Abstract
The endometrium is a dynamic target organ in a woman’s reproductive life. It undergoes cyclical change regulated by the fine balance between oestrogen and progesterone. The endometrial thickness (ET) varies according to the phases of the menstrual cycle. Endometrium contains both oestrogen and progesterone receptors, which respond to above hormones, irrespective of whether the woman is in reproductive or menopausal phase. Abundance of oestrogen leads to endometrial hyperplasia, and paucity causes endometrial atrophy. The initial best modality of assessing ET or aberration is high resolution transvaginal ultrasonogram. Thickened endometrium is always a clinical conundrum. Dilemma does remain as to the thickness of endometrium which requires intervention, mostly in symptomatic pre and perimenopausal women. In post-menopausal women with bleeding, the cut-off of ET that warrants investigation is almost defined. However, the cut-off value of ET in asymptomatic postmenopausal women; beyond which intervention is required, is still debated. Dilemma also exists about the cut-off of ET in both symptomatic and asymptomatic women on HRT and Tamoxifen. This article will discuss the above issues and reach at some consensus about the cut-off of ET after critical analysis of evidence and experience and will help clinicians in arriving at a proper decision in dealing with such clinically confounding situations.
Keywords: Endometrial thickness, TVS, AUB, Perimenopause/menopause, Intervention
In the reproductive years of women’s life, the endometrium is a dynamic target organ, regulated by the hypothalamic- pituitary- ovarian axis leading to its cyclical changes mediated through oestrogen and progesterone. Endometrium contains both oestrogen and progesterone receptors, which bind and respond to the above hormones respectively, irrespective of whether the woman is in reproductive or menopausal phase of her life. In post-menopausal women with bleeding, the cut-off of endometrial thickness (ET) that warrants investigation is almost defined. However, there is no consensus as to the cut-off value of endometrial thickness in asymptomatic postmenopausal women. This article will discuss about the cut-off of endometrial thickness (ET) in symptomatic premenopausal and perimenopausal women, postmenopausal women with or without bleeding episode(s), and women under HRT and Tamoxifen. This will help to reach at some consensus, when to intervene, so that the diagnosis of endometrial hyperplasia (EH) and endometrial carcinoma (EC) is not delayed or missed.
Transvaginal ultrasonography (TVS) is preferred as the first modality of imaging, to assess endometrial milieu. Other imaging modalities like, Saline Infusion Sonohysterography (SIS), sonohysterography, hysterosalpingography, Computerized Tomography and Magnetic Resonance Imaging are sometimes used to confirm or add-on the findings of USG when deemed necessary. To avoid overestimation of endometrial thickness, the measurement should be made by USG on midline sagittal image, not oblique image or too close to uterine cornua. To ascertain that the imaging plane is midline, the endometrial echo should be seen continuous with the edocervical canal. Typically, endometrial thickness is measured and reported as the sum of the two adjacent layers of the endometrium, a measurement called the endometrial echo complex (EEC). If the complex is indistinct, heterogeneous or focally thickened, the double thickness measurement of the thickest segment of EEC should be measured. It is pertinent to know about patient’s age, day of menstrual cycle and any history of drug intake like HRT, Tamoxifen etc., in addition to clinical history and physical examination. Ideally, ultrasound scan should be performed after cessation of period or in periovulatory period as focal endometrial pathology can easily be detected during this period [1].
Endometrium in Paediatric Age
In the new born, the endometrium appears as a thin echogenic line, representing stimulated endometrium. About one fourth of them do have small amount of fluid collection inside the uterine cavity. An endometrial thickness of 6–8 mm implies imminent menarche [2]. At puberty, the endometrium develops approximately to that of adult and varies with the period of menstruation. USG finding of a tubular, cystic midline mass with internal echo suggests haematocolpos or haematometrocolpos in young adult.
Endometrium in Pre and Peri-menopause
At this stage, it will be prudent to define pre-menopause and peri-menopause [3].
Pre-menopause is a phase of women’s life when cycles are usually regular, may be irregular, but with no noticeable changes in the body, but hormonal changes may start to occur, and she is still in her reproductive phase of life.
Peri-menopause is a transition phase of women’s life which begins several years before natural menopause and the average duration being about 5 years. Cycles become irregular in terms of length, frequency and amount of flow. This is due to fluctuation of oestrogen levels and other sex hormones. They experience menopausal symptoms like, hot flushes, night sweats and mood swing etc.
Phases of Menstrual Cycle and Endometrial Thickness
During menstruation- endometrium appears as a thin, echogenic line 1–4 mm in thickness. During the proliferative phase of cycle (day-5–14), the endometrium develops a trilaminar or striated appearance and measures 12–13 mm (10–16 mm) at ovulation. During secretory phase (Days 15–28), the endometrium measures 16–18 mm and is more echogenic [4]. In a study of 111 premenopausal women with abnormal uterine bleeding who underwent TVS and D&C, it was observed that the median ET was 10.5 mm in all women and cut-off ET value was 8 mm with sensitivity and specificity of 83.9%, and 58.8%, respectively, and 90.4% negative predictive value for abnormal endometrium. There were only five women who had abnormal endometrium with thickness less than 8 mm. Four had simple hyperplasia, and only one had complex hyperplasia without atypia [5]. Another study of 144 women had almost similar result of median ET of 9.4 mm in all women and cut-off of 8 mm with sensitivity and specificity of 83.6% and 56.4%, respectively [6]. Minagawa et al. [7] analysed 367 premenopausal women and observed that the median ET was 12.2 mm in the women with abnormal uterine bleeding (AUB), compared with 9.4 mm in the asymptomatic women. They detected endometrial carcinoma (EC) in four patients with ET ≥ 20 mm and one case of EC in ET of 17.3 mm [7].
