Abstract
Objectives
The aim was to assess the performance of antigen-based rapid diagnostic tests (Ag-RDTs) for SARS CoV-2 when implemented for large-scale universal screening of asymptomatic individuals.
Methods
This study was a pragmatic implementation study for universal Ag-RDT-based screening at a tertiary care hospital in Germany where patients presenting for elective procedures and selected personnel without symptoms suggestive of SARS-CoV-2 were screened with an Ag-RDT since October 2020. Test performance was calculated on an individual patient level.
Results
In total, 49 542 RDTs were performed in 27 421 asymptomatic individuals over a duration of 5 and a half months. Out of 222 positive results, 196 underwent in-house confirmatory testing with PCR, out of which 170 were confirmed positive, indicating a positive predictive value of 86.7% (95% CI 81.2–91.1%). Negative Ag-RDTs were not routinely tested with PCR, but a total of 94 cases of false negative Ag-RDTs were detected due to PCR tests being performed within the following 5 days with a median cycle threshold value of 33 (IQR 29–35).
Discussion
This study provides evidence that Ag-RDTs can have a high diagnostic yield for transmission relevant infections with limited false positives when utilized at the point of care on asymptomatic patients and thus can be a suitable public health test for universal screening.
Keywords: Ag-RDTs, Diagnostic yield, Positive predictive value, SARS-CoV-2, Screening
Introduction
Several researchers and policy makers, supported by evidence from modelling studies, have recently argued to increase large-scale screening for SARS-CoV-2 to curb transmission from patients with minimal or no symptoms [[1], [2], [3], [4], [5]]. Antigen detection point-of-care rapid diagnostic tests (Ag-RDTs) have shown very good sensitivity (88%) in persons with high viral load (cycle threshold (CT) < 30) along with high specificity (>99%) [6,7]. With their favourable ease of use, rapid turnaround and good (although suboptimal) performance, Ag-RDTs meet the characteristics for a test for public health use and could allow for better control of transmission if implemented in well-designed universal screening strategies [8,9]. However, one frequently raised concern has been the potentially imperfect specificity, leading to large numbers of false positives when using Ag-RDTs in large-scale screening strategies with low prevalence [10], which could conceivably disrupt workflows and undermine trust in the test. Furthermore, in a setting where high-risk persons are present (e.g., hospital), concerns exist regarding imperfect sensitivity leading to secondary cases and substantial morbidity and mortality. Data from large-scale screening implementation efforts that would allow to gauge diagnostic yield and issues with false positives are limited.
Materials and methods
We performed a large-scale, pragmatic implementation study of Ag-RDTs in the context of a universal screening programme at one of Germany's largest tertiary care hospitals (Heidelberg University Hospital, Germany) serving over 100 000 inpatients and 1.3 million outpatients per year [11]. The study was conducted between 20 September 2020 and 7 March 2021 during Germany's second wave of COVID-19. Over the duration of the study, SARS-CoV-2 incidence in the region served by the hospital ranged from 11.6 cases per 100 000 inhabitants in the last 7 days (23 September) to 254.2 (22 December) [12]. Patients without SARS-CoV-2-associated symptoms presenting for elective procedures, including outpatient treatment requiring close contact or longer presence (e.g., dialysis), were screened with an Ag-RDT. Additionally, depending on setting and local SARS-CoV-2 infection dynamics, other external personnel (e.g., craftspeople, visitors, translators) were similarly screened. The test used was the STANDARD Q COVID-19 Ag Test (SD Biosensor, Inc. Gyeonggi-do, Korea), a WHO recommended and independently validated instrument-free lateral flow assay for SARS-CoV-2 detection [[13], [14], [15]] performed at point of care using the nasopharyngeal swabs provided in the kit. Prior to screening initiation, nursing staff were trained to perform the test and had to pass a competency test implemented by members of the hospital hygiene department. In patients with a positive Ag-RDT, an additional nasopharyngeal swab was collected for confirmatory SARS-CoV-2 PCR using one of the following commercially available PCRs: the SARS CoV-2 assay from TibMolbiol (Berlin, Germany), the Allplex SARS-CoV-2 Assay from Seegene (Seoul, South Korea) or the Abbott (Illinois, USA) RealTime 2019-nCoV assay. Ag-RDT results were confirmed with PCR in selected departments (e.g., haematology) prior to ward admission, prior to planned procedures associated with high levels of aerosol production, when a patient developed SARS-CoV-2 associated symptoms, or for contact screening when a case was detected on hospital wards. To analyse diagnostic yield of Ag-RDTs and false positives, we systematically extracted results of the Ag-RDTs performed from the hospital internal laboratory system, as well as results of PCR tests (with CT values) that were done within 5 days after Ag-RDT screening. Positive predictive value (PPV), sensitivity, and 95% confidence intervals were computed using the confirmatory PCR result as the reference standard. Because tests performed in the same individual are expected to be correlated, sensitivity and predictive values were calculated on individual patient rather than test level. Analysis was conducted using R v4.0.3 (The R Foundation). The ethics review board at Heidelberg University approved this study (S-811/2020).
