4). Both sexes of RLL and RRL produced haploid gametes from the genomes they had twice, while RRL also made 10% LL gametes by automixis. LR frogs showed much variation in their gamete production. In RRL-rich populations, their RL sperm production was high (22%) to explain the observed proportion of RRL males [172]. Populations with biased sex ratio were long known in this complex. Such populations include P. ridibundus of both sexes associated with exclusively diploid hybrid males [201] that produce either the LY genome or the RX, leading to the emergence of only hybrid (esculentus) males or P. ridibundus females after crosses with P. ridibundus females [204]. To date, the studies by Christiansen [172,275] appear the most comprehensive ones to include sex chromosome information and sex determination in water frogs. Nevertheless, knowledge on master sex-determining genes, potential intraspecific variation (as observed in other ranid frogs [276]) and on their molecular genetic interactions in the hybrids is lacking.
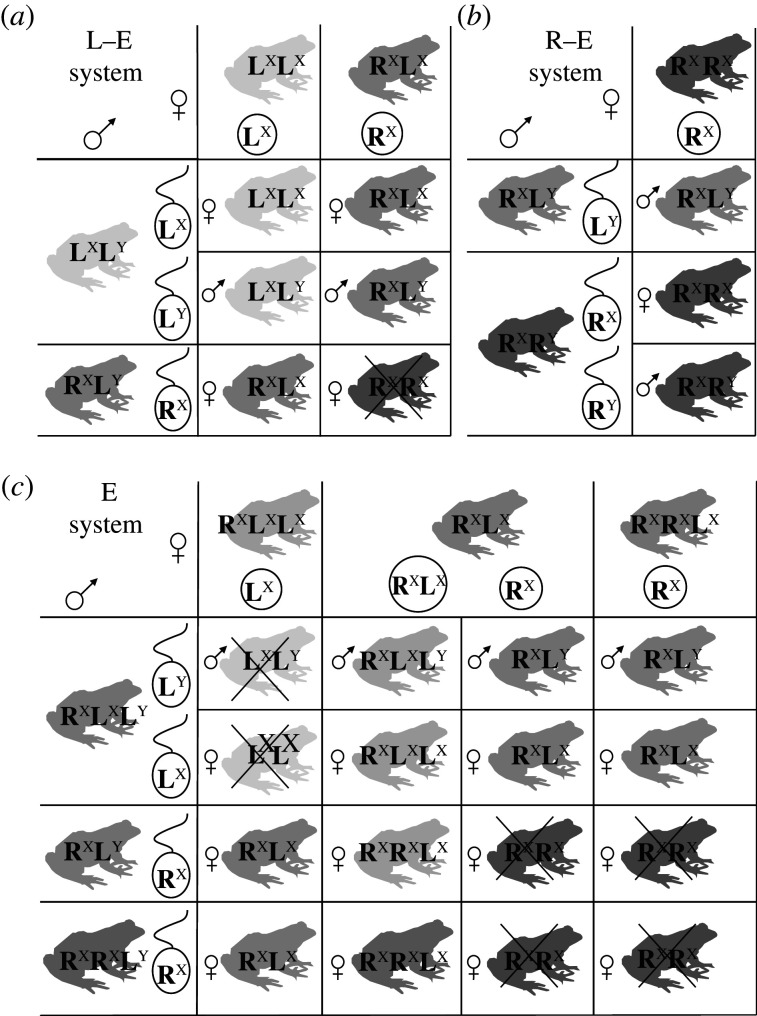
Figure 4.
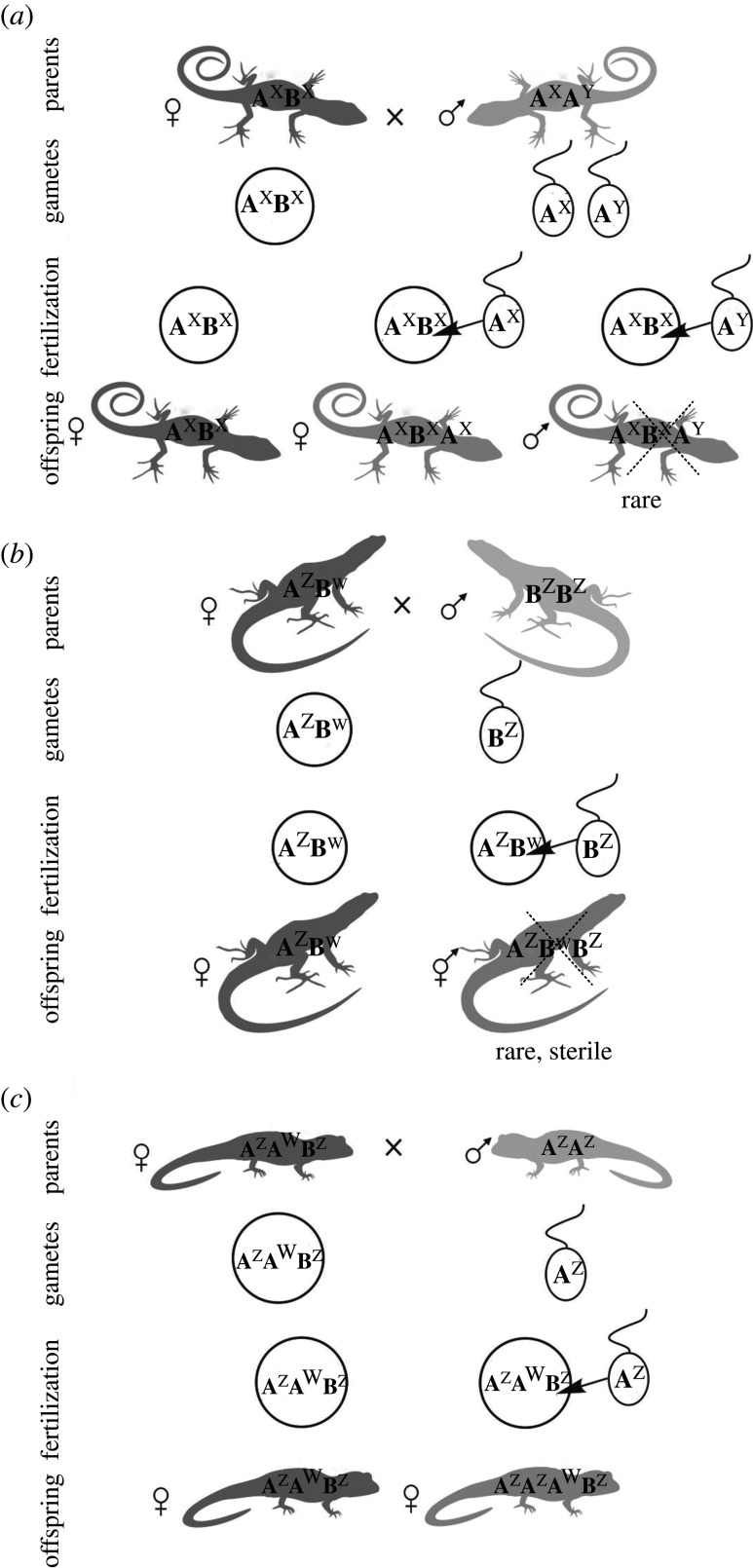
Inferred inheritance of male heterogametic (XY) sex determination loci in three hybridogenetic population systems of Western Palearctic water frogs (Pelophylax esculentus complex). Reproduction and inheritance of sex-determining loci in water frogs in three different population systems with diploid and/or triploid hybridogenetic (hemiclonal and meroclonal, box 1) hybrids. (a) In the L–E system (gonochoristic P. lessonae reproduce with hybridogenetic P. esculentus), the Y-factor, restricted to the L-genome, produces female excess among RxLx-hybrid offspring. RxRx-offspring typically die before reaching sexual maturity (crossed out). (b) In the R–E system (gonochoristic P. ridibundus reproduce with hybridogenetic P. esculentus), diploid RxLy hybrid genotypes are all-male. (c) In the E system (‘pure' diploid and triploid hybrid P. esculentus hybrids only), the dominant male-determining Y factor supposedly only occurs in males' L-genomes (Ly). Any resulting non-hybrid offspring (LL, RR) do not survive to sexual maturity. Male RRL offspring (RxRxLy) are usually not formed by the crosses of this population system. Frog silhouettes, their gametes and resulting offspring are shown. P. lessonae (LL): light grey; P. ridibundus (RR): dark grey; diploid (RL) and triploid hybrids (RRL and RLL): intermediate grey; redrawn from Christiansen [172].
(iii) . Bufonidae
In Palearctic green toads, Stöck et al. [277,278] have identified secondary contact and hybrid zones in a phylogeographic framework. In diploid/diploid contacts, introgression scales with divergence; i.e. with the degree of speciation [15,16,279]. A range-wide multi-locus phylogeny [35] involved 15 green toad taxa and showed that at least five separate allotriploid and allotetraploid taxa evolved in the Pleistocene. The maternal and paternal ancestors of hybrid polyploids exclusively stem from two deeply diverged (6 Ma, 3.1–9.6 Ma) nuclear clades, with distinctly greater divergence than the parental species of diploid hybrids, found at secondary contact zones. Presumably in all allotriploid forms (electronic supplementary material, table S1), but best examined in Batura toads (Bufo(tes) baturae), two conspecific genomes (NOR+) and a deeply diverged allospecific one (NOR–) are found, suggesting that genomic imbalance and divergence are the reasons for their meroclonal reproductive mode: ‘pre-equalizing hybrid meiosis' (figure 1) [50,73]. The maternal and paternal genome contributions appear asymmetric, with the maternal nuclear (and mitochondrial) genomes of all polyploids constantly stemming from the same clade, and the paternal genome from the other, pointing to a potential role of Darwin's corollary (§4b). Using cytogenetics and inheritance patterns, Stöck et al. [280] and Betto-Colliard et al. [281] established that diploid and allotetraploid toads reproduce meiotically. At least the imbalanced allotriploid species B. baturae reproduces partly clonally [50,73]. Sex chromosomes of diploid toads have been characterized using microsatellites and nuclear sequence markers [282–285], showing that the linkage group, homologous to autosomal LG1 in X. tropicalis and harboring Dmrt1, is sex-linked in several diploid species of green toads. Male heterogamety (XY) exhibits drastically reduced X–Y recombination in green toads in general, but occasional X–Y recombination occurs on evolutionary time scales [283]. LG1 appears to represent the sex chromosomes in all so far tested diploid green toad species (Bufo siculus, B. shaartusiensis, B. balearicus, B. turanensis, B. variabilis, B. viridis and probably B. boulengeri). Phylogenetic analyses of a 600 bp fragment of Dmrt1 furthermore showed that X and Y alleles of this gene cluster by species and not by gametologue. This suggests that XY-sequence similarity stems from occasional XY-recombination involving Dmrt1, which preliminarily rejects its role as the master sex determination gene, pending future extension of this evidence to the entire Dmrt1 gene [285]. The details of sex determination in the allopolyploids have not been examined.
(d) . Reptiles
Approximately 40 species complexes (full list: electronic supplementary material, table S1), i.e. only 0.4% of known squamate reptiles [151,286], are obligately parthenogenetic (box 1), and with few potential exceptions arose via hybridization between sexually reproducing progenitors [46]. Hybrid-origin parthenogenesis is known, for example, in the families Gymnophthalmidae (Loxopholis, formerly Leposoma [287,288], Gekkonidae (see below), Lacertidae (Darevskia [289] and Teiidae (Aspidoscelis, Cnemidophorus) [70,290,291]). Many parthenogenetic reptile species are clonal hybrid triploids, while tetraploids, with few exceptions [40,292], were only produced by laboratory crosses [291].
(i) . Teiidae
Parthenogens in Aspidoscelis (formerly Cnemidophorus, [293]) evolved by hybridization of two diverged mtDNA clades [294]. Gonochoristic Aspidoscelis seem to exhibit XY sex determination with slightly heteromorphic sex chromosomes [295]; male and female de-novo F1-hybrids remain sterile or have unknown fertility [295]. Unisexual Aspidoscelis reproduce by premeiotic endomitosis and sister chromatid pairing (figure 1) [69,70]. While natural tetraploids with three parental genomes (trihybrids), resulting from hybridization of triploid lineages with sexual males, are sterile or their fertility remains unknown [296], a self-sustaining 4n lineage was produced in the laboratory [291], raising the even nowadays unresolved question of what constrains development of cascading polyploid series as seen in some invertebrates [41]. That all-female reptiles apparently have evolved in a male heterogametic system appears an exception, since most well-examined cases seem based on ZW-systems with a recessive or dominant W. Initially, the rise of diploid hybrid parthenogenetic lineages [42] would be consistent with the expectation that homogametic XX female hybrids are fitter than male hybrids. Fertilization of parthenogenetic XX females by XY males of a parental or even third species [46,297] elevates these unisexual lineages to triploids [298]. Under dominant male heterogamety, XXY genotypes would be males and possibly also suffer from Haldane-effects (unfit, inviable, infertile), while XXX genotypes would be female and, if so, tri-hybridity of diverged genomes increases the heterozygosity and may even reinforce or ensure clonal oogenesis owing to mismatched chromosomes. Despite the great efforts to reveal the cytogenetic mechanisms of gametogenesis [69] and research on ploidy elevation [291] in Aspidoscelis, the elucidation of their sex chromosomal situation remains to be done.
