Abstract
Purpose of Study
To study the role of uterine artery Doppler pulsatility index (UtA-PI), serum pregnancy-associated plasma protein-A (PAPP-A) and free beta human chorionic gonadotropin (fβ-hCG) levels, individually and in combination with each other, at 11–14 weeks of gestation for prediction of preeclampsia (PE).
Methods
In a prospective observational study, a total of 100 low-risk gravid females were recruited at 11–14-weeks of gestation. UtA-PI, PAPP-A and fβ-hCG levels were estimated. These women were followed up until delivery for the development of PE and gestational hypertension (GH).
Results
The best individual marker for screening PE and GH was UtA-PI with ROC AUC (± standard error) = 0.934 ± 0.028, p < 0.0001. UtA-PI at a cutoff value of ≥ 2.8 (95th percentile) had 77.8% sensitivity, 98.9% specificity, 97.8% NPV and 87.5% PPV in detecting PE. PAPP-A (MoM) at a cutoff value of ≤ 0.27 (5th percentile) demonstrated 44.4% sensitivity, 95.6% specificity, 94.5% NPV and 50% PPV. fβ-hCG (MoM) at a cutoff value of ≤ 0.5 (5th percentile) had a specificity of 94.5%. Among the combined markers, UtA-PI along with PAPP-A estimation served best with a sensitivity and specificity of 44% and 100%, respectively. Addition of fβ-hCG to either UtA-PI or PAPP-A levels was not found sensitive for detecting PE but yielded 100% specificity and 96% NPV.
Conclusion
UtA-PI as a stand-alone test was found most useful for the prediction of PE. Addition of either or both of PAPP-A and fβ-hCG to UtA-PI did not improve the sensitivity of combined test with only a slight improvement in specificity and NPV. Their routine addition to UtA-PI studies is not recommended for prediction of PE at 11–14 weeks of gestation in low- and lower-middle-income countries (LMIC).
Keywords: Uterine artery pulsatility index, Serum PAPP-A, β-hCG, Preeclampsia
Background
Preeclampsia (PE) is a hypertensive disorder specific to pregnancy with grave maternal, fetal and neonatal prognosis. It usually presents after twenty weeks of gestation and up to six weeks postpartum. It affects 3–10% of all pregnancies. In India, the reported incidence is 8–10% [1].
In PE, the defective deep placentation during the second wave of trophoblastic invasion (11–14 weeks of gestation) leads to faulty cytotrophoblastic invasion of myometrial segments of spiral arteries due to failure of expression of invasive and adhesive signal molecules. This halts progressive conversion of high resistance uteroplacental flow to low resistance high flow circulation which might be represented by a high resistance flow pattern on the uterine artery (UtA) Doppler study at 11–14 weeks [2–6]. Most of these studies have documented a high specificity (> 90%) but low sensitivity (< 50%) of uterine artery pulsatility index (UtA-PI) in predicting PE.
Recent literature has shown a promising role of placental biochemical markers in predicting PE. Pregnancy-associated plasma protein A (PAPP-A) and free beta human chorionic gonadotropin (fβ-hCG) glycoprotein are among many such biochemical markers. Low levels of PAPP-A have been consistently associated with defective placenta formation [7–15]. It was hence hypothesized that the addition of these two biochemical markers would improve the predictive value of UtA-PI in detecting PE at 11–14 weeks of gestation. This study was therefore conducted to determine whether the combined test would improve the overall predictive value of UtA-PI as a stand-alone marker in screening PE in low- and lower-middle-income countries (LMIC) such as the Indian subcontinent.
Materials and Methods
We did a prospective observational study on 100 low-risk women at a tertiary care hospital located in New Delhi over fourteen months after approval from clinical research ethics committee of the institute. Gravid females with a single viable intrauterine pregnancy and at 11–14 weeks of gestation were recruited after a written and informed consent. Exclusion criteria were obesity, extremes of age, preexisting medical or surgical illness, abnormal ultrasonographic placental appearance, fetal malformations or history of first-trimester bleeding. Sample size was determined using the formula:
where N = total study population; t = confidence level at 95% (1.96); p = prevalence (0.07); m = margin of error (0.05).
After an initial history taking and examination, women underwent transabdominal ultrasonography (TAS) and 10 ml of blood sample for PAPP-A and fβ-hCG estimation was withdrawn in the same setting.
TAS was done using a 3.5 MHz curvilinear transducer with women in a semi-recumbent position. Crown rump length (CRL) was measured first to confirm the period of gestation (POG). The transducer was then placed longitudinally over the lower lateral quadrant of abdomen angled medially. Color flow mapping was done to identify uterine artery as it crossed the external iliac artery. Sample volume was placed 1 cm downstream to the cross over point. Three consecutive waveforms were obtained. UtA-PI was measured on both sides and a mean value was recorded.
The blood sample was subjected to centrifugation at room temperature for 10 min at 400 rpm. The serum was analyzed for PAPP-A and fβ-hCG using ELISA immunoassay technique and values were expressed in multiples of medians (MoM).
