Abstract
We report a case of spontaneous coronary artery dissection located next to a myocardial bridge in a patient with concomitant takotsubo cardiomyopathy. A fusion image with multidetector-row computed tomography and single-photon emission computed tomography played an important role in the diagnosis of these lesions. (Level of Difficulty: Advanced.)
Key Words: apical ballooning, myocardial bridge, spontaneous coronary artery dissection, takotsubo cardiomyopathy
Abbreviations and Acronyms: 123I-BMIPP, iodine-123 beta-methyl iodophenyl pentadecanoic acid; CAG, coronary angiography; ECG, electrocardiogram; IVUS, intravascular ultrasonography; LAD, left anterior descending artery; MB, myocardial bridge; MDCT, multidetector-row computed tomography; SCAD, spontaneous coronary artery dissection; SPECT, single-photon emission computed tomography; TC, takotsubo cardiomyopathy
Graphical abstract
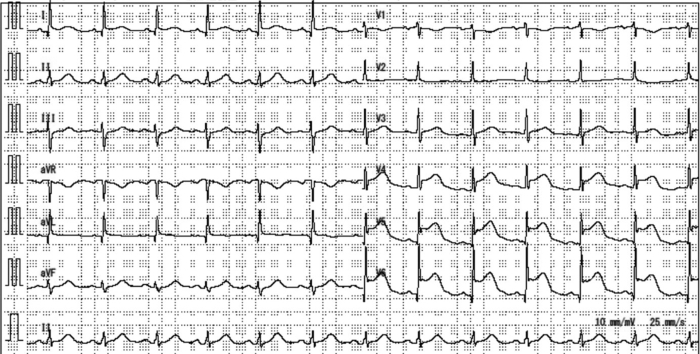
A 68-year-old woman visited the emergency department for sudden onset chest pain at rest. She reported feeling more fatigued than usual over the past few months. Furthermore, she had been caring for her grandchildren for a few days, and this had caused considerable physical and mental exhaustion. There were no other activities or unusual events, such as exercise and trauma. Physical examination revealed blood pressure of 178/109 mm Hg and a regular pulse (79 beats/min). The electrocardiogram (ECG) showed ST-segment elevation in the anterolateral leads (Figure 1). Echocardiography showed apical ballooning and basal hyperkinesis without ventricular outflow tract obstruction; the ejection fraction was 60%, without evidence of valvular disease. The troponin T level was 0.086 ng/ml (normal value <0.01 ng/ml).
Learning Objectives
-
•
To present this case of SCAD with concomitant TC and MB, which has never been reported before.
-
•
To present the usefulness of multimodality imaging techniques other from CAG, such as SPECT, MDCT, and IVUS, for detecting these diseases.
Figure 1.
12-Lead Electrocardiogram
ST-segment elevation is visible in the anterolateral leads.
Past Medical History
She had a medical history of hypertension and dyslipidemia and had been taking a statin.
Differential Diagnoses
In view of her symptoms, ST-segment elevation on ECG, echocardiography findings, and elevated troponin T, we proposed myocardial infarction, takotsubo cardiomyopathy (TC), and pericarditis as differential diagnoses.
Investigations
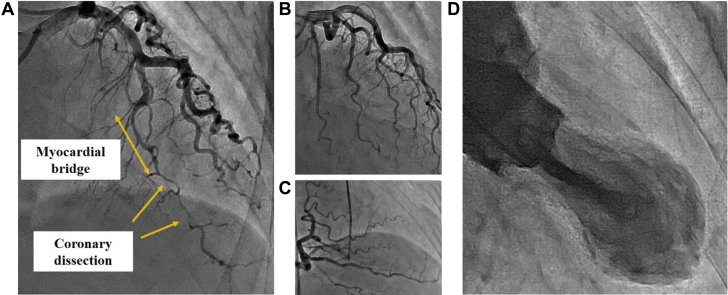
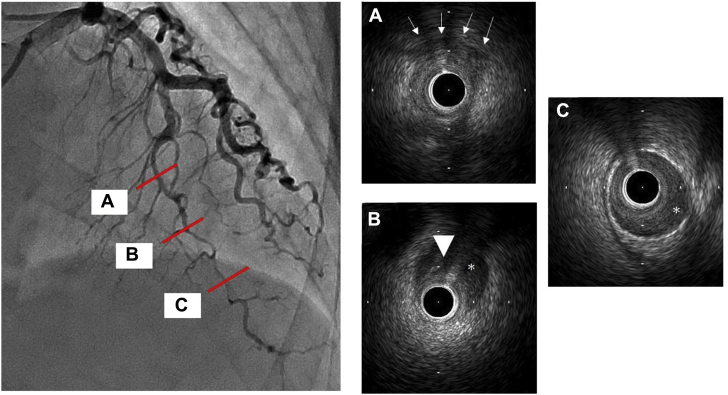
Emergency coronary angiography (CAG) revealed type 2b spontaneous coronary artery dissection (SCAD) in the distal left anterior descending artery (LAD) (Figure 2A, Video 1). No significant stenosis was found in other arteries (Figures 2B and 2C). Intravascular ultrasonography (IVUS) revealed narrowing of the lumen secondary to extensive coronary artery dissection. Its entry point was on the distal end of a myocardial bridge (MB) (Figures 3A to 3C). Left ventriculography revealed apical ballooning of the middle to apical segments, with hyperkinesis in the basal segments, suggestive of TC (Figure 2D, Video 2).