In a multicentric study of premenopausal (median age 38.5 years) participants, the observation was that in the women with a menstrual cycle length of 28–30 days, the average endometrial thickness (ET) was 7 mm on days 1–6, 5.4 mm immediately after menstruation (day 7 or 8) and 9.2 mm on days 13–14. During secretory phase of cycle, on day 18, the ET increased further to 11.1 mm and became thinner afterwards. In their study, they found that the median ET was 8.6/8.8/8.4 mm in all the women/women with AUB/women without AUB, respectively [8].
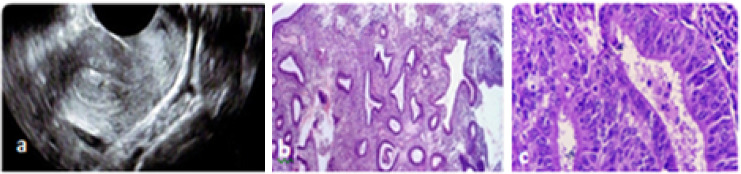
In another study, by Meenakshi et al. [9], 120 women of 40–45 years with heavy menstrual bleeding (HMB) were subjected to TVS and endometrial biopsy. It was observed that endometrial hyperplasia (Fig. 1) was detected in women with ET > 11 mm, and simple hyperplasia with atypia was detected in ET ≥ 11–16 mm; complex hyperplasia without atypia was detected when ET was ≥ 16–20 mm. There was no endometrial pathology in cases when ET was < 11 mm [9].
Fig. 1.
a Thickened Endometrium-14.7 mm. b Simple hyperplasia, back to back arrangements of glands with the little stroma lined by normal epithelium. c Proliferative endometrium, endometrial glands lined by pseudo-stratified columnar epithelium
USG Features in Endometrial Hyperplasia and Carcinoma (EH/EC)
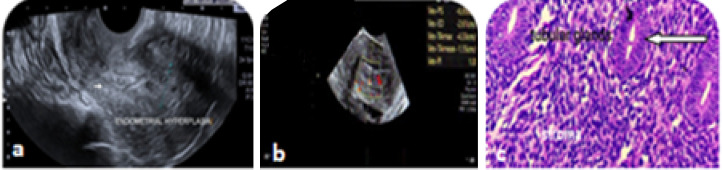
Diffuse echogenic and homogenous thickening of endometrial echo complex with well-defined myometrial interface are typically demonstrated in EH. It is not only about endometrial thickness, but good resolution transvaginal transducer can characterise endometrium beyond thickness. Even if the ET is below threshold, focal thickening (Fig. 2) and heterogeneity of endometrium should be considered abnormal and intervention planned [10]. Ultrasound features of heterogeneous and irregular endometrial thickening, polypoid mass lesion, intrauterine fluid collection and frank myometrial invasion (characterised by disruption of a sub endometrial halo) are suggestive of endometrial carcinoma rather than endometrial hyperplasia [11] (Fig. 3). Hyperplastic endometrium in PCOD, obese etc. needs histological evaluation of endometrium to be on safer side even in young.
Fig. 2.
a Focal thickening, b increased vascularity, c EIN-glands lined by atypical cells with enlarged hyperchromatic nucleus
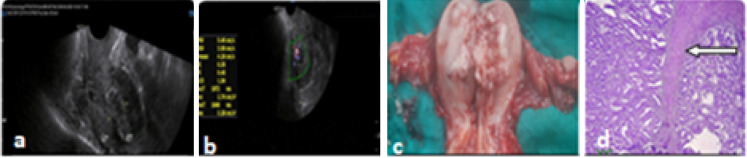
Fig. 3.
a Endometrial hyperplasia, ET-16.7 mm in PMB with myoinvasion, b increased vascularity in myometrium, c postoperative picture of myoinvasion, d adenocarcinoma with myoinvasion marked by arrow
The cut-off of ET in premenopausal women with abnormal bleeding for endometrial biopsy is yet to be defined. However, diffuse homogenous thickening of endometrium > 16 mm in secretory phase is taken as cut-off, though sensitivity and specificity of 67% and 75%, respectively, are regarded as suboptimal. In fact, a thickened endometrium in late secretory phase of cycle is usually normal and to minimize false positive result, a routine ultrasound should be preferably done in early proliferative phase, though the accepted threshold value of endometrial thickness is yet to be defined in this phase of cycle [12, 13]. Moreover, thickened endometrium alone does not always predict endometrial pathology. Various associated risk factors should be taken into consideration before planning for endometrial biopsy. The risk of premalignant/malignant endometrial pathology increases by 25% in AUB in premenopausal diabetic, obese women with ET > 11 mm [14].
