Abstract
With increasing atrial septal defect (ASD) repairs, more women of childbearing age will have ASD closure devices. Current ASD closure trials have excluded women planning pregnancy, making their management challenging. We present a pregnant woman, with a repaired ASD, who presented with device-related infective endocarditis. (Level of Difficulty: Beginner.)
Key Words: atrial septal defect, echocardiography, endocarditis, pregnancy
Abbreviations and Acronyms: ASD, atrial septal defect; CHD, congenital heart disease; IE, infective endocarditis; PFO, patent foramen ovale; TEE, transesophageal echocardiogram; TTE, transthoracic echocardiogram
Graphical abstract
A 29-year-old G1P0 woman at 20 weeks’ gestation, with a history of atrial septal defect (ASD) closure, presented with fevers and myalgias. Physical examination and imaging demonstrated Janeway lesions and patchy bilateral pulmonary infiltrates concerning for septic emboli. She was treated with vancomycin and ceftriaxone starting on day 2 of hospitalization and blood cultures grew methicillin-sensitive Staphylococcus aureus.
Learning Objectives
-
•
To review the current indications for prophylactic antibiotic therapy after ASD closure.
-
•
To understand the challenges of treating IE during pregnancy.
Past Medical History
The patient’s medical history was significant only for an ASD, which was closed percutaneously using an Amplatzer device (Abbott Medical, Plymouth, Minnesota) 7 months before presentation, at another institution, for primary stroke prevention. Before device placement, she described episodes of migraines, which were attributed to her ASD. In addition, her father had been treated with an ASD closure device after a cryptogenic stroke. During the year following ASD closure, she underwent multiple dental cleanings and received prophylactic antibiotics during the first 6 months after ASD closure, in accordance with American Heart Association guidelines (1).
Differential Diagnosis
Given the patient’s history of ASD closure with a prosthetic device, positive blood cultures, and bilateral pulmonary infiltrates suggestive of septic emboli, the leading differential diagnosis was infective endocarditis (IE). Other differential diagnoses included bacteremia from complicated pneumonia and infectious vasculitis.
Investigations
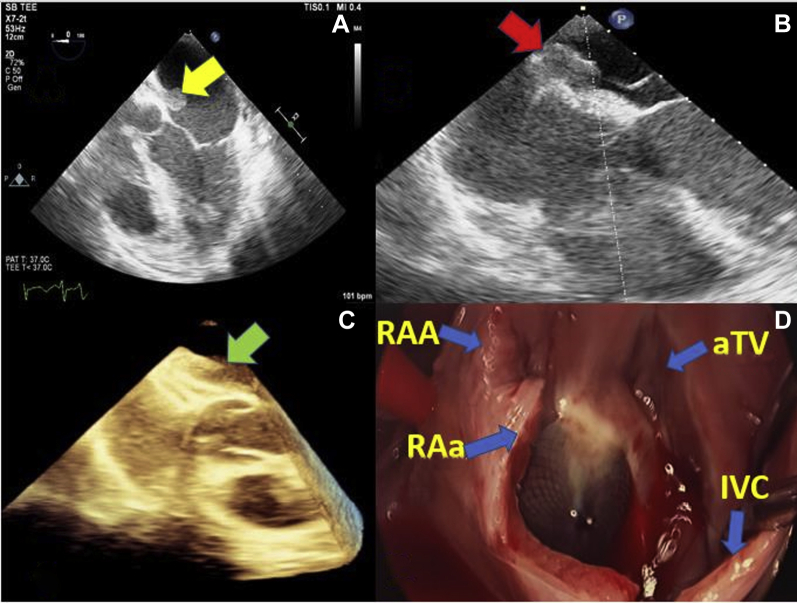
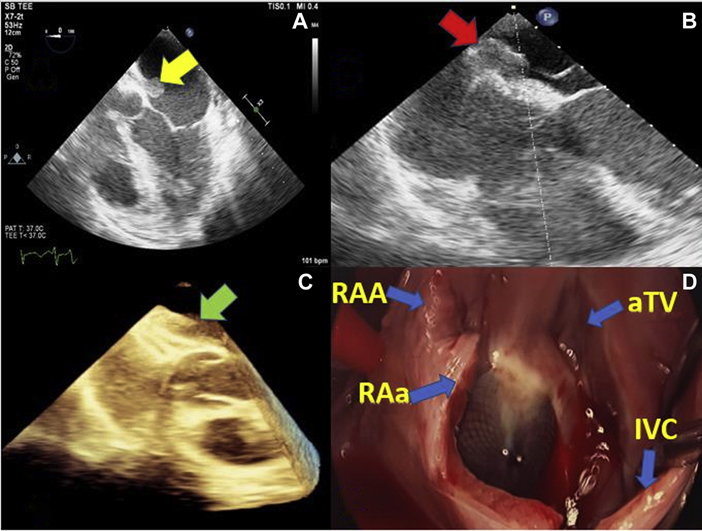
Initial transthoracic echocardiogram (TTE) showed a well-seated Amplatzer device with no residual shunt and no vegetations. Because of persistent bacteremia and high suspicion of IE, a transesophageal echocardiogram (TEE) was obtained that demonstrated a 2.3 × 1.1-cm. mobile tissue density on the left atrial surface of the device, consistent with a vegetation (Figure 1, Video 1).