(ii) . Lacertidae
The Caucasian parthenogenetic lacertid Darevskia present allo-diploid hybrids (figure 1) [299–301], with known hybrid compositions [39,294,302]. Only certain combinations of inter-clade hybridizations of bisexual species (caucasica clade and rudis clade) led to diploid parthenogenetic lineages, despite numerous records of natural within-clade hybridizations [295]. The ancestral clades show deep divergences (discussed by Avise [46]) and possibly can all be traced back to a few initial hybridization events [39], seemingly supporting the ‘rare formation hypothesis' (see §3(a)). Murphy et al. [289] proposed sex chromosomes to play key roles in the formation of unisexual Darevskia, which like most lacertid lizards [303] feature female heterogamety (ZW). Murphy et al. [289] stated that unisexual D. dahli and D. armeniaca express the micro-heteromorphic W chromosome from their maternal ancestry, D. mixta [304,305], while D. unisexualis expresses the derived micro-heteromorphic chromosome from its maternal lineage, D. raddei [295,304]). Furthermore, the W chromosome in the maternal gonochoristic D. raddei appeared polymorphic while the W chromosome of D. rostombekowi is more similar in size and heterochromatin patterns to the paternal ancestor D. portschinskii than to the maternal ancestor [295,304]. Likewise, most recently, Spangenberg [306] suggested the recessive w chromosome in unisexual D. rostombekowi to be inherited from the maternal ancestor D. raddei. Murphy et al. [289] further assumed that genes on the highly derived W chromosome might be a prerequisite for unisexuality, as suggested by the sister-relationship of both maternal ancestors. Accordingly, the combination between W-chromosomal genes of the maternal clade (caucasica) and Z chromosomal ones from the paternal clade (rudis) interrupts normal meiosis and produces unreduced viable eggs. Based on a single parthenogenetic female, Spangenberg et al. [197] confirmed synapsis of autosomes during meiotic prophase I, but asynaptic Z and recessive w, and suggested automixis with homeologous autosomes and Zw-sex chromosomes (figure 5), restoring diploidy by central fusion [71] (figure 1). Interestingly, triploid Darevskia remain sterile, perhaps because of dosage complications at higher ploidy hybrids, although meiotic instability owing to unpaired chromosomes could also explain the rarity of fertile triploids [152]. In sympatric populations of parthenogenetic D. unisexualis—from matings with males of the gonochoristic D. valentini—natural triploid hybrids result [197]. A single triploid (D. unisexualis × D. valentini) ZZw-male showed distorted synapsis, disturbed meiotic prophase I, passing of meiosis II, but spermatogenesis produced abnormal spermatids [197]. Sexual genotypes and phenotypes in Darevskia appear consistent with a recessive w sex determination, in which the number of Z chromosomes in ZZw-triploid hybrids affects femaleness.
Figure 5.
Reproduction and sex determination systems in three parthenogenetic lizards with different sex-determining systems. (a) Aspidoscelis with male heterogamety (XX/XY): all-female diploid hybrids (AB, left) normally reproduce parthenogenetically; rare fertilization by gonochoristic parental species' males (right) leads to ploidy elevation to a new XXX-triploid parthenogenetic form (X-sperm) but possibly to rare or inviable XXY-males (right, Y-sperm); inferred from Moritz & Bi [41]. (b) Darevskia with female heterogamety under a recessive w chromosome: occasional fertilization of a diploid parthenogenetic hybrid Zw female by sperm with a dominant male Z factor from a gonochoristic species leads to sterile triploid ZZw intersex genotypes; redrawn from Spangenberg et al. [197]. (c) Heteronotia with female heterogamety under a dominant W chromosome: occasional fertilization of allotriploid parthenogenetic zzW females (left) by sperm with a recessive male z-factor leads to rare tetraploid zzzW genotypes that also develop into females (right); drawn after Moritz [292]. Lizard silhouettes, their gametes and the resulting offspring are shown; arrows indicate occasional fertilization causing ploidy elevation.
(iii) . Gekkonidae
In Gekkonidae, five all-female species complexes in five different genera are obligate parthenogens [307], Lepidodactylus [307,308], Hemidactylus [308,309], Heteronotia [310], Hemiphyllodactylus [311] and Nactus [312]. Interestingly, in the molecular phylogeny of squamates (e.g. [313]), all belong to one of two major subclades of Gekkonidae, and all appear to be female heterogametic (ZW). It would be very interesting to elucidate whether their sex chromosomes are homologous. While the knowledge about sex chromosomes in Gekkonidae has strongly increased in the past years [314,315], several reported cases of sex chromosome heteromorphism (e.g. Lepidodactylus lugubris [316] that may also have a complex origin according to Trifonov et al. [307]; males are infertile: [317], and Hemidactylus vietnamensis [309]), may not present sex chromosomes but fixed heterozygosities in certain clonal lineages [318].
Triploid parthenogenetic Heteronotia binoei have independent reciprocal hybrid origins from two cytogenetically characterized sexual lineages (CA6, SM6) [292]. While the triploid parthenogenetic form (3N1) has mtDNAs derived from CA6 sexual females, 3N2 parthenogens share mtDNAs with bisexual SM6 [319]. Therefore, some triploids have two CA6 nuclear genomes copies (form A) and others two SM6 nuclear genomes (form BC). The split between the sexual ancestral lineage, which gave rise to multiple hybridizations, was estimated at 5.7–6.5 Ma [320]. Despite the existence of a heteromorphic sex chromosome pair in some diploid populations, parthenogenetic Heteronotia have homomorphic sex chromosomes but show different C-banding patterns between Z and W. The W is also cytogenetically polymorphic in several parthenogens [310]. Moritz [292] stated the existence of a dominant W, since ZZW-triploids but also four tetraploid individuals (ZZZW) that arose by fertilization of a parthenogenetic triploid from a sexual species' male [292] were females (figure 5).
6. Conclusion
Well-differentiated sex-chromosomes in mammals and birds tend to evolve with unequal rates, potentially causing Haldane-effects in hybrids, presumably owing to relatively well-examined dosage imbalances in the heterogametic hybrid sexes (XY-males, ZW-females). With few exceptions, heteromorphic sex chromosomes in hybrid zones introgress less easily than autosomes into the other species' gene pools. Judged from the limited examples, undifferentiated vertebrate sex chromosomes in earlier stages of divergence, when involved in hybridization and introgression, exhibit a variety of evolutionary outcomes. They may contribute to the emergence of multiple-sex chromosome systems with genetic interactions in hardly predictable dominance hierarchies, where multiple sex loci and/or chromosomes may drive diversification and potentially reinforce the speciation process. Empirical data further suggest that introgression of sex chromosomes under early divergence may not only result in evolutionary genetic interactions (e.g. in hybrid zones) but even lead to the evolution of new sex-determining systems in the affected lineages.
Under greater divergences in the ‘extended speciation continuum', just before most vertebrate hybrids already exhibit complete intrinsic reproductive isolation, a few interspecific hybrids show sex-specific distortion of gametogenesis towards female clonality, which may be caused or influenced by hybrids' genotypic sex. Analysing 41 hybrid ‘asexual' fish (17), amphibian (9) and reptile (15) taxa, we show that K2P-corrected distances, based on different mtDNA fragments of parental species, are larger than approximately 5% reaching up to approximately 22% (figure 2). This supports the hypothesis that ancestral divergence is of major importance in evolving a natural ‘asexual’ vertebrate.
Up to now, the technological limitations in detecting undifferentiated sex chromosomes, sex determination loci and thereby systems in these taxa have caused scarcity of this kind of data for most such vertebrate complexes. Likewise, dominance and recessiveness of sex chromosomes in hybrids remain widely underexplored in many diploid ancestral groups of ‘asexual taxa'.
Although most ‘asexual' vertebrates probably feature genetic sex determination, the evidence (§5), in line with theory (§4c), shows that most ‘asexual' as well as meiotic allopolyploid vertebrates, with the exception of a few lizards, possess undifferentiated sex chromosomes.
The fields of sex chromosomes and sex determination in the ancestral lineages of ‘asexual' as well as some meiotic allopolyploid vertebrate complexes remain widely underexplored (see above; electronic supplementary material, table S1). Hybrid ‘asexual' vertebrates can emerge from parental species with either XY or ZW sex determination systems. However, based on limited data (electronic supplementary material, table S2), it seems more likely to evolve an all-female hybrid form parents with female heterogamety (ZW). Diploid ‘asexual’ Darevskia possess a recessive w, and thus rare triploid ZZw genotypes are infertile. A dominant W, as inferred in triploid (and rare tetraploid) Heteronotia, and triploid to pentaploid Ambystoma ensures that sex chromosome dosage increase by Z-chromosome additions under ploidy elevation is possible without compromising the femaleness and thus fertility of these ‘asexuals' (figure 5). From the scarce data (§4b), out of 52 ‘asexual' taxa with known ancestry, information on sex chromosomes is entirely missing for 36. Of the remaining 16, ten known parental species are female heterogametic (ZZ/ZW), whereas only six are male heterogametic (XX/XY), suggesting that it might be easier to evolve an ‘asexual vertebrate' in a ZZ/ZW system (§4b: hypothesis ii). This could mean that Haldane's rule might be relevant to understand ‘asexual' vertebrate evolution, since such ZW females present the heterogametic sex and their premeiotic or meiotic aberrations could be Haldane-effects that promoted the evolution of these ‘asexual' females, while hybrid males got lost over time since either they could not reproduce by themselves or owing to backcrosses.
We further hypothesize that under male heterogamety (XY), the evolution of all-female polyploid taxa may be generally less probable since a dominant Y male determiner (cf. [215]) would not lead to female hybrids but to males only (XXY, XXXY), whereas a recessive y may lead to Xy diploid male hybrids, possibly XXy intersexes and perhaps XXXy females. More complex evolutionary interactions and potential dominance hierarchies in hybrids, resulting from XX/XY and ZZ/ZW parental forms, which generate complex sex chromosomal hybrid polyploid situations (e.g. XWY, XZW, etc.) may lead to individual and hardly predictable outcomes, further contributing to the theoretically [215] and empirically [147] shown examples.
Importantly, molecular information on the sex determination loci and/or master genes is so far only available in very few hybrid allodiploid or allopolyploid systems. Such data as well as mechanistic insights into sex chromosomal evolutionary effects under hybridization may be keys for a full future understanding of the field. Sex chromosome and sex determination research in ‘asexual' and allopolyploid vertebrates in context to speciation appears underexplored and calls for integrative approaches combining rigorous crossing experiments, and the application of cutting-edge techniques reaching from cellular biology, cytogenetics and genomics, to sexomics [321], to close these research gaps for a comprehensive understanding of their evolution (see also [286]).
Acknowledgements
We thank Jim Bogart, Lukáš Kratochvíl and Jörg Plötner for references, Manfred Schartl for occasional discussion, Maya Counot for patience and technical support, and two anonymous reviewers for valuable feedback on a previous version of this paper.
Contributor Information
Matthias Stöck, Email: matthias.stoeck@igb-berlin.de.
Karel Janko, Email: janko@iapg.cas.cz.
Data accessibility
Data and materials have been previously published or can be found in the electronic supplemental material.
The data are provided in the electronic supplementary material [322].
Authors' contributions
M.S. and K.J. conceived and coordinated the study. M.S., K.J., R.R., D.D., D.K.L. and Z.S. wrote the paper. D.D. prepared the figures, electronic supplementary material, table S1 and file S1, supported by Z.S., D.K.L., and M.S. D.K.L. prepared file S2 and figure 2. Z.S. prepared the K2P analyses, supported by all authors. All authors critically read the manuscript, provided feedback and gave final approval for publication.
Competing interests
We declare we have no competing interests.
Funding
M.S. was in part supported by COFASP/ERANET (STURGEoNOMICS) from the German Federal Ministry of Food and Agriculture through the Federal Office for Agriculture and Food (grant no. 2816ERA04G), and in part by the project ‘Breaking down the wall between human health and environmental testing of endocrine disruptors’: EndocRine Guideline Optimization (ERGO), grant 825753. D.D. was funded by the Czech Science Foundation (grant no. PPLZ L200452002). R.R. was funded by the Czech Science Foundation (grant nos. 18-14325S and 20-23794S), and the Charles University, grant no. PRIMUS/19/SCI/008. This study was also supported by the Czech Science Foundation (grant nos. 19-21552S and 21-25185S), the Ministry of Education, Youth and Sports of the Czech Republic (grant no. 539 EXCELLENCE CZ.02.1.01/0.0/0.0/15_003/0000460 OP RDE) for K.J. and D.D.