The Royal Australian and New Zealand College of Radiologist and Cancer Australia National Centre for Gynaecological cancers in their guideline in 2011 suggested endometrial biopsy when ET is > 12 mm in premenopausal women and ≥ 5 mm in perimenopausal women with persistent erratic menstrual bleeding or in cases of anovulatory AUB [3]. It is estimated that in 10% of premenopausal women with AUB, histological finding is suggestive of endometrial hyperplasia [15]. ACOG committee opinion number 557, states that all women with AUB do not need to undergo endometrial sampling/biopsy, unless there is high risk of hyperplasia or malignancy. The committee recommended endometrial biopsy in women with AUB aged 45 years or more and in women < 45 years, in cases of anovulatory AUB (cases of PCOD, Obesity and Diabetes etc.); when they do not respond to conservative treatment [16]. The UK NICE guideline 2007 upgraded in 2018 recommends considering endometrial biopsy for women who are at high risk of endometrial pathology, such as: women with persistent intermenstrual or persistent irregular bleeding, and women with infrequent heavy bleeding who are obese or have polycystic ovary syndrome, women taking Tamoxifen, women for whom treatment for HMB has been unsuccessful [17]. ACOG practical bulletin 128 recommend endometrial biopsy in premenopausal women with; persistent intermenstrual bleeding, or for women over the age of 45 with heavy menstrual bleeding (HMB), after the failure of medical treatment [18]. However, some studies do not support these views, as this may lead to undue invasive procedures with inherent complications and cost. As the risk of detection of atypical endometrial hyperplasia and endometrial carcinoma in this group of patients is as low as 1.31%, the routine use of endometrial biopsy can be avoided, or be done in selected cases when indicated as per NICE guideline [19].
We need to recognise that “one size does not fit all”. In our opinion, each patient should be individualized and considering the above recommendations and guidelines, perhaps, endometrial tissue evaluation should at least be offered to patients with AUB above 45 years, below 45 years in any woman with risk factors of EC, women with persistent intermenstrual or persistent irregular bleeding, and women for whom conservative treatment for HMB has been unsuccessful, before deciding upon definitive management.
Addition of Power Doppler to grayscale TVS can improve diagnostic accuracy and differentiate benign from malignant lesions. In a study of 100 women (54 premenopausal with AUB and 46 women with PMB), the authors concluded that, when power Doppler was added to grey scale TVS, the specificity and negative predictive value improved and was comparable to hysteroscopy. The negative predictive value of ET in predicting premalignant and malignant condition was 92.9 per cent. The addition of power Doppler can be of great value in premenopausal women with AUB, as no consensus has been reached as to cut-off of ET [20].
Postmenopausal Endometrium
Normal Appearance
After menopause endometrium is typically thin due to the lack of oestrogen and appears on ultrasound as thin, homogenous and echogenic measuring < 3 mm [21]. In a large study involving 1182 menopausal women, the observation was, that ET decreased during the first 5 years since menopause (YSM) by 0.03 mm/year (p < 0.01) than remained almost static till 13 years after menopause. After that there was slight increase in ET by 0.01 mm/year (p 0.05). The reason for this increase in ET in the late menopause is not understood. This may probably be due to age related increase in BMI and altered fat distribution [22].
Asymptomatic Postmenopausal Women
Though Ultrasound is not routinely advocated, the incidental finding of thickened endometrium in ultrasound mostly by TVS, poses dilemma in deciding further procedure(s) to rule out endometrial pathology. In an article analyzing 266 asymptomatic post-menopausal women, thickened endometrium > 6 mm detected by TVS (6 mm as baseline thickness), ET was found to be; 6–10 mm, 11–15 mm, 16–20 mm and > 20 mm in 57.1%, 26.3%,10.5% and 6.1% cases, respectively. On histopathologic evaluation, no cases of atypical hyperplasia(AEH) was found in ET from 6 to 15 mm. 7.1% and 12.5% cases of AEH were detected when the ET was 16–20 mm and > 20 mm, respectively. Endometrial carcinoma was found in 2.6%, 2.9%, 12.5% cases when ET was 6–10 mm, 11–15 mm and > 20 mm respectively. Diagnosis of endometrial carcinoma in women without vaginal bleeding in this group with ET ranging from 6 to 10 mm was found to be 3.5%. The authors concluded the cut-off value of ET to be 10.5 mm for further evaluation. However, they also suggested evaluation of the cases with less thickened endometrium in presence of other risk factors [23]. Increased vascularity and fluid accumulation in association with endometrial thickening with inhomogeneity raise suspicion of malignancy [24].
A review article by Smith-Bindman et al. [25], concluded that in asymptomatic postmenopausal woman, a biopsy should be considered, if the endometrium measures > 11 mm, as the risk of cancer is 6.7%, and biopsy is not required, if ET is < 11 mm, as the risk of cancer is very low [25]. In a prospective study with 1024 women, the authors opined that cut-off of > 11 mm be considered as pathological [26]. According to SOGC guide line, women with thickened endometrium > 11 mm or increased vascularity, inhomogeneity of endometrium, particulate fluid, should undergo further evaluation [27].
Symptomatic Postmenopausal Women
With advancing age after menopause, the chance of occurrence of endometrial intraepithelial neoplasia/endometrial carcinoma (EIN/EC) also increases when the woman presents with PMB. It is observed that the incidence is low below age of 50 years, but prevalence is progressively higher at 4%, 12.4%, 14.2% and 18.9% as age advances from 50 to 54.9 years, 55–59.9 years, 60–69.9 years and > 70 years, respectively. In postmenopausal bleeding YSM plays a vital role in terms of occurrence of EIN/EC. The incidence of EIN/EC is 4.9%, 10.6%, 17%,11.5%,19.7% in case of YSM is < 3 years, 3–6.9 years, 7–9.9 years, 10–19.9 years and > 20 years, respectively [28].
Different cut-off values of endometrial thickness have been suggested by many authors.As suggested by Smith-Bindman et al. [25], the risk of carcinoma is around 7.3%, if the endometrium is > 5 mm and 0.07%, if the endometrium is < 5 mm.