Results
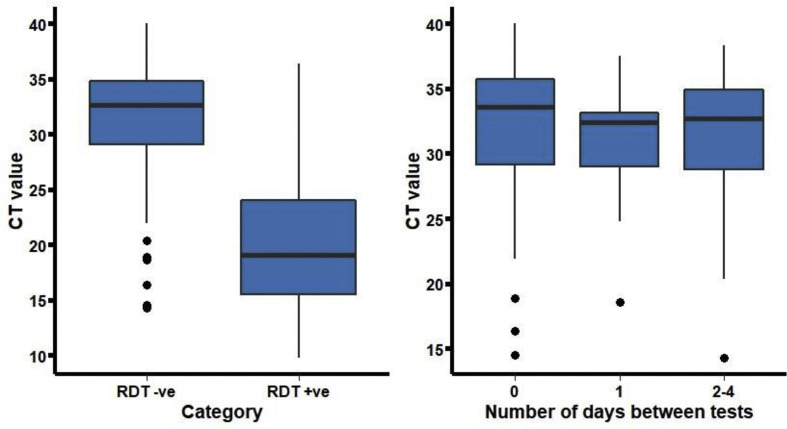
Between 20 September 2020 and 7 March 2021, 49 542 Ag-RDTs were performed in 27 421 asymptomatic individuals. Of the total 27 421 patients tested, 19 712 (72%) were only tested once. Ag-RDTs were positive in 222 individuals and 49 320 Ag-RDTs were negative in 27 199 individuals. Out of the 222 individuals with positive Ag-RDTs, 196 (88.3%) were also tested using PCR in-house. The PPV for the Ag-RDTs was 170/196 (86.7%, 95% CI 81.2–91.1%). Among patients with a positive confirmatory PCR performed within 5 days of a positive Ag-RDT, the median CT value was 19 (interquartile range, IQR 15–24, Fig. 1 ). Additionally, 7165 PCRs were negative within 5 days of a negative RDT.
Fig. 1.
SARS-CoV-2 PCR CT-values according to Ag-RDT result (left) and for false-negative Ag-RDT results according to the number of days between discordant tests (right). Left panel: CT values were higher among patients with negative versus positive RDTs (p < 0.001). Right panel: among patients with a negative RDT and a positive PCR, the CT values did not differ between the different groups (p = 0.339).
Of 27 421 patients with a negative Ag-RDT, 94 had a positive PCR in the 5 days following the Ag-RDT. The median number of days between the tests was 1 (IQR 0–1). Based on these false-negative cases identified via PCR, the overall sensitivity of the Ag-RDT can be estimated to be 170/264 (64.4%, 95% CI 58.3–70.2). The median CT value of patients who were missed using Ag-RDTs was 33 (IQR 29–35). In total, only 12/94 (12.8%) Ag-RDT false-negative patients had a PCR with a CT value < 25, and 10/12 were identified on the same or following day.