References
- 1.Darwin C. 1859. On the origin of species by means of natural selection or the preservation of favoured races in the struggle for life. London, UK: J. Murray. [PMC free article] [PubMed] [Google Scholar]
- 2.Mayr E. 1942. Systematics and the origin of species from the viewpoint of a zoologist. New York, NY: Columbia University Press. [Google Scholar]
- 3.Via S. 2009. Natural selection in action during speciation. Proc. Natl Acad. Sci. USA 106, 9939-9946. ( 10.1073/pnas.0901397106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coyne JA, Orr HA. 2004. Speciation. Sunderland, MA: Sinauer Associates. [Google Scholar]
- 5.Petit RJ, Excoffier L. 2009. Gene flow and species delimitation. Trends Ecol. Evol. 24, 386-393. ( 10.1016/j.tree.2009.02.011) [DOI] [PubMed] [Google Scholar]
- 6.Abbott R, et al. 2013. Hybridization and speciation. J. Evol. Biol. 26, 229-246. ( 10.1111/j.1420-9101.2012.02599.x) [DOI] [PubMed] [Google Scholar]
- 7.Marques D, Meier J, Seehausen O. 2019. A combinatorial view on speciation and adaptive radiation. Trends Ecol. Evol. 34, 531-544. ( 10.1016/j.tree.2019.02.008) [DOI] [PubMed] [Google Scholar]
- 8.McGee MD, et al. 2020. The ecological and genomic basis of explosive adaptive radiation. Nature 586, 75-79. ( 10.1038/s41586-020-2652-7) [DOI] [PubMed] [Google Scholar]
- 9.Matute DR, Cooper BS. 2021. Comparative studies on speciation: 30 years since Coyne and Orr. Evolution 75, 764-778. ( 10.1111/evo.14181) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nosil P. 2012. Ecological speciation. New York, NY: Oxford University Press. [Google Scholar]
- 11.Wu C. 2001. The genic view of the process of speciation. J. Evol. Biol. 14, 851-865. (doi:10.1046/ j.1420-9101.2001.00335.x) [Google Scholar]
- 12.Frankel N, Erezyilmaz D, McGregor A, Wang S, Payre F, Stern DL. 2011. Morphological evolution caused by many subtle-effect substitutions in regulatory DNA. Nature 474, 598-603. ( 10.1038/nature10200) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nosil P, Schluter D. 2011. The genes underlying the process of speciation. Trends Ecol. Evol. 26, 160-167. ( 10.1016/j.tree.2011.01.001) [DOI] [PubMed] [Google Scholar]
- 14.Mallet J. 2008. Hybridization, ecological races and the nature of species: empirical evidence for the ease of speciation. Phil. Trans. R. Soc. B 363, 2971-2986. ( 10.1098/rstb.2008.0081) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dufresnes C, Bonato L, Novarini N, Betto-Colliard C, Perrin N, Stöck M. 2014. Inferring the degree of incipient speciation in secondary contact zones of closely related lineages of Palearctic green toads (Bufo viridis subgroup). Heredity 113, 9-20. ( 10.1038/hdy.2014.26) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dufresnes C, Lymberakis P, Kornilios P, Savary R, Perrin N, Stöck M. 2018. Phylogeography of Aegean green toads (Bufo viridis subgroup): continental hybrid swarm vs. insular diversification with discovery of a new island endemic. BMC Evol. Biol. 18, 67. ( 10.1186/s12862-018-1179-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singhal S, Moritz C. 2013. Reproductive isolation between phylogeographic lineages scales with divergence. Proc. R. Soc. B 280, 20132246. ( 10.1098/rspb.2013.2246) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Poelstra JW, et al. 2014. The genomic landscape underlying phenotypic integrity in the face of gene flow in crows. Science 20, 1410-1414. ( 10.1126/science.1253226) [DOI] [PubMed] [Google Scholar]
- 19.McDevitt AD, Mariani S, Hebblewhite M, Decesare NJ, Morgantini L, Seip D, Weckworth BV, Musiani M. 2009. Survival in the Rockies of an endangered hybrid swarm from diverged caribou (Rangifer tarandus) lineages. Mol. Ecol. 18, 665-679. ( 10.1111/j.1365-294X.2008.04050.x) [DOI] [PubMed] [Google Scholar]
- 20.Kallman KD. 1975. The platyfish Xiphophorus maculatus. In Handbook of genetics , vol. 4 (ed. King RC), pp. 81-132. New York, NY: Plenum Press. [Google Scholar]
- 21.Volff JN, Schartl M. 2002. Sex determination and sex chromosome evolution in the medaka, Oryzias latipes, and the platyfish, Xiphophorus maculatus. Cytogenet. Genome Res. 99, 170-177. ( 10.1159/000071590) [DOI] [PubMed] [Google Scholar]
- 22.Lee BY, Hulata G, Kocher TD. 2004. Two unlinked loci controlling the sex of blue tilapia (Oreochromis aureus). Heredity 92, 543-549. ( 10.1038/sj.hdy.6800453) [DOI] [PubMed] [Google Scholar]
- 23.Cnaani A, et al. 2008. Genetics of sex determination in tilapiine species. Sex. Dev. 2, 43-54. ( 10.1159/000117718) [DOI] [PubMed] [Google Scholar]
- 24.Ser JR, Roberts RB, Kocher TD. 2010. Multiple interacting loci control sex determination in Lake Malawi cichlid fish. Evolution 64, 486-501. ( 10.1111/j.1558-5646.2009.00871.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dufresnes C, Majtyka T, Baird SJE, Gerchen J, Borzée A, Savary R, Ogielska M, Perrin N, Stöck M. 2016. Empirical evidence for large X-effects in animals with undifferentiated sex chromosomes. Sci. Rep. 6, 21029. ( 10.1038/srep21029) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gerchen JF, Dufresnes C, Stöck M. 2018. Introgression across hybrid zones is not mediated by large X-effects in green toads with undifferentiated sex chromosomes. Am. Nat. 192, E178-E188. ( 10.1086/699162) [DOI] [PubMed] [Google Scholar]
- 27.Carling MD, Brumfield RT. 2008. Haldane's rule in an avian system: using cline theory and divergence population genetics to test for differential introgression of mitochondrial, autosomal, and sex-linked loci across the Passerina bunting hybrid zone. Evolution 62, 2600-2615. ( 10.1111/j.1558-5646.2008.00477.x) [DOI] [PubMed] [Google Scholar]
- 28.Storchová R, Reif J, Nachman MW. 2010. Female heterogamety and speciation: reduced introgression of the Z chromosome between two species of nightingales. Evolution 64, 456-471. ( 10.1111/j.1558-5646.2009.00841.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Janoušek V, et al. 2012. Genome-wide architecture of reproductive isolation in a naturally occurring hybrid zone between Mus musculus musculus and M. m. domesticus. Mol. Ecol. 21, 3032-3047. ( 10.1111/j.1365-294X.2012.05583.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carneiro M, et al. 2014. The genomic architecture of population divergence between subspecies of the European rabbit. PLoS Genet. 10, e1003519. ( 10.1371/journal.pgen.1003519) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kottler VA, et al. 2020. Independent origin of XY and ZW sex determination mechanisms in mosquitofish sister species. Genetics 14, 193-209. ( 10.1534/genetics.119.302698) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miura I. 2007. An evolutionary witness: the frog Rana rugosa underwent change of heterogametic sex from XY male to ZW female. Sex. Dev. 1, 323-331. ( 10.1159/000111764) [DOI] [PubMed] [Google Scholar]
- 33.Ogata M, Lambert M, Ezaz T, Miura I. 2018. Reconstruction of female heterogamety from admixture of XX-XY and ZZ-ZW sex chromosome systems within a frog species. Mol. Ecol. 27, 4078-4089. ( 10.1111/mec.14831) [DOI] [PubMed] [Google Scholar]
- 34.Ogata M, Suzuki K, Yuasa Y, Miura I. 2021. Sex-chromosome evolution from a heteromorphic to a homomorphic system by inter-population hybridization in a frog. Phil. Trans. R. Soc. B 376, 20200105. ( 10.1098/2020.0105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Betto-Colliard C, Hofmann S, Sermier R, Perrin N, Stöck M. 2018. Profound genetic divergence and asymmetric parental genome contributions as hallmarks of hybrid speciation in polyploid toads. Proc. R. Soc. B 285, 20172667. ( 10.1098/rspb.2017.2667) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saetre G-P, Borge T, Lindroos K, Haavie J, Sheldon BC, Primmer C, Syvänen AC. 2003. Sex chromosome evolution and speciation in Ficedula flycatchers. Proc. R. Soc. B 270, 53-59. ( 10.1098/rspb.2002.2204) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bolfíková B, Hulva P. 2012. Microevolution of sympatry: landscape genetics of hedgehogs Erinaceus europaeus and E. roumanicus in Central Europe. Heredity 108, 248-255. ( 10.1038/hdy.2011.67) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Janko K, et al. 2018. Hybrid asexuality as a primary postzygotic barrier between nascent species: on the interconnection between asexuality, hybridization and speciation. Mol. Ecol. 27, 248-263. ( 10.1111/mec.14377) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tarkhnishvili D, et al. 2020. Genotypic similarities among the parthenogenetic Darevskia rock lizards with different hybrid origins. BMC Evol. Biol. 20, 122. ( 10.1186/s12862-020-01690-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kupriyanova LA. 2014. Concept of hybridogeneous speciation of vertebrate animals: Complex studies of unisexual species of reptilia. Proc. Zool. Inst. 318, 382-390. [Google Scholar]
- 41.Moritz C, Bi K. 2011. Spontaneous speciation by ploidy elevation: laboratory synthesis of a new clonal vertebrate. Proc. Natl Acad. Sci. USA 108, 9733-9734. ( 10.1073/pnas.1106455108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cole CJ, Dessauer HC, Paulissen MA, Walker JM. 2020. Hybridization between whiptail lizards in Texas: Aspidoscelis laredoensis and A. gularis, with notes on reproduction of a hybrid. Am. Mus. Nov. 3947, 1-13. ( 10.1206/3947.1) [DOI] [Google Scholar]
- 43.Stöck M, Roth P, Podloucky R, Grossenbacher K. 2008b. Wechselkröten – unter Berücksichtigung von Bufo viridis Laurenti, 1768; Bufo variabilis (Pallas, 1769); Bufo boulengeri Lataste, 1879; Bufo balearicus Böttger, 1880 und Bufo siculus Stöck, Sicilia, Belfiore, Lo Brutto, Lo Valvo und Arculeo, 2008, pp. 413-498. In Handbuch der amphibien und reptilien europas, vol. 5 (ed. Grossenbacher K). (Froschlurche II) [Handbook of the Amphibians and Reptiles of Europe. vol. 5 (Anura II)], pp. 413-498. Wiesbaden, Germany: AULA-Verlag. [Google Scholar]
- 44.Randler C. 2006. Extrapair paternity and hybridization in birds. J. Avian Biol. 37, 1-5. ( 10.1111/j.2006.0908-8857.03592.x) [DOI] [Google Scholar]
- 45.Zechner U, Reule M, Orth A, Bonhomme F, Strack B, Guénet JL, Hameister H, Fundele R. 1996. An X-chromosome linked locus contributes to abnormal placental development in mouse interspecific hybrids. Nat. Gen. 12, 398-403. ( 10.1038/ng0496-398) [DOI] [PubMed] [Google Scholar]
- 46.Avise IJ. 2008. Clonality: The genetics, ecology, and evolution of sexual abstinence in vertebrate animals. New York, NY: Oxford University Press. [Google Scholar]
- 47.Hubbs CL, Hubbs LC. 1932. Apparent parthenogenesis in nature, in a form of fish of hybrid origin. Science 76, 628-630. ( 10.1126/science.76.1983.628) [DOI] [PubMed] [Google Scholar]
- 48.Schartl M, Nanda I, Schlupp I, Wilde B, Eppenlen JT, Schmid M, Parzefall J. 1995. Incorporation of subgenomic amounts of DNA as compensation for mutational load in a gynogenetic fish. Nature 373, 68-71. ( 10.1038/373068a0) [DOI] [Google Scholar]
- 49.Lamatsch DK, Stöck M. 2009. Sperm-dependent parthenogenesis and hybridogenesis in teleost fishes. In Lost sex—The evolutionary biology of parthenogenesis (eds Schön I, Martens K, van Dijk P.), pp. 399-432. Heidelberg, Berlin, Germany: Springer. [Google Scholar]
- 50.Stöck M, Ustinova J, Betto-Colliard C, Schartl M, Moritz C, Perrin N. 2012. Simultaneous Mendelian and clonal genome transmission in a sexually reproducing, all-triploid vertebrate. Proc. R. Soc. B 279, 1293-1299. ( 10.1098/rspb.2011.1738) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schultz RJ. 1967. Gynogenesis and triploidy in the viviparous fish Poeciliopsis. Science 157, 1564-1567. ( 10.1126/science.157.3796.1564) [DOI] [PubMed] [Google Scholar]
- 52.Tunner HG. 1973. Demonstration of the hybrid origin of the common green frog Rana esculenta. Naturwissenschaften 60, 481-482. ( 10.1007/BF00592872) [DOI] [PubMed] [Google Scholar]
- 53.Tunner HG, Heppich S. 1981. Premeiotic genome exclusion during oogenesis in the common edible frog, Rana esculenta. Naturwissenschaften 68, 207. ( 10.1007/bf01047207) [DOI] [PubMed] [Google Scholar]
- 54.Ogielska M. 1994. Nucleus-like bodies in gonial cells of Rana esculenta (Amphibia, Anura) tadpoles—a putative way of chromosome elimination. Zool. Pol. 39, 461-474. [Google Scholar]
- 55.Dedukh D, Riumin S, Chmielewska M, Rozenblut-Kościsty B, Kolenda K, Kaźmierczak M, Dudzik A, Ogielska M, Krasikova A. 2020. Micronuclei in germ cells of hybrid frogs from Pelophylax esculentus complex contain gradually eliminated chromosomes. Sci. Rep. 10, 1-13. ( 10.1038/s41598-020-64977-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cimino MC. 1972. Egg-production, polyploidization and evolution in a diploid all-female fish of the genus Poeciliopsis. Evolution 26, 294-306. ( 10.2307/2407039) [DOI] [PubMed] [Google Scholar]