Figure 1.
TEE and Intraoperative Images Showing Large Vegetation on ASD Closure Device
(A) TEE mid esophageal 5-chamber view showing large, mobile vegetation attached to the left atrial surface of an Amplatzer device. (B) TEE mid esophageal 4-chamber view showing point of attachment of the vegetation to the device. (C) Three-dimensional TEE image showing thickening and tissue densities between the 2 arms of the device consistent with an infectious process. (D) Intraoperative image showing epithelialization of the Amplatzer device in the center of the image. ASD = atrial septal defect; aTV = anterior tricuspid valve; IVC = inferior vena cava; RAA = right atrial appendage; RAa = right atriotomy; TEE = transesophageal echocardiogram.
Management
On day 5 of hospitalization, she developed visual field deficits concerning for septic emboli. Although the patient hoped to delay surgery until the infant was viable, the obstetrics, infectious disease, and cardiothoracic surgery teams concluded that the balance of risks favored urgent surgery, given the persistent bacteremia and visual disturbances due to systemic embolization. After extensive multidisciplinary discussions with the patient, a decision was made to pursue surgery. At 20 weeks’ gestation, she was taken for emergent surgery where the vegetation and Amplatzer device were successfully removed and the interatrial septum was repaired with a pericardial patch.
Discussion
Cardiac disease complicates 1% to 4% of all pregnancies in the United States and accounts for 10% to 25% of maternal mortality (2). IE is an infection of damaged endothelial cells, particularly valve leaflets, with an incidence of 5 to 7 per 100,000 patient-years and has a poor prognosis, with in-hospital mortality rates as high as 15% to 20% at 1 year (3). A systematic review looking at IE in pregnancy estimated maternal and fetal mortality rates of 11% and 14%, respectively; however, this was thought to be an underestimation, as not all cases of pregnancy-related IE are published. Major risk factors for pregnancy-related IE are intravenous drug use, congenital heart disease (CHD), and rheumatic heart disease. Underlying heart disease remains a significant risk factor, as 34% of the included patients in the systematic review had predisposing cardiac conditions (4). Incidence of IE in adults with CHD is much higher, at approximately 0.9 to 1.3 cases per 1,000 patient-years, with a 20% recurrence rate (5). Lifelong antibiotic prophylaxis before dental procedures is indicated in unrepaired cyanotic congenital heart defects and in repaired defects with residual shunt or valvular regurgitation. Prophylaxis before dental procedures is also recommended for the first 6 months following successful defect repair (1). Prosthetic device IE at 1 year after ASD closure is extremely rare. In a recent case series of 22 patients with IE, none were reported at or after the 1-year mark (6). Bacterial seeding that causes infection likely occurs before complete neo-endothelialization of the prosthetic device, which is thought to occur between 1 and 3 months after implantation (7). Our patient developed IE 7 months after ASD closure, at that point complete neo-endothelization was expected to be complete, especially because no residual shunt was seen on TTE. Although our patient received appropriate antibiotic prophylaxis in the high-risk period after device placement, her presentation of IE suggests that some patients may require prophylactic antibiotics beyond 6 months. It is possible pregnancy may potentially lead to immunocompromised state, therefore increasing the risk of IE.
ASDs and patent foramen ovales (PFOs) occur when there is a failure to close the communication between the right and left atria. Although an ASD is a deficiency in the atrial septum resulting in failure to overlap, causing continuous left-to-right shunting, a PFO is not a defect of the true septum but rather a failure of fusion of the primum and secundum atrial septa. This failure creates a flap valve opening that results in a transient right-to-left shunt when right atrial pressures exceed left atrial pressures. Although recent trials have demonstrated that PFO closure, as compared with antiplatelet or anticoagulation alone, reduces the risk of recurrent cryptogenic stroke, PFO closure has not been shown to be an effective migraine treatment (8,9). Although studies of transcatheter closure of ASDs did not indicate whether or not pregnant women were included, PFO closure trials specifically excluded women who were pregnant, lactating, or had a desire to become pregnant (9,10). The RESPECT (Randomized Evaluation of Recurrent Stroke Comparing PFO Closure to Established Current Standard of Care Treatment) trial excluded women who intended to become pregnant within the next year and the Gore REDUCE (GORE HELEX Septal Occluder/GORE CARDIOFORM Septal Occluder and Antiplatelet Medical Management for Reduction of Recurrent Stroke or Imaging-Confirmed TIA in Patients With Patent Foramen Ovale) trial excluded women planning to become pregnant within the next 2 years (8). Each of the PFO closure trials had strict entry criteria and therefore clinical application is most appropriate for patients who are not planning pregnancy in the near future and are younger than 60 years, with moderate to large interatrial shunt and/or an atrial septal aneurysm (8).