In a retrospective study of 192 patients of histologically proven endometrial carcinoma at our centre, 94% of the patients had appreciable endometrial thickening > 5 mm and/or polypoid lesion by TVS, whereas in 6% cases no endometrial abnormality could be detected [29].
The SRU (Society of Radiologists in Ultrasound) consensus statement recommends that the sonogram should be interpreted as abnormal if the double thickness of the endometrium is greater than 5 mm, because of the fact that at 4 mm cut-off, the false positivity is higher with negligible alteration in sensitivity [30]. However, all the participants of SRU consensus panel were not of uniform opinion and dissenters recommended 4 mm as a threshold of ET. The ACOG (American College of Obstetricians and Gynecologists) committee recommends the threshold of ET as ≤ 4 mm, beyond which there is need for evaluation in PMB [31]. In a study of 304 women with PMB, by Patel et al., there was about 4% incidence of endometrial carcinoma with ET < 5 mm [32]. The Abnormal Uterine Bleeding Working Group (AUBWG) of the Southern California in 2012 changed the recommended threshold of ET from 5 to 4 mm [33] which was unanimously approved by Southern California Permanente Medical group (SCPMG) in 2013.
Considering above evidences, it is wise to consider threshold of ET as ≤ 4 mm, beyond which steps for further evaluation are warranted.
In women with thin endometrium, invasive procedures to evaluate endometrium for histopathology can be avoided. But in symptomatic women with persistent or recurrent bleeding episodes, even if the endometrial echo complex is less than 4 mm, endometrial biopsy is warranted [31, 34], and thin endometrium does not exclude presence of type 2 endometrial carcinomas [35].
Cases of PMB with strong suspicion of malignancy on TVS or with persistent/recurrent bleeding should be evaluated further by hysteroscopy and endometrial biopsy, even when primary blind biopsy shows no abnormality [31].
Power Doppler Study of Endometrium
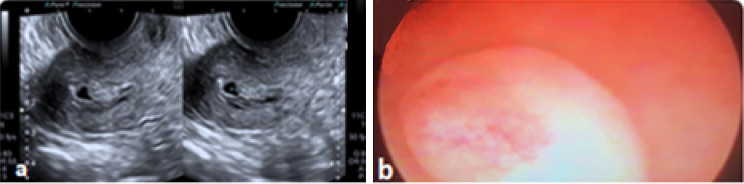
Alcazar JL et al. described three different vascular patterns in the Power Doppler flow mapping of endometrium [36]. 1. Multiple vessel patterns (pattern-A), which is suggestive of endometrial carcinoma, 2. Single vessel pattern (pattern B), which is the characteristic feature of endometrial polyp, (Fig. 4) and 3. Scattered Vessel pattern (pattern-C), the features suggest diagnosis of endometrial hyperplasia.
Fig. 4.
a Endometrial polyp with fluid in cavity. b endometrial polyp on hysteroscopy
In a prospective study of 80 women with PMB, the authors opined that use of Power Doppler as an adjunct to TVS in cases of PMB not on HRT, with ET ≤ 5 mm, is useful in differentiating between endometrial carcinoma and other causes of thickened endometrium. Addition of Power Doppler to TVS might be a helpful tool in cases of asymptomatic postmenopausal women for further evaluation to rule out EH/EC, where the cut-off of ET is not well defined [37].
When dilemma in endometrial imaging arises between thickened endometrium, and endometrial polyp, hysteroscopic evaluation and polypectomy may be curative and save her from stress of major surgical procedure.
Women on Tamoxifen
Tamoxifen, a selective oestrogen receptor modulator has antioestrogenic activity on breast and oestrogenic activity on endometrium. With the oestrogenic activity, it is associated with endometrial proliferation, endometrial hyperplasia, endometrial polyp formation, carcinoma endometrium and uterine sarcoma. The risk of developing EC is 2–3 times more than that of general population. Moreover, the risk is dependent on dose and duration of therapy [38]. Up to one-half of breast cancer patients who are treated with this medication may develop an endometrial lesion within 6–36 months [39].
The women planned for Tamoxifen should have pretreatment screening with TVS and or endometrial biopsy before Tamoxifen therapy to rule out pre-existing endometrial pathology. Screening asymptomatic women (both pre and post menopausal) on Tamoxifen, with TVS or endometrial biopsy, or both has no benefit in terms of detecting endometrial pathology [40]. Abnormal uterine bleeding in any women on Tamoxifen, is associated with high risk of EH/EC, irrespective of age, BMI and duration of treatment [41]. Hence, only symptomatic women on Tamoxifen should be evaluated with TVS and endometrial biopsy [27]. As Tamoxifen induces sub-epithelial stromal hypertrophy, TVS measurement of ET and histological finding do have poor correlation in Tamoxifen users which accounts for false positive ET [42]. Normal endometrial thickness despite Tamoxifen use in postmenopausal women should be < 5 mm, although ~ 50% of those receiving Tamoxifen have been reported to have a thickness of > 8 mm [43].
Though ET of > 8 mm is taken as cut-off in Tamoxifen users, the evidence suggests not to subject pre- and post-menopausal asymptomatic women for endometrial evaluation.
Women on HRT
Gupta A et al. in response to query by Thomas C. Winter stated that women on HRT with ET > 8 mm be taken as threshold in asymptomatic postmenopausal women and ET of > 5 mm with in cases of postmenopausal bleeding, for further evaluation, whether she is on HRT, Tamoxifen or otherwise [11]. An observational study conducted in 2000 women by Sturdee et al. showed that use of continuous combined HRT did not increase the risk of EH as opposed to sequential HRT, rather caused reversal of hyperplasia [44]. Smith-Bindman et al. in their article stated that for detecting endometrial atypia in women on HRT with no vaginal bleeding, the acceptable range of endometrial thickness is less well defined; cut-off values of 8–11 mm have been suggested. The risk of carcinoma is ~ 7%, if the endometrium is > 11 mm, and 0.002%, if the endometrium is < 11 mm [25].