At subsequent antenatal visits, body weight, blood pressure and urine albumin levels (using a reagent-based dipstick) were recorded. PE was defined as blood pressure ≥ 140/90 mm Hg on any two occasions, at least 4 h apart and urine albumin levels ≥ 1 + (≥ 30 mg/dL). In the absence of proteinuria, it was labeled as gestational hypertension (GH). Women were followed up until delivery for the development of PE and GH (primary outcomes). Preterm delivery (delivery before 37 weeks of gestation), delivery of low birth weight babies (LBW, birth weight less than 2500 g) and fetal growth retardation (FGR, fetal weight less than 10th centile for gestational age with abnormal umbilical artery Doppler findings) were taken as secondary outcomes.
Receiver operating characteristics (ROC) curve analysis was done to define the best predictor for outcomes examined. The area under the curve (AUC) and its 95% CI (confidence interval) were calculated. Sensitivities, specificities, positive predictive values (PPV), negative predictive values (NPV) and accuracy ratios for cutoff points of UtA-PI, PAPP-A and fβ-hCG in the prediction of PE were calculated individually and in combination with each other. Descriptive and inferential statistical analysis was performed using Microsoft Excel 2007, Xlstat 2015.1.03.16112 and Medcalc software version 15.4.
Results
A total of 118 women were recruited; however, 18 were lost to follow-up. Hence, records of 100 patients were available for the final analysis.
Women were divided into four groups according to the POG—11–11+6, 12–12+6, 13–13+6 and 14+0 weeks. Out of the total 100 women, nine women developed PE (9%) and five women developed GH (5%). Two women developed abruption (2%), and 13 women delivered prematurely (13%). Nine women delivered LBW baby (9%), and one woman developed FGR (1%).
Most women (33%) belonged to the gestational age group of 12 + 0–12 + 6 weeks. The majority (57%) were primigravida. Most (88%) were in the age group of 20–25 years. 92% of women had body mass index (BMI) values in the range of 18.9–24.9 (normal range).
UtA-PI was defined as maximum uterine artery velocity excursion upon mean uterine artery velocity. The majority (29%) of women had UtA-PI in the range of 1.6–2.
The mean UtA-PI value between 11 to 14 weeks was calculated as 1.56 ± 1.5. The median value was 0.71. The mean UtA-PI decreased significantly over POG[r = (−)0.0258; p < 0.012] from 11–14 weeks. It was 1.82 ± 0.68 at 11–11+6 weeks, 1.6 ± 0.63 at 12–12+6 weeks, 1.41 ± 0.41 at 13–13+6 weeks and 1.4 ± 0.99 at 14+0 weeks (Fig. 1). This trend represents decreasing impedance to flow in UtA with increasing gestation secondary to trophoblastic invasion of spiral arteries [2].
Fig.1.
Scatter plot showing distribution of UtA-PI, PAPP-A and fβ-hCG according to POG (in days) between 11 to 14 weeks. UtA-PI values decreased significantly between 11 to 14 weeks of gestation [(r = (−)0.0229; p = 0.03] (a). PAPP-A values increased [r = ( +)0.021; p = 0.01) and fβ-hCG values decreased [r = (−)0.05; p < 0.001] over the same POG (b, c)
PAPP-A values consistently increased during 11–14 weeks of POG[r = ( + )0.021; p = 0.01] (Fig. 1).
The majority (43%) of women had PAPP-A values between 1.1 and 1.5. The mean value of PAPP-A was 1.15 ± 0.54 and the median value reported was 1.14.
fβ-hCG values declined significantly during 11–14 weeks of POG[r = (−)0.05; p < 0.0010] (Fig. 1).
The mean fβ-hCG value was 1.55 ± 0.60. The median value was 1.64.
Values of individual markers were analyzed using logistic regression models. ROC curves were generated for UtA-PI, PAPP-A and fβ-hCG for PE and GH (Fig. 2). The cutoff values of significance at 95% CI for each marker were defined. For UtA-PI the cutoff value was defined at ≥ 2.8 (95th percentile). For PAPP-A it was defined at ≤ 0.27 MoM (5th percentile) and for fβ-hCG at ≤ 0.5 MoM (5th percentile). Sensitivities, specificities, PPVs and NPVs of individual and combined markers were calculated for screening purposes of PE.
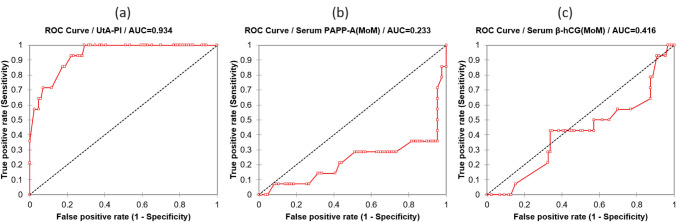
Fig.2.