Figure 2.
Coronary Angiography
(A) Myocardial bridge in the middle left anterior descending artery and distal left anterior descending coronary dissection are visible. (B and C) No significant stenosis is seen in the left circumflex and right coronary arteries. (D) Left ventriculography shows apical ballooning akinesia and basal hyperkinesia.
Figure 3.
Intravascular Ultrasound Imaging
(A) View of the myocardial bridge segment (arrows). (B and C) Intravascular ultrasound imaging showing coronary artery dissection (asterisks = pseudo-lumen; arrowhead = entry point).
Management
We performed balloon angioplasty for the flow-limited LAD lesion because of the patient’s persistent chest pain and abnormal ECG findings. The creatine phosphokinase level was 376 IU/l (normal range 41 to 153 IU/l). Antiplatelet agents, beta-blockers, and an angiotensin-converting enzyme inhibitor were added. Because IVUS revealed atherosclerotic changes separate from the coronary dissection lesion, the statin dose was increased after the procedure.
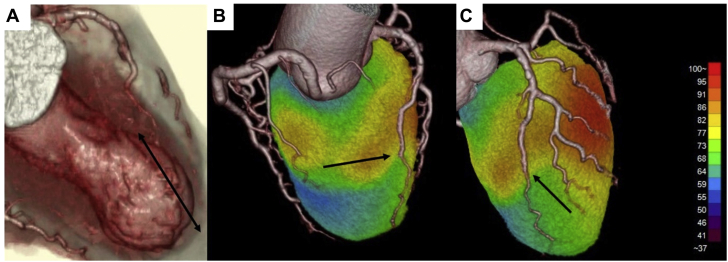
Multidetector-row computed tomography (MDCT), performed on day 2 post-CAG to observe the lesion and to evaluate the relationship between the MB site and the coronary artery dissection origin, showed almost complete resolution of the blood flow and dissection in the LAD. The 3-dimensional volume image of the MDCT showed that the hinge point between the basal hyperkinesis and apical ballooning was coincident with the entry point of the coronary artery dissection and distal end of an MB (Figure 4A). On postoperative day 4, myocardial viability and ischemic memory were assessed by thallium-201 chloride and iodine-123 beta-methyl iodophenyl pentadecanoic acid (123I-BMIPP) single-photon emission computed tomography (SPECT). When compared with thallium-201 chloride SPECT, 123I-BMIPP SPECT revealed much more reduced uptake in the middle to apical segments beyond the LAD, a finding suggesting preserved viability and the impact of ischemia related to TC. In addition, 123I-BMIPP SPECT and computed tomography fusion imaging showed coincidence of the proximal end of the coronary artery dissection and the reduced uptake region (Figures 4B and 4C). To understand the clinical pathology of SCAD, TC, and MB, SPECT was obtained for viability and ischemic memory; however, the territory was small, and the findings did not change clinical management. On day 6, an invasive acetylcholine provocation test was performed to evaluate possible coronary spasm, which has been reported to be associated with SCAD; this test demonstrated positive spastic findings at the mid-LAD. Calcium-channel blockers were administered to prevent coronary spasm.
Figure 4.
Rendered 3-Dimensional Volume Image of Multidetector Computed Tomography and Iodine-123 Beta-Methyl Iodophenyl Pentadecanoic Acid Single-Photon Emission Computed Tomography Fusion Imaging
(A) Apical ballooning and abrupt caliber change noted in the distal left anterior descending artery (arrow). (B and C) Iodine-123 beta-methyl iodophenyl pentadecanoic acid single-photon emission computed tomography and computed tomography fusion imaging revealing coincidence of the proximal end of the coronary artery dissection (arrows) and reduced uptake region.
Discussion
SCAD rarely causes acute coronary syndrome and is often found in young women with relatively few arteriosclerotic risk factors. Fibromuscular dysplasia, collagen tissue defects, and chronic inflammatory systemic diseases have been related to SCAD. Proposed precipitating factors include hormonal effects, pregnancy, and coronary spasm (1). Although CAG is the gold standard for SCAD diagnosis (2), IVUS and optical coherence tomography are important for the definitive diagnosis and detailed evaluation of this condition. SCADs are classified into 3 subtypes according to CAG findings; on the basis of the patient’s CAG and IVUS, the present case was classified as type 2.
Conversely, TC often shows clinical symptoms similar to those of acute coronary syndrome and manifests with transient local wall motion abnormalities mainly in the apex without obstructive coronary artery lesions; these findings do not correspond with the perfusion territory of a single coronary artery (3). To date, several reports have been published on the coexistence of SCAD and TC. The culprit lesions of SCAD are more frequently visible in the LAD, including the septal and diagonal branches, than in the left circumflex and right coronary arteries (4,5). TC-like apical ballooning that is unexplained by the LAD; that does not wrap around the cardiac apex, diagonal branch, and left circumflex arteries, and that resembles other perfusion areas can be considered coexistent SCAD and TC. This patient had SCAD that was associated with the LAD and that did not wrap around the cardiac apex. These findings and the patient’s emotional stress suggested that SCAD and TC coexisted in this patient.