Conclusion
There is lack of consensus on cut-off thickness of endometrium in dealing with thickened endometrium detected incidentally by USG/TVS except in cases of postmenopausal bleeding. Either a repeat transvaginal ultrasound or intervention is a reasonable alternative. Endomyo interface study and addition of Power Doppler mapping for abnormal vascularity over TVS detected thickened endometrium to differentiate benign from malignant is a step ahead. Myometrial echogenicity and appearance sometimes point towards some pathology in symptomatic women even if endometrium is thin.
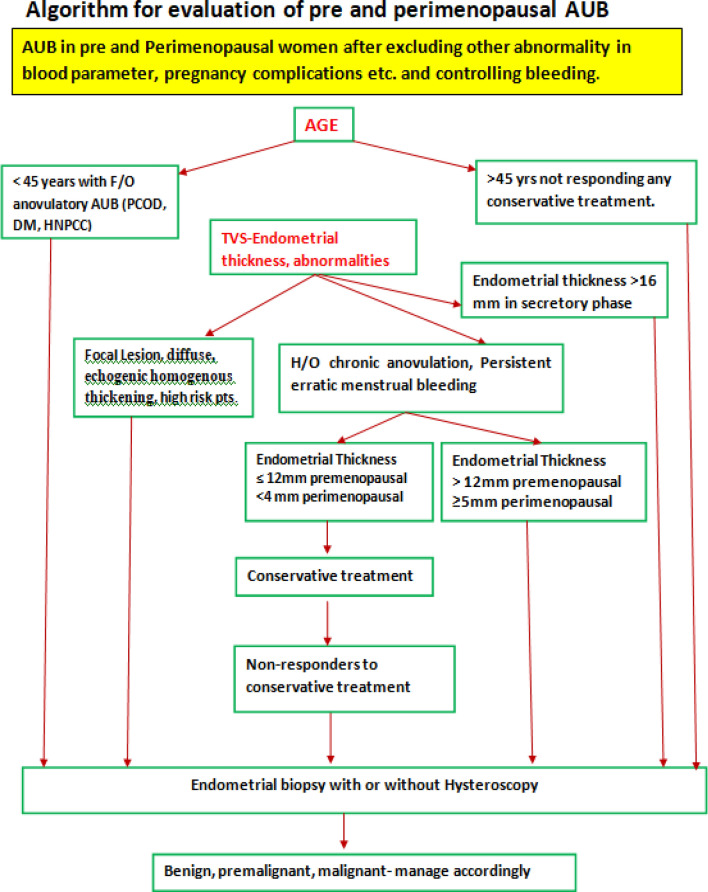
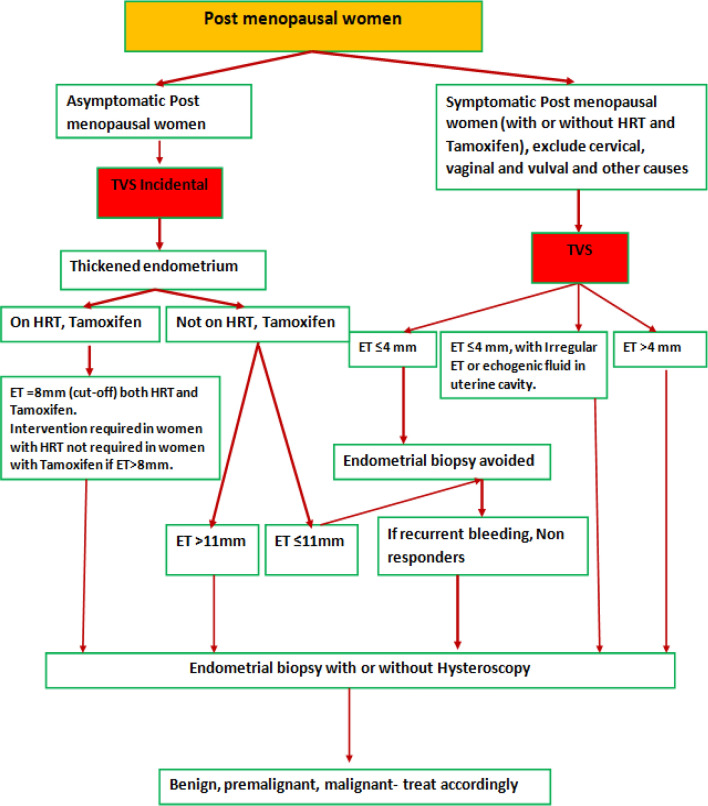
However, looking in to the observations of different authors and guidelines as discussed above, some working principles have to be adopted in regard to ET to balance between undue interventions and missing out EH/EC. However, every woman is unique, and personalized decision is need of the situation (Figs. 5, 6).
- In pre and perimenopausal women with AUB
- Endometrial biopsy is advocated in ovulatory AUB aged ≥ 45 years and in anovulatory AUB < 45 years to rule out endometrial hyperplasia.
- It is preferable to advise ultrasonogram in early proliferative phase to detect endometrial abnormalities and avoid false positive result.
- Diffuse homogenous thickening of endometrium > 16 mm in secretory phase has been recommended as cut-off.