Discussion
This pragmatic implementation study of a universal screening programme of asymptomatic patients at a tertiary care hospital where quick turnaround and detection of cases with high risk of secondary transmission were essential showed the benefit of Ag-RDTs identifying SARS-CoV-2-infected persons who would have otherwise entered a high-risk environment leading to potential secondary transmission. The sensitivity estimated in this study is higher than that observed in other studies of asymptomatic infections [7]. Although the data on accuracy in asymptomatic patients is limited, we acknowledge that the sensitivity estimate is likely an overestimate (i.e., at most 64.4%), as not all patients with asymptomatic infections were PCR tested in this pragmatic study. However, as viral load kinetics have been confirmed to be largely similar in asymptomatic and symptomatic patients, judging from performance in symptomatic patients, one would expect to capture most infections in the first week of illness in asymptomatic persons when viral load is high and thus prevent most secondary transmissions [7,16,17]. This is supported by the few new cases observed in the hospital during the study period. The imperfect sensitivity however highlights the need for the continuation of other protective measures. Furthermore, authorities have recommended against clinical extrapolation of infectiousness based exclusively on CT [18]. Missed cases with CT < 25 on PCR testing were identified within 24 hours and are likely attributable to a negative Ag-RDT in the early phase of disease when the viral load is increasing rapidly [19]. Our study also showed a very high PPV of the Ag-RDT, thus confirming the high reliability of a positive result from an Ag-RDT shown in accuracy studies [7].
To the best of our knowledge, this is the first large-scale implementation study of a universal screening programme of asymptomatic individuals to analyse the diagnostic yield of SARS-CoV-2 Ag-RDTs. The central limitation of this study, inherent to a pragmatic implementation study, is the limited confirmatory PCR testing for negative Ag-RDTs, and potential selection bias associated with confirmatory testing being only performed in high-risk or subsequently symptomatic patients. Additionally, the delay in confirmatory PCR-based testing merits caution when interpreting the sensitivity and specificity estimates.
In conclusion, this study provides evidence that an Ag-RDT can be a suitable test for large-scale universal screening in a hospital setting and adds the important component of a public health test to our diagnostic armamentarium. Even as vaccination rates continue to increase, given the emergence of novel virus variants, resulting immune escape despite vaccination, and the high risk associated with undetected infections in clinical settings, we expect universal screening to remain necessary as the pandemic progresses.
Transparency declaration
Mr Wachinger, Dr Olaru, Mrs Horner, Dr Schnitzler and Dr Heeg have nothing to disclose; Dr Denkinger reports grants from Ministry of Science, Research and the Arts, State of Baden-Wuerttemberg, Germany, during the conduct of the study. The authors did not receive any industry support for conducting this study. Within the previous 3 years, C.M.D. has received support from SAP SE, and P.S. has received support from Janssen-Cilag, MSD Sharp & Dohme and RAM Group DE GmbH for work not related to this study. J.W., I.D.O., S.H. and K.H. have nothing to disclose. This study was funded in part by the Ministry of Science, Research and the Arts, State of Baden-Wuerttemberg, Germany, as well as hospital-internal funds.
Author contributions
J.W., K.H. and C.M.D. conceptualized the study. I.D.O. analysed the data, supported by J.W., S.H., P.S. and C.M.D. J.W., I.D.O. and C.M.D. drafted the manuscript. All co-authors critically revised and approved the final version of the research note.
Editor: F. Allerberger
References
- 1.Moghadas S.M., Fitzpatrick M.C., Sah P., Pandey A., Shoukat A., Singer B.H., et al. The implications of silent transmission for the control of COVID-19 outbreaks. Proc Natl Acad Sci USA. 2020;117:17513–17515. doi: 10.1073/pnas.2008373117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization . 2020. SARS-CoV-2 antigen-detecting rapid diagnostic tests: an implementation guide.https://www.who.int/publications/i/item/9789240017740 (cited 9 July 2021). Available from: [Google Scholar]