ROC for prediction of PE and GH by UtA-PI, PAPP-A and fβ-hCG (MoM) at 11–14 weeks of gestation. The AUC ± standard error was maximum for UtA-PI at 0.934 ± 0.028 (p < 0.0001) in screening PE and GH (a). The AUC for PAPP-A and fβ-hCG was 0.233 ± 0.86(p = 0.002) and 0.416 ± 0.086 (p = 0.330), respectively (b, c)
UtA-PI was the best overall marker for predicting PE with a sensitivity of 79% and a specificity of 98.9%. It also had the highest sensitivity (83.3%) as a stand-alone parameter in the prediction of early onset PE (onset before 32-weeks of gestation; EOPE). It was followed in line by PAPP-A with a sensitivity of 44.4% and a specificity of 95.6%. fβ-hCG was not found sensitive for PE but had a specificity of 94.5% in predicting PE (Table1).
Table 1.
Sensitivities, specificities, NPV and PPV of various markers individually and in combination with each other in predicting PE and GH
| Markers | Sensitivity (%) | Specificity (%) | NPV (%) | PPV (%) | LR + | LR- | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| PE | GH | PE | GH | PE | GH | PE | GH | PE | PE | |
| UtA-PI |
77.8 83* |
20 |
98.9 97.8* |
89.4 |
97.8 98.9* |
95.5 |
87.5 71.4* |
9.01 |
78 39* |
0.22 |
| PAPP-A (MoM) |
44.4 50* |
– |
95.6 94.6* |
91.5 |
94.5 96.7* |
94.5 |
50 37.5* |
– |
10 9.4* |
0.6 |
| fβ-hCG (MoM) | – | 20 |
94.5 91.4* |
92.6 |
90.5 93.4* |
95.7 | – | 12.5 | 0 | 1 |
| PAPP-A(MoM) + fβ-hCG (MoM) | – | – |
100 100* |
100 |
91 94* |
95 | – | – | ||
| PAPP-A (MoM) + UtA-PI |
44 50* |
– |
100 98.9* |
95.7 |
94.7 96.9* |
94.7 |
100 75* |
- | 47* | 0.5* |
| fβ-hCG (MoM) with UtA-PI | – | 20 | 98.9–100 | 100 | 90–91 | 95.9 | – | 100 | ||
| PAPP-A (MoM) + fβ-hCG (MoM) + UtA-PI | – | – | 100 | 100 | 91 | 95 | – | – | ||
*EOPE; LR + (positive likelihood ratio) = sensitivity/(1−specificity); LR−(negative likelihood ratio) = (1−sensitivity)/specificity
When we studied the effect of combined markers, the combination of PAPP-A and UtA-PI demonstrated 44% sensitivity and 100% specificity for PE. While the sensitivity was not superior to UtA-PI alone in predicting PE (44.4% vs. 77.8%), specificity was raised only marginally (100% vs. 98.9%). When fβ-hCG was added to either or both of UtA-PI and PAPP-A, the combined test had no sensitivity for PE. Also, the reported specificity for PE was similar to or only slightly raised as compared to UtA-PI alone (98.9–100% vs.98.9%) (Table 1).
Discussion
We reported a prevalence rate of 9% for PE in our study. The reported mean first-trimester UtA-PI value of 1.5 in our study was lower than that reported by Gomez et al. [4] (1.7, 50th centile). However, it was higher than that reported by Yasmin Casmod et al. [16]. Nevertheless first-trimester UtA-PI values of > 1.5 is considered raised. The cutoff value for UtA-PI at 95th percentile was 2.8 in our study (Table.2). This was higher (2.35–2.6) as compared to the values reported by other authors [3–5, 14, 17, 18]. This difference in cutoff value could be attributed to racial and ethnic differences between the study populations. We reported that UtA-PI had 77.7% sensitivity and 98.9% specificity in predicting PE. Numerous authors have documented high specificity (≥ 90%) and low sensitivity (< 50%) of UtA-PI for PE, including Cnossen et al. in a meta-analysis (Table2) [3–6, 14–17, 19–22]. In contrast in a recent study, Abdel et al. [22] reported 56% specificity and 100% sensitivity of UtA-PI in detecting PE (Table2). When we studied the EOPE group, the sensitivity of UtA-PI increased to 83.3%; however, the specificity was not much altered (Tables1, 2). The positive likelihood ratio (LR +) of UtA-PI for detecting PE, EOPE and GH was 70.7, 24.5 and 1.9, respectively (Table1). This trend was in concurrence to that reported by Cnossen et al. and Poon et al. [4, 14, 19] who had demonstrated that sensitivity of UtA-PI increases with the severity of the hypertensive disorder.
Table 2.