- When premenopausal vaginal bleeding occurs in diabetic obese women with ET > 11 mm, the risk of premalignant/malignant endometrial pathology increases by 25%.
- Endometrial biopsy is indicated when ET is > 12 mm in premenopausal women and ≥ 5 mm in perimenopausal women with persistent erratic menstrual bleeding.
- Asymptomatic postmenopausal women
- Endometrial biopsy should be considered, if the endometrium measures > 11 mm, or with other risk features.
- Symptomatic Postmenopausal women
- ET > 4 mm endometrial biopsy is required
- Persistent or recurrent bleeding, even with endometrial echo complex less than ≤ 4 mm warrants endometrial biopsy to rule out Type II EC.
- Women on Tamoxifen
- Pretreatment screening with TVS and or endometrial biopsy before Tamoxifen therapy to rule out pre-existing endometrial pathology which may aggravate during treatment with Tamoxifen.
- There is no known added risk of endometrial cancer in premenopausal women on Tamoxifen; hence these women need no stringent follow-up.
- Though ET of > 8 mm is taken as cut-off in Tamoxifen users, the evidence suggests not to go for endometrial evaluation in asymptomatic women both pre and post-menopausal.
- In patients on Tamoxifen presenting with irregular vaginal bleeding endometrial biopsy is recommended.
- Woman with PMB should have TVS done and if ET is > 4 mm, endometrial biopsy should be done regardless of any drug use.
- Women on HRT
- The acceptable range of endometrial thickness is less well established in this group; cut-off values of 8 mm have been suggested in asymptomatic women.
- The risk of carcinoma is ~ 7%, if the endometrium is > 11 mm, and 0.002%, if the endometrium is < 11 mm
- Women on HRT the ET of > 8 mm should be treated as thickened and endometrial evaluation be done in asymptomatic women.
- Women on HRT with PMB endometrial evaluation should be done if ET is > 4 mm
Fig. 5.
Algorithm of evaluation for pre and perimenopausal AUB
Fig. 6.
Algorithm for evaluation of postmenopausal women
Dr. S. K. Giri
is a former Professor, Dept. Gynaecologic Oncology, A.H. Postgraduate Institute of Cancer (A.H.PGIC), Cuttack and former director of the same institute. He has been the President of the Association of Gynaecologic Oncologists of India, 2007–2009. He has more than 40 publications in national and international journals and contributed chapters in Gynaecologic Oncology in different books including FOGSI publications. He has delivered Dr. Subodh Mitra Memorial oration at Bengal Obstetrics and Gynaecological society in 2015 and ICOG oration at Thrissur Obst. and Gyn. Society in 2016. He is also MCh examiner in Gynaecologic Oncology at TMH, Mumbai under Homi Bhabha National Institute, KMIO and St. John’s Medical College Banglore under Rajiv Gandhi University of Health Sciences, Karnataka, Gujarat Cancer Research Institute under Gujarat University, CMC, Vellore under MGR, Medical University, Chennai. He is a peer reviewer in Indian Journal of Gynaecologic Oncology and Indian Journal of Surgical Oncology
Compliance with Ethical Standards
Conflict of interest
None of the authors have any conflicts of interest to declare.
Ethical Approval
This article does not contain any studies with human participants performed by any of the authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
S. K. Giri, Email: drskgiri@gmail.com
B. L. Nayak, Email: blnayak2266@gmail.com
Janmejay Mohapatra, Email: dr.janmejaya.mohapatra@hotmail.com.
References
- 1.Okaro E, Condous G, Bourne T. The role of transvaginal ultrasound in the management of abnormal uterine bleeding. Gynecol Surg. 2004;1:119–126. doi: 10.1007/s10397-004-0012-5. [DOI] [Google Scholar]
- 2.Landes CJ, Blair JC. Normal growth and puberty. In: Mann G, Blair JS, Garden A, editors. Imaging of gynecological disorders in infants and children. Berlin: Springer; 2012. pp. 81–113. [Google Scholar]
- 3.Abnormal vaginal bleeding in pre- and peri-menopausal women. A diagnostic guide for General Practitioners and Gynaecologists. http://canceraustralia.gov.au/sites/default/files/publications/ncgc-vaginal-bleeding-flowchartsmarch-20111_504af02038614.pdf.