Special anesthetic considerations were made before both TEE and surgery for our patient. Before the TEE, pre-procedural planning with the cardiac team enabled a focused examination to obtain views of the suspected area of IE to minimize procedure time and discomfort. Only topical, rather than systemic, anesthesia was used to avoid reduction in cardiac output and fetal hypoperfusion. During surgery, the most important consideration was the avoidance of sudden changes in cardiac output and early implementation of extracorporeal membrane oxygenation to maintain perfusion of the fetus, if necessary.
Follow-up
The patient had an uncomplicated postoperative course and went on to deliver a healthy infant without any sequelae of IE 17 weeks after surgery. Repeat TTE at 6 and 12 months post-procedure demonstrated no evidence of interatrial shunt. Both mother and child continue to do well 1 year after surgery. The subject of this case report provided consent to publication, which was obtained through our institution’s standard protocol.
Conclusions
IE of an intracardiac closure device is rare and can present unique challenges in pregnant patients. Although indications for ASD and PFO closure are increasing with recent trials, they largely exclude women planning pregnancy. Duration of antibiotic prophylaxis may need to be prolonged beyond 6 months in those with prosthetic intracardiac devices for CHD.
Funding Support and Author Disclosures
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For a supplemental video, please see the online version of this paper.
Appendix
TEE views of the patient’s ASD closure device and associated vegetation.
References
- 1.Lafaurie G.I., Noriega L.A., Torres C.C. Impact of antibiotic prophylaxis on the incidence, nature, magnitude, and duration of bacteremia associated with dental procedures: a systematic review. J Am Dent Assoc. 2019;150:948–959.e4. doi: 10.1016/j.adaj.2019.06.017. [DOI] [PubMed] [Google Scholar]
- 2.Petersen E.E., Davis N.L., Goodman D. Racial/ethnic disparities in pregnancy-related deaths - United States, 2007–2016. MMWR Morb Mortal Wkly Rep. 2019;68:762–765. doi: 10.15585/mmwr.mm6835a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cahill T.J., Prendergast B.D. Infective endocarditis. Lancet. 2016;387:882–893. doi: 10.1016/S0140-6736(15)00067-7. [DOI] [PubMed] [Google Scholar]
- 4.Kebed K.Y., Bishu K., Al Adham R.I. Pregnancy and postpartum infective endocarditis: a systematic review. Mayo Clin Proc. 2014;89:1143–1152. doi: 10.1016/j.mayocp.2014.04.024. [DOI] [PubMed] [Google Scholar]
- 5.Montanaro C., Dimopoulos K., Shore D.F. Infective endocarditis in patients with congenital heart disease: when, where and how. Int J Cardiol. 2017;249:171–172. doi: 10.1016/j.ijcard.2017.09.186. [DOI] [PubMed] [Google Scholar]
- 6.Amedro P., Soulatges C., Fraisse A. Infective endocarditis after device closure of atrial septal defects: case report and review of the literature. Catheter Cardiovasc Interv. 2017;89:324–334. doi: 10.1002/ccd.26784. [DOI] [PubMed] [Google Scholar]
- 7.Lock J.E., Rome J.J., Davis R. Transcatheter closure of atrial septal defects. Experimental studies. Circulation. 1989;79:1091–1099. doi: 10.1161/01.cir.79.5.1091. [DOI] [PubMed] [Google Scholar]
- 8.Osteraas N.D., Vargas A., Cherian L., Song S. Role of PFO closure in ischemic stroke prevention. Curr Treat Options Cardiovasc Med. 2019;21:63. doi: 10.1007/s11936-019-0775-7. [DOI] [PubMed] [Google Scholar]
- 9.Majunke N., Bialkowski J., Wilson N. Closure of atrial septal defect with the Amplatzer septal occluder in adults. Am J Cardiol. 2009;103:550–554. doi: 10.1016/j.amjcard.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 10.Fraisse A., Latchman M., Sharma S.R. Atrial septal defect closure: indications and contra-indications. J Thorac Dis. 2018;10:S2874–S2881. doi: 10.21037/jtd.2018.08.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
TEE views of the patient’s ASD closure device and associated vegetation.