Studies on screening characteristics of UtA-PI (at ≥ 95th percentile) at 11–14 weeks of gestation for PE and GH
| N | (n/N%) Prevalence of PE | 95th percentile | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | LR + | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GH | PE | EOPE | PE | EOPE | FGR | PE | EOPE | PE | EOPE | PE | ||||
| Martin et al. [3] | 3045 | 1.5 | 2.35 | – | 27 | 50 | 95.4 | 95.1 | – | 11 | 4.5 | 99.8 | – | |
| Gomez et al. [4] | 999 | 2.2 | – | 24 | 30 | 50 | 95.1 | – | 95.4 | 11.3 | – | 97.9- | – | – |
| Pilalis et al. [5] | 893 | 2.1 | 2.52 | – | 23 | 60 | – | – | – | 6.7 | – | – | – | – |
| Cnossen et al. [19] | 4966 (PE low risk) | – | – | – | 25 | – | 95 | – | – | – | – | 5.4 | – | 5.4 |
| 433 (PE low risk) | – | – | – |
SPE 40 |
– |
SPE 90 |
– | – | – | – |
SPE 4 |
– | SPE4 | |
|
3045 (FGR low risk) |
– | – | – | – | – | – | – | 75 | – | – | – | – | – | |
| 999 (FGR low risk) | – | – | – | – | – | – | SFGR 95 | – | – | – | – | – | ||
| 785 (high risk) | – | – | – | – | – | – | – | 76 | – | – | – | – | – | |
| Poon et al. [14] | 7977 | 2.9 | 2.45 | 18 | 36* | 37–84* | – | – | – | – | – | – | – | – |
| Napolitano et al. [6] | 6221 | 2.9 | – | 20.7 | – | – | – | – | – | – | – | – | – | |
| Woschitz et al. [20] |
139 (high risk) |
18 | 2.5 | – | 22 | – | 88 | – | – | 29 | – | – | – | – |
| Marcolin et al. [17] | 162 (low risk) | 9.25 | – | – | – | – | – | – | – | – | – | – | – | – |
| Yasmin Casmod et al. [16] | 144 (low risk) | 5.8 | 2.7 | – | – | – | – | – | – | – | – | – | – | – |
| Neravi et al. [21] | 100 (low risk) | 22(45% of these had severe PE) | With preeclampsia the mean PI at 12–16 weeks was 0.9573 and at 24–26 weeks was 0.7968, which was statistically significant (p < 0.0001) as compared to non-preeclamptic women. Hence along with uterine artery diastolic notching and UtA-RI, UtA-PI can be used as a Doppler index for detecting preeclampsia | |||||||||||
| Abdel Moety GAF et al. [22] | 100 (low risk) | – | 2.37 | – | 100 | – | 56 | – | – | – | – | – | – | – |
| Present study | 100 | 9 | 2.8 | 20 | 77.7 | 83.3 | 98.9 | 97.87 | 94.25 | 87.5 | 71.42 | 97.8 | 98.2 | 70.7 |
N = sample size; n = total affected cases; SPE severe preeclampsia; SFGR severe fetal growth retardation;* The estimated detection rate of EOPE, for a 5% false positive rate, was increased from 37% in screening by maternal factors alone to 78% with combined screening by maternal factors and biophysical markers. This was further improved to 84% with serum PAPP-A. However, there was no significant positive contribution from addition of serum PAPP-A to maternal factors, MAP and UtA−lowest PI in the prediction of late PE and GH
With defective deep placentation and resulting ischemia, the placental synthetic function is also disrupted and so is the production of PAPP-A. Low PAPP-A concentration at 11–13+6-weeks of gestation has been associated with subsequent development of PE, SGA infants, and spontaneous preterm delivery [23]. In our study, the mean ± SD and median value of PAPP-A were 1.15 ± 0.54 and 1.14 MoM, respectively. Pilalis et al. [5] had reported a similar mean (1.19MoM) and median (1.11MoM) values for PAPP-A levels between 11 to 14 weeks of gestation. PAPP-A concentration increases exponentially with a doubling time of 3–4 days during the first trimester. It rises throughout pregnancy thereafter [7, 8, 24]. It also increased significantly during 11–14 weeks of gestation in our study (Fig. 1). The cutoff value used for PAPP-A at 5th percentile in our study was ≤ 0.27MoM. A total of 8% of women had PAPP-A values ≤ 0.27MoM. In studies conducted by Ozdamar et al. and others, the cutoff value used at 5th percentile was ≥ 0.4MoM (Table 3) [5, 7, 8–12, 14, 15, 23, 25–28]. This value was higher than the cutoff value used in our study (0.27MoM). This difference in cutoff values can be attributed to differences in laboratory standards for processing samples and disparity in race and ethnicity of subjects. The reported sensitivity and specificity of PAPP-A for PE was 44.4% and 95.6%, respectively. Most authors support low (< 20%) sensitivity of PAPP-A in predicting PE at this POG (Table3). Similar to UtA-PI, PAPP-A demonstrated an increasing sensitivity (50% vs.44.4%) for more and severe EOPE than late PE. Poon et al. [14] reported high sensitivity (84%, at 5% false positive rate/FPR) of PAPP-A for screening EOPE when it was combined with maternal factors. In a recent study, Yliniemi et al. [29] reported that at 9–13+6weeks of gestation, a combination of PAPPA, free β-hCG (fβ-hCG), other serum biomarkers and maternal characteristics can detect 48% of women with EOPE in a low-risk population.