- 4.Langer EJ. Normal anatomy of the female pelvis and transvaginal sonography. In: Norton EM, Scout L, Feldstein VA, editors. Callen’s ultrasonography in obstetrics and gynecology. 6. Philadelphia: Elsevier; 2017. pp. 805–834. [Google Scholar]
- 5.Getpook C, Wattanakumtornkul S. Endometrial thickness screening in premenopausal women with abnormal uterine bleeding. J Obstet Gynaecol Res. 2006;32:588–592. doi: 10.1111/j.1447-0756.2006.00455.x. [DOI] [PubMed] [Google Scholar]
- 6.Ozdemir S, Celik C, Gezginç K, Kireşi D, Esen H. Evaluation of endometrial thickness with transvaginal ultrasonography and histopathology in premenopausal women with abnormal vaginal bleeding. Arch Gynecol Obstet. 2010;282:395–399. doi: 10.1007/s00404-009-1290-y. [DOI] [PubMed] [Google Scholar]
- 7.Minagawa Y, Sato S, Ito M, Onohara Y, Nakamoto S, Kigawa J. Transvaginal ultrasonography and endometrial cytology as a diagnostic schema for endometrial cancer. Gynecol Obstet Investig. 2005;59:149–154. doi: 10.1159/000083089. [DOI] [PubMed] [Google Scholar]
- 8.Tsuda H, Ito MY, Todo Y, Iba T, Tasaka K, et al. Measurement of endometrial thickness in premenopausal women in office gynecology. Reprod Med Biol. 2018;17(1):29–35. doi: 10.1002/rmb2.12062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meenakshi S, Rekha S, Amrita Y. Significance of endometrial thickness on transvaginal sonography in heavy menstrual bleeding. J Clin Res Sci Med. 2019;5(1):28–32. [Google Scholar]
- 10.Rezvani M, Winter CT. Abnormal uterine bleeding: the role of ultrasound. In: Norton EM, Scout L, Feldstein VA, editors. Callen’s ultrasonography in obstetrics and gynecology. 6. Philadelphia: Elsevier; 2017. pp. 835–845. [Google Scholar]
- 11.Gupta A, Desai A, Bhatt S. Imaging of the endometrium: physiologic changes and diseases. RadioGraphics. 2017;37:2206–2207. doi: 10.1148/rg.2017170008. [DOI] [PubMed] [Google Scholar]
- 12.Shi AA, Lee SI. Radiological reasoning: algorithmic workup of abnormal vaginal bleeding with transvaginal sonography and sonohysterography. AJR Am J Roentgenol. 2008;191(6 Suppl):S68–S73. doi: 10.2214/AJR.07.7067. [DOI] [PubMed] [Google Scholar]
- 13.Rezvani M, Winter CT. Abnormal uterine bleeding: the role of ultrasound. In: Norton EM, Scout L, Feldstein VA, editors. Callen’s ultrasonography in obstetrics and gynecology. 6. Philadelphia: Elsevier; 2017. pp. 805–834. [Google Scholar]
- 14.Giannella L, Cerami LB, Setti T, Bergamini E, Boselli F. Prediction of endometrial hyperplasia and cancer among premenopausal women with abnormal uterine bleeding. BioMed Res Int. 2019. 10.1155/2019/8598152. [DOI] [PMC free article] [PubMed]
- 15.Armstrong AJ, Hurd WW, Elguero S, Barker NM, Zanotti KM. Diagnosis and management of endometrial hyperplasia. J Minim Invasive Gynecol. 2012;19:562–571. doi: 10.1016/j.jmig.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 16.American College of Obstetricians and Gynecologists ACOG committee opinion no. 557: management of acute abnormal uterine bleeding in nonpregnant reproductive-aged women. Obstet Gynecol. 2013;121(4):891–896. doi: 10.1097/01.AOG.0000428646.67925.9a. [DOI] [PubMed] [Google Scholar]
- 17.National Institute for health and clinical excellence CG44. Heavy menstrual bleeding. NICE, 2007. upgraded in 2018
- 18.Committee on Practice Bulletins—Gynecology Practice bulletin no 128: diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstet Gynecol. 2012;120(1):197–206. doi: 10.1097/AOG.0b013e318262e320. [DOI] [PubMed] [Google Scholar]
- 19.Pennant ME, Mehta R, Moody P, et al. Premenopausal abnormal uterine bleeding and risk of endometrial cancer. BJOG Int J Obstet Gynaecol. 2017;124(3):404–411. doi: 10.1111/1471-0528.14385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Veena P, Baskaran D, Maurya DK, Kubera NS, Dorairaj J. Addition of power Doppler to grey scale transvaginal ultrasonography for improving the prediction of endometrial pathology in perimenopausal women with abnormal uterine bleeding. J Indian J Med Res. 2018;148(3):302–308. doi: 10.4103/ijmr.IJMR_96_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Breijer MC, Peeters JA, Opmeer BC, et al. Capacity of endometrial thickness measurement to diagnose endometrial carcinoma in asymptomatic PM women; a systematic review and meta-analysis. Ultrasound Obstet Gyencol. 2012;40:621. doi: 10.1002/uog.12306. [DOI] [PubMed] [Google Scholar]
- 22.Warming L, Ravn P, Skouby S, Christiansen C. Measurement precision and normal range of endometrial thickness in a postmenopausal population by transvaginal ultrasound. Ultrasound Obstet Gynecol. 2002;20:492–495. doi: 10.1046/j.1469-0705.2002.00828.x. [DOI] [PubMed] [Google Scholar]
- 23.Ozelci R, Dilbaz B, Akpinar F, Kinay T, et al. The significance of sonographically thickened endometrium in asymptomatic postmenopausal women. Obstet Gynecol Sci. 2019;62(4):273–279. doi: 10.5468/ogs.2019.62.4.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Opolskiene G, Sladkevicius P, Valentin L. Ultrasound assessment of endometrial morphology and vascularity to predict endometrial malignancy in women with postmenopausal bleeding and sonographic endometrial thickness > 4.5 mm. Ultrasound Obstet Gynecol. 2007;30:332–340. doi: 10.1002/uog.4104. [DOI] [PubMed] [Google Scholar]