- 57.Berger L. 1968. Morphology of the F1 generation of various crosses within Rana esculenta complex. Acta Zool. Cracov. 13, 301-324. [Google Scholar]
- 58.Heppich S, Tunner HG, Greilhuber J. 1982. Premeiotic chromosome doubling after genome elimination during spermatogenesis of the species hybrid Rana esculenta. Theor. Appl. Genet. 61, 101-104. ( 10.1007/BF00273874) [DOI] [PubMed] [Google Scholar]
- 59.Christiansen DG, Fog K, Pedersen BV, Boomsma JJ. 2005. Reproduction and hybrid load in all-hybrid populations of Rana esculenta waterfrogs in Denmark. Evolution 59, 1348-1361. ( 10.1111/j.0014-3820.2005.tb01784.x) [DOI] [PubMed] [Google Scholar]
- 60.Günther R. 1983. Zur Populationsgenetik der Mitteleuropäischen Wasserfrösche des Rana esculenta-Synkleptons (Anura, Ranidae). Zool. Anz. 211, 43-54 [in German]. [Google Scholar]
- 61.Bogart JP, Bi K, Fu J, Noble DW, Niedzwiecki J. 2007. Unisexual salamanders (genus Ambystoma) present a new reproductive mode for eukaryotes. Genome 50, 119-136. ( 10.1139/G06-152) [DOI] [PubMed] [Google Scholar]
- 62.MacGregor H, Uzzell T. 1964. Gynogenesis in salamanders related to Ambystoma jeffersonianum. Science 143, 1043-1045. ( 10.1126/science.143.3610.1043) [DOI] [PubMed] [Google Scholar]
- 63.Bi K, Bogart JP. 2010. Probing the meiotic mechanism of intergenomic exchanges by genomic in situ hybridization on lampbrush chromosomes of unisexual Ambystoma (Amphibia: Caudata). Chromosome Res. 18, 371-382. ( 10.1007/s10577-010-9121-3) [DOI] [PubMed] [Google Scholar]
- 64.Monaco PJ, Rasch EM, Balsano JS. 1984. Apomictic reproduction in the Amazon molly, Poecilia formosa, and its triploid hybrids. In Evolutionary genetics of fishes. Monographs in evolutionary biology (ed. Turner BJ), pp. 311-328. Boston, MA: Berlin, Germany: Springer. ( 10.1007/978-1-4684-4652-4_6) [DOI] [Google Scholar]
- 65.Yamashita M, Jiang J, Onozato H, Nakanishi T, Nagahama Y. 1993. A tripolar spindle formed at meiosis I assures the retention of the original ploidy in the gynogenetic triploid crucian carp, ginbuna Carassius auratus langsdorfii. Dev. Growth Differ. 35, 631-636. ( 10.1111/j.1440-169X.1993.00631.x) [DOI] [PubMed] [Google Scholar]
- 66.Dedukh D, et al. 2020. Parthenogenesis as a solution to hybrid sterility: the mechanistic basis of meiotic distortions in clonal and sterile hybrids. Genetics 215, 975-987. ( 10.1534/genetics.119.302988) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bohlen J, Rab P. 2001. Species and hybrid richness in spined loaches of the genus Cobitis L. (Teleostei: Cobitidae), with a checklist of European forms and suggestions for their conservation. J. Fish Biol. 59, 79-85. ( 10.1111/j.1095-8649.2001.tb01380.x) [DOI] [Google Scholar]
- 68.Juchno D, Arai K, Boroń A, Kujawa R. 2016. Meiotic chromosome configurations in oocytes of Cobitis taenia and its polyploid hybrids. Ichthyol. Res. 64, 240-243. ( 10.1007/s10228-016-0556-1) [DOI] [Google Scholar]
- 69.Lutes AA, Neaves WB, Baumann DP, Wiegraebe W, Baumann P. 2010. Sister chromosome pairing maintains heterozygosity in parthenogenetic lizards. Nature 464, 283-286. ( 10.1038/nature08818) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cuellar O. 1971. Reproduction and the mechanism of meiotic restitution in the parthenogenetic lizard Cnemidophorus uniparens. J. Morphol. 133, 139-165. ( 10.1002/jmor.1051330203) [DOI] [PubMed] [Google Scholar]
- 71.Spangenberg V, et al. 2020. Cytogenetic mechanisms of unisexuality in rock lizards. Sci. Rep. 10, 8697. ( 10.1038/s41598-020-65686-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang Q, Arai K, Yamashita M. 1998. Cytogenetic mechanisms for triploid and haploid egg formation in the triploid loach Misgurnus anguillicaudatus. J. Exp. Zool. 281, 608-619. () [DOI] [Google Scholar]
- 73.Stöck M, et al. 2002. A bisexually reproducing all-triploid vertebrate. Nat. Gen. 30, 325-328. ( 10.1038/ng839) [DOI] [PubMed] [Google Scholar]
- 74.Nei M, Kumar S. 2000. Molecular evolution and phylogenetics. Oxford, UK and New York, NY: Oxford University Press. [Google Scholar]
- 75.Sinclair EA, Pramuk JB, Bezy RL, Crandall KA, Sites JW Jr. 2009. DNA evidence for nonhybrid origins of parthenogenesis in natural populations of vertebrates. Evolution 64, 1346-1357. ( 10.1111/j.1558-5646.2009.00893.x) [DOI] [PubMed] [Google Scholar]
- 76.Lampert KP. 2008. Facultative parthenogenesis in vertebrates: reproductive error or chance? Sex. Dev. 2, 290-301. (doi:10..1159/000195678) [DOI] [PubMed] [Google Scholar]
- 77.Booth W, Schuett GW. 2016. The emerging phylogenetic pattern of parthenogenesis in snakes. Biol. J. Linn. Soc. 118, 172-186. ( 10.1111/bij.12744) [DOI] [Google Scholar]
- 78.Straube N, Lampert K, Geiger M, Weib J, Kirchhauser J. 2016. First record of second-generation facultative parthenogenesis in a vertebrate species, the white spotted bamboo shark Chiloscyllium plagiosum. J. Fish Biol. 88, 668-675. ( 10.1111/jfb.12862) [DOI] [PubMed] [Google Scholar]
- 79.Dobzhansky T. 1937. Genetics and the origin of species. New York, NY: Columbia University. [Google Scholar]
- 80.Muller HJ. 1942. Isolating mechanisms, evolution, and temperature. Biol. Symp. 6, 71-125. [Google Scholar]
- 81.Turelli M, Orr HA. 2000. Dominance, epistasis and the genetics of postzygotic isolation. Genetics 154, 1663-1679. ( 10.1093/genetics/154.4.1663) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Orr HA, Masly JP, Presgraves DC. 2004. Speciation genes. Curr. Opin. Genet. Dev. 14, 675-679. ( 10.1016/j.gde.2004.08.009) [DOI] [PubMed] [Google Scholar]
- 83.Presgraves DC. 2010. The molecular evolutionary basis of species formation. Nat. Rev. Genet. 11, 175-180. ( 10.1038/nrg2718) [DOI] [PubMed] [Google Scholar]
- 84.Ting CT, Tsaur SC, Wu ML, Wu CI. 1998. A rapidly evolving homeobox at the site of a hybrid sterility gene. Science 282, 1501-1504. ( 10.1126/science.282.5393.1501) [DOI] [PubMed] [Google Scholar]
- 85.Phadnis N, Orr HA. 2009. A single gene causes both male sterility and segregation distortion in Drosophila hybrids. Science 323, 376-379. ( 10.1126/science.1163934) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Oliver PL, et al. 2009. Accelerated evolution of the Prdm9 speciation gene across diverse metazoan taxa. PLoS Genet. 5, e1000753. ( 10.1371/journal.pgen.10007539) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gregorova S, et al. 2018. Modulation of Prdm9-controlled meiotic chromosome asynapsis overrides hybrid sterility in mice. eLife 7, e34282. ( 10.7554/eLife.34282) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bateson W. 1909. Heredity and variation in modern lights. In Darwin and modern science, vol. 1909 (ed. Seward AC), pp. 85-101. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 89.Forsdyke DR. 2003. William Bateson, Richard Goldschmidt, and non-genic modes of speciation. J. Biol. Syst. 11, 341-350. ( 10.1142/S0218339003000932) [DOI] [Google Scholar]
- 90.White MJD. 1978. In Modes of speciation. San Francisco, CA: WH Freeman. [Google Scholar]
- 91.Rieseberg LH. 2001. Chromosomal arrangements and speciation. Trends Ecol. Evol. 16, 351-358. ( 10.1016/S0169-5347(01)02187-5) [DOI] [PubMed] [Google Scholar]
- 92.Tulchinsky AY, Johnson NA, Watt WB, Porter AH. 2014. Hybrid incompatibility arises in a sequence-based bioenergetic model of transcription factor binding. Genetics 198, 1155-1166. ( 10.1093/genetics/198.3.NP) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Petrov DA, Schutzman JL, Hartl DL, Lozovskaya ER. 1995. Diverse transposable elements are mobilized in hybrid dysgenesis in Drosophila virilis. Proc. Natl Acad. Sci. USA 92, 8050-8054. ( 10.1073/pnas.92.17.8050) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.O'Neill RJ, O'Neill MJ, Graves JAM. 1998. Undermethylation associated with retroelement activation and chromosome remodeling in an interspecific mammalian hybrid. Nature 393, 68-72. ( 10.1038/29985) [DOI] [PubMed] [Google Scholar]
- 95.Hill T, Schlötterer C, Betancourt AJ. 2016. Hybrid dysgenesis in Drosophila simulans associated with a rapid invasion of the P-element. PLoS Genet. 12, e1006058. ( 10.1371/journal.pgen.1006058) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Peona V, et al. 2021. The avian W chromosome is a refugium for endogenous retroviruses with likely effects on female-biased mutational load and genetic incompatibilities. Phil. Trans. R. Soc. B 376, 20200186. ( 10.1098/rstb.2020.0186) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Charlesworth B, Coyne J, Barton N. 1987. The relative rates of evolution of sex chromosomes and autosomes. Am. Nat. 130, 113-146. ( 10.1086/284701) [DOI] [Google Scholar]
- 98.Masly JP, Presgraves DC. 2007. High-resolution genome-wide dissection of the two rules of speciation in Drosophila. PLoS Biol. 5, e243. ( 10.1371/journal.pbio.0050243) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Payseur BA, Presgraves DC, Filatov DA. 2018. Introduction: sex chromosomes and speciation. Mol. Ecol. 27, 3745-3748. ( 10.1111/mec.14828) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Haldane JBS. 1922. Sex ratio and unisexual sterility in hybrid animals. J. Genet. 12, 101-109. ( 10.1007/BF02983075) [DOI] [Google Scholar]
- 101.Orr HA. 1997. Haldane's Rule. Annu. Rev. Ecol. Syst. 28, 195-218. ( 10.1146/annurev.ecolsys.28.1.195) [DOI] [Google Scholar]
- 102.Coyne JA, Orr HA. 1989. Two rules of speciation. In Speciation and Its consequences (eds Otte D, Endler J), pp. 180-207. Sunderland, MA: Sinauer Associates. [Google Scholar]
- 103.Turelli M, Moyle LC. 2007. Asymmetric postmating isolation: Darwin's corollary to Haldane's rule. Genetics 176, 1059-1088. ( 10.1093/genetics/176.2.NP) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Llopart A. 2012. The rapid evolution of X-linked male-biased gene expression and the large-X effect in Drosophila yakuba, D. santomea, and their hybrids. Mol. Biol. Evol. 29, 3873-3886. ( 10.1093/molbev/mss190) [DOI] [PubMed] [Google Scholar]
- 105.Meisel RP, Connallon T. 2013. The faster-X effect: integrating theory and data. Trends Genet. 29, 537-544. ( 10.1016/j.tig.2013.05.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Janoušek V, Fischerová J, Mořkovský L, Reif J, Antczak M, Albrecht T, Reifová R. 2019. Postcopulatory sexual selection reduces Z-linked genetic variation and might contribute to the large Z effect in passerine birds. Heredity 122, 622-635. ( 10.1038/s41437-018-0161-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hurst LD, Pomiankowski A. 1991. Causes of sex ratio bias may account for unisexual sterility in hybrids: a new explanation of Haldane's rule and related phenomena. Genetics 128, 841-858. ( 10.1093/genetics/128.4.841) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bhattacharyya T, Gregorova S, Mihola O, Anger M, Sebestova J, Denny P, Simecek P, Forejt J. 2013. Mechanistic basis of infertility of mouse intersubspecific hybrids. Proc. Natl Acad. Sci. USA 110, E468-E477. ( 10.1073/pnas.1219126110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bhattacharyya T, Reifova R, Gregorova S, Simecek P, Gergelits V, Mistrik M, Martincova I, Pialek J, Forejt J. 2014. X chromosome control of meiotic chromosome synapsis in mouse inter-subspecific hybrids. PLoS Genet. 10, e1004088. ( 10.1371/journal.pgen.1004088) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Filatov DA. 2018. The two ‘rules of speciation’ in species with young sex chromosomes. Mol. Evol. 27, 3799-3810. ( 10.1111/mec.14721) [DOI] [PubMed] [Google Scholar]
- 111.Beukeboom L, Perrin N. 2014. The evolution of sex determination. New York, NY: Oxford University Press. [Google Scholar]
- 112.Roux C, Fraïsse C, Romiguier J, Anciaux Y, Galtier N, Bierne N. 2016. Shedding light on the grey zone of speciation along a continuum of genomic divergence. PLoS Biol. 14, e2000234. ( 10.1371/journal.pbio.2000234) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Melander SL, Mueller RL. 2020. Comprehensive analysis of salamander hybridization suggests a consistent relationship between genetic distance and reproductive isolation across tetrapods. Copeia 108, 987-1003. ( 10.1643/CH-19-319) [DOI] [Google Scholar]
- 114.Barton NH, Charlesworth B. 1984. Genetic revolutions, founder effects and speciation. Annu. Rev. Ecol. Evol. Syst. 15, 133-164. ( 10.1146/annurev.es.15.110184.001025) [DOI] [Google Scholar]
- 115.Hendry AP, Bolnick DI, Berner D, Peichel CL. 2009. Along the speciation continuum in sticklebacks. J. Fish. Biol. 75, 2000-2036. ( 10.1111/j.1095-8649.2009.02419.x) [DOI] [PubMed] [Google Scholar]