Table 3.
Studies on screening characteristics of PAPP-A and fβ-hCG (MoM) (at ≤ 5th percentile) at 11–14 weeks of gestation for PE and GH
| N | Prevalence (n/N%) | PAPP-A (MoM) ≤ 5th percentile |
Sensitivity (%) | Specificity (%) | PPV(%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GH | PE | EOPE | GH | PE | EOPE | PE | EOPE | PE | EOPE | |||
| Charas Ong et al. [7] | 5297 | 1.5 | 2.54 | – |
< 5th centile < 10th centile |
11 20 |
15 26 |
– | – | – | 5.4 | – |
| Yaron et al.[9] | 1622 | – | – | – | 0.5 | – | 22.2 | – | – | – | 2.5 | – |
| Smith et al. [8] | 8839 | – | 3.7 | – | 0.963 | – | 7.6 | – | – | – | – | – |
| Tul et al. [25] | 1136 | – | – | – | 0.5 | – | – | 19.6 | – | – | – | – |
| Kwik and Morris et al. [26] | 827 | – | – | – | 0.5 | – | – | 32.7 | – | – | – | – |
| Dugoff et al. [10] | 33,395 | – | 2.28 | – | 1.54 | – | 7.8 | – | – | – | 3.5 | – |
| Krantz et al. [27] | 6276 | – | – | – | – | – | – | – | – | – | – | – |
| Spencer et al. [11] | 4295 | – | 1.45 | – | 0.84 | – | 14.1;62.1* | – | – | – | – | – |
| Kavak et al. [12] | 490 | – | – | – | – | 73 | 65 | - | – | |||
| Pilalis et al. [5] | 878 | – | 1.5 | 0.7 | 0.41 | 23.1 | – | – | – | 6.7 | – | |
| Poon et al. [14] | 8366 | 1.7 | 1.9 | 1.5 | – | 18** | 40** | 84** | – | – | – | – |
| Ozdamar et al. [15] | 6822 | – | 0.8 | – | 0.956 | – | 70 | – | 65.6 | – | 67 | – |
| Balci S et al. [28] | 158 | GH/PE3.16 | – | 0.72 | – | 82.4*** | – | 29.8*** | – | 67*** | – | |
| N Yu et al. [23] | 500 | 9.-8 | – | – | – | 26 (AUC 0.63) | – | – | – | – | – | |
| Present study | 100 | 5 | 9 | 6 | 0.27 | 0 | 44.4 | 50 | 95.6 | 94.6 | 50 | 37.5 |
| N | Prevalence (%) | Cutoff levels of fβ-hCG | Sensitivity (%) | Specificity (%) | PPV (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Charas et al. [7] | 5297 | 1.5 | 2.54 | – |
< 5th centile < 10th centile |
7 12 |
10 22 |
– | – | – | – | |
| Ozdamar et al. [15] | 6822 | – | 0.8 | – | fβ-hCG values showed no statistically significant difference (Mann–Whitney U test, p = 0.882) in PE groups and non-PE groups | |||||||
| Abdel Moety GAF et al. [22] | 100 | – | – | – | 43,792 miu/ml | – | 50 | – | 78 | – | – | – |
| Present study | 100 | 5 | 9 | 6 | 0.5 MoM (≤ 5th percentile) | 20 | 0 | 0 | 94 | 91.4 | 0 | 0 |
*PAPP-A when combined with second trimester UtA−PI at 5% FPR
** Sensitivity of combined maternal factors, lower UtA−PI, MAP and serum PAPP-A at 5% FPR
***Poor outcomes combined
We used a cutoff value of ≤ 0.5MoM at 5th percentile for fβ-hCG in our study. In concurrence with Ong et al. [7] our mean fβ-hCG values decreased significantly over 11–14 weeks of gestation (Fig. 1). We reported that as a stand-alone marker, fβ-hCG was not a sensitive test for the detection of PE. This was again in concurrence to Ong et al. [7] who had demonstrated low (10%) sensitivity of fβ-hCG for proteinuric pregnancy-induced hypertension. Recently, Yu et al. [23] also agreed to no significant differences in fβ-hCG values in PE group compared to controls (0.92 vs.1.00; p¼−0.536) (Table 3).
Numerous articles have described roles of first-trimester UtA-PI, maternal characteristics and biochemical markers like PAPP-A, PlGF (placental growth factor), PP13 (placental protein 13), sEndoglin, Inhibin-A, Activin-A, Pentraxin 3 and P-selectin in predicting PE with varying results. Till date only a single study by Abdel et al. [22] from Egypt has tested the three parameters together (UtA-PI, PAPP-A and fβ-hCG) similar to our study design. Our study was first such study being conducted in the Indian subcontinent.