- 25.Smith-Bindman R, Weiss E, Feldstein V. How thick is too thick? When endometrial thickness should prompt biopsy in postmenopausal women without vaginal bleeding. Ultrasound Obstet Gynecol. 2004;24:558–565. doi: 10.1002/uog.1704. [DOI] [PubMed] [Google Scholar]
- 26.Hefler L, Lafleur J, Kickmaier S, Leipold H, et al. Risk of endometrial cancer in asymptomatic postmenopausal patients with thickened endometrium: data from the FAME-Endo Study: an observational register study. Arch Gynecol Obstet. 2018;298(4):813–820. doi: 10.1007/s00404-018-4885-3. [DOI] [PubMed] [Google Scholar]
- 27.Wolfman W, Toronto ON, et al. SOGC clinical practice guideline: asymptomatic endometrial thickening. J Obstet Gynaecol Can. 2018;40(5):e367–e377. doi: 10.1016/j.jogc.2018.03.005. [DOI] [PubMed] [Google Scholar]
- 28.Van Doorn CH, Opmeer BC, Jitze Duk M, Kruitwagen RFMP, et al. The relation between age, time since menopause, and endometrial cancer in women with postmenopausal bleeding. Int Jr Gynecol Cancer. 2007;17:1118–1123. doi: 10.1111/j.1525-1438.2007.00925.x. [DOI] [PubMed] [Google Scholar]
- 29.Nayak B, Parida S, Rautray NP, Mohapatra J, Samantaray S, Giri SK. Transvaginal sonography (TVS) in evaluation of endometrial carcinoma and its correlation with histopathology: a retrospective analysis. Indian J Gynecol Oncol. 2017;15:12. doi: 10.1007/s40944-016-0095-8. [DOI] [Google Scholar]
- 30.Ruth BG, Robert LB, Carol BB, Beryl RB, et al. Evaluation of the woman with postmenopausal bleeding: society of radiologists in ultrasound-sponsored consensus conference statement. Ultrasound Q. 2002;18(1):61–69. doi: 10.1097/00013644-200203000-00007. [DOI] [PubMed] [Google Scholar]
- 31.The role of transvaginal ultrasonography in evaluating the endometrium of women with postmenopausal bleeding. ACOG Committee Opinion No. 734. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2018;131:e124–9. [DOI] [PubMed]
- 32.Patel V, Wilkinson EJ, Chamala S, Lu X, Castagno J, Rush D. Endometrial thickness as measured by transvaginal ultrasound and the corresponding histopathologic diagnosis in women with postmenopausal bleeding. Int J Gynecol Pathol. 2017;36:348–355. doi: 10.1097/PGP.0000000000000344. [DOI] [PubMed] [Google Scholar]
- 33.Munro GM. Investigation of women with postmenopausal uterine bleeding: clinical practice recommendations. Perm J. 2014;18(1):55–70. doi: 10.7812/TPP/13-072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Endometrial cancer. Practice Bulletin No. 149. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2015;125:1006–26 [DOI] [PubMed]
- 35.Wang J, Wieslander C, Hansen G, Cass I, Vasilev S, Holschneider CH. Thin endometrial echo complexon ultrasound does not reliably exclude type 2 endometrial cancers. Gynecol Oncol. 2006;101(1):120–125. doi: 10.1016/j.ygyno.2005.09.042. [DOI] [PubMed] [Google Scholar]
- 36.Alcazar JL, Castillo G, Minguez JA, Galan MJ. Endometrial blood flow mapping using transvaginal power Doppler sonography in women with postmenopausal bleeding and thickened endometrium. Ultrasound Obstet Gynecol. 2003;21:583–588. doi: 10.1002/uog.143. [DOI] [PubMed] [Google Scholar]
- 37.El-Morsi Aboul-Fotouh M, Hani Mosbeh M, Fathi El-Gebaly A, Nageeb MA. Transvaginal power Doppler sonography can discriminate between benign and malignant endometrial conditions in women with postmenopausal bleeding. Middle East Fertil Soc J. 2012;17:22–29. doi: 10.1016/j.mefs.2011.07.003. [DOI] [Google Scholar]
- 38.Fisher B, Costantino JP, Wickerham DL, Redmond CK, Kavanah M, Cronin WM, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90:1371–1388. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- 39.De Muylder X, Neven P, De Somer M, et al. Endometrial lesions in patients undergoing tamoxifen therapy. Int J Gynecol Obstet. 1991;36:127–130. doi: 10.1016/0020-7292(91)90767-Y. [DOI] [PubMed] [Google Scholar]
- 40.American College of Obstetricians and Gynecologists Committee on Gynecologic Practice. ACOG Committee Opinion No. 336: Tamoxifen and uterine cancer. Obstet Gynecol 2006; 107:1475–8, Number 601, Reaffirmed on 2017. [DOI] [PubMed]
- 41.Lee M, Piao J, Jeon JM. Risk factors associated with endometrial pathology in premenopausal breast cancer patients treated with tamoxifen. Yonsei Med J. 2020;61(4):317–322. doi: 10.3349/ymj.2020.61.4.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Achiron R, Lipitz S, Sivan E, Goldenberg M, Horovitz A, Frenkel Y, et al. Changes mimicking endometrial neoplasia in postmenopausal, Tamoxifen-treated women with breast cancer: a transvaginal Doppler study. Ultrasound Obstet Gynecol. 1995;6:116–120. doi: 10.1046/j.1469-0705.1995.06020116.x. [DOI] [PubMed] [Google Scholar]
- 43.Hann LE, Giess CS, Bach AM, et al. Endometrial thickness in tamoxifen-treated patients: correlation with clinical and pathologic findings. AJR Am J Roentgenol. 1997;168(3):657–661. doi: 10.2214/ajr.168.3.9057510. [DOI] [PubMed] [Google Scholar]
- 44.Sturdee DW, Ulrich LG, Barlow DH, Wells M, Campbell MJ, Vessey MP, et al. The endometrial response to sequential and continuous combined oestrogen–progestogen replacement therapy. BJOG. 2000;107:1392–1400. doi: 10.1111/j.1471-0528.2000.tb11654.x. [DOI] [PubMed] [Google Scholar]