- 116.Peccoud J, Ollivier A, Plantegenest M, Simon J-C. 2009. A continuum of genetic divergence from sympatric host races to species in the pea aphid complex. Proc. Natl Acad. Sci. USA 106, 7495-7500. ( 10.1073/pnas.0811117106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.De Queiroz K. 2007. Species concepts and species delimitation. Syst. Biol. 56, 879-886. ( 10.1080/10635150701701083) [DOI] [PubMed] [Google Scholar]
- 118.Galtier N. 2019. Delineating species in the speciation continuum: a proposal. Evol. Appl. 12, 657-663. ( 10.1111/eva.12748) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Malinsky M, Challis RJ, Tyers AM, Schiffels S, Terai Y, Ngatunga BP, Turner GF. 2015. Genomic islands of speciation separate cichlid ecomorphs in an East African crater lake. Science 350, 1493-1498. ( 10.1126/science.aac9927) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wolf JB, Ellegren H. 2016. Making sense of genomic islands of differentiation in light of speciation. Nat. Rev. Genet. 18, 87-100. ( 10.1038/nrg.2016.133) [DOI] [PubMed] [Google Scholar]
- 121.Mořkovský L, Janoušek V, Reif J, Rídl J, Pačes J, Choleva L, Janko K, Nachman MW, Reifová R. 2018. Genomic islands of differentiation in two songbird species reveal candidate genes for hybrid female sterility. Mol. Ecol. 27, 949-958. ( 10.1111/mec.14479) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hey J, Pinho C. 2012. Population genetics and objectivity in species diagnosis. Evolution 66, 1413-1429. ( 10.1111/j.1558-5646.2011.01542.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Orr HA, Turelli M. 2001. The evolution of postzygotic isolation: accumulating Dobzhansky-Muller incompatibilities. Evolution 55, 1085-1094. ( 10.1111/j.0014-3820.2001.tb00628.x) [DOI] [PubMed] [Google Scholar]
- 124.Matute DR, Butler IA, Turissini DA, Coyne JA. 2010. A test of the snowball theory for the rate of evolution of hybrid incompatibilities. Science 329, 1518-1521. ( 10.1126/science.1193440) [DOI] [PubMed] [Google Scholar]
- 125.Coyne JA, Orr HA. 1997. Patterns of speciation in Drosophila revisited. Evol. Int. J. Org. Evol. 51, 295-303. ( 10.2307/2410984) [DOI] [PubMed] [Google Scholar]
- 126.Presgraves DC. 2002. Patterns of postzygotic isolation in Lepidoptera. Evol. Int. J. Org. Evol. 56, 1168-1183. ( 10.1111/j.0014-3820.2002.tb01430.x) [DOI] [PubMed] [Google Scholar]
- 127.`Ravinet M, Yoshida K, Shigenobu S, Toyoda A, Fujiyama A, Kitano J. 2018. The genomic landscape at a late stage of stickleback speciation: high genomic divergence interspersed by small localized regions of introgression. PLoS Genet. 14, e1007358. ( 10.1371/journal.pgen.1007358) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sciuchetti L, Dufresnes C, Cavoto E, Brelsford A, Perrin P. 2018. Dobzhansky–Muller incompatibilities, dominance drive, and sex-chromosome introgression at secondary contact zones: a simulation study. Evolution 72, 1350-1361. ( 10.1111/evo.13510) [DOI] [PubMed] [Google Scholar]
- 129.Turelli M, Begun DJ. 1997. Haldane's rule and X-chromosome size in Drosophila. Genetics 147, 1799-1815. ( 10.1093/genetics/147.4.1799) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lima TG. 2014. Higher levels of sex chromosome heteromorphism are associated with markedly stronger reproductive isolation. Nat. Commun. 5, 4743. ( 10.1038/ncomms5743) [DOI] [PubMed] [Google Scholar]
- 131.Roco AS, Olmstead AW, Degitz SJ, Amano T, Zimmerman LB, Bullejos M. 2015. Coexistence of Y, W, and Z sex chromosomes in Xenopus tropicalis. Proc. Natl Acad. Sci. USA 112, E4752-E4761. ( 10.1073/pnas.1505291112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Mitros T, et al. 2019. A chromosome-scale genome assembly and dense genetic map for Xenopus tropicalis. Dev. Biol. 452, 8-20. ( 10.1016/j.ydbio.2019.03.015) [DOI] [PubMed] [Google Scholar]
- 133.Schartl M. 2015. Sex determination by multiple sex chromosomes in Xenopus tropicalis. Proc. Natl Acad. Sci. USA 112, 10575-10576. ( 10.1073/pnas.1513518112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Furman BLS, Cauret CMS, Knytl M, Song X-Y, Premachandra T, Ofori-Boateng C, Jordan DC, Horb ME, Evans BJ. 2020. A frog with three sex chromosomes that co-mingle together in nature: Xenopus tropicalis has a degenerate W and a Y that evolved from a Z chromosome. PLoS Genet. 16, e1009121. ( 10.1371/journal.pgen.1009121) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hall DW, Kirkpatrick M. 2006. Reinforcement and sex linkage. Evolution 60, 908-921. ( 10.1111/j.0014-3820.2006.tb01170.x) [DOI] [PubMed] [Google Scholar]
- 136.Wilk RJ, Horth L. 2016. A genetically distinct hybrid zone occurs for two globally invasive mosquito fish species with striking phenotypic resemblance. Ecol. Evol. 6, 8375-8388. ( 10.1002/ece3.2562) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Johannesson K, Le Moann A, Perini S, André C. 2020. A Darwinian laboratory of multiple contact zones. Trends Ecol. Evol. 35, 1021-1036. ( 10.1016/j.tree.2020.07.015) [DOI] [PubMed] [Google Scholar]
- 138.Runemark A, Eroukhmanoff F, Nava-Bolaños A, Hermansen JS, Meier JI. 2018. Hybridization, sex-specific genomic architecture and local adaptation. Phil. Trans. R. Soc. B 373, 20170419. ( 10.1098/rstb.2017.0419) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Dixon G, Kitano J, Kirkpatrick M. 2018. The origin of a new sex chromosome by introgression between two stickleback fishes. Mol. Biol. Evol. 36, 28-38. ( 10.1093/molbev/msy181) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Takahashi H, Nagai T, Goto A. 2005. Hybrid male sterility between the fresh- and brackish-water types of ninespine stickleback Pungitius pungitius (Pisces, Gasterosteidae). Zool. Sci. 22, 35-40. ( 10.2108/zsj.22.35) [DOI] [PubMed] [Google Scholar]
- 141.Uno Y, Nishida C, Oshima Y, Yokoyama S, Miura I, Matsuda Y, Nakamura M. 2008. Comparative chromosome mapping of sex-linked genes and identification of sex chromosomal rearrangements in the Japanese wrinkled frog (Rana rugosa, Ranidae) with ZW and XY sex chromosome systems. Chrom. Res. 16, 637-647. ( 10.1007/s10577-008-1217-7) [DOI] [PubMed] [Google Scholar]
- 142.Ito M. 2018. Sex determination and differentiation in frogs. In Reproductive and developmental strategies. Diversity and commonality in animals (eds Kobayashi K, Kitano T, Iwao Y, Kondo M), pp. 349-366. Tokyo, Japan: Springer. [Google Scholar]
- 143.Ogata M, Hasegawa Y, Ohtani H, Mineyama M, Miura I. 2008. The ZZ/ZW sex-determining mechanism originated twice and independently during evolution of the frog, Rana rugosa. Heredity 100, 92-99. ( 10.1038/sj.hdy.6801068) [DOI] [PubMed] [Google Scholar]
- 144.Runemark A, Vallejo-Marin M, Meier JI. 2019. Eukaryote hybrid genomes. PLoS Genet. 15, e1008404. ( 10.1371/journal.pgen.1008404) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ernst A. 1918. Bastardierung als ursache der apogamie im pflanzenreich. Eine Hypothese zur experimentellen Vererbungs- und Abstammungslehre. Jena, Germany: Fischer-Verlag; [in German]. [Google Scholar]
- 146.Wetherington JD, Kotora KE, Vrijenhoek RC. 1987. A test of the spontaneous heterosis hypothesis for unisexual vertebrates. Evolution 41, 721-723. ( 10.1111/j.1558-5646.1987.tb05848.x) [DOI] [PubMed] [Google Scholar]
- 147.Moritz C, Brown WM, Densmore LD, Wright JW, Vyas D, Donnellan S, Adams M, Baverstock P. et al. 1989. Genetic diversity and the dynamics of hybrid parthenogenesis in Cnemidophorus (Teiidae) and Heteronotia (Gekkonidae). In Evolution and ecology of unisexual vertebrates), pp. 268-280. Albany, NY: New York State Museum. [Google Scholar]
- 148.Stöck M, Lampert KP, Möller D, Schlupp I, Schartl M. 2010. Monophyletic origin of multiple clonal lineages in an asexual fish (Poecilia formosa). Mol. Ecol. 19, 5204-5215. ( 10.1111/j.1365-294X.2010.04869.x) [DOI] [PubMed] [Google Scholar]
- 149.Warren W, et al. 2018. Clonal polymorphism and high heterozygosity in the celibate genome of the Amazon molly. Nat. Ecol. Evol. 2, 669-679. ( 10.1038/s41559-018-0473-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Hojsgaard D, Schartl M. 2021. Skipping sex: a non-recombinant genomic assemblage of complementary reproductive modules. Bioessays 43, e2000111. ( 10.1002/bies.202000111) [DOI] [PubMed] [Google Scholar]
- 151.Kearney M, Fujita MK, Ridenour J. 2009. Lost sex in the reptiles: constraints and correlations. In Lost sex—The evolutionary biology of parthenogenesis (eds Schön I, Martens K, van Dijk P.), pp. 447-474. Heidelberg, Berlin, Germany: Springer. ( 10.1007/978-90-481-2770-2_21) [DOI] [Google Scholar]
- 152.Fujita MK, Moritz C. 2009. Origin and evolution of parthenogenetic genomes in lizards: current state and future directions. Cytogenet. Genome Res. 127, 261-272. ( 10.1159/000295177) [DOI] [PubMed] [Google Scholar]
- 153.Choleva L, Janko K. 2013. Rise and persistence of animal polyploidy: evolutionary constraints and potential. Cytogenet. Genome Res. 140, 151-170. ( 10.1159/000353464) [DOI] [PubMed] [Google Scholar]
- 154.Simon J-C, Delmotte F, Rispe C, Crease T. 2003. Phylogenetic relationships between parthenogens and their sexual relatives: the possible routes to parthenogenesis in animals. Biol. J. Linn. Soc. 79, 151-163. ( 10.1046/j.1095-8312.2003.00175.x) [DOI] [Google Scholar]
- 155.Bogart JP. 1980. Evolutionary implications of polyploidy in amphibians and reptiles. In Polyploidy: biological relevance (ed. Lewis WH), pp. 341-378. New York, NY: Plenum Press. [DOI] [PubMed] [Google Scholar]
- 156.Schmid M, Evans BJ, Bogart JP. 2015. Polyploidy in amphibia. Cytogenet. Gen. Res. 145, 315-330. ( 10.1159/000431388) [DOI] [PubMed] [Google Scholar]
- 157.De Storme N, Mason A. 2014. Plant speciation through chromosome instability and ploidy change: cellular mechanisms, molecular factors and evolutionary relevance. Curr. Plant Biol. 1, 10-33. ( 10.1016/j.cpb.2014.09.002) [DOI] [Google Scholar]
- 158.Faria R, Navarro A. 2010. Chromosomal speciation revisited: rearranging theory with pieces of evidence. Trends Ecol. Evol. 25, 660-669. ( 10.1016/j.tree.2010.07.008) [DOI] [PubMed] [Google Scholar]
- 159.Carman JG. 1997. Asynchronous expression of duplicate genes in angiosperms may cause apomixis, bispory, tetraspory, and polyembryony. Biol. J. Linn. Soc. 61, 51-94. ( 10.1111/j.1095-8312.1997.tb01778.x) [DOI] [Google Scholar]
- 160.Husband BC. 2000. Constraints on polyploid evolution: a test of the minority cytotype exclusion principle. Proc. R. Soc. B 267, 217-223. ( 10.1098/rspb.2000.0990) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Neaves WB, Baumann P. 2011. Unisexual reproduction among vertebrates. Trends Genet. 27, 81-88. ( 10.1016/j.tig.2010.12.002) [DOI] [PubMed] [Google Scholar]
- 162.Choleva L, Janko K, De Gelas K, Bohlen J, Šlechtová V, Rábová M, Ráb P. 2012. Synthesis of clonality and polyploidy in vertebrate animals by hybridization between two sexual species. Evolution 66, 2191-2203. ( 10.1111/j.1558-5646.2012.01589.x) [DOI] [PubMed] [Google Scholar]
- 163.Schultz RJ. 1969. Hybridization, unisexuality, and polyploidy in the teleost Poeciliopsis (Poeciliidae) and other vertebrates. Am. Nat. 103, 605-619. ( 10.1086/282629) [DOI] [Google Scholar]
- 164.Dawley RM, Bogart JP (eds). 1989. Evolution and ecology of unisexual vertebrates (eds RM Dawley, JP Bogart). New York State Museum Bulletin 466. Albany, NY: New York State Museum. [Google Scholar]
- 165.Butlin R, Schön I, Griffiths HI. 1998. Introduction to reproductive modes: sex and parthenogenesis. In Evolutionary ecology of reproductive modes in non-marine ostracods (ed. Martens K), pp. 1-24. Leiden, The Netherlands: Backhuys Publ. [Google Scholar]
- 166.Stenberg P, Saura A. 2009. Cytology of asexual animals. In Lost sex (eds Schön I, Martens K, Dijk P), pp. 63-74. Dordrecht, The Netherlands: Springer. ( 10.1007/978-90-481-2770-2_4) [DOI] [Google Scholar]
- 167.Stenberg P, Saura A. 2013. Meiosis and its deviations in polyploid animals. Cytogenet. Genome Res. 140, 185-203. ( 10.1159/000351731) [DOI] [PubMed] [Google Scholar]
- 168.Mason AS, Pires C. 2015. Unreduced gametes: meiotic mishap or evolutionary mechanism? Trends Genet. 31, 3-10. ( 10.1016/j.tig.2014.09.011) [DOI] [PubMed] [Google Scholar]
- 169.Cunha C, Doadrio I, Coelho MM. 2008. Speciation towards tetraploidization after intermediate processes of non-sexual reproduction. Phil. Trans. R. Soc. B 363, 2921-2929. ( 10.1098/rstb.2008.0048) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Hojsgaard D, Hörandl E. 2015. Apomixis as a facilitator of range expansion and diversification in plants. In Evolutionary biology: biodiversification from genotype to phenotype (ed. Pontarott P), pp. 305-327. Cham, Switzerland: Springer International Publishing. ( 10.1007/978-3-319-19932-0_16) [DOI] [Google Scholar]