In a case–control study by Staboulidou et al. [13] when UtA-PI was combined with PAPP-A, the former could differentiate between low PAPP-A levels due to trisomy 21 and EOPE. While UtA-PI was higher in the late PE and EOPE group, it was not in trisomy 21 group. When we combined PAPP-A with UtA-PI, the combination had 44.4% sensitivity in predicting PE. This was lower (44.4 vs.77.8%) when compared with the sensitivity of UtA-PI alone (Table 1). Poon et al. [14] demonstrated that PAPP-A does not add to the sensitivity of the combined test comprising of maternal factors, UtA-lowest PI and mean arterial pressure (MAP) for detection of late PE (with onset after 32 weeks of gestation). O'Gorman et al. [30] documented that combined screening by maternal factors, UtA-PI, MAP, and placental growth factors could predict 75% of preterm PE, but only 47% of term PE (at 10% FPR). The reported specificity of combined UtA-PI and PAPP-A was 100% in our study. Abdel et al. [22] reported 94.4% specificity for the same (Table 4).
Table 4.
Studies on screening characteristics of UtA-PI (at ≥ 95th centile), PAPP-A (at ≤ 5th percentile) and fβ-hCG (at ≤ 5th percentile) at 11–14 weeks of gestation for PE and GH
| N | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| GH | PE | EOPE | PE | EOPE | PE | EOPE | PE | EOPE | |||
| Abdel Moety GAF et al. [22] | 100 | UtA-PI and PAPP-A | – |
100 100 |
– |
94.44 88.89 |
– |
44.44 28.57 |
– |
100 100 |
– |
| UtA-PI and fβ-hCG | – | ||||||||||
| Present study | 100 | UtA-PI and PAPP-A | 44 | 50 | 100 | 98.9 | 100 | 75 | 94.7 | 96.9 | |
| UtA-PI and fβ-hCG | 20 | – | – | 98.9–100 | – | – | – | 90–91 | – | ||
The combination of fβ-hCG to UtA-PI and/or PAPP-A was not found sensitive for detection of PE. The specificity of this test was either unaltered (98.9%) or was raised only slightly (100%). However, Abdel et al. [22] had reported a lower specificity of 88.89% for UtA-PI in combination with fβ-hCG. Wright et al. [31] demonstrated that at 11–13 weeks of POG PAPP‐A, but not fβ‐hCG, along with maternal factors improved prediction of preterm PE (those delivered at < 37week gestation) (Table 4)
Conclusion
The limitations of our study were the small sample size and study type. We conclude that at a cutoff value of 0.28 (95th percentile), UtA-PI is an important screening tool for detecting PE at an early gestational age of 11–14 weeks with a sensitivity of 77.7%. When PAPP-A was added to UtA-PI, the sensitivity of the combined test decreased to 44.4% with only a slight improvement in specificity (100% vs. 98.9%; PAPP-A + UtA-PI vs.UtA-PI). Addition of fβ-hCG was not found sensitive for detecting PE with an almost similar specificity (98.9–100% vs. 98.9%; fβ-hCG + UtA-PI vs.UtA-PI). Hence, our hypothesis that the addition of PAPP-A and fβ-hCG to UtA-PI adds to the predictive value of UtA-PI studies for PE was proven wrong. Moreover, high cost of biochemical tests remains a limiting factor for their routine application in LMIC. Hence, we do not recommend their routine addition to UtA-PI studies for screening of PE at 11–14 weeks of gestation in LMIC. However, there remains scope for large-scale randomized controlled trials combining uterine artery Doppler studies with various biochemical markers to detect PE at its inception.
Acknowledgements
None
Zeba Khanam
Zeba Khanam is a young gynecologist working in the Department of Obstetrics and Gynaecology, V.M.M.C and Safdarjung Hospital, New Delhi, as a senior resident. She did her postgraduation from the same college. She has worked as a consultant gynecologist in the Indian Railways. She has keen interest in the field of fetal medicine, perinatology and high-risk pregnancy. This is her first research article. She has chapters to her name in the AOGD monthly bulletin. Many of her articles are awaiting acceptance in various obstetrics and gynecology journals.
Funding
This study has no funding source.
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no conflict of interest.
Consent to Participate
Informed consent was obtained from all individual participants included in the study.