Multiple hypotheses explaining the causal link between SCAD and TC have been proposed. First, acute myocardial ischemia secondary to SCAD induces post-ischemic myocardial stunning, and physical stress associated with clinical symptoms, such as acute chest pain, and myocardial infarction resulting from SCAD may result in TC. Second, some common precipitating factors and similarities in clinical courses exist; these can lead to both SCAD and TC. Third, when TC precedes the other conditions, the mechanical stress in the hinge point between the basal hyperkinesia and the middle to apical akinesia may induce SCAD (6).
MB is a congenital anomaly in which part of the coronary artery, typically on the epicardial side, tunnels into the myocardium. Coexisting cases of MB and SCAD have been reported. A previous report summarized 5 case reports and a cohort study (7). Similar to the association between SCAD and TC, several possible causal links have been proposed between MB and SCAD, such as endothelial-intimal injury associated with vasospasm and blood flow dynamics.
Although the causal link among SCAD, TC, and MB is difficult to confirm, we propose the following hypothesis on the basis of the multimodal imaging findings of this case. SPECT and MDCT fusion imaging and CAG findings indicated that the hinge point between the hyperkinesia and akinesia segments and the distal end of the MB was a coincidence and that the SCAD started from that point. Furthermore, the acetylcholine provocation test result was positive. These findings suggest that the mechanical and functional stresses in the hinge point caused by wall motion abnormalities and progression of endothelial-intimal injury associated with vasospasm and pressure overload could have become triggers, which may have induced SCAD.
Follow-Up
The patient was discharged on day 7. She is receiving medications, and her further clinical course has been uneventful.
Conclusions
To our knowledge, this is the first report of a patient with concurrent SCAD, TC, and MB detected by multimodality imaging. No unified view of the causal link among these diseases exists to date. Therefore, multimodality imaging with SPECT, MDCT, and IVUS may be useful for elucidating the relationships among SCAD, TC, and MB.
Funding Support and Author Disclosures
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Acknowledgments
The authors gratefully acknowledge the work of the past and present members of Fukuyama Cardiovascular Hospital. The authors confirm that written consent for submission and publication of this case report, including images and the associated text, has been obtained from the patient in line with COPE guidance.
Footnotes
Nupoor Narula, MD, served as the Guest Associate Editor for this paper.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental videos, please see the online version of this article.
Appendix
Preprocedural Coronary Angiography. Coronary angiography revealed slight squeezing, with a myocardial bridge in the mid-left anterior descending artery and distal left anterior descending coronary artery dissection.
Left Ventriculography. Left ventriculography showed middle to apical akinesis and basal hyperkinesis.
References
- 1.Garcia-Guimarães M., Bastante T., Antuña P. Spontaneous coronary artery dissection: mechanisms, diagnosis and management. Eur Cardiol. 2020;26:1–8. doi: 10.15420/ecr.2019.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saw J. Coronary angiogram classification of spontaneous coronary artery dissection. Catheter Cardiovasc Interv. 2014;84:1115–1122. doi: 10.1002/ccd.25293. [DOI] [PubMed] [Google Scholar]
- 3.Madhavan M., Prasad A. Proposed Mayo Clinic criteria for the diagnosis of tako-tsubo cardiomyopathy and long-term prognosis. Herz. 2010;35:240–244. doi: 10.1007/s00059-010-3339-x. [DOI] [PubMed] [Google Scholar]
- 4.Chou A.Y., Sedlak T., Aymong E. Spontaneous coronary artery dissection misdiagnosed as takotsubo cardiomyopathy: a case series. Can J Cardiol. 2015;31:1073.e5–1073.e8. doi: 10.1016/j.cjca.2015.03.018. [DOI] [PubMed] [Google Scholar]
- 5.Ghafoor H.U., Bose A., El-Meligy A., Hannah J. A case report of recurrent spontaneous coronary artery dissection and takotsubo cardiomyopathy: a treatment dilemma. Eur Heart J Case Rep. 2020;4:1–6. doi: 10.1093/ehjcr/ytaa004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Y-Hassan S. Spontaneous coronary artery dissection and takotsubo syndrome: an often overlooked association; review. Cardiovasc Revasc Med. 2018;19:717–723. doi: 10.1016/j.carrev.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 7.Tajrishi F.Z., Ahmad A., Jamil A. Spontaneous coronary artery dissection and associated myocardial bridging: current evidence from cohort study and case reports. Med Hypotheses. 2019;128:50–53. doi: 10.1016/j.mehy.2019.05.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Preprocedural Coronary Angiography. Coronary angiography revealed slight squeezing, with a myocardial bridge in the mid-left anterior descending artery and distal left anterior descending coronary artery dissection.
Left Ventriculography. Left ventriculography showed middle to apical akinesis and basal hyperkinesis.