- 171.Husband BC. 2004. The role of triploid hybrids in the evolutionary dynamics of mixed-ploidy populations. Biol. J. Linn. Soc. 82, 537-546. ( 10.1111/j.1095-8312.2004.00339.x) [DOI] [Google Scholar]
- 172.Christiansen GR. 2009. Gamete types, sex determination and stable equilibria of all-hybrid populations of diploid and triploid edible frogs (Pelophylax esculentus). BMC Evol. Biol. 9, 135. ( 10.1186/1471-2148-9-135) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Stöck M, Ustinova J, Lamatsch DK, Schartl M, Perrin N, Moritz C. 2010. A vertebrate reproductive system involving three ploidy levels: hybrid origin of triploids in a contact zone of diploid and tetraploid Palearctic green toads (Bufo viridis subgroup). Evolution 64, 944-959. ( 10.1111/j.1558-5646.2009.00876.x) [DOI] [PubMed] [Google Scholar]
- 174.Collares-Pereira MJ, Matos I, Morgado-Santos M, Coelho MM. 2013. Natural pathways towards polyploidy in animals: the Squalius alburnoides fish complex as a model system to study genome size and genome reorganization in polyploids. Cytogenet. Genome Res. 140, 47-116. ( 10.1159/000351729) [DOI] [PubMed] [Google Scholar]
- 175.Alves MJ, Coelho MM, Collares-Pereira MJ. 2001. Evolution in action through hybridisation and polyploidy in an Iberian freshwater fish: a genetic review. Genetica 111, 375-385. ( 10.1023/a:1013783029921) [DOI] [PubMed] [Google Scholar]
- 176.Lloyd A, Bomblies K. 2016. Meiosis in autopolyploid and allopolyploid Arabidopsis. Curr. Opin. Plant Biol. 30, 116-122. ( 10.1016/j.pbi.2016.02.004) [DOI] [PubMed] [Google Scholar]
- 177.Lenormand T, Engelstädter J, Johnston SE, Wijnker E, Haag CR. 2016. Evolutionary mysteries in meiosis. Phil. Trans. R. Soc. B 371, 20160001. ( 10.1098/rstb.2016.0001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Mallet J. 2007. Hybrid speciation. Nature 446, 279-283. ( 10.1038/nature05706) [DOI] [PubMed] [Google Scholar]
- 179.Chapman MA, Burke JM. 2007. Genetic divergence and hybrid speciation. Evolution 61, 1773-1780. ( 10.1111/j.1558-5646.2007.00134.x) [DOI] [PubMed] [Google Scholar]
- 180.Lukhtanov VA, Dincă V, Friberg M, Šíchová J, Olofsson M, Vila R, Marec F, Wiklund C. 2018. Versatility of multivalent orientation, inverted meiosis, and rescued fitness in holocentric chromosomal hybrids. Proc. Natl Acad. Sci. USA 115, 9610-9619. ( 10.1073/pnas.1802610115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Jančúchová-Lásková J, Landová E, Frytna D. 2015. Are genetically distinct lizard species able to hybridize? A review. Curr. Zool. 61, 155-180. ( 10.1093/czoolo/61.1.155) [DOI] [Google Scholar]
- 182.Hernández Chávez C, Turgeon J. 2007. Asexual and sexual hybrids between Fundulus diaphanus and F. heteroclitus in the Canadian Atlantic region. Mol. Ecol. 16, 1467-1480. ( 10.1111/j.1365-294X.2007.03239.x) [DOI] [PubMed] [Google Scholar]
- 183.Shimizu Y, Shibata N, Yamashita M. 1997. Spermiogenesis without preceding meiosis in the hybrid medaka between Oryzias latipes and O. curvinotus. J. Exp. Zool. 279, 102-112. () [DOI] [Google Scholar]
- 184.Ward RD, Zemlak TS, Innes BH, Last PR, Hebert PDN. 2005. DNA barcoding Australia's fish species. Phil. Trans. R. Soc. B 360, 1847-1857. ( 10.1098/rstb.2005.1716) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Mabragana E, Diaz de Astarloa JM, Hanner R, Zhang J, Gonzallez Castro M. 2011. DNA barcoding identifies Argentine fishes from marine and brackish waters. PLoS ONE 6, e28655. ( 10.1371/journal.pone.0028655) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Chang C-H, Shao K-T, Lin H-Y, Chiu Y-C, Lee M-Y, Liu S-H, Lin P-L. 2017. DNA barcodes of the native ray-finned fishes in Taiwan. Mol. Ecol. Res. 17, 796-805. ( 10.1111/1755-0998.12601) [DOI] [PubMed] [Google Scholar]
- 187.Che J, Chen H-M, Yang J-X, Jin J-Q, Jiang K, Yuan ZY, Murphy RW, Zhang YP. 2012. Universal COI primers for DNA barcoding amphibians. Mol. Ecol. Res. 12, 247-258. ( 10.1111/j.1755-0998.2011.03090.x) [DOI] [PubMed] [Google Scholar]
- 188.Xia Y, Gu H-F, Peng R, Chen Q, Zheng Y-C, Murphy RW, Zeng XM. 2012. COI is better than 16S rRNA for DNA barcoding Asiatic salamanders (Amphibia: Caudata: Hynobiidae). Mol. Evol. Res. 12, 48-56. ( 10.1111/j.1755-0998.2011.03055.x) [DOI] [PubMed] [Google Scholar]
- 189.Jeong TJ, Jun J, Han S, Kim HT, Oh K, Kwak M. 2013. DNA barcode reference data for the Korean herpetofauna and their applications. Mol. Eol. Res. 13, 1019-1132. ( 10.1111/1755-0998.12055) [DOI] [PubMed] [Google Scholar]
- 190.Perl BRG, Nagy Z, Sonet G, Glaw F, Wollenberg Valero KC, Vences M. 2014. DNA barcoding Madagascar's amphibian fauna. Amphibia-Reptilia 35, 197-206. ( 10.1163/15685381-00002942) [DOI] [Google Scholar]
- 191.Chambers EA, Hebert PDN. 2016. Assessing DNA barcodes for species identification in North American reptiles and amphibians in natural history collections. PLoS ONE 11, e0154363. ( 10.1371/journal.pone.0154363) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Vrijenhoek RC, Dawley RM, Cole CJ, Bogart JP. 1989. A list of the known unisexual vertebrates. In Evolution and ecology of unisexual vertebrates (eds Dawley RM, Bogart JP), pp. 19-23. New York State Museum Bulletin 466. Albany, NY: New York State Museum. [Google Scholar]
- 193.Vrijenhoek RC. 1994. Unisexual fish: model systems for studying ecology and evolution. Annu. Rev. Ecol. Syst. 25, 71-96. ( 10.1146/annurev.es.25.110194.000443) [DOI] [Google Scholar]
- 194.Komaru A, Konishi K. 1999. Non-reductional spermatozoa in three shell color types of the freshwater clam Corbicula fluminea in Taiwan. Zoolog. Sci. 16, 105-108. ( 10.2108/zsj.16.105) [DOI] [Google Scholar]
- 195.Morgado-Santos M, Carona S, Vicente L, Collares-Pereira MJ. 2017. First empirical evidence of naturally occurring androgenesis in vertebrates. R. Soc. Open Sci. 4, 170200. ( 10.1098/rsos.170200) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Park J-Y, Kim I-S, Ko M-H. 2011. Characteristics of rare males in the cobitid unisexual complex, Cobitis hankugensis-Iksookimia longicorpa. Folia Zool. Praha 60, 290-294. ( 10.25225/fozo.v60.i4.a4.2011) [DOI] [Google Scholar]
- 197.Spangenberg V, Arakelyan M, Galoyan E, Matveevsky S, Petrosyan R, Bogdanov Y, Danielyan F, Kolomiets O. 2017. Reticulate evolution of the rock lizards: meiotic chromosome dynamics and spermatogenesis in diploid and triploid males of the genus Darevskia. Genes 8, 149. ( 10.3390/genes8060149) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Kuroda M, Fujimoto T, Murakami M, Yamaha E, Arai K. 2019. Aberrant meiotic configurations cause sterility in clone-origin triploid and inter-group hybrid males of the Dojo loach, Misgurnus anguillicaudatus. Cytogenet. Genome Res. 158, 46-54. ( 10.1159/000500303) [DOI] [PubMed] [Google Scholar]
- 199.Kuroda M, Fujimoto T, Murakami M, Yamaha E, Arai K. 2018. Clonal reproduction assured by sister chromosome pairing in Dojo loach, a teleost fish. Chromosome Res. 26, 243-253. ( 10.1007/s10577-018-9581-4) [DOI] [PubMed] [Google Scholar]
- 200.Shimizu Y, Shibata N, Sakaizumi M, Yamashita M. 2000. Production of diploid eggs through premeiotic endomitosis in the hybrid medaka between Oryzias latipes and O. curvinotus. Zoolog. Sci. 17, 951-958. () [DOI] [Google Scholar]
- 201.Graf J-D, Polls Pelaz M. 1989. Evolutionary genetics of the Rana esculenta complex. In Evolution and ecology of unisexual vertebrates (eds Dawley RM, Bogart JP), pp. 289-302. New York State Museum Bulletin 466. Albany, NY: New York State Museum. [Google Scholar]
- 202.Uzzell T, Günther R, Berger L. 1976. Rana ridibunda and Rana esculenta: a leaky hybridogenetic system (Amphibia Salientia). Proc. Acad. Nat. Sci. Phila. 128, 147-171. [Google Scholar]
- 203.Dedukh D, Litvinchuk S, Rosanov J, Shabanov D, Krasikova A. 2017. Mutual maintenance of di- and triploid Pelophylax esculentus hybrids in R-E systems: results from artificial crossings experiments. BMC Evol. Biol. 17, 220. ( 10.1186/s12862-017-1063-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Doležálková-Kaštánková M, Pruvost NBM, Plötner J, Reyer H-U, Janko K, Choleva L. 2018. All-male hybrids of a tetrapod Pelophylax esculentus share its origin and genetics of maintenance. Biol. Sex Differ. 9, 13. ( 10.1186/s13293-018-0172-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Mateos M, Sanjur OI, Vrijenhoek RC. 2002. Historical biogeography of the livebearing fish genus Poeciliopsis (Cyprinodontiformes). Evolution 56, 972-984. ( 10.1111/j.0014-3820.2002.tb01409.x) [DOI] [PubMed] [Google Scholar]
- 206.Mateos M. 2005. Comparative phylogeography of livebearing fishes in the genera Poeciliopsis and Poecilia (Poeciliidae: Cyprinodontiformes) in central Mexico. J. Biogeo. 32, 775-780. ( 10.1111/j.1365-2699.2005.01236.x) [DOI] [Google Scholar]
- 207.Cole CJ, Taylor HL, Neaves WB, Baumann DP, Newton A, Schnittker R, Baumann P. 2017. The second known tetraploid species of parthenogenetic tetrapod (Reptilia: Squamata: Teiidae): description, reproduction, comparisons with ancestral taxa, and origins of multiple clones. Bull. Mus. Comp. Zool. 161, 285-321. ( 10.3099/MCZ37.1) [DOI] [Google Scholar]
- 208.Yoshikawa H, Morishima K, Fujimoto T, Saito T, Kobayashi T, Yamaha E, Arai K. 2009. Chromosome doubling in early spermatogonia produces diploid spermatozoa in a natural clonal fish. Biol. Reprod. 80, 973-979. ( 10.1095/biolreprod.108.075150) [DOI] [PubMed] [Google Scholar]
- 209.Schilthuizen M, Giesbers MC, Beukeboom LW. 2011. Haldane's rule in the 21st century. Heredity 107, 95-102. ( 10.1038/hdy.2010.170) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Brandvain Y, Pauly GB, May M, Turelli M. 2014. Explaining Darwin's corollary to Haldane's Rule: the role of mitonuclear interactions in asymmetric postzygotic isolation among toads. Genetics 197, 743-747. ( 10.1534/genetics.113.161133) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Muller HJ. 1925. Why polyploidy is rarer in animals than in plants. Am. Nat. 59, 346-353. [Google Scholar]
- 212.Orr A. 1990. "Why polyploidy is rarer in animals than in plants" revisited. Am. Nat. 136, 759-770. ( 10.1086/285130) [DOI] [Google Scholar]
- 213.Otto SP, Whitton J. 2000. Polyploid incidence and evolution. Annu. Rev. Genet. 34, 401-437. ( 10.1146/annurev.genet.34.1.401) [DOI] [PubMed] [Google Scholar]
- 214.Mable BK. 2004. ‘Why polyploidy is rarer in animals than in plants': myths and mechanisms. Biol. J. Linn. Soc. 82, 453-466. ( 10.1111/j.1095-8312.2004.00332.x) [DOI] [Google Scholar]
- 215.Wertheim B, Beukeboom LW, van de Zande L. 2013. Polyploidy in animals: effects of gene expression on sex determination, evolution and ecology. Cytogenet. Genome Res. 140, 256-269. ( 10.1159/000351998) [DOI] [PubMed] [Google Scholar]
- 216.Evans BJ, Pyron RA, Wiens JJ. 2012. Polyploidization and sex chromosome evolution in Amphibians. In Polyploidy and genome evolution (eds Soltis PS, Soltis DE), pp. 385-410. Berlin, Heidelberg, Germany: Springer. ( 10.1007/978-3-642-31442-1_18) [DOI] [Google Scholar]
- 217.Mawaribuchi S, et al. 2017. Sex chromosome differentiation and the W- and Z-specific loci in Xenopus laevis. Dev. Biol. 426, 393-400. ( 10.1016/j.ydbio.2016.06.015) [DOI] [PubMed] [Google Scholar]
- 218.Morishima K, Horie S, Yamaha E, Arai K. 2002. A cryptic clonal line of the loach Misgurnus anguillicaudatus (Teleostei: Cobitidae) evidenced by induced gynogenesis, interspecific hybridization, microsatellite genotyping and multilocus DNA fingerprinting. Zoolog. Sci. 19, 565-575. ( 10.2108/zsj.19.565) [DOI] [PubMed] [Google Scholar]
- 219.Morishima K, Yoshikawa H, Arai K. 2012. Diploid clone produces unreduced diploid gametes but tetraploid clone generates reduced diploid gametes in the Misgurnus loach. Biol. Reprod. 86, 33, 1–8. ( 10.1095/biolreprod.111.093302) [DOI] [PubMed] [Google Scholar]
- 220.Saitoh K, Kim I-S, Lee E-H. 2004. Mitochondrial gene introgression between spined loaches via hybridogenesis. Zoolog. Sci. 21, 795-798. ( 10.2108/zsj.21.795) [DOI] [PubMed] [Google Scholar]
- 221.Itono M, Morishima K, Fujimoto T, Bando E, Yamaha E, Arai K. 2006. Premeiotic endomitosis produces diploid eggs in the natural clone loach, Misgurnus anguillicaudatus (Teleostei: Cobitidae). J. Exp. Zoolog. A Comp. Exp. Biol. 305A, 513-523. ( 10.1002/jez.a.283) [DOI] [PubMed] [Google Scholar]
- 222.Itono M, Okabayashi N, Morishima K, Fujimoto T, Yoshikawa H, Yamaha E, Arai K. 2007. Cytological mechanisms of gynogenesis and sperm incorporation in unreduced diploid eggs of the clonal loach, Misgurnus anguillicaudatus (Teleostei: Cobitidae). J. Exp. Zool. Part Ecol. Genet. Physiol. 307, 35-50. ( 10.1002/jez.a.344) [DOI] [PubMed] [Google Scholar]