Consent to Publication
Patients signed informed consent regarding publishing their data. All authors give consent for publication. We have not submitted/published this study previously. The manuscript represents original work, and it is not under consideration for publication elsewhere. All authors meet the criteria for authorship and have signed a statement attesting authorship, disclosing all potential conflicts of interest, and releasing the copyright for publication.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study was approved by the Government of India Institute Ethics Committee/Ethics Sub-Committee Safdarjung Hospital & VMMC, New Delhi-110029 S.No. IEC/VMMC/SJH/Thesis/Nov-13/34, dated 20/12/2013. This article does not contain any studies with animals performed by any of the authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rastogi AP, New Delhi: Zahid, Centre for health informatics, National Institute of Health and Family Welfare. Available from 1 June 2016https://www.nhp.gov.in/disease/gynaecology-and-obstetrics/preeclampsia
- 2.Dascau V, Cristina O, Puschita M. Uterine artery doppler flow indices in pregnant women during the 11 weeks + 0 days and 13 weeks + 6 days gestational ages: a study of 258 patients. Eur J Obstet Gynecol Reprod Biol. 2019;234:e50. doi: 10.1016/j.ejogrb.2018.08.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martin AM, Bindra R, Curcio P, et al. Screening for pre-eclampsia and fetal growth restriction by uterine artery doppler at 11–14 weeks’ gestation. Ultrasound Obstet Gynecol. 2001;18:583–586. doi: 10.1046/j.0960-7692.2001.00594.x. [DOI] [PubMed] [Google Scholar]
- 4.Gómez O, Martínez JM, Figueras F, et al. Uterine artery doppler at 11–14 weeks of gestation to screen for hypertensive disorders and associated complications in an unselected population. Ultrasound Obstet Gynecol. 2005;26(5):490–494. doi: 10.1002/uog.1976. [DOI] [PubMed] [Google Scholar]
- 5.Pilalis A, Souka AP, Antsaklis P, et al. Screening for pre-eclampsia and fetal growth restriction by uterine artery doppler and PAPP-A at 11–14 weeks' gestation. Ultrasound Obstet Gynecol. 2007;29(2):135–140. doi: 10.1002/uog.3881. [DOI] [PubMed] [Google Scholar]
- 6.Napolitano R, Rajakulasingam R, Memmo A, et al. Uterine artery doppler screening for pre-eclampsia: comparison of the lower, mean and higher first-trimester pulsatility indices. Ultrasound Obstet Gynecol. 2011;37(5):534–537. doi: 10.1002/uog.8848. [DOI] [PubMed] [Google Scholar]
- 7.Ong CYT, Liao AW, Spencer K, et al. First trimester maternal serum free β human chorionic gonadotrophin and pregnancy-associated plasma protein A as predictors of pregnancy complications. Br J Obstet Gynaecol. 2000;107(10):1265–1270. doi: 10.1111/j.1471-0528.2000.tb11618.x. [DOI] [PubMed] [Google Scholar]
- 8.Smith GCS, Stenhouse EJ, Crossley JA, et al. Early pregnancy levels of pregnancy-associated plasma protein A and the risk of intrauterine growth restriction, premature birth, preeclampsia, and stillbirth. J Clin Endocrinol Metab. 2002;87(4):1762–1767. doi: 10.1210/jcem.87.4.8430. [DOI] [PubMed] [Google Scholar]
- 9.Yaron Y, Heifetz S, Ochshorn Y, et al. Decreased first trimester PAPP-A is a predictor of adverse pregnancy outcome. Prenat Diagn. 2002;22(9):778–782. doi: 10.1002/pd.407. [DOI] [PubMed] [Google Scholar]
- 10.Dugoff L, Hobbins JC, Malone FD, et al. First-trimester maternal serum PAPP-A and free-beta subunit human chorionic gonadotropin concentrations and nuchal translucency are associated with obstetric complications: a population-based screening study (the FASTER Trial) Am J Obstet Gynecol. 2004;191(4):1446–1451. doi: 10.1016/j.ajog.2004.06.052. [DOI] [PubMed] [Google Scholar]
- 11.Spencer K, Yu CKH, Cowans NJ, et al. Prediction of pregnancy complications by first-trimester maternal serum PAPP-A and free fβ-hCG and with second-trimester uterine artery doppler. Prenat Diagn. 2005;25(10):949–953. doi: 10.1002/pd.1251. [DOI] [PubMed] [Google Scholar]
- 12.Kavak ZN, Basgul A, Elter K, et al. The efficacy of first-trimester PAPP-A and free beta hCG levels for predicting adverse pregnancy outcome. J Perinat Med. 2006;34(2):145–148. doi: 10.1515/JPM.2006.026. [DOI] [PubMed] [Google Scholar]
- 13.Staboulidou I, Galindo A, Maiz N, et al. First-trimester uterine artery doppler and serum pregnancy-associated plasma protein-A in preeclampsia and chromosomal defects. Fetal Diagn Ther. 2009;25(3):336–339. doi: 10.1159/000235880. [DOI] [PubMed] [Google Scholar]
- 14.Poon LC, Stratieva V, Piras S, et al. Hypertensive disorders in pregnancy: combined screening by uterine artery doppler, blood pressure and serum PAPP-A at 11–13 weeks. Prenat Diagn. 2010;30(3):216–223. doi: 10.1002/pd.2440. [DOI] [PubMed] [Google Scholar]