- 3.Smith D.R.M., Duval A., Pouwels K.B., Guillemot D., Fernandes J., Huynh B.-T., et al. Optimizing COVID-19 surveillance in long-term care facilities: a modelling study. BMC Med. 2020;18:386. doi: 10.1186/s12916-020-01866-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peeling R.W., Olliaro P. Rolling out COVID-19 antigen rapid diagnostic tests: the time is now. Lancet Infect Dis. 2021;21:1052–1053. doi: 10.1016/S1473-3099(21)00152-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson-León M., Caplan A.L., Kenny L., Buchan I., Fesi L., Olhava P., et al. Executive summary: it's wrong not to test: the case for universal, frequent rapid COVID-19 testing. E Clin Med. 2021;33:100759. doi: 10.1016/j.eclinm.2021.100759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dinnes J., Deeks J.J., Berhane S., Taylor M., Adriano A., Davenport C., et al. Rapid, point-of-care antigen and molecular-based tests for diagnosis of SARS-CoV-2 infection. Cochrane Database Syst Rev. 2021;3 doi: 10.1002/14651858.CD013705.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brümmer LE, Katzenschlager S, Gaeddert M, Erdmann C, Schmitz S, Bota M, et al. The accuracy of novel antigen rapid diagnostics for SARS-CoV-2: a living systematic review and meta-analysis. medRxiv 2021:2021.02.26.21252546. Available from: https://www.medrxiv.org/content/10.1101/2021.02.26.21252546v1.full.pdf. [DOI] [PMC free article] [PubMed]
- 8.Boehme C., Hannay E., Sampath R. SARS-CoV-2 testing for public health use: core principles and considerations for defined use settings. Lancet Glob Health. 2021;9:e247–e249. doi: 10.1016/S2214-109X(21)00006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Larremore D.B., Wilder B., Lester E., Shehata S., Burke J.M., Hay J.A., et al. Test sensitivity is secondary to frequency and turnaround time for COVID-19 screening. Sci Adv. 2021;7 doi: 10.1126/sciadv.abd5393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peeling R.W., Olliaro P.L., Boeras D.I., Fongwen N. Scaling up COVID-19 rapid antigen tests: promises and challenges. Lancet Infect Dis. 2021 doi: 10.1016/S1473-3099(21)00048-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heidelberg University Hospital . 2020. Annual report 2019.https://report.ukhd-mfhd.de/2019/ (cited 9 July 2021). Available from: [Google Scholar]
- 12.Landratsamt Rhein-Neckar-Kreis . 2021. Fallzahlen aus dem Rhein-Neckar-Kreis und dem Stadtgebiet Heidelberg.https://www.rhein-neckar-kreis.de/start/landratsamt/coronavirus+fallzahlen.html (cited 5 July 2021). Available from: [Google Scholar]
- 13.Berger A., Nsoga M.T.N., Perez-Rodriguez F.J., Aad Y.A., Sattonnet-Roche P., Gayet-Ageron A., et al. Diagnostic accuracy of two commercial SARS-CoV-2 antigen-detecting rapid tests at the point of care in community-based testing centers. PLoS ONE. 2021;16 doi: 10.1371/journal.pone.0248921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krüger LJ, Gaeddert M, Köppel L, Brümmer LE, Gottschalk C, Miranda IB, et al. Evaluation of the accuracy, ease of use and limit of detection of novel, rapid, antigen-detecting point-of-care diagnostics for SARS-CoV-2. medRxiv. 2020. 2020.10.01.20203836. Available from: https://www.medrxiv.org/content/10.1101/2020.10.01.20203836v1.full.pdf.
- 15.World Health Organization . 2020. WHO emergency use assessment coronavirus disease (COVID-19) IVDs: public report.https://www.who.int/diagnostics_laboratory/eual/201019_final_pqpr_eul_0563_117_00_standard_q_covid19_ag_test.pdf?ua=1 (cited 8 July 2021). Available from: [Google Scholar]
- 16.Lee S., Kim T., Lee E., Lee C., Kim H., Rhee H., et al. Clinical course and molecular viral shedding among asymptomatic and symptomatic patients with SARS-CoV-2 infection in a community treatment center in the Republic of Korea. JAMA Intern Med. 2020;180:1447–1452. doi: 10.1001/jamainternmed.2020.3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones T.C., Biele G., Mühlemann B., Veith T., Schneider J., Beheim-Schwarzbach J., et al. Estimating infectiousness throughout SARS-CoV-2 infection course. Science. 2021;373:eabi5273. doi: 10.1126/science.abi5273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention . 2021. Clinical questions about COVID-19: questions and answers.https://www.cdc.gov/coronavirus/2019-ncov/hcp/faq.html#Testing,-Isolation,-and-Quarantine-for-Persons-Who-Have-Recovered-from-Previous-SARS-CoV-2-Infection (cited 5 July 2021). Available from: [Google Scholar]
- 19.Kissler S.M., Fauver J.R., Mack C., Olesen S.W., Tai C., Shiue K.Y., et al. Viral dynamics of acute SARS-CoV-2 infection and applications to diagnostic and public health strategies. PLOS Biol. 2021;19 doi: 10.1371/journal.pbio.3001333. [DOI] [PMC free article] [PubMed] [Google Scholar]