- 223.Janko K, Bohlen J, Lamatsch D, Flajšhans M, Epplen JT, Ráb P, Kotlík P, Šlechtová V. 2007. The gynogenetic reproduction of diploid and triploid hybrid spined loaches (Cobitis: Teleostei), and their ability to establish successful clonal lineages—on the evolution of polyploidy in asexual vertebrates. Genetica 131, 185-194. ( 10.1007/s10709-006-9130-5) [DOI] [PubMed] [Google Scholar]
- 224.Oshima K, Morishima K, Yamaha E, Arai K. 2005. Reproductive capacity of triploid loaches obtained from Hokkaido Island, Japan. Ichthyol. Res. 52, 1-8. ( 10.1007/s10228-004-0245-3) [DOI] [Google Scholar]
- 225.Arias-Rodriguez L, Yasui GS, Kusuda S, Arai K. 2010. Reproductive and genetic capacity of spermatozoa of inter-populational hybrid males in the loach, Misgurnus anguillicaudatus. J. Appl. Ichthyol. 26, 653-658. ( 10.1111/j.1439-0426.2010.01534.x) [DOI] [Google Scholar]
- 226.Juchno D, Pecio A, Boroń A, Leska A, Jablonska O, Cejko BI, Kowalski RK, Judycka S, Przybylski M. 2017. Evidence of the sterility of allotetraploid Cobitis loaches (Teleostei, Cobitidae) using testes ultrastructure. J. Exp. Zool. Part Ecol. Integr. Physiol. 327, 66-74. ( 10.1002/jez.2071) [DOI] [PubMed] [Google Scholar]
- 227.Suzuki R, Oshiro T, Nakanishi T. 1985. Survival, growth and fertility of gynogenetic diploids induced in the cyprinid loach, Misgurnus anguillicaudatus. Aquaculture 48, 45-55. ( 10.1016/0044-8486(85)90051-1) [DOI] [Google Scholar]
- 228.Saitoh K. 1989. Multiple sex-chromosome system in a loach fish. Cytogenet. Cell Genet. 52, 62-64. ( 10.1159/000132840) [DOI] [PubMed] [Google Scholar]
- 229.Vasil'ev VP. 1995. Karyological diversity and taxonomic heterogeneity of Cobitis taenia (Pisces, Cobitidae). Doklady Biol. Sci. 342, 308-311. [Google Scholar]
- 230.Vasil'eva ED, Vasil'ev VP. 1998. Sibling species in genus Cobitis (Cobitidae). Cobitis rossomeridionalis sp. nova. J. Ichthyol. 38, 580-590. [Google Scholar]
- 231.Arai K, Fujimoto T. 2013. Genomic constitution and atypical reproduction in polyploid and unisexual lineages of the Misgurnus loach, a teleost fish. Cytogenet. Genome Res. 140, 226-240. ( 10.1159/000353301) [DOI] [PubMed] [Google Scholar]
- 232.Boroń A. 2003. Karyotypes and cytogenetic diversity of the genus Cobitis (Pisces, Cobitidae) in Poland: a review. Cytogenetic evidence for a hybrid origin of some Cobitis triploids. Folia Biol. (Praha) 51(Suppl.), 49-54. [PubMed] [Google Scholar]
- 233.Majtánová Z, Choleva L, Symonová R, Ráb P, Kotusz J, Pekárik L, Janko K. 2016. Asexual reproduction does not apparently increase the rate of chromosomal evolution: karyotype stability in diploid and triploid clonal hybrid fish (Cobitis, Cypriniformes, Teleostei). PLoS ONE 11, e0146872. ( 10.1371/journal.pone.0146872) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Marta A, Dedukh D, Bartoš O, Majtánová Z, Janko K. 2020. Cytogenetic characterization of seven novel satDNA markers in two species of spined loaches (Cobitis) and their clonal hybrids. Genes 11, 617. ( 10.3390/genes11060617) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Sola L, Rossi AR, Iaselli V, Rasch EM, Monaco PJ. 1992. Cytogenetics of bisexual/unisexual species of Poecilia. II. Analysis of heterochromatin and nuclear organizer regions in Poecilia mexicana mexicana by C-banding, DAPI, Chromomycin A3, and Ag-staining. Cytogen. Cell Genet. 60, 229-235. ( 10.1159/000133346) [DOI] [PubMed] [Google Scholar]
- 236.Avise J, Trexler J, Travis J, Nelson W. 1991. Poecilia mexicana is the recent female parent of the unisexual fish P. formosa. Evolution 46, 1530-1533. ( 10.2307/2409901) [DOI] [PubMed] [Google Scholar]
- 237.Lampert KP, Lamatsch DK, Fischer P, Epplen JT, Nanda I, Schmid M, Schartl M. 2007. Automictic reproduction in interspecific hybrids of poeciliid fish. Curr. Biol. 17, 1948-1953. ( 10.1016/j.cub.2007.09.064) [DOI] [PubMed] [Google Scholar]
- 238.Schartl M, Schlupp I, Schartl A, Meyer MK, Nanda I, Schmid M, Epplen JT, Parzefall J. 1991. On the stability of dispensable constituents of the eukaryotic genome: stability of coding sequences versus truly hypervariable sequences in a clonal vertebrate, the Amazon molly, Poecilia formosa. Proc. Natl Acad. Sci. USA 88, 8759-8763. ( 10.1073/pnas.88.19.8759) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Rasch EM, Balsano JS. 1973. Biochemical and cytogenetic studies of Poecilia from eastern Mexico. II. Frequency, perpetuation, and probable origin of triploid genomes in females associated with Poecilia formosa. Rev. Biol. Trop. 21, 351-381. [Google Scholar]
- 240.Lamatsch DK, Steinlein C, Schmid M, Schartl M. 2000. Non-invasive determination of genome size and ploidy level in fishes by flow cytometry detection of triploid Poecilia formosa. Cytometry 39, 91-95. () [DOI] [PubMed] [Google Scholar]
- 241.Lamatsch DK, Stöck M, Fuchs R, Döbler M, Wacker R, Parzefall J, Schlupp I, Schartl M. 2010. Morphology, testes development and behaviour of unusual triploid males in microchromosome-carrying clones of Poecilia formosa. J. Fish Biol. 77, 1459-1487. ( 10.1111/j.1095-8649.2010.02766.x) [DOI] [PubMed] [Google Scholar]
- 242.Lu Y, Bierbach D, Ormanns J, Warren WC, Walter RB, Schartl M. 2021. Fixation of allelic gene expression landscapes and expression bias pattern shape the transcriptome of the Amazon molly. Genome Res. 31, 1-8. ( 10.1101/gr.268870.120) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Bogart JP. 2019. Unisexual salamanders in the genus Ambystoma. Herpetologica 75, 259-267. ( 10.1655/Herpetologica-D-19-00043.1) [DOI] [Google Scholar]
- 244.Bogart JP, Bartoszek J, Noble DWA, Bi K. 2009. Sex in unisexual salamanders: discovery of a new sperm donor with ancient affinities. Heredity 103, 483-449. ( 10.1038/hdy.2009.83) [DOI] [PubMed] [Google Scholar]
- 245.Bogart JP. 2019. A family study to examine clonal diversity in unisexual salamanders (genus Ambystoma). Genome 62, 549-561. ( 10.1139/gen-2019-0034) [DOI] [PubMed] [Google Scholar]
- 246.Bogart J. 2003. Genetics and systematics of hybrid species. In Reproductive biology and phylogeny of urodela (ed. Sever DM), pp. 109-134. Enfield, NH: M/s Science Inc. [Google Scholar]
- 247.Bi K, Bogart JP. 2010. Time and time again: unisexual salamanders (genus Ambystoma) are the oldest unisexual vertebrates. BMC Evol. Biol. 10, 238. ( 10.1186/1471-2148-10-238) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Sessions SK. 1982. Cytogenetics of diploid and triploid salamanders of the Ambystoma jeffersonianum complex. Chromosoma 84, 599-621. ( 10.1007/BF00286329) [DOI] [Google Scholar]
- 249.Bi K, Bogart JP. 2006. Identification of intergenomic recombinations in unisexual salamanders of the genus Ambystoma by genomic in situ hybridization (GISH). Cytogenet. Genome Res. 112, 307-312. ( 10.1159/000089885) [DOI] [PubMed] [Google Scholar]
- 250.Robertson AV, Ramsden C, Niedzwiecki J, Fu J, Bogart JP. 2006. An unexpected recent ancestor of unisexual Ambystoma. Mol. Ecol. 15, 3339-3351. ( 10.1111/j.1365-294X.2006.03005.x) [DOI] [PubMed] [Google Scholar]
- 251.Bi K, Bogart JP, Fu J. 2008. The prevalence of genome replacement in unisexual salamanders of the genus Ambystoma (Amphibia, Caudata) revealed by nuclear gene genealogy. BMC Evol. Biol. 8, 158. ( 10.1186/1471-2148-8-158.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Bi K, Bogart JP, Fu J. 2007. Intergenomic translocations in unisexual salamanders of the genus Ambystoma (Amphibia, Caudata). Cytogenet. Genome Res. 116, 289-297. ( 10.1159/000100413) [DOI] [PubMed] [Google Scholar]
- 253.Keinath MC, Timoshevskaya N, Timoshevskiy VA, Voss R, Smith JJ. 2018. Miniscule differences between sex chromosomes in the giant genome of a salamander. Sci. Rep. 8, 17882. ( 10.1038/s41598-018-36209-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Tymowska J. 1991. Polyploidy and cytogenetic variation in frogs of the genus Xenopus. In Amphibian cytogenetics and evolution (eds Green DM, Sessions SK), pp. 259-297. San Diego, CA: Academic Press. [Google Scholar]
- 255.Session AM, et al. 2016. Genome evolution in the allotetraploid frog Xenopus laevis. Nature 538, 336-343. ( 10.1038/nature19840) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Furman BLS, Evans BJ. 2018. Divergent evolutionary trajectories of two young, homomorphic, and closely related sex chromosome systems. Genome Biol. Evol. 10, 742-755. ( 10.1093/gbe/evy045) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Yoshimoto S, Ito M. 2011. A ZZ/ZW-type sex determination in Xenopus laevis. FEBS J. 278, 1020-1026. ( 10.1111/j.1742-4658.2011.08031.x) [DOI] [PubMed] [Google Scholar]
- 258.Bewick AJ, Anderson DW, Evans BJ. 2011. Evolution of the closely related, sex-related genes DM-W and DMRT1 in African clawed frogs (Xenopus). Evolution 65, 698-712. ( 10.1111/j.1558-5646.2010.01163.x) [DOI] [PubMed] [Google Scholar]
- 259.Furman BLS, Evans BJ. 2016. Sequential turnovers of sex chromosomes in African clawed frogs (Xenopus) suggest some genomic regions are good at sex determination. G3 (Bethesda) 6, 3625-3633. ( 10.1534/g3.116.033423) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Furman BLS, Dang UJ, Evans BJ, Golding GB. 2018. Divergent subgenome evolution after allopolyploidization in African clawed frogs (Xenopus). J. Evol. Biol. 31, 1945-1858. ( 10.1111/jeb.13391) [DOI] [PubMed] [Google Scholar]
- 261.Song X-Y, et al. 2021. Sex chromosome degeneration, turnover, and sex-biased expression of sex-linked transcripts in African clawed frogs (Xenopus). Phil. Trans. R. Soc. B 376, 20200095. ( 10.1098/20200095) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Günther R. 1990. Die Wasserfrösche Europas. [The water frogs of Europe]. Die Neue Brehm-Bücherei 600. Wittenberg, Germany: Ziemsen. [In German.] [Google Scholar]
- 263.Plötner J. 2005. Die westpaläarktischen Wasserfrösche. Von Märtyrern der Wissenschaft zur biologischen Sensation. [The Western-Palearctic water frogs. From martyrs of science to biological sensation]. Beiheft, Zeitschrift f. Feldherpetologie, pp. 1–166. Bielefeld, Germany: Laurenti Verlag. [In German.] [Google Scholar]
- 264.Graf J-D, Karch F, Moreillon M-C. 1977. Biochemical variation on the Rana esculenta complex: a new hybrid form related to Rana perezei and Rana ridibunda. Experientia 33, 1582-1584. ( 10.1007/BF01934010) [DOI] [PubMed] [Google Scholar]
- 265.Uzzell T, Hotz H. 1979. Electrophoretic and morphological evidence for two forms of green frog (Rana esculenta complex) in peninsular Italy (Amphibia: Salentia). Mitteilungen aus dem Zoologischen Museum in Berlin 55, 13-27 [Google Scholar]
- 266.Holsbeek G, Jooris R. 2010. Potential impact of genome exclusion by alien species in the hybridogenetic water frogs (Pelophylax esculentus complex). Biol. Inv. 12, 1-13. ( 10.1007/s10530-009-9427-2) [DOI] [Google Scholar]
- 267.Berger L. 1990. On the origin of genetic systems of European waterfrogs. Zool. Pol. 35, 5-27. [Google Scholar]
- 268.Heppich S, Tunner HG. 1979. Chromosomal constitution and C-banding in homotypic Rana esculenta crosses. Mitt. Zool. Mus. Berlin 55, 111-114. [Google Scholar]
- 269.Miura I. 1995. The late replication banding patterns of chromosomes are highly conserved in the genera Rana, Hyla, and Bufo (Amphibia: Anura). Chromosoma 103, 567-574. ( 10.1007/BF00355322) [DOI] [PubMed] [Google Scholar]
- 270.Jeffries DL, et al. 2018. A rapid rate of sex-chromosome turnover and non-random transitions in true frogs. Nat. Commun. 9, 4088. ( 10.1038/s41467-018-06517-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Berger L, Uzzell T, Hotz TZ. 1988. Sex determination and sex ratios in western Palearctic water frogs: XX and XY female hybrids in the Pannonian Basin? Proc. Acad. Nat. Sci. Phila. 140, 220-239. [Google Scholar]
- 272.Hotz H, Uzzell T, Berger L. 1997. Linkage groups of protein-coding genes in western Palearctic water frogs reveal extensive evolutionary conservation. Genetics 147, 255-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Schempp W, Schmid M. 1981. Chromosome banding in Amphibia. VI. BrdU-replication patterns in Anura and demonstration of XX/XY sex chromosomes in Rana esculenta. Chromosoma (Berlin) 83, 697-710. ( 10.1007/BF00328528) [DOI] [PubMed] [Google Scholar]
- 274.Berger L, Günther R. 1991–1992 Inheritance patterns of water frog males from the environments of nature reserve Steckby, Germany. Zool. Pol. 37, 87-100. [Google Scholar]
- 275.Christiansen DG, Reyer HU. 2009. From clonal to sexual hybrids: genetic recombination via triploids in all-hybrid populations of water frogs. Evolution 63, 1754-1768. ( 10.1111/j.1558-5646.2009.00673.x) [DOI] [PubMed] [Google Scholar]
- 276.Perrin N. 2021. Sex-chromosome evolution in frogs: What role for sex-antagonistic genes? Phil. Trans. R. Soc. B 376, 20200094. ( 10.1098/rstb.2020.0094) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Stöck M, Moritz C, Hickerson M, Frynta D, Dujsebayeva T, Eremchenko V, Macey JR, Papenfuss TJ, Wake DB. 2006. Evolution of mitochondrial relationships and biogeography of Palearctic green toads (Bufo viridis subgroup) with insights in their genomic plasticity. Mol. Phylogenet. Evol. 41, 663-689. ( 10.1016/j.ympev.2006.05.026) [DOI] [PubMed] [Google Scholar]
- 278.Stöck M, Sicilia A, Belfiore N, Buckley D, Lo Brutto S, Lo Valvo M, Arculeo M. 2008. Post-Messinian evolutionary relationships across the Sicilian channel: mitochondrial and nuclear markers link a new green toad from Sicily to African relatives. BMC Evol. Biol. 8, 56. ( 10.1186/1471-2148-8-56) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Colliard C, Sicilia A, Turrisi GF, Arculeo M, Perrin N, Stöck M. 2010. Strong reproductive barriers in a narrow hybrid zone of West-Mediterranean green toads (Bufo viridis subgroup) with Plio-Pleistocene divergence. BMC Evol. Biol. 10, 232. ( 10.1186/1471-2148-10-232) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Stöck M, Steinlein C, Lamatsch DK, Schartl M, Schmid M. 2005. Multiple origins of tetraploid taxa in the Eurasian Bufo viridis subgroup. Genetica 124, 255-272. ( 10.1007/s10709-005-3085-9) [DOI] [PubMed] [Google Scholar]
- 281.Betto-Colliard C, Sermier R, Litvinchuk S, Perrin N, Stöck M. 2015. Origin and genome evolution of polyploid green toads in Central Asia: evidence from microsatellite markers. Heredity 114, 300-308. ( 10.1038/hdy.2014.100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Stöck M, Croll D, Dumas Z, Biollay S, Wang J, Perrin N. 2011. A cryptic heterogametic transition revealed by sex-linked DNA markers in Palearctic green toads. J. Evol. Biol. 24, 1064-1070. ( 10.1111/j.1420-9101.2011.02239.x) [DOI] [PubMed] [Google Scholar]
- 283.Stöck M, Savary R, Betto-Colliard C, Biollay S, Jourdan-Pineau H, Perrin N. 2013. Low rates of X-Y recombination, not turnovers, account for homomorphic sex chromosomes in several diploid species of Palearctic green toads (Bufo viridis subgroup). J. Evol. Biol. 3, 674-682. ( 10.1111/jeb.12086) [DOI] [PubMed] [Google Scholar]
- 284.Brelsford A, et al. 2013. Homologous sex chromosomes in three deeply divergent anuran species. Evolution 67, 2434-2440. ( 10.1111/evo.12151) [DOI] [PubMed] [Google Scholar]
- 285.Tamschick S, Rozenblut-Kościsty B, Bonato L, Dufresnes C, Lymberakis P, Kloas W, Ogielska M, Stöck M. 2015. Sex chromosome conservation, Dmrt1-phylogeny and gonad morphology in diploid Palearctic green toads (Bufo viridis subgroup). Cytogenet. Genome Res. 144, 315-324. ( 10.1159/000380841) [DOI] [PubMed] [Google Scholar]
- 286.Fujita MK, Singhal S, Brunes TO, Maldonado JA. 2020. Evolutionary dynamics and consequences of parthenogenesis in vertebrates. Annu. Rev. Ecol. Evol. Syst. 51, 191-214. ( 10.1146/annurev-ecolsys-011720-114900) [DOI] [Google Scholar]
- 287.Pellegrino KCM, Rodrigues MT, Harris DJ, Yonenaga-Yassuda Y, Sites JW Jr. 2011. Molecular phylogeny, biogeography and insights into the origin of parthenogenesis in the Neotropical genus Leposoma (Squamata: Gymnophthalmidae): ancient links between the Atlantic Forest and Amazonia. Mol. Phylogenet. Evol. 61, 446-459. ( 10.1016/j.ympev.2011.07.010) [DOI] [PubMed] [Google Scholar]
- 288.Brunes TO, da Silva AJ, Marques-Souza S, Rodrigues MT, Pellegrino KCM. 2019. Not always young: the first vertebrate ancient origin of true parthenogenesis found in an Amazon leaf litter lizard with evidence of mitochondrial haplotypes surfing on the wave of a range expansion. Mol. Phylogenet. Evol. 135, 105-122. ( 10.1016/j.ympev.2019.01.023) [DOI] [PubMed] [Google Scholar]
- 289.Murphy RW, Fu J, MacCulloch RD, Darevsky IS, Kupriyanova LA. 2000. A fine line between sex and unisexuality: the phylogenetic constraints on parthenogenesis in lacertid lizards. Zool. J. Linn. Soc. 130, 527-549. ( 10.1111/j.1096-3642.2000.tb02200.x) [DOI] [Google Scholar]
- 290.Serena M. 1984. Distribution and habitats of parthenogenetic and sexual Cnemidophorus lemniscatus (Sauria: Teiidae) in Surinam. Copeia 1984, 713-719. ( 10.2307/1445154) [DOI] [Google Scholar]
- 291.Lutes AA, Baumann DP, Neaves WB, Baumann P. 2011. Laboratory synthesis of an independently reproducing vertebrate species. Proc. Natl Acad. Sci. USA 108, 9910-9915. ( 10.1073/pnas.1102811108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Moritz C. 1990. Patterns and processes of sex chromosome evolution in Gekkonid lizards (Sauria: Reptilia). In Cytogenetics of amphibians and reptiles (ed. Olmo E), pp. 205-219. Berlin, Germany: Birkhäuser-Verlag. [Google Scholar]
- 293.Reeder TW, Cole CJ, Dessauer HC. 2002. Phylogenetic relationships of whiptail lizards of the genus Cnemidophorus (Squamata: Teiidae): a test of monophyly, reevaluation of karyotypic evolution, and review of hybrid origins. Am. Mus. Novit. 3365, 1-61. () [DOI] [Google Scholar]
- 294.Moritz C, Uzzell T, Spolsky C, Hotz H, Darevsky I, Kupriyanova L, Danielyan F. 1992. The maternal ancestry and approximate age of parthenogenetic species of Caucasian rock lizards (Lacerta: Lacertidae). Genetica 87, 53-62. ( 10.1007/BF00128773) [DOI] [Google Scholar]
- 295.Darevsky IS, Kupriyanova LA, Uzzell TM. 1985. Parthenogenesis in reptiles. In Biology of the reptilia (eds Gans C, Billett F), pp. 412-526. New York, NY: John Wiley and Sons Inc. [Google Scholar]
- 296.Cole JC, Painter CW, Dessauer HC, Taylor HL. 2007. Hybridization between the endangered unisexual gray-checkered whiptail lizard (Aspidoscelis dixoni) and the bisexual western whiptail lizard (Aspidoscelis tigris) in southwestern New Mexico. Am. Mus. Nov. 3555, 1-31. (doi:10.1206/0003-0082(2007)3555 [1:HBTEUG]2.0.CO;2) [Google Scholar]
- 297.Densmore LD III, Wright JW, Brown WM. 1989. Mitochondrial DNA analyses and the origin and relative age of parthenogenetic lizards (genus Cnemidophorus). II. C. neomexicanus and the C. tesselatus complex. Evolution 43, 943-957. ( 10.1111/j.1558-5646.1989.tb02541.x) [DOI] [PubMed] [Google Scholar]
- 298.Parker ED Jr, Selander RK. 1976. The organisation of genetic diversity in the parthenogenetic lizard Cnemidophorus tesselatus. Genetics 84, 791-805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Uzzell T, Darevsky IS. 1974. The evidence of the hybrid origin of parthenogenetic Caucasian rock lizards of the Lacerta genus. Zhurnal Obshchei Biol. 35, 553-561. [PubMed] [Google Scholar]
- 300.Darevsky IS, Kupriyanova LA, Danielyan FD. 1986. New evidence of hybrid males of parthenogenetic species. In Studies in herpetology (ed. Rocek Z), pp. 207-212. Prague, Czechoslovakia: Charles University. [Google Scholar]
- 301.Uzzell T, Darevsky IS. 1975. Biochemical evidence for the hybrid origin of the parthenogenetic species of the Lacerta saxicola complex (Sauria, Lacertidae), with a discussion of some ecological and evolutionary implications. Copeia 1975, 204-222. ( 10.2307/1442879) [DOI] [Google Scholar]
- 302.Kupriyanova LA. 1999. Genetic variations in hybrid unisexual species and forms of the genus Lacerta (Lacertidae, Reptilia): possible cytogenetic mechanisms, cytogenetics of meiosis in natural polyploidy forms. Tsytologia 41, 1038-1047. [Google Scholar]
- 303.Rovatsos M, Vukic J, Altmanová M, Johnson Pokorná M, Moravec J, Kratochvíl L. 2016. Conservation of sex chromosomes in lacertid lizards. Mol. Ecol. 25, 3120-3126. ( 10.1111/mec.13635) [DOI] [PubMed] [Google Scholar]
- 304.Kupriyanova LA. 1989. Cytogenetic evidence for genome interaction in hybrid lacertid lizards. In Evolution and ecology of unisexual vertebrates (eds Dawley RM, Bogart JP), pp. 236-240. New York State Museum Bulletin 466. Albany, NY: New York State Museum. [Google Scholar]
- 305.Kupriyanova LA. 1992. Diversity in parthenogenetic lacertid lizards: cytogenetic studies. In Proceedings of the Sixth Ordinary General Meeting of Societas Europaea Herpetologica (eds Korsós Z, Kiss I), pp. 273-279, Budapest, Hungary, 19–23 August 1991. [Google Scholar]
- 306.Spangenberg V et al. 2020. Evolution of the parthenogenetic rock lizard hybrid karyotype: Robertsonian translocation between two maternal chromosomes in Darevskia rostombekowi. Chromosoma 129, 275-283. ( 10.1007/s00412-020-00744-7) [DOI] [PubMed] [Google Scholar]
- 307.Trifonov VA, Paoletti A, Caputo Barucchi V, Kalinina T, O'Brien PCM, Ferguson-Smith MA, Giovannotti M. 2015. Comparative chromosome painting and NOR distribution suggest a complex hybrid origin of triploid Lepidodactylus lugubris (Gekkonidae). PLoS ONE 10, e0132380. ( 10.1371/journal.pone.0132380) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Moritz C, Case TJ, Bolger DT, Donnellan SC. 1993. Genetic diversity and the history of some Pacific island house geckos (Hemidactylus and Lepidodactylus). Biol. J. Linn. Soc. 48, 113-133. ( 10.1111/j.1095-8312.1993.tb00882.x) [DOI] [Google Scholar]
- 309.Darevsky IS, Kupriyanova LA, Roshchin V. 1984. A new all-female triploid species of gecko and karyological data on the bisexual Hemidactylus frenatus from Vietnam. J. Herpetol. 18, 277-284. ( 10.2307/1564081) [DOI] [Google Scholar]
- 310.Moritz C. 1984. The origin and evolution of parthenogenesis in Heteronotia binoei (Gekkonidae) I. Chromosome banding studies. Chromosoma 89, 151-162. ( 10.1007/BF00292899) [DOI] [Google Scholar]
- 311.Zug GR. 2010. Speciation and dispersal in a low diversity taxon: the slender geckos Hemiphyllodactylus (Reptilia, Gekkonidae). Smithson. Contrib. Zool. 631, 1-70. ( 10.5479/si.00810282.631) [DOI] [Google Scholar]
- 312.Moritz C. 1987. Parthenogenesis in the tropical gekkonid lizard, Nactus arnouxii (Sauria, Gekkonidae). Evolution 41, 1252-1266. ( 10.1111/j.1558-5646.1987.tb02464.x) [DOI] [PubMed] [Google Scholar]
- 313.Pyron RA, Burbrink FT, Wiens JJ. 2013. A phylogeny and revised classification of Squamata, including 4161 species of lizards and snakes. BMC Evol. Biol. 13, 93. ( 10.1186/1471-2148-13-93) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Gamble T, Coryell J, Ezaz T, Lynch J, Scantlebury DP, Zarkower D. 2015. Restriction site-associated DNA sequencing (RAD-seq) reveals an extraordinary number of transitions among gecko sex-determining systems. Mol. Biol. Evol. 32, 1296-1309. ( 10.1093/molbev/msv023) [DOI] [PubMed] [Google Scholar]
- 315.Rovatsos M, Farkačová K, Altmanová M, Johnson Pokorná M, Kratochvíl L. 2019. The rise and fall of differentiated sex chromosomes in geckos. Mol. Ecol. 28, 3042-3052. ( 10.1111/mec.15126) [DOI] [PubMed] [Google Scholar]
- 316.Volobouev V, Pasteur G. 1988. Presumptive sex chromosomes of a unisexual homomorphic species of lizards, Lepidodactylus lugubris. Heredity 60, 463-467. ( 10.1038/hdy.1988.65) [DOI] [PubMed] [Google Scholar]
- 317.Röll B, von Düring MUG. 2008. Sexual characteristics and spermatogenesis in males of the parthenogenetic gecko Lepidodactylus lugubris (Reptilia, Gekkonidae). Zoology 111, 385-400. ( 10.1016/j.zool.2007.09.004) [DOI] [PubMed] [Google Scholar]
- 318.Gamble T. 2010. A review of sex determining mechanisms in geckos (Gekkota: Squamata). Sex. Dev. 4, 88-103. ( 10.1159/000289578) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Kearney M, Moussalli A, Strasburg JL, Lindenmayer D, Moritz C. 2003. Geographic parthenogenesis in the Australian arid zone: I. A climatic analysis of the Heteronotia binoei complex (Gekkonidae). Evol. Ecol. Res. 5, 953-976. [Google Scholar]
- 320.Strasburg JL, Kearney M. 2005. Phylogeography of sexual Heteronotia binoei (Gekkonidae) in the Australian arid zone: climatic cycling and repetitive hybridization. Mol. Ecol. 14, 2755-2772. ( 10.1111/j.1365-294X.2005.02627.x) [DOI] [PubMed] [Google Scholar]
- 321.Stöck M, et al. 2021. A brief review of vertebrate sex evolution with a pledge for integrative research - towards ‘sexomics’. Phil. Trans. R. Soc. B. 376, 20200426. ( 10.1098/rstb.2020.0426). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Stöck M, Dedukh D, Reifová R, Lamatsch DK, Starostová Z, Janko K. 2021. Sex chromosomes in meiotic, hemiclonal, clonal and polyploid hybrid vertebrates: along the ‘extended speciation continuum’. Figshare. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Stöck M, Dedukh D, Reifová R, Lamatsch DK, Starostová Z, Janko K. 2021. Sex chromosomes in meiotic, hemiclonal, clonal and polyploid hybrid vertebrates: along the ‘extended speciation continuum’. Figshare. [DOI] [PMC free article] [PubMed]
Data Availability Statement
Data and materials have been previously published or can be found in the electronic supplemental material.
The data are provided in the electronic supplementary material [322].