- 15.Ozdamar O, Gun I, Keskin U, et al. The role of maternal serumbeta-HCG and PAPP-A levels at gestational weeks 10–14 in the prediction of pre-eclampsia. Pak J Med Sci. 2014;30(3):568–573. doi: 10.12669/pjms.303.4554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Casmod Y, Van Dyk Barbara, Nicolaou E. Uterine artery Doppler screening as a predictor of pre-eclampsia. Health SA Gesondheid. 2016;21:391–396. doi: 10.1016/j.hsag.2016.06.004. [DOI] [Google Scholar]
- 17.Marcolin AC, Scandiuzzi RM, Quintana SM, et al. [299-POS]: First-trimester screening combining maternal risk factors and uterine artery doppler indices is useful to predict hypertension in low-risk pregnant woman. Pregnancy Hypertens. 2015;5:148. doi: 10.1016/j.preghy.2014.10.305. [DOI] [Google Scholar]
- 18.Plasencia W, Maiz N, Bonino S, et al. Uterine artery Doppler at 11 + 0 to 13 + 6 weeks in the prediction of pre-eclampsia. Ultrasound Obstet Gynecol. 2007;30(5):742–749. doi: 10.1002/uog.5157. [DOI] [PubMed] [Google Scholar]
- 19.Cnossen JS, Morris RK, ter Riet G, et al. Use of uterine artery doppler ultrasonography to predict pre-eclampsia and intrauterine growth restriction: a systematic review and bivariable meta-analysis. CMAJ. 2008;178(6):701–711. doi: 10.1503/cmaj.070430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Woschitz T. Uterine artery doppler in women with history of previous pre eclampsia and women with chronic hypertension reevaluation of a prognostic value in a high risk population. Gynecol Obstet. 2014 doi: 10.4172/2161-0932.1000206. [DOI] [Google Scholar]
- 21.Neravi A, Udayashree V. Role of uterine artery doppler at 12–16 weeks of gestation in prediction of pre-eclampsia an observational study. Int J Reprod Contracept Obstet Gynecol. 2018;7(8):3162–3167. doi: 10.18203/2320-1770.ijrcog20183310. [DOI] [Google Scholar]
- 22.Abdel Moety GA, Almohamady M, Sherif NA, et al. Could first-trimester assessment of placental functions predict preeclampsia and intrauterine growth restriction? A prospective cohort study. J Matern Fetal Neonatal Med. 2016;29(3):413–417. doi: 10.3109/14767058.2014.1002763. [DOI] [PubMed] [Google Scholar]
- 23.Yu N, Cui H, Chen X, Chang Y. First trimester maternal serum analytes and second trimester uterine artery doppler in the prediction of preeclampsia and fetal growth restriction. Taiwan J Obstet Gynecol. 2017;56(3):358–361. doi: 10.1016/j.tjog.2017.01.009. [DOI] [PubMed] [Google Scholar]
- 24.Fialova L, Malbohan IM. Pregnancy-associated plasma protein A (PAPP-A): theoretical and clinical aspects. Bratisl Lek Listy. 2002;103(6):194–205. [PubMed] [Google Scholar]
- 25.Tul N, Pusenjak S, Osredkar J, et al. Predicting complications of pregnancy with first-trimester maternal serum free-betahCG PAPP-A and inhibin-A. Prenat Diagn. 2003;23(12):990–996. doi: 10.1002/pd.735. [DOI] [PubMed] [Google Scholar]
- 26.Kwik M, Morris J. Association between first trimester maternal serum pregnancy associated plasma protein-A and adverse pregnancy outcome. Aust N. Z. Obstet Gynaecol. 2003;43(6):438–442. doi: 10.1046/j.0004-8666.2003.00126.x. [DOI] [PubMed] [Google Scholar]
- 27.Krantz D, Goetzl L, Simpson JL, et al. Association of extreme first-trimester free human chorionic gonadotropin-beta, pregnancy-associated plasma protein A, and nuchal translucency with intrauterine growth restriction and other adverse pregnancy outcomes. Am J Obstet Gynecol. 2004;191(4):1452–1458. doi: 10.1016/j.ajog.2004.05.068. [DOI] [PubMed] [Google Scholar]
- 28.Balcı S. Predictive values of maternal serum PAPP-A level, uterine artery doppler velocimetry, and fetal biometric measurements for poor pregnancy and poor neonatal outcomes in pregnant women. J Turk Ger Gynecol Assoc. 2016;17(3):143–149. doi: 10.5152/jtgga.2016.16040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yliniemi A, Makikallio K, Korpimaki T, et al. Combination of PAPPA, fhCGβ, AFP, PlGF, sTNFR1, and maternal characteristics in prediction of early-onset preeclampsia. Clin Med Insights Reprod Health. 2015;11(9):13–20. doi: 10.4137/CMRH.S21865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O'Gorman N, Wright D, Syngelaki A, et al. Competing risks model in screening for preeclampsia by maternal factors and biomarkers at 11–13 weeks gestation. Am J Obstet Gynecol. 2016;214(1):103.e1–103.e12. doi: 10.1016/j.ajog.2015.08.034. [DOI] [PubMed] [Google Scholar]
- 31.Wright A, Guerra L, Pellegrino M, et al. Maternal serum PAPP-A and free fβ-hCG at 12, 22 and 32 weeks' gestation in screening for pre-eclampsia. Ultrasound Obstet Gynecol. 2016;47(6):762–767. doi: 10.1002/uog.15849. [DOI] [PubMed] [Google